Abstract
Background
To date, only monoclonal antibodies have been shown to be effective for outpatients with COVID-19. Interferon lambda-1 is a type III interferon involved in innate antiviral responses with activity against respiratory pathogens. We aimed to investigate the safety and efficacy of peginterferon lambda in the treatment of outpatients with mild-to-moderate COVID-19.
Methods
In this double-blind, placebo-controlled trial, outpatients with laboratory-confirmed COVID-19 were randomly assigned to a single subcutaneous injection of peginterferon lambda 180 μg or placebo within 7 days of symptom onset or first positive swab if asymptomatic. Participants were randomly assigned (1:1) using a computer-generated randomisation list created with a randomisation schedule in blocks of four. At the time of administration, study nurses received a sealed opaque envelope with the treatment allocation number. The primary endpoint was the proportion of patients who were negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA on day 7 after the injection, analysed by a χ2 test following an intention-to-treat principle. Prespecified analysis of the primary endpoint, adjusted for baseline viral load, using bivariate logistic regression was done. The trial is now complete. This trial is registered with ClinicalTrials.gov, NCT04354259.
Findings
Between May 18, and Sept 4, 2020, we recruited 30 patients per group. The decline in SARS-CoV-2 RNA was greater in those treated with peginterferon lambda than placebo from day 3 onwards, with a difference of 2·42 log copies per mL at day 7 (p=0·0041). By day 7, 24 (80%) participants in the peginterferon lambda group had an undetectable viral load, compared with 19 (63%) in the placebo group (p=0·15). After controlling for baseline viral load, patients in the peginterferon lambda group were more likely to have undetectable virus by day 7 than were those in the placebo group (odds ratio [OR] 4·12 [95% CI 1·15–16·73; p=0·029). Of those with baseline viral load above 106 copies per mL, 15 (79%) of 19 patients in the peginterferon lambda group had undetectable virus on day 7, compared with six (38%) of 16 in the placebo group (OR 6·25 [95% CI 1·49–31·06]; p=0·012). Peginterferon lambda was well tolerated, and adverse events were similar between groups with mild and transient aminotransferase, concentration increases more frequently observed in the peginterferon lambda group. Two individuals met the threshold of grade 3 increase, one in each group, and no other grade 3 or 4 laboratory adverse events were reported.
Interpretation
Peginterferon lambda accelerated viral decline in outpatients with COVID-19, increasing the proportion of patients with viral clearance by day 7, particularly in those with high baseline viral load. Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding.
Funding
The Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, continues to be a global health threat. To date, only remdesivir and dexamethasone have shown efficacy in randomised trials of hospitalised patients with COVID-19 and an interim analysis of a monoclonal antibody infusion was shown to accelerate viral clearance in outpatients.1, 2, 3 As with other acute viral infections, early initiation of antiviral therapy for COVID-19 might improve clinical outcomes;4 however, few studies among outpatients have been completed. In addition to halting clinical progression, early treatment might shorten the duration of viral shedding, potentially reducing onward transmission.5
Interferons are produced as part of the innate immune response to viral infections, driving induction of genes with antiviral, antiproliferative, and immunoregulatory properties.6 The broad array of genes induced by interferons limits the risk of antiviral resistance and makes interferons optimal agents for novel viral pathogens.7 Interferon lambdas, known as type III interferons, promote a similar antiviral state to that of interferon alfa or beta, but use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, resulting in fewer systemic side-effects.7 Interferon lambda-1 controls respiratory viral infections without the risk of promoting cytokine storm syndrome, as has been seen in mice with type I interferon treatment.8 Additionally, interferon lambda inhibits SARS-CoV-2 replication in cell culture and mouse models.9, 10 Peginterferon lambda, a long-acting form of interferon lambda-1, has been assessed in over 3000 patients with viral hepatitis infections, showing similar antiviral efficacy to that of interferon alfa, but with an improved side-effect profile.11, 12
Research in context.
Evidence before this study
A comprehensive literature review was completed before the development of the initial protocol (PubMed search March 20, to March 30, 2020, using the search terms “COVID-19 OR SARS-CoV-2” AND “treatment OR interferon”). We found almost no studies on the use of antiviral therapy for outpatients with COVID-19. Indeed, at the time of manuscript submission, treatment trials for patients with COVID-19 had largely focused on patients treated in hospital, with no therapies approved for outpatients. Several studies in ambulatory populations have been registered and an interim analysis of a trial of a monoclonal antibody was reported, showing faster viral clearance and reduced hospitalisation in treated patients compared with placebo, leading to its emergency use authorisation by the US Federal Drug Administration in the USA (PubMed search up to Nov 10, 2020, using the terms “COVID-19 treatment” and “controlled trials”). A monoclonal antibody cocktail has also been reported to accelerate viral clearance in outpatients. Uncontrolled case series of hydroxychloroquine with or without azithromycin have been reported with mixed results but no clear signal of efficacy and some concerns raised about cardiac toxicity. Small non-randomised trials with interferon beta and other co-interventions in hospitalised patients have suggested that interferon might accelerate viral clearance. Treatment in the outpatient setting has potential to prevent infected individuals from deteriorating and, perhaps more importantly, might shorten the duration of viral shedding, reducing the risk of transmission and the duration required for self-isolation, with substantial public health effects.
Added value of this study
This study shows that a single subcutaneous injection of 180 μg peginterferon lambda has an antiviral effect in outpatients with COVID-19. The decline in viral load was greater with peginterferon lambda treatment than with placebo. The more rapid viral load decline and higher clearance rate were most pronounced in those with high viral loads, a finding also reported with monoclonal antibody therapies in patients with COVID-19. However, the magnitude of the viral load decline compared with that of placebo was much greater with peginterferon lambda than has been reported with monoclonal antibody therapies to date. Peginterferon lambda was safe and well tolerated in outpatients with mild-to-moderate COVID-19, with a similar side-effect profile to that of placebo and no concerning laboratory adverse events.
Implications of all the available evidence
No approved therapy exists for outpatients with COVID-19. This study showed that peginterferon lambda accelerated viral clearance, particularly in those with high baseline viral loads, highlighting the importance of quantitative viral load testing in the assessment of antiviral agents for patients with COVID-19. Treatment early in the course of disease might prevent clinical deterioration and shorten the duration of viral shedding, which might have an important public health effect by reducing transmission and reducing the duration of self-isolation.
The in-vitro and in-vivo efficacy of interferon lambda against SARS-CoV-2 provided strong rationale for investigation in humans. As such, we aimed to investigate the efficacy of a single 180 μg subcutaneous injection of peginterferon lambda or placebo in outpatients with COVID-19.
Methods
Study design and participants
In this randomised, double-blind, placebo-controlled study, individuals were recruited from outpatient testing centers at six institutions in Toronto, Canada, between May 18, and Sept 4, 2020, and referred to a single ambulatory site for enrolment and randomisation. Individuals with SARS-CoV-2 infection confirmed by nasopharyngeal swab were eligible if they were within 7 days of symptom onset or first positive test if asymptomatic. Study coordinators consented and enrolled the participants. The main exclusion criteria included pregnancy and pre-existing immunosuppressive or other medical conditions that could be worsened by peginterferon lambda (see protocol for complete inclusion and exclusion criteria). In line with institutional COVID-19 restrictions, all individuals provided witnessed informed verbal consent. The research ethics boards of all participating institutions approved the study, which was registered (NCT04354259) and done under a Clinical Trial Application approved by Health Canada.
Randomisation and masking
All participants attended a single study site following enrollment. Eligible consenting adults were randomly assigned (1:1) to a single subcutaneous injection of 180 μg of peginterferon lambda or saline placebo. A computer-generated randomisation list was created by the study statistician (BEH) with a randomisation schedule in blocks of four. At the time of randomisation, the study personnel received a sealed opaque envelope with the treatment allocation number that indicated which vial to administer to the participant. Study medications were stored in individual, numbered opaque bags in the study refrigerator until use. Because we did not have an identical matching placebo, one of two study personnel administering the medication was aware of the treatment allocation. All participants were instructed to look away during the administration and the syringe had no identifiable features on to unmask allocation to the participant. After administering the medication, all further follow-up (phone calls and study visits) was completed by study personnel unaware of treatment allocation. A second copy of sealed envelopes with treatment allocation was stored in a locked cabinet for emergency purposes in case of the need for unmasking, and the study statistician maintained the masked randomisation list on a secure server. Aside from the unblinded nurse administering the study medication, all other study personnel and study participants remained masked to treatment allocation until unmasking of the study. Unmasked data were provided to the data and safety monitoring committee for scheduled safety review. No interim efficacy review was done. Analysis of study results was done after the statistical analysis plan was finalised, at which point the randomisation list linking data to study identification numbers was unmasked.
Procedures
Before receiving the study medication, participants were taught proper self-collection technique for mid-turbinate nasal swabs followed by witnessed collection of the initial swab. Following the administration of the study medication, participants were observed for 30–60 min. Participants were given written instructions and access to a video showing proper self-collection technique, and self-collection was observed by the study staff during remote follow-up visits using videoconferencing software whenever possible. Temperature monitoring and assessments of adverse events were done remotely by study personnel who were masked to study group along with self-collected mid-turbinate swabs on days 0, 1, 2, 3, 5, 7, 10, and 14. Haematological and biochemical tests were obtained on days 0, 3, 7, and 14. Quantitative SARS-CoV-2 results were generated for mid-turbinate swabs (appendix p 3). Anti-SARS-CoV-2 IgG antibodies to spike protein were measured on days 0, 3, 7, and 14 (using the Liason assay according to the manufacturer's instructions [DiaSorin, Saluggia, Italy]). The interferon lambda-4 genotype, associated with response to interferon alfa and spontaneous hepatitis C virus clearance, was assessed by sequencing rs368234815 in genomic DNA from whole blood and categorised as TT or non-TT (_G/T or _G/_G).13, 14, 15
Outcomes
The primary efficacy outcome was the proportion of individuals with a negative mid-turbinate swab for SARS-CoV-2 at day 7, which was chosen on the basis of available data at the time of study conception of clearance rates by day 7 with interferon beta16 and a pragmatic consideration that clearance beyond 7 days in an outpatient population would be of little practical benefit. The primary safety outcome was the incidence of treatment-emergent serious adverse events by day 14. Secondary outcomes included time to undetectable SARS-CoV-2 RNA, quantitative change in SARS-CoV-2 RNA over time, anti-SARS-CoV-2 IgG antibody positivity, the incidence and severity (mild, moderate, or severe) of adverse events, and the proportion of patients admitted to hospital by day 14 (a complete list of secondary outcomes is given in the appendix [pp 10–11]). Detailed directed and open-ended symptoms were assessed serially by phone. Because of overlap between symptoms of COVID-19 and potential peginterferon lambda-related adverse events, all symptoms were recorded and categorised, and any symptoms outside of the directed symptom assessment were considered adverse events. Laboratory adverse event severity was graded using the Common Terminology Criteria for Adverse Events version 5.0. An independent data and safety monitoring committee reviewed safety data after 10, 20, and 30 patients completed 7 days of post-treatment follow-up.
Statistical analysis
Given the results of early COVID-19 studies,16, 17 we estimated 40% viral clearance by day 7 in the placebo group and 80% in the peginterferon lambda group, requiring 30 patients per group, to achieve 80% power with an alpha of 0·05, accounting for 10% dropout.
Demographic and baseline clinical characteristics were summarised using means with standard errors or medians with IQRs for continuous variables, and proportions for categorical variables. The main efficacy outcome was analysed by a χ2 test following an intention-to-treat principle. Prespecified analysis of the primary endpoint adjusted for baseline viral load using bivariate logistic regression was done. Secondary outcomes were compared using χ2 tests for proportions, or Wilcoxon or linear regression controlling for baseline values. The effect of treatment, baseline factors, and viral load on clearance by day 7 were assessed using logistic regression and time-to-clearance was assessed using Kaplan-Meier analysis. Generalised estimating equations were used to analyse differences between the peginterferon lambda and placebo groups in symptom and adverse event severity grade, and generalised linear models were used for differences in laboratory and viral load patterns over time. All statistical analyses were done using SAS (version 9.4).
This trial is registered with ClinicalTrials.gov, NCT04354259.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
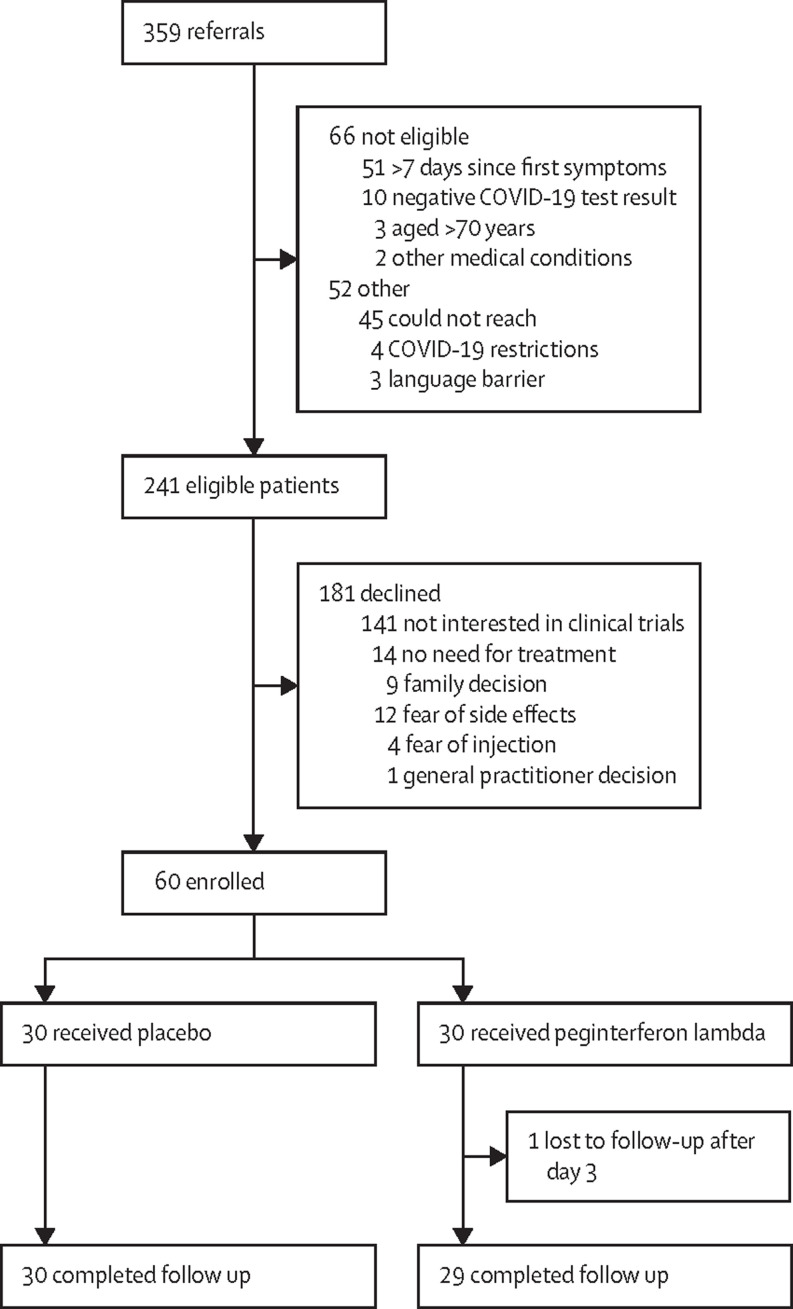
Between May 10, and Sept 4, 2020, of 359 individuals approached for the study, 118 (33%) did not meet inclusion or met exclusion criteria, and 181 (50%) eligible individuals declined to participate (figure 1 ). All 60 randomly assigned individuals (30 [50%] in the peginterferon lambda group and 30 [50%] in the placebo group) received an injection and 59 (98%) completed follow-up, with one lost to follow-up after day 3 in the peginterferon lambda group (figure 1). The median age was 46 years (IQR 32–54), 35 (58%) were female, and 11 (19%) were asymptomatic (table 1 ). 31 (52%) participants in both groups were white, with more non-Hispanic Black participants in the placebo group than in the peginterferon group (17% vs 3%; table 1). The mean time from symptom onset to randomisation was 4·5 days (SD 1·7). The median baseline SARS-CoV-2 RNA concentration was 6·71 log copies per mL (IQR 1·92–8·01), with ten (33%) individuals in the placebo group and five (17%) in the peginterferon lambda group having undetectable viral load on the day of randomisation. Other baseline characteristics were similar between groups (table 1).
Figure 1.
CONSORT diagram
Table 1.
Baseline characteristics
| Peginterferon lambda (n=30) | Placebo (n=30) | ||
|---|---|---|---|
| Sex | |||
| Female | 18 (60%) | 17 (57%) | |
| Male | 12 (40%) | 13 (43%) | |
| Age, years | 48 (30–53) | 39 (33–55) | |
| Race or ethnicity | |||
| White | 15 (50%) | 16 (53%) | |
| Black | 1 (3%) | 5 (17%) | |
| Asian | 8 (27%) | 7 (23%) | |
| Other | 6 (20%) | 2 (7%) | |
| Comorbidity* | 5 (17%) | 4 (13%) | |
| Body-mass index, kg/m2 | 27·3 (5·2) | 26·1 (4·2) | |
| Body-mass index category | |||
| <25 kg/m2 | 9 (30%) | 11 (37%) | |
| 25–30 kg/m2 | 15 (50%) | 13 (43%) | |
| >30 kg/m2 | 6 (20%) | 6 (20%) | |
| Interferon lambda 4 genotype | |||
| TT | 18 (60%) | 16 (57%) | |
| Non-TT | 12 (40%) | 12 (43%) | |
| Asymptomatic | 5 (17%) | 6 (20%) | |
| Time from symptom onset to injection, days | 4·3 (1·7) | 4·7 (1·7) | |
| Time from positive SARS-CoV-2 test to injection, days | 3·2% (1·1) | 3·3 (1·2) | |
| Baseline laboratory results | |||
| Haemoglobin, g/L | 14·7 (1·4) | 14·9 (1·6) | |
| White blood cells, ×109/L | 4·9 (2·1) | 5·1 (1·7) | |
| Lymphocytes, ×109/L | 1·5 (0·4) | 1·5 (0·5) | |
| Neutrophils, ×109/L | 2·9 (1·8) | 3·1 (1·6) | |
| Platelets, ×109/L | 221 (62) | 213 (64) | |
| Creatinine, μmol/L | 80 (14) | 81 (18) | |
| Alanine aminotransferase, U/L | 32 (16) | 39 (52) | |
| Aspartate aminotransferase, U/L | 28 (11) | 32 (24) | |
| Total bilirubin, μmol/L | 10 (5) | 12 (10) | |
| Baseline SARS-CoV-2 viral load, log copies per mL | 6·16 (3·14) | 4·87 (3·68) | |
| SARS-CoV-2 RNA undetectable at baseline | 5 (17%) | 10 (33%) | |
| SARS-CoV-2 RNA ≥106 copies per mL at baseline | 19 (63%) | 16 (53%) | |
| Anti-SARS-CoV-2 S protein IgG antibody at baseline† | 0/27 | 5/24 (21%) | |
Data are n (%), n/N (%), median (IQR), or mean (SD). SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Comorbidities were hypertension (n=6), diabetes (n=3), and heart disease (n=2).
Baseline antibody results were available in 51 participants (27 [53%] in the peginterferon lambda group and 24 [47%] in the placebo group).
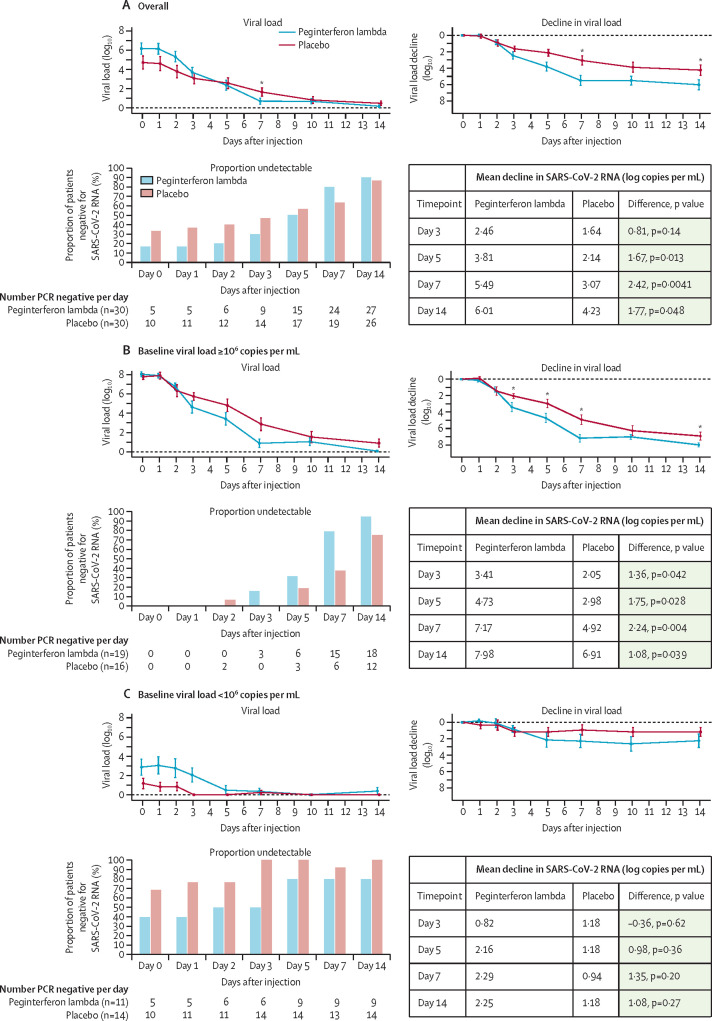
The baseline SARS-CoV-2 RNA concentration was higher in the peginterferon lambda group and was significantly associated with the probability of clearance by day 7 (odds ratio [OR] 0·69 [95% CI 0·51–0·87]; p=0·001). The mean decline in SARS-CoV-2 RNA was significantly greater in the peginterferon lambda group than in the placebo group (figure 2 ). By day 3, viral load decline was 0·81 log greater in the treatment group (p=0·14) than in the placebo group and this difference increased to 1·67 log copies per mL by day 5 (p=0.013) and 2·42 log copies per mL by day 7 (p=0·0041; figure 2). In absolute terms, by day 7 the viral concentration decreased by 5·5 log copies per mL in the treatment group compared with 3·1 log copies per mL in the placebo group (figure 2). At day 14, the difference in viral decline was 1·77 log copies per mL greater in the peginterferon lambda group than in the placebo group (p=0·048; figure 2). The difference in viral load decline between groups was greatest in individuals with baseline viral load at or above 106 copies per mL, with a decline by day 7 of 7·17 log copies per mL in the peginterferon lambda group compared with 4·92 log copies per mL in the placebo group (p=0·004; figure 2). A similar effect was observed when restricted to those with detectable virus at baseline (appendix p 4). Viral decline and clearance was rapid in participants with low viral load in both groups (figure 2).
Figure 2.
Proportion of patients negative for SARS-CoV-2 RNA and mean absolute and change in SARS-CoV-2 viral load
The proportion of patients negative for SARS-CoV-2 RNA per day after the injection, including all treated patients (A) and stratified by baseline viral load, above 106 copies per mL (B), and below 106 copies per mL (C). Figure shows the mean SARS-CoV-2 viral load and viral load decline from baseline for the peginterferon lambda and placebo groups per day after the injection and stratified by baseline viral load above or below 106 copies per mL. The error bars represent standard error. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *p<0·05 at indicated timepoints.
Overall, by day 7, 24 (80%) of 30 participants in the peginterferon lambda group were negative for SARS-CoV-2 RNA compared with 19 (63%) of 30 in the placebo group (p=0·15; figure 2). After adjusting for baseline viral load, peginterferon lambda treatment was significantly associated with clearance by day 7 (OR 4·12 [95% CI 1·15–16·73]; p=0·029; table 2 ).
Table 2.
Crude and adjusted odds of undetectable SARS-CoV-2 RNA at day 7 with peginterferon lambda compared with placebo treatment
|
All (n=60) |
≥106 copies per mL (n=35) |
||||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | ||
| Unadjusted | |||||
| Peginterferon lambda vs placebo | 2·32 (0·74–7·81) | 0·15 | 6·25 (1·49–31·06) | 0·012 | |
| Adjusted for individual covariates (bivariate) | |||||
| Baseline viral load | |||||
| Peginterferon lambda vs placebo | 4·12 (1·15–16·73) | 0·029 | 8·16 (1·76–51·5) | 0·0061 | |
| Viral load at baseline | 0·69 (0·51–0·87) | 0·001 | 0·60 (0·26–1·21) | 0·16 | |
| Baseline viral load by log increases | |||||
| 103 copies per mL | 1·45 (0·12–23·96) | 0·76 | NA | NA | |
| 104 copies per mL | 1·88 (0·24–18·8) | 0·53 | NA | NA | |
| 105 copies per mL | 2·43 (0·47–15·5) | 0·28 | NA | NA | |
| 106 copies per mL | 3·14 (0·81–14·0) | 0·096 | NA | NA | |
| 107 copies per mL | 4·05 (1·15–15·8) | 0·029 | NA | NA | |
| 108 copies per mL | 5·23 (1·32–23·5) | 0·018 | NA | NA | |
| 109 copies per mL | 6·76 (1·29–41·7) | 0·023 | NA | NA | |
| Duration of symptoms before randomisation | |||||
| Peginterferon lambda vs placebo | 2·57 (0·78–8·48) | 0·11 | 12·05 (1·83–79·3) | 0·0028 | |
| Day (each day increase) | 1·23 (0·88–1·73) | 0·23 | 1·71 (1·00–2·90) | 0·029 | |
| Sex | |||||
| Peginterferon lambda vs placebo | 2·36 (0·75–8·04) | 0·14 | 6·44 (1·50–33·5) | 0·012 | |
| Female vs male | 1·52 (0·47–5·23) | 0·49 | 2·13 (0·47–10·9) | 0·33 | |
| Age | |||||
| Peginterferon lambda vs placebo | 2·48 (0·78–8·61) | 0·13 | 9·42 (1·91–66·78) | 0·0047 | |
| Age (1-year increase) | 0·97 (0·93–1·02) | 0·23 | 0·95 (0·87–1·02) | 0·15 | |
| IFN4L genotype | |||||
| Peginterferon lambda vs placebo | 2·24 (0·70–7·72) | 0·18 | 7·66 (1·65–45·7) | 0·0085 | |
| IFN4L _G and TT/_G vs TT | 0·78 (0·22–2·54) | 0·68 | 0·46 (0·08–2·24) | 0·34 | |
| Body-mass index | |||||
| Peginterferon lambda vs placebo | 2·37 (0·75–8·06) | 0·14 | |||
| <25 kg/m2vs ≥30 kg/m2 | 1·59 (0·31–8·09) | 0·57 | |||
| 25·0–29·9 kg/m2vs ≥30 kg/m2 | 1·22 (0·26–5·31) | 0·79 | |||
| Comorbidity* | |||||
| Peginterferon lambda vs placebo | 2·35 (0·75–7·98) | 0·14 | 10·46 (2·05–82·6) | 0·0036 | |
| No vs yes | 1·41 (0·26–6·46) | 0·67 | 7·39 (0·78–99·7) | 0·082 | |
| Asymptomatic at presentation | |||||
| Peginterferon lambda vs placebo | 2·91 (0·88–10·8) | 0·081 | NA | NA | |
| Yes vs no | 2·13 (0·45–15·6) | 0·36 | NA | NA | |
IFN4L=Interferon lambda 4. NA=not applicable. SARS-CoV-2 RNA=severe acute respiratory syndrome coronavirus 2.
Comorbidities were hypertension, diabetes, chronic obstructive pulmonary disease, and heart disease.
The odds of viral clearance by day 7 with peginterferon lambda compared with placebo increased with every log increase in baseline viral load (figure 3 ). For those with baseline RNA of 106 copies per mL or greater, 15 (79%) of 19 individuals had undetectable virus at day 7 in the peginterferon lambda group, compared with six (38%) of 16 in the placebo group (OR 6·25 [95% CI 1·49–31·06]; p=0·012; figure 2), translating to a median time to viral clearance of 7 days (95% CI 6·2–7·8) with peginterferon lambda, compared with 10 days (7·8–12·2) with placebo (p=0·038; appendix p 4). Of those with baseline viral load at least 106 copies per mL who were still positive at day 7, participants in the peginterferon lambda group had lower viral loads than did those in the placebo group, with three (75%) of four at 104 copies per mL or lower, compared with six (60%) of ten above 105 copies per mL in the placebo group (appendix p 9). By contrast, in those with baseline viral load below 106 copies per mL, both groups cleared quickly; nine (82%) of 11 participants in the peginterferon lambda group and 13 (93%) of 14 in the placebo group had undetectable virus at day 7 (OR 0·35 [0·01–4·15]; p=0·40; figure 2).
Figure 3.
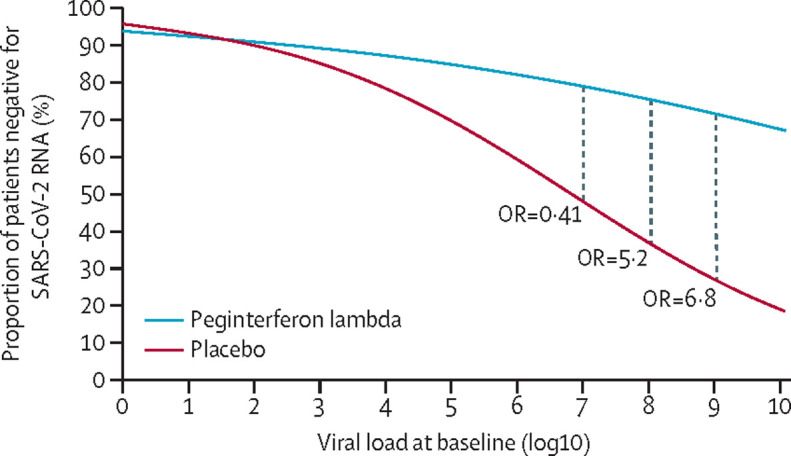
Probability of SARS-CoV-2 clearance by day 7 according to baseline viral load
The odds of clearance by day 7 in the peginterferon lambda group compared with in the placebo group for each baseline viral load (log copies per mL). OR=odds ratio. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
No baseline covariates modified the association between baseline viral load and treatment assignment with clearance by day 7 (table 2; appendix p 6). Participants who were asymptomatic were more likely to have baseline viral loads below 106 copies per mL than were those with symptoms (ten [91%] of 11 vs 13 [27%] of 49; p<0·0001). At randomisation, five (10%) of 51 participants with available samples were seropositive for SARS-CoV-2 S IgG antibodies, of whom four had undetectable SARS-CoV-2 RNA. Antibody positivity increased in both groups in the 14 days following drug administration (appendix p 7). The presence of antibodies at any timepoint was associated with a corresponding lower viral load, with the association weakening with time as more participants cleared virus (figure 2; appendix p 7).
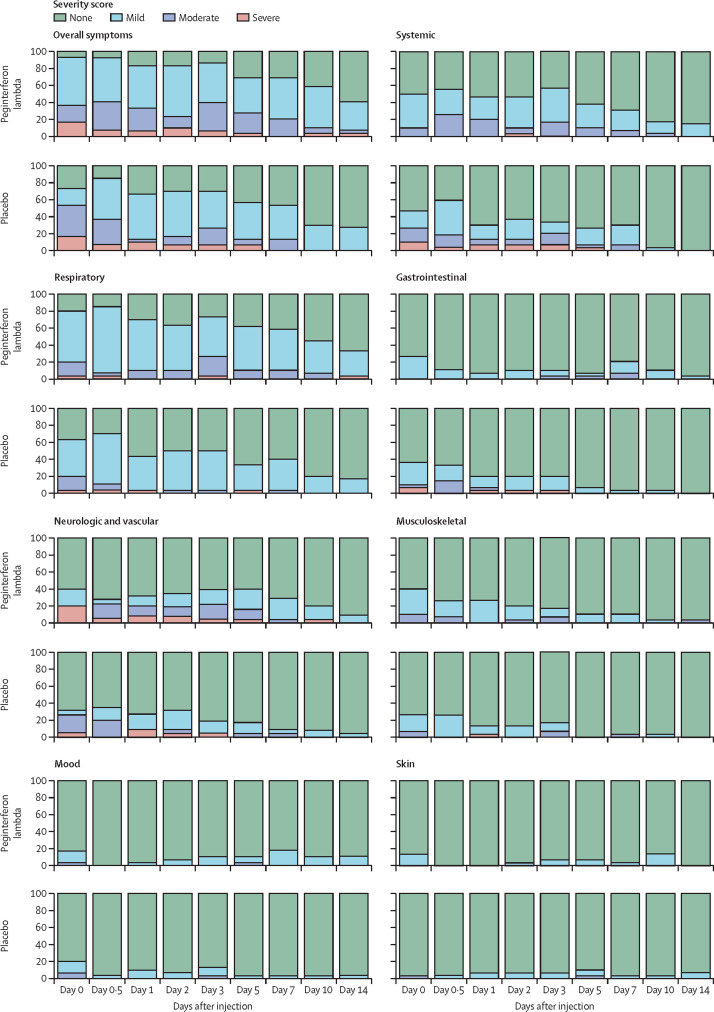
Symptoms were grouped into seven categories (appendix p 10) and reported as absent, mild, moderate, or severe. Respiratory and fever or systemic symptoms were most common in both groups (figure 4 ). Documented temperature above 38°C was rare and only reported beyond day 2 in the peginterferon lambda group (appendix p 8). Overall, most symptoms in both groups were mild to moderate and we found no difference in frequency or severity of any of the seven symptom categories between treatment groups (figure 4). Participants with high baseline viral loads (≥106 copies per mL) had higher symptom scores than did those with low baseline viral loads in all categories, except skin, respiratory, and mood symptoms (table 3 ).
Figure 4.
Symptoms in the peginterferon lambda and placebo groups per day
The proportion of participants reporting no, mild, moderate, or severe symptoms is shown for both groups. Symptoms were grouped into categories and the most severe ranking of any symptom in the specific category was used for each participant at each day. Overall significant declines of symptom severity over time were observed in all categories in both groups (p<0·0001), except skin and mood. No significant difference between treatments and no significant difference of decline of symptom severity between treatments were observed.
Table 3.
Association between symptoms and a viral load of 106 copies per mL or higher
| Odds ratio (95% CI)* | p value | |
|---|---|---|
| Overall | 5·88 (1·37–25·00) | 0·017 |
| Fever or systemic | 6·06 (1·48–25·00) | 0·012 |
| Respiratory | 4·93 (0·94–25·64) | 0·060 |
| Gastrointestinal | 11·9 (2·24–62·50) | 0·038 |
| Musculoskeletal | 5·81 (1·31–25·64) | 0·020 |
| Skin | 0·37 (0·05–2·99) | 0·36 |
| Mood | 8·00 (0·98–66·67) | 0·052 |
| Neurological and vascular | 52·63 (1·93–infinity) | 0·019 |
Association with high viral load (yes vs no).
A symptom was graded as severe on 20 occasions by seven (23%) patients in the peginterferon lambda group and on 30 occasions by seven (23%) patients in the placebo group with different patterns. In the peginterferon lambda group, severe symptoms were most commonly loss of taste and smell, whereas in the placebo group, fever and systemic symptoms were most frequently rated as severe (appendix p 12). Symptoms improved over time in both groups (appendix p 11). Using a linear generalised linear mixed model for time to improvement, the rate of improvement was generally similar between groups (appendix p 11); however, respiratory symptoms improved faster with peginterferon lambda treatment compared with placebo (OR 4·67 [95% CI 0·91–23·91]; p=0·064). This effect was more evident in those with higher viral load (≥106 copies per mL) for whom the OR with treatment compared with placebo was 5·88 (0·81–42·46; p=0·079) compared with those with low viral loads, for whom the OR was 3·67 (0·19–70·11; p=0·39).
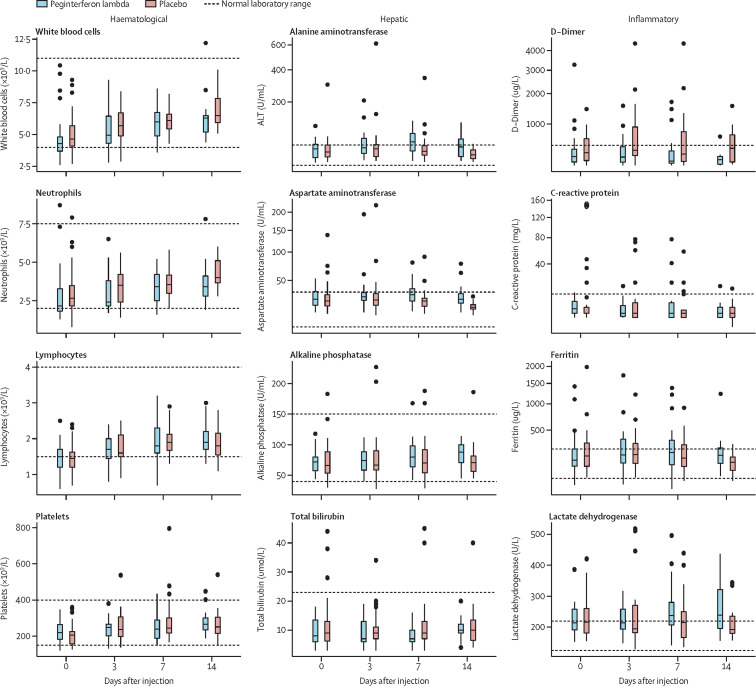
Laboratory adverse events were mild and similar between groups (table 4 ). Aminotransferase concentrations were increased at baseline in three (11%) participants in both groups and increased mildly, more so in the peginterferon lambda group (table 4). However, only two individuals met the threshold of grade 3 increase, one in each group. No other grade 3 or 4 laboratory adverse events were reported in the peginterferon lambda group (table 4). We found no increases in bilirubin concentrations that were associated with increases in aminotransferase concentrations. Haemoglobin, white blood cell count, and platelet counts were similar, with no episodes of myelosuppression in either group (table 4). D-dimer concentrations were increased in both groups at baseline but declined over time only in the peginterferon lambda group (at day 7, 841 μ/L in the placebo group vs 437 μ/L in the peginterferon lambda group; p=0·017; figure 5 ). Other inflammatory markers including ferritin and C-reactive protein concentrations were increased at baseline in both groups and changed minimally over time (figure 5).
Table 4.
Summary of AEs and SAEs by treatment group.
| Peginterferon lambda (n=30) | Placebo (n=30) | |
|---|---|---|
| Severe symptoms | ||
| Reports (number of participants*) | 20 (7) | 30 (7) |
| AEs | 2 | 1 |
| SAEs | 1 | 1 |
| Treatment-related AEs | 0 | 0 |
| Treatment-related SAEs | 0 | 0 |
| Emergency room visits | 1 | 4 |
| Hospital admissions | 1 | 1 |
| Laboratory abnormalities (grade 3 or 4) | ||
| Haemoglobin | 0 | 0 |
| White blood cells | 0 | 0 |
| Lymphocytes | 0 | 0 |
| Neutrophils | 0 | 1 |
| Platelets | 0 | 0 |
| Creatinine | 0 | 0 |
| Alanine aminotransferase | 1 | 3 |
| Aspartate aminotransferase | 1 | 1 |
| Total bilirubin | 0 | 0 |
AE=adverse event. SAE=serious adverse event.
Some participants reported multiple severe symptoms.
Figure 5.
Laboratory values in the peginterferon lambda and placebo groups per day
The median and IQRs for haematological, hepatic, and inflammatory markers at days 0, 3, 7, and 14 in each group are shown.
Adverse events outside of the directed symptom categories occurred in one participant in the placebo group (rectal bleeding) and in two who received peginterferon lambda (confusion and pneumonia). All were deemed unrelated to treatment. Five participants sought care in the emergency room by day 14; four in the placebo group and one in the peginterferon lambda group (table 4). All were seen for worsening respiratory symptoms and one person in each group was admitted to hospital, accounting for the one serious adverse event in each group. A participant in the placebo group was admitted to hospital on day 1 post-injection with progressive dyspnoea attributed to worsening COVID-19, which improved over time leading to discharge on day 3. One participant in the peginterferon lambda group was admitted to hospital on day 14 with dyspnoea and was found to have a pulmonary embolism necessitating anticoagulation. No deaths occurred in either group.
Discussion
Treatment with a single subcutaneous injection of peginterferon lambda accelerated viral load decline and, after controlling for baseline viral load, reduced time to viral clearance in outpatients with COVID-19. The treatment effect was most apparent in those with high baseline viral loads. Peginterferon lambda was well tolerated, with similar symptoms to those treated with placebo.
Results for SARS-CoV-2 diagnostic testing are routinely reported dichotomously as positive or negative, without viral load quantification. Cycle threshold (Ct) values are sometimes reported, but are only semi-quantitative, and vary by assay and even by run, limiting comparisons between samples. Our use of plasmid-derived cDNA standards with every PCR run allowed for quantification of SARS-CoV-2 RNA in each specimen, permitting direct comparison between samples. Quantification is useful clinically because higher viral levels have been correlated with greater severity of COVID-1918, 19 and increased infectivity.20 When individuals have recovered from infection, they might have persistently very low levels of RNA detected at very high Ct values (>33), which are not infectious.21
We found a clear antiviral effect of peginterferon lambda. Type III interferons lead to a slower but more sustained induction of interferon-stimulated genes, with peak responses seen after approximately 72 h, aligning with the onset of the antiviral response.22 Studies of monoclonal antibodies for outpatients with COVID-19 have also shown antiviral effects. In the early reports of the Regeneron monoclonal antibody cocktail, the difference in the decline in viral load with treatment compared with placebo by day 7 was 0·51 log copies per mL for the high dose and 0·23 log for the low dose group.23 The differences were greater in those who were seronegative for SARS-CoV-2 antibodies at baseline, with a difference of 0·60 log copies per mL with the high dose and 0·51 log copies per mL with the low dose, compared with the placebo group at day 7.23 Similar to our findings, greater antiviral effects were seen in those with high baseline viral load. Chen and colleagues reported that an intravenous infusion of neutralising antibody LY-CoV555 led to a reduction in viral load that was 0·53 log copies per mL greater with treatment than with placebo at day 11.3 Notably, in both of these studies, clinical benefits were seen with treatment in terms of reduced medical visits or hospitalisation,3, 23 highlighting the importance of even modest acceleration in viral load decline.
We found that the odds of clearance were greater in all study participants with peginterferon lambda than with placebo after controlling for baseline viral load. The effect of peginterferon lambda was most evident when the baseline viral load was above 106 copies per mL. Although the specific threshold for transmissible virus is unknown, using a standard infectivity assay, Bullard and colleagues reported that at Ct values above 24, corresponding to approximately 106–107 copies per mL, infectious virus could not be detected.20 We observed that in individuals with low viral load, irrespective of their assigned group, spontaneous clearance occurred rapidly and almost universally by day 7. This finding does not indicate a lack of effectiveness of peginterferon lambda at low viral loads, but rather that when low levels of virus were detected, treatment was not required because clearance was imminent. Rapid clearance in those with low baseline viral loads explains the apparent absence of an antiviral effect seen by Jagannathan and colleagues24 in another trial of peginterferon lambda in outpatients, as the median Ct value at baseline was 30 with at least 75% of viral loads below 5·5 log copies per mL in their study compared with median baseline Ct value of 23·7 and 35 (58%) of 60 patients with viral loads above 6·0 log copies per mL in our trial.
Five (10%) of 51 participants, all in the placebo group, had already developed SARS-CoV-2-specific antibodies by the day of randomisation. Similarly, in the REGN-COV2 monoclonal antibody cocktail trial,23 45% of the study population was positive for SARS-CoV-2 IgG antibodies by day 0, which was associated with low viral loads and no benefit from therapy. Understanding why some but not all infected individuals develop rapid IgG antibodies that are associated with milder disease course clearly warrants further investigation. Antivirals will probably be most effective early in infection and maximally beneficial to those with high viral loads. Ideally, antivirals would be given shortly after disease onset because rapid reduction of viral load is likely to lower the risk of clinical deterioration and might reduce transmission, translating into important public health benefits.
Several studies have investigated the interaction between SARS-CoV-2 and the endogenous interferon response. Like many viral pathogens, SARS-CoV-2 appears to impair induction of interferon, with low levels of type I and type III interferon production seen with ex-vivo infection of lung slices by severe acute respiratory syndrome coronavirus and to a greater extent with SARS-CoV-2.25 This finding is consistent with detailed immune profiling in patients showing an impaired type I interferon response in patients with severe, compared with mild, COVID-19.26 Patients with inborn errors of interferon production and those with antibodies to interferon alfa also have a much higher risk of severe disease with SARS-CoV-2 infection.27, 28 On the basis of the rationale that low interferon production is associated with severe disease, interferon treatment has been proposed. Early studies from China investigated subcutaneous interferon beta treatment. Although studies found a suggestion of accelerated viral clearance with interferon beta treatment, the studies were uncontrolled, non-randomised, and included co-interventions with other drugs (eg, hydroxychloroquine, lopinavir and ritonavir, umifenovir), making drawing strong conclusions difficult.16, 29, 30 A randomised open-label study of lopinavir and ritonavir with or without interferon beta in patients admitted to hospital showed that interferon treatment decreased the duration of viral shedding and shortened the duration of symptoms.31 However, early reports from the WHO SOLIDARITY trial32 found no clinical benefit to interferon beta treatment in hospitalised patients. Whether the lack of benefit in individuals with more severe manifestations of COVID-19 relates to introduction of therapy late in the course of illness or possibly to the proinflammatory side-effects of type I interferon is unknown. In mice infected with severe influenza, treatment with interferon alfa increased mortality compared with control mice, by promoting cytokine release syndrome, whereas treatment with interferon lambda was associated with improved survival.8 To diminish the risk of systemic inflammation from type I interferon use, Monk and colleagues33 investigated inhaled nebulised interferon beta-1a in patients admitted to hospital with moderate COVID-19. The authors showed a substantial reduction in clinical worsening by day 15 with good tolerability.33 Antiviral effects were not reported. Because of the limited distribution of the interferon lambda receptor, we felt that interferon lambda might provide a safer approach to interferon treatment in patients with COVID-19 with similar antiviral effects but a reduced risk of potentially harmful proinflammatory responses.7
Despite the clear antiviral effect of peginterferon lambda, we did not see a marked difference in clinical outcomes. To translate acceleration of viral clearance to clear clinical benefit, the study would likely need to be enriched for those at higher risk of severe disease, such as individuals older than 65 years and those with comorbidities.34 Notably, the antiviral effect of peginterferon lambda compared with that of placebo was similar in those with comorbidities and the entire study population. Interestingly, among those still RNA positive at day 7, the viral concentrations were lower in the peginterferon lambda group than in the placebo group, which might have clinical relevance given the finding in the monoclonal antibody outpatient trial that patients with a higher viral load at day 7 were more likely to require hospitalisation.3 In addition to the risk of disease progression, lowering viral loads might reduce the risk of transmission. In those with high baseline viral load, most participants treated with placebo had detectable virus at day 7, with most of these continuing to exceed 105 copies per mL, raising concern of persistent shedding of competent virus. By contrast, few participants who received peginterferon lambda had detectable virus at day 7, all with viral loads below 106 copies per mL. To identify those most likely to benefit from this therapy, either quantitative testing could be introduced or a qualitative assay, ideally a point-of-care test, could be titrated to achieve an analytical sensitivity of approximately 105–106 copies per mL, allowing for immediate risk stratification and identification of the need for treatment. Indeed, this quantitative detection of SARS-CoV-2 could probably already be achieved using available rapid antigen tests, with detection sensitivities in the range of 10–50 000 copies per mL, safely below the infectious threshold but avoiding those with very low viral loads who are unlikely to require any intervention.35 More Black participants were in the placebo group than in the treatment group, a population typically shown to have reduced responsiveness to type I interferon for treatment of viral hepatitis.36, 37 However, similar proportions of patients in each group had the treatment-responsive interferon lambda genotype (TT), which is strongly associated with response to interferon alfa for hepatitis C infection and thought to explain most of the differential response to interferon by race.14 No effect of the interferon lambda genotype was observed on baseline viral load or response to treatment in the interferon lambda group. A high proportion of eligible individuals declined to participate in the study, probably because of the listed adverse event profile, which reflected weekly injections for a year of treatment for hepatitis B and C infections.11, 12
Peginterferon lambda was well tolerated with no identified safety concerns. Side-effects of peginterferon lambda overlap with COVID-19 symptoms, making distinguishing whether adverse events were related to treatment or the infection difficult. As has been reported previously,3 symptoms were more prominent in those with higher viral loads. With detailed serial symptom assessment, we found that symptoms improved in both groups over time. Notably, among those who were asymptomatic at baseline, we found no difference in the number of adverse events between the treatment and placebo groups. Mild and transient aminotransferase increases were seen more frequently in the peginterferon lambda group than in the placebo group, which has been reported previously.11 D-dimer concentrations reduced with peginterferon lambda treatment, which might be relevant given the association of high concentrations with more severe disease and increased all-cause mortality.34, 38, 39 The side-effect profile and absence of haematological toxicity is consistent with the improved tolerability of type III interferons compared with that of type I interferons.11
Study limitations include the small sample size, although clearance rates in those with high viral loads were consistent with the power calculations. Based on viral load and antibody data at the baseline visit, several participants were probably clearing the infection, an observation reported in other studies of outpatients with COVID-19.3, 23 Ideally with the introduction of point-of-care testing, treatment could be initiated promptly at the time of diagnosis, which was not possible in this study because of delays in reporting times of positive results and required time for recruitment and consent. The benefit of treatment was more pronounced in the group with high baseline viral load than in those with low baseline viral load. Quantitative assays or calibrated qualitative tests for COVID-19 could identify those most likely to benefit from therapy. As a phase 2 trial, the study was not powered to show differences in transmission, which are hard to document, or hospitalisation and mortality, which would require a larger study enriched for those at high risk of complications. However, as a first step to confirm efficacy, viral clearance is a key relevant endpoint. Having shown safety and efficacy in an ambulatory cohort, we and others are now investigating the clinical benefit of peginterferon lambda in patients admitted to hospital with COVID-19.
In conclusion, peginterferon lambda is among the first antiviral therapies to show benefit among outpatients with COVID-19. Peginterferon lambda accelerated viral clearance, particularly in those with high baseline viral load. This treatment might have potential to avert clinical deterioration, shorten the duration of infectiousness, and reduce isolation time, with substantial public health and societal effects.
Data sharing
Deidentified data, a data dictionary, and the full protocol will be made available upon publication to investigators after approval of a relevant protocol by the principal investigator (JJF) and signing of a data use agreement. Requests for data should be sent to jordan.feld@uhn.ca.
Acknowledgments
Acknowledgments
This study was supported by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund. Medication was supplied by Eiger BioPharmaceuticals. We thank the volunteers who participated in study, as well as the clinical, laboratory, and administrative teams from the following referral centres: University Health Network, Michael Garron Hospital, William Osler Health System, Trillium Health Partners, St Michael's Hospital, North York General Hospital, and Sunnybrook Health Sciences Centre, ON, Canada.
Contributors
JJF, CK, MJB, DHST, AH, BC, JSG, and BEH contributed to study conception and design. JJF, CK, MJB, RAK, CL, SMB, AKB, JP, JM, DHST, TC, DK, AC, BO, SN, JB, RH, DS, AP, WA, BB, DMS, JC, RH, HM, and MAZ contributed to data acquisition. JJF, CK, MJB, RAK, WA, BB, and BEH verified the data. JJF, CK, MJB, RAK, BEH, and HLAJ contributed to the drafting of the manuscript. CK, MJB, RAK, SMB, AKB, JP, DHST, BC, BO, WA, IC, CH, DMS, DLT, JSG, AJG, HLAJ, and BEH contributed to critical revision of the manuscript. JJF, CK, MJB, HLAJ, and BEH did the statistical analysis. JJF, CK, MJB, RAK, SN, JB, RH, DS, WA, AP, BB, and DMS supervised the study. JJF, CK, MJB, and BEH verified the data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
JJF reports research support unrelated to this work from Eiger BioPharmaceuticals; grants and personal fees from AbbVie, Gilead; personals fees from Abbott, Enanta, and Roche; and grants from Janssen and Wako/Fujifilm, all outside of the submitted work. MJB has received consulting fees and grants from AbbVie and Gilead, outside of the submitted work. DHST reports grants from Canada Research Chair programmes conducted during the study; and grants from Gilead, ViiV Healthcare, and AbbVie, outside of the submitted work. JP reports grants from Gilead, outside of the submitted work. BC reports grants from Nubiyota and Sanofi, outside of the submitted work. IC and CH are employees of Eiger BioPharmaceuticals. JSG is a board member and founder of Eiger BioPharmaceuticals, in which he has an equity interest, and is an inventor on a patent application for the use of interferon lambda to treat coronavirus infections. AJG reports grants from Gilead and Janssen; personal fees from Roche and SQZ Biotech; and grants and personal fees from GlaxoSmithKine (GSK). HLAJ reports grants from AbbVie and Bristol Myers Squibb; personal fees from Vir Biotechnology, Viroclinics, Enyo, Arena, and GSK; and grants and personal fees from Arbutus, Gilead, Janssen, Medimmune, Merck, and Roche all outside of the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2029849. NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki FY, Macleod MD, Paggiaro P, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123–129. doi: 10.1093/jac/dkg007. [DOI] [PubMed] [Google Scholar]
- 5.Carrat F, Duval X, Tubach F, et al. Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households. Antivir Ther. 2012;17:1085–1090. doi: 10.3851/IMP2128. [DOI] [PubMed] [Google Scholar]
- 6.Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prokunina-Olsson L, Alphonse N, Dickenson RE, et al. COVID-19 and emerging viral infections: the case for interferon lambda. J Exp Med. 2020;217 doi: 10.1084/jem.20200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson S, McCabe TM, Crotta S, et al. IFNλ is a potent anti-influenza therapeutic without the inflammatory side effects of IFNα treatment. EMBO Mol Med. 2016;8:1099–1112. doi: 10.15252/emmm.201606413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinnon KH, 3rd, Leist SR, Schäfer A, et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586:560–566. doi: 10.1038/s41586-020-2708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderheiden A, Ralfs P, Chirkova T, et al. Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol. 2020;94:e00985–e001020. doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muir AJ, Arora S, Everson G, et al. A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection. J Hepatol. 2014;61:1238–1246. doi: 10.1016/j.jhep.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Chan HLY, Ahn SH, Chang TT, et al. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: a randomized phase 2b study (LIRA-B) J Hepatol. 2016;64:1011–1019. doi: 10.1016/j.jhep.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song S, Zhang D, Qian Z, Li T, Shen Y, Lu H. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang University. 2020 doi: 10.3785/j.issn.1008-9292.2020.03.03. htps://doi.org.10.3785/j.issn.1008-9292.2020.03.03 published online March 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8:e70. doi: 10.1016/S2213-2600(20)30354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bullard J, Dust K, Funk D, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syedbasha M, Egli A. Interferon lambda: modulating immunity in infectious diseases. Front Immunol. 2017;8:119. doi: 10.3389/fimmu.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinreich DM, Sivapalasingam S, Norton T. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagannathan P, Andrews JR, Bonilla H, et al. Peginterferon lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. medRxiv. 2020 doi: 10.1101/2020.11.18.20234161. published online Nov 23. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu H, Chan JF, Wang Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020;71:1400–1409. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b treatment for COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie X, Jiang Y, Zeng Y, Liu H. Combination antiviral therapy with lopinavir/ritonavir, arbidol and interferon-α1b for COVID-19. Antivir Ther. 2020 doi: 10.3851/IMP3362. published online June 4. [DOI] [PubMed] [Google Scholar]
- 31.Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan H, Peto R, Quarraisha AK, et al. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity trial results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. published online Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9:196–206. doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scohy A, Anantharajah A, Bodéus M, Kabamba-Mukadi B, Verroken A, Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy KR, Hoofnagle JH, Tong MJ, et al. Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group. Hepatology. 1999;30:787–793. doi: 10.1002/hep.510300319. [DOI] [PubMed] [Google Scholar]
- 37.Luo S, Cassidy W, Jeffers L, Rajender Reddy KR, Bruno C, Howell CD. Interferon-stimulated gene expression in black and white hepatitis C patients during peginterferon alfa-2a combination therapy. Clin Gastroenterol Hepatol. 2005;3:499–506. doi: 10.1016/s1542-3565(04)00615-9. [DOI] [PubMed] [Google Scholar]
- 38.Vidali S, Morosetti D, Cossu E, et al. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review. ERJ Open Res. 2020;6:00260–02020. doi: 10.1183/23120541.00260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naymagon L, Zubizarreta N, Feld J, et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb Res. 2020;196:99–105. doi: 10.1016/j.thromres.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data, a data dictionary, and the full protocol will be made available upon publication to investigators after approval of a relevant protocol by the principal investigator (JJF) and signing of a data use agreement. Requests for data should be sent to jordan.feld@uhn.ca.