Abstract
Minimal phenotyping refers to the reliance on the use of a small number of self-reported items for disease case identification, increasingly used in genome-wide association studies (GWAS). Here we report differences in genetic architecture between depression defined by minimal phenotyping and strictly defined major depressive disorder (MDD): the former has a lower genotype-derived heritability that cannot be explained by inclusion of milder cases and a higher proportion of the genome contributing to this shared genetic liability with other conditions than for strictly defined MDD. GWAS based on minimal phenotyping definitions preferentially identifies loci that are not specific to MDD, and, although it generates highly predictive polygenic risk scores, the predictive power can be explained entirely by large sample sizes rather than by specificity for MDD. Our results show that reliance on results from minimal phenotyping may bias views of the genetic architecture of MDD and impede the ability to identify pathways specific to MDD.
A key requisite for robust identification of genetic risk loci underlying psychiatric disease is the use of an appropriately large sample. However, the high cost of phenotyping limits sample collection1. One solution for reducing the burden of case identification is to use information from hospital registers2 or individuals’ self-reported symptoms, help seeking, diagnoses or medication. We refer to the latter strategy as ‘minimal phenotyping’, as it minimizes phenotyping costs and reduces data to a single or few self-reported answers.
However, apart from detecting more GWAS) loci3-5 (Supplementary Table 1), the consequences of sacrificing symptomatic information for genetic analyses have rarely been investigated. The consequences may be particularly important for MDD because of its phenotypic and likely etiological heterogeneity6, its high degree of comorbidity with other psychiatric diseases7 and the substantial discrepancies between self-assessment using symptom scales and diagnoses made with full diagnostic criteria8. While a majority of the population self-identifies as having one or two depressive symptoms at any one time, only between 9% and 20% of the population has sufficient symptoms to meet criteria for lifetime occurrence of MDD8-10. Furthermore, there are high rates of false positives when diagnoses are made without applying diagnostic criteria11, and antidepressants are prescribed for a wide range of conditions other than MDD12-14. As such, a cohort of MDD cases obtained either through the use of either self-reported illness or prescribed treatment may yield a sample that is not representative of the clinical disorder but enriched in those with nonspecific subclinical depressive symptoms and depression secondary to a comorbid disease.
By comparing the genetic architecture of minimal phenotyping definitions of depression with those using full diagnostic criteria for MDD in the UK Biobank15, a community-based survey of half a million men and women, we assess the implications of a minimal phenotyping strategy for GWAS in MDD. We find that MDD defined by minimal phenotyping has a large nonspecific component, and if GWAS loci from these definitions are chosen for follow-up molecular characterization, they may not be informative about biology specific to MDD.
Results
Definitions of depression in UK Biobank.
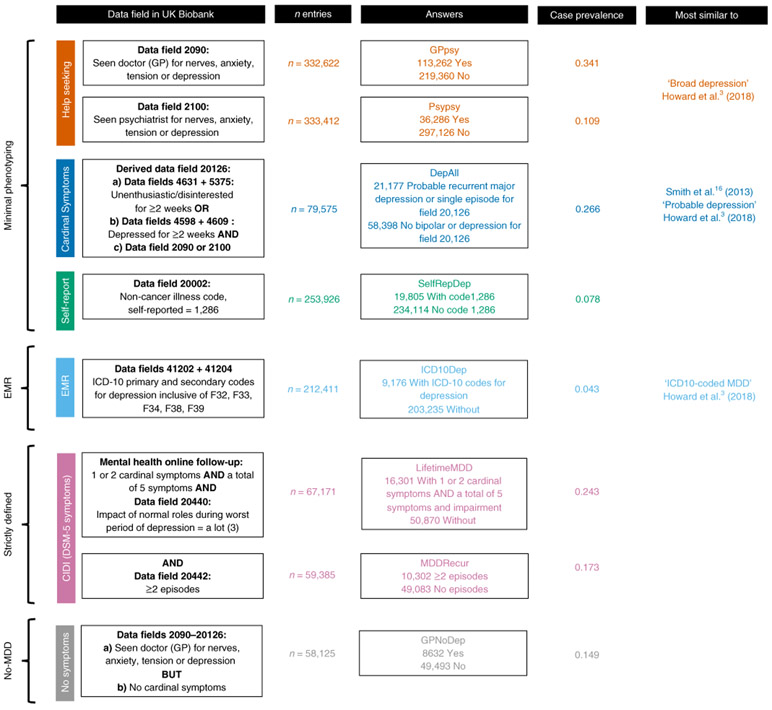
We identified five ways that MDD could be defined in the UK Biobank. First, self-reports of participants seeking medical attention for depression or related conditions provided ‘help-seeking’ definitions of MDD (referred to as ‘broad depression’ in a previous GWAS3). Second, participants were diagnosed with ‘symptom-based’ MDD if, in addition to meeting help-seeking criteria, they reported ever experiencing one or more of the two cardinal features of depression (low mood or anhedonia) for at least 2 weeks16. Third, a ‘self-report’ definition of MDD was based on participants’ self-reports of all past and current medical conditions to trained nurses. Fourth, an electronic medical record (EMR) definition was derived from the International Classification of Diseases, Tenth Revision (ICD-10) primary and secondary illness codes in electronic health records. Finally, a ‘CIDI-based’ diagnosis of lifetime MDD was available from individuals who answered an online ‘Mental Health Follow-up’ questionnaire (MHQ)17 based on the Composite International Diagnostic Interview Short Form (CIDI-SF)18, which included the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for MDD (Supplementary Note, Supplementary Fig. 1 and Supplementary Table 2). None of the definitions used trained interviewers applying structured clinical interviews, and only the last applied operationalized criteria, including symptoms, length of episode (more than 2 weeks) and impaired social, occupational or educational function. From here on, we refer to the first three definitions as ‘minimal’, the fourth as ‘EMR-based, and the fifth as ‘strictly’ defined MDD (Supplementary Note). We also included a category of participants who met the help-seeking definition (part of broad depression in Howard et al.3) but failed to meet the symptom-based definition (as they had neither of the two cardinal symptoms of depression: depressed mood or a loss of interest or pleasure in daily activities for more than 2 weeks). We refer to this group as ‘no-MDD’ (described in detail in the Supplementary Note and Supplementary Table 3). Figure 1 outlines the different diagnostic categories and the number of samples that each group contained.
Fig. 1 ∣. Definitions of depression in UK Biobank.
This figure shows the different definitions of MDD in the UK Biobank and the color coding used consistently in this paper. The minimal phenotyping definitions of depression are shown in red for help-seeking definitions derived from the Touchscreen Questionnaire; blue for symptom-based definitions derived from the Touchscreen Questionnaire; and green for the self-report-based definition derived from the Verbal Interview. The EMR definition of depression is shown in orange for definitions based on ICD-10 codes. Strictly defined MDD is shown in purple for CIDI-based definitions derived from the Online Mental Health Follow-up. The no-MDD definition is shown in brown for GPNoDep, containing cases in help-seeking definitions that did not have cardinal symptoms for MDD. The data fields in the UK Biobank relevant for defining each phenotype are shown in ‘Data field in UK Biobank’; the number of individuals with non-missing entries for each definition are shown in ‘n entries’; the qualifying answers for cases and controls are shown in ‘Answers’; the case prevalence in each definition is shown in ‘Case prevalence’; and the study and definitions of depression most similar to our definitions are shown in ‘Most similar to’. The similarities and differences between help-seeking, EMR and symptom-based definitions in comparison to previously reported definitions of depression can be found in the Supplementary Note.
All definitions were based on recall of episodes or symptoms of depression by participants in the UK Biobank. As priming of recall by current mood affects the reliability of such reports19-21, we emphasize that each definition is noisy and can be interpreted as being enriched for individuals truly fulfilling its criteria. We explore further characteristics of all definitions and considerations in their GWAS in the Supplementary Note, Supplementary Figs. 2-5 and Supplementary Tables 2-11.
Minimal phenotyping definitions of depression are epidemiologically different from strictly defined MDD.
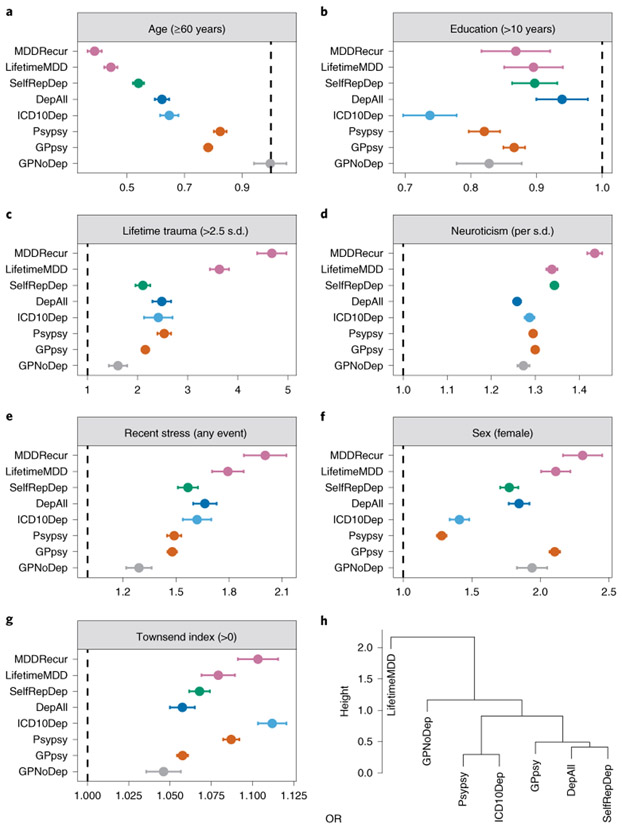
We assessed whether known risk factors for MDD were similar between definitions of depression22. Figure 2a-g shows the mean effect (odds ratio, OR) with confidence intervals of each of the following: sex23,24, age25, educational attainment26-28, socioeconomic status29, neuroticism24,30, experience of stressful life events in the 2 years leading up to the baseline assessment and cumulative traumatic life events preceding assessment31,32 (Supplementary Note and Supplementary Table 12). Estimates of the risk factor effect sizes differed substantially, and often highly significantly, as shown by the confidence intervals in Fig. 2. These may reflect differences in methods of ascertainment or underlying pathology between definitions of depression. Next, we asked whether differences in risk factors could be used to classify definitions of depression. We applied a clustering algorithm and found that all minimal phenotyping definitions of depression clustered separately from strictly defined MDD (Fig. 2h).
Fig. 2 ∣. Relationship between definitions of depression and environmental risk factors.
a–g, Forest plots of ORs of known environmental risk factors and different types (categories) of definitions of depression in the UK Biobank (Definition) from logistic regression, using UK Biobank assessment center, age, sex and years of education as covariates to control for potential geographic and demographic differences between environmental risk factors, except when they were tested. The lifetime trauma measure was derived from the Online Mental Health Follow-up (Supplementary Note and Supplementary Table 7); the Townsend deprivation index, years of education, sex, age, recent stress and neuroticism were derived from Touchscreen Questionnaire (Supplementary Note). h, Hierarchical clustering of definitions of depression in the UK Biobank using ORs with environmental risk factors, performed using the hclust function in R; ‘height’ refers to the Euclidean distance between MDD definitions at the ORs of all six risk factors. MDDRecur was not included in this clustering analysis as it is a subset of the LifetimeMDD definition. The statistics used to generate these plots are presented as source data.
Minimal definitions of depression are not just milder or noisier versions of strictly defined MDD.
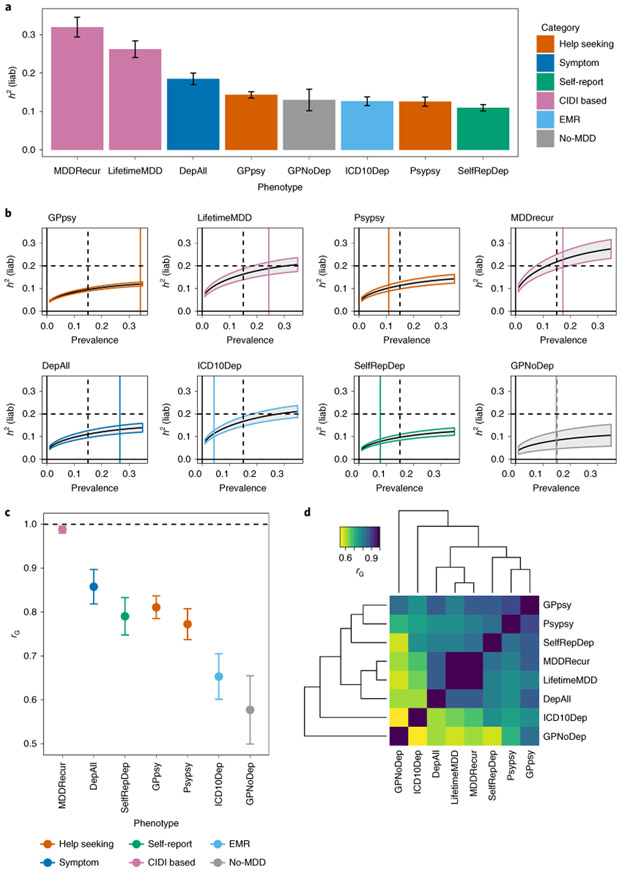
Depression defined by minimal phenotyping had lower SNP-based heritabilities (h2SNP) than more strictly defined versions (Fig. 3a). Self-report(SelfRepDeph2SNP = 11%, standard error (s.e.) = 0.85%) and help-seeking (Psypsy h2SNP = 13%, s.e. = 1.18%; GPpsy h2SNP = 14%, s.e. = 0.81%) definitions had heritabilities of 15% or less. By contrast, strictly defined MDD (LifetimeMDD) had a much higher h2SNP of 26% (s.e. = 2.15%); imposing the further criterion of recurrence brought the h2SNP up to 32% (s.e. = 2.56%). Other definitions had intermediate h2SNP. All h2SNP values were estimated on the liability scale using phenotype correlation-genotype correlation (PCGC)33 (Supplementary Note), and the trend held regardless of the method used33-36 (Supplementary Note and Supplementary Table 13). We further verified that the trend could not be explained by potential case prevalence misestimations (Fig. 3b, Supplementary Note, Supplementary Fig. 3 and Supplementary Table 13) and was not affected by regions of high linkage disequilibrium (LD) or complexity37 (Supplementary Note and Supplementary Fig. 3). We compared h2SNP estimates from previous studies of MDD4,38,39 (Supplementary Fig. 6) with our results and found that they fit squarely into the trend we observed: the less strict the criteria used to diagnose MDD, the lower the h2SNP.
Fig. 3 ∣. SNP heritability and genetic correlation estimates among definitions of MDD in UK Biobank.
a, h2SNP estimates from PCGC18 on each of the definitions of MDD in the UK Biobank (Methods). h2SNP (represented as h2(liab)) was converted to the liability scale40,63 using the observed prevalence of each definition of depression in the UK Biobank as both population and sample prevalence (Supplementary Table 4). Error bars show the s.e. of the estimates. b, h2SNP estimates of definitions of MDD in the UK Biobank from LDSC using logistic regression summary statistics on all SNPs with minor allele frequency (MAF) > 5% (Methods), transformed to the liability scale assuming a range of population case prevalence values, from 0 to 0.5. We do not show results for case prevalence from 0.5 to 1, as they would mirror those from 0 to 0.5, with shaded area representing the s.e. of the estimates. We indicate with a black vertical dashed line the population prevalence of 0.15, used in PGC1-MDD; a colored vertical line shows the population prevalence of each definition of depression in the UK Biobank. We also indicate with a black horizontal dashed line the arbitrary liability-scale h2SNP of 0.2, previously estimated for MDD in PGC1-MDD. Using this, we show that at no prevalence would minimal phenotyping-defined depression such as GPpsy (help-seeking definition) reach this estimate. c, Genetic correlation ‘rG’ between CIDI-based LifetimeMDD and all other definitions of MDD in the UK Biobank, estimated using PCGC. Error bars show the s.e. of the estimates. d, Pairwise rG between all definitions of depression in the UK Biobank, also detailed in Supplementary Table 15.
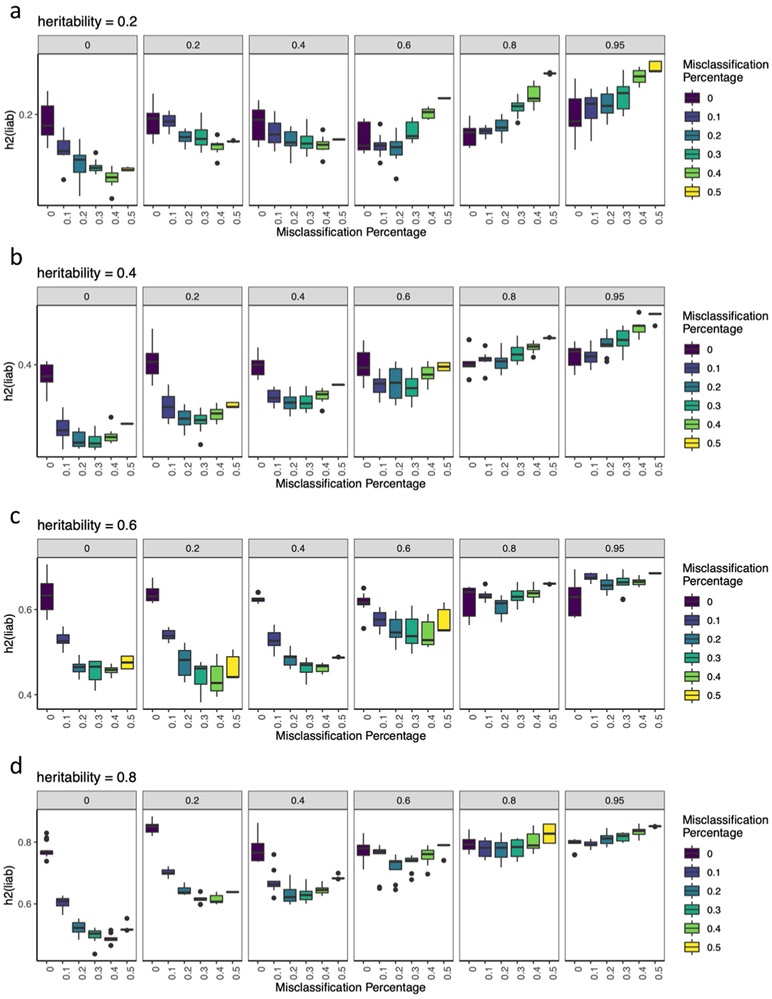
We examined the roles of a number of additional factors for the lower h2SNP of minimal phenotyping definitions of MDD. First, minimal phenotyping definitions did not simply have a higher environmental contribution to MDD than the stricter definitions. When we assessed h2SNP in MDD cases with high and low exposure to environmental risk factors40, we found that minimal phenotyping definitions of depression (GPpsy and SelfRepDep) showed no significant difference between exposures, which were similar to or lower than those for strictly defined MDD (LifetimeMDD and MDDRecur) (Supplementary Note and Supplementary Table 14). Second, the minimal phenotyping definitions did not merely include milder cases of MDD as previously hypothesized41. Inclusion of milder cases is equivalent to lowering the threshold for disease liability in the population above which ‘cases’ for MDD are defined. Under the liability threshold model42, this did not reduce the h2SNP (Supplementary Note and Extended Data Fig. 1). Instead, we showed through simulations that the lower h2SNP of minimal phenotyping definitions of depression may be due to misdiagnosis of controls as cases of MDD and misclassification of those with other conditions as cases of MDD (Extended Data Figs. 1 and 2).
Genetic correlations between definitions of depression and other diseases.
We found that the genetic correlation (rG) between minimal and strictly defined MDD included a large proportion of nonspecific liability to mental ill health. The rG between GPpsy (minimally defined MDD) and LifetimeMDD (strictly defined MDD) was 0.81 (s.e. = 0.03), significantly different than unity (Fig. 3c,d, Supplementary Table 15, Supplementary Fig. 6 and Supplementary Note). One interpretation of this finding is that the correlation represents shared genetic liability to MDD4,5. However, the majority of the genetic liability of LifetimeMDD due to GPpsy (approximately rG2 = 0.812 = 66%) was shared with the no-MDD definition, GPNoDep, and the genetic liability of GPNoDep explained approximately 70% of the genetic liability of GPpsy (rG = 0.84, s.e. = 0.05), and 34% of that of LifetimeMDD (rG = 0.58, s.e. = 0.08).
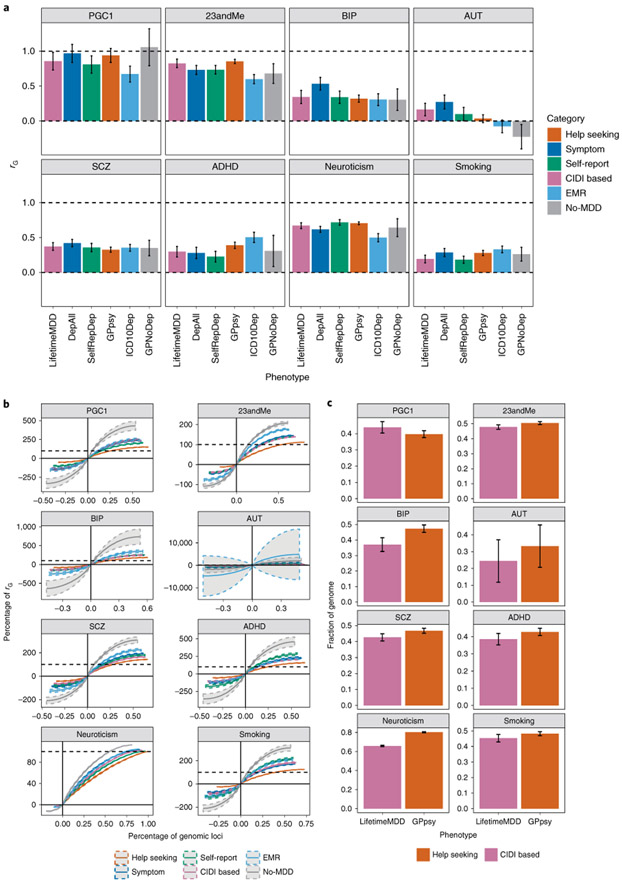
We next examined rG between different definitions of MDD and comorbid diseases, using cross-trait LD score regression (LDSC)43 to estimate rG with neuroticism and smoking (Extended Data Fig. 3 and Supplementary Tables 16 and 17) in the UK Biobank, as well as with all psychiatric conditions in the Psychiatric Genomics Consortium (PGC)44, including PGC1-MDD39, and depression defined in 23andMe4 (Supplementary Table 1). Figure 4a and Supplementary Table 18 show few differences in rG estimates between other psychiatric disorders and the different definitions of MDD in the UK Biobank, consistent with previous reports45.
Fig. 4 ∣. Genetic correlation between definitions of MDD and other psychiatric conditions.
a, The genetic correlation estimated by cross-trait LDSC43 on the liability scale between definitions of MDD in the UK Biobank and other psychiatric conditions in both the UK Biobank (smoking and neuroticism) and PGC44 (Supplementary Table 1), including schizophrenia49 (SCZ) and bipolar disorder50 (BIP) (Supplementary Table 1). Error bars show the s.e. of the estimates. AUT, autism; ADHD, attention deficit/hyperactivity disorder. b, The cumulative fraction of regional genetic correlation (out of the sum of regional genetic correlation across all loci) between definitions of MDD in the UK Biobank and schizophrenia in 1,703 independent loci in the genome64 estimated using rho-HESS46, plotted against the percentage of independent loci. CIDI-based LifetimeMDD is shown in purple, while help-seeking-based GPpsy is shown in red. The steeper the curve, the smaller the number of loci explaining the total genetic correlation. The dashed colored curves around each solid line represent the s.e. of the estimate computed using a jackknife approach as described in Shi et al.36 The dashed black line represents 100% of the sum of genetic correlation between each definition of MDD in the UK Biobank and schizophrenia. The cumulative sums of positive regional genetic correlations (right of y axis) go beyond 100%; this is mirrored by the negative regional genetic correlations (left of y axis) that go below 0%. c, We ranked all 1,703 loci by their magnitude of genetic correlation and asked what fraction of loci summed to 90% of total genetic correlation. This figure shows the percentage of loci summing to 90% of total genetic correlation between either LifetimeMDD (in purple) or GPpsy (in red) and all psychiatric conditions tested, with s.e. estimated using the same jackknife approach. The higher the percentage, the higher the number of genetic loci contributing to 90% of total genetic correlation. Error bars show the s.e. of the estimates.
Similar rG estimates can result from different genetic architectures, indexed by the extent to which genetic liability is spread across the genome. We estimated local rG and the percentage of the genome contributing to total rG using rho-HESS46 (Methods and Fig. 4b). Approximately 65.8% (s.e. = 0.6%), 37.1% (s.e. = 4.5%) and 42.7% (s.e. = 2.3%) of the genome explained 90% of the total rG between strictly defined MDD (LifetimeMDD) and neuroticism, bipolar disorder and schizophrenia, respectively. In comparison, 80.2% (s.e. = 0.6%), 47.3% (s.e. = 2.4%) and 46.8% (s.e. = 0.2%) of the genome was needed to explain the same percentage of total rG between help-seeking-based GPpsy and the same conditions (Fig. 4c). In other words, minimal phenotyping definitions of depression share more genetic loci with other psychiatric conditions than strictly defined MDD does.
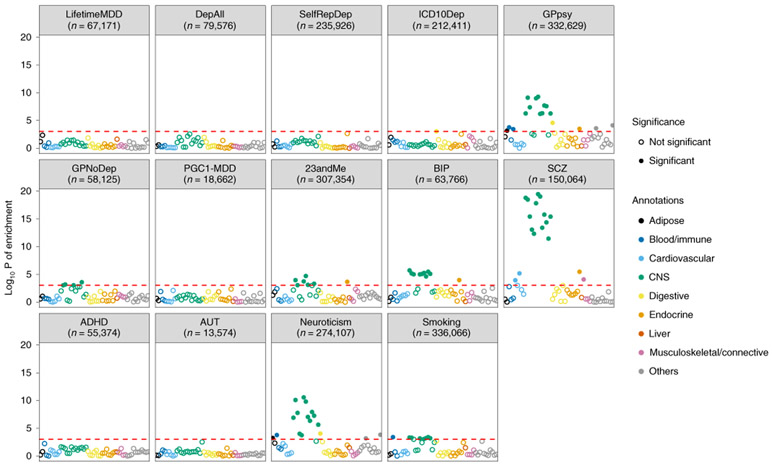
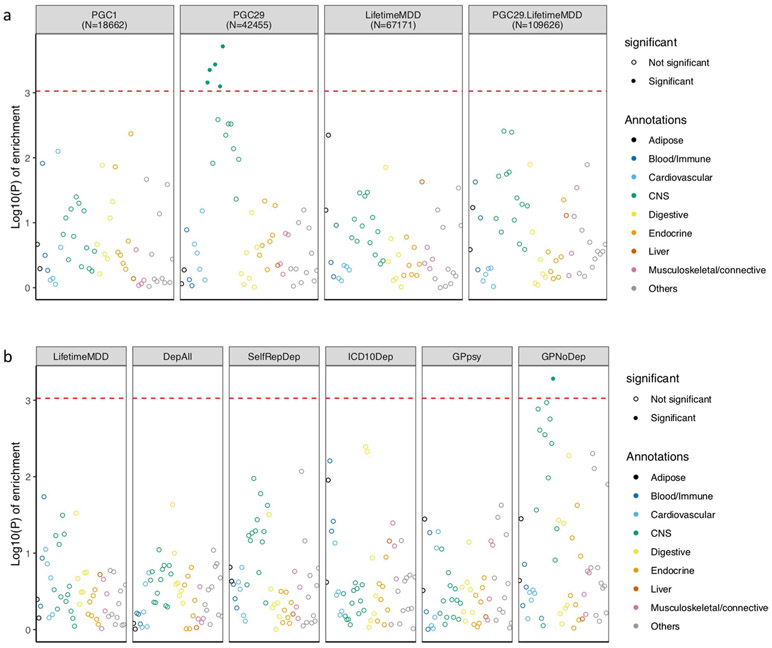
Previous work4 reported that depression defined through minimal phenotyping shows enrichment of h2SNP in regions of the genome encoding genes specifically and highly expressed in central nervous system (CNS) tissues represented in Genotype-Tissue Expression (GTEx)47 project. We assessed this in the definitions of depression in the UK Biobank using LDSC-SEG48. As shown in Fig. 5, neither strictly defined MDD (LifetimeMDD) nor MDD defined on the basis of structured clinical assessments in PGC1-MDD showed significant CNS enrichments, even though larger and more heterogeneous cohorts did (Methods, Supplementary Note, Supplementary Table 1 and Extended Data Fig. 4). Notably, the minimal phenotyping definition GPpsy showed a significant CNS enrichment, as did the no-MDD help-seeking definition GPNoDep, neuroticism, smoking, and other disorders in the PGC44, such as schizophrenia49 and bipolar disorder50. Our analysis shows that the degree of CNS enrichment does not relate to the strictness of the definition of MDD and is neither sufficient nor valid evidence that any particular definition of depression better represents MDD or captures the biological mechanisms behind MDD.
Fig. 5 ∣. Tissue-specific gene expression enrichment in definitions of MDD.
The −log10 P value is shown for enrichment in h2SNP in genes specifically expressed in 44 GTEx tissues, estimated using partitioned h2SNP in LDSC; the help-seeking based definition of MDD (GPpsy), as well as its constituent no-MDD phenotype (GPNoDep), showed enrichment of h2SNP in genes specifically expressed in CNS tissues, similarly to an independent cohort of help-seeking-based MDD (23andMe4) and other psychiatric conditions such as bipolar disorder50, schizophrenia49, autism, personality dimension neuroticism, and the behavioral trait smoking. We indicate the sample size (n) for each definition of depression and psychiatric condition.
GWAS hits from minimal phenotyping are not specific to MDD.
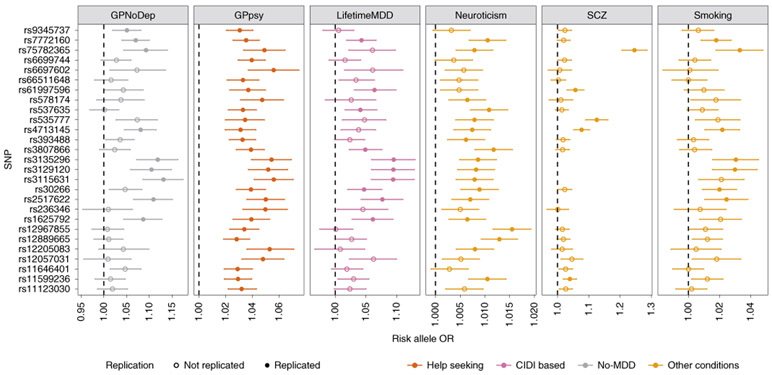
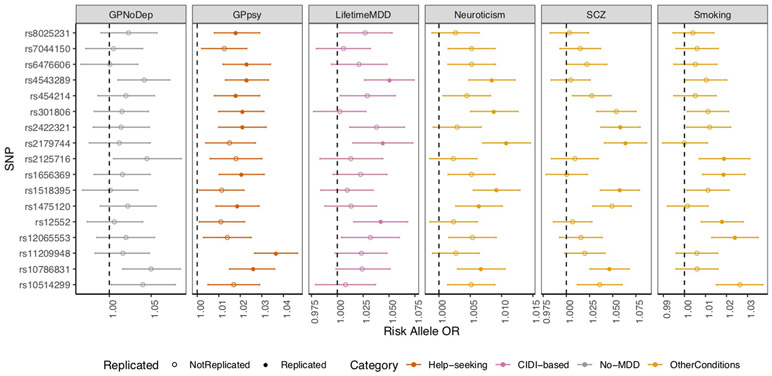
We next examined the specificity of the action of individual genetic loci found in GWAS of each definition of MDD. We found that the help-seeking definitions gave the greatest number of genome-wide-significant loci (27 from GPpsy and Psypsy; Supplementary Table 10) in GWAS, consistent with their larger sample sizes and statistical power for finding associations. We examined whether these loci could be detected in strictly defined MDD. Of the 27 loci from minimal phenotyping definitions, 10 showed significant effects (at P < 0.05 after multiple-testing correction for 27 loci) on LifetimeMDD, despite the latter’s much smaller sample size, consistent with the hypothesis that risk loci for minimal phenotyping MDD also act in strictly defined MDD. However, all ten loci also showed significant effects in neuroticism, smoking, schizophrenia and the no-MDD help-seeking condition (GPNoDep; Supplementary Table 19). Furthermore, all significant SNPs in minimal phenotyping definitions of depression had the same directions of effect on no-MDD phenotypes (Fig. 6).
Fig. 6 ∣. GWAS hits from minimal phenotyping definition of MDD in the UK Biobank are not specific to MDD.
ORs are shown for the risk alleles at 27 loci significantly associated with help-seeking definitions of MDD in the UK Biobank (GPpsy and Psypsy), in logistic regression GWAS conducted using MDD definitions based on on CIDI (LifetimeMDD, in purple), help seeking (GPpsy, in red) and no-MDD (GPNoDep, in brown) based definitions of MDD. For comparison, we show the same in conditions other than MDD: neuroticism, smoking and schizophrenia (all in pink). SNPs missing in each panel were not tested in the respective GWAS. For clarity of display, scales on different panels vary to accommodate the different magnitudes of ORs of SNPs in different conditions. ORs at all 27 loci were highly consistent across phenotypes, being completely aligned in direction of effect, regardless of whether it was a definition of MDD or a risk factor or condition other than MDD. All results are shown in Supplementary Table 14. Error bars show the s.e. of the estimates.
We found the same pattern of results when we used loci identified from a minimal phenotyping strategy in an independent study by 23andMe that used a minimal phenotyping definition4. Of the 17 loci, 10 replicated in GPpsy (at P < 0.05, after multiple testing correction for 17 loci) and 3 replicated in LifetimeMDD. All significant SNPs had the same directions of effect on neuroticism, smoking and schizophrenia (Extended Data Fig. 5 and Supplementary Table 20) and are therefore not specific to MDD, consistent with our analysis of minimal phenotyping definitions in the UK Biobank. In summary, GWAS of minimal phenotyping definitions of depression primarily enables the discovery of pathways that are shared with other conditions. It is not currently possible to assess the specificity of GWAS loci from strictly defined MDD in the same way, given that the sample size for strictly defined MDD remains relatively small and GWAS hits relatively few.
Out-of-sample prediction of MDD.
Finally, we explored how well the definitions of depression in the UK Biobank predict strictly defined, CIDI-based MDD in independent cohorts, using data from 23 MDD cohorts in the latest data freeze from the MDD Working Group of the Psychiatric Genomics Consortium (PGC29-MDD5,51; Supplementary Note, Supplementary Table 21 and Supplementary Fig. 7). We constructed polygenic risk scores (PRSs) on each definition of depression in the UK Biobank (Methods) and examined their prediction in each of the PGC29-MDD cohorts. Of note, PRS from all definitions of depression in the UK Biobank, whether minimally or strictly phenotyped, accounted for a small proportion of variation in disease status in PGC29-MDD (Supplementary Table 22). We observed the following features.
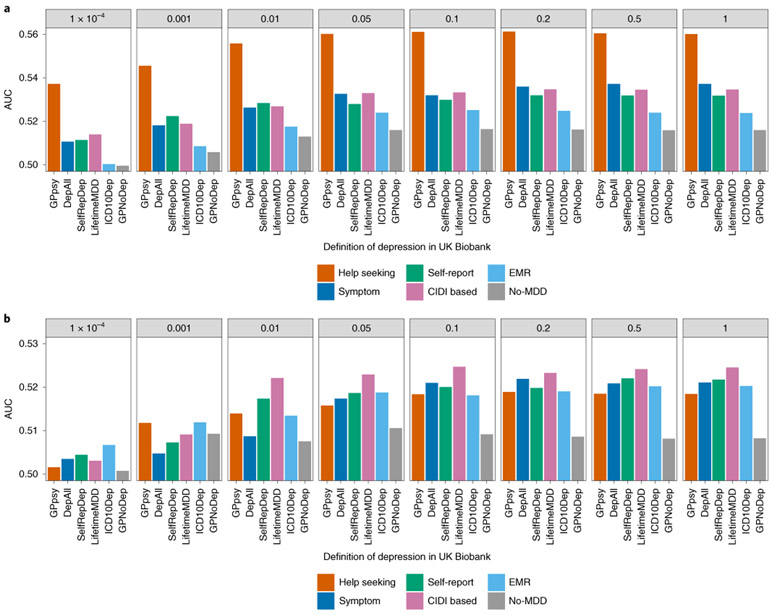
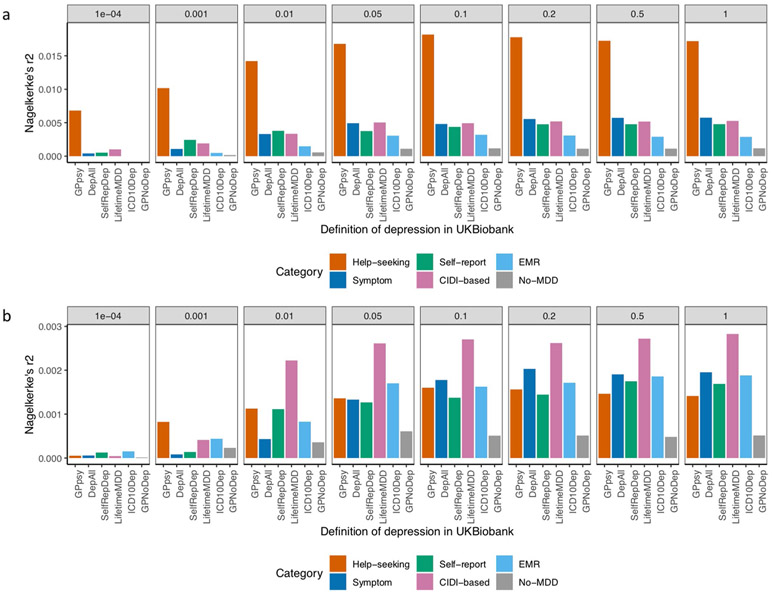
First, the PRS obtained using the full sample of GPpsy performed best at predicting MDD status in independent cohorts from PGC29-MDD (Nargelkerke’s r2=0.018, area uncer the curve (AUC) = 0.56 at a P-value threshold of 0.1; Fig. 7a and Extended Data Fig. 6). However, when equal sample sizes were used (randomly downsampled to 50,000 and case prevalence of 0.15; Methods), GPpsy no longer performed best at predicting MDD status in PGC29-MDD cohorts (Fig. 7b). Rather, the PRS from strictly defined CIDI-based MDD (LifetimeMDD) best predicted MDD disease status (Nargelkerke’s r2 = 0.0027, AUC = 0.52 at a P-value threshold of 0.1; Extended Data Fig. 6).
Fig. 7 ∣. Out-of-sample prediction of MDD in PGC cohorts.
a, The AUC of PRSs calculated for each definition of depression in the UK Biobank and MDD status indicated in 19 PGC29-MDD cohorts5, while controlling for cohort-specific effects. PRSs were calculated using effect sizes at independent (LD r2 < 0.1) SNPs passing P-value thresholds of 10−4, 0.001, 0.01, 0.05, 0.01, 0.2, 0.5 and 1, in GWAS performed on all definitions of depression in the UK Biobank. b, This figure shows the same analysis performed on downsampled data (7,500 cases and 42,500 controls) for each definition of depression.
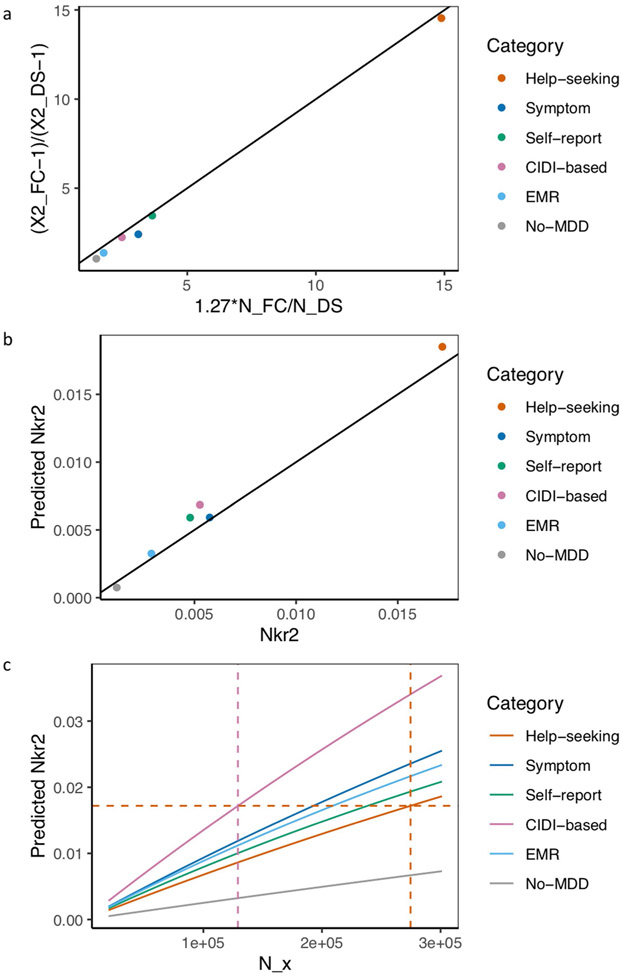
Second, the higher prediction accuracy of the PRS obtained using the full sample of GPpsy could be entirely explained by the larger sample size52 (113,260 cases and 219,362 controls; effective sample size = 298,677; Supplementary Note and Extended Data Fig. 7). We calculated the effective sample size needed for other definitions to have the same predictive power: for strictly defined LifetimeMDD, we would need an effective sample size of 129,106 (Supplementary Note and Extended Data Fig. 7), less than half of that of GPpsy.
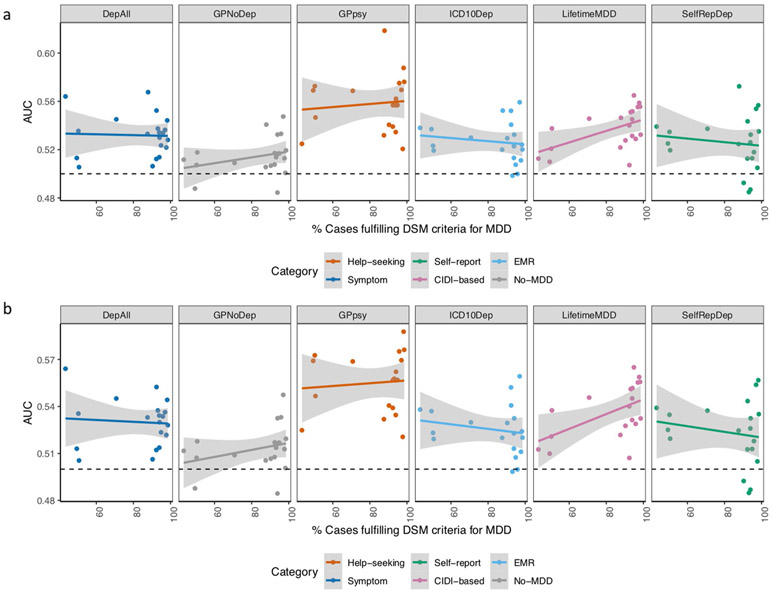
Third, the PRS from strictly defined LifetimeMDD predicted MDD disease status better in the PGC29-MDD cohorts, which had a higher percentage of cases fulfilling DSM-5 symptom criteria (Supplementary Table 21 and Extended Data Fig. 8; Pearson r2 between the AUC and percentage of cases in PGC29-MDD cohorts fulfilling DSM-5 symptom criteria = 0.26, P = 0.025 at PRS P value = 0.1). This is consistent with the interpretation that LifetimeMDD captures signals specific to MDD. We did not observe such a trend for GPpsy (Pearson r = 0.02, P = 0.57 at PRS P value = 0.1) or any other definition of depression (Supplementary Table 23), suggesting their lower specificity for MDD.
Discussion
Our study demonstrates that the genetic architecture of minimal phenotyping definitions of depression is different from that of strictly defined MDD and is enriched for nonspecific effects on MDD. Using a range of definitions of MDD in the UK Biobank, from self-reported help seeking to a full assessment of the DSM-5 criteria for MDD through self-reported symptoms from the MHQ, we made five key observations.
First, the heritabilities of depression defined by minimal phenotyping strategies are lower than those of MDD defined by full DSM-5 criteria using the CIDI questionnaire. Second, although there is substantial genetic correlation between definitions, much of the shared genetic liability is not specific to MDD and significant differences remain, indicating the presence of genetic effects unique to each definition. Third, a larger percentage of the genome contributes to the shared genetic liability between minimal phenotyping definitions of depression and other psychiatric conditions than that between CIDI-based MDD and other conditions, likely driven by misdiagnosis due to nonspecific phenotyping. Fourth, all GWAS hits from the GPpsy minimal definition of depression are shared with genetically correlated conditions such as neuroticism and smoking. Finally, while minimal phenotyping definitions enable greater predictive power for MDD status in independent cohorts, this is due to the large sample size rather than indexing of MDD-specific effects. These results point to the nonspecific nature of genetic factors identified in minimal phenotyping definitions of depression.
A number of factors need to be borne in mind when interpreting the above observations. Importantly, none of the definitions of depression in the UK Biobank were obtained from structured clinical interviews with an experienced rater (the gold standard for diagnosing MDD). The closest to that standard in the UK Biobank is the online MHQ17, based on the CIDI-SF18. Our results suggest that self-reported diagnoses using CIDI-SF or other diagnostic questionnaire with full DSM-5 criteria lie on the same genetic liability continuum as MDD. This would argue that MDD cases identified through self-report using a full diagnostic questionnaire will be enriched for more strictly defined forms, with the consequence that results from genetic analysis will include loci that contribute to strictly defined MDD disease risk53,54.
Minimal definitions of MDD do not simply include cases with lower genetic liability to MDD. This is consistent with a recent study of three large twin cohorts, which asked whether a combination of MDD, depressive symptoms and neuroticism could capture all genetic liability of MDD55 and showed that 65% of the genetic effects contributing to MDD are specific, and minimally defined depression (inclusive of MDD, depressive symptoms and neuroticism) can index only around one-third of the genetic liability to MDD. Similarly, previously reported high degrees of genetic correlation between MDD and depressive symptoms (rG = 0.7, implying that roughly rG2 = 49% of genetic factors contributing to liability of the former is attributable to that of the latter)22 need to be put in perspective of even higher degrees of sharing between depressive symptoms and other traits such as neuroticism (rG = 0.79–0.94, implying that roughly rG2=62–88% of genetic variance of the former is attributable to that of the latter, especially if both were assayed at a single time point56).
Our findings have important implications for downstream investigations. One interpretation is that the nonspecific effects found through using minimal phenotyping approaches will still advance understanding of the biology of psychiatric disorders and their treatment5,57. A recent report used the ‘quasi-replication’ of GWAS loci between depressive symptoms and neuroticism as validation of their functional significance56. An alternative view is that these loci reflect the ways in which depressive symptoms can develop as secondary effects, including through susceptibility to adverse life events58, personality types24 and use of or exposure to psychoactive agents like cigarettes59,60—in which case, while useful for understanding the basis of mental ill health, they are not informative about the genetic etiology of MDD and are not useful for developing disease-specific treatment.
Our findings indicate the need for ways to integrate both strict and minimal phenotyping approaches to determine which loci to prioritize for follow-up functional analyses. They also indicate a need for means to assess symptoms for diagnosing MDD with specificity at scale, rather than reliance on minimal phenotyping. Fast and accurate diagnostic methods that use a limited number of questionnaire items are becoming available: for example, computerized adaptive diagnostic screening may be as effective for the diagnosis of MDD as an hour-long face-to-face clinician diagnostic interview61. There are ongoing attempts to convert behavioral health tracking data from phones or wearable devices into diagnostic information62. If successful, these attempts may lead to a dramatic expansion in the ability to collect data appropriate for psychiatric genetics.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41588-020-0594-5.
Methods
Genome-wide associations.
To obtain and access the difference between ORs of associations in different definitions of depression in the UK Biobank, as well as for smoking (data field 20160) and neuroticism (data field 20127), we performed logistic regression (or linear regression with –standard-beta for neuroticism) on all 5,276,842 common SNPs (MAF > 5% in all 337,198 White-British unrelated samples) in PLINK65 (version 1.9) with 20 principal components and genotyping array as covariates.
Estimation of SNP heritability and genetic correlation among definitions of MDD.
All estimates of h2SNP were computed with the PCGC66 approach implemented with PCGC-ss33, using 5,276,842 common SNPs (MAF > 5% in all 337,198 White-British unrelated samples). LD scores at SNPs were computed with LDSC34 in 10,000 random samples drawn from the White-British samples in the UK Biobank as an LD reference, as well as the MAF at all 5,276,842 common SNPs in all 337,198 White-British samples as a MAF reference. Covariates were genotyping array and 20 principal components computed using samples in each definition of MDD with flashPCA67. Where we stratified each definition of MDD in the UK Biobank into two strata by risk factors such as sex (Supplementary Note), we computed specific principal components for each definition and stratum (see also the Supplementary Note and Supplementary Table 13).
Estimation of genetic correlation between definitions of MDD and other conditions.
Summary statistics for other psychiatric conditions from previous GWAS studies were obtained as described in Supplementary Table 1. Association summary statistics for smoking and neuroticism in the UK Biobank were generated by GWAS (Supplementary Table 15 and 16, and Extended Data Fig. 3). We estimated the genetic correlation between definitions of MDD in the UK Biobank and each of these conditions using LDSC43, with an LD reference panel generated with European (EUR) individuals from 1000 Genomes68. To obtain regional rG, we partitioned the genome into 1,703 independent loci64 and estimated regional rG with rho-HESS46, using an LD reference panel generated with EUR individuals from 1000 Genomes68. We estimated s.e. for each regional rG and the total rG across the genome using a jackknife approach implemented in HESS36. To assess the percentage of genome contributing to total rG, we ranked all independent loci by their absolute value of regional rG, and asked how many loci would contribute 90% of the total rG.
Enrichment of SNP heritability in genes specifically expressed in tissues.
We estimated the enrichment of h2SNP in genes specifically expressed in 44 tissues in the GTEx47 project using the partitioned h2SNP framework in LDSC-SEG46 and an LD reference panel generated with EUR individuals from 1000 Genomes68. We obtained tissue-specific gene expression annotations in GTEx tissues from LDSC-SEG and then estimated the enrichment of h2SNP in annotations that corresponded to each of the tissues together with 52 annotations in the baseline model69. We report the P value of the one-sided test of enrichment of h2SNP in genes specifically expressed in each tissue against the baseline.
Out-of-sample predictions of MDD.
We performed out-of-sample prediction using individual-level genotype and phenotype data from the PGC29-MDD cohorts5. We obtained permissions from 20 cohorts with sample sizes greater than 500, among which 17 recorded endorsement of DSM-5 criteria A for MDD (Supplementary Note and Supplementary Table 21). We obtained PRSs from GWAS for each definition of depression in the UK Biobank, using LD-clumped (LD r2 < 0.1) independent SNPs with P values for association below eight thresholds (P < 10−4, 0.001, 0.01, 0.05, 0.1, 0.2, 0.5 and 1), and predicted MDD status in the 20 PGC cohorts using the Ricopili pipeline70-82. We obtained Nagelkerke’s r2 between the PRSs and MDD status, the AUC of the prediction and the variance of MDD status explained by the PRSs for each cohort. We also obtained the same measures for MDD status pulling data from all cohorts, controlling for cohort differences by including cohort as a covariate.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Extended Data
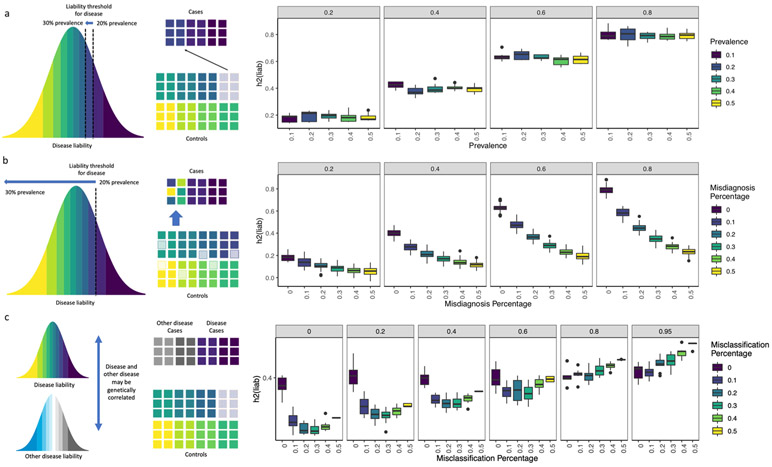
Extended Data Fig. 1 ∣. Simulations of misdiagnosis and misclassification.
a-c, Each boxplot show h2SNP estimates from 10 simulated phenotypes, with upper and lower boundaries of boxes represent the first to third quartiles of all estimates, and the whiskers extends to 1.5 times the interquartile range of the estimates. a, This figure shows that liability scale h2SNP does not change with shifting of liability threshold Ki∈{0.1, 0.2, 0.3, 0.4, 0.5} for simulated heritabilities ∈ {0.2, 0.4, 0.6, 0.8}. b, The figure shows that liability scale h2SNP is deflated with increasing percentage of controls being misdiagnosed as cases, when prevalence of diagnosed cases is kept constant at Ki=0.2, for simulated heritabilities ∈ {0.2, 0.4, 0.6, 0.8}. c, This figure shows liability scale h2SNP is deflated with increasing percentage of misclassification of cases of “other” disease as cases of focal disease, if rG between the two diseases are moderate to low, for simulated , for each of which all cases at prevalence Ki,1=0.2 are correctly identified as cases.
Extended Data Fig. 2 ∣. Simulations of misclassification at different heritabilities.
a-d, These figures shows the estimated h2SNP using-pcgc option with-prevalence K in LDAK, plotted on the y-axis) of binary traits (yi,1, where i ∈{1..10}) with simulated 0.2, 0.4, 0.6, and 0.8, for each of which all cases (at prevalence Ki,1 = 0.2) are correctly identified as cases, while varying numbers of cases misclassified from a genetically correlated binary trait (yi,2, where i∈{1..10}) of equal and prevalence as cases of yi,1. Genetic correlations between yi,1 and yi,2 (rGi∈{0, 0.2, 0.4, 0.6, 0.8, 0.95}) are shown in the grey bars above each panel. Each boxplot show h2SNP estimates from 10 simulated phenotypes, with upper and lower boundaries of boxes represent the first to third quartiles of all estimates, and the whiskers extends to 1.5 times the interquartile range of the estimates.
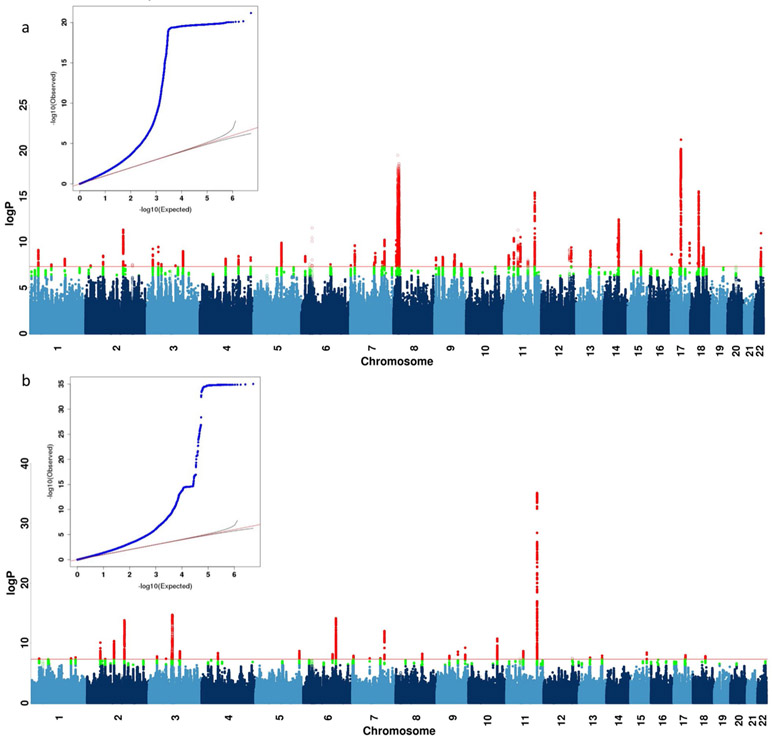
Extended Data Fig. 3 ∣. GWAS on neuroticism and smoking in UK Biobank.
a, b, This figure shows the Manhattan plot of neuroticism score (data field 20127, quantitative trait from 0 to 12) in 274,107 individuals and ever smoked status (data field 20160, binary trait of 0 for “No”, and 1 for “Yes”) in 336,066 individuals in UK Biobank using linear regression on all 8,968,716 common SNPs (MAF > 5% in all 337,198 White-British, unrelated samples) for all the above analyses in PLINK (version 1.9)32 with 20 PCs and genotyping array as covariates. We report all associations with P-values smaller than 5×10−8 as genome-wide significant (red). We indicated the SNPs in SVs and the MHC in all Manhattan plots as hollow points instead of solid points due to lack of control for population structure in these regions, and show all top SNPs within peaks (1-Mb regions) in Supplementary Tables 10 and 11.
Extended Data Fig. 4 ∣. LDSC-SEG analysis of tissue-specific enrichment of h2SNP.
a, This figure shows −log10(P) of enrichment in heritability in genes specifically expressed in 44 GTEx tissues, estimated using partitioned heritability in LDSC-SEG, on LifetimeMDD (n = 67,171), PGC1-MDD (n = 18,759), PGC29 (n = 42,455) and a meta-analysis of LifetimeMDD and PGC29 (n = 109,626, PC29.LifetimeMDD, Methods). While PGC29 shows CNS enrichment, neither LifetimeMDD nor the meta-analysis shows the same enrichment. This suggests sample size and differences in genetic architecture and cohort heterogeneity affects results from LDSC-SEG. b, This figure shows the same analysis performed on down-sampled data for each definition of depression. Each definition is randomly down-sampled to 7,500 cases and 42,500 controls, a constant prevalence of 0.15, to remove confounding from sample size and difference in statistical power on the enrichment analysis. This figure shows that at equal sample size and prevalence, GPNoDep (no-MDD Help-seeking phenotype) is the only one showing CNS enrichment, suggesting it may be driving the CNS enrichment signal in GPpsy in Fig. 5.
Extended Data Fig. 5 ∣. GWAS hits from 23andMe are not specific to MDD.
This figure shows the odds ratios of risk alleles (Risk Allele ORs) at 17 loci significantly associated with help-seeking based definitions of MDD in 23andMe27, in GWAS conducted on CIDI-based (LifetimeMDD, in purple), help-seeking (GPpsy in red) and no-MDD (GPNoDep, in orange) based definitions of MDD, as well as conditions other than MDD: neuroticism, smoking and SCZ (all in brown). SNPs missing in each panel are not tested in the respective GWAS. For clarity of display, scales on different panels vary to accommodate the different magnitudes of ORs of SNPs in different conditions. ORs at all 17 loci are highly consistent across phenotypes, regardless of whether it is a definition or MDD or a risk factor or condition other than MDD. All results are shown in Supplementary Table 20. Error bars show the standard errors of the estimates.
Extended Data Fig. 6 ∣. Out-of-sample prediction in PGC cohorts.
a, This figure shows the Nagelkerke’s r2 of polygenic risk scores (PRS) calculated for each definition of depression in UK Biobank and MDD status indicated in 19 PGC29-MDD cohorts, while controlling for cohort specific effects. PRS were calculated using effect sizes at independent (LD r2 < 0.1) SNPs passing P-value thresholds 10−4, 0.001, 0.01, 0.05, 0.01, 0.2, 0.5 and 1 respectively, in GWAS performed on all definitions of depression in UK Biobank. b, This figure shows the same analysis performed on down-sampled data (7,500 cases, 42,500 controls) for each definition of depression.
Extended Data Fig. 7 ∣. Relationship between effective sample size and prediction accuracy.
a, This figure shows the relationship between the ratio of effective sample sizes between the full cohort (NFC) and down-sampled (NDS) data for each definition of depression and the ratio of their mean Chi-square (χ2) statistic from GWAS, with black line x = y for reference. Across all definitions of depression, is highly correlated with (Pearson r2 = 0.999, P = 5.50×10−7), and has an effect of beta = 1.27 (s.e. = 0.02) on . b, This figure shows the Nagelkerke’s r2 (Nkr2) for MDD status in PGC29 cohorts predicted for PRS of different definitions of depression at NFC, plotted against their respective empirical Nkr2 at NFC, both at P-value threshold = 1. The Pearson correlation r2 between predicted and actual NKr2 across all definitions were 0.989 (P = 4.46×10−5). c, This figure shows for each definition of depression the effective sample size NX required for each predicted Nkr2 in out-of-sample prediction of MDD status in PGC29 cohorts. While Nx= 274,677 (indicated with orange vertical dotted line) for GPpsy to achieve a Nkr2 of 0.0172 (indicated with orange horizontal dotted line), a smaller Nx= 129,106 (indicated with pink vertical dotted line) is needed to achieve the same Nkr2 for LifetimeMDD.
Extended Data Fig. 8 ∣. Prediction accuracy in cohorts with different percentage of DSM MDD cases.
a, This figure shows the area under the curve (AUC) of polygenic risk scores (PRS) calculated for each definition of depression in UK Biobank and MDD status indicated in 20 PGC29-MDD cohorts at P-value threshold of 0.1 (using all SNPs after LD-clumping, see results at all P-value thresholds in Supplementary Table 23), plotting AUC for each cohort against their respective percentage of cases fulfilling DSM-5 criteria A for MDD (see Supplementary Table 21). It shows that strictly defined CIDI-based LifetimeMDD is the only definition of depression in UK Biobank that shows increases in AUC as percentage of cases fulfilling DSM-5 criteria A for MDD in PGC cohorts increases, despite not giving the highest AUC. b, This figure shows the same analysis removing the PGC29-MDD cohort rad3, which is the outlier giving AUC > 0.6 in GPpsy in a. As this is a UK-based cohort, it is possible it contains relatives of individuals in UK Biobank that upwardly biased prediction accuracy in it. For all analysis shown in Fig. 7, Extended Data Figs. 6 and 7 and Supplementary Table 23, we have removed this cohort.
Supplementary Material
Acknowledgements
We thank O. Weissbrod, A. Dahl, H. Shi and V. Zuber for insightful discussions. N.C. is supported by the ESPOD Fellowship from European Bioinformatics (EMBL-EBI) and Wellcome Sanger Institute. A.V. is supported by the Swedish Brain Foundation. C.M.L. and G.B. are funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London Maudsley Foundation Trust and King’s College London. In the last 3 years, M.M.W. has received research funds from the US National Institute of Mental Health (NIMH), the Templeton Foundation and the Sackler Foundation and has received royalties for publication of books on interpersonal psychotherapy from Perseus Press and Oxford University Press, on other topics from the American Psychiatric Association Press and royalties on the social adjustment scale from Multihealth Systems. The CoLaus∣PsyCoLaus study was and is supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne and the Swiss National Science Foundation (grants 3200B0-105993, 3200B0-118308, 33CSCO-122661, 33CS30-139468, 33CS30-148401 and 33CS30-177535/1). The PGC has received major funding from the US NIMH and the US National Institute of Drug Abuse (U01 MH109528 and U01 MH1095320). This research was conducted using the UK Biobank resource under application no. 28709 and with the support and collaboration from all investigators who make up the MDD Working Group of the PGC (full list in the Supplementary Note). We are greatly indebted to the hundreds of thousands of individuals who have shared their life experiences with the UK Biobank and PGC investigators.
Footnotes
A list of members and affiliations appears in the Supplementary Note.
Competing interests
C.M.L. is on the scientific advisory board of Myriad Neuroscience. H.J.G. has received travel grants and speaker’s honoraria from Fresenius Medical Care, Neuraxpharm and Janssen Cilag as well as research funding from Fresenius Medical Care. B.W.J.H.P. has received (non-related) research grants from Jansen Research and Boehringer Ingelheim.
Extended data is available for this paper at https://doi.org/10.1038/s41588-020-0594-5.
Supplementary information is available for this paper at https://doi.org/10.1038/s41588-020-0594-5.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability
Genotype and phenotype data used in this study are from the full release (imputation version 2) of the UK Biobank resource obtained under application no. 28709. We used publicly available summary statistics from other studies downloadable from the website of the Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc/results-and-downloads), the references for which can be found in Supplementary Table 1. We also referenced the 2011 Census aggregate data from the UK Data Service (https://doi.org/10.5257/census/aggregate-2011-2).
References
- 1.Lu JT, Campeau PM & Lee BH Genotype–phenotype correlation: promiscuity in the era of next-generation sequencing. Obstet. Gynecol. Surv 69, 728–730 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Ripke S et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet 45, 1150–1159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard DM et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun 9, 1470 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyde CL et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet 48, 1031–1036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wray NR et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet 50, 668–681 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flint J & Kendler KS The genetics of major depression. Neuron 81, 484–503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–3105 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Boyd JH, Weissman MM, Thompson WD & Myers JK Screening for depression in a community sample. Understanding the discrepancies between depression symptom and diagnostic scales. Arch. Gen. Psychiatry 39, 1195–1200 (1982). [DOI] [PubMed] [Google Scholar]
- 9.Breslau N Depressive symptoms, major depression, and generalized anxiety: a comparison of self-reports on CES-D and results from diagnostic interviews. Psychiatry Res. 15, 219–229 (1985). [DOI] [PubMed] [Google Scholar]
- 10.Weissman MM & Myers JK Rates and risks of depressive symptoms in a United States urban community. Acta Psychiatr. Scand 57, 219–231 (1978). [DOI] [PubMed] [Google Scholar]
- 11.Mitchell AJ, Vaze A & Rao S Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 374, 609–619 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Mojtabai R Clinician-identified depression in community settings: concordance with structured-interview diagnoses. Psychother. Psychosom 82, 161–169 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Druss BG et al. Understanding mental health treatment in persons without mental diagnoses: results from the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 64, 1196–1203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus SC & Olfson M National trends in the treatment for depression from 1998 to 2007. Arch. Gen. Psychiatry 67, 1265–1273 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Sudlow C et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DJ et al. Prevalence and characteristics of probable major depression and bipolar disorder within UK Biobank: cross-sectional study of 172,751 participants. PLoS One 8, e75362 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis KAS et al. Mental health in UK Biobank: development, implementation and results from an online questionnaire completed by 157 366 participants. BJPsych Open 4, 83–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Kessler RC & Ustun TB The World Mental Health (WMH) Survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int. J. Meth. Psych. Res 13, 93–121 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bromet EJ, Dunn LO, Connell MM, Dew MA & Schulberg HC Long-term reliability of diagnosing lifetime major depression in a community sample. Arch. Gen. Psychiatry 43, 435–440 (1986). [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS, Neale MC, Kessler RC, Heath AC & Eaves LJ The lifetime history of major depression in women. Reliability of diagnosis and heritability. Arch. Gen. Psychiatry 50, 863–870 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Rice JP, Rochberg N, Endicott J, Lavori PW & Miller C Stability of psychiatric diagnoses. An application to the affective disorders. Arch. Gen. Psychiatry 49, 824–830 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Foley DL, Neale MC & Kendler KS Genetic and environmental risk factors for depression assessed by subject-rated symptom check list versus structured clinical interview. Psychol. Med 31, 1413–1423 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Kendler KS, Gardner CO, Neale MC & Prescott CA Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol. Med 31, 605–616 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Kendler KS, Gatz M, Gardner CO & Pedersen NL Personality and major depression: a Swedish longitudinal, population-based twin study. Arch. Gen. Psychiatry 63, 1113–1120 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulos GS et al. ‘Vascular depression’ hypothesis. Arch. Gen. Psychiatry 54, 915–922 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Kessler RC et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Kessler RC, Foster CL, Saunders WB & Stang PE Social consequences of psychiatric disorders. I: Educational attainment. Am. J. Psychiatry 152, 1026–1032 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Lorant V et al. Socioeconomic inequalities in depression: a meta-analysis. Am. J. Epidemiol 157, 98–112 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Kessler RC Epidemiology of women and depression. J. Affect. Disord 74, 5–13 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Neale MC, Kessler RC, Heath AC & Eaves LJ A longitudinal twin study of personality and major depression in women. Arch. Gen. Psychiatry 50, 853–862 (1993). [DOI] [PubMed] [Google Scholar]
- 31.Kessler RC The effects of stressful life events on depression. Ann. Rev. Psychol 48, 191–214 (1997). [DOI] [PubMed] [Google Scholar]
- 32.Mazure CM Life stressors as risk factors in depression. Clinical Psychology: Science and Practice 5, 291–313 (1998). [Google Scholar]
- 33.Weissbrod O, Flint J & Rosset S Estimating SNP-based heritability and genetic correlation in case–control studies directly and with summary statistics. Am. J. Hum. Genet 103, 89–99 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulik-Sullivan BK et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet 47, 291–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loh P-R et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat. Genet 47, 1385–1392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, Kichaev G & Pasaniuc B Contrasting the genetic architecture of 30 complex traits from summary association data. Am. J. Hum. Genet 99, 139–153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price AL et al. Long-range LD can confound genome scans in admixed populations. Am. J. Hum. Genet 83, 132–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CONVERGE consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 523, 588–591 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry 18, 497–511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson RE et al. Molecular genetic analysis subdivided by adversity exposure suggests etiologic heterogeneity in major depression. Am. J. Psychiatry 175, 545–554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Northern Ireland Statistics and Research Agency: 2011 Census aggregate data. UK Data Service 10.5257/census/aggregate-2011-1 (2016). [DOI] [Google Scholar]
- 42.Dempster ER & Lerner IM Heritability of threshold characters. Genetics 35, 212–236 (1950). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulik-Sullivan B et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet 47, 1236–1241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381, 1371–1379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brainstorm Consortium et al. Analysis of shared heritability in common disorders of the brain. Science 360, eaap8757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi H, Mancuso N, Spendlove S & Pasaniuc B Local genetic correlation gives insights into the shared genetic architecture of complex traits. Am. J. Hum. Genet 101, 737–751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet 45, 580–585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finucane HK et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet 50, 621–629 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Psychiatric Genomics Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet 43, 977–998 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trzaskowski M et al. Quantifying between-cohort and between-sex genetic heterogeneity in major depressive disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet 180, 439–447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turley P et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat. Genet 50, 229–237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corfield EC, Yang Y, Martin NG & Nyholt DR A continuum of genetic liability for minor and major depression. Transl. Psychiatry 7, e1131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Direk N et al. An analysis of two genome-wide association meta-analyses identifies a new locus for broad depression phenotype. Biol. Psychiatry 82, 322–329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kendler KS et al. Shared and specific genetic risk factors for lifetime major depression, depressive symptoms and neuroticism in three population-based twin samples. Psychol. Med 49, 2745–2753 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okbay A et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet 48, 624–633 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McIntosh AM, Sullivan PF & Lewis CM Uncovering the genetic architecture of major depression. Neuron 102, 91–103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendler KS & Karkowski-Shuman L Stressful life events and genetic liability to major depression: genetic control of exposure to the environment? Psychol. Med 27, 539–547 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Fluharty M, Taylor AE, Grabski M & Munafo MR The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob. Res 19, 3–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wootton RE et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol. Med 6, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gibbons RD et al. The computerized adaptive diagnostic test for major depressive disorder (CAD-MDD): a screening tool for depression. J. Clin. Psychiatry 74, 669–674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freimer NB & Mohr DC Integrating behavioural health tracking in human genetics research. Nat. Rev. Genet 20, 129–130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bycroft C et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berisa T & Pickrell JK Approximately independent linkage disequilibrium blocks in human populations. Bioinformatics 32, 283–285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang CC et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golan D, Lander ES & Rosset S Measuring missing heritability: inferring the contribution of common variants. Proc. Natl Acad. Sci. USA 111, E5272–E5281 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abraham G & Inouye M Fast principal component analysis of large-scale genome-wide data. PLoS One 9, e93766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.1000 Genomes Project Consortium et al. A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finucane HK et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet 47, 1228–1235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam M et al. RICOPILI: Rapid Imputation for COnsortias PIpeLIne. Bioinformatics 10.1093/bioinformatics/btz633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berardi D et al. Increased recognition of depression in primary care. Comparison between primary-care physician and ICD-10 diagnosis of depression. Psychother. Psychosom 74, 225–230 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Fry A et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol 186, 1026–1034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adams MJ et al. Factors associated with sharing email information and mental health survey participation in large population cohorts. Int. J. Epidemiol 10.1101/471433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mullins N & Lewis CM Genetics of depression: progress at last. Curr. Psychiatry Rep 19, 43 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sullivan PF et al. Psychiatric genomics: an update and an agenda. Am. J. Psychiatry 175, 15–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coyne JC, Schwenk TL & Smolinski M Recognizing depression: a comparison of family physician ratings, self-report, and interview measures. J. Am. Board Fam. Pract 4, 207–215 (1991). [PubMed] [Google Scholar]
- 77.Nevin RL Low validity of self-report in identifying recent mental health diagnosis among U.S. service members completing Pre-Deployment Health Assessment (PreDHA) and deployed to Afghanistan, 2007: a retrospective cohort study. BMC Public Health 9, 376 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clarke DE et al. DSM-5 field trials in the United States and Canada. Part I: study design, sampling strategy, implementation, and analytic approaches. Am. J. Psychiatry 170, 43–58 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Spitzer RL, Forman JB & Nee J DSM-III field trials. I. Initial interrater diagnostic reliability. Am. J. Psychiatry 136, 815–817 (1979). [DOI] [PubMed] [Google Scholar]
- 80.Keller MB et al. Results of the DSM-IV mood disorders field trial. Am. J. Psychiatry 152, 843–849 (1995). [DOI] [PubMed] [Google Scholar]
- 81.McCarthy S et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet 48, 1279–1283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willer CJ, Li Y & Abecasis GR METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genotype and phenotype data used in this study are from the full release (imputation version 2) of the UK Biobank resource obtained under application no. 28709. We used publicly available summary statistics from other studies downloadable from the website of the Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc/results-and-downloads), the references for which can be found in Supplementary Table 1. We also referenced the 2011 Census aggregate data from the UK Data Service (https://doi.org/10.5257/census/aggregate-2011-2).