Abstract
The biological functions of SIRT6 (e.g. deacetylation, defatty-acylation and mono-ADP-ribosylation) play a pivotal role in regulating lifespan and several fundamental processes controlling aging such as DNA repair, gene expression, and telomeric maintenance. Over the past decades, aberration of SIRT6 has been extensively observed in diverse life-threatening human diseases. In this comprehensive review, we summarize the critical roles of SIRT6 in the onset and progression of human diseases including cancer, inflammation, diabetes, steatohepatitis, arthritis, cardiovascular diseases, neurodegenerative diseases, viral infections, renal and corneal injuries, as well as the elucidation of the related signaling pathways. Moreover, we discuss the advances in the development of small molecule SIRT6 modulators including activators and inhibitors as well as their pharmacological profiles towards potential therapeutics for SIRT6-mediated diseases.
Keywords: SIRT6, deacetylation, defatty-acylation, mono-ADP-ribosylation, human diseases, small molecule modulators, activators, inhibitors
1. INTRODUCTION
The yeast silent information regulator two (Sir2), a NAD+-dependent histone deacetylase, is a key regulator of lifespan and cellular aging that was discovered in Saccharomyces cerevisiae.1 Sirtuin (SIRT) family is the mammalian homologues of yeast Sir2, which so far have identified seven members, from SIRT1 to SIRT7.2 They are characterized by highly conserved catalytic domain and NAD+ binding site, but varied N- and C-terminal. These proteins localize to different cellular compartments to exert a broad range of physiological functions. SIRT1 is present both in the nucleus and cytoplasm whereas SIRT2 predominantly resides in the cytoplasm. SIRT3, SIRT4 and SIRT5 are mitochondrial proteins, while SIRT6 and SIRT7 are exclusively localized in the nucleus.3,4 Among the seven SIRT family members, SIRT6 specifically modifies on histone H3 to regulate lifespan and several fundamental processes controlling aging such as DNA repair, gene expression, and telomeric maintenance.5–8 Studies using SIRT6-deficient mice revealed aging associated degenerative phenotypes that include severe lymphopenia, loss of subcutaneous fat, lordokyphosis, severe metabolic defects, and eventual animal death at about 4 weeks in a C57BL/6 background.9 Conversely, transgenic (TC) male mice with SIRT6 overexpression have a profoundly longer lifespan than the wild-type mice, indicating beneficial effects of SIRT6 in aging.10
SIRT6 contributes to DNA repair and SIRT6-knockout (KO) cells exhibit genomic instability and DNA damage hypersensitivity. In response to DNA double‐strand breaks (DSBs), SIRT6 associates dynamically with chromatin and acutely decreases global cellular H3K9 acetylation, which consequently forms a macromolecular complex with the DNA DSBs repair factor DNA‐PK (DNA‐dependent protein kinase) and promotes DNA DSBs repair.11 In addition, SIRT6 may recruit the chromatin remodeler sucrose nonfermenting 2 homolog (SNF2H) to DSBs, and is critical to enable SNF2H to open chromatin through H3K56 deacetylation, which is an early step in the DNA damage response.7 Recent studies also observed that c-Jun NH2-terminal kinase (JNK) phosphorylates SIRT6 to stimulate DNA DSBs repair in response to oxidative stress. Upon this posttranslational modification, SIRT6 recruits Poly(ADP-Ribose) Polymerase 1 (PARP1) to DNA breaks and activates it through mono-ADP-ribosylation, which in turn promotes homologous recombination (HR) and nonhomologous DNA end joining (NHEJ) and enhances DNA breaks repair.5,12,13 Additionally, SIRT6 deacetylating H3K9 and H3K56 on telomeric chromatin exhibits a role in telomeric maintenance. SIRT6-dependent deacetylation impacts the propagation of a specialized chromatin state at mammalian telomeres and consequently maintains proper telomere metabolism and function.14,15 In addition to the clear role in DSBs repair and telomeric maintenance, the main physiological function of SIRT6 as a histone deacetylase is regulating the expression of various genes such as glycolytic genes, Myc-target ribosomal protein genes, lipid metabolism genes, and NF-κB-dependent inflammatory genes.4,16–20 Gene expression regulated by SIRT6 is involved in maintenance and differentiation of stem cells.16,21–24 SIRT6 H3K56 deacetylation in embryonic stem cells (ESCs) may repress the expression of pluripotent genes (Oct4, Sox2 and Nanog) to regulate proper differentiation of ESCs.16,24 Given the important physiological function of SIRT6, it is not surprising that dysregulation of SIRT6 activity has been found associated with the onset of diverse pathologies. Several excellent reviews have provided comprehensive accounts for the cellular and physiological functions of SIRT6.4,25–27 Here, we summarize the critical roles of SIRT6 and the relevant signaling pathways in the onset and progression of various human diseases, including cancer, inflammation, diabetes, steatohepatitis, arthritis, cardiovascular diseases, neurodegenerative disease, viral infections, renal and corneal injuries. Moreover, we also discuss the advances in developing small molecule SIRT6 modulators and their in vitro and in vivo pharmacological profiles towards potential therapeutics for the SIRT6-mediated human diseases.
2. STRUCTURES
The SIRT family proteins consist of a conserved enzymatic core of about 250 amino acids, and are flanked with different length of N-terminal extension (NTE) and C-terminal extension (CTE).28,29 SIRT6 has a total length of 355 amino acids, including NTE residues 1–42, the enzymatic core domain residues 43–276 and the CTE residue 277–355.30 The NTE domain plays a pivotal role not only to the intrinsic catalytic activity of SIRT6, but also to the nucleosome binding which is essential for cellular chromatin association. Thus, the mutant with N-terminal deletion significantly abrogates its ability to regulate global levels of H3K9 and H3K56 acetylation in cells. In contrast, the CTE is not required for the catalytic activity but is largely indispensable for nuclear localization of SIRT6 protein. The terminal seven-amino acid sequence of CTE at residue 345 – 345PKRVKAK351 resembles a canonical nuclear localization signal, which plays an indistinguishable role with the CTE for proper nuclear localization. As expected, the enzymatic core domain is the foundation of the enzymatic activity and chromatin association of SIRT6, as the mutation H133Y at this domain results in the abrogation of these functions.30
The structures of SIRT6 protein have been solved in complex with ADP-ribose (ADPr) and myristoyl peptide by Denu and other groups (PDB codes: 3PKI, 3ZG6).31,32 Similar to other three-dimensional structures of SIRT family protein,29,33 SIRT6 exhibits two globular domains: a large Rossmann fold and a small splayed zinc-binding motif, which consist of eight α-helices and nine β-strands (Figure 1a). The Rossmann fold domain is composed of six parallel β-sheet (β1, β2, β3, β7, β8, and β9) sandwiched between two α-helices (α6 and α7) on one side and four α-helices (α1, α4, α5, and α8) on the other side. The zinc-binding domain is formed by three antiparallel β-sheet (β4, β5, and β6) connected to the extending loops of two α-helices (α3 and α6). As an NAD+ dependent enzyme, SIRT6’s particular large Rossmann fold renders two binding pockets for both NAD+ and the acyl substrates.31 Unlike other SIRT family protein, SIRT6 lacks a conserved, high flexible loop, instead of a stable single helix for NAD+ binding.31,33 The unique structure of SIRT6 provides the basis for binding to NAD+ with relative high affinity (Kd = 27 ± 1 μM) even in the absence of substrates. The adenosine moiety of NAD+ is bound in a site near α8 and β8 (A site), and nicotinamide (NAM) moiety located in open pocket around the N-terminal loop (C site). The acylated peptide myristoyl H3K9 (Myr-H3K9) was reported to bind to SIRT6 through many hydrogen-bonding interactions with the Rossmann fold, while the long-chain Myr moiety is accommodated in a hydrophobic channel towards the N-terminal loop. This channel is formed by hydrophobic residues from several flexible loops, including A11, P60, F62, W69, P78, F80, F84, V113, L130, L184 and I217 (Figure 1b).32 A continuous study form Denu group revealed relatively low in vitro deacetylase activity of SIRT6, which probably stems from a unique splayed configuration between the Rossmann fold and the zinc binding domain.31,34 However, long chain fatty acids (FAs) were found to stimulate the deacetylase activity of SIRT6. FAs competitively bind to the hydrophobic channel occupied by acyl moiety of acylated substrate, which may change the SIRT6 protein to catalytical favorable conformation, and thus enhance the deacetylase activity of SIRT6 by increasing the binding affinity of SIRT6 with an acetylated substrate up to 35 folds.34,35
Figure 1.
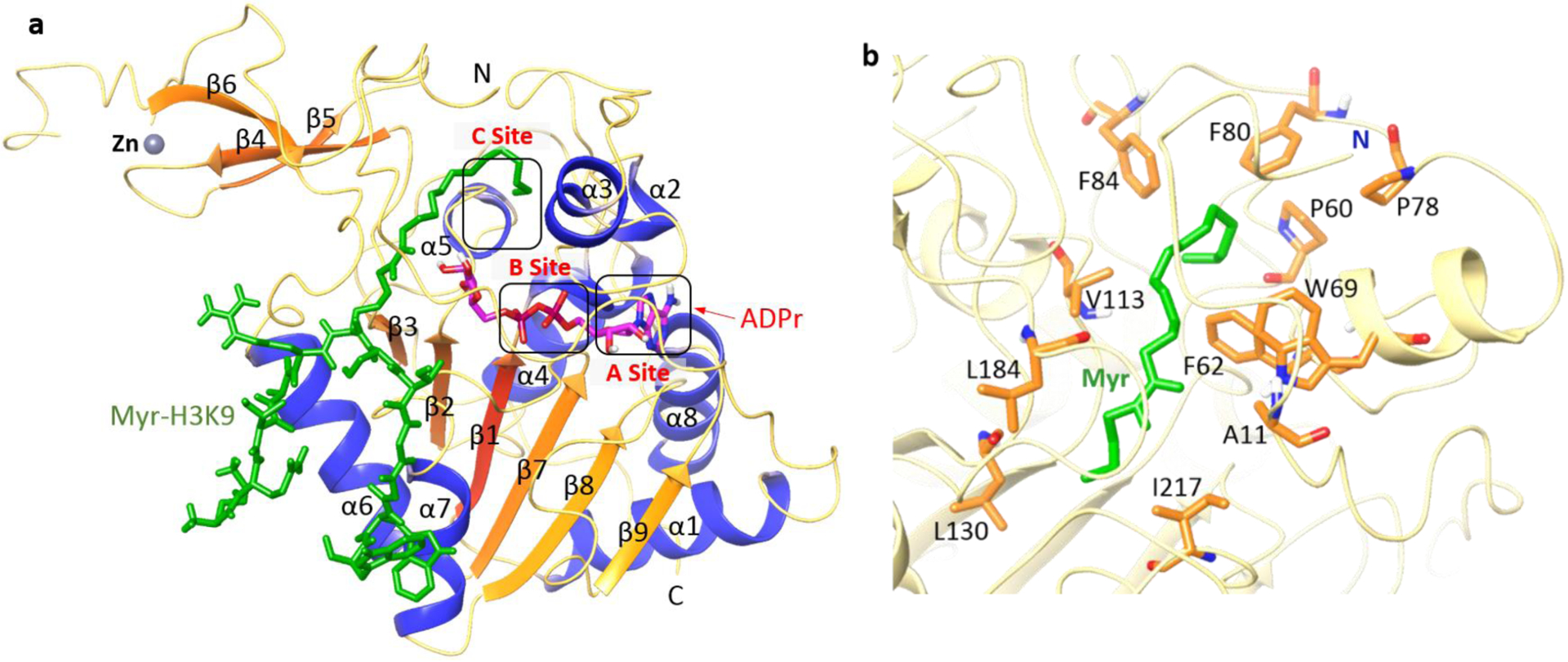
(a) Overall structure of SIRT6 with Myr-H3K9 (green) peptide and ADPr (magenta) bound (PDB code: 3ZG6). (b) Hydrophobic residues in SIRT6 that accommodate the Myr group.
3. ENZYMATIC FUNCTION
3.1. Deacetylation
Among various post-translational modifications, histone deacetylation is one of the most studied modification, closely associated with gene expression, transcriptional regulation, cell cycle control, DNA damage repair, telomeric maintenance and cellular metabolism.36–39 SIRT6 has been well-defined as an NAD+-dependent histone deacetylase targeting on specific sites on histone H3.4,40 As mentioned above, the in vitro deacetylase activity of SIRT6 is much lower than other members of Sirtuins family in spite of its significant cellular function.31 The stimulated catalytic efficiency by long chain FAs indicates that SIRT6 might be activated through an endogenous activation manner.4 Another explanation of the low in vitro activity is that SIRT6 exerts its deacetylation depending on the packaged nucleosomes instead of free histones.41 The H3K9 in telomeric chromatin was firstly biochemically characterized as a substrate of SIRT6. Removal of the H3K9 acetylation helps preventing the telomere from aberrant end-to-end chromosomal fusions and abnormal replication, which in turn maintains proper mammalian telomere metabolism.14 In addition, SIRT6 can interact with the NF-κB RELA submit and repress the expression of NF-κB-dependent gene by H3K9 deacetylation at NF-κB gene promoter.17 By H3K9 deacetylation, SIRT6 also suppresses the expression of multiple glycolytic genes, such as Ldhb, and acts as corepressor of transcription factor HIF1α to regulate glucose homeostasis.42
H3K56 has been determined to be another substrate of SIRT6 and the cellular efficiency of SIRT6 H3K56 deacetylation is higher than that catalyzed by SIRT1.15 H3K56Ac was found dramatically elevated both in SIRT6-KO cells and in vivo, and reconstitution with wild-type (WT) SIRT6 decreased the H3K56 hyperacetylation in SIRT6 deficient cells. The physiological function of H3K56 deacetylation was proven to be highly relevant to the regulation of DNA repair and the stability of telomeric chromatin and gene expression.7,15,43 Recently, SIRT6 was also identified to control the release of paused Pol II to modulate transcriptional pausing and elongation through the deacetylation of both H3K9 and H3K56.44 Besides the frequent H3K9 and H3K56 deacetylation, SIRT6 was demonstrated to promote deacetylation of a new substrate, H3K18, at pericentric chromatin, which is required for protecting against mitotic errors, genomic instability, and cellular senescence. The transcriptional co-repressor KRAB-interacting protein 1 (KAP1) was found to be a significant intermediate for the biological function of H3K18 deacetylation, although the exact mechanism needs further verification.45
Given the critical role of SIRT6 deacetylation on the physiological function, it is not surprising that some non-histone proteins as the substrate of SIRT6 could also be deacetylated to regulate a specific signaling pathway. For example, SIRT6 is associated with general control nonrepressed protein 5 (GCN5) and deacetylated it on K549 residue, and subsequently repressed function of transcriptional coactivator peroxisome proliferator-activated receptor-g coactivator 1- α (PGC-1α) to regulate the hepatic gluconeogenesis.46 SIRT6 has also been shown to bind to pyruvate kinase M2 (PKM2) and deacetylate it on K433 residue, thereby suppressing its oncogenic function.47 Deacetylation of Ku70 on lysine 542 by SIRT6 was found to block BCL2-associated X protein (Bax) mitochondrial translocalization to abolish the apoptosis of hepatocellular carcinoma (HCC) cell.48 SIRT6 may also regulate the enzymatic activity of nicotinamide phosphoribosyltransferase (NAMPT) and the secretion of extracellular NAMPT (eNAMPT) by modifying the acetylation level on K53 and K369, respectively, which results in the regulation of intracellular nicotinamide adenine dinucleotide phosphate (NADPH) levels and conferring resistance to oxidative stress.49 In addition, X-box-binding protein 1 (XBP1) was deacetylated by SIRT6 at Lys257 and Lys297 residues to promote its degradation and consequently attenuate ER stress-induced hepatic steatosis.50 Moreover, SIRT6-mediated activation of SOD2/Prdx6 pathway is pivotal for antidepressant response, which requires directing binding of SIRT6 to superoxide dismutase 2 (SOD2) and peroxiredoxin 6 (Prdx6), subsequently deacetylating them at Lys68/122 and Lys63/209, respectively.51 Recently, SIRT6 has also been observed to suppress β–Smad family members (SMADs) 2 and 3 through deacetylation at Lys54 and Lys333/378, respectively, to protect against liver fibrosis.52,53 Last but not least, SIRT6 was demonstrated to deacetylate p53 on K382,54 which may significantly affect various physiological functions such as cancer prevention and stress resistance.54–57
3.2. Mono-ADP-ribosylation
Although we know SIRT6 has robust histone deacetylation activity, the first enzymatic function of SIRT6 was defined as NAD+-dependent mono-ADP-ribosyltransferase.58 An early in vitro assay showed that the recombinant mouse SIRT6 (mSIRT6) could catalyze the transfer of radiolabel from [32P] NAD+ to the protein itself, a reaction of ADP-ribosyltransferase activity. High labeling efficiency (15%) was detected in WT of mSIRT6 and two key mutations abolished it, suggesting that the transfer of radiolabel was depending on the enzymatic mechanism and excluded covalent bonding of [32P] NAD+ with mSIRT6. Surprisingly, this function was accomplished by an intra-molecular mechanism, indicating the SIRT6 may auto-regulate its activity by utilizing ADP-ribosylation.58 A specific physiological function of SIRT6 mono-ADP-ribosylation evolved significant role in DNA DSBs repair. SIRT6 is physically associated with PARP1 and activates it through catalyzing ADP-ribosylation on K521 residue, thereby prompting the DSBs repair.5 In addition, SIRT6 ADP-ribosylation may also be relevant to cancer cell apoptosis. Van Meter et al. has reported that the mono-ADP-ribosyltransferase activity of SIRT6 is required to promote the apoptosis of cancer cells by the activation of p53 and p73 signaling cascades, although the exact site remains to be elucidated.59 More recently, several ADP-ribosylation substrates have been identified. SIRT6 was revealed to bind to 5’-UTR of long interspersed element 1 (L1) loci and mono-ADP ribosylate the nuclear corepressor protein, KAP1, and thus suppress the activity of L1 retrotransposon, which may be relevant in the course of aging.20 In addition, SIRT6 has been shown to ADP-ribosylate on the K312 residues of BAF170, a subunit of BAF chromatin remodeling complex, and then enhance the transcription of a subset of NF-E2–related factor 2 (Nrf2) responsive genes such as HO-1, upon oxidative stress.60 Moreover, SIRT6 mono-ADP ribosylation on the lysine demethylase KDM2A can locally increase H3K36me2 level at DNA damage sites and subsequently promote H3K9 tri-methylation and enhance DSBs repair.61
3.3. Defatty-acylation
It is well known that some SIRT proteins, such as SIRT5, are preferentially to hydrolyze long chain acyl substrate rather than acetylated lysine, indicating that SIRT6 with weak deacetylase activity may have alternative substrates as well.32 The in vitro deacetylase activity of SIRT6 is hundreds-fold less potent than its defatty-acylase activity. This attracts researchers to investigate the defatty-acylase activity of SIRT6 as well as its related physiological functions.34 Notably, SIR6 G60 is considered as a significant residue to differentiate the deacetylase and defatty-acylase activity of SIRT6 and the mutant G60A was found to abolish deacetylase but retain defatty-acylase activity on peptide substrates. Mechanistically, the fatty-acyl but not acetyl peptides reverse the conformational change induced by G60A mutation, and thus restore the binding affinity of SIRT6 G60A to NAD+.62 To date, the most pivotal role of SIRT6 defatty-acylation was discovered to modulate the secretion of secretory proteins. Pioneer works from Lin group has revealed that SIRT6 was able to promote the secretion of proinflammation cytokine, tumor necrosis factor-α (TNF-α), by catalyzing the NAD+ dependent long chain defatty-acylation.32 Residues K19 and K20 were confirmed to be the defatty-acylation sites of TNF-α. The regulation of TNF-α secretion by SIRT6 indicates that SIRT6 might be present in some secretory organelles when exerting its defatty-acylation function such as the endoplasmic reticulum (ER). This SIRT6-related regulation mechanism may be generalized to other secretory proteins such as insulin-like growth factor 1 (IGF1), which is in lower expression in SIRT6−/− mice. The regulation ability of SIRT6 defatty acylation also contributes to other hundreds of secretory proteins, including ribosomal proteins such as RPL17 and RPS7. It was demonstrated that defatty-acylation of SIRT6 may suppress the secretion of ribosomal proteins via inhibiting them sorting to the exosomes.62 In addition to secretory proteins, SIRT6 has been shown to regulate R-Ras2, a member of Ras family. Lysine fatty-acylation on R-Ras2 facilitates it to localize at the plasma membrane, subsequently interacts with an isoform of the catalytic subunit of PI3K, p110a, and then activates the PI3K/Akt signaling pathway to induce cell proliferation. SIRT6 was able to defatty-acylate R-Ras2 on the C-terminal residues. When removing the fatty-acyl group, R-Ras2 is suppressed to localize at plasma membrane, thus inhibiting downstream cell proliferation.63 Until now, histones have seldomly been determined as the substrates of SIRT6 defatty-acylation from some preliminary in vitro analysis. By using synthesized histone peptides as substrate, SIRT6 may efficiently catalyze the defatty-acylation of octanoyl, myristoyl and palmitoyl on H3K9 and myristoyl on H2B12.32 Moreover, a chemical biology study revealed that the SIRT6 actively removed fatty-acylation from H3K9, H3K18, and H3K27 in fatty-acyl-modified nucleosome, but showed sluggish defatty-acylation rate on H3K14, H3K36, H3K56, and H3K79, while the exact physiological functions remain to be further elucidated.64
4. HUMAN DISEASES
4.1. Cancer
Over the past decades, SIRT6 has been extensively investigated for its biological functions in diverse life-threatening human diseases such as cancer, inflammation, diabetes and neurogenerative disease.4,27,65–69 In the context of cancer, SIRT6 was considered as a double-edged sword due to its dual role of both tumor suppression and promotion, depending on the type of tumors (Table 2).70 Observations in the functions of controlling DNA damage repair, genomic stability, cellular metabolic homeostasis and apoptosis suggest that SIRT6 may protect against tumor growth.25,70,71 Specifically, a significant mechanism referring to the tumor suppressive role of SIRT6 was the inhibition of aerobic glycolysis (a.k.a. Warburg effect), an important factor for supporting the rapid growth of cancer cells, by co-repressing Myc transcriptional activity.72 Downregulated expression of SIRT6 in various human cancers is associated with increased tumor progression, and poor clinical prognosis has been observed in breast, hepatocellular, lung, ovarian and glioma cancers. However, other studies also revealed an opposite function of SIRT6 in human cancers. The elevated expression of SIRT6 along with poor clinical outcomes indicates the oncogenic function of SIRT6 in some other types of cancers such as hepatocellular and colon cancers.
Table 2.
The role of SIRT6 in various human cancers.
| Cancer type | Function | Cell line | In vivo | Mechanism(s) |
|---|---|---|---|---|
| HCC | suppressor | HepG2 | Inhibit phosphorylation of ERK1/2.77 | |
| HepG2, HCCLM3 | Transfected HepG2 xenograft | Reduce cyclin D1 and p-ERK expression.75 | ||
| HepG2, Huh7 | FOXA2 and SIRT6 coordinately suppress the expression of ZEB2.74 | |||
| HepG2, H1299, HCT116, U2OS, 293A | Transfected HepG2 xenograft | UBE3A-mediated SIRT6 degradation promotes the proliferation.78 | ||
| oncogene | PLC/PRF/5, SMMC-7721, Huh-7, SK-Hep-1 | Transfected SK-Hep-1 xenograft | Inhibit activation of Bax by H3K9 deacetylation.79 | |
| SK-Hep-1, Huh-7 | Deacetylation of Ku70 to enhance the Ku70-Bax interaction.48 | |||
| Hep3B, Huh-7, MHcc-97H, -97l, -lM6, -lM3, YY-8103, SK-hep-1 | Upregulate Bcl-2, pERK and decrease Bax.81 | |||
| Hep3B | Hep3B xenograft | Stimulate degradation of E-cadherin.82 | ||
| HepG2, Huh7, HeLa | Regulate FoxO3 activity.84 | |||
| Hep3B, HepG2, SNU475, SK-Hep1, SNU449, Huh-7 | SIRT6-KO Hep3B xenograft | Prevent DNA damage and cellular senescence.80 | ||
| HepG2, Huh7 | HepG2 and Huh7 xenografts | Enable TGF-β1/H2O2 /HOCl.83 | ||
| Colon cancer | suppressor | HCT116, RKO | HCT116 xenograft | USP10 inhibits c-Myc through SIRT6 stabilization.87 |
| RKO, HT-29, SW620, COLO 205, HCT116 | MiR-34c-5p inhibits SIRT6 to activate JAK2/STAT3.90 | |||
| HCT116, HT29 | SW620 xenograft | Modulate PTEN/AKT signaling.89 | ||
| LoVo, HCT-116, SW48, HT-29, DLD1, SW480 | Transfected LoVo xenograft | Akt inactivation increases FoxO3a to elevate SIRT6 expression.88 | ||
| oncogene | HCT116, RCO | Promote EMT process.91 | ||
| Lung cancer | suppressor | A549 | SIRT6 plays radiosensitization effect on A549 cells.99 | |
| NCI-H1299, HCC827, A549 | Astragaloside IV sensitizes tumor cells to gefitinib via regulation of SIRT6.100 | |||
| A549 | Inhibit Twist1 expression.96 | |||
| A549 | Decrease expression of PKM, LDHA, and HK.98 | |||
| A549 | Transfected A549 xenograft | Inhibits HIF1α expression.97 | ||
| oncogene | A549, SPC-A1, GLC82, PC9 | Activate ERK1/2/MMP9 pathway.102 | ||
| A549 and H1299 | Drive EMT via transrepression of KLF4.104 | |||
| A549 | transfected A549 xenograft | MiR-186 inhibits SIRT6 to antagonize cancer progression.103 | ||
| Breast cancer | suppressor | MCF-7, MDA-MB-231 | MDA-MB-231 xenografts and orthotopic model | AKT phosphorylate SIRT6 to induce MDM2-mediated degradation.106 |
| MCF7, Hs578t | RUNX2-mediated repression of the SIRT6 regulates metabolic pathways.105 | |||
| oncogene | MCF-7 | Decrease FoxO acetylation and expression.109 | ||
| BT474, BT474-LapR | Regulate FoxO3 acetylation and lapatinib sensitivity.108 | |||
| MCF7, T47D, MDA-MB231, 293 | CSNK2A1 mediates phosphorylation of SIRT6.107 | |||
| Glioma | suppressor | T98G, U87MG, A172, U251, CCF-STTG1 | Suppress expression of RNA-binding protein PCBP2.115 | |
| U87-MG, T98G | Inhibit JAK2/STAT3 signaling pathway.112 | |||
| U87, T98, A172, U251 | MiR-33a suppresses SIRT6 facilitates tumor growth through apoptosis and oxidative stress resistance.113 | |||
| U251, U87, LN18, A172 | Downregulate NOTCH3 expression.110 | |||
| U251, U87 | Transfected U87 xenograft | FoxO3a upregulates SIRT6 to inhibit Warburg effect.114 | ||
| A-172, U-138 MG, U-251 MG | MST1 upregulates SIRT6 expression via FoxO3a.111 | |||
| Bone cancer | suppressor | SAOS-2, MG-63 | Downregulate N-cadherin.118 | |
| U2OS, MG-63 | FoxN3 transcriptionally regulates SIRT6, suppresses cancer migration and invasion.119 | |||
| U2-OS, MG-63 | Transfected U2-OS xenograft | MiR-654–5p inhibits tumor growth and represses SIRT6 expression.120 | ||
| oncogene | NCI-H929, U266, KMS-28, RPMI-8226/S, MM.1S | Transfected NCI-H929 xenografts | Inhibit MAPK pathway signaling via H3K9 deacetylation.116 | |
| U2OS, MG-63, Saos-2, 143B | Regulate ERK1/2/MMP9 pathway.117 | |||
| Skin Cancer | suppressor | SKMel-239, 501Mel, Mel888, SKMel-147, STCs, SKMel-28 | L-C-B, S6.2–7, and S4.1–1 xenografts | SIRT6 inhibition activates IGF-AKT to promote melanoma drug resistance.125 |
| MV3 | Transfected MV3 xenograft | FoxO3a‑SIRT6 axis suppresses aerobic glycolysis in melanoma.126 | ||
| oncogene | NHEK | SIRT6-WT and skin-specific homozygous SIRT6-KO mice | Repress AMPK signaling to promote COX-2 expression.121 | |
| A375, Hs 294T | SIRT6-KD induces G1-phase arrest and increase senescence-like type.122 | |||
| HKC, SCC13 | Sirt6 down-regulation reproduces the miR-34a pro-differentiation effects.127 | |||
| Cervical carcinoma | suppressor | HeLa | Driving apoptosis is mediated by activation p53 and p73 apoptotic signaling cascades.59 | |
| Fibrosarcoma | suppressor | HT1080 | Driving apoptosis is mediated by activation p53 and p73 apoptotic signaling cascades.59 | |
| Nasopharyngeal carcinoma | suppressor | 5–8 F, CNE1 | Inhibit NF-κB to induce apoptosis.128 | |
| Renal cancer | oncogene | 786-O | SIRT6 silence in RCC leads to G1/S phase arrest.129 | |
| Prostate cancer | oncogene | PC-3, DU145, 22RV1, LNCaP | SIRT6 knockdown leads to cell cycle arrest, increases apoptosis and DNA damage.130 | |
| Ovarian cancer | suppressor | SKOV3, OVCAR3 | Downregulate Notch 3 expression.132 | |
| oncogene | OVCAR3, OVCAR5 | Active β-catenin and increase EMT.133 | ||
| Pancreatic Cancer | suppressor | Panc-1, BxPc3, YAPC, ASPC-1, SW1990, psn1 | PDAC xenograft | SIRT6 regulates Lin28b through promoting histone deacetylation.131 |
| Leukemia | suppressor | Jurkat cells, RS4:11 | SIRT6-PARP1 affects HMGB1 polyADP-ribosylation and acetylation and promotes chemotherapy-induced autophagy.137 | |
| oncogene | U937, MOLM-14, MV4–11, HL60, HEL, THP-1, NOMO-1, OCI-AML2, OCI-AML3, NB4 | SIRT6-depleted xenograft | Depletion of SIRT6 enzymatic activity increases AMLcells’ vulnerability to DNA-damaging agents.134 |
4.11. Hepatocellular carcinoma (HCC)
SIRT6 was considered as tumor suppressor and highly relevant to the initiation and progression of hepatocellular carcinoma.73,74 The remarkable downregulation of SIRT6 mRNA and protein levels was observed in the human HCC tissue compared with normal adjacent tissue, and overexpression of SIRT6 was able to inhibit the proliferation of liver cancer cells in vitro and tumor formation in nude mice.74–76 Early studies by using genetic mouse models specific for liver cancer initiation revealed that transcriptional activation of SIRT6 may repress the anti-apoptotic activity of survivin through reduction of H3K9 and NF-κB activation, thus markedly impairing liver cancer development at the initiation stage.73 This apoptosis-related anticancer manner was regulated by other factors such as extracellular signal-regulated kinases (ERK). Overexpression of SIRT6 in HepG2 cells represses the phosphorylation of ERK1 and ERK2 (pERK1/2), as well as inhibits the expression of cyclin D1, hence to induce cancer cell apoptosis or the cycle arrest in the G1 phase.75,77 In addition, SIRT6 was also disclosed to directly interact with the mammalian forkhead transcription factor FOXA2 and coordinately inhibit the expression of tumor metastasis regulator, ZEB2, to decrease HCC cells proliferation and invasion.74 Notably, the tumor suppressive function of SIRT6 could be regulated by E3 ubiquitin ligase UBE3A.78 UBE3A ubiquitylates SIRT6 on K160 residue to trigger its degradation, and therefore exerts tumorigenesis function, including promoting the proliferative capacity, migration potential, and invasiveness of HCC cells.78
With respect to the role of SIRT6 in HCC, it is somewhat controversial. In other studies, aberrantly increased SIRT6 expression was also observed in HCC cell lines and clinical HCC tissues samples, and the high SIRT6 expression was significantly correlated with poor survival rate.79–81 Indeed, SIRT6 has been determined to play oncogenic role in HCC through several manners, including inhibiting Bax-mediated apoptosis,48,79,81 promoting epithelial–mesenchymal transition (EMT) and suppressing cellular senescence.80,82,83 Mechanistically, SIRT6 can suppress the activation of Bax either by direct H3K9 deacetylation at the promoter to potentiate apoptosis evasion, or by deacetylation of Ku70 to enhance the Ku70-Bax interaction, which finally block the mitochondrial translocation of Bax and decrease the apoptotic ratio of HCC cell.48,79 Opposite from the previous results,75,77 studies on other HCC cells (e.g. Huh-7) found upregulated expression of pERK and decreased Bax expression in response to SIRT6 overexpression.81 In SK-Hep-1 xenograft mouse model, SIRT6-knockdown (KD) observed significantly repressed tumor growth and induced apoptosis in vivo, which further demonstrated the oncogenic function of SIRT6 in HCC.79 In addition, SIRT6 downregulation is required for the chemosensitivity of HCC cells to doxorubicin treatment, which works through increasing the nuclear localization and activation of forkhead box O3 (FoxO3) to trigger pro-apoptotic target gene expression. When FoxO3 is absent, SIRT6 overexpression was found not able to prevent HCC cell death.84,85 Independently, SIRT6 was demonstrated to potentiate the EMT, which is considered as the leading cause of poor prognosis for HCC, by deacetylating Beclin-1 to promote of the autophagic degradation of E-cadherin.82 By preventing DNA damage and cellular senescence, SIRT6 also acts as a tumorigenic factor in HCC through regulating senescence-related signaling pathways such as the p16/Rb- and p53/p21-pathway, and TGF-β1/H2O2/HOCl.80,83 The effects induced by SIRT6 overexpression can be rescued by the MicroRNAs miR-125b, which suppresses the expression of SIRT6 by directly targeting the seed-matching region of its 3’UTR.86
4.1.2. Colon cancer
Extensive evidence supports that SIRT6 acts as a tumor suppressor in colon cancers. Early studies observed that decreased expression of SIRT6 was detected in the early stage of human colon cancer cells.72,87 It was further determined by an analysis of human colon tissues, showing downregulated expression of SIRT6, while patients with higher SIRT6 expression have a better overall survival and prognosis.88 For enacting the tumor suppressor role, SIRT6 positively regulates the expression of tumor suppressors PTEN and PIP2, as well as decreases expression of oncogenic AKT1, mTOR, cyclin d1, and c-myc.89 SIRT6 also acts as an intermediate factor that can be upregulated in the process of colon cancer inhibition. Specifically, ubiquitin-specific peptidase USP10 suppresses SIRT6 ubiquitination to promote the expression of SIRT6, and then antagonizes the transcriptional activity of the c-myc oncogene, thereby inducing colon cell cycle arrest and tumoral inhibition.87 Similarly, SIRT6 expression was found positively regulated by the transcriptional factor FoxO3a, which in turn activates Bax and mitochondrial pathway to promote apoptosis.88 On the contrary, SIRT6 was found downregulated by miR-34c-5p to promote colon cancer cell proliferation by activation of the JAK2/STAT3 signaling pathway.90
Opposite to its role as tumor suppressor, recent studies also revealed tumor promotive function of SIRT6.91 In the studies, SIRT6 was found to be higher expressed in the colon tumor tissues of 196 colon carcinoma patients compared to the non-tumor tissues, meanwhile poor prognosis and worse overall survivals were highly depended on the increased level of SIRT6. Mechanistic analysis suggests involvement of H3K9 deacetylation, by which SIRT6 not only interacts with snail to regulate EMT process, but also suppresses tet methylcytosine dioxygenase 1 (TET1) transcription to facilitate tumorigenesis and metastasis of colon cancer cells.91
4.1.3. Lung cancer
Lung cancer is by far the leading cause of cancer-related death worldwide and non-small cell lung cancer (NSCLC) accounts for about 85% of lung cancer cases.92 Elaborating of the relationship between SIRT6 and NSCLC makes great sense for discovering a new biomarker for tumor targeting therapy.93 Both the mRNA and protein SIRT6 levels were found downregulated in NSCLC tissue versus noncancerous tissue, and high expressions of SIRT6 appear to be favorable prognostic factors, indicating a tumor suppressor role of SIRT6 in NSCLC.94–96 SIRT6 was firstly shown to suppress cell proliferation through down-regulating the expression of oncogene Twist1 in NSCLC.96 Further, glycolysis-mediated tumor energy metabolism was observed to be regulated by SIRT6 in NSCLC. Overexpression of SIRT6 may decrease the expression of HIF1α and VEGF, promoting prolyl hydroxylase-2 (PHD) expression, consequently resulting in inhibition of angiogenesis and tumor growth.97 Other enzymes such as pyruvate kinase (PKM), lactate dehydrogenase (LDHA), and hexokinase (HK) were also shown to be downregulated in A549 cells to inhibit glycolysis, thus leading to G0/G1 phase arrest as well as cell apoptosis.98 Moreover, SIRT6 highly impacts the radiosensitivity and chemosensitivity to other stimuli. The overexpression of SIRT6 induces a radiosensitization effect in NSCLC, as well as increased chemosensitivity of NSCLC to gefitinib, thereby resulting in decreased cell growth and cell cycle arrest.98–100
Although SIRT6 is clearly a tumor suppressor from the above discussion, there exists a discrepancy from other studies.101 For instance, upregulation of SIRT6 may promote metastasis and invasion of NSCLC via activating the ERK1/2/MMP9 pathway, while SIRT6 downregulation by miR-186 suppresses the progression of A549 cell line, indicating the tumor promotive role of SIRT6.102,103 In addition, SIRT6 was observed to induce NSCLC cell migration and invasion through driving EMT by regulating snail-dependent transrepression of KLF4, similar to that in colon cancer.91,104 The mRNA analysis from NSCLS tissue has also revealed higher cytoplasmic SIRT6 level associated with more aggressive cancer, shorter overall survival and recurrence-free survival,93 further suggesting that SIRT6 may act as a oncogene in certain cases.
4.1.4. Breast cancer (BC)
Lower expression of SIRT6 was found in malignant BC tissues and the survival of BC patients was positively correlated with the expression level of SIRT6, suggesting a protective role of SIRT6 against tumorigenesis.105,106 SIRT6 manipulating breast cancer growth heavily relies on its phosphorylation.106,107 Early observation indicates that the kinase AKT1 phosphorylates SIRT6 at residue Ser338, subsequently induces the MDM2-mediated ubiquitination and degradation of SIRT6, thereby promoting tumorigenesis and drug-resistance in breast cancer.106 In agreement with this manner, overexpression of wild-type MDM2 but not its mutant decreases the endogenous SIRT6 in cells, whereas knocking down MDM2 increases SIRT6 abundance.106 Later studies from Bae et al. also suggest that SIRT6 ser338 phosphorylation regulated by protein kinase CK2α1/CSNK2A1 is involved in the progression of breast carcinoma. However, SIRT6 and phosphorylated SIRT6 might be both oncogene in this study due to the reasons that SIRT6 overexpression increases proliferation of MCF7 cells, whereas SIRT6 deletion or mutation at the Ser338 site inhibits its proliferation.107 In addition, SIRT6-mediated repression of glucose metabolism was found to be reversed by runt-related transcription factor 2 (RUNX2)-induced SIRT6 inhibition, which in turn suppresses mitochondrial respiration and promotes BC progression.105 Besides, SIRT6 confers resistance of breast cancer cells to chemotherapies such as paclitaxel, epirubicin, doxorubicin and lapatinib. Indeed, higher level of SIRT6 was observed in paclitaxel- and epirubicin-resistant MCF-7 cells than in the parental sensitive cells, while SIRT6-KO, depletion or catalytically inactive increases the sensitivity to chemotherapies.49,108,109 Mechanistic analysis suggests that acetylation of tumor suppressor FoxO3a finely tuned by SIRT6 may be relevant to the enhanced DNA repair of chemotherapies-induced DNA damage, thus leading to drug resistance.109
4.1.5. Glioma cancer
The available studies to date suggest that SIRT6 may play a single suppressive activity in glioma cancer, since only downregulated expression of SIRT6 was observed in human glioma tissue samples.110,111 SIRT6 overexpression largely induces the apoptosis of glioma cells by reducing oxidative stress and suppressing the activation of the JAK2/STAT3 signaling pathway in glioma.112 Consistent results indicate that the miR-33a may downregulate SIRT6. Loss of miR-33a can lead to upregulation of SIRT6, and the resulting apoptosis of U251 cells is derived from increased levels of Bax and cleaved caspase-8, decreased expression of Bcl-2, as well as the inhibition of the JAK2/STAT3 pathway.113 SIRT6-controlled glycolysis has also been explored in glioblastoma (GBM). FoxO3a may bind to the promoter region of SIRT6, and the subsequent enhanced expression of SIRT6 impairs aerobic glycolysis, thereby inhibiting the Warburg effect and blocking glioblastoma growth.114 On the upstream, the mammalian sterile 20-like 1 (MST1) positively regulates transcriptional factor FoxO3a to increase expression of SIRT6 to promote apoptosis of glioma cells.111 In addition, SIRT6 may also suppress the expression of the RNA-binding protein PCBP2 and notch receptor 3 (NOTCH3) signaling pathways to inhibit cancer cell proliferation, migration, and invasion in glioma.110,115
4.1.6. Bone cancer
Recent studies defined SIRT6 as oncogene to promote the migration and invasion in multiple myeloma and osteosarcoma.116,117 SIRT6 was observed to be notably overexpressed in osteosarcoma tissue, and high expression of SIRT6 is associated with adverse prognosis for osteosarcoma (OS) patients.117 ERK1/2 signaling pathway, which is highly related to the progression of HCC and NSCLC, plays a similar role to that in bone cancer. SIRT6 overexpression positively modulates the levels of pERK1/2, subsequently increases the activation of the mitogen-activated protein kinase kinase (MEK)–ERK1/2 pathway and MMP9 level, thereby promoting tumor growth. SIRT6-KD or treating with MEK inhibitors remarkably decreases the levels of phosphorylated ERK1/2 and MMP9, further suggesting that SIRT6 serves as tumor promoter by modulating ERK1/2/MMP9 pathway.117 Conversely, observation from Gao et al. by analyzing primary OS tissue samples collected from 112 patients indicates that the expression of SIRT6 is downregulated in OS cell lines and tissues, and the survival of patients with high expression of SIRT6 is significantly longer compared to patients expressing low levels of SIRT6.118 Mechanistically, N‑cadherin as a direct target of SIRT6 may be reduced to regulate progression of OS cells,118 meanwhile miR654–3p and forkhead Box N3 (FOXN3) acting on the upstream may transcriptionally downregulate SIRT6 to suppress migration and invasion in OS.119,120
4.1.7. Skin cancer
SIRT6 was observed to be upregulated, both at mRNA and protein levels, in skin cancer cell lines, and clinical tissue samples of human squamous cell carcinoma and melanoma.121,122 SIRT6-KD in A375 and Hs294T human melanoma cells significantly decreases cell growth, colony formation, induces G1-phase arrest and increases senescence-like type.122,123 Wang and colleagues have discovered that SIRT6 may suppress the growth of primary melanoma, while promote metastatic melanoma development relying on an autophagy-dependent way in vitro, indicating a complicated role of autophagy in melanoma.124 In addition, SIRT6 also regulates melanoma growth via the IGF-AKT signaling pathway. Downregulation of SIRT6 promotes H3K56 acetylation at the IGFBP2 locus, and consequently activates the IGF-1 receptor and downstream AKT signaling, which propels melanoma drug resistance.125 Enhanced aerobic glycolysis has previously been identified as an important hallmarks of tumor growth. In melanoma, FoxO3a expression was reported to be negatively correlated with the expression aerobic glycolysis-related genes such as HK, HK3, phosphofructokinase (PFK) fructobiphosphatase 3, PKM and LDHA. SIRT6 overexpression inhibits FoxO3a deficiency-induced upregulation of glycolysis and cancer cell proliferation, indicating a tumor suppression role of SIRT6 in melanoma through tumor glycolysis inhibition.126 Besides the oncogenic role in melanoma, Sirt6 positive cells were massively observed in premalignant actinic keratoses and cutaneous squamous cell carcinomas (SCCs), indicating the apparent oncogenic role of SIRT6 in SCCs. In the miR-34a-SIRT6 network, miR-34a is pivotal for squamous cell differentiation and is suppressed in skin and oral SCCs. Sirt6 is a critical target of miR-34a, and its down-regulation is sufficient to restore the pro-differentiation effects of miR-34a.127 In addition, SIRT6 may act as an oncogene of SCCs by repressing AMPK signaling to promote expression of pro-survival protein cyclooxygenase-2 (COX-2). UVB radiation activates the AKT pathway to upregulate SIRT6 and COX-2, in a manner that SIRT6 and AKT/COX-2 signaling cascade facilitates skin carcinogenesis.121
4.1.8. Other cancers
SIRT6 is highly correlated with cell apoptosis. As aforementioned, overexpression of SIRT6 may decrease anti-apoptotic Bcl-2 levels, increase pro-apoptotic Bax and cleaved caspase-3 levels, as well as inhibit NF-κB signaling, thus leading to apoptosis in various tumors such as nasopharyngeal and renal carcinoma.59,128,129 However, opposite results from Liu and colleagues revealed that SIRT6 was overexpressed in prostate tumors and SIRT6-KD was beneficial to cancer cell apoptosis and cell cycle arrest, unraveling a oncogenic function of SIRT6 in prostate cancer.130 In pancreatic ductal adenocarcinoma (PDAC), low SIRT6 expression was found associated with poor prognosis.131 SIRT6 down-regulation results in histone hyperacetylation at the promoter of Lin28b, which acts as a negative regulator of the let-7 microRNA. The following increased expression of Lin28b and the downstream let-7-target genes, HMGA2, IGF2BP1, and IGF2BP3 promote tumor growth in vitro and in vivo.131 In ovarian cancer, SIRT6 inhibits the proliferation of cancer cells by suppressing the expression of Notch 3 both at the mRNA and protein levels.132 However, SIRT6 may also promote the invasiveness of ovarian cancer cells via activation of EMT-related signaling pathway.133 The role of SIRT6 is controversial and not well understood in leukemia.134–137 A typical biological relevance comparison from Cagnetta et al. observed that acute myeloid leukemia (AML) patients with SIRT6 overexpression showed features of genomic instability and poor prognosis, and SIRT6-KD made AML blasts more sensitive to daunorubicin treatment in NSG mice. These results provide a proof-of-concept study for AML treatment by inhibiting SIRT6 to enhance their sensitivity to DNA-damage agents.70,134
4.2. Inflammation
TNF-α and NF-κB are two most important pro-inflammatory factors involved in inflammation.138,139 The unique regulatory roles of SIRT6 in inflammatory diseases mainly depends on the enzymatic activity on TNF- α and NF-κB, as well as some other factors within regulation function on TNF-α and NF-κB. As a defatty acylation enzyme, SIRT6 was found able to remove the fatty acyl modification on K19 and K20 of TNF-α, and thus to promote the secretion of TNF-α.32,62 In addition, a separate study also identified that SIRT6 could increase the mRNA level of inflammatory cytokines, such as TNF, by activating Ca2+ signaling through its deacetylation function, hence to enhance the production of TNF.140 Consistently, the secretion efficiency of TNF-α is obviously higher in SIRT6-WT than that in SIRT6-KO macrophages, and intracellular NAD+ is beneficial for TNF-α synthesis.32,141 The above results indicate SIRT6 is able to promote inflammation by increasing the secretion of TNF-α in an NAD+-dependent manner. However, a controversial study by Kawahara and colleagues revealed an anti-inflammatory role of SIRT6, through regulating of another pro-inflammatory factor, NF-κB.142 SIRT6 physically interacts with the NF-κB RelA submit and repress the expression of NF-κB-dependent gene by H3K9 deacetylation at NF-κB gene promoter.17 To our best knowledge, SIRT6 has been mainly defined as an anti-inflammation protein by inhibiting the expression of NF-κB target gene and other pro-inflammatory cytokines, whereas the pro-inflammation role has been little reported.142
Evidence suggests that SIRT6 overexpression was observed to suppress various inflammatory responses such as inflammations induced by collagen-induced arthritis (CIA) and hypoxia in human osteoblasts.143,144 Meanwhile, SIRT6-KD mice were found to evolve inflammation in organs. For instance, mice with the SIRT6 ablation exhibit chronic inflammation in the liver and increased inflammation in the adipose tissue.145,146 Mechanistically, SIRT6-KD in human umbilical vein endothelial cells (HUVECs) increases the expression of NF-κB and proinflammatory cytokines (e.g. IL-1β, IL-6, IL-8), whereas overexpression of SIRT6 is associated with decreased NF-κB transcriptional activity (Figure 2).147 Consistently, SIRT6 mitigates lipopolysaccharide (LPS)-induced HUVECs inflammatory responses through increasing the nuclear factor Nrf2 expression, consequently inhibits the release of TNF-α and IL‐6, and enhances the NQO1 and HO1.148 In addition, SIRT6 plays an anti-inflammatory role in chronic liver inflammation by physically interacting with c-Jun, subsequently deacetylating H3K9 at the promoter of proinflammatory genes, monocyte chemotactic protein-1 (MCP-1), IL-6 to repress the transcription of these genes.145 In gut inflammation, colitis is associated with decreased levels of intestinal SIRT6. SIRT6 may plays a protection role by preserving the expression of R-spondin 1 (Rspo1), an important trophic factor for intestinal epithelial cell growth.149 In the dextran sulfate sodium (DSS)-induced colitis model, SIRT6 overexpression suppresses the activated NF-κB and c-Jun signaling, thereby alleviating the colitis in terms of clinical manifestations, histopathological damage, loss of tight junction function and imbalanced intestinal microenvironment.150 For combating Propionibacterium acnes-induced inflammation, SIRT6 overexpression was found to downregulate the expression of toll‐like receptor (TLR4) and consequently inhibit the phosphorylation of nuclear NF-κB subunit, p65.151 Obesity epidemic always accompanies with macrophages accumulation and inflammation.152 SIRT6 deletion in macrophages promotes the activation of nuclear NF-κB and endogenous production of IL-6, which triggers STAT3 activation by phosphorylation to promote inflammation.153 A fat mouse model with SIRT6-KD driven by SIRT6f/f:Fabp4-Cre-KO exhibits increased expression of various inflammatory genes including F4/80, TNF-α, IL-6 and MCP-1 in both white and brown adipose tissues.154 The above findings provide a promising approach to inhibiting inflammation by activating SIRT6. Recently, He et al. demonstrated that an approached drug, Sitagliptin, exerts its anti-inflammation function via regulating the SIRT6-dependent signaling pathway. Specifically, sitagliptin could significantly suppress the production of MCP-1, IL-6 and IL-1β, but upregulate SIRT6 expression, both in TNF-α-stimulated endothelial cells and in vivo, while SIRT6-KO abolishes these effects. It has been revealed that the modulatory effect of sitagliptin on inflammation is mediated by certain SIRT6-related manner, although the exact target remains to be elucidated.155
Figure 2.
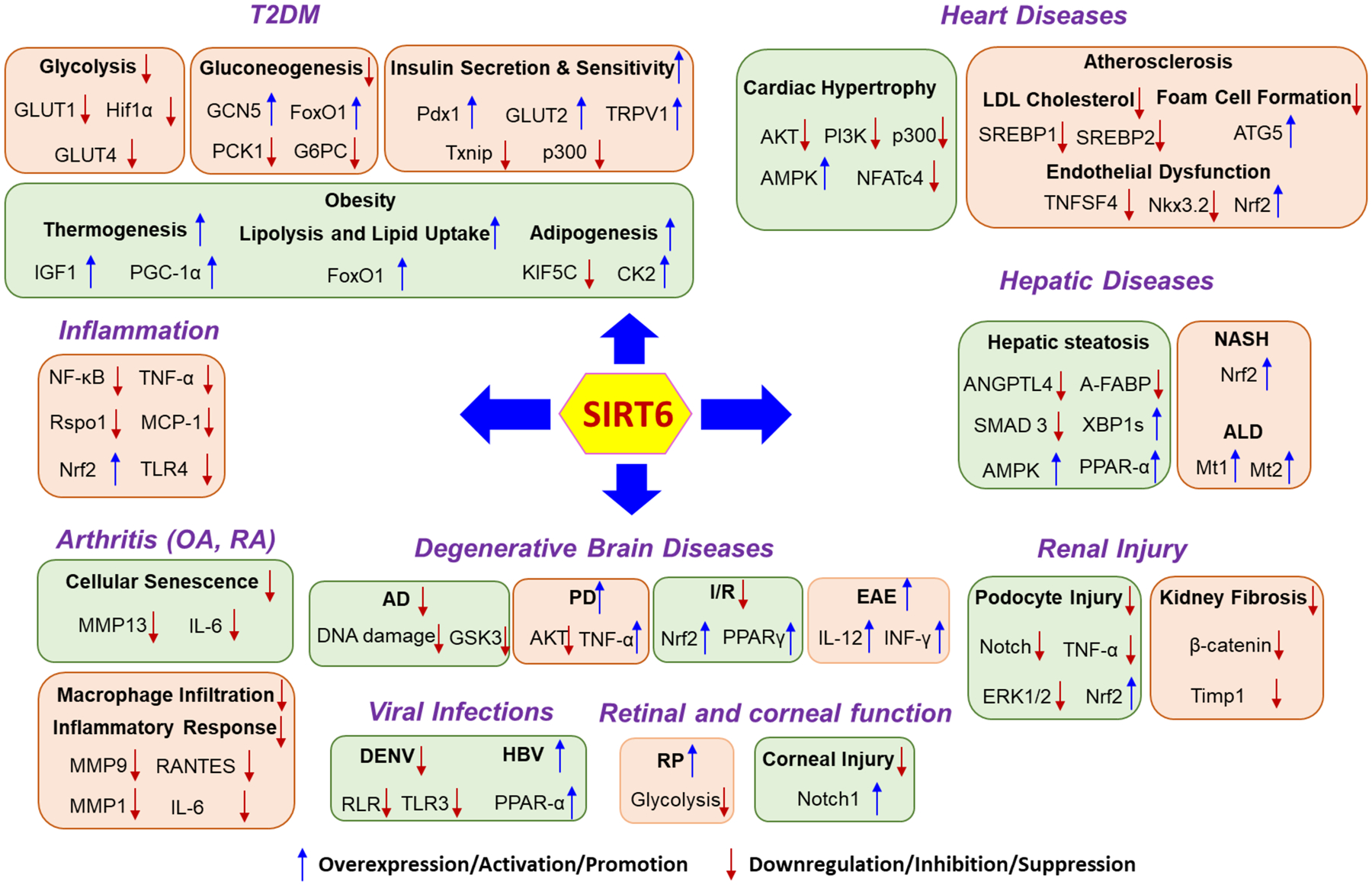
The role of SIRT6 in various human diseases other than cancer. T2DM, Type 2 diabetes mellitus; OA, osteoarthritis; RA, Rheumatoid arthritis; AD, Alzheimer’s disease; PD, Parkinson’s disease ALD, alcohol-related liver disease; NASH, nonalcoholic steatohepatitis; EAE, experimental autoimmune encephalomyelitis; RP, retinitis pigmentosa; DENV, dengue virus; HBV, hepatitis B virus.
4.3. Osteoarthritis and rheumatoid arthritis
Osteoarthritis (OA) is the most common condition of arthritis that is generally caused by a joint damage from cartilage degeneration in aging people.156 SIRT6 is involved in OA prevention, either by suppressing cellular senescence or inhibiting inflammatory responses.157–162 Studies showed that SIRT6 expression was significantly down regulated in the articular chondrocytes of OA patients, as well as in knee joints of aging mice and senescent chondrocytes,160 indicating positive correlation between SIRT6-related senescence and OA. Mechanistically, the downregulation of SIRT6 is accompanied by increased levels of MMP13 and decreased expression of collagen II in IL-1β-induced chondrocytes degeneration model, while this effect can be reversed by SIRT6 overexpression. Meanwhile, SIRT6 overexpression can also lead to decreased expression of NF-κB-dependent genes such as IL-6, MMP9, RANTES. As a result, overexpression of SIRT6 effectively suppresses replicative senescence of chondrocytes, and attenuates cartilage degradation in mature mice.160 Observation from Matsushita et al. also showed that SIRT6 inhibition increased MMP-1 and MMP-13 expression, and caused DNA damage, telomere dysfunction and subsequent cellular senescence in human chondrocytes, supporting the finding that the protective role of SIRT6 in cell senescence prevents the progression of OA.158,159 Interestingly, a well-defined SIRT6 activator, cyanidian, exhibits anti-inflammatory effects by suppression of NF-κB pathway and the expression of an array of inflammatory mediators (e.g. NO, PGE2, TNF-α, IL-6, iNOs, COX-2, ADAMTS5 and MMP13), and reducing the degradation of collagen II in IL-1β-induced human OA chondrocytes. This was further verified by the in vivo amelioration of OA development in surgical destabilization of the medial meniscus (DMM) mouse models of OA.163
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by the proliferation of fibroblast-like synoviocytes (FLS) and cartilage destruction.164 As a result of its anti-inflammation role, SIRT6 activation may suppress disease progression in RA.143 Early observation suggests that cigarette smoke extracts stimulate inflammation and matrix-destructive responses in RA synovial fibroblasts (RASF) by upregulating pro-inflammatory cytokine IL8 and the matrix-destructive enzyme MMP1, respectively. SIRT6 overexpression reduces the production of MMP1, revealing protective function of SIRT6 to the cigarette smoke-induced RA.165 Later studies indicate the distinct manner of protecting myeloid from RA by SIRT6, through suppression of macrophage infiltration and polarization in joint synovium, which plays a prominent role in the development and progression of RA.166 Mechanistically, SIRT6 deficiency in macrophages stabilizes FoxO1 protein by regulating its acetylation, and then causes inflammation. Conversely, overexpression of SIRT6 in the synovial fluid macrophages of RA patients reduces related inflammatory responses. In addition, transcriptionally activating SIRT6 by inhibition of its methylation has also been demonstrated to play a pivotal role in restraining RA progression.167
4.4. Diabetes
Type 2 diabetes mellitus (T2DM), characterized by high glucose concentration, is a major human disease caused by β-cell dysfunction and insulin resistance (IR).168,169 Glucose control to maintain stable blood sugar levels has critical importance to reduce the risk of developing diabetes and diabetic complication. SIRT6, as a principal regulator of glucose homeostasis, has been shown to regulate glucose metabolism and impact T2DM through an array of biological processes including glucose uptake, gluconeogenesis, insulin secretion and sensitivity (Figure 2).65,69
Early study on SIRT6-KD mice exhibits lethal hypoglycemia in early life, while feeding the mice with glucose-containing water increases blood glucose and saves the life of mutant mice, indicating blood glucose regulation by SIRT6 deletion in glucose homeostasis.9,170 SIRT6 functions as repressor of multiple glycolytic genes through H3K9 deacetylation at the promoter. From the SIRT6-KD study, glycolytic genes (GLUT1, PDK1, LDHA) are highly expressed and the transcription factor Hif1α, a critical regulator of nutrient stress responses, is activated, accompanied by enhanced glucose uptake with upregulation of glycolysis and diminished mitochondrial respiration.42 Concurrently, the absence of SIRT6 may trigger abundant membrane association of glucose transporters (GLUT) 1 and 4, as well as activate AKT signaling pathway through increasing expression of multiple insulin receptors such as IRS1, IRS2, thus to enhance glucose uptake.170 In the context of hypoxia condition, SIRT6 was also found to suppress hypoxia-induced autophagy through inhibition of glycolysis.171 On the upstream, SIRT1 may form a SIRT1-FoxO3a-NRF1 (SFN) protein complex on the SIRT6 promoter and positively regulate expression of SIRT6.18 Hence, SIRT6 inhibition is able to lower blood glucose by enhancing glycolysis and glucose uptake. Based on the ability of repressing the expression of glucose transporters and glycolytic enzymes, SIRT6 inhibition has been considered as an alternative approach for T2DM treatment. Recently, a proof-of-concept study for assessing the viability of small molecule SIRT6 inhibitor for T2DM treatment indicates that administration of the SIRT6 inhibitor SYN17739303 may improve oral glucose tolerance in vivo, upregulate the expression of the glucose transporters GLUT1 and 4 in the muscle and enhance the activity of the glycolytic pathway.172 More recently, a phenylpiperazines SIRT6 inhibitor was also reported to increase GLUT1 protein expression in BxPC-3cell, as well as reduce the blood glucose content in a T2DM mouse model.173 These studies provide a promising strategy for improving glycemic control in T2DM by inhibiting SIRT6. However, SIRT6 may modulate the gluconeogenesis and insulin secretion, acting a contradictory role in glucose metastasis.
Multiple studies suggest that SIRT6 may suppress gluconeogenesis, a function that can lead to reduction of the blood glucose. SIRT6 can interact with and modify the acetyltransferase GCN5 to enhance its activity. This enhancement may increase the acetylation of PGC-1α, a key mediator of gluconeogenic gene transcription, thus leading to the suppression of gluconeogenic gene expression and hepatic glucose production (HGP).46 The glucose reduction role of SIRT6 was further demonstrated in vivo. Increased hepatic SIRT6 expression suppresses the gluconeogenic transcriptional program and blood glucose levels in diabetic mouse model, suggesting that liver-targeted activation of SIRT6 may be beneficial for T2DM diabetes control. Meanwhile, the gluconeogenesis inhibition controlled by SIRT6 is mediated by hepatic FoxO1/3/4 genes.174–177 Specifically, p53-mediated activation of SIRT6 increases the interaction with and deacetylation of FoxO proteins and subsequently induces nuclear exclusion of FoxO1. When exporting to the cytoplasm, the expression of phosphoenolpyruvate carboxy-kinase 1 (PCK1) and glucose-6-phosphatase (G6PC) is suppressed, thereby encoding rate-limiting enzymes in gluconeogenesis.176
Extensive evidence supports that SIRT6 also regulates glucose metabolism by enhancing insulin secretion and sensitivity. SIRT6-overexpression mice (SIRT6BAC mice) were shown to be prevented from hyperglycemia and glucose intolerance induced by high-caloric-diet (HCD). The physiological overexpression of SIRT6 drives improved insulin sensitivity in skeletal muscle and liver, supporting putative anti-T2DM function from SIRT6 activation.178 SIRT6 plays an essential role in pancreatic β-cell function. Impairment of glucose-stimulated insulin secretion (GSIS) is commonly found in SIRT6 KO pancreatic β-cells and in vivo.177,179–181 Mechanistic analysis indicates that SIRT6-stimulated nuclear extrusion of FoxO1 subsequently relieves a molecular blockage on the expression of glucose sensing genes Pdx1, which in turn augments GLUT2 expression and GSIS.177 In addition, SIRT6 was found to inhibit RNA polymerase II at the promoter region of thioredoxin-interacting protein (Txnip) through deacetylation. The downregulation of Txnip in β-cells partially restores the insulin secretion and largely rescues the glucose intolerance.182 In contrast to the positive correlation of SIRT6 to insulin sensitivity from above discussion, a recent study from Tang et al. observed the opposite regulation of SIRT6 to insulin sensitivity in a hepatocyte‐specific SIRT6 deletion model. In this context, hepatic SIRT6 reduced p300 to downregulate the protein level of estrogen receptor ERα, and thus attenuated estrogen‐induced activation of PI3K and blocked insulin signal transduction to eventually reduce insulin sensitivity in the liver. Consistently, SIRT6 deletion in hepatocytes significantly increased insulin sensitivity, especially in female mice, by improving the expression of ERα.183
Obesity accounts for the majority cases of type 2 diabetes.184 Obese people who are accompanied with insulin resistance due to reduced efficiency of insulin action in the periphery may dramatically increase the prevalence and incidence for the development of T2DM.184,185 Multiple evidence suggests that SIRT6 overexpression accumulates less fat in high fat diet (HFD)-induced obesity model, while SIRT6 levels are significantly decreased in HFD-fed mice and other adipose tissues, indicating the protective function of SIRT6 for forming obesity.68,186–190 SIRT6 enhancement by genetic manipulation was observed to increase the insulin sensitivity and glucose uptake in HFD-induced obese mice and IR adipocytes, respectively. In this model, the abated expression of transient receptor potential vallinoid 1 (TRPV1), which works as a transporter of Calcitonin gene-related peptide (CGRP) and regulator of GLUT expression, was restored by SIRT6 overexpression to promote CGRP production and GLUT4 level, hence to increase glucose uptake and decrease insulin resistance.68 In addition, TG mice with SIRT6 overexpression protect against pathological damage caused by diet-induced obesity. In this context, SIRT6 overexpression downregulates a selective set of peroxisome proliferator-activated receptor-responsive genes, lipid storage-associated genes, and is concomitant with less visceral fat, LDL-cholesterol, and triglycerides.190 On the other hand, neural SIRT6-deleted mice lead to growth retardation and ultimately cause obesity through down-regulating of growth hormone (GH) and IGF1 levels, indicating the important role of SIRT6 in preventing obesity.191 Mechanistically, SIRT6 may regulate obesity formation due to its critical role in thermogenesis in brown adipocytes. PGC-1α, a central regulator of brown fat thermogenesis, is activated by phospho-ATF29 (p-ATF2) binding to its promoter. Depletion of SIRT6 expression in brown adipocytes impedes p-ATF2-mediated activation PGC-1α, subsequently inhibits the thermogenic program of brown fat and leads to obesity.186 In addition, regulating lipid metabolism through mTORC2-SIRT6-FoxO1 pathway may protect against obesity at thermoneutrality in brown adipocyte. Inhibition of mTORC2 was found able to trigger FoxO1 deacetylation by SIRT6 and the resulting activated FoxO1 may stimulate lipolysis and lipid uptake.192 However, with respect to adipocyte differentiation, SIRT6 plays positive regulation in adipogenesis and conceivable negative role in obesity prevention. SIRT6 deficiency impairs their adipogenesis in preadipocytes and in vivo.154,193 SIRT6 negatively regulates KIF5C expression and its interaction with CK2α’, hence to release CK2α’ nuclear translocation and provoke CK2 kinase activity, and consequently promote mitotic clonal expansion during adipogenesis.193
4.5. Heart diseases
SIRT6 has been implicated as a significant factor in heart-related diseases due to its complex regulation functions in multiple molecular pathways. Accumulating studies reported the cardioprotection role of SIRT6.194–196 Cardiomyocytes from TG mice with SIRT6 overexpression showed higher levels of survival when subjected to prolonged hypoxia, indicating that SIRT6 prevents heart from hypoxic damage.197 The mechanism involves the activation of pAMPK-α pathway, increased expression of Bcl2, inhibition of NF-κB, decreased generation of reactive oxygen species (ROS) and reduction of pAkt during hypoxia.197 SIRT6 appears to also provide protection role against transverse aortic constriction (TAC)-induced heart dysfunction. In TAC treated mice, telomerase reverse transcriptase (TERT), and telomere repeat binding factor (TRF)-1 are obviously downregulated. SIRT6 overexpression increases TERT and TRF1 and consequently promotes mouse survival after TAC treatment.198 In addition, SIRT6 is able to modulate cardiac glucose metabolism via transcriptionally regulation of FoxO1-PDK4 signaling, a function that prevents the heart from cardiac metabolic dysfunction.199 More recently, SIRT6 has also been observed to protect the cardiomyocyte from doxorubicin-caused apoptosis through epigenetic activation of SIRT6–Tip60–Gata4 axis signaling, highlighting a cooperative strategy for avoiding chemotherapeutic cardiotoxicity.200
4.5.1. SIRT6 in cardiac hypertrophy, myocardial fibrosis and failure
Cardiac hypertrophy is an adaptive response to pressure or volume overload, which typically precedes the onset of heart failure.201 As aforementioned, SIRT6 transcriptionally represses AKT signaling pathway by H3K9 deacetylation.125,202 SIRT6 deleting in mice exhibits aberrant activation of IGF-AKT signaling and develops cardiac hypertrophy and heart failure, whereas TG mice with SIRT6 abundant are protected from hypertrophic stimuli, indicating that SIRT6 blocks IGF-AKT signaling to antagonize the development of cardiac hypertrophy and failure.203 Along this line, the protective function of SIRT6 is regulated by nicotinamide mononucleotide adenylyltransferase 2 (Nmnat2), whose overexpression increases the intracellular NAD+ level and then augments cardiomyocytes hypertrophic prevention by activating SIRT6.204 SIRT6 was also observed to suppress PI3K/Akt signaling to reduce p300 expression via promoting its degradation.183,205,206 The downregulation of p300 protein subsequently inhibited the transcriptional activity of NF-κB p65 subunit, hence protecting cardiomyocytes from hypertrophy by angiotensin II (Ang II) or phenylephrine stimulation.205,207 In addition to inhibiting AKT signaling, SIRT6 acts as a negative regulator in cardiomyocyte hypertrophy by suppression of NFATc4 expression and transcriptional activity, as well as preventing the activation of STAT3 signaling.208,209 SIRT6-induced autophagy also plays a protective role in isoproterenol-induced cardiac hypertrophy. SIRT6-mediated AKT inhibition may promote nuclear retention of FoxO3 transcription factor, which is responsible for autophagy activation and subsequent cardiomyocytes protection.210 Differentiation of cardiac fibroblasts (CFs) into myofibroblasts resulting in cardiac fibrosis represents a significant hallmark of heart failure. By blocking NF-κB signaling, SIRT6 may prevent CFs from differentiation into myofibroblasts.211 The angiotensin-converting enzyme 2 (ACE2) is a critical enzyme in the metabolism of Ang II. ACE2-deficient rat showed low level of SIRT6 and phosphorylated AMPKα as well as increased myocardial fibrosis. The protective function of SIRT6 in Ang II-induced myocardial fibrosis and injury is mainly attributed to the activation of AMPK-ACE2 signaling pathway.212
4.5.2. Atherosclerosis, myocardial infarction and coronary heart disease
Coronary artery disease (CAD), including its complication myocardial infarction (MI), is a complex disease which commonly ascribes to atherosclerosis.213,214 Atherosclerosis is a progressive heart disease characterized by the accumulation of cholesterol-engorged macrophages (called foam cells) and fatty streaks in the large arteries.215 The pathogenesis and progression of CAD and its complication disease are highly relevant to SIRT6, and sequence variants of SIRT6 gene are frequently observed in CAD patient.216,217 SIRT6 has been demonstrated to exert protective effects in atherosclerosis mainly depending on three manners through decreasing low-density lipoprotein (LDL) cholesterol, reducing macrophage foam cell formation or preventing endothelial dysfunction (Figure 2).215,218–220 The regulation of SIRT6 in lipid metabolism, particularly in LDL cholesterol, represents an effective manner for the protection of atherosclerosis. Early study found that SIRT6 inhibits cholesterol levels via reducing the lipogenic transcription factors SREBP1 and SREBP2 and activating AMPK pathway.221 In supporting of this role, SIRT6 was observed to be recruited to Srebp2 gene by FoxO3, creating a suppressive chromatin state via H3K9 and H3K56 deacetylation to inhibit expression of Srebp2 and its target genes.222 In addition, the recruitment of SIRT6 by FoxO3 also results in the repression of proprotein convertase subtilisin kexin type 9 (Pcsk9) gene expression, thereby decreasing LDL-cholesterol levels. In accordance with this function, overexpression of SIRT6 in high fat diet-fed mice lowers LDL-cholesterol.223 By regulating FoxO3, SIRT6 may also protect the heart from ischemia/reperfusion (I/R) injury through AMPK-mediated antioxidant defense mechanisms.224 In term of foam cell formation, SIRT6 plays a suppression role relying on an autophagy-dependent pathway. SIRT6 overexpression induces autophagy by regulating the key autophagy initiation gene ATG5 and promotes cholesterol efflux by decreasing the expression of miR-33 as well as its host gene Srebp2.220 A recent study also revealed that SIRT6 could promote macrophage autophagy and reduce interaction with endothelial cells to reduce the infiltration of macrophages, thus contributing to the stability of atherosclerosis plaques.219 Of note, SIRT6-induced autophagy may be reversed by myeloid cells trigger receptors 1 (TREM-1)-mediated pyroptosis, causing endothelial inflammation and atherosclerosis development.225
Endothelial cells (ECs) dysfunction is an early pathological feature of atherogenesis.226 Early studies indicate SIRT6 deaccelerates endothelial senescence in vitro and in vivo, revealing a pivotal role in regulating endothelial function.227 Predictably, impairment of endothelium‐dependent vasorelaxation displays in both global and endothelium‐specific SIRT6-KO and SIRT6+/− haploinsufficient mice.228 Conversely, SIRT6 overexpression promotes H3K9 deacetylation, down regulates the expression of multiple atherosclerosis‐related genes such as proatherogenic gene TNFSF4, a function related to atherosclerosis protection.228 Consistent inferences were made when studying the implication of SIRT6 to atherosclerotic lesion development. Downregulation of SIRT6 in apolipoprotein E–deficient (ApoE−/−) mice exhibits impairment of endothelium-dependent vasodilation, increase of plaque size and more vulnerable plaque.229 In another model, ECs treated by cholesterol crystal (CC) develop an array of endothelial dysfunction pathological features, including inhibition of nitric oxide (NO) and endothelial nitric oxide synthase (eNOS), upregulation of adhesion molecules and enhancement of monocyte adhesion to ECs. SIRT6 Overexpression was observed to alleviate minute CC-induced endothelial dysfunction via Nrf2 activation-involved manners.230 In addition, SIRT6 also prevents endothelial injury by transcriptionally inhibiting NK3 homeobox 2 (Nkx3.2) to enhance the expression of novel regulator of blood pressure, GATA5, which is also a protective role related to hypertension.231 Taken together, the prevention of SIRT6 on ECs, as well as its regulation of lipid metabolism and macrophage infiltration may indicate protective role in the pathogenesis and progression of CAD. Therefore, pharmacological activation of SIRT6 provides a feasible therapeutic strategy for atherogenesis treatment.232
4.6. Degenerative brain diseases
SIRT6 is highly expressed in cortical and hippocampal regions of brain tissues and SIRT6-deficient animals exhibit developmental retardation, which not only reveals a pivotal role in brain development, but also predicts a close relationship between the aberration of SIRT6 with pathological features of human neurodegenerative diseases (Figure 2).233,234 In Alzheimer’s disease (AD) patients, both SIRT6 mRNA and protein of brain are obviously lower compared to normal subjects, with the same trend as observed in AD mouse model.235–237 SIRT6 plays AD-protective function via maintaining genomic stability and preventing DNA damage in brain.236,237 As exemplified in Aβ42-treated HT22 mouse hippocampal neurons, SIRT6 and p53 decrease, consequently resulting in DNA damage. SIRT6 overexpression rescues Aβ42-induced DNA damage, indicating a promising therapeutic approach for AD treatment.236 In addition, SIRT6 KO mice exhibit accelerated DNA damage accumulation, and hyperphosphorylation of Tau in brain. Specifically, SIRT6 depletion in cells activates glycogen synthase kinase 3 (GSK3) to increase Tau stability and phosphorylation, which may be rescued by GSK3 inhibition.237,238 Moreover, a recent study observed that supplementation of NAD+, which has been determined to activate SIRT6, rescues the key Alzheimer’s features and DNA damage responses in AD mouse model, further verifying the neuroprotection role of SIRT6.239
However, opposite to the lower level of SIRT6 in AD patients, SIRT6 protein in Parkinson’s disease (PD) patient brains are higher than that in healthy controls. SIRT6 plays pathogenic and pro-inflammatory role in PD, which attributes to the promotion of the pro-apoptotic TNF-α pathway and suppression of pro-survival AKT signaling. Therefore, brain-specific SIRT6-KO mice confer neuroprotection from MPTP-induced PD.240 Additionally, the suppression of AKT by SIRT6 is also associated with depressive disorders. In chronic unpredictable mild stress (CUMS) rat model, SIRT6 significantly increases in the hippocampal region. The overexpression of SIRT6 inhibits AKT/GSK3β/CRMP2 signaling pathway that can result in depression-like behaviors. On the contrary, downregulation of hippocampal SIRT6 exhibits antidepressant-like effect in mice.241,242 However, it is notable that overexpression of SIRT6 has been shown to be detrimental to memory formation. Studies from Yin et al. reported that SIRT6 overexpression in the CA1 region impaired the formation of long-term fear memory via inhibiting the IGF2 and related signaling pathway.243 Similarly, a study focusing on the role of SIRT6 in excitatory neurons observed enhanced contextual fear memory in SIRT6 genetic depletion mice, while spatial memory was not affected.244
Considering that ischemic stroke shares common characteristics and pathophysiological mechanisms with myocardial I/R injury, SIRT6 might play a similar protective role in cerebral ischemia.224,245,246 The anti-inflammatory effects of SIRT6 have been demonstrated in brain via regulating various signaling. Ischemia mouse brain shows reduced SIRT6 expression accompanied with inflammation, while retinoid X receptor (RXR) agonist bexarotene ameliorates neuroinflammation via activation of PPAR-γ/SIRT6/FoxO3a pathway in subarachnoid hemorrhage rats.247,248 Antioxidant role of SIRT6 also applies to I/R injury protection. SIRT6 was found to protect the brain from cerebral I/R by reducing oxidative stress via enhancing the antioxidant Nrf2 signaling. On the contrary, miR-370, which may inhibit SIRT6 expression, exhibits aggravated cerebral I/R injury by regulating Nrf2/ARE signal pathway.246,249 Due to protective role mentioned above, SIRT6 has been demonstrated to be necessitated for sodium sulfide-mediated cytoprotective effect in cerebral I/R.250 Interestingly, it is recently reported that endothelial SIRT6 exerts protective role in cerebral I/R injury by preserving blood–brain barrier integrity.251 Specifically, endothelial-specific SIRT6 deletion mice show increased blood–brain barrier disruption and poor post-stroke outcome, while post-ischemic SIRT6 overexpression rescues the cerebral I/R injury damage caused by SIRT6-KD. Importantly, in ischemic stroke patients, SIRT6 expression is significantly higher in those showing short-term neurological improvement as compared to unfavorable short-term outcome, correlating with clinical stroke outcome.251
Regarding the role in controlling neuroinflammation, SIRT6 inhibition was also reported to delay the onset of a neuroinflammatory and demyelinating disease experimental autoimmune encephalomyelitis (EAE).252,253 EAE, a common murine model of multiple sclerosis (MS), is predominantly considered a T helper 1-driven autoimmune disease and the activation of dendritic cells (DCs) significantly contributes to its pathogenesis.252,254 A previous study has reported that SIRT6 promotes conventional DC differentiation and function, and SIRT6-KO DCs exhibit low differentiation and immunostimulatory capacity and an overall reduction in their ability to produce IL‐12, TNF‐α and IL‐6 secretion via stimuli.255 A recent proof-of-concept study by using SIRT6 inhibitor SYN17739303 to evaluate the impact of SIRT6 inhibition to the EAE revealed that pharmacologically inhibiting SIRT6 effectively delayed EAE disease onset by reducing the representation of CXCR4-positive and of CXCR4/CCR7-double-positive DCs in lymph nodes. In addition, SIRT6 inhibition correlated with decreased production of the autoimmunity-promoting cytokines such as IFN-γ and IL-12, and increased production of the anti-inflammatory cytokine IL-10.252
4.7. Hepatic diseases
The critical role of SIRT6 in hepatic fat metabolism protects liver from formation of steatohepatitis. Liver-specific SIRT6-KO in mice indicates increased glycolysis, triglyceride synthesis, and reduced β oxidation, which lead to fat accumulation and thus cause fatty liver formation.18 Comparing the SIRT6-KO mice with wild types when simultaneously feeding with a high-fat and high-fructose (HFHF) diet, the SIRT6-KO group shows increased hepatic steatosis and inflammation as well as aggravated glucose intolerance and insulin resistance.256 On the contrary, TG mice overexpressing SIRT6 accumulate less visceral fat, LDL-cholesterol, and triglycerides than the wild type, and are more prone to be protected against HFD-induced hepatic lipidosis and other metabolic damages.257 Consistently, lower expression of SIRT6 was observed in human fatty liver samples than in normal controls. Collectively, these results indicate a positive role of SIRT6 in steatohepatitis prevention.18 Mechanistically, overexpression of SIRT6 was observed to downregulate a set of peroxisome proliferator-activated receptor gamma (PPAR-γ) target genes such as angiopoietin-like protein 4 (ANGPTL4), and adipocyte fatty acid-binding protein (A-FABP), which are highly associated with lipid metabolism, lipid transport and adipogenesis. The transcription level of PPAR-γ itself has no difference between the TG and wildtype mice.257 A separate and relevant study reported that activation of PPAR-γ by the agonist rosiglitazone could ameliorate hepatic steatosis by stimulation of the SIRT6-AMPK-mediated pathway.258 In addition, SIRT6 can promote hepatic fatty acid β-oxidation and inhibit pyruvate oxidation through activating PPAR-α by binding to PPAR-α coactivator NCOA2 and decreasing its acetylation. Through this manner, SIRT6 properly regulates the whole-body respiratory exchange ratio and liver fat content, thereby preventing from fatty liver and other metabolic dysregulation diseases.259 Consistent with this finding, SIRT6 and miR-122 negatively regulate each other to co-regulate the fatty acid β-oxidation.260 Moreover, SIRT6 deacetylation may also exert liver protection by transcriptionally activating XBP1s, which confer resistance to ER stress-induced hepatic steatosis.50 In addition, SIRT6 activation may alleviate liver fibrosis by suppressing SMAD 2/3 signaling to affect hepatic stellate cells (HSCs) activation or transcriptionally inhibiting activity of orphan nuclear receptor estrogen-related receptor (ERR)γ to impact bile acid production, representing a new therapeutic potential for treating nonalcoholic steatohepatitis (NASH) and cholestatic liver injury, respectively.52,53,261 In terms of attenuating NASH progression, SIRT6 can also curb inflammation and oxidative stress through enhancing the nuclear import of nuclear factor Nrf2 and transcriptionally activating phase II/antioxidant genes.256 By combating with oxidative stress, SIRT6 may also protect the liver from alcohol-related liver disease (ALD). SIRT6 expression is decreased in the livers of alcoholic cirrhosis (AC) patients and ALD mice, and SIRT6-KO mice exacerbate liver injury and ALD. This phenomenon is highly correlated with dysregulation of lipid homeostasis, increased oxidative stress and inflammation in SIRT6-KO ALD model, which may be reversed by TC mice with hepatic SIRT6 overexpression. Several antioxidative stress-related genes such as metallothionein 1 and 2 (Mt1 and Mt2) are positively regulated by SIRT6, and its overexpression in liver may ameliorate ethanol-induced injury and ALD in SIRT6-deficient mice.262
4.8. Renal injury
The significant role of SIRT6 in glucose homeostasis maintenance, cell apoptosis and autophagy regulation, anti-inflammation and anti-fibrosis may also show influence in renal function.263 Recent evidence has demonstrated the importance of SIRT6 in kidney protection. SIRT6 is downregulated in renal biopsies from podocytopathies patients, while SIRT6 depletion plays a direct pathophysiologic role in the human kidney disease.264,265 It is reported that SIRT6-KD in mice can induce podocyte injury and proteinuria. SIRT6 transcriptionally inhibits Notch1 and Notch4 signaling pathways to exert pleiotropic protective actions in podocytes.264,265 Along with this observation, SIRT6 overexpression was found to protect kidney from various damages via stimuli such as cisplatin-induced acute kidney injury, sepsis-induced acute kidney injury, hypoxia‐induced tubular epithelial cell injury, cadmium-induced kidney toxicity and Ang II-induced urinary albumin excretion, glomerulosclerosis and podocyte injury.266–271 Mechanistically, the protective effect of SIRT6 in kidney acts on several pathways, including inhibiting ERK1/2 expression by H3K9 deacetylation to reduce inflammation and apoptosis,266 down-regulating the secretion of TNF-α and IL-6 to promote apoptosis and reduce autophagy,267 activating Nrf2 to regulate oxidative stress, inflammation and apoptosis,268 attenuating hypoxia‐triggered damage and G2/M phase arrest,269 and promoting M2 macrophage transformation to alleviate renal inflammatory injury.272
Moreover, recent studies also unraveled a protective role of SIRT6 against kidney fibrosis. SIRT6 was observed to be gradually upregulated during the progression of fibrogenesis induced by unilateral ureteral obstruction (UUO) and renal I/R, while SIRT6-KD aggravates TGF-β–Smad 3–induced fibrotic changes in renal tubular cells. SIRT6 deficiency enhances β-catenin activation during TGF-β treatment, and thus promotes the expression of fibrotic genes and renal fibrosis, indicating antifibrotic role of SIRT6 in kidneys by epigenetically repressing β-catenin signaling.273,274 A separate study in diabetic nephropathy (DN) disease suggests that expression of Nampt protein is decreased in proximal tubular cells, which is associated with renal fibrotic changes. Nampt inhibition causes the downregulation of downstream SIRT6, leading to upregulation of the pro-fibrotic factor Timp1, which in turn inhibits fibrous tissue degradation and promotes fibrogenesis.275 Collectively, these findings supports that activating SIRT6 may offer a potential therapeutic strategy to treat or prevent the progression of renal fibrosis.
4.9. Retinal and corneal function
SIRT6 has been identified to play an essential role in maintaining proper retinal function. SIRT6 is highly expressed in the retina. SIRT6-KO mice show profound impairment in retina, including major retinal transmission defects and elevated apoptosis in inner retina cells, which is accompanied by changes in expression of glycolytic genes and glutamate receptors.276 Given the critical role of glucose availability to retinal function, SIRT6 expression level was found to decrease, along with hyperacetylation of its chromatin substrates and regulation of related signaling participated in early diabetic retinas.277 However in retinitis pigmentosa (RP) disease, SIRT6 inhibition is considered to provide electrophysiological and anatomic rescue of both rod and cone photoreceptors in a preclinical model of RP and eventually attenuates retinal degeneration.278 Outer segment (OS) of photoreceptors, whose dysgenesis is an early manifestation of RP disease, are continuously regenerated in a process that generates phospholipids from the pentose phosphate pathway (PPP).279,280 Inhibition of SIRT6 promotes the accumulation of biosynthetic intermediates of glycolysis, thereby improving OS length, enhancing photoreceptor survival, and preserving cellular OS.278 In addition, corneal injury, including corneal scarring, vascularization, and inflammation was also observed in SIRT6-KO mice.281 Consistent with this finding, it is reported that SIRT6 deficiency causes delayed and incomplete wound healing in the corneal epithelial wound healing mouse model. At the molecular level, SIRT6 deficiency may result in the inhibition of Notch1 singling, thereby leading to dysregulation of corneal epithelial homeostasis and associated excessive inflammation in the corneal stroma.282
4.10. Viral infections
In the context of antiviral immunity, the role of SIRT6 is little known.283 Early observation from a loss-of-function study suggests that small interfering RNA (siRNA)-mediated SIRT6-KD in infected fibroblast MRC5 cells results in significant increased viral titers such as hCMV, HSV1, Adenovirus, and Influenza A, indicating an antiviral role by SIRT6 upregulation.284 SIRT6 participates in immune activation of macrophages interferon antiviral response, and SIRT6-KD promotes cytomegalovirus growth in both fibroblasts and macrophages, providing the protective function of SIRT6 to viral infections.285 Moreover, SIRT6 was observed to play a suppressive role in dengue virus (DENV)-induced inflammatory responses, as elucidated by the increased transcription level of proinflammatory chemokines and cytokines such as IL6, TNF-α, CCL2 and CCL5, in SIRT6-silenced murine Raw264.7 macrophages after DENV infection. Mechanistically, overexpression of SIRT6 inhibits RIG-I-like receptor (RLR) and TLR3 signaling pathway, which in turn decreases the activation of associated pro-inflammatory cytokine NF-κB. Notably, DENV replication was found to be reduced when SIRT6 was silenced.286 On the contrary, SIRT6 has also been shown to positively regulate hepatitis B virus (HBV) transcription and replication. SIRT6 overexpression may upregulate the expression of PPAR-α to promote the activation of HBV core promoter. The treatment with SIRT6 inhibitor OSS_128167 blocks the positive effect of SIRT6 overexpression on HBV transcription, indicating a therapeutic potential for HBV.287
5. SIRT6 MODULATORS
Given the critical roles of SIRT6 in a variety of human diseases including cancer, diabetes, inflammation, hepatic diseases, neurodegenerative disorders, renal injury and retinal dysfunction and viral infections, regulating the activity of SIRT6 using small modulators may offer therapeutic benefits for blocking the initiation and progression of these diseases.288,289 Small molecules targeting a well-defined target have been reported to indirectly act on SIRT6 by regulating its expression or modulating SIRT6-mediated signaling pathways,290–295 exemplified by the DPP-4 inhibitor Sitagliptin, which inhibits vascular inflammation by upregulating SIRT6 expression.155 Due to the complex role of SIRT6 in multiple molecular pathways, manipulating SIRT6 by directly binding to it with small molecules can be viewed as more specific and efficient approach for relevant disease therapy. There are two different pockets in SIRT6 protein for small molecule binding, one defined as the active site and the other as the allosteric site.
To the best of our knowledge, small molecule modulators including both inhibitors and activators which bind in the active site, are reported to act on SIRT6 by binding to the pocket locating at the distal end of SIRT6’s extended acyl binding channel near the N-terminal loop.296–298 Several amino acid residues (e.g. Ile 61, Pro62, Phe64/82/86, Val70, Asn114, Val115 and Asp116) form a hydrophobic pocket to anchor the molecules in the pocket with polar contact and potential H-bonds. Most modulators binding in the active site have partial overlap with the pocket where NAM moiety of NAD+ occupies (Figure 1a, C site), and thus no ADPr competition but statistically significant NAD+ or NAM competition with them are detected in the activity assays.54,296–300 Importantly, SIRT6 activators binding in this pocket primarily exerts concentration-dependent deacetylation activity. Some of them has no improvement to or even inhibits the SIRT6-dependent demyristoylation activity, likely due to the compatible binding of activators with that of acetylated substrates and overlapping with longer acylated substrate.34,35,296,300,301
Although these activators and inhibitors bind to the same active site of SIRT6, different activation or inhibition activity reflects by distinguished interactions from the exact binding of modulators. As demonstrated by comparing quercetin to catechin gallate (CG), it has been reported that natural product quercetin binds to the extended SIRT6 acyl channel to stimulate SIRT6 deacetylation activity, whereas its close analogues CG occupies the same pocket but exerts inhibitory action. The co-structure analysis revealed that CG distinguishes itself from quercetin by offering a tilted position due to the saturated chroman ring, thus orienting chroman ring and its trihydroxybenzoate substituent towards N-terminus and V154/G155, respectively (Figure 3a).300 Such a characteristic also applies to inhibitor trichostatin A (TSA), which is anchored in C site with the water-mediated hydrogen bonds and places the phenyl ring in the chroman fragment of CG occupied pocket thus orienting towards the N-terminus (Figure 3b).297 The SIRT6 activator UBCS039 shares the similar binding pocket of its pyrrolo[1,2-a]quinoxaline with chromene of the activators quercetin and cyanidin, which supports the relevance of this orientation for activation.296 Collectively, we may rationally speculate that SIRT6 binding by the activators or inhibitors in the same active site but appearing distinct orientations stabilizes it to catalytically favorable or unfavorable conformation, thereby leading to the catalysis improvement or inhibition, respectively.
Figure 3. Co-crystal structure of SIRT6 with modulators.
(a) Superimposition of SIRT6-CG (PDB code: 6QCJ) and SIRT6-Quercetin (PDB code: 6QCD) in ribbon representation. (b) Superimposition of SIRT6-CG (PDB code: 6QCJ), SIRT6-quercetin (PDB code: 6QCD), SIRT6-cyanidin (PDB code: 6QCH), SIRT6-UBCS039 (PDB code: 5MF6), and SIRT6-trichostatin A (PDB code: 6HOY) in ribbon representation. Ligands and key residues on the binding site are shown in stick representation and water molecule is shown as a red ball. H-bond is shown as purple dotted line. CG, quercetin, cyanidin, UBCS039 and trichostatin A are colored in magenta, cyan, yellow, green and teal, respectively. (c) Superimposition of SIRT6-MDL-801 (PDB code: 5Y2F) and SIRT6-UBCS039 (PDB code: 5MF6) in surface representation. MDL-801 is shown in magenta stick and UBCS039 in green stick. Key residues of SIRT6-MDL-801 allosteric binding site are highlighted in magenta sticks. H-bond is shown in purple dotted line and π stacking is shown in blue dotted line. (d) SIRT6-MDL-801 MD simulation snapshot in surface presentation. MDL-801 is shown in magenta stick and Key residues of SIRT6-MDL-801 allosteric binding site are highlighted in magenta sticks.
Allostery is a biological process that the effect of binding with protein at one site can be transmitted to another, often distal, functional site, allowing for regulation of the biological function.302,303 Modulators binding in the allosteric site may exhibit significant pharmacological advantages such as higher selectivity and lower toxicity compared to that in the orthosteric site.304 Pioneer efforts from Huang et al. revealed series of benzenesulfonamide analogues as selective SIRT6 allosteric activators with the high binding potency.301 Among them, MDL-800 and MDL-801 were claimed as allosteric activators given the following reasons. First, dramatically increased kcat and moderately decreased Km were observed in the kinetic study, suggesting that MDL compounds not only increase the binding affinity of substrate but also elevate the catalytic efficiency of SIRT6. Second, a structural comparison of SIRT6 with or without MDL-801 observed conformational changes on the backbone of the N-terminal residues after binding to the compound. Third, mutations of Phe86 and Phe82 from the allosteric pocket markedly lowered the potency of MDL compounds. Last, UBCS039, which has been validated as a SIRT6 activator, did not reverse MDL-801 activated SIRT6 deacetylation, indicating no competitive effect between these two activators. Moreover, UBCS039 exhibited a competitive binding with fatty acids, while MDL-800/801 do not compete with fatty acids, which further defined different binding site of these compounds.301 Indeed, superimposition of SIRT6-UBCS039 (PDB code: 5MF6) and SIRT6-MDL-801 (PDB code: 5Y2F) shows that MDL-801 binds to the exit of hydrophobic channel, and is very close to but has little overlap with UBCS039 binding pocket (Figure 3c). The allosteric mechanism was further elucidated by using molecular dynamic simulations. Specifically, MDL-801 may allosterically activates SIRT6 through rotating the 2-methyl-4-fluoro-5-bromo phenyl moiety to the interior of the allosteric site, which consequently forms additional hydrophobic interactions between the 5-Br atom and Met136 at the base of the allosteric site (Figure 3d). In addition, either the M136A mutant, or modifications on the phenyl ring by removal of the 5-Br or restraining the rotation of the phenyl ring markedly decrease the activated deacetylation activity by MDL-801 binding, strongly supporting that the 5-Br atom is critical for the ligand allosteric efficacy.304
5.1. Inhibitors
5.1.1. Quinazolinediones
A structure-based in silico screening by using available SIRT6 X-ray structure (PDB code: 3K35) has identified a collection of quinazolinedione analogues as SIRT6 inhibitors (Figure 4). Docking analysis reveals that compound 1 (SYN17739303) allocates at the NAM binding pocket and part of the substrate binding pocket. In the fluorescence assay, compound 1 shows moderate SIRT6 inhibition in deacetylation activity with an IC50 of 106 μM, which is 3-fold more potent than that against SIRT1 but comparable with no selectivity towards SIRT2. Meanwhile, compound 1 increases H3K9 acetylation in BxPC3 cell, indicating the cell permeability and activation in cultured cells.305 Further optimization through virtual screening results in compounds 2–4 with improved potency and better selectivity.299 In biological function studies, all these compounds reduce the production of TNF-α in BxPC-3 cells, especially compounds 1 and 3, which exhibit more than 50% inhibition at 100 μM. While in cellular glucose uptake assay, only compounds 1, 3, and 4 display obvious (1.3–1.9 folds) increasement of glucose uptake in BxPC-3 cells. SIRT6 deletion enhances the sensitivity of cancer cells to chemotherapeutics.109,130 For verification of this function, compounds 2 and 3 exhibit improved anticancer activity of gemcitabine against pancreatic cancer cells, indicating that SIRT6 inhibitors may increase the sensitivity of tumor cells to commonly used anticancer agents.299 Glucose uptake enhancement by SIRT6 inhibition is involved in the potential for T2DM treatment.68,170 To test the anti-T2DM effects of these analogues, compound 1 was evaluated in HFD-fed mouse model due to its suitable preliminary ADME data. Treatment with compound 1 decreases mouse weight and orally improves glucose tolerance, accompanied by increased expression of glucose transporters and p-Akt level. Notably, SIRT6 inhibition by compound 1 reduces the insulin revel, suggesting that the anti-diabetic effects of SIRT6 inhibitors rely on the improvement of cellular glucose uptake and glycolysis, instead of the insulin secretion.172 Besides the function in anti-T2DM, compound 1 also showed the ability to reduce DC migration and delay the onset of autoimmune disease EAE.252
Figure 4.
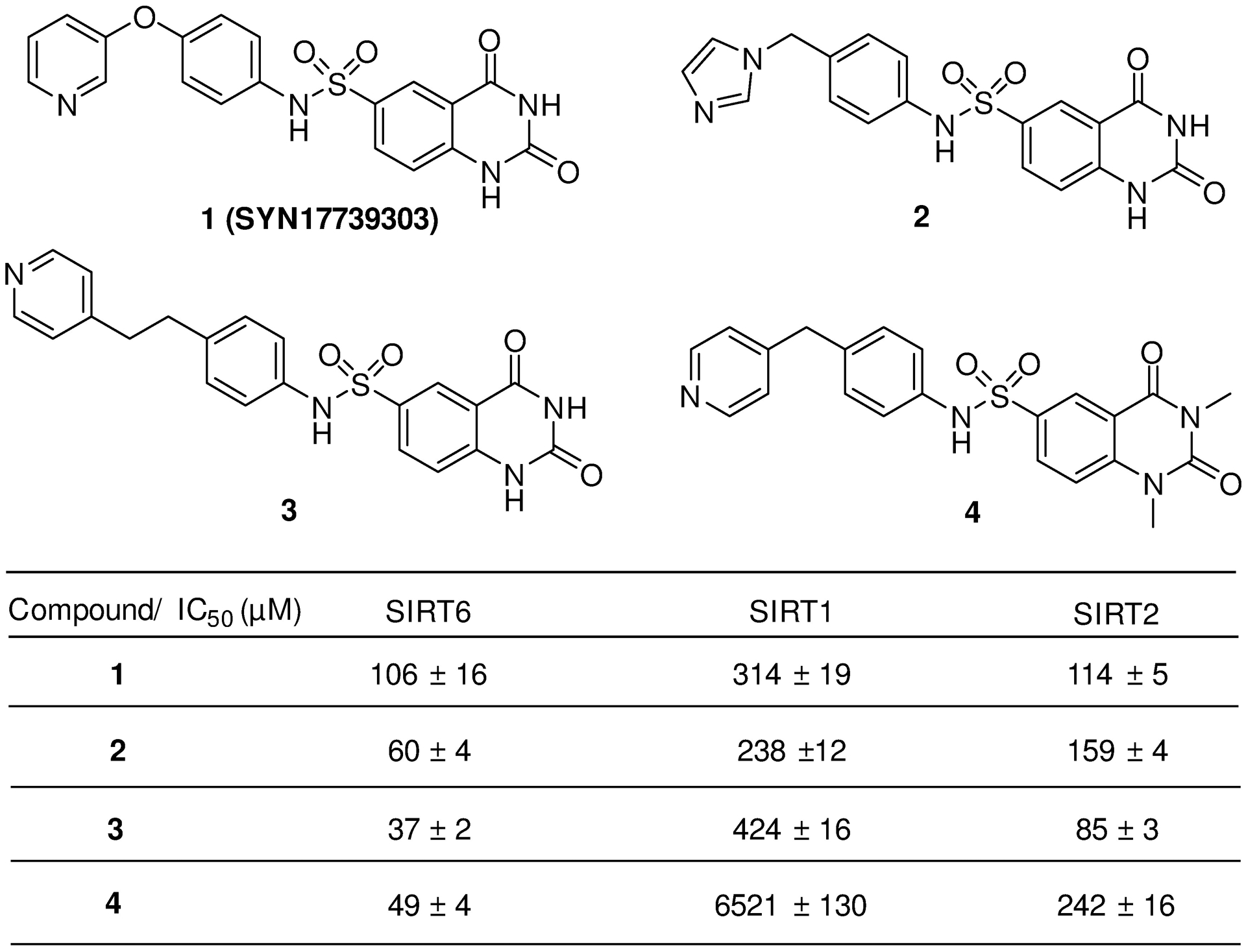
The structures and potency of quinazolinediones as SIRT6 inhibitors.
5.1.2. Salicylates
As shown in Figure 5, compound 5 (OSS-128167) was the first selective SIRT6 inhibitor discovered from the in silico screening with a moderate potency of micromolar range, but a decent selectivity over SIRT1 and SIRT2. Likewise, the salicylic moiety forms key hydrogen bonds and stacking interaction with residues in NAM binding pocket, through hydroxyl and carboxyl groups and aromatic phenyl ring, respectively. It exhibits time-dependent increased H3K9 acetylation, cellular glucose uptake enhancement and TNF-α inhibition.305 It induces chemo-sensitization in primary and drug-resistant multiple myeloma cells to DNA damage agents. SIRT6-KD abolishes the increased anticancer activity by co-administration with compound 5.116 SIRT6 may promote HBV transcription and replication by upregulating the PPAR-α expression.287 Both the HBV core deoxyribonucleic acid (DNA) and the 3.5-Kb (RNA) were reduced in response to compound 5 treatment. Most importantly, the suppression function was further determined in HBV TG mice, providing a proof-of-concept study towards anti-virus through SIRT6 inhibition.287 From the same in silico screening reported for quinazolinedione-like hits, compounds 6 and 7 were reported with increased SIRT6 inhibitory activity and comparable selectivity to that of compound 5. Biological evaluation revealed the glucose uptake increasement, TNF-α inhibition and synergistic chemo-sensitization of pancreatic cancer cells to gemcitabine, by the treatment with these inhibitors.306
Figure 5.
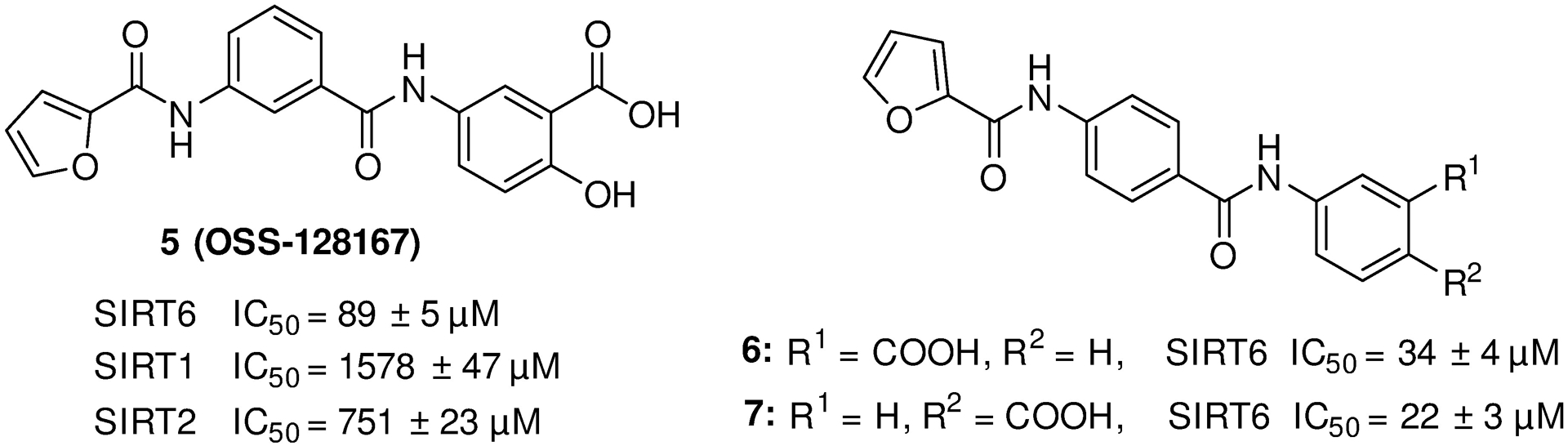
The structures of salicylate analogs as SIRT6 inhibitors.
5.1.3. Trichostatin A
Trichostatin A (TSA, 8 shown in Figure 6) was initially identified as a potent and selective class I/II HDAC inhibitor with low nanomolar potency, and found to also suppress SIRT6 transcription by upregulating acetylated E2F1.307 It is reported as a direct SIRT6 inhibitor by significantly inhibiting H3K9 deacetylation in a dose-dependent manner with a Ki value of 2.02 μM. It is selective to SIRT6 with no inhibition over other SIRT subtypes (e.g. SIRT1, SIRT2, SIRT3, SIRT5) at 50 μM concentration. TSA induces noticeable H3K9 acetylation in HEK293 cells at the low concentration of 1 μM.54 The co-crystal structural analysis of SIRT6/TSA complex shows a perfect overlay of hydroxamate with NAM carbamide. TSA inhibits SIRT6 by directly binding to it with a Kd of 227 μM, while more extensive mechanistic studies remain to be elucidated.297
Figure 6.
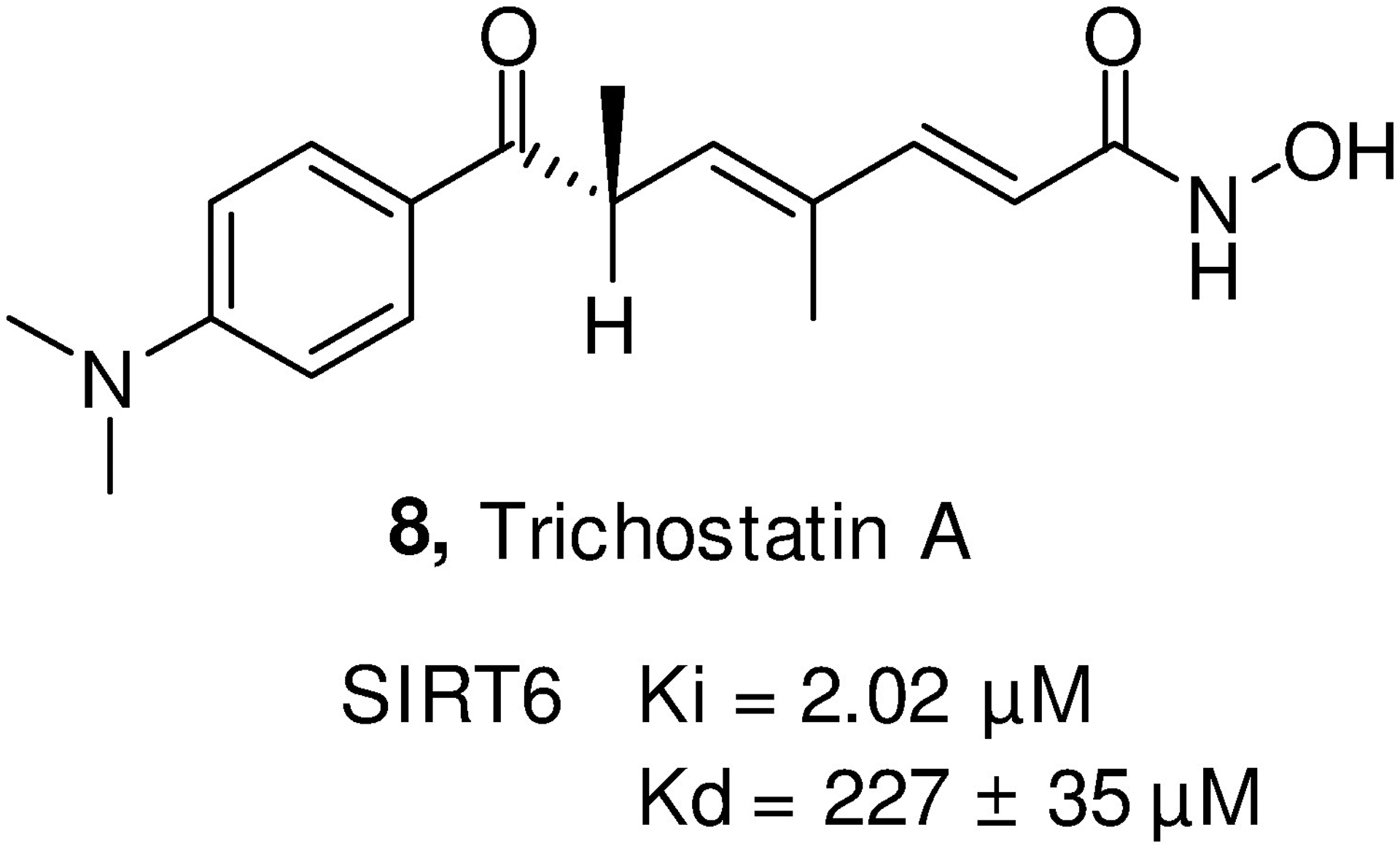
The structure of trichostatin A.
5.1.4. Natural polyphenols
Natural polyphenol analogues (Figure 7) were identified as either SIRT6 activators or inhibitors.300,308–312 There are three structural features that distinct some of them as potent inhibitors. First, the catechol moiety assumes a position overlapping with NAM moiety in the binding pocket with a slightly rotated pose compared to that for activators. Second, the saturated chroman ring with trans-configuration offers a tilted position in the binding pocket that drives SIRT6 protein to a favorable conformation for inhibitors. Third, the galloyl moiety linked to hydroxyl extends the molecule to a deeper area towards V154 residues likely to enhance the inhibitory activity.300,310 Among them, CG (compound 12) and gallocatechin gallate (compound 13) are the most potent analogues with an IC50 value of 2.5 μM and 5.4 μM, respectively, in the HPLC-based SIRT6 deacetylation assays.310,313 In addition, CG was detected to successfully increase the H3K56 acetylation at 25 μM in SIRT6-WT human U2OS cells, but not in SIRT6-KO cells.300
Figure 7.
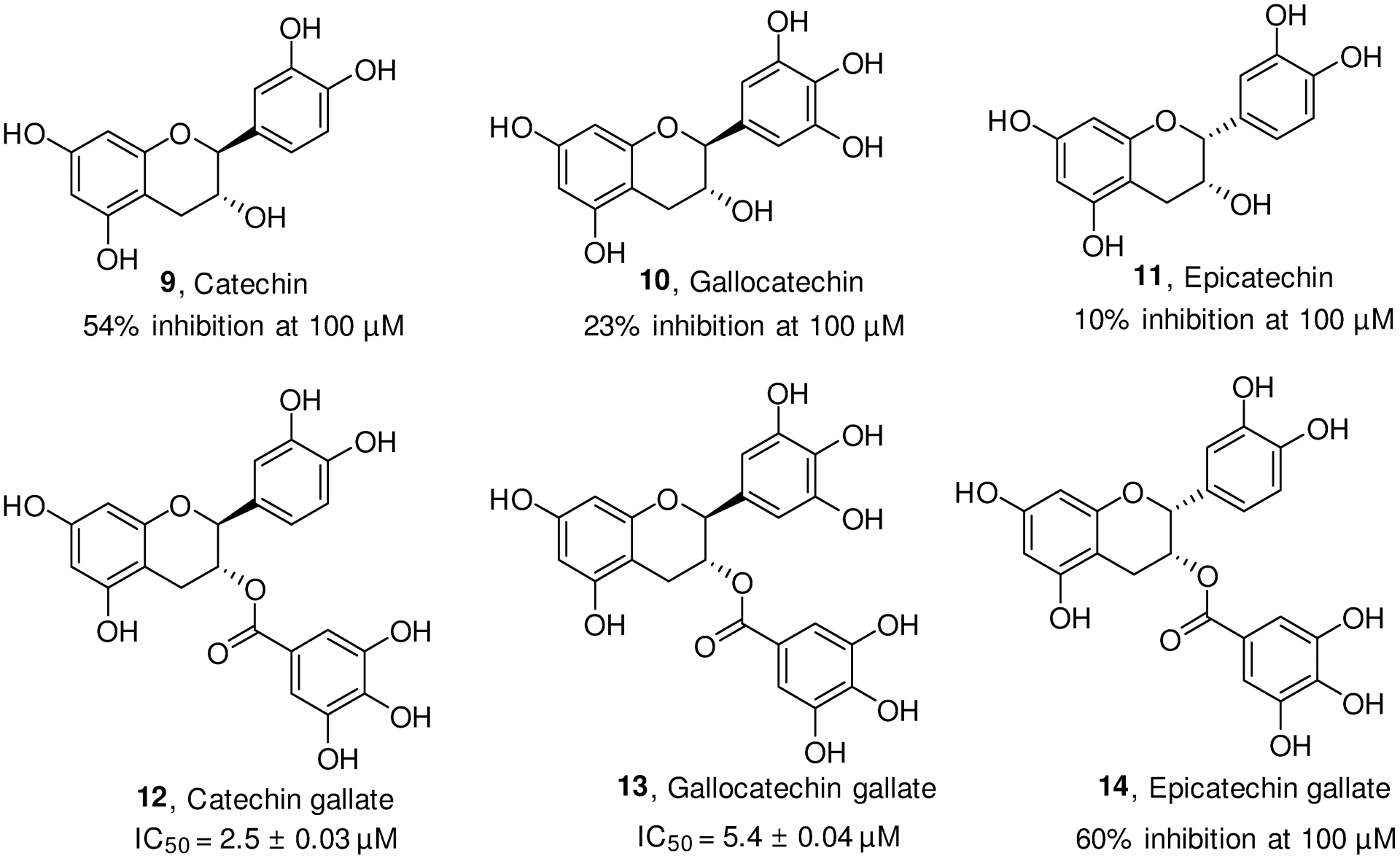
The structures of natural polyphenols as SIRT6 modulators.
5.1.5. Lysine-based peptides
Lysine-based peptides were known to inhibit various SIRT subtypes including SIRT6. As shown in Figure 8, the characteristic of these peptides is the unique Nε-modified lysine residue, which mimics the endogenous substrates to occupy the conserved Nε-acetyl lysine binding site.314,315 The catalytic mechanism-based design provides these modulators with more chance to possess good activity. The first effort from Kokkonen et al. designed a series of pentapeptides and shortened peptides containing Nε-thioacetyl lysine. The pentapeptide, AKK(thioAc)LM (compound 15) was the most potent compound with an IC50 value of 47 μM for SIRT6, but no selectivity over SIRT1 and SIRT2.316 However, the shortened peptides such as compounds 18 and 19 show very weak activity on both H3K9 and H3K56 deacetylation.316,317 Later, Lin and colleagues developed a fluorogenic high-throughput assay for screening the anti-deacylation activity of SIRT6 modulators based on its ability to hydrolyze myristoyl lysine.318 With this method, they and other researchers discovered a series of Nε-fattyacyl lysine-contained pentapeptides with low micromolar anti-demyristoylation activity. Compound 16 (BH-TM4) effectively inhibits SIRT6 in both the demyristoylation and deacetylation activity assays with only 1.5–3 folds of selectivity in inhibiting other SIRTs.315,319 The cyclic peptide inhibitor compound 17 was developed with sub-micromolar potency against SIRT6 demyristoylation and moderate selectivity over other subtypes. Notably, compound 17 exhibits no obvious inhibitory potency on H3K9 deacetylation in pancreatic cancer BxPC-3 cells, likely due to the unmatched intracellular function assay or its poor cell permeability and incapability to inhibit SIRT6 inside cells.320 An interesting study on anti-deacetylation and pro-demyristoylation activity was carried out with the lysine-based short peptides. Specifically, compound 20 with dodecylamide at the tail shows significant inhibition against SIRT6 deacetylation, but 1.52-fold activation to its demyristoylation. Length extension of the tail to 18 carbons results in compound 21 with an abolished anti-deacetylation but a retained pro-demyristoylation potency. Moreover, compounds 20 and 21 increase the basal secretion of TNF-α in THP-1 cells, which is most probably ascribed to the promotion of demyristoylation activity.321
Figure 8.
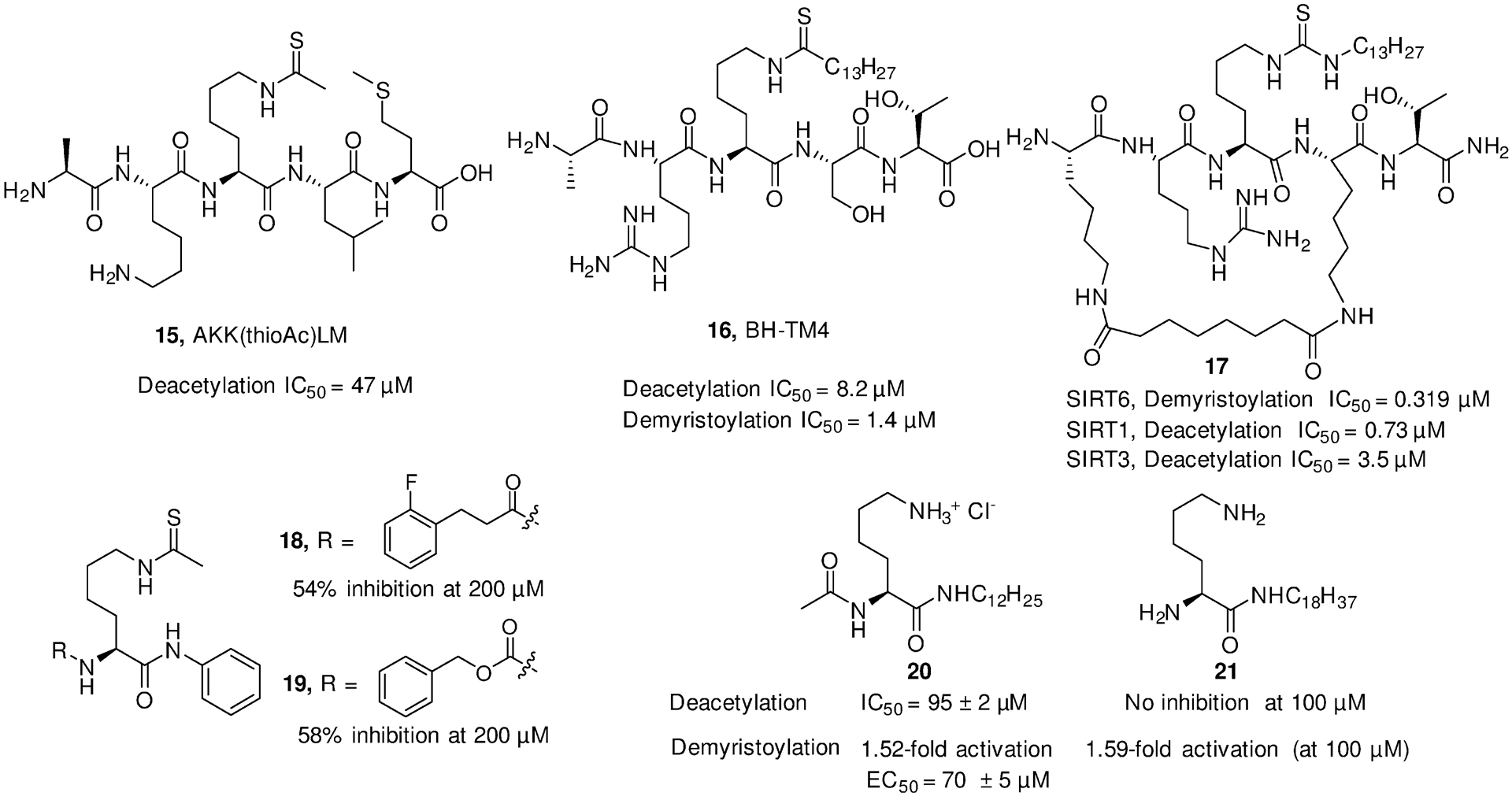
The structures of lysine-based peptides as SIRT6 modulators.
5.1.6. Phenylpiperazines
Recently, an in-house drug screening program has identified Hit01 (compound 22, Figure 9) with an IC50 of 35 μM in the H3K9 deacetylation fluorescence assay, which is considered as a promising hit of SIRT6 inhibitors. Structural optimization focused on the substituents of piperazine and phenyl significantly improves the activity, affording compounds 23–25 with low micromolar IC50 values. The most potent compound 23 binds with SIRT6 with a Kd value of 9.76 μM. In BxPC-3 cells, compound 23 increases H3K9 and H3K18 acetylation, as well as upregulates the expression of GLUT-1 in a dose-dependent manner. Its SIRT6 inhibitory activity was further demonstrated in a T2DM animal model, indicating that it significantly reduced the blood glucose content in HFD-fed mice at a dosage of 30 mg/kg with no obvious body weight loss.173
Figure 9.
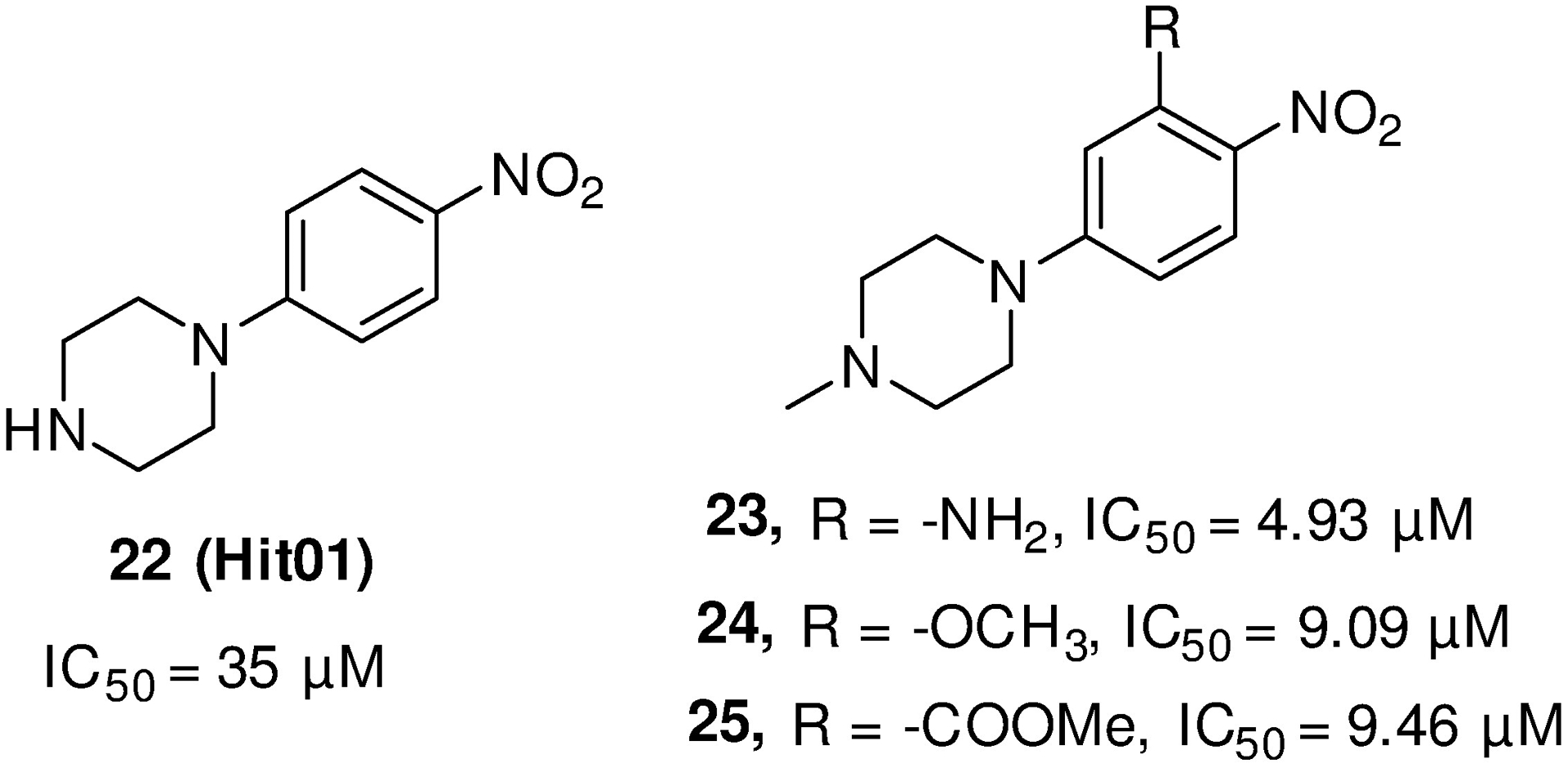
The structures of phenylpiperazines as SIRT6 inhibitors.
5.2. Activators
5.2.1. Fatty acids and fatty ethanolamides
Studies from Denu and colleagues indicate that long-chain FAs (Figure 10) can stimulate deacetylase activity but inhibit the demyristoylase of SIRT6. FAs competitively bind to the hydrophobic pocket shared with myristoylated peptide substrates and increase the affinity of SIRT6 for acetylated substrates.34 Oleic acid (compound 27) exhibits a higher maximum activation fold (FAmax) than that of myristic acid (compound 26), whereas dodecanoic acid was found unable to stimulate SIRT6, indicating a limitation of shorter chain length for deacetylation activation. Modification of the acids leading to the ethanolamide analogues increases the activation efficiency but reduces the FAmax.322 The most potent analogues of this series are lysophosphatidic acid and N-linoleoylglycine, exhibiting dozens of activation folds to SIRT6 deacetylation.35
Figure 10.
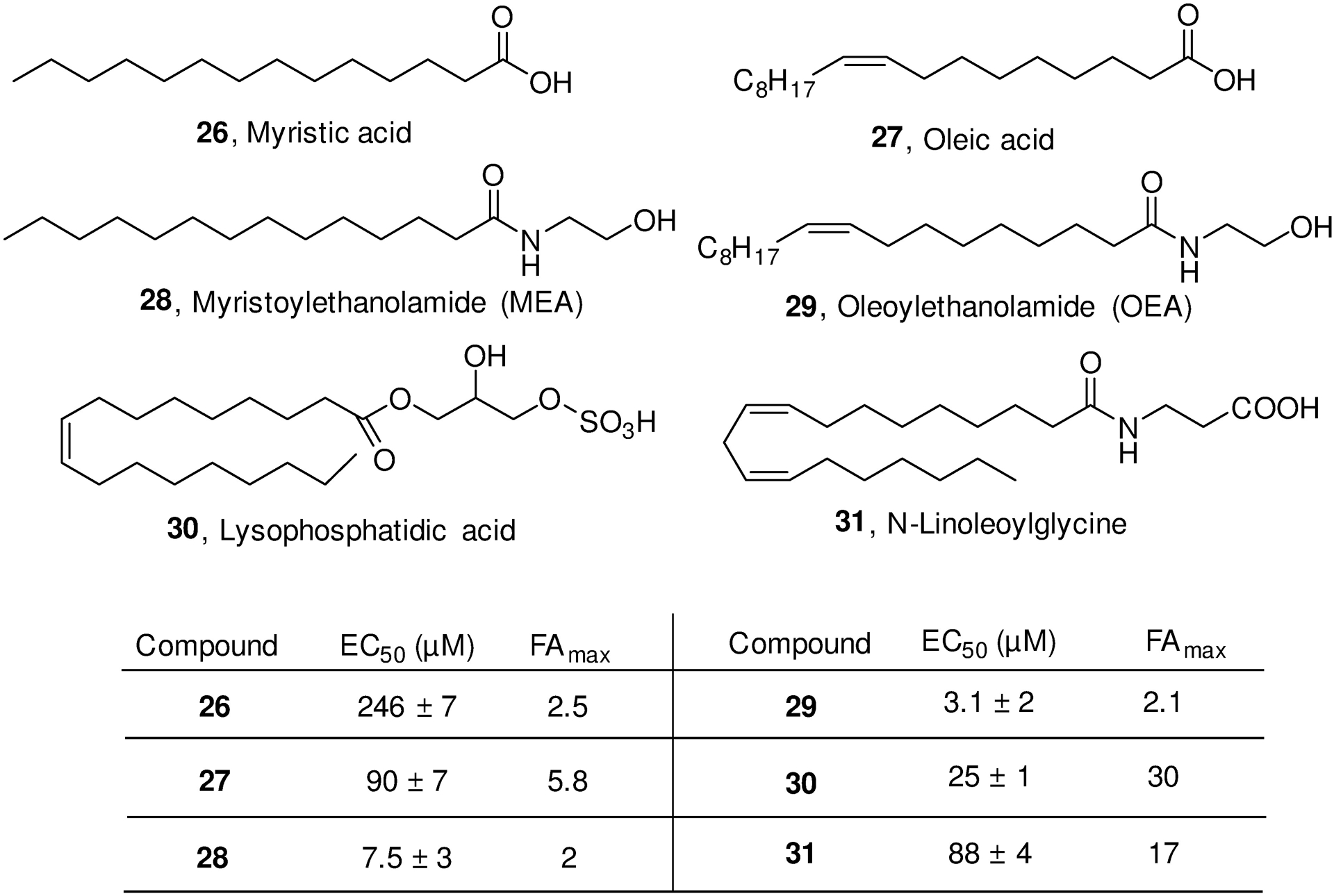
The structures of fatty acids and fatty ethanolamides as SIRT6 activators.
5.2.2. Aromatic acids
In addition to fatty acid, Denu group has also identified several aromatic acids (Figure 11) as SIRT6 H3K9 deacetylation activators.35 Among them, TTNPB (compound 32) and CL-4 (compound 33) display the best biological activities and highest FAmax values, as well as good selectivity over other SIRT subtypes (e.g. SIRT1, SIRT2, SIRT3, SIRT5). A brief structural optimization based on CL-4 suggests the importance of chlorine atom and benzamide moiety, as elucidated by the abolished activity in CL-5A (compound 34) and the improved potency in CL-5D (compound 35). Notably, CL-5D induces 4-fold activation at a low concentration of 3 μM, while the same effect shown in CL-4 is at 35 μM, indicating a feasibility for further improving the potency and the physicochemical profiles by optimizing on the right side of the lead CL-4. In addition, CL-5D was determined to bind the same hydrophobic pocket as that for long chain FAs and inhibit demyristoylation with a Ki value of 13.4 μM.35
Figure 11.
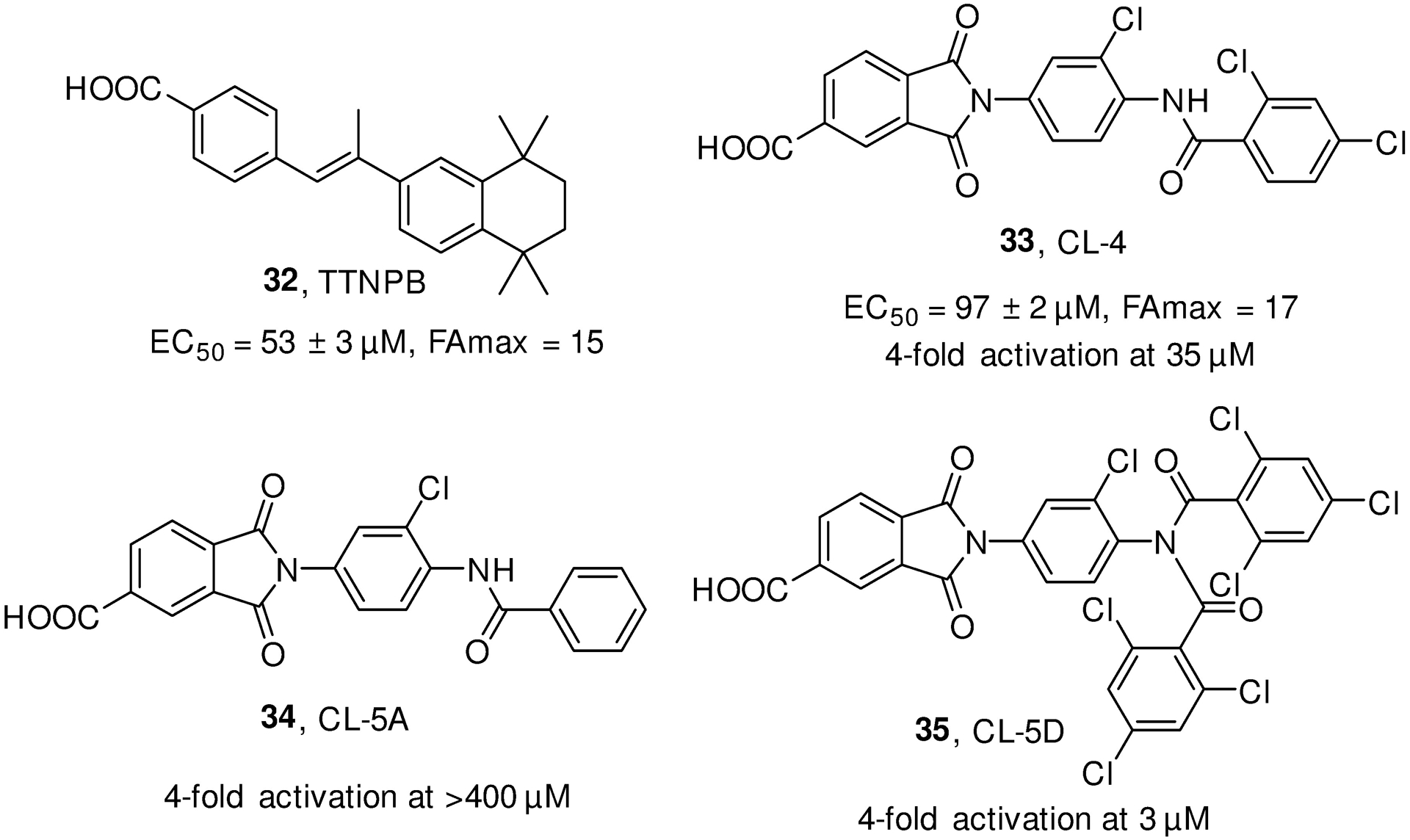
The structures of aromatic acids as SIRT6 activators.
5.2.3. UBCS039
UBCS039 (Compound 36, Figure 12) was identified as a direct SIRT6 activator with a binding affinity of 44 μM, which dose-dependently increases SIRT6 deacetylation with a maximum about 2-fold stimulation and an EC50 value of 38 μM. The first co-crystal structure of SIRT6/activator complex was solved by soaking UBCS039 with the truncated SIRT6 protein. The pyridine moiety locates at the hydrophobic NAM binding pocket and acts as the major anchoring point by interacting with Pro62 residues through polar contact or H-bond.296 In Hela and HCT116 cancer cells, UBCS039 efficiently induces H3K9 and H3K56 deacetylation in a time-dependent manner. Further, it induces autophagy-associated cell deaths in SIRT6 wild type instead of SIRT6 deleted cancer cells, revealing the potential of pharmacological activation of SIRT6 by small molecule activators for cancer treatment. Despite the relatively low potency, UBCS039 may be considered as a good hit for further structural optimization to treat autophagy-related diseases.323
Figure 12.
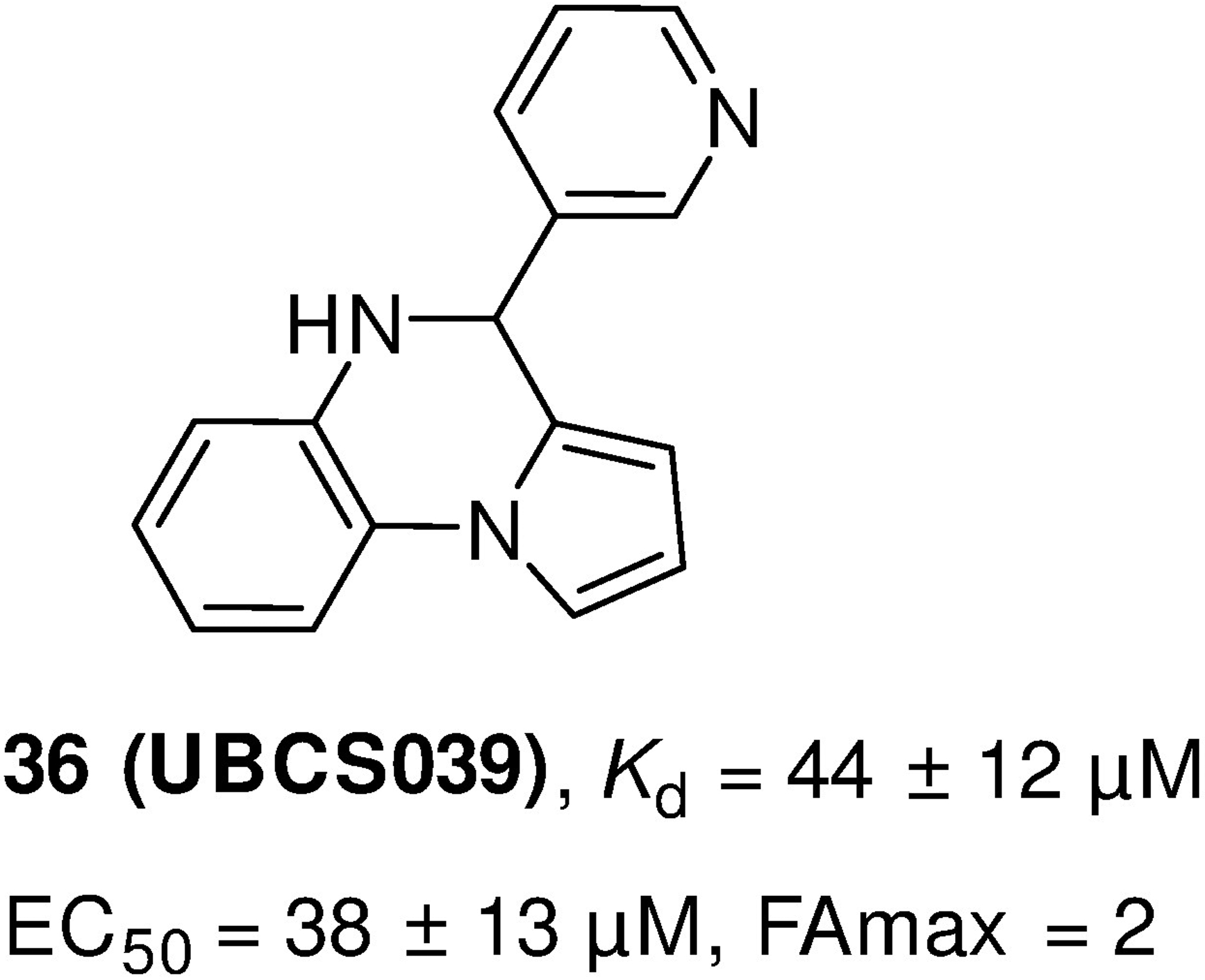
The structure of SIRT6 activator UBCS039.
5.2.4. Flavonoids
As shown in Figure 13, both quercetin (compound 37) and luteolin (compound 38) were initially identified as weak SIRT6 inhibitors with approximately 50% inhibition at 100 μM, but increased H3K9 deacetylation with maximal activation of 10-fold at higher concentrations.309,310 Another MS-based assay with the H3K9Ac substrate found a single dose-dependent increase in SIRT6 activity and no evidence for the reported SIRT6 inhibition by lower quercetin concentrations.300 Quercetin can increase the H3K18 deacetylation in Hela nucleosomes, and H3K9 and H3K18 deacetylation in free histones at 5 μM. The activity was found significantly increased in cyanidin (compound 39) with an EC50 of 460 μM and FAmax of 55-fold, which may be ascribed to the removal of unfavorable interaction of keto group in quercetin with M157 residue of SIRT6 (Figure 3). Isoquercetin (compound 40) shows a retained SIRT6 activation, with a lower potency (about 2.3-fold of FAmax), but a significant selectivity over SIRT1, SIRT2, SIRT3 and SIRT5. According to the co-crystal structural analysis, the sugar moiety at isoquercetin shows no obvious interactions for the SIRT6 binding but clashes with the alternative binding site of the other SIRT isoforms.300 The stimulation of SIRT6 by cyanidin was further verified in ameliorating the progression of osteoarthritis. Treatment of IL-1β-induced human OA chondrocytes with cyanidin may be associated with the activation of the SIRT6 expression, thereby suppressing the expression of inflammatory cytokines to inhibit OA, while SIRT6 silencing abolishes the protective effects of cyanidin. Moreover, cyanidin significantly alleviates the aggressive course of OA in surgical DMM mouse models of OA.163
Figure 13.
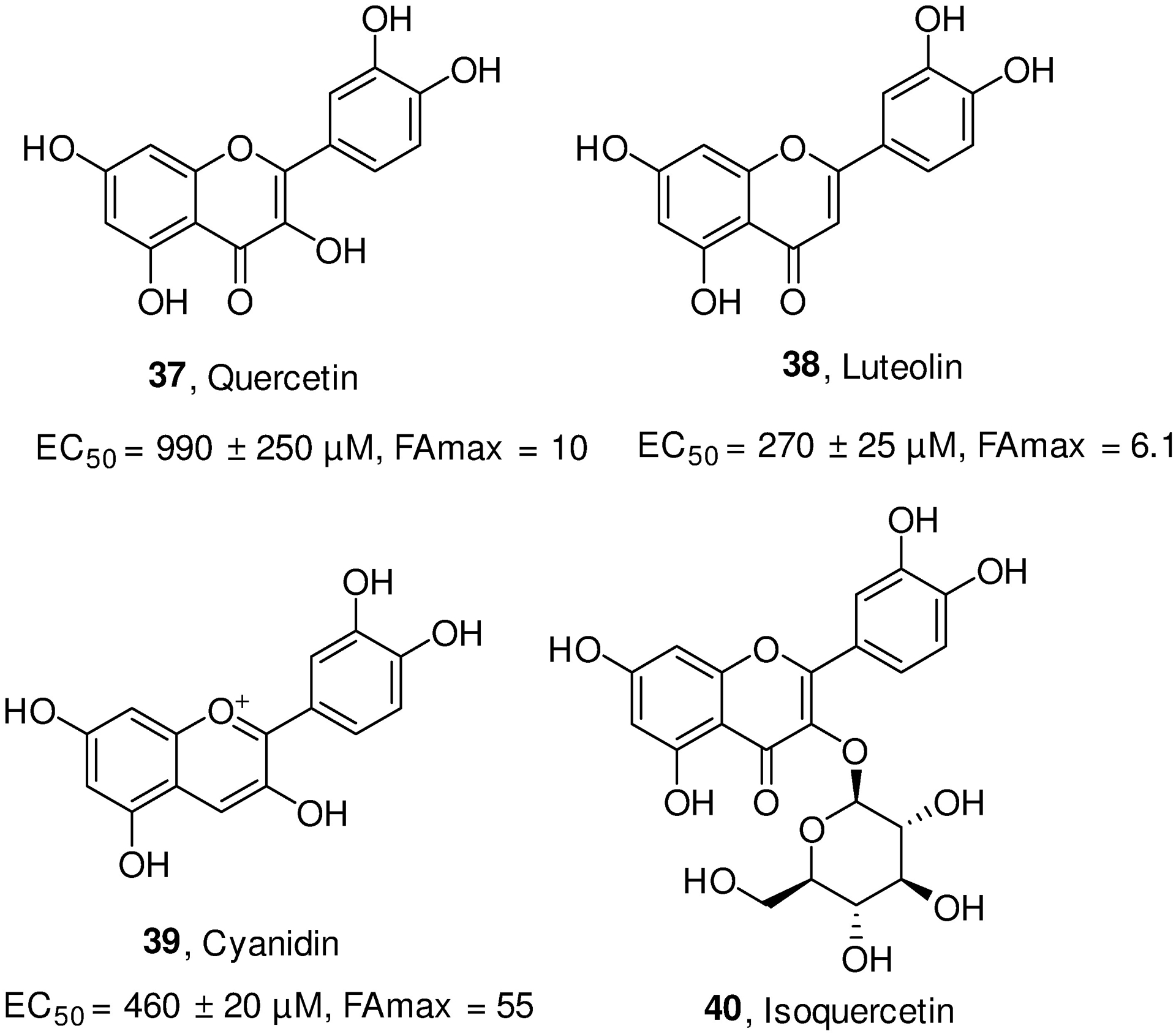
The structures of flavonoids as SIRT6 activators.
5.2.5. Auinoline-4-carboxamides
A recent virtual screening study by Chen et al. reported a series of compounds containing scaffold of quinoline-4-carboxamide as SIRT6 activators. Structure-based optimization identified the most potent compound, 2-(1-benzofuran-2-yl)-N-(diphenylmethyl) quinoline-4-carboxamide (compound 41, Figure 13), which exhibited EC1.5 value of 0.58 ± 0.12 μM (EC50 value of 5.35 ± 0.69 μM) and 0.72 ± 0.25 μM (EC50 value of 8.91 ± 1.81 μM) against SIRT6-dependent peptide deacetylation and demyristoylation, respectively. This compound also showed excellent selectivity for SIRT6 against other tested SIRT family members and HDACs. In addition, it exhibited antiviability and antiproliferation activities against PDAC cells (PANC-1, BXPC-3, MIAPaCa-2, and AsPC-1), as well as decreased levels of H3K9ac, H3K18ac, and H3K56ac in PANC-1 and BXPC-3 cells. In vivo, compound 41 significantly inhibited the tumor growth at the dose of 100 mg/kg in the subcutaneous PANC-1 tumor xenograft model. Despite the obvious poor water solubility and only 4.24% bioavailability, this compound exhibits a therapeutic potential by activating SIRT6 for PDAC therapy and is worthy of further in-depth optimization as the lead molecule.
5.2.6. Allosteric activators
To identify allosteric SIRT6 activators, Huang et al. recently virtually docked more than 5,000,000 compounds into a predicted allosteric pocket around Phe82 and Phe86 on the surface of SIRT6, and consequently verified two benzenesulfonamide analogues with substantial activity in stimulating SIRT6 deacetylation (Figure 3c).301,324 Structural optimization on the hit led to MDL-800 (compound 42) and MDL-801 (compound 43, Figure 15), both of which show a relatively high efficiency with an EC50 value of 10.3 μM and 5.7 μM, respectively. In addition, both compounds exhibit a significantly enhanced SIRT6 deacetylase activity at the concentration of 100 μM in the fluorescence-based H3K9 deacetylation assay (more than 22-fold activation), as well as in HPLC- and MS-based assays. Moreover, MDL-800 was evaluated for 18 diverse HDAC members, showing more than ten-fold selectivity over SIRT1–5, SIRT7 and HDAC1–11.
Figure 15.
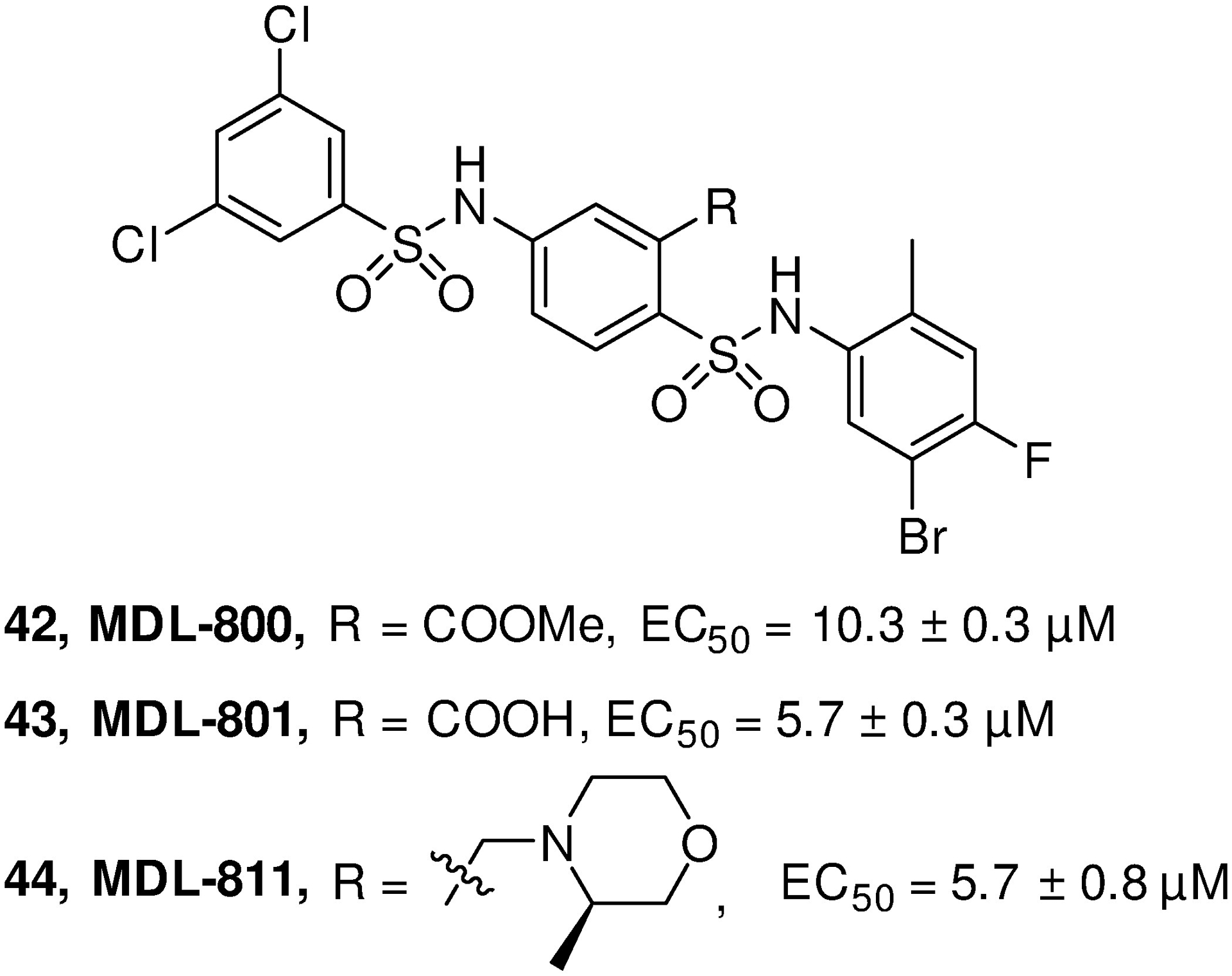
The structures of MDL-800, MDL-801 and MDL-811.
The biological function of MDL-800 was further characterized due to its proper permeability coefficient for cellular uptake and accumulation in cells. MDL-800 was shown to significantly promote H3K9 and H3K56 deacetylation in a dose-dependent manner in human HCC Bel7405, PLC/PRF/5 and Bel 7402 cell lines. The decreased acetylation in HCC cells causes cell-cycle arrest at G0-G1 phase that results in cancer proliferation inhibitory effects of MDL compounds. Conversely, negligible effects on both histone deacetylation and cell-cycle arrest were observed in SIRT-KO cell lines after MDL-800 treatment. The anticancer activity of MDL-800 was further evaluated in vivo using immunocompromised mice engrafted with Bel7405 cells. Moderate antitumor efficacy was observed at the doses of 150 mg/kg with treatment of MDL-800 for 14 days. Western blotting of the tumor tissues shows decreased expression of H3K9ac and H3K56ac, as well as properly regulated cell-cycle biomarkers. Consistently, SIRT6-KO xenograft mice showed no obvious tumor shrink when treating with MDL-800.301 In addition, MDL-800 was also demonstrated to dose-dependently increase SIRT6 deacetylase activity in 12 NSCLC cell lines and markedly suppress the tumor growth in HCC827 cell-derived xenograft model at the dose of 80 mg/kg via intraperitoneal route.325 A structural modification focused on improving the characteristics by introducing water-soluble fragments to the central phenyl ring of MDL-801 results in compound MDL-811 with an EC50 value of 5.7 μM (compound 44). MDL-811 significantly inhibits proliferation on diverse colorectal cancer cell lines and patient-derived organoids. Moreover, the in vivo anti-tumor efficacy of MDL-811 was further demonstrated in HCT116 cell line- and patient-derived xenografts, as well as in the APCmin/+ spontaneous colorectal cancer model while no obvious body weight loss and other abnormal behavioral signs were observed.326 Despite only modest in vivo activity, these results support a therapeutic benefit for cancer treatment by activating SIRT6.
6. CONCLUSIONS AND FUTURE PERSPECTIVE
Accumulating evidence demonstrates the critical roles of SIRT6 in regulating a variety of human diseases. For cancer, the regulation of SIRT6 in DNA damage repair, glycolysis, cell senescence and apoptosis bring its double-edged sword role in both cancer prevention and promotion. For diabetes, SIRT6 downregulation in T2DM model promotes the expression of glycolytic genes (e.g. GLUT1, GLUT4, PDK1 and LDHA) and consequently increases glucose uptake and lowers blood sugar, ascertaining anti-T2DM function by SIRT6 suppression. However, the activity involved in suppressing gluconeogenesis and enhancing insulin secretion and sensitivity from SIRT6 activation may also result in reduction of the blood glucose. In addition, SIRT6 prevents obesity formation by modulating the homeostasis of visceral fat, LDL-cholesterol, and triglycerides, as well as thermogenesis in brown adipocytes, thereby decreasing the prevalence and incidence for T2DM. Simultaneously, the positive regulation in hepatic fat metabolism by SIRT6 activation also protects liver from formation of steatohepatitis. For inflammation, the enzymatic activity of SIRT6 may transcriptionally increase the secretion of TNF-α and repress the expression of NF-κB-dependent genes, which are critical to pro- and anti-inflammatory functions, respectively. The physiological functions of SIRT6 primarily define it as an anti-inflammatory protein given its important biological role in either directly or indirectly inhibiting the expression of NF-κB target genes and other pro-inflammatory cytokines. The anti-inflammatory role of SIRT6, as well as its function in suppressing cellular senescence and inhibiting macrophage infiltration and polarization synergistically exert prevention in OA and RA diseases. For heart diseases, SIRT6 plays a positive role in protecting heart from cardiac hypertrophy, myocardial fibrosis and failure by repressing the AKT and activating AMPK and the related downstream signaling pathways (e.g. p300, NFATc4, ACE2). In addition, the physiological function of SIRT6 activation in decreasing LDL cholesterol, reducing macrophage foam cell formation and preventing endothelial dysfunction exerts protective effects in atherosclerosis and coronary heart diseases. For neurodegenerative diseases, SIRT6 plays protective function in AD via maintaining genomic stability and preventing Aβ42- and pTau-induced DNA damage in brain. In addition, SIRT6 functions as a preventor in cerebral ischemia through its activity in anti-inflammatory and antioxidant effects and preserving blood–brain barrier integrity. However, the suppression of AKT in brain by SIRT6 activation accounts for the pathogenic role in PD and depression disorder, and SIRT6 depletion confers neuroprotection from MPTP-induced PD and antidepressant-like effects in CUMS rat model, respectively. In addition, SIRT6 was found to play a role in controlling neuroinflammation with effects in delaying the onset of EAE disease. SIRT6 has also been verified to exert pleiotropic protective actions in renal and corneal injury through transcriptionally regulating a number of inflammation- and apoptosis-related signaling pathways (e.g. ERK1/2, TNF-α, Nrf2, β-catenin, Notch1 and Notch4). Moreover, SIRT6-KD is believed beneficial to viral infections, revealing its antiviral role by SIRT6 upregulation. SIRT6 overexpression promotes HBV transcription and replication and the effects can be reversed by treating with SIRT6 inhibitor OSS_128167 as aforementioned.
Collectively, the growing understanding of SIRT6 reveals its complex roles in human diseases. For example, SIRT6 works as a gatekeeper of DNA repair in healthy conditions that prevents cancer initiation, but the same function in tumors promotes cancer cell proliferation. SIRT6 concurrently regulates multiple signaling pathways in a disease model that may exert different functions. In A549 cancer cells, SIRT6 exhibits anticancer activity by decreasing Twist1 expression but drives EMT to promote cancer proliferation. Moreover, SIRT6 plays an opposite role in regulating the same signaling pathways in different disease models such as ERK, which is inhibited in HepG2 cells but stimulated in Huh-7. In addition to cancer, SIRT6 deficiency in renal results in upregulation of Notch1 and Notch4 and exacerbates podocytes injury, while the Notch singling is impaired in injury cornea by SIRT6 deletion. Thus, the exact role of SIRT6 in human disease depends on the exact pathogenic types, as well as the dominant signaling pathways.
Despite the wealth of knowledge regarding the significance of SIRT6 in human diseases, the discovery of potent SIRT6 modulators is in its infancy. To date, only limited modulators are identified to be active from in vitro assays and very few of them have been further observed to successfully modulate deacetylation in cells and show in vivo efficacy in animal models of SIRT6-related diseases. Despite limited in vitro activity and moderate selectivity, inhibitor SYN17739303 has demonstrated to be efficacious in vivo against T2DM, as well as the onset of EAE, representing the first proof-of-concept research to understand therapeutic potential of SIRT6 for human disease treatment. Later, OSS-128167 was found to inhibit HBV transcription and replication in HBV TG mouse model with no obvious hepatotoxicity, indicating a promising therapeutic strategy for HBV treatment by SIRT6 inhibition. The more potent lysine-based peptides, especially cyclic peptide inhibitor 17, shows sub-micromolar inhibition against SIRT6 demyristoylation and moderate selectivity over other subtypes. However, its weak activity in cellular H3K9 deacetylation and the foreseeable poor physicochemical properties hinder it for further preclinical development. Among the activators, small molecules exhibit an improved capability of overcoming the limitations of long-chain fatty acids and fatty ethanolamides such as poor cellular permeability, aqueous solubility, and metabolic stability, and may be worthwhile for further studies. Intriguingly, the allosteric activator MDL-800 efficiently inhibits tumor growth in Bel7405 xenograft HCC model at the doses of 150 mg/kg and decreases expression of H3K9ac and H3K56ac in tumor tissues. Given this breakthrough finding in vivo, the target-specific molecules that directly activate SIRT6 may offer great therapeutic potential and hold promise for cancer treatment. Although challenges exist, such as the poor potency and low selectivity, difficulty in separating the activation and inhibition property and not well-established in vivo assays in some disease models, opportunities appear to be exciting for the development of direct SIRT6 modulators. First, only a very limited number of small molecule modulators are available, leaving the door open for the chemists to discover more potent diverse modulators with novel scaffolds, representing an unmet medicinal endeavor. Second, given the available co-crystal structures and the elucidation of specific amino acid residues for small molecular binding, computer-aided virtual screening and computer-assisted rational drug design may significantly facilitate the chemical probe and drug discovery to enhance the structural diversity and potency as well as drug properties of SIRT6 modulators. The discovery of SYN17739303 and OSS-128167 appears to be exciting examples of structure-based drug design. Third, several obtained modulators (e.g. SYN17739303, OSS-128167 and MDL-800) that have been validated in vivo may be considered as promising lead compounds for further structural optimization to achieve more potent modulators with better druglike properties. Last but not least, despite the moderate potency, modulators with low small molecular weight such as the aforementioned phenylpiperazines and UBCS039 should not be ignored, which may offer wide chemical space for further structural optimization through fragment-based drug design327–329 to yield enhanced pharmacological profiles and physicochemical properties of new chemical entities as SIRT6 modulators.
Figure 14.
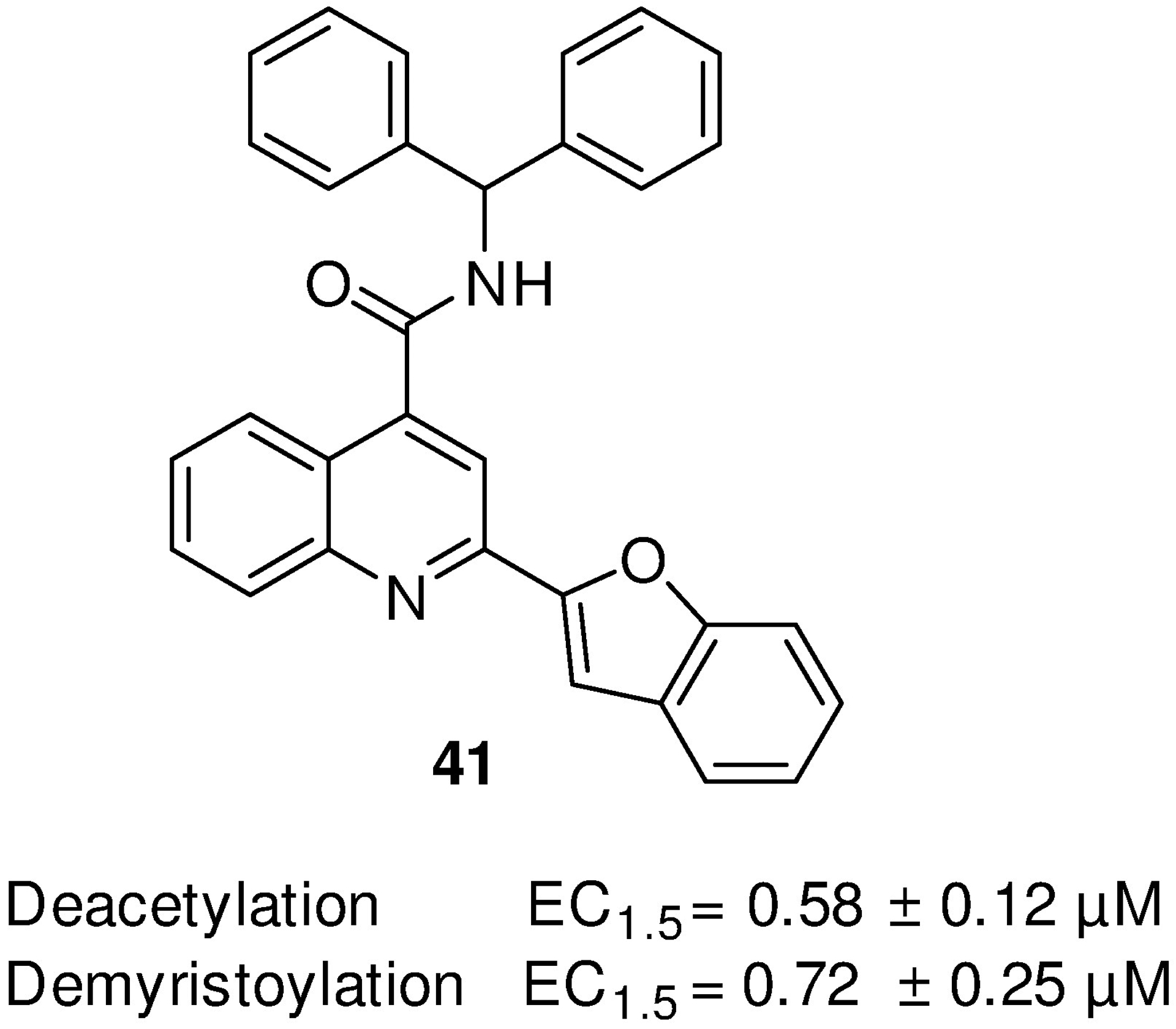
The structure of compound 41 as a SIRT6 activator.
Table 1.
The substrates of SIRT6.
| Enzymatic action | Substrates | Site | Physiological function | |
|---|---|---|---|---|
| Deacetylation | Histones | H3K9 | K9 | Modulate telomeric chromatin,14 NF-κB-dependent gene expression.17 Control glycolytic genes expression.42 Regulate cancer cell apoptosis.79 |
| H3K56 | K56 | DSB repair and genomic stability.7 | ||
| H3K18 | K18 | Pericentric transcriptional silencing.45 | ||
| Nonhistones | GCN5 | K549 | Regulate the transcription of gluconeogenic gene PGC-1α.46 | |
| PKM2 | K433 | Suppress PKM2 nuclear localization and oncogenic functions.47 | ||
| Ku70 | K542 | Attenuate Bax-mediated apoptosis in HCC.48 | ||
| NAMPT | K53/369 | Regulate the enzymatic activity of NAMPT and the secretion of eNAMPT, respectively.49 | ||
| XBP1s | K257/297 | Promote XBP1s protein degradation and confers resistance to hepatic steatosis.50 | ||
| SOD2 | K68/122 | Regulation of antidepressant response.51 | ||
| Prdx6 | K63/209 | Regulation of antidepressant response.51 | ||
| SMAD3 | K333/378 | Protect against liver fibrosis.53 | ||
| SMAD2 | K54 | Protect against liver fibrosis.52 | ||
| P53 | K382 | Regulation of apoptosis and stress resistance.54,55 | ||
| Mono-ADP-ribosylation | Nonhistones | SIRT6 | Mono-ADP-ribosylation SIRT6.58 | |
| PARP1 | K521 | Prompt the DSB repair.5 | ||
| KAP1 | Repress LINE1 retrotransposons.20 | |||
| BAF170 | K312 | Protect cells from oxidative stress.60 | ||
| KDM2A | Promote DNA repair.61 | |||
| Defatty-acylation | Histones | H3K9, H3K18, H3K2732,64 | K9/18/27 | |
| Nonhistones | TNF-α | K19/20 | Regulate the secretion of TNF-α.32 | |
| R-Ras2 | Suppress the plasma membrane localization of R-Ras2.63 | |||
ACKNOWLEDGEMENTS
This work was partially supported by the Breakthrough Award W81XWH-17-1-0071 (J.Z.) from the Department of Defense (DoD), grants EY022694 (W.Z.) and EY031054 (H.L.) from the National Institutes of Health. J.Z. is also supported by the John D. Stobo, M. D. Distinguished Chair Endowment Fund, and the John Sealy Memorial Endowment Fund at UTMB.
Abbreviations:
- Sir2
silent information regulator two
- SIRT
Sirtuin
- KO
knockout
- DSBs
double strand breaks
- SNF2H
sucrose nonfermenting 2 homolog
- JNK
c-Jun NH2-terminal kinase
- PARP1
poly(ADP-ribose) polymerase 1
- WT
wild type
- HR
homologous recombination
- NHEJ
nonhomologous DNA end joining
- ESCs
embryonic stem cells
- NTE
N-terminal extension
- CTE
C-terminal extension
- ADPr
ADP-ribose
- NAM
nicotinamide
- FAs
fatty acids
- KAP1
KRAB-interacting protein 1
- GCN5
general control nonrepressed protein 5
- PGC-1α
coactivator peroxisome proliferator-activated receptor-g coactivator 1- α
- PKM2
pyruvate kinase M2
- Bax
BCL2-associated X protein
- NAMPT
nicotinamide phosphoribosyltransferase
- eNAMPT
extracellular NAMPT
- NADPH
nicotinamide adenine dinucleotide phosphate
- HCC
hepatocellular carcinoma
- XBP1s
X-box-binding protein 1
- SOD2
superoxide dismutase 2
- Prdx6
peroxiredoxin 6
- SMAD3
β–Smad family member 3
- mSIRT6
mouse SIRT6
- Nrf2
NF-E2–related factor 2
- KDM2A
lysine demethylase 2A
- TNF-α
tumor necrosis factor-α
- IGF1
insulin-like growth factor 1
- ERK
extracellular signal-regulated kinases
- pERK
phosphorylation of ERK
- FOXA2
forkhead Box A2
- UBE3A
ubiquitin protein ligase E3A
- ZEB2
zinc finger E-box binding homeobox 2
- EMT
epithelial–mesenchymal transition
- FoxO3
forkhead box O3
- ER
endoplasmic reticulum
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- PIP2
PI-4,5-biphosphate
- mTOR
mammalian target of rapamycin
- JAK
Janus kinase
- STAT3
Signal transducer and activator of transcription 3
- TET1
tet methylcytosine dioxygenase 1
- NSCLC
non-small cell lung cancer
- PHD
prolyl hydroxylase-2
- PKM
pyruvate kinase
- LDHA
lactate dehydrogenase
- HK
hexokinase
- MMP9
Matrix Metallopeptidase 9
- KLF4
krüppel-like factor 4
- BC
breast cancer
- MDM2
mouse double minute 2 homolog
- RUNX2
runt-related transcription factor 2
- GBM
glioblastoma
- MST1
mammalian sterile 20-like 1
- PCBP2
poly(RC) binding protein 2
- NOTCH3
notch receptor 3
- OS
osteosarcoma
- MEK
mitogen-activated protein kinase kinase
- FOXN3
forkhead Box N3
- PCBP2
poly(RC) binding protein 2
- COX-2
cyclooxygenase-2
- PEK
phosphofructokinase
- PEK
phosphofructokinase
- SCCs
squamous cell carcinomas
- PDAC
pancreatic ductal adenocarcinoma
- AML
acute myeloid leukemia
- CIA
collagen-induced arthritis
- HUVECs
human umbilical vein endothelial Cells
- LPS
lipopolysaccharide
- TLR4
toll‐like receptor
- Rspo1
R-Spondin 1
- MCP1
monocyte chemotactic protein-1
- IL
interleukin
- OA
osteoarthritis
- RANTES
regulated upon activation, normal T cell expressed and secreted
- DMM
destabilization of the medial meniscus
- RA
Rheumatoid arthritis
- FLS
fibroblast-like synoviocytes
- RASF
RA synovial fibroblasts
- T2DM
Type 2 diabetes mellitus
- KD
knockdown
- IR
insulin resistance
- GLUT
glucose transporter
- PDK
pyruvate dehydrogenase kinase
- SFN
SIRT1-FoxO3a-NRF1
- HFD
high fat diet
- TRPV1
transient receptor potential vanilloid 1
- CGRP
calcitonin gene-related peptide
- HCP
hepatic glucose production
- PCK1
phosphoenolpyruvate carboxy-kinase 1
- G6PC
glucose-6-phosphatase
- HCD
high-caloric-diet
- GSIS
glucose-stimulated insulin secretion
- Txnip
thioredoxininteracting protein
- TG
transgenic
- GH
growth hormone
- p-ATF2
phospho-ATF29
- KIF5C
kinesin family member 5C
- CK2
the ser/thr kinase casein kinase 2
- pAMPK
phosphorylated AMP-activated protein kinase
- ROS
reactive oxygen species
- TAC
transverse aortic constriction
- TERT
telomerase reverse transcriptase
- TRF
telomere repeat binding factor
- Nmnat2
nicotinamide mononucleotide adenylyltransferase 2
- PI3K
phosphoinositide 3-kinases
- NFATc4
nuclear factor of activated T cells 4
- CFs
cardiac fibroblasts
- Ang II
angiotensin II
- HSCs
hepatic stellate cells
- ERR
estrogen-related receptor
- ACE2
angiotensin-converting enzyme
- CAD
Coronary artery disease
- MI
myocardial infarction
- LDL
low-density lipoprotein
- SREBP
sterol regulatory element-binding protein
- Pcsk9
proprotein convertase subtilisin kexin type 9
- ATG5
autophagy related 5
- TREM-1
triggering receptor expressed on myeloid cells-1
- ECs
endothelial cells
- TNFSF4
TNF superfamily member 4
- CC
cholesterol crystal
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- Nkx3.2
NK3 homeobox 2
- GATA5
GATA binding protein 5
- AD
Alzheimer’s disease
- GSK3
glycogen synthase kinase-3
- PD
Parkinson’s disease
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- CUMS
chronic unpredictable mild stress
- CRMP2
collapsin response mediator protein 2
- I/R
ischemia/reperfusion
- RXR
retinoid X receptor
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- HFHF
high-fat and high-fructose
- ANGPTL4
angiopoietin-like protein 4
- A-FABP
adipocyte fatty acid-binding protein
- NCOA2
nuclear receptor coactivator 2
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- DC
dendritic cell
- NASH
nonalcoholic steatohepatitis
- ALD
alcohol-related liver disease
- AC
alcoholic cirrhosis
- Mt
metallothionein
- UUO
unilateral ureteral obstruction
- DN
diabetic nephropathy
- RP
retinitis pigmentosa
- OS
outer segment
- PPP
pentose phosphate pathway
- Timp1
TIMP metallopeptidase inhibitor 1
- siRNA
small interfering RNA
- DENV
dengue virus
- RLR
RIG-I-like receptor
- HBV
hepatitis B virus
- CG
catechin gallate
Author Biosketch
Gang Liu graduated in College of Pharmaceutical Sciences and Chinese Medicine, Southwest University, China in 2010. Then he joined Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences (CAS), Shanghai, China and worked as an assistant experimentalist for two years. He obtained his Ph.D. degree in 2017 from SIMM, CAS, China under the supervision of Professor Ao Zhang. Dr. Liu is currently pursuing his postdoctoral training at the Chemical Biology Program, Department of Pharmacology and Toxicology at University of Texas Medical Branch (UTMB) under the supervision of Professor Jia Zhou. His research interests currently focus on the rational design and chemical synthesis of target-based small molecules as novel pharmacological probes and therapeutics for cancer and other human diseases.
Haiying Chen received her Bachelor of Science degree in Engineering from Tianjin University (Branch) in 1995. She worked as an engineer in Tianjin Research Institute of Construction Machinery associated with designing and programming computer testing systems. She joined Professor Jia Zhou’s drug discovery program in 2014 as a Research Associate. Her research interests focus on computer-assisted rational drug design of small molecule modulators and the understanding of key interactions and binding modes with the drug targets for CNS disorders, cancer, and other human diseases.
Hua Liu received her Ph.D. in vascular biology from Medical College of Georgia in 2010. She is currently a tenure-track Assistant Professor in the Departments of Ophthalmology & Visual Sciences at the University of Texas Medical Branch. Her research interests are to understand the role of aging in human diseases. Her previous work provides the first evidence that loss of anti-aging molecule Sirt6 results in progression of glomerular injury in the kidney and impairs corneal epithelial wound healing.
Wenbo Zhang received his Ph.D. in cell biology from Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, China in 2003. Then he served as the chief manager for one and a half years at the Department of Drug Screening, Shanghai Target Drug Pharmaceutical Company, China. He started his postdoctoral training at Medical College of Georgia in 2004 and was promoted to a junior faculty in 2009. Dr. Zhang is currently a tenured Professor in the Departments of Ophthalmology & Visual Sciences, and Neuroscience, Cell Biology & Anatomy at the University of Texas Medical Branch. His research field is focused on the mechanisms of retinal neuronal and vascular injury, and pathological angiogenesis.
Jia Zhou received his Ph.D. in organic chemistry from Nankai University, China in 1997. Then he joined the chemistry faculty in the same university and was promoted to Associate Professor there. He started his postdoctoral research in organic chemistry with Dr. Sidney M. Hecht at the University of Virginia in 1999. After further postdoctoral training in medicinal chemistry with Dr. Alan P. Kozikowski at Georgetown University Medical Center, he worked in pharmaceutical industry at Acenta Discovery, and PsychoGenics, Inc. as a Senior Principal Scientist for 7 years. Dr. Zhou is currently a tenured Professor and also a faculty member of the Center for Addiction Research, Center for Biodefense and Emerging Infectious Diseases, Sealy Center for Structural Biology and Biophysics, and Sealy Center for Molecular Medicine at UTMB. He is an author of more than 180 peer-reviewed papers, 7 book chapters, and an inventor of 26 patents.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interest.
REFERENCES
- 1.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–2921. [DOI] [PubMed] [Google Scholar]
- 4.Chang AR, Ferrer CM, Mostoslavsky R. SIRT6, a mammalian deacylase with multitasking abilities. Physiol Rev. 2020;100(1):145–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332(6036):1443–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z, Zhang L, Zhang W, et al. SIRT6 rescues the age related decline in base excision repair in a PARP1-dependent manner. Cell Cycle. 2015;14(2):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toiber D, Erdel F, Bouazoune K, et al. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Mol Cell. 2013;51(4):454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masri S Sirtuin-dependent clock control: new advances in metabolism, aging and cancer. Curr Opin Clin Nutr Metab Care. 2015;18(6):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–329. [DOI] [PubMed] [Google Scholar]
- 10.Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. [DOI] [PubMed] [Google Scholar]
- 11.McCord RA, Michishita E, Hong T, et al. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging (Albany NY). 2009;1(1):109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao Z, Tian X, Van Meter M, Ke Z, Gorbunova V, Seluanov A. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc Natl Acad Sci U S A. 2012;109(29):11800–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Meter M, Simon M, Tombline G, et al. JNK phosphorylates SIRT6 to stimulate DNA double-strand break repair in response to oxidative stress by recruiting PARP1 to DNA breaks. Cell Rep. 2016;16(10):2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452(7186):492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michishita E, McCord RA, Boxer LD, et al. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle. 2009;8(16):2664–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etchegaray JP, Chavez L, Huang Y, et al. The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nat Cell Biol. 2015;17(5):545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawahara TL, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136(1):62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Xiao C, Wang RH, et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12(3):224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos-Barriopedro I, Bosch-Presegué L, Marazuela-Duque A, et al. SIRT6-dependent cysteine monoubiquitination in the PRE-SET domain of Suv39h1 regulates the NF-κB pathway. Nat Commun. 2018;9(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Meter M, Kashyap M, Rezazadeh S, et al. SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat Commun. 2014;5:5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Zhang Y, Yehuda-Shnaidman E, et al. Liver X receptor alpha is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Mol Cell Biol. 2008;28(7):2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Wu Y, Fu D, Liu Y, Huang C. SIRT6 regulates osteogenic differentiation of rat bone marrow mesenchymal stem cells partially via suppressing the nuclear factor-κB signaling pathway. Stem Cells. 2014;32(7):1943–1955. [DOI] [PubMed] [Google Scholar]
- 23.Xie W, Song C, Young NL, et al. Histone h3 lysine 56 acetylation is linked to the core transcriptional network in human embryonic stem cells. Mol Cell. 2009;33(4):417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan Y, Xue Y, Song C, Grunstein M. Acetylated histone H3K56 interacts with Oct4 to promote mouse embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2013;110(28):11493–11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitiello M, Zullo A, Servillo L, et al. Multiple pathways of SIRT6 at the crossroads in the control of longevity, cancer, and cardiovascular diseases. Ageing Res Rev. 2017;35:301–311. [DOI] [PubMed] [Google Scholar]
- 26.Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem Sci. 2014;39(2):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoh A, Imai SI, Guarente L. The brain, sirtuins, and ageing. Nat Rev Neurosci. 2017;18(6):362–374. [DOI] [PubMed] [Google Scholar]
- 28.Sacconnay L, Carrupt PA, Nurisso A. Human sirtuins: Structures and flexibility. J Struct Biol. 2016;196(3):534–542. [DOI] [PubMed] [Google Scholar]
- 29.Sanders BD, Jackson B, Marmorstein R. Structural basis for sirtuin function: what we know and what we don’t. Biochim Biophys Acta. 2010;1804(8):1604–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tennen RI, Berber E, Chua KF. Functional dissection of SIRT6: identification of domains that regulate histone deacetylase activity and chromatin localization. Mech Ageing Dev. 2010;131(3):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan PW, Feldman JL, Devries MK, Dong A, Edwards AM, Denu JM. Structure and biochemical functions of SIRT6. J Biol Chem. 2011;286(16):14575–14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang H, Khan S, Wang Y, et al. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496(7443):110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moniot S, Weyand M, Steegborn C. Structures, substrates, and regulators of Mammalian sirtuins - opportunities and challenges for drug development. Front Pharmacol. 2012;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldman JL, Baeza J, Denu JM. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 2013;288(43):31350–31356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein MA, Liu C, Kuznetsov VI, Feltenberger JB, Tang W, Denu JM. Mechanism of activation for the sirtuin 6 protein deacylase. J Biol Chem. 2020;295(5):1385–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drazic A, Myklebust LM, Ree R, Arnesen T. The world of protein acetylation. Biochim Biophys Acta. 2016;1864(10):1372–1401. [DOI] [PubMed] [Google Scholar]
- 37.Shakespear MR, Iyer A, Cheng CY, et al. Lysine deacetylases and regulated glycolysis in macrophages. Trends Immunol. 2018;39(6):473–488. [DOI] [PubMed] [Google Scholar]
- 38.Schizas D, Mastoraki A, Naar L, et al. Concept of histone deacetylases in cancer: Reflections on esophageal carcinogenesis and treatment. World J Gastroenterol. 2018;24(41):4635–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu S, Cho EH, Lee JY. Histone deacetylase 9: Its role in the pathogenesis of diabetes and other chronic diseases. Diabetes Metab J. 2020;44(2):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tasselli L, Zheng W, Chua KF. SIRT6: Novel mechanisms and links to aging and disease. Trends Endocrinol Metab. 2017;28(3):168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gil R, Barth S, Kanfi Y, Cohen HY. SIRT6 exhibits nucleosome-dependent deacetylase activity. Nucleic Acids Res. 2013;41(18):8537–8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong L, D’Urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140(2):280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang B, Zwaans BM, Eckersdorff M, Lombard DB. The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle. 2009;8(16):2662–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etchegaray JP, Zhong L, Li C, et al. The histone deacetylase SIRT6 restrains transcription elongation via promoter-proximal pausing. Mol Cell. 2019;75(4):683–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tasselli L, Xi Y, Zheng W, et al. SIRT6 deacetylates H3K18ac at pericentric chromatin to prevent mitotic errors and cellular senescence. Nat Struct Mol Biol. 2016;23(5):434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominy JE Jr., Lee Y, Jedrychowski MP, et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol Cell. 2012;48(6):900–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhardwaj A, Das S. SIRT6 deacetylates PKM2 to suppress its nuclear localization and oncogenic functions. Proc Natl Acad Sci U S A. 2016;113(5):E538–E547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao NN, Ren JH, Tang H, et al. Deacetylation of Ku70 by SIRT6 attenuates Bax-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;485(4):713–719. [DOI] [PubMed] [Google Scholar]
- 49.Sociali G, Grozio A, Caffa I, et al. SIRT6 deacetylase activity regulates NAMPT activity and NAD(P)(H) pools in cancer cells. FASEB J. 2019;33(3):3704–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bang IH, Kwon OK, Hao L, et al. Deacetylation of XBP1s by sirtuin 6 confers resistance to ER stress-induced hepatic steatosis. Exp Mol Med. 2019;51(9):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, Zhu Y, Liu X, et al. Phencynonate mediates antidepressant response by activating sirtuin 6-SOD2/Prdx6 pathway. Biochem Biophys Res Commun. 2018;505(3):898–904. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Li Y, Liu Q, et al. Sirt6 alleviated liver fibrosis by deacetylating conserved lysine 54 on Smad2 in hepatic stellate cells. Hepatology. 2020: 10.1002/hep.31418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong X, Huang M, Kim HG, et al. SIRT6 protects against liver fibrosis by deacetylation and suppression of smad3 in hepatic stellate cells. Cell Mol Gastroenterol Hepatol. 2020;10(2):341–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood M, Rymarchyk S, Zheng S, Cen Y. Trichostatin A inhibits deacetylation of histone H3 and p53 by SIRT6. Arch Biochem Biophys. 2018;638:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reed SM, Quelle DE. p53 Acetylation: Regulation and consequences. Cancers (Basel). 2014;7(1):30–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell. 2011;2(6):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi H, Woods NT, Piluso LG, et al. p53 acetylation is crucial for its transcription-independent proapoptotic functions. J Biol Chem. 2009;284(17):11171–11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280(22):21313–21320. [DOI] [PubMed] [Google Scholar]
- 59.Van Meter M, Mao Z, Gorbunova V, Seluanov A. SIRT6 overexpression induces massive apoptosis in cancer cells but not in normal cells. Cell Cycle. 2011;10(18):3153–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rezazadeh S, Yang D, Tombline G, et al. SIRT6 promotes transcription of a subset of NRF2 targets by mono-ADP-ribosylating BAF170. Nucleic Acids Res. 2019;47(15):7914–7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rezazadeh S, Yang D, Biashad SA, et al. SIRT6 mono-ADP ribosylates KDM2A to locally increase H3K36me2 at DNA damage sites to inhibit transcription and promote repair. Aging (Albany NY). 2020;12(12):11165–11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Khan S, Jiang H, et al. Identifying the functional contribution of the defatty-acylase activity of SIRT6. Nat Chem Biol. 2016;12(8):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Spiegelman NA, Nelson OD, Jing H, Lin H. SIRT6 regulates Ras-related protein R-Ras2 by lysine defatty-acylation. Elife. 2017;6:e25158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang WW, Zeng Y, Wu B, Deiters A, Liu WR. A chemical biology approach to reveal Sirt6-targeted histone H3 sites in nucleosomes. ACS Chem Biol. 2016;11(7):1973–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bae EJ. Sirtuin 6, a possible therapeutic target for type 2 diabetes. Arch Pharm Res. 2017;40(12):1380–1389. [DOI] [PubMed] [Google Scholar]
- 66.Lerrer B, Gertler AA, Cohen HY. The complex role of SIRT6 in carcinogenesis. Carcinogenesis. 2016;37(2):108–118. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Peterson LM, Guzman-Perez G, Krier CR, Ahmad N. The sirtuin 6: An overture in skin cancer. Exp Dermatol. 2020;29(2):124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang W, Fan Y. SIRT6 as a potential target for treating insulin resistance. Life Sci. 2019;231:116558. [DOI] [PubMed] [Google Scholar]
- 69.Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne). 2019;10:00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desantis V, Lamanuzzi A, Vacca A. The role of SIRT6 in tumors. Haematologica. 2018;103(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan RI, Nirzhor SSR, Akter R. A review of the recent advances made with SIRT6 and its implications on aging related processes, major human diseases, and possible therapeutic targets. Biomolecules. 2018;8(3):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sebastian C, Zwaans BM, Silberman DM, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151(6):1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Min L, Ji Y, Bakiri L, et al. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol. 2012;14(11):1203–1211. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Yu Z, Xiao Y, Meng Q, Wang Y, Chang W. Coordination of FOXA2 and SIRT6 suppresses the hepatocellular carcinoma progression through ZEB2 inhibition. Cancer Manag Res. 2018;10:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Yadong, Pan Teng, Wang Haiyu, et al. Overexpression of SIRT6 attenuates the tumorigenicity of hepatocellular carcinoma cells. Oncotarget. 2017;8(44):76223–76230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marquardt JU, Fischer K, Baus K, et al. Sirtuin-6-dependent genetic and epigenetic alterations are associated with poor clinical outcome in hepatocellular carcinoma patients. Hepatology. 2013;58(3):1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang ZG, Qin CY. Sirt6 suppresses hepatocellular carcinoma cell growth via inhibiting the extracellular signalregulated kinase signaling pathway. Mol Med Rep. 2014;9(3):882–888. [DOI] [PubMed] [Google Scholar]
- 78.Kohli S, Bhardwaj A, Kumari R, Das S. SIRT6 is a target of regulation by UBE3A that contributes to liver tumorigenesis in an ANXA2-dependent manner. Cancer Res. 2018;78(3):645–658. [DOI] [PubMed] [Google Scholar]
- 79.Ran LK, Chen Y, Zhang ZZ, et al. SIRT6 overexpression potentiates apoptosis evasion in hepatocellular carcinoma via BCL2-associated X protein-dependent apoptotic pathway. Clin Cancer Res. 2016;22(13):3372–3382. [DOI] [PubMed] [Google Scholar]
- 80.Lee N, Ryu HG, Kwon JH, et al. SIRT6 depletion suppresses tumor growth by promoting cellular senescence induced by DNA damage in HCC. PLoS One. 2016;11(11):e0165835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang C, Yu Y, Huang Q, Tang K. SIRT6 regulates the proliferation and apoptosis of hepatocellular carcinoma via the ERK1/2 signaling pathway. Mol Med Rep. 2019;20(2):1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han LL, Jia L, Wu F, Huang C. Sirtuin6 (SIRT6) promotes the EMT of hepatocellular carcinoma by stimulating autophagic degradation of E-cadherin. Mol Cancer Res. 2019;17(11):2267–2280. [DOI] [PubMed] [Google Scholar]
- 83.Feng XX, Luo J, Liu M, et al. Sirtuin 6 promotes transforming growth factor-beta1/H2O2/HOCl-mediated enhancement of hepatocellular carcinoma cell tumorigenicity by suppressing cellular senescence. Cancer Sci. 2015;106(5):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu JQ, Deng F, Hu XP, Zhang W, Zeng XC, Tian XF. Histone deacetylase SIRT6 regulates chemosensitivity in liver cancer cells via modulation of FOXO3 activity. Oncol Rep. 2018;40(6):3635–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27(16):2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song S, Yang Y, Liu M, et al. MiR-125b attenuates human hepatocellular carcinoma malignancy through targeting SIRT6. Am J Cancer Res. 2018;8(6):993–1007. [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Z, Yang H, Tan C, et al. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013;5(6):1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Nie L, Xu K, et al. SIRT6, a novel direct transcriptional target of FoxO3a, mediates colon cancer therapy. Theranostics. 2019;9(8):2380–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian J, Yuan L. Sirtuin 6 inhibits colon cancer progression by modulating PTEN/AKT signaling. Biomed Pharmacother. 2018;106:109–116. [DOI] [PubMed] [Google Scholar]
- 90.Li N, Mao D, Cao Y, Li H, Ren F, Li K. Downregulation of SIRT6 by miR-34c-5p is associated with poor prognosis and promotes colon cancer proliferation through inhibiting apoptosis via the JAK2/STAT3 signaling pathway. Int J Oncol. 2018;52(5):1515–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geng CH, Zhang CL, Zhang JY, Gao P, He M, Li YL. Overexpression of Sirt6 is a novel biomarker of malignant human colon carcinoma. J Cell Biochem. 2018;119(5):3957–3967. [DOI] [PubMed] [Google Scholar]
- 92.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Azuma Y, Yokobori T, Mogi A, et al. SIRT6 expression is associated with poor prognosis and chemosensitivity in patients with non-small cell lung cancer. J Surg Oncol. 2015;112(2):231–237. [DOI] [PubMed] [Google Scholar]
- 94.Gong J, Wang H, Lou W, et al. Associations of sirtuins with clinicopathological parameters and prognosis in non-small cell lung cancer. Cancer Manag Res. 2018;10:3341–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu B, Yan Y, Shao B, Tian L, Zhou W. Downregulation of SIRT6 is associated with poor prognosis in patients with non-small cell lung cancer. J Int Med Res. 2018;46(4):1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han Z, Liu L, Liu Y, Li S. Sirtuin SIRT6 suppresses cell proliferation through inhibition of Twist1 expression in non-small cell lung cancer. Int J Clin Exp Pathol. 2017;7(8):4774–4781. [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Sheng Z, Cai Y. SIRT6 overexpression inhibits HIF1α expression and its impact on tumor angiogenesis in lung cancer. Int J Clin Exp Pathol. 2018;11(6):2940–2947. [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Y, Sheng Z, Chen Y, Wang J. Influence of SIRT6 regulation of cellular glycometabolism on radiosensitivity of non-small-cell lung cancer A549 cells. Int J Clin Exp Pathol. 2018;11(3):1575–1580. [PMC free article] [PubMed] [Google Scholar]
- 99.Cai Y, Sheng ZY, Liang SX. Radiosensitization effect of overexpression of adenovirus-mediated SIRT6 on A549 non-small cell lung cancer cells. Asian Pac J Cancer Prev. 2014;15(17):7297–72301. [DOI] [PubMed] [Google Scholar]
- 100.Dai PC, Liu DL, Zhang L, et al. Astragaloside IV sensitizes non-small cell lung cancer cells to gefitinib potentially via regulation of SIRT6. Tumour Biol. 2017;39(4):1010428317697555. [DOI] [PubMed] [Google Scholar]
- 101.Krishnamoorthy V, Vilwanathan R. Silencing Sirtuin 6 induces cell cycle arrest and apoptosis in non-small cell lung cancer cell lines. Genomics. 2020;112(5):3703–3712. [DOI] [PubMed] [Google Scholar]
- 102.Bai L, Lin G, Sun L, et al. Upregulation of SIRT6 predicts poor prognosis and promotes metastasis of non-small cell lung cancer via the ERK1/2/MMP9 pathway. Oncotarget. 2016;7(26):40377–40386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruan L, Chen J, Ruan L, Yang T, Wang P. MicroRNA-186 suppresses lung cancer progression by targeting SIRT6. Cancer Biomark. 2018;21(2):415–423. [DOI] [PubMed] [Google Scholar]
- 104.Li Z, Huang J, Shen S, et al. SIRT6 drives epithelial-to-mesenchymal transition and metastasis in non-small cell lung cancer via snail-dependent transrepression of KLF4. J Exp Clin Cancer Res. 2018;37(1):323. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Choe M, Brusgard JL, Chumsri S, et al. The RUNX2 transcription factor negatively regulates SIRT6 expression to alter glucose metabolism in breast cancer cells. J Cell Biochem. 2015;116(10):2210–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thirumurthi U, Shen J, Xia W, et al. MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Sci Signal. 2014;7(336):ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bae JS, Park SH, Jamiyandorj U, et al. CK2alpha/CSNK2A1 phosphorylates SIRT6 and is involved in the progression of breast carcinoma and predicts shorter survival of diagnosed patients. Am J Pathol. 2016;186(12):3297–3315. [DOI] [PubMed] [Google Scholar]
- 108.Mahmud Z, Gomes AR, Lee HJ, et al. EP300 and SIRT1/6 co-regulate lapatinib sensitivity via modulating FOXO3-acetylation and activity in breast cancer. Cancers (Basel). 2019;11(8):11081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khongkow M, Olmos Y, Gong C, et al. SIRT6 modulates paclitaxel and epirubicin resistance and survival in breast cancer. Carcinogenesis. 2013;34(7):1476–1486. [DOI] [PubMed] [Google Scholar]
- 110.Chen X, Li D, Gao Y, Cao Y, Hao B. Histone deacetylase SIRT6 inhibits glioma cell growth through down-regulating NOTCH3 expression. Acta Biochim Biophys Sin (Shanghai). 2018;50(4):417–424. [DOI] [PubMed] [Google Scholar]
- 111.Zhu D, Sun C, Qian X. MST1 suppresses viability and promotes apoptosis of glioma cells via upregulating SIRT6 expression. J Integr Neurosci. 2019;18(2):117–126. [DOI] [PubMed] [Google Scholar]
- 112.Feng J, Yan PF, Zhao HY, Zhang FC, Zhao WH, Feng M. SIRT6 suppresses glioma cell growth via induction of apoptosis, inhibition of oxidative stress and suppression of JAK2/STAT3 signaling pathway activation. Oncol Rep. 2016;35(3):1395–1402. [DOI] [PubMed] [Google Scholar]
- 113.Chang M, Qiao L, Li B, et al. Suppression of SIRT6 by miR-33a facilitates tumor growth of glioma through apoptosis and oxidative stress resistance. Oncol Rep. 2017;38(2):1251–1258. [DOI] [PubMed] [Google Scholar]
- 114.Dong Z, Zhong X, Lei Q, Chen F, Cui H. Transcriptional activation of SIRT6 via FKHRL1/FOXO3a inhibits the Warburg effect in glioblastoma cells. Cell Signal. 2019;60:100–113. [DOI] [PubMed] [Google Scholar]
- 115.Chen X, Hao B, Liu Y, et al. The histone deacetylase SIRT6 suppresses the expression of the RNA-binding protein PCBP2 in glioma. Biochem Biophys Res Commun. 2014;446(1):364–369. [DOI] [PubMed] [Google Scholar]
- 116.Cea M, Cagnetta A, Adamia S, et al. Evidence for a role of the histone deacetylase SIRT6 in DNA damage response of multiple myeloma cells. Blood. 2016;127(9):1138–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin H, Hao Y, Zhao Z, Tong Y. Sirtuin 6 contributes to migration and invasion of osteosarcoma cells via the ERK1/2/MMP9 pathway. FEBS Open Bio. 2017;7(9):1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Gao Y, Qu Y, Zhou Q, Ma Y. SIRT6 inhibits proliferation and invasion in osteosarcoma cells by targeting N-cadherin. Oncol Lett. 2019;17(1):1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xue W, Ma L, Wang Z, Zhang W, Zhang X. FOXN3 is downregulated in osteosarcoma and transcriptionally regulates SIRT6, and suppresses migration and invasion in osteosarcoma. Oncol Rep. 2019;41(2):1404–1414. [DOI] [PubMed] [Google Scholar]
- 120.Xu XZ, Song H, Zhao Y, Zhang L. MiR-654–5p regulated cell progression and tumor growth through targeting SIRT6 in osteosarcoma. Eur Rev Med Pharmacol Sci. 2020;24(7):3517–3525. [DOI] [PubMed] [Google Scholar]
- 121.Ming M, Han W, Zhao B, et al. SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer. Cancer Res. 2014;74(20):5925–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garcia-Peterson LM, Ndiaye MA, Singh CK, Chhabra G, Huang W, Ahmad N. SIRT6 histone deacetylase functions as a potential oncogene in human melanoma. Genes Cancer. 2017;8(9–10):701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia-Peterson LM, Ndiaye MA, Chhabra G, et al. CRISPR/Cas9-mediated knockout of SIRT6 imparts remarkable antiproliferative response in human melanoma cells in vitro and in vivo. Photochem Photobiol. 2020:doi: 10.1111/php.13305.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang L, Guo W, Ma J, et al. Aberrant SIRT6 expression contributes to melanoma growth: Role of the autophagy paradox and IGF-AKT signaling. Autophagy. 2018;14(3):518–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Strub T, Ghiraldini FG, Carcamo S, et al. SIRT6 haploinsufficiency induces BRAF(V600E) melanoma cell resistance to MAPK inhibitors via IGF signalling. Nat Commun. 2018;9(1):3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dong Z, Yang J, Li L, et al. FOXO3a-SIRT6 axis suppresses aerobic glycolysis in melanoma. Int J Oncol. 2020;56(3):728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lefort K, Brooks Y, Ostano P, et al. A miR-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 2013;32(16):2248–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ouyang L, Yi L, Li J, et al. SIRT6 overexpression induces apoptosis of nasopharyngeal carcinoma by inhibiting NF-kappaB signaling. Onco Targets Ther. 2018;11:7613–7624. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Ding Y, Wu S, Huo Y, et al. Inhibition of Sirt6 suppresses tumor growth by inducing G1/S phase arrest in renal cancer cells. Int J Clin Exp Pathol. 2019;12(7):2526–2535. [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y, Xie QR, Wang B, et al. Inhibition of SIRT6 in prostate cancer reduces cell viability and increases sensitivity to chemotherapeutics. Protein Cell. 2013;4(9):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kugel S, Sebastián C, Fitamant J, et al. SIRT6 suppresses pancreatic cancer through control of Lin28b. Cell. 2016;165(6):1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang J, Yin XJ, Xu CJ, et al. The histone deacetylase SIRT6 inhibits ovarian cancer cell proliferation via down-regulation of Notch 3 expression. Eur Rev Med Pharmacol Sci. 2015;19(5):818–824. [PubMed] [Google Scholar]
- 133.Bae JS, Noh SJ, Kim KM, et al. SIRT6 is involved in the progression of ovarian carcinomas via beta-catenin-mediated epithelial to mesenchymal transition. Front Oncol. 2018;8:00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cagnetta A, Soncini D, Orecchioni S, et al. Depletion of SIRT6 enzymatic activity increases acute myeloid leukemia cells’ vulnerability to DNA-damaging agents. Haematologica. 2018;103(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gaal Z, Olah E, Rejto L, Balint BL, Csernoch L. Expression levels of warburg-effect related microRNAs correlate with each other and that of histone deacetylase enzymes in adult hematological malignancies with emphasis on acute myeloid leukemia. Pathol Oncol Res. 2017;23(1):207–216. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Y, Huang Z, Sheng F, Yin Z. MYC upregulated LINC00319 promotes human acute myeloid leukemia (AML) cells growth through stabilizing SIRT6. Biochem Biophys Res Commun. 2019;509(1):314–321. [DOI] [PubMed] [Google Scholar]
- 137.Kong Q, Li Y, Liang Q, Xie J, Li X, Fang J. SIRT6-PARP1 is involved in HMGB1 polyADP-ribosylation and acetylation and promotes chemotherapy-induced autophagy in leukemia. Cancer Biol Ther. 2020;21(4):320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zelová H, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013;62(7):641–651. [DOI] [PubMed] [Google Scholar]
- 139.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bauer I, Grozio A, Lasigliè D, et al. The NAD+-dependent histone deacetylase SIRT6 promotes cytokine production and migration in pancreatic cancer cells by regulating Ca2+ responses. J Biol Chem. 2012;287(49):40924–40937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Gool F, Galli M, Gueydan C, et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15(2):206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mendes KL, Lelis DF, Santos SHS. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev. 2017;38:98–105. [DOI] [PubMed] [Google Scholar]
- 143.Lee HS, Ka SO, Lee SM, Lee SI, Park JW, Park BH. Overexpression of sirtuin 6 suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis. Arthritis Rheum. 2013;65(7):1776–1785. [DOI] [PubMed] [Google Scholar]
- 144.Hou KL, Lin SK, Chao LH, et al. Sirtuin 6 suppresses hypoxia-induced inflammatory response in human osteoblasts via inhibition of reactive oxygen species production and glycolysis-A therapeutic implication in inflammatory bone resorption. Biofactors. 2017;43(2):170–180. [DOI] [PubMed] [Google Scholar]
- 145.Xiao C, Wang RH, Lahusen TJ, et al. Progression of chronic liver inflammation and fibrosis driven by activation of c-JUN signaling in Sirt6 mutant mice. J Biol Chem. 2012;287(50):41903–41913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kuang J, Zhang Y, Liu Q, et al. Fat-specific sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes. 2017;66(5):1159–1171. [DOI] [PubMed] [Google Scholar]
- 147.Lappas M Anti-inflammatory properties of sirtuin 6 in human umbilical vein endothelial cells. Mediators Inflamm. 2012;2012:597514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qin Y, Cao L, Hu L. Sirtuin 6 mitigated LPS-induced human umbilical vein endothelial cells inflammatory responses through modulating nuclear factor erythroid 2-related factor 2. J Cell Biochem. 2019;120:11305–11317. [DOI] [PubMed] [Google Scholar]
- 149.Liu F, Bu HF, Geng H, et al. Sirtuin-6 preserves R-spondin-1 expression and increases resistance of intestinal epithelium to injury in mice. Mol Med. 2017;23:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu K, Guo Y, Ping L, et al. Protective effects of SIRT6 overexpression against DSS-induced colitis in mice. Cells. 2020;9(6): 10.3390/cells9061513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang R, Li H, Guo Q, Zhang L, Zhu J, Ji J. Sirtuin6 inhibits c-triggered inflammation through TLR4 abrogation regulated by ROS and TRPV1/CGRP. J Cell Biochem. 2018;119(11):9141–9153. [DOI] [PubMed] [Google Scholar]
- 152.Lauterbach MA, Wunderlich FT. Macrophage function in obesity-induced inflammation and insulin resistance. Pflugers Archiv : European journal of physiology. 2017;469(3–4):385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee Y, Ka SO, Cha HN, et al. Myeloid Sirtuin 6 deficiency causes insulin resistance in high-fat diet-fed mice by eliciting macrophage polarization toward an M1 phenotype. Diabetes. 2017;66(10):2659–2668. [DOI] [PubMed] [Google Scholar]
- 154.Xiong X, Zhang C, Zhang Y, Fan R, Qian X, Dong XC. Fabp4-Cre-mediated Sirt6 deletion impairs adipose tissue function and metabolic homeostasis in mice. J Endocrinol. 2017;233(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He Y, Yang G, Yao F, et al. Sitagliptin inhibits vascular inflammation via the SIRT6-dependent signaling pathway. Int Immunopharmacol. 2019;75:105805. [DOI] [PubMed] [Google Scholar]
- 156.Loeser RF. The Role of aging in the development of osteoarthritis. Transactions of the American Clinical and Climatological Association. 2017;128:44–54. [PMC free article] [PubMed] [Google Scholar]
- 157.Ailixiding M, Aibibula Z, Iwata M, et al. Pivotal role of Sirt6 in the crosstalk among ageing, metabolic syndrome and osteoarthritis. Biochem Biophys Res Commun. 2015;466(3):319–326. [DOI] [PubMed] [Google Scholar]
- 158.Duarte JH. Osteoarthritis: SIRT6 prevents chondrocyte senescence and DNA damage. Nat Rev Rheumatol. 2015;11(5):260. [DOI] [PubMed] [Google Scholar]
- 159.Nagai K, Matsushita T, Matsuzaki T, et al. Depletion of SIRT6 causes cellular senescence, DNA damage, and telomere dysfunction in human chondrocytes. Osteoarthritis Cartilage. 2015;23(8):1412–1420. [DOI] [PubMed] [Google Scholar]
- 160.Wu Y, Chen L, Wang Y, et al. Overexpression of Sirtuin 6 suppresses cellular senescence and NF-kappaB mediated inflammatory responses in osteoarthritis development. Sci Rep. 2015;5:17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blaney Davidson EN, van Caam AP, van der Kraan PM. Osteoarthritis year in review 2016: biology. Osteoarthritis Cartilage. 2017;25(2):175–180. [DOI] [PubMed] [Google Scholar]
- 162.Zhi LQ, Yao SX, Liu HL, Li M, Duan N, Ma JB. Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via SIRT6-mediated autophagy. Mol Med Rep. 2018;17(3):4035–4042. [DOI] [PubMed] [Google Scholar]
- 163.Jiang C, Sun ZM, Hu JN, et al. Cyanidin ameliorates the progression of osteoarthritis via the Sirt6/NF-kappaB axis in vitro and in vivo. Food Funct. 2019;10(9):5873–5885. [DOI] [PubMed] [Google Scholar]
- 164.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Engler A, Niederer F, Klein K, et al. SIRT6 regulates the cigarette smoke-induced signalling in rheumatoid arthritis synovial fibroblasts. J Mol Med (Berl). 2014;92(7):757–767. [DOI] [PubMed] [Google Scholar]
- 166.Woo SJ, Noh HS, Lee NY, et al. Myeloid sirtuin 6 deficiency accelerates experimental rheumatoid arthritis by enhancing macrophage activation and infiltration into synovium. EBioMedicine. 2018;38:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang CW, Wu X, Liu D, et al. Long non-coding RNA PVT1 knockdown suppresses fibroblast-like synoviocyte inflammation and induces apoptosis in rheumatoid arthritis through demethylation of sirt6. J Biol Eng. 2019;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne). 2013;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Saisho Y β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6(1):109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xiao C, Kim HS, Lahusen T, et al. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem. 2010;285(47):36776–36784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shun CT, Lin SK, Hong CY, Lin CF, Liu CM. Sirtuin 6 modulates hypoxia-induced autophagy in nasal polyp fibroblasts via inhibition of glycolysis. Am J Rhinol Allergy. 2016;30(3):179–185. [DOI] [PubMed] [Google Scholar]
- 172.Sociali G, Magnone M, Ravera S, et al. Pharmacological Sirt6 inhibition improves glucose tolerance in a type 2 diabetes mouse model. FASEB J. 2017;31(7):3138–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun W, Chen X, Huang S, et al. Discovery of 5-(4-methylpiperazin-1-yl)-2-nitroaniline derivatives as a new class of SIRT6 inhibitors. Bioorg Med Chem Lett. 2020;30(16):127215. [DOI] [PubMed] [Google Scholar]
- 174.Xiong X, Tao R, DePinho RA, Dong XC. Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PLoS One. 2013;8(8):e74340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ohtake F, Takeyama K, Matsumoto T, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423(6939):545–550. [DOI] [PubMed] [Google Scholar]
- 176.Zhang P, Tu B, Wang H, et al. Tumor suppressor p53 cooperates with SIRT6 to regulate gluconeogenesis by promoting FoxO1 nuclear exclusion. Proc Natl Acad Sci U S A. 2014;111(29):10684–10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Song MY, Wang J, Ka SO, Bae EJ, Park BH. Insulin secretion impairment in Sirt6 knockout pancreatic beta cells is mediated by suppression of the FoxO1-Pdx1-Glut2 pathway. Sci Rep. 2016;6:30321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anderson JG, Ramadori G, Ioris RM, et al. Enhanced insulin sensitivity in skeletal muscle and liver by physiological overexpression of SIRT6. Mol Metab. 2015;4(11):846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xiong X, Sun X, Wang Q, et al. SIRT6 protects against palmitate-induced pancreatic beta-cell dysfunction and apoptosis. J Endocrinol. 2016;231(2):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xiong X, Wang G, Tao R, et al. Sirtuin 6 regulates glucose-stimulated insulin secretion in mouse pancreatic beta cells. Diabetologia. 2016;59(1):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Song MY, Kim SH, Ryoo GH, et al. Adipose sirtuin 6 drives macrophage polarization toward M2 through IL-4 production and maintains systemic insulin sensitivity in mice and humans. Exp Mol Med. 2019;51(5):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Qin K, Zhang N, Zhang Z, et al. SIRT6-mediated transcriptional suppression of Txnip is critical for pancreatic beta cell function and survival in mice. Diabetologia. 2018;61(4):906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tang C, Liu P, Zhou Y, Jiang B, Song Y, Sheng L. Sirt6 deletion in hepatocytes increases insulin sensitivity of female mice by enhancing ERα expression. J Cell Physiol. 2019;234(10):18615–18625. [DOI] [PubMed] [Google Scholar]
- 184.Eckel RH, Kahn SE, Ferrannini E, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. 2011;96(6):1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. [DOI] [PubMed] [Google Scholar]
- 186.Yao L, Cui X, Chen Q, et al. Cold-inducible SIRT6 regulates thermogenesis of brown and beige fat. Cell Rep. 2017;20(3):641–654. [DOI] [PubMed] [Google Scholar]
- 187.Martinez-Jimenez V, Cortez-Espinosa N, Rodriguez-Varela E, et al. Altered levels of sirtuin genes (SIRT1, SIRT2, SIRT3 and SIRT6) and their target genes in adipose tissue from individual with obesity. Diabetes Metab Syndr. 2019;13(1):582–589. [DOI] [PubMed] [Google Scholar]
- 188.Carreira MC, Izquierdo AG, Amil M, Casanueva FF, Crujeiras AB. Oxidative stress induced by excess of adiposity is related to a downregulation of hepatic SIRT6 expression in obese individuals. Oxid Med Cell Longev. 2018;2018:6256052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Palmer NO, Fullston T, Mitchell M, Setchell BP, Lane M. SIRT6 in mouse spermatogenesis is modulated by diet-induced obesity. Reproduction, Fertility and Development. 2011;23:929–939. [DOI] [PubMed] [Google Scholar]
- 190.Kanfi Y, Peshti V, Gil R, et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9(2):162–173. [DOI] [PubMed] [Google Scholar]
- 191.Schwer B, Schumacher B, Lombard DB, et al. Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and causes obesity. Proc Natl Acad Sci U S A. 2010;107(50):21790–21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jung SM, Hung CM, Hildebrand SR, et al. Non-canonical mTORC2 signaling regulates brown adipocyte lipid catabolism through SIRT6-FoxO1. Mol Cell. 2019;75(4):807–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen Q, Hao W, Xiao C, et al. SIRT6 is essential for adipocyte differentiation by regulating mitotic clonal expansion. Cell Rep. 2017;18(13):3155–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36(48):3404–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.D’Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. 2018;28(8):711–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yuan X, Chen G, Guo D, Xu L, Gu Y. Polydatin alleviates septic myocardial injury by promoting SIRT6-mediated autophagy. Inflammation. 2020;43(3):785–795. [DOI] [PubMed] [Google Scholar]
- 197.Maksin-Matveev A, Kanfi Y, Hochhauser E, Isak A, Cohen HY, Shainberg A. Sirtuin 6 protects the heart from hypoxic damage. Exp Cell Res. 2015;330(1):81–90. [DOI] [PubMed] [Google Scholar]
- 198.Li Y, Meng X, Wang W, et al. Cardioprotective effects of SIRT6 in a mouse model of transverse aortic constriction-induced heart failure. Front Physiol. 2017;8:00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khan D, Sarikhani M, Dasgupta S, et al. SIRT6 deacetylase transcriptionally regulates glucose metabolism in heart. J Cell Physiol. 2018;233(7):5478–5489. [DOI] [PubMed] [Google Scholar]
- 200.Peng L, Qian M, Liu Z, et al. Deacetylase-independent function of SIRT6 couples GATA4 transcription factor and epigenetic activation against cardiomyocyte apoptosis. Nucleic Acids Res. 2020: 10.1093/nar/gkaa1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Archives of toxicology. 2015;89(9):1401–1438. [DOI] [PubMed] [Google Scholar]
- 202.Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: Its implication in cardiac hypertrophy and aging. Circ Res. 2014;114(2):368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sundaresan NR, Vasudevan P, Zhong L, et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18(11):1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cai Y, Yu SS, Chen SR, et al. Nmnat2 protects cardiomyocytes from hypertrophy via activation of SIRT6. FEBS Lett. 2012;586(6):866–874. [DOI] [PubMed] [Google Scholar]
- 205.Shen P, Feng X, Zhang X, et al. SIRT6 suppresses phenylephrine-induced cardiomyocyte hypertrophy though inhibiting p300. J Pharmacol Sci. 2016;132(1):31–40. [DOI] [PubMed] [Google Scholar]
- 206.Tang C, Liu P, Zhou Y, Jiang B, Song Y, Sheng L. Sirt6 deletion in hepatocytes increases insulin sensitivity of female mice by enhancing ERalpha expression. J Cell Physiol. 2019;234(10):18615–18625. [DOI] [PubMed] [Google Scholar]
- 207.Yu SS, Cai Y, Ye JT, et al. Sirtuin 6 protects cardiomyocytes from hypertrophy in vitro via inhibition of NF-kappaB-dependent transcriptional activity. Br J Pharmacol. 2013;168(1):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang X, Li W, Shen P, et al. STAT3 suppression is involved in the protective effect of SIRT6 against cardiomyocyte hypertrophy. J Cardiovasc Pharmacol. 2016;68:204–214. [DOI] [PubMed] [Google Scholar]
- 209.Li Z, Zhang X, Guo Z, et al. SIRT6 suppresses NFATc4 expression and activation in cardiomyocyte hypertrophy. Front Pharmacol. 2018;9:01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lu J, Sun D, Liu Z, et al. SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl Res. 2016;172:96–112. [DOI] [PubMed] [Google Scholar]
- 211.Tian K, Liu Z, Wang J, Xu S, You T, Liu P. Sirtuin-6 inhibits cardiac fibroblasts differentiation into myofibroblasts via inactivation of nuclear factor kappaB signaling. Transl Res. 2015;165(3):374–386. [DOI] [PubMed] [Google Scholar]
- 212.Zhang Z-Z, Cheng Y-W, Jin H-Y, et al. The sirtuin 6 prevents angiotensin II-mediated myocardial fibrosis and injury by targeting AMPK-ACE2 signaling. Oncotarget. 2017;8(42):72302–72314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shao C, Wang J, Tian J, Tang YD. Coronary artery disease: From mechanism to clinical practice. Adv Exp Med Biol. 2020;1177:1–36. [DOI] [PubMed] [Google Scholar]
- 214.Li X, Liu M, Sun R, Zeng Y, Chen S, Zhang P. Atherosclerotic coronary artery disease: The accuracy of measures to diagnose preclinical atherosclerosis. Exp Ther Med. 2016;12(5):2899–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sosnowska B, Mazidi M, Penson P, Gluba-Brzozka A, Rysz J, Banach M. The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis. 2017;265:275–282. [DOI] [PubMed] [Google Scholar]
- 216.Tang SS, Xu S, Cheng J, et al. Two tagSNPs rs352493 and rs3760908 within SIRT6 Gene Are Associated with the Severity of Coronary Artery Disease in a Chinese Han Population. Dis Markers. 2016;2016:1628041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang L, Ma L, Pang S, Huang J, Yan B. Sequence variants of SIRT6 gene promoter in myocardial infarction. Genet Test Mol Biomarkers. 2016;20(4):185–190. [DOI] [PubMed] [Google Scholar]
- 218.Arsiwala T, Pahla J, van Tits LJ, et al. Sirt6 deletion in bone marrow-derived cells increases atherosclerosis - Central role of macrophage scavenger receptor 1. J Mol Cell Cardiol. 2020;139:24–32. [DOI] [PubMed] [Google Scholar]
- 219.Wang T, Sun C, Hua L, et al. Sirt6 stabilizes atherosclerosis plaques by promoting macrophage autophagy and reducing contact with endothelial cells. Biochem Cell Biol. 2020;98:120–129. [DOI] [PubMed] [Google Scholar]
- 220.He J, Zhang G, Pang Q, et al. SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. FEBS J. 2017;284(9):1324–1337. [DOI] [PubMed] [Google Scholar]
- 221.Elhanati S, Kanfi Y, Varvak A, et al. Multiple regulatory layers of SREBP1/2 by SIRT6. Cell Rep. 2013;4(5):905–912. [DOI] [PubMed] [Google Scholar]
- 222.Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6. J Lipid Res. 2013;54(10):2745–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J Biol Chem. 2013;288(41):29252–29259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang XX, Wang XL, Tong MM, et al. SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3alpha-dependent antioxidant defense mechanisms. Basic Res Cardiol. 2016;111(2):13. [DOI] [PubMed] [Google Scholar]
- 225.Zi Y, Yi-An Y, Bing J, et al. Sirt6-induced autophagy restricted TREM-1-mediated pyroptosis in ox-LDL-treated endothelial cells: relevance to prognostication of patients with acute myocardial infarction. Cell Death Discov. 2019;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gimbrone MA Jr., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shen J, Ma W, Liu Y. Deacetylase SIRT6 deaccelerates endothelial senescence. Cardiovasc Res. 2013;97(3):391–392. [DOI] [PubMed] [Google Scholar]
- 228.Xu S, Yin M, Koroleva M, et al. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. AGING. 2016;8(5):1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Liu Z, Wang J, Huang X, Li Z, Liu P. Deletion of sirtuin 6 accelerates endothelial dysfunction and atherosclerosis in apolipoprotein E-deficient mice. Transl Res. 2016;172:18–29. [DOI] [PubMed] [Google Scholar]
- 230.Jin Z, Xiao Y, Yao F, et al. SIRT6 inhibits cholesterol crystal-induced vascular endothelial dysfunction via Nrf2 activation. Exp Cell Res. 2020;387(1):111744. [DOI] [PubMed] [Google Scholar]
- 231.Guo J, Wang Z, Wu J, et al. Endothelial SIRT6 is vital to prevent hypertension and associated cardiorenal injury through targeting Nkx3.2-GATA5 signaling. Circ Res. 2019;124(10):1448–1461. [DOI] [PubMed] [Google Scholar]
- 232.Yepuri G, Ramasamy R. Significance and mechanistic relevance of SIRT6-mediated endothelial dysfunction in cardiovascular disease progression. Circ Res. 2019;124(10):1408–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cardinale A, de Stefano MC, Mollinari C, Racaniello M, Garaci E, Merlo D. Biochemical characterization of sirtuin 6 in the brain and its involvement in oxidative stress response. Neurochem Res. 2015;40(1):59–69. [DOI] [PubMed] [Google Scholar]
- 234.Zhang W, Wan H, Feng G, et al. SIRT6 deficiency results in developmental retardation in cynomolgus monkeys. Nature. 2018;560(7720):661–665. [DOI] [PubMed] [Google Scholar]
- 235.Griñán‐Ferré C, Sarroca S, Ivanova A, et al. Epigenetic mechanisms underlying cognitive impairment and Alzheimer disease hallmarks in 5XFAD mice. AGING. 2016;8(4):664–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jung ES, Choi H, Song H, et al. p53-dependent SIRT6 expression protects Abeta42-induced DNA damage. Sci Rep. 2016;6:25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kaluski S, Portillo M, Besnard A, et al. Neuroprotective functions for the histone deacetylase SIRT6. Cell Rep. 2017;18(13):3052–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tamura M, Mukai J, Gordon JA, Gogos JA. Developmental inhibition of Gsk3 rescues behavioral and neurophysiological deficits in a mouse model of schizophrenia predisposition. Neuron. 2016;89(5):1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hou Y, Lautrup S, Cordonnier S, et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A. 2018;115(8):E1876–E1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nicholatos JW, Francisco AB, Bender CA, et al. Nicotine promotes neuron survival and partially protects from Parkinson’s disease by suppressing SIRT6. Acta Neuropathol Commun. 2018;6(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li W, Liu X, Qiao H. Downregulation of hippocampal SIRT6 activates AKT/CRMP2 signaling and ameliorates chronic stress-induced depression-like behavior in mice. Acta Pharmacol Sin. 2020:doi: 10.1038/s41401-41020-40387-41405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mao Q, Gong X, Zhou C, et al. Up-regulation of SIRT6 in the hippocampus induced rats with depression-like behavior via the block Akt/GSK3beta signaling pathway. Behav Brain Res. 2017;323:38–46. [DOI] [PubMed] [Google Scholar]
- 243.Yin X, Gao Y, Shi HS, et al. Overexpression of SIRT6 in the hippocampal CA1 impairs the formation of long-term contextual fear memory. Sci Rep. 2016;6:18982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kim H, Kim HS, Kaang BK. Elevated contextual fear memory by SIRT6 depletion in excitatory neurons of mouse forebrain. Mol Brain. 2018;11(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Meloux A, Bejot Y, Rochette L, Cottin Y, Vergely C. Brain-heart interactions during ischemic processes: Clinical and experimental evidences. Stroke. 2020;51(2):679–686. [DOI] [PubMed] [Google Scholar]
- 246.Zhang W, Wei R, Zhang L, Tan Y, Qian C. Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through NRF2 activation. Neuroscience. 2017;366:95–104. [DOI] [PubMed] [Google Scholar]
- 247.Lee OH, Kim J, Kim JM, et al. Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia. Biochem Biophys Res Commun. 2013;438(2):388–394. [DOI] [PubMed] [Google Scholar]
- 248.Zuo Y, Huang L, Enkhjargal B, et al. Activation of retinoid X receptor by bexarotene attenuates neuroinflammation via PPARgamma/SIRT6/FoxO3a pathway after subarachnoid hemorrhage in rats. J Neuroinflammation. 2019;16(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ruan ZF, Xie M, Gui SJ, Lan F, Wan J, Li Y. MiR-370 accelerated cerebral ischemia reperfusion injury via targeting SIRT6 and regulating Nrf2/ARE signal pathway. Kaohsiung J Med Sci. 2020: 10.1002/kjm1002.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hu Y, Li R, Yang H, Luo H, Chen Z. Sirtuin 6 is essential for sodium sulfide-mediated cytoprotective effect in ischemia/reperfusion-stimulated brain endothelial cells. J Stroke Cerebrovasc Dis. 2015;24(3):601–609. [DOI] [PubMed] [Google Scholar]
- 251.Liberale L, Gaul DS, Akhmedov A, et al. Endothelial SIRT6 blunts stroke size and neurological deficit by preserving blood-brain barrier integrity: a translational study. Eur Heart J. 2020;41(16):1575–1587. [DOI] [PubMed] [Google Scholar]
- 252.Ferrara G, Benzi A, Sturla L, et al. Sirt6 inhibition delays the onset of experimental autoimmune encephalomyelitis by reducing dendritic cell migration. J Neuroinflammation. 2020;17(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011;164(4):1079–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Quintana FJ, Yeste A, Mascanfroni ID. Role and therapeutic value of dendritic cells in central nervous system autoimmunity. Cell Death Differ. 2015;22(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lasigliè D, Boero S, Bauer I, et al. Sirt6 regulates dendritic cell differentiation, maturation, and function. Aging. 2016;8(1):34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ka SO, Bang IH, Bae EJ, Park BH. Hepatocyte-specific sirtuin 6 deletion predisposes to nonalcoholic steatohepatitis by up-regulation of Bach1, an Nrf2 repressor. Faseb j. 2017;31(9):3999–4010. [DOI] [PubMed] [Google Scholar]
- 257.Kanfi Y, Peshti V, Gil R, et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 2010;9:162–173. [DOI] [PubMed] [Google Scholar]
- 258.Yang SJ, Choi JM, Chae SW, et al. Activation of peroxisome proliferator-activated receptor gamma by rosiglitazone increases sirt6 expression and ameliorates hepatic steatosis in rats. PLoS One. 2011;6(2):e17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Naiman S, Huynh FK, Gil R, et al. SIRT6 promotes hepatic beta-oxidation via activation of PPARalpha. Cell Rep. 2019;29(12):4127–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Elhanati S, Ben-Hamo R, Kanfi Y, et al. Reciprocal Regulation between SIRT6 and miR-122 Controls Liver Metabolism and Predicts Hepatocarcinoma Prognosis. Cell Rep. 2016;14(2):234–242. [DOI] [PubMed] [Google Scholar]
- 261.Hao L, Bang IH, Wang J, et al. ERRγ suppression by Sirt6 alleviates cholestatic liver injury and fibrosis. JCI insight. 2020: 10.1172/jci.insight.137566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kim HG, Huang M, Xin Y, et al. The epigenetic regulator SIRT6 protects the liver from alcohol-induced tissue injury by reducing oxidative stress in mice. J Hepatol. 2019;71(5):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond). 2013;124(3):153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Huang W, Liu H, Zhu S, et al. Sirt6 deficiency results in progression of glomerular injury in the kidney. AGING. 2017;9(3):1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liu M, Liang K, Zhen J, et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun. 2017;8(1):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Li Z, Xu K, Zhang N, et al. Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int. 2018;93(4):881–892. [DOI] [PubMed] [Google Scholar]
- 267.Zhang Y, Wang L, Meng L, Cao G, Wu Y. Sirtuin 6 overexpression relieves sepsis-induced acute kidney injury by promoting autophagy. Cell Cycle. 2019;18(4):425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fan X, Wei W, Huang J, Liu X, Ci X. Isoorientin attenuates cisplatin-induced nephrotoxicity through the inhibition of oxidative stress and apoptosis via activating the SIRT1/SIRT6/Nrf-2 pathway. Front Pharmacol. 2020;11:00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gao Z, Chen X, Fan Y, Zhu K, Shi M, Ding G. Sirt6 attenuates hypoxia-induced tubular epithelial cell injury via targeting G2/M phase arrest. J Cell Physiol. 2020;235(4):3463–3473. [DOI] [PubMed] [Google Scholar]
- 270.So KY, Park BH, Oh SH. Cytoplasmic sirtuin 6 translocation mediated by p62 polyubiquitination plays a critical role in cadmium-induced kidney toxicity. Cell Biol Toxicol. 2020: 10.1007/s10565-10020-09528-10562. [DOI] [PubMed] [Google Scholar]
- 271.Yang Q, Hu J, Yang Y, et al. Sirt6 deficiency aggravates angiotensin II-induced cholesterol accumulation and injury in podocytes. Theranostics. 2020;10(16):7465–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ji L, Chen Y, Wang H, et al. Overexpression of Sirt6 promotes M2 macrophage transformation, alleviating renal injury in diabetic nephropathy. Int J Oncol. 2019;55(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cai J, Liu Z, Huang X, et al. The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking beta-catenin target gene expression. Kidney Int. 2020;97(1):106–118. [DOI] [PubMed] [Google Scholar]
- 274.Gewin LS. Sirtuin 6 and renal injury: another link in the beta-catenin chain? Kidney Int. 2020;97(1):22–23. [DOI] [PubMed] [Google Scholar]
- 275.Muraoka H, Hasegawa K, Sakamaki Y, et al. Role of Nampt-Sirt6 axis in renal proximal tubules in extracellular matrix deposition in diabetic nephropathy. Cell Rep. 2019;27(1):199–212. [DOI] [PubMed] [Google Scholar]
- 276.Silberman DM, Ross K, Sande PH, et al. SIRT6 is required for normal retinal function. PLOS ONE 2014;9(6):e98831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zorrilla-Zubilete MA, Yeste A, Quintana FJ, Toiber D, Mostoslavsky R, Silberman DM. Epigenetic control of early neurodegenerative events in diabetic retinopathy by the histone deacetylase SIRT6. J Neurochem. 2018;144(2):128–138. [DOI] [PubMed] [Google Scholar]
- 278.Zhang L, Du J, Justus S, et al. Reprogramming metabolism by targeting sirtuin 6 attenuates retinal degeneration. J Clin Invest. 2016;126(12):4659–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Venkatesh A, Ma S, Le YZ, Hall MN, Rüegg MA, Punzo C. Activated mTORC1 promotes long-term cone survival in retinitis pigmentosa mice. J Clin Invest. 2015;125(4):1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Peshti V, Obolensky A, Nahum L, et al. Characterization of physiological defects in adult SIRT6−/− mice. PLoS One. 2017;12(4):e0176371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hu X, Zhu S, Liu R, et al. Sirt6 deficiency impairs corneal epithelial wound healing. AGING 2018;10(8):1932–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Huarachi Olivera RE, Lazarte Rivera A. [Coronavirus disease (COVID-19) and sirtuins]. Rev Fac Cien Med Univ Nac Cordoba. 2020;77(2):117–125. [DOI] [PubMed] [Google Scholar]
- 284.Koyuncu E, Budayeva HG, Miteva YV, et al. Sirtuins are evolutionarily conserved viral restriction factors. mBio. 2014;5(6):e02249–02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Dantoft W, Robertson KA, Watkins WJ, Strobl B, Ghazal P. Metabolic regulators nampt and sirt6 serially participate in the macrophage interferon antiviral cascade. Front Microbiol. 2019;10:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Li P, Jin Y, Qi F, et al. SIRT6 acts as a negative regulator in dengue virus-induced inflammatory response by targeting the DNA binding domain of NF-κB p65. Front Cell Infect Microbiol. 2018;8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jiang H, Cheng ST, Ren JH, et al. SIRT6 inhibitor, OSS_128167 restricts hepatitis B virus transcription and replication through targeting transcription factor peroxisome proliferator-activated receptors alpha. Front Pharmacol. 2019;10:01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mautone N, Zwergel C, Mai A, Rotili D. Sirtuin modulators: where are we now? A review of patents from 2015 to 2019. Expert Opin Ther Pat. 2020;30(6):389–407. [DOI] [PubMed] [Google Scholar]
- 289.Klein MA, Denu JM. Biological and catalytic functions of sirtuin 6 as targets for small-molecule modulators. J Biol Chem. 2020: 10.1074/jbc.REV1120.011438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang Z, Liang Y, Zhang L, Zhang N, Liu Q, Wang Z. Phosphodiesterase 4 inhibitor activates AMPK‐SIRT6 pathway to prevent aging‐related adipose deposition induced by metabolic disorder. AGING. 2018;10(9):2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen Y, Sun T, Wu J, et al. Icariin intervenes in cardiac inflammaging through upregulation of SIRT6 enzyme activity and inhibition of the NF-kappa B pathway. Biomed Res Int. 2015;2015:895976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Li Y, Li X, Cole A, McLaughlin S, Du W. Icariin improves Fanconi anemia hematopoietic stem cell function through SIRT6-mediated NF-kappa B inhibition. Cell Cycle. 2018;17(3):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kim JH, Lee JM, Kim JH, Kim KR. Fluvastatin activates sirtuin 6 to regulate sterol regulatory element-binding proteins and AMP-activated protein kinase in HepG2 cells. Biochem Biophys Res Commun. 2018;503(3):1415–1421. [DOI] [PubMed] [Google Scholar]
- 294.Wencel PL, Lukiw WJ, Strosznajder JB, Strosznajder RP. Inhibition of Poly(ADP-ribose) Polymerase-1 Enhances Gene Expression of Selected Sirtuins and APP Cleaving Enzymes in Amyloid Beta Cytotoxicity. Mol Neurobiol. 2018;55(6):4612–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yang SJ, Choi JM, Chang E, Park SW, Park CY. Sirt1 and Sirt6 mediate beneficial effects of rosiglitazone on hepatic lipid accumulation. PLoS ONE. 2014;9(8):e105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.You W, Rotili D, Li TM, et al. Structural basis of sirtuin 6 activation by synthetic small molecules. Angew Chem Int Ed Engl. 2017;56(4):1007–1011. [DOI] [PubMed] [Google Scholar]
- 297.You W, Steegborn C. Structural basis of Sirtuin 6 inhibition by the hydroxamate trichostatin A: Implications for protein deacylase drug development. J Med Chem. 2018;61(23):10922–10928. [DOI] [PubMed] [Google Scholar]
- 298.Bolivar BE, Welch JT. Studies of the binding of modest modulators of the human enzyme, Sirtuin 6, by STD NMR. Chembiochem. 2017;18(10):931–940. [DOI] [PubMed] [Google Scholar]
- 299.Sociali G, Galeno L, Parenti MD, et al. Quinazolinedione SIRT6 inhibitors sensitize cancer cells to chemotherapeutics. Eur J Med Chem. 2015;102:530–539. [DOI] [PubMed] [Google Scholar]
- 300.You W, Zheng W, Weiss S, Chua KF, Steegborn C. Structural basis for the activation and inhibition of Sirtuin 6 by quercetin and its derivatives. Sci Rep. 2019;9(1):19176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Huang Z, Zhao J, Deng W, et al. Identification of a cellularly active SIRT6 allosteric activator. Nat Chem Biol. 2018;14(12):1118–1126. [DOI] [PubMed] [Google Scholar]
- 302.Motlagh HN, Wrabl JO, Li J, Hilser VJ. The ensemble nature of allostery. Nature. 2014;508(7496):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wodak SJ, Paci E, Dokholyan NV, et al. Allostery in its many disguises: From theory to applications. Structure. 2019;27(4):566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lu S, Chen Y, Wei J, et al. Mechanism of allosteric activation of SIRT6 revealed by the action of rationally designed activators. Acta Pharmaceutica Sinica B. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Parenti MD, Grozio A, Bauer I, et al. Discovery of novel and selective SIRT6 inhibitors. J Med Chem. 2014;57(11):4796–4804. [DOI] [PubMed] [Google Scholar]
- 306.Damonte P, Sociali G, Parenti MD, et al. SIRT6 inhibitors with salicylate-like structure show immunosuppressive and chemosensitizing effects. Bioorg Med Chem. 2017;25(20):5849–5858. [DOI] [PubMed] [Google Scholar]
- 307.Wu M, Seto E, Zhang J. E2F1 enhances glycolysis through suppressing Sirt6 transcription in cancer cells. Oncotarget. 2015;6:11252–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Yasuda M, Wilson DR, Fugmann SD, Moaddel R. Synthesis and characterization of SIRT6 protein coated magnetic beads: identification of a novel inhibitor of SIRT6 deacetylase from medicinal plant extracts. Anal Chem. 2011;83(19):7400–7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Singh N, Ravichandran S, Norton DD, Fugmann SD, Moaddel R. Synthesis and characterization of a SIRT6 open tubular column: predicting deacetylation activity using frontal chromatography. Anal Biochem. 2013;436(2):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rahnasto-Rilla M, Tyni J, Huovinen M, et al. Natural polyphenols as sirtuin 6 modulators. Sci Rep. 2018;8(1):4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sinha S, Patel S, Athar M, et al. Structure-based identification of novel sirtuin inhibitors against triple negative breast cancer: An in silico and in vitro study. Int J Biol Macromol. 2019;140:454–468. [DOI] [PubMed] [Google Scholar]
- 312.Zhao S, Zhu YY, Wang XY, et al. Structural insight into the interactions between structurally similar inhibitors and SIRT6. Int J Mol Sci. 2020;21(7):2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Rahnasto-Rilla M, Lahtela-Kakkonen M, Moaddel R. Sirtuin 6 (SIRT6) Activity Assays. Methods Mol Biol. 2016;1436:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Huhtiniemi T, Salo HS, Suuronen T, et al. Structure-based design of pseudopeptidic inhibitors for SIRT1 and SIRT2. J Med Chem. 2011;54(19):6456–6468. [DOI] [PubMed] [Google Scholar]
- 315.He Y, Yan L, Zang W, Zheng W. Novel sirtuin inhibitory warheads derived from the N(epsilon)-acetyl-lysine analog L-2-amino-7-carboxamidoheptanoic acid. Org Biomol Chem. 2015;13(42):10442–10450. [DOI] [PubMed] [Google Scholar]
- 316.Kokkonen P, Rahnasto-Rilla M, Kiviranta PH, et al. Peptides and Pseudopeptides as SIRT6 Deacetylation Inhibitors. ACS Med Chem Lett. 2012;3(12):969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kokkonen P, Rahnasto-Rilla M, Mellini P, Jarho E, Lahtela-Kakkonen M, Kokkola T. Studying SIRT6 regulation using H3K56 based substrate and small molecules. Eur J Pharm Sci. 2014;63:71–76. [DOI] [PubMed] [Google Scholar]
- 318.Hu J, He B, Bhargava S, Lin H. A fluorogenic assay for screening Sirt6 modulators. Org Biomol Chem. 2013;11(32):5213–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.He B, Hu J, Zhang X, Lin H. Thiomyristoyl peptides as cell-permeable Sirt6 inhibitors. Org Biomol Chem. 2014;12(38):7498–7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Liu J, Zheng W. Cyclic peptide-based potent human SIRT6 inhibitors. Org Biomol Chem. 2016;14(25):5928–5935. [DOI] [PubMed] [Google Scholar]
- 321.Sociali G, Liessi N, Grozio A, et al. Differential modulation of SIRT6 deacetylase and deacylase activities by lysine-based small molecules. Mol Divers. 2019: 10.1007/s11030-11019-09971-11032. [DOI] [PubMed] [Google Scholar]
- 322.Rahnasto-Rilla M, Kokkola T, Jarho E, Lahtela-Kakkonen M, Moaddel R. N-acylethanolamines bind to SIRT6. Chembiochem. 2016;17(1):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Iachettini S, Trisciuoglio D, Rotili D, et al. Pharmacological activation of SIRT6 triggers lethal autophagy in human cancer cells. Cell Death Dis. 2018;9(10):996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lu S, Shen Q, Zhang J. Allosteric methods and their applications: facilitating the discovery of allosteric drugs and the investigation of allosteric mechanisms. Acc Chem Res. 2019;52(2):492–500. [DOI] [PubMed] [Google Scholar]
- 325.Shang JL, Ning SB, Chen YY, Chen TX, Zhang J. MDL-800, an allosteric activator of SIRT6, suppresses proliferation and enhances EGFR-TKIs therapy in non-small cell lung cancer. Acta Pharmacol Sin. 2020: 10.1038/s41401-41020-40442-41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Shang J, Zhu Z, Chen Y, et al. Small-molecule activating SIRT6 elicits therapeutic effects and synergistically promotes anti-tumor activity of vitamin D(3) in colorectal cancer. Theranostics. 2020;10(13):5845–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Chen H, Zhou X, Wang A, Zheng Y, Gao Y, Zhou J. Evolutions in fragment-based drug design: the deconstruction-reconstruction approach. Drug Discov Today. 2015;20(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Chen H, Yang Z, Ding C, et al. Fragment-based drug design and identification of HJC0123, a novel orally bioavailable STAT3 inhibitor for cancer therapy. Eur J Med Chem. 2013;62:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hajduk PJ, Greer J. A decade of fragment-based drug design: strategic advances and lessons learned. Nat Rev Drug Discov. 2007;6(3):211–219. [DOI] [PubMed] [Google Scholar]


