Abstract
Candidate phyla radiation (CPR) bacteria and DPANN archaea are unisolated, small-celled symbionts that are often detected in groundwater. The effects of groundwater geochemistry on the abundance, distribution, taxonomic diversity and host association of CPR bacteria and DPANN archaea has not been studied. Here, we performed genome-resolved metagenomic analysis of one agricultural and seven pristine groundwater microbial communities and recovered 746 CPR and DPANN genomes in total. The pristine sites, which serve as local sources of drinking water, contained up to 31% CPR bacteria and 4% DPANN archaea. We observed little species-level overlap of metagenome-assembled genomes (MAGs) across the groundwater sites, indicating that CPR and DPANN communities may be differentiated according to physicochemical conditions and host populations. Cryogenic transmission electron microscopy imaging and genomic analyses enabled us to identify CPR and DPANN lineages that reproducibly attach to host cells and showed that the growth of CPR bacteria seems to be stimulated by attachment to host-cell surfaces. Our analysis reveals site-specific diversity of CPR bacteria and DPANN archaea that coexist with diverse hosts in groundwater aquifers. Given that CPR and DPANN organisms have been identified in human microbiomes and their presence is correlated with diseases such as periodontitis, our findings are relevant to considerations of drinking water quality and human health.
Subject terms: Microbiology, Environmental sciences
Metagenomics and electron microscopy are combined to analyse the diversity of episymbiotic CPR bacteria and DPANN archaea in eight groundwater communities.
Main
Metagenome-enabled phylogenomic analyses have led to the classification of two groups of organisms that lack pure culture representatives—the CPR bacteria and DPANN archaea1–4. Although diverse, CPR and DPANN organisms share conserved traits that are indicative of a symbiotic lifestyle, being ultrasmall in size with small genomes and minimal biosynthetic capabilities5–9. Episymbiosis (surface attachment) with bacterial or archaeal hosts has been observed in co-cultures of Saccharibacteria with Actinobacteria10,11, Nanoarchaeota with Crenarchaeota12–14, and Nanohaloarchaeota and archaeal Richmond Mine acidophilic nanoorganisms (ARMAN) with Euryarchaeota15–17, and one case of endosymbiosis has been reported in which a member of the CPR superphylum Parcubacteria lives inside a protist18. CPR and DPANN organisms are ubiquitous and can be abundant in groundwater, in which they are predicted to contribute to biogeochemical cycling2,4,8,9,19–24. CPR bacteria can persist in drinking water through multiple treatment methods25–27, posing the question of whether groundwater is a source of CPR10,11,28–30 and DPANN31 organisms detected in human microbiomes.
The variation in the abundance and distribution of CPR and DPANN organisms in groundwater environments, their roles and their relationships with host organisms are not well characterized. Subsurface environments such as groundwater are difficult to sample and are poorly characterized compared with surface environments, despite harbouring an estimated 90% of all bacterial biomass32. CPR/DPANN organism abundance is likely to have been underestimated in genomic surveys because they are small enough to pass through 0.2 µm filters, which are widely used to collect cells. Furthermore, ‘universal’ primers to divergent or intron-containing 16S rRNA genes2,12,15 are unlikely to detect many members of both groups. Most of the available near-complete CPR and DPANN genomes are from just two aquifers2,19,22. In this Article, to investigate the roles that CPR and DPANN organisms may have in groundwater ecosystems, we applied genome-resolved metagenomics to analyse eight groundwater communities in Northern California, and cryogenic transmission electron microscopy (cryo-TEM) to image the community with the highest abundance of CPR/DPANN organisms.
Results
Metagenome sampling and MAG assembly
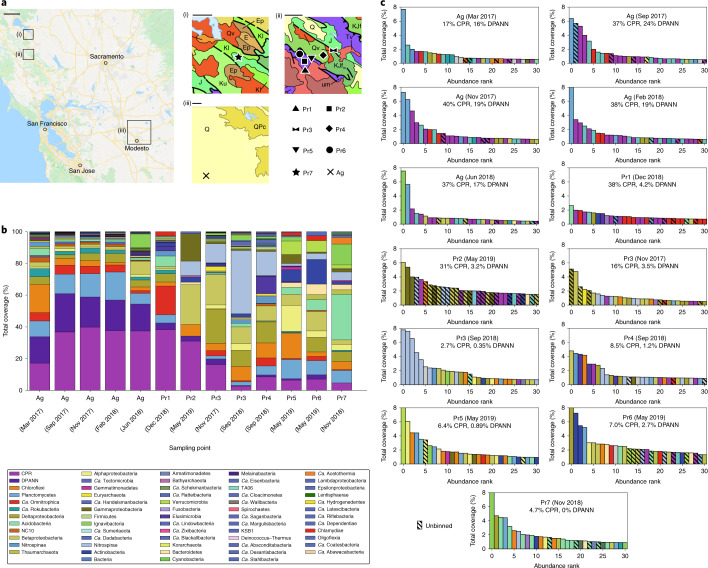
The planktonic fractions of eight groundwater communities in Northern California were sampled during 2017–2019 (Fig. 1a) using bulk filtration (0.1 µm filter). Some sites were also sampled using serial size filtration (2.5 µm, 0.65 µm, 0.2 µm and 0.1 µm filters) in parallel. This enterprise required pumping 400–1,200 l of groundwater from each site through a purpose-built sequential filtration apparatus to recover sufficient biomass for deep sequencing of each size fraction (Methods). One site (Ag) is an agriculturally impacted, river sediment-hosted aquifer and the remaining sites are pristine groundwater aquifers hosted in a mixture of sedimentary and volcanic rocks (Pr1–Pr7, numbered in decreasing order of total CPR and DPANN organism abundance). On the basis of the high abundance and diversity of CPR/DPANN organisms found at the Ag site in a previous metagenomics study33, we sampled five time points over 15 months.
Fig. 1. Sampling and overview of groundwater communities.
a, Map of eight Northern California groundwater sites sampled in this study (image from Google Maps). Insets: geological maps (i)–(iii) show the sampled areas (black boxes). J, marine sedimentary and metasedimentary rocks (Jurassic); KJfm, marine sedimentary and metasedimentary rocks (Cretaceous–Jurassic); Q, marine and non-marine (continental) sedimentary rocks (Pleistocene–Holocene); Qv, marine sedimentary and metasedimentary rocks (Cretaceous–Jurassic); um, plutonic (Mesozoic); K, marine sedimentary rocks (Pliocene); Kl, marine sedimentary and metasedimentary rocks (Lower Cretaceous); Ep, marine sedimentary rocks (Paleocene); E, marine sedimentary rocks (Eocene); QPc, non-marine (continental) sedimentary rocks (Pliocene–Pleistocene); Tv, volcanic rocks (Tertiary). Scale bars, 23.3 km (large map), 3.2 km (i and ii) and 9.7 km (iii). b, Phylum-level breakdown (with the exception of CPR and DPANN superphyla) of rpS3 genes detected in each site. The sampling dates for each site are indicated. c, Rank abundance curves showing the 30 rpS3 genes with highest relative coverage identified for each site. The hatched bars indicate an unbinned rpS3 gene.
Binning of bacterial and archaeal genomes from metagenomic data was performed using four different binning algorithms/techniques on the basis of GC content, coverage, the presence/copies of ribosomal proteins and single-copy genes, tetranucleotide frequencies and patterns of coverage across samples (Methods). The highest quality bins were chosen using DASTool34 and then manually curated. All bins for a given site were dereplicated at 99% average nucleotide identity (ANI)35, resulting in a dereplicated set of 2,007 genomes across all sites (≥70% completeness and ≤10% contamination). The median genome completeness was >90% and up to 58% of metagenomic reads mapped to each site’s dereplicated genome set (assembly and binning statistics are provided in Supplementary Tables 1 and 2). Of this dereplicated set, 540 and 206 genomes were classified as CPR bacteria and DPANN archaea, respectively.
Abundance and diversity of CPR/DPANN organisms
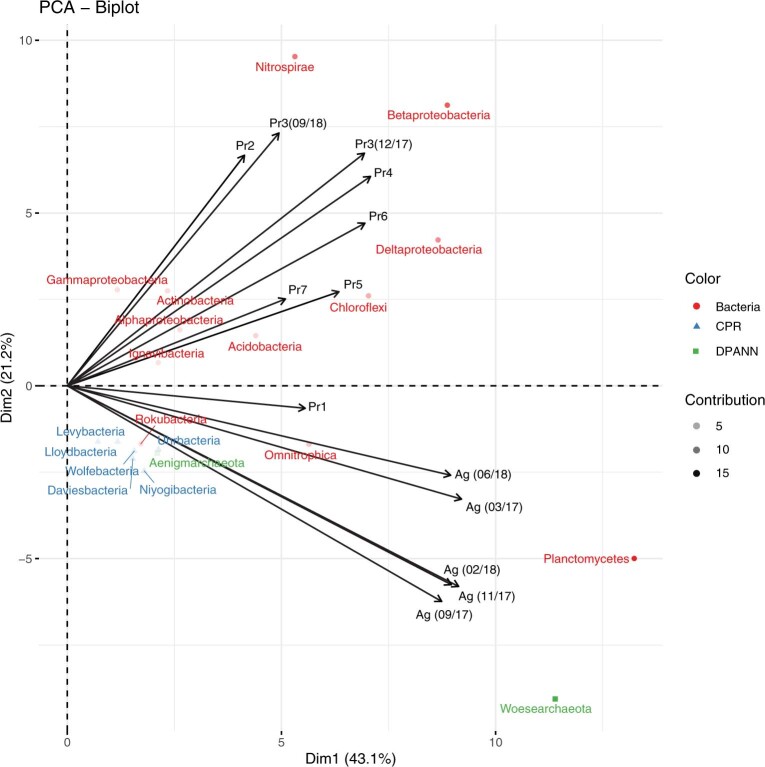
We first sought to characterize and compare compositions of the eight groundwater communities, with a particular focus on CPR and DPANN organisms. To broadly survey microbial community composition, we used the ribosomal protein uS3 (encoded by rpS3) as a single-copy marker gene due to its strong phylogenetic signal36. A comparison of rpS3 genes against recovered genomes indicated that, with the exception of the Pr2 site, the majority of the most abundant organisms at each site are represented by genome bins (Fig. 1c, the hatched bars indicate unbinned rpS3 genes). We found that all of the groundwater communities are distinct in phylum-level composition (Fig. 1b,c), with a strong divide between the Ag site and the pristine sites on the basis of principal component analysis (Extended Data Fig. 1). Change over time of the Ag groundwater community is examined in further detail in the ‘An agriculturally impacted groundwater site rich in CPR/DPANN’ section.
Extended Data Fig. 1. A biplot representation of phylum-level lineages in ordination space.
Arrows show the direction of greatest gradient change according to site. The transparency of the points reflects the contribution of the phylum to the principal components.
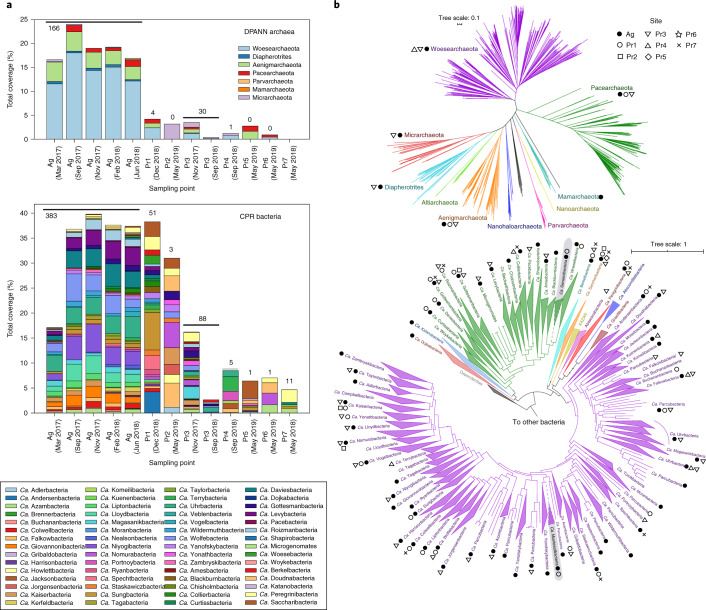
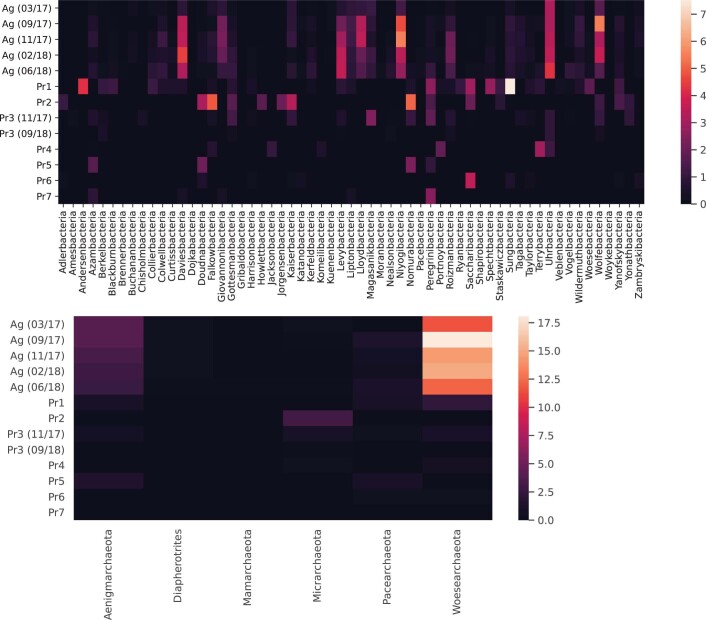
Specifically, the populations of CPR and DPANN organisms are quite distinct between sites (Fig. 2a), although a few CPR and DPANN lineages are fairly ubiquitous across sites (Extended Data Fig. 2). Across all of the sites, CPR and DPANN organisms represent 3–40% and 0–24% of the communities (measured by bulk filtration onto a 0.1 µm filter), respectively. The abundance of DPANN archaea in Ag groundwater (10–24%) is much higher compared to the pristine sites, where DPANN organisms comprise <5% of the community. Across all of the sites, genomes were recovered from 58 out of 73 currently identified phylum-level lineages within the CPR4 and from 6 out of 10 currently identified phylum-level lineages within the DPANN radiation (Fig. 2b). In particular, recovered CPR genomes from Ag groundwater span most of the diversity within the CPR (Fig. 2b, filled black circles). On the basis of the criteria for 16S rRNA gene sequence identity (<76% for phylum-level21,37) and concatenated ribosomal protein phylogenetic placement2, we defined two new phylum-level lineages within the CPR, each consisting of sequences from Ag and Pr1 groundwater (Supplementary Table 3). We propose the names ‘Candidatus Genascibacteria’ and ‘Candidatus Montesolbacteria’ for these new phylum-level lineages (Fig. 2b, highlighted in grey) on the basis of the two sites at which the representative sequences were found.
Fig. 2. Distribution of CPR and DPANN organisms across groundwater sites.
a, Abundances (relative coverage of scaffolds containing rpS3 marker genes) of phylum-level lineages within DPANN (above) and CPR (below) in all sites. The numbers above the bars indicate the number of draft-quality (>70% complete, <10% contamination) dereplicated DPANN or CPR genomes that were recovered from metagenomic reads. b, Maximum likelihood phylogenetic tree of the DPANN radiation (top), based on 14 concatenated ribosomal proteins, and of the CPR (bottom), based on 15 concatenated ribosome proteins. Phylum-level lineages within the CPR (as previously defined2) are collapsed. Markers next to each lineage indicate the groundwater sites where at least one representative genome from that lineage was recovered. New CPR lineages ‘Candidatus Genascibacteria’ (within the Microgenomates superphylum in green) and ‘Candidatus Montesolbacteria’ (within the Parcubacteria superphylum in purple) are highlighted in grey.
Extended Data Fig. 2. Distribution of CPR (top) and DPANN (bottom) phylum-level lineages across groundwater sites.
Color/legend indicate relative coverage values (percentages).
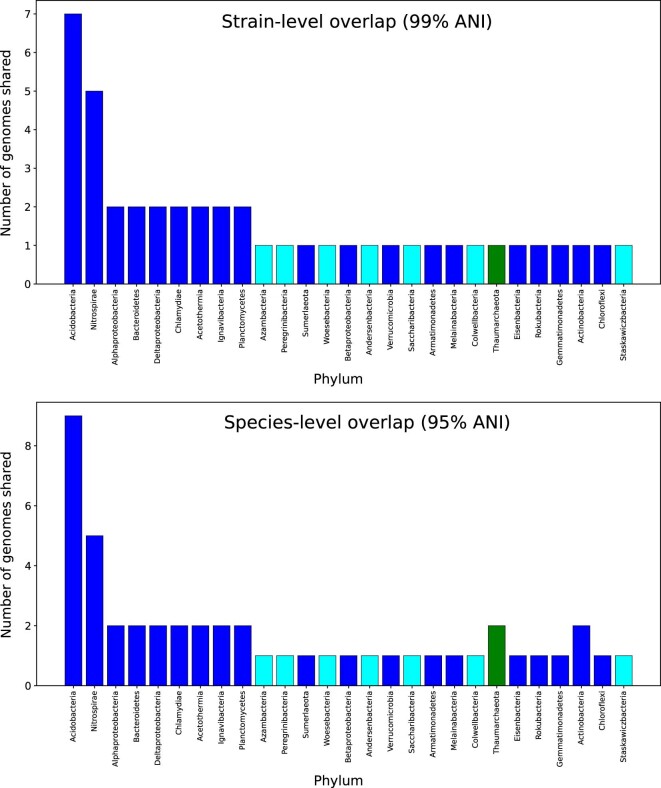
To assess groundwater community similarity at the genome level, we used ANI to cluster35 the 2,007 genomes from this study with 3,044 genomes from previous studies of two groundwater sites rich in CPR/DPANN organisms: Crystal Geyser in Utah22,38,39 and an aquifer adjacent to the Colorado River in Rifle, Colorado2,19. At the strain level (>99% ANI), there is very little similarity between genomes of the analysed sites; most pairs of sites share one or no strains despite the fact that sites Pr1 to Pr6 are located in close proximity (~1 km between neighbouring sites) and multiple sites are hosted in plutonic rock. The sole pair of sites that share more than a few strains (>99% ANI) is Pr1 and Pr7, which share 44 strains, including 7 CPR bacterial strains (Extended Data Fig. 3). It is unlikely that the aquifers of these two sites are connected as they lie on separate sides of Putah Creek, a major hydrological feature. Furthermore, we do not attribute this observed genome similarity to index hopping during sequencing (Methods). Even at the species level (>95% ANI40), most pairs of analysed sites share no more than one species in common (Extended Data Fig. 3). The overall lack of genomic similarity between these ten groundwater communities—at the phylum, species and strain levels—indicates that there is a high degree of specialization based on local hydrogeochemical conditions.
Extended Data Fig. 3. Genome similarity at the strain (>99% ANI) and species (>95% ANI) level between Pr1 and Pr7 genomes.
Blue bars indicate non-CPR bacteria, aqua bars indicate CPR bacteria, and green bars indicate archaea. The magnitude of the y-axis indicates the number of genomes shared according between the two sites according to the ANI threshold.
Roles of CPR/DPANN organisms in biogeochemical cycling
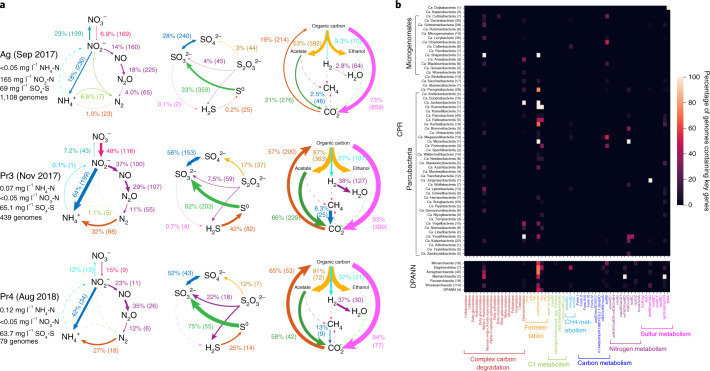
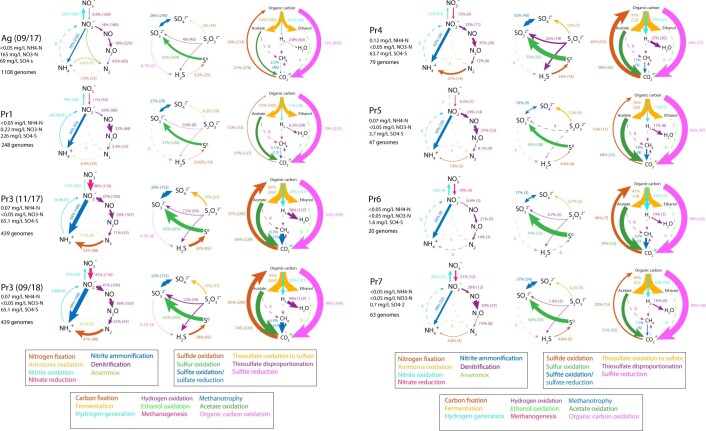
Next, given the abundance of CPR and DPANN organisms, we sought to investigate the potential metabolic roles these organisms have in these eight groundwater communities. As most CPR/DPANN organisms are predicted to be symbionts, it is probable that their metabolic roles within a community vary with the metabolic capacities of their host organisms. To investigate this relationship, we profiled all recovered genomes against a curated set of protein hidden Markov models (HMMs)41 (Methods) and utilized genome relative coverage values to compare metabolic profiles of whole communities (Fig. 3a and Extended Data Fig. 4). The metabolic profile of Ag groundwater is clearly differentiated from that of the pristine sites33 (Extended Data Fig. 5). In Ag groundwater, which receives heavy nitrogen input from neighbouring agricultural activity, ammonia is oxidized by seven Planctomycetes that are capable of anammox (comprising 8% of the community), resulting in low levels of ammonia in Ag groundwater (Fig. 3a). The Ag community encodes greater capacity for nitrite oxidation than nitrate reduction, consistent measurements of high nitrate (165 mg l−1 NO3-N) and low nitrite (<0.05 mg l−1 NO2-N) levels (Fig. 3a). Most of the groundwater communities sampled have an incomplete capacity for denitrification (Extended Data Fig. 4), with far fewer genomes encoding the required genes for the final step of nitrous oxide reduction compared with the previous steps. Pr3 and Pr4 are two sites with a greater capacity for nitrous oxide reduction compared with the other groundwater communities, in addition to nitrogen fixation, thiosulfate disproportionation, sulfide oxidation and carbon fixation (Fig. 3a and Extended Data Fig. 4). Although Pr3 and Pr4 have little species-level overlap, their similarity in community-level metabolic capacities may reflect their proximity (<1 km) and similar groundwater chemistry (levels of NH4-N, NO3-N, NO2-N and SO4-S).
Fig. 3. Metabolic profile of groundwater communities.
a, Biogeochemical cycling diagrams profiling the community-level metabolic potential of three groundwater sites sampled in this study (all of the sites are shown in Extended Data Fig. 4). The total relative abundance of all genomes capable of carrying out the step, as well as the number of genomes containing the capacity for that step, are listed next to each metabolic step. Arrow sizes are drawn proportional to the total relative abundance of genomes capable of carrying out the metabolic step. b, Heat map of 746 CPR and DPANN genomes from this study (rows are phylum-level lineages, and the numbers in parentheses are the number of genomes recovered), showing the percentage of genomes containing key genes required for various metabolic and biosynthetic functions (columns).
Extended Data Fig. 4. Community-level cycling of nitrogen, sulfur, and carbon in the eight groundwater communities sampled in this study.
Listed next to each metabolic step are the total relative abundance of all genomes capable of carrying out the step, and the number of genomes containing the capacity for that step. Arrow sizes are drawn proportional to the total relative abundance of genomes capable of carrying out the metabolic step.
Extended Data Fig. 5. Depiction of total relative coverages of different metabolic capacities, in principal component space.
Principal component analysis was performed on the relative abundance of organisms with specific metabolic capacities across all sites.
We specifically examined key metabolic marker genes in CPR and DPANN genomes to assess what metabolic roles that they may have (Fig. 3b). The presence of the nitrite reductase nirK in 19 CPR and 4 DPANN genomes as well as the presence of nosD in 11 DPANN genomes across sites suggest a complementary or accessory role of many CPR/DPANN lineages in denitrification (consistent with previous identification of nirK genes in Parcubacteria21,42). Furthermore, 13 DPANN genomes in Ag groundwater encode the small subunit of nitrite reductase (nirD) but lack the catalytic large subunit nirB, suggesting that DPANN organisms have an accessory role in nitrite reduction to ammonia. At Ag, Pr1 and Pr3, we found that 30 DPANN genomes and 3 CPR genomes encode sulfur dioxygenase sdo, while dozens of diverse CPR and DPANN genomes encode sat, cysC and cysN, which are involved in sulfate reduction, suggesting a potential role of CPR and DPANN organisms in transformations to sulfite.
An agriculturally impacted groundwater site rich in CPR/DPANN organisms
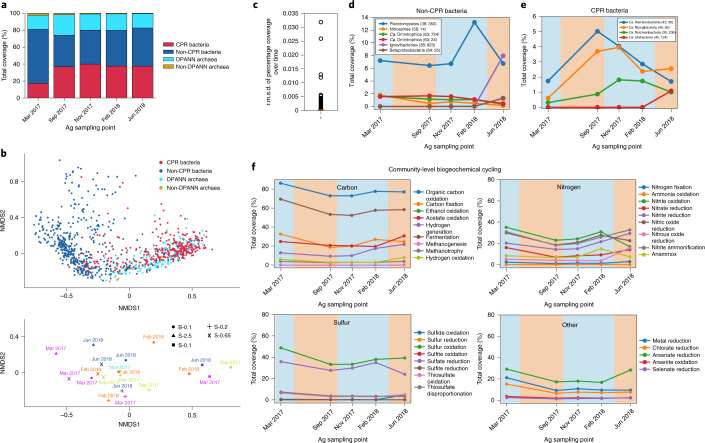
After establishing the prevalence and metabolic roles of CPR and DPANN organisms in groundwater communities, we performed temporal and size filtration sampling of Ag groundwater (Fig. 4a) to investigate how these characteristics change with time and environmental factors. Non-metric multidimensional scaling (NMDS) ordination (Methods) shows that, as expected, most CPR and DPANN genomes cluster together and away from other bacteria and archaea, distinguished by prevalence in the 0.1–0.2 µm fraction (Fig. 4b). There is no observable clustering of genomes by sampling time in ordination space and the median root mean square deviation (r.m.s.d.) of genome relative abundances over time is ~0.002 (Fig. 4c), indicating a very stable community at the strain level (genomes dereplicated at 99% ANI). Inspection of abundance patterns in individual genomes with an r.m.s.d. > 0.004 (Fig. 4d,e) show a coabundance pattern between a Planctomycetes organism and several CPR bacteria that, although certainly not conclusive, may result from a parasitic CPR–host relationship. The co-occurrence of two Ignavibacteria and Betaproteobacteria organisms with an Uhrbacteria organism (Fig. 4d) may be an indication of a commensal or mutualistic CPR–host relationship. These observed temporal trends merit further investigation to determine whether they reflect symbiotic relationships.
Fig. 4. Ag groundwater microbial community over time.
a, Relative abundances of non-CPR bacteria, CPR bacteria, DPANN archaea and non-DPANN archaea genomes (1,103 in total) in Ag over time. b, NMDS analysis of 1,103 Ag genome relative abundances in all size fractions over all time points. The positions of genomes in ordination space are shown in the top graph, while the positions of the samples in ordination space are shown in the bottom graph. In the bottom graph, B-0.1 refers to bulk filtration on a 0.1 µm filter (circles), S-2.5 refers to 2.5+ µm size fractions (triangles), S-0.65 refers to 0.65–2.5 µm size fractions (crosses), S-0.2 refers to 0.2–0.65 µm size fractions (plus signs), and S-0.1 refers to 0.1–0.2 µm size fractions (closed squares). c, Box plot showing the r.m.s.d. of the relative abundance of all of the genomes in the bulk filter (whole community on a 0.1 µm filter) over time. The median r.m.s.d. (orange line) is <0.001, indicating that there is little variation in relative abundance over time for individual genomes in Ag. d,e, The relative abundance over time for non-CPR bacteria (d) and CPR bacteria (e) that have an r.m.s.d. > 0.004. Genomes are identified in the legend by phylum, percentage GC and coverage in the original time point that the representative genome was derived from (the latter two in parentheses). f, The variation in Ag community-level capacity (total relative abundance of all genomes capable of a broad metabolic function) for carbon, nitrogen, sulfur and miscellaneous element cycling over time. For d–f, the blue and orange backgrounds indicate the rainy and dry seasons in Northern California, respectively.
Examination of the changes in metabolic cycling capacities in Ag groundwater over time indicate that there is a higher community capacity (5–10% relative abundance) for organic carbon oxidation, carbon fixation, fermentation, nitrite oxidation, nitric oxide reduction and sulfate reduction during the rainy season (Fig. 4f, blue background) compared with the dry season (Fig. 4f, orange background). Furthermore, we see a greater increase in these metabolic capacities during the 2016–2017 rainy season compared with the 2017–2018 rainy season (Fig. 4f), which may reflect a major difference in rainfall (more than 25 cm more in 2016–2017 versus 2017–2018)43. Overall, we found that Ag groundwater is an extremely stable incubator for high abundance and diversity of both CPR and DPANN organisms, but the microbial community is not stagnant in its metabolic capacities, which vary between rainy and dry seasons.
Pili-mediated episymbiotic interactions between ultrasmall cells and hosts
Fundamental to understanding the wider role of CPR and DPANN organisms in groundwater communities is characterizing their relationships with hosts. With few exceptions, host organisms have not been conclusively identified and only a handful of studies have performed high-resolution microscopy to directly image the physical associations between CPR/DPANN organisms and hosts in natural environments15,44,45. To observe CPR/DPANN–host interactions in the Ag groundwater community in a near-native state, we used tangential flow filtration (TFF) to gently concentrate cells from groundwater and preserved them by cryo-plunging them in liquid ethane on-site for later characterization by cryo-TEM (Methods).
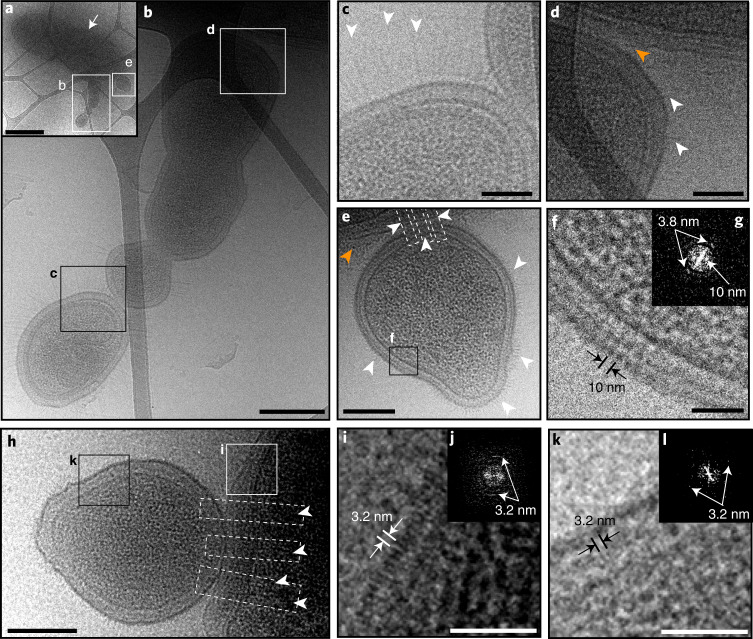
Many ultrasmall cells (longest dimension <500 nm) were observed attached to the surface of larger host cells (Fig. 5a). The ultrasmall cells have cell envelopes that are decorated by pili (Fig. 5, white arrows; a magnified view is shown in Extended Data Fig. 6), some of which extend into the corresponding host cell, potentially mediating episymbiont–host interaction (Fig. 5, white dashed boxes). At the ultrasmall cell–host contact region shown in Fig. 5e,h, the host cell envelope appears to be thickened, whereas the episymbiont cell envelope is thinned. For multiple pairs of ultrasmall cells and hosts, a line of higher density is observed at the cell interface (Fig. 5d,e,i, orange arrows), similar to what has previously been observed at tight interfaces between archaeal ARMAN (DPANN) cells and their Thermoplasmatales hosts44. The host in Fig. 5a has multiple ultrasmall cells directly attached to its cell envelope that appear to be in the process of dividing (Fig. 5b,e,f), raising the possibility that CPR/DPANN replication is correlated with host attachment (discussed in the next section). Overall, cryo-TEM imaging of TFF-concentrated groundwater shows that some ultrasmall cells in Ag groundwater—which are likely to be CPR or DPANN organisms on the basis of size—are episymbionts of prokaryotic hosts, attaching through pili-like structures.
Fig. 5. Imaging of Ag groundwater cells concentrated with tangential flow filtration.
a, Image of larger host cell (white arrow) with multiple ultrasmall cells (magnified in b and e) attached. b, Magnification of the indicated area in a (white box) showing a chain of ultrasmall cells attached to the surface of the larger host cell. c, Magnification of the indicated area in b (black box) showing the contact region between two ultrasmall cells and the pili-like appendages decorating their surfaces (white arrowheads). d, Magnification of the indicated area in b (white box) showing the contact region between an ultrasmall cell and host cell. Pili-like appendages are indicated by white arrowheads. e, Magnification of the indicated area in a (white box) showing a single ultrasmall cell decorated by pili-like appendages (white arrowheads) attached to a host cell. Attachment may be mediated by pili-like appendages that extend from the ultrasmall cell into the host cell (dashed white boxes). f,g, The membrane from the ultrasmall cell in e (black box), with the membrane structure showing a clear periodicity measured to be 10 nm (f) as well as a periodicity of 3.8 nm that is evident in Fourier space (g; white arrows indicate repeating structure spacings). h, Image of a host cell with a single ultrasmall cell attached. Several pili-like appendages extend from the ultrasmall cell into the host cell (dashed white boxes and arrowheads). i,j, Magnification of the indicated area in h (white box) showing the host cell envelope, the outer layer of which exhibits a periodicity of 3.2 nm; a Fourier-transformed image is shown (j; white arrows indicate repeating structure spacings). k,l, Magnification of the indicated area in h (black box) showing the ultrasmall cell envelope, which also exhibits a periodicity of 3.2 nm in the outer layer; a Fourier-transformed image is shown (l; white arrows indicate repeating structure spacings). For d and e, the orange arrowheads indicate lines of high density observed at the contact interface. Scale bars, 1 μm (a), 200 nm (b, e and h) and 50 nm (c, d, f, i and k).
Extended Data Fig. 6. Zoomed in view of cryo-TEM images of ultra-small cells connected to host cells with pili-like appendages in Ag groundwater (Fig. 5) concentrated by tangential flow filtration.
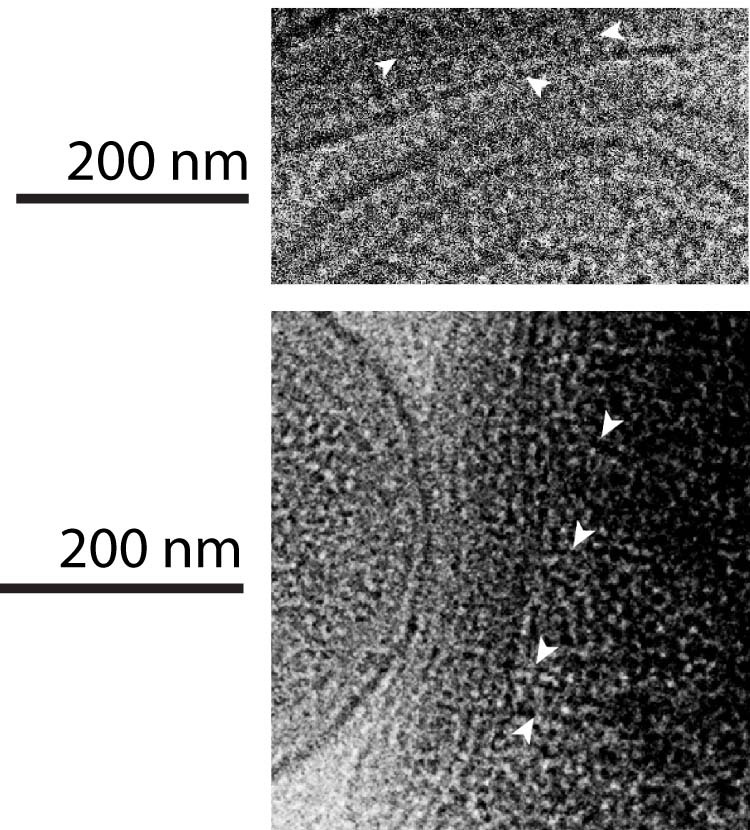
White arrows indicate pili-like appendages extending into the host from the ultra-small cell.
An important question regarding the biology of CPR bacteria relates to the nature of their cell envelope and the degree to which it resembles that of their host cells. Genomic analysis indicates that CPR bacteria cannot de novo synthesize fatty acids4 but do possess fatty-acid-based membrane lipids46, raising the possibility that CPR bacteria receive lipids or lipid building blocks from host organisms. The ultrasmall cell in Fig. 5e has a surface layer with a periodicity of 3.8 nm and 10 nm, but lacks an outer membrane expected for Gram-negative bacteria (Fig. 5f,g), consistent with previous cryo-TEM images of groundwater CPR bacteria45. Meanwhile, the host’s cell envelope appears to have two lipid layers, suggesting a Gram-negative structure (Fig. 5d,e). In Fig. 5h, from a different ultrasmall cell–host pair, we also resolve two lipid layers in the host cell envelope and no outer membrane in the ultrasmall cell. Interestingly, in this case, a periodicity of 3.2 nm is detected in the outermost layers of both the host cell (Fig. 5i) and the attached ultrasmall cell (Fig. 5k), but it is unclear whether the outer layers of the two cells have the same structure. On the basis of an apparent lack of an outer membrane, the ultrasmall cells observed in Ag groundwater have cell envelopes that do not resemble those of Gram-negative bacteria, but seemingly can attach to Gram-negative hosts.
Host attachment and replication of CPR/DPANN cells
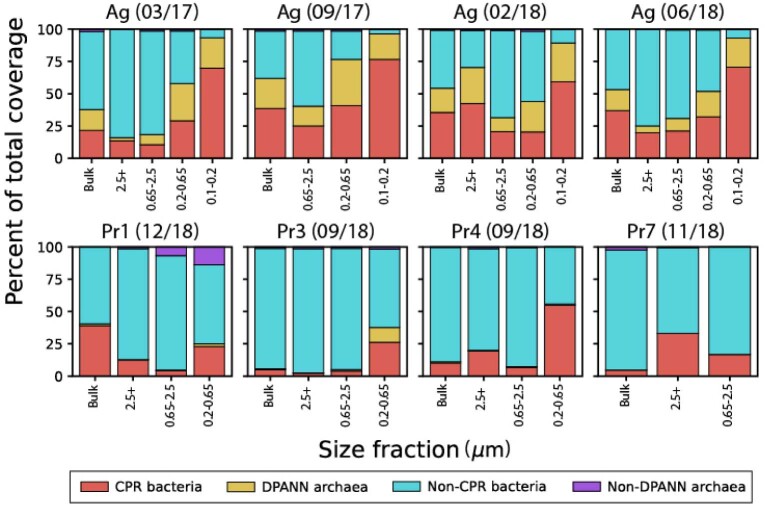
Imaging of likely CPR/DPANN organisms directly attached to host cells led us to investigate how widespread physical attachment is across the diversity of both radiations. We analysed the distribution of CPR/DPANN organisms among size fractions across five sites, which should reflect two factors—cell size and attachment to host cells. Most microorganisms outside the CPR and DPANN groups are present in the 0.65–2.5 µm or 2.5+ µm fractions (Fig. 4b). Owing to their small cell size (average ~0.2 µm diameter47), a CPR/DPANN cell present in the 2.5+ µm or 0.65–2.5 µm fraction is probably attached to a larger organism, whereas a CPR/DPANN cell present in the 0.1–0.2 µm fraction is probably unattached. Substantial coverage of CPR/DPANN genomes in the 2.5+ µm and 0.65–2.5 µm fractions indicate that a fair number of CPR/DPANN cells retain host attachment throughout the filtration process, and we consider it probable that pili penetrating from ultrasmall cells into the host (Fig. 5) are strong enough to resist disruption. We therefore consider the distribution of CPR/DPANN organisms among size fractions as indicative of the degree of host attachment.
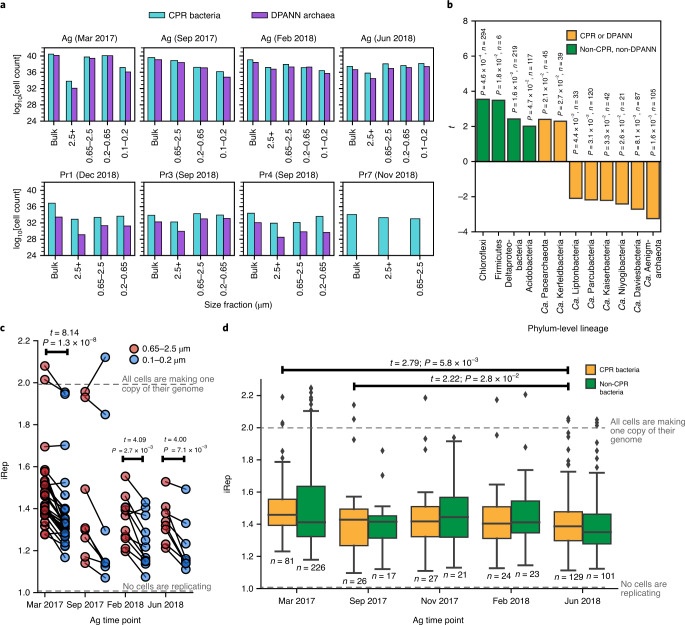
To assess the distribution of organisms among size fractions, the absolute number of cells represented by each genome was estimated from the genome relative abundance and the mass of DNA extracted (Methods). We observed high cell counts (>1028 cells) of CPR and DPANN genomes in 2.5+ µm and 0.65–2.5 µm fractions (Fig. 6a), representing a diverse range of lineages (Supplementary Table 7). In the case of Ag on March 2017 and September 2017, cell counts of diverse CPR and DPANN genomes (Extended Data Fig. 7) were several orders of magnitude higher in the 0.65–2.5 µm fraction than in the 0.1–0.2 µm fractions (Fig. 6a). These cell count distributions suggest that a host-attached lifestyle is common across diverse CPR and DPANN lineages and across groundwater sites.
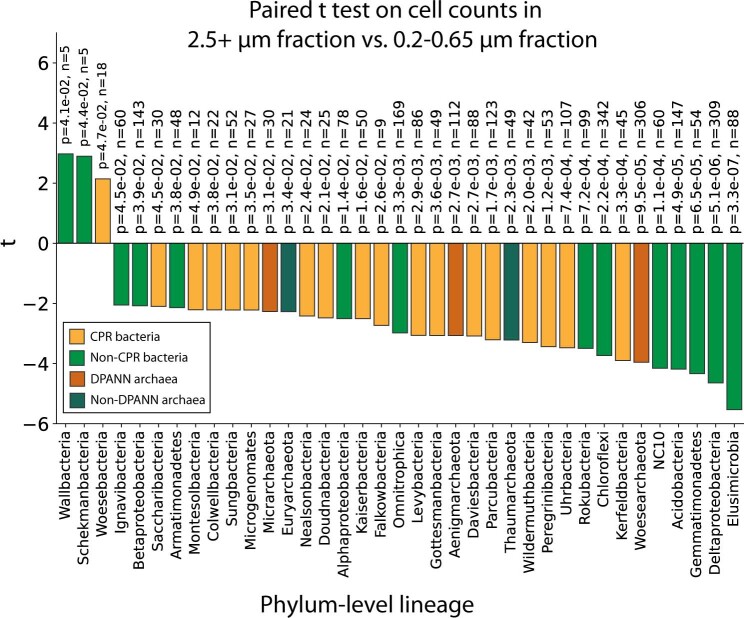
Fig. 6. Analysis of host attachment and growth rates of CPR/DPANN organisms.
a, Estimated cell counts (log transformed) for all size fraction data collected in this study. Each size fraction shown corresponds to a single sampling event. It was logistically infeasible to perform size filtration at some sites, and some filters collected did not contain enough biomass for DNA sequencing. b, Results from a two-sided paired t-test on estimated cell counts of genomes in the largest (2.5+ µm) and smallest (0.1–0.2 µm) size fractions after serial size filtration of Ag groundwater. A positive t statistic indicates enrichment of cells in the 2.5+ µm compared with the 0.1–0.2 µm fraction. Values listed above each bar are the calculated P value and sample size (n, number of genomes tested) for each phylum-level lineage. c, Calculated iRep values for CPR bacteria genomes in the 0.65–2.5 µm fraction versus the 0.1–0.2 µm fraction, across all Ag sampling points. n = 28 (March 2017), n = 8 (September 2017), n = 11 (February 2018) and n = 8 (June 2018) genomes tested. Note that iRep values represent the average replication state of the cell population represented by a genome. An iRep value of 1.0 indicates that, on average, no cells in the population are actively replicating, whereas an iRep value of 2.0 indicates that, on average, every cell is actively creating one copy of its genome. The statistically significant results (P < 0.05) of a two-sided paired t-test on iRep values between the two size fractions are shown above the box plots. Note that the November 2017 time point was excluded because only bulk filtration (no size filtration) was performed. d, Calculated iRep values for Ag bacteria caught in the bulk 0.1 µm filter (whole-community filtration). The statistically significant results (P < 0.05) of independent two-sided t-tests on iRep values of CPR bacteria between all possible pairs of sampling points are shown above the box plots. For the box plot, the centre line is the median; the top and bottom lines are the first and third quartiles, respectively; and the whiskers show 1.5× the interquartile range; individual dots are outliers; n values (number of genomes tested) are indicated on the plot.
Extended Data Fig. 7.
Relative coverage for CPR bacteria, non-CPR bacteria, DPANN archaea, and non-DPANN archaea genomes in all size fractions sequenced in this study.
For most CPR and DPANN lineages, estimated cell counts were significantly higher in the 0.1–0.2 µm fractions compared with the 2.5+ µm fractions, whereas the other bacterial and archaeal lineages exhibited the reverse trend (paired t-test; Fig. 6b). Two notable exceptions are the CPR lineage ‘Candidatus Kerfeldbacteria’ and the DPANN lineage ‘Candidatus Pacearchaeota’, which were enriched in the 2.5+ µm fraction relative to the 0.1–0.2 µm fraction (paired t-test, P = 0.027 and 0.021; Fig. 6b), indicating that a high fraction of these populations is host-attached and/or the attachment is more resistant to the disruptive effects of filtration compared with other CPR/DPANN lineages. Ca. Pacearchaeota genomes encode especially minimal metabolic capacities among DPANN lineages (Fig. 3b), suggesting a heavy dependence on host resources48. An additional CPR lineage, ‘Candidatus Woesebacteria’, was found to have significantly higher cell counts in the 2.5+ µm fraction versus the 0.2–0.65 µm fraction (Extended Data Fig. 8).
Extended Data Fig. 8. Results from a paired t-test (two-tailed) on estimated cell counts of genomes in the 2.5+ µm versus the 0.2–0.65 µm size fractions after serial size filtration.
A positive t statistic indicates enrichment on the 2.5+ µm compared to the 0.2–0.65 µm size fraction. Values listed by each bar are the calculated p value (top value) and sample size (bottom value) for each phylum-level lineage.
Cryo-EM images of dividing, host-attached ultrasmall cells (Fig. 5a,b) suggest that attachment to a host may stimulate CPR/DPANN cell division. To investigate this hypothesis, we calculated instantaneous replication rates (iRep values49) for CPR genomes in Ag groundwater (archaeal genomes were excluded as archaeal replication is not generally bidirectional). For reference, iRep = 1.0 indicates that, on average, no cells represented by a genome are actively replicating, whereas iRep = 2.0 indicates that, on average, every cell represented by a genome is creating one copy of its genome. We found that at three Ag sampling points—March 2017, February 2018 and June 2017—CPR organisms exhibit significantly higher replication rates in the 0.65–2.5 µm fraction than the 0.1–0.2 µm fraction (Fig. 6c), suggesting that host-attached CPR bacteria consistently exhibit a higher replication rate than non-host-attached CPR bacteria. We found that CPR bacteria as a whole (measured in the bulk filtered community) exhibited higher replication rates during the height of the 2016–2017 rainy season (March 2017) and the beginning of the next rainy season (September 2018) compared with during the height of the 2018 dry season (June 2018; Fig. 6d). Significant differences in bulk filtration replication rates were not observed between any point and the height of the 2017–18 rainy season (February 2018; Fig. 6d), which may be explained by the >25 cm more rainfall during the 2016–2017 rainy season compared with during the 2017–2018 rainy season43. Together, these findings support the deduction that CPR cell replication is stimulated by host attachment and may be more prevalent during the rainy season compared with the dry season.
Discussion
We sampled one agricultural and seven pristine groundwater sites in Northern California that are situated in a range of rock types and sourced from multiple aquifers. We recovered a total of 746 draft quality CPR and DPANN genomes that derive from most of the major lineages within both radiations and from two apparently new phylum-level lineages within the CPR, hereafter named ‘Candidatus Genascibacteria’ and ‘Candidatus Montesolbacteria’. To our knowledge, only two previous studies have recovered and compared CPR bacterial genomes across multiple groundwater sites21,50, and neither reported DPANN genomes. Very little species-level overlap (defined as >95% ANI) exists between genomes recovered from this study and previous studies of Crystal Geyser22,39 and Rifle2,19 aquifers, a finding that may reflect a combination of species adaptation to different geochemical conditions of the groundwater system51–53, bottleneck effects and/or founder effects. Our findings suggest that characterization of microbiomes of additional groundwater sites—using 0.1 µm filters rather than 0.22 µm filters and binning of MAGs to capture maximum CPR/DPANN diversity—is likely to reveal further diversity in the CPR and DPANN radiations.
The pristine sites that we sampled serve as sources of local drinking water. Notably, at the time of sampling, the Pr2 site (Rattlesnake Spring), which has been a popular source of public drinking water for over a century, contained more than 30% CPR bacteria and 3% DPANN archaea, raising the possibility that CPR/DPANN organisms in groundwater are the source for human-associated members. CPR bacteria have been detected in multiple human body sites and correlated with inflammatory bowel disease28, vaginosis54, periodontitis55–57 and herpes viral titres30,58, and DPANN archaea have been detected in lung fluids31. However, few genomes of human-associated CPR or DPANN organisms exist, giving limited information about their role in human microbiomes and their relationship with environmental counterparts31. One recent study found remarkably low variation and high synteny between human-associated and groundwater Saccharibacteria59, suggesting the possibility that drinking water is a source of CPR bacteria in the human oral cavity25–27. However, all of these human-associated Saccharibacteria genomes derive from people who use tap water (although CPR bacteria can persist in drinking water after treatment25–27) rather than groundwater as a drinking source. To investigate whether groundwater is a source of human-associated CPR bacteria, it will be necessary to sequence groundwater sites together with the microbiomes of specific humans who use the groundwater versus tap water as their primary source of drinking water.
The Ag groundwater microbial community, which includes organisms from most CPR and DPANN lineages, is extremely stable at the strain level (>99% ANI). This stability may be due to consistent, heavy input of carbon and nitrogen from agricultural waste (cow manure) collected on-site in lagoons, dried piles and used to fertilize the on-site corn field33. Although the Ag community composition is stable, increases in community metabolic capacities and CPR bacterial replication rates occur during rainy seasons. Several factors may contribute to these changes during the rainy season: the onset of more anoxic conditions in the groundwater, greater runoff from agricultural waste piles, increased volume of and changes in microbial composition of the cow manure after calves are born in the spring, and soil changes associated with the adjacent corn field that supplies much of the recharge to the sampled Ag well33. Analysis of coabundance patterns over time indicated potential parasitic as well as commensal/mutualistic relationships between several CPR lineages and Planctomycetes, Ignavibacteria and Betaproteobacteria hosts, although more investigation is required to directly connect these observations to symbiotic relationships. These observations provide a starting point for targeted cultivation of CPR and DPANN organisms based on conditions favourable to growth of putative hosts. The recovery of diverse DPANN but few non-DPANN archaeal genomes from Ag groundwater poses the intriguing question of whether bacteria may serve as hosts for DPANN archaea.
An important aspect of our study was the use of cryo-TEM, a technique that has only rarely been applied to study environmental communities in a near-native state15,44,45, to observe physical attachment between the ultrasmall cells and hosts in Ag groundwater. Combined with genomic analysis of CPR and DPANN cell counts in serial size fractions, our data suggest that physical attachment to host organisms is a common lifestyle in both radiations, with the lineages Ca. Kerfeldbacteria in the CPR and Ca. Pacearchaeota within DPANN exhibiting particularly strong physical attachment to hosts relative to other CPR and DPANN organisms. On the basis of replication-rate analysis and with cryo-TEM imaging of dividing host-attached ultrasmall cells, higher CPR instantaneous replication rates are associated with physical attachment to hosts, suggesting that the availability of host-supplied resources may stimulate replication of CPR organisms. One recent study instead concluded that there is no widespread attachment of CPR bacteria to hosts on the basis of failure to detect co-occurring CPR and host genomic signatures in SAGs47. However, we believe the incompleteness of the reported SAGs and the small absolute number of organisms analysed per site render the results inconclusive. Our study highlights the need for high-quality MAGs and high-resolution microscopy to assess interactions among community members in a robust fashion.
Methods
Groundwater sampling, chemistry measurements and surface geology determination
All groundwater sites were sampled at shallow depths (<100 m below the surface). Groundwater was pumped from each well using a submersible pump (Geotech Environmental Equipment) into a sterile container, and then pumped using a peristaltic pump into an apparatus that was custom built for filtering high volumes of water (Harrington Industrial Plastics) at a rate of 3.8–7.6 l min−1. Before filtration, at least 100 l of water was pumped to purge the well volume and to flush the system. Polyethersulfone membrane filter cartridges designed for high-volume filtration (Graver Technologies) from the ZTEC G series (0.1 µm and 0.2 µm), ZTEC B series (0.65 µm) and PMA series (2.5 µm) were used. When a sufficient volume of water had been filtered (400 l for bulk filtration and an additional 800 l for serial size filtration), filters were removed and stored on dry ice. Filters were stored in a −80 °C freezer until processed. The surface geology of each sampling site was determined from the California Department of Conservation’s 2010 geological map of California (https://maps.conservation.ca.gov/cgs/gmc/), rock fragments recovered during drilling (Pr4) and by on-site geological surveys. Pumped groundwater was shipped on dry ice to the UC Davis Analytical Laboratory for water chemistry measurements of electrical conductivity (EC), sodium adsorption ratio (SAR), total organic carbon (TOC), dissolved organic carbon (DOC), NH4-N, NO3-N, SO4-S (soluble S), HCO3, CO3, soluble Zn, Cu, Mn, Fe, Cd, Cr, Pb, Ni, K, Ca, Mg, Na, Cl and B. Water chemistry measurements are shown in Supplementary Table 5.
DNA extraction and sequencing
The plastic housing was removed from the filter cartridges under sterile conditions and the filters were retained for DNA extraction. To extract DNA, either a quarter or a half of a filter was placed in PowerBead solution from the Qiagen DNeasy PowerSoil kit (no bead-beating was performed), then vortexed for 10 min with massaging to remove cells from the entire filter surface. After vortexing, the filter was removed, solution C1 (Qiagen DNeasy PowerSoil Kit) was added to the PowerBead solution and the solution was placed in a 65 °C water bath for 30 min. The rest of the DNA extraction procedure was performed according to the Qiagen DNeasy PowerSoil kit manufacturer’s instructions, beginning with the addition of solution C2. Ethanol precipitation was performed to concentrate and purify the extracted DNA before sequencing. Genomic DNA was quantified using the Qubit dsDNA High Sensitivity assay and, when quantity permitted, DNA quality was assessed using agarose gel electrophoresis. Library preparation and sequencing were performed at the California Institute for Quantitative Biosciences’ (QB3) genomics facility and the Chan Zuckerberg BioHub’s sequencing facility. Libraries were prepared with target insert sizes of 400–600 bp. Samples were sequenced using 150 bp paired-end reads on either a HiSeq 4000 platform or a NovaSeq 6000 platform, with a read depth of ~10 Gbp per sample except for Ag March 2017 samples, which were sequenced at 150 Gbp.
Metagenomic assembly
BBTools (v.38.78) was used to remove Illumina adapters as well as PhiX and other Illumina trace contaminants60. Reads were trimmed using Sickle61 (v.1.33) using the default quality threshold of 20 (quality type set to sanger, which is CASAVA v.1.8 or higher). Each physical filter was considered to be an independent sample, that is, metagenomic reads from a single filter were assembled together, rather than coassembing total reads from all filters/size fractions. Assembly was performed using MEGAHIT (v.1.2.9) with the default parameters62. Assembled contigs were then scaffolded using the scaffolding function from IDBA-UD63 (v.1.1.3). Scaffold coverage values were calculated as the ratio of total length of mapped reads to the total length of the scaffold, using bowtie2 (v.2.3.5.1)64 for mapping. Only scaffolds of >1 kb in length were considered for gene prediction and genome binning. Gene prediction was performed using Prodigal (v.2.6.3) using the ‘meta’ option65 and genes were annotated using USEARCH66 (v.10.0.240) against the KEGG67,68, Uniref100 (ref. 69) and UniProt70 databases. 16S rRNA genes were identified using a custom HMM2 (16SfromHMM.py, available at GitHub (https://github.com/christophertbrown/bioscripts)) and insertions of 10 bp or greater were removed. Prediction of tRNA genes was performed using tRNAscan-SE71 (v.1.3.1).
Genome binning, curation, dereplication and coverage calculation
Scaffolds longer than 1 kb only were considered for protein annotation and binning. Scaffolds were binned on the basis of GC content, coverage, presence/copies of ribosomal proteins and single-copy genes, taxonomic profile, tetranucleotide frequency and patterns of coverage across samples. On ggKbase (https://ggkbase.berkeley.edu/), protein annotations were performed using USEARCH (v.10.0.240) against the KEGG, UniRef100 and UniProt databases as well as against an internal database comprised of publicly available genomes from NCBI. Scaffold taxonomic profiles were then determined on the basis of a voting scheme, whereby the winning taxonomic profile had to have more than 50% of protein ‘votes’ for each taxonomic rank on the basis of protein annotations. A combination of manual binning on ggKbase (https://ggkbase.berkeley.edu/) and automated binning using CONCOCT72 (v.1.1.0), Maxbin273 (v.2.2.7) and Abawaca2 (v.1.07) was used to generate candidate bins for each sample. The best bins were determined using DASTool34 (v.1.1.1) and manually checked using ggKbase to remove incorrectly assigned scaffolds according to the criteria listed above. Bacterial genomes were then filtered for completeness (>70%) using a set of 43 single copy genes previously used for the CPR2,19, and archaeal genomes were filtered using 48 single-copy genes for DPANN. Contamination was assessed using checkM74 (<10%; Supplementary Table 2). The program dRep35 (v.2.5.3) was used to dereplicate genomes from each site at 99% ANI (strain level), resulting in a representative set of 2,007 genomes across all sites. The median estimated genome completeness of each site’s representative set is over 90%, with 18–58% of each site’s raw reads mapping back to the representative set (Supplementary Table 1). Singlefold coverage values for genomes were calculated as the ratio of the total length of mapped reads (bowtie2 v.2.3.5.1) to the total length of the genome.
Phylogenetic classification
Genomes with a clear taxonomic classification on the basis of the internal ggKbase database (>50% of the genome sequence had a clear scaffold-level taxonomic winner, based on best matches of protein sequences to those in genomes of a taxonomically comprehensive database) were classified according to their predicted ggKbase taxonomy. For genomes without a clear predicted ggKbase taxonomy, phylogenetic analysis was performed using several marker sets as follows: concatenated ribosomal proteins (encoded by a syntenic block of genes and selected to avoid binning error chimaeras), rpS3 proteins and 16S rRNA genes (for CPR bacteria). Reference sequences for all of the phylogenetic trees were taken from previously published studies that recovered many high-quality CPR and DPANN genomes2,3,19,22.
The concatenated ribosomal protein set for bacteria includes 15 proteins (L2, L3, L4, L5, L6, L14, L15, L18, L22, L24, S3, S8, S10, S17 and S19), whereas the archaeal set includes 14 proteins (the bacterial set without S10, which is missing from many archaeal genomes). Ribosomal proteins were identified by searching predicted open reading frames (ORFs) against ribosomal protein databases using USEARCH66. For each individual ribosomal protein, hits and reference sequences were aligned to the Pfam HMM model using hmmalign from HMMer75 (v.3.3), alignments were converted from the Stockholm format to FASTA and insertions added by hmmalign were stripped. All individual ribosomal protein alignments were concatenated together, and concatenated sequences with an ungapped length of greater than 1,100 amino acid residues were combined with reference sequences to build a maximum-likelihood tree using IQ-Tree (v.1.6.12; iqtree -s <alignmentfile> -st AA -nt 48 -bb 1000 -m LG+G4+FO+I).
For rpS3 gene phylogenetic analysis, rpS3 genes were identified using a custom HMM with an HMM alignment score cut-off of 40 (ref. 36). Identified rpS3 genes were aligned with rpS3 reference sequences using mafft76 (using the default parameters) and columns with >95% gaps were removed with trimal77. The alignment was used to build a maximum likelihood tree using IQ-Tree (iqtree -s <alignmentfile> -st AA -nt 48 -bb 1000 -m LG+G4+FO+I).
For 16S rRNA gene phylogenetic analysis of CPR bacterial genomes, 16S rRNA genes were identified using a custom HMM2 (using 16SfromHMM.py, available at GitHub (https://github.com/christophertbrown/bioscripts)) and insertions of 10 bp or greater were removed (using strip_masked.py from https://github.com/christophertbrown/bioscripts). Sequences with lengths of >800 bp were used for phylogenetic analysis. SSU-align was used to align 16S sequences from this study with reference sequences from the previous studies mentioned above as well as CPR bacteria sequences from SILVA database78. The resulting alignment was used to build a maximum-likelihood tree using RAxML-HPC BlackBox79 (v.8.2.12) on the CIPRES Science Gateway80 with the general time reversible model of nucleotide substitution (raxmlHPC-HYBRID -T 4 -s infile -N autoMRE -n result -f a -p 12345 -x 12345 -m GTRCAT).
Genomes forming the new phylum-level lineages ‘Candidatus Genascibacteria’ and ‘Candidatus Montesolbacteria’ were identified on the basis of the following criteria: (1) they formed a monophyletic group in the 16S rRNA gene phylogeny; (2) 16S rRNA genes shared less than 76% sequence identity to the closest representatives; (3) they were also supported by the concatenated ribosomal protein phylogeny; and (4) more than one representative draft genome was available. A list of ‘Candidatus Genascibacteria’ and ‘Candidatus Montesolbacteria’ genomes and ANI with closest 16S rRNA hits from SILVA78 is provided in Supplementary Table 3.
Ordination analysis
Principal component analysis was performed on rpS3 relative coverage values and on the metabolic capacities of whole communities (the summed relative coverage values of genomes encoding a particular metabolic transformation). Principal component analysis was performed using the FactoMineR package81 and visualized using factoextra82. Relative abundance values were scaled to unit variance before the calculation of the principal components. NMDS analysis was performed on normalized read counts (reads per million total reads) for all genomes from Ag groundwater, based on read mapping with BBMap60. NMDS analysis was performed using the metaMDS function in the Vegan package for R83, using the default parameters. In brief, the data were transformed using Wisconsin double standardization of the square root of the matrix, followed by construction of a Bray–Curtis dissimilarity matrix, then an NMDS with 20 random starts. Finally, the results were scaled to maximize variation to the first principal component. Results were visualized using the ggplot2 package for R84.
Assessing index hopping between Pr1 and Pr7
Pr1 and Pr7 were the only pair of analysed sites that shared more than a few strains (44 pairs of genomes with >99% ANI). These two sites are separated by Putah Creek, a major hydrological feature, and so are unlikely to be fed from the same aquifer. Although DNA from Pr1 and Pr7 was sequenced on the same NovaSeq 6000 lane, we do not attribute this strain overlap to index hopping, as dual indexing was used and reads with mismatched indices were not analysed, reducing the already low incidence of index hopping (<2% of reads). Furthermore, although the 44 genome pairs share >99% ANI, they are not identical, differing in sequence by up to 10,000 bp per Mb of genome.
Genome and community-level metabolic predictions
To analyse the metabolic capacity of the sampled groundwater communities at both the genome and community level, the program METABOLIC41 (v.4.0) was used to search predicted ORFs against a curated set of KEGG, TIGRfam, Pfam and custom HMM profiles corresponding to key marker genes for biogeochemical cycling. For specific sets of proteins that are often misannotated due to high sequence similarity despite divergent function (for example, amoABC/pmoABC), an additional motif-validation step was performed in which sequences were searched for conserved residue patterns indicative of either amoABC or pmoABC. On the basis of the presence/absence of this manually curated set of marker genes, the presence/absence of metabolic capacities encoded by each genome was determined, and the number and relative abundance of genomes in the community that encode a metabolic capacity were calculated. The biogeochemical cycling diagrams shown in Fig. 4 and Extended Data Fig. 4 are based off this manually curated set of key marker genes.
In addition to marker gene analysis, METABOLIC was also used to evaluate the completeness of KEGG modules for key biogeochemical cycling processes. In brief, the capacity of a genome for a broad metabolic function (for example, carbon fixation) was determined using the following steps:
The presence/absence of relevant genes (for example, either the large or small RuBisCo subunit, phosphoribulokinase, phosphoglycerate kinase) was determined by profiling against a custom set of HMMs, utilizing Kofam-suggested cut-off values for Kofam HMMs and custom cut-off values for TIGRfam, Pfam and custom HMMs. Custom cut-offs were chosen by adjusting noise cut-offs and trusted cut-offs to avoid potential false-positive hits19.
The presence/absence of each reaction in the relevant KEGG module was determined by combinations of key genes (as defined by the KEGG database). For example, the KEGG reaction R00024 (the carboxylation of RuBP by RuBisCo) in the KEGG module M00165 (the Calvin–Benson–Bassham cycle) is considered present only if the genome contains a hit for either the large or small subunits of RuBisCo (KEGG entries K01601–K01602).
A given KEGG module was considered to be present if genes identified for >75% of the reactions in the module were present. This 75% cut-off was chosen to reflect the fact that MAGs, which are in most cases neither complete nor circularized (in our case, we have a 70% cut-off for genome completeness), will have incomplete metabolic pathways.
Finally, a genome was considered to have broad metabolic capacity (carbon fixation) if any relevant KEGG module was present (CBB pathway, 3HP cycle, 3HP/4HB cycle, Wood Ljungdahl pathway or reverse tricarboxylic acid cycle). The results from METABOLIC for each site are provided in Supplementary Tables 8–15.
Cryo-TEM sample preparation in the field
Cryo-TEM samples were prepared onsite at the Ag dairy farm on 5 February 2018. Approximately 30 l of pumped Ag groundwater was concentrated to a final volume of ~5 ml, using TFF (Millipore Pellicon Cassette Standard Acrylic Holder) with a 30 kDa ultrafiltration cassette (Millipore Pellicon 2 Biomax). Aliquots of 5 μl were taken directly from the suspensions and deposited onto 300 mesh lacey carbon coated Cu-grids (Ted Pella, 01895) that had been treated by glow discharge within 24 h. Grids were blotted with filter paper and plunged into liquid ethane held at liquid nitrogen temperatures using a portable, custom-built cryo-plunging device85. Plunged grids were stored in liquid nitrogen before transfer to the microscope and maintained at 80 K during acquisition of all datasets.
Cryo-TEM imaging
Imaging was performed using a JEOL–3100-FFC electron microscope (JEOL) equipped with a FEG electron source operating at 300 kV. An Omega energy filter (JEOL) attenuated electrons with energy losses that exceeded 30 eV of the zero-loss peak before detection by a Gatan K2 Summit direct electron detector. Dose-fractionated images were acquired with a pixel size of 3.41 Å px−1 using a dose of 7.27 e− Å−2 per frame. Data were collected using the Gatan Microscopy Suite (v.3.4.1) and SerialEM (v.3.7). Up to 30 frames per image were aligned and averaged using IMOD86 (v.4.9) and image contrast was adjusted in ImageJ (v.2.0.0).
Analysis of cell distribution across serial size filters
To analyse the distribution of Ag cells across size fractions, we needed to estimate total cell counts, whereas sequencing data can generate only relative abundance values (in the absence of an internal standard). We began with the general equation: total cell count of a genome = relative abundance from sequencing × microbial load, an approach that has been discussed and tested in depth previously87. Our method takes the form: c = x × l × m where c is the total cell count of a genome; x is the relative coverage of a genome; l is the total cell counts of all community members per ng of DNA in the community; and m is the ng of DNA extracted from the size fraction. The term l × m estimates microbial load, that is, the total cell count of all members in a community.
In our method, we utilize DNA yield (measured variable m in our equation) as an estimate of microbial load in a sample. DNA yield is an imperfect estimate of true microbial load for a number of reasons, including potential ploidy88 and bias in sequencing representation depending on the DNA extraction method89. However, there are also limitations and problems with other estimates of microbial load, such as flow cytometry-based cell counting90. Given that we extracted all samples in this study using the same DNA extraction kit and have fluorometry-based measurements of DNA yield (Qubit dsDNA HS Assay), we chose to use DNA yield as the best available measurement of microbial load.
Fluorometry-based quantification of DNA yield measures DNA mass (that is, the number of double-stranded DNA base pairs). Meanwhile, the relative abundance of a genome (relative coverage) is proportional to the relative fraction of total cells represented by the genome, rather than the relative fraction of total DNA represented by the genome. For example, a CPR genome with a relative abundance of 1% will constitute less than 1% of the total DNA yield from a groundwater community, owing to its smaller genome size than other members of the community. To account for genome-size-dependent DNA yield, we calculated how many microbial cells would correspond to 1 ng of DNA on the basis of the genome sizes of each genome recovered from the community (parameter l in our equation). The molecular weight of each genome calculated as number of base pairs × 650 Da per base pair. The relative coverage of a genome in a given size fraction was calculated as the total length of reads mapping to the genome divided by the total length of the genome (mapping was performed with bowtie2)64.
To find significant differences in cell counts between two given size fractions (that is, 2.5+ µm versus 0.1–0.2 µm), paired t-tests were performed on cell counts from each phylum with more than 5 representative genomes and with cell count distributions in each size fraction that did not deviate significantly from normality (assessed by plotting cell count distributions and performing a Shapiro–Wilks test).
iRep analysis
Instantaneous replication rates were calculated for Ag bacterial genomes using iRep49 (v.1.1.14) with a tolerance of three mismatches per read. Reads from each size fraction were mapped to the bacterial genomes using bowtie2 (ref. 64).
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Newick tree of concatenated ribosomal proteins from DPANN genomes.
Alignment of concatenated ribosomal proteins from DPANN genomes.
Newick tree of concatenated ribosomal proteins from CPR genomes.
Alignment of concatenated ribosomal proteins from CPR genomes.
Sequencing, assembly, and genome summary statistics.
Completeness and contamination estimates for all MAGs.
Relative abundance (coverage) of rpS3 marker genes.
MAGs in Candidatus Genascibacteria’ and ‘Candidatus Montesolbacteria’ lineages.
Groundwater chemistry measurements.
Taxonomy of all MAGs.
Relative abundance (coverage) of MAGs.
Metabolic profile of Ag groundwater community.
Metabolic profile of Pr1 groundwater community.
Metabolic profile of Pr2 groundwater community.
Metabolic profile of Pr3 groundwater community.
Metabolic profile of Pr4 groundwater community.
Metabolic profile of Pr5 groundwater community.
Metabolic profile of Pr6 groundwater community.
Metabolic profile of Pr7 groundwater community.
Estimated cell counts for MAGs.
Results of two-tailed paired t-tests on MAG cell abundances across size fractions.
NCBI accession numbers.
Acknowledgements
We thank E. Genasci, A. Book, K. Kritikos, K. Fults, D. Fults, P. Smith and J. Smith for access to their groundwater wells; C. J. Castelle, L. Valentin, B. Al-Shayeb, K. Lane, M. Olm, N. Oberleitner, R. Méheust, A. Jaffe, J. West-Roberts, A. Probst and D. Geller-McGrath for their assistance with field work; C. J. Castelle and A. Jaffe for assistance with archaeal and CPR phylogenetic analysis, respectively; and E. Montabana for help with collection of cryo-TEM data. C.H. was supported by a Camille and Henry Dreyfus Foundation Postdoctoral Fellowship in Environmental Chemistry. Funding for groundwater sampling and sequencing was provided by the Innovative Genomics Institute, the Allen Foundation and the Chan Zuckerberg BioHub.
Extended data
Author contributions
C.H. and J.F.B. designed the study. C.H., R.K. and I.F.F. collected samples, extracted DNA and performed manual binning. C.H. and R.K. performed on-site TFF filtration. C.H. performed automated binning, bin selection, bin curation and dereplication, phylogenetic analysis, abundance and community comparison analysis, metabolic analysis, cell count distribution analysis and on-site TFF filtration. M.L.W. prepared cryo-TEM grids and collected cryo-TEM data. R.K. performed ordination analysis. C.H. and J.F.B. drafted the manuscript. All of the authors reviewed the manuscript.
Data availability
NCBI accession numbers for metagenome reads and metagenome assembled genomes (BioProject: PRJNA640378) are provided in Supplementary Table 18. Metagenome assembled genomes are also available online (http://ggkbase.berkeley.edu/all_nc_groundwater_genomes; please note that it is necessary to register for an account by provision of an email address before download).
Code availability
Identification of 16S rRNA genes and removal of insertions was performed using the custom scripts 16SfromHMM.py and strip_masked.py, which are available in the ctbBio Python package (https://github.com/christophertbrown/bioscripts/blob/master/ctbBio/16SfromHMM.py and https://github.com/christophertbrown/bioscripts/blob/master/ctbBio/strip_masked.py). Identification of rpS3 genes was performed using an HMM trained as previously described36 on a published alignment of rpS3 sequences from across the tree of life3. The custom HMMs used to identify key genes in metabolic cycling are available in the METABOLIC program (https://github.com/AnantharamanLab/METABOLIC).
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Microbiology thanks Kirsten Kusel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41564-020-00840-5.
Supplementary information
is available for this paper at 10.1038/s41564-020-00840-5.
References
- 1.Rinke C, et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature. 2013;499:431–437. doi: 10.1038/nature12352. [DOI] [PubMed] [Google Scholar]
- 2.Brown CT, et al. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature. 2015;523:208–211. doi: 10.1038/nature14486. [DOI] [PubMed] [Google Scholar]
- 3.Hug LA, et al. A new view of the tree of life. Nat. Microbiol. 2016;1:16048. doi: 10.1038/nmicrobiol.2016.48. [DOI] [PubMed] [Google Scholar]
- 4.Castelle CJ, Banfield JF. Major new microbial groups expand diversity and alter our understanding of the tree of life. Cell. 2018;172:1181–1197. doi: 10.1016/j.cell.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Wrighton KC, et al. Fermentation, hydrogen, and sulfur metabolism in multiple uncultivated bacterial Phyla. Science. 2012;337:1661–1665. doi: 10.1126/science.1224041. [DOI] [PubMed] [Google Scholar]
- 6.Kantor RS, et al. Small genomes and sparse metabolisms of sediment-associated bacteria from four candidate phyla. mBio. 2013;4:e00708-13. doi: 10.1128/mBio.00708-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wrighton KC, et al. Metabolic interdependencies between phylogenetically novel fermenters and respiratory organisms in an unconfined aquifer. ISME J. 2014;8:1452–1463. doi: 10.1038/ismej.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson WC, Stegen JC. The reduced genomes of Parcubacteria (OD1) contain signatures of a symbiotic lifestyle. Front. Microbiol. 2015;6:713. doi: 10.3389/fmicb.2015.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castelle CJ, et al. Genomic expansion of domain archaea highlights roles for organisms from new phyla in anaerobic carbon cycling. Curr. Biol. 2015;25:690–701. doi: 10.1016/j.cub.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 10.He X, et al. Cultivation of a human-associated TM7 phylotype reveals a reduced genome and epibiotic parasitic lifestyle. Proc. Natl Acad. Sci. USA. 2015;112:244–249. doi: 10.1073/pnas.1419038112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross, K. L. et al. Targeted isolation and cultivation of uncultivated bacteria by reverse genomics. Nat. Biotechnol. 10.1038/s41587-019-0260-6 (2019). [DOI] [PMC free article] [PubMed]
- 12.Huber H, et al. A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature. 2002;417:63–67. doi: 10.1038/417063a. [DOI] [PubMed] [Google Scholar]
- 13.Wurch L, et al. Genomics-informed isolation and characterization of a symbiotic Nanoarchaeota system from a terrestrial geothermal environment. Nat. Commun. 2016;7:12115. doi: 10.1038/ncomms12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St John, E. et al. A new symbiotic nanoarchaeote (Candidatus Nanoclepta minutus) and its host (Zestosphaera tikiterensis gen. nov., sp. nov.) from a New Zealand hot spring. Syst. Appl. Microbiol. 10.1016/j.syapm.2018.08.005 (2018). [DOI] [PubMed]
- 15.Baker BJ, et al. Enigmatic, ultrasmall, uncultivated Archaea. Proc. Natl Acad. Sci. USA. 2010;107:8806–8811. doi: 10.1073/pnas.0914470107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golyshina OV, et al. ‘ARMAN’ archaea depend on association with euryarchaeal host in culture and in situ. Nat. Commun. 2017;8:60. doi: 10.1038/s41467-017-00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamm JN, et al. Unexpected host dependency of Antarctic Nanohaloarchaeota. Proc. Natl Acad. Sci. USA. 2019;116:14661–14670. doi: 10.1073/pnas.1905179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong J, Qing Y, Guo X, Warren A. ‘Candidatus Sonnebornia yantaiensis’, a member of candidate division OD1, as intracellular bacteria of the ciliated protist Paramecium bursaria (Ciliophora, Oligohymenophorea) Syst. Appl. Microbiol. 2014;37:35–41. doi: 10.1016/j.syapm.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Anantharaman K, et al. Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nat. Commun. 2016;7:13219. doi: 10.1038/ncomms13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spang A, Caceres EF, Ettema TJG. Genomic exploration of the diversity, ecology, and evolution of the archaeal domain of life. Science. 2017;357:eaaf3883. doi: 10.1126/science.aaf3883. [DOI] [PubMed] [Google Scholar]
- 21.Danczak RE, et al. Members of the candidate phyla radiation are functionally differentiated by carbon- and nitrogen-cycling capabilities. Microbiome. 2017;5:112. doi: 10.1186/s40168-017-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Probst AJ, et al. Differential depth distribution of microbial function and putative symbionts through sediment-hosted aquifers in the deep terrestrial subsurface. Nat. Microbiol. 2018;3:328–336. doi: 10.1038/s41564-017-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann M, et al. Predominance of Cand. Patescibacteria in groundwater is caused by their preferential mobilization from soils and flourishing under oligotrophic conditions. Front. Microbiol. 2019;10:1407. doi: 10.3389/fmicb.2019.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vigneron A, et al. Ultra-small and abundant: candidate phyla radiation bacteria are potential catalysts of carbon transformation in a thermokarst lake ecosystem. Limnol. Oceanogr. 2019;7:13219. [Google Scholar]
- 25.Pinto AJ, Schroeder J, Lunn M, Sloan W, Raskin L. Spatial-temporal survey and occupancy-abundance modeling to predict bacterial community dynamics in the drinking water microbiome. mBio. 2014;5:e01135-14. doi: 10.1128/mBio.01135-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bautista-de los Q, et al. Emerging investigators series: microbial communities in full-scale drinking water distribution systems—a meta-analysis. Environ. Sci. Water Res. Technol. 2016;2:631–644. [Google Scholar]
- 27.Bruno A, et al. Exploring the under-investigated ‘microbial dark matter’ of drinking water treatment plants. Sci. Rep. 2017;7:44350. doi: 10.1038/srep44350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehbacher T, et al. Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J. Med. Microbiol. 2008;57:1569–1576. doi: 10.1099/jmm.0.47719-0. [DOI] [PubMed] [Google Scholar]
- 29.Kowarsky M, et al. Numerous uncharacterized and highly divergent microbes which colonize humans are revealed by circulating cell-free DNA. Proc. Natl Acad. Sci. USA. 2017;114:9623–9628. doi: 10.1073/pnas.1707009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urbaniak C, et al. The influence of spaceflight on the astronaut salivary microbiome and the search for a microbiome biomarker for viral reactivation. Microbiome. 2020;8:56. doi: 10.1186/s40168-020-00830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koskinen K, et al. First insights into the diverse human archaeome: specific detection of archaea in the gastrointestinal tract, lung, and nose and on skin. mBio. 2017;8:e00824-17. doi: 10.1128/mBio.00824-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA. 2018;115:6506. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludington WB, et al. Assessing biosynthetic potential of agricultural groundwater through metagenomic sequencing: A diverse anammox community dominates nitrate-rich groundwater. PLoS ONE. 2017;12:e0174930. doi: 10.1371/journal.pone.0174930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieber CMK, et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat. Microbiol. 2018;3:836–843. doi: 10.1038/s41564-018-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olm MR, Brown CT, Brooks B, Banfield JF. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 2017;11:2864–2868. doi: 10.1038/ismej.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond S, et al. Mediterranean grassland soil C–N compound turnover is dependent on rainfall and depth, and is mediated by genomically divergent microorganisms. Nat. Microbiol. 2019;1:1356–1367. doi: 10.1038/s41564-019-0449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarza P, et al. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014;12:635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 38.Emerson JB, Thomas BC, Alvarez W, Banfield JF. Metagenomic analysis of a high carbon dioxide subsurface microbial community populated by chemolithoautotrophs and bacteria and archaea from candidate phyla. Environ. Microbiol. 2016;18:1686–1703. doi: 10.1111/1462-2920.12817. [DOI] [PubMed] [Google Scholar]
- 39.Probst AJ, et al. Genomic resolution of a cold subsurface aquifer community provides metabolic insights for novel microbes adapted to high CO2 concentrations. Environ. Microbiol. 2017;19:459–474. doi: 10.1111/1462-2920.13362. [DOI] [PubMed] [Google Scholar]
- 40.Olm MR, et al. Consistent metagenome-derived metrics verify and delineate bacterial species boundaries. mSystems. 2020;5:e00731-19. doi: 10.1128/mSystems.00731-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou, Z., Tran, P., Liu, Y., Kieft, K. & Anantharaman, K. METABOLIC: a scalable high-throughput metabolic and biogeochemical functional trait profiler based on microbial genomes. Preprint at bioRxiv10.1101/76164 (2019).
- 42.Speth, D. R., In’t Zandt, M. H., Guerrero-Cruz, S., Dutilh, B. E. & Jetten, M. S. M. Genome-based microbial ecology of anammox granules in a full-scale wastewater treatment system. Nat. Commun. 7, 11172 (2016). [DOI] [PMC free article] [PubMed]
- 43.Historical Rainfall Data for Years 1888 to 2020 (Modesto Irrigation District, accessed March 2020); https://www.mid.org/weather/historical.jsp
- 44.Comolli LR, Banfield JF. Inter-species interconnections in acid mine drainage microbial communities. Front. Microbiol. 2014;5:367. doi: 10.3389/fmicb.2014.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luef B, et al. Diverse uncultivated ultra-small bacterial cells in groundwater. Nat. Commun. 2015;6:6372. doi: 10.1038/ncomms7372. [DOI] [PubMed] [Google Scholar]
- 46.Probst, A. J. et al. Lipid analysis of CO2-rich subsurface aquifers suggests an autotrophy-based deep biosphere with lysolipids enriched in CPR bacteria. ISME J. 10.1038/s41396-020-0624-4 (2020). [DOI] [PMC free article] [PubMed]
- 47.Beam JP, et al. Ancestral absence of electron transport chains in patescibacteria and DPANN. Front. Microbiol. 2020;11:1848. doi: 10.3389/fmicb.2020.01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castelle CJ, et al. Biosynthetic capacity, metabolic variety and unusual biology in the CPR and DPANN radiations. Nat. Rev. Microbiol. 2018;16:629–645. doi: 10.1038/s41579-018-0076-2. [DOI] [PubMed] [Google Scholar]
- 49.Brown CT, Olm MR, Thomas BC, Banfield JF. Measurement of bacterial replication rates in microbial communities. Nat. Biotechnol. 2016;34:1256–1263. doi: 10.1038/nbt.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian R, et al. Small and mighty: adaptation of superphylum Patescibacteria to groundwater environment drives their genome simplicity. Microbiome. 2020;8:51. doi: 10.1186/s40168-020-00825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amalfitano S, et al. Groundwater geochemistry and microbial community structure in the aquifer transition from volcanic to alluvial areas. Water Res. 2014;65:384–394. doi: 10.1016/j.watres.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Ben Maamar S, et al. Groundwater isolation governs chemistry and microbial community structure along hydrologic flowpaths. Front. Microbiol. 2015;6:1457. doi: 10.3389/fmicb.2015.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnabosco C, et al. The biomass and biodiversity of the continental subsurface. Nat. Geosci. 2018;11:707–717. [Google Scholar]
- 54.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 55.Paster BJ, et al. Bacterial diversity in necrotizing ulcerative periodontitis in HIV-positive subjects. Ann. Periodontol. 2002;7:8–16. doi: 10.1902/annals.2002.7.1.8. [DOI] [PubMed] [Google Scholar]
- 56.Kumar PS, et al. New bacterial species associated with chronic periodontitis. J. Dent. Res. 2003;82:338–344. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 57.Liu B, et al. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS ONE. 2012;7:e37919. doi: 10.1371/journal.pone.0037919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewhirst FE, et al. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLean JS, et al. Acquisition and adaptation of ultra-small parasitic reduced genome bacteria to mammalian hosts. Cell Rep. 2020;32:107939. doi: 10.1016/j.celrep.2020.107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bushnell, B. BBTools Software Package (2014); http://sourceforge.net/projects/bbmap
- 61.Joshi, N. A., Fass, J. N. Sickle: a sliding-window, adaptive, quality-based trimming tool for FastQ files v.1.33 (2011).
- 62.Li D, Liu C-M, Luo R, Sadakane K, Lam T-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 63.Peng Y, Leung HCM, Yiu SM, Chin FYL. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012;28:1420–1428. doi: 10.1093/bioinformatics/bts174. [DOI] [PubMed] [Google Scholar]
- 64.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hyatt D, et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 67.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzek BE, et al. UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics. 2015;31:926–932. doi: 10.1093/bioinformatics/btu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res.45, D158–D169 (2017). [DOI] [PMC free article] [PubMed]
- 71.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alneberg J, et al. Binning metagenomic contigs by coverage and composition. Nat. Methods. 2014;11:1144–1146. doi: 10.1038/nmeth.3103. [DOI] [PubMed] [Google Scholar]
- 73.Wu Y-W, Simmons BA, Singer SW. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32:605–607. doi: 10.1093/bioinformatics/btv638. [DOI] [PubMed] [Google Scholar]
- 74.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- 77.Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller, M. A., Pfeiffer, W. & Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proc.2010 Gateway Computing Environments Workshop (GCE) 1–8 (IEEE, 2010).
- 81.Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 2008;25:1–18. [Google Scholar]
- 82.Kassambara, A. & Mundt, F. Factoextra: Extract and visualize the results of multivariate data analyses. R package version 1 (2017); https://www.rdocumentation.org/packages/factoextra/versions/1.0.7
- 83.Oksanen, J., Kindt, R., Legendre, P. & O’Hara, B. The vegan package version 2.4.2 (2017).
- 84.Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
- 85.Comolli LR, et al. A portable cryo-plunger for on-site intact cryogenic microscopy sample preparation in natural environments. Microsc. Res. Tech. 2012;75:829–836. doi: 10.1002/jemt.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mastronarde DN, Held SR. Automated tilt series alignment and tomographic reconstruction in IMOD. J. Struct. Biol. 2017;197:102–113. doi: 10.1016/j.jsb.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morton JT, et al. Establishing microbial composition measurement standards with reference frames. Nat. Commun. 2019;10:2719. doi: 10.1038/s41467-019-10656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soppa J. Polyploidy in archaea and bacteria: about desiccation resistance, giant cell size, long-term survival, enforcement by a eukaryotic host and additional aspects. J. Mol. Microbiol. Biotechnol. 2014;24:409–419. doi: 10.1159/000368855. [DOI] [PubMed] [Google Scholar]
- 89.Knudsen BE, et al. Impact of sample type and DNA isolation procedure on genomic inference of microbiome composition. mSystems. 2016;1:e00095-16. doi: 10.1128/mSystems.00095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kunin V, Copeland A, Lapidus A, Mavromatis K, Hugenholtz P. A bioinformatician’s guide to metagenomics. Microbiol. Mol. Biol. Rev. 2008;72:557–578. doi: 10.1128/MMBR.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Newick tree of concatenated ribosomal proteins from DPANN genomes.
Alignment of concatenated ribosomal proteins from DPANN genomes.
Newick tree of concatenated ribosomal proteins from CPR genomes.
Alignment of concatenated ribosomal proteins from CPR genomes.
Sequencing, assembly, and genome summary statistics.
Completeness and contamination estimates for all MAGs.
Relative abundance (coverage) of rpS3 marker genes.
MAGs in Candidatus Genascibacteria’ and ‘Candidatus Montesolbacteria’ lineages.
Groundwater chemistry measurements.
Taxonomy of all MAGs.
Relative abundance (coverage) of MAGs.
Metabolic profile of Ag groundwater community.
Metabolic profile of Pr1 groundwater community.
Metabolic profile of Pr2 groundwater community.
Metabolic profile of Pr3 groundwater community.
Metabolic profile of Pr4 groundwater community.
Metabolic profile of Pr5 groundwater community.
Metabolic profile of Pr6 groundwater community.
Metabolic profile of Pr7 groundwater community.
Estimated cell counts for MAGs.
Results of two-tailed paired t-tests on MAG cell abundances across size fractions.
NCBI accession numbers.
Data Availability Statement
NCBI accession numbers for metagenome reads and metagenome assembled genomes (BioProject: PRJNA640378) are provided in Supplementary Table 18. Metagenome assembled genomes are also available online (http://ggkbase.berkeley.edu/all_nc_groundwater_genomes; please note that it is necessary to register for an account by provision of an email address before download).
Identification of 16S rRNA genes and removal of insertions was performed using the custom scripts 16SfromHMM.py and strip_masked.py, which are available in the ctbBio Python package (https://github.com/christophertbrown/bioscripts/blob/master/ctbBio/16SfromHMM.py and https://github.com/christophertbrown/bioscripts/blob/master/ctbBio/strip_masked.py). Identification of rpS3 genes was performed using an HMM trained as previously described36 on a published alignment of rpS3 sequences from across the tree of life3. The custom HMMs used to identify key genes in metabolic cycling are available in the METABOLIC program (https://github.com/AnantharamanLab/METABOLIC).