Summary sentence:
Discussion on the emerging evidence of phenotypic and functional PMN heterogeneity in tissue and implications for health and disease outcomes.
Introduction
Immune cells have evolved as highly plastic cells that can adapt to challenges and environmental cues and in the process acquire new functional phenotypes. This flexible malleable adaptation is critical for maintaining tissue homeostasis and cellular function in health and disease. Such concepts are exemplified by the well-recognized CD4 cellular plasticity, which is required for maintenance of immunocompetence, or macrophage plasticity and phenotypic switch, which defines initiation and resolution of inflammation and tissue regeneration [1]. Here we will discuss the emerging phenotypical and functional plasticity of neutrophils (PMNs).
Breaking the dogma.
PMN longevity and plasticity.
PMNs are an important component of the innate immune system and provide the first line of defense against physical or pathogenic insult [2]. PMNs are commonly viewed as short-lived, terminally differentiated cells that are fully committed to phagocytic functionality and act during the initiation state of the inflammatory response. However, this view is rapidly changing with the emerging evidence demonstrating increased PMN life-span, plasticity and phenotypic heterogeneity. PMN life span in the circulation can extend beyond 5 days (which is significantly longer than what was once assumed) and likely even longer in the tissue. Environmental cues such as cytokines, hypoxia or pathogen encounters promote PMN survival, allowing/supporting the idea of PMN transitional stages and acquisition of new phenotypes [3]. Indeed, PMN heterogeneity and distinct subsets have been identified in the bone marrow (including putative proliferating progenitors that give rise to committed immature and mature PMNs) and in the circulation (decreased CD62L expression and gain of CD11b and CXCR4 and function in aged PMNs) [3]. The PMN heterogeneity during development and in the bone marrow and later in the circulation likely stems the phenotypic and functional differences in tissue (illustrated by the schematic, Fig 1). It is important to note that thus far, PMN classification into subsets was primarily based on the identification of phenotypically and/or functionally distinct PMN populations in various tissues. This perhaps indicates cell polarity rather than bona fide cell subsets. As such, whether these PMN subpopulation are indeed truly committed cell subsets or the same cell population caught at different transitional stages due to varying local activation/polarization cues remains to be determined.
Figure 1: Emerging PMN plasticity.
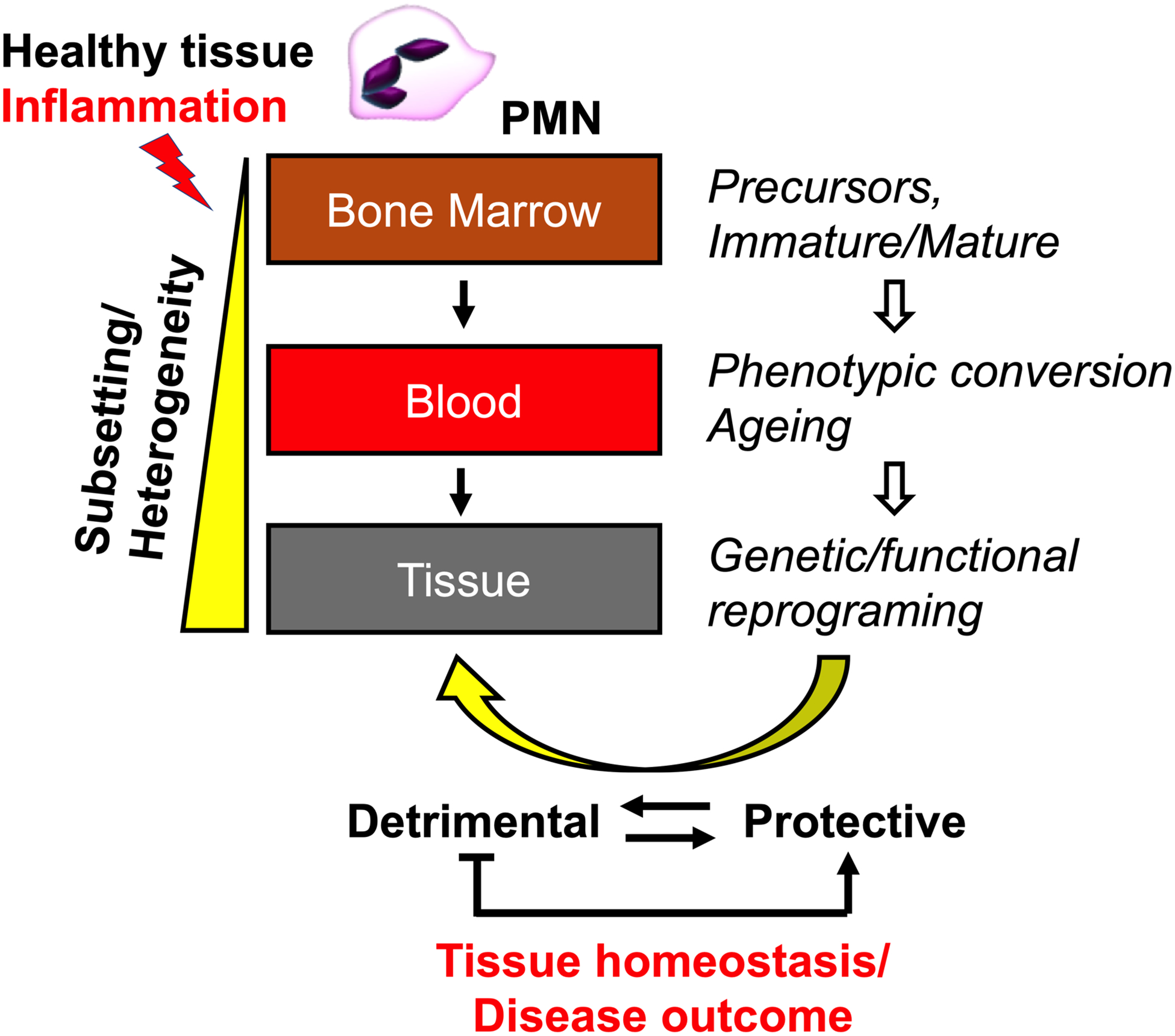
PMN heterogeneity initiates in the bone marrow and further potentiated in the circulation, leading to phenotypically and functionally distinct PMN subsets in the tissue. This dictates the PMN impact on tissue homeostasis and disease outcomes, providing exciting new therapeutic opportunities.
Neutrophil functions the “bad and good”.
PMN presence in the tissue is often viewed as detrimental and is associated with exacerbated inflammation and tissue injury [4]. While this is true in the setting of acute inflammation accompanied by heightened numbers of infiltrating PMNs, this view is also changing with the emerging evidence of PMN homeostatic and pro-resolution functions. PMN can contribute to tissue repair by multiple mechanisms, including, clearance of cellular debris, immune regulation, resolution of inflammation and tissue remodeling. Specific examples include the release of matrix metallopeptidase 9 (MMP-9), which degrades damage-associated molecular patterns (DAMP) proteins to dampen recruitment of other immune cells. Via the release of NETs or vascular endothelial growth factor (VEGF) and dynamic interactions with macrophages, PMNs can promote vascular growth and neovascularization [5]. Via the release of pro-resolving soluble mediators, such as Annexin A1, IL-10, IL-22 and IL1ra, or via engagement of surface adhesive receptors, such as intercellular adhesion molecule-1 (ICAM-1), PMNs can promote tissue regeneration and injury resolution [6, 7].
PMN sub-setting in the olfactory neuroepithelium may lead to neuroprotection.
The work by Ogawa et al., is one of latest examples of PMN plasticity. In their work authors describe a distinct PMN population that originates from the BM and upon homing to the nasal olfactory neuroepithelial tissue acquire characteristics of a hybrid neutrophil-eosinophil cell type. Ogawa et al. elegantly showed that these cells were distinct from eosinophils, but expressed classical eosinophil marker SigelcF, as well as the PMN marker Ly6G, thus were termed double positive cells (DPCs). DPCs were also morphologically distinct from eosinophils (lacked typical eosinophilic granules) and had more lobulated nuclei as compared to healthy PMNs. It is important to note that these observations support similar findings from a different group, showing Ly-6G+/Siglec-F+ PMNs in nasal mucosa [8]. The distinct and more pronounced lobulation in DPCs may be an important indicator of a particular functional state and have significant functional consequences. For example, nuclear deformability is a limiting step in PMN migration, and increased lobulation may indicate improved flexibility and migratory capacity. Similarly, PMN NETosis requires chromatin release and extensive nuclear remodeling and may be impacted by hyper lobulation [9]. Although authors have not observed differences in NETosis between DPCs and PMNs, these ideas merit more careful future investigations.
Intriguingly, Ogawa et al. further observed that DPCs underwent genetic reprograming upon reaching the neuroepithelial tissue. Gene expression analyses revealed that DPCs turned on several genes encoding inflammatory cytokines and chemokines, suggesting transition to a more activated cellular state. In parallel the expression of potentially neuroprotective genes, including Sox11and Mtap1b and IL33 were also increased in these cells, implying that BM-derived PMNs upon homing to olfactory neuroepithelial tissues acquire “neuro-supportive” phenotype that sustain tissue homeostasis.
Future directions and relevance of PMN plasticity in disease.
One of the key findings by Ogawa et al. is that upon entering the target tissue PMNs are capable of undergoing rapid genetic reprogramming, which supports the idea of PMN plasticity. Using RNA-sequencing of DPCs authors demonstrate changes in the “neuro-supportive” genes, however, whether these changes in gene expression are indeed translated into PMN neuroprotective function remains to be determined. To address this a more detailed phenotypic characterization of DPCs and identification of additional markers, which will perhaps allow for specific depletion of this population is required. Another important question that arises from these observations, is how the neuroepithelial cell niche (or cellular niches in other organs) fosters such reprograming and phenotypic conversions of PMNs. To identify specific factors that may drive this process, detailed analyses of the olfactory neuroepithelium microenvironment should be performed.
Authors further demonstrate that at baseline, DPCs localization was restricted to the olfactory neuroepithelial tissue and was not found in other organs. Although authors found that DPCs were expanded with inflammation in the olfactory, whether their localization remains restricted to this organ remains to be determined. Supporting the idea that perhaps under certain conditions this distinct PMN population could be found in other organs, SiglecF+ PMNs were previously observed in lung adenocarcinomas, where they exhibited pro-tumorigenic properties and underwent reprogramming upon entry into the lung [10].
Many diseases are driven by PMNs and their effect on the surrounding tissue. As such, there is continued interest in understanding mechanisms that regulate PMN recruitment and function. Importantly, since PMNs can facilitate both “good” and “bad” outcomes, dissecting the mechanisms that drive phenotypic and functional heterogeneity of PMNs in different anatomical niches, including the bone marrow, blood and tissue will offer new avenues for therapeutic intervention.
Once better defined, PMN subsets with distinct effector functions could be either selectively suppressed/attenuated or reinforced to achieve optimal therapeutic outcome.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) DK124199, American Cancer Society Research Scholar Award and Crohn’s & Colitis Foundation Senior Research Award.
Footnotes
Author declared no conflict of interest.
REFERENCES
- 1.Sica A and Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122, 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L (2000) Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest 80, 617–53. [DOI] [PubMed] [Google Scholar]
- 3.Ng LG, Ostuni R, Hidalgo A (2019) Heterogeneity of neutrophils. Nat Rev Immunol 19, 255–265. [DOI] [PubMed] [Google Scholar]
- 4.Silvestre-Roig C, Hidalgo A, Soehnlein O (2016) Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 127, 2173–81. [DOI] [PubMed] [Google Scholar]
- 5.Peiseler M and Kubes P (2019) More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest 129, 2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumagin R, Brazil JC, Nava P, Nishio H, Alam A, Luissint AC, Weber DA, Neish AS, Nusrat A, Parkos CA (2016) Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol 9, 1151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, Catz SD (2018) Neutrophils: New insights and open questions. Sci Immunol 3. [DOI] [PubMed] [Google Scholar]
- 8.Matsui M, Nagakubo D, Satooka H, Hirata T (2020) A novel Siglec-F(+) neutrophil subset in the mouse nasal mucosa exhibits an activated phenotype and is increased in an allergic rhinitis model. Biochem Biophys Res Commun 526, 599–606. [DOI] [PubMed] [Google Scholar]
- 9.Manley HR, Keightley MC, Lieschke GJ (2018) The Neutrophil Nucleus: An Important Influence on Neutrophil Migration and Function. Front Immunol 9, 2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, Rickelt S, Severe N, Baryawno N, Faget J, Savova V, Zemmour D, Kline J, Siwicki M, Garris C, Pucci F, Liao HW, Lin YJ, Newton A, Yaghi OK, Iwamoto Y, Tricot B, Wojtkiewicz GR, Nahrendorf M, Cortez-Retamozo V, Meylan E, Hynes RO, Demay M, Klein A, Bredella MA, Scadden DT, Weissleder R, Pittet MJ (2017) Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science 358. [DOI] [PMC free article] [PubMed] [Google Scholar]


