Abstract
SMARCA4-deficient thoracic sarcomatoid tumors were characterized by inactivating mutations of SMARCA4 and often found in the chest of young and middle-aged males with a smoking history. Recently, SMARCA4-deficient thoracic sarcomatoid tumors were reported to represent primarily smoking-associated undifferentiated/de-differentiated carcinomas rather than primary thoracic sarcomas. The main complication of this tumor is compression of the respiratory tract and/or blood vessels. A 39-year-old man presented with a 2-month history of fever and dyspnea. Computed tomography revealed a mediastinal tumor invading the right and left pulmonary arteries. Because of severe right heart failure, we considered him ineligible for bronchoscopy. We scheduled palliative irradiation with 40 Gy/20 Fr to improve hemodynamics and perform endobronchial ultrasound transbronchial needle aspiration later. However, irradiation was ineffective, and his general condition deteriorated quickly and he died after a 7-week hospitalization. An autopsy revealed that the diagnosis was SMARCA4-deficient thoracic undifferentiated carcinoma. It has been reported that this tumor is insensitive to radiotherapy and there were some cases which responded to an immune checkpoint inhibitor. Therefore, when caring for patients with mediastinal tumors that invade and compress the trachea and large vessels, it is important to consider this tumor as a differential diagnosis and try to make a pathological diagnosis as soon as possible.
Keywords: SMARCA4-Deficient thoracic undifferentiated carcinoma, Radiation therapy, Right heart failure, SMARCA4, Sarcomatoid tumor, Radio-insensitive
Abbreviations: SWI/SNF, switch/sucrose non-fermentable; SD-TSTs, SMARCA4-deficient thoracic sarcomatoid tumors; SMARCA4-DTC, SMARCA4-deficient thoracic undifferentiated carcinoma; ICI, Immune checkpoint inhibitors; ECOG PS, Eastern Cooperative Oncology Group performance status
1. Introduction
SMARCA4 is a subunit of the switch/sucrose non-fermentable (SWI/SNF) complex [1]. It plays an important role in chromatin remodeling and, thus, in the regulation of vital cellular processes and functions, such as proliferation and differentiation [2]. SMARCA4-deficient thoracic sarcomatoid tumors (SD-TSTs) are characterized by inactivating mutations of SMARCA4 and are often found in the chest of young and middle-aged males with a smoking history [3,4]. The neoplasms are mainly located in the mediastinum, followed by the pleura and lungs. The main complication of SD-TSTs is compression of the respiratory tract and/or blood vessels [5]. Recently, Rekhtman et al. reported that SD-TSTs primarily represented smoking-associated undifferentiated/de-differentiated carcinomas rather than primary thoracic sarcomas [6]. The initial choice of therapy for invasive tumors is critical as failure is likely to deteriorate the patient's general condition, with the only subsequent option being palliative care. Herein, we report a case of radio-insensitive SMARCA4-deficient thoracic undifferentiated carcinoma (SMARCA4-DTC) with severe pulmonary artery invasion.
2. Case presentation
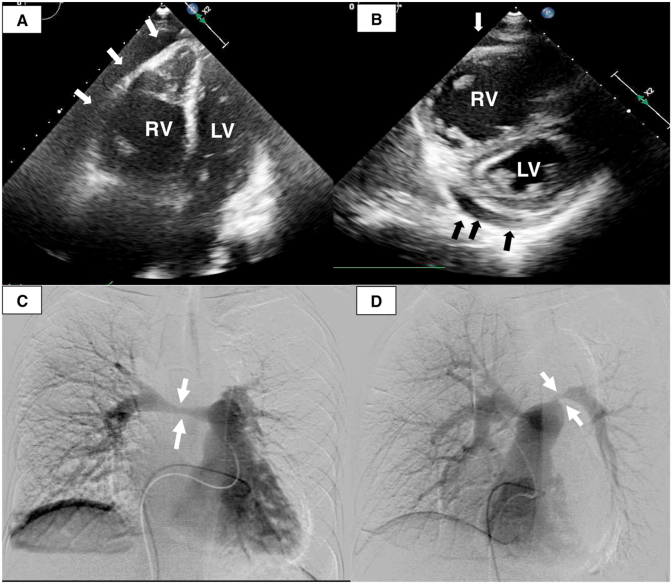
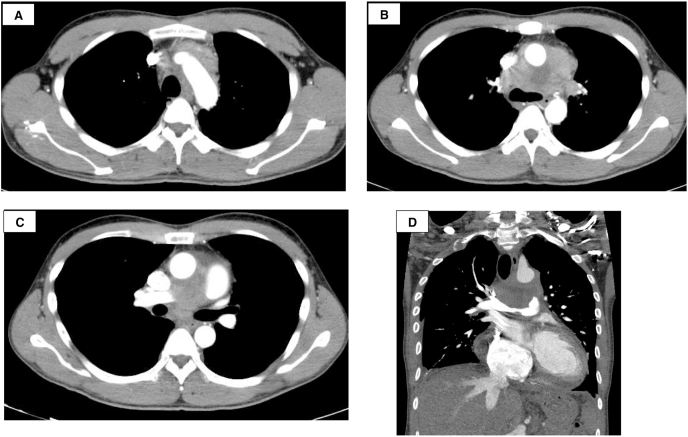
A 39-year-old man presented at the outpatient clinic of a community hospital with a 2-month history of fever and dyspnea and no relevant past history. He had a smoking history of 19-pack-years. Cardiomegaly was seen on the chest radiograph. Right ventricular enlargement and flattening of the interventricular septum of the left ventricle with pericardial effusion (Fig. 1A–B) were seen on echocardiography. Computed tomography (CT) revealed a mediastinal tumor invading the right and left pulmonary arteries (Fig. 2A–D). Pulmonary angiography showed severe pulmonary artery stenosis (Fig. 1C–D).
Fig. 1.
(A) Apical four-chamber echogram showing an enlarged right ventricle (RV) and compressed left ventricle (LV) with pericardial effusion (arrows). (B) Parasternal short axis echocardiogram showing flattening of the interventricular septum and compression of the LV by the severely enlarged RV with pericardial effusion (arrows). (C) Pulmonary angiography showing 75% stenosis of the right pulmonary artery (arrows). (D) Oblique pulmonary angiography showing 99% stenosis of the left pulmonary artery (arrows).
Fig. 2.
Computed tomography (CT) images of the thorax. (A) (B) (C) The CT images are axial images taken in the arterial phase. (D) The CT image is a coronal image taken in the pulmonary artery phase.
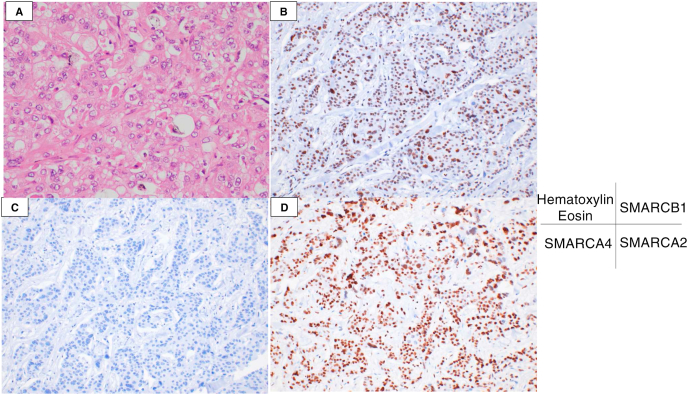
He was then referred to our department for further management. Due to severe right heart failure, his Eastern Cooperative Oncology Group performance status (ECOG PS) was 3, and he could not lie on his back for more than 30 minutes despite the use of morphine and catecholamine. Therefore, bronchoscopy could not be performed. We scheduled palliative irradiation with 40 Gy/20 Fr to improve the hemodynamics and perform Endobronchial ultrasound transbronchial needle aspiration later. However, irradiation was ineffective, and his general condition deteriorated. He died after a 7-week hospitalization. An autopsy was performed after obtaining informed consent from the patient's family. All procedures were in accordance with the Declaration of Helsinki. The tumor was a poorly differentiated carcinoma with a partial glandular structure. Immunohistochemical staining revealed that the tumor cells were positive for SMARCA2 and negative for SMARCA4. The final diagnosis was SMARCA4-DTC (Fig. 3A–D). Programmed death-ligand 1 (PD-L1) expression was observed in 1% of the tumor cells. Molecular analysis using QIAGEN comprehensive panel detected no SMARCA4 mutation, a reduced SMARCA4 copy number to 1, and loss of SMARCA4 heterozygosity.
Fig. 3.
Histopathological analysis. (A) The tumor is composed of a poorly differentiated carcinoma (Hematoxylin and eosin). (B–D) On immunohistochemical analyses, the tumor cells are SMARCB1-positive (B), SMARCA4-negative (C), and SMARCA2-positive (D).
3. Discussion
The stepwise inactivation of SMARCA2 from preexisting SMARCA4-deficient carcinomatous elements might cause SD-TSTs [6]. In our patient, the tumor cells lacked typical rhabdoid-like features and were SMARCA2-positive, leading to the final diagnosis of SMARCA4-DTC. The clinical characteristics and radio-insensitivity were consistent with SD-TSTs [5]. Recently, correlations between response to immune checkpoint inhibitors (ICIs) and loss of the SWI/SNF complex have been reported [7]. A patient with a previously SMARCA4-deficient thoracic tumor responded remarkably well to an immune checkpoint inhibitor (ICI) though the tumor cells were PD-L1-negative on immunohistochemistry [8]. In our case, the patient could be administered only palliative radiation because his ECOG PS was 3. However, a recent PePS2 trial showed that pembrolizumab could be safely administered in patients with advanced non-small-cell lung cancer and ECOG PS of 2 [9]. Therefore, if the ECOG PS of the patient is equal to or more than 2, ICIs might be a potentially effective treatment option.
The differential diagnoses of mediastinal tumors include radiosensitive tumors like malignant lymphomas and germ cell tumors. Therefore, it is important to know the imaging features of SMARCA4-DTC. Calcification and cystic component are usually not seen with SMARCA4-DTC. Unlike germ cell tumors, SMARCA4-DTCs are heterogeneous on post-contrast computed tomography. Compared to malignant lymphoma, which is a radiosensitive tumor, SMARCA4-DTC has a higher probability of causing vascular encasement [[10], [11], [12]]. In patients like ours, invasion and compression of the large vessels or the trachea by mediastinal tumors could be indicative of tumor stiffness, and SMARCA4-DTC should be considered [10].
4. Conclusion
When mediastinal tumors without calcification and cystic component invade and compress large vessels or the trachea aggressively, SMARCA4-DTC should be considered as a differential diagnosis, and biopsy should be attempted. Palliative irradiation before the pathological diagnosis is an important therapeutic strategy but might be ineffective. If the tumor is insensitive to palliative irradiation and the ECOG PS of the patient is ≤ 2 after the pathological diagnosis, treatment with ICI may be considered as an option. Overall, it is important to make a pathological diagnosis as soon as possible when there is a possibility of SMARCA4-DTC.
Author contributions
Shotaro Ito and Hajime Asahina had full access to the case and drafted the original manuscript. Naoko Yamaguchi, Utano Tomaru, Tadashi Hasegawa, Yutaka Hatanaka, Kanako C Hatanaka, Hiroshi Taguchi, Taisuke Harada, Hiroshi Ohira, Daisuke Ikeda, Hidenori Mizugaki, Eiki Kikuchi, Junko Kikuchi, Jun Sakakibara-Konishi, Naofumi Shinagaw, and Satoshi Konno critically reviewed the manuscript for intellectual contents. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Authors declare that they have no conflict of interest.
References
- 1.Roberts C.W., Orkin S.H. The SWI/SNF complex--chromatin and cancer. Nat. Rev. Canc. 2004;4(2):133–142. doi: 10.1038/nrc1273. [DOI] [PubMed] [Google Scholar]
- 2.Wilson B.G., Roberts C.W. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Canc. 2011;11(7):481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 3.Le Loarer F., Watson S., Pierron G., de Montpreville V.T., Ballet S., Firmin N., Auguste A., Pissaloux D., Boyault S., Paindavoine S., Dechelotte P.J., Besse B., Vignaud J.M., Brevet M., Fadel E., Richer W., Treilleux I., Masliah-Planchon J., Devouassoux-Shisheboran M., Zalcman G., Allory Y., Bourdeaut F., Thivolet-Bejui F., Ranchere-Vince D., Girard N., Lantuejoul S., Galateau-Sallé F., Coindre J.M., Leary A., Delattre O., Blay J.Y., Tirode F. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat. Genet. 2015;47(10):1200–1205. doi: 10.1038/ng.3399. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida A., Kobayashi E., Kubo T., Kodaira M., Motoi T., Motoi N., Yonemori K., Ohe Y., Watanabe S.I., Kawai A., Kohno T., Kishimoto H., Ichikawa H., Hiraoka N. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod. Pathol. 2017;30(6):797–809. doi: 10.1038/modpathol.2017.11. [DOI] [PubMed] [Google Scholar]
- 5.Perret R., Chalabreysse L., Watson S., Serre I., Garcia S., Forest F., Yvorel V., Pissaloux D., Thomas de Montpreville V., Masliah-Planchon J., Lantuejoul S., Brevet M., Blay J.Y., Coindre J.M., Tirode F., Le Loarer F. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am. J. Surg. Pathol. 2019;43(4):455–465. doi: 10.1097/PAS.0000000000001188. [DOI] [PubMed] [Google Scholar]
- 6.Rekhtman N., Montecalvo J., Chang J.C., Alex D., Ptashkin R.N., Ai N., Sauter J.L., Kezlarian B., Jungbluth A., Desmeules P., Beras A., Bishop J.A., Plodkowski A.J., Gounder M.M., Schoenfeld A.J., Namakydoust A., Li B.T., Rudin C.M., Riely G.J., Jones D.R., Ladanyi M., Travis W.D. SMARCA4-Deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J. Thorac. Oncol. 2020;15(2):231–247. doi: 10.1016/j.jtho.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao D., Margolis C.A., Gao W., Voss M.H., Li W., Martini D.J., Norton C., Bossé D., Wankowicz S.M., Cullen D., Horak C., Wind-Rotolo M., Tracy A., Giannakis M., Hodi F.S., Drake C.G., Ball M.W., Allaf M.E., Snyder A., Hellmann M.D., Ho T., Motzer R.J., Signoretti S., Kaelin W.G., Jr., Choueiri T.K., Van Allen E.M. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359(6377):801–806. doi: 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henon C., Blay J.Y., Massard C., Mir O., Bahleda R., Dumont S., Postel-Vinay S., Adam J., Soria J.C., Le Cesne A. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann. Oncol. 2019;30(8):1401–1403. doi: 10.1093/annonc/mdz160. [DOI] [PubMed] [Google Scholar]
- 9.Middleton G., Brock K., Savage J., Mant R., Summers Y., Connibear J., Shah R., Ottensmeier C., Shaw P., Lee S.-M., Popat S., Barrie C., Barone G., Billingham L. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir. Med. 2020;8(9):895–904. doi: 10.1016/s2213-2600(20)30033-3. [DOI] [PubMed] [Google Scholar]
- 10.Crombé A., Alberti N., Villard N., Pilleul F., Buy X., Le Loarer F., Kind M. Imaging features of SMARCA4-deficient thoracic sarcomas: a multi-centric study of 21 patients. Eur. Radiol. 2019;29(9):4730–4741. doi: 10.1007/s00330-019-06017-x. [DOI] [PubMed] [Google Scholar]
- 11.Gu L., Zhang L., Hou N., Li M., Shen W., Xie X., Teng Y. Clinical and radiographic characterization of primary seminomas and nonseminomatous germ cell tumors. Niger. J. Clin. Pract. 2019;22(3):342–349. doi: 10.4103/njcp.njcp_448_18. [DOI] [PubMed] [Google Scholar]
- 12.Tateishi U., Müller N.L., Johkoh T., Onishi Y., Arai Y., Satake M., Matsuno Y., Tobinai K. Primary mediastinal lymphoma: characteristic features of the various histological subtypes on CT. J. Comput. Assist. Tomogr. 2004;28(6):782–789. doi: 10.1097/00004728-200411000-00009. [DOI] [PubMed] [Google Scholar]