Abstract
Background
There is a paucity of evidence to support safe and effective management of patients with acute severe ulcerative colitis during the COVID-19 pandemic. We sought to identify alterations to established conventional evidence-based management of acute severe ulcerative colitis during the early COVID-19 pandemic, the effect on outcomes, and any associations with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe COVID-19 outcomes.
Methods
The PROTECT-ASUC study was a multicentre, observational, case-control study in 60 acute secondary care hospitals throughout the UK. We included adults (≥18 years) with either ulcerative colitis or inflammatory bowel disease unclassified, who presented with acute severe ulcerative colitis and fulfilled the Truelove and Witts criteria. Cases and controls were identified as either admitted or managed in emergency ambulatory care settings between March 1, 2020, and June 30, 2020 (COVID-19 pandemic period cohort), or between Jan 1, 2019, and June 30, 2019 (historical control cohort), respectively. The primary outcome was the proportion of patients with acute severe ulcerative colitis receiving rescue therapy (including primary induction) or colectomy. The study is registered with ClinicalTrials.gov, NCT04411784.
Findings
We included 782 patients (398 in the pandemic period cohort and 384 in the historical control cohort) who met the Truelove and Witts criteria for acute severe ulcerative colitis. The proportion of patients receiving rescue therapy (including primary induction) or surgery was higher during the pandemic period than in the historical period (217 [55%] of 393 patients vs 159 [42%] of 380 patients; p=0·00024) and the time to rescue therapy was shorter in the pandemic cohort than in the historical cohort (p=0·0026). This difference was driven by a greater use of rescue and primary induction therapies with biologicals, ciclosporin, or tofacitinib in the COVID-19 pandemic period cohort than in the historical control period cohort (177 [46%] of 387 patients in the COVID-19 cohort vs 134 [36%] of 373 patients in the historical cohort; p=0·0064). During the pandemic, more patients received ambulatory (outpatient) intravenous steroids (51 [13%] of 385 patients vs 19 [5%] of 360 patients; p=0·00023). Fewer patients received thiopurines (29 [7%] of 398 patients vs 46 [12%] of 384; p=0·029) and 5-aminosalicylic acids (67 [17%] of 398 patients vs 98 [26%] of 384; p=0·0037) during the pandemic than in the historical control period. Colectomy rates were similar between the pandemic and historical control groups (64 [16%] of 389 vs 50 [13%] of 375; p=0·26); however, laparoscopic surgery was less frequently performed during the pandemic period (34 [53%] of 64] vs 38 [76%] of 50; p=0·018). Five (2%) of 253 patients tested positive for SARS-CoV-2 during hospital treatment. Two (2%) of 103 patients re-tested for SARS-CoV-2 during the 3-month follow-up were positive 5 days and 12 days, respectively, after discharge from index admission. Both recovered without serious outcomes.
Interpretation
The COVID-19 pandemic altered practice patterns of gastroenterologists and colorectal surgeons in the management of acute severe ulcerative colitis but was associated with similar outcomes to a historical cohort. Despite continued use of high-dose corticosteroids and biologicals, the incidence of COVID-19 within 3 months was low and not associated with adverse COVID-19 outcomes.
Funding
None.
Introduction
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has challenged conventional treatment strategies for inflammatory bowel disease (IBD), including management of patients with acute severe ulcerative colitis. Acute severe ulcerative colitis is most commonly defined by the Truelove and Witts criteria,1 which combines the frequency of bloody stools (≥6 per day) with markers of systemic toxicity. Around 20–30% of patients with ulcerative colitis require admission to hospital at some point during their disease course for an acute severe flare,2, 3 and before the COVID-19 pandemic acute severe ulcerative colitis was associated with a mortality of 1·0–2·9%.2, 4
Research in context.
Evidence before this study
Expert consensus exercises have indicated a paucity of evidence to support safe and effective management of acute severe ulcerative colitis during the COVID-19 pandemic. Based on the immunomodulatory properties of standard treatments for acute severe ulcerative colitis, there are theoretical concerns around the vulnerability of patients to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and poorer outcomes from COVID-19. Potential risk factors relevant to acute severe ulcerative colitis included severely active mucosal inflammation, nosocomial infection, and the use of corticosteroids and immunosuppressants. We searched PubMed from Dec 1, 2019, to Jan 23, 2021, with the terms “acute severe ulcerative colitis” AND (“SARS-CoV-2” OR “COVID-19”), with no language restrictions.
Added value of this study
To our knowledge, we report one of the largest series of patients diagnosed with acute severe ulcerative colitis to date. This cohort will have relevance to the contemporary management of ulcerative colitis beyond the COVID-19 pandemic period. During the first COVID-19 pandemic wave there were adaptations to acute severe ulcerative colitis practice, including use of ambulatory pathways, greater use of rescue therapy, and reduced use of laparoscopic surgery. Outcomes from medical and surgical management of acute severe ulcerative colitis during the first wave of the COVID-19 pandemic were similar to a pre-pandemic control cohort. There was low incidence of COVID-19 in hospitalised and ambulant patients with acute severe ulcerative colitis treated with steroids with or without biologics or small molecules during the acute episode and up to 90 days from acute severe ulcerative colitis diagnosis. In this group of patients with a high inflammatory burden treated with powerful immunosuppression, no severe COVID-19 outcomes were observed.
Implications of all the available evidence
Our data provide reassurance for clinicians during subsequent waves of the COVID-19 pandemic regarding the conventional management of patients with acute severe ulcerative colitis using immune modifying drugs, including use of intravenous corticosteroids and rescue therapies. Adaptations to care pathways and the effect of SARS-CoV-2 have not been detrimental to overall patient outcomes in acute severe ulcerative colitis and support the shaping of future care pathways in subsequent waves of the pandemic. Prospective studies are recommended to embed current and innovative changes to care pathways during the pandemic and to determine pathway use in acute severe ulcerative colitis treatment during the post-pandemic period.
Data from small cohorts during the first wave of the COVID-19 pandemic suggested that disease activity might be a predictor for adverse COVID-19 outcomes in patients with IBD.5, 6 Despite this, clinicians might have used a higher clinical threshold to determine which patients required emergency hospital admission because of concerns regarding nosocomial spread of SARS-CoV-2,7 particularly in those thought to be most vulnerable to severe COVID-19 outcomes, in whom shielding and isolation was recommended. These concerns were shared by patients, whose reluctance for treatment in hospital might have led to failure to attend for infusions and delayed presentation even with severe IBD symptoms.8, 9
Pandemic-related challenges persisted after presentation to secondary care with acute severe ulcerative colitis. Recommended early endoscopic mucosal assessment might have been affected by uncertainty and delays regarding potential viral shedding in faeces, pre-endoscopic viral screening, availability of personal protective equipment, endoscopic capacity, and staffing shortages.10, 11
Conflicting evidence concerning the effect of high-dose steroids in SARS-CoV-2 infection and COVID-1912, 13 challenged conventional steroid treatment dosing strategies. Data to inform discussions and decisions regarding the risk to benefit ratio of drugs used as rescue therapy, such as infliximab and ciclosporin, in the pandemic era, are still emerging.13, 14 Furthermore, early evidence from the pandemic showed that contracting COVID-19 in the peri-operative period increased mortality substantially, and this might subsequently have encouraged surgeons to set higher thresholds for considering colectomy15 and debate the role of laparoscopic surgery.16
Many of the current guidance documents relating to IBD care during the COVID-19 pandemic, including acute severe ulcerative colitis, are based on expert consensus supported by few, if any, published data.17, 18 The impact of potential changes to conventional management pathways for acute severe ulcerative colitis outcomes is uncertain. A RAND consensus panel from the British Society of Gastroenterology issued an expert consensus19 pending evidence, acknowledging that there are considerable areas of uncertainty in relation to risk stratifying and managing patients with acute severe ulcerative colitis in the era of the COVID-19 pandemic. The panel also suggested that this might contribute to variability in practice patterns and differences in patient outcomes.
The aim of this study was to identify alterations to established conventional evidence-based management of acute severe ulcerative colitis as a consequence of the first wave of the COVID-19 pandemic in the UK, and to evaluate the effect on patient outcomes and COVID-19 acquisition and severity.
Methods
Study cohorts
The COVID-19 pandemic response of assessment, endoscopy, and treatment in acute severe ulcerative colitis (PROTECT-ASUC) study was a multicentre, observational, case-control study in 60 acute secondary care hospitals throughout the UK. We included adult patients (≥18 years) with either ulcerative colitis or IBD unclassified presenting with acute severe ulcerative colitis who fulfilled the Truelove and Witts criteria.1
Cases and controls were identified as either admitted or managed in emergency ambulatory care settings between March 1, 2020, and June 30, 2020 (COVID-19 pandemic period cohort), or between Jan 1, 2019, and June 30, 2019 (historical control cohort), respectively. Sites were asked to identify consecutive patients. Patients with Crohn's disease, infective colitis, cytomegalovirus, or Clostridioides difficile infections were excluded. Patients were identified from the participating site admission clinical records and IBD databases.
This study was registered with research governance teams at all hospital sites to approve access to patient records. The study was approved by Leeds and Bradford ethics committee (Integrated Research Approval System no 284030, Research Ethics Committee reference 20/HRA/2578). As no additional study procedures were done, the need for written informed consent was waived by the ethics committee. The clinical protocol is available online.
Data collection
We collected baseline clinical information including demographics (age, sex, ethnicity, body-mass index, and smoking status), disease characteristics (disease duration, disease extent, and previous treatments, including steroid, immunomodulatory, and biological therapies), disease severity markers (C-reactive protein, serum albumin, haemoglobin, C-reactive protein to albumin ratio, and endoscopic severity), and testing for SARS-CoV-2.
After diagnosis of acute severe ulcerative colitis, details of steroid therapy, including preparation, dose duration, and clinical setting where instituted and continued (ambulatory outpatient care or inpatient), need for, and drug(s) prescribed as, rescue therapy, as well as need for emergency colectomy during index admission were recorded. Data on therapies, including 5-aminosalicylic acids (5-ASAs), steroids, and immunomodulators either discontinued or initiated during the episode of acute severe ulcerative colitis were recorded. Follow-up data were collected at 3 months, with day of initial admission marked as day 0, and included clinical and biomarker remission status of IBD (where available), change in therapy during follow-up, and need for colectomy.
COVID-19 diagnoses at the point of acute severe ulcerative colitis diagnosis, nosocomial development of COVID-19, and COVID-19 acquisition between hospital discharge and 3-month follow up were recorded, including whether a diagnosis was based on symptoms, SARS-CoV-2 serology, or quantitative PCR.
All clinical data were collected, pseudo-anonymised, and entered into a secure central REDCap server hosted at the Royal Devon and Exeter NHS Foundation Trust (Exeter, UK).
Outcomes
The primary outcome was the proportion of patients with acute severe ulcerative colitis receiving rescue therapy (including primary induction) or colectomy. Secondary outcome measures, both during an acute severe ulcerative colitis episode and at 3-month follow up, were time to rescue therapy or surgery, time to colectomy, new drugs before hospital discharge, length of hospital admission, death during acute severe ulcerative colitis episode, adverse events (post-operative complications and mortality), positive PCR for SARS-CoV-2, and serious outcomes from COVID-19 (defined as the need for mechanical ventilation, intensive care unit treatment, or death).
Statistical analysis
The study was analysed and reported according to STROBE methodology20 and SAMPL.21 Non-parametric data were summarised as medians and IQRs and differences between current pandemic cohort cases and historic cohort controls were analysed using the Mann-Whitney U test. Categorical variables were summarised as proportions and analysed by Fisher's exact test or χ2 test as appropriate, first, for initial outcomes after acute severe ulcerative colitis and second, for 3-month follow-up data.
Kaplan-Meier survival curves were plotted for rescue therapy or colectomy and colectomy rates in the cases and controls in the first 30 days after diagnosis of acute severe ulcerative colitis. We used a combined outcome of rescue therapy or colectomy in preference of rescue therapy alone to avoid the incorrect assignment of patients who went straight to surgery as having survived without rescue therapy, when no such therapy would be possible. All tests were two-sided and p values of less than 0·05 were considered to indicate a significant difference, with no correction made for multiple tests.
Clinically plausible and previously reported markers of disease severity22 in acute severe colitis (stool frequency, C-reactive protein, haemoglobin, albumin, and C-reactive protein to albumin ratio) were selected for univariable and multivariable logistic regression models for the primary outcome of interest: rescue therapy (including primary induction) or surgery. In the multivariable analysis, we present all terms without a reductive model as our intention was to establish if case-control status influenced outcome independently of markers for disease severity. These findings informed a sensitivity analysis using complete cases and after propensity score matching using the MatchIt package.23 The covariates included were day 0 stool frequency, log(C-reactive protein), and albumin.
The study is registered with ClinicalTrials.gov, NCT04411784. Analyses were done using R version 4.0.2 and the survival package.
Role of the funding source
There was no funding source for this study. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Results
834 consecutive patients fulfilling the criteria for acute severe ulcerative colitis were submitted by 60 UK centres. 52 patients were excluded from the final analysis, one patient with COVID-19 at baseline, six patients who were admitted to hospital outside the specified periods, 19 patients who received neither intravenous steroids nor rescue therapy, ten patients with acute cytomegalovirus colitis, and 16 patients with C difficile associated diarrhoea. Data from 782 patients were included in the final analysis (398 COVID-19 pandemic cases and 384 historical controls; appendix p 11).
The baseline demographic and disease characteristics of the two cohorts were similar (table 1 ). At the time of presentation with acute severe ulcerative colitis, a higher proportion of patients during the COVID-19 period were receiving oral steroids, rectal steroids, and biological or small molecule therapies than were patients in the historical cohort (table 1). The median duration of oral steroids before meeting acute criteria was 14·0 days (IQR 7·0–28·2) for the COVID-19 cohort versus 13·5 days (7·0–25·0) for the historical cohort (p=0·21). We observed no difference in the use of oral or topical mesalazines or thiopurines between the two groups (table 1). Additionally, among patients receiving oral steroids, we observed no difference in the type of steroid (prednisolone vs poorly bioavailable steroid) used between the cohorts (table 1).
Table 1.
Summary of baseline characteristics and therapies
| COVID-19 pandemic period cohort (n=398) | Historical control cohort (n=384) | p value | |||
|---|---|---|---|---|---|
| Age (years)* | 38·0 (27·0–54·8) | 36·0 (26·0–52·0) | 0·12 | ||
| Sex | .. | .. | 0·83 | ||
| Male | 193 (48%) | 190 (49%) | .. | ||
| Female | 205 (52%) | 194 (51%) | .. | ||
| Body-mass index (kg/m2)† | 24·4 (21·9–27·4) | 24·4 (20·9–28·4) | 0·90 | ||
| Smoking status | .. | .. | 0·69 | ||
| Non-smoker | 212/312 (68%) | 186/283 (66%) | .. | ||
| Ex-smoker | 78/312 (25%) | 72/283 (25%) | .. | ||
| Current smoker | 22/312 (7%) | 25/283 (9%) | .. | ||
| Ethnicity | .. | .. | 0·18 | ||
| White | 294/363 (81%) | 253/323 (78%) | .. | ||
| Asian | 42/363 (12%) | 44/323 (14%) | .. | ||
| Black | 14/363 (4%) | 12/323 (4%) | .. | ||
| Arab | 4/363 (1%) | 4/323 (1%) | .. | ||
| Mixed | 1/363 (<1%) | 7/323 (2%) | .. | ||
| Other | 8/363 (2%) | 3/323 (10%) | .. | ||
| Comorbidities | |||||
| Hypertension | 40 (10%) | 37 (10%) | 0·90 | ||
| Diabetes | 26 (7%) | 30 (8%) | 0·49 | ||
| Cardiovascular disease‡ | 22 (6%) | 25 (7%) | 0·65 | ||
| Chronic kidney disease | 3 (1%) | 5 (1%) | 0·50 | ||
| Chronic obstructive pulmonary disease | 12 (3%) | 5 (1%) | 0·14 | ||
| Asthma | 35 (9%) | 32 (8%) | 0·90 | ||
| Chronic liver disease§ | 3 (1%) | 3 (1%) | 1·0 | ||
| Current malignancy | 4 (1%) | 3 (1%) | 1·0 | ||
| Solid organ transplant | 0 | 1 (<1%) | 1·0 | ||
| Stroke | 5 (1%) | 2 (1%) | 0·45 | ||
| Number of comorbidities | .. | .. | 0·81 | ||
| 0 | 279 (70%) | 277 (72%) | .. | ||
| 1 | 85 (21%) | 72 (19%) | .. | ||
| 2 | 21 (5%) | 23 (6%) | .. | ||
| >2 | 13 (3%) | 12 (3%) | .. | ||
| Time since diagnosis (years)¶ | 1·0 (0·0–5·0) | 2·0 (0·0–6·0) | 0·14 | ||
| IBD subtype | .. | .. | 1·0 | ||
| Ulcerative colitis | 18 (5%) | 18 (5%) | .. | ||
| IBD unclassified | 380 (95%) | 366 (95%) | .. | ||
| Disease extent | .. | .. | 0·57 | ||
| Proctitis | 25/338 (7%) | 33/371 (9%) | .. | ||
| Left-sided colitis | 169/338 (50%) | 172/371 (46%) | .. | ||
| Extensive colitis | 144/338 (43%) | 166/371 (45%) | .. | ||
| Therapies before acute severe ulcerative colitis | |||||
| No treatment | 105 (26%) | 114 (30%) | 0·34 | ||
| Oral mesalazine | 200 (50%) | 191 (50%) | 0·94 | ||
| Rectal mesalazine | 55 (14%) | 49 (13%) | 0·67 | ||
| Rectal steroids | 19 (5%) | 8 (2%) | 0·049 | ||
| Any oral steroid | 148/382 (39%) | 95/368 (26%) | 0·0015 | ||
| Type of oral steroid | .. | .. | 0·17 | ||
| Poorly bioavailable corticosteroids‖ | 23/147 (16%) | 8/92 (9%) | .. | ||
| Prednisolone | 124/147 (84%) | 84/92 (91%) | .. | ||
| Thiopurines** | 65 (16%) | 56 (15%) | 0·55 | ||
| All biologicals or small molecules†† | 107 (27%) | 70 (18%) | 0·0047 | ||
| Anti-tumour necrosis factor drugs‡‡ | 65 (16%) | 48 (13%) | 0·15 | ||
| Vedolizumab | 27 (7%) | 17 (4%) | 0·16 | ||
| Ustekinumab | 3 (1%) | 0 | 0·25 | ||
| Tofacitinib | 12 (3%) | 6 (2%) | 0·23 | ||
| Number of previous admissions with acute severe ulcerative colitis | .. | .. | 0·70 | ||
| 0 | 192/364 (53%) | 174/309 (56%) | .. | ||
| 1 | 92/364 (25%) | 78/309 (25%) | .. | ||
| 2 | 48/364 (13%) | 33/309 (11%) | .. | ||
| >2 | 32/364 (9%) | 24/309 (8%) | .. | ||
Data are median (IQR), n (%), or n/N (%), when N differs from the total in the table heading. p values were calculated by Fisher's exact test or Mann-Whitney U test for discrete and continuous variables, respectively. IBD=inflammatory bowel disease.
N=782 (398 cases and 384 controls).
N=436 (236 cases and 200 controls).
Coronary artery disease, heart failure, and arrythmia.
Primary sclerosing cholangitis, non-alcoholic fatty liver disease, and cirrhosis.
N=739 (379 cases and 360 controls).
Beclometasone dipropionate and budesonide.
Azathioprine, mercaptopurine, or tioguanine.
Infliximab, adalimumab, golimumab, vedolizumab, tofacitinib, or ustekinumab.
Infliximab, adalimumab, and golimumab.
We observed no difference at day 1, day 3, or day 5 in any established markers of acute severe ulcerative colitis severity (stool frequency, C-reactive protein, haemoglobin, albumin, C-reactive protein to albumin ratio) between the two cohorts, with the exception of serum albumin levels, which were lower in the COVID-19 pandemic period cohort (appendix p 6).
A greater proportion of patients in the COVID-19 pandemic cohort than patients in the historical control cohort were managed initially on an ambulatory pathway (51 [13%] of 385 patients vs 19 [5%] of 360 patients; p=0·00023; table 2 ). However, 43 (84%) of 51 ambulatory patients in the COVID-19 cohort required inpatient admission, compared with 18 (95%) of 19 ambulatory patients in the historical cohort (p=0·43). Patients were less likely to present emergently to the accident and emergency department in the COVID-19 period compared with the historical cohort (table 2).
Table 2.
Hospital care pathways for acute severe ulcerative colitis
| COVID-19 pandemic period cohort (n=398) | Historical control cohort (n=384) | p value | ||
|---|---|---|---|---|
| Patient initially managed on an ambulatory pathway* for intravenous steroids | 51/385 (13%) | 19/360 (5%) | 0·00023 | |
| Attended accident and emergency department with acute severe ulcerative colitis | 295/394 (75%) | 322/381 (85%) | 0·00095 | |
| Ward patient first managed when diagnosed with acute severe ulcerative colitis | .. | .. | 0·42† | |
| Dedicated gastrointestinal ward | 200/380 (53%) | 212/378 (56%) | .. | |
| Non-gastrointestinal ward | 148/380 (39%) | 166/378 (44%) | .. | |
| Gastrointestinal ward converted to general medicine during COVID-19 period | 32/380 (8%) | .. | .. | |
| Reviewed by consultant gastroenterologist within 24 h of admission to hospital | 314/389 (81%) | 287/372 (77%) | 0·25 | |
| Clinician responsible for patient after first 24 h | .. | .. | 0·35‡ | |
| IBD specialist | 238/390 (61%) | 216/376 (57%) | .. | |
| Non-IBD gastroenterologist | 94/390 (24%) | 94/376 (25%) | .. | |
| Non-gastroenterology physician | 41/390 (11%) | 54/376 (14%) | .. | |
| Colorectal surgeon | 15/390 (4%) | 9/376 (2%) | .. | |
| Other general surgeon | 2/390 (1%) | 3/376 (1%) | .. | |
| Patient discussed at IBD multidisciplinary team meeting | 150/393 (38%) | 140/366 (38%) | 1·0 | |
Data are n/N (%). IBD=inflammatory bowel disease.
Daily outpatient visits for intravenous steroids instead of admission to hospital.
p value for comparison of gastrointestinal versus non-gastrointestinal ward.
p value for Fisher's exact test comparison of clinician responsible for patient after first 24 h in hospital.
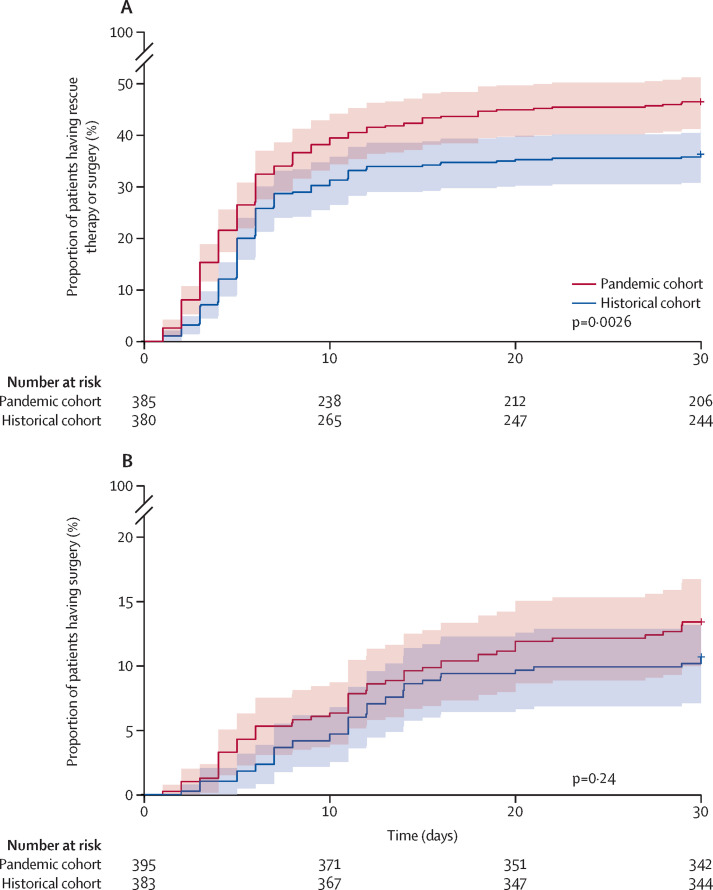
The proportion of patients receiving rescue therapy (including primary induction) or surgery was higher during the pandemic period compared with the historical period (217 [55%] of 393 patients vs 159 [42%] of 380 patients; p=0·00024; table 3 ; appendix p 12). This difference was driven by a greater use of rescue and primary induction therapies with biologicals (anti-tumour necrosis factor [TNF], anti-IL12/23, and anti-α4β7 integrin), ciclosporin, or tofacitinib in the COVID-19 pandemic period cohort than in the historical control period cohort (177 [46%] of 387 patients in the COVID-19 cohort vs 134 [36%] of 373 patients in the historical cohort; p=0·0064). By contrast, we found no difference in the requirement for emergency surgery between the cohorts (64 [16%] of 389 patients in the COVID-19 cohort vs 50 [13%] of 375 patients in the historical cohort; p=0·26).
Table 3.
Primary medical, surgical, intensive care unit, and mortality outcomes
| COVID-19 pandemic period cohort (n=398) | Historical control cohort (n=384) | p value | ||
|---|---|---|---|---|
| Primary endpoint | ||||
| Rescue (including primary induction) or surgery | 217/393 (55%) | 159/380 (42%) | 0·00024 | |
| Medical therapy outcomes | ||||
| Received intravenous steroids | 380/393 (97%) | 369/375 (98%) | 0·16 | |
| Responded to intravenous steroids | 264/384 (69%) | 282/377 (75%) | 0·065 | |
| Received rescue or primary induction therapy | 177/387 (46%) | 134/373 (36%) | 0·0064 | |
| Responded to rescue therapy | 139/171 (81%) | 105/132 (80%) | 0·77 | |
| Surgical outcomes | ||||
| Required emergency surgery for acute severe ulcerative colitis | 64/389 (16%) | 50/375 (13%) | 0·26 | |
| Surgery type | .. | .. | 0·50 | |
| Subtotal colectomy | 62/64 (97%) | 52/52 (100%) | .. | |
| Diversion | 2/64 (3%) | 0 | .. | |
| Surgery method | .. | .. | 0·018 | |
| Open | 30/64 (47%) | 12/50 (24%) | .. | |
| Laparoscopic | 34/64 (53%) | 38/50 (76%) | .. | |
| Post-operative complications | 22/59 (37%) | 14/48 (29%) | 0·42 | |
| Acute severe ulcerative colitis outcomes | ||||
| Length of stay (days)* | 7·0 (5·0–13·0) | 7·0 (5·0–12·0) | 0·99 | |
| Intensive care unit admissions† | 12/382 (3%) | 11/380 (3%) | 0·32 | |
| Invasive ventilation | 3 (1%) | 3 (1%) | 1·0 | |
| Non-invasive ventilation | 2/384 (1%) | 4/380 (1%) | 0·45 | |
| Extracorporeal membrane oxygenation | 0 | 0 | .. | |
| Death | 5/392 (1%) | 5/379 (1%) | 1·0 | |
| Composite intensive care unit, non-invasive ventilation, death, and extracorporeal membrane oxygenation | 17 (4%) | 14 (4%) | · | |
Data are n/N (%), median (IQR), or n (%).
N=673.
Not including planned post-operative intensive care unit admission.
The response to intravenous corticosteroid in the pandemic period cohort did not differ from that of the historical cohort (264 [69%] of 384 patients in the COVID-19 cohort vs 282 [75%] of 377 patients in the historical cohort; p=0·065). In patients requiring rescue or induction therapies, the choice of agents differed between the two cohorts, with greater use of non-infliximab and non-ciclosporin based treatments (42 [24%] of 177 patients in the COVID-19 cohort vs 17 [13%] of 134 in the historical cohort; p=0·019) in the pandemic period cohort (table 4 ). Time to rescue therapy or surgery was shorter in the pandemic cohort compared with the historical cohort (p=0·0026; figure 1A ). Rescue therapy or surgery happened both at a higher rate and more quickly during the COVID-19 pandemic compared with the historical control period. The overall response to rescue therapy was similar within the two cohorts (table 4). High first infliximab dose (10 mg/kg) induction loading was used in 27 (23%) of 115 patients in the COVID-19 period cohort and 17 (18%) of 92 patients in the historical control cohort, and an accelerated infliximab dosing schedule with a second dose administered before discharge was used in 23 (19%) of 120 patients in the COVID-19 cohort and 24 (23%) of 104 patients in the historical cohort (table 4).
Table 4.
Treatments during hospitalisation and before discharge
| COVID-19 pandemic period cohort (n=398) | Historical control cohort (n=384) | p value | ||
|---|---|---|---|---|
| Any rescue therapy | 177/387 (46%) | 134/373 (36%) | 0·0064 | |
| Infliximab | 124/176 (70%) | 106/133 (80%) | 0·021* | |
| Adalimumab | 1/176 (1%) | 3/133 (2%) | .. | |
| Ciclosporin | 11/176 (6%) | 11/133 (8%) | .. | |
| Tofacitinib | 13/176 (7%) | 3/133 (2%) | .. | |
| Ustekinumab | 7/176 (4%) | 0 | .. | |
| Vedolizumab | 20/176 (11%) | 10/133 (8%) | .. | |
| Data not available | 1 | 1 | ||
| Dose of infliximab (mg/kg) | .. | .. | 0·40 | |
| 10 | 27/115 (23%) | 17/92 (18%) | .. | |
| 5 | 88/115 (77%) | 75/92 (82%) | .. | |
| Second dose of infliximab given before discharge | 23/120 (19%) | 24/104 (23%) | 0·51 | |
Data are n/N (%).
Comparison of which drug was given as rescue therapy in each cohort (ie, the proportion of patients receiving each type of rescue therapy was significantly different between the two groups).
Figure 1.
Time to initiation of rescue therapy or surgery for acute severe ulcerative colitis within the first 30 days (A) and time to surgery (B)
We observed no difference in time to surgery between the two cohorts (p=0·24; figure 1B). However, laparoscopic surgery was done less often in the pandemic period cohort than in the control cohort (table 3). We observed no difference between the two cohorts in the need for postoperative intensive care unit stay (18 [32%] of 57 patients in the COVID-19 cohort vs 14 [31%] of 45 patients in the historical cohort; p=1·0) nor in the overall complication rate (22 [37%] of 59 patients in the COVID-19 cohort vs 14 [29%] of 48 patients in the historical cohort; p=0·42) or specific complications (appendix p 7). Furthermore, we observed no difference in mortality between the two cohorts (five [1%] of 392 patients in the COVID-19 cohort vs five [1%] of 379 in the historical cohort; p=1·0)
In multivariable logistic regression analysis, the odds of rescue therapy or surgery were lower in the historical cohort than in the COVID-19 pandemic cohort (odds ratio [OR] 0·63, 95% CI 0·44–0·89; p=0·0083); this result was independent of day 1 biomarkers for disease severity including stool frequency, C-reactive protein, haemoglobin, albumin, and albumin to C-reactive protein ratio (appendix p 8). Therefore, to ascertain if cohort type influenced our primary and secondary endpoints, we did propensity score matching using stool frequency, C-reactive protein, haemoglobin, albumin, and albumin to C-reactive protein ratio. We found that results for our primary outcomes remained significant after matching for these variables. In the matched cohort of 281 cases and 266 controls, day 0 albumin was no longer different (p=0·080). In univariable logistic regression of the primary outcome in the matched cohort, the univariable OR was 0·62 (95% CI 0·44–0·87; p=0·006), favouring the historical cohort.
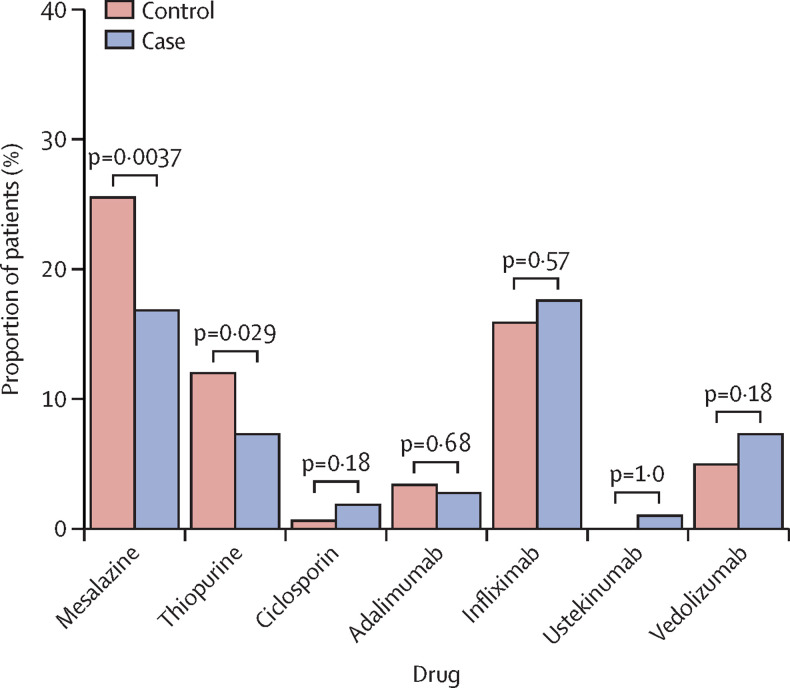
We observed differences in the type of new drugs initiated during hospital treatment and at discharge during the two periods. There were higher rates of initiation of biologicals and small molecules and lower rates of initiation of azathioprine and mesalazine (table 4; figure 2 ).
Figure 2.
Maintenance treatments started during treatment in hospital
3-month follow-up data were available for 697 patients (322 from the COVID-19 period and 375 from the control period; table 5 ). At 3 months, we found no difference in the proportion of cases or controls in symptomatic, biochemical, or endoscopic remission (table 5). We found no difference in the proportion of patients in the two cohorts who had a flare of their IBD (table 5). The proportions of patients in each cohort who were initiated on oral or topical mesalazines, oral steroids, and thiopurines did not differ (table 5). Furthermore, with regard to key acute severe ulcerative colitis outcome measures, the proportion of patients requiring readmission for active disease, intravenous steroids, and surgery was not significantly different between the two cohorts (table 5).
Table 5.
Changes to treatment at 3-month follow-up
| COVID-19 pandemic period cohort (n=322) | Historical control cohort (n=375) | p value | |||
|---|---|---|---|---|---|
| Patient disease status | |||||
| Symptomatic remission | 125/290 (45%) | 143/340 (42%) | 0·96 | ||
| Biochemical remission | 163/255 (64%) | 191/307 (62%) | 0·73 | ||
| Endoscopic remission | 15/45 (33%) | 25/81 (31%) | 0·85 | ||
| Flare in the past 3 months | 79/307 (26%) | 100/365 (27%) | 0·29 | ||
| New IBD therapies | |||||
| Oral mesalazine | 9 (3%) | 13 (3%) | 0·67 | ||
| Topical mesalazine | 13 (4%) | 23 (6%) | 0·23 | ||
| Topical steroids | 6 (2%) | 7 (2%) | 1·0 | ||
| Intravenous steroids | 19 (6%) | 23 (6%) | 1·0 | ||
| Oral steroid | 35 (11%) | 34 (9%) | 0·45 | ||
| Oral prednisolone | 31/35 (89%) | 32/34 (94%) | 0·67 | ||
| Poorly bioavailable corticosteroids* | 4/35 (11%) | 2/34 (6%) | .. | ||
| Thiopurine† monotherapy | 15 (5%) | 25 (7%) | 0·33 | ||
| Anti-TNF monotherapy | 17 (5%) | 27 (7%) | 0·35 | ||
| Anti-TNF and immunomodulator‡ | 7 (2%) | 10 (3%) | 0·81 | ||
| Vedolizumab | 19 (6%) | 17 (5%) | 0·49 | ||
| Ustekinumab | 2 (1%) | 1 (<1%) | 0·60 | ||
| Tofacitinib | 6 (2%) | 7 (2%) | 1·0 | ||
| Readmitted to hospital with active disease | 75/307 (24%) | 81/363 (22%) | 0·32 | ||
| Active IBD and COVID-19 symptoms | 4/79 (5%) | .. | .. | ||
| Active IBD and no COVID-19 symptoms | 71/79 (90%) | 81/81 (100%) | .. | ||
| COVID-19 symptoms and no active IBD | 4/79 (5%) | .. | .. | ||
| Surgery | 26/301 (9%) | 19/358 (5%) | 0·12 | ||
| Emergency surgery | 16/26 (62%) | 9/19 (47%) | 0·38 | ||
| Elective surgery | 10/26 (38%) | 10/19 (53%) | .. | ||
Data are n/N (%) or n (%). p values were calculated by Fisher's exact test or Mann-Whitney U test for discrete and continuous variables, respectively. IBD=inflammatory bowel disease. TNF=tumour necrosis factor.
Beclometasone dipropionate and budesonide.
Azathioprine, mercaptopurine, or tioguanine.
Thiopurine or methotrexate.
SARS-CoV-2 nasopharyngeal swab testing was done in 253 (64%) of 398 included patients. Five (2%) of 253 patients tested PCR-positive during their hospital visit for acute severe ulcerative colitis. There were no serious COVID-19 outcomes. 103 patients were re-tested for SARS-CoV-2 infection by PCR during the 3-month follow-up and two (2%) patients tested positive 5 days and 12 days, respectively, after discharge from index admission for acute severe ulcerative colitis. Both patients recovered without serious outcomes. Details of SARS-CoV-2 PCR-positive patients and therapies are included in the appendix (p 9).
Shielding data after discharge from hospital were available in 292 patients included in the pandemic period cohort (appendix p 10). 51 (17%) of 292 patients were confirmed to have shielded and 31 (11%) were confirmed not to have shielded. A further 102 (35%) patients were advised to shield but were not confirmed to have followed the advice.
Discussion
To our knowledge, we report one of the largest series of patients diagnosed with acute severe ulcerative colitis to date. This cohort will have relevance to the contemporary management of ulcerative colitis beyond the COVID-19 pandemic period. We identified adaptations to treatment pathways during the first pandemic wave relative to a 2019 pre-pandemic cohort in the UK. During the COVID-19 era, we observed a greater proportion of patients receiving rescue therapy (including primary induction) or surgery. This difference was driven by a greater use of rescue and primary induction therapies with biologicals, ciclosporin, or tofacitinib. We also observed a reduction in use of immunomodulators and 5-ASAs during both the acute episode and at the point of discharge. Our study identified increased use of ambulatory (outpatient) pathways for initial administration of intravenous steroids, although most of these patients were still admitted to hospital. We found that conventional use of corticosteroids during the early pandemic remained prevalent and was not associated with either an increased incidence of SARS-CoV-2 infection, nor with adverse outcomes in the small number of patients diagnosed with COVID-19. The incidence of surgery for acute severe ulcerative colitis was not higher during the pandemic. However, surgical practice for medically refractory patients during the pandemic was modified, with a reduction in laparoscopic colectomy rates. Reassuringly, the immediate and 3-month outcomes of acute severe ulcerative colitis during the pandemic were similar to the historical control cohort. Furthermore, during a 3-month follow-up period, we found no increase in risk of flares, readmissions to hospital, or colectomies and in the pandemic cohort, only two COVID-19 diagnoses occurred among 103 tested patients.
Consensus statements and expert opinion in the early stages of the COVID-19 pandemic cautioned against use of high-dose corticosteroids (≥20 mg of prednisolone per day) in patients with IBD because of concerns regarding adverse outcomes of COVID-19 infection.17, 18, 24 This caution was largely based on extrapolation and lessons from historical cohorts in previous coronavirus pandemics.25 Patients with IBD have a higher seasonal flu risk and corticosteroids are an independent risk factor.26 Steroids are also a risk factor for serious or opportunistic infection, particularly when combined with thiopurines.27, 28, 29 Conversely, both dexamethasone and hydrocortisone, which have potent immune modifying effects, have been shown to be beneficial in severe COVID-19, an infection characterised by an exaggerated systemic inflammatory response in some patients.12, 30
Steroids were reported to be a risk factor for adverse COVID-19 outcomes in the SECURE-IBD registry,13, 14 which includes physician-reported cases of COVID-19, and also in a small cohort from Italy,5 both of which hold potential for reporting bias and neither of which systematically controlled for disease activity. These reports have understandably led to concerns regarding the management of patients with acute severe ulcerative colitis, for whom intravenous high-dose corticosteroids remain the cornerstone of first-line management.19 However, despite intense immunosuppression, including use of intravenous and high-dose oral corticosteroids, we observed low numbers of patients in our study with concurrent SARS-CoV-2 infection during admission or during the 3-month follow-up period. In our study, there was no reduction in the use of intravenous steroids in patients with acute severe ulcerative colitis during the COVID-19 era compared with the historical cohort, and in longitudinal follow-up we found no increase in the risk of SARS-CoV-2 infection or serious adverse outcomes secondary to COVID-19 in this cohort.
There is increasing interest in the role of cytokine-directed therapies as a treatment for severe COVID-19 outside the IBD setting.31, 32 Furthermore, a report suggested low prevalence of SARS-CoV-2 seroconversion in patients with immune-mediated inflammatory diseases on cytokine therapies, including IBD.33 In the present study, we observed an overall increase in the use of rescue therapies during the COVID-19 pandemic, in particular, the use of Janus kinase inhibitors and biologicals. The reasons for this increase are likely to be multifactorial and could relate to delayed presentation and advanced disease,34 concerns regarding prolonged steroid use in early expert consensuses,18 and wider availability and physician confidence in use of newer biologicals over time. Initial SECURE-IBD registry data13 potentially supported the use of biologicals in patients with acute severe ulcerative colitis by showing an inverse association with risk of admission to hospital and death in patients with COVID-19 and IBD on anti-TNF monotherapy (adjusted OR 0·9, 95% CI 0·4–2·2). Furthermore, an update from the SECURE-IBD registry14 suggests increased risk of severe COVID-19 outcomes with thiopurine monotherapy and in combination with anti-TNFs. Consistent with concern regarding thiopurine use and susceptibility to viral infection, we observed lower azathioprine use during the pandemic study period. Additionally, and perhaps in response to the as yet unclear mechanisms underpinning the association with 5-ASA and severe COVID-19 outcomes in the SECURE-IBD registry,13, 14 we also witnessed lower use of 5-ASA at the point of discharge during the pandemic study period. In addition to these safety concerns, logistical issues such as infusion unit capacity and the need for regular blood monitoring during the pandemic35 might have had a role in the reduction in the use of thiopurines, combination therapy, and infusion-based biologicals.
There is increasing debate about the timing of rescue therapy in patients who are refractory to intravenous corticosteroids as current practice is guided by a criterion36 developed before the era of biologicals. Our study suggests increasing and more varied use of rescue therapy and primary induction agents, although we observed no difference in overall colectomy rates. The use of early risk stratification tools and their effect on guiding the timing of rescue therapy is being evaluated in an ongoing study (ELEVATE ASUC, NCT03907631).
Enhanced adherence to well publicised public health measures, including patient access to a self-risk identification tool18 in our cohort (see appendix p 10 for shielding data), might have reduced the risk of SARS-CoV-2 acquisition; nevertheless, our data provide some reassurance regarding the use of intravenous corticosteroids and induction doses of biologicals during subsequent waves of the pandemic. Although we did not systematically analyse the seroconversion rates in all patients in this study (the subject of the recently launched UK CLARITY IBD programme), the low rates in tested patients and low rates of serious COVID-19 outcomes in the 3-month longitudinal follow-up period is reassuring and in line with previous observations.37, 38 We will seek to extend this follow-up period in a forthcoming amendment to the existing study to capture longer-term COVID-19 risk, IBD outcomes, and surveillance for the emergence of so-called long COVID and IBD immunological phenomena.
In the non-COVID-19 setting, clinically active IBD is reported to be an independent risk factor for serious viral or opportunistic infections.28, 29 Our study does not support the assumption that inflamed mucosa in acute severe ulcerative colitis is associated with an increased risk of SARS-CoV-2 infection. Additionally, although the number of patients with COVID-19 was small in our cohort, our study does not support data from small cohorts that active IBD is a risk factor for serious COVID-19 outcomes.5, 6 The effect of disease activity in IBD on risk of COVID-19 acquisition in different IBD phenotypes is being evaluated in another study from our group (PREPARE-IBD).
Colectomy is required in up to 20% of patients with acute severe ulcerative colitis.3, 4 Emerging data from the COVIDSurg cohort15 indicate significant mortality in patients who acquire SARS-CoV-2 in the perioperative period, with an understandable increase in the threshold to undertake surgery. COVIDSurg has not reported outcomes in emergency surgery for patients with IBD. In the present study, we observed no difference in colectomy rates during the pandemic study period, and there were no new infections with SARS-CoV-2 in patients requiring colectomy. Additionally, we found no difference in mortality. We observed a reduction in the number of colectomies using a laparoscopic approach in the pandemic period, reflecting initial concerns of transmission risk to health-care professionals.16
We observed increased use of ambulatory patient pathways during the COVID-19 period compared with the historical control group. This could reflect concern regarding nosocomial transmission of COVID-19 during hospital admission, particularly in patients requiring surgery.15 More frequent use of ambulatory acute severe ulcerative colitis pathways, using single daily dose methylprednisolone with close monitoring by the specialist teams in day care centres or infusion units,39 in the COVID-19 cohort could mitigate this risk, but a large proportion of patients subsequently required hospital admission, therefore this practice should be further evaluated in randomised studies.
Our study has several strengths. To our knowledge, we report the largest cohort of patients with acute severe ulcerative colitis treated during the early COVID-19 pandemic worldwide. We collected detailed metadata on clinical and biochemical disease activity markers to assess association with COVID-19 outcomes and included a matched cohort of patients treated for acute severe ulcerative colitis before the pandemic onset. However, we acknowledge that our study also has several limitations in relation to study design. PROTECT-ASUC was retrospective, and although requested patient selection was consecutive, it is possible that not all patients with acute severe ulcerative colitis from each centre were captured, which might lead to selection bias. However, baseline patient and clinical disease phenotypic data, as well as disease severity indices apart from serum albumin levels and steroid intake, were all well matched, and therefore justify comparison across the two time periods. Furthermore, using univariable and multivariable analyses, we identified potential confounding factors associated with the need for rescue therapy or colectomy among the two cohorts. We used nearest neighbour matching to confirm that our principal findings remained significant. Although the proportion of patients on steroids before acute severe ulcerative colitis was similar in the two cohorts, we did not have data on the dose or duration of steroids. Only five (1%) of 385 patients in the pandemic cohort were diagnosed with SARS-CoV-2 infection. Although we did not identify any severe outcomes, larger cohorts to further investigate study associations are desirable. Due to the retrospective nature of this study, we acknowledge there are some missing data. Adverse events from rescue therapy, surgery, and post-operative infections might not have been captured if not systematically recorded in local electronic recorded data. Therefore, our results might underestimate the incidence of adverse events.
Despite theoretical concerns regarding acute severe ulcerative colitis treatments and risk of SARS-CoV-2 acquisition or severe COVID-19 outcomes, our data show two reassuring important conclusions. First, although there have been some adaptations to conventional management of patients during the pandemic, with regard to intravenous steroids, choice and frequency of biological and small molecule induction or rescue therapy, and surgical approaches to colectomy, these did not lead to different acute severe ulcerative colitis outcomes for patients. Second, use of cornerstone medications, such as high-dose intravenous steroids and biologicals, in acute severe ulcerative colitis appears to pose a low risk of nosocomial and post-discharge acquisition of SARS-CoV-2 and of developing severe COVID-19.
Additional large-scale prospective studies during the COVID-19 pandemic are recommended to confirm the low incidence of COVID-19 in this patient group and to further investigate COVID-19 outcomes. The challenges faced during the pandemic might also provide the impetus for more formal randomised trials to evaluate the safety and effectiveness of alternative acute severe ulcerative colitis treatment strategies, including the use of ambulatory pathways and non-conventional biological rescue therapy.
Data sharing
De-identified participant data will be made available to others for meta-analysis upon request following Study Steering Group discussion and signing of a data access agreement. Requests for access to data should be made to the corresponding author and the first authors via the corresponding email given.
Acknowledgments
Acknowledgments
We thank the clinical and research teams at the participating sites for identification of patients, data collection, and data entry. UK gastroenterology trainees and trainee networks (MaGNET—Mersey Gastroenterology Network, GLINT—Gastro London Investigative Network for Trainees, WMRIG—West Midlands Research In Gastroenterology, GasTRIN NoW—Gastroenterology Trainee Research and Improvement Network North-West, OxYGEN—The Oxford and Thames Valley Young Gastroenterologists Network, and TReNDD NI—Trainee Research Network in Digestive Diseases Northern Ireland) were integral in data collection for this study. CAL acknowledges support from the National Institute for Health Research Newcastle Biomedical Research Centre. We appreciate support from Crohn's & Colitis UK and the British Society of Gastroenterology for promotion of this study.
Contributors
All authors formed the study steering group. SSe was responsible for initial study design, which was further developed by the steering group. NAK led methodological development and all members of the steering group contributed to subsequent protocol development. SSe led regulatory approvals and study co-ordination. The PROTECT-ASUC study group and steering group were responsible for local site approvals, data acquisition, and data entry. NAK and GJW verified the data and led the statistical analysis, supported by all members of the steering group. SSe, GJW, and CAL led the writing group. SSe, GJW, NAK, and CAL verified the underlying data. All members of the steering group contributed to manuscript redrafting, editing, and review and approved the final version.
Declaration of interests
SSe reports grants from Biogen, Takeda, AbbVie, Tillotts Pharma, Ferring, and Biohit, advisory board fees from Takeda, AbbVie, Pharmacocosmos, Ferring, Falk Pharma, Cellgene, Tillots Pharma, Biohit, and Janssen, and personal speaker fees from AbbVie, Biogen, Janssen, Merck, Tillotts, and Falk Pharma, outside the submitted work. GJW reports personal fees from AbbVie, Falk, Janssen, and Norgine, during the conduct of the study. NAK reports personal fees from Dr Falk, Janssen, Takeda, and Tillots and grants and personal fees from Pharmacosmos, outside the submitted work. KVP reports personal fees and non-financial support from Janssen, AbbVie, Takeda, and Dr Falk and non-financial support from Ferring, outside the submitted work. SSu reports personal fees from Celltrion, Janssen, Takeda, Vifor Pharma, and Boehringer-Ingelheim, outside the submitted work. AJK reports personal fees from Pfizer, Celgene-BMS, Tillotts, and Janssen and personal fees and non-financial support from Dr Falk and Takeda, outside the submitted work. MJB reports grants from Vifor International and Tillotts Pharma, travel costs and meeting expenses from Tillotts Pharma, and personal fees from Vifor International, outside the submitted work. CAL reports grants from Genentech, AbbVie, Eli Lilly, Pfizer, Roche, UCB Biopharma, Sanofi Aventis, Biogen IDEC, Orion OYJ, and AstraZeneca, grants and personal fees from Janssen and Takeda, and personal fees from Ferring and Dr Falk Pharma, outside the submitted work. All other authors report no competing interests.
Contributor Information
PROTECT-ASUC Study Group:
Shukri Abdale, Abdullah Abbasi, Anwar Abusrewil, Precious Aghimien, Saeed Ahmed, Akram Ali, Amjad Ali, Jad Alkhoury, Patrick Allen, Ammar Al-Rifaie, Richard Appleby, Ramesh Arasaradnam, Naila Arebi, Bradley Arms-Williams, Muteeb Ashraf, Andrea Au, Tamar Avades, Homira Ayubi, Saleha Azhar, Samantha Baillie, Sharmili Balarajah, Aaron Bancil, Abdul Basit, Murad Bayati, Andrew Bell, Alexander Berry, Shivaram Bhat, Joya Bhattacharyya, Sophia Bishop, Laura Blackmore, Ashley Bond, Simon Borg-Bartolo, Emma Botwright, Sonia Bouri, Stephen Boyle, Neil Bradley, Fiona Brailsford, Deborah Britton, Caitlin Brown, Rhys Butcher, Jeffrey Butterworth, Rachel Campbell, Roisin Campbell, Iona Campbell, Ruth Carr, Josiah Carter, Peter Cartlidge, Rajiv Chandy, Kelly Chatten, Rakesh Chaudhary, Desmond Chee, Jonathan Cheesbrough, Antonia Churchhouse, Sara Chughtai, Jennie Clough, Alexander Cole, Johannah Cook, Rachel Cooney, Sarah Cotton, Archibald Coulter, Tamsin Critchlow, Frederic Cuison, Chris Curran, Ana-Maria Darie, Robin Dart, Pantong Davwar, Kasamu Kabiru Dawa, Anjan Dhar, Shahida Din, Kok Leong Diong, Benjamin Disney, Emma Dooks, Louise Downey, Anita D'Souza, Lovesh Dyall, Ali El Rida El Masri, Mary Elias, Holli Evans, Richard Felwick, Michael Finegan, Paul Flanagan, Rishi Fofaria, Steven Chung Ming Fong, Richard Fox, Aileen Fraser, Christian Frunza, Alhassan Ghodeif, Nivedita Ghosh, Leah Gilroy, Larissa Good, John Gordon, Nicola Grasso, Aurelién M Guéroult, James Gulliver, Sarah Guthrie, Markus Gwiggner, Mina Hanna, Christopher Harlow, Wendy Harrison, Ailsa Hart, Barney Hawthorne, Julie Henshaw, Rosaleen Herdman-Grant, Patricia Hooper, Willow Howard, Nasir Hussain, Thomas Hutton, Aye Mya Htun, Peter Irving, Reema Jagdish, Anum Javed, Asima Javed, Nishani Jayasooriya, Matthew Johnson, Emma Johnston, Gareth-Rhys Jones, Cynthia Kanagasundaram, Fotein Karagkouni, Karen Kemp, Cheryl Kemp, Hesham Khalil, Najeebullah Khan, Mais Khasawneh, Bilal Khurshid, Andrew King, Beverley Kirkham, Fiona Kirkham, Flora Kokwaro, Mohamed Korani, Ioannis Koumoutsos, Aditi Kumar, Anish John Kuriakose Kuzhiyanjal, Martyn Lakeland, Sophie Laverick, Charlie Lees, Emma Levell, Scott Levison, Samuel Lim, Yuen-Hui Lim, Jimmy Limdi, James Oliver Lindsay, Jessica Lisle, Alan Lobo, Raphael Luber, Laura Lucaciu, Holly Lyne, Jonathan MacDonald, Aarani Mahalingam, Sara Mahgoub, Ridhima Malakar, Fenella Marley, Joy Mason, Zia Mazhar, Hannah McCaughan, Tracy Naughton, Adam McCulloch, Stuart McIlwaine, Nirmol Meah, Leila Mebarek, Mike Mendall, Radharetnas Meiarasu, Nasir Mir, Tilly Mills, Jentus Milton, Victoria Moffat, Gordon W Moran, Liam Morris, Gary Morrison, Graham Morrison, Robert Mulligan, Charles Murray, Jennifer Murray, Mutwakil Musharaf, Sally Myers, Pineshwari Naeck-Boolauky, Andres Naranjo, Janardhan Navaratnam, Deanna Naylor, Emma Nixon, Kirsty Nixon, Hesam Ahmadi Nooredinvand, Uche Nosegbe, Olaolu Olabintan, Elaine Ong Ming San, Comfort Okpeh, Hayley Owen, Ruth Owen, Christopher Palmer-Jones, Kalyan Peddada, Mohammad Peerally, Rebecca Perkins, Frank Phillips, Keith Pohl, Richard Pollok, Nick Powell, Farah Qayyum, Maria Qurashi, Mohammed Nabil Quraishi, Elizabeth Ratcliffe, Shellie Radford, Sohail Rahmany, Hanin Ramadan, Arvind Ramadas, Anne Reddington, Tom Riley, Peter Rimmer, Susan Ritchie, Jacqueline Roscoe, Konstantina Rosiou, Siobhan Rowland, Joseph Sabine, Aamir Saifuddin, Mark Samaan, Priya Sarkar, Shahzad Sarwar, Ayodele Sasegbon, Jayne Saunders, Gregory Sebepos-Rogers, John Paul Seenan, Christian Selinger, Solange Serna, Sonika Sethi, Matthew Shale, Richard Shenderey, Achuth Shenoy, Yousuf Sherifat, Roosey Sheth, Spyros Siakavellas, Rafid Sikafi, Amar Singh, Salil Singh, Updesh Singh, Ganesh Sivaji, Philip Smith, R Alexander Speight, Andy Spence, Catherine Stansfield, Helen Steed, Kishaani Suseeharan, Maria Tabuso, Donatas Taucius, Joanne Taylor, Amit Thakor, Tony Tham, Gill Townsend, Tristan Townsend, Thomas Troth, Ruth Tunney, Kelly Turner, Nosheen Umar, Vithushan Vakeeswarasarma, Ajay M Verma, Hazel Wallace, Katharina Wallis, Hannah Walton, Bo Wang, Eleanor Warner, Callum Watson, Eleanor Watson, Susie Wen, Monika Widlak, Maureen Williams, Amy Woods, Lisa Younge, and Mansoor Zafar
Supplementary Material
References
- 1.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. BMJ. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitton A, Buie D, Enns R, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107:179–194. doi: 10.1038/ajg.2011.386. [DOI] [PubMed] [Google Scholar]
- 3.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(suppl 3):s1–106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch RW, Lowe D, Protheroe A, Driscoll R, Rhodes JM, Arnott ID. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther. 2013;38:935–945. doi: 10.1111/apt.12473. [DOI] [PubMed] [Google Scholar]
- 5.Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- 6.Lukin DJ, Kumar A, Hajifathalian K, et al. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology. 2020;159:1541–1544. doi: 10.1053/j.gastro.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Amico F, Rahier JF, Leone S, Peyrin-Biroulet L, Danese S. Views of patients with inflammatory bowel disease on the COVID-19 pandemic: a global survey. Lancet Gastroenterol Hepatol. 2020;5:631–632. doi: 10.1016/S2468-1253(20)30151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H-G, Xie R, Ma TH, Yang XZ. Excessive anxiety in IBD patients is unnecessary for COVID-19. Clin Res Hepatol Gastroenterol. 2020;44:e121–e122. doi: 10.1016/j.clinre.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo T, Liu Q, Zhang F, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-322294. published online July 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Occhipinti V, Saibeni S, Sampietro GM, Pastorelli L. Impact of COVID-19 outbreak on the management of patients with severe IBD: a domino effect. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.027. published online May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2020 doi: 10.1136/gutjnl-2020-322539. published online Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nepogodiev D, Bhangu A, Glasbey JC, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Simone B, Chouillard E, Di Savario S, et al. Emergency surgery during the COVID-19 pandemic: what you need to know for practice. Ann R Coll Surg Engl. 2020;102:323–332. doi: 10.1308/rcsann.2020.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allez M, Fleshner P, Geary R, Lakatos PL, Rubin DT. Care of the patient with IBD requiring hospitalization during the COVID-19 pandemic. J Crohns Colitis. 2020;14(suppl 3):S774–S779. doi: 10.1093/ecco-jcc/jjaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy NA, Jones GR, Lamb CA, et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69:984–990. doi: 10.1136/gutjnl-2020-321244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Din S, Kent A, Pollok RC, et al. Adaptations to the British Society of Gastroenterology guidelines on the management of acute severe UC in the context of the COVID-19 pandemic: a RAND appropriateness panel. Gut. 2020;69:1769–1777. doi: 10.1136/gutjnl-2020-321927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharaibeh A, Koppikar S, Bonilla-Escobar FJ. Strengthening the reporting of observational studies in epidemiology (STROBE) in the International Journal of Medical Students. Int J Med Stud. 2014;2014:36–37. [Google Scholar]
- 21.Lang TA, Altman DG. Basic statistical reporting for articles published in biomedical journals: the “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines. Int J Nurs Stud. 2015;52:5–9. doi: 10.1016/j.ijnurstu.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Sebastian S, Myers S, Nadir S, Subramanian S. Systematic review: efficacy and safety of accelerated induction regimes in infliximab rescue therapy for hospitalized patients with acute severe colitis. Dig Dis Sci. 2019;64:1119–1128. doi: 10.1007/s10620-018-5407-7. [DOI] [PubMed] [Google Scholar]
- 23.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2015;42:22–29. [Google Scholar]
- 24.Rubin DT, Abreu MT, Rai V, et al. Management of patients with Crohn's disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an international meeting. Gastroenterology. 2020;159:6–13. doi: 10.1053/j.gastro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastian S, Gonzalez HA, Peyrin-Biroulet L. Safety of drugs during previous and current coronavirus pandemics: lessons for inflammatory bowel disease. J Crohns Colitis. 2020;14:1632–1643. doi: 10.1093/ecco-jcc/jjaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinsley A, Navabi S, Williams ED, et al. Increased risk of influenza and influenza-related complications among 140 480 patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:369–376. doi: 10.1093/ibd/izy243. [DOI] [PubMed] [Google Scholar]
- 27.Naganuma M, Kunisaki R, Yoshimura N, Takeuchi Y, Watanabe M. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol. 2013;48:595–600. doi: 10.1007/s00535-012-0686-9. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisniewski A, Kirchgesner J, Seksik P, et al. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United European Gastroenterol J. 2020;8:303–313. doi: 10.1177/2050640619889763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angus CD, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson PC, Richards D, Tanner HL, Feldman M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2:e633–e655. doi: 10.1016/S2665-9913(20)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon D, Tascilar K, Krönke G, et al. Patients with immune-mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS-CoV-2 seroconversion. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Occhipinti V, Pastorelli L. Challenges in the care of IBD patients during the COVID-19 pandemic: report From a “red zone” area in Northern Italy. Inflamm Bowel Dis. 2020;26:793–796. doi: 10.1093/ibd/izaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy NA, Hansen R, Younge L, et al. Organisational changes and challenges for inflammatory bowel disease services in the UK during the COVID-19 pandemic. Frontline Gastroenterol. 2020;11:343–350. doi: 10.1136/flgastro-2020-101520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905–910. doi: 10.1136/gut.38.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fumery M, Matias C, Brochot E. Seroconversion of immunoglobulins to SARS-CoV2 in patients with inflammatory bowel disease patients treated by biologics. J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa154. published online July 22. [DOI] [PubMed] [Google Scholar]
- 38.Norsa L, Cosimo P, Indriolo A, Sansotta N, D'Antiga L, Callegaro A. Asymptomatic severe acute respiratory coronavirus 2 infection in patients with inflammatory bowel disease under biologic treatment. Gastroenterology. 2020;159:2229–2231. doi: 10.1053/j.gastro.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Townsend T, Fiske J, Collins P, et al. Ambulatory management of acute severe ulcerative colitis: a pandemic-driven initiative. Inflamm Bowel Dis. 2020;26:e112–e113. doi: 10.1093/ibd/izaa231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data will be made available to others for meta-analysis upon request following Study Steering Group discussion and signing of a data access agreement. Requests for access to data should be made to the corresponding author and the first authors via the corresponding email given.