Abstract
Background/objectives
Bile acids (BA) act as detergents in intestinal fat absorption and as modulators of metabolic processes via activation of receptors such as FXR and TGR5. Elevated plasma BA as well as increased intestinal BA signalling to promote GLP-1 release have been implicated in beneficial health effects of Roux-en-Y gastric bypass surgery (RYGB). Whether BA also contribute to the postprandial hypoglycaemia that is frequently observed post-RYGB is unknown.
Methods
Plasma BA, fibroblast growth factor 19 (FGF19), 7α-hydroxy-4-cholesten-3-one (C4), GLP-1, insulin and glucose levels were determined during 3.5 h mixed-meal tolerance tests (MMTT) in subjects after RYGB, either with (RYGB, n = 11) or without a functioning gallbladder due to cholecystectomy (RYGB-CC, n = 11). Basal values were compared to those of age, BMI and sex-matched obese controls without RYGB (n = 22).
Results
Fasting BA as well as FGF19 levels were elevated in RYGB and RYGB-CC subjects compared to non-bariatric controls, without significant differences between RYGB and RYGB-CC. Postprandial hypoglycaemia was observed in 8/11 RYGB-CC and only in 3/11 RYGB. Subjects who developed hypoglycaemia showed higher postprandial BA levels coinciding with augmented GLP-1 and insulin responses during the MMTT. The nadir of plasma glucose concentrations after meals showed a negative relationship with postprandial BA peaks. Plasma C4 was lower during MMTT in subjects experiencing hypoglycaemia, indicating lower hepatic BA synthesis. Computer simulations revealed that altered intestinal transit underlies the occurrence of exaggerated postprandial BA responses in hypoglycaemic subjects.
Conclusion
Altered BA kinetics upon ingestion of a meal, as frequently observed in RYGB-CC subjects, appear to contribute to postprandial hypoglycaemia by stimulating intestinal GLP-1 release.
Subject terms: Dietary carbohydrates, Metabolic diseases
Introduction
Bariatric surgery, commonly Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy, is currently the most effective long-term treatment of morbid obesity and its metabolic comorbidities [1], with metabolic benefits that actually precede reductions in body weight [2]. The mechanisms underlying these metabolic improvements, particularly of the weight-independent ones, are incompletely understood. A role for bile acids (BA), now known to exert hormone-like functions via activation of nuclear receptors such as Farnesoid X Receptor (FXR, NR1H4) and the membrane-bound Takeda G Protein-coupled receptor 5 (TGR5), has been suggested. Indeed, fasting plasma BA concentrations have repeatedly been reported to be 2–3 times elevated after RYGB in human subjects [3, 4]. Preclinical studies have indicated a specific role for intestinal BA, by their ability to induce a TGR5-mediated GLP-1 release from intestinal L cells [5]. The relevance of factors such a BA–GLP-1 axis for metabolic control in humans that have undergone bariatric surgery is supported by recent reports [6]. Yet, other factors such as alterations in microbiome composition [7], delayed intestinal glucose absorption [8] as well as an acute reduction in caloric intake [9] likely all contribute to improved metabolic control after bariatric surgery.
It is increasingly recognised that a substantial number of RYGB patients develop postprandial hypoglycaemia. Prior studies have established that particularly females without a history of diabetes mellitus or hypertension and with high insulin sensitivity are susceptible to develop this highly undesirable response to food intake [10]. An exaggerated postprandial GLP-1 response and altered β-cell function have been suggested as contributing factors [11, 12] but a clear pathophysiological mechanism has not been elucidated. To our knowledge, a possible contribution of altered postprandial BA kinetics, which could potentially translate into excess GLP-1 release, to this invalidating pathophysiological response has not been evaluated yet.
RYGB induces distinct anatomic alterations of the GI tract which causes orally ingested food to bypass part of the stomach and duodenum and to be rapidly delivered to the jejunum. The mixing of food with BA, delivered by the gallbladder to the upper part of the bilio-pancreatic limb, is therefore postponed to the common limb where alimentary and bilio-pancreatic limbs merge. The gallbladder evidently has an important function in BA physiology, as it contracts in response to a meal and then delivers a pulse of BA into the intestinal lumen. Removal of the gallbladder has been shown to differentially modulate synthesis of the two primary BA (cholic and chenodeoxycholic acids, CA and CDCA, respectively) without major effects on human BA pool size and composition [13]. Cholecystectomy influences fasting and postprandial concentrations of BA and fibroblast growth factor (FGF)19, which is secreted by the ileocytes upon FXR activation by BA, as well as of C4, an established marker of hepatic BA synthesis [14]. Interestingly, and logically in view of the well-known relationship between obesity and gallstone disease [15], the prevalence of cholecystectomy is high in subjects prior and after bariatric surgery [16, 17]. In this study, we have made use of this fact by evaluating the relationships between postprandial BA kinetics and glucose excursions in RYGB subjects with and without a functioning gallbladder.
The primary aim of this study was thus to explore the associations between postprandial BA kinetics, i.e. the concentrations of total BA and individual BA species as well as of FGF19 and C4, and the occurrence of postprandial hypoglycaemia during a mixed-meal tolerance test (MMTT) in RYGB subjects. Specifically, we explored whether cholecystectomy (CC), which is expected to alter BA kinetics, is associated with higher risk for postprandial hypoglycaemia after RYGB.
Subjects and methods
Description of subjects of the MMTT study and the LOWER study
The primary aim of the MMTT study was to investigate the prevalence of dumping syndrome in a random sample of non-diabetic subjects who underwent primary RYGB between 2008 and 2011 [11, 18]. Subjects between the ages of 18 and 75 years were recruited in 2014 and 2015 at the Medical Centre Leeuwarden, The Netherlands. Details on the selection procedure were previously described [18]. For the current study we selected two equally large subgroups, one group with subjects that had undergone cholecystectomy (RYGB-CC) and one group with a functioning gallbladder (RYGB). The first group (RYGB-CC, n = 11) determined the final group sizes studied and for the RYGB group a random selection out of the remaining subjects of the MMTT study was performed with SPSS. Written informed consent was obtained from all subjects and the study was approved by the regional Medical Ethics committee in Leeuwarden, The Netherlands. The study has been registered on www.isrctn.com as ISRCTN11738149.
Out of 313 subjects of the LOWER study [19], 22 non-bariatric control subjects were selected for comparison of fasting parameters with those of the bariatric subjects. In brief, the LOWER study recruited subjects ranging in BMI from 27 to 60 kg/m2 for a randomised study with four parallel energy-restricted intervention diets differing in protein and/or carbohydrate content. For the current study, only baseline samples collected prior to the intervention diets were used. These control subjects were matched for BMI, age and sex to the bariatric subjects via SPSS Fuzzy Case-Control matching with accepted differences of 5.5, 10 and 0, respectively.
Study protocol of the MMTT study
Subjects underwent the MMTT in the morning at the clinical research centre after an overnight fast. Anthropometric measurements and blood samples were collected before the test meal (t = 0). Blood was withdrawn by means of an indwelling catheter in the antecubital vein. The mixed meal consisted of 200 mL Ensure® Plus (Abbott Laboratories, North Chicago, IL, USA) containing 300 kcal, 12.5 g protein, 40.4 g carbohydrate, 9.8 g fat and 154.9 g water. The subjects had to finish the test meal within 10 min. Blood samples were subsequently obtained at 10, 20, 30, 60, 90, 120, 150, 180 and 210 min after the start of the test meal.
Analytical procedures
Measurements of insulin, glucose, GLP-1 and FGF19 concentrations were performed by routine procedures as detailed in Supplementary Methods. For the quantification of 7α-hydroxy-4-cholesten-3-one (C4) in plasma, we used a sensitive and highly specific automated on-line solid-phase extraction method coupled to high performance liquid chromatography-tandem mass spectrometry (XLC–MS/MS) as detailed in Out et al. [20]. The concentrations of 15 individual BA species were determined by LC–MS/MS as described [21]. The detection limit applied for the individual BA concentrations was 0.025 µmol/L except for UDCA, TCA, GDCA and GDCA, in which case the limit was set at 0.05 µmol/L.
Computer simulations
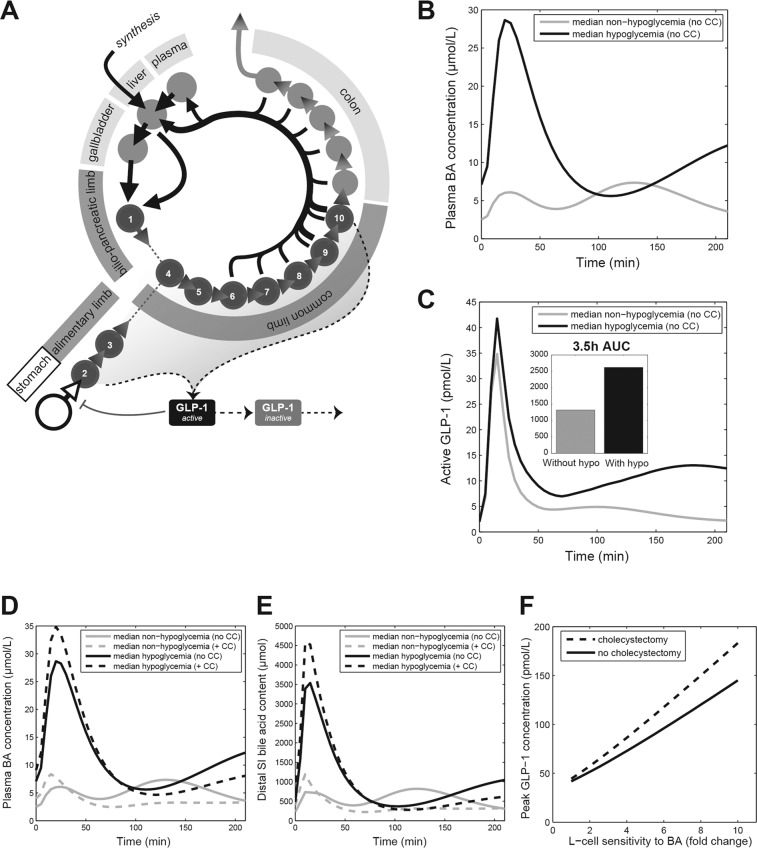
For simulations of BA and GLP-1 kinetics after RYGB, the computational model of the enterohepatic circulation of BA developed by Sips et al. [22] was adapted for RYGB and expanded with stomach and GLP-1 kinetics (Fig. 3A, Supplementary Material, Github 10.5281/zenodo.3984980). In the GLP-1 kinetics model, secretion of GLP-1 is mainly stimulated by uptake of nutrients from the intestinal lumen. However, above-basal BA concentrations can also induce GLP-1 secretion. To be able to simulate the impact of RYGB on BA metabolism the anatomy of the small intestine was changed to reflect the roux limb (2 compartments/about 100 cm) and the bilio-pancreatic limb (1 compartment/about 50 cm). To be able to simulate the differences in postprandial dynamics before and after RYGB published in previous studies [22], parameters reflecting intestinal nutrient and BA transport (including stomach emptying, gallbladder emptying, intestinal transit and bacterial transformation) had to be changed (Fig. 3A, Supplementary Material). Simulations were performed with Matlab (MATLAB. version 7.14.0 (R2012a). Natick, Massachusetts: The MathWorks Inc.), using differential equation solver ode15s.
Fig. 3. . Mathematical modelling analysis of the bile acid and GLP-1 response to RYGB.
A Overview of post-RYGB model structure (B) Simulated plasma BA responses mirroring the hypoglycemia and non-hypoglycemia groups. To recreate the observed BA and GLP-1 concentration curves, simulations were performed for a range of values for fasting transit speeds (here, all three fasting intestinal transit parameters are changed by the same factor) and the postprandial transit response. The responses that most closely recreated the median fasting and peak postprandial BA concentrations were chosen for comparison and simulation. For more information, see the Supplementary Figure. C Simulated active GLP-1 concentrations (D) Plasma bile acid concentrations for selected simulations and associated cholecystectomized simulation. E Distal BA content for simulations introduced in D. F To illustrate how the difference in distal BA between non-cholecystectomized subjects and cholecystectomized subjects propagates to the GLP-1 response, the peak GLP-1 concentration is given for a range of sensitivities of L cells for BA. The chosen values range from the assumed L-cell sensitivity for BA in the normal situation, to ×10 that value (fold change). CC cholecystectomy.
Statistical analysis
Results are presented as mean ± standard deviation or median [IQR], unless specified otherwise. Changes over time are graphically presented as median values [IQR]. Additional information about the statistical analyses is presented in the Supplementary Methods.
Statistical analyses were performed in IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA). Due to the exploratory nature of this study, statistical analyses were not corrected for multiple testing and occurrence of statistically significant differences was interpreted as hypothesis-generating rather than hypothesis-confirming. GraphPad Prism, version 8.0 (Graphpad Software Inc., La Jolla, CA, USA) was used for graphic presentations.
Results
Participant characteristics
Out of a total of 51 enroled subjects, 44 subjects completed the original MMTT study [11] of which 33 subjects were included in the selection process of the current study based on availability of sufficient plasma for the planned analyses. Eleven of these subjects had undergone CC prior to the MMTT study (RYGB-CC) and, therefore, out of the remaining 22 subjects eleven subjects with their gallbladder in situ (RYGB) were randomly selected. A total of 22 control subjects from the LOWER study [19], with participants from the same region and with the same ethnicity, were selected to provide baseline control values, matched on age, BMI and gender. The subject characteristics are presented in Table 1. Age and gender were not different between subjects with and without CC and the time between the RYGB and MMTT was similar. Weight (+6.8%) and BMI (+8.4%) were somewhat higher in the controls compared to all RYGB subjects. Mean fasting concentration of glucose was higher (+27%) in the control subjects compared to all RYGB subjects, with no differences observed in fasting insulin and HOMA2-IR.
Table 1.
Subject characteristicsa.
| Bariatric subjects | |||||
|---|---|---|---|---|---|
| with CC | without CC | p valueb | Non-bariatric subjects | p valuec | |
| n | 11 | 11 | 22 | ||
| Age at MMTT, y | 46.4 ± 12.8 | 47.8 ± 9.4 | 0.78 | 48.3 ± 13.0 | 0.37 |
| Men, n (%) | 1 (9) | 5 (45) | 0.149 | 6 (27.3) | 1.00 |
| Post-operative time to MMTT, mo | 50.0 ± 7.1 | 53.0 ± 13.8 | 0.53 | ||
| Weight preoperative, kg | 135.0 ± 20.5 | 141.8 ± 14.0 | 0.37 | ||
| BMI preoperative, kg/m2 | 45.5 ± 6.1 | 45.2 ± 3.8 | 0.89 | ||
| Weight at MMTT, kg | 92.1 ± 15.6 | 94.2 ± 17.0 | 0.77 | 99.5 ± 10.3 | 0.029 |
| BMI at MMTT, kg/m2 | 31.0 ± 4.5 | 29.9 ± 4.9 | 0.59 | 33.0 ± 3.1 | 0.001 |
| TWL at MMTT, % | 31.6 ± 5.8 | 33.7 ± 9.8 | 0.56 | ||
| EWL at MMTT, % | 73.2 ± 15.7 | 76.1 ± 20.9 | 0.72 | ||
| Fasting glucose, mmol/L | 4.2 ± 0.7 | 4.3 ± 0.8 | 0.75 | 5.4 ± 0.7 | 0.000 |
| Fasting insulin, mU/L | 7.9 ± 4.0 | 9.7 ± 6.3 | 0.43 | 9.4 ± 5.1 | 0.63 |
| HOMA2-IR | 1.0 ± 0.5 | 1.2 ± 0.8 | 0.42 | 1.2 ± 0.7 | 0.40 |
CC cholecystectomy, EWL excess weight loss, MMTT mixed–meal tolerance test, TWL total weight loss.
aCholecystectomy was performed in 7/11 subjects prior to and in 4/11 after bariatric surgery, and at least 12 months before execution of MMTT.
bData were compared with an independent T test between the bariatric subject with CC (n = 11) and the bariatric subjects without CC (n = 11) except for gender and hypoglycemia which were compared with a Fishers Exact Test.
cData were compared with a paired T test between the bariatric subjects (n = 22) and the non-bariatric subjects (n = 22) except for gender which was compared with an McNemar Test.
Fasting plasma concentrations of bile acids, FGF19 and C4 after RYGB: limited effects of cholecystectomy
Fasting BA concentrations in all bariatric subjects were more than 3 times higher than those in the matched non-bariatric controls (Table 2). This concentration difference was evident for all quantified classes of BA analysed, i.e., the primary, secondary, conjugated and unconjugated species. Additionally, the median fasting concentration of FGF19 was significantly higher in the RYGB subjects while that of C4 was similar between the two groups. The glycine-to-taurine conjugation ratio of plasma BA was higher in the bariatric subjects than in the non-bariatric controls, while the ratio 12α-hydroxylated/non-12α-hydroxylated BA was lower (Table 2).
Table 2.
Comparison of fasting plasma concentrations of bile acids, FGF19 and C4 between bariatric subjects and non-bariatric subjects.
| Non-bariatric | Bariatric (all) | p valuea | BDL (%) | |
|---|---|---|---|---|
| n | 22 | 22 | ||
| Primary BA | 0.087 [0.052; 0.325] | 1.208 [0.108; 5.085] | 0.003 | |
| CA | 0.035 [0.025; 0.104] | 0.561 [0.043; 1.245] | 0.006 | 25.0 |
| CDCA | 0.054 [0.027; 0.201] | 0.588 [0.066; 3.855] | 0.003 | 15.9 |
| Secondary BA | 0.317 [0.195; 0.412] | 0.665 [0.317; 2.095] | 0.006 | |
| LCA* | 0.025 [0.025; 0.025] | 0.025 [0.025; 0.025] | 0.75 | 85.0 |
| DCA | 0.209 [0.120; 0.335] | 0.581 [0.213; 1.750] | 0.004 | 0.0 |
| UDCA | 0.050 [0.050; 0.050] | 0.050 [0.050; 0.157] | 0.017 | 70.5 |
| Taurine conjugated BA | 0.158 [0.150; 0.199] | 0.256 [0.154; 0.354] | 0.009 | |
| TCA | 0.050 [0.050; 0.050] | 0.050 [0.050; 0.050] | 0.60 | 77.3 |
| TCDCA | 0.031 [0.025; 0.066] | 0.087 [0.029; 0.137] | 0.005 | 29.5 |
| TLCA | 0.025 [0.025; 0.025] | 0.025 [0.025; 0.025] | – | 100 |
| TDCA | 0.025 [0.025; 0.035] | 0.031 [0.025; 0.092] | 0.011 | 43.2 |
| TUDCA | 0.025 [0.025; 0.025] | 0.025 [0.025; 0.025] | 0.32 | 97.7 |
| Glycine-conjugated BA | 0.584 [0.297; 0.838] | 1.546 [0.726; 2.544] | 0.001 | |
| GCA | 0.058 [0.049; 0.132] | 0.179 [0.073; 0.445] | 0.010 | 4.5 |
| GCDCA | 0.282 [0.122; 0.416] | 0.861 [0.376; 1.168] | 0.001 | 2.3 |
| GLCA | 0.025 [0.025; 0.025] | 0.025 [0.025; 0.034] | 0.173 | 75.0 |
| GDCA | 0.117 [0.056; 0.222] | 0.260 [0.136; 0.485] | 0.011 | 9.1 |
| GUDCA | 0.025 [0.025; 0.049] | 0.038 [0.025; 0.076] | 0.079 | 43.2 |
| Unconjugated BA | 0.416 [0.322; 0.684] | 2.022 [0.392; 8.046] | 0.004 | |
| Conjugated BA | 0.785 [0.452; 1.160] | 1.742 [0.876; 2.874] | 0.001 | |
| Total BA | 1.204 [0.906; 1.541] | 3.919 [2.410; 13.843] | 0.001 | |
| Ratios | ||||
| G:T | 3.07 [1.93; 4.03] | 5.84 [3.22; 8.25] | 0.010 | |
| Conjugated:unconjugated BA | 1.46 [1.03; 2.16] | 0.86 [0.26; 2.32] | 0.57 | |
| CA:DCA | 0.55 [0.33; 0.82] | 0.78 [0.45; 1.62] | 0.200 | |
| 12α:non-12α hydroxylated BA | 1.44 [1.04; 1.62] | 1.06 [0.76; 1.36] | 0.042 | |
| FGF19 | 74.85 [41.56; 126.74] | 131.19 [68.20; 181.23] | 0.039 | |
| C4 | 42.00 [29.78; 66.63] | 40.10 [22.03; 77.68] | 0.96 |
BA concentrations in µmol/L, FGF19 concentrations in pg/mL and C4 concentrations in nmol/L. Data are median [IQR].
BA bile acid, BDL below detection limit, G:T glycine-conjugated bile acids to taurine conjugated bile acids.
*n = 20 versus n = 20 because of four unintegrated peaks of LCA.
aData were compared with a related samples Wilcoxon rank Test between non-bariatric subjects and bariatric subjects.
Comparison of bariatric subjects with (RYGB) and without gallbladder (RYGB-CC) revealed no significant differences in fasting total BA concentrations and FGF19 (Table 3). However, the concentrations of all glycine-conjugated BAs as well as the glycine–taurine conjugation ratio were significantly higher in the RYGB-CC subjects as was the concentration of C4, indicative for a higher hepatic BA synthesis in these subjects.
Table 3.
Comparison of fasting plasma concentrations of bile acids, FGF19 and C4 between bariatric subjects with (RYGB-CC) or without (RYGB) prior cholecystectomy.
| RYGB-CC | RYGB | p valuea | BDL (%) | |
|---|---|---|---|---|
| n | 11 | 11 | ||
| Primary BA | 1.358 [0.130; 7.440] | 1.028 [0.084; 2.440] | 0.48 | |
| CA | 0.752 [0.042; 1.410] | 0.369 [0.043; 1.150] | 0.75 | 9.1 |
| CDCA | 0.715 [0.088; 6.030] | 0.516 [0.041; 1.290] | 0.52 | 9.1 |
| Secondary BA | 0.848 [0.293; 4.208] | 0.573 [0.325; 1.105] | 0.48 | |
| LCA* | 0.025 [0.025; 0.029] | 0.025 [0.025; 0.025] | 0.23 | 77.3 |
| DCA | 0.773 [0.208; 3.880] | 0.498 [0.250; 1.030] | 0.52 | 0.0 |
| UDCA | 0.068 [0.050; 0.236] | 0.050 [0.050; 0.050] | 0.171 | 59.1 |
| Taurine conjugated BA | 0.266 [0.163; 0.458] | 0.173 [0.150; 0.313] | 0.171 | |
| TCA | 0.050 [0.050; 0.050] | 0.050 [0.050; 0.050] | 1.00 | 77.3 |
| TCDCA | 0.088 [0.037; 0.253] | 0.038 [0.025; 0.132] | 0.24 | 22.7 |
| TLCA | 0.025 [0.025; 0.025] | 0.025 [0.025; 0.025] | – | 100 |
| TDCA | 0.032 [0.025; 0.107] | 0.025 [0.025; 0.078] | 0.24 | 36.4 |
| TUDCA | 0.025 [0.025; 0.025] | 0.025 [0.025; 0.025] | 0.75 | 95.5 |
| Glycine-conjugated BA | 1.662 [1.463; 3.188] | 0.809 [0.369; 1.873] | 0.034 | |
| GCA | 0.241 [0.154; 0.512] | 0.077 [0.041; 0.263] | 0.088 | 4.5 |
| GCDCA | 0.958 [0.786; 2.300] | 0.432 [0.195; 1.080] | 0.116 | |
| GLCA | 0.033 [0.025; 0.058] | 0.025 [0.025; 0.025] | 0.047 | 68.2 |
| GDCA | 0.391 [0.221; 0.739] | 0.195 [0.080; 0.456] | 0.116 | 9.1 |
| GUDCA | 0.039 [0.028; 0.161] | 0.034 [0.025; 0.072] | 0.193 | 27.3 |
| Unconjugated BA | 2.101 [0.375; 12.248] | 1.727 [0.397; 3.301] | 0.61 | |
| Conjugated BA | 2.177 [1.711; 3.549] | 0.959 [0.519; 2.186] | 0.047 | |
| Total BA | 5.256 [3.812; 13.964] | 2.783 [1.370; 5.982] | 0.065 | |
| Ratios | ||||
| G:T | 8.19 [5.71; 9.89] | 5.33 [2.46; 5.98] | 0.001 | |
| Conjugated:unconjugated BA | 1.08 [0.14; 6.20] | 0.81 [0.28; 2.32] | 0.65 | |
| CA:DCA | 0.88 [0.34; 1.84] | 0.71 [0.53; 1.26] | 0.85 | |
| 12α:non-12α hydroxylated BA | 1.00 [0.70; 1.51] | 1.14 [0.89; 1.31] | 0.40 | |
| FGF19 | 88.84 [64.79; 186.25] | 135.80 [79.96; 179.56] | 0.90 | |
| C4 | 75.70 [29.20; 99.00] | 27.20 [14.10; 56.60] | 0.047 |
BA concentrations in µmol/L, FGF19 concentrations in pg/mL, C4 concentrations in nmol/L. Data are median [IQR].
BA bile acid, BDL below detection limit, G:T glycine-conjugated bile acids to taurine conjugated bile acids.
*n = 9 with CC versus n = 11 without CC because of 2 unintegrated peaks of LCA.
aData were compared with a Mann–Whitney U Test between the bariatric subject with CC (n = 11) and the bariatric subjects without CC (n = 11).
Comparison of postprandial insulin, GLP-1, bile acid, FGF19 and C4 excursions in RYGB subjects subdivided on basis of the occurrence of hypoglycaemia
Postprandial excursions of glucose, insulin and GLP-1 of subjects participating in the MMTT study have been presented elsewhere for the whole group (n = 44) [10]. The results described below were obtained in a subgroup of this patient population (n = 22), primarily selected on the basis of prior cholecystectomy (RYGB-CC, n = 11) and randomly with a gallbladder in situ (RYGB, n = 11). Subsequently, these 22 subjects were re-distributed based on the occurrence of hypoglycaemia during the MMTT, defined as a postprandial glucose concentration <3.3 mmol/L. Hypoglycaemia tended to occur more frequently in RYGB-CC (8/11 subjects) than in RYGB (3/11 subjects) (p < 0.086). Characteristics of the groups thus obtained are presented in Table 4. Subjects with postprandial hypoglycaemia also presented with significantly lower fasting concentrations of glucose and insulin and a lower value for calculated HOMA2-IR. Fasting plasma BA parameters were, however, very similar between the hypo- and normoglycaemic subjects (Supplementary Table 3).
Table 4.
Characteristics of the bariatric subjects subdivided on the basis of occurrence of postprandial hypoglycemiaa.
| Bariatric subjects | p valueb | ||
|---|---|---|---|
| with HYPO | without HYPO | ||
| n | 11 | 11 | |
| Age at MMTT, y | 46.7 ± 11.7 | 47.5 ± 10.8 | 0.88 |
| Men, n (%) | 2 (18) | 4 (36) | 0.64 |
| Cholecystectomy, n (%) | 8 (73) | 3 (27) | 0.086 |
| Post-operative time to MMTT, mo | 47.5 ± 7.0 | 55.4 ± 12.7 | 0.087 |
| Weight preoperative, kg | 132.3 ± 20.0 | 144.5 ± 12.7 | 0.101 |
| BMI preoperative, kg/m2 | 44.7 ± 5.1 | 46.0 ± 5.0 | 0.54 |
| Weight at MMTT, kg | 87.5 ± 15.8 | 98.7 ± 14.6 | 0.101 |
| BMI at MMTT, kg/m2 | 29.5 ± 4.0 | 31.4 ± 5.2 | 0.35 |
| TWL at MMTT, % | 33.7 ± 6.5 | 31.5 ± 9.3 | 0.53 |
| EWL at MMTT, % | 78.8 ± 15.1 | 70.5 ± 20.5 | 0.29 |
| Fasting glucose, mmol/L | 4.0 ± 0.6 | 4.6 ± 0.7 | 0.037 |
| Fasting insulin, mU/L | 6.7 ± 4.2 | 11.0 ± 5.4 | 0.053 |
| HOMA2-IR | 0.8 ± 0.5 | 1.4 ± 0.7 | 0.047 |
EWL excess weight loss, HYPO hypoglycemia, MMTT mixed-meal tolerance test, TWL total weight loss.
aAll subjects were from West-European descent.
bData were compared with an independent T test between the bariatric subject with HYPO (n = 11) and the bariatric subjects without HYPO (n = 11) except for gender and hypoglycemia which were compared with a Fishers Exact Test.
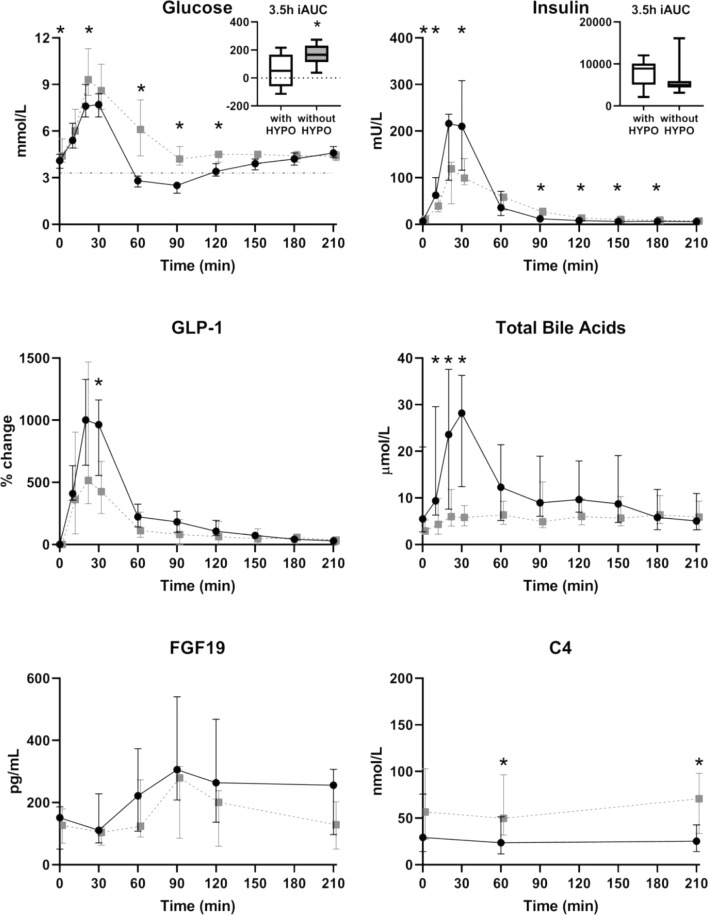
As shown in Fig. 1, postprandial glucose concentrations were significantly lower at 20, 60, 90 and 120 min after the meal in the subjects classified as hypoglycaemic compared to the subjects without hypoglycaemia during the MMTT (p = 0.047, p < 0.001, p < 0.001, p = 0.001) and, consequently, the 3.5 h iAUC was also lower (p = 0.023). Already in the first 30 min after the meal, hypoglycaemic subjects presented with higher concentrations of insulin, GLP-1 and total BA (Fig. 1). All individual BA species measured contributed to the exaggerated total BA response associated with hypoglycaemia (data not shown). Additionally, the concentrations of C4 were significantly higher at 60 and 210 min in subjects experiencing hypoglycaemia (p = 0.020, p = 0.010) while plasma FGF19 concentrations did not differ at any time point during the MMTT (Fig. 1).
Fig. 1. Glucose, insulin, GLP-1, total bile acids, FGF19 and C4 over time in response to a mixed meal in patients after Roux-en-Y gastric bypass surgery, subdivided between subjects with hypoglycemia (black) and without hypoglycemia (grey).
Data are median [IQR] and boxplots with min–max whiskers. HYPO hypoglycemia, iAUC incremental area under the curve. *p < 0.05 with a Mann–Whitney U Test between the bariatric subject with HYPO (n = 11) and the bariatric subjects without HYPO (n = 11). Due to large inter-assay variations in GLP-1 measurements (11, 18), within-subject changes from baseline were used for this analysis since all measurements from each individual were carried out in the same run.
In the complete set of 22 bariatric subjects, the peak (p = 0.061) and the iAUC (p = 0.035) of total plasma BA concentrations during the first hour after the mixed meal were inversely correlated with the nadir of the glucose concentrations (Fig. 2).
Fig. 2. Relationships between postprandial elevations of plasma bile acids and plasma glucose responses.
Correlation between the nadir of glucose during 30–120 min and the peak (left) and the iAUC (right) of the total bile acids in the first 60 min in response to a mixed meal in patients after Roux-en-Y gastric bypass surgery, subdivided between subjects with cholecystectomy and without cholecystectomy. CC cholecystectomy, iAUC incremental area under the curve.
Computer simulation of enterohepatic cycling of bile acids in relation to occurrence of postprandial hypoglycaemia
To gain a better understanding of the physiological parameters that may underlie the observed inter-individual differences in the plasma BA responses, simulations were performed with a previously developed mathematical model of the enterohepatic circulation of BA (Fig. 3A) [22]. This model has been shown to accurately describe BA metabolism in healthy subjects and was adjusted to include the anatomical changes induced by RYGB. Local sensitivity analysis of the model parameters revealed that the postprandial BA excursion in plasma is mainly determined by gallbladder emptying and intestinal transit time. It was therefore no surprise that simulation of literature data describing effects of RYGB on plasma BA excursions could be best accomplished by adjusting parameters reflecting stomach and gallbladder emptying and intestinal transit. Attempts to simulate the diverse postprandial BA profiles in the current studies revealed that variation in postprandial transit time (increased early transit, delayed post-absorptive transit (Fig. 3B)) can account for the observed variation. In silico, cholecystectomy augments the effect of the altered transit time by further increasing the early peak of the BA profile.
Coupling of intestinal BA kinetics to GLP-1 release by L cells enabled testing of the relationships between plasma BA and GLP-1 concentrations (Fig. 3B, C). The simulations show that the observed increase in GLP-1 excursion is the result of the RYGB-induced changes in postprandial dynamics, which propel both nutrients and BA faster and further down the intestine. Although the propulsion of nutrients is the main contributing factor to increased GLP-1 release, this effect is exaggerated by the co-occurrence of increased BA concentrations (which also stimulate GLP-1 secretion). Next, simulations were performed in which the changes in transit time were selected such that plasma BA excursions closely match the median plasma BA excursions of both the hypoglycaemia and non-hypoglycemia groups, in order to pinpoint underlying differences (Fig. 3D, E).
The computational model of BA dynamics includes a detailed description of intestinal transit speed. To be able to control transit in the different parts of the intestine separately, the intestinal trajectory between stomach and the end of the colon has been divided into three distinct transit zones (proximal small intestinal, distal small intestinal and colon). After RYGB, gastric emptying of liquid meals is increased dramatically [23], and so early postprandial transit is increased. However, distal small intestinal transit has been found to be delayed after RYGB [24]. Early appearance of nutrients and bile in the distal small intestine may lead to regulatory slowing down of transit speeds. Based on these observations, simulations were performed with several values for decreased early transit and for increased relative postprandial transit. Simulations with fasting transit speeds at 60% of the original value (i.e. 1.67 × slower) and a 5 times faster postprandial increase of transit (relative to fasting) were found to correspond well with the fasting and postprandial plasma BA concentrations of the normoglycemia group, whereas in the group with exaggerated BA response a ten times faster postprandial transit time compared to fasting led to a good correspondence with the data. Simulation of CC produced an extra, albeit relatively modest effect, the effect of these changes on plasma and distal intestinal BA concentrations and on the resulting plasma GLP-1 concentrations are shown in (Fig. 3D–F). More extreme increases in postprandial transit, resulting in higher plasma BA concentrations, were shown to correspond with a higher GLP-1 response (Fig. 3F).
Discussion
This study provides support for a distinct role of the currently well-established BA–GLP-1–insulin axis [5, 25] in the occurrence of postprandial hypoglycaemia in subjects who have experienced successful weight loss upon RYGB, often referred to as post-bariatric hypoglycaemia (PBH). So far the underlying causes of PBH, that may occur in up to 50% of cases depending on definition and diagnostic tool used, have largely remained elusive. We hypothesised that altered kinetics of enterohepatic BA cycling might be involved in generating this adverse event, as BA are released into the intestine upon ingestion of a meal and show postprandial plasma responses, reflecting their enterohepatic cycling, within the same timeframe as glucose and insulin do [26]. BA are known to exert a number of signalling functions in cells of the intestinal wall, i.e. FXR activation in ileocytes and TGR5-mediated stimulation of GLP-1 release by L cells, that may impact on postprandial glucose metabolism [25]. To test this hypothesis, we have analyzed postprandial responses of individual BA species in RYGB subjects after a standardised test meal and evaluated the relationships hereof with the GLP-1 and insulin responses as well as the occurrence of hypoglycaemia. A priori, we reasoned that the absence of a gallbladder due to prior cholecystectomy for gallstone disease might alter postprandial BA kinetics in RYGB subjects and, hence, might impact on the incidence of postprandial hypoglycaemia. Indeed, our analysis revealed that postprandial hypoglycaemia after a standardised meal test was associated with exaggerated postprandial responses of total and individual BA, GLP-1 and insulin and that the prevalence hereof appeared to be higher in cholecystectomized subjects (8/11) than in subjects with a functional gallbladder (3/11). The area under the postprandial BA curve correlated with the severity of hypoglycaemia, indicative for a functional role of circulating BA in control of glucose excursions. Intriguingly, mathematical modelling of BA kinetics using a recently developed method to analyze the dynamics of the enterohepatic circulation of BA revealed that a very rapid early and delayed distal intestinal transit and, consequently, high abundance of BA in the terminal ileum directly after a meal preceded hypoglycaemia. Rapid transit appeared more evident in simulations without a functioning gallbladder. Collectively, our data support the concept that high BA prevalence and enhanced BA signalling in the lower intestine upon ingestion of a meal strongly promotes GLP-1 secretion by L cells which, in turn, stimulates insulin secretion by pancreatic β-cells [27] and, concomitantly, suppresses glucagon secretion by α-cells [28]. The ensuing hyperinsulinemia can lead to hypoglycaemia, either by promoting peripheral glucose uptake or suppression of hepatic glucose production or by a combination of both.
Confirming a wealth of earlier studies, summarised in [29], fasting plasma BA levels were 2–3 times higher after RYGB compared to the matched non-bariatric cohort: elevated levels were evident for virtually all quantifiable BA species. The glycine–taurine conjugation ratio was higher in RYGB subjects while the ratio between conjugated and unconjugated BA was similar in both groups, which, in view of the preference for taurine of the human hepatic BA conjugation machinery [25], indicates a relatively lower taurine availability in RYGB subjects. Fasting FGF19 levels were higher in RYGB subjects than in controls, confirming earlier observations [4], but this was not accompanied by altered C4 concentrations indicating absence of differences in hepatic BA synthesis between the groups. Yet, it should be realised that BA synthesis and plasma C4 levels show strong day–night variation [25] that may not be adequately captured by single point measurements of FGF19 and C4 in the morning. Fasting plasma BA concentrations were similar in RYGB subjects with and without functional gallbladder, but plasma C4 levels were higher in the latter group suggesting induction of hepatic BA synthesis by cholecystectomy. This is supported by the higher glycine–taurine conjugation ratio in these subjects, as this ratio usually reflects a condition with accelerated BA turnover with consequently increased use of ubiquitous glycine molecules for the conjugation reaction. Elevated plasma C4 levels have recently also been reported in cholecystectomized subjects without RYGB [14]. This study demonstrated high expression of FGF19 mRNA in human cholangiocytes and very high FGF19 concentrations in gallbladder bile: reduced plasma FGF19 coinciding with elevated C4 at days 10 and 90 after cholecystectomy were interpreted to reflect strong impact of gallbladder-derived FGF19 on hepatic BA synthesis in humans. In contrast, we found no differences in neither fasting nor postprandial plasma FGF19 levels between RYGB subjects with and without gallbladder, indicating that FGF19 produced by the gallbladder does not contribute to appearance of FGF19 in plasma under these conditions. Furthermore, we did not find significant differences in FGF19 levels between the groups with and without hypoglycaemia during the mixed-meal test. FGF19 has been found to be elevated in patients with symptomatic PBH after RYGB [30]. We propose that the BA-GLP-1 and the BA-FGF19 axes are upregulated after bariatric surgery with a spectrum of activities ranging from beneficial effects on glucose handling to pathophysiological effects leading to PBH. In our asymptomatic cohort, we observed a tendency towards slightly higher postprandial levels of FGF19 in the hypoglycaemic group (data not shown). In the study of Mulla et al. [30] postprandial BA were unfortunately not measured.
The simulations presented in Fig. 3 demonstrate how alterations to gastrointestinal physiology resulting from RYGB with and without cholecystectomy can underlie the post-RYGB changes in BA and GLP-1 kinetics. The characteristic sharp increase in plasma BA concentrations in the first 30 min observed following RYGB can result from an increase in proximal small intestinal transit, which may result from accelerated stomach emptying [23]. This will result in a higher BA concentration in the terminal ileum (Fig. 3F) and hence a more rapid BA absorption via the Apical Sodium-dependent Bile acid Transporter (ASBT, encoded by SLC10A2) [24]. Interestingly, the simulations support our hypothesis that cholecystectomy predisposes RYGB patients to develop exaggerated BA and GLP-1 responses. This observation alone does not preclude the presence of other mechanisms underlying or contributing to the changes in plasma BA levels. However, a number of additional simulations were performed, comparing the effects of a wide array of RYGB-induced physiological changes on BA metabolism. The only parameter tested that induced increases of plasma BA concentrations without simultaneous perturbation of the composition of the BA pool was the slowing of distal post-absorptive transit, leading us to conclude this mechanism is a strong candidate for the driving force behind the RYGB-induced increase in BA concentration [22].
In conclusion, this explorative study supports a role for altered intestinal BA fluxes in the development of PBH after RYGB through activation of the intestinal GLP-1-pancreatic islet axis. In addition, our data suggest that cholecystectomized subjects are more prone to develop postprandial BA-induced hypoglycaemia upon RYGB. In fact, a recent study on self-reported complaints of dumping syndrome and post-bariatric hypoglycemia in 590 patients who underwent bariatric surgery in the period 2008-2011 in the same institution showed that cholecystectomy indeed increases the relative risk of hypoglycemia, particularly in subgroups defined by gender, age, presence of diabetes and % weight loss [31]. Based on these results, it might be worthwhile to evaluate the potential beneficial effects of BA sequestration as treatment of postprandial hypoglycaemia after RYGB.
Supplementary information
Acknowledgements
University of Groningen Campus Fryslân (MvdB, TvZ), the Netherlands Heart Foundation (CVON2018-27) (AKG, FK) and EU’s Seventh Programme for Research, Technological Development and Demonstration under grant agreement No. 305707 (AKG, NAWvR). The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41366-020-00726-w) contains supplementary material, which is available to authorised users.
References
- 1.Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:2279–89. doi: 10.1007/s11695-017-2666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–07. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Nemati R, Lu J, Dokpuang D, Booth M, Plank LD, Murphy R. Increased bile acids and FGF19 after sleeve gastrectomy and Roux-en-Y gastric bypass correlate with improvement in type 2 diabetes in a randomized trial. Obes Surg. 2018;28:2672–86. doi: 10.1007/s11695-018-3216-x. [DOI] [PubMed] [Google Scholar]
- 5.Albaugh VL, Banan B, Antoun J, Xiong Y, Guo Y, Ping J, et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2019;156:1041–51. doi: 10.1053/j.gastro.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen S, Svane MS, Kuhre RE, Clausen TR, Kristiansen VB, Rehfeld JF, et al. Chenodeoxycholic acid stimulates glucagon-like peptide-1 secretion in patients after Roux-en-Y gastric bypass. Physiol Rep. 2017;5:e13140. [DOI] [PMC free article] [PubMed]
- 7.Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68:70–82. doi: 10.1136/gutjnl-2018-316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baud G, Daoudi M, Hubert T, Raverdy V, Pigeyre M, Hervieux E, et al. Bile diversion in Roux-en-Y gastric bypass modulates sodium-dependent glucose intestinal uptake. Cell Metab. 2016;23:547–53. doi: 10.1016/j.cmet.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–42. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emous M, Wolffenbuttel BHR, Totté E, van Beek AP. The short- to mid-term symptom prevalence of dumping syndrome after primary gastric-bypass surgery and its impact on health-related quality of life. Surg Obes Relat Dis. 2017;13:1489–1500. doi: 10.1016/j.soard.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Emous M, van den Broek M, Wijma RB, de Heide LJM, van Dijk G, Laskewitz A, et al. Prevalence of hypoglycaemia in a random population after Roux-en-Y gastric bypass after a meal test. Endocr Connect. 2019;8:969–78. doi: 10.1530/EC-19-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–5. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 13.Berr F, Stellaard F, Pratschke E, Paumgartner G. Effects of cholecystectomy on the kinetics of primary and secondary bile acids. J Clin Investig. 1989;83:1541–50. doi: 10.1172/JCI114050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrera F, Azocar L, Molina H, Schalper KA, Ocares M, Liberona J, et al. Effect of cholecystectomy on bile acid synthesis and circulating levels of fibroblast growth factor 19. Ann Hepatol. 2015;14:710–21. doi: 10.1016/S1665-2681(19)30766-5. [DOI] [PubMed] [Google Scholar]
- 15.Erlinger S. Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol. 2000;12:1347–52. doi: 10.1097/00042737-200012120-00015. [DOI] [PubMed] [Google Scholar]
- 16.Amstutz S, Michel JM, Kopp S, Egger B. Potential benefits of prophylactic cholecystectomy in patients undergoing bariatric bypass surgery. Obes Surg. 2015;25:2054–60. doi: 10.1007/s11695-015-1650-6. [DOI] [PubMed] [Google Scholar]
- 17.Wanjura V, Sandblom G, Österberg J, Enochsson L, Ottosson J, Szabo E. Cholecystectomy after gastric bypass—incidence and complications. Surg Obes Relat Dis. 2017;13:979–87. doi: 10.1016/j.soard.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Wijma RB, Emous M, van den Broek M, Laskewitz A, Kobold ACM, van Beek AP. Prevalence and pathophysiology of early dumping in patients after primary Roux-en-Y gastric bypass during a mixed-meal tolerance test. Surg Obes Relat Dis. 2018;15:73–81. doi: 10.1016/j.soard.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Soenen S, Bonomi AG, Lemmens SG, Scholte J, Thijssen MA, van Berkum F. Relatively high-protein or ‘low-carb’ energy-restricted diets for body weight loss and body weight maintenance? Physiol Behav. 2012;107:374–80. doi: 10.1016/j.physbeh.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Out C, Dikkers A, Laskewitz A, Boverhof R, van der Ley C, Kema IP, et al. Prednisolone increases enterohepatic cycling of bile acids by induction of Asbt and promotes reverse cholesterol transport. J Hepatol. 2014;61:351–7. doi: 10.1016/j.jhep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Eggink HM, Tambyrajah LL, van den Berg R, Mol IM, van den Heuvel JK, Koehorst M, et al. Chronic infusion of taurolithocholate into the brain increases fat oxidation in mice. J Endocrinol. 2018;236:85–97. doi: 10.1530/JOE-17-0503. [DOI] [PubMed] [Google Scholar]
- 22.Sips FLP, Eggink HM, Hilbers PAJ, Soeters MR, Groen AK, van Riel NAW. In silico analysis identifies intestinal transit as a key determinant of systemic bile acid metabolism. Front Physiol. 2018;9:631. doi: 10.3389/fphys.2018.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horowitz M, Harding PE, Maddox A, Maddern GJ, Collins PJ, Chatterton BE, et al. Gastric and oesophageal emptying in insulin-dependent diabetes mellitus. J Gastroenterol Hepatol. 1986;1:97–113. doi: 10.1111/j.1440-1746.1986.tb00104.x. [DOI] [Google Scholar]
- 24.Dirksen C, Damgaard M, Bojsen-Møller KN, Jørgensen NB, Kielgast U, Jacobsen SH, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25:346–55. doi: 10.1111/nmo.12087. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling - mechanisms and research needs. Nat Rev Endocrinol. 2019;15:701–12. doi: 10.1038/s41574-019-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med. 2018;215:383–96. doi: 10.1084/jem.20171965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;8571:1300–4. doi: 10.1016/S0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Parajuli KR, Fava GE, Gupta R, Xu W, Nguyen LU, et al. GLP-1 receptor in pancreatic α-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes. 2019;68:34–44. doi: 10.2337/db18-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chavez-Talavera O, Haas J, Grzych G, Tailleux A, Staels B. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes: what do the human studies tel? Curr Opin Lipidol. 2019;30:244–54. doi: 10.1097/MOL.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 30.Mulla CM, Goldfine AB, Dreyfuss JM, Houten S, Pan H, Pober DM, et al. Plasma FGF-19 levels are increased in patients with post-bariatric hypoglycemia. Obes Surg. 2019;29:2092–9. doi: 10.1007/s11695-019-03845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Furth AM, van den Broek M, Emous M, de Heide LJM, Kuipers F, van Beek AP. Cholecystectomy increases the risk of dumping syndrome andpost-bariatric hypoglycemia after bariatric surgery. Surg. Obes. Rel. Dis. 2020;16:1939−47. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.