Abstract
Here we synthesize current understanding of the magnitudes and methods for assessing human and wildlife exposures to poly- and perfluoroalkyl substances (PFAS). Most human exposure assessments have focused on two to five legacy PFAS and wildlife assessments are typically limited to targeted PFAS (up to ~30 substances). However, shifts in chemical production are occurring rapidly and targeted methods for detecting PFAS have not kept pace with these changes. Total fluorine (TF) measurements complemented by suspect screening using high resolution mass spectrometry are thus emerging as essential tools for PFAS exposure assessment. Such methods enable researchers to better understand contributions from precursor compounds that degrade into terminal perfluoroalkyl acids (PFAA). Available data suggest that diet is the major human exposure pathway for some PFAS but there is large variability across populations and PFAS compounds. Additional data on TF in exposure media and the fraction of unidentified organofluorine are needed. Drinking water has been established as the major exposure source in contaminated communities. As water supplies are remediated, and for the general population, exposures from dust, personal care products, indoor environments and other sources may be more important. A major challenge for exposure assessments is the lack of statistically representative population surveys. For wildlife, bioaccumulation processes differ substantially between PFAS and neutral lipophilic organic compounds, prompting a revaluation of traditional bioaccumulation metrics. There is evidence that both phospholipids and proteins are important for the tissue partitioning and accumulation of PFAS. New mechanistic models for PFAS bioaccumulation are being developed that will assist in wildlife risk evaluations.
Keywords: organofluorine, exposure assessment, bioaccumulation, drinking water, toxicants, wildlife
Graphical Abstract:
Methods for assessing human and wildlife exposures to per- and polyfluoroalkyl substances are reviewed along with current understanding of exposure sources and pathways.
1. INTRODUCTION
Poly- and perfluoroalkyl substances (PFAS) are a class of thousands of anthropogenic substances containing an aliphatic fluorinated carbon chain (Buck 2011; OECD 2018). The PFAS family includes: (a) perfluoroalkyl substances, mainly perfluoroalkyl acids (PFAA) such as perfluoroalkyl carboxylates (PFCA) and perfluoroalkyl sulfonates (PFSA), as well as perfluoroalkane sulfonamide substances, and (b) polyfluoroalkyl substances such as fluorotelomer monomers, including fluorotelomer alcohols (FTOH), fluorotelomer olefins (FTO) and fluorotelomer iodides (FTI), and polyfluoroalkyl ether acids. Both perfluoroalkyl substances and polyfluoroalkyl substances can be polymers or non-polymers. The extraordinary strength of the C-F bond in the perfluoroalkyl moiety imparts unique properties (Smart 2001; Biffinger 2004) and has led to widespread industrial and commercial uses of PFAS. With over 7800 PFAS chemical structures identified to date (US EPA 2020a) and thousands registered for regulatory purposes (e.g., chemical inventories) (OECD 2018), they play a prominent role in modern society.
PFAS present a societal challenge. On one hand, PFAS represent some of the most innovative developments in materials chemistry and provide innumerable societal benefits (Johns 2000). However, following decades of widespread global use, and because many PFAS are highly persistent and mobile, concerns have been raised about the ecological and human health impacts of PFAS exposures. PFAS use categories include personal care products (PCP), cosmetics, ski wax, aqueous film forming foams (AFFF), textile treatments for stain and water repellency, food contact materials, medical devices, membranes in fuel cells, and membranes in chlor-alkali processes. This list of PFAS applications, though not encompassing of the full scale of PFAS use, captures their diversity. Prior work established the concept of “essential uses” of PFAS by first reviewing where they are abundantly used and when such uses can be replaced by safer alternatives (Cousins 2019).
Multiple strategies have been implemented to reduce emissions, production and use of specific PFAS. Manufacturers have phased out production of certain PFAS and in some cases replaced them with new PFAS or chemical substitutes. For example in textile treatments, many polymers containing long perfluoroalkyl side chains (more than seven perfluorinated carbons) were replaced by analogs containing short perfluoroalkyl side chains (six or four perfluorinated carbons) or fluorine-free moieties (e.g., siloxanes and hydrocarbon polymers) (Schellenberger 2019a). Further efforts are underway to constrain leachable content in fluoropolymers and side-chain fluorinated polymers. This leachable content consists of unbound monomers (such as fluorotelomer alcohols), oligomers, and other non-polymeric PFAS used during the polymer manufacturing process (such as surfactants and chain transfer reagents). Governments have implemented plans to restrict the usage, manufacture and import of certain PFAS, typically on a chemical-by-chemical basis and with certain exemptions. However, based on the recalcitrance of PFAS terminal products, their ubiquitous presence, and continued usage, PFAS exposure to humans and wildlife continues (Scheringer 2014).
The volume of research publications over the past decade identifying PFAS in environmental media, humans and wildlife is staggering with several comprehensive review publications (Houde 2011b; Jian 2018; Sunderland 2019; Wang 2019). However, our ability to quantify how production and environmental releases of PFAS translate into PFAS tissue burdens in humans and wildlife is still limited. PFAS exposure science was a key area explored in the SETAC Focused Topic Meeting on Environmental Risk Assessment of PFAS held in Durham, NC, USA August 12-15, 2019.
Here we synthesize current understanding of PFAS exposure sources for humans and wildlife and identify key knowledge gaps that inhibit the development of informed regulatory decisions based on sound science. Specifically, we review: (a) PFAS sources and environmental transport pathways; (b) analytical methods used to assess PFAS exposures and their strengths and limitations; (c) methods for assessing human exposures to PFAS; (d) current understanding of the relative importance of different human exposure pathways; and (e) current understanding of PFAS bioaccumulation in wildlife.
2. OVERVIEW OF PFAS SOURCES AND ENVIRONMENTAL PATHWAYS
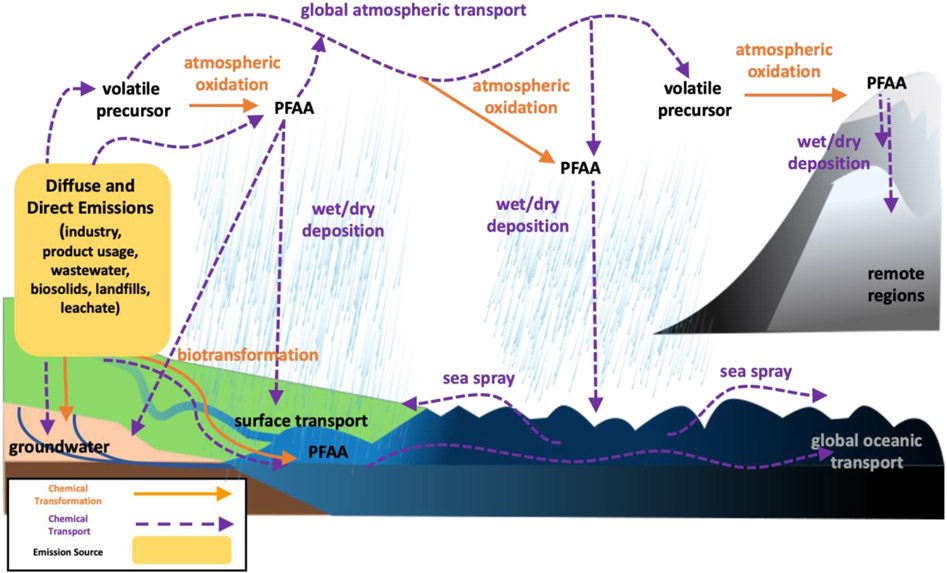
Figure 1 provides a schematic of the sources and spatial scales of PFAS transport and accumulation. Releases to the environment occur during the production, use, and disposal of materials containing PFAS. For example, legacy emissions of perfluorooctanoic acid (PFOA) were dominated by its manufacture and use to manufacture fluoropolymer products (Prevedouros 2006), whereas emissions of PFOS were dominated by its release during use of consumer and industrial products (e.g., surface treatments, AFFF, insecticides) (Armitage 2009b; Paul 2009; Wang 2017a).
Figure 1.
Conceptual representation of key emission sources and global transport pathways of perfluoroalkyl acids (PFAA) and their polyfluorinated precursors.
Existing chemical production and release inventories for PFAS have largely focused on PFCA, PFSA and their precursor compounds (e.g., FTOH and perfluorooctane- sulfonamides and -sulfonamidoethanols: FASA) (Armitage 2006; Prevedouros 2006; Yarwood 2007; Armitage 2009a; Armitage 2009b; Paul 2009; Wang 2014a; Wang 2014b; Kotthoff 2015; Shi 2015; Wang 2017b; Boucher 2019). A major shift in chemical production occurred between 2000-2002 with the voluntary phase-out of the base chemical POSF (perfluorooctane sulfonyl fluoride) to PFOS and perfluorooctane sulfonamide-based chemistry by 3M, the major global manufacturer at the time (3M Company 1999). Stewardship programs for PFOA in the United States and Europe have similarly been very successful at phasing out releases of this compound. We now know PFOS, PFOA and other long-chain legacy PFAS compounds represent only a small proportion of the total number of PFAS (US EPA 2020a). Understanding of the global environmental production and distribution of the legacy PFAS has been greatly facilitated by academic partnerships with industry that allowed the development of global emissions inventories for some compounds (Prevedouros 2006; Armitage 2009a; Armitage 2009b; Paul 2009; Wang 2014a; Wang 2017a, 2017b). We thus recommend greater transparency and collaboration between academia, industry and government to prioritize and improve understanding of global environmental releases of the thousands of PFAS structures on US EPA Master List (US EPA 2020a).
The effects of the phase out in chemical production of PFOS and its precursors as well as the PFOA stewardship program are well documented and illustrate the potential benefits of coordinated action curbing chemical releases. For example, ocean modeling studies forced by changes in riverine discharges to the North Atlantic show a large decline in surface (0-10 m depth) seawater PFOS concentrations at their peak (median > 60 pg L−1) around the year 2000 to <40 pg/L in 2020 (Zhang 2017). In juvenile North Atlantic pilot whales, perfluorooctane sulfonamide: FOSA (a precursor to PFOS) accounted for 84% of the 15 targeted PFAS measured in the year 2000 but declined to 34% by 2013 (Dassuncao 2017). Human cohort studies in Denmark, Australia, Japan, Germany, and the Faroe Islands all indicate large declines in PFAS exposure for the general population outside of contaminated areas over this same time period (Olsen 2012; Okada 2013; Yeung 2013a; Toms 2014; Bjerregaard-Olesen 2016; Dassuncao 2018).
Sources of local scale contamination include fluorochemical manufacturing facilities, other manufacturing facilities where PFAS are used, PFAS-containing AFFF, wastewater treatment plants, and landfills. High environmental concentrations of PFAS result in exposure through consumption of contaminated drinking water, agricultural products, or fish and game. For more diffuse PFAS exposures driven predominantly by use of consumer products, population has been established as a good proxy for contamination of surface waters and coastal ecosystems (Paul 2012; Xie 2013; Li 2015; Zhang 2017).
Environmental concentrations and human and wildlife exposures to PFAS are typically highest at contaminated sites (Paustenbach 2007; Pistocchi 2009; Hoffman 2011; Shin 2011a; Shi 2015). Fluorochemical manufacturing sites are responsible for a large proportion of global emissions of certain types of PFAS such as PFCA, but there are relatively few of these sites. One study reported the presence of 16 fluorochemical manufacturing plants in the U.S. (Hu 2016) and another estimated that there were 33 fluoropolymer production plants worldwide as of 2002 (Prevedouros 2006). High volume emissions from such facilities can impact large geographic areas and correspondingly large populations. For example, releases into the Ohio River from a fluoropolymer production plant in the U.S. state of West Virginia resulted in elevated level of PFAS in drinking water in communities hundreds of miles downstream (Herrick 2017).
Use of AFFF containing PFAS for fire suppression or training activities at military bases, commercial airports, and fire-training areas around the globe has contaminated many aquatic environments (Moody 1999; Barzen-Hanson 2017). Landscapes and water systems adjacent to areas of AFFF use often have high levels of PFAS in soil, sediment, groundwater, surface water, or drinking water (Karrman 2011; Houtz 2013; Anderson 2016; US DOD 2017). PFAS concentrations measured in different environmental media at ten active United States Air Force installations were highest for PFOS and included 4,300 μg/L in groundwater, 8,970 μg/L in surface water, 190,000 μg/kg in sediments, 9,700 μg/kg in surface soil and 1,700 μg/kg in subsurface soil (Anderson 2016).
Incidents of localized contamination have been linked to facilities that employ PFAS to produce goods such as plastic and textile coating facilities (VTDEC 2016; NHDES 2020; NYDEC 2020), and leather tanneries (US EPA 2020b). Numerous other industries are users of PFAS, and all of these have the potential to cause localized contamination. For example, major sources of PFAS contamination in surface waters in New York State and Rhode Island, USA included mixed industrial sources that predominately release PFOS and PFOA, metal plating industry sites, and landfills (Zhang 2016). PFAS enter landfills as components of residual materials and can be released to the environment in leachate, and may also contribute to elevated concentrations in wastewater (Huset 2011; Lang 2017; Masoner 2020). Concentrations of PFAS in municipal solid waste vary substantially depending on the waste source (Solo-Gabriele 2020).
Wastewater treatment facilities receive PFAS in influent and discharge PFAS in treated effluent and biosolids (Sinclair 2006; Coggan 2019). Treated effluent can be a source of PFAS exposure if it is discharged to a water body that is used as a drinking water source. For example, the probability of detecting PFAS in US public drinking supplies was significantly associated with higher numbers of wastewater treatment plants within a watershed (Hu 2016). Land application of biosolids or irrigation using reclaimed water can result in accumulation of PFAS in soils and underlying groundwater, and uptake into food or fodder crops (Choi 2019; Coggan 2019; Lazcano 2019; Letcher 2020). Concentrations of PFAS are highest in effluent and biosolids for treatment plants that receive wastewater from industrial plants that use PFAS or facilities that use AFFF (3M 2001; Houtz 2016).
Following decades of releases (ca. 1958 to present), PFAS are now ubiquitous in the global environment and the ocean is thought to be the final sink for the terminal products (PFAA) associated with most global production (Armitage 2009a; Armitage 2009b; Zhang 2017). The spatial distributions of PFAS in the environment following releases reflect their physical-chemical properties (propensity for sorption vs. transport in air and water) and types of releases (air, water, soil) during manufacturing, use, and disposal. The relative importance of the atmospheric transport/precursor degradation pathway versus the oceanic transport/terminal end product pathway has been assessed for some PFAA. Generally, aquatic discharges and oceanic transport are more relevant next to source regions in the United States, Europe, and Japan (Prevedouros 2006; Armitage 2009a; Zhang 2017) while atmospheric transport is important in remote regions such as the Arctic and Southern Ocean for many compounds (Wang 2015; Dassuncao 2017; MacInnis 2017; Yeung 2017; Pickard 2018). Accumulation of PFAS in the oceans reflects both contemporary and historic PFAS production because terminal PFAA are not known to appreciably degrade under environmental conditions and the timescales associated with PFAS removal through burial in coastal and deep-sea sediment are long (Yamashita 2008).
A major focus of PFAS research is better understanding the releases and environmental degradation pathways of precursor compounds that degrade into terminal PFAA. The majority of precursor compounds studied to date are neutral organics with appreciable vapor pressures. This means that they have a greater propensity for atmospheric transport, whereas most PFAA have low pKa values and exist as stable ions in solution (Cheng 2009). Significant effort is being dedicated to better understanding point source releases of atmospheric PFAS (both PFAA and precursor compounds), but stack testing methods and inventories are still limited. Ionizable precursors also exist but data are scarce. Surface deposition of atmospheric PFAA emissions followed by leaching of PFAS to groundwater has been demonstrated at multiple industrial sites (Guelfo 2018; NHDES 2020; NYDEC 2020). Integration of PFAS measurements into routine atmospheric monitoring for pollutants by programs like the North American Deposition Program (ww.nadp.org) would thus be valuable for measuring changes in atmospheric PFAS concentrations, assisting atmospheric PFAS modeling efforts (Thackray 2020), and identifying regions vulnerable to atmospheric PFAS contamination.
3. ANALYTICAL TECHNIQUES FOR MEASURING PFAS EXPOSURES
The ability of scientists and regulators to identify and rank the importance of different PFAS exposure sources is directly contingent on analytical methods available for measuring PFAS in a variety of environmental matrices and biological tissues. Analytical techniques for PFAS have advanced rapidly from an early focus on PFOA and PFOS to routine measurements of a suite of approximately 40 PFAS with commercially available analytical standards for detection using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Recent methods have been evolving for detecting volatile species, new compounds, and total fluorine in environmental and human samples. These advances have been critical for keeping pace with the rapidly changing chemical landscape of PFAS exposure sources.
3.1. Targeted PFAS analysis
Most studies report concentrations of non-volatile PFAS measured using LC-MS/MS. This is commonly referred to as “targeted” analysis because it is based on setting up the instrumental analysis to collect data on specific substances, while confirmation of these substances relies on specific mass-to-charge (m/z) ratios and retention times based on parameters determined using commercially available chemical standards. Nonetheless, this approach enables quantitative PFAS determination with high precision and high sensitivity. Targeted PFAS analysis is used for water (drinking water, surface water, groundwater), air/ airborne particulate, food, solids (soil, sediment, house dust), and consumer products. The same techniques are also used to determine PFAS in diverse biological tissues including plasma, sera, whole blood, urine, breast milk, muscle, and other tissues. Over the past few decades, targeted PFAS measurements has improved substantially with improved instrument sensitivity and lower detection limits, numerous laboratory intercomparisons and standard operating procedures, and the addition of more PFAS that can be detected (Guelfo 2020). A major analytical challenge is that synthesis of analytical standards for newer PFAS has lagged their production and release and a lack of reference materials for the major PFAS in routine analysis (Xiao 2017; Land 2018).
3.2. Suspect screening and non-targeted analysis
While targeted analysis is limited to a finite number of PFAS analytes, a comprehensive understanding of PFAS exposure calls for innovative techniques. Specifically, techniques that reveal the presence of emerging PFAS produced intentionally and unintentionally in various industrial processes, as well as PFAS transformation products formed in natural and engineered systems. Suspect screening and nontargeted analysis using high-resolution mass spectrometry (HRMS) allow discovery and characterization of unidentified PFAS in the environment. HRMS provides highly accurate mass to charge ratio of the analytes (< ±0.001 m/z), its fragments, and their isotopic patterns. Comparing such information with chemical databases that contain thousands of PFAS compounds enables identification of the molecular structure of analytes without analytical standards. Suspect screening and nontargeted analyses have led to the identification of emerging anionic, zwitterionic, cationic, and neutral PFAS in water (Strynar 2015; Barzen-Hanson 2017; Gebbink 2017; Newton 2017), sediment (Newton 2017), soil (Baduel 2017; Lin 2017), airborne particulate matter (Yu 2018), and biological samples (Rotander 2015b; Liu 2018).
A drawback of HRMS methods is that such analyses are typically qualitative, due to the absence of analytical standards. In addition, the PFAS congener can only be determined if the sample preparation has adequately recovered the analyte. Analyte spike and recovery validation is not possible without analytical standards. In addition, HRMS data analysis is labor intensive and require specialized analysts. However, it is increasingly recognized for its data-banking value, in which archived HRMS spectra for routine analyses can be re-analyzed when emerging analytes become a priority.
3.3. New methods for closing the fluorine mass balance in exposure studies
A major challenge in PFAS exposure assessment is that most studies employ targeted LC-MS/MS analyses and thus cannot assess the total burden of PFAS in environmental and biological samples. Even HRMS studies are limited by the sample preparation technique which can be discriminatory towards certain classes of PFAS. Also, because HRMS analyses are qualitative or semi-quantitative in nature, they cannot be used to develop mass budgets for total PFAS in the environment or the total burden of PFAS exposures. This has led to the development and use of several total fluorine (TF) measurements to better characterize the total burden of known and unknown PFAS in a sample.
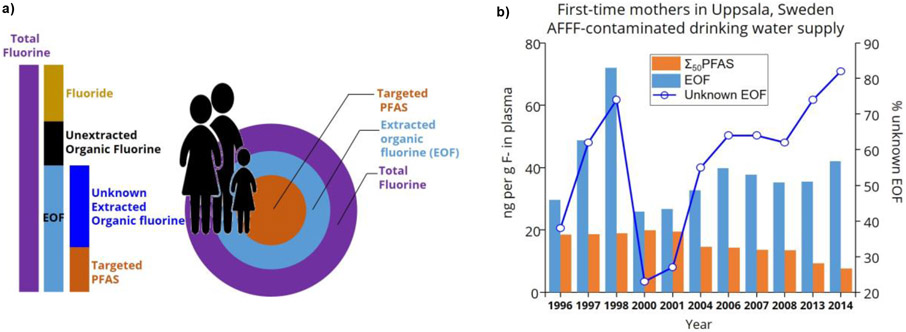
TF methods rely on determinations of the concentration of atomic fluorine. Several strategies have been developed for the determination of the total organofluorine (TOF), extractable organofluorine (EOF), or adsorbable organofluorine (AOF) content in order to assess the total PFAS content, as reviewed in prior work (Koch 2020). Known PFAS are typically determined using targeted LC-MS/MS techniques on the same sample extracts analyzed for EOF or AOF analyzed using combustion ion chromatography (CIC). The presence of organofluorine is indicative of anthropogenic substances, since organofluorines are very rare in nature as a consequence of the high energy bond between carbon and fluorine. By contrast, inorganic fluoride is the most abundant halogen on Earth and must be quantified to assess the organoflourine content. The portion of unidentified organofluorine in a sample is calculated by subtracting the concentration of target PFAS, converted to moles of fluorine, from the total organofluorine content in the same extract (Figure 2). Because the quantity of unidentified organofluorine is dependent on the targeted analysis, it is challenging to compare unidentified organofluorine among different studies. In Figure 2b, the unidentified organofluorine is shown relative to the targeted analysis of 50 PFAS congeners in human blood. In general, studies on TOF and EOF/AOF indicate a significant portion of organofluorine in biota and the environment is not captured by monitoring of the typical suite of PFAA congeners.
Figure 2.
Components of the fluorine mass balance. Panel a) shows a conceptual diagram representing the relative fractions of total fluorine (TF) including fluoride, unextracted organofluorine, extractable organofluorine (EOF), and targeted PFAS. Panel b) shows actual data where unknown EOF was determined using targeted analysis of 50 PFAS congeners in blood plasma of first time mothers from Uppsala, Sweden (data from Miaz 2020).
Different methods have been developed for total fluorine or organofluorine measurements in environmental samples and consumer products, including defluorination with sodium biphenyl (Musijowski 2007), 19F Nuclear Magnetic Resonance (NMR) (Moody 2000), continuum source molecular absorption spectrometry (CS-MAS) (Qin 2012), inductively coupled plasma tandem mass spectrometry (ICP-MS/MS) (Jamari 2018), instrumental neutron activation analysis (INAA) (Schultes 2019), particle-induced gamma-ray emission spectrometry (PIGE) (Ritter 2017), CIC (Miyake 2007a), and X-ray photoelectron spectroscopy (XPS) (Tokranov 2019). While some methods can distinguish between organofluorine and inorganic fluoride (NMR, XPS), others give the total concentration of fluorine in a sample (for example PIGE and CIC), therefore a pre-extraction of inorganic fluorine is needed.
Assessing PFAS in consumer products presents an important challenge for fully understanding human exposure. Some studies have measured the fluorine concentration at the material surface or sub-surface (PIGE, XPS, and INAA), while others consider the average concentrations throughout the whole sample analyzed (CIC). The unknown organofluorine content is useful for understanding TF analysis in human tissues. Concentrations of organofluorine in human serum have been measured using the CIC method, both total fluorine before extraction (Miyake 2007b) and EOF after extraction (Yeung 2016). As an example of this application, one study targeted 52 PFAS congeners in human blood from Münster, Germany including PFSA, PFCA, FASA, fluorotelomer acids (FTUCA & FTCA), and polyfluorinated phosphate esters (Yeung 2016). Through TF and EOF analysis, these PFAS accounted for approximately 80% of EOF from 1982-2006 and the unknown EOF increased to 50% from 2007 to 2009, further emphasizing that humans are being increasingly exposed to new organofluorine substances. Caution must be applied in ascribing the unknown EOF to PFAS because many pharmaceuticals contain fluorine. It may be possible to adapt methods to avoid coextraction of non-PFAS organofluorine. For example, a recent study (Figure 2b) used acetonitrile extraction followed by a dispersive graphitized carbon clean-up for human plasma (Miaz 2020), which is likely to sorb the fluorine in pharmaceuticals that is typically in the form of a –F or –CF3 moiety on an aromatic ring.
The Total Oxidizable Precursor Assay (TOP) assay provides another method for estimating unknown precursors. The method has been mainly applied to aqueous samples which are subjected to oxidation via hydroxyl radicals formed in a persulfate thermolysis, to transform PFAA precursors to their terminal products (Houtz 2013; Houtz 2016). The oxidized extracts and un-oxidized extracts are analyzed by LC-MS/MS for PFAS in order to quantify the concentrations of oxidizable precursors. The TOP assay highlights the relevance of PFAA precursors to PFAA concentrations in the environment. For example, one study determined that precursors in AFFF had significantly contributed to PFCA and PFSA in groundwater from a firefighting training area (Houtz 2013).
3.4. Strengths and limitations of analytical techniques for assessing exposures
Table 1 compares the strengths and limitations of different analytical methods used to assess PFAS exposures. Comprehensive human exposure assessments need to consider a wider range of PFAS than available from targeted LC-MS/MS measurements, including precursors that transform to terminal PFAA. Non-targeted screening for a variety of matrices and the TOP assay in aqueous samples have been proven useful for detecting additional PFAS. The utility of non-targeted analysis depends on identification strategies and available suspect lists. The TOP assay provides quantitative data on the contribution of oxidizable precursors and can provide some insight on the precursor structure (e.g. functional group and chain lengths). However, the TOP assay has only reliably been performed on aqueous samples due to matrix interference in soil and biota. Further, it is not able to oxidize certain emerging PFAS like per- and polyfluoroalkyl ether acids (PFEA) such as F-53B (6:2 Cl-PFESA) and GenX (hexafluoropropylene oxide dimer acid). Non-specific TF methods add information on the magnitude of unknown PFAS that are not quantifiable using targeted analysis. However, some of the detected total fluorine or organofluorine may include compounds outside of those PFAS according to the current definition by the Organization for Economic Cooperation and Development (OECD).
Table 1.
Comparison of analytical techniques for assessing PFAS exposure.a
| Methods | Applicable matrices |
Advantages | Limitations |
|---|---|---|---|
| Targeted analysis | All matrices |
|
|
| Non-targeted analysisb | All matrices |
|
|
| TOP | Mainly aqueous samples |
|
|
| EOF (CIC) | All matrices |
|
|
| AOF (CIC) | Water |
|
|
| PIGE | Solids |
|
|
| XPS | Solids |
|
|
A more comprehensive discussion of the strengths and limitations of different analytical techniques is reviewed elsewhere (Guelfo 2020).
For a more detailed discussion of the strengths and limitations of non-targeted analysis please see the following viewpoints and response (Hites 2018, 2019; Samanipour 2019).
Pharmaceuticals and pesticides with a low atomic fraction of fluorine could result in moderate or high organofluorine content in water if present in high concentrations. Other PFAS classes could be excluded from the analysis during conventional sample extraction. For example, the non-polar PFAS like perfluorobutyl side-chain fluorinated co-polymers surfactant with molecular weight >1600 g/mol was only extracted from soil, wastewater sludge and sediment using 1:1 hexane/acetone, whereas much lower recoveries were obtained using methanol or acetonitrile (Chu 2017; Letcher 2020). The removal of inorganic fluoride also needs to be validated, especially for natural waters and drinking water where fluoride concentrations can be orders of magnitude higher than PFAS concentrations. Non-specific TF methods that target atomic fluorine thus risk overestimating the PFAS content of samples but nonetheless provide a useful screening metric for identifying the magnitude of unidentified organofluorine in an environmental sample.
4. Methods for assessing human exposure to PFAS
Exposure pathways for PFAS can be examined as a chain of events shown in Figure 3, linking sources to media (via fate and transport) to external exposure (via behavioral factors) to concentrations in blood, the body’s central compartment (via toxicokinetics). Exposure routes that are typically examined for PFAS include: dietary ingestion, water ingestion (particularly in contaminated communities), and inhalation of air and dust particles. Hand-to-mouth contact and dermal absorption can also be relevant pathways.
Figure 3.
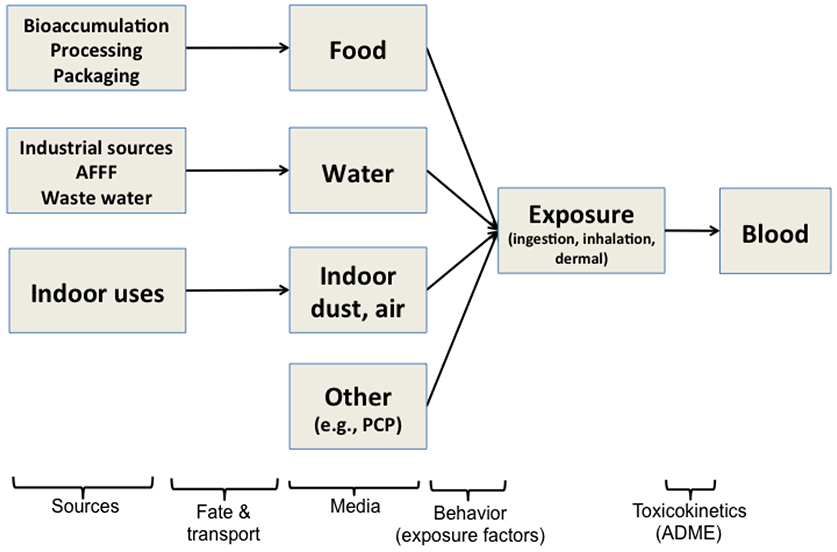
Schematic of exposure assessment steps for humans that relates PFAS sources to exposure media, and internal concentrations of PFAS in blood. Not all possible exposure routes (e.g., outdoor air) or arrows are shown. ADME = Absorption, distribution, metabolism and excretion.
4.1. Two approaches to PFAS exposure assessment
Exposures are typically estimated using two complementary approaches. The exposure factor (“bottom up”) approach relies on measured concentrations of certain PFAS in exposure media (e.g., food, water, air, dust) and uses estimates of exposure frequency and duration to estimate external exposure (mass/kg body weight/time). PFAS levels in media (dietary items, drinking water, and the indoor environment) can also be estimated using multi-media modeling assessments of global or local sources, transport and accumulation. An alternate method is the epidemiologic (“top down”) approach to exposure assessment, which typically involves regressing serum/blood levels against measured concentrations in different media (e.g., water, air, dust), and/or behavioral data (e.g., food consumption) to estimate the strength of association with one or more sources. With sufficient data regarding multiple pathways, either method can estimate the relative importance of different exposure routes.
Several studies have used the exposure factor approach to quantify the contribution of PFAS in the indoor environment, seafood, drinking water, and food packaging to total exposures in humans (Trudel 2008; Vestergren 2008; Harrad 2010; Gebbink 2015; Dassuncao 2018). Exposure to precursors has been linked to increased bioaccumulation in food webs (Kelly 2009; Dassuncao 2017; Boisvert 2019; Zhang 2019). Associations between serum PFAS concentrations and exposure behaviors such as water district of residence, consumption of tap water, and fish consumption have also been characterized in prior work (Christensen 2017; Herrick 2017; Dassuncao 2018; Barton 2020).
The two exposure assessment methods each have strengths and weaknesses and are complementary. In the exposure factor approach, one or both elements may be uncertain. For example, while measuring PFAS in food can be analytically challenging with many non-detects, average food consumption rates are better known. In contrast, measuring PFAS in house dust is easier, but dust ingestion rates are uncertain, particularly for adults. Carrying out statistically representative (and therefore extrapolatable) sampling and characterizing the fraction of exposure originating from precursors is challenging for many exposure routes. One strength of the exposure factor approach is the potential for a direct link to chemical production and environmental concentrations that drive exposures (Armitage 2009a). This information is critical for designing interventions that mitigate human and wildlife exposures.
The epidemiologic approach integrates both exposure and toxicokinetics, but often must take into account potential confounding between exposure routes as well as exposure measurement error. For example, the latter can arise when regressing serum concentrations of persistent PFAS with media measured at one point in time. The long half-life of many PFAS in humans means that serum concentrations reflect cumulative exposures over a relatively long time period, while external exposure measurements may often reflect a short exposure window. Hybrid models can be used to integrate and compare the two approaches. For example, one can use toxicokinetic models to estimate serum levels from exposure estimates (or vice versa), comparing the estimated and measured serum levels to determine how much of the total exposure has been captured (Trudel 2008; Thompson 2010; Haug 2011; Lorber 2011; Dassuncao 2018; Hu 2019).
4.2. Toxicokinetic models
Simplified one-compartment toxicokinetic (TK) models include three main parameters for each PFAS considered: absorption efficiency, elimination half-life, and volume of distribution (Trudel 2008; Thompson 2010; Lorber 2011; Hu 2019). Limited data from human studies are available to characterize the absorption efficiency and the volume of distribution in TK models. Instead these have been estimated by extrapolating animal data, which can be problematic. Reliable PFAS elimination half-lives needed for one-compartment TK modeling still only exist for four PFAS: PFOS, PFOA, PFNA, PFHxS. Median elimination half-lives for these four PFAS range between 2.1 and 8.5 years (Hu 2019).
Many exposure assessments rely on relatively simple one-compartment TK models to convert external doses to internal concentrations or the reverse. This is often done by assuming steady-state. For individuals with changing metabolism and elimination processes for PFAS such as infants, children, pregnant or lactating women, the steady state assumption may be invalid depending on half-life of a given PFAS and recent shifts in exposure. For example, time dependent exposure assessments are needed for individuals who live in proximity to contaminated sites where exposure concentrations have changed either due to releases or site cleanup (Balk et al. 2019; Shin et al. 2011a; Verner et al. 2016). Given the long human half-lives of PFAS, constant exposures over years to decades (3-4 half-lives) are needed to reach approximate steady state.
One compartment TK models can provide reasonable estimates of serum concentrations. Additional modeling is needed to estimate tissue specific PFAS concentrations (e.g., brain, liver, kidney) that may be relevant for interpreting different health outcomes associated with exposures. Empirical TK data needed to describe PFAS partitioning among tissues, dermal absorption, facilitated transport and elimination half-lives are still limited or non-existent for many PFAS and represent an important research need. As the focus of PFAS research shifts toward issues associated with emerging compounds, additional data for parameterizing TK models represent a critical research need.
4.3. Biomonitoring
PFAS concentrations have been measured in plasma, serum, and whole blood (Olsen 2003a; Olsen 2003b; Kannan 2004; Olsen 2005; Kärrman 2006; Ehresman 2007; Olsen 2007). Non-invasive measurement for internal exposure, for example dried blood spots, hair and nails, are not typically reported for PFAS but have been measured in some studies and show promise for future work (Kim 2017; Jian 2018; Wang 2018; Poothong 2019). Measured PFAS concentrations in human plasma and serum vary across different populations, from single- or double-digit μg/L levels in the general population (Kannan 2004; Olsen 2005; Kärrman 2006) to hundreds or even thousands of μg/L in occupationally exposed workers and residents near contaminated sites (Olsen 2003a; Olsen 2003b; Olsen 2007). Observed concentrations also vary by geography, PFAS types, sex, and age.
The US National Health and Nutrition Examination Survey (NHANES) has included serum PFAS monitoring since 1999, which can now be used to describe nationally representative temporal changes in exposures. However, the limited sample size (about 2k per cycle) means only a limited number of US counties were included each year. Developing countries have even fewer serum measurements. Most published studies are focused on communities that live near a manufacturing plant, or individuals who have come to the hospital to seek care for another condition (usually pregnancy) and thus are not statistically extrapolatable to the general population or for specific demographic groups (Jiang 2014; Ramli 2020). Future PFAS researchers may benefit from establishing collaborations with existing representative population surveys such as the Demographics and Health Survey for developing countries (United States Agency for International Development), and the Multiple Indicator Cluster Surveys (United Nations Children’s Fund), which includes environmental health monitoring metrics such as PFAS exposure (Boerma 2001; Corsi 2012; Fabic 2012).
In addition to representative sampling there are a number of challenges in using biomonitoring data for assessing exposure to PFAS. Binding affinities of different PFAS vary (Ng 2013), which affects how some PFAS partition between serum and whole blood (Poothong 2017). PFAS concentrations in human blood/serum are the result of external exposures to a much larger mixture of compounds, including many PFAS precursors, but only a small subset of PFAS are routinely targeted in studies (Vestergren 2008; Gebbink 2015). For example, neutral volatile atmospheric precursors such as FTOH and perfluoroalkyl sulfonamides (FASA) can biotransform in humans and wildlife contributing to overall exposures of the terminal end products such as PFOS and PFOA. Without considering precursors, PFAS exposures and risks are likely underestimated. However, directly quantifying exposures to precursors is difficult because of in vivo biotransformation and the large number of unidentified compounds (Benskin 2009; Ross 2012; Yeung 2016).
New analytical methods that measure TF provide insights into the amount of PFAS that are not accounted for with targeted approaches (see Section 3). Studies with these newer analytical tools have shown that routinely monitored PFAS often comprise only a small fraction (<50%) of total PFAS in human exposure media such as textiles (Robel 2017), food packaging (Schultes 2019), and drinking water (Hu 2019). EOF measurements in sediments and river water have shown similar results in the environment (Yeung 2013b; Koch 2019). In another study focusing on the liver of marine mammals, targeted PFAS were observed to account for almost all EOF in tissues from Greenland, Iceland, and Sweden but only 30-75% of the EOF in tissues from the eastern US (Spaan 2020). An integrated approach using a combination of targeted, non-targeted, EOF, and TF analytical techniques showed that while identifiable PFAS have decreased over the past two decades (Figure 2b), the percent of unidentifiable EOF has increased (Miaz 2020). These studies reinforce the importance of accounting for precursors when assessing biological exposures to PFAS. Additional research is needed to establish the link between precursor levels in exposure media (soil, dust, air, water, food, consumer products) and their overall contributions to biological exposures. The role of fluorinated polymers as a source of PFAS exposure to humans and wildlife continues to elude researchers due to the challenge of characterizing the polymers and isolating the contribution of PFAA from polymers versus the residual unbound PFAS content in polymers (Rankin 2014; Rankin 2015; Washington 2015; Li 2017). In addition, questions about the presence of fluoropolymers in microplastics that are globally prevalent remain. Recent studies have reported the release of fluorinated polymers containing microplastic fibers during cleaning of outdoor jackets and the capacity of microplastics to sorb PFAS under environmental conditions (Schellenberger 2019b; Llorca 2020).
4.4. Estimating dietary PFAS exposures
Dietary exposure to PFAS has primarily been estimated using the exposure factor approach by measuring PFAS concentrations in various foods and multiplying by food consumption rates for a given population or demographic group. Food consumption rates vary by age, geographically and culturally but typical exposure factors are relatively well known (US EPA 2011). PFAS concentrations have been reported in milk, meat, vegetables, fruits, and bread in the sub- to low ng/g range, while the majority of food samples analyzed contained PFAS below detection limits (Ericson 2007; Tittlemier 2007). In homogenized whole meals, a similar concentration range was reported, although the maximum concentration observed was 118 ng PFOA per gram of fresh food (Fromme 2007). As discussed in Section 5, a number of studies have estimated dietary exposure to PFAS using the exposure factor approach, almost all European. However, the US Food and Drug Agency is undertaking a study of PFAS in food (de Jager 2019).
One challenge in extrapolating measured PFAS concentrations in foods to estimated exposures is that random sampling of foods in a statistically representative manner is generally unavailable. Sampling of PFAS concentrations in consumed food or individual ingredients can result in different exposure estimates because food contact materials (FCM) and cooking potentially alter PFAS concentrations. Early studies tended to focus on PFOA and PFOS, while later studies have started to report concentrations of other PFAS and precursors. New data on TOF and EOF would be useful.
Several studies have used the epidemiologic approach to associate serum PFAS concentrations with different food sources. For example, one study found associations between serum concentrations of several PFAS and fish/seafood consumption in Norway (Haug 2010). In a cohort of 941 American adults with blood sampled between 1996 and 1999, investigators reported positive associations of several PFAS in plasma with consumption of “meat/fish/shellfish (especially fried fish, and excluding omega-3 fatty acid rich fish), low-fiber and high-fat bread/cereal/rice/pasta, and coffee/tea,” but inverse associations with some other foods such as vegetables and fruit (Lin 2020). Another study reported associations between serum PFOA and PFNA and fast food consumption and take-out coffee in the USA using data from the US National Health and Nutrition Examination Survey (NHANES), suggesting a role for FCM (Nelson 2010). A different study also based on NHANES data reported associations between serum PFAS concentrations and fast food restaurant meals as well as microwave popcorn (Susmann 2019). A small (n=61) but remarkable Norwegian study examined food consumption using several approaches but report few significant correlations with PFAS in blood (Poothong 2020). While diet is likely an important route of exposure for many people, it is difficult to estimate and thus uncertain. Statistically representative surveys of dietary exposure to PFAS are therefore needed as well as better data on the sources of PFAS found in food and links to those present in FCM.
4.5. Indoor exposure via inhalation and dust ingestion
Most North Americans spend approximately 90% of their time in indoor environments. PFAS are used extensively in products designed for indoor use such as stain resistant coatings for carpet and furniture. They are found in indoor air and dust, although so far the connection to specific indoor sources has received little attention (Beesoon 2012). Thus, there is the potential for indoor exposures to PFAS via inhalation, ingestion of dust and dermally.
Investigation of indoor exposure to PFAS is more complicated than for many groups of compounds (e.g., polybrominated diphenyl ethers: PBDEs) due to the vast variety of physical-chemical properties for PFAS and the existence of precursors and polymers. Semivolatile organic compounds (SVOCs) will tend to partition between the vapor phase, suspended particulate, dust, and indoor surfaces (including skin and clothing), depending in part on their octanol/air partition coefficients (Weschler 2008). Some PFAS such as FTOH and FOSE are relatively volatile and are found in the vapor phase indoors. Other PFAS such as PFOA and PFOS are found at high concentrations in dust. There is little information available about the indoor presence and fate of fluorinated polymers (e.g., side-chain fluoropolymers used in some stain resistance formulations). In part this is due to analytical difficulties (Rankin 2015; Letcher 2020). They may be released from products to dust via physical abrasion—as has been shown for other low volatility compounds (Webster 2009)—or potentially gradually breakdown over time, releasing the fluorinated side chains as may occur in outdoor environments (Washington 2015; Letcher 2020). An important question is the amount of unidentified organic fluorine in air and dust. For example, TF analysis in conjunction with HRMS may be a useful first step in examining polymers or other unmeasured compounds in dust.
PFAS concentrations in indoor environments are usually measured through filtration or adsorption to a solid phase (filters or sorbents) using either active air pumping or passive samples, followed by extraction of the solid phase to recover PFAS for quantification (Martin 2002; Shoeib 2008; Padilla-Sánchez 2017; Guo 2018; Rauert 2018; Wong 2018; Yao 2018). In indoor air, concentrations can be an order of magnitude higher (ng/m3 levels) than outdoor environments (Shoeib 2004; Shoeib 2005; Yao 2018). Indoor air sampling has tended to focus on the more volatile compounds such as the FTOHs and FASA.
PFAS concentrations in dust can be very high and have been reported at the μg/g level (Moriwaki 2003; Kubwabo 2005; Shoeib 2005; Strynar 2008; Eriksson 2015a; Eriksson 2015b; Lankova 2015; Winkens 2018). Dust sampling has found PFAA and other PFAS, including relatively large amounts of polyfluorinated phosphate esters (diPAP) (De Silva 2012; Eriksson 2015a; Makey 2017). Methods for dust sampling and processing—e.g., where and how to sample dust in homes, sieving size—are less standardized than for air. Bigger issues are the variety of indoor environments—home, workplace, childcare facilities, vehicles, etc. (with homes being a main focus)—and the difficulty of doing representative sampling (Goosey 2012; Fraser 2013; Zheng 2020). Unlike the extraordinary efforts that have been made for representative biomonitoring (e.g., NHANES in the USA), most indoor sampling is convenience sampling that may not be representative of exposures across the general population. Exposure factors for inhalation are well known, but exposure factors for dust ingestion are quite uncertain (US EPA 2011). As a result, inhalation estimates are likely more reliable than those for dust ingestion. An additional source of uncertainty is the amount of conversion of precursors (e.g., FTOH, FASA) into terminal end products (e.g., PFCA, PFSA) found in serum/blood (Poothong 2020). This issue is particularly important when trying to compare inhalation with dust ingestion, or indoor routes of exposure to diet and other sources.
Two North American studies have found associations between serum levels of some PFAA and the precursors FTOH and FASA, respectively, in indoor air (Fraser 2012; Fraser 2013; Makey 2017), but little association with PFAS concentrations in dust. A Norwegian study found associations between certain PFAS in whole blood and indoor air and/or dust (Poothong 2020). The latter study also estimated that diet was more important than indoor exposure on average but that inhalation and dust ingestion dominated for some study participants, particularly the people with the highest blood concentrations. Some studies have found that serum PFAS concentrations increase with socioeconomic status indicating that indoor and dietary exposure may be partly correlated due to purchasing decisions (Nelson 2012). Relatively few studies have used epidemiologic techniques to examine air and dust or other pathways simultaneously (Haug 2011; Fraser 2013; Makey 2017; Poothong 2020).
In summary, some epidemiologic evidence suggests indoor exposure is important enough to be empirically associated with serum/blood levels, and may be the dominant exposure route for some people (Fraser 2013; Makey 2017; Poothong 2020). More research is needed on the differences in indoor exposure patterns between people as well as differences between countries and time trends. Relatively little research has been conducted on the connection between indoor levels and putative sources, total organic fluorine, the contribution of fluorine-containing polymers, and exposure of children.
4.6. Outdoor air exposures
Reported PFAS concentrations in outdoor air range from non-detectable or sub pg/m3 levels to hundreds of pg/m3 (Martin 2002; Stock 2004; Barber 2007; Jahnke 2007; Fromme 2009; Rauert 2018; Wong 2018). PFAS concentrations in urban areas are typically higher than in rural areas (Martin 2002; Stock 2004; Barber 2007; Jahnke 2007). A few studies of general populations report that outdoor air PFAS concentrations are one to two orders of magnitude lower than indoors (Shoeib 2011), presumably due to indoor sources. For such populations, we expect inhalation exposure indoors to exceed outdoor exposures.
Little is currently known about communities with major atmospheric point sources. In Parkersburg, West Virginia, USA, local drinking water sources were contaminated primarily by air emissions of PFOA emitted by the Washington Works facility followed by deposition and groundwater transport (Davis 2007). In Fayetteville, NC, precipitation monitoring to assess the deposition of GenX via air emissions has been ongoing since 2018. Communities in Hoosick Falls, NY, Bennington, VT and Merrimack, NH have had varying extent and types of monitoring conducted in response to concerns or documentation of groundwater/drinking water contamination. An important question in these scenarios is the relative importance of exposure via inhalation vs. water ingestion. Using a sophisticated fate and transport model, researchers estimated PFOA concentrations in ambient air and water in the communities surrounding the Washington Works facility in West Virginia, USA over time (Shin 2011b; Shin 2011c). Their results compared well with measured water values and indoor air was assumed to be 10% of outdoor air due to partial infiltration. Transport of PFAS in air is faster than in soil and groundwater. Thus, for people living in areas with contaminated air, estimated inhalation exposure exceeded that via water ingestion in the early time period but was less than water ingestion later on (Shin 2011b; Shin 2011c). These results suggest that inhalation exposure to PFAS might exceed water ingestion in areas with continuing air emissions but mitigation of drinking water (e.g., via filtration).
4.7. Dermal exposures to PFAS
Dermal exposure to PFAS can result from contact with house dust, PCP, and other consumer products. It has received relatively little attention, with two exceptions: dust and, more recently, cosmetics/personal care products (PCP). For example, one study estimated dermal exposure of children to PFAS in dust in childcare settings using measured dust concentrations and an exposure factor for the amount of dust adhering to skin (Zheng 2020). Poothong et al (2020) used an alternative method for skin contact: measuring PFAS on hands using handwipes. Both then used the fraction dermal absorption approach, a model commonly used in risk assessment (Kissel 2011).
One study examined selected PCP with product labeling indicating PFAS ingredients such as polyfluorinated phosphate esters (PAPs) or other fluorinated compounds and reported PFCAs in the ug/g range but did not determine the levels of PAPs (Fujii 2013). Another study that measured 39 PFAS, as well as EOF and total fluorine, detected PAPs at up to 470 ug/g in cosmetic products (Schultes 2018). The measured PFAA accounted for only a small fraction of the EOF and TF, implying the presence of unidentified compounds, potentially including polymers or inorganic fluorine. Skin contact with PCP can depend on a number of factors including the amount of the product applied per unit area (loading), the surface area of exposed skin, the duration of use and the frequency of washing the skin. Prior work has estimated dermal absorption of PFOA in foundation cosmetics, leading to an absorbed dose through dermal exposure of <0.006-3.1 ng/kg/day, with the high end exceeding dietary exposure in Sweden (Schultes 2018). This dermal exposure estimate for PFOA does not include indirect exposure via PAP .
5. Ranking sources of PFAS exposure for human populations
A major question discussed by the exposure assessment panel at the SETAC PFAS topic meeting was whether it is possible to rank the relative importance of PFAS exposure sources for different human populations at this time. Researchers and decision-makers seek to understand the relative contributions of different exposure pathways to human exposure in order to inform risk assessments and prioritize interventions. The panel concluded that there were many gaps remaining in this area, especially for general populations that have diverse exposure pathways for PFAS. A summary of present understanding is provided below.
5.1. Occupational exposures
Occupational health effects associated with human PFAS exposures have been reported for individuals who worked in fluorochemical production plants in the US (Olsen 1999), Italy (Girardi 2019) and China (Fu 2016). Occupational exposure to PFAS occurs mainly through inhalation and dermal contact (Franko 2012). Inhalation exposure to PFAS in a workplace situation can be important due to sublimation into the gaseous phase of volatile manufacturing intermediates that are hydrolyzed to end product PFAS (Kaiser 2010). Firefighters working and training with AFFF did not show a relationship between internal exposure of PFOS and the self-reported frequency of direct skin contact with AFFF, indicating that dermal contact may not be an important pathway of exposure (Rotander 2015a). However, they had elevated blood levels of PFOS and PFHxS, which is consistent with the composition of legacy electrochemical fluorination (ECF) AFFF. Elevated levels of PFNA and other long-chain PFCAs have been connected to occupational exposure for firefighters (Laitinen 2014; Trowbridge 2020). Professional ski wax technicians showed elevated blood levels of PFCAs, which was associated with number of working years. Ski wax contains both precursor semifluorinated n-alkanes and PFCA (Plassmann 2013; Carlson 2020) and is often applied by using heat (130-220°C) which results in gaseous compounds and particles. An exposure study with internal dose measurement and personal and ambient air including airborne particles showed that inhalation of both precursor compounds and terminal end products contributed to the internal dose (Nilsson 2010).
5.2. Communities near contaminated sites
PFAS have been detected in both surface water and groundwater sources near facilities that manufacture or process fluorochemicals. Concentrations in aqueous matrices in areas without point sources generally occur below detection limits or at the sub ng/L (parts per trillion) level but are commonly detected at the μg/L (parts per billion) level or higher in contaminated areas (Chen 2017b; Cai 2018; Pan 2018; Park 2018; Janda 2019). These include sites with historical releases of AFFF, such as military bases and airports, and wastewater treatment plants that have received industrial wastes (Herrick 2017; Worley 2017; Barton 2020). These sites have been associated with contamination of drinking water across the U.S. with at least six million people estimated to have been exposed to levels above EPA health advisories for PFOS and PFOA of 70 ng/L (Hu 2016). The total number of people exposed to PFAS in the U.S. is greater because this estimate does not include populations exposed through small drinking water systems or private wells or exposure to other PFAS.
Many biomonitoring studies have shown PFAS in drinking water near contaminated sites have led to population blood levels that are much greater than background levels (Daly 2018; Ingelido 2018; Li 2018). In such areas, serum levels are associated with concentrations in drinking water (Hoffman 2011). Drinking water has been estimated to contribute up to 75% of exposures near contaminated sites (Vestergren 2009). The U.S. Agency for Toxics Substances and Disease Registry (ATSDR) is currently conducting biological monitoring at over 15 sites with contaminated drinking water across the country following comparable methods for collecting blood and administering exposure questionnaires. This work will generate further information about the contributions of drinking water exposures across a diverse set of communities near contaminated sites.
Production and use of the most common legacy PFAS (PFOS, PFOA, PFHxS) have decreased in the U.S. but an increasing number of sites contaminated with these compounds have been identified over the past decade. Data compiled by the Northeastern University’s Social Science Environmental Health Research Institute (SSEHRI) show that as of May 2020 there were 393 known contaminated public drinking water systems in the U.S. (SSEHRI 2020). As background levels of legacy PFAS decrease, the relative contribution of their exposure from drinking water sources near these contaminated sites will increase (Bao 2017). Furthermore, replacement compounds, such as PFEA, including GenX, have already been detected in drinking water near manufacturing facilities (Hopkins 2018). Newer methods that measure EOF and TF are beginning to be used on drinking water with the goal of quantifying the exposure of unidentified PFAS (Hu 2019). However, the large-scale implications of changes in production have yet to be fully understood.
5.3. General population PFAS exposures
The relative importance of different PFAS exposure sources varies dramatically across general populations with diverse PFAS exposure sources. Table 2 summarizes some prior literature estimates of source contributions to overall PFAA exposures for adult populations without occupational exposure and not living in close proximity to point sources of PFAS contamination. Large variability in the relative importance of different exposure sources across studies reflects variable concentrations in environmental media and differing assumptions regarding exposure sources, frequencies, duration, and consideration of precursors.
Table 2.
Literature estimates of source contributions (%) to adult exposures to PFASa
| PFAS | Carbon length |
Exposure Mediumb | Exposure Routeb | Study Location |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Dust | Water | Consumer goods |
Inhalation | Dermal | Indirect | Ref. | |||
| PFBA | 4 | 4 | 96 | NA | c | |||||
| PFHxA | 6 | 38 | 4 | 38 | 8 | 12 | NA | c | ||
| PFHxA | 6 | 87 | 4 | 2 | Norway | d | ||||
| PFHxS | 6 | 57 | 38 | 5 | Finland | e | ||||
| PFHxS | 6 | 94 | 1 | Norway | d | |||||
| PFHpA | 7 | 93 | 1 | Norway | d | |||||
| PFHpS | 7 | 100 | Norway | d | ||||||
| PFOA | 8 | 16 | 11 | 58 | 14 | NA & EU | f | |||
| PFOA | 8 | 85 | 6 | 1 | 3 | 4 | Germany Japan | g | ||
| PFOA | 8 | 77 | 8 | 11 | 4 | Norway | h | |||
| PFOA | 8 | 66 | 9 | 24 | <1 | <1 | US | i | ||
| PFOA | 8 | 41 | 37 | 22 | Korea | j | ||||
| PFOA | 8 | 99 | <1 | China | k | |||||
| PFOA | 8 | 47 | 8 | 12 | 6 | 27 | NA | c | ||
| PFOA | 8 | 95 | <2.5 | <2.5 | Finland | e | ||||
| PFOA | 8 | 89 | 3 | 2 | Norway | d | ||||
| PFOA | 8 | 91 | 3 | 5 | Ireland | l | ||||
| PFOS | 8 | 66 | 10 | 7 | 2 | 16 | NA | c | ||
| PFOS | 8 | 72 | 6 | 22 | <1 | <1 | US | m | ||
| PFOS | 8 | 96 | 1 | 1 | 2 | Norway | h | |||
| PFOS | 8 | 81 | 15 | 4 | NA & EU | f | ||||
| PFOS | 8 | 93 | 4 | 3 | Korea | j | ||||
| PFOS | 8 | 100 | <1 | China | k | |||||
| PFOS | 8 | 95 | <2.5 | <2.5 | Finland | e | ||||
| PFOS | 8 | 75 | 3 | Norway | d | |||||
| PFOS | 8 | 100 | Ireland | l | ||||||
| PFOPA | 8 | 100 | Norway | d | ||||||
| PFNA | 9 | 79 | 5 | 1 | Norway | d | ||||
| PFDA | 10 | 51 | 2 | 4 | 15 | 28 | NA | c | ||
| PFDA | 10 | 78 | 1 | 2 | Norway | d | ||||
| PFDS | 10 | 89 | 4 | Norway | d | |||||
| PFUnDA | 11 | 61 | 4 | 1 | Norway | d | ||||
| PFDoDA | 12 | 86 | 2 | 2 | 4 | 5 | NA | c | ||
| PFDoDA | 12 | 48 | 15 | Norway | d | |||||
| PFTrDA | 13 | 89 | 1 | Norway | d | |||||
NA = North America; EU = Europe.
Adapted from (Sunderland 2019), and updated with more recent publications.
Where available, central tendency values are presented.
Data from (Gebbink 2015). Data shown here are based on the intermediate exposure scenario in Fig. 3 in their manuscript.
Data from (Poothong 2020)
Data from (Balk 2019); Values represent modeled exposures for children at 10.5 years of age.
Data from (Trudel 2008). Values shown here are based on the high exposure scenarios from Figs. 2 and 5.
Data from (Vestergren 2009). Values shown here are for the background population exposure from Figure 4a in their manuscript.
Data from (Haug 2011). Values shown here are based on the 50th percentile exposure scenario for women and the mid-range scenario for dust exposure.
Data from (Lorber 2011). Data shown here are based on pathway specific intake estimates for adults.
Data from (Tian 2016). Data shown here are for adult exposures based on Fig. 4 in their manuscript.
Data from (Shan 2016). Data shown here are based on summed estimated daily intakes.
Data from (Harrad 2019).
Data from (Egeghy 2011). Values shown here represent the typical environmental exposure scenario shown in Fig. 3 in their manuscript.
Several studies have shown that the exposure to PFAS in children differs from adults due to behavioral and dietary variability. Breast feeding is known to be an important source of early-life exposure to PFAS (Mogensen 2015; Kang 2016; Papadopoulou 2016). A study from the Faroe Islands showed hand-to-mouth contact with carpeting was an important exposure source for children but not adults based on the contrasting composition of PFAS measured in serum (Hu 2018). The 2020 European Food Safety Authority (EFSA) report found that toddlers/children had a two-fold higher exposure than adults, in part due to maternal exposure (European Food Safety Authority 2020).
There is general agreement that dietary exposure is the major contributor to population exposure for PFOS and PFOA (Table 2), but more limited evidence for PFHxA, PFHpA, PFNA, PFDA, and PFHxS. In 2018, EFSA estimated that the main contributors to adult dietary exposure to PFOS were fish, meat, eggs and products with these ingredients (European Food Safety Authority 2018). The greatest sources of exposure to PFOA were eggs and dairy and products containing these ingredients. The youngest population groups were estimated to have the highest dietary exposure to PFOA and PFOS. In 2020, EFSA expanded its analysis of exposures to PFAS in foods and reported that the sources contributing to PFAS exposure to adults and children were consistent with the findings from 2018. They concluded that PFOA contributed 21%, PFNA 4%, PFHxS 10% and PFOS 66% to the sum of PFAS exposures, based on the median of the mean lower-bound estimates. EFSA was unable to draw meaningful conclusions about the contributions of PFAS from FCM.
Comparing water and serum samples from 1989-90, one study estimated that drinking water contributed about 20% of total exposure of several legacy PFAS in the general U.S. population (Hu et al 2019). There are still large uncertainties related to the fraction of PFAS exposure in the general population that originates from dust, consumer products, inhalation, and other pathways. For example, exposures to PFAS precursors in dust that degrade into terminal PFAAs have not been well-characterized (Balk 2019; de la Torre 2019; Harrad 2019). Table 2 suggests that for the general population, indoor exposures to PFAS through inhalation, dermal contact or incidental ingestion of dust and air contribute less to exposure than dietary ingestion on average, although the balance may be different for subpopulations. Without rigorously conducted exposure studies it is challenging to rank order the most important human exposure pathways and without these data, our ability to design evidence-based exposure intervention strategies will be limited.
6. CURRENT UNDERSTANDING OF PFAS EXPOSURE IN WILDLIFE
6.1. PFAS occurrence and temporal trends in wildlife
Elevated exposures of wildlife to PFAS represent a concern for their health directly and for human populations that consume wildlife (Fair 2019; Guillette 2020). In 2001, the first report on the global occurrence of PFOS in wildlife was released illustrating widespread presence in biological tissues even in remote regions such as the Arctic (Giesy 2001). Concentrations of PFOS and other PFAA have been detected in invertebrates, fish, amphibians, reptiles, birds, and mammals worldwide (Ahrens 2011; Reiner 2015; Penland 2020). Several comprehensive reviews (Houde 2011a; Reiner 2015; Muir 2019) have synthesized data from available biomonitoring studies.
The highest PFAS concentrations in wildlife tend to be associated with proximity to contaminated sites. For example, one of the highest reported fish PFOS concentrations (maximum 9349 ng/g dry weight in whole fish tissue) was from an AFFF-impacted site downstream from Barksdale Air Force Base in Louisiana (Lanza 2017). Many biomonitoring studies have identified elevated exposures to legacy and emerging PFAS as the result of industrial activities (Custer 2012; Custer 2014; Liu 2017; Groffen 2019; Lopez-Antia 2019; Guillette 2020). Legacy PFAS such as PFOS are still abundant at many contaminated sites and novel PFAS are increasingly being detected. For example, one study reported PFOS was the predominant compound in fish (mean 263-348 ng/g wet weight (ww) in muscle) adjacent to a major fluorochemical production facility in Wuhan, China (Zhou 2013). Suspect and non-target screening subsequently revealed a suite of 330 novel fluorinated structures belonging to 10 different chemical classes in fish liver from the same region (Liu 2018). The profile of specific PFAS released at each contaminated site affects the accumulation of different compounds in biota and may also be relevant for determining exposure risks such as near AFFF contaminated regions (Yeung 2013c; Custer 2014; Munoz 2017; Larson 2018; Salice 2018; Langberg 2019; Munoz 2020).
Biological time series data for specific ecosystems suggest variable temporal changes in PFAS across compounds and ecosystems. In the Arctic, there is sustained or increasing (post-2010) PFAA levels in some wildlife (Muir et al. 2019). Following the phase out of the parent chemical to PFOS and its precursors ca. 2000-2002, several studies have noted rapid declines in perfluorooctane sulfonamide (FOSA), an atmospheric precursor to PFOS that is biotransformed by most mammals, but less consistent declines or even increases in PFOS (Smithwick 2006; Ahrens 2009a; Dassuncao 2017; Sun 2019; Schultes 2020). This likely reflects the rapid response of atmospheric concentrations to changes in chemical production but a lagged response of most aquatic ecosystems. Most time series studies have focused on targeted PFAS but recent work has shown that trends in TF indicated by EOF and other methods differ from the legacy compounds (Schultes 2020). Understanding temporal and spatial trends in emerging PFAS compounds in biota is thus an important research need (Spaan 2020).
6.2. PFAS bioaccumulation metrics
The bioaccumulation potential of persistent organic pollutants (POPs) is commonly reported based on several metrics: bioconcentration factor (BCF: the direct uptake of a chemical by an organism from water or air, i.e., BCF = Cfish/Cwater in a controlled laboratory experiment with no dietary intake); biomagnification factor (BMF: the concentration of an organism relative to their diet, i.e., Cfish/Cprey); bioaccumulation factor (BAF: the combined effects of all uptake pathways). BCFs are commonly measured in laboratory experiments, while BMFs and BAFs are typically field-based measurements. The trophic magnification factor (TMF) is an indicatory of dietary biomagnification and is generally established empirically using slope of the relationship between trophic position in a food web based on stable nitrogen isotopes and chemical concentrations in organisms from a field-based food web. A large body of work has established that for neutral, hydrophobic POPs, simple partitioning between lipid and water indicated by their octanol-water partition coefficient (Kow) or octanol-air partition coefficient (Koa) provides a reasonable proxy for bioaccumulation propensity.
By contrast, processes governing the uptake of PFAS into organisms and partitioning across tissues are less well-understood, even for commonly studied PFAA (Figure 4). The pKa of long-chain PFAA are thought to range from less than zero to 1, indicating that they are almost completely ionized at environmentally and biologically relevant pH (Goss 2008a, 2008b). Despite being ionized, PFAS are bioavailable, and long-chain PFAA can accumulate in specific biological media to levels equivalent to lipid accumulation of neutral POPs. Given these observations, the question emerges of whether and how existing metrics for chemical accumulation in wildlife can be used to describe diverse PFAS. This formidable data gap hampers efforts to develop mechanistic models for exposure and risk assessment of PFAS, and stands as a major limitation to ecological exposure assessment of PFAS.
Figure 4.
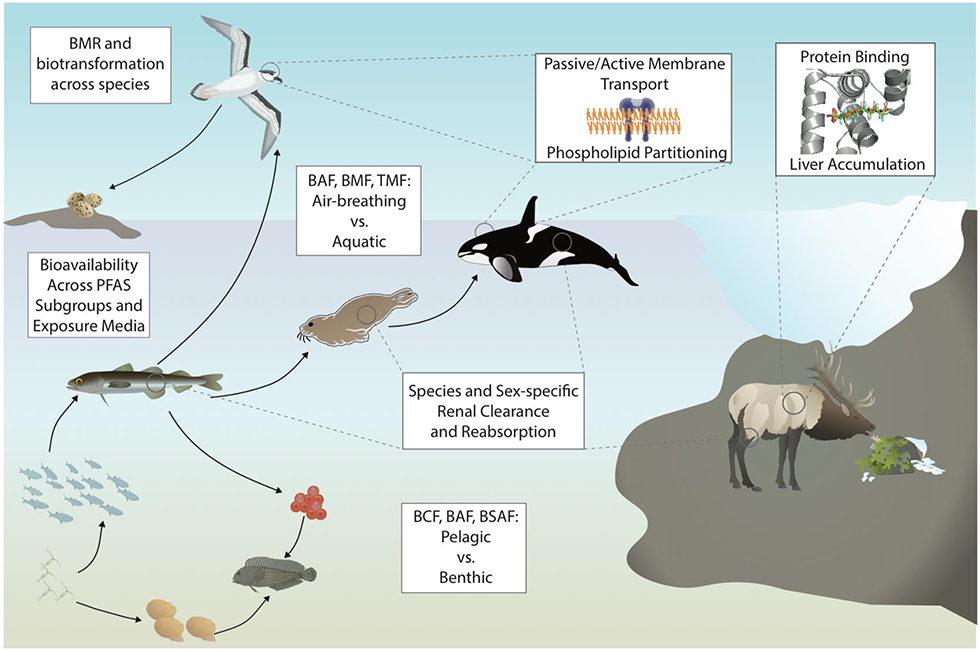
Key bioaccumulation processes, metrics and gaps associated with PFAS in wildlife. BMR = basal metabolic rate; BAF = bioaccumulation factor; BMF = biomagnification factor; TMF = trophic magnification factor; BCF = bioconcentration factor; BSAF = biota-sediment accumulation factor.
A synthesis of 513 laboratory-based and 931 field-based measurements indicates long-chain PFCA with 12-14 carbon-chain length generally exhibit the highest bioaccumulation potential, with whole-body BCF values ranging between 18,000-40,000 L/kg (Gobas 2020). Laboratory-based whole-body BCFs of PFCA with 8 to 11 carbon-chain lengths are generally much lower (BCF range: 4.0-4,900 L/kg). Similarly, PFOS exhibits relatively low laboratory-based whole-body BCFs, generally in the range of 100 to 1,000 L/kg. Field-based BAFs are generally in agreement with the laboratory-derived BCF values, but in some cases are somewhat higher, reflecting dietary accumulation. Generally, BCFs and BAFs (L/kg) of individual PFAAs in plankton, aquatic gill-ventilating invertebrates, and fish increase with increasing perfluoroalkyl chain length and hydrophobicity (Condor 2008), though exceptions have been identified (Munoz 2017; Zhang 2019).
Avian and marine mammalian food webs exhibit the highest reported TMFs for PFAA (Kelly 2009; Tomy 2009). For example, the TMF for PFOS in these relatively long food webs containing air-breathing wildlife (e.g., marine birds and mammals) is approximately 20. TMFs in aquatic piscivorous food webs tend to be much lower. For example, TMFs of PFOS in the Lake Ontario aquatic piscivorous food webs range between 1.9 to 5.9 (Martin 2004; Houde 2008). Other studies have reported negligible biomagnification of PFOS in aquatic piscivorous food webs, with TMFs not substantially different than one (Loi 2011; Penland 2020). This behavior mirrors previous observations of food web-specific biomagnification of low KOW-high KOA moderately hydrophobic organic chemicals (Kelly 2007). In particular, PFOS and several other PFAS of concern, which are likewise moderately hydrophobic and poorly metabolizable substances, may not biomagnify extensively in aquatic food webs due to efficient respiratory elimination to water via gills. Conversely, these substances can biomagnify to a high degree in food webs containing air-breathing animals because elimination of these substances via lung-air exchange is negligible. More data are needed to refine these hypotheses and address variability across current data sets. Observed variability in TMFs for PFAA is likely due to selection of species, tissues, concentration normalization techniques, as well as the influence of site-specific conditions, life history stage, trophic condition of sampled individuals, and tissues and techniques used for stable isotope analyses.
The contribution of PFAA precursors to field-based measurements of BAFs represents a major gap in understanding of PFAS bioaccumulation. For example, one study noted higher than expected accumulation of PFCA with five and six carbons in marine plankton from the Northwestern Atlantic and posited this reflects the accumulation of degraded precursor compounds (Zhang 2019). Another study that included liver tissues from marine mammals from the same region found a large fraction (30-75%) of unidentified organofluorine (Spaan 2020). Shrimp from a subtropical food-web in Hong Kong were similarly noted to have a high fraction of unknown fluorinated compounds (Loi 2011). Some precursor compounds behave more similarly to traditional POPs and may thus have enhanced bioaccumulation propensity (Dassuncao 2017). Additional data on bioaccumulation potential and health risks associated with unidentified organofluorine are thus needed, particularly as chemical manufacturing has shifted away from the legacy PFAS typically detected in biota (Section 2).
6.3. Modifications to bioaccumulation metrics for POPs needed for PFAS
Table 3 illustrates some potential modifications to key bioaccumulation metrics that better reflect the behavior of PFAS in biological systems. In deciding the appropriate metric to use and how it is to be defined (in what tissue, with what type of normalization) a key guiding question is: “For what purpose?” If a TMF is being calculated to understand the exposure of a predator organism to PFAS in its prey, it makes the most sense to use the concentration in the portion of the prey consumed by that organism to define the denominator. For example, one study calculated the BMF for PFAA in polar bears using the ratio of polar bear liver to ringed seal blubber concentrations (Boisvert 2019). Similarly, when considering BCFs in sport fish, PFAS concentrations in muscle tissue may be most relevant in establishing dietary guidelines for humans, but liver-specific BCFs may provide better insight for potential health consequences to the fish population itself.
Table 3.
Modifications to persistent organic pollutant bioaccumulation metrics for PFAS.
| Metric | Traditional Indicator | PFAS-Specific Recommendation |
|---|---|---|
| KOW, KOA | Used as surrogates for equilibrium partitioning of neutral organic chemicals to lipid tissues of aquatic and air-breathing organisms. | DOW: Octanol-water distribution ratio (takes degree of ionization into account); DMW: Membrane-water partition ratio; Chemical activity ratios; KPW: Protein-water partition coefficient; KA, KD: Equilibrium association and dissociation constants for specific proteins (e.g. albumin, LFABP) Key gap: relevant metric for air-breathing organisms. |
| BCF: bioconcentration factor (waterborne or airborne exposure only) BAF: bioaccumulation factor (waterborne/airborne and/or dietary exposure) |
Concentration in organism (whole body, lipid-normalized)/concentration in water (freely dissolved) | Concentration (chemical activity)1 in serum/ concentration in water; Concentration in liver/ concentration in water; Concentration in organism (whole body)/concentration in water. Key gap: (1) Selecting appropriate tissue to represent accumulation in organism. (2) Accounting for contributions of precursors to field based BAFs. |
| BMF: biomagnification factor TMF: trophic magnification factor |
Concentration in predator (whole body, lipid-normalized)/concentration in prey (whole body, lipid-normalized) | Concentration in predator liver /concentration in prey liver; Concentration in predator (whole body) / concentration in prey (whole body); Key gaps: Selecting appropriate predator and prey tissues across food webs. |
Emerging approach: activity-based metrics. See section 6.4.
Refining metrics to better capture the behavior of PFAS in biological systems requires a better understanding of how PFAS are transferred from external exposure sources to the internal environment, and how, once internalized, they distribute to different tissues and are eliminated (Figure 5). Presently there is little consensus on the tissue type (e.g., liver, kidney, muscle) that best represents PFAS bioaccumulation in wildlife and whether normalization of concentrations to protein or phospholipid is needed. For example, using tissue-specific measurements in fish, there were large variations in blood BCF and blood BAF compared to the analogous whole-body BAF and BCF whereas liver-based BCF and BAF were in the same order of magnitude for the whole body accumulation parameters (Martin 2003; Shi 2018).
Figure 5.
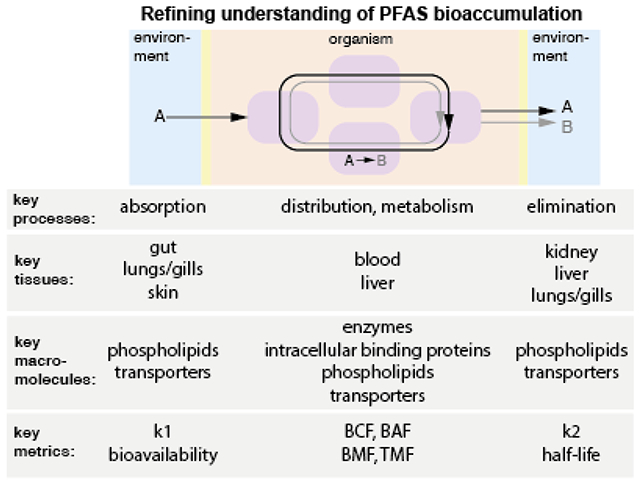
Key elements for predicting the relationship between external exposure and internal dose of wildlife and human to PFAS. The internal distribution and dose are driven by the balance of absorption, distribution, metabolism, and elimination (ADME). For PFAS, unlike for neutral organic chemicals, internal distribution is substantially influenced by protein binding and transporter uptake and efflux, leading to accumulation in the liver and blood and species and sex-specific elimination half-lives.
PFAA are acidic compounds with low pKa, which means they are often associated with proteins (e.g., serum albumin) and phospholipids rather than storage lipids (Armitage 2012; Armitage 2013; Ng 2013, 2014; Chen 2016; Armitage 2017; Dassuncao 2019). As a result, PFAA accumulate primarily in the blood, liver, kidney, and brain of organisms rather than adipose tissue (Martin 2003; Ahrens 2009b; Borg 2010; Kowalczyk 2013; Dassuncao 2019). The affinity of different PFAA for proteins varies widely, suggesting binding-site-specific interactions and facilitated transport of some compounds. PFAA interact with protein molecules through a combination of polar, non-polar, and electrostatic interactions and not through covalent binding. Hence, sorption and desorption are dependent on thermodynamic gradients (i.e., chemical activity differences) in biological systems (Bischel 2010). Distribution of PFAA to structural (muscle) proteins is generally low, while binding to specific proteins (e.g. serum albumin, liver fatty acid binding protein) leads to tissue-specific accumulation patterns (Luebker 2002; Jones 2003; Martin 2003; Ahrens 2009b; Ng 2014). Phospholipids also play an important role in tissue specific partitioning (Armitage 2012). This is particularly true for the long chain PFAS and brain tissue, which is very high in phospholipid content (Dassuncao 2019). Thus, more refined approaches to account for tissue-specific uptake in bioaccumulation assessments are needed.
6.4. PFAS bioaccumulation modeling
Mechanistic bioaccumulation (i.e., toxicokinetic) models developed for neutral lipophilic contaminants such as polychlorinated biphenyls (PCBs) and organochlorine pesticides have been widely used by academics, risk assessment professionals, and regulatory authorities (Arnot 2004). Applications of these models include substance prioritization, screening-level risk assessments, development of environmental quality guidelines, total maximum daily loads, and other purposes. They use information on the physical-chemical properties of the compound of interest, as well as environmental and biological properties of the site and organisms. The success of these existing mechanistic approaches relies on good data quality on the partitioning properties of neutral compounds in biological media and tissues. Thus, a major challenge for PFAS is suitable data on their partitioning in biological compartments.
One available approach for modeling fish bioaccumulation of ionizable organic compounds (IOCs, including PFAA) takes into account the role of thermodynamic gradients in tissue partitioning for PFAS (Armitage 2013). Specifically, the model is based on pH-dependent distribution ratios, including the chemical’s octanol-water distribution ratio (DOW) and membrane-water distribution ratio (DMW), as well as the estimated distribution ratio between non-lipid organic matter (NLOM) and water. It provides a simple partitioning-based equation to predict steady-state concentrations of PFAA and other IOCs in aquatic organisms.
Strong relationships between empirically derived bioaccumulation metrics (BCF, BAF, TMF) and distribution ratios for protein-water (DPW) and membrane-water (DMW) of individual PFAA have been demonstrated for some ecosystems (Kelly 2009; Chen 2016). The relatively high degree of bioaccumulation of long-chain PFCA with 12-14 carbons in fish is likely attributable to high DMW and DPW (estimated values of 105 to 106 DMW and 104 to 106 DPW). Thus, DPW and DMW are two key parameters that may be useful for predicting PFAS bioaccumulation potential (Table 3).
The NLOM component of the IOC model represents endogenous protein (Armitage 2013). While it performs well for many IOCs, it tends to underestimate the bioaccumulation capacity of some PFAA. This is likely due to the complex association of PFAS with proteins. Thus, a key to parameterizing this model is the difference in distribution ratios between neutral and ionized forms of the specific PFAS congener, which may require challenging experimental determinations. Further, different protein types can exhibit different sorptive capacities for IOCs (Henneberger 2016). For some compounds, protein-water distribution ratios (Dpw) in plasma protein (e.g., albumin) can be orders of magnitude greater than those in structural proteins (e.g., muscle protein). Thus, application of simple equilibrium partitioning-based models may require utilizing a series of distribution coefficients for different proteins (e.g., transporter protein-water distribution ratios (DTP-W) and structural protein-water distribution ratios (DSP-W). This approach was used to assess tissue-specific bioaccumulation of PFAA and other IOCs in laboratory exposed fish (Chen 2016, 2017a).
A chemical activity-based approach to ecological risk assessment bridges some gaps between traditional empirical modeling efforts and mechanistic models (Gobas 2017; Gobas 2018). This approach was used to assess bioaccumulation and exposure risks of several PFAS in wildlife at AFFF-impacted sites (Gobas 2020). The chemical activities of PFOS and other PFAA indicated that these compounds tend to primarily biomagnify in food webs composed of air-breathing wildlife (birds, mammals, terrestrial reptiles), compared to those comprising only aquatic organisms. For example, activity-based biomagnification factors in upper trophic wildlife (bird and mammals) were found to range between 10 and 20 and in aquatic organisms were close to 1, indicating negligible biomagnification. An advantage of this approach that is particularly relevant to PFAS is that it can be used effectively for both neutral and ionic substances, including anionic, cationic and zwitterionic compounds. A limitation of this approach is that solubility estimates and hence calculated activities are based on numerous assumptions regarding physicochemical properties, phase partitioning, protein-binding and toxicokinetics.
Beyond simple partitioning-based models for substance screening, more sophisticated approaches may be required for higher resolution modeling. For example, IOC binding to intra- and extracellular protein (serum albumin, liver fatty acid binding protein), as well as membrane-associated organic anion transporters, may act to provide both enhanced sorption capacity and advective transport across biological membranes (Ng 2013). This affects uptake and elimination rates as well as tissue distribution, and helps explain the long elimination half-lives of PFAA in organisms.
Physiologically-based toxicokinetic (PBTK) models incorporating absorption, distribution, metabolism, and excretion (ADME) metrics have been developed to assess toxicokinetics of PFOS and PFOA in various animal models, including fish and mammals (Andersen 2006; Tan 2008; Consoer 2014; Fabrega 2014; Consoer 2016; Fabrega 2016; Cheng 2017; Khazaee 2018). PBTK models are particularly useful for assessing the influence of membrane transporters, and revealing important challenges in equilibrium modeling of PFAS bioaccumulation. For example, laboratory-based evaluations of bioconcentration (e.g. the OECD 305 test), a kinetic BCF can be defined as the ratio of uptake and elimination rates (k1/k2). However, the elimination rate for PFAS can be affected by saturable transporter-mediated renal uptake and clearance, as has been observed in rats (Han 2012) and monkeys (Andersen 2006), so that the k2 rate becomes dose-dependent. Therefore, observations both in the laboratory and in the field (e.g. at highly contaminated sites) may be concentration dependent and thus not representable by first-order kinetics.
6.5. In vitro and in silico methods to support modeling and assessment
As more focus is placed on molecular interactions between PFAS and biomolecules including proteins, transporters, and phospholipids, a variety of in vitro and analytical methods have been developed to support both laboratory assessments and the parameterization of mechanistic models. In vitro methods include cell- and vesicle-based assays to estimate and compare uptake rates into cells via passive diffusion and active transport (Luebker 2002; Katakura 2007; Nakagawa 2008; Weaver 2010; Herédi-Szabó 2012), and protein-binding assays using equilibrium dialysis (Bischel 2010; Bischel 2011), fluorescence displacement (Chen 2009; MacManus-Spencer 2010; Yang 2020), and other methods (MacManus-Spencer 2010). A key challenge of these approaches is high variability across studies, which can lead to order of magnitude differences in estimated binding affinities for the same PFAS-protein combinations (MacManus-Spencer 2010; Ng 2014). Recently, a hybrid approach used size exclusion column co-elution (SECC) with non-target mass spectrometry to identify L-FABP (liver fatty acid binding protein) ligands from complex PFAS mixtures (Yang 2020). The method identified 31 new L-FABP ligands from AFFF. Given the known importance of this protein to liver accumulation of PFAS in diverse organisms, this method illustrates the potential for combining non-target analysis with bioaccumulation potential screening.
In silico methods have been developed to predict the behavior of PFAS at the molecular level in biological systems. These methods have an advantage of high throughput testing and are not hindered by the lack of available standards and samples. Molecular docking and molecular dynamics are computational approaches originally developed for drug discovery, and are powerful tools for the prediction of protein-ligand interactions.
They have now been used to screen a number of legacy and emerging PFAS for binding with serum albumin (Salvalaglio 2010; Ng 2015), L-FABP (Zhang 2013; Ng 2015; Cheng 2018; Yang 2020), and peroxisome proliferator activated receptors (PPARs), which are thought to be linked to some of the toxic effects of PFAS (Li 2019). Predicted binding affinities from these kinds of studies are highly correlated with experimental observations and can be used to help parameterize mechanistic PBTK models. However, we note the predicted binding affinities are overestimated for perfluoroalkylsulfonamide and may still require anchoring to some experimental binding data.
The role of phospholipids and related application of DMW in PFAS toxicokinetics and tissue has not been fully realized. It vitro assay protocols to assess phospholipid partitioning using laboratory-prepared liposomes (Escher 2000) are applicable to determining DMW for PFAS. Solid-supported phosphatidylcholine (PC) membranes can also be applied for generating empirical data for DMW for PFAS such as TRANSIL-XL membrane affinity assay kits (Droge 2019).
Finally, more empirical computational methods attempt to build predictive relationships between PFAS structure and their bioaccumulation potential or toxicity based on available data rather than mechanistic process-based approaches. These include quantitative structure activity relationships (QSARs) for predicting key physicochemical properties for PFAS, as recently reviewed (Lampic 2020), and a machine learning-based approach to classifying the potential bioactivity of thousands of PFAS (Cheng 2019). Hybrid in vitro/in silico approaches that generate data with which to develop a QSAR have also been used, for example to tackle the important subject of PFAS mixture toxicity (Hoover 2019).
7. Summary and Key Research Gaps
In this manuscript, we reviewed current understanding of: (a) PFAS sources and environmental transport pathways, (b) analytical methods used to assess PFAS exposures and their strengths and limitations, (c) methods for assessing human exposures and the relative importance of different sources and pathways, and (d) PFAS bioaccumulation in wildlife. Changing geographic locations of PFAS manufacturing and the shifting chemical landscape poses a number of challenges for exposure assessments for humans and wildlife. Synthesis of analytical standards for newer PFAS has lagged their production and release and thus non-targeted methods, suspect screening and total fluorine assessments have all emerged as essential tools for understanding the total burden of PFAS in the environment, humans and wildlife. Although such data are now appearing across all media, more data are needed, particularly for food items, PCPs, dust, drinking water, and wildlife tissues that contain PFAS. For both humans and wildlife, additional research is needed to characterize the fraction of exposure originating from precursors that degrade into terminal PFAA that have been linked to adverse health effects. Existing literature on time trends in PFAS in biological tissues suggest that while some legacy PFAS have decreased over the past two decades, the percent of unidentifiable EOF has increased. There is a need for greater constraints on residual content (i.e. unbound fluorinated surfactants, monomers) in fluoropolymers, side-chain polymers and ether-based polymers, and for more data on their contribution to PFAS exposure. Measurement of polymers in environmental media remains an important challenge.
A major focus of past research has been contaminated sites where the highest concentrations in the environment and biota are often found. Moving forward, a better understanding of atmospheric PFAS sources and potential resulting exposures is needed, particularly as treatment systems are introduced for contaminated drinking water supplies. Integration of PFAS measurements into long-term atmospheric monitoring for pollutants would be valuable for measuring changes in atmospheric PFAS concentrations, aiding model development, and identifying vulnerable regions.
Occupational health effects associated with human PFAS exposures have been reported for individuals who worked in fluorochemical production plants. There is a reasonable consensus that near contaminated sites, drinking water is often the main pathway for human exposure to PFAS. However, data are still sparse on exposure sources for the general population and for communities where drinking water contamination has been remediated. Presently, there is a paucity of data on the indoor environment where many PFAS-containing products are found. Dermal exposures to PFAS from dust and PCP warrant additional consideration given the extremely high concentrations reported for some PCP and dust. There is general agreement that dietary exposure is the major contributor to population exposure for PFOS and PFOA, but more limited evidence for other PFAS, including that contributed by food processing and packaging. More information is needed about the contribution of food processing and packaging to PFAS in food.
Most prior work on human exposure pathways has not included statistically representative population surveys. Future PFAS researchers may benefit from establishing collaborations with existing representative populations. The field needs total exposure studies seeking to characterize all pathways and routes of exposure to PFAS including precursors. Empirical TK data needed to describe PFAS partitioning among tissues, facilitated transport and elimination half-lives are still limited or non-existent for many PFAS and represent an important research need. As the focus of PFAS research shifts toward issues associated with emerging compounds, additional data for parameterizing TK models represent a critical research need.
For wildlife exposures, a major challenge is adapting existing metrics for chemical accumulation to describe diverse PFAS. This data gap hampers efforts to develop mechanistic models for exposure and risk assessment of PFAS. Early PFAS bioaccumulation modeling suggested that some PFAS are efficiently eliminated though the gills of water breathing organisms but biomagnify to a greater degree in air-breathing organisms due to limited lung-air exchange (Kelly 2007). Mechanistic bioaccumulation models for PFAS are needed to understand the tissue accumulation patterns for novel PFAS, including neutral precursors and intermediates. Non-traditional approaches to probing molecular mechanisms of bioaccumulation such as protein association have important impacts on predicting tissue distribution and internal dose but also can have organismal effects if PFAS are able to perturb endogenous functions. Tissue and cellular partitioning of PFAS can be used to inform models; current wildlife monitoring is primarily limited to liver or muscle or blood.
Early research on PFAS releases and global transport was conducted as a partnership between industry and academia. We thus recommend greater transparency and collaboration between academia, industry and government to prioritize and then improve understanding of global environmental releases of the thousands of PFAS structures present in global commerce. One example of improved transparency could be a commitment by industry to release information on the chemical structures and analytical standards for new PFAS used in commerce. Better characterizing PFAS exposure sources for humans and wildlife is an essential first step in the design of effective risk mitigation strategies.
ACKNOWLEDGEMENTS
This manuscript was developed by participants in the Exposure Assessment Panel as part of the SETAC North America Focused Topic Meeting on Environmental Risk Assessment of PFAS held August 12-15, 2019. WH-B acknowledges funding from the NIEHS Superfund Research Program (P42ES007381). CN acknowledges support from the U.S. National Science Foundation (NSF: 1845336). AR acknowledges financial support from the U.S. National Oceanic and Atmospheric Administration (NOAA) Dr. Nancy Foster Scholarship program (NA17NOS4290028) and the NIEHS Superfund Research Program (P42ES027706). EMS acknowledges support from NIEHS (P42ES027706) and the Strategic Environmental Research and Development Program (SERDP, ER18-1280). TFW was supported in part by NIEHS (R01ES027813, R01ES028800). MS acknowledges financial support from the North Carolina PFAS Testing Network through the North Carolina Policy Collaboratory. Positions or statements expressed herein do not necessarily reflect organizational views of the authors. All coauthors contributing to synthesizing information and writing this review. AOD and EMS led the synthesis team.
References
- 3M. 2001. Environmental Monitoring-Multi-City Study (Water, Sludge, Sediment, POTW Effluent and Landfill Leachate Samples). U.S. Environmental Protection Agency, Office of Pollution and Prevention and Toxic Substances, Washington, DC. Docket AR-226-1030a. [Google Scholar]
- 3M Company. 1999. Fluorochemical Use, Distribution and Release Overview. US EPA Public Docket AR226-0550, 3M Company: St Paul, MN. [Google Scholar]
- Ahrens L 2011. Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. Journal of Environmental Monitoring 13:20–31. DOI: 10.1039/c0em00373e. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Siebert U, Ebinghaus R. 2009a. Temporal trends of polyfluoroalkyl compounds in harbor seals (Phoca vitulina) from the German Bight, 1999-2008. Chemosphere 76:151–158. DOI: 10.1016/j.chemosphere.2009.03.053. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Siebert U, Ebinghaus R. 2009b. Total body burden and tissue distribution of polyfluorinated compounds in harbor seals (Phoca vitulina) from the German Bight. Mar Pollut Bull 58:520–525. DOI: 10.1016/j.marpolbul.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Andersen ME, Clewell HJ, Tan YM, Butenhoff JL, Olsen GW. 2006. Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys - Probing the determinants of long plasma half-lives. Toxicology 227:156–164. DOI: 10.1016/j.tox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Anderson R, Long G, Porter R, Anderson J. 2016. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 150:678–685. [DOI] [PubMed] [Google Scholar]
- Armitage J, Cousins IT, Buck RC, Prevedouros K, Russell MH, MacLeod M, Korzeniowski SH. 2006. Modeling Global-Scale Fate and Transport of Perfluorooctanoate Emitted from Direct Sources. Environmental Science & Technology 40:6969–6975. DOI: 10.1021/es0614870. [DOI] [PubMed] [Google Scholar]
- Armitage J, Macleod M, Cousins I. 2009a. Comparative assessment of the global fate and transport pathways of long-chain perfluorocarboxylic acids (PFCAs) and perfluorocarboxylates (PFCs) emitted from direct sources. Environmental Science & Technology 43:5830–5836. [DOI] [PubMed] [Google Scholar]
- Armitage J, Schenker U, Scheringer M, Martin J, Macleod M, Cousins I. 2009b. Modeling the global fate and transport of perfluorooctane sulfonate (PFOS) and precursor compounds in relation to temporal trends in wildlife exposure. Environmental Science & Technology 43:9274–9280. [DOI] [PubMed] [Google Scholar]
- Armitage JM, Arnot JA, Wania F. 2012. Potential Role of Phospholipids in Determining the Internal Tissue Distribution of Perfluoroalkyl Acids in Biota. Environmental Science & Technology 46:12285–12286. DOI: 10.1021/es304430r. [DOI] [PubMed] [Google Scholar]
- Armitage JM, Arnot JA, Wania F, Mackay D. 2013. Development and evaluation of a mechanistic bioconcentration model for ionogenic organic chemicals in fish. Environmental Toxicology and Chemistry 32:115–128. DOI: 10.1002/etc.2020. [DOI] [PubMed] [Google Scholar]
- Armitage JM, Erickson RJ, Luckenbach T, Ng CA, Prosser RS, Arnot JA, Schirmer K, Nichols JW. 2017. Assessing the bioaccumulation potential of ionizable organic compounds: Current knowledge and research priorities. Environmental Toxicology and Chemistry 36:882–897. DOI: 10.1002/etc.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot JA, Gobas F. 2004. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environmental Toxicology and Chemistry 23:2343–2355. DOI: 10.1897/03-438. [DOI] [PubMed] [Google Scholar]
- Baduel C, Mueller JF, Rotander A, Corfield J, Gomez-Ramos MJ. 2017. Discovery of novel per- and polyfluoroalkyl substances (PFASs) at a fire fighting training ground and preliminary investigation of their fate and mobility. Chemosphere 185:1030–1038. DOI: 10.1016/j.chemosphere.2017.06.096. [DOI] [PubMed] [Google Scholar]
- Balk FGP, Puetz KW, Ribbenstedt A, Gomis MI, Filipovic M, Cousins IT. 2019. Children's exposure to perfluoroalkyl acids - a modelling approach. Environ Sci-Process Impacts 21:1875–1886. DOI: 10.1039/c9em00323a. [DOI] [PubMed] [Google Scholar]
- Bao WW, Qian ZM, Geiger SD, Liu E, Liu YM, Wang SQ, Lawrence WR, Yang BY, Hu LW, Zeng XW, Dong GH. 2017. Gender-specific associations between serum isomers of perfluoroalkyl substances and blood pressure among Chinese: Isomers of C8 Health Project in China. Science of the Total Environment 607:1304–1312. DOI: 10.1016/j.scitotenv.2017.07.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JL, Berger U, Chaemfa C, Huber S, Jahnke A, Temme C, Jones KC. 2007. Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. Journal of Environmental Monitoring 9:530–541. DOI: 10.1039/B701417A. [DOI] [PubMed] [Google Scholar]
- Barton KE, Starling AP, Higgins CP, McDonough CA, Calafat AM, Adgate JL. 2020. Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. Int J Hyg Environ Health 223:256–266. DOI: 10.1016/j.ijheh.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzen-Hanson KA, Roberts SC, Choyke S, Oetjen K, McAlees A, Riddell N, McCrindle R, Ferguson PL, Higgins CP, Field JA. 2017. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environmental Science & Technology 51:2047–2057. DOI: 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Beesoon S, Genuis SJ, Benskin JP, Martin JW. 2012. Exceptionally High Serum Concentrations of Perfluorohexanesulfonate in a Canadian Family are Linked to Home Carpet Treatment Applications. Environmental Science & Technology 46:12960–12967. DOI: 10.1021/es3034654. [DOI] [PubMed] [Google Scholar]
- Benskin JP, Holt A, Martin JW. 2009. Isomer-Specific Biotransformation Rates of a Perfluorooctane Sulfonate (PFOS)-Precursor by Cytochrome P450 Isozymes and Human Liver Microsomes. Environmental Science & Technology 43:8566–8572. DOI: 10.1021/es901915f. [DOI] [PubMed] [Google Scholar]
- Biffinger JC, Kim HW, DiMagno SG. 2004. The polar hydrophobicity of fluorinated compounds. ChemBioChem 5:622–627. [DOI] [PubMed] [Google Scholar]
- Bischel HN, MacManus-Spencer LA, Luthy RG. 2010. Noncovalent Interactions of Long-Chain Perfluoroalkyl Acids with Serum Albumin. Environmental Science & Technology 44:5263–5269. DOI: 10.1021/es101334s. [DOI] [PubMed] [Google Scholar]
- Bischel HN, MacManus-Spencer LA, Zhang CJ, Luthy RG. 2011. STRONG ASSOCIATIONS OF SHORT-CHAIN PERFLUOROALKYL ACIDS WITH SERUM ALBUMIN AND INVESTIGATION OF BINDING MECHANISMS. Environmental Toxicology and Chemistry 30:2423–2430. DOI: 10.1002/etc.647. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, Bach CC, Long M, Ghisari M, Bossi R, Bech BH, Nohr EA, Henriksen TB, Olsen J, Bonefeld-Jorgensen EC. 2016. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environment International 91:14–21. [DOI] [PubMed] [Google Scholar]
- Boerma JT, Holt E, Black R. 2001. Measurement of biomarkers in surveys in developing countries: Opportunities and problems. Popul Dev Rev 27:303-+. DOI: 10.1111/j.1728-4457.2001.00303.x. [DOI] [Google Scholar]
- Boisvert G, Sonne C, Riget FF, Dietz R, Letcher RJ. 2019. Bioaccumulation and biomagnification of perfluoroalkyl acids and precursors in East Greenland polar bears and their ringed seal prey. Environmental Pollution 252:1335–1343. DOI: 10.1016/j.envpol.2019.06.035. [DOI] [PubMed] [Google Scholar]
- Borg D, Bogdanska J, Sundstrom M, Nobel S, Hakansson H, Bergman A, DePierre JW, Halldin K, Bergstrom U. 2010. Tissue distribution of S-35-labelled perfluorooctane sulfonate (PFOS) in C57Bl/6 mice following late gestational exposure. Reprod Toxicol 30:558–565. DOI: 10.1016/j.reprotox.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Boucher J, Cousins I, Scheringer M, Hungerbühler K, Wang Z. 2019. Toward a Comprehensive Global Emission Inventory of C4–C10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C6- and C10-Based Products. . Environmental Science & Technology Letters 6:1–7. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environmental Assessment and Management 7:513–541. DOI: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Wang X, Wu Y, Zhao S, Li Y, Ma L, Chen C, Huang J, Yu G. 2018. Temporal trends and transport of perfluoroalkyl substances (PFASs) in a subtropical estuary: Jiulong River Estuary, Fujian, China. Science of The Total Environment 639:263–270. DOI: 10.1016/j.scitotenv.2018.05.042. [DOI] [PubMed] [Google Scholar]
- Carlson G, Tupper S. 2020. Ski wax contributes to environmental contamination by per- and polyfluoroalkyl substances. Chemosphere 261:128078. [DOI] [PubMed] [Google Scholar]
- Chen FF, Gong ZY, Kelly BC. 2016. Bioavailability and bioconcentration potential of perfluoroalkyl-phosphinic and -phosphonic acids in zebrafish (Danio rerio): Comparison to perfluorocarboxylates and perfluorosulfonates. Science of the Total Environment 568:33–41. DOI: 10.1016/j.scitotenv.2016.05.215. [DOI] [PubMed] [Google Scholar]
- Chen FF, Gong ZY, Kelly BC. 2017a. Bioaccumulation Behavior of Pharmaceuticals and Personal Care Products in Adult Zebrafish (Danio rerio): Influence of Physical-Chemical Properties and Biotransformation. Environmental Science & Technology 51:11085–11095. DOI: 10.1021/acs.est.7b02918. [DOI] [PubMed] [Google Scholar]
- Chen H, Han J, Zhang C, Cheng J, Sun R, Wang X, Han G, Yang W, He X. 2017b. Occurrence and seasonal variations of per- and polyfluoroalkyl substances (PFASs) including fluorinated alternatives in rivers, drain outlets and the receiving Bohai Sea of China. Environmental Pollution 231:1223–1231. DOI: 10.1016/j.envpol.2017.08.068. [DOI] [PubMed] [Google Scholar]
- Chen YM, Guo LH. 2009. Fluorescence study on site-specific binding of perfluoroalkyl acids to human serum albumin. Arch Toxicol 83:255–261. DOI: 10.1007/s00204-008-0359-x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Psillakis E, Hoffmann MR, Colussi AJ. 2009. Acid dissociation versus molecular association of perfluoroalkyl oxoacids: environmental implications. J Phys Chem A 113:8152–8156. [DOI] [PubMed] [Google Scholar]
- Cheng WX, Ng CA. 2017. A Permeability-Limited Physiologically Based Pharmacokinetic (PBPK) Model for Perfluorooctanoic acid (PFOA) in Male Rats. Environmental Science & Technology 51:9930–9939. DOI: 10.1021/acs.est.7b02602. [DOI] [PubMed] [Google Scholar]
- Cheng WX, Ng CA. 2018. Predicting Relative Protein Affinity of Novel Per- and Polyfluoroalkyl Substances (PFASs) by An Efficient Molecular Dynamics Approach. Environmental Science & Technology 52:7972–7980. DOI: 10.1021/acs.est.8b01268. [DOI] [PubMed] [Google Scholar]
- Cheng WX, Ng CA. 2019. Using Machine Learning to Classify Bioactivity for 3486 Per- and Polyfluoroalkyl Substances (PFASs) from the OECD List. Environmental Science & Technology 53:13970–13980. DOI: 10.1021/acs.est.9b04833. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Lazcano RK, Yousefi P, Trim H, Lee LS. 2019. Perfluoroalkyl Acid Characterization in US Municipal Organic Solid Waste Composts. Environmental Science & Technology Letters 6:372–377. DOI: 10.1021/acs.estlett.9b00280. [DOI] [Google Scholar]
- Christensen K, Raymond M, Blackowicz M, Liu Y-J, Thompson B, Anderson H, Turyk M. 2017. Perfluoroalkyl substances and fish consumption. Environmental Research 154:145–151. [DOI] [PubMed] [Google Scholar]
- Chu SG, Letcher RJ. 2017. Side-chain fluorinated polymer surfactants in aquatic sediment and biosolid-augmented agricultural soil from the Great Lakes basin of North America. Science of The Total Environment 607:262–270. [DOI] [PubMed] [Google Scholar]
- Coggan TL, Moodie D, Kolobaric A, Szabo D, Shimeta J, Crosbie ND, Lee E, Fernandes M, Clarke BO. 2019. An investigation into per- and polyfluoroalkyl substances (PFAS) in nineteen Australian wastewater treatment plants (WWTPs). Heliyon 5:e02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condor J, Hoke R, Wolf W, Russell MH, Buck R. 2008. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environmental Science & Technology 42:995–1003. [DOI] [PubMed] [Google Scholar]
- Consoer DM, Hoffman AD, Fitzsimmons PN, Kosian PA, Nichols JW. 2014. Toxicokinetics of perfluorooctanoate (PFOA) in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 156:65–73. DOI: 10.1016/j.aquatox.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Consoer DM, Hoffman AD, Fitzsimmons PN, Kosian PA, Nichols JW. 2016. TOXICOKINETICS OF PERFLUOROOCTANE SULFONATE IN RAINBOW TROUT (ONCORHYNCHUS MYKISS). Environmental Toxicology and Chemistry 35:717–727. DOI: 10.1002/etc.3230. [DOI] [PubMed] [Google Scholar]
- Corsi DJ, Neuman M, Finlay JE, Subramanian SV. 2012. Demographic and health surveys: a profile. Int J Epidemiol 41:1602–1613. DOI: 10.1093/ije/dys184. [DOI] [PubMed] [Google Scholar]
- Cousins I, Goldenman G, Herzke D, Lohmann R, Miller M, Ng CA, Patton S, Scheringer M, Trier X, Vierke L, Wang Z, DeWitt JC. 2019. The concept of essential use for determining when uses of PFASs can be phased out. Environmental Science Processes & Impacts 21:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer CM, Custer TW, Dummer PM, Etterson MA, Thogmartin WE, Wu Q, Kannan K, Trowbridge A, McKann PC. 2014. Exposure and Effects of Perfluoroalkyl Substances in Tree Swallows Nesting in Minnesota and Wisconsin, USA. Arch Environ Contam Toxicol 66:120–138. DOI: 10.1007/s00244-013-9934-0. [DOI] [PubMed] [Google Scholar]
- Custer CM, Custer TW, Schoenfuss HL, Poganski BH, Solem L. 2012. Exposure and effects of perfluoroalkyl compounds on tree swallows nesting at Lake Johanna in east central Minnesota, USA. Reprod Toxicol 33:556–562. DOI: 10.1016/j.reprotox.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Daly ER, Chan BP, Talbot EA, Nassif J, Bean C, Cavallo SJ, Metcalf E, Simone K, Woolf AD. 2018. Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int J Hyg Environ Health 221:569–577. DOI: 10.1016/j.ijheh.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Dassuncao C, Hu XC, Nielsen F, Weihe P, Grandjean P, Sunderland EM. 2018. Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Environmental Science & Technology. DOI: 10.1021/acs.est.7b06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassuncao C, Hu XC, Zhang X, Bossi R, Dam M, Mikkelsen B, Sunderland EM. 2017. Temporal shifts in poly-and perfluoroalkyl substances (PFASs) in North Atlantic pilot whales indicate large contribution of atmospheric precursors. Environmental Science & Technology 51:4512–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassuncao C, Pickard H, Pfohl M, Tokranov AK, Li ML, Mikkelsen B, Slitt A, Sunderland EM. 2019. Phospholipid Levels Predict the Tissue Distribution of Poly- and Perfluoroalkyl Substances in a Marine Mammal. Environmental Science & Technology Letters 6:119–125. DOI: 10.1021/acs.estlett.9b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K, Aucoin M, Larsen B, Kaiser M, Hartten A. 2007. Transport of ammonium perfluorooctanoate in environmental media near a floropolymer manufacturing facility. Chemosphere 67:2011–2019. [DOI] [PubMed] [Google Scholar]
- de Jager L 2019. Investigation of PFAS concentrations in US food products. https://www.fda.gov/food/cfsan-constituent-updates/fda-makes-available-testing-method-pfas-foods-and-final-results-recent-surveys. . SETAC Europe 29th Annual Meeting TH069. Helsinki, Sweden. [Google Scholar]
- de la Torre A, Navarro I, Sanz P, Martinez MD. 2019. Occurrence and human exposure assessment of perfluorinated substances in house dust from three European countries. Science of the Total Environment 685:308–314. DOI: 10.1016/j.scitotenv.2019.05.463. [DOI] [PubMed] [Google Scholar]
- De Silva A, Allard C, Spencer C, Webster G, Shoeib M. 2012. Phosphorus-containing fluorinated organics: Polyfluoroalkyl phosphoric acid diesters (diPAPs), perfluorophosphonates (PFPAs), and perfluorophosphinates (PFPIAs) in residential indoor dust. Environmental Science & Technology 46:12575–12582. [DOI] [PubMed] [Google Scholar]
- Droge STJ. 2019. Membrane-Water Partition Coefficients to Aid Risk Assessment of Perfluoroalkyl Anions and Alkyl Sulfates. Environmental Science & Technology 53:760–770. DOI: 10.1021/acs.est.8b05052. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Lorber M. 2011. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. Journal of exposure science & environmental epidemiology 21:150–168. DOI: 10.1038/jes.2009.73. [DOI] [PubMed] [Google Scholar]
- Ehresman DJ, Froehlich JW, Olsen GW, Chang S-C, Butenhoff JL. 2007. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environmental Research 103:176–184. DOI: 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Ericson I, Gómez M, Nadal M, van Bavel B, Lindström G, Domingo JL. 2007. Perfluorinated chemicals in blood of residents in Catalonia (Spain) in relation to age and gender: A pilot study. Environment International 33:616–623. DOI: 10.1016/j.envint.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Karrman A. 2015a. World-Wide Indoor Exposure to Polyfluoroalkyl Phosphate Esters (PAPs) and other PFASs in Household Dust. Environmental Science & Technology 49:14503–14511. DOI: 10.1021/acs.est.5b00679. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Kärrman A. 2015b. World-Wide Indoor Exposure to Polyfluoroalkyl Phosphate Esters (PAPs) and other PFASs in Household Dust. Environmental Science & Technology 49:14503–14511. DOI: 10.1021/acs.est.5b00679. [DOI] [PubMed] [Google Scholar]
- Escher BI, Schwarzenbach RP, Westall JC. 2000. Evaluation of liposome-water partitioning of organic acids and bases. 2. Comparison of experimental determination methods. Environmental Science & Technology 34:3962–3968. DOI: 10.1021/es0010711. [DOI] [Google Scholar]
- European Food Safety Authority. 2018. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food (draft). . EFSA J 16:1–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority. 2020. Risks to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal 18:e06223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabic MS, Choi Y, Bird S. 2012. A systematic review of Demographic and Health Surveys: data availability and utilization for research. Bull World Health Organ 90:604–612. DOI: 10.2471/blt.11.095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrega F, Kumar V, Schuhmacher M, Domingo JL, Nadal M. 2014. PBPK modeling for PFOS and PFOA: Validation with human experimental data. Toxicol Lett 230:244–251. DOI: 10.1016/j.toxlet.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Fabrega F, Nadal M, Schuhmacher M, Domingo JL, Kumar V. 2016. Influence of the uncertainty in the validation of PBPK models: A case-study for PFOS and PFOA. Regul Toxicol Pharmacol 77:230–239. DOI: 10.1016/j.yrtph.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Fair PA, Wolf B, White ND, Arnott SA, Kannan K, Karthikraj R, Vena JE. 2019. Perfluoroalkyl substances (PFASs) in edible fish species from Charleston Harbor and tributaries, South Carolina, United States: Exposure and risk assessment. Environmental Research 171:266–277. DOI: 10.1016/j.envres.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko J, Meade BJ, Frasch HF, Barbero AM, Anderson SE. 2012. Dermal pentration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. J Toxicol Env Health Part A 75:50–62. DOI: 10.1080/15287394.2011.615108. [DOI] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, Kato K, Shoeib M, Vieira VM, McClean MD. 2012. Polyfluorinated Compounds in Serum Linked to Indoor Air in Office Environments. Environmental Science & Technology 46:1209–1215. DOI: 10.1021/es2038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, Watkins DJ, Strynar MJ, Kato K, Calafat AM, Vieira VM, McClean MD. 2013. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers' serum. Environment International 60:128–136. DOI: 10.1016/j.envint.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Schlummer M, Möller A, Gruber L, Wolz G, Ungewiss J, Böhmer S, Dekant W, Mayer R, Liebl B, Twardella D. 2007. Exposure of an Adult Population to Perfluorinated Substances Using Duplicate Diet Portions and Biomonitoring Data. Environmental Science & Technology 41:7928–7933. DOI: 10.1021/es071244n. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. 2009. Perfluorinated compounds – Exposure assessment for the general population in western countries. International Journal of Hygiene and Environmental Health 212:239–270. DOI: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Fu JJ, Gao Y, Cui L, Wang T, Liang Y, Qu GB, Yuan B, Wang YW, Zhang AQ, Jiang GB. 2016. Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China. Sci Rep 6:10. DOI: 10.1038/srep38039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Harada KH, Koizumi A. 2013. Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compounding agents. Chemosphere 93:538–544. DOI: 10.1016/j.chemosphere.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Berger U, Cousins IT. 2015. Estimating human exposure to PFOS isomers and PFCA homologues: The relative importance of direct and indirect (precursor) exposure. Environment International 74:160–169. DOI: 10.1016/j.envint.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, van Asseldonk L, van Leeuwen SPJ. 2017. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environmental Science & Technology 51:11057–11065. DOI: 10.1021/acs.est.7b02488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy J, Kannan K. 2001. Global distribution of perfluoroctane sulfonate in wildlife. Environmental Science & Technology 35:1339–1342. [DOI] [PubMed] [Google Scholar]
- Girardi P, Merler E. 2019. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environmental Research 179:10. DOI: 10.1016/j.envres.2019.108743. [DOI] [PubMed] [Google Scholar]
- Gobas F 2020. A Framework for Assessing Bioaccumulation and Exposure Risks of Per- and Polyfluoroalkyl Substances in Threatened and Endangered Species on Aqueous Film Forming Foam (AFFF)-Impacted Sites. https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/ER18-1502 [Accessed June 21, 2020].
- Gobas F, Mayer P, Parkerton TF, Burgess RM, van de Meent D, Gouin T. 2018. A Chemical Activity Approach to Exposure and Risk Assessment of Chemicals. Environmental Toxicology and Chemistry 37:1235–1251. DOI: 10.1002/etc.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobas F, Otton SV, Tupper-Ring LF, Crawford MA, Clark KE, Ikonomou MG. 2017. Chemical activity-based environmental risk analysis of the plasticizer di-ethylhexyl phthalate and its main metabolite mono-ethylhexyl phthlalate. Environmental Toxicology and Chemistry 36:1483–1492. DOI: 10.1002/etc.3689. [DOI] [PubMed] [Google Scholar]
- Goosey E, Harrad S. 2012. Perfluoroalkyl substances in UK indoor and outdoor air: Spatial and seasonal variation, and implications for human exposure. Environment International 45:86–90. [DOI] [PubMed] [Google Scholar]
- Goss KU. 2008a. The pK(a) values of PFOA and other highly fluorinated carboxylic acids. Environmental Science & Technology 42:456–458. DOI: 10.1021/es702192c. [DOI] [PubMed] [Google Scholar]
- Goss KU. 2008b. The pK(a) values of PFOA and other highly fluorinated carboxylic acids (vol 73, pg 2323, 1951). Environmental Science & Technology 42:5032–5032. DOI: 10.1021/es8011904. [DOI] [PubMed] [Google Scholar]
- Groffen T, Lasters R, Lopez-Antia A, Prinsen E, Bervoets L, Eens M. 2019. Limited reproductive impairment in a passerine bird species exposed along a perfluoroalkyl acid (PFAA) pollution gradient. Science of the Total Environment 652:718–728. DOI: 10.1016/j.scitotenv.2018.10.273. [DOI] [PubMed] [Google Scholar]
- Guelfo JL, Korzeniowski S, Mills M, Anderson J, Anderson R, Arblaster J, Conder J, Cousins IT, Dasu K, Henry B, Lee L, Liu J, McKenzie E, Willey J. 2020. Environmental sources, chemistry, fate and transport of per- and polyfluoroalkyl substances: State of the science, key knowledge gaps, and recommendations presented at the August 2019 SETAC focus topic meeting. Environmental Toxicology and Chemistry in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfo JL, Marlow T, Klein DM, Savitz DA, Frickel S, Crimi M, Suuberg EM. 2018. Evaluation and Management Strategies for Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water Aquifers: Perspectives from Impacted US Northeast Communities. Environmental Health Perspectives 126:13. DOI: 10.1289/ehp2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette TC, McCord J, Guillette M, Polera ME, Rachels KT, Morgeson C, Kotlarz N, Knappe DRU, Reading BJ, Strynar M, Belcher SM. 2020. Elevated levels of per- and polyfluoroalkyl substances in Cape Fear River Striped Bass (Morone saxatilis) are associated with biomarkers of altered immune and liver function. Environment International 136:10. DOI: 10.1016/j.envint.2019.105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Lyu Y, Xu T, Yao B, Song W, Li M, Yang X, Cheng T, Li X. 2018. Particle size distribution and respiratory deposition estimates of airborne perfluoroalkyl acids during the haze period in the megacity of Shanghai. Environmental Pollution 234:9–19. DOI: 10.1016/j.envpol.2017.10.128. [DOI] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. 2012. Renal Elimination of Perfluorocarboxylates (PFCAs). Chem Res Toxicol 25:35–46. DOI: 10.1021/tx200363w. [DOI] [PubMed] [Google Scholar]
- Harrad S, de Wit C, Abdallah M, Bergh C, Bjorklund J, Covaci A, Darnerud P, de Boer J, Diamond M, Huber S, Leonards P, Mandalakis M, Ostman C, Haug L, Thomsen C, Webster T. 2010. Indoor contamination with hexabromocyclododecanes, polybrominated diphenyl ethers, and perfluoroalkyl compounds: an important exposure pathway for people? Environmental Science & Technology 44:3221–3231. [DOI] [PubMed] [Google Scholar]
- Harrad S, Wemken N, Drage DS, Abdallah MAE, Coggins AM. 2019. Perfluoroalkyl Substances in Drinking Water, Indoor Air and Dust from Ireland: Implications for Human Exposure. Environmental Science & Technology 53:13449–13457. DOI: 10.1021/acs.est.9b04604. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C. 2011. Characterisation of human exposure pathways to perfluorinated compounds — Comparing exposure estimates with biomarkers of exposure. Environment International 37:687–693. DOI: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM, Knutsen HK. 2010. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environment International 36:772–778. DOI: 10.1016/j.envint.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Henneberger L, Goss KU, Endo S. 2016. Partitioning of Organic Ions to Muscle Protein: Experimental Data, Modeling, and Implications for in Vivo Distribution of Organic Ions. Environmental Science & Technology 50:7029–7036. DOI: 10.1021/acs.est.6b01417. [DOI] [PubMed] [Google Scholar]
- Herédi-Szabó K, Kis E, Krajcsi P. 2012. The Vesicular Transport Assay: Validated In Vitro Methods to Study Drug-Mediated Inhibition of Canalicular Efflux Transporters ABCB11/BSEP and ABCC2/MRP2. Current Protocols in Toxicology 54:23.24.21–23.24.16. [DOI] [PubMed] [Google Scholar]
- Herrick RL, Buckholz J, Biro FM, Calafat AM, Ye X, Xie C, Pinney SM. 2017. Polyfluoroalkyl substance exposure in the Mid-Ohio River Valley, 1991-2012. Environmental Pollution 228:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites R, Jobst K. 2018. Is nontargted screening reproducible? Environmental Science & Technology 52:11975–11976. [DOI] [PubMed] [Google Scholar]
- Hites R, Jobst K. 2019. Response to “Letter to the Editor: Optimism for nontarget analysis in environmental chemistry”. Environmental Science & Technology 53:5531–5533. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Webster T, Bartell S, Weisskopf M, Fletcher T, Vieira V. 2011. Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ Health Perspect 119:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover G, Kar S, Guffey S, Leszczynski J, Sepulveda MS. 2019. In vitro and in silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere 233:25–33. DOI: 10.1016/j.chemosphere.2019.05.065. [DOI] [PubMed] [Google Scholar]
- Hopkins ZR, Sun M, DeWitt JC, Knappe DRU. 2018. Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J Am Water Work Assoc 110:13–28. DOI: 10.1002/awwa.1073. [DOI] [Google Scholar]
- Houde M, Czub G, Small JM, Backus S, Wang XW, Alaee M, Muir DCG. 2008. Fractionation and Bioaccumulation of Perfluorooctane Sulfonate (PFOS) Isomers in a Lake Ontario Food Web. Environmental Science & Technology 42:9397–9403. DOI: 10.1021/es800906r. [DOI] [PubMed] [Google Scholar]
- Houde M, De Silva A, Muir DC, Letcher R. 2011a. Monitoring of perfluorinated compounds in aquatic biota: An updated review. Environmental Science & Technology 45:7962–7973. [DOI] [PubMed] [Google Scholar]
- Houde M, De Silva AO, Muir DCG, Letcher RJ. 2011b. Monitoring of perfluorinated compounds in aquatic biota: An updated review. Environmental Science & Technology 45:7962–7973. [DOI] [PubMed] [Google Scholar]
- Houtz E, Higgins C, Field J, Sedlak D. 2013. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environmental Science & Technology 47:8187–8195. [DOI] [PubMed] [Google Scholar]
- Houtz EF, Sutton R, Park JS, Sedlak M. 2016. Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res 95:142–149. DOI: 10.1016/j.watres.2016.02.055. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM. 2016. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environmental Science & Technology Letters 3:344–350. DOI: 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XC, Dassuncao C, Zhang X, Grandjean P, Weihe P, Webster GM, Nielsen F, Sunderland EM. 2018. Can profiles of poly-and Perfluoroalkyl substances (PFASs) in human serum provide information on major exposure sources? Environmental Health 17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XC, Tokranov AK, Liddie J, Zhang XM, Grandjean P, Hart JE, Laden F, Sun Q, Yeung LWY, Sunderland EM. 2019. Tap Water Contributions to Plasma Concentrations of Poly- and Perfluoroalkyl Substances (PFAS) in a Nationwide Prospective Cohort of US Women. Environmental Health Perspectives 127:11. DOI: 10.1289/ehp4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huset CA, Barlaz MA, Barofsky DF, Field JA. 2011. Quantitative determination of fluorochemicals in municipal landfill leachates. Chemosphere 82:1380–1386. DOI: 10.1016/j.chemosphere.2010.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelido AM, Abballe A, Gemma S, Dellatte E, Iacovella N, De Angelis G, Zampaglioni F, Marra V, Miniero R, Valentini S, Russo F, Vazzoler M, Testai E, De Felip E. 2018. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environment International 110:149–159. DOI: 10.1016/j.envint.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Jahnke A, Ahrens L, Ebinghaus R, Temme C. 2007. Urban versus Remote Air Concentrations of Fluorotelomer Alcohols and Other Polyfluorinated Alkyl Substances in Germany. Environmental Science & Technology 41:745–752. DOI: 10.1021/es0619861. [DOI] [PubMed] [Google Scholar]
- Jamari NLA, Behrens A, Raab A, Krupp EM, Feldmann J. 2018. Plasma processes to detect fluorine with ICPMS/MS as M-F (+): an argument for building a negative mode ICPMS/MS. J Anal At Spectrom 33:1304–1309. DOI: 10.1039/c8ja00050f. [DOI] [Google Scholar]
- Janda J, Nödler K, Brauch H-J, Zwiener C, Lange FT. 2019. Robust trace analysis of polar (C2-C8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: method development and application to surface water, groundwater and drinking water. Environmental Science and Pollution Research 26:7326–7336. DOI: 10.1007/s11356-018-1731-x. [DOI] [PubMed] [Google Scholar]
- Jian J-M, Chen D, Han F-J, Guo Y, Zeng L, Lu X, Wang F. 2018. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Science of The Total Environment 636:1058–1069. [DOI] [PubMed] [Google Scholar]
- Jiang WW, Zhang YF, Zhu LY, Deng JM. 2014. Serum levels of perfluoroalkyl acids (PFAAs) with isomer analysis and their associations with medical parameters in Chinese pregnant women. Environment International 64:40–47. DOI: 10.1016/j.envint.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Johns K, Stead G. 2000. Fluoroproducts: the extremophiles. Journal of Fluorine Chemistry 104:5–18. [Google Scholar]
- Jones PD, Hu WY, De Coen W, Newsted JL, Giesy JP. 2003. Binding of perfluorinated fatty acids to serum proteins. Environmental Toxicology and Chemistry 22:2639–2649. DOI: 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- Kaiser MA, Dawson BJ, Barton CA, Botelho MA. 2010. Understanding Potential Exposure Sources of Perfluorinated Carboxylic Acids in the Workplace. Ann Occup Hyg 54:915–922. DOI: 10.1093/annhyg/meq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Choi K, Lee H-S, Kim D-H, Park N-Y, Kim S-K, Kho Y. 2016. Elevated levels of short carbon-chain PFCAs in breast milk among Korean women: Current status and potential challenges. Environmental Research 148:351–359. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Wouwe NV, Yang JH, Aldous KM. 2004. Perfluorooctanesulfonate and Related Fluorochemicals in Human Blood from Several Countries. Environmental Science & Technology 38:4489–4495. DOI: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Karrman A, Elgh-Dalgren K, Lafossas C, Møskeland T. 2011. Environmental levels and distribution of structural isomers of perfluoroalkyl acids after aqueous fire-fighting foam (AFFF) contamination. Environmental Chemistry 8:372–380. [Google Scholar]
- Kärrman A, van Bavel B, Järnberg U, Hardell L, Lindström G. 2006. Perfluorinated chemicals in relation to other persistent organic pollutants in human blood. Chemosphere 64:1582–1591. DOI: 10.1016/j.chemosphere.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Katakura M, Kudo N, Tsuda T, Hibino Y, Mitsumoto A, Kawashima Y. 2007. Rat organic anion transporter 3 and organic anion transporting polypeptide 1 mediate perfluorooctanoic acid transport. J Health Sci 53:77–83. DOI: 10.1248/jhs.53.77. [DOI] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas F. 2007. Food web-specific biomagnification of persistent organic pollutants. Science 317:236–239. DOI: 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Surridge B, Hoover D, Grace R, Gobas F. 2009. Perfluoroalkyl Contaminants in an Arctic Marine Food Web: Trophic Magnification and Wildlife Exposure. Environmental Science & Technology 43:4037–4043. DOI: 10.1021/es9003894. [DOI] [PubMed] [Google Scholar]
- Khazaee M, Ng CA. 2018. Evaluating parameter availability for physiologically based pharmacokinetic (PBPK) modeling of perfluorooctanoic acid (PFOA) in zebrafish. Environ Sci-Process Impacts 20:105–119. DOI: 10.1039/c7em00474e. [DOI] [PubMed] [Google Scholar]
- Kim D-H, Oh J-E. 2017. Development and validation of an extraction method for the anlaysis of perfluoroalkyl substances in human hair. Chemosphere 175:446–451. [DOI] [PubMed] [Google Scholar]
- Kissel JC. 2011. The mismeasure of dermal absorption. J Expo Sci Environ Epidemiol 21:302–309. DOI: 10.1038/jes.2010.22. [DOI] [PubMed] [Google Scholar]
- Koch A, Aro R, Wang T, Yeung LWY. 2020. Towards a comprehensive analytical workflow for the chemical characterisation of organofluorine in consumer products and environmental samples. Trac-Trends in Analytical Chemistry 123:9. DOI: 10.1016/j.trac.2019.02.024. [DOI] [Google Scholar]
- Koch A, Karrman A, Yeung LWY, Jonsson M, Ahrens L, Wang T. 2019. Point source characterization of per- and polyfluoroalkyl substances (PFASs) and extractable organofluorine (EOF) in freshwater and aquatic invertebrates. Environ Sci-Process Impacts 21:1887–1898. DOI: 10.1039/c9em00281b. [DOI] [PubMed] [Google Scholar]
- Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D. 2015. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environmental Science and Pollution Research 22:14546–14559. DOI: 10.1007/s11356-015-4202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk J, Ehlers S, Oberhausen A, Tischer M, Furst P, Schafft H, Lahrssen-Wiederholt M. 2013. Absorption, Distribution, and Milk Secretion of the Perfluoroalkyl Acids PFBS, PFHxS, PFOS, and PFOA by Dairy Cows Fed Naturally Contaminated Feed. Journal of Agricultural and Food Chemistry 61:2903–2912. DOI: 10.1021/jf304680j. [DOI] [PubMed] [Google Scholar]
- Kubwabo C, Stewart B, Zhu J, Marro L. 2005. Occurrence of perfluorosulfonates and other perfluorochemicals in dust from selected homes in the city of Ottawa, Canada. Journal of Environmental Monitoring 7:1074–1078. DOI: 10.1039/B507731C. [DOI] [PubMed] [Google Scholar]
- Laitinen JA, Koponen J, Koikkalainen J, Kiviranta H. 2014. Firefighters' exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams. Toxicol Lett 231:227–232. DOI: 10.1016/j.toxlet.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Lampic A, Parnis JM. 2020. Property Estimation of Per- and Polyfluoroalkyl Substances: A Comparative Assessment of Estimation Methods. Environmental Toxicology and Chemistry 39:775–786. DOI: 10.1002/etc.4681. [DOI] [PubMed] [Google Scholar]
- Land M, de Wit CA, Bignert A, Cousins IT, Herzke D, Johansson JH, Martin JW. 2018. What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environmental Evidence 7:4. DOI: 10.1186/s13750-017-0114-y. [DOI] [Google Scholar]
- Lang JR, Allred BM, Field JA, Levis JW, Barlaz MA. 2017. National Estimate of Per- and Polyfluoroalkyl Substance (PFAS) Release to US Municipal Landfill Leachate. Environmental Science & Technology 51:2197–2205. DOI: 10.1021/acs.est.6b05005. [DOI] [PubMed] [Google Scholar]
- Langberg HA, Breedveld GD, Gronning HM, Kvennas M, Jenssen BM, Hale SE. 2019. Bioaccumulation of Fluorotelomer Sulfonates and Perfluoroalkyl Acids in Marine Organisms Living in Aqueous Film-Forming Foam Impacted Waters. Environmental Science & Technology 53:10951–10960. DOI: 10.1021/acs.est.9b00927. [DOI] [PubMed] [Google Scholar]
- Lankova D, Svarcova A, Kalachova K, Lacina O, Pulkrabova J, Hajslova J. 2015. Multi-analyte method for the analysis of various organohalogen compounds in house dust. Analytica Chimica Acta 854:61–69. DOI: 10.1016/j.aca.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Lanza HA, Cochran RS, Mudge JF, Olson AD, Blackwell BR, Maul JD, Salice CJ, Anderson TA. 2017. Temporal monitoring of perfluoroactane sulfonate accumulation in aquatic biota downstream of historical aqueous film forming foam use areas. Environmental Toxicology and Chemistry 36:2022–2029. DOI: 10.1002/etc.3726. [DOI] [PubMed] [Google Scholar]
- Larson ES, Conder JM, Arblaster JA. 2018. Modeling avian exposures to perfluoroalkyl substances in aquatic habitats impacted by historical aqueous film forming foam releases. Chemosphere 201:335–341. DOI: 10.1016/j.chemosphere.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Lazcano RK, de Perre C, Mashtare ML, Lee LS. 2019. Per- and polyfluoroalkyl substances in commercially available biosolid-based products: The effect of treatment processes. Water Environ Res 91:1669–1677. DOI: 10.1002/wer.1174. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Chu SG, Smyth SA. 2020. Side-chain fluorinated polymer surfactants in biosolids from wastewater treatment plants. Journal of Hazardous Materials 388:8. DOI: 10.1016/j.jhazmat.2020.122044. [DOI] [PubMed] [Google Scholar]
- Li CH, Ren XM, Cao LY, Qin WP, Guo LH. 2019. Investigation of binding and activity of perfluoroalkyl substances to the human peroxisome proliferator-activated receptor beta/delta. Environ Sci-Process Impacts 21:1908–1914. DOI: 10.1039/c9em00218a. [DOI] [PubMed] [Google Scholar]
- Li L, Liu J, Hu J, Wania F. 2017. Degradation of fluorotelomer-based polymers contributes to the global occurrence of fluorotelomer alcohol and perfluoroalkyl carboxylates: A combined dynamic substance flow and environmental fate modeling analysis. Environmental Science & Technology 51:4461–4470. [DOI] [PubMed] [Google Scholar]
- Li L, Zhai Z, Liu J, Hu J. 2015. Estimating industrial and domestic environmental releases of perfluorooctanoic acid and its salts in China from 2004 to 2012. Chemosphere 129:100–109. [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, Jakobsson K. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational and Environmental Medicine 75:46–51. DOI: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PID, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, Fleisch AF, Calafat AM, Sanchez-Guerra M, Osorio-Yanez C, Webster TF, Horton ES, Oken E. 2020. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: Cross-sectional results from the Diabetes Prevention Program Trial. Environment International 137:10. DOI: 10.1016/j.envint.2019.105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YF, Ruan T, Liu AF, Jiang GB. 2017. Identification of Novel Hydrogen-Substituted Polyfluoroalkyl Ether Sulfonates in Environmental Matrices near Metal-Plating Facilities. Environmental Science & Technology 51:11588–11596. DOI: 10.1021/acs.est.7b02961. [DOI] [PubMed] [Google Scholar]
- Liu YN, Qian ML, Ma XX, Zhu LY, Martin JW. 2018. Nontarget Mass Spectrometry Reveals New Perfluoroalkyl Substances in Fish from the Yangtze River and Tangxun Lake, China. Environmental Science & Technology 52:5830–5840. DOI: 10.1021/acs.est.8b00779. [DOI] [PubMed] [Google Scholar]
- Liu YW, Ruan T, Lin YF, Liu AF, Yu M, Liu RZ, Meng M, Wang YW, Liu JY, Jiang GB. 2017. Chlorinated Polyfluoroalkyl Ether Sulfonic Acids in Marine Organisms from Bohai Sea, China: Occurrence, Temporal Variations, and Trophic Transfer Behavior. Environmental Science & Technology 51:4407–4414. DOI: 10.1021/acs.est.6b06593. [DOI] [PubMed] [Google Scholar]
- Llorca M, Schirinzi G, Martínez M, Barceló D, Farré M. 2020. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environmental Pollution 235:680–691. [DOI] [PubMed] [Google Scholar]
- Loi EIH, Yeung LWY, Taniyasu S, Lam PKS, Kannan K, Yamashita N. 2011. Trophic Magnification of Poly- and Perfluorinated Compounds in a Subtropical Food Web. Environmental Science & Technology 45:5506–5513. DOI: 10.1021/es200432n. [DOI] [PubMed] [Google Scholar]
- Lopez-Antia A, Groffen T, Lasters R, AbdElgawad H, Sun JC, Asard H, Bervoets L, Eens M. 2019. Perfluoroalkyl Acids (PFAAs) Concentrations and Oxidative Status in Two Generations of Great Tits Inhabiting a Contamination Hotspot. Environmental Science & Technology 53:1617–1626. DOI: 10.1021/acs.est.8b05235. [DOI] [PubMed] [Google Scholar]
- Lorber M, Egeghy PP. 2011. Simple intake and pharmacokinetic modeling to characterize exposure of Americans to perfluoroctanoic acid, PFOA. Environmental science & technology 45:8006–8014. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, Hansen KJ, Bass NM, Butenhoff JL, Seacat AM. 2002. Interactions of flurochemicals with rat liver fatty acid-binding protein. Toxicology 176:175–185. DOI: 10.1016/s0300-483x(02)00081-1. [DOI] [PubMed] [Google Scholar]
- MacInnis JJ, French K, Muir DCG, Spencer C, Criscitiello A, De Silva AO, Young CJ. 2017. Emerging investigator series: a 14-year depositional ice record of perfluoroalkyl substances in the High Arctic. Environ Sci-Process Impacts 19:22–30. DOI: 10.1039/c6em00593d. [DOI] [PubMed] [Google Scholar]
- MacManus-Spencer LA, Tse ML, Hebert PC, Bischel HN, Luthy RG. 2010. Binding of Perfluorocarboxylates to Serum Albumin: A Comparison of Analytical Methods. Anal Chem 82:974–981. DOI: 10.1021/ac902238u. [DOI] [PubMed] [Google Scholar]
- Makey CM, Webster TF, Martin JW, Shoeib M, Harner T, Dix-Cooper L, Webster GM. 2017. Airborne Precursors Predict Maternal Serum Perfluoroalkyl Acid Concentrations. Environmental Science & Technology 51:7667–7675. DOI: 10.1021/acs.est.7b00615. [DOI] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR, Muir DCG. 2003. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry 22:189–195. DOI: 10.1002/etc.5620220125. [DOI] [PubMed] [Google Scholar]
- Martin JW, Muir DCG, Moody CA, Ellis DA, Kwan WC, Solomon KR, Mabury SA. 2002. Collection of Airborne Fluorinated Organics and Analysis by Gas Chromatography/Chemical Ionization Mass Spectrometry. Analytical Chemistry 74:584–590. DOI: 10.1021/ac015630d. [DOI] [PubMed] [Google Scholar]
- Martin JW, Whittle DM, Muir DCG, Mabury SA. 2004. Perfluoroalkyl contaminants in a food web from lake Ontario. Environmental Science & Technology 38:5379–5385. DOI: 10.1021/es049331s. [DOI] [PubMed] [Google Scholar]
- Masoner JR, Kolpin DW, Cozzarelli IM, Smalling KL, Bolyard SC, Field JA, Furlong ET, Gray JL, Lozinski D, Reinhart D, Rodowa A, Bradley PM. 2020. Landfill leachate contributes per-/poly-fluoroalkyl substances (PFAS) and pharmaceuticals to municipal wastewater. Environ Sci: Water Res Technol 6:1300–1311. [Google Scholar]
- Miaz LT, Plassmann MM, Gyllenhammar I, Bignert A, Sandblom O, Lignell S, Glynn A, Benskin JP. 2020. Temporal trends of suspect- and target-per/polyfluoroalkyl substances (PFAS), extractable organic fluorine (EOF) and total fluorine (TF) in pooled serum from first-time mothers in Uppsala, Sweden, 1996–2017. Environmental Science Processes & Impacts 22:1071–1083. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Kato M, Urano K. 2007a. A method for measuring semi- and non-volatile organic halogens by combustion ion chromatography. Journal of Chromatography A 1139:63–69. DOI: 10.1016/j.chroma.2006.10.078. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Yamashita N, Rostkowski P, So MK, Taniyasu S, Lam PKS, Kannan K. 2007b. Determination of trace levels of total fluorine in water using combustion ion chromatography for fluorine: A mass balance approach to determine individual perfluorinated chemicals in water. Journal of Chromatography A 1143:98–104. DOI: 10.1016/j.chroma.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Mogensen U, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. 2015. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environmental Science & Technology 49:10466–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C, Field J. 1999. Determination of perfluorocarboxylates in groundwater impacted by fire-fighting activity. Environmental Science & Technology 33:2800–2806. [Google Scholar]
- Moody CA, Field JA. 2000. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environmental Science & Technology 34:3864–3870. DOI: 10.1021/es991359u. [DOI] [Google Scholar]
- Moriwaki H, Takata Y, Arakawa R. 2003. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in vacuum cleaner dust collected in Japanese homes. Journal of Environmental Monitoring 5:753–757. DOI: 10.1039/B307147M. [DOI] [PubMed] [Google Scholar]
- Muir D, Bossi R, Carlsson P, Evans M, De Silva A, Halsall C, Rauert C, Herzke D, Hung H, Letcher R, Riget F, Roos A. 2019. Levels and trends of poly- and perfluoroalkyl substances in the Arctic environment: An update. Emerging Contaminants 5:240–271. [Google Scholar]
- Munoz G, Desrosiers M, Duy SV, Labadie P, Budzinsk H, Liu JX, Sauve S. 2017. Environmental Occurrence of Perfluoroalkyl Acids and Novel Fluorotelomer Surfactants in the Freshwater Fish Catostomus commersonii and Sediments Following Firefighting Foam Deployment at the Lac-Megantic Railway Accident. Environmental Science & Technology 51:1231–1240. DOI: 10.1021/acs.est.6b05432. [DOI] [PubMed] [Google Scholar]
- Munoz G, Desrosiers M, Vetter L, Duy SV, Jarjour J, Liu JX, Sauve S. 2020. Bioaccumulation of Zwitterionic Polyfluoroalkyl Substances in Earthworms Exposed to Aqueous Film-Forming Foam Impacted Soils. Environmental Science & Technology 54:1687–1697. DOI: 10.1021/acs.est.9b05102. [DOI] [PubMed] [Google Scholar]
- Musijowski J, Trojanowicz M, Szostek B, Lima J, Lapa R, Yamashita H, Takayanagi T, Motomizu S. 2007. Flow-injection determination of total organic fluorine with off-line defluorination reaction on a solid sorbent bed. Anal Chim Acta 600:147–154. DOI: 10.1016/j.aca.2007.03.068. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Hirata T, Terada T, Jutabha P, Miura D, Harada KH, Inoue K, Anzai N, Endou H, Inui KI, Kanai Y, Koizumi A. 2008. Roles of organic anion transporters in the renal excretion of perfluorooctanoic acid. Basic Clin Pharmacol Toxicol 103:1–8. DOI: 10.1111/j.1742-7843.2007.00155.x. [DOI] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General US Population. Environmental Health Perspectives 118:197–202. DOI: 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Scammell MK, Hatch EE, Webster TF. 2012. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003-2006. Environmental Health 11:15. DOI: 10.1186/1476-069x-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S, McMahen R, Stoeckel JA, Chislock M, Lindstrom A, Strynar M. 2017. Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama. Environmental Science & Technology 51:1544–1552. DOI: 10.1021/acs.est.6b05330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CA, Hungerbuehler K. 2015. Exploring the Use of Molecular Docking to Identify Bioaccumulative Perfluorinated Alkyl Acids (PFAAs). Environmental Science & Technology 49:12306–12314. DOI: 10.1021/acs.est.5b03000. [DOI] [PubMed] [Google Scholar]
- Ng CA, Hungerbuhler K. 2013. Bioconcentration of Perfluorinated Alkyl Acids: How Important Is Specific Binding? Environmental Science & Technology 47:7214–7223. DOI: 10.1021/es400981a. [DOI] [PubMed] [Google Scholar]
- Ng CA, Hungerbuhler K. 2014. Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environmental Science & Technology 48:4637–4648. DOI: 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- NHDES. 2020. New Hampshire Department of Environmental Services. NH PFAS investigation, St. Gobain. New Hampshire Department of Environmental Services: Concord, NH. https://www4.des.state.nh.us/nh-pfas-investigation/?cat=8 [accessed 05 May 2020]. [Google Scholar]
- Nilsson H, Karrman A, Rotander A, van Bavel B, Lindstrom G, Westberg H. 2010. Inhalation Exposure to Fluorotelomer Alcohols Yield Perfluorocarboxylates in Human Blood? Environmental Science & Technology 44:7717–7722. DOI: 10.1021/es101951t. [DOI] [PubMed] [Google Scholar]
- NYDEC. 2020. New York Department of Environmental Conservation, Hoosick Falls area, information for communities impacted by per- and poly-fluoroalkyl substances (PFAS). New York Department of Environmental Conservation: Albany, NY. https://www.dec.ny.gov/chemical/108791.html [accessed 05 May 2020]. [Google Scholar]
- OECD. 2018. Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per and Polyfluoroalkyl Substances (PFASs). . [Google Scholar]
- Okada E, Kashino I, Matsuura H, Sasaki S, Miyashita C, Yamamoto J, Ikeno T, Ito YM, Matsumura T, Tamakoshi A, Kishi R. 2013. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environment International 60:89–96. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Burlew MM, Mandel JH. 2003a. Epidemiologic Assessment of Worker Serum Perfluorooctanesulfonate (PFOS) and Perfluorooctanoate (PFOA) Concentrations and Medical Surveillance Examinations. Journal of Occupational and Environmental Medicine 45:260–270. DOI: 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Mandel JH, Zobel LR. 1999. Serum perfluorooctane sulfonate and hepatic and lipid clinical chemistry tests in fluorochemical production employees. Journal of Occupational and Environmental Medicine 41:799–806. DOI: 10.1097/00043764-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Huang H-Y, Helzlsouer KJ, Hansen KJ, Butenhoff JL, Mandel JH. 2005. Historical Comparison of Perfluorooctanesulfonate, Perfluorooctanoate, and Other Fluorochemicals in Human Blood. Environmental Health Perspectives 113:539–545. DOI: doi: 10.1289/ehp.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Lange CC, Ellefson ME, Mair DC, Church TR, Goldberg CL, Herron RM, Medhdizadehkashi Z, Nobiletti JB, Rios JA, Reagen WK, Zobel LR. 2012. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environmental Science & Technology 46:6830–6838. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Logan PW, Hansen KJ, Simpson CA, Burris JM, Burlew MM, Vorarath PP, Venkateswarlu P, Schumpert JC, Mandel JH. 2003b. An Occupational Exposure Assessment of a Perfluorooctanesulfonyl Fluoride Production Site: Biomonitoring. AIHA Journal 64:651–659. DOI: 10.1080/15428110308984859. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Zobel LR. 2007. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. International Archives of Occupational and Environmental Health 81:231–246. DOI: 10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- Padilla-Sánchez JA, Papadopoulou E, Poothong S, Haug LS. 2017. Investigation of the Best Approach for Assessing Human Exposure to Poly- and Perfluoroalkyl Substances through Indoor Air. Environmental Science & Technology 51:12836–12843. DOI: 10.1021/acs.est.7b03516. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Sun Y, Guo Y, Dai J. 2018. Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environmental Science & Technology 52:7621–7629. DOI: 10.1021/acs.est.8b00829. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Sabaredzovic A, Namork E, Nygaard U, Granum B, Haug L. 2016. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): The association with breastfeeding and maternal PFAS concentrations. Environment International 94:687–694. [DOI] [PubMed] [Google Scholar]
- Park H, Choo G, Kim H, Oh J-E. 2018. Evaluation of the current contamination status of PFASs and OPFRs in South Korean tap water associated with its origin. Science of The Total Environment 634:1505–1512. DOI: 10.1016/j.scitotenv.2018.04.068. [DOI] [PubMed] [Google Scholar]
- Paul A, Scheringer M, Hungerbuhler K, Loos R, Jones K, Sweetman A. 2012. Estimating the aquatic emissions and fate of perfluorooctane sulfonate (PFOS) into the river Rhine. Journal of Environmental Monitoring 14:524–530. [DOI] [PubMed] [Google Scholar]
- Paul AG, Jones KC, Sweetman AJ. 2009. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environmental Science & Technology 43:386–392. [DOI] [PubMed] [Google Scholar]
- Paustenbach D, Panko J, Scott P, Unice K. 2007. A methodology for estimating human exposure to perfluorooctanoic acid (PFOA): a retrospective exposure assessment of a community (1951-2003). J Toxicol Environ Health A 70:28–57. [DOI] [PubMed] [Google Scholar]
- Penland T, Cope G, Kwak T, Strynar M, Greishaber C, Heise R, Sessions F. 2020. Trophodynamics of Per- and Polyfluoroalkyl Substances in the Food Web of a Large Atlantic Slope River. Environmental Science & Technology 54:6800–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard HM, Criscitiello AS, Spencer C, Sharp MJ, Muir DCG, De Silva AO, Young CJ. 2018. Continuous non-marine inputs of per- and polyfluoroalkyl substances to the High Arctic: a multi-decadal temporal record. Atmospheric Chemistry and Physics 18:5045–5058. DOI: 10.5194/acp-18-5045-2018. [DOI] [Google Scholar]
- Pistocchi A, Loos R. 2009. A map of European emissions and concentrations of PFOS and PFOA. Environmental Science & Technology 43:9237–9244. [DOI] [PubMed] [Google Scholar]
- Plassmann MM, Berger U. 2013. Perfluoroalkyl carboxylic acids with up to 22 carbon atoms in snow and soil samples from a ski area. Chemosphere 91:832–837. DOI: 10.1016/j.chemosphere.2013.01.066. [DOI] [PubMed] [Google Scholar]
- Poothong S, Papadopoulou E, Lundanes E, Padilla-Sanchez J, Thomsen C, Haug LS. 2019. Dried blood spots for reliable biomonitoring of poly- and perfluoroalkyl substances (PFASs). Science of The Total Environment 655:1420–1426. [DOI] [PubMed] [Google Scholar]
- Poothong S, Papadopoulou E, Padilla-Sanchez JA, Thomsen C, Haug LS. 2020. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environment International 134:9. DOI: 10.1016/j.envint.2019.105244. [DOI] [PubMed] [Google Scholar]
- Poothong S, Thomsen C, Padilla-Sanchez JA, Papadopoulou E, Haug LS. 2017. Distribution of Novel and Well-Known Poly- and Perfluoroalkyl Substances (PFASs) in Human Serum, Plasma, and Whole Blood. Environmental Science & Technology 51:13388–13396. DOI: 10.1021/acs.est.7b03299. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins I, Buck R, Korzeniowski S. 2006. Sources, fate and transport of perfluorocarboxylates. Environmental Science & Technology 40:32–44. [DOI] [PubMed] [Google Scholar]
- Qin ZW, McNee D, Gleisner H, Raab A, Kyeremeh K, Jaspars M, Krupp E, Deng H, Feldmann J. 2012. Fluorine Speciation Analysis Using Reverse Phase Liquid Chromatography Coupled Off-Line to Continuum Source Molecular Absorption Spectrometry (CS-MAS): Identification and Quantification of Novel Fluorinated Organic Compounds in Environmental and Biological Samples. Anal Chem 84:6213–6219. DOI: 10.1021/ac301201y. [DOI] [PubMed] [Google Scholar]
- Ramli MR, Yoneda M, Mohd MA, Haron DEM, Ahmad ED. 2020. Level and determinants of serum perfluoroalkyl acids (PFAAs) in a population in Klang Valley, Malaysia. Int J Hyg Environ Health 223:179–186. DOI: 10.1016/j.ijheh.2019.09.005. [DOI] [PubMed] [Google Scholar]
- Rankin K, Lee H, Tseng P, Maybury S. 2014. Investigating the biodegradability of a fluorotelomer-based acrylate polymer in a soil-plant microcosm by inndirect and direct analysis. Environmental Science & Technology 48:12783–12790. [DOI] [PubMed] [Google Scholar]
- Rankin K, Maybury S. 2015. Matrix normalized MALDI-TOF quantification of a fluorotelomer-based acrylate polymer. Environmental Science & Technology 49:6093–6101. [DOI] [PubMed] [Google Scholar]
- Rauert C, Shoieb M, Schuster JK, Eng A, Harner T. 2018. Atmospheric concentrations and trends of poly- and perfluoroalkyl substances (PFAS) and volatile methyl siloxanes (VMS) over 7 years of sampling in the Global Atmospheric Passive Sampling (GAPS) network. Environmental Pollution 238:94–102. DOI: 10.1016/j.envpol.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Reiner JL, Place BJ. 2015. Perfluorinated Alkyl Acids in Wildlife. In DeWitt JC, ed, Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. -Molecular and Integrative Toxicology. Springer-Verlag London Ltd, Godalming, pp 127–150. [Google Scholar]
- Ritter EE, Dickinson ME, Harron JP, Lunderberg DM, DeYoung PA, Robel AE, Field JA, Peaslee GF. 2017. PIGE as a screening tool for Per- and polyfluorinated substances in papers and textiles. Nucl Instrum Methods Phys Res Sect B-Beam Interact Mater Atoms 407:47–54. DOI: 10.1016/j.nimb.2017.05.052. [DOI] [Google Scholar]
- Robel AE, Marshall K, Dickinson M, Lunderberg D, Butt C, Peaslee G, Stapleton HM, Field JA. 2017. Closing the Mass Balance on Fluorine on Papers and Textiles. Environmental Science & Technology 51:9022–9032. DOI: 10.1021/acs.est.7b02080. [DOI] [PubMed] [Google Scholar]
- Ross MS, Wong CS, Martin JW. 2012. Isomer-Specific Biotransformation of Perfluorooctane Sulfonamide in Sprague-Dawley Rats. Environmental Science & Technology 46:3196–3203. DOI: 10.1021/es204028v. [DOI] [PubMed] [Google Scholar]
- Rotander A, Karrman A, Toms LM, Kay M, Mueller JF, Gomez Ramos MJ. 2015a. Novel fluorinated surfactants tentatively identified in firefighters using liquid chromatography quadrupole time-of-flight tandem mass spectrometry and a case-control approach. Environ Sci Technol 49:2434–2442. DOI: 10.1021/es503653n. [DOI] [PubMed] [Google Scholar]
- Rotander A, Karrman A, Toms LML, Kay M, Mueller JF, Ramos MJG. 2015b. Novel Fluorinated Surfactants Tentatively Identified in Firefighters Using Liquid Chromatography Quadrupole Time-of-Flight Tandem Mass Spectrometry and a Case-Control Approach. Environmental Science & Technology 49:2434–2442. DOI: 10.1021/es503653n. [DOI] [PubMed] [Google Scholar]
- Salice CJ, Anderson TA, Anderson RH, Olson AD. 2018. Ecological risk assessment of perfluooroctane sulfonate to aquatic fauna from a bayou adjacent to former fire training areas at a US Air Force installation. Environmental Toxicology and Chemistry 37:2198–2209. DOI: 10.1002/etc.4162. [DOI] [PubMed] [Google Scholar]
- Salvalaglio M, Muscionico I, Cavallotti C. 2010. Determination of Energies and Sites of Binding of PFOA and PFOS to Human Serum Albumin. J Phys Chem B 114:14860–14874. DOI: 10.1021/jp106584b. [DOI] [PubMed] [Google Scholar]
- Samanipour S, Martin J, Lamoree M, Reid M, Thomas K. 2019. Letter to the Editor: Optimism for nontarget analysis in environmental chemistry. Environmental Science & Technology 53:5529–5530. [DOI] [PubMed] [Google Scholar]
- Schellenberger S, Hill PJ, Levenstam O, Gillgardd P, Cousins IT, Taylor M, Blackburn RS. 2019a. Highly fluorinated chemicals in functional textiles can be replaced by re-evaluating liquid repellency and end-user requirements. Journal of Cleaner Production 217:134–143. [Google Scholar]
- Schellenberger S, Jönsson C, Mellin P, Levenstam OA, Liagkouridis I, Ribbenstedt A, Hanning A-C, Schultes L, Plassmann M, Persson C, Cousins I, Benskin J. 2019b. Release of side-chain fluorinated polymer-containing microplastic fibers from functional textiles during washing and first estimates of perfluoroalkyl acid emissions. Environmental Science & Technology 53:14329–14338. [DOI] [PubMed] [Google Scholar]
- Scheringer M, Trier X, Cousins I, de Voogt P, Fletcher T, Wang Z, Webster T. 2014. Helsingør Statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere 114:337–339. [DOI] [PubMed] [Google Scholar]
- Schultes L, Peaslee GF, Brockman JD, Majumdar A, McGuinness SR, Wilkinson JT, Sandblom O, Ngwenyama RA, Benskin JP. 2019. Total Fluorine Measurements in Food Packaging: How Do Current Methods Perform? Environmental Science & Technology Letters 6:73–78. DOI: 10.1021/acs.estlett.8b00700. [DOI] [Google Scholar]
- Schultes L, Sandblom O, Broeg K, Bignert A, Benskin JP. 2020. Temporal Trends (1981-2013) of Per- and Polyfluoroalkyl Substances and Total Fluorine in Baltic cod (Gadus morhua). Environmental Toxicology and Chemistry 39:300–309. DOI: 10.1002/etc.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes L, Vestergren R, Volkova K, Westberg E, Jacobson T, Benskin JP. 2018. Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: implications for environmental emissions and human exposure. Environ Sci-Process Impacts 20:1680–1690. DOI: 10.1039/c8em00368h. [DOI] [PubMed] [Google Scholar]
- Shan G, Wang Z, Zhou L, Du P, Luo X, Wu Q, Zhu L. 2016. Impacts of daily intakes on the isomeric profiles of perfluoroalkyl substances (PFASs) in human serum. Environment International 89-90:62–70. DOI: 10.1016/j.envint.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vestergren R, Nost TH, Zhou Z, Cai Y. 2018. Probing the differential tissue distribution and bioaccumulation behavior of per- and polyfluoroalkyl substances of varying chain-lengths, isomeric structures and functional groups in crucian carp. Environmental Science & Technology 52:4592–4600. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vestergren R, Xu L, Song X, Niu X, Zhang C, Cai Y. 2015. Characterizing direct emissions of perfluoroalkyl substances from ongoing fluoropolymer production sources: A spatial trend study of Xiaoqing River, China. Environ Pollut 206:104-112. Environmental Pollution 206:104–112. [DOI] [PubMed] [Google Scholar]
- Shin H, Vieira V, Ryan P, Detwiler R, Sanders B, Steenland K, Bartell S. 2011a. Environmental fate and transport modeling for perfluorooctanoic acid emitted from the Washington Works Facility in West Virginia. Environmental Science & Technology 45:1435–1442. [DOI] [PubMed] [Google Scholar]
- Shin HM, Vieira VM, Ryan PB, Detwiler R, Sanders B, Steenland K, Bartell SM. 2011b. Environmental Fate and Transport Modeling for Perfluorooctanoic Acid Emitted from the Washington Works Facility in West Virginia. Environmental Science & Technology 45:1435–1442. DOI: 10.1021/es102769t. [DOI] [PubMed] [Google Scholar]
- Shin HM, Vieira VM, Ryan PB, Steenland K, Bartell SM. 2011c. Retrospective Exposure Estimation and Predicted versus Observed Serum Perfluorooctanoic Acid Concentrations for Participants in the C8 Health Project. Environmental Health Perspectives 119:1760–1765. DOI: 10.1289/ehp.1103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Ikonomou M, Kannan K. 2004. Indoor and Outdoor Air Concentrations and Phase Partitioning of Perfluoroalkyl Sulfonamides and Polybrominated Diphenyl Ethers. Environmental Science & Technology 38:1313–1320. DOI: 10.1021/es0305555. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Lee SC, Lane D, Zhu J. 2008. Sorbent-Impregnated Polyurethane Foam Disk for Passive Air Sampling of Volatile Fluorinated Chemicals. Analytical Chemistry 80:675–682. DOI: 10.1021/ac701830s. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Webster GM, Lee SC. 2011. Indoor Sources of Poly- and Perfluorinated Compounds (PFCS) in Vancouver, Canada: Implications for Human Exposure. Environmental Science & Technology 45:7999–8005. DOI: 10.1021/es103562v. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Wilford BH, Jones KC, Zhu J. 2005. Perfluorinated Sulfonamides in Indoor and Outdoor Air and Indoor Dust: Occurrence, Partitioning, and Human Exposure. Environmental Science & Technology 39:6599–6606. DOI: 10.1021/es048340y. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Kannan K. 2006. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environmental Science & Technology 40:1408–1414. [DOI] [PubMed] [Google Scholar]
- Smart B 2001. Fluorine substituent effects (on bioactivity). Journal of Fluorine Chemistry 109:3–11. [Google Scholar]
- Smithwick M, Norstrom RJ, Mabury SA, Solomon K, Evans TJ, Stirling I, Taylor MK, Muir DCG. 2006. Temporal trends of perfluoroalkyl contaminants in polar bears (Ursus maritimus) from two locations in the North American Arctic, 1972-2002. Environmental Science & Technology 40:1139–1143. DOI: 10.1021/es051750h. [DOI] [PubMed] [Google Scholar]
- Solo-Gabriele HM, Jones AS, Lindstrom AB, Lang JR. 2020. Waste type, incineration, and aeration are associated with per- and polyfluoroalkyl levels in landfill leachates. Waste Manage 107:191–200. DOI: 10.1016/j.wasman.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan KM, van Noordenburg C, Plassmann MM, Schultes L, Shaw S, Berger M, Heide-Jorgensen MP, Rosing-Asvid A, Granquist SM, Dietz R, Sonne C, Riget F, Roos A, Benskin JP. 2020. Fluorine Mass Balance and Suspect Screening in Marine Mammals from the Northern Hemisphere. Environmental Science & Technology 54:4046–4058. DOI: 10.1021/acs.est.9b06773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SSEHRI. 2020. Social Science Environmental Health Research Institute. PFAS Contamination Site Tracker. https://pfasproject.com/pfas-contamination-site-tracker/ Updated April 6, 2020. Accessed June 25, 2020.
- Stock NL, Lau FK, Ellis DA, Martin JW, Muir DCG, Mabury SA. 2004. Polyfluorinated Telomer Alcohols and Sulfonamides in the North American Troposphere. Environmental Science & Technology 38:991–996. DOI: 10.1021/es034644t. [DOI] [PubMed] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, McMillan L, Thurman M, Ferrer I, Ball C. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environmental Science & Technology 49:11622–11630. DOI: 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Strynar MJ, Lindstrom AB. 2008. Perfluorinated Compounds in House Dust from Ohio and North Carolina, USA. Environmental Science & Technology 42:3751–3756. DOI: 10.1021/es7032058. [DOI] [PubMed] [Google Scholar]
- Sun JC, Bossi R, Bustnes JO, Helander B, Boertmann D, Dietz R, Herzke D, Jaspers VLB, Labansen AL, Lepoint G, Schulz R, Sonne C, Thorup K, Tottrup AP, Zubrod JP, Eens M, Eulaers I. 2019. White-Tailed Eagle (Haliaeetus albicilla) Body Feathers Document Spatiotemporal Trends of Perfluoroalkyl Substances in the Northern Environment. Environmental Science & Technology 53:12744–12753. DOI: 10.1021/acs.est.9b03514. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu X, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of exposure science & environmental epidemiology 29:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmann HP, Schaider LA, Rodgers KM, Rudel R. 2019. Dietary Habits Related to Food Packaging and Population Exposure to PFASs. Environmental Health Perspectives 127:10. DOI: 10.1289/ehp4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YM, Clewell HJ, Andersen ME. 2008. Time dependencies in perfluorooctylacids disposition in rat and monkeys: A kinetic analysis. Toxicol Lett 177:38–47. DOI: 10.1016/j.toxlet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Thackray CPP, Selin NEE, Young CJJ. 2020. A global atmospheric chemistry model for the fate and transport of PFCAs and their precursors. Environ Sci-Process Impacts 22:285–293. DOI: 10.1039/c9em00326f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Lorber M, Toms LML, Kato K, Calafat AM, Mueller JF. 2010. Use of simple pharmacokinetic modeling to characterize exposure of Australians to perfluorooctanoic acid and perfluorooctane sulfonic acid. Environment International 36:390–397. DOI: 10.1016/j.envint.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Tian Z, Kim S-K, Shoeib M, Oh J-E, Park J-E. 2016. Human exposure to per- and polyfluoroalkyl substances (PFASs) via house dust in Korea: Implication to exposure pathway. Science of The Total Environment 553:266–275. DOI: 10.1016/j.scitotenv.2016.02.087. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao X-L, Dabeka RW. 2007. Dietary Exposure of Canadians to Perfluorinated Carboxylates and Perfluorooctane Sulfonate via Consumption of Meat, Fish, Fast Foods, and Food Items Prepared in Their Packaging. Journal of Agricultural and Food Chemistry 55:3203–3210. DOI: 10.1021/jf0634045. [DOI] [PubMed] [Google Scholar]
- Tokranov AK, Nishizawa N, Amadei CA, Zenobio JE, Pickard HM, Allen JG, Vecitis CD, Sunderland EM. 2019. How Do We Measure Poly- and Perfluoroalkyl Substances (PFASs) at the Surface of Consumer Products? Environmental Science & Technology Letters 6:38–43. DOI: 10.1021/acs.estlett.8b00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms LM, Thompson J, Rotander A, Hobson P, Calafat AM, Kato K, Ye X, Broomhall S, Harden F, Mueller JF. 2014. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environment International 71:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomy GT, Pleskach K, Ferguson SH, Hare J, Stern G, Macinnis G, Marvin CH, Loseto L. 2009. Trophodynamics of Some PFCs and BFRs in a Western Canadian Arctic Marine Food Web. Environmental Science & Technology 43:4076–4081. DOI: 10.1021/es900162n. [DOI] [PubMed] [Google Scholar]
- Trowbridge J, Gerona RR, Lin T, Rudel RA, Bessonneau V, Buren H, Morello-Frosch R. 2020. Exposure to Perfluoroalkyl Substances in a Cohort of Women Firefighters and Office Workers in San Francisco. Environmental Science & Technology 54:3363–3374. DOI: 10.1021/acs.est.9b05490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K. 2008. Estimating Consumer Exposure to PFOS and PFOA. Risk Analysis 28:251–269. DOI: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- US DOD. 2017. Aqueous Film Forming Foam Report to Congress. Reprot No. 18-C-0270. 13 pp. [Google Scholar]
- US EPA. 2011. Exposure Factors Handbook: 2011 Edition. US EPA Office of Research and Development, Washington DC. EPA/600/R-090/052F. 1466 pp. [Google Scholar]
- US EPA. 2020a. PFAS Master List of PFAS Substances: https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster. US EPA. [Google Scholar]
- US EPA. 2020b. United States Environmental Protection Agency. Wolverine World Wide Tannery. United States Environmental Protection Agency: Washington, D.C. https://www.epa.gov/mi/wolverine-world-wide-tannery [accessed 05 May 2020]. [Google Scholar]
- Vestergren R, Cousins IT. 2009. Tracking the Pathways of Human Exposure to Perfluorocarboxylates. Environmental Science & Technology 43:5565–5575. DOI: 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- Vestergren R, Cousins IT, Trudel D, Wormuth M, Scheringer M. 2008. Estimating the contribution of precursor compounds in consumer exposure to PFOS and PFOA. Chemosphere 73:1617–1624. DOI: 10.1016/j.chemosphere.2008.08.011. [DOI] [PubMed] [Google Scholar]
- VTDEC. 2016. Vermont Department of Environmental Conservation. Vermont PFOA contamination response, information for impacted communities. Vermont Department of Environmental Conservation: Montpelier, VT. http://dec.vermont.gov/commissioners-offcie/pfoa/communities [accessed 05 May 2020]. [Google Scholar]
- Wang Y, Chang W, Wang L, Zhang Y, Zhang Y, Wang M, Wang Y, Li P. 2019. A review of sources, multimedia distribution and health risks of novel fluorinated alternatives. Ecotoxicology and Environmental Safety 182:109402. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shi Y, Vestergren R, Zhou Z, Liang Y, Cai Y. 2018. Using hair, naril and urine samples for human exposure assessment of legacy and emerging per- and polyfluoroalkyl substances. Science of The Total Environment 636:318–391. [DOI] [PubMed] [Google Scholar]
- Wang Z, Boucher J, Scheringer M, Cousins I, Hungerbühler K. 2017a. Toward a Comprehensive Global Emission Inventory of C4–C10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C8-Based Products and Ongoing Industrial Transition. Environmental Science & Technology 51:4482–4493. [DOI] [PubMed] [Google Scholar]
- Wang Z, Boucher J, Scheringer M, Cousins I, Hungerbühler K. 2017b. Toward a Comprehensive Global Emission Inventory of C4–C10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C8-Based Products and Ongoing Industrial Transition. Environ Sci Technol 51:4482–4493. Environmental Science & Technology 51:4482-4493. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins I, Scheringer M, Buck R, Hungerbuhler K. 2014a. Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environment International 70:62–75. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins I, Scheringer M, Buck R, Hungerbühler K. 2014b. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: The remaining pieces of the puzzle. Environment International 69:166–176. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xie ZY, Mi WY, Moller A, Wolschke H, Ebinghaus R. 2015. Neutral Poly/Per-Fluoroalkyl Substances in Air from the Atlantic to the Southern Ocean and in Antarctic Snow. Environmental Science & Technology 49:7770–7775. DOI: 10.1021/acs.est.5b00920. [DOI] [PubMed] [Google Scholar]
- Washington JW, Jenkins TM, Rankin K, Naile JE. 2015. Decades-Scale Degradation of Commercial, Side-Chain, Fluorotelomer-Based Polymers in Soils and Water. Environmental Science & Technology 49:915–923. DOI: 10.1021/es504347u. [DOI] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. 2010. Roles of Rat Renal Organic Anion Transporters in Transporting Perfluorinated Carboxylates with Different Chain Lengths. Toxicological Sciences 113:305–314. DOI: 10.1093/toxsci/kfp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster TF, Harrad S, Millette JR, Holbrook RD, Davis JM, Stapleton HM, Allen JG, McClean MD, Ibarra C, Abdallah MAE, Covaci A. 2009. Identifying Transfer Mechanisms and Sources of Decabromodiphenyl Ether (BDE 209) in Indoor Environments Using Environmental Forensic Microscopy. Environmental Science & Technology 43:3067–3072. DOI: 10.1021/es803139w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW. 2008. Semivolatile organic compounds in indoor environments. Atmos Environ 42:9018–9040. DOI: 10.1016/j.atmosenv.2008.09.052. [DOI] [Google Scholar]
- Winkens K, Giovanoulis G, Koponen J, Vestergren R, Berger U, Karvonen AM, Pekkanen J, Kiviranta H, Cousins IT. 2018. Perfluoroalkyl acids and their precursors in floor dust of children's bedrooms – Implications for indoor exposure. Environment International 119:493–502. DOI: 10.1016/j.envint.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Wong F, Shoeib M, Katsoyiannis A, Eckhardt S, Stohl A, Bohlin-Nizzetto P, Li H, Fellin P, Su Y, Hung H. 2018. Assessing temporal trends and source regions of per- and polyfluoroalkyl substances (PFASs) in air under the Arctic Monitoring and Assessment Programme (AMAP). Atmospheric Environment 172:65–73. DOI: 10.1016/j.atmosenv.2017.10.028. [DOI] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye XY, Calafat AM, Campbell S, Woudneh MB, Fisher J. 2017. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environment International 106:135–143. DOI: 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F 2017. Emerging poly- and perfluoroalkyl substances in the aquatic environment: A review of current literature. Water Research 124:482–495. DOI: 10.1016/j.watres.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Xie S, Lu Y, Wang T, Liu S, Jones K, Sweetman A. 2013. Estimation of PFOS emission from domestic sources in the eastern coastal region of China. Environment International 59:336–343. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Taniyasu S, Petrick G, Wei S, Gamo T, Lam PKS, Kannan K. 2008. Perfluorinated acids as novel chemical tracers of global circulation of ocean waters. Chemosphere 70:1247–1255. DOI: 10.1016/j.chemosphere.2007.07.079. [DOI] [PubMed] [Google Scholar]
- Yang DW, Han JJ, Hall DR, Sun JX, Fu J, Kutarna S, Houck KA, LaLone CA, Doering JA, Ng CA, Peng H. 2020. Nontarget Screening of Per- and Polyfluoroalkyl Substances Binding to Human Liver Fatty Acid Binding Protein. Environmental Science & Technology 54:5676–5686. DOI: 10.1021/acs.est.0c00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Zhao Y, Sun H, Chang S, Zhu L, Alder AC, Kannan K. 2018. Per- and Polyfluoroalkyl Substances (PFASs) in Indoor Air and Dust from Homes and Various Microenvironments in China: Implications for Human Exposure. Environmental Science & Technology 52:3156–3166. DOI: 10.1021/acs.est.7b04971. [DOI] [PubMed] [Google Scholar]
- Yarwood G, Kemball-Cook S, Keinath M, Waterland R, Korzeniowski S, Buck R, Russell M, Washburn S. 2007. High-resolution atmospheric modeling of fluorotelomer alcohols and perfluorocarboxylic acids in the North American troposphere. Environmental Science & Technology 41:5756–5762. [DOI] [PubMed] [Google Scholar]
- Yeung LW, Mabury SA. 2016. Are humans exposed to increasing amounts of unidentified organofluorine? Environmental Chemistry 13:102–110. [Google Scholar]
- Yeung LW, Robinson SJ, Koschorreck J, Mabury SA. 2013a. Part II. A temporal study of PFOS and its precursors in human plasma from two German cities in 1982–2009. Environmental Science & Technology 47:3875–3882. [DOI] [PubMed] [Google Scholar]
- Yeung LWY, Dassuncao C, Mabury S, Sunderland EM, Zhang XM, Lohmann R. 2017. Vertical Profiles, Sources, and Transport of PFASs in the Arctic Ocean. Environmental Science & Technology 51:6735–6744. DOI: 10.1021/acs.est.7b00788. [DOI] [PubMed] [Google Scholar]
- Yeung LWY, De Silva AO, Loi EIH, Marvin CH, Taniyasu S, Yamashita N, Mabury SA, Muir DCG, Lam PKS. 2013b. Perfluoroalkyl substances and extractable organic fluorine in surface sediments and cores from Lake Ontario. Environment International 59:389–397. DOI: 10.1016/j.envint.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Yeung LWY, Mabury SA. 2013c. Bioconcentration of Aqueous Film-Forming Foam (AFFF) in Juvenile Rainbow Trout (Oncorhyncus mykiss). Environmental Science & Technology 47:12505–12513. DOI: 10.1021/es403170f. [DOI] [PubMed] [Google Scholar]
- Yu NY, Guo HW, Yang JP, Jin L, Wang XB, Shi W, Zhang XW, Yu HX, Wei S. 2018. Non-Target and Suspect Screening of Per- and Polyfluoroalkyl Substances in Airborne Particulate Matter in China. Environmental Science & Technology 52:8205–8214. DOI: 10.1021/acs.est.8b02492. [DOI] [PubMed] [Google Scholar]
- Zhang LY, Ren XM, Guo LH. 2013. Structure-Based Investigation on the Interaction of Perfluorinated Compounds with Human Liver Fatty Acid Binding Protein. Environmental Science & Technology 47:11293–11301. DOI: 10.1021/es4026722. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lohmann R, Dassuncao C, Hu XC, Weber AK, Vecitis CD, Sunderland EM. 2016. Source Attribution of Poly- and Perfluoroalkyl Substances (PFASs) in Surface Waters from Rhode Island and the New York Metropolitan Area. Environmental Science & Technology Letters 3:316–321. DOI: 10.1021/acs.estlett.6b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang Y, Dassuncao C, Lohmann R, Sunderland EM. 2017. North Atlantic deep water formation inhibits high Arctic contamination by continental perfluorooctane sulfonate discharges. 2017 Global Biogeochemical Cycles:8. [Google Scholar]
- Zhang XM, Lohmann R, Sunderland EM. 2019. Poly- and Perfluoroalkyl Substances in Seawater and Plankton from the Northwestern Atlantic Margin. Environmental Science & Technology 53:12348–12356. DOI: 10.1021/acs.est.9b03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng GM, Boor BE, Schreder E, Salamova A. 2020. Indoor exposure to per- and polyfluoroalkyl substances (PFAS) in the childcare environment. Environmental Pollution 258:8. DOI: 10.1016/j.envpol.2019.113714. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liang Y, Shi YL, Xu L, Cai YQ. 2013. Occurrence and Transport of Perfluoroalkyl Acids (PFAAs), Including Short-Chain PFAAs in Tangxun Lake, China. Environmental Science & Technology 47:9249–9257. DOI: 10.1021/es402120y. [DOI] [PubMed] [Google Scholar]