Abstract
Background
The first national severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serosurvey in India, done in May–June, 2020, among adults aged 18 years or older from 21 states, found a SARS-CoV-2 IgG antibody seroprevalence of 0·73% (95% CI 0·34–1·13). We aimed to assess the more recent nationwide seroprevalence in the general population in India.
Methods
We did a second household serosurvey among individuals aged 10 years or older in the same 700 villages or wards within 70 districts in India that were included in the first serosurvey. Individuals aged younger than 10 years and households that did not respond at the time of survey were excluded. Participants were interviewed to collect information on sociodemographics, symptoms suggestive of COVID-19, exposure history to laboratory-confirmed COVID-19 cases, and history of COVID-19 illness. 3–5 mL of venous blood was collected from each participant and blood samples were tested using the Abbott SARS-CoV-2 IgG assay. Seroprevalence was estimated after applying the sampling weights and adjusting for clustering and assay characteristics. We randomly selected one adult serum sample from each household to compare the seroprevalence among adults between the two serosurveys.
Findings
Between Aug 18 and Sept 20, 2020, we enrolled and collected serum samples from 29 082 individuals from 15 613 households. The weighted and adjusted seroprevalence of SARS-CoV-2 IgG antibodies in individuals aged 10 years or older was 6·6% (95% CI 5·8–7·4). Among 15 084 randomly selected adults (one per household), the weighted and adjusted seroprevalence was 7·1% (6·2–8·2). Seroprevalence was similar across age groups, sexes, and occupations. Seroprevalence was highest in urban slum areas followed by urban non-slum and rural areas. We estimated a cumulative 74·3 million infections in the country by Aug 18, 2020, with 26–32 infections for every reported COVID-19 case.
Interpretation
Approximately one in 15 individuals aged 10 years or older in India had SARS-CoV-2 infection by Aug 18, 2020. The adult seroprevalence increased approximately tenfold between May and August, 2020. Lower infection-to-case ratio in August than in May reflects a substantial increase in testing across the country.
Funding
Indian Council of Medical Research.
Introduction
As of Sept 30, 2020, India reported the second highest number of COVID-19 cases in the world, amounting to nearly 6·3 million cases and more than 97 000 deaths.1 Case reporting is influenced by strategies implemented for case finding, testing, and contact tracing, and might underestimate the true burden of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Population-based data can supplement case-based surveillance to inform public health measures. Population-based seroepidemiological studies are useful to measure the extent of SARS-CoV-2 infection and the effect of ongoing public health responses in controlling the pandemic.2
The first nationwide SARS-CoV-2 serosurvey in India was done in May–June, 2020, when the entire country was under stringent lockdown, with the exception of conditional relaxation in areas deemed to be minimally affected.3 It found a low seroprevalence of 0·73% (95% CI 0·34–1·13) among the general adult population aged 18 years or older.4 Notably, this serosurvey found a high infection-to-case ratio (81·6–130·1 infections per reported COVID-19 case), suggesting the need for a further expansion of testing, and a low infection-fatality ratio (0·27–15·04 deaths per 10 000 infections). From June, 2020, onwards, India had various phases of relaxation of lockdown measures that varied across the states, depending on the local epidemic situation.3
Research in context.
Evidence before this study
The seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies is important to understand the transmission dynamics of the virus; estimate total infections, including mild and asymptomatic individuals who might not receive testing; and inform the possibility of transmission interruption through the depletion of susceptible individuals, if seroconversion is associated with robust immunity. We reviewed the evidence for the seroprevalence of SARS-CoV-2 available as of Sept 30, 2020, by searching the National Library of Medicine article database and medRxiv for preprint publications, published in English, using the terms “serology”, “seroconversion”, “serosurveillance”, “seroepidemiology”, “seroprevalence”, “seropositivity”, “SARS-CoV-2”, and “COVID-19”. Several studies describing the seroprevalence of SARS-CoV-2 had been done across various geographical areas, using different sampling and recruitment strategies, as well as a range of testing approaches. Most studies were limited to smaller subnational areas, few were representative of the population as a whole, and potential sources of bias included the method of participant selection, non-response rates, and misclassification resulting from test specificity, particularly when the prevalence was low. The first national SARS-CoV-2 serosurvey in India indicated an overall low seroprevalence among adults by May, 2020, and the majority of infections were in people living in urban areas, with an estimated 82–130 infections for every reported COVID-19 case.
Added value of this study
India represents one of the largest populations at risk of COVID-19 and as of Sept 30, 2020, had reported the second highest number of confirmed cases globally. Because of India's large size, geographical diversity, and population heterogeneity, it is difficult to understand the extent of transmission of SARS-CoV-2 using case-based surveillance data alone. Furthermore, Indian cities represent challenging conditions for COVID-19 control, with some of the world's highest population densities and contact rates. This population-based study represents seroprevalence at the national level, covering many areas across India's large expanse. Our findings indicate an overall seroprevalence of around 7% among individuals aged 10 years or older, with a tenfold increase in adult seroprevalence between May and August, 2020. We estimated that for every reported case of COVID-19 there were 26–32 infections, and the infection-fatality ratio in surveyed districts was 0·09–0·11%. We found no difference in seropositivity by age group, sex, or occupation. Our findings indicate a substantial transmission in rural areas, although seroprevalence continues to be higher in urban slum and non-slum areas. We also found evidence of seroconversion among those without symptoms or known exposure, highlighting the limitations of symptom-directed or exposure-directed testing.
Implications of all the available evidence
The increasing national seroprevalence in India suggests a growing epidemic moving from urban to rural areas, but most of the population remain susceptible to infection. Continued expansion of testing capacity and stringent application of infection control measures remain warranted. Further rounds of the national serosurvey are planned and should provide crucial information on the rate of seroconversion, informing overall public health strategy and action.
We aimed to do a second national household serosurvey to measure changes in the epidemiology of SARS-CoV-2 infection, compare changes in population-based indicators for infection, and assess the effect of the public health response to the epidemic in India. The objectives of the second serosurvey were to estimate the nationwide seroprevalence of SARS-CoV-2 antibodies in the general population, including by age group, sex, area of residence, occupation, and COVID-19-related characteristics, and determine the trends in infections since the previous serosurvey.
Methods
Study design and participants
We did a cross-sectional serosurvey in the same 700 clusters (villages in rural areas and wards in urban areas) from 70 districts in 21 states across India that were included in the first nationwide serosurvey (appendix pp 4–8), between Aug 18 and Sept 20, 2020. Within each of the selected clusters, four random locations were selected. In each location, the survey teams chose a random starting point and visited a minimum of four consecutive households. The survey teams listed all household members aged 10 years or older who were permanent residents, and all eligible individuals present in the household at the time of the survey team visit were invited to participate. Individuals aged younger than 10 years and households that did not respond at the time of survey were excluded. From each random location, at least ten individuals were enrolled in the serosurvey. By selecting ten clusters per district, a minimum of 400 individuals were enrolled from each district.
We obtained written informed consent from individuals aged 18 years or older, or assent from children aged between 10 and 17 years, with written informed consent from their parents or guardians, before the survey. The study protocol was approved by the Central Ethics Committee of Health Research of the Indian Council of Medical Research (ICMR) and the Institutional Human Ethics Committee of the ICMR National Institute of Epidemiology, Chennai.
Procedures
Eligible participants were interviewed to collect information about sociodemographic details, symptoms suggestive of COVID-19 since March 1, 2020 (eg, fever, cough, shortness of breath, sore throat, new loss of taste or smell, fatigue), exposure history to laboratory-confirmed COVID-19 cases, and history of COVID-19 illness using the Open Data Kit mobile phone application. 3–5 mL of venous blood was collected from each participant, and centrifuged serum samples were transported to ICMR National Institute of Epidemiology, Chennai under cold chain.
Participant serum samples were tested for the presence of SARS-CoV-2 specific IgG antibodies on the Abbott Architect i2000SR automated analyser using the Abbott SARS-CoV-2 IgG assay (Abbott Park, IL, USA) as per the manufacturer's instructions. This assay detects IgG antibodies against the SARS-CoV-2 nucleocapsid protein, and has a sensitivity of 100·0% and specificity of 99·6%.5 The assay was calibrated with positive and negative quality controls before analyses. Assay results higher than or equal to the cutoff index value of 1·4 were interpreted as positive for SARS-CoV-2 antibodies. As a part of quality control, 10% of positive serum samples and an equal number of negative serum samples were re-tested using the same assay.
Statistical analysis
We described the characteristics of study participants as percentages, means, and SDs. We categorised the reported occupations into high-risk and low-risk categories, on the basis of the potential risk of exposure to a known or unknown COVID-19 case. For example, occupations such as health-care workers, police or security personnel, shopkeepers, bus or taxi drivers, or bank employees were considered as high-risk occupations; whereas, for example, farmers, retired employees, students, or information technology professionals were considered as being at lower risk of exposure. The information about occupation of the participants was captured as open-ended text and was categorised into high and low risk by the investigators. The data were analysed to estimate the seroprevalence of IgG antibodies against SARS-COV-2 with 95% CI, using a random-effects model to account for cluster sampling. To estimate the weighted seroprevalence, we calculated sampling weights as a product of the inverse of the sampling fraction for the selection of districts and the selection of villages or wards from each district. The weighted seroprevalence was further adjusted for the sensitivity and specificity of the assay.6 We also calculated the seroprevalence by age group, sex, area of residence, and COVID-19-related characteristics of study participants. In the first serosurvey, only one adult aged 18 years or older was randomly selected from each household,4 whereas in this second serosurvey, all consenting individuals aged 10 years or older were sampled. To compare the seroprevalence between the first and second nationwide surveys, we randomly selected one adult per household from the survey database, and estimated the adjusted seroprevalence among these adults. We calculated the infection doubling time among adults using the observed seroprevalence, and difference in time between the median survey dates of the two serosurveys.7 We obtained a non-linear correlation coefficient by fitting polynomial curves for the IgG positivity and cumulative incidence of reported COVID-19 cases by districts.
To estimate the total number of SARS-CoV-2 infections among individuals aged 10 years or older, we multiplied the adjusted seroprevalence in the population aged 10 years or older in the selected 70 districts, by the total population of the entire country aged 10 years or older. We divided the estimated number of infections by the number of reported COVID-19 cases detected by RT-PCR or rapid antigen test, at 1 week (Aug 18, 2020) and 2 weeks (Aug 10, 2020) before the median survey date (Aug 25, 2020) to estimate the infection-to-case ratio. As the number of COVID-19 cases reported nationally are not specified by the type of tests (eg, positive by RT-PCR, positive by rapid antigen test, or negative by rapid antigen test but RT-PCR positive), it was not possible to adjust the total number of reported cases to account for the lower sensitivity of the rapid antigen test. We estimated the infection-to-case ratio at two different timepoints because studies indicate that IgG antibodies against SARS-CoV-2 start appearing between 7 and 14 days after symptom onset.8
We calculated the infection-fatality ratio for the 70 districts by dividing the number of deaths reported 3 weeks after symptom onset (assuming a 3 week lag time from infection to death),9 by the estimated number of SARS-CoV-2 infections in the selected 70 districts. The data were analysed using STATA version 16.1, and R version 3.5.1 (appendix p 2).
Role of the funding source
The funder of the study was involved in reviewing the study design, writing of the manuscript, and the decision to submit the paper for publication. All authors had access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
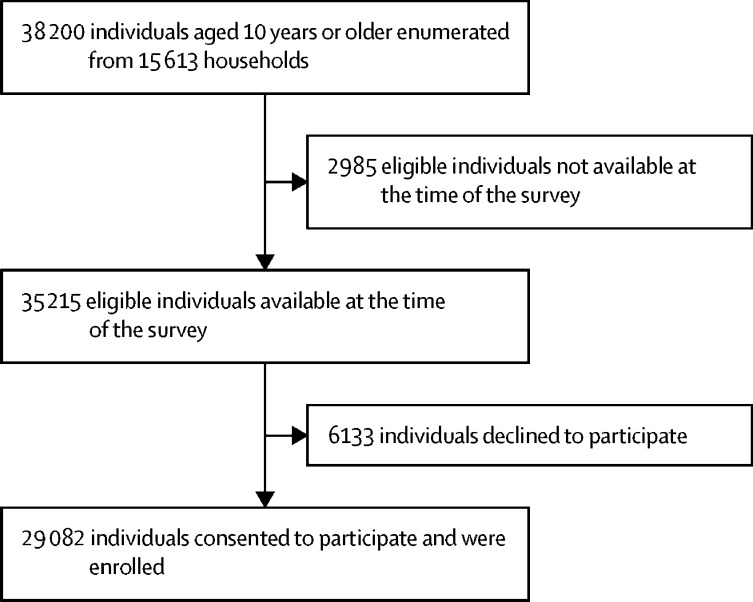
Between Aug 18 and Sept 20, 2020, we enumerated 38 200 individuals aged 10 years or older from 15 613 households in the 700 clusters. Approximately 26% of clusters were located in urban areas. 35 215 eligible individuals (92·2%) were available at the time of survey, of whom 29 082 (82·6%) consented to participate and were enrolled (figure 1 ).
Figure 1.
Flowchart of participant enrolment
Of the 29 082 survey participants, 16 663 (57·3%) were in the age group 18–44 years, 6630 (22·8%) were aged 45–60 years, 3021 (10·4%) were aged 10–17 years, and 2768 (9·5%) were aged older than 60 years (table 1 ). 14 191 participants (48·8%) were female, 21 524 (74·0%) were residing in rural areas, and 4263 (14·7%) had an occupation with a high risk of exposure to people potentially infected with COVID-19. 546 participants (1·9%) reported symptoms suggestive of COVID-19 since March, 2020, of whom 191 (35·0%) reported seeking medical care. 747 individuals (2·6%) reported having been tested for SARS-CoV-2 previously, of whom 47 (6·3%) previously had a positive COVID-19 test.
Table 1.
Participant characteristics
| Participants (n=29 082) | ||
|---|---|---|
| Age, years | ||
| 10–17 | 3021 (10·4%) | |
| 18–44 | 16 663 (57·3%) | |
| 45–60 | 6630 (22·8%) | |
| >60 | 2768 (9·5%) | |
| Mean age, years (SD) | 37·0 (16·4) | |
| Sex | ||
| Male | 14 870 (51·1%) | |
| Female | 14 191 (48·8%) | |
| Other | 21 (0·1%) | |
| Area of residence | ||
| Rural | 21 524 (74·0%) | |
| Urban non-slum | 4932 (17·0%) | |
| Urban slum | 2626 (9·0%) | |
| Occupation with high risk of exposure to COVID-19 (n=29 033) | 4263 (14·7%) | |
| History of COVID-19-related symptoms since March 1, 2020 | 546 (1·9%) | |
| Symptomatic individuals who sought medical care (n=545) | 191 (35·0%) | |
| Symptomatic individuals who were hospitalised (n=191) | 31 (16·2%) | |
| History of contact with a known COVID-19 case (n=29 044) | 215 (0·7%) | |
| Previously tested for COVID-19 (n=29 044) | 747 (2·6%) | |
| Previous positive COVID-19 test (n=747) | 47 (6·3%) | |
Data are n (%) unless otherwise stated.
Of the 29 082 participants, 3135 tested positive for the presence of IgG antibodies against SARS-CoV-2; resulting in an unweighted seroprevalence of 10·8% (95% CI 9·8–11·8; table 2 ). The seropositivity across districts ranged from 0·5% (Palakkad in Kerala; Kullu in Himachal Pradesh) to 42·6% (Ganjam in Odisha; appendix pp 4–8). The weighted seroprevalence adjusted for test performance was 6·6% (95% CI 5·8–7·4).
Table 2.
Seroprevalence by demographic characteristics
| Participants tested, n | Seropositive participants, n | Unweighted seroprevalence, % (95% CI)* | Weighted seroprevalence, % (95% CI)† | Weighted seroprevalence adjusted for test performance, % (95% CI)‡ | ||
|---|---|---|---|---|---|---|
| Overall | 29 082 | 3135 | 10·8% (9·8–11·8) | 7·0% (6·2–7·8) | 6·6% (5·8–7·4) | |
| Sex | ||||||
| Male | 14 870 | 1673 | 11·2% (10·2–12·4) | 7·1% (6·3–7·9) | 6·7% (5·9–7·5) | |
| Female | 14 191 | 1462 | 10·3% (9·3–11·4) | 6·9% (6·1–7·7) | 6·5% (5·7–7·3) | |
| Other | 21 | 0 | .. | .. | .. | |
| Age, years | ||||||
| 10–17 | 3021 | 271 | 9·0% (7·7–10·5) | 5·8% (4·9–6·8) | 5·4% (4·5–6·4) | |
| 18–44 | 16 663 | 1820 | 10·9% (9·9–12·0) | 7·3% (6·5–8·1) | 6·9% (6·1–7·7) | |
| 45–60 | 6630 | 753 | 11·4% (10·1–12·7) | 6·9% (6·1–7·9) | 6·5% (5·7–7·5) | |
| >60 | 2768 | 291 | 10·5% (9·0–12·3) | 6·6% (5·6–7·7) | 6·2% (5·2–7·3) | |
| Area of residence | ||||||
| Rural | 21 524 | 1889 | 8·8% (7·8–9·8) | 5·6% (5·0–6·4) | 5·2% (4·6–6·0) | |
| Urban non-slum | 4932 | 672 | 13·6% (11·4–16·2) | 9·4% (7·5–11·7) | 9·0% (7·1–11·3) | |
| Urban slum | 2626 | 574 | 21·9% (17·7–26·6) | 17·2% (13·2–22·0) | 16·9% (12·9–21·7) | |
| Occupation with high risk of exposure to COVID-19 (n=29 033) | ||||||
| Yes | 4263 | 519 | 12·2% (10·4–14·2) | 6·9% (6·0–8·0) | 6·5% (5·6–7·6) | |
| No | 24 770 | 2608 | 10·5% (9·6–11·5) | 7·0% (6·2–7·8) | 6·6% (5·8–7·4) | |
| History of COVID-19-related symptoms since March 1, 2020 (n=29 045) | ||||||
| Yes | 546 | 99 | 18·1% (14·1–23·0) | 11·6% (9·2–14·6) | 11·2% (8·8–14·3) | |
| No | 28 499 | 3029 | 10·6% (9·7–11·6) | 6·9% (6·2–7·7) | 6·5% (5·8–7·3) | |
| History of contact with a known COVID-19 case (n=29 044) | ||||||
| Yes | 215 | 57 | 26·5% (18·4–36·6) | 13·0% (9·9–17·1) | 12·7% (9·5–16·8) | |
| No | 25 013 | 2690 | 10·8% (9·8–11·8) | 6·7% (6·0–7·5) | 6·3% (5·6–7·1) | |
| Not known | 3816 | 381 | 10·0% (8·1–12·2) | 8·3% (6·9–9·9) | 7·9% (6·5–9·5) | |
| Previously tested for COVID-19 (n=29 044) | ||||||
| Yes | 747 | 173 | 23·2% (18·5–28·6) | 12·2% (10·0–14·9) | 11·8% (9·6–14·6) | |
| No | 28 297 | 2955 | 10·4% (9·5–11·4) | 6·2% (5·5–7·0) | 5·8% (5·1–6·6) | |
| Previous COVID-19 test result (n=747)§ | ||||||
| Positive | 47 | 38 | 80·9% (64·5–90·7) | .. | .. | |
| Negative | 665 | 132 | 19·9% (15·4–25·2) | .. | .. | |
| Not known | 35 | 3 | 8·6% (2·4–26·7) | .. | .. | |
Adjusted for clustering.
Weighted for sampling weights.
Adjusted for test performance as reported by manufacturer (sensitivity 100·0% and specificity 99·6%).
Weighted and adjusted seroprevalence not estimated because of small sample number.
Seroprevalence was lowest among children aged 10–17 years (5·4% [95% CI 4·5–6·4]), and highest among adults aged 18–44 years (6·9% [6·1–7·7]), but did not differ significantly between age groups, and was similar among males (6·7% [5·9–7·5]) and females (6·5% [5·7–7·3]; table 2). Seroprevalence was higher in urban slums (16·9% [12·9–21·7]) and urban non-slum areas (9·0% [7·1–11·3]) than in rural areas (5·2% [4·6–6·0]). There was no difference in seroprevalence between occupations categorised as high or low risk based on potential exposure to COVID-19. Among individuals who reported a history of symptoms suggestive of COVID-19, seroprevalence was 11·2% (8·8–14·3), compared with 6·5% (5·8–7·3) among individuals who did not report symptoms suggestive of COVID-19. However, among seropositive individuals, 99 (3·2%) reported a history of symptoms consistent with COVID-19 but 3029 (96·6%) reported no symptoms. Seroprevalence was higher among those who reported history of contact with a laboratory-confirmed COVID-19 case (12·7% [9·5–16·8]) and among those who had previously been tested for SARS-CoV-2 (11·8% [9·6–14·6]).
Individuals who were positive on previous SARS-CoV-2 testing had a higher seroprevalence (80·9% [64·5–90·7]) than those who tested negative (19·9% [15·4–25·2]) or were not aware of their result (8·6% [2·4–26·7]; table 2). Of the nine individuals with laboratory-confirmed SARS-CoV-2 infection but who were seronegative, the duration between PCR and serology testing was 1 day for two individuals, 8 days for one, 20–40 days for four, and more than 90 days for two. After excluding the three individuals with an interval of less than 2 weeks (to account for up to 2 weeks’ delay in the development of IgG antibodies between the date of laboratory confirmation and serological testing), 38 (86%) of 44 previous COVID-19 patients had IgG antibodies. The remaining six seronegative individuals were tested for COVID-19 because of their contact with a confirmed case, and none of them reported symptoms during illness. For two (33%) of these six seronegative individuals, the duration between laboratory confirmation and serological testing was more than 90 days.
Among 15 084 randomly selected adults (one from each household), 1696 were seropositive for SARS-CoV-2 IgG antibodies, resulting in an unweighted seroprevalence of 11·2% (95% CI 10·2–12·4). The weighted and adjusted seroprevalence among adults was 7·1% (6·2–8·2). Using an interval of 99 days between the two nationwide surveys, the infection doubling time was 30·2 days (95% CI 23·6–34·6).
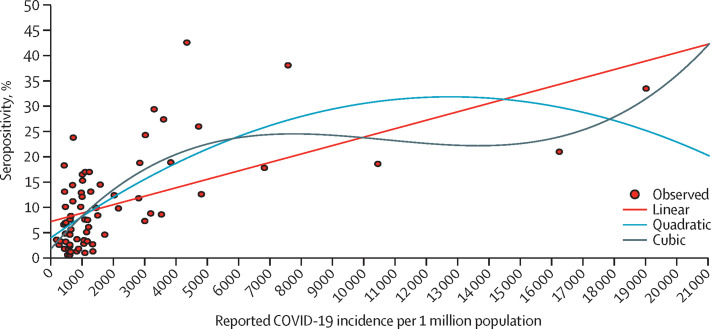
We estimated that, among individuals aged 10 years or older, a cumulative 10 663 677 infections (95% CI 9 371 110–11 956 244) had occurred by Aug 18, 2020, in the selected 70 districts. Extrapolation to the entire country resulted in an estimation of 74 326 463 infections (65 317 195–83 335 732). Considering there were 2 339 112 reported COVID-19 cases by Aug 10, 2020, and 2 856 248 by Aug 18, 2020, we estimated there were 31·8 (95% CI 27·9–35·6) and 26·0 (22·9–29·2) infections per reported case by these respective dates. Among the 70 surveyed districts, there were 10 058 COVID-19 deaths by Aug, 31, 2020, and 11 358 deaths by Sept 8, 2020, with an infection-fatality ratio ranging from 9·43 (95% CI 8·41–10·73) to 10·65 (9·50–12·12) COVID-19 deaths per 10 000 infections. The seroprevalence had a positive non-linear correlation with the cumulative incidence of reported COVID-19 cases (correlation coefficient 0·702) in the selected 70 districts (figure 2 ).
Figure 2.
SARS-CoV-2 IgG seropositivity and incidence of reported COVID-19 cases per 1 million population by district, fitted with polynomial curves
Points on the graph represent the 70 surveyed districts. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Discussion
Our findings from the second nationwide serosurvey indicate that nearly 7% of India's population aged 10 years or older had been exposed to SARS-CoV-2 infection by August, 2020, with an estimated 74 million infections. Seroprevalence did not differ by age group or sex, but was higher in urban areas, especially in the slums, than in rural areas.
Seroprevalence among adults increased by about ten times, from 0·7% in May, 2020, to 7·1% in August, 2020. All 70 surveyed districts showed a rise in IgG seropositivity between the two serosurveys, although the change was highly variable. Despite the study not being powered to provide reliable district-level estimates, some of the variation observed between districts matches the known context. For example, the largest increase in seropositivity was recorded in the Ganjam district, which also reported the highest number of COVID-19 cases in Odisha State, subsequent to migration of interstate and intrastate informal workers, and challenges in facility-based quarantine. Interstate migration of informal workers is also thought to explain the substantial rise in seroprevalence in all six districts in Bihar, and Kamrup Metropolitan district in Assam.10 The increase in seropositivity among adults between the first and second serosurveys also indicates widespread infection in all districts, except for Palakkad and Kullu.
The seroprevalence of SARS-CoV-2 infection did not differ by age group or sex, indicating similar exposure and susceptibility between these groups. This absence of a significant difference was despite school closures and other non-pharmaceutical infection-control interventions (eg, washing hands, wearing masks, physical distancing) during the survey period, suggesting household-level exposure of children and adults aged older than 60 years to other household members who are more mobile, socially active, and perhaps non-adherent to the prescribed non-pharmaceutical measures. Seroprevalence was reported to be similar across age groups in Brazil (first survey),11 and Spain,12 with the seroprevalence among adults aged older than 65 years being lower than in those aged 5–65 years in Santa Clara County, CA, USA,9 and higher in older adults in Greece13 and Iceland.14 Children had a lower seroprevalence compared with adults aged younger than 60 years in the second survey in Brazil.11 A similar seroprevalence by sex to that observed in our study has been reported from serosurveys in Santa Clara County9 and Spain,12 but a serosurvey in Geneva, Switzerland15 showed a higher seroprevalence among males.
During June–October, 2020, a number of serosurveys have been done in various Indian cities or states (appendix p 11). The higher seroprevalence in urban slum and non-slum areas observed in our study is consistent with that of other serosurveys in densely populated urban areas, where the prevalence ranged from 7·8% to 51·5%. The seroprevalence was also higher in slum areas of Mumbai (54·1%) compared with non-slum areas (16·1%).16 Although population density, coupled with high mobility and challenges in safe physical distancing and hand hygiene are the main drivers of spread of infection in urban areas, especially in urban slums,17 our findings also indicate substantial transmission among the rural population later in the epidemic, by contrast with the first serosurvey.4 Transmission is likely to increase further in these rural areas in the coming months, emphasising the need to implement non-pharmaceutical interventions, as well as strengthening health-care facilities for the effective management of infections.18
One in nine individuals who reported no COVID-19-related symptoms were seropositive for SARS-CoV-2 IgG antibodies (adjusted seroprevalence 6·5% [95% CI 5·8–7·3]), indicating asymptomatic seroconversion among the general population in India. Seroconversion was also documented among individuals without a history of known contact with a COVID-19 case, and among those without any previous SARS-CoV-2 testing. These data support the expansion of testing strategies to include individuals who do not have known exposure or symptoms.19 Only 3% of seropositive individuals reported symptoms suggestive of COVID-19, highlighting the limitations of symptom-directed testing and the importance of universal prevention methods.
Among the laboratory-confirmed patients with COVID-19 identified in our survey, only 81% of patients had SARS-CoV-2 IgG antibodies. The reasons for absence of IgG antibodies in recovered COVID-19 individuals might be due to poor B-cell response,20, 21 false-negative testing, or waning immunity over time.22
The laboratory infrastructure for the diagnosis of COVID-19 has been rapidly built up from one laboratory in January, 2020, to 1511 laboratories by August, 2020.23 With the addition of rapid point-of-care antigen-detection tests and the expansion of testing criteria, test capacity and use saw further growth, resulting in more than 34 million tests having been done as of Aug 21, 2020.23, 24, 25 The decrease in infection-to-case ratio from 82–131 in May, 2020, to 26–32 in August, 2020, is a consequence of the growth of testing outpacing the growth of infection rate. The ratio of estimated infections to reported cases in Brazil was 10·3, based on the serosurveys done in May–June, 2020.11
Population-based seroprevalence data are useful in understanding the current and future course of the COVID-19 pandemic. The overall seroprevalence of less than 10% in India indicates that a large proportion of the population remains susceptible to SARS-CoV-2 infection. The transmission of infection is expected to continue in most states in India until the herd immunity threshold is achieved, either by natural infection or vaccination. Although this threshold is unknown, most estimates place it at higher than 50% of the population.26 Heterogeneity in individual susceptibility or exposure to infection, pre-existing immunity in the population, and use of non-pharmaceutical infection-control interventions might alter the required prevalence for herd immunity.27, 28, 29 The infection doubling time at the national level was estimated to be 30·2 days (95% CI 23·6–34·6). Assuming the same rate of infection continued, the required herd immunity threshold could be estimated to be reached as of November–December, 2020. However, the duration of persistence of IgG antibodies14 and memory B cells, and the contribution and durability of cell-mediated immunity against SARS-CoV-2 is still uncertain.29 It is pertinent to note that the reported number of COVID-19 cases in India has been declining since October, 2020.
The infection-fatality ratio indicates the probability of death among those infected. In our study, the infection-fatality ratio ranged from 0·09% to 0·11%. A systematic review and meta-analysis of published studies on COVID-19 as of July, 2020, indicated an infection-fatality ratio of 0·68% (95% CI 0·53–0·82).30 Another study based on the seroprevalence data from 51 locations indicated substantial variation in infection-fatality ratios, ranging from 0·00% to 1·54%, with a median of 0·23%.31 The lower infection-fatality ratio in our study could be accounted for by several factors, including the completeness of death reporting, variation in the prevalence of comorbidities, and the age structure of the population.32, 33 Due to the absence of age-stratified death data from these 70 districts, and as the study was not powered for age-stratified seroprevalence, we could not calculate age-stratified infection-fatality ratios.
Our study has several limitations. The representation of children aged 10–17 years in the surveyed sample was lower than the census-based age distribution in India. According to Census of India projections, about 14% of the population are aged 10–17 years,34 whereas 10·4% of the study population were aged 10–17 years. The under-representation of children and over-representation of adults in the survey could lead to overestimation of the true seroprevalence, if we expect a real difference in the risk of exposure to SARS-CoV-2 across age groups. Although the required sample size was achieved, about 17% of the eligible population declined to participate in the survey. If this non-response was not at random, then this could introduce selection bias. Individuals who declined to participate were more likely to be male and younger than 60 years of age (appendix p 9). We adjusted our weighted seroprevalence estimate as per the manufacturer specified sensitivity (100·0%) and specificity (99·6%) of the Abbott SARS-CoV-2 IgG assay. According to an external evaluation, the sensitivity is reported as 92·7% and the specificity as 100·0%.35 Adjusting for these figures, our estimated overall seroprevalence was 7·6% (95% CI 6·7–8·4; appendix p 10). In the first nationwide serosurvey, we used a laboratory assay which detected IgG antibodies against whole cell antigen, and positive serum samples were re-tested with an assay that detects antibodies against the S1 domain of the spike protein of SARS-CoV-2, to improve the specificity of testing.4 In this second serosurvey, we used a laboratory assay which detected IgG antibodies against the nucleocapsid protein of the virus. Although we used different assays in the two serosurveys, we adjusted the seroprevalence to account for each assay's sensitivity and specificity. Additionally, as antibodies to the nucleocapsid protein of SARS-CoV-2 virus have been shown to reduce over time,36 we might have underestimated the seroprevalence and number of infections. For the same reason, we might have underestimated the true difference in seroprevalence between the two serosurveys, as we used antibody assays for different viral proteins. Finally, we might have overestimated the infection-to-case ratio by using COVID-19 cases reported at 1 week and 2 weeks before the median date of survey for all clusters. About half of the 700 clusters were surveyed within the first 8 days of the study period. The remaining clusters were surveyed over the next 3 weeks, and the number of cases reported from these clusters at 1 or 2 weeks before the actual date of survey would have been higher.
In conclusion, our findings indicate that nearly one in 15 individuals aged 10 years or older were exposed to SARS-CoV-2 in India by Aug 18, 2020. Although the seroprevalence among adults increased approximately tenfold between May and August, 2020, a large proportion of the population remains susceptible to SARS-CoV-2. These findings, in combination with the first national serosurvey and other serosurvey data, give a clear picture of the epidemic in India; there is high seroprevalence in urban slums, as well as non-slum urban areas, and seroprevalence is now increasing in the vast rural areas of the country. Although the epidemic was successfully contained to the cities at the outset, the current general trend presents many forthcoming challenges. We recommend continued expansion of testing capacity to improve the infection-to-case ratio, especially in districts with high seroprevalence but low case reporting; continued application of interventions to control transmission of the virus; and health facility planning for increased caseloads throughout the country, with particular focus in rural areas. Finally, we recommend further rounds of the national serosurvey, to continue providing strategic insight into the epidemiology of the SARS-CoV-2 pandemic, and to inform public health action.
Data sharing
A subset of the key anonymised individual participant data collected during the study, along with a data dictionary, is available upon request to the corresponding author, after approval of a proposal with a signed data access agreement.
Acknowledgments
Acknowledgments
We acknowledge the field supervision and support provided by WHO India; Ministry of Health and Family Welfare, Government of India; State and district health officials; primary health-care staff in the planning and conduct of the serosurvey; and the following ICMR team members: E B Arun Prasath, S Sarath Kumar, D Sudha Rani, Jasmine Farzana, G Kiruthika, E Michaelraj (National Institute of Epidemiology, Chennai); Naveen Kumar Mandal, Kumar Gautam, Sanjeev Gupta, Ujjwal Prakash, Sahdeo Mandal, Kumar Ayush, Saurav Kumar, Santosh Kumar, Rahul Kumar, Ranjeet Kumar, Paras Kumar, Kumar Mandal, Satish Kumar Thakur, Amit Kumar, Amit Lakra, Ashish Kumar, Binod Kumar, Amit Ranjan, Prateek Raushan, Vikas Kumar, Mumtaz Alam Khan, Baidyanath Roy, Alok Kumar, Ajeet Kumar Ram, Sakaldeep Kumar, Ajeet Kumar, Aaditya Panday, Umesh Kumar, Dhirendra Kumar, Abhay Kumar, Sanjeet Kumar, Bhoop Dhakar, Kamlesh Kumar, Alupt Kumar, Vikash Kumar Roy, Kundan Kunal, Vikash Kumar, Siyanand Sah, Vivek Kumar, B P Subramanya, Geetika Shankar, Anand Gautam, Susheel Gautam, Adarsh Varghese, Kunnal Kuvalekar, K R Rakesh Mandal, Bnuop Singh Dhalean (Bihar); Sunil Kumar Pankaj, Irshad Khan, Rahul Roy, Chetan Ravishankar Raut, Kunjbihari Patel, Chandra Kishore Thakre, Pekhan Kumar Sahu, Nand Kumar Modi, Nand Kumar Sahu, Champu Ratre, Mahesh Gopal Patel, Bhoopendra Thakur, Awadh Baghel, Hemant Bawthande, Dev Kumar Sahu, Rajesh Kumar Soni, Devdas Joshi, Vokesh Yadu, Gulshan Sahu, Anshuman Choudhary, Archana Nagwanshi (Chhattisgarh); A M Kadri, Harsh N Bakshi, Pranav G Patel, Arthur Mcwan, Anand Santoke, Monark Vyas, S A Aarya, Pankaj Nimawat, Shabbir Ali Dedhrotiya, Yagvalky Jani, Jitendra Patel, Hasmukh Parmar, Hardik Nakshiwala, Vaidehi Gohil, Jagdish Patel, Parulben Patel, Jigneshbhai Tadvi, Piyushbhai Parasar, Vinodbhai Valvi, Jagdishbhai Padvi, Dhawal Patel, Divyaben Zala, Mayurbhai Vasava, Manmitbhai Solanki, Darshnaben Patel, Chetnaben Chaudhari, Aartiben Rathva, Riyaben Mistry, Nikiben Bhau, Jyotsnaben Bariya, Tejasbhai Patel, Kartikbhai Prajapati, Babita Roy, Pareshbhai Parmar, Manojbhai Bhagora, Pareshbhai Patel, Hemantbhai Kalasva, Shardaben Vankar, Divya Patel, Ravisinh Chauhan, Nimisha Patel, Misha Patel, Harsha Sadat, Puja Patel, Girish Shah, Partapsinh Taviyad, Raginiben Gosai, Krutikaben Rana, Imtiyazbhai Shaikh, Madhuben Mahera, Bhavikaben Patel, Prakashakumar Patel, Sangitaben Patel, Geetaben Patel, Pratapbhai Pagi, Bharatbhai Rana, Jinalben Patel, Archanaben Pandavi, Dilipbhai Baria, IshavarSinh Rathod, Sharmishtha Patel, Sunitaben Solanki, Bhavesh Vaghela, Moinuddin Mansuri, Nitesh Rathore, Purvi Nayak, Hardeep Khair, Rajendra Acharya, Vijyaben Amin, Nirmal Prajapati, V J Pargi, Asmitaben Kharadi, Rajubhai Patel, Komalben, Hemangini Baria, Meenaben Bamaniya, Shantaben Prajapati, Rameshbhai Patel, Imran Mansuri, Yashvantbhai Nayak, K K Parmar, Rahul Siroi, Krunal Darji, Mahavir Solanki, Shivani Joshi, Mahesh Gavit (Gujarat); Iqra Nisar Chowdri, Tanzeela Bashir Qazi, Rafiya Kousar, Iram Sabah, Shahroz, Abdul Aziz, Ishtiyaq Ahmad Sumji, Mehvish Khan, Shaista Ismail, Anjum Asma, Sameer Ahmad, Danish Fayaz, Nayra, Owais, Saima, Humaira, Suhail, Jazia Majeet, Saba, Rumaisa, Zarka, Nadeem, Nafeesa, Tawseef, Hassina Mir, Syed Arshad Rafiq, Zahoor Ahmad, Mohammad Ashraf, Gulzar Ahmad Sheikh, Riyaz Ahmad, Mohammad Rameez, Zaffar Iqbal, Aijaz Bashir, Zahoor Ahmed, Faisal Bin Ayoub, Ashraf, Imran Shafi, Tabasum, Mohammad Mansoor, Abdul Majeed, Ghulam Mohiuddin Najar, Satnam Singh, Shahzada Manzoor, Muhammad Yousuf Rather, Jameel Ahmad Baba, Jabbar Sofi, Farooq Ahmad Ahangar, Fayaz Ahmad Wani, Jarnail Singh, Farooq Ahmad, Shanipal Singh, Rameez Ahmad, Firdousa Akhtar, Naseeb Singh, Mohammad Akram, Feroze Ahmad, Tufail Ahmad Qazi, Mushtaq Ahmad, Abdul Rashid Ganai (Jammu and Kashmir); Anushil Anand, Swagata Lakshmi Tarafdar, Alok Kumar, Aman Gupta, Vikash Kumar Sinha, Mukesh Kumar Aggarwal, Viresh Kumar Mishra, Nilesh Kumar, Abdul Kalam Azad, Raushan Raj, Mamta Kumari, Puja Kachhap, Soni Khatun, Sudhanshu Munda, Jayaram Mehta, Suraj Mahto, Jyoti Anant, Pratima Kumari, Ajay Kerketta (Jharkhand); Jawaid Akhtar, Arundathi Chandrashekar, Patil Om Prakash, Rameshchandra Reddy, Pankaj Kumar Pandey, Kiran K, Sarika Jain Agrawal, M V Kumar, H P Arundathi Das, Ranganath Vivekanand Reddy, G Hamsaveni, Lokesh Alahari, R S Sreedhar, K R Nischit, Mishba Hani, Anil Talikoti, N T Nagraj, Satish Ghatage, Shoba, Sandeep, Ravi Shankar, Savitha, Sujatha, Govardhan, Babu Mahendra, Vinay Kumar, Harish, Vishwas, Srinivas, Premasudha, Vanitha, Suhail, Rakesh, Sahana, Sunitha, Mustapa, Krishnamurthy, Rekha, Minaz-ul-Islam, Manohar, Sanjeev Patil, K Archana, Amir Pasha, Renuka Katti, Mantappa Halamalli, Sunil Serikar, Siddu Patil, Nagendra, N Vijayalakshmi, Y G Srikantha, R Hariprashanth, M N Prasanna, Lal Kumar, R Bhuvaneshwari, R Ragapriya, Dinesh Kumar B, Praveen Pujar, Ullera Ashoka, A N Sunil, Umar Farooq M Dalawai, N Narasimharaju, Bheema Zakheer Hussain, Namewar Hanmanth, Somashekharayya S Hiremath, Charan Raj Rao (Karnataka); P S Rakesh, V G Vinod Kumar, Suja Aloysius, A K Anitha, Sharath G Rao, Nikilesh Menon Ravikumar, D A Nithin Dev, Arun Raj, M R Abhirami, A Shilna, T Nikhilamol, Anumol Raju S, A S Asitha, M Manoj, R Sindhya, G Jaicy, Neethu Sugathan, K Sumi, Peneena Varghese, M P Anupranam, V Prakash Jaison, B S Vishnu Raj, V S Venoth, Gladson, Refic D (Kerala); Debjit Chakraborty, Suman Kanungo, Subrata Biswas, Malay Kumar Saha, Ajay Chakraborty, Jayesh Mehta, Amitabha Sarkar, Bipra Bishnu, Abhijit Dey, Arup Chakrabortty, Amlan Datta, Debasish Roy, Shyamal Soren, Jagannath Sarkar, Somnath Mukherjee, Prakash Chandra Mridha, Girish Chandra Bera, Nitai Mandal, Santanu Sahu, Atrayee Chakraborty, Rabiul Islam Gayen, Dilip Biswas, Samudra Sengupta, Subarna Goswami, Saptarshi Bannerjee, Soumen Jana, Joyeeta Bhattacharyya, Medhavi Manish, Biswajit Namasharma, Chandan Ghosh, Debarati Chakraborty, Kunal Maiti, Milan Barman, Pintu Manik, Priya Rana, Purnima Roy, Rajani Kurmi, Rocky Ansari, Sanglap Maity, Somobrota Naskar, Souptik Jana, Sourav Pradhan, Bishakha Pramanik, Dipannita Sardar, Sujit Kumar Shreshta, Arpita Das, Shrikant Shankar Gawali, Sriparna Garai, Swarnendu Sasmal, Ujjal Maitra, Saiful Gazi, Joydeep Banerjee, Rupali Ghosh, Nawaid Ali, Pokhraj Dey, Chandan Ghosh, Susanta Bera, Sk Monirul Jaman, Shrija Ghosh, Dev Kumar Dolai, Purnima Das, Wasim Reza, Rajesh Das (West Bengal); Bhagwan Harkal, Amit Patil, Archana Gaikwad, Namrata Hajari, Hema Vishwakarma, Rahul Arke, Vivek Yengade, Avinash Shinde, Dhiraj Panpatil, Aditya Bengale, Padmakar Kendre, Suraj Rakhunde, Prathamesh Chavan, Pramod Jamale, Inayat Nadaf, Tejas Phale, Sunil Shirke, Ajit bachude, Anupkumar Yadav, N Ramaswami, Satish Pawar, Archana Patil, Padmaja Jogewar, Sumedh Andurkar, Abhijeet Raut, Deepak Madhukar Mugalikar, Vipin Itankar, Rahul Dwivedi, Rahul Rekhawar, Abhijeet Chaudhari, Rajesh Deshmukh, Nagurao S Chavan, Balasaheb Nagargoje, Nilkanth Bhosikar, Ashok Thorat, Pradeep Morambikar, Sanjay Salunkhe, Dilip Patode, Shankar Rao Deshmukh, Balaji Shinde, Radhakrishna Pawar, Sandip Sangale, Bhupal Girigosai, Sandip Bharaswadkar, Rajabhau Yeole, Harshad Lande, Sandip Shinde, Prakash Nandapurkar, Mujeeb Sayyed, Chetan Khade, Amol Gaikwad, Anil Rathod, Akshay Phulari (Maharashtra); M P Sharma, Shivendra Mishra, A S Malviya Mahavir Khandelwal, Devendra Bhothwal, C K Gupta, Seema Jaiswal, Jyoti Ahirwar, Ganesh Damor, Hemant Singh Thakur, Pratipal Singh, Hemant Pancheshwar, Bhagwansingh Patil, Rameshwar Uikey, Sheetal Sariyam, Akaksha Kushram, Shashibhushan Dubey, Sandip Sharma, Saurabh Bhadoriya, Himmat Singh, Yogendra Mourya, Shashank Kesharwani, Prahlad Soni, Pushpendra Rajput, Ankita Sharma, Ashish Namdeo, Jitendra Kumar, Priyanka Birha, Monu Sen, Rekha Prajapati, Priyanka Singore, Lipi Jain, Ashok Solanki, Kalpana Patel (Madhya Pradesh); Spandan Kumar Bhanjadeo, Nutan Dwibedi, Sushree Sukanya Samantray Dinabandhu Padhan, Satyabrata Rout, Sagarkanta Pradhan, Sadruddin Khan, Kanhu Charan Sahoo, Nirupama Sahoo, Subrat Kumar Nayak, Manas Bhoi, Jeevan Kumar Mohanta, Rojalin Das, Janaki Biswal, Ashis Kumar Mohapatra (Odisha); P K Anand, Vikas Dhikav, Suresh Yadav, Elantamilan D, Ramesh Kumar Hudda, Mohendra Thakor, Anil Purohit, Pankaj Kumar, Sharwan Kumar, Trilok Kumar, Jogaram Choudhary, Praveen Bhaghel, Suresh Kumar, Sandeep Kumar Yadav, Mohan Lal Meena, Sunil Kumar (Rajasthan); John Arokyadoss Y, M Magesh Kumar, P Kumaravel, Vasudevan, Anbarasan, J Chitra, Jagannathan, N Santhanakumar, Udhayakumar, Murugesan, Rajmohan, Nandhakumar, G Preethi, Chandrabalu, Akshitha, Hari Vignesh, Sentrayan, Suresh, Senbagavalli, Chandrakumar, Santhosh, K Ponmalaiselvan, M Thirumalai (Tamil Nadu); R Ananthan, Anwar Basha, Blessy, J P Deva Raj, S Devendra, Mahesh Kumar, I I Meshram, Raja Sriswan, P Raghavendra, Ravindranath, G Sarika, Santosh Kumar Banjara, Srinivas Rao J, V Surekha, Subba Rao G, F Sylvia, Ronald Rose D, A Rajesham, B Jagdish, Rajyalaxmi, Raji Reddy, Jhansi, Indraja, Venkataramana, B V Nancharamma, Hrusikesh Panda, G L Stephen, Roja Sreenu, P Bhavani, Aruna Harish, Sree Ramakrishna, Narsimhulu Chandrababu, G Neeraja, Sheela Srinivas, Myadara Satyanarayana, S P V Prasad, P Sunu, Anitha, Rani, Sai Babu, Ch Pallavi, G Vijaya Lakshmi, D Swarupa, Tulasi Bai G, Deepak Kumar, Bhagya Sri A, Sai Kumar Kommu, Laxman, Anjaiah, Venkatamma, Ravi, Nagesh, B Vijay, Sathaiah P Nagendra (Telangana); Akhileshwar Sharda, Dilip Singh, Vijay Kumar, Naresh Dhakar, Vinay Kumar, Akash Yadav, Deshdeepak Gautam, Swati Singh, Brijesh Maurya, Shaurabh Kumar, Manisha Dhakar, Sheena Singh, Rahul Yadav, Sonu Yadav, Narendra Yadav, Mevaram, Himalaya Kumar, Raju, Balijeet Sodhi, Rajesh Jain, Shivanka Gaur, Deepak Ohari, Tikam Singh, Saubhagya Prakash, Haridutt Nemi, Nandan Kumar Mishra, Dechen Yangdol, Upendra Singh, Harshit Kumar, Amit Yadav, Mohit Tiwari, Gopal Prasad, Sapna Yadav, Basudev Singh, Deepak Babu, Sushil Kumar Pal, Rahul Kumar,Mahaveer, Pushpendra Kumar, Rahul Gond, Prabhat Kumar, Hariom Kushwah, Gani Aftridi, Nistha Verma, Veer Vishal, Rakesh Sharma, Uday, Saurabha Yadav, Iftikhar Uddin, Raju Kashyap, Navneet Rajput, Satya Prakash, Mohit Sharma, Sunny Sharma, Santosh Kushwah, Akhalesh Yadav, Vikas Sabharwal, Ravinder Singh, Sushil Chander, Rajesh Guleri, Sukhwant Singh, Satyvrat Vaidya, Raman Sharma, Pankaj Singh, Manu Jain, Archana Srivastava, Manoj Bahukhandi, Ramesh Kunwar, Ashish Gusain, Arjit Kumar, Dhruv Gopal Prashant Upadhyay, Shishir Puri, Vineet Kumar Shukla, R K Gautam, Sukhwant Singh, S K Garg, Anil Gautam, Ramayya Dora, C K J S Rawat, Bhupinder Singh, Himanshu Sharma, Madhu Kumari, Rajesh Morya, R S Yadav, Surinder Singh, Agam Jain, Raju Kumavat, Sandeep Patil, Rakhi Bhargav, Sanjay Chopra, Jyoti Mishra, Mohammed Husain, Debilal, Amit Mohan Prasad, Madhu Gairola, Vinay Dange, Ghanshyam Singh, Atul Kumar Singhal, Shri Prakash Agrawal, Satish Chandra Singh, Ramesh Chandra Pandey, Birendra Panchal, Vishal Yadav, Mukesh Kumar Mishra, Sonal Rajput, Jaibardhan Siddharth, Rohit Baghel, Punit Kumar, Md Afroj, Abhishek Kumar Mishra, Awdhesh Kumar, Kiran, Akash Kushwaha, Deepak Kumar, Vinod Kumar, Vipul Kumar, Vijay Kumar Prasad, and Mayank Badola (Uttar Pradesh).
Contributors
TB, JWVT, and MSaK did the literature search. MVM, TB, KR, MSaK, NS, DCSR, and BB did the study design. SSe, RSa, SA, RB, SDB, AKB, JB, VC, DD, AKD, KRD, GRD, SMSK, MSuK, AL, MM, AMa, SSM, CR, AT, DKB, ASC, FD, IH, AK, SK, JSK, GGJNL, AMi, ARN, GVP, MAQ, SeS, RKS, KS, VKS, PKS, PS, RaS, DSV, AV, and SP did the data collection. CPGK and KS did the laboratory investigations. VS, JWVT, MVM, TB, RSa, and MSaK did the data analysis. MVM, TB, KR, NS, DCSR, and BB did the data interpretation. MVM, TB, VS, JWVT, and MSaK accessed and verified the data. MVM, TB, JWVT, NS, VS, and MSaK wrote the first draft of the manuscript. All authors approved the final version of the manuscript.
ICMR Serosurveillance Group members
Site coordinators: Rushikesh Andhalkar, Anshuman Chaudhury, Hirawati Deval, Sarang Dhatrak, Rajeev Ranjan Gupta, Ezhilarasan Ilayaperumal, Babu Jagjeevan, Ramesh Chandra Jha, K Kiran, Nivethitha N Krishnan, Alok Kumar, VG Vinoth Kumar, K Nagbhushanam, Arlappa Nimmathota, Ashok Kumar Pandey, Harpreet Singh Pawar, Kushal Singh Rathore, Aby Robinson, Hari Bhan Singh, Vimith Cheruvathoor Wilson, Ashwini Yadav, Rajiv Yadav, Kamran Zaman. Surveillance laboratory group: T Karunakaran, Josephine Pradhan, R Sivakumar, Annamma Jose, K Kalaiyarasi, Sauvik Dasgupta, R Anusha. Epidemiology and surveillance group: Tanu Anand, Giridhara R Babu, Himanshu Chauhan, Tanzin Dikid, Raman R Gangakhedkar, Shashi Kant, Sanket Kulkarni, J P Muliyil, Ravindra Mohan Pandey, Swarup Sarkar, Aakash Shrivastava, Sujeet K Singh, Sanjay Zodpey. Institutional coordinators: Aparup Das, Pradeep Das, Shanta Dutta, Rajni Kant, Kanwar Narain, Somashekar Narasimhaiah, Sanghamitra Pati, Shripad Patil, Hemalatha Rajkumar, Tekumalla Ramarao, Kamalesh Sarkar, Shalini Singh, Gurudayal S Toteja.
Declaration of interests
We declare no competing interests.
Contributor Information
ICMR Serosurveillance Group:
Rushikesh Andhalkar, Anshuman Chaudhury, Hirawati Deval, Sarang Dhatrak, Rajeev Ranjan Gupta, Ezhilarasan Ilayaperumal, Babu Jagjeevan, Ramesh Chandra Jha, K Kiran, Nivethitha N Krishnan, Alok Kumar, VG Vinoth Kumar, K Nagbhushanam, Arlappa Nimmathota, Ashok Kumar Pandey, Harpreet Singh Pawar, Kushal Singh Rathore, Aby Robinson, Hari Bhan Singh, Vimith Cheruvathoor Wilson, Ashwini Yadav, Rajiv Yadav, T Karunakaran, Josephine Pradhan, T Sivakumar, Annamma Jose, K Kalaiyarasi, Sauvik Dasgupta, R Anusha, Tanu Anand, Giridhara R Babu, Himanshu Chauhan, Tanzin Dikid, Raman R Gangakhedkar, Shashi Kant, Sanket Kulkarni, J P Muliyil, Ravindra Mohan Pandey, Swarup Sarkar, Aakash Shrivastava, Sujeet K Singh, Sanjay Zodpey, Aparup Das, Pradeep Das, Shanta Dutta, Rajni Kant, Kanwar Narain, Somashekar Narasimhaiah, Sanghamitra Pati, Shripad Patil, Hemalatha Rajkumar, Tekumalla Ramarao, Kamalesh Sarkar, Shalini Singh, Gurudayal S Toteja, and Kamran Zaman
Supplementary Material
References
- 1.Government of India COVID-19 dashboard. Sept 30, 2020. https://www.mygov.in/covid-19
- 2.WHO . World Health Organization; Geneva: 2020. A coordinated global research roadmap: 2019 novel coronavirus; March 2020. [Google Scholar]
- 3.Wikipedia COVID-19 pandemic lockdown in India. Oct 2, 2020. https://en.wikipedia.org/wiki/COVID-19_pandemic_lockdown_in_India
- 4.Murhekar MV, Bhatnagar T, Selvaraju S. Prevalence of SARS-CoV-2 infection in India: findings from the national serosurvey, May–June 2020. Indian J Med Res. 2020;152:48–60. doi: 10.4103/ijmr.IJMR_3290_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food & Drug Administration EUA authorized serology test performance. 2020. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 6.Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol. 1996;25:1107–1116. [PubMed] [Google Scholar]
- 7.Galvani AP, Lei X, Jewell NP. Severe acute respiratory syndrome: temporal stability and geographic variation in case-fatality rates and doubling times. Emerg Infect Dis. 2003;9:991–994. doi: 10.3201/eid0908.030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long QX, Liu BZ, Deng HJ. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 9.Bendavid E, Mulaney B, Sood N. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv. 2020 doi: 10.1101/2020.04.14.20062463. published online April 30. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saikia A. How Ganjam — and not capital Bhubaneshwar — became Odisha's COVID-19 hotspot. Aug 4, 2020. https://scroll.in/article/969139/how-ganjam-and-not-capital-bhubaneshwar-became-odishas-covid-19-hotspot
- 11.Hallal PC, Hartwig FP, Horta BL. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollán M, Pérez-Gómez B, Pastor-Barriuso R. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogogiannidou Z, Vontas A, Dadouli K. Repeated leftover serosurvey of SARS-CoV-2 IgG antibodies, Greece, March and April 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.31.2001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudbjartsson DF, Norddahl GL, Melsted P. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stringhini S, Wisniak A, Piumatti G. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malani A, Shah D, Kang G. Seroprevalence of SARS-CoV-2 in slums versus non-slums in Mumbai, India. Lancet Glob Health. 2021;9:e110–e111. doi: 10.1016/S2214-109X(20)30467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhury P, Rao SP. Reviving the post COVID-19 Indian economy and the twin challenges of informal workers and slums. May 1, 2020. https://landportal.org/blog-post/2020/05/reviving-post-covid-19-indian-economy-and-twin-challenges-informal-workers-and
- 18.Radhakrishnan V, Sen S, Singaravelu N. The Hindu explains: is COVID-19 intensifying in rural India? Sept 5, 2020. https://www.thehindu.com/news/national/the-hindu-explains-is-covid-19-intensifying-in-rural-india/article32476163.ece
- 19.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long QX, Tang XJ, Shi QL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 21.Sekine T, Perez-Potti A, Rivera-Ballesteros O. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158. doi: 10.1016/j.cell.2020.08.017. 68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vabret N. Antibody responses to SARS-CoV-2 short-lived. Nat Rev Immunol. 2020;20:519. doi: 10.1038/s41577-020-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Indian Council of Medical Research Press release — In the fight against COVID-19, India scales a new peak in daily testing, achieves record ten lakhs test per day. Aug 22, 2020. https://www.icmr.gov.in/pdf/press_realease_files/PR_ICMR_tenLakhs_Testing_per_Day22082020.pdf
- 24.Indian Council of Medical Research Strategy for COVID-19 testing in India (version 5) May 18, 2020. https://www.icmr.gov.in/pdf/covid/strategy/Testing_Strategy_v5_18052020.pdf
- 25.Indian Council of Medical Research Advisory — Newer additional strategies for COVID-19 testing. June 23, 2020. https://www.icmr.gov.in/pdf/covid/strategy/New_additional_Advisory_23062020_3.pdf
- 26.Britton T, Ball F, Trapman P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science. 2020;369:846–849. doi: 10.1126/science.abc6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguas R, Corder RM, King JG, Gonçalves G, Ferreira MU, Gomes MGM. Herd immunity thresholds for SARS-CoV-2 estimated from unfolding epidemics. medRxiv. 2020 doi: 10.1101/2020.07.23.20160762. published online Nov 16. (preprint) [DOI] [Google Scholar]
- 28.Lourenço J, Pinotti F, Thompson C, Gupta S. The impact of host resistance on cumulative mortality and the threshold of herd immunity for SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.07.15.20154294. published online Oct 1. (preprint) [DOI] [Google Scholar]
- 29.Simoneaux R, Shafer SL. Can herd immunity save us from COVID-19? ASA Monitor. 2020;84:18–19. [Google Scholar]
- 30.Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis. 2020;101:138–148. doi: 10.1016/j.ijid.2020.09.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannidis JPA. The infection fatality rate of COVID-19 inferred from seroprevalence data. medRxiv. 2020 doi: 10.1101/2020.05.13.20101253. published online May 19. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallapaty S. How deadly is the coronavirus? Scientists are close to an answer. Nature. 2020;582:467–468. doi: 10.1038/d41586-020-01738-2. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Saez J, Lauer SA, Kaiser L. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30584-3. published online July 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Commission on Population Population projections for India and States 2011–2036: report of the technical group on population projections. 2019. https://nhm.gov.in/New_Updates_2018/Report_Population_Projection_2019.pdf
- 35.Public Health England Evaluation of the Abbott SARS-CoV-2 IgG for the detection of anti-SARSCoV-2 antibodies. June 8, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/890566/Evaluation_of_Abbott_SARS_CoV_2_IgG_PHE.pdf
- 36.Ripperger TJ, Uhrlaub JL, Watanabe M. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925. doi: 10.1016/j.immuni.2020.10.004. 33.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A subset of the key anonymised individual participant data collected during the study, along with a data dictionary, is available upon request to the corresponding author, after approval of a proposal with a signed data access agreement.