Abstract
Proteolysis-targeting chimaera (PROTAC) technology is an emerging approach for achieving targeted degradation of a protein of interest (POI) intracellularly. However, the cell permeability of PROTACs is limited by their high molecular weight and total polar surface area. Moreover, the activation of the proteasome-mediated degradation by PROTAC requires the formation of a ternary (three-component) complex, composed of the PROTAC, the POIs, and E3-ligases related proteins (E3Ps). Simplifying the three-component system to two-component system could theoretically increase the efficiency of the formation of ternary complex and enhance the protein degradation efficiency. Herein, we demonstrate that pre-fusion of PROTACs with E3Ps (called “pre-fused PROTACs”) before administration could transform the original PROTAC system to two-component system. After delivery by lipid nanoparticles, the degradation of POI by pre-fused PROTACs is dramatically increased and accelerated compared with standard PROTACs. Moreover, we demonstrate that this approach can be generalized to another hydrophobic tag (HyT) degrader by demonstrating the improved targeted protein degradation after pre-fusion the HyT degrader with heat shock protein 70 (HSP70).
Keywords: PROTAC, Lipid-like nanoparticle (LNP), Drug delivery, Protein degradation
Graphical Abstract
1. Introduction
Targeted degradation of specific proteins via the ubiquitin–proteasome system (UPS) by small molecules proteolysis-targeting chimaera (PROTAC) has emerged as a promising strategy for treating various diseases, owing to their high efficiency and selectivity [1–3]. PROTAC is a hetero bifunctional molecule composed of two components, a ligand targeting a protein of interest (POI) and an UPS activating ligand, connected by a linker [4]. Nowadays, ARV-110, an oral administrated PROTACs for androgen receptor (AR) degradation, is currently in clinic trials for the treatment of prostate cancer, demonstrating the great clinical potential of technologies based on the UPS-mediated degradation of POI [5]. Even though PROTACs showed significant advantages compared with small molecule inhibitors, there were still two obstacles hindering their further development. First, the cell permeability of the PROTAC molecules decreased significantly owing to the large molecule weight (MW > 800) and the presence of multiple hydrogen bond donors and acceptors [6, 7]. Second, the degradation of POIs by PROTACs requires the formation of an active ternary complex, which is a typical three-component system. As a result, the degradation ability of PROTAC decreased dramatically when applied at a high concentration due to the ‘hook effect’ of the three-component system [8, 9]. However, there was few studies about the enhancement of the cell permeability and the avoidance of “hook effect” so far.
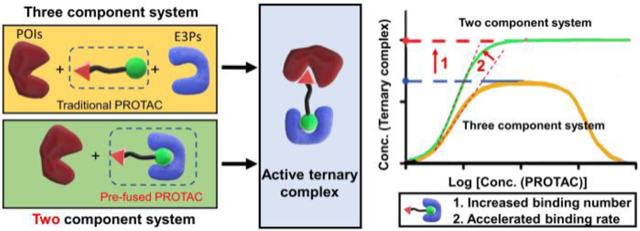
Apart from the low cell permeability, the “hook effect” caused by the distinct mechanism of three-component system remained another limitation of current PROTACs. In the three-component system, the successful formation of activated ternary complex by PROTACs requires the recognition of both protein of interest (POI) and E3-ligases related proteins (E3Ps) together. In the theoretical model, two component system shows higher binding amount and rate (Scheme 1A) [9]. If the original three-component PROTAC system can be transformed to a two-component system, the degradation efficiency is predicted to be significantly increased. However, there was no works related to the transformation of traditional PROTACs to two-component system.
Scheme 1.
(A) The formation of active ternary complex of two or three component system calculated by theoretical models. (B) The LNP delivers the pre-fused PROTAC for enhanced activation of the ubiquitin–proteasome system through one step binding instead of two step binding with free PROTAC.
Herein, we designed a lipid nanoparticle (LNP) platform to deliver the incorporated E3Ps and PROTACs (termed pre-fused PROTACs) and demonstrated the enhanced degradation of POI comparing with that treated with PROTACs only. Based on the pre-fusion, the original three-component system including PROATC, POI, and E3P changes to two-component system consisting of pre-fused PROTAC and POI. As predicted by theoretical model, the pre-fused PROTAC could form ternary complex more effectively than traditional PROTAC, which would theoretically enhance the efficiency of ubiquitination and further degradation of POI. As shown in Scheme 1B, the pre-fused PROTAC was then encapsulated into LNP and could escape from the endo/lysosome after the endocytosis [10]. The pre-fused PROTAC can form the active ternary complex through one-step binding, which is predictive to be more efficient than the traditional PROATC undergoing two-step binding. Such delivery system could benefit the intracellular delivery of E3P pre-fused PROTAC.
2. Materials and Methods
2.1. Materials
ARV-771 was bought from MedChemExpress (Monmouth Junction, NJ, USA) and ARV-825 was purchased from LLCChemietek (Indianapolis, IN, USA). SARD279 was synthesized according to previous report [11]. The VHL and HSP70 proteins were both bought from Novus Biologicals (Centennial, CO, USA). 80-O14B lipid was synthesized according to the previous publication (Figure S2) [12]. HeLa and LNCaP cell lines were all purchased from ATCC (Manassas, VA, USA) and cultured in Eagle’s minimal essential medium (DMEM) and Roswell Park Memorial Institute Medium (RPMI 1640) with 10% fetal bovine serum (FBS) and 100 μg/mL penicillin-streptomycin (Gibco Laboratories, Grand Island, NY, USA), respectively. Recombinant Anti-Brd4 (ab128874) and anti-AR (ab133273) antibodies were purchased from Abcam (Cambridge, MA, USA). The anti-GAPDH, HRP-labelled goat anti-rabbit, and HRP-labelled rabbit anti mouse secondary antibodies were bought from Invitrogen (Carlsbad, CA, USA).
2.2. Preparation and encapsulation of pre-fused PROATC
Generally, ARV-771 was firstly dissolved in DMSO and diluted to 100 nM in phosphate-buffered saline (PBS). Then VHL protein was added to the solution with a final concentration of 2.4 μg/mL. The mixture was incubated for 30 min at room temperature to form pre-fused ARV-771. 80-O14B LNP was formulated by directly dissolving the lipidoid into 25 mM sodium acetate solution (pH = 5.5). The 80-O14B LNP was added into the solution with the final concentration of 5 μg/mL and the solution was incubated at room temperature for another 15 min before administration. The pre-fused ARV-825 or SARD279 were prepared through the similar route.
2.3. Characterization of pre-fused PROTAC
The encapsulation efficiency of ARV-771 and pre-fused ARV-771 were measured using high performance liquid chromatography (HPLC). The ARV-771 and pre-fused ARV-771 LNPs were dialyzed (MWCO=3500) with water for 24 h. The LNPs were then freeze-dried and re-dissolved in acetonitrile. The concentration of ARV-771 encapsulated in the LNPs were measured by HPLC using the acetonitrile-water (90/10, V/V) as the mobile phase. The size and zeta potential of LNPs were characterized by Zetaplus analyzer (Zetaplus, Brookhaven, USA).
2.4. Degradation of POI by pre-fused PROTAC
1 × 106 Hela cells were cultured in 6 well plate for 24 h before administration. The pre-fused ARV-771 was encapsulated into 80-O14B LNP and then diluted to different concentration. After 24 h incubation, the proteins were isolated from Hela cells using the radioimmunoprecipitation assay (RIPA) lysis buffer and quantitated by bicinchoninic acid assay (BCA assay). Then the proteins were diluted to the sample concentration using NuPAGE™ LDS sample buffer (Invitrogen, Carlsbad, CA, USA) and denatured at 95 °C for 5 min. The study of targeted protein degradation by ARV-825 and SARD279 were carried out in a similar way.
2.5. Western blot
General western blot was carried out on the Invitrogen Novex SureLock Mini-Cell system. All the supplies needed for the system were bought from Invitrogen (Carlsbad, CA, USA).10 μL sample was loaded onto 4–12% bis-tris protein gel and the gel was run with a stable voltage of 120 V for 80 min. Then the gel was cut according to the protein ladder and transferred to PVDF membrane with a stable current of 250 mA for 3 h. The membrane was blocked by 5% skimmed milk for 1 h at room temperature and then incubated with primary antibody overnight. After 3 times of washing by TBST (tris-buffered saline, 0.1% tween 20), the membrane was incubated with secondary antibody for another 1 h and then washed by TBST for 3 times. The final membrane was then imaged using enhanced chemiluminescence (ECL) substrate.
3. Results and discussion
3.1. Enhanced degradation of BRD4 by delivery of pre-fused ARV-771.
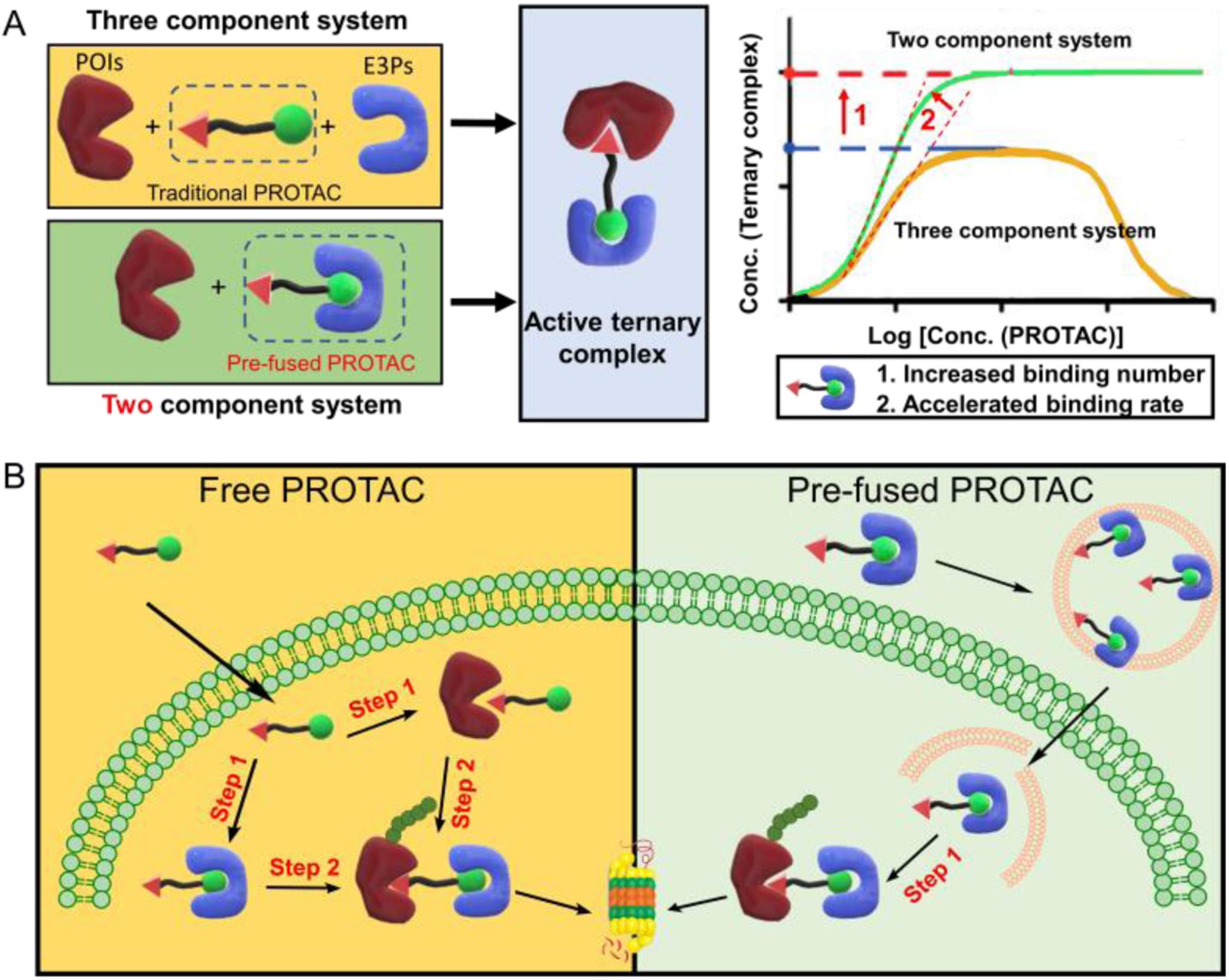
To evaluate this hypothesis, we firstly chose a reported PROTAC, that is ARV-771, as a model molecule [13]. There are two binding sites of ARV-771, one to bind a specific E3P known as von Hippel-Lindau (VHL) protein, and one to bind the target protein, bromodomain-containing protein 4 (BRD4). BRD4, which promotes progression and regulation in different cancer types including neuroblastoma and glioblastoma, is considered as an important therapeutic target [14]. The PROTAC recruits and binds both VHL and BRD4, bringing the two compounds into close proximity. BRD4 is then ubiquitinated and subsequently degraded by proteasome. In traditional three-component PROTAC system, the ternary complex formed by binding of both BRD4 and VHL to AVR-771 is necessary for the successful degradation of BRD4. We hypothesize that if we complex the VHL to ARV-711 first before delivery, the efficiency of BRD4 degradation would increase owing to the simplified two-component system.
As a proof of concept, ARV-771 was firstly incubated with VHL protein in PBS for 30 min to from the pre-fused ARV-771 (Figure 1A). Then the pre-fused ARV-771 was loaded into a synthetic lipidoid nanoparticle (LNP: 80-O14B). 80-O14B is a synthetic lipid nanoparticle that we have previously shown for efficiently intracellular protein delivery (structure shown in Figure S1) [12, 15]. Moreover, after the encapsulation of pre-fused ARV-771, the LNP exhibited an increased particle size and reduced zeta potential, suggesting the successful interaction between LNP and pre-fused VHL/ARV-771 complex (Figure S2 and Table S1). The encapsulation efficiency of ARV-771 in 80-O14B LNP is 27.2% in free version comparing with 60.8% in pre-fused version. The dramatical increase in the ARV-771 encapsulation may be attributed to the high binding rate of VHL-protein to the cationic LNPs.
Figure 1.
(A) The preparation of pre-fused ARV-771 and encapsulation into 80-O14B LNP. (B) Western blot of the BRD4 protein remained in Hela cells after treatment with different formulations.
HeLa cells, which express BRD4 at high levels, were selected as a model cell line to evaluate the efficiency of pre-fused ARV-771-induced BRD4 degradation (Figure 1B) [16]. After 24 h of treatment with unfused ARV-771 (pure ARV-771) at low concentration of 25 and 50 nM, the BRD4 still remained at high level (more than 80% remained). The control cells treated with blank LNP didn’t show significant degradation of BRD4, indicating that as expected, our lipid nanoparticles themselves have no proteolytic activity. Notably, the unfused ARV-771-loaded LNP showed an enhancement of BRD4 degradation, due to the improved delivery of large PROTAC molecule (ARV-771) by LNP. However, based on the mechanism of PROTACs, the molecules (ARV-771) are recyclable for protein degradation, providing the possibility that even a single molecule could lead to total degradation of the POI, thus this dose is theoretically capable of the complete degradation of BRD4 under optimal conditions. We hypothesized that the modest efficiency of ARV-771 in BRD4 degradation at low concentration might be due to the low efficiency of the required simultaneous binding of POIs and E3Ps in the three-component system and may be improved by our pre-fused system. Interestingly, after fusing ARV-771 with VHL and subsequently loading the fused molecule into LNPs for intracellular delivery, we observed both the BRD4 were almost completely degraded (more than 90% reduction) after 24 h of treatment (Figure 1, lane 8 and 9). Such dramatically increased efficacy proved the hypothesis that the pre-fused PROTAC transformed the three-component PROTAC system to two-component PROTAC system, leading to the degradation of the POI with higher efficiency.
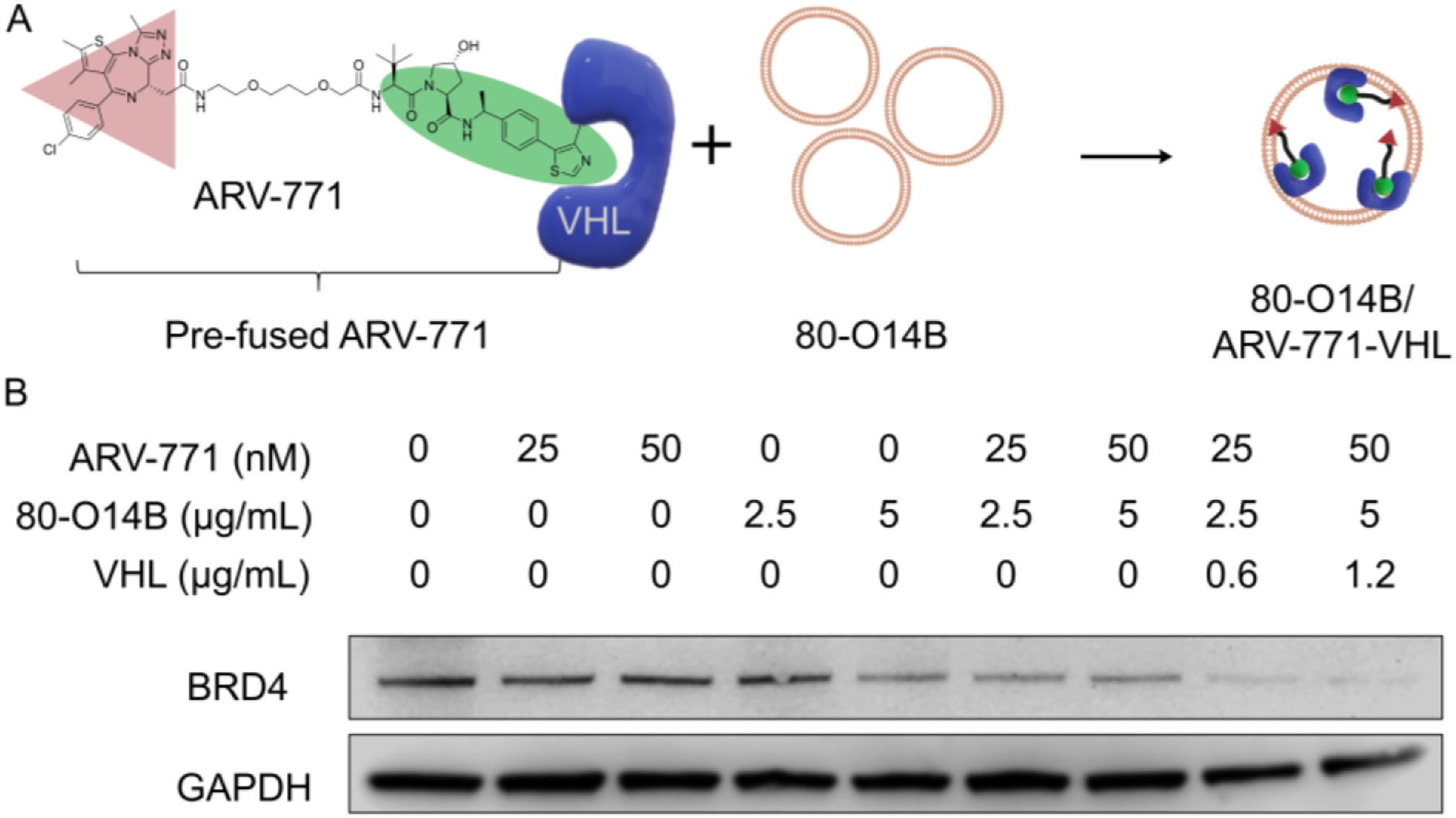
The degradation of BRD4 at different concentration of VHL was determined by Western blot. As shown in Figure S3, the VHL pre-fused ARV-711 exhibited increased degradation of BRD4 when the concentration of ARV-711 increases in the range of 0.16 – 0.63 μg/mL. However, the continuous increasing of VHL concentration to 5 μg/mL didn’t further enhance the BRD4 degradation. The reason might be attributed to the saturation of the binding of VHL and ARV-771 as well as the encapsulation capability of 80-O14B LNP. Nonetheless, the influence of prefusing VHL to ARV-711 on the degradation of BRD4 further proved the important role of VHL in the pre-fusion system.
To investigate whether the enhanced protein degradation was truly induced by the pre-fusion of PROTAC with its corresponding E3P, we selected another PROTAC (ARV-825) [17]. ARV-825 is also a BRD4 degrader, but its UPS-activating ligand binds to cereblon instead of VHL. As ARV-825 lacks a VHL binding site, the incubation of ARV-825 with VHL should not form stable pre-fused PROTAC (Figure S4A). As shown in Figure S4B, the pre-incubation of ARV-825 with VHL didn’t show obvious enhanced BRD4 degradation compared with that of free ARV-825 even at the high concentration of 100 nM, which is consistent with our prediction. As expected, the delivery of VHL protein alone also showed no effect on protein degradation. These results demonstrated the enhanced degradation of POI happens only when the PROTAC is pre-fused with the correct E3P.
Overall, the pre-fusion of ARV-771 by VHL could increase the BRD4 degradation efficiency compared with free ARV-771. Moreover, the enhancement of the degradation was also proved to be mediated by the pre-fusion of corresponding E3P.
3.2. Increased level and rate of protein degradation by delivery of pre-fused PROTAC.
The pre-fused ARV-771 delivered by LNP was proved to enhance the protein degradation of BRD4 according to above results, which is benefited from both the enhanced cell permeability by LNP and the two-component system after pre-fusion. Based on it, we further evaluated the degradation kinetics of free ARV-771 and pre-fused ARV-771. As shown in Figure 2A, free AVR-771 showed modest degradation of BRD4 with the half degradation concentration (DC50) of about 100 nM. However, after pre-fusion with VHL and delivered by 80-O14B LNP, the degradation ability of BRD4 increased significantly (Figure 2B). The BRD4 was totally degraded (>95%) at the concentration of 25 nM. The dramatically enhancement of degradation ability of PROTACs confirms the two-component system through pre-fusion of VHL to ARV-771 could greatly enhance the degradation ability comparing with the three-component system.
Figure 2.
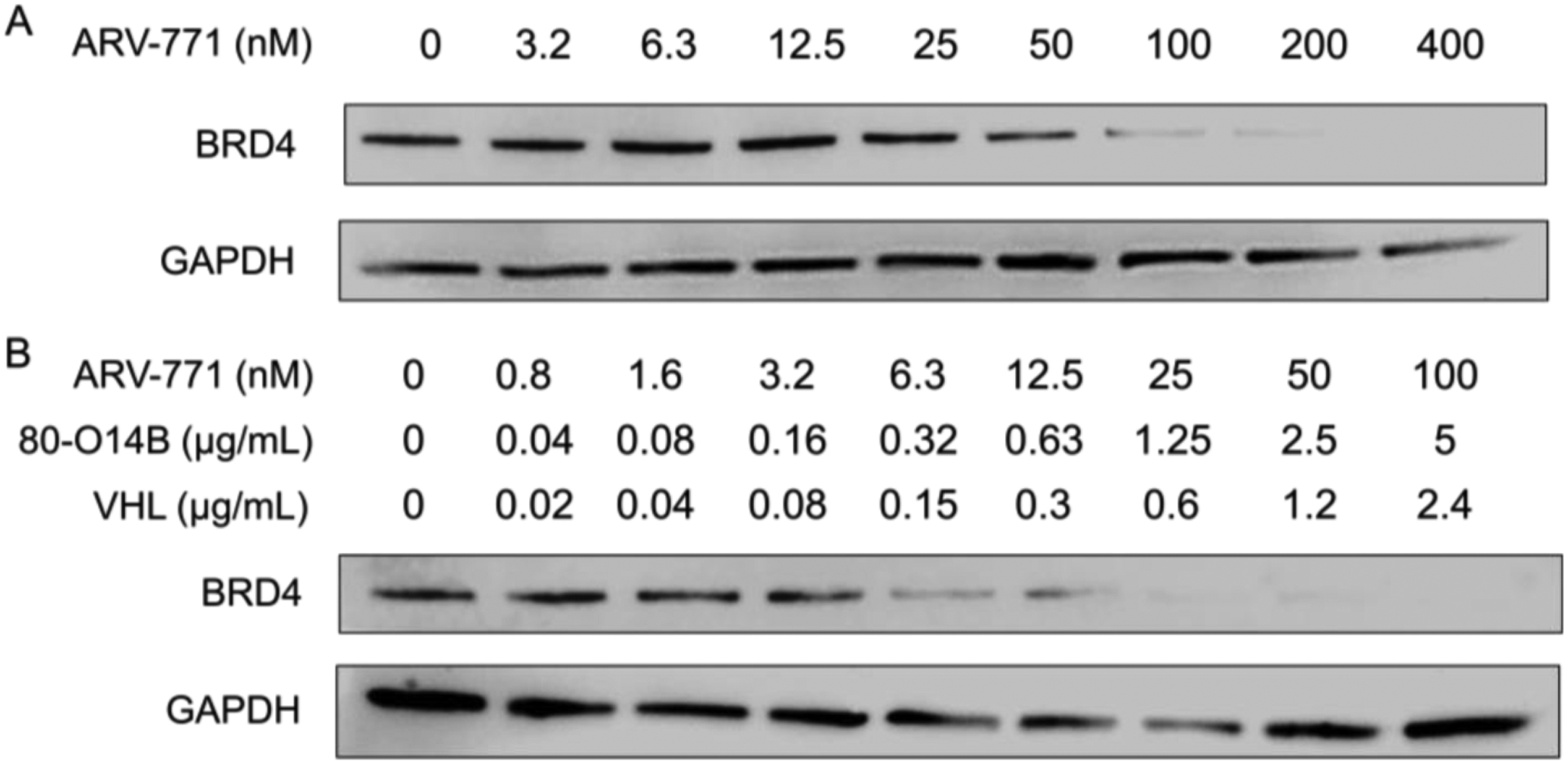
Western blot of BRD4 protein in Hela cells treated with free ARV-771 (A) or pre-fused ARV-771 (B).
The two-component PROTAC system not only increases the degradation amount but also accelerates the degradation rate owing to the simplified recognition route comparing with that of three-component system (Scheme 1B). For traditional three-component PROTAC system, there are at least two steps before the formation of the ternary complex required for ubiquitination and degradation of POIs. More specifically, both the POIs and E3Ps should bind to the same PROTAC molecule, which significantly decreases and decelerates the successful recognition. However, in the two-component system, after the pre-fusion with E3Ps, only the binding of the targeted proteins is required for the formation of ternary complex before the successful ubiquitination and degradation. Such simplified binding route might accelerate the recognition and then increase the speed of degradation. To test the hypothesis, we conducted a time-dependent degradation assay in Figure 3. HeLa cells were treated with free ARV-771 or VHL pre-fused ARV-771 for different time periods. For free ARV-771, there was no obvious degradation of BRD4 during the first 6 hours. The free ARV-771 seemed to be active after 8 hours and the total degradation occurred only after 24 hours of incubation. However, the degradation rate was significantly accelerated in the cells by treating with the VHL pre-fused ARV-771 delivered using 80-O14B LNP. The protein level showed about 50% reduction after only 6 hours of treatment and almost disappeared (> 90% reduction) after 8 hours of treatment (Figure 3). The results showed that the two-component PROTAC system by pre-fusion of VHL to ARV-771 could accelerate the degradation rate.
Figure 3.
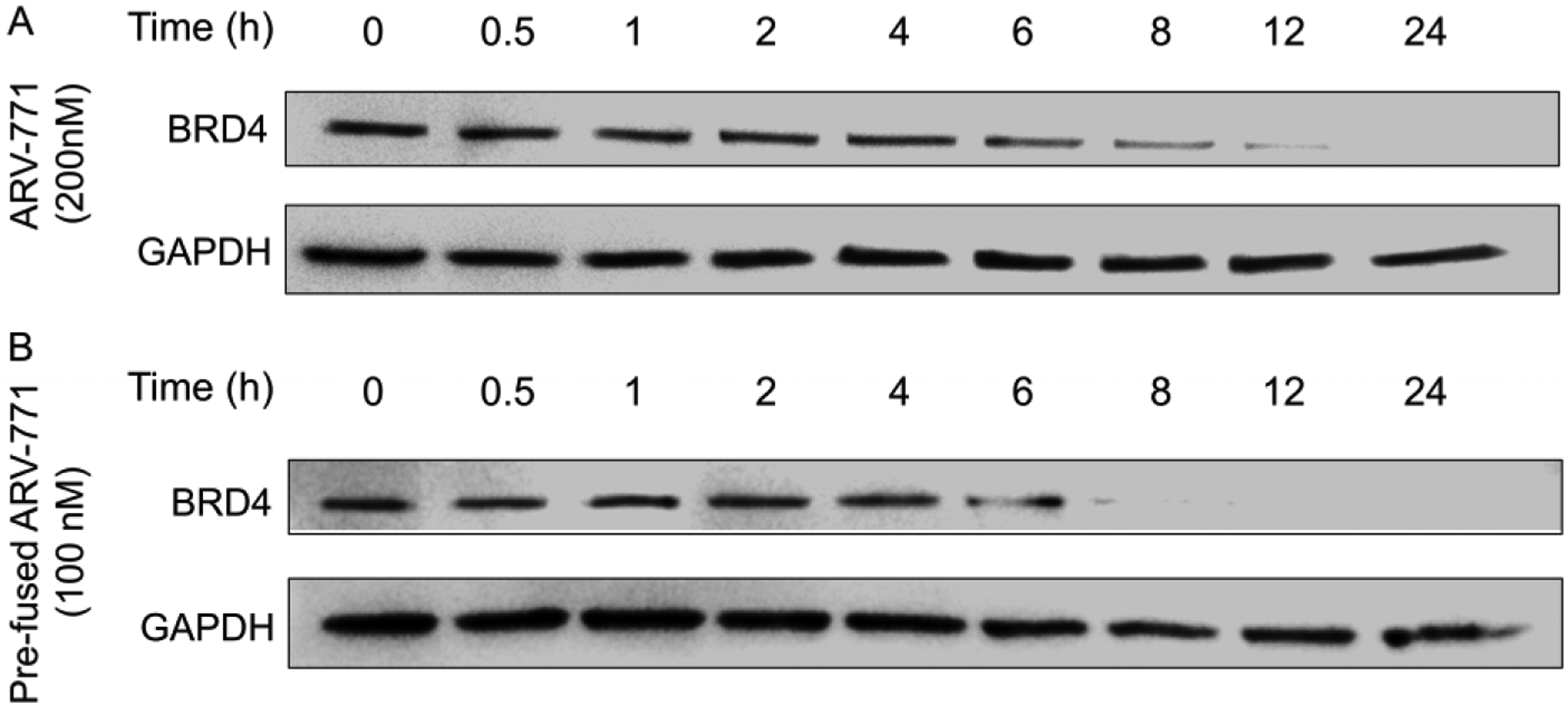
Western blot of the time-dependent degradation of BRD4 by free ARV-771 (A) or pre-fused ARV-771 (B) after incubation for different period of time.
The pre-fusion of PROTAC not only increased the degradation level of BRD4 protein, but also accelerated the degradation rate. These results were consistent with the prediction of the theoretical models, which might also be applied in other three-component systems.
3.3. Pre-fusion as a general method for enhancing the efficiency of other three-component systems.
To further investigate whether the pre-fusion method is a general strategy for enhancing the small-molecule mediated protein degradation, we studied SARD279, a HyT (hydrophobic tag) degrader for androgen receptor (AR).[11] Different from PROTAC, the UPS-activating ligands of HyT degraders are hydrophobic molecules which can recruit HSP70 for targeted protein degradation [18]. As shown in Figure 4A, free SARD279 showed no AR degradation in LNCaP cells at the concentration from 0.125 to 0.5 μM. The addition of LNP to the system did not increase the AR degradation efficiency. However, after pre-fusing SARD279 with HSP70 and delivering using LNP, the complex showed more than 80% degradation of AR at 0.5 μM. Moreover, the degradation of AR by pre-fused SARD279 showed a concentration dependency. In Figure 4B, the DC50 of SARD279 after pre-fusion decreased to about 0.06 μM, about 16 times lower than that of the free SARD279. The successful enhancement of SARD279 by the pre-fusion of HSP70 further demonstrated the pre-fusion of E3P to PROTACs could be a universal method to increase efficiency of extensive PROTACs.
Figure 4.
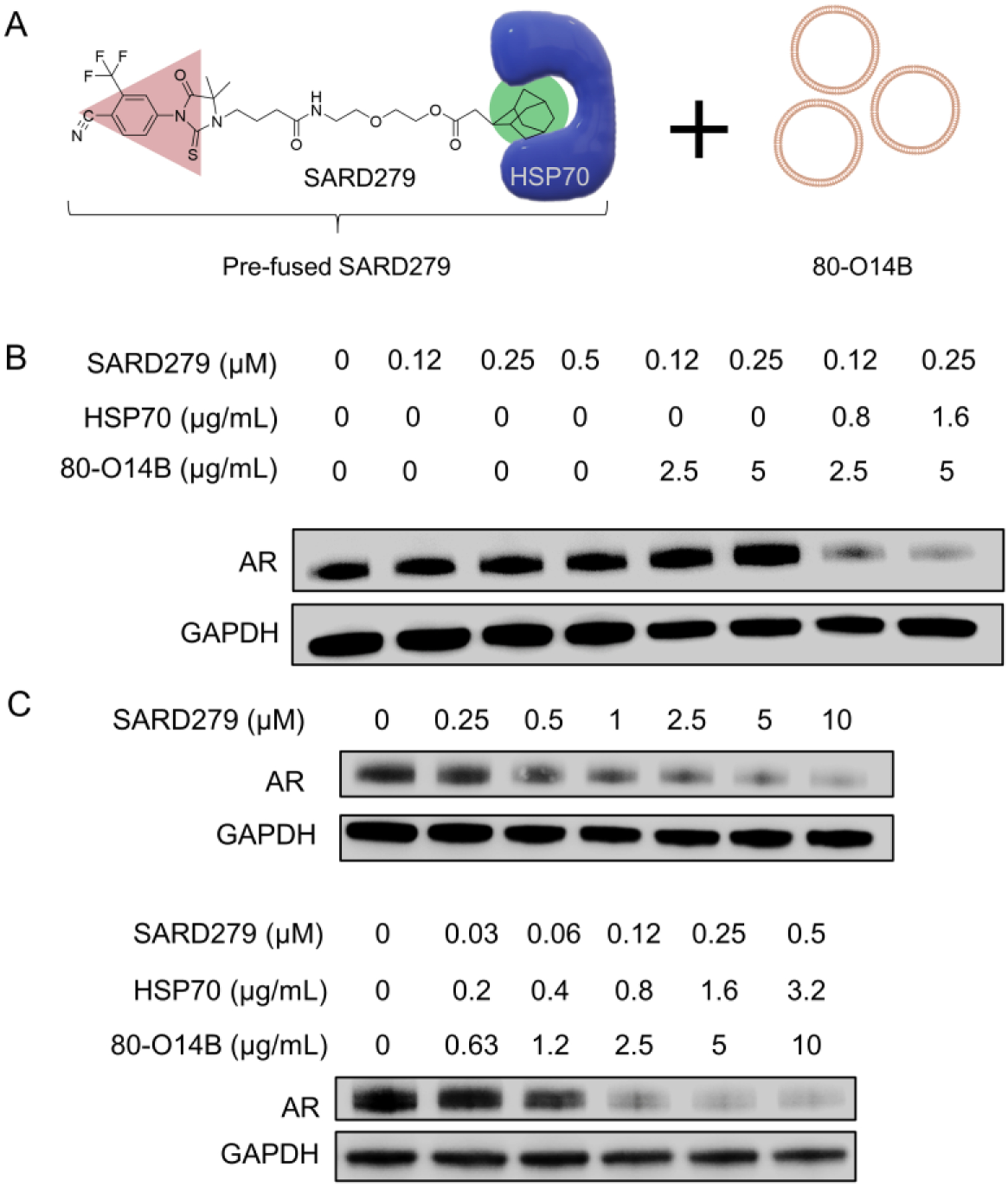
(A) SARD279 was pre-fused with HSP70 and then encapsulated into 80-O14B LNP. (B) AR expression in LNCaP cells under different formulations of LNP, SARD279, and HSP70. (C) Concentration depended AR degradation by free or pre-fused SARD279.
Conclusion
In summary, we designed a novel two-component PROTAC system by the pre-fusing E3Ps to PROTACs before administration and delivered it to the targeted cells by LNPs for enhanced degradation of POIs. As a proof of concept, we pre-fused the VHL to ARV-771 and delivered it to Hela cells for degradation of BRD4. We proved that the two-component PROTAC system showed significantly increased efficiency in degradation of POI than the traditional three component system even at rather low concentration. Moreover, the rate of degradation was also accelerated in the two-component system. Finally, the efficiency of this strategy was also confirmed in Hyts degraders, revealing the versatility of this method. Further studies focusing on the active and passive targeting of tumor sites in vivo is needed to compare the perfused PROTACs and free form in cancer treatment.
Supplementary Material
Highlights:
The pre-fused PROTACs conveted the tranditional three component PROTACs to two component systems
The pre-fused ARV-771 delivered by 80-O14B significanlty enhanced the degradation of BRD4
The pre-fusion were proved to be a general method for enhancment of the effiencicy of three-component systems for protein degradation
Acknowledgements:
This work was supported by National Institutes of Health (NIH) Grant R01 EB027170-01.
References
- [1].Toure M, Crews CM, Small-Molecule PROTACS: New Approaches to Protein Degradation, Angewandte Chemie International Edition, 55 (2016) 1966–1973. [DOI] [PubMed] [Google Scholar]
- [2].Ottis P, Crews CM, Proteolysis-targeting chimeras: induced protein degradation as a therapeutic strategy, ACS chemical biology, 12 (2017) 892–898. [DOI] [PubMed] [Google Scholar]
- [3].Toure M, Crews CM, Small‐molecule PROTACS: new approaches to protein degradation, Angewandte Chemie International Edition, 55 (2016) 1966–1973. [DOI] [PubMed] [Google Scholar]
- [4].Gadd MS, Testa A, Lucas X, Chan K-H, Chen W, Lamont DJ, Zengerle M, Ciulli A, Structural basis of PROTAC cooperative recognition for selective protein degradation, Nature Chemical Biology, 13 (2017) 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Neklesa TK, Snyder LB, Bookbinder M, Chen X, Crew AP, Crews CM, Dong H, Gordon D, Macaluso J, Raina K, An oral androgen receptor PROTAC degrader for prostate cancer, Cancer Res, 77 (2017) 5637. [Google Scholar]
- [6].Foley CA, Potjewyd F, Lamb KN, James LI, Frye SV, Assessing the Cell Permeability of Bivalent Chemical Degraders Using the Chloroalkane Penetration Assay, ACS Chemical Biology, 15 (2020) 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Scott DE, Rooney TPC, Bayle ED, Mirza T, Willems HMG, Clarke JH, Andrews SP, Skidmore J, Systematic Investigation of the Permeability of Androgen Receptor PROTACs, ACS Medicinal Chemistry Letters, 11 (2020) 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bondeson DP, Mares A, Smith IED, Ko E, Campos S, Miah AH, Mulholland KE, Routly N, Buckley DL, Gustafson JL, Zinn N, Grandi P, Shimamura S, Bergamini G, Faelth-Savitski M, Bantscheff M, Cox C, Gordon DA, Willard RR, Flanagan JJ, Casillas LN, Votta BJ, den Besten W, Famm K, Kruidenier L, Carter PS, Harling JD, Churcher I, Crews CM, Catalytic in vivo protein knockdown by small-molecule PROTACs, Nature Chemical Biology, 11 (2015) 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Douglass EF, Miller CJ, Sparer G, Shapiro H, Spiegel DA, A Comprehensive Mathematical Model for Three-Body Binding Equilibria, Journal of the American Chemical Society, 135 (2013) 6092–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xiong H, Liu S, Wei T, Cheng Q, Siegwart DJ, Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo, Journal of Controlled Release, 325 (2020) 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gustafson JL, Neklesa TK, Cox CS, Roth AG, Buckley DL, Tae HS, Sundberg TB, Stagg DB, Hines J, McDonnell DP, Norris JD, Crews CM, Small-Molecule-Mediated Degradation of the Androgen Receptor through Hydrophobic Tagging, Angewandte Chemie International Edition, 54 (2015) 9659–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang M, Zuris JA, Meng F, Rees H, Sun S, Deng P, Han Y, Gao X, Pouli D, Wu Q, Georgakoudi I, Liu DR, Xu Q, Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles, Proceedings of the National Academy of Sciences, 113 (2016) 2868–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AMK, Wang J, Chen X, Dong H, Siu K, PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer, Proceedings of the National Academy of Sciences, 113 (2016) 7124–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang Q, Kumar V, Lin F, Sethi B, Coulter DW, McGuire TR, Mahato RI, ApoE mimetic peptide targeted nanoparticles carrying a BRD4 inhibitor for treating Medulloblastoma in mice, Journal of Controlled Release, 323 (2020) 463–474. [DOI] [PubMed] [Google Scholar]
- [15].Arellano CL, Bioreducible lipid-like nanoparticles for intracellular protein delivery. M.Sc. Thesis, Tufts University (2014) UMI 1567011. [Google Scholar]
- [16].Zengerle M, Chan K-H, Ciulli A, Selective small molecule induced degradation of the BET bromodomain protein BRD4, ACS chemical biology, 10 (2015) 1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lebraud H, Wright DJ, Johnson CN, Heightman TD, Protein degradation by in-cell self-assembly of proteolysis targeting chimeras, ACS central science, 2 (2016) 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Neklesa TK, Tae HS, Schneekloth AR, Stulberg MJ, Corson TW, Sundberg TB, Raina K, Holley SA, Crews CM, Small-molecule hydrophobic tagging–induced degradation of HaloTag fusion proteins, Nature Chemical Biology, 7 (2011) 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


