Abstract
The greatest obstacle to an HIV cure is the persistence of latently infected cellular reservoirs in people living with HIV (PLWH) taking antiretroviral therapy (ART). However, no consensus exists on the direct link between local tissue inflammation and the HIV burden. Herein, we have compared the levels of local inflammation, epithelial integrity and HIV DNA between inflamed and non-inflamed colon tissue in a PLWH who underwent a colectomy due to ulcerative colitis. We have observed a 27-fold higher frequency of cells harboring HIV DNA in inflamed compared to non-inflamed colon tissue. Analysis of the expression of occludin-1 and claudin-3 confirmed our macroscopic characterization of inflamed and non-inflamed colon. Our results confirm that increased gut permeability and inflammation are associated with a higher frequency of infected cells and suggest that restoring gut barrier integrity may be used as a strategy to reduce inflammation and HIV persistence in the gut.
Keywords: HIV DNA, Reservoir, Colon, Ulcerative colitis, Inflammation, Gut barrier integrity
Introduction
The greatest challenge to an HIV cure is the persistence of latently infected cellular reservoirs in memory CD4+ T cells and macrophages in people living with HIV (PLWH) taking antiretroviral therapy (ART). One of the main reservoirs lies in the gastrointestinal (GI) tract which contains a large proportion of T cells and macrophages.1 HIV RNA, HIV DNA and infectious virus have been detected in the gut of ART-suppressed individuals.2, 3, 4 Moreover, higher levels of in vitro proinflammatory cytokine production were found in gut biopsies of HIV-infected patients when compared with uninfected controls.5 Persistent gut inflammation is associated with HIV replication, gut epithelial barrier dysfunction and microbial translocation, even in PLWH taking ART.6 However, no consensus exists on the direct link between local tissue inflammation and HIV burden.
There is evidence that HIV infection breaches the gut barrier in a way similar to inflammatory bowel disease (IBD).7 The exact cause of IBD remains unknown but an important role of CD4+ T cells is suggested in its pathophysiology. Some studies showed that the incidence of IBD in individuals with HIV is increased, either due to local inflammation, or immune changes induced by HIV such as loss of CD4+ T cell number and function which may facilitate its development.8 On the other hand, it is purported that a progressive decline in the CD4+ T cell count caused by HIV may reduce IBD disease activity and contribute to remission.9
As availability of tissue samples is sparse, comparison of different sections of the same tissue is challenging in living people. Most studies comparing HIV reservoir frequency in different organs use animal models or autopsy-obtained specimens. Herein, we have compared the levels of local inflammation, epithelial integrity and HIV DNA between inflamed and non-inflamed colon tissue in a woman living with HIV who underwent a colectomy due to treatment-resistant ulcerative colitis UC).
Methods and materials
Participant
A 36-year-old, Canadian-born Caucasian woman was diagnosed with HIV in 2008 with a normal CD4+ T cell count at 627/mm3, a CD4:CD8 ratio of 0.9 and low viral load at 1089 HIV-1 copies/mL. No ART was initiated due to the normal CD4+ T cell count. After four years with a viral load below 5000 copies, viremia increased to 78011 copies and a rapid CD4+ T cell count drop to 443 cells/mm3 with a decrease of the CD4:CD8 ratio to 0.3 were observed. Treatment with tenofovir disoproxil 300 mg/emtricitabine 200 mg orally once daily and raltegravir 400 mg orally twice daily was initiated in September 2012. Her HIV viral load was below the detection level from November 2012. A few months later, she presented with abdominal cramps and melena; a colonoscopy examination was compatible with ulcerative colitis (UC). The biopsy results confirmed chronic idiopathic inflammatory bowel disease, while CMV was negative by immunohistochemistry. Due to the severity of her UC, she was initially treated with oral prednisone (50 mg daily). In the following years, she had several flare-ups and received the tumor necrosis factor-α blockers infliximab and adalimumab, α4β7 integrin blocker vedolizumab and Janus kinase inhibitor tofacitinib.10 In June 2018, due to recurrent episodes of intestinal bleeding and pain, a total colectomy with ileal pouch was suggested. Her CD4+ T cell count was then at 531 cells/mm3 with a viral load below the level of detection. She underwent surgery in June 2018 and after signing an informed consent, she agreed to have a portion of her resected colon used for research purposes including tissue biobanking and HIV reservoir research.
Tissue samples
Blood samples were collected from the participant before surgery to isolate peripheral blood mononuclear cells (PBMCs) and plasma, which were then stored in liquid nitrogen and at −80 °C, respectively. Quantification of HIV plasma viral load (VL) was done using the Abbott Real Time HIV-1 assay (Abbott Laboratories, Abbott Park, Illinois, USA). CD4+ and CD8+ T cell counts were measured in blood using 4-colour flow cytometry. Immediately after surgery, a pathologist examined the specimen, and separated inflamed sections from non-inflamed tissue (Supplementary Fig. 1). Both tissue sections were kept in medium (RPMI + HEPES + Glucose, Wisent, Canada) and processed within 1 hour post-surgery. Small tissue sections were flash frozen in liquid nitrogen and stored at −80 °C until use. Cells were extracted from fresh tissue with multiple liberase treatments as previously described.11 Percentages of CD8+ and CD8 negative considered as CD4+ T cells among CD45+CD3+ hematopoietic T-cells were assessed by flow cytometry as previously described.11
HIV-DNA quantification
The frequency of cells harboring HIV DNA in peripheral blood and gut tissue was measured using a PCR-based assay for the gag gene using a technique previously described that can detect a single copy of viral genome per PCR reaction.12
Markers of inflammation measured by real-time quantitative PCR
RNA was extracted from flash frozen pieces of tissue by using the RNeasy Mini Kit (Qiagen) and reversed transcribed by using the RT2 First Strand Kit (Qiagen). Transcripts for GAPDH, tumor necrosis factor α (TNF-α), IL-6, CD4, occludin and claudin-3 were measured by using the RT2 SYBR Green Mastermixes (Qiagen). All measurements were done in triplicate on a BioRad CFX384 and analyzed using CFX Maestro.
Study results
No difference in CD4+ T cell frequency between inflamed and non-inflamed colon tissue
In order to assess the infiltration of CD4+ T cells in both the inflamed and non-inflamed parts of the colon, CD4+ T cell frequency was measured by flow cytometry; the expression of CD4 was assessed by real-time quantitative PCR. A low percentage of CD4+ T cells was detected in both the inflamed (1.8%) and non-inflamed (4.9%) gut sections (Table 1) by flow cytometry. Furthermore, levels of CD4 transcripts were similar in the inflamed and non-inflamed tissues (Fig. 1C).
Table 1.
The percents of CD4+ T cell in PBMC and different gut tissues.
| CD4+ % | CD8+ % | CD3+ % | CD45+ % | Total HIV DNA (median) normalized by CD4 percents | |
|---|---|---|---|---|---|
| PBMC | 47 | 40 | 88 | 100 | 250 |
| Inflamed gut | 65.7 | 30.9 | 26.5 | 10.8 | 1482 |
| Non-inflamed gut | 59.6 | 38.8 | 42.2 | 19.6 | 27 |
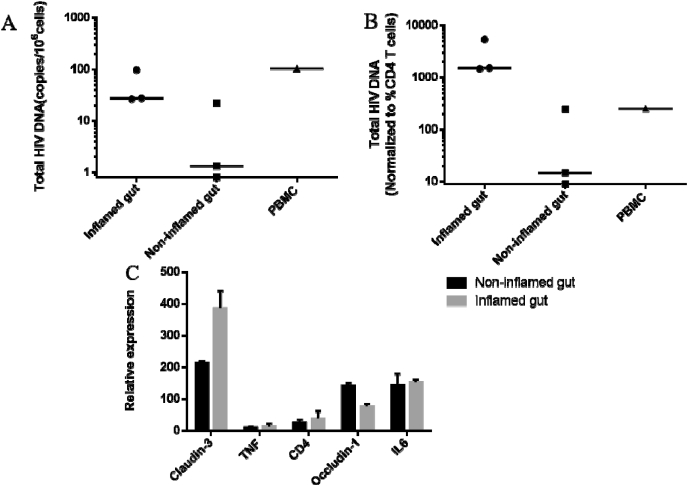
Fig. 1.
Total HIV DNA and relative expression of claudin-3, CD4, IL-6, TNF and occludin-1 in inflamed and non-inflamed gut tissue. Median of HIV DNA levels in 3 inflamed and non-inflamed tissue samples. PBMCs were measured by nested qPCR. A. HIV DNA copies per million cells. B. HIV DNA levels normalized to the percentage of CD4+ T cells detected in both tissues and PBMCs. Unpaired nonparametric test (Mann-Whitney tests) was used for statistical analysis. C. Normalized expression of IL-6, TNF-α and occludin-1 according to GAPDH expression.
Higher levels of HIV DNA in the inflamed compared to non-inflamed colon
HIV DNA level in PBMCs was 103 copies/106 cells. In three biopsies of the inflamed part of the colon, we detected a median of 27 (range 26–97) copies/106 cells of HIV DNA, while HIV DNA level in the adjacent non-inflamed gut tissue was only 1 copy (0–22)/106 cells (Fig. 1A). HIV DNA levels normalized to the CD4+ T cell percentage in each type of gut tissue also showed higher levels in the inflamed section with 1482 (1400–5142) compared to 27 (0–451) HIV copies/106 CD4+ T cells in the non-inflamed section (Fig. 1B).
Decreased occludin-1 and increased claudin-3 expression in inflamed colon
Claudin-3 is a marker of gut epithelium which is increased during inflammation. We detected a higher expression of claudin-3 in the inflamed gut tissue compared to the non-inflamed part (Fig. 1C). Conversely, occludin-1 is a marker of epithelium integrity which decreases during inflammation. The expression of occludin-1 was decreased in inflamed tissue. As expected, the ratio of claudin:occludin expression was higher in the inflamed than non-inflamed gut sections.13 However, the expression of IL-6 and TNF-α in inflamed and non-inflamed gut tissues was not different, possibly due to the long-term immune suppression with anti-TNF-α medication and prednisone.
Discussion
In an ART-treated adult woman who underwent colectomy for UC, we have observed a higher frequency of cells harboring HIV DNA in the inflamed than non-inflamed colon tissue. Previous studies have shown that HIV DNA levels per million CD4+ T cells were on average 2–5 times higher in the gut than blood in ART-suppressed PLWH,14,15 a trend we have also observed in this participant.
To confirm our macroscopic evaluation of inflamed and non-inflamed colon sections, we have performed RT-qPCR to quantify markers of inflammation and epithelial integrity. The gut barrier is composed of a single layer of epithelial cells joined at their apical side by tight junctions (TJs). The TJ is constituted by transmembrane proteins such as occludin, tricellulin and claudins.16 TJ dysfunction can lead to the disruption of the intestinal barrier integrity, manifested by epithelial hyperpermeability as observed in patients with IBD. Moreover, increased macromolecular flux in the intestine has been suggested as a predictor for inflammatory relapse in UC patients in remission.17 As expected, we have detected a lower expression of occludin-1 in the inflamed compared to the non-inflamed colon sections. Although the influence of UC on claudin-3 expression is unclear, we have found a higher ratio of claudin-3 over occludin-1 expression, as previously described.13 There is solid evidence that HIV infection breaches the gut barrier in a way similar to UC.6,7,18
There are several explanations for the differences observed in the frequency of infected cells between inflamed and non-inflamed gut tissue. Local inflammation in the context of HIV infection would activate mucosal CD4+ T cells and recruit new cells that become infected and remain on site as tissue residents. This is particularly documented for Th17 CD4+ T cells, a population of helper T cells resident in the gut.19 Through the expression of CCR6, they can migrate into the intestinal mucosa. CCR6 is also a marker for colon and blood CD4+ T cells enriched for replication-competent HIV DNA.4 Th17 cells could contribute to the difference in levels of HIV reservoir between inflamed and non-inflamed tissue.
In this well-documented case, our results confirm that increased gut permeability and inflammation could be associated with a higher frequency of HIV infected cells. Moreover, when gut epithelial integrity is preserved, there was no evidence of increased microbial translocation in a study of early SIV infection in natural hosts’ infection.20 This study and our results both suggest that a gut epithelial damage enhances the persistence of HIV reservoirs and increases inflammation-associated comorbidities.
Conclusions
Taken together, our results suggest that local gut inflammation may facilitate the persistence of a pool of infected cells as compared to non-inflamed colon tissue. Our work provides further evidence of the heterogeneous distribution of HIV infected cells within the same organ. Novel therapies that restore gut barrier integrity may reduce inflammation and HIV reservoir size in the gut of ART-treated PLWH.18
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Angie Massicotte, Josée Girouard and Cezar Iovi for study coordination and assistance. They authors are grateful to Dr Patrick Charlebois for providing fresh tissues during surgery. The authors thank the patient for agreeing to participate in this research.
This work was funded by the Fonds de la Recherche du Québec-Santé (FRQ-S): Réseau 218 SIDA/Maladies infectieuses and Thérapie cellulaire; the Canadian Institutes of Health Research (CIHR; Grants HOP 103230 and PTJ 166049); the Vaccines & Immunotherapies Core of the CIHR Canadian HIV Trials Network (CTN; Grant CTN 247); the Canadian Foundation for AIDS Research (CANFAR; Grant 02–512); CIHR-funded Canadian HIV Cure Enterprise (CanCURE) Team Grant HB2-164064 and China Scholarship Council (No.201906325018).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jve.2021.100033.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
macroscopic view of non-inflamed and inflamed colon tissue sections
References
- 1.Mowat A.M., Viney J.L. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 2.Yukl S.A., Gianella S., Sinclair E. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202(10):1553–1561. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Telwatte S., Lee S., Somsouk M. Gut and blood differ in constitutive blocks to HIV transcription, suggesting tissue-specific differences in the mechanisms that govern HIV latency. PLoS Pathog. 2018;14(11) doi: 10.1371/journal.ppat.1007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gosselin A., Wiche Salinas T.R., Planas D. HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy. AIDS. 2017;31(1):35–48. doi: 10.1097/QAD.0000000000001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Stefano M., Favia A., Monno L. Intracellular and cell-free (infectious) HIV-1 in rectal mucosa. J Med Virol. 2001;65(4):637–643. doi: 10.1002/jmv.2084. [DOI] [PubMed] [Google Scholar]
- 6.Isnard S., Ramendra R., Dupuy F.P. Plasma levels of C-type lectin REG3alpha and gut damage in people with HIV. J Infect Dis. J Infect Dis. 2019;221(1):110–121. doi: 10.1093/infdis/jiz423. 2020 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzahrani J., Hussain T., Simar D. Inflammatory and immunometabolic consequences of gut dysfunction in HIV: parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine. 2019;46:522–531. doi: 10.1016/j.ebiom.2019.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpstone D.R., Duggal A., Gazzard B.G. Inflammatory bowel disease in individuals seropositive for the human immunodeficiency virus. Eur J Gastroenterol Hepatol. 1996;8(6):575–578. doi: 10.1097/00042737-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Skamnelos A., Tatsioni A., Katsanos K.H., Tsianos V., Christodoulou D., Tsianos E.V. CD4 count remission hypothesis in patients with inflammatory bowel disease and human immunodeficiency virus infection: a systematic review of the literature. Ann Gastroenterol. 2015;28(3):337–346. [PMC free article] [PubMed] [Google Scholar]
- 10.Costiniuk C.T., Bessissow T., Isnard S., Routy J.-P. Use of various immunotherapies for refractory ulcerative colitis in a person living with HIV: a case report. Oxford Med Case Rep. 2021;(1):2021. doi: 10.1093/omcr/omaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattin A., Wiche Salinas T.R., Gosselin A. HIV-1 is rarely detected in blood and colon myeloid cells during viral-suppressive antiretroviral therapy. AIDS. 2019;33(8):1293–1306. doi: 10.1097/QAD.0000000000002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandergeeten C., Fromentin R., Merlini E. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol. 2014;88(21):12385–12396. doi: 10.1128/JVI.00609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poritz L.S., Harris L.R., 3rd, Kelly A.A., Koltun W.A. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci. 2011;56(10):2802–2809. doi: 10.1007/s10620-011-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poles M.A., Boscardin W.J., Elliott J. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr. 2006;43(1):65–68. doi: 10.1097/01.qai.0000230524.71717.14. [DOI] [PubMed] [Google Scholar]
- 15.d’Ettorre G., Paiardini M., Zaffiri L. HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr HIV Res. 2011;9(3):148–153. doi: 10.2174/157016211795945296. [DOI] [PubMed] [Google Scholar]
- 16.Landy J., Ronde E., English N. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22(11):3117–3126. doi: 10.3748/wjg.v22.i11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu L.C. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. 2018;25(1):79. doi: 10.1186/s12929-018-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang J., Isnard S., Lin J. Treating from the inside out: relevance of fecal microbiota transplantation to counteract gut damage in GVHD and HIV infection. Front Med. 2020 doi: 10.3389/fmed.2020.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wacleche V.S., Goulet J.P., Gosselin A. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology. 2016;13(1):59. doi: 10.1186/s12977-016-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raehtz K.D., Barrenas F., Xu C. African green monkeys avoid SIV disease progression by preventing intestinal dysfunction and maintaining mucosal barrier integrity. PLoS Pathog. 2020;16(3) doi: 10.1371/journal.ppat.1008333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
macroscopic view of non-inflamed and inflamed colon tissue sections