Highlights
-
•
Diaphragmatic clear cell carcinoma (CCC) from endometriosis is extremely rare.
-
•
Diaphragmatic CCC from endometriosis shows cystic mass with solid component.
-
•
MR and serial images are useful in the diagnosis of diaphragmatic CCCs.
Keywords: Clear cell carcinoma, Diaphragm, Endometriosis, Computed tomography (CT), Magnetic resonance imaging (MRI)
Abstract
Diaphragmatic endometriosis is extremely rare. Although endometriosis is considered generally benign, malignant transformation of endometriosis was reported in 1925. Multiple studies have since described clear cell carcinoma (CCC) or endometrioid carcinoma arising from ovarian endometriosis. Previously, only two reports of primary diaphragmatic CCC were reported, in which coexistent endometriosis with CCC was not histologically proven. We report a case of a 55-year-old postmenopausal woman who was admitted to Kindai university hospital for the examination of a cystic mass with papillary components in the right diaphragm. On her past medical history, abdominal hysterectomy and bilateral salpingo-oophorectomy was performed for high-grade cervical intraepithelial neoplasia, uterine myoma, and bilateral ovarian endometriosis 5 years ago. Unenhanced CT performed 5 years ago, showed a nodular lesion with low density in the right diaphragm, consistent with diaphragmatic endometriosis. Magnetic resonance imaging during this admission, showed a cystic mass with papillary components in the right diaphragm and a T2*-weighted gradient echo imaging showed partial low signal intensity in the papillary components and cyst wall, which was suspected to represent hemosiderin deposition. Based on these serial images, malignant transformation of diaphragmatic endometriosis was suspected. Under, open abdominal combined resection of the mass and part of the diaphragm was performed. Endometriosis implants were detected on the pelvic peritoneum. Histopathological examination revealed clear cell carcinoma associated with endometriosis and hemosiderin deposition in the cyst wall. T2*-weighted gradient echo imaging was useful in the detection of hemosiderin deposition caused by the coexistent endometriosis. When a cystic mass with papillary components and cyst wall with hemosiderin deposits are encountered on MR images, malignant transformation of endometriosis is suspected and a detailed medical history should be determined and the possibility of concurrent endometriosis or adenomyosis should be investigated, as should the potential existence of diaphragmatic endometriosis in previous images.
1. Introduction
Endometriosis is commonly located in the pelvic cavity including the ovaries, uterosacral ligaments, and pouch of Douglas, and diaphragmatic endometriosis is rare. It was reported that ovarian endometriosis was associated with an increased risk of malignant transformation developing clear cell carcinoma (CCC) and endometrioid carcinoma (Jimbo et al., 1997, Stamp et al., 2016). Previously, only two reports of primary diaphragmatic CCC were reported, but coexistent endometriosis was not histologically proven (Fujiu et al., 2010, Harimoto et al., 2018). We herein report a first case of histologically proven primary diaphragmatic CCC associated with endometriosis that presented characteristic imaging findings with radiologic–pathologic correlation.
2. Case presentation
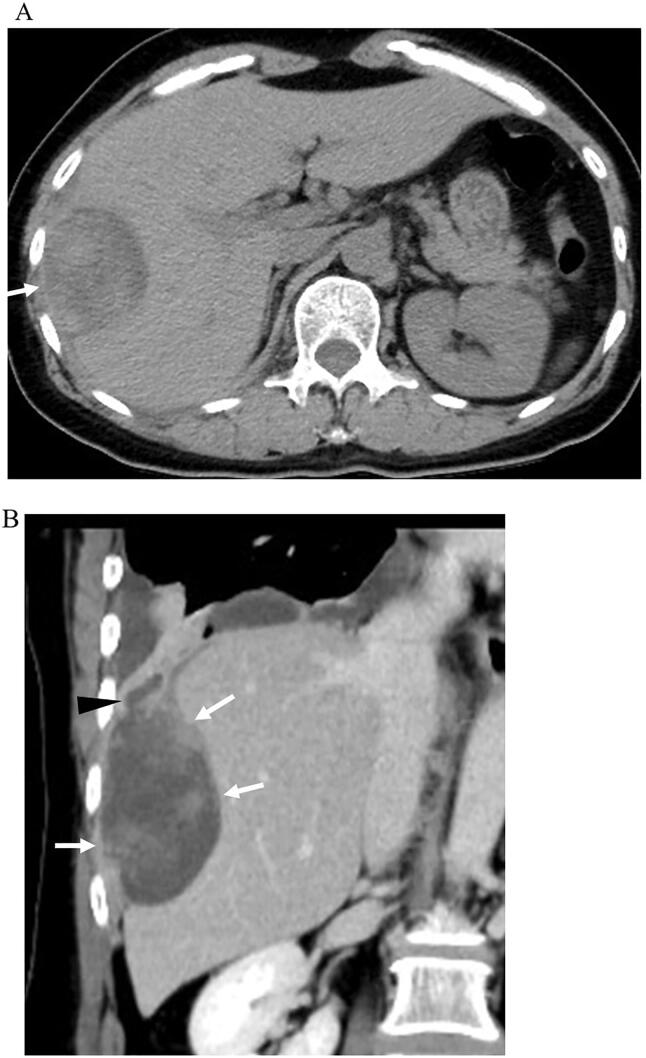
A 55-year-old postmenopausal woman, gravida 3, para 3 was found to have an asymptomatic right pleural effusion on routine chest radiography for periodic medical examination. She was admitted to a nearby general hospital for further examination of right pleural effusion. Unenhanced computed tomography (CT) showed a 5.5 × 4.8 cm round mass with heterogenous high density in the right diaphragm (Fig. 1A). Enhanced CT showed a cystic mass with enhanced papillary components, which had a fistula to the pleural cavity complicated with pleural effusion and passive atelectasis (Fig. 1B). Ultrasonography-guided percutaneous aspiration of the cystic mass was performed. The size of the mass was transiently reduced, but it immediately regrew. Fluid cytology analysis revealed neutrophil-dominant leukocytosis and no malignant cells. She was referred to our hospital for surgery.
Fig. 1.
A. Unenhanced computed tomography (CT) shows a round mass (arrow) with heterogenous high density in the right diaphragm. B. Enhanced CT with coronal reconstruction shows a cystic mass with enhanced papillary components (arrows), which had a fistula (arrowhead) to the pleural cavity complicated with pleural effusion and passive atelectasis.
On her past medical history, abdominal hysterectomy and bilateral salpingo-oophorectomy was performed for high-grade cervical intraepithelial neoplasia, uterine myoma, and bilateral ovarian endometriosis at our hospital 5 years ago. At that time, unenhanced CT scan was performed for screening of other diseases. Endometriosis in the right diaphragm was not identified on unenhanced CT images and during the surgery, However, on retrospective review of the unenhanced CT images, a nodular lesion with low density in the right diaphragm, consistent with diaphragmatic endometriosis (Fig. 2) was detected.
Fig. 2.
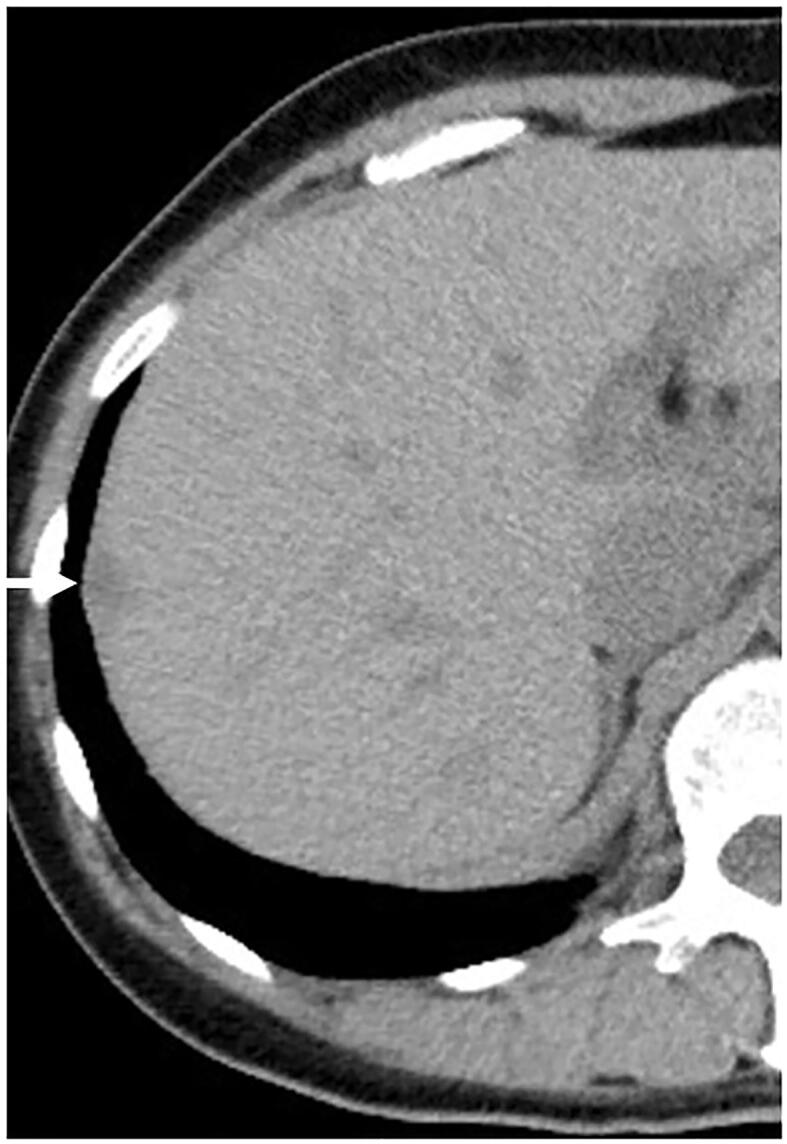
Unenhanced computed tomography performed 5 years ago, shows a nodular lesion (arrow) with low density in the right diaphragm, consistent with diaphragmatic endometriosis.
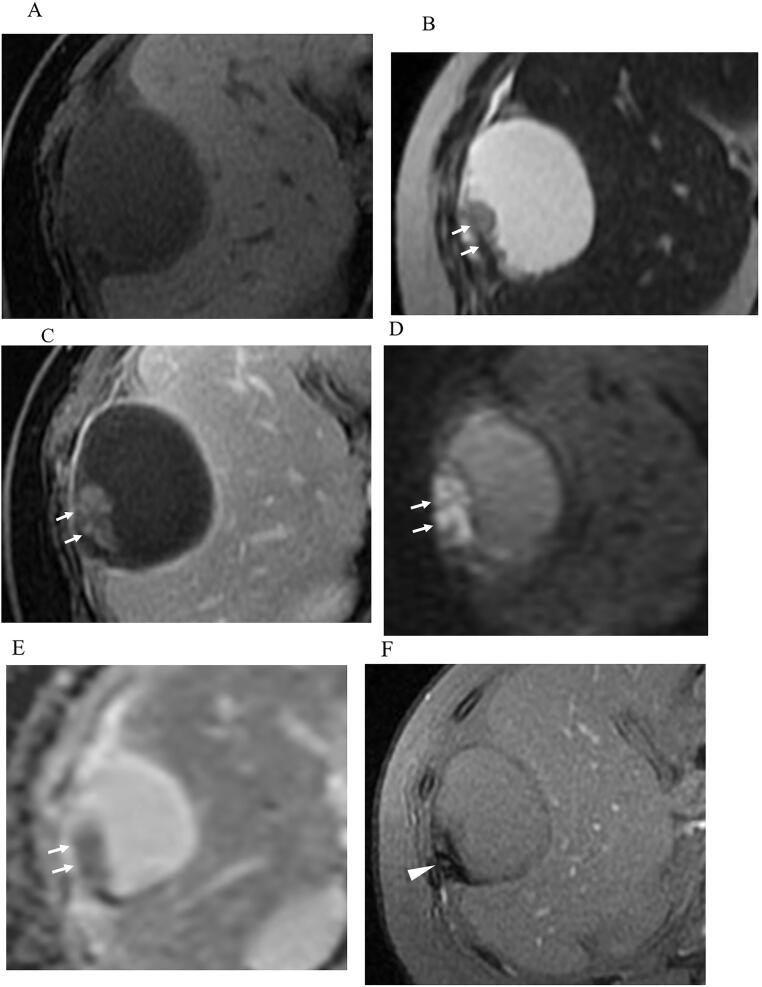
Magnetic resonance imaging was performed during this admission. The cystic mass contained a serous fluid with low signal intensity on T1-weighted imaging and high signal intensity on T2-weighted imaging (Fig. 3A, B). The papillary components exhibited low signal intensity on T1-weighted imaging, high signal intensity on T2-weighted imaging, enhancement on gadolinium T1-weighted imaging, and high-signal restricted diffusion on diffusion-weighted imaging with apparent diffusion coefficient (ADC) mapping (mean ADC value 0.96 × 10 × 10–3 mm2/s) (Fig. 3A-E). T2*-weighted gradient echo imaging showed partial low signal intensity in the papillary components and cyst wall, which was suspected to represent hemosiderin deposition (Fig. 3F).
Fig. 3.
T1-weighted (A) and T2-weighted (B) imaging shows a cystic mass with papillary projection (arrows). The cystic mass contains serous fluid and exhibited low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The papillary components exhibits enhancement on gadolinium T1-weighted imaging (C: arrow), and restricted diffusion on diffusion-weighted imaging (D: arrow) and apparent diffusion coefficient (ADC) map (E: arrow). The mean ADC value is 0.96x10 × 10–3 mm2/s. T2*-weighted gradient echo imaging (F) shows partial low signal intensity (arroehead) in the papillary components and cyst wall, which is suspected to be hemosiderin deposition.
Based on these serial images, malignant transformation of diaphragmatic endometriosis was suspected. Open abdominal surgery was performed, and right diaphragmatic cystic mass with a fistula to the pleural cavity was found. The mass adhered to the liver, but the adhesion was easily removed. Combined resection of the mass and part of the diaphragm, and repair of the diaphragmatic defect using a Gore-tex mesh were performed. Endometriosis implants were detected on the pelvic peritoneum during the surgery.
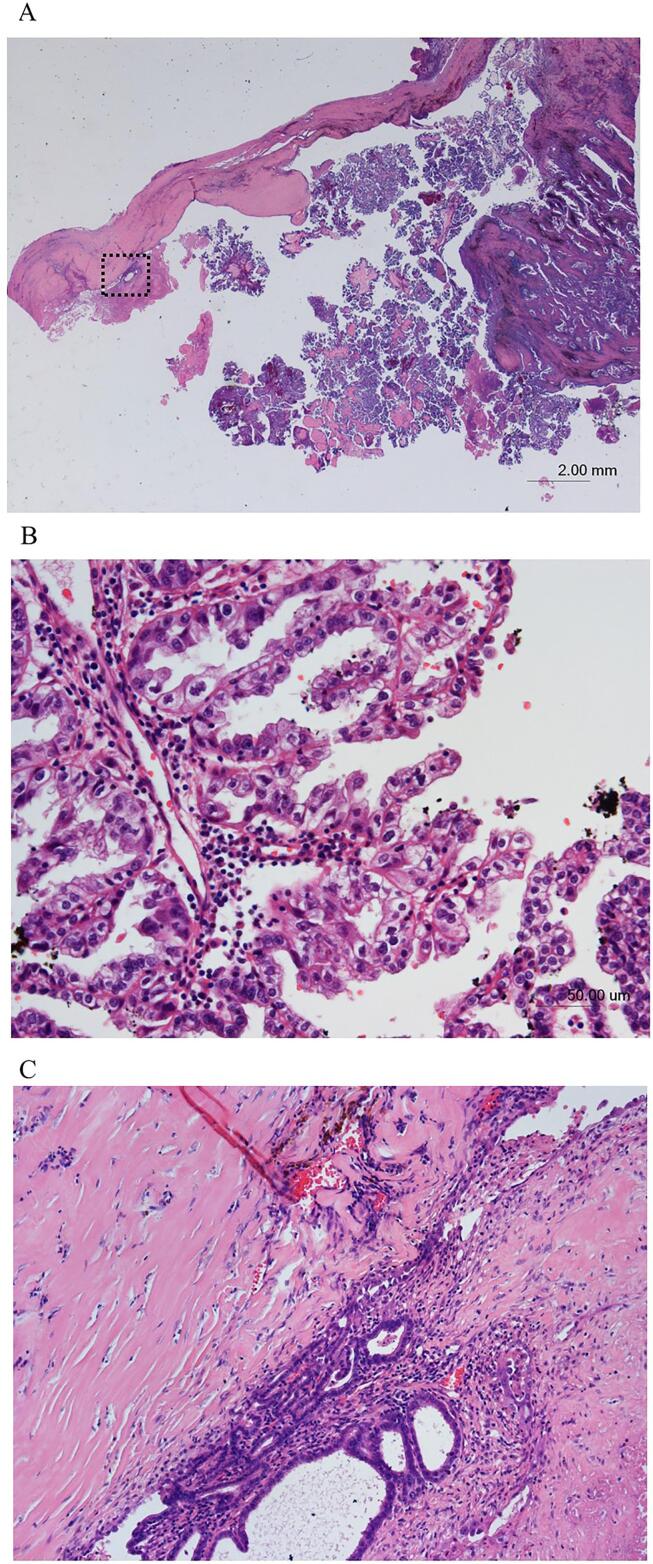
Histopathological examination revealed papillary structures and hemosiderin deposition in papillary components and cyst wall (Fig. 4A). The papillary structures were lined by columnar or hobnail cells with clear cytoplasm and nuclei of various sizes (Fig. 4B), and the tumor cells invading a diaphragmatic muscle. In immunohistochemistry analysis the tissue was hepatocyte nuclear factor 1 beta (HNF1β)-positive and estrogen receptor-negative. Thus, clear cell carcinoma (CCC) was diagnosed. Moreover, endometrial glands and endometrial stroma were detected in the cyst wall (Fig. 4C), resulting in a diagnosis of endometriosis. Based on histopathology and serial imaging, a primary diaphragmatic CCC associated with endometriosis was diagnosed.
Fig. 4.
Histopathological examination, Hematoxylin and eosin (H&E) staining. A. Low-power of view (x5). reveals papillary structures and hemosiderin deposition in the papillary components and cyst wall. B. On high-power of view (x200), the papillary structures are lined by columnar or hobnail cells with clear cytoplasm and nuclei of various sizes, resulting in a diagnosis of clear cell carcinoma. C. On high-power of view (x100), endometrial glands and endometrial stroma are detected in the cyst wall, resulting in a diagnosis of endometriosis.
She received six courses of TC adjuvant chemotherapy (paclitaxel 180 mg/m2 and carboplatin AUC 6 mg/m × minute) at 3-week intervals and is alive without evidence of disease 15 months after the surgery.
3. Discussion and conclusions
Endometriosis is characterized by the extrauterine growth of endometrial glandular epithelial and stromal cells. It is commonly located in the pelvic cavity including the ovaries, uterosacral ligaments, and pouch of Douglas, and more rarely in remote locations such as the abdominal peritoneum, diaphragm, pericardium, pleura, lung, and brain. It affects 10% of all women of reproductive age, and 30% of infertile women. It is a benign chronic gynecological disease and is clinically associated with dysmenorrhea, dyspareunia, pelvic pain, and infertility. Its etiology is yet to be clearly elucidated, but it is considered to involve a combination of anatomical, hormonal, immunological, reactive, estrogenic, genetic, epigenetic, and environmental factors. With regard to pathogenesis, Sampson’s retrograde menstruation theory that endometriosis originates from implantation and invasion of menstrual endometrial cells due to reflux through the fallopian tubes into the peritoneal cavity is widely accepted. And the Coelomic metaplasia theory postulates that endometriosis originates from the metaplasia of specialized cells that are present in the mesothelial lining of the visceral and abdominal peritoneum.
Diaphragmatic endometriosis is rare, and it usually occurs in the right side of the diaphragm. The higher prevalence in the right diaphragm supports Sampson’s retrograde menstruation theory. On the left side of the pelvis, the sigmoid colon covers part of the left adnexa and is fixed to the pelvic side wall by adhesions. Conversely the right side of the pelvis has no such protective barrier, therefore on that side menstrual blood is carried away to the right subphrenic space by the flow of peritoneal fluid. Catamenial pneumothorax and hemothorax can be caused. Typical symptoms of diaphragmatic endometriosis include chest pain, dyspnea, epigastric pain, shoulder pain, and upper abdomen pain. The symptoms are usually periodic, but some patients have continual symptoms that are not associated with the menstrual cycle. Moreover, it is often asymptomatic as it was in the present case. On CT imaging, it often appears as a nodular or plaque-type lesion in the right diaphragm, as it was in the present case (Posniak et al., 1990). On T1-weighted magnetic resonance imaging the lesion often demonstrates hyperintensity, reflecting hemorrhage. In the treatment, medical treatment is the first line for symptomatic cases. Surgery is indicated in the symptomatic patients with failure of medical treatment. For some groups, it is recommended that asymptomatic implants incidentally founded during laparoscopy must be treated to avoid, theoretically, a progression to deep endometriotic nodules.
It was reported that the rates of synchronous endometriosis in conjunction with ovarian cancer were reportedly 40.6% in patients with CCC and 23.1% in patients with endometrioid carcinoma (Jimbo et al., 1997). In another report, endometriosis was identified in the final pathology reports in 51% of patients with CCC and 43% with endometrioid carcinoma (Stamp et al., 2016). It has been suggested that hemosiderin, heme, or iron deposition trigger oxidative stress in patients with endometriosis, and the oxidative stress may contribute to subsequent carcinogenesis of endometriosis (Kobayashi et al., 2009). Endometriosis-associated ovarian carcinogenesis is considered to involve aberrant expression of the AT-rich interactive domain-containing protein 1A (ARID1A) tumor suppressor gene, and driver genes including phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and Kirsten rat sarcoma viral oncogene homologs (KRAS) (Anglesio et al., 2017), which are promoted under oxidative stress. HNF1β appears to play a key role in anti-oxidative defense mechanisms. Overexpression of HNF1β is evident in nearly all CCCs, it may play a role in the development of CCCs in stressful environments, and it can be used as a biomarker to assist the histopathological diagnosis of CCC (Shigetomi et al., 2012).
The origin of CCCs in the peritoneum and diaphragm is currently thought to be associated with Müllerian metaplasia and endometriosis. To our knowledge 15 cases of primary peritoneal or diaphragmatic CCCs have previously been reported, and they are summarized in Table 1 (Terada and Kawaguchi, 2005, Takano et al., 2009, Fujiu et al., 2010, Harimoto et al., 2018, Shigeta et al., 2014, Insabato et al., 2015, Peiro et al., 2020). Only two cases of diaphragmatic CCC were identified (Fujiu et al., 2010, Harimoto et al., 2018). The mean age of the patients was 55 years (range 45–67 years), and 6/15 cases had a history of endometriosis or adenomyosis, or concurrent endometriosis and adenomyosis. In treatment, it is reported that TC (paclitaxel and carboplatin) adjuvant chemotherapy after debulking surgery can be effective.
Table 1.
Summary of primary peritoneal and diaphragmatic clear cell carcinomas reported in the literature.
| Case | Authors | Age | Past history or concurrence of endometriosis or adenomyosis | Serum CA125 (U/mL) | Tumor size | Location | Peritoneal dissemination | CT findings | MR findings | Macroscopic findings | Coexistent endometriosis with CCC on the histopatlogy | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Evans et al. | 54 | Ov EM, AM | NA | 18 × 13 cm | Sigmoid mesocolon | No | Multilocular cyst with nodules | NA | Multilocular cyst with nodules | No | DS followed by RT | NA |
| 2 | Lee et al. | 67 | None | 2218 | 6 cm | Abdomen, Pelvs | Yes | NA | NA | NA | No | DS with TAH + BSO | NA |
| 3 | Tziortzioti et al. | 62 | None | 313.9 | 0.5–2 cm | Subdiaphragm, Abdomen, Omentum | Yes | NA | NA | NA | No | DS with TAH + BSO, CTx | DOD at 6 months |
| 4 | Ichimura et al. | 45 | Ov EM | 28 | NA | Pelvis | No | Cyst with solid components | NA | NA | No | DS with TAH + BSO, CTx | RD at 32 months |
| 5 | Hama et al. | 53 | EM | 467 | NA | Pelvis, Liver surface | Yes | Cystic mass with solid coponents | Multicystic mass with hemorrhage and heterogenous solid protrusions | NA | No | DS and BSO | DOD at 5 months |
| 6 | Terada et al. | 49 | None | NA | 3 cm, 2 cm | Greatur curvature of the stomach, Splenic hilus | No | NA | NA | Cystic tumor with solid and papillary components | No | DS | DOD at 6 months |
| 7 | Takano et al. | 53 | None | 467 | 13 × 5 cm | Between liver and disphargm, Omentum, Abdomen | Yes | NA | NA | NA | No | DS | DOD at 5 months |
| 8 | Takano et al. | 66 | None | 347 | 20 × 15 cm | Omentum | No | NA | Cystic and solid mass | NA | No | DS with TAH + BSO, omentectomy, lymph node dissection, CTx | NED at 20 months |
| 9 | Muezzinoglu et al. | 54 | pEM | 42.3 | 25 × 25 × 4 cm | Abdomen | No | NA | Cystic mass with solid nodules | Mulicystic tumor with papillary structures and solid nodules | Yes | DS with TAH + BSO, CTx | NED at 12 months |
| 10 | Johnson et al. | 54 | None | NA | 5.6 × 3.7 × 3.5 cm | Pelvis | No | Soft tissue mass | NA | NA | No | CTx and RTx | RD (bone and liver) |
| 11 | Shigeta et al. | 59 | AM, pEM | 76 | 7 cm | Pelvis | No | NA | Heterogenous tumor | Multilocular cystic tumor with solid components | No | DS with TAH + BSO, PLD, CTx | NED at 5 months |
| 12 | Insabato et al. | 49 | None | NA | 9.5 × 9 × 7 cm | Adherent to the ileum | No | Solid and cystic mass | NA | Microcystc and solid tumor | No | DS with left oophorectomy | RD at 6 months(Inguinal lymphnode), NED at 5 months after second surgery and CTx |
| 13 | Peiro et al. | 48 | None | 57 | 25 × 15 cm | Abdomen | No | Cystic mass wth papillary projection | NA | Multilocular cystic tumor with papillary components | No | DS with T1H + BSO + omentectomy | RD at 8 months (peritoneum), stable at 28 months after initial surgery by CTx |
| 14 | Fujiu et al. | 65 | Ov EM | 48 | 2.5 cm | Right diaphragm | No | Thick-walled cystic mass with peripheral enhancement | Thick-walled cystic mass with peripheral enhancement | Thick-walled unilocular cyst with protruded components | No | DS | RD at 12 months (lung) |
| 15 | Harimoto et al. | 55 | None | NA | 3 cm | Right diaphragm | No | Lobulated cystic mass | Lobulated cystic mass | Lobulated cystic tumor with protruded components and intracystic hemorrhage | No | DS | NA |
| 16 | Present case | 55 | Ov EM, pEM | 40 | 5.5 × 4.8 cm | Right diaphragm | No | Cyst with solid and papillary components | Cyst with solid and papillary components | Cyst with solid and papillary components | Yes | DS and Cx | NED at 15 months |
Ov EM, ovarian endometriosis; pEM, peritoneal endometriosis; AM, adenomyosis; CCC, clear cell carcinoma; NA, not available DS, debulking surgery; TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; CTx, chemotherapy; RTx, radiotherapy; DOD, death of disease; NED, no evidence of isease; RD, recurrence of disease.
On imaging, most appeared as unilocular or multilocular cystic masses with solid or papillary components, which is similar to malignant transformation of ovarian endometriosis (Tanaka et al., 2000, Takeuchi et al., 2006). In the present case, T2*-weighted gradient echo images showed partial low signal intensity in the papillary components and cyst wall, reflecting hemosiderin deposition. Such findings can be useful in the detection of the coexistence of endometriosis with hemosiderin deposition (Tanaka et al., 2000, Takeuchi et al., 2006).
In conclusion, we report a first case of histologically proven primary diaphragmatic CCC associated with endometriosis. Imaging showed a cystic mass with papillary components in the right diaphragm. T2*-weighted gradient echo imaging detected hemosiderin deposition caused by the coexistent endometriosis. When these characteristic imaging features are encountered, a detailed medical history should be determined and the possibility of concurrent endometriosis or adenomyosis should be investigated, as should the potential existence of diaphragmatic endometriosis in previous images.
Informed consent
Written informed consent was obtained from the patient to publish this data.
CRediT authorship contribution statement
Mitsuru Matsuki: Conceptualization, Writing - original draft. Isao Numoto: Data curation. Takefumi Hamakawa: Data curation. Kazunari Ishii: Writing - review & editing. Takaaki Chikugo: Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Jimbo H., Yoshikawa H., Onda T., Yasugi T., Sakamoto A., Taketani Y. Prevalence of ovarian endometriosis in epithelial ovarian cancer. Int. J. Gynaecol. Obstet. 1997;59:245–250. doi: 10.1016/s0020-7292(97)00238-5. [DOI] [PubMed] [Google Scholar]
- Stamp J.P., Gilks C.B., Wesseling M., Eshragh S., Ceballos K., Anglesio M.S. BAF250a expression in atypical endometriosis and endometriosis-associated ovarian cancer. Int. J. Gynecol. Cancer. 2016;26:825–832. doi: 10.1097/IGC.0000000000000698. [DOI] [PubMed] [Google Scholar]
- Terada T., Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J. Exp. Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- Takano M., Yoshikawa T., Kato M., Aida S., Goto T., Furuya K., Kikuchi Y. Primary clear cell carcinoma of the peritoneum: report of two cases and a review of the literature. Eur. J. Gynaecol. Oncol. 2009;30:575–578. [PubMed] [Google Scholar]
- Fujiu K., Miyamoto H., Hashimoto S., Suzuki N., Takano Y., Teranishi Y. A case of diaphragmatic clear cell carcinoma in a patient with a medical history of ovarian endometriosis. Int. J. Clin. Oncol. 2010;15:489–492. doi: 10.1007/s10147-010-0052-y. [DOI] [PubMed] [Google Scholar]
- Harimoto N., Hagiwara K., Yamanaka T., Ishii N., Igarashi T., Watanabe A. Fairly rare clear cell adenocarcinoma mimicking liver cancer: a case report. Surg. Case. Rep. 2018;4:97. doi: 10.1186/s40792-018-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posniak H.V., Keshavarzian A., Jabamoni R. Diaphragmatic endometriosis: CT and MR findings. Gastrointest. Radiol. 1990;15:349–351. doi: 10.1007/BF01888817. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Yamada Y., Kanayama S., Furukawa N., Noguchi T., Haruta S. The role of hepatocyte nuclear factor-1beta in the pathogenesis of clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer. 2009;19:471–479. doi: 10.1111/IGC.0b013e3181a19eca. [DOI] [PubMed] [Google Scholar]
- Anglesio M.S., Papadopoulos N., Ayhan A., Nazeran T.M., Noë M., Horlings H.M. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 2017;376:1835–1848. doi: 10.1056/NEJMoa1614814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi H., Tsunemi T., Haruta S., Kajihara H., Yoshizawa Y., Tanase Y. Molecular mechanisms linking endometriosis under oxidative stress with ovarian tumorigenesis and therapeutic modalities. Cancer Invest. 2012;30:473–480. doi: 10.3109/07357907.2012.681821. [DOI] [PubMed] [Google Scholar]
- Shigeta N., Yoshino K., Matsuzaki S., Morii E., Ueda Y., Kimura T. Clear cell adenocarcinoma of the peritoneum: a case report and literature review. J. Ovarian. Res. 2014;7:86. doi: 10.1186/s13048-014-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insabato L., Natella V., Somma A., Persico M., Camera L., Losito N.S. Primary peritoneal clear cell carcinoma versus ovarian carcinoma versus malignant transformation of endometriosis: a vexing issue. Int. J. Surg. Pathol. 2015;23:211–216. doi: 10.1177/1066896915573567. [DOI] [PubMed] [Google Scholar]
- Peiro G., Silva-Ortega S., Garcia-Espasa C., Sala-Ferichola M., Perez-Vicente S., Castellon-Molla E. Primary peritoneal clear cell carcinoma. A case report and literature review. Gynecol. Oncol. Rep. 2020;32:100551. doi: 10.1016/j.gore.2020.100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y.O., Yoshizako T., Nishida M., Yamaguchi M., Sugimura K., Itai Y. Ovarian carcinoma in patients with endometriosis: MR imaging findings. Am. J. Roentgenol. 2000;175:1423–1430. doi: 10.2214/ajr.175.5.1751423. [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Matsuzaki K., Uehara H., Nishitani H. Malignant transformation of pelvic endometriosis: MR imaging findings and pathologic correlation. Radiographics. 2006;26:407–417. doi: 10.1148/rg.262055041. [DOI] [PubMed] [Google Scholar]