Abstract
Introduction:
Innate and adaptive immunity play a critical role in the underlying pathological mechanisms of atherosclerosis and potential target sites of sterile inflammation open opportunities to develop novel therapeutics. In response to oxidized LDL in the intimal layer, T cell subsets are recruited and activated at the site of atheroma to upregulate pro-atherogenic cytokines which exacerbate plaque formation instability.
Areas covered:
A systematic search of PubMed and the Web of Science was performed between January 2001- September 2020 and relevant articles in sterile inflammation and atherosclerosis were critically reviewed. The original information was collected on the interconnection between danger associated molecular patterns (DAMPs) as the mediators of sterile inflammation and the receptor complex of CD36-TLR4-TLR6 that primes and activates inflammasomes in the pathophysiology of atherosclerosis. Mediators of sterile inflammation are identified to target therapeutic strategies in the management of atherosclerosis.
Expert opinion:
Sterile inflammation via NLRP3 inflammasome is perpetuated by the activation of IL-1β and IL-18 and induction of pyroptosis resulting in the release of additional inflammatory cytokines and DAMPs. Challenges with current inhibitors of the NLRP3 inflammasome lie in the specificity, stability, and efficacy in targeting the NLRP3 inflammasome constituents without ameliorating upstream or downstream responses necessary for survival.
Keywords: Atherosclerosis, CD36, DAMPs, HMGB1, Inflammasomes, NLRP3, oxidized LDL, Pyroptosis, Sterile Inflammation, Toll-like receptors
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death throughout the world. In the United States, nearly 48% adults (121.5 million) suffer from CVD [1]. Also, one in three deaths is due to CVD conditions, including coronary artery disease, myocardial infarction, heart failure, and stroke. Atherosclerosis is the primary cause of CVD where fatty deposits in the luminal lining of arterial blood vessels lead to plaque formation [2]. Traditionally, hypercholesterolemia is considered as the major risk factor for CVD. However, other metabolic conditions such as type II diabetes mellitus accelerate atherosclerotic lesions [3]. Atherosclerosis focuses mainly on low-density lipoprotein (LDL), as it is more atherogenic than its counterparts: very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and high-density lipoprotein (HDL) [4]. Moreover, the underlying challenge with atherosclerosis is the lengthy asymptomatic phase [5]. Generally, the atherosclerotic plaques are covered by a fibrous membrane that is made up of proliferative vascular smooth muscle cells of the tunica media layer. However, the accumulation of components that make up the plaque overwhelms the fibrous cap leading to rupture and prothrombotic downstream effects [6]. Immunological activity has been detected at every stage of the underlying pathophysiology. This review article focuses on the process of atherosclerotic formation and its regulation by the inflammatory processes within the body.
A systematic search of PubMed and the Web of Science was performed between January 2001- September 2020 in the area of sterile inflammation and atherosclerosis using the key words: AGE, atherosclerosis, cardiovascular disease, CD36, danger associated molecular patterns, HMGB-1, immune cells, inflammasomes, NLRP3, ox-LDL, pyroptosis, RAGE, sterile inflammation, Toll-like receptors. Article titles and abstracts were screened, with full-text review ultimately determining inclusion status. Only articles in English discussing original studies investigating the role of danger associated molecular patterns, including AGE, HMGB1, ox-LDL, RAGE, TLRs in sterile inflammation and atherosclerosis were included.
2. Mediators and immunological events in the pathology of atherosclerosis
2.1. Role of ox-LDL and LOX-1
Insoluble LDL is associated with apolipoprotein (Apo)B-100 which transports LDL to various tissues. In a study aimed at determining the association between inflammatory markers and lipoprotein subfractions, a positive association was found between small LDL cholesterol and LDL score with systemic inflammatory markers including white blood cell count, neutrophil count, lymphocyte count, C-reactive protein (CRP), fibrinogen and erythrocyte sedimentation rate; large LDL cholesterol had no significant link with systemic inflammatory markers in the study [7]. LDL transports mainly cholesterol esters and small amounts of triglycerides that lead to deposits within the tunica intima layer. The formation of such deposits is due to the interaction between positively charged ApoB with the negatively charged proteoglycans of the vessel wall [8]. Following uptake into the intima layer, LDL is subjected to oxidation through its interaction with reactive oxygen species (ROS) [9]. Accumulation of oxidized-LDL (ox-LDL) induces proinflammatory conditions including the upregulation of adhesion molecules P-selectin, vascular cell adhesion molecule-1 (VCAM-1), intracellular cell adhesion molecule-1 (ICAM-1), and lectin-like ox-LDL receptor-1 (LOX-1) on endothelial cells [10]. Adhesion molecules assist in the rolling and adhesion of leukocytes, including monocytes, facilitating their migration into the intima layer in response to chemokines. Such chemokines include factors such as C-X-C motif chemokine ligand 1 (CXCL1) which is also released from endothelial cells in response of ox-LDL to mobilize monocytes and neutrophils to inflammatory sites [11,12]. The integrin present on the cell surface of monocyte, relevant for atherosclerosis, is VLA-4 which binds to VCAM-1 on the endothelium. In order to continue the recruitment of leukocytes to the site of inflammation, ox-LDL and monocyte chemotactic protein-1 (MCP-1) act as chemo-attractants. The CCR2 receptor for MCP-1 on monocyte/macrophages is reported to be upregulated in plaque development. MCP-1 attracts monocytes and T cells and has a role in stimulating proliferation of smooth muscle cells. In turn, the smooth muscle cells of the intima layer proliferate and differentiate to accelerate the development of atherosclerosis [13,14].
Scavenger receptors on endothelial cells are involved in lipid transport across the membrane [15]. LOX-1 is expressed on endothelial cells, macrophages, and smooth muscle cells. It is the major receptor for ox-LDL and its expression is upregulated following proinflammatory conditions [16]. Ox-LDL leads to downstream effects through LOX-1 to activate arginase-II that causes further endothelial dysfunction due to the downregulation of endothelial nitric oxide synthase (eNOS). Damaged endothelial cells lead to the production of ROS through actions of myeloperoxidase, 15-lipooxygenase, and/or eNOS that induce oxidative stress promoting LDL oxidation. In addition, the ROS released from activated leukocytes within the tunica intima aggravate pro-atherogenic events [17].
Within the intima layer, ox-LDL acts as a ligand and interacts with scavenger receptors, SR-A1 and CD36 which are expressed on macrophages [18,19]. Subsets of monocytes have been identified and classified based on cell surface epitope recognition using flow cytometry as classical (CD14high/CD16−), intermediate (CD14high/CD16+), and non-classical (CD14low/CD16+) in humans [20]. The “intermediate” monocytes which express high levels of CD14 and intermediate CD16 (CD14high/CD16+) have been associated with cardiovascular death, myocardial infarction, and death [21,22]. In response to macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) released from endothelial cells, the monocytes differentiate into macrophages [23]. Binding of ox-LDL with scavenger receptors, SR-A1 and CD36, results in further activation of macrophages and uptake of ox-LDL. Macrophages within the tunica intima layer uptake ox-LDL and form foam cells leading to lipid-rich plaque. The plaque includes other inflammatory cells that forward a cascade of immune response leading to vulnerability of the fibrous cap, eventual rupture, and thrombotic events [11,24].
2.2. Adaptive immune response in atherosclerosis
B-lymphocytes expressing MHC class II present plaque-derived antigens to CD4+ T cells. As such, there was a reduction in the development of atherosclerosis in ApoE−/− mice lacking both B and T cells compared to their immunocompetent counterparts. ApoE −/− mice model is a widely used and well-established model to investigate the pathophysiology of atherosclerosis and treatment strategies. ApoE−/− mice have poor lipoprotein clearance and increased levels of cholesterol ester enriched particles in blood. ApoE−/− mice show high levels of total cholesterol level and develop severe atherosclerotic lesions within a few weeks after birth. Subsequently, there is an increase in cytokine and protease secretion as there is an imbalance of cholesterol loading specifically in macrophages which triggers inflammation [25]. Immunodeficient ApoE−/− mice that receive LDL-specific CD4+ T cells develop severe atherosclerosis [26,27]. The recruitment of CD4+ T cells to the site of atherosclerotic lesion involves mechanisms similar to the recruitment of monocytes. Atherosclerosis is a Th1-dominant disease [28] as the Th1 cells recognize antigens and secrete proinflammatory cytokines such as IL-2, interferon-γ (IFN-γ), and tumor necrosis factor-alpha (TNF-α). Injection of IL-2 in ApoE−/− mice enhances atherosclerosis, whereas anti-IL-2 antibodies reduce atherosclerosis [29]. In a similar fashion, IFN-γ deficient receptor in ApoE−/− models reduces atherosclerotic lesion size and the deficiency in IFN-γ attenuates atherosclerosis in ApoE−/− and LDLr−/− models [30]. In addition, the ApoE−/− pigs exhibit severe hypercholesteremia under conditions of high fat and cholesterol diet [31]. Th1-derived proinflammatory cytokines, IL-2 and IFN-γ, have been found in large proportion of plaques. IFN-γ promotes the proatherogenic inflammation including monocyte extravasation, macrophage activation, and foam cell formation. Moreover, the IFN-γ counteracts fibrous cap formation by enhancing collagen degradation and inhibiting smooth muscle cell proliferation. This leads to a weak fibrous cap creating a rupture and causing thrombotic events. The IFN-γ-deficient mice and mice that lack Th1 transcription factor T-bet exhibited reduced atherosclerotic development suggesting a potential role of IFN-γ in atherosclerosis [32,33]. Similarly, the cytokines IL-12 and IL-18 produced by macrophages drive the Th1 phenotype of CD4+ T cells. In IL-12−/− and ApoE−/− mice, there was a reduction in atherosclerosis and similar results were obtained while administering anti-IL-12 antibodies. In a similar fashion, IL-18−/− and ApoE−/− mice demonstrate a concomitant reduction in atherosclerosis. These results consistently support the idea that Th1-related cytokines are overall pro-atherogenic and accelerate the development of atherosclerosis [34].
Also, Th2 cells play a role in atherosclerosis as the Th2 cells produce IL-4, IL-5, and IL-13. Both IL-5 and IL-13 have protective effects against atherosclerosis whereas IL-5 has both pro- and anti-atherogenic effects [35,36]. Overall, the Th2 cytokines have a complex role in the pathophysiology of atherosclerosis. In addition, the Tregs are specialized in maintaining immune homeostasis by inhibiting T cells through anti-inflammatory cytokines such as TGF-β, IL-10, and IL-35. Since manifestation of atherosclerosis is linked to Th1 cells, Tregs are believed to be involved in the stabilization of the disease. Tregs control proinflammation and limit Th1 cell response in the plaque. Tregs are protective and their reduction increases the events of atherosclerosis in hypercholesterolemia mice [37].
The differentiation of helper T cell to Th17 is induced by TGF-β and IL-6. It mainly produces IL-17A which plays a role in autoimmune diseases. Th17 is pro-atherogenic especially in the early phase of atherosclerotic development by modulating the production of IFN-γ and IL-5 from CD4+ T cells [38]. Administration of anti-IL-17A as a neutralizing factor in the treatment of ApoE−/− mice resulted in reduction of plaque size [39]. However, other studies suggest anti-atherogenic effects as a result of TGF-β signaling in T cells [40]. IL-17 does so by enhancing collagen deposition of smooth muscles leading to increased fibrous cap formation [41]. Patients with low IL-17A levels have an increased risk of current cardiovascular events [42]. These conflicting results raise concern on the use of IL-17 inhibitors in clinical settings due to cardiovascular risk. Controversial findings may be attributed to the fact that IL-17 may not have any effect on lesion size. Madur et. al. demonstrated that IL-17 deficiency did not affect plaque burden, percentage of stenosis, but reduced outward vessel remodeling and had no correlation with the level of thickness in the medial layer of human carotid arteries [43]. Controversial results may be due to differences in study protocols such as methods for eliminating IL-17.
Classically, the role of platelets has been attributed to hemostasis and coagulation. Recent evidence has emerged for its diverse role in immunological processes such as enhancing the adaptive immune response, though it has not been clearly elucidated. Platelets express functional CD40L, an important modulator of the adaptive immune response. Expression of CD40L that is classically described on CD4+ T cells provide the second signal for B cell activation. Mutations in CD40L on T cells can result in hyper IgM syndrome as a result of defective class-switching. CD40L on T cells also ligate with CD40 on dendritic cells to promote dendritic cell activation and enhance antigen presentation. In the same manner, platelet derived CD40L promotes dendritic cell maturation and activation. Without proper antigen presentation, both the humoral and cell mediated immune responses are affected. Additionally, platelets are able to process and present antigens through major histocompatibility complex class I (MHC I) indicating its role in its interaction with CD8+ T cells. Furthermore, platelets modulate T cell immunity via cytokine secretion or cell-cell contact. Platelets have a complicated function in this manner as one study demonstrated constant promotion of Treg cell responses whereas Th1/Th17 had a transient enhancement phase followed by a suppression phase. Other studies have proposed Th1 differentiation and their function is enhanced by platelet-derived RANTES and suppressed by platelet-derived TGF-β. Chemokine platelet factor 4 attenuates Th1 and enhances Treg responses [44,45]. The role of platelets in the adaptive immune response is complex as it promotes pro- and anti-inflammatory responses of CD4+ T cells.
3. Sterile inflammation in atherosclerosis
3.1. Role of danger associated molecular patterns (DAMPs)
Sterile inflammation is an immunological defense response in either acute conditions such as ischemic reperfusion injuries or chronic diseases such as atherosclerosis. Nonetheless, it is termed sterile inflammation since it occurs in the absence of pathogen associated molecular patterns (PAMPs), but it is a response due to damage associated molecular patterns (DAMPs). DAMPs are endogenous mediators that may sequester intracellularly under normal physiological or pathological conditions (Table 1) [46-58]. For example, damaged mitochondria are involved in sterile inflammation as they release many DAMPs including mitochondrial DNA, peptides, and lipids that trigger inflammation through pattern recognition receptors (PRRs). In addition, ox-LDL in atherosclerotic plaque may serve as a DAMP. These inflammatory responses are similar to those during infection as there is recruitment of neutrophils, macrophages, cytokines, and T cells.
Table 1:
Recognized DAMPs and their role in inflammation and possible pathological complications.
| Location | DAMPs | Receptors | Role in inflammation |
Pathological implications |
Ref |
|---|---|---|---|---|---|
| Nucleus | Histones | TLR2, TLR4, TLR9 | Immunological self-tolerance (autoimmunity) | Atherosclerosis, autoinflammatory diseases, cancer, Alzheimer’s disease, infertility | [46-48] |
| HMGB1 | TLR2, TLR4, TLR9, CD36, RAGE | Activate innate immune response, inflammasome activation | Atherosclerosis, autoimmune disorders, renal diseases | [49] | |
| IL-1α | IL-1R | Innate and acquired immunity; both pro- and anti-inflammatory | Autoinflammatory diseases | [50] | |
| IL-33 | ST2 | Innate and acquired immunity | Autoimmune diseases, cardiovascular, allergic | [50] | |
| DNA | TLR9, AIM2 | Innate immune response, dendritic cell maturation | Carcinogenesis, neurodegeneration, aging | [51] | |
| Cytosol | ATP | P2Y2, P2X7 | Activate NLRP3 | IL-1β and IL-18 production | [52] |
| Heat shock proteins | CD91, TLR2, TLR4, SREC1, FEEL1 | T cell modulated immune response | Carcinogenesis, autoimmune diseases | [53] | |
| Uric acid | P2X7 | Neutrophil recruitment, adaptive immune response, dendric cell maturation | Atherosclerosis, obesity, metabolic syndrome, gout, kidney disease | [54] | |
| F-actin | DNGR1 | CD8+ T cell response | Mucosal inflammation (Crohn’s Disease, Irritable Bowel Syndrome) | [55] | |
| S100 Proteins | TLR2, TLR4, RAGE, CD36 | Regulate immune homeostasis, post traumatic injury, inflammation | Rheumatic diseases, atherosclerosis | [56] | |
| Mitochondria | Mitochondrial DNA | TLR9 | Activate macrophages and neutrophils | Atherosclerosis, neurodegenerative diseases | [57] |
| Mitochondrial Transcriptional Factor A | RAGE, TLR9 | Activate dendritic cells, interferon release, cytokine production | Mitochondrial genome transcription | [58] |
Mitochondrial DNA damage accelerates atherosclerosis as ox-LDL promotes mitochondrial dysfunction and cell death in human macrophages. Free radical damage leading to ROS decreases the mitochondrial membrane potential and disrupts the respiratory chain leading to further ROS formation and further oxidative stress. Mitochondrial dysfunction also leads to increased lipolysis resulting in increased free fatty acids and glycerol. Post-mortem human studies involving detection of mitochondrial DNA have found increased mutation in arterial cells. Furthermore, mitochondrial mutations that were discovered in human blood samples correlated with lower overall content of mitochondrial DNA in peripheral blood lymphocytes in CAD patients compared to healthy subjects. Many mitochondrial DNA mutations have been linked to atherogenesis. Exact mechanisms are unclear but could be due to impairment of electron transfer and protein synthesis in mitochondria [59].
Pentraxin 3 (PTX3) is an acute phase protein that belongs to a superfamily of multimeric proteins including short and long pentraxins. PTX3 is a PRR and plays a critical role in innate immunity. Levels of PTX3 are elevated in elderly hypertensive patients and clinical data have suggested PTX3 to be a valid biomarker in atherosclerosis as it is directly correlated with the severity of carotid stenosis and in patients with acute coronary syndromes. PTX3 increases tissue factor, an upstream initiator of the coagulation cascade [60]. Surprisingly, there are conflicting reports concerning PTX3 in cardiovascular disease. In a study, lesions in the aorta were increased in PTX3−/− and ApoE−/− mice that were fed atherogenic diet for 16 weeks. Mice lacking PTX3 demonstrated a higher inflammatory profile suggesting an atheroprotective effect of PTX3 [61].
Cellular stress or injury to the intima layer of blood vessels due to ox-LDL also releases DAMPs into the extracellular environment to be recognized by macrophages to induce inflammation triggering atherosclerotic process [62].The triggers of sterile inflammation are broadly categorized into either the intracellular or extracellular matrix. One of the most critical intracellular proteins is High mobility group box 1 (HMGB1), a nuclear protein that regulates gene transcription under normal conditions and acts as an endogenous danger signal through activation of the innate immune system when released as a result of cellular damage. HMBG1 is organized into A box and B box domains with an acidic C-terminal tail. HMGB1 translocates from the nucleus to the cytosol where it is packaged in vesicles prior to extracellular secretion to act as a cytokine [63]. HMGB1 is released passively and almost instantaneously in response to cellular damage and actively by the secretion via cells such as monocytes, macrophages, dendritic cells, natural killer cells, endothelial cells, and tumor cells. HMGB1 activates the innate immune response via initiation of cellular signal transduction through interactions with extracellular pathogens, cytokines, and plasma membrane receptors [64]. Active secretion is much slower than passive secretion as the nuclear HMGB1 requires translocation to the cytoplasm via the JAK-STAT signaling pathway that hyperacetylates lysine residues located on two nuclear localization sequence sites thus, preventing bidirectional shuttling of HMGB1 and allowing accumulation of HMGB1 in the cytosol. HMGB1 is then released via exocytosis or through pyroptosis, a proinflammatory cell death mechanism which protects against infection, to reach the extracellular space. Furthermore, HMGB1 exhibits feed-forward release as a mechanism through which it can induce its own release thereby maintaining a positive-feedback proinflammatory cascade [65]. Attenuated HMGB1 translocation from the nucleus to the cytoplasm reduces inflammation. Studies where recombinant HMGB1 is administered to disease-free mice or during renal ischemia reperfusion injury reported the exacerbation of inflammatory response supporting evidence for elevated HMGB1 in human atherosclerotic plaque, but not in normal arteries [66] creating inflammatory conditions. Importantly, the smooth muscle cells located in atherosclerotic arteries proliferate, migrate, and activate to secrete more HMGB1 in response to HMGB1 since HMGB1 can act through an autocrine loop [67]. Also, HMGB1 increases adhesion molecules including ICAM-1, VCAM-1, and induces inflammatory mediators such as IL-8, MCP-1, and TNF-α [68].
3.2. Interaction of HMGB-1 with toll-like receptors (TLRs) and RAGE
HMGB1 interacts with a variety of receptors that are usually categorized to interact with either exogenous or endogenous ligands via TLR2/TLR4/TLR9 or receptor for advanced glycation end products (RAGE) [69]. TLR mRNA transcripts in human arteries were quantified and demonstrated that normal arteries showed low levels of TLR expression with a relatively higher level of expression of TLR4 mRNA. In atherosclerotic lesions, the abundance of TLR expression was elevated in TLR1, TLR2 and TLR4 transcripts and elevated expression was associated with inflammatory activation of endothelial cells and macrophages [70].
RAGE is a transmembrane, cell surface receptor that is expressed on macrophages, smooth muscle cells, and endothelial cells [71,72]. Advanced glycation end products (AGEs) interact with RAGEs which increase the expression and release of proinflammatory cytokines and reactive oxygen species. Other ligands that bind to RAGEs include S100 protein, HMGB1, and β-amyloid peptide [73]. RAGE, similar to toll like receptors (TLRs), are expressed on immune cells such as macrophages as well as endothelial cells. In addition, they recognize PAMPS and DAMPS triggering the inflammatory responses. In essence, RAGE and TLRs are PRRs that share similar signaling pathways, however TLRs respond to infection whereas RAGE is involved with endogenous molecules. Both signaling pathways enhance receptor expression leading to a positive feedback loop. This loop creates several signaling pathways that activate proinflammatory transcription factor NF-κB resulting in the production of cytokines, adhesive molecules, and matrix metalloproteinases [74,75]. Other signal transduction pathways downstream of RAGE include MAPKs, PI3K, Akt/PKB, Rho GTPases, Jak/STAT, and Src family kinases.
HMGB1 binds to RAGE mediating the chemotaxis, cell growth, immune cell differentiation, migration of immune and smooth muscle cells, and upregulation of cell surface expression of RAGE and TLR4 [65,76]. TLR4 is a requirement for cytokine release when HMGB1 binds to RAGE as macrophages do not secrete cytokines if HMGB1 binds to RAGE in macrophages with absent or functionally inactivated TLR4 [77]. The TLR4 signaling pathway occurs through MyD88-dependent nuclear translocation of NF-κB which upregulates gene transcriptions for cytokines such as TNF-α, IL-1, IL-6, and IL-8 [78]. In intestinal epithelial cells, HMGB1-RAGE activates NOX2 and peroxynitrite generation which upregulates TLR4, therefore, inducing proinflammatory cytokines IL-1β and IL-6 production [79]. In endothelial cells, there is upregulation of cell surface adhesion molecules including ICAM-1 and VCAM-1 as well as further cytokine production.
Generally, all TLRs associate either directly or indirectly with adaptor protein MyD88 which leads to activation of PI3K, except for TLR3 which associates with Toll/IL-1R domain containing adapter including IFN- β (TRIF). Signaling downstream of MyD88 requires association with IL-1R associated kinase (IRAK) family. Furthermore, the MyD88 activates TNF-receptor associated factor 6 (TRAF-6). TGF-β-activated Kinase 1 (TAK1) activation causes binding with TAK1-binding protein to form a complex to activate MAPK and NF-κB signaling pathways. Ultimately, the downstream effects are mediated through NF-κB pathway resulting in the activation of a number of genes coding for proinflammatory cytokines including TNF- α, IL-1, and IL-6 [80].
TLR4-activated inflammatory cascades are greatly implicated in the pathogenesis of atherosclerosis via the NF-κB pathway. Ox-LDL upregulates TLR4 expression in atherosclerotic plaque cells as TLR4 expression is necessary for macrophages to differentiate into foam cells. Studies involving ApoE−/− mice with deficient TLR4 demonstrate attenuated atherosclerosis. Moreover, MyD88 plays a significant role in TLR4 signal cascade. Lack of TLR4 or MyD88 reduces atherosclerosis via decreased macrophage recruitment and chemokine levels. In studies involving ApoE−/− and MyD88−/− mice, there was a reduction in aortic atherosclerosis and macrophage accumulation compared to ApoE−/− mice alone [81]. These studies demonstrate the relevance of MyD88 pathway in atherosclerosis and the inhibition of MyD88 prevents atherosclerosis via regulating inflammatory responses [82]. Ox-LDL-mediated upregulation of TLR4 in smooth muscle cells induces secretion of proinflammatory cytokines including IL-1β, TNF-α, MCP-1, and MMP-2. It was proposed that TLR4 plays a crucial role in SMCs inflammatory response [83] indicating its role in plaque instability [84]. In addition, the ox-LDL increases HMGB1 secretion via oxidative stress increasing the HMGB1-TLR4 interaction which depend on reduced form of Cys106 on HMGB1 with a disulfide bond between Cys23 and Cys45 [85]. Specifically, this isoform of HMGB1 binds myeloid differentiation factor-2 (MD-2) leading to the activation of TLR4 complex by binding two TLR4 chains together and subsequently leading to the MyD88-dependent activation of NF-κB [86]. HMGB1-TLR4 axis upregulates Caveolin-1, disrupting the balance between constitutive eNOS and inducible iNOS in endothelial cells leading to endothelial cell dysfunction [87,88]. Also, HMGB1 and TLR4 have been implicated in intimal hyperplasia in the mouse model of carotid wire injury [89]. Although TLR4 is a receptor for HMGB1, it is also responsible for the secretion of HMGB1 [90].
Further studies have demonstrated that the HMBG1-TLR4 association induces atherosclerosis by inhibiting PPARγ/LXRα-ABCA1 axis. ATP-binding cassette transporter 1 (ABCA-1) has anti-inflammatory effects and an anti-atherogenic role via cholesterol efflux that is regulated by peroxisome proliferator-activated receptor gamma (PPARγ)-nuclear receptor liver X receptor alpha (LXRα) pathway [91,92]. Cholesterol efflux from macrophages in atherosclerotic lesions and transport back to liver is an important mechanism of reverse cholesterol transport (RCT) that is mediated by HDL and its apolipoprotein A1. It plays a critical role in not only maintaining intracellular cholesterol levels, but it also prevents macrophage-derived foam cell formation in atherosclerotic plaques [93]. The findings from a study found a 67% reduction in cardiovascular events in the highest quartile of cholesterol efflux capacity versus the lowest quartile in an adjusted model that included traditional risk factors, HDL cholesterol level, and HDL particle concentration. Cholesterol efflux capacity had an inverse association with the incidence of cardiovascular events [94].
TLR2 expression is upregulated in aortic regions of disturbed blood flow in LDLr−/− mice that are disease free. Furthermore, TLR2 expression continues to be upregulated in LDLr−/− mice with early progression of atherosclerosis. In LDLR−/− mice, a total deletion of TLR2 in hyperlipidemic mice was shown to be atheroprotective [95]. Arterial endothelial cells demonstrating laminar flow express lower levels of TLR2 compared to endothelial cells that have been exposed to a disturbed flow. The atheroprotective effects of TLR2 extend to the foam cells where a TLR2 deficiency resulted in diminished accumulation of foam cells in the aorta of ApoE−/− mice [96]. Moreover, the disruption of TLR2-MyD88 pathway inhibits inflammation, similar to TLR4 [97]. Furthermore, the TLR2 is involved in promoting vascular smooth muscle cell migration from the tunica media to the tunica intima layer mediated through IL-6 [98]. Interestingly, the HMGB1 binds to TLR2 to trigger proinflammatory conditions which has been linked to CD36, a class B scavenger receptor functioning in the binding of long-chain fatty acids and ox-LDL [99].
3.3. Interaction of S100 proteins with toll-like receptors (TLRs) and RAGE
S100 proteins have similar characteristics to that of HMGB1. S100 proteins act as DAMPs that interact with TLR2, TLR3, TLR4, and RAGE following their release from phagocytes and modulate cellular pro-inflammatory response via NF-κB pathway [100]. S100 proteins have a wide range of intra- and extracellular functions to regulate cell growth and death. It belongs to a calcium-binding cytosolic protein family comprising the subtypes S100A8, S100A9, and S100A12 which mediate vascular inflammation and atherosclerosis [101] where S100A12 is the most predictive of coronary heart disease in a prospective population-based cohort study [102]. However, the increased S100A8/A9 levels are the predicting factors for the future myocardial infarction, stroke, and cardiovascular death [103]. ApoE−/− S100A9−/− double knockout mice demonstrated reduction in aortic atherosclerosis [104]. Though neutrophils are relatively lower in number than monocytes/macrophages in atherosclerotic lesions, there is a larger abundance of S100A8/A9 in neutrophils than monocytes/macrophages.
In a study focused on lifestyle changes in coronary artery disease, S100A12 mRNA in peripheral blood cells was one of the most significantly reduced genes [105]. In transgenic mice studies, targeted expression of S100A12 demonstrated increase coronary calcifications and plaque vulnerability. One mechanism in which S100A12 mediates vascular calcification is via the upregulation of Nox-1 mRNA suggesting a critical role of NADPH-derived ROS in the calcification effects of S100A12 in smooth muscle cells [106]. Furthermore, activation of mast cell and monocyte recruitment via S100A12 promoted atherosclerosis via the secretion of proinflammatory cytokines [107]. While S100A8/A9 has been known to bind fatty acids and CD36, more recently, S100A12 has also been observed to bind to CD36 as well. Previously, it was described that the activation of RAGE-S100A12 and TLR4-S100A12 axes are the major mechanisms by which S100A12 exhibits inflammatory effects in the pathogenesis of atherosclerosis. The fatty acid binding ability of S100 family of proteins suggests the possibility of S100A12 facilitating uptake of ox-LDL through CD36 [108]. Furthermore, association for RAGE and CD36 signaling has been established via knockdown experiments [109]. In addition, S100A12 possibly upregulates CD36 via RAGE which is depicted in Figure 1.
Figure 1:
Inflammation in atherosclerosis. LDL migrates into the intima layer after endothelial cell damage and will undergo uptake via macrophages to form foam cells after being oxidized by ROS. Ox-LDL induces secretion of CXCL1 recruiting monocytes. MCP-1 sends out a chemotactic signal for further recruitment of monocytes whereby VLA4 adheres to VCAM-1 on the endothelium. Within the intima layer, ox-LDL interacts with scavenger receptors and pattern recognition receptors on macrophages inducing foam cell formation as well as secretion of DAMPs. In addition, the secretion of cytokines from mainly Th1 cells will exacerbate the inflammatory response leading to worsening plaque formation.
4. Inflammasome pathway in atherosclerosis
Unlike the adaptive immune response, the innate immune response detects pathogens, including DAMPs, using the corresponding PRRs [110]. Inflammasomes are involved in the innate immunity and sterile inflammation resulting in the release of proinflammatory cytokines into circulation and pyroptosis. The canonical inflammasome is associated with the conversion of procapase-1 to caspase-1, whereas noncanonical inflammasome converts procaspase-11 to caspase-11 [111]. While many inflammasomes have been identified including NLR family pyrin domains NLRP1, NLRP3, NLRC4, NLRP6, and absent in melanoma 2 (AIM2), NLRP3 is the most extensively studied as it has been linked with various autoimmune and inflammatory diseases [112].
The NLRP3 inflammasome is a multi-subunit protein complex that is inactive in its monomer form in the cytosol. NLRP3 has leucine rich repeats that are blocked by chaperone proteins. Once activated, it recruits adapter ASC and binds through oligomerization where the PYD domain of ASC binds to PYD domain of NLRP3. Using its CARD domain, ASC recruits procaspase-1 and cleaves to active caspase-1, which induces the inflammatory response through release of IL-1β and IL-18 [113]. The inflammasome activation in regard to atherosclerosis has been spotlighted on monocytes/macrophages, endothelial cells, smooth muscle cells, and other immune cells such as dendritic cells and T cells [114]. Generally, the NLRP3 activation requires both a priming signal and an activation signal. A priming signal occurs through the recognition of danger signals which induce expression of NLRP3 and pro-IL-1β at the transcriptional level through activation of NF-κB pathway. NLRP3 is de-ubiquinated which is mediated by TLR4 receptor through MyD88 ensuring the stability of NLRP3 [115]. Among the diverse events activating NLRP3, the DAMPs and potassium efflux are the major contributing factors in the activation of inflammasomes. The potassium efflux is caused by bacterial toxins or by extracellular ATP which engages with potassium channel [116]. DAMPs and PAMPs from ruptured lysosome activate inflammasome, likely through the effect of reactive oxygen species. In addition, Cathepsin-B, released from ruptured lysosomes and the poor integrity of cellular microtubule network including F-actin due to shear stress, leads to NLRP3 inflammasome activation [117]. Mitochondrial damage due to metabolic stress induces oxidation of mitochondrial DNA, which is liberated into the cytosol or extracellular space and activates NLRP3 inflammasome [118].
HMGB1, S100A12 proteins, or mtDNA activate the NLRP3 inflammasome increasing the pool of IL-1β [119]. The interaction between HMGB1, RAGE, and the activation of the NLRP3 inflammasome is implicated in the pathology of atherosclerosis driving foam cell formation [120]. HMGB1-induced NLRP3 inflammasome is dependent on the expressions of TLR2, TLR4, and RAGE and the vascular smooth muscle cells significantly attenuated production of IL-1β via inflammasome pathway with decreased expression of TLR2, TLR4, and RAGE. In vascular smooth muscle cells treated with IL-1β to increase P2Y2 receptor (G-protein coupled receptor that is involved with recruiting monocytes), concomitant upregulation of RAGE and HMGB1 was evident demonstrating IL-1β-accelerated atherosclerosis through P2Y2 receptor [121]. HMGB1-driven activation of the NLRP3 inflammasome induces foam cell formation, coupled with CD36 mediated uptake of ox-LDL.
Ox-LDL acts to both prime and activate the NLRP3 inflammasome. As previously mentioned CD36 uptakes ox-LDL in the intima layer. The CD36−/− mice model demonstrated protective effects against atherosclerosis [122]. The inflammasome is primed and activated by CD36-TLR4-TLR6 complex. Uptake of cholesterol crystals occur through sequestering by CD36, which induces intracellular CD36-TLR4-TLR6 heteromerization [123]. Subsequent activation of NF-κB leads to lysosomal damage and NLRP3 activation, whereby production of ROS, proinflammatory responses, and increased CD36 expression are stimulated. NLRP3 protein levels including all its associated components, ASC, caspase-1, IL-1β, and IL-18, correlate with severity of coronary artery stenosis [124]. In studies involving ApoE−/− mice, the mice develop atherosclerosis on either chow or high fat diet. In LDLr−/− mice, atherosclerosis was developed when fed with high fat diet [125]. However, LDLr−/− mice with NLRP3−/−, ASC−/−, or IL1α−/− IL1β−/− bone marrow significantly reduced the development of plaque [126]. Also, the LDLr−/− with caspase-1−/− bone marrow decreased plaque size [127]. In addition, the inflammasome components deleted in the ApoE−/− background similarly led to a reduction in atherosclerosis [115]. ApoE−/−/Nlrp3−/−, ApoE−/−/Asc−/−, and ApoE−/−/Caspase-1−/− double knockout mice resulted in atherogenesis, indicating that the atherosclerosis is a complex pathway involving processes other than the inflammasome activation [128]. Nonetheless, epidemiological studies have found correlations between NLRP3 expression and CVD severity [129]. Finally, the caspase-1 proteolytically activates Gasdermin D resulting in the formation of pores in the cell membrane and damaging cell integrity [130] which allows the release of pro-inflammatory cytokines IL-1β and IL-18 [131,132]. DAMPs released from pyroptotic cells include IL-1α, HMGB1, and ATP which lead to further immune response differing from apoptosis, as apoptotic cells retains HMGB1 [115] (Figure 2).
Figure 2:
Sterile inflammation and formation of inflammasome. Ox-LDL increases HMGB1 and S100A12 secretion which can bind to TLRs and RAGE to modulate inflammatory response via NF-κB pathway. Ox-LDL also interacts with CD36 resulting in further activation of macrophages. HMGB1 and S100 are DAMPs that activate the NLRP3 inflammasome through CD36-TLR4-TLR6 complex. Activation requires a priming signal which occurs via NF-κB. Many processes including K+ efflux, ATP release, ROS, Cathepsin-B all play a role in activating NLRP3 inflammasome. NLRP3 recruits caspase 1 which cleaves Pro-IL-1B, Pro-IL-18, and Gasdermin D. Gasdermin D leads to pyroptosis and release of proinflammatory DAMPs and cytokines further exacerbating the immune response.
5. Interventions targeting sterile inflammation
The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) study determined that canakinumab, a monoclonal antibody that targets IL-1β, reduced inflammatory markers such as C-reactive protein (CRP) and IL-6 and reduced cardiovascular events in patients with prior myocardial infarction. There was no significant reduction in cholesterol levels, but the magnitude of effect on cardiovascular events was similar to proprotein convertase subtilisin–kexin type 9 (PCSK9) therapy. There was also a reduction in cancer mortality receiving canakinumab, a finding consistent with IL-1 related progression of tumors, particularly lung cancer [133]. Of note, there was higher incidence of fatal infection and sepsis compared to placebo group which warrants consideration for further studies into risk of attenuating immune response versus the benefit of reduced cardiovascular events.
Methotrexate is used as a first line treatment in inflammatory diseases such as rheumatoid arthritis. While its mechanism of action is not entirely clear apart from hyperhomocysteinemia and its antiproliferative effects, the Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) trial suggested that methotrexate treatment in different combinations corrected the HDL function [134]. Other studies suggested anti-atherosclerotic effects via activation of adenosine A2A receptor by methotrexate leading to limited foam cell formation and upregulating RCT [135]. The Cardiovascular Inflammation Reduction Trial (CIRT) study aimed to shed more light in regard to methotrexate and cardiovascular risk. Patients in trial had increased cardiovascular risk either with metabolic syndrome, type 2 diabetes, or previous myocardial infarction. Unlike the CANTOS trial, CIRT determined that low-dose methotrexate did not reduce IL-1β, IL-6, or CRP [136].
HMGB1 has significant relevance for drug targeted therapies. Release of HMGB1 from nucleus to cytosol requires JAK/STAT pathway. Resveratrol has been suggested to reduce HMGB1 release via modulating the JAK/STAT signaling pathway and is considered a potential useful agent for atherosclerosis and inflammatory diseases [137]. Lu et al. reported that the pharmacological inhibition of JAK/STAT pathway did not decrease proinflammatory cytokines suggesting a potential therapy to inhibit HMGB1 release without affecting the innate immune system [138]. However, the JAK/STAT pathway plays a role in many fundamental processes and the faulty JAK/STAT signaling may result in autoimmune disorders or cancer. Irey et al. demonstrated that although JAK/STAT inhibition exhibits antitumor effects, the inhibition increases pro-tumorigenic inflammatory factors in the tumor microenvironment promoting therapeutic resistance [139]. Therefore, while there are anti-inflammatory effects by inhibiting the JAK/STAT pathway, further investigations are warranted to elucidate the risks and benefits of targeting the JAK/STAT pathway in atherosclerosis.
HMGB1 function requires the specific redox state of reduced Cys106 to bind TLR4 leading to downstream cascades. When Cys106 (or Cys45) is substituted by an alanine residue, HMGB1 loses the ability to bind MD-2 and subsequently activate TLR4 [85]. Macrophages deficient in MD-2 have markedly reduced HMGB1-mediated NF-κB translocation and TNF-α release [140]. P5779 is a peptide that binds exclusively at the MD-2 binding site for HMGB1 inhibiting HMGB1 induced TNF-α release and protecting against hepatic ischemia/reperfusion-induced injury, chemical toxicity, and sepsis [86]. Furthermore, TLR4, HMGB1, and IL-6 expression in arterial injury-induced intimal hyperplasia models were reduced by administering P5779. Moreover, the folic acid-derived drugs function as P5779 mimetics and are capable of inhibiting HMBG1 and MD-2 binding [141]. In addition, silencing of TLR4 leads to a reduction in atherosclerosis and is another target for therapy. While statins are used for their ability to inhibit HMG-CoA reductase activity, atorvastatin specifically inhibits the TLR4/Myd88/NF-κB pathway and RAGE signaling [142]. These strategies ultimately decrease NLRP3 inflammasome assembly and activation [143]. Even when HMGB1 binds to RAGE, inactivation or absence of TLR4 on macrophages inhibits cytokine production via NF–κB translocation [82]. While inhibition of TLR4 and MD-2 has gained evidence for its role in attenuating atherosclerosis, clinical translation remains more complex. TLR4 is significant in the recognition of exogenous as well as endogenous pathogens. Blocking the TLR4 cascade can lead to suppression of immune responses as it is expressed on T cells. Hence, it is ideal to target MD-2 and block TLR4/MD-2, rather than blocking TLR4 directly.
TLR4/MyD88-dependent pathway signals through IL-1 receptor associated kinase-4 (IRAK-4) to form a complex to recruit IRAK-1 and IRAK-2. Phosphorylation of IRAKs leads to the interaction of MyD88 to with tumor necrosis factor receptor-associated factor 6 (TRAF6). Although IRAK-1 and IRAK-4 only share 31% of their sequence identity, their sequence identity is >90% along the ATP binding pocket where inhibitors typically bind. Despite this, most inhibitors currently are selective for IRAK-4, as IRAK-1 inhibitors have proven more elusive. Also, IRAK-4 is the initial kinase recruited that leads to multiple downstream signaling molecules. In studies involving type II diabetic ApoE−/− mice, IRAK-4 inhibitor demonstrated a protective effect on vascular smooth muscle cells as well as decreased expression of MCP-1 [144]. Furthermore, IRAK-1 is involved in regulating the NLRP3 inflammasome and functions to regulate IL-1β activation [145]. Since TRAF6 is associated with IRAKs, targeting TRAF6 signaling in macrophages demonstrated an anti-inflammatory effect and a reduction in atherosclerosis [146].
Activation of the NF-κB pathway leads to regulation of many proinflammatory genes linked to atherosclerosis including that of activating NLRP3 inflammasome. Studies have demonstrated that inactivating the NF-κB pathway attenuates atherosclerosis [147]. However, genetic deficiency in the NF-κB pathway is a type of Mendelian primary immunodeficiency disease where functions of cell proliferation, cell survival, innate immunity, and inflammation can be severely affected [148]. Autosomal recessive deficiencies in IRAK-4 and MyD88 affect patients by predisposing them to recurrent pyogenic bacterial infections. Specifically, in IRAK-4 deficiency, the most common infections are due to Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa bacteria. While there seems to be no defect in leukocyte development or most responses involving T and B cells, the deficiency of IRAK-4 and MyD88 result in the impairment of TLR4 response and NF-κB-mediated cellular responses.
Currently, there are several pharmacological agents that can either target the NLRP3 inflammasome indirectly (i.e. via inhibiting ASC aggregation) or inhibit NLRP3 directly. While there is no pharmacological intervention to disrupt pyroptosis directly, necrosulfonamide has been identified to bind directly to and inhibit Gasdermin D [149]. Aside from necrosulfonamide, salidroside has inhibitory effects on Gasdermin D. Salidroside decreases atherosclerotic plaques through the inhibition of endothelial cell pyroptosis via the inhibition of caspase-1, IL-1β, and the decreased expression of Gasdermin D [150]. Careful evaluation of these pharmacological agents suggests that the specific targeting of the NLRP3 inflammasome may be a better option. Given the current evidence accompanied with atherosclerosis being an age-related disease, targeting specifically the sterile inflammasome components may be beneficial as a clinical therapeutic strategy (Figure 3). However, further investigation is warranted for translating these strategies to clinical arena.
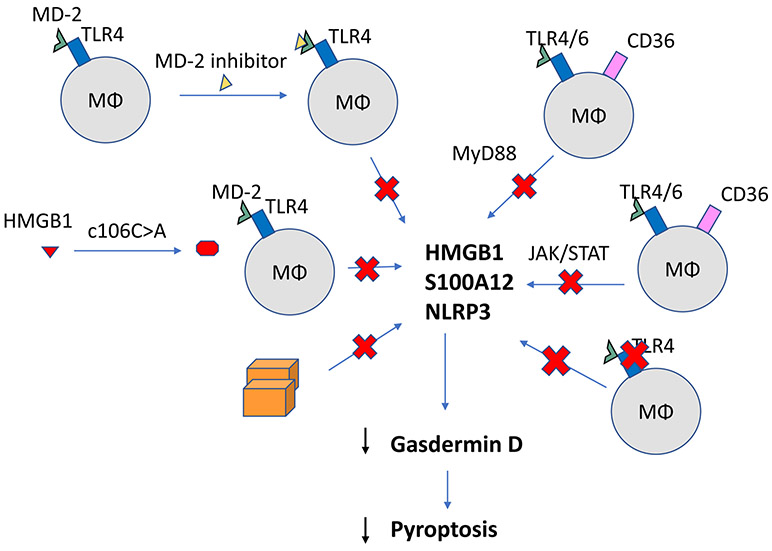
Figure 3:
Potential targets for therapy. Inhibition of JAK/STAT pathway can attenuate nuclear translocation of HMGB1 to cytosol. However, JAK/STAT inhibition increases pro-tumorigenic inflammatory factors. Substitution of alanine for cysteine 106 prevents HMGB1 from binding MD-2. Using an MD-2 inhibitor itself or inhibiting TLR4 can prevent downstream MyD88 cascade and NLRP3 activation. However, genetic deficiency in MyD88 pathway is an immunodeficiency that affects cell proliferation, cell survival, and inflammation. Targeting NLRP3 directly or Gasdermin-D is also a potential site to inhibit pyroptosis is release of DAMPs and pro-inflammatory cytokines. Overall, targeting the sterile inflammasome components may be beneficial as a clinical therapeutic strategy.
6. Conclusion
Atherosclerotic process is critically dependent on LDL migrating into the intima layer of endothelial cells where they become oxidized by ROS. Ox-LDL interacts with SR-A1 and CD36 on macrophages resulting in the formation of foam cells, creating a positive feedback loop recruiting more monocytes and ox-LDL in the intima layer forming an atherosclerotic plaque. Several subsets of macrophages have been identified, and particularly the “intermediate” macrophages which express high levels of CD14 and intermediate levels of CD16 are associated with high cardiovascular death.
Both innate and adaptive immune response play a role in the pathogenesis of atherosclerosis. Atherosclerosis has been described as a Th1-dominant disease, as Th1 secretes atherogenic cytokines including IL-2, interferon-γ, and TNF-α. A reduction in Th1 or related cytokines led to a reduction in atherosclerosis. Th2 response due to IL-4, IL-5, and IL-13 in atherosclerosis has been more complex. IL-5 and IL-13 had protective effects whereas IL-5 had both pro-atherogenic and anti-atherogenic effects. Similarly, Th17 response in atherosclerosis was complicated as it demonstrated a pro-atherogenic modulation via IFN-γ and IL-5, but an anti-atherogenic effect through TGF-β signaling. Moreover, Tregs are protective and reduce atherosclerosis in hypercholesterolemic mice by controlling Th1 response.
Sterile inflammation is an innate immune response that occurs in the presence of DAMPs. Ox-LDL increases the secretion of DAMPs including HMGB1 and S100 proteins triggering inflammation via binding RAGE, TLR4, and CD36, and modulating the inflammatory response via the NF-κB pathway. Downstream cascade of NF-κB pathway leads to activation of the NLRP3 inflammasome. The NLRP3 inflammasome is a multi-subunit protein complex that requires recruitment and activation of several domains. Following priming and activation through the CD36-TLR4-TLR6 complex, NLRP3 inflammasome releases IL-1β and IL-18 leading to pyroptosis via Gasdermin D. This pathway has high clinical relevance for targeted therapies. Inhibiting JAK/STAT release of HMGB1 or any pathway involved in priming and activating NLRP3 inflammasome is subject to study. For example, while statins are commonly known for inhibiting HMG-CoA reductase activity, atorvastatin inhibits the TLR4/Myd88/NF-κB pathway and RAGE signaling. Given the current research, agents targeting the sterile inflammasome components seem promising as we continue to elucidate such interventions.
7. Expert opinion
While there are stable therapies for atherosclerosis, it remains one of the leading causes of the death. The ability to reduce complications and take prophylactic measures have remained as frontline treatment. Our understanding of this disease as an inflammatory process has led to the recent developments and possibilities of managing the pathogenesis of atherosclerosis by targeting the sterile inflammation axis. DAMP-induced activation of NLRP3 inflammasome via CD36-TLR4-TLR6 complex leading to downstream inflammatory cascades including pyroptosis and release of IL-1β and IL-18 is a complex process that has many potential sites for targeted therapies. Currently, a multitude of pharmacological inhibitors of NLRP3 inflammasome have gained interest. To date, the current clinical treatments of canakinumab and anakinra target IL-1β and IL-18. Even then, the cardiovascular indications of canakinumab seem to be less relevant as the US Food and Drug Administration issued a response in regard to the efficacy of this drug as insufficient. Challenges also arise when elucidating inhibitors for constituents of the NLRP3 inflammasome. Parthenolide inhibits caspase-1 activation, however, has poor solubility and bioavailability. VX-740 also blocks caspase-1, however, demonstrated hepatic toxicity in animals after long-term exposure. On the other hand, molecules such as CY-09 and Tranilast have anti-inflammatory effects on NLRP3 inflammasome directly. CY-09 inhibits the ATPase activity of the NLRP3 inflammasome preventing its ability for oligomerization and activation. It is reportedly the first compound that has been recognized to offer both in vitro and in vivo inhibitory effects on the NLRP3 inflammasome. Tranilast is an anti-allergic drug that was identified to inhibit NLRP3 activation through an ATPase-independent manner by blocking NACHT domain to inhibit NLRP3-NLRP3 interaction and subsequent ASC oligomerization. Like many identified NLRP3 inhibitors, it does not interfere with the upstream signaling pathways including expression of pro-IL-1β, ROS production, K+ efflux, or mitochondrial damage.
The challenge with current inhibitors either being used in clinical practice or at the phase II clinical trials lies in the search for an NLRP3 inflammasome inhibitor that can efficiently inhibit NLRP3 itself to provide clinical therapy. However, while therapies targeting the NLRP3 inflammasome pathway are promising, the goal of ameliorating atherosclerosis is complicated. As we continue to learn more about the NLRP3 inflammasome, the field will continue to evolve with emerging evidence for targeted therapy with strong efficacy for inhibiting the constituents of the NLRP3 inflammasome or the NLRP3 inflammasome itself. Furthermore, it is critical to keep in mind the balance of targeted therapies versus simultaneous inhibition of several cytokines or pathways that regulate the immune response necessary for survival. For example, why is there a redundancy in pathways regarding HMGB1 and S100 proteins with TLR4 and CD36 that lead to inflammasome activation? What is the role of DAMPs-induced activation of complement system and crosstalk with downstream activation of inflammatory cells and inflammasomes? What combination of therapies are ideal to inhibit the right marker in the sterile inflammation pathway to ameliorate atherosclerosis without leading to immunocompromised patients? Future research expanding into clinical areas should take advantage of the current understanding of NLRP3 inflammasome and related cytokines to guide direct inhibition with improved specificity for increased efficacy and potency without ameliorating the innate immune response.
highlights.
Atherosclerosis is a complicated disease mediated by the innate and adaptive immune response. Macrophages express CD36 to facilitate ox-LDL uptake leading to the release of danger associated molecular patterns (DAMPs). “Intermediate” monocytes which express high levels of CD14 and intermediate CD16 have been associated with cardiovascular death.
Atherosclerosis was described as a Th1 dominant disease whereby IL-2, interferon-γ, and TNF-α has led to upregulation of atheroma formation. Th2 and Th17 have pro-atherogenic and anti-atherogenic effects. Tregs are protective of atherosclerosis.
Recent research has focused on the triggers of sterile inflammation and the activation of the NLRP3 inflammasome driving atheroma formation.
HMGB1 and S100 proteins are DAMPs release from damaged cells or immune cells trigger cascade of inflammatory response via binding TLR4, RAGE, and CD36. HMGB1 and S100 proteins drive atherosclerosis through CD36-TLR4-TLR6 complex that primes and activates the inflammasome.
The pro-inflammatory response is further perpetuated by activation of IL-1β and IL-18 leading to pyroptosis and releases inflammatory cytokines.
The authors critically discussed the recent developments and novel strategies in the management of atherosclerosis by targeting the mediators of sterile inflammation.
Acknowledgments
Funding
The research work of DK Agrawal is supported by research grants R01HL144125 and R01HL147662 from the National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial or non-financial involvement with any organization or entity with financial or non-financial interest or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
*Of interest
**Of considerable interest
- [1].Virani Salim S, Alonso Alvaro, Benjamin Emelia J, et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139–596. 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- [2].Vilahur G, Badimon JJ, Bugiardini R, et al. Perspectives: The burden of cardiovascular risk factors and coronary heart disease in Europe and worldwide. Eur Heart J Suppl 2014;16:A7–11. 10.1093/eurheartj/sut003. [DOI] [Google Scholar]
- [3].Katakami N Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J Atheroscler Thromb 2018;25:27–39. 10.5551/jat.RV17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hernáez Á, Soria-Florido MT, Schröder H, et al. Role of HDL function and LDL atherogenicity on cardiovascular risk: A comprehensive examination. PLOS ONE 2019;14:e0218533. 10.1371/journal.pone.0218533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hatzidakis A, Savva E, Perisinakis K, et al. CT coronary angiography in asymptomatic male patients with high atherosclerosis risk: Is it justified? Hellenic J Cardiol 2020:S110996662030066X. 10.1016/j.hjc.2020.04.004. [DOI] [PubMed] [Google Scholar]
- [6].Wu M-Y, Li C-J, Hou M-F, et al. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int J Mol Sci 2017;18:2034. 10.3390/ijms18102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang Y, Li S, Xu R-X, et al. Systemic Inflammatory Markers Are Closely Associated with Atherogenic Lipoprotein Subfractions in Patients Undergoing Coronary Angiography. Mediators Inflamm 2015;2015:1–9. 10.1155/2015/235742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Otsuka F, Kramer MCA, Woudstra P, et al. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis 2015;241:772–82. 10.1016/j.atherosclerosis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nowak WN, Deng J, Ruan XZ, et al. Reactive Oxygen Species Generation and Atherosclerosis. Arterioscler Thromb Vasc Biol 2017;37. 10.1161/ATVBAHA.117.309228. [DOI] [PubMed] [Google Scholar]
- [10].Woollard KJ, Chin-Dusting J. Therapeutic targeting of p-selectin in atherosclerosis. Inflamm Allergy Drug Targets 2007;6:69–74. 10.2174/187152807780077345. [DOI] [PubMed] [Google Scholar]
- [11].van der Vorst EPC, Döring Y, Weber C. Chemokines and their receptors in Atherosclerosis. J Mol Med 2015;93:963–71. 10.1007/s00109-015-1317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Varona JF, Ortiz-Regalón R, Sánchez-Vera I, et al. Soluble ICAM 1 and VCAM 1 Blood Levels Alert on Subclinical Atherosclerosis in Non Smokers with Asymptomatic Metabolic Syndrome. Arch Med Res 2019;50:20–8. 10.1016/j.arcmed.2019.05.003. [DOI] [PubMed] [Google Scholar]
- [13].Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 2016;118:692–702. 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hasanov Z, Ruckdeschel T, König C, et al. Endosialin Promotes Atherosclerosis Through Phenotypic Remodeling of Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol 2017;37:495–505. 10.1161/ATVBAHA.116.308455. [DOI] [PubMed] [Google Scholar]
- [15].Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul Pharmacol 2016;84:1–7. 10.1016/j.vph.2016.05.013. [DOI] [PubMed] [Google Scholar]
- [16].Kattoor AJ, Goel A, Mehta JL. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019;8:218. 10.3390/antiox8070218.*This article highlights LOX-1 as an important glycoprotein on endothelial cells that internalizes ox-LDL leading to further upregulation of inflammation, oxidative stress, and atherosclerosis.
- [17].Burtenshaw D, Kitching M, Redmond EM, et al. Reactive Oxygen Species (ROS), Intimal Thickening, and Subclinical Atherosclerotic Disease. Front Cardiovasc Med 2019;6:89. 10.3389/fcvm.2019.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger Receptors Class A-I/II and CD36 Are the Principal Receptors Responsible for the Uptake of Modified Low Density Lipoprotein Leading to Lipid Loading in Macrophages. J Biol Chem 2002;277:49982–8. 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- [19].Qin M, Wang L, Li F, et al. Oxidized LDL activated eosinophil polarize macrophage phenotype from M2 to M1 through activation of CD36 scavenger receptor. Atherosclerosis 2017;263:82–91. 10.1016/j.atherosclerosis.2017.05.011.*This study determined that ox-LDL signaling increased the expression of CD36. Additionally, CD36 signaling in eosinophils did not affect IL-1β expression unlike that of macrophages.
- [20].Ong S-M, Hadadi E, Dang T-M, et al. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis 2018;9:1–12. 10.1038/s41419-018-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gupta Rajat M, Lee-Kim Vivian S, Libby Peter. The March of Monocytes in Atherosclerosis. Circ Res 2020;126:1324–6. 10.1161/CIRCRESAHA.120.316981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wildgruber M, Aschenbrenner T, Wendorff H, et al. The “Intermediate” CD14++CD16+ monocyte subset increases in severe peripheral artery disease in humans. Sci Rep 2016;6:39483. 10.1038/srep39483.*This article highlights "intermediate monocytes," expressing high levels of CD14 and intermediate levels of CD16, as the particular subset elevated in atherosclerosis.
- [23].Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 2016;100:481–9. 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Costopoulos C, Huang Y, Brown AJ, et al. Plaque Rupture in Coronary Atherosclerosis Is Associated With Increased Plaque Structural Stress. JACC Cardiovasc Imaging 2017;10:1472–83. 10.1016/j.jcmg.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lo Sasso G, Schlage WK, Boué S, et al. The Apoe−/− mouse model: a suitable model to study cardiovascular and respiratory disease s in the context of cigarette smoke exposure and harm reduction. J Transl Med 2016;14:146. 10.1186/s12967-016-0901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kimura T, Kobiyama K, Winkels H, et al. Regulatory CD4 + T Cells Recognize Major Histocompatibility Complex Class II Molecule–Restricted Peptide Epitopes of Apolipoprotein B. Circulation 2018;138:1130–43. 10.1161/CIRCULATIONAHA.117.031420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tay C, Kanellakis P, Hosseini H, et al. B Cell and CD4 T Cell Interactions Promote Development of Atherosclerosis. Front Immunol 2020;10:3046. 10.3389/fimmu.2019.03046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res 2019;124:315–27. 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Upadhya S, Mooteri S, Peckham N, et al. Atherogenic Effect of Interleukin-2 and Antiatherogenic Effect of Interleukin-2 Antibody in Apo-E-Deficient Mice: Angiology 2016. 10.1177/000331970405500308.*This study demonstrated that anti-IL-2 has protective effects against atherosclerosis in apo-E-deficient mice; supporting the role of adaptive immune response in enhancing atherogenesis.
- [30].Cole JE, Navin TJ, Cross AJ, et al. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci 2011;108:2372–7. 10.1073/pnas.1018515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fang B, Ren X, Wang Y, et al. Apolipoprotein E deficiency accelerates atherosclerosis development in miniature pigs. Dis Model Mech 2018;11:dmm036632. 10.1242/dmm.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Herrero-Fernandez, Gomez-Bris, Somovilla-Crespo, et al. Immunobiology of Atherosclerosis: A Complex Net of Interactions. Int J Mol Sci 2019;20:5293. 10.3390/ijms20215293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li J, McArdle S, Gholami A, et al. CCR5 + T-bet + FoxP3 + Effector CD4 T Cells Drive Atherosclerosis. Circ Res 2016;118:1540–52. 10.1161/CIRCRESAHA.116.308648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yasuda K, Nakanishi K, Tsutsui H. Interleukin-18 in Health and Disease. Int J Mol Sci 2019;20:649. 10.3390/ijms20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fatkhullina AR, Peshkova IO, Koltsova EK. The role of cytokines in the development of atherosclerosis. Biochem Mosc 2016;81:1358–70. 10.1134/S0006297916110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Foks AC, Kuiper J. Immune checkpoint proteins: exploring their therapeutic potential to regulate atherosclerosis. Br J Pharmacol 2017;174:3940–55. 10.1111/bph.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest 2013;123:1323–34. 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Danzaki K, Matsui Y, Ikesue M, Ohta D, et al. Interleukin-17A Deficiency Accelerates Unstable Atherosclerotic Plaque Formation in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol 2012;32:273–80. 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- [39].Gao Q, Jiang Y, Ma T, et al. A Critical Function of Th17 Proinflammatory Cells in the Development of Atherosclerotic Plaque in Mice. J Immunol 2010;185:5820–7. 10.4049/jimmunol.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gisterå A, Robertson A-KL, Andersson J, et al. Transforming Growth Factor–β Signaling in T Cells Promotes Stabilization of Atherosclerotic Plaques Through an Interleukin-17–Dependent Pathway. Sci Transl Med 2013;5:196ra100–196ra100. 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- [41].Taleb S, Romain M, Ramkhelawon B, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med 2009;206:2067–77. 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Simon T, Taleb S, Danchin N, et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J 2013;34:570–7. 10.1093/eurheartj/ehs263. [DOI] [PubMed] [Google Scholar]
- [43].Madhur MS, Funt SA, Li L, et al. Role of Interleukin 17 in Inflammation, Atherosclerosis, and Vascular Function in Apolipoprotein E–Deficient Mice. Arterioscler Thromb Vasc Biol 2011;31:1565–72. 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ali RA, Wuescher LM, Worth RG. Platelets: essential components of the immune system 2016:16. [PMC free article] [PubMed] [Google Scholar]
- [45].Morrell CN, Aggrey AA, Chapman LM, et al. Emerging roles for platelets as immune and inflammatory cells. Blood 2014;123:2759–67. 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Araki Y, Mimura T. The Histone Modification Code in the Pathogenesis of Autoimmune Diseases. Mediators Inflamm 2017;2017:1–12. 10.1155/2017/2608605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Szatmary P, Huang W, Criddle D, et al. Biology, role and therapeutic potential of circulating histones in acute inflammatory disorders. J Cell Mol Med 2018;22:4617–29. 10.1111/jcmm.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ye X, Feng C, Gao T, et al. Linker Histone in Diseases. Int J Biol Sci 2017;13:1008–18. 10.7150/ijbs.19891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chi W, Chen H, Li F, et al. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J Neuroinflammation 2015;12:137. 10.1186/s12974-015-0360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 2018;281:8–27. 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bertheloot D, Latz E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol 2017;14:43–64. 10.1038/cmi.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roh JS, Sohn DH. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw 2018;18:e27. 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hauet-Broere F, Wieten L, Guichelaar T, et al. Heat shock proteins induce T cell regulation of chronic inflammation. Ann Rheum Dis 2006;65:iii65–8. 10.1136/ard.2006.058495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schaefer L Complexity of Danger: The Diverse Nature of Damage-associated Molecular Patterns. J Biol Chem 2014;289:35237–45. 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Srinivasan N, Gordon O, Ahrens S, et al. Actin is an evolutionarily-conserved damage-associated molecular pattern that signals tissue injury in Drosophila melanogaster. ELife 2016;5:e19662. 10.7554/eLife.19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xia C, Braunstein Z, Toomey AC, et al. S100 Proteins As an Important Regulator of Macrophage Inflammation. Front Immunol 2018;8:1908. 10.3389/fimmu.2017.01908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Riley JS, Tait SW. Mitochondrial DNA in inflammation and immunity. EMBO Rep 2020;21. 10.15252/embr.201949799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Little JP, Simtchouk S, Schindler SM, et al. Mitochondrial transcription factor A (Tfam) is a pro-inflammatory extracellular signaling molecule recognized by brain microglia. Mol Cell Neurosci 2014;60:88–96. 10.1016/j.mcn.2014.04.003. [DOI] [PubMed] [Google Scholar]
- [59].Volobueva A, Grechko A, Yet S-F, et al. Changes in Mitochondrial Genome Associated with Predisposition to Atherosclerosis and Related Disease. Biomolecules 2019;9:377. 10.3390/biom9080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fornai F The inflammatory protein Pentraxin 3 in cardiovascular disease 2016:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Norata GD, Marchesi P, Pulakazhi Venu VK, et al. Deficiency of the Long Pentraxin PTX3 Promotes Vascular Inflammation and Atherosclerosis. Circulation 2009;120:699–708. 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- [62].Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu Rev Pathol Mech Dis 2020;15:493–518. 10.1146/annurev-pathmechdis-012419-032847. [DOI] [PubMed] [Google Scholar]
- [63].Magna M, Pisetsky DS. The Role of HMGB1 in the Pathogenesis of Inflammatory and Autoimmune Diseases. Mol Med 2014;20:138–46. 10.2119/molmed.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lu B, Wang H, Andersson U, et al. Regulation of HMGB1 release by inflammasomes. Protein Cell 2013;4:163–7. 10.1007/s13238-012-2118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Andersson U, Tracey KJ. HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection. Annu Rev Immunol 2011;29:139–62. 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang B, Wei G, Liu B, et al. The Role of High Mobility Group Box 1 Protein in Interleukin-18-Induced Myofibroblastic Transition of Valvular Interstitial Cells. Cardiology 2016;135:168–78. 10.1159/000447483. [DOI] [PubMed] [Google Scholar]
- [67].Schmidt AM. 2016 ATVB Plenary Lecture: Receptor for Advanced Glycation Endproducts and Implications for the Pathogenesis and Treatment of Cardiometabolic Disorders: Spotlight on the Macrophage. Arterioscler Thromb Vasc Biol 2017;37:613–21. 10.1161/ATVBAHA.117.307263.*This article highlights the interaction of receptor advanced glycation end products (RAGE) with macrophages and the subsequent pro-inflammatory signaling response.
- [68].Fiuza C, Bustin M, Talwar S, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003;101:2652–60. 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- [69].Yang H, Wang H, Czura CJ, et al. The cytokine activity of HMGB1. J Leukoc Biol 2005;78:1–8. 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- [70].Edfeldt K, Swedenborg J, Hansson GK, et al. Expression of Toll-Like Receptors in Human Atherosclerotic Lesions: A Possible Pathway for Plaque Activation. Circulation 2002;105:1158–61. 10.1161/circ.105.10.1158. [DOI] [PubMed] [Google Scholar]
- [71].Ott C, Jacobs K, Haucke E, et al. Role of advanced glycation end products in cellular signaling. Redox Biol 2014;2:411–29. 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shi D, Chang JW, Choi J, et al. Receptor for Advanced Glycation End Products (RAGE) is Expressed Predominantly in Medium Spiny Neurons of tgHD Rat Striatum. Neuroscience 2018;380:146–51. 10.1016/j.neuroscience.2018.03.042. [DOI] [PubMed] [Google Scholar]
- [73].Fritz G RAGE: a single receptor fits multiple ligands. Trends Biochem Sci 2011;36:625–32. 10.1016/j.tibs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- [74].Chen Y-J, Chan D-C, Chiang C-K, et al. Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-κB pathway activation. J Orthop Res 2016;34:791–800. 10.1002/jor.23083. [DOI] [PubMed] [Google Scholar]
- [75].Senatus LM, Schmidt AM. The AGE-RAGE Axis: Implications for Age-Associated Arterial Diseases. Front Genet 2017;8. 10.3389/fgene.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhong H, Li X, Zhou S, et al. Interplay between RAGE and TLR4 Regulates HMGB1-Induced Inflammation by Promoting Cell Surface Expression of RAGE and TLR4. J Immunol 2020. 10.4049/jimmunol.1900860. [DOI] [PubMed] [Google Scholar]
- [77].Yang H, Hreggvidsdottir HS, Palmblad K, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci 2010;107:11942–7. 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Björkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 2004;10:416–21. 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- [79].Chandrashekaran V, Seth RK, Dattaroy D, et al. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biol 2017;13:8–19. 10.1016/j.redox.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Roshan MHK, Tambo A, Pace NP. The Role of TLR2, TLR4, and TLR9 in the Pathogenesis of Atherosclerosis. Int J Inflamm 2016;2016:1–11. 10.1155/2016/1532832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci 2004;101:10679–84. 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen T, Luo W, Wu G, et al. A novel MyD88 inhibitor LM9 prevents atherosclerosis by regulating inflammatory responses and oxidative stress in macrophages. Toxicol Appl Pharmacol 2019;370:44–55. 10.1016/j.taap.2019.03.012. [DOI] [PubMed] [Google Scholar]
- [83].Yin Y-W, Liao S-Q, Zhang M-J, et al. TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACAT1 expression. Cell Death Dis 2014;5:e1574–e1574. 10.1038/cddis.2014.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gargiulo S, Gamba P, Testa G, et al. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 2015;14:569–81. 10.1111/acel.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yang H, Lundbäck P, Ottosson L, et al. Redox Modification of Cysteine Residues Regulates the Cytokine Activity of High Mobility Group Box-1 (HMGB1). Mol Med 2012;18:250–9. 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [86].Yang H, Wang H, Ju Z, et al. MD-2 is required for disulfide HMGB1–dependent TLR4 signaling. J Exp Med 2015;212:5–14. 10.1084/jem.20141318.** This article demonstrated that HMGB1 binds to TLR4 via the myeloid differentiation factor 2 (MD-2). This novel finding suggests a potential site of intervention in sterile inflammation while preserving immune response to microbes.
- [87].Gliozzi M, Scicchitano M, Bosco F, et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int J Mol Sci 2019;20:3294. 10.3390/ijms20133294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lin F, Pei L, Zhang Q, et al. Ox-LDL induces endothelial cell apoptosis and macrophage migration by regulating caveolin-1 phosphorylation. J Cell Physiol 2018;233:6683–92. 10.1002/jcp.26468. [DOI] [PubMed] [Google Scholar]
- [89].Cai J, Yuan H, Wang Q, et al. HMGB1-Driven Inflammation and Intimal Hyperplasia After Arterial Injury Involves Cell-Specific Actions Mediated by TLR4. Arterioscler Thromb Vasc Biol 2015;35:2579–93. 10.1161/ATVBAHA.115.305789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in Inflammation and Cancer. Annu Rev Immunol 2010;28:367–88. 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- [91].Cao X, Zhang L, Chen C, et al. The critical role of ABCG1 and PPARγ/LXRα signaling in TLR4 mediates inflammatory responses and lipid accumulation in vascular smooth muscle cells. Cell Tissue Res 2017;368:145–57. 10.1007/s00441-016-2518-3. [DOI] [PubMed] [Google Scholar]
- [92].Lake NJ, Taylor RL, Trahair H, et al. TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis. Eur Heart J 2017;38:3579–87. 10.1093/eurheartj/ehx315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nakaya K, Tohyama J, Naik SU, et al. Peroxisome Proliferator-Activated Receptor-α Activation Promotes Macrophage Reverse Cholesterol Transport Through a Liver X Receptor–Dependent Pathway. Arterioscler Thromb Vasc Biol 2011;31:1276–82. 10.1161/ATVBAHA.111.225383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rohatgi A, Khera A, Berry JD, et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N Engl J Med 2014;371:2383–93. 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mullick AE, Soldau K, Kiosses WB, et al. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med 2008;205:373–83. 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Liu X, Ukai T, Yumoto H, et al. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis 2008;196:146–54. 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Rangasamy SB, Jana M, Roy A, et al. Selective disruption of TLR2-MyD88 interaction inhibits inflammation and attenuates Alzheimer’s pathology. J Clin Invest 2018;128:4297–312. 10.1172/JCI96209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lee G-L, Chang Y-W, Wu J-Y, et al. TLR 2 Induces Vascular Smooth Muscle Cell Migration Through cAMP Response Element–Binding Protein–Mediated Interleukin-6 Production. Arterioscler Thromb Vasc Biol 2012;32:2751–60. 10.1161/ATVBAHA.112.300302. [DOI] [PubMed] [Google Scholar]
- [99].Yokoi H, Yanagita M. Targeting the fatty acid transport protein CD36, a class B scavenger receptor, in the treatment of renal disease. Kidney Int 2016;89:740–2. 10.1016/j.kint.2016.01.009. [DOI] [PubMed] [Google Scholar]
- [100].Xiao X, Yang C, Qu S-L, et al. S100 proteins in atherosclerosis. Clin Chim Acta 2020;502:293–304. 10.1016/j.cca.2019.11.019. [DOI] [PubMed] [Google Scholar]
- [101].Oesterle A, Bowman MAH. S100A12 and the S100/Calgranulins - Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets 2016:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ligthart S, Sedaghat S, Ikram MA, et al. EN-RAGE: A Novel Inflammatory Marker for Incident Coronary Heart Disease. Arterioscler Thromb Vasc Biol 2014;34:2695–9. 10.1161/ATVBAHA.114.304306. [DOI] [PubMed] [Google Scholar]
- [103].Averill MM, Kerkhoff C, Bornfeldt KE. S100A8 and S100A9 in Cardiovascular Biology and Disease. Arterioscler Thromb Vasc Biol 2012;32:223–9. 10.1161/ATVBAHA.111.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Croce K, Gao H, Wang Y, et al. Myeloid-Related Protein-8/14 Is Critical for the Biological Response to Vascular Injury. Circulation 2009;120:427–36. 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ellsworth DL, Croft DT, Weyandt J, et al. Intensive Cardiovascular Risk Reduction Induces Sustainable Changes in Expression of Genes and Pathways Important to Vascular Function. Circ Cardiovasc Genet 2014;7:151–60. 10.1161/CIRCGENETICS.113.000121. [DOI] [PubMed] [Google Scholar]
- [106].Gawdzik J, Mathew L, Kim G, et al. Vascular Remodeling and Arterial Calcification Are Directly Mediated by S100A12 (EN-RAGE) in Chronic Kidney Disease. Am J Nephrol 2011;33:250–9. 10.1159/000324693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med 2007;13:719–24. 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- [108].Farokhzadian J, Mangolian Shahrbabaki P, Bagheri V. S100A12-CD36 axis: A novel player in the pathogenesis of atherosclerosis? Cytokine 2019;122:154104. 10.1016/j.cyto.2017.07.010. [DOI] [PubMed] [Google Scholar]
- [109].Tondera C, Laube M, Pietzsch J. Insights into binding of S100 proteins to scavenger receptors: class B scavenger receptor CD36 binds S100A12 with high affinity. Amino Acids 2017;49:183–91. 10.1007/s00726-016-2349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Takeuchi O, Akira S. Pattern Recognition Receptors and Inflammation. Cell 2010;140:805–20. 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- [111].Lamkanfi M, Dixit VM. Inflammasomes and Their Roles in Health and Disease. Annu Rev Cell Dev Biol 2012;28:137–61. 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- [112].Zeng C, Wang R, Tan H. Role of Pyroptosis in Cardiovascular Diseases and its Therapeutic Implications. Int J Biol Sci 2019;15:1345–57. 10.7150/ijbs.33568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lamkanfi M, Dixit VM. Mechanisms and Functions of Inflammasomes. Cell 2014;157:1013–22. 10.1016/j.cell.2014.04.007.**The findings in this article demonstrate binding between S100A12 and CD36 suggest a potential role for translocation of fatty acids as well as regulation of PPARγ.
- [114].Bruchard M, Rebé C, Derangère V, et al. The receptor NLRP3 is a transcriptional regulator of T H 2 differentiation. Nat Immunol 2015;16:859–70. 10.1038/ni.3202. [DOI] [PubMed] [Google Scholar]
- [115].Baldrighi M, Mallat Z, Li X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 2017;267:127–38. 10.1016/j.atherosclerosis.2017.10.027. [DOI] [PubMed] [Google Scholar]
- [116].Muñoz-Planillo R, Kuffa P, Martínez-Colón G, et al. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013;38:1142–53. 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Chevriaux A, Pilot T, Derangère V, et al. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front Cell Dev Biol 2020;8. 10.3389/fcell.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Shimada K Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis n.d.:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kim EJ, Park SY, Baek SE, et al. HMGB1 Increases IL-1β Production in Vascular Smooth Muscle Cells via NLRP3 Inflammasome. Front Physiol 2018;9:313. 10.3389/fphys.2018.00313.**This article demonstrated HMGB1 induced production of IL-1β in vascular smooth muscle cells in relation to expression of TLR2, TLR4, and RAGE expression.
- [120].Wang R, Wu W, Li W, et al. Activation of NLRP3 Inflammasome Promotes Foam Cell Formation in Vascular Smooth Muscle Cells and Atherogenesis Via HMGB1. J Am Heart Assoc 2018;7. 10.1161/JAHA.118.008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Eun SY, Ko YS, Park SW, et al. IL-1β enhances vascular smooth muscle cell proliferation and migration via P2Y2 receptor-mediated RAGE expression and HMGB1 release. Vascul Pharmacol 2015;72:108–17. 10.1016/j.vph.2015.04.013. [DOI] [PubMed] [Google Scholar]
- [122].Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 2013;14:812–20. 10.1038/ni.2639.**This article identifies the role of CD36 with TLR4-TLR6 as an important regulator of the inflammasome in several disease processes including atherosclerosis.
- [123].Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 2010;11:155–61. 10.1038/ni.1836.**The findings in this article demonstrate pharmacological intervention via necrosulfonamide to inhibit Gasdermin D attenuates pyroptotic pore formation, cell death and the release of IL-1β.
- [124].Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, et al. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J Am Heart Assoc 2016;5. 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Getz GS, Reardon CA. Do the Apoe −/− and Ldlr −/− Mice Yield the Same Insight on Atherogenesis? Arterioscler Thromb Vasc Biol 2016;36:1734–41. 10.1161/ATVBAHA.116.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–61. 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hendrikx T, Jeurissen MLJ, van Gorp PJ, et al. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in Ldlr−/− mice. FEBS J 2015;282:2327–38. 10.1111/febs.13279. [DOI] [PubMed] [Google Scholar]
- [128].Menu P, Pellegrin M, Aubert J-F, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis 2011;2:e137–e137. 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Shi X, Xie W-L, Kong W-W, et al. Expression of the NLRP3 Inflammasome in Carotid Atherosclerosis. J Stroke Cerebrovasc Dis 2015;24:2455–66. 10.1016/j.jstrokecerebrovasdis.2015.03.024. [DOI] [PubMed] [Google Scholar]
- [130].Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016;535:111–6. 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- [131].Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016;535:153–8. 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sborgi L, Rühl S, Mulvihill E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 2016;35:1766–78. 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377:1119–31. 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- [134].Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of High-Density Lipoprotein Function in Patients With Early Rheumatoid Arthritis Treated With Methotrexate Monotherapy or Combination Therapies in a Randomized Controlled Trial. Arthritis Rheumatol 2017;69:46–57. 10.1002/art.39833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Reiss AB, Carsons SE, Anwar K, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum 2008;58:3675–83. 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ridker PM, Everett BM, Pradhan A, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med 2019;380:752–62. 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ma C, Wang Y, Dong L, et al. Anti-inflammatory effect of resveratrol through the suppression of NF- B and JAK/STAT signaling pathways. Acta Biochim Biophys Sin 2015;47:207–13. 10.1093/abbs/gmu135. [DOI] [PubMed] [Google Scholar]
- [138].Lu B, Antoine DJ, Kwan K, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci 2014;111:3068–73. 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Irey EA, Lassiter CM, Brady NJ, et al. JAK/STAT inhibition in macrophages promotes therapeutic resistance by inducing expression of protumorigenic factors. Proc Natl Acad Sci 2019;116:12442–51. 10.1073/pnas.1816410116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yang H, Wang H, Chavan SS, et al. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol Med 2015;21:S6–12. 10.2119/molmed.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sun S, He M, Wang Y, et al. Folic acid derived-P5779 mimetics regulate DAMP-mediated inflammation through disruption of HMGB1:TLR4:MD-2 axes. PLOS ONE 2018;13:e0193028. 10.1371/journal.pone.0193028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zhou F, Tan Y. Atorvastatin improves plaque stability in diabetic atherosclerosis through the RAGE pathway n.d.:8. [DOI] [PubMed] [Google Scholar]
- [143].Peng S, Xu L-W, Che X-Y, et al. Atorvastatin Inhibits Inflammatory Response, Attenuates Lipid Deposition, and Improves the Stability of Vulnerable Atherosclerotic Plaques by Modulating Autophagy. Front Pharmacol 2018;9:438. 10.3389/fphar.2018.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Cao L, Pan D, Li D, Zhang Y, et al. Relation between anti-atherosclerotic effects of IRAK4 and modulation of vascular smooth muscle cell phenotype in diabetic rats n.d.:12. [PMC free article] [PubMed] [Google Scholar]
- [145].Singer JW, Fleischman A, Al-Fayoumi S, et al. Inhibition of interleukin-1 receptor-associated kinase 1 (IRAK1) as a therapeutic strategy. Oncotarget 2018;9:33416–39. 10.18632/oncotarget.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Seijkens TTP, van Tiel CM, Kusters PJH, et al. Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. J Am Coll Cardiol 2018;71:527–42. 10.1016/j.jacc.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Gareus R, Kotsaki E, Xanthoulea S, et al. Endothelial Cell-Specific NF-κB Inhibition Protects Mice from Atherosclerosis. Cell Metab 2008;8:372–83. 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- [148].Paciolla M, Pescatore A, Conte MI, et al. Rare mendelian primary immunodeficiency diseases associated with impaired NF-κB signaling. Genes Immun 2015;16:239–46. 10.1038/gene.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Rathkey JK, Zhao J, Liu Z, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol 2018;3:eaat2738. 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Xing S-S, Yang J, Li W, et al. Salidroside Decreases Atherosclerosis Plaque Formation via Inhibiting Endothelial Cell Pyroptosis. Inflammation 2020;43:433–40. 10.1007/s10753-019-01106-x. [DOI] [PubMed] [Google Scholar]