Abstract
Objective: To design and validate a novel murine model of full-thickness (FT) vaginal wound healing that mirrors postinjury tissue repair and underscores the impact of estrogen signaling-driven healing kinetics, inflammation, and neovascularization.
Approach: Five-week-old female CD1 mice were subjected to two 1-mm FT wounds. To assess wound healing kinetics, vaginas were harvested at 6, 12, 18, 24, 48, and 72 h and 7 days postinjury. Wounds from all time points were analyzed by hematoxylin and eosin and trichrome to, respectively, assess the rate of wound closure and tissue deposition. Inflammatory leukocyte (CD45), neutrophil (Ly6G), and macrophage (F480 and CD206) infiltration was examined by immunohistochemistry (IHC) and the resulting anti-inflammatory M2 (CD206)/total (F480) macrophage ratio quantified. Neovascularization (CD31) and estrogen receptor-α (ERα) expression levels were similarly determined by IHC.
Results: We observed rapid healing with resolution of mucosal integrity by 48 h (p < 0.05), and overall neutrophils and polarized type 2 macrophages (M2) apexed at 12 h and reduced to near control levels by day 7 postinjury. Tissue repair was virtually indistinguishable from the surrounding vagina. CD31+ vessels increased between 12 h and day 7 and ERα trended to decrease at 12 h postinjury and rebound at day 7 to uninjured levels.
Innovation: A proof-of-concept murine model to study vaginal wound healing kinetics and postinjury regenerative repair in the vagina was developed and verified.
Conclusion: We surmise that murine vaginal mucosal repair is accelerated and potentially regulated by estrogen signaling through the ERα, thus providing a cellular and molecular foundation to understand vaginal healing responses to injury.
Keywords: vaginal injury, regenerative wound healing, estrogen receptor, wound kinetics, novel murine injury model

Julie C.-E. Hakim, MD, FRCSC, FACOG
Introduction
Vaginal atresia, congenital adrenal hyperplasia, obstructive Mullerian anomalies, and Mayer–Rokitansky–Kuster–Hauser syndrome (vaginal aplasia) are prevalent disorders in young girls and adolescents, which ultimately require vaginal surgery with an incidence that ranges from 1/5,000 to 1/2,500.1–3 Notably, surgical vaginal reconstruction leads to increased tissue fibrosis within the mucosal canal in 73% of patients,4,5 which inevitably causes lifelong scarring sequelae or requires reoperation. What determines whether postsurgical vaginal wound healing undergoes regenerative or fibrotic tissue repair is not known and mechanisms that govern vaginal wound healing remain to be elucidated, which requires development of robust in vivo models.
Previous research in vaginal wound healing has mainly utilized rats, rabbits, and guinea pigs as model animals to simulate full-thickness vaginal wounds6–8 where initial work compared full-thickness (FT) wounds (injuries that breach the muscularis) between vaginal and dermal tissues.9 For instance, Abramov et al. showed that FT vaginal wounds in rabbits follow the same histological sequence of dermal wound healing, but at an accelerated pace,9 which equally leads to a similar fibrosis pattern. Interestingly, while the mRNA profiling of FT vaginal wounds followed similar gene regulation as dermal wounds, the mucosal wound mRNA profiles reflected molecular differences underscoring reduced proinflammatory (TNFα, IL1α, and IL1β) and profibrotic (TGFβ) gene expression.10 However, rabbits do not experience an estrus cycle, thereby undermining the comparison with human vaginal tissue repair pathophysiology.9 On the other hand, Balgobin et al.7 systemically replaced estradiol in oophorectomized guinea pigs and created FT vaginal injuries to study the effects of estrogen on vaginal tissue at 4 and 21 days postinjury. While estradiol appeared to increase smooth muscle and thickness of the vagina with increased total and cross-linked collagen, the authors did not assess vaginal healing kinetics or inflammatory and angiogenic profiles. Furthermore, while rabbits and guinea pigs provide adequate vaginal size to create full-thickness injury models, their use is limited for in vivo applications given the cost, federal regulations, and lack of transgenic lines. Moreover, subsequent studies on FT vaginal (epithelium and stroma) injury models in rats from the introitus to the cervix have been previously described.11 Although these studies demonstrated the role of acute postoperative administration of vaginal estrogen on epithelial and stromal healing in the context of biomechanical properties of the healed vaginal tract, the impact of immune cells and healing kinetics was overlooked.
Recent literature has highlighted the effects of estrogen on vaginal wound healing where postoperative vaginal estrogen increases tissue compliance through promotion of matrix components such as collagen types I and III.11 The observations are supported by the strong effects of estrogen on the vaginal mucosa such as the increase in cellular proliferation and differentiation.12 Notably, other studies showed that low-dose conjugated equine estrogen (Premarin; Pfizer, New York, NY) could improve vaginal weight through increased vaginal thickness in ovariectomized rats while enhancing epithelial layer proliferation and inhibiting mucosal proteases.6 In this study, we sought to understand the dynamics of estrogen signaling and vaginal physiological responses to injury using an innovative, in vivo murine model of mucosal wound healing to investigate the mechanistic relevance of estrogen in vaginal tissue repair.
The primary objectives of this project were, first, to establish a murine model of FT vaginal wound healing to define vaginal cell and molecular reactions to injury in the context of kinetics, inflammatory burden, and angiogenic events. Second, in view of recent literature, which highlights the effects of estrogen on progressive stages of vaginal wound healing, including proliferation, epithelial closure, and matrix turnover,6,7 we evaluated the significance of estrogen receptor α (ERα) density during wound healing.
Clinical Problem Addressed
The high incidence of postsurgical fibrosis is at odds with the notion that vaginal mucosa heals regeneratively. There are no established postoperative procedures to reduce vaginal tissue fibrosis. Therefore, understanding how the vagina heals and uncovering the mechanisms that dictate the direction of vaginal tissue repair toward either regenerative or fibrotic wound healing are fundamental to improve patient care and surgical outcomes.
Materials and Methods
Ethics statement
Female CD1 pre-estrus mice between 4 and 5 weeks old were purchased from the Center for Comparative Medicine at Baylor College of Medicine and used for these experiments according to the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines.13 Animals were socially housed under a 12-h light–12-h dark cycle with free access to standard food and water. These studies were carried out in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH). All animal research was conducted according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Baylor College of Medicine (assurance number AN-7475).
In vivo FT vaginal wound
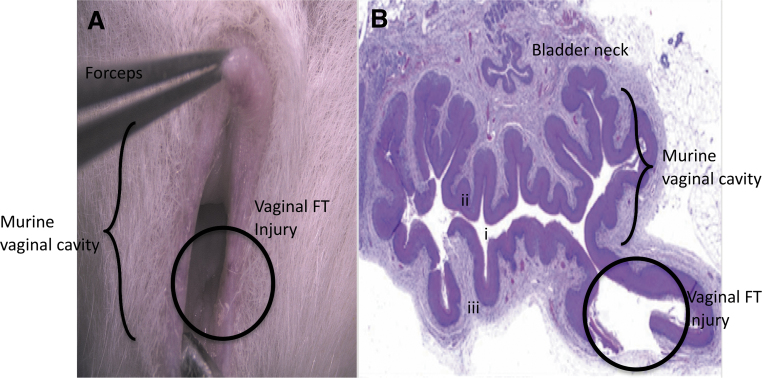
We first established and validated an in vivo, FT vaginal wound model. Mice were sedated using isoflurane at a rate of 0.5 L/min and underwent a bilateral, FT vaginal wound broaching the vaginal smooth muscle layer using a 1-mm biopsy punch at the dorsolateral aspects of the vaginal canal at 500 μm distal to the introitus by inverting the vaginal mucosa using smooth flat forceps (Fig. 1). Injuries were marked using a 29-gauge insulin needle tip dipped in green tissue dye (Davidson's Histology Tissue Dye, Catalog No. 1008-P; Bradley Products, Bloomington, MN). Mice were allowed to recover after surgeries and heal postoperatively. Mice were euthanized at 0, 6, 12, 24, 48, and 72 h and 7 days postinjury. Vaginal tissue was explanted and fixed in 10% neutral buffered formalin (n = 2–4 animals per time point comprising biological and technical replicates).
Figure 1.
Murine vaginal wound model. (A) Minimally invasive intravaginal injury method. (B) H&E staining of a vaginal cross section demonstrates full-thickness injury (circle) where (i) represents the vaginal lumen, (ii) indicates the vaginal epithelial layer, and (iii) depicts vaginal stroma and smooth muscle layers. H&E, hematoxylin and eosin.
Histology of wounds
Formalin-fixed paraffin-embedded (FFPE) sections were cut to 5-μm thickness, rehydrated, and stained with hematoxylin and eosin (H&E) and trichrome staining on a Leica autostainer (ST5020). Vaginal tissue sections were double-blind examined by two pathologists for assessment of vaginal injury depth for validation of our injury model.
To determine wound healing kinetics, H&E-stained wound sections were imaged at 5 × using a Leica DM2000 microscope with a Leica DFC450 camera (Buffalo Grove, IL) and Leica Application Suite X (LAS-X). Measurements (in μm) of the epithelial gap between encroaching epithelial margins were taken using LAS-X software. ImageJ software (NIH, Bethesda, MD) was used to determine total cell density within the injured area of the submucosa using four 40 × images taken around the injury, which was determined to be equal across treatment groups (Supplementary Fig. S1). To evaluate extracellular matrix deposition, 5-μm sections were stained with trichrome and four 40 × images were taken per slide and quantified using ImageJ as the area of submucosal tissue above a threshold, which remained constant for all images.
Immunohistochemistry staining
Immunohistochemistry (IHC) was used to evaluate angiogenesis and inflammation following FT wounding. FFPE sections were cut to 5 μm and rehydrated, followed by antigen retrieval using either citrate buffer at pH 6.0 or low-pH antigen solution (K8005; Agilent, Santa Clara, CA) and the PT Link antigen retrieval system (Dako). IHC was completed either by hand or by using an autostainer (Dako Autostainer Link 48; Agilent). The following primary and secondary antibodies were used for IHC analysis: CD31 (550274, 1:100; BD Pharmingen, San Jose, CA), CD45 (ab10558, 1:2,000; Abcam, Cambridge, MA), CD206 (ab64693, 1:500; Abcam), F4/80 (MF480000, 1:5,000; Thermo Fisher, San Francisco, CA), ERα (ab32063, 1:50; Abcam), Ly6G (551459, 1:200; BD Pharmingen), anti-rabbit (K4009; Agilent), and anti-rat (D35-110; GBI Labs, Bothell, WA). 3,3′-Diaminobenzidine (DAB) or aminoethyl carbazole (AEC) was used to detect positive staining and hematoxylin was used as a counterstain. Four 40 × images per slide were taken using a Leica DM2000 microscope outfitted with a Leica DFC450 camera using Leica Application Suite X software. ImageJ was used to count positive cells within the submucosal tissue, reported as number of cells/submucosal area.
Statistical analyses
Microsoft Excel (v16.22) and Prism (version 6.0e) were used to conduct all statistical analyses. One-way analysis of variance with multiple comparisons post-testing between groups was performed using Dunnett's correction to determine the significance between noninjured control tissue and injured tissue at different time points. Tukey's tests were performed to determine differences between time points. Sample size was considered adequate to determine the difference from controls, for 0 and 48 h postinjury for epithelial gap and 0 and 12 h postinjury for cellular fluctuations, calculated for 95% confidence and power of 80%. Outliers were removed using Q test analyses.
Results
Murine model validation for intravaginal FT wounding
In this study, we established a murine, FT, vaginal wound healing model using an intravaginal minimally invasive approach. One-millimeter punch biopsy wounds were created in the vagina. Grossly, H&E staining of the vaginal cross section at the injury site revealed tissue breaches extending through the epithelial, submucosal, and smooth muscle layers (Fig. 1B), which were consistent across all the animals tested.
Vaginal wounds in a murine model are regenerated by 48 h after injury
We observed that in the majority of mice evaluated, the epithelial layer of vaginal tissue is completely closed and regenerated by 48 h postwounding (Fig. 2A, B). The epithelial gap was widest (p < 0.05) at 12 h and showed progressive decrease between 12 and 72 h. The submucosal tissue layer regenerated more slowly, with large tissue breaches persisting until 72 h postwounding. Despite regeneration of the submucosal tissue defect at 7 days postinjury, with healing indistinguishable from the surrounding uninjured vaginal tissue, there were no statistically significant differences in the total (quantified) collagen presence over time in the injury reaction area (Fig. 2A, C). See Supplementary Figure S1 for H&E and trichrome image acquisition localization and quantification of uninjured vaginal tissue and vaginal tissue at 12 h and 7 days postinjury.
Figure 2.
Murine vaginal wound healing. (A) Representative H&E and trichrome images of murine vaginal wound healing kinetics. (B) Epithelial gap (in μm) of murine vaginal wound healing. (C) Total collagen (% positive area) in injured murine vaginal tissue healing. Data represent mean ± SEM (NI group n = 2, 6–72-h group n = 4, and 7-day group n = 3), 4 images/mouse. *Indicates p < 0.05 compared with uninjured controls, as determined by ANOVA and Dunnett's post hoc test. Scale bar represents 100 μm. ANOVA, analysis of variance; NI, no injury.
Inflammation is resolved and vascular lumen density is restored by day 7 postwounding
We analyzed inflammatory cellular infiltration and vascular lumen density at 12 h and 7 days postinjury as these were the time points that corresponded to highest and minimal epithelial gaps, respectively. We compared inflammation and vascular density at 12 h and 7 days with that of the uninjured control, no injury (NI) vaginal tissue.
We first evaluated angiogenesis by staining for CD31, a marker for endothelial cells. By counting the number of vessels (within the submucosal area) that were positive for CD31, we found that there was a steady, but nonsignificant, increase in the number of CD31+ vessels between the control tissue and injured tissue both 12 h and 7 days postinjury (Fig. 3A, B).
Figure 3.
Inflammatory and angiogenic changes over time in murine vaginal wound resolution. (A) Representative images of wild-type uninjured female and injured mice at 12 h and 7days postinjury. (B) Quantification of CD31+ vessel numbers per area of the submucosa. (C) Quantification of total CD45+ leukocytes, (D) Ly6G+ neutrophils, (E) F4/80+ macrophages, and (F) anti-inflammatory CD206+ M2 macrophages per area of submucosa. (G) Anti-inflammatory M2 macrophage burden represented by the CD206+/F480+ ratio. Collective quantification data represent mean ± SEM (NI group n = 2, 12-h group n = 4, and 7-day group n = 3), 4 images per mouse. *Indicates p < 0.05 compared to uninjured controls as determined by ANOVA and Dunnett's post hoc test. Scale bar represents 100 μm. ANOVA, analysis of variance; NI, no injury.
We next quantified the overall cellular inflammatory burden and macrophage subpopulations using IHC. CD45 was used to reveal total leukocyte populations near the FT wound, and Ly6G was used to detect infiltrating neutrophils. Following tissue injury, we observed that while the number of CD45+ cells was significantly decreased around the wound bed (p = 0.0061), the population recovered by 7 days postinjury (Fig. 3A, C). We noted the opposite pattern for first-responder neutrophil Ly6G+ cells, with the greatest Ly6G+ influx seen at 12 h postinjury (p = 0.0044), which decreased to near-uninjured tissue levels at 7 days postinjury (Fig. 3A, D). Moreover, the total F480+ macrophage population and anti-inflammatory CD206+ M2 macrophages exhibited a similar trend as the overall CD45+ leukocyte population with a significant decrease at 12 h postinjury (CD206, p = 0.0131), which returned to nearly uninjured levels by 7 days (Fig. 3A, E, F). The ratio of M2 (CD206+)/total macrophages (F480+) showed opposite trends and increased markedly at 12 h postinjury and decreased to near-uninjured levels at 7 days postinjury (Fig. 3G). Taken together, these data support the concept that there is a population of resident vaginal leukocytes (CD45+) and that upon injury, there is an acute and rapid influx of neutrophils (Ly6G+) to the vaginal space. Although the overall macrophage population decreases at 12 h, the M2 burden appears to increase at 12 h and supports a shift toward a prohealing environment based on F480 and the CD206:F480 ratio at the earlier time point.
Estrogen receptor expression levels change during vaginal wound healing
To investigate whether estrogen signaling plays a role in vaginal wound healing, we compared ERα levels between uninjured controls and mice at 12 h and 7 days postinjury. We found that at 12 h postinjury, there was an almost 60% reduction in the number ERα-positive cells within the submucosa (Fig. 4A, B), which returned to uninjured control values at 7 days postinjury, thus revealing a transient injury-driven reduction of ERα signaling. This suggests that while estrogen is known to play a role in vaginal wound healing, signaling through ERα could be transient in the healing process since ERα levels returned to uninjured control levels by 7 days postinjury.
Figure 4.
Estrogen plays a role in murine vaginal wound healing. ERα density measuring vaginal wound resolution between uninjured mice and mice at 12 h and 7 days postinjury, respectively, illustrated by IHC images (A) and quantification per area of submucosa (B) of ERα. Data represent mean ± SEM (NI group n = 2, 12-h group n = 4, and 7-day group n = 4). ERα, estrogen receptor alpha; IHC, immunohistochemistry.
Our collective data support two important and novel concepts, namely that the 1-mm, murine, FT vaginal wound heals in an accelerated and regenerative manner by day 3 postinjury and that injury impacts inflammatory and angiogenic cell populations, which could be influenced by estrogen signaling.
Discussion
The present study established an unappreciated wound repair model of murine vaginal mucosa, which uncovers unique healing kinetics, inflammatory patterns, and angiogenic profiles that connect estrogen signaling with vaginal responses to injury.
Model, kinetics, and extracellular matrix
We devised and validated a minimally invasive intravaginal approach of FT vaginal wounding, which enables a robust means to inquire how the vagina and other mucosal tissues such as oral and gastrointestinal microenvironments heal. The model is innovative, minimally invasive, and cost-effective while circumventing the limitations of previous larger animal models, opening new options for state-of-the-art, genetically engineered research approaches and generation of unique reagents for virtually unlimited experimental manipulations. We also demonstrate that murine vaginal wound healing occurs in an accelerated manner with primary epithelial closure long before submucosal integrity is restored. This mirrors established knowledge regarding healing in other mucosally lined tissues, with an absence of the hyperproliferative epithelial appearance seen in dermal tissue repair.
Role of inflammation and angiogenesis in wound healing
When a new wound is created, destruction of the extracellular matrix (ECM) and introduction of microbes lead to an influx of polymorphonuclear leukocytes (PMN or neutrophils), which compose the primary responders of innate immunity. The initial influx of neutrophils to the site after wounding with subsequent reduction follows established patterns of inflammatory postwounding behavior. Neutrophils are recruited to the site immediately after injury to reduce tissue damage associated with the release of proinflammatory mediators,14,15 reactive oxygen species, proteases, and antimicrobials. Consistent with this notion, we demonstrated that the overall cellular inflammatory burden from neutrophils was highest at 12 h postinjury and returned to near-uninjured tissue levels by 7 days postwounding. Interestingly, we did note decreases in total leukocytes and total and anti-inflammatory M2 macrophages at 12 h, with the levels returning to near-uninjured tissue levels by day 7. Our findings are further supported by other tissue injury models16,17 that have observed a similar shift in leukocyte:neutrophil ratios with comparable M1 to M2 macrophage polarization after injury and over time. Moreover, M1 to M2 polarization from early phase wound repair to later healing processes in dermal wounds has been confirmed in alterations implicated in the pathogenesis of chronic wounds. Furthermore, although neutrophil decontamination of the wound bed in the early phases of repair is critical, their efficient removal by macrophages is vital to nondisordered wound healing.16 Importantly, neutrophils are noted to be absent or markedly reduced in nonscarring tissues such as fetal skin, oral mucosa, and Acomys nonscarring mice.17,18 Notably, emerging evidence suggests that macrophages are the central arbiters in all stages of the wound healing response, which exhibit remarkable polarization plasticity present at varying levels during all stages of wound healing.19 The initial proinflammatory environment at 12 h postinjury observed in our murine model of vaginal wound healing undergoes polarizing M1 to M2 shifts into a prohealing stage by 7 days postinjury.
Role of angiogenesis in wound healing
The intertwined nature of inflammation and angiogenesis can be understood through the dynamics of our vaginal model of wound healing. Migration and proliferation of endothelial cells within the proliferative phase of the vaginal tissue repair responses to injury are critical to formation of new capillary beds for nutrient and waste transport and subsequent resolution of the wound site. During the wound healing cycle, macrophages release vascular endothelial growth factors to recruit endothelial cells into the granular tissue.20,21 This continues throughout the proliferative phase and into the latent stages of vascular remodeling, with wound healing progressing in coordination with an increase of M2 macrophages. While we observed no significant rise in the number of CD31+ vessels near the wound, there is a slight upward trend between 12 h and 7 days, suggesting that similar to other wound healing models, angiogenesis follows the inflammatory repair phase, which promotes wound resolution.
Wound healing as an estrogen-dependent phenomenon
The role of estrogen is a potent anti-inflammatory mediator, which has been studied in specific mechanisms of wound healing that include angiogenesis, M1 to M2 macrophage polarization, and fibroblast inhibition. Consistent with those studies, patients undergoing hormone replacement therapy exhibit improved cutaneous wound healing, through reduced PMN recruitment, decreased elastase production, and subsequent increase in collagen deposition.22–25 Exposure to physiological levels of 17β-estradiol (E2) reduces PMN migration by inhibiting chemotaxis through an estrogen receptor-dependent mechanism.26 In this context, estrogen receptors (ERs) are important in many cellular functions, and the expression levels of these receptors concomitantly vary with systemic estrogen levels in a dose-dependent manner.27,28 However, what remains to be elucidated is how the fluctuations in endogenous estrogen affect ER sensitivity, location, and/or density, which are known to be affected by injury.29 In this study, we found that ERα density decreases immediately after injury and rebounds to uninjured levels when injured tissues are regenerated at day 3. Our collective data are supported by previous studies, which show that ERα density is inversely correlated with wound healing in rodent vaginal tissue.6,30–32 Future studies will not only evaluate the connection of ERα density but also unveil how estrogens regulate vaginal mucosa responses to injury as they relate to inflammation, angiogenesis, and collagen deposition. In sum, the emerging concepts from our work and that of others surmise that ERα signaling is mechanistically influenced by injury, which warrants further exploration to leverage our discoveries on the bench with bedside applications that improve regenerative wound repair, standard of clinical care, and patient's quality of life.
Conclusion
The generation, validation, and study of a mouse model for FT vaginal wound healing are vital to understand how estrogen and ER interactions transduce signals initiated by vaginal cell and molecular responses to injury. In this study, we found that our minimally invasive model heals regeneratively, setting a foundation to decipher how vaginal mucosa mediators regulate regenerative tissue repair, which will inevitably lead to identification of next-generation biomarkers to translate into clinical practice. Future FT vaginal wound healing studies of oophorectomized and genetically engineered, conditional cell-specific ERα and/or estrogen receptor beta (ERβ)-deficient mice (commercially available) could uncover genetic and epigenetic mechanisms of estrogen and ER signaling of vaginal wound healing. The proposed studies address the following: (a) a critical knowledge gap underpinning estrogen-driven vaginal responses to injury and (b) unmet clinical needs for the treatment of vaginal scar tissues. We conclude that our present data on postinjury variations in ERα density, dynamics, and sensitivity underscore an opportune postoperative window for the application of estrogen-targeted adjuvant therapies to optimize wound healing outcomes of pediatric, adolescent, and adult female patients.
Innovation
The mechanisms underpinning the cross talk of estrogen-dependent and inflammation–angiogenesis-driven vaginal wound repair are largely unknown. Their elucidation could lead to creation of personalized precision therapies to improve wound healing, promote regenerative healing, and reduce fibrosis by establishing vaginal wound healing kinetics using our groundbreaking murine model. We have validated histopathological methods of quantifying vaginal fibrosis and investigated the relationship of estrogen signaling in wound progression. These discoveries will shed new light onto the pathogenesis of vaginal fibrosis, which will lead to the identification and design of personalized, biomarker-guided precision therapies for vaginal wound healing.
Key Findings
Murine vaginal wound healing occurs in an accelerated manner compared with dermal wound healing, with primary epithelial closure (by day 3 postinjury) occurring long before submucosal integrity is restored.
There is an initial vaginal proinflammatory environment at 12 h postinjury that shifts to a prohealing state by 7 days postinjury.
Overall expression of ERα is directly related to wound resolution progression.
Supplementary Material
Acknowledgments and Funding Sources
The authors thank the TCH Office of Surgical research, especially Drs. Hector Martinez-Valdez and Monica Farenholtz, for their editorial support. Additionally, the entire CV Pathology Team at the Texas Heart Institute (especially Drs. Vela and Segura) was instrumental in this work. This work was supported, in part, through seed funding by the Department of Obstetrics and Gynecology at Baylor College of Medicine, the Clinical Scientist Training Program (CSTP) at Baylor College of Medicine, and through the Texas Children's Hospital Basic Science Surgical Research Clayton Award.
Abbreviations and Acronyms
- AEC
3-amino-9-ethyl carbazole
- ANOVA
analysis of variance
- ARRIVE
Animal Research: Reporting In Vivo Experiments
- CD206
cluster of differentiation 206, Mannose Receptor
- CD31
cluster of differentiation 31, platelet endothelial cell adhesion molecule
- CD45
cluster of differentiation 45, protein tyrosine phosphatase, receptor type, C
- DAB
3-3′-diaminobenzidine
- ECM
extracellular matrix
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- ERs
estrogen receptors
- F480
epidermal growth factor (EGF)-like module-containing mucin-like hormone receptor 1 (EMR1)
- FFPE
formalin-fixed paraffin-embedded
- FT
full-thickness
- H&E
hematoxylin and eosin
- IACUC
Institutional Animal Care and Use Committee
- IHC
immunohistochemistry
- IL1α
interleukin 1 alpha
- IL1β
interleukin 1 beta
- Ly6G
lymphocyte antigen 6 complex locus G6D
- M1
macrophage type 1
- M2
macrophage type 2
- NI
no injury control
- NIH
National Institutes of Health
- PMN
polymorphonuclear neutrophil
- TGFβ
transforming growth factor beta
- TNFα
tumor necrosis factor alpha
Author Disclosure and Ghostwriting
No competing financial interests exist. No ghostwriters were enlisted.
About the Authors
Jennifer M. McCracken, PhD, joined the Laboratory for Regenerative Tissue Repair at Texas Children's Hospital in 2019 as a Research Associate. She completed her PhD in toxicology in 2017. Her research interests are aimed to understand the role of high-molecular-weight hyaluronan and estrogen in regulation of vaginal wound repair. Swathi Balaji, PhD, is an Assistant Professor and Codirector of the Laboratory for Regenerative Tissue Repair in the Department of Pediatric Surgery at Baylor College of Medicine and Texas Children's Hospital. Dr. Balaji's research is focused on understanding the role of exosomes in regulation of the fibrogenic fibroblast phenotype in scar formation and persistence in dermal injuries. Sundeep G. Keswani, MD, is a Professor of Pediatric and Fetal Surgery and Director of the Laboratory for Regenerative Tissue Repair in the Department of Pediatric Surgery at Baylor College of Medicine and Texas Children's Hospital. His research is focused on understanding inflammatory and ECM cross talk in the pathogenesis of fibrosis and scarring in multiorgan fibrosis models. Julie C.-E. Hakim, MD, FRCSC, FACOG, is a Pediatric Gynecologist at Texas Children's Hospital. Her NIGMS K08-funded studies are focused on understanding the mechanisms of vaginal wound healing and medical device innovation to improve vaginal surgical reconstruction outcomes in pediatric and adult patients.
Supplementary Material
References
- 1. Vaginal Agenesis. USNational Libr Med. https://ghr.nlm.nih.gov/condition/mayer-rokitansky-kuster-hauser-syndrome#statistics (accessed July31, 2019)
- 2. Androgen Insensitivity Syndrome. USNational Libr Med 2017 [cited Oct 2, 2017]. https://ghr.nlm.nih.gov/condition/androgen-insensitivity-syndrome (accessed July31, 2019)
- 3. Mayer-Rokitansky-Küster-Hauser syndrome. USNational Libr Med 2017 [cited Oct 2, 2017]. https://ghr.nlm.nih.gov/condition/mayer-rokitansky-kuster-hauser-syndrome (accessed July31, 2019)
- 4. Raya-Rivera A, Esquiliano D, Fierro-Pastrana R, et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 2014;384:329–336 [DOI] [PubMed] [Google Scholar]
- 5. Godbole P, Koyle M, Wilcox D, eds. Pediatric Urology: Surgical Complications and Management. Hoboken, NJ: Second Edi Wiley Blackwell, 2015 [Google Scholar]
- 6. Montoya TI, Maldonado PA, Acevedo JF, Word RA. Effect of vaginal or systemic estrogen on dynamics of collagen assembly in the rat vaginal wall. Biol Reprod 2015;92:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balgobin S, Montoya TI, Shi H, et al. Estrogen alters remodeling of the vaginal wall after surgical injury in guinea pigs. Biol Reprod 2013;89:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abramov Y, Webb AR, Botros SM, Goldberg RP, Ameer GA, Sand PK. Effect of bilateral oophorectomy on wound healing of the rabbit vagina. Fertil Steril 2011;95:1467–1470 [DOI] [PubMed] [Google Scholar]
- 9. Abramov Y, Webb AR, Miller JJR, et al. Biomechanical characterization of vaginal versus abdominal surgical wound healing in the rabbit. Am J Obstet Gynecol 2006;194:1472–1477 [DOI] [PubMed] [Google Scholar]
- 10. Gallant-Behm CL, Du P, Lin S, Marucha P, DiPietro L, Mustoe T. Epithelial regulation of mesenchymal tissue behavior. J Invest Dermatol 2011;131:892–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ripperda CM, Maldonado PA, Acevedo JF, et al. Vaginal estrogen: a dual-edged sword in postoperative healing of the vaginal wall. Menopause 2017;24:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cotreau MM, Chennathukuzhi VM, Harris HA, et al. A study of 17B-estradiol-regulated genes in the vagina of postmenopausal women with vaginal atrophy. Maturitas 2007;58:366–376 [DOI] [PubMed] [Google Scholar]
- 13. NC3Rs ARRIVE Guidelines Checklist 2014. https://www.nc3rs.org.uk/arrive-guidelines (accessed December22, 2018)
- 14. Mak K, Manji A, Gallant-Behm C, et al. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci 2009;56:168–180 [DOI] [PubMed] [Google Scholar]
- 15. Johnson A, Francis M, Dipietro LA. Differential apoptosis in mucosal and dermal wound healing. Adv Wound Care 2014;3:751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landen N, Dongqing L, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci 2016;3861–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bucur M, Dinca O, Vladan C, et al. Variation in expression of inflammation-related signaling molecules with profibrotic and antifibrotic effects in cutaneous and oral mucosa scars. J Immunol Res 2018;1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larouche J, Sheoran S, Maruyama K. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care 2018;7:209–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44:450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greb RR, Heikinheimo O, Williams RF, Hodgen GD, Goodman AL. Vascular endothelial growth factor in primate endometrium is regulated by oestrogen-receptor and progesterone-receptor ligands in vivo. Hum Reprod 1997;12:1280–1292 [DOI] [PubMed] [Google Scholar]
- 21. Gagliardi A, Hadd H, Collins DC. Inhibition of angiogenesis by suramin. Cancer Res 1992;52:5073–5075 [PubMed] [Google Scholar]
- 22. Ashcroft GS, Dodsworth J, Van BE, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-b1 levels. Nature 1996;4:303–308 [DOI] [PubMed] [Google Scholar]
- 23. Son ED, Lee JY, Lee S, et al. Topical application of 17beta-estradiol increases extracellular matrix protein synthesis by stimulating TGF-Beta signaling in aged human skin in vivo. J Invest Dermatol 2005;124:1149–1161 [DOI] [PubMed] [Google Scholar]
- 24. Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol 1999;155:1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen B, Wen Y, Yu X, Polan ML. Elastin metabolism in pelvic tissues: is it modulated by reproductive hormones? Am J Obstet Gynecol 2005;192:1605–1613 [DOI] [PubMed] [Google Scholar]
- 26. Ito I, Hayashi T, Yamada K, Kuzuya M, Naito M, Iguchi A. Physiological concentration of estradiol inhibits polymorphonuclear leukocyte chemotaxis via a receptor mediated system. Life Sci 1995;56:2247–2253 [DOI] [PubMed] [Google Scholar]
- 27. Press M, Nousek-Goebl N, Bur M, Greene G. Estrogen receptor localization in the female genital tract. Am J Pathol 1986;123:280–292 [PMC free article] [PubMed] [Google Scholar]
- 28. Buchanan DL, Kurita T, Taylor JA, Lubahn DB, Cunha GR, Cooke PS. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology 1998;139:4345–4352 [DOI] [PubMed] [Google Scholar]
- 29. Dubal D, Rau S, Shughrue P, et al. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERA in estradiol- mediated protection against delayed cell death. Endocrinology 2006;147:3076–3084 [DOI] [PubMed] [Google Scholar]
- 30. Campbell L, Emmerson E, Davies F, et al. Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its antiinflammatory activities. J Exp Med 2010;207:1825–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilliver SC, Ruckshanthi JPD, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology 2008;149:5747–5757 [DOI] [PubMed] [Google Scholar]
- 32. Hardman MJ, Emmerson E, Campbell L, Ashcroft GS. Selective estrogen receptor modulators accelerate cutaneous wound healing in ovariectomized female mice. Endocrinology 2008;149:551–557 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.