Abstract
COVID-19 took a heavy toll on older adults. In Belgium, by the end of August, 93% of deaths due to COVID-19 were aged 65 or older. Similar trends were observed in other countries. As a consequence, older adults were identified as a group at risk, and strict governmental restrictions were imposed on them. This has caused concerns about their mental health. Using an online survey, this study established the impact of the COVID-19 pandemic on adults aged 65 years or older, and which factors moderate this impact. Participants reported a significant decrease in activity level, sleep quality and wellbeing during the COVID-19 pandemic. Depression was strongly related to reported declines in activity level, sleep quality, wellbeing and cognitive functioning. Our study shows that the COVID-19 pandemic had a severe impact on the mental health of older adults. This implies that this group at risk requires attention of governments and healthcare.
Subject terms: Geriatrics, Quality of life, Ageing, Human behaviour
Introduction
The COVID-19 pandemic took a heavy toll on older adults. For example in Belgium, approximately 41% of the cases were aged 60 or older, but 93% of deaths due to COVID-19 were aged 65 or older1. Similar trends were observed in other countries (e.g.,2). This implied that older adults became a specific focus of several governmental COVID-19 regulations. For example, in Belgium they were no longer allowed to look after their grandchildren, visitors and external services (e.g., meal delivery, cleaning) were not allowed in assisted living facilities and nursing homes for a long time, and people were regularly warned when measures were introduced or relaxed to pay specific attention to older and vulnerable people.
Hence, during the COVID-19 pandemic, older adults were considered as a group at risk. As a response, concerns have risen about the mental health of older adults. The World Health Organization (WHO) has warned that the impact on mental and psychosocial wellbeing of vulnerable groups, such as older adults, will be large and enduring3. The United Nations (UN) stressed that, although COVID-19 is in the first place a physical health crisis, it has the seeds of a major mental health crisis as well, especially for specific populations such as older adults, if action is not taken4. It has been suggested that the measures taken by governance regarding social distancing and isolation, especially targeting groups at risk, can result in social isolation and loneliness5,6. The latter variables are known to decrease wellbeing and increase the risk for depression and cognitive dysfunction7. Brooke and Jackson7 pointed out that a decline in activity and mobility in older adults during lockdown can also lead to more frailty and a lower wellbeing in older adults. Moreover, in response to stress, sleep quality can decline and increase the risk for depression8. Finally, as older adults already face cognitive decline as part of normal aging9,10, which is moderated by several lifestyle variables such as physical activity, engagement in stimulating activities and social network11, the COVID-19 pandemic causing social isolation and loss of activity, might also impact cognitive functioning.
These concerns warrant a thorough assessment of how older adults are currently doing. However, little is hitherto known about the impact of the COVID-19 pandemic on the general population of older adults. A few first studies are being published now, and seem to indicate that older adults’ wellbeing was not severely impacted by the COVID-19 pandemic. However, as the authors indicated, these studies were either conducted in the very early stages of the pandemic when social distancing was recommended by the government but no lockdown was yet installed12,13, or in countries such as the Netherlands where at the time of the study the lockdown was not restrictive14. Findings of disaster studies might be relevant in this respect. For example, studies on the effect of natural disasters on older adults showed a disaster-related decline in cognitive function15. Results on the effect on wellbeing are mixed, and indicated that being older did not necessarily increase psychological vulnerability (16; see also the “wellbeing paradox”17). However, socialising and social participation were found to be crucial to reduce the risk of cognitive decline and decrease in wellbeing after such disasters18, which is precisely what has been severely limited during the COVID-19 pandemic, especially for risk groups such as older adults.
Given these concerns and the lack of studies focused on older adults during the peak of the COVID-19 pandemic, the goal of this study was to establish how adults aged 65 years or older are responding to the COVID-19 pandemic. Using self-report measures in an online survey, the impact of the COVID-19 period on wellbeing, activity level, sleep quality and cognitive functioning was studied. Based on the raised concerns and previous studies on the impact of disasters on older adults, we hypothesised that the COVID-19 pandemic had a detrimental effect on the wellbeing, activity level, sleep quality and cognitive functioning of older adults. Furthermore, we examined possible vulnerability and protective factors that might have influenced the impact of the COVID-19 period on the wellbeing and cognitive functioning of older adults.
Results
Participant characteristics and Pearson correlations
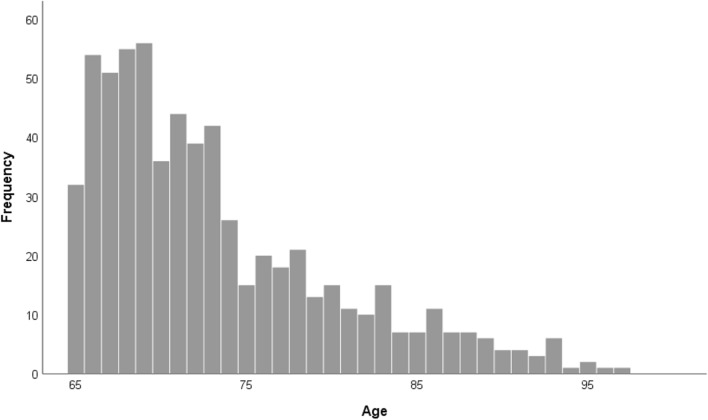
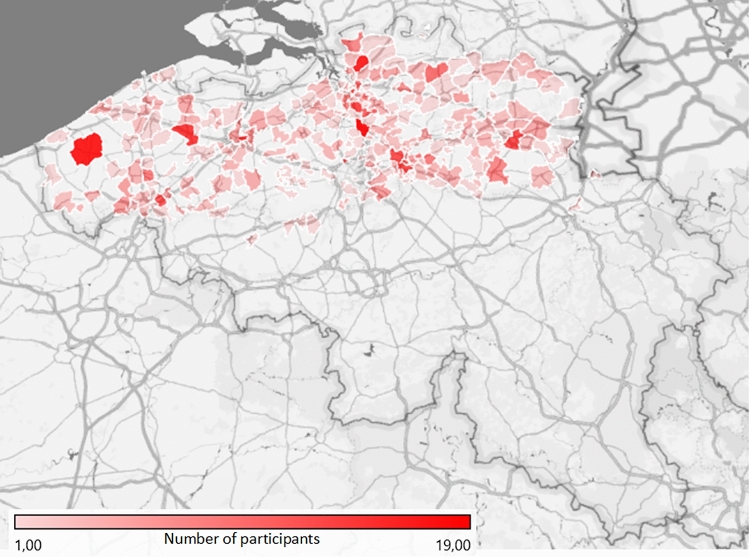
Supplementary Table 1 provides a more detailed overview of the participant characteristics. Participants were on average 73 years old (SD = 6.99, range 65–97). Figure 1 displays the frequency distribution of age. Two hundred sixty-two were male, 377 were female, and one identified themselves with another gender. Geographically, participants were spread across Flanders (i.e., the northern, Dutch-speaking part of Belgium), as can be seen on Fig. 2. The majority of the participants (81%) lived in their own home and 19% lived in an assisted living facility or nursing home (jointly referred to as care facility). 28% lived alone, 54% with one cohabitant and 18% with two or more cohabitants. Most participants (respectively 29%, 41%, 32% and 25%) reported that during the past week they had 3 to 4 contacts (not taking into account cohabitants) in real life outside, no contact in real life inside, 3 to 4 contacts by telephone and more than 9 contacts via the internet (e.g., skype, whatsapp). Most participants (52%) had a university or high school degree and received a monthly individual net income between 1001 and 1500 euro (25%), 1501 and 2000 euro (30%) or 2001 and 2500 euro (17%). The majority of participants (88%) did not suffer from Parkinson’s disease, dementia, stroke, diabetes and/or epilepsy. Only 4% reported that they had been contaminated with COVID-19. 15% reported that at least one of their close relatives or friends had been contaminated with COVID-19.
Figure 1.
Frequency distribution of age.
Figure 2.
Spreading of participants across Flanders. Map generated with Microsoft Excel (2016).
The means (SD) of the total scores of the questionnaires and the Pearson correlations between age and the total scores of the questionnaires in the survey can be found in Table 1 (see also Supplementary Fig. 1 for a matrix scatterplot containing all two-by-two scatter plots).
Table 1.
Means (standard deviations) and Pearson correlations between age and the total scores of the questionnaires in the survey.
| Valid N | Mean (SD) | Age | CFQ | GDS-15 | PWI-A_pre | PWI-A_current | LSNS-6 | BRS | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 640 | 73 (6.99) | – | − 0.19*** | − 0.005 | − 0.045 | 0.020 | − 0.17*** | − 0.009 |
| CFQ | 623 | 22.43 (12.02) | – | 0.28*** | − 0.29*** | − 0.31*** | − 0.035 | − 0.29*** | |
| GDS-15 | 640 | 3.00 (3.01) | – | − 0.47*** | − 0.70*** | − 0.32*** | − 0.45*** | ||
| PWI-A_pre | 640 | 78.05 (11.20) | – | 0.74*** | 0.33*** | 0.42*** | |||
| PWI-A_current | 640 | 70.67 (14.84) | – | 0.35*** | 0.43*** | ||||
| LSNS-6 | 636 | 16.52 (5.65) | – | 0.20*** | |||||
| BRS | 633 | 3.33 (0.67) | - |
CFQ Cognitive Failures Questionnaire total score, GDS-15 Geriatric Depression Scale-15 total score, PWI-A_pre Personal Wellbeing Index-Adults total score retrospectively assessed for the period before COVID-19, PWI-A_current Personal Wellbeing Index-Adults total score assessed for the current month (i.e., during the COVID-19 period); LSNS-6 Lubben Social Network Scale-6 total score, BRS Brief Resilience Scale mean score; ***p < 0.001.
Comparison before and during the COVID-19 period
Table 2 reports the number of participants who reported a decrease, no change or an increase with regards to wellbeing, activity level, sleep quality and cognitive functioning during the COVID-19 period.
Table 2.
Mean (SD) of the reported changes in wellbeing, activity level and sleep quality (all based on the calculated difference scores) and cognitive functioning (based on the subjective cognitive change questions) and the number of participants who reported a decrease (Ndecrease), no change (Nnochange) or an increase (Nincrease) in wellbeing, activity level, sleep quality and cognitive functioning based on the difference scores and the subjective cognitive change questions.
| M(SD) | Ndecrease | Nnochange | Nincrease | ||
|---|---|---|---|---|---|
| Change in wellbeing (PWI-A) | General life satisfaction | − 9.63 (15.34) | 317 | 299 | 24 |
| Standard of living | − 3.98 (11.41) | 140 | 479 | 21 | |
| Health | − 2.88 (9.39) | 130 | 484 | 26 | |
| Achieving in life | − 3.88 (11.09) | 131 | 491 | 18 | |
| Relationships | − 7.09 (15.09) | 213 | 398 | 29 | |
| Safety | − 10.39 (16.10) | 291 | 340 | 9 | |
| Community connectedness | − 10.89 (17.70) | 290 | 323 | 27 | |
| Future security | − 10.39 (14.69) | 306 | 328 | 6 | |
| Subjective wellbeing (PWI-A total score) | − 7.39 (10.02) | 483 | 113 | 44 | |
| Change in activity level | − 0.94 (1.61) | 321 | 276 | 43 | |
| Change in sleep quality | − 0.28 (1.19) | 122 | 472 | 46 | |
| Change in cognitive functioning | Cognitive functioning | 1.94 (0.31) | 50 | 576 | 14 |
| Remembering | 2.93 (0.40) | 53 | 575 | 12 | |
| Concentration | 2.91 (0.44) | 78 | 539 | 23 | |
| Doing two things at the same time | 2.97 (0.35) | 37 | 584 | 19 | |
| Recalling | 2.92 (0.41) | 63 | 560 | 17 | |
| Forgetfulness | 2.92 (0.40) | 63 | 560 | 17 | |
For the subjective cognitive change questions assessing problems with remembering, concentration, doing two things at the same time, recalling and forgetfulness, the response options 1 = ”a lot more problems than before” and 2 = ”more problems than before” were grouped together and the response options 4 = ” less problems than before” and 5 = ”a lot less problems than before” were grouped together in the reported numbers of participants.
With regards to wellbeing, the most prominent decreases were reported for general life satisfaction, safety, community connectedness and future security (see Table 2). The subjective wellbeing score indicated that the majority of the participants (76%) reported a decrease in wellbeing in one or more domains. Half of the participants reported that their activity level had decreased and 19% reported a decreased sleep quality during the COVID-19 period. A minority of the participants (8%) reported that their cognitive functioning in general had decreased during the COVID-19 period, with 8%, 12%, 6%, 10% and 10% indicating increases in problems with remembering things, concentrating on something, doing two things at the same time, recalling things or forgetfulness, respectively.
Paired-samples t-tests with Bonferroni correction (α = 0.0045) indicated that participants reported that they had been significantly less active during the past week (i.e., during the COVID-19 period) compared to before the COVID-19 period (Mcurrent = 6.65; Mbefore = 7.58), t(639) = − 14.69, p < 0.001, d = 0.58. They also reported that their quality of sleep had been poorer during the past week compared to before COVID-19 (Mcurrent = 6.88; Mbefore = 7.16), t(639) = − 5.87, p < 0.001, d = 0.23. Furthermore, life satisfaction in all seven domains, general life satisfaction and subjective wellbeing were significantly lower currently compared to before COVID-19: t(639) = 8.84, p < 0.001, d = 0.35 for standard of living (Mcurrent = 76; Mbefore = 80), t(639) = 7.75, p < 0.001, d = 0.31 for health (Mcurrent = 73; Mbefore = 76), t(639) = 8.84, p < 0.001, d = 0.35 for achieving in life (Mcurrent = 75; Mbefore = 78), t(638) = 11.88, p < 0.001, d = 0.47 for relationships (Mcurrent = 71; Mbefore = 79), t(639) = 16.33, p < 0.001, d = 0.64 for safety (Mcurrent = 70; Mbefore = 81), t(639) = 15.57, p < 0.001, d = 0.61 for community connectedness (Mcurrent = 66; Mbefore = 77), t(639) = 17.90, p < 0.001, d = 0.71 for future security (Mcurrent = 66; Mbefore = 76), t(639) = 15.88, p < 0.001, d = 0.63 for general life satisfaction (Mcurrent = 69; Mbefore = 78) and t(639) = 18.66, p < 0.001, d = 0.74 for the subjective wellbeing index (Mcurrent = 71; Mbefore = 78). Note that when participants filled in the survey (i.e., week 1 to 5; see also Supplementary Table 1) did not lead to significant systematic fluctuations with regards to the reported changes in wellbeing, activity level, sleep quality and cognitive functioning.
Linear and ordinal logistic regression analyses
Supplementary Tables 2 and 3 report the Pearson correlations between the difference scores and subjective cognitive change questions and potential continuous vulnerability and protective variables, as well as the means (SD) for the difference scores and subjective cognitive change questions for each level of the potential categorical vulnerability and protective variables. Linear and ordinal logistic regression analyses were conducted to study possible vulnerability and protective factors. Table 3 provides the model fit of all models and the statistics for the predictors who made a significant contribution to the models. No problems of multicolinearity arose (all VIF < 1.78).
Table 3.
Statistical results of the 17 linear and ordinal logistic regression analyses conducted for changes in wellbeing, activity level and sleep quality (all based on the calculated difference scores) and cognitive functioning (based on the subjective cognitive change questions) with gender, age, whether the participant lived alone or not, whether the participant lived in a care facility or not, monthly individual net income, depression (i.e., GDS-15 total score), social network (i.e., LSNS-6 total score), general susceptibility to cognitive failures (i.e., CFQ total score) and resilience (i.e. BRS total score) as predictors.
| Group of analyses | Dependent variable | Model fit | Predictor | Test statistics |
|---|---|---|---|---|
| Changes in wellbeing (α = 0.0055) | General life satisfaction | F(9,605) = 21.76, p < 0.001, = 0.25 | Depression | β = − 2.77, 95% CI = [− 3.20, − 2.34], t(605) = − 12.66*** |
| Resilience | β = − 2.70, 95% CI = [− 4.56, − 0.85], t(605) = − 2.86** | |||
| Standard of living | F(9,605) = 13.09, p < 0.001, = 0.15 | Depression | β = − 1.29, 95% CI = [− 1.62, − 0.95], t(605) = − 7.48*** | |
| Living in a care facility or not | β = − 7.07, 95% CI = [− 10.02, − 4.12], t(605) = − 4.70*** | |||
| Health | F(9,605) = 8.00, p < 0.001, = 0.093 | Depression | β = − 0.87, 95% CI = [− 1.16, − 0.59], t(605) = − 5.99*** | |
| Achieving in life | F(9,605) = 12.41, p < 0.001, = 0.14 | Depression | β = − 1.43, 95% CI = [− 1.76, − 1.10], t(605) = − 8.55*** | |
| Relationships | F(9,604) = 10.38, p < 0.001, = 0.12 | Depression | β = − 1.72, 95% CI = [− 2.17, − 1.27], t(604) = − 7.47*** | |
| Safety | F(9,605) = 13.38, p < 0.001, = 0.15 | Depression | β = 1.83, 95% CI = [− 2.30, − 1.36], t(605) = − 7.62*** | |
| Community connectedness | F(9,605) = 13.24, p < 0.001, = 0.15 | Depression | β = − 2.53, 95% CI = [− 3.05, − 2.01], t(605) = − 9.50*** | |
| Future security | F(9,605) = 15.46, p < 0.001, = 0.18 | Depression | β = − 1.87, 95% CI = [− 2.29, − 1.44], t(605) = − 8.70*** | |
| Subjective wellbeing | F(9,605) = 25.27, p < 0.001, = 0.26 | Depression | β = − 1.79, 95% CI = [− 2.06, − 1.51], t(605) = − 12.78*** | |
| Changes in activity level and sleep quality (α = 0.025) | Activity level | F(9,605) = 12.54, p < 0.001, = 0.15 | Depression | β = − 0.22, 95% CI = [− 0.27, − 0.17], t(605) = − 9.02*** |
| Sleep Quality | F(9,605) = 8.39, p < 0.001, = 0.098 | Depression | β = − 0.12, 95% CI = [–0.16, − 0.084], t(605) = − 6.60*** | |
| Gender | β = 0.25, 95% CI = [0.055,0.45], t(598) = 2.52* | |||
| Changes in cognitive functioning (α = 0.0083) | Cognitive functioning | χ2(9) = 94.49, p < 0.001, Nagelkerke R2 = 0.27 | Depression | β = − 0.28, 95% CI = [− 0.38, − 0.18]; OR = 0.76; Wald(1) = 30.03*** |
| CFQ | β = − 0.061, 95% CI = [− 0.086, − 0.035]; OR = 0.94; Wald(1) = 22.06*** | |||
| Remembering | χ2(9) = 94.10, p < 0.001, Nagelkerke R2 = 0.25 | Depression | β = − 0.24, 95% CI = [− 0.34, − 0.14]; OR = 0.79; Wald(1) = 22.19*** | |
| CFQ | β = − 0.076, 95% CI = [− 0.10, − 0.050]; OR = 0.93; Wald(1) = 32.66*** | |||
| Concentration | χ2(9) = 96.92, p < 0.001, Nagelkerke R2 = 0.22 | Depression | β = − 0.24, 95% CI = [− 0.33, − 0.16]; OR = 0.79; Wald(1) = 32.83*** | |
| CFQ | β = − 0.049, 95% CI = [− 0.069, − 0.028]; OR = 0.95; Wald(1) = 22.13*** | |||
| Doing two things at the same time | χ2(9) = 49.50, p < 0.001, Nagelkerke R2 = 0.15 | Depression | β = − 0.20, 95% CI = [− 0.31, − 0.09]; OR = 0.82; Wald(1) = 12.94*** | |
| CFQ | β = − 0.048, 95% CI = [− 0.074, − 0.022]; OR = 0.95; Wald(1) = 12.75*** | |||
| Recalling | χ2(9) = 74.79, p < 0.001, Nagelkerke R2 = 0.19 | Depression | β = − 0.14, 95% CI = [− 0.23, − 0.049]; OR = 0.87; Wald(1) = 9.30** | |
| CFQ | β = − 0.062, 95% CI = [− 0.084, − 0.039]; OR = 0.94; Wald(1) = 29.74*** | |||
| Forgetfulness | χ2(9) = 96.24, p < 0.001, Nagelkerke R2 = 0.24 | Depression | β = − 0.22, 95% CI = [− 0.31, − 0.13]; OR = 0.80; Wald(1) = 21.94*** | |
| CFQ | β = − 0.065, 95% CI = [− 0.088, − 0.041]; OR = 0.94; Wald(1) = 30.09*** |
Both the fit of the full model (Model fit) and the test statistics for the significant predictors are reported.
Only predictors that contributed significantly to the model are reported; OR = odds-ratio; *p < 0.02; **p < 0.005; ***p < 0.001.
Changes in wellbeing (α = 0.0055)
With regards to general life satisfaction, the model indicated that at least one predictor significantly contributed to explaining changes in this wellbeing item. Changes in general life satisfaction showed a significant negative linear relation with depression. This implies that an increase in depression was associated with a (stronger) decrease in satisfaction with standard of living during the COVID-19 period. Changes in general life satisfaction also showed a significant negative linear relation with resilience. However, as this association is contrary to the positive correlation coefficient observed between these two variables (r = 0.11, see Supplementary Table 2), this could rather indicate a suppressor situation (see19,20), leading us to be cautious about this effect. With regards to satisfaction with standard of living, the model indicated that at least one predictor significantly contributed to explaining changes in this wellbeing item. Changes in satisfaction with standard of living showed a significant negative linear relation with both depression and whether the participant lived in a care facility or not. This implies that an increase in depression and living in a care facility as compared to not living in a care facility (as “not living in a care facility” was set as the reference category of our dummy variable) were associated with a (stronger) decrease in satisfaction with standard of living during the COVID-19 period. For all other PWI-A items and total score, models always indicated that at least one predictor significantly contributed to explaining changes in these items or total score. For all these models, there was only a significant negative linear relation between depression and changes in these wellbeing items and total score. This implies that an increase in depression was associated with a decrease in satisfaction with all domains and in subjective wellbeing during COVID-19. None of the other predictors contributed significantly to the models (all p > 0.007).
Changes in activity and sleep (α = 0.025)
The model indicated that at least one predictor significantly contributed to explaining changes in activity level. There was a significant negative linear relation between depression and changes in activity. This implies that an increase in depression was associated with a decrease in activity level during the COVID-19 period. None of the other predictors contributed significantly to the model (all p ≥ 0.12). With regards to sleep quality, the model indicated that at least one predictor significantly contributed to explaining changes in sleep quality. There was a significant negative linear relation between depression and changes in sleep quality and a positive relation between gender and changes in sleep quality. This implies that an increase in depression and being male as compared to female (as “male” was set as the reference category of our dummy variable) were associated with a (stronger) decrease in sleep quality during COVID-19. None of the other predictors contributed significantly to the model (all p > 0.12).
Changes in cognitive functioning (α = 0.0083)
For all six subjective cognitive change questions, the ordinal logistic models had a significantly better fit compared to the null model. In all models, two predictors always contributed significantly to explaining the changes in cognitive functioning. When depression increased with 1 unit, the ordered log-odds of being in a higher category of the response scale of the subjective cognitive change questions (with a higher category indicating fewer problems, see “Methods” section) decreased by 0.28 for general cognitive functioning and by 0.24, 0.24, 0.20, 0.14 and 0.22 for remembering, concentration, doing two things at the same time, recalling and forgetfulness, respectively, while the other variables in the model are held constant. When the susceptibility to cognitive failures (i.e., CFQ total score) increased with 1 unit, the ordered log-odds of being in a higher category of the response scale of the subjective cognitive change questions decreased by 0.061 for general cognitive functioning and by 0.076, 0.049, 0.048, 0.061 and 0.065 for remembering, concentration, doing two things at the same time, recalling and forgetfulness, while the other variables in the model are held constant. None of the other predictors contributed significantly to the models (all p > 0.015).
We note that including the four variables describing the number of contacts participants had had during the past week (see Supplementary Table 1) as additional predictors in the models, did not lead to significant effects (all p ≥ 0.045) and did not change the pattern of results, except that the association between resilience and changes in general life satisfaction was no longer significant with the set α (p = 0.008). For reasons of parsimony we therefore opted not to include these additional predictors.
Discussion
The COVID-19 pandemic had an enormous impact on older adults aged 65 years or older. The risk of social isolation and loneliness due to governmental regulations raises concerns about the mental health and cognitive functioning of this population. Based on recent editorials about the impact of the COVID-19 pandemic on older adults and literature on the impact of disasters, we hypothesised that wellbeing, level of activity, quality of sleep and cognitive functioning would be severely impacted. To address this, we assessed how older adults are currently doing. Using an online survey with self-report measures, we studied the impact of the COVID-19 period on wellbeing, level of activity, quality of sleep and cognitive functioning of a general population of older adults aged 65 years or older. In addition, vulnerability and protective factors were examined that might have influenced the impact of the COVID-19 period.
In line with our hypothesis, it became clear that the COVID-19 period had a significant impact on older adults. We observed a significant decrease in wellbeing, activity level and sleep quality during the COVID-19 period as compared to before COVID-19. This is not in line with the first studies being published on how older adults responded to the pandemic, which did not observe a severe impact on older adults’ wellbeing12–14. But as these studies were completed during the early onset of the COVID-19 pandemic, this might highlight that the severity of the impact increased when the pandemic (and the imposed restrictions) further progressed. Most older adults indicated that their cognitive functioning had not changed during the COVID-19 period, although a subgroup of 8% indicated a decrease.
When examining possible vulnerability and protective factors, reported changes in wellbeing, activity level, sleep quality and cognitive functioning were especially related to depression. These findings suggest that depression might be a vulnerability factor that influenced the impact of the COVID-19 period on older adults. However, given the cross-sectional nature of our data, it is not clear if depression was a precursor, already present before the COVID-19 period, or if depression was triggered and/or intensified during the COVID-19 period. Previous studies showed that depression in itself is negatively correlated with wellbeing, activity and sleep21–23. However, the socioemotional selectivity theory24 could also explain why depression increased as a consequence of the COVID-19 period. This theory proposes that older adults use their social network as a buffer against negative experiences25. As the COVID-19 pandemic led to a decrease of older adults’ social network and contacts, this emotional buffer might have disappeared, which in turn could have paved the way for depression. In order to shed more light on the exact role of depression in the observed COVID-19 changes, we aim to longitudinally follow a substantial subsample of these older adults to study how their reported changes in wellbeing, activity level, sleep quality and cognitive functioning, and the role of depression therein, evolve with the fluctuating COVID-19 situation over time.
Next to depression, other variables such as living in a care facility or not and gender were related to changes in one specific domain of wellbeing and sleep quality, respectively. Susceptibility to cognitive failures was related to changes in cognitive functioning during the COVID-19 period.
Some limitations of this study need to be addressed. First of all, the sample of older adults in this study is rather homogenous in some respects. Participants were all Dutch-speaking older adults living in Flanders. Moreover, most were in good health and their socioeconomic status was high, as indexed by educational level and income. We have compared basic demographics of the sample of older adults in this study with demographic information of the older population in Flanders from 2020 that is available from the Belgian government. Overall, the sample of older adults in this study was younger than the older population in Flanders. The age distribution in Flanders in 2020 was 27.4%, 24.3%, 17.6%, 15.2%, 10.2% and 5.2% respectively for the age ranges 65–69, 70–74, 75–79, 80–84, 85–89 and 90 years or older. The age distribution of our sample was 38.7%, 29.2%, 13.6%, 0.09%, 0.06% and 0.03%, respectively. In addition, the female to male ratio in our sample was slightly different from the female to male ratio in Flanders: 58.9% of our participants were female, compared to 54.6% in the general population of older adults26. These differences in the demographics of the sample urge some caution in generalising the results of this study to the general population of older adults in Flanders and additional studies in different countries, and more heterogeneous samples of older adults, especially targeting the oldest age groups, are needed. Moreover, since this study was administered online, a part of this older adult population could probably not be reached. Third, the design of this study was cross-sectional and administered during the COVID-19 period. Questions regarding the period before COVID-19 were therefore necessarily retrospective, which might lead to biased self-reports27. In addition, this study relied on subjective self-report which can differ from objective states (e.g.28). For example, it has already been shown that cognitive ability tends to be overestimated with increasing age29. During the ongoing COVID-19 pandemic, experimental studies administered to a sample of older adults repeatedly over time (e.g., when the pandemic activity decreases or flares up again in new waves) could shed more light on the actual effect of the COVID-19 period on cognitive functioning.
To summarize, the COVID-19 pandemic had a severe impact on the wellbeing, activity level and sleep quality of older adults. Only a small group of participants reported a decline in cognitive functioning. All changes reported during the COVID-19 period were strongly related to depression. Findings of this study are important because they give a first, thorough assessment of the mental health of older adults during this COVID-19 pandemic. This study showed that the concerns raised about the wellbeing of older adults are justified, and that this group at risk requires the attention of governments and healthcare. Furthermore, this study exposed that when we are faced with extreme stressors, such as COVID-19, in the future, prevention and intervention strategies are needed to aid older adults to prepare for and cope with them, especially for those at risk of depression (see also suggestions by Armitage and Nellums30 and Tian et al.31). Specifically, since social networks can act as a buffer against negative events according to the socioemotional selectivity theory24, it might be beneficial to devote more attention to the importance of maintaining strong social relationships during major stressors, such as the COVID-19 pandemic. Social media usage and telephone contact could increase social interactions among the older adult population30,32. Media actions might help in stressing the importance of maintaining such interactions for older adults. In addition, improving social skills could aid in preventing loneliness and decreased wellbeing, as for example suggested by Masi et al.33. Psychological counselling could play an important role here. Finally, as COVID-19 has complicated the accessibility of this population through social distancing, nursing home restrictions, and an overwhelmed health system, we are urged to explore novel ways to reach older adults34.
Methods
Participants
Participants were recruited through social media, radio news, targeted newsletters and discussion fora and electronic mailings to all directors of assisted living facilities and nursing homes in Flanders. Only adults aged 65 years or older with a thorough knowledge of Dutch and living in Belgium could participate in the study. Only data of participants who filled in at least 50% of the survey were retained for analysis to ensure sufficient data quality. If a participant completed the survey more than once (N = 5), only the most complete, or, in case all entries were equally complete, the first entry was retained for analyses. In total, 640 participants meeting these criteria took part in the survey study.
We have complied with all relevant ethical regulations. All participants provided written informed consent. This study was approved by the Social and Societal Ethics Committee (SMEC) from KU Leuven (G-2020-1987). For their participation, participants could win one of 16 gift certificates via a random draft.
Material
The online survey was created and data were generated using Qualtrics software35. The language of the survey was Dutch. The survey existed of several general and demographic questions and questionnaires.
General and demographic questions
We asked participants several general questions assessing their year of birth, gender, country of residence, nationality, postal code, living situation, educational level, current and previous work situation, monthly individual net income, age-related diseases and whether the participant and/or any of their close relatives or friends had been infected with the corona virus.
Subjective cognitive functioning
To assess subjective cognitive functioning, the Dutch version of the Cognitive Failures Questionnaire (CFQ36,37) was used. The CFQ exists of 25 items assessing self-reported frequency of failures in several cognitive domains (e.g., perception, attention, memory and action). In our study, participants indicated how often these failures occurred during the past month (i.e., during the COVID-19 period) on a 5-point scale ranging from “very often” (= 4) to “never” (= 0). We added a response option “not applicable”, since some situations described in the questionnaire might not have occurred during the COVID-19 period for some participants (e.g., “Do you fail to see what you want in a supermarket (although it’s there)?”). A total score across all items, varying between 0 and 100, provides a measure of the general susceptibility to cognitive failures with a higher score indicating a higher susceptibility. The “not applicable” scores were not included to calculate the total score. For participants who indicated “not applicable” on more than 50% of the items (N = 15), no CFQ total score was computed. The Dutch version of the CFQ has been validated across the adult life span, including older adults38. The internal consistency of the CFQ in the current study was high with Cronbach’s α = 0.92.
Subjective cognitive change
Additionally, we assessed whether participants felt that their general cognitive functioning had changed during the COVID-19 period using a 3-point scale: Yes, it has decreased (= 1); No, it has not changed (= 2); Yes, it has improved (= 3). We also asked them whether they had experienced the following cognitive problems during the COVID-19 period: problems to remember things, to concentrate on something, to do two things at the same time, to recall things and forgetfulness. They indicated the frequency of these problems in comparison to the period before the COVID-19 period on a 5-point scale with labels “a lot more than before” (= 1), “more than before” (= 2), “not more or less than before” (= 3), “less than before” (= 4), “a lot less than before” (= 5). The internal consistency of these six subjective cognitive change questions was high with Cronbach’s α = 0.86.
Depression
To assess self-reported depression, the Geriatric Depression Scale-15 (GDS-1539,40) was used. This scale exists of 15 Yes/No items and is designed to assess depressive symptoms in older populations. Using a score key, each item receives a score of 0 or 1 and these scores are then summed leading to a total score between 0 and 15, with higher scores indicating more depressive symptoms. The psychometric qualities of the GDS-15 have been supported by numerous studies, also in Dutch older populations (e.g.41). The internal consistency of the GDS-15 in the current study was high with Cronbach’s α = 0.81.
Activity and sleep
Participants were asked to evaluate their activity level and quality of sleep both before the COVID-19 period (retrospectively), and during the past week (i.e., during the COVID-19 period). These four questions were answered on an 11-point scale ranging from 0 (i.e., “not active at all” and “very poor quality of sleep” respectively) to 10 (i.e., “extremely active” and “very good quality of sleep” respectively).
Wellbeing
Wellbeing was assessed using the Dutch version of the Personal Wellbeing Index-Adults (PWI-A42,43). The PWI-A is a multidimensional scale measuring life satisfaction in seven different domains (standard of living, health, achieving in life, relationships, safety, community connectedness and future security). An additional item assessing general life satisfaction can also be administered. All eight items are scored on an 11-point scale ranging from “no satisfaction at all” (= 0) to “completely satisfied” (= 10). The raw scores are converted to a 0–100 scale, with higher scores indicating more satisfaction. The scores for each domain can be studied individually, and the seven domain items (excluding the general life satisfaction item) can be summed to provide an index of “subjective wellbeing”. The psychometric properties of the PWI-A have been reported as satisfactory for older adults44 and for a Dutch sample43. In the current study, participants filled in the PWI-A twice: they indicated their satisfaction for each domain once (retrospectively) for the period before COVID-19 and once for the past month (i.e., during the COVID-19 period). The internal consistency in the current study was high for the PWI-A total score (i.e., 7 items) relating to the pre-COVID-19 period (Cronbach’s α = 0.91) and to the past month (Cronbach’s α = 0.91).
Social network
The Lubben Social Network Scale-6 (LSNS-645), specifically constructed for older adults, was used to assess social network, support and isolation. It consists of six items: three items evaluating family ties and three comparable items evaluating non-family ties. These items are scored on a 6-point scale where participants indicate the number of ties (i.e., 0, 1, 2, 3 or 4, 5 to 8, 9 or more). A sum score can be calculated, ranging between 0 and 30, with a higher score indicating more social engagement. The psychometric properties of the LSNS-6 are good45, although we were unable to obtain data for the Dutch version specifically. The internal consistency of the LSNS-6 in the current study was high with Cronbach’s α = 0.82. Next to the LSNS-6, we also asked participants how many contacts they had (not taking into account cohabitants) during the past week in real life outside, in real life inside, by telephone and via the internet (e.g., skype, whatsapp), using the same response scale of the LSNS-6.
Resilience
Resilience, or the ability to bounce back or recover from stress, was assessed using the Dutch version of the Brief Resilience Scale (BRS46,47). The BRS exists of six items measuring the degree of individual resilience. The items are scored on a 5-point scale ranging from “strongly disagree” to “strongly agree”. After reversing items 2, 4 and 6, a mean score is calculated which ranges between 1 and 5, with a higher score indicating more resilience. The validity of the BRS has been confirmed in older adults48 and for the Dutch version47. The internal consistency of the BRS in the current study was high with Cronbach’s α = 0.81.
Procedure
The survey existed of a first page providing participants with information about the study and an informed consent form. After participants had read the instructions and informed consent, they were asked to explicitly check a box if they agreed to participate and to confirm that they were 65 years or older.
Afterwards, participants first completed the general and demographics questions. Next, the questionnaires were administered in the following order: CFQ, subjective cognitive change questions, GDS-15, questions regarding activity level and sleep quality, PWI-A, additional social network question, LSNS-6 and BRS. Finally, participants could provide their contact details to contact them for follow-up surveys or in case they had won a gift certificate. After completing the survey, we provided participants with web links and a telephone number where they could obtain more information about COVID-19 and psychological care if needed. The median number of minutes it took participants to complete the survey was 20. The survey was launched on May 19 2020 and closed on June 22 2020.
Statistical analyses
First, we summarized the participant characteristics and the Pearson correlations between the main constructs. We considered correlations as small, medium or strong when the correlation coefficient was below 0.30, between 0.30 and 0.50 and above 0.50, respectively (based on Cohen49).
Second, we compared participants’ report of their current wellbeing, activity level, sleep quality and cognitive functioning (i.e., during the COVID-19 period) with their retrospective report of these variables before COVID-19. We calculated difference scores between the reports during and before the COVID-19 period for activity level, sleep quality and the PWI-A items and total score (i.e., reportduring – reportbefore, where negative scores indicate a decrease in the report during the COVID-19 period compared to before). From now on, we will refer to these as the difference scores. We reported the descriptive statistics for the difference scores and the subjective cognitive change questions. Furthermore, we conducted two-sided paired-samples t-tests with Bonferroni correction to compare the reports during and before the COVID-19 period. As 11 comparisons were conducted (i.e., for the eight PWI-A items and total score, activity level and sleep quality). Cohen’s d (calculated as the difference between the means divided by the standard deviation) were reported. We considered effect sizes as small, medium or strong when the Cohen’s d was around 0.20, 0.50 and 0.80, respectively (based on Cohen49).
Finally, we conducted linear or ordinal logistic regression analyses to determine the contribution of potential vulnerability and protective factors, in explaining changes in wellbeing, activity level and sleep quality (i.e., the 11 difference scores) and cognitive functioning (i.e., the six subjective cognitive change questions). This led to a total of 17 dependent variables and hence 17 separate regression analyses. These analyses were grouped in three parts focused on changes in wellbeing (i.e., nine analyses), changes in activity and sleep (two analyses) and changes in cognitive functioning (i.e., six analyses). Bonferroni correction was applied for each group of analyses, leading to α being set at 0.0055 for wellbeing, 0.025 for activity and sleep and 0.0083 for cognitive functioning. For changes in wellbeing, activity level and sleep quality, linear regression analyses were performed. Given the ordinal nature of the subjective cognitive change questions, we conducted ordinal linear regression analyses for changes in cognitive functioning. The following variables were included in each regression analysis as predictors: gender, age, whether the participant lived alone or not (derived based on the reported number of cohabitants, see Supplementary Table 1), whether the participant lived in a care facility or not (derived based on the reported living situation, see Supplementary Table 1), the reported monthly individual net income (to index socio-economic status), depression (i.e., GDS-15 total score), social network (i.e., LSNS-6 total score), general susceptibility to cognitive failures (i.e., CFQ total score) and resilience (i.e. BRS total score). Categorical predictors were dummy recoded. The presence of multicolinearity was examined using a cut-off of < 5 for the Variance Inflation Factor (VIF50).
Supplementary Information
Acknowledgements
The authors wish to thank all participants for completing the survey and all nursing staff who helped participants to complete the survey.
Author contributions
S.D.P., C.G. and E.V.D.B. designed the study; S.D.P. and E.V.D.B. created the survey; S.D.P., C.G. and E.V.D.B. recruited the participants; S.D.P. and E.V.D.B. analyzed the data and wrote the manuscript; C.G., E.D., M.-A.V. and R.D.R. provided feedback on previous drafts of the manuscript, and provided suggestions for the analysis strategy.
Data availability
The anonymized, raw data that support the findings of this study are available in the Open Science Framework (OSF, https://osf.io/re7sm/) with the identifier “https://doi.org/10.17605/OSF.IO/RE7SM”.
Code availability
The SPSS code used to generate results that are reported in the paper is available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84127-7.
References
- 1.Sciensano. COVID-19. https://epistat.wiv-isp.be/covid/ (2020).
- 2.Guterres, A. Secretary-General’s policy brief: The impact of COVID-19 on older persons. United Nationshttps://unsdg.un.org/sites/default/files/2020-05/Policy-Brief-The-Impact-of-COVID-19-on-Older-Persons.pdf (2020).
- 3.World Health Organization (WHO). Health care considerations for older people during COVID-19 pandemic. WHO. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/technical-guidance/health-care-considerations-for-older-people-during-covid-19-pandemic (2020).
- 4.United Nations (UN). Policy brief: COVID-19 and the need for action on mental health. UNhttps://unsdg.un.org/sites/default/files/2020-05/UN-Policy-Brief-COVID-19-and-mental-health.pdf (2020).
- 5.Banerjee D. The impact of Covid-19 pandemic on elderly mental health. Int. J. Geriatr. Psychiatry. 2020;35:1466–1467. doi: 10.1002/gps.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vahia IV. COVID-19, aging, and mental health: Lessons from the first six months. Am. J. Geriatr. Psychiatry. 2020;28:691–694. doi: 10.1016/j.jagp.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooke J, Jackson D. Older people and COVID-19: Isolation, risk and ageism. J. Clin. Nurs. 2020;29:2044–2046. doi: 10.1111/jocn.15274. [DOI] [PubMed] [Google Scholar]
- 8.Stanton R, et al. Depression, anxiety and stress during COVID-19: Associations with changes in physical activity, sleep, tobacco and alcohol use in australian adults. Int. J. Environ. Res. Public Health. 2020;17:4065. doi: 10.3390/ijerph17114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013;2013:1–8. doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murman D. The impact of age on cognition. Semin. Hear. 2015;3:111–121. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clare L, et al. Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLOS Med. 2017;14:e1002259. doi: 10.1371/journal.pmed.1002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivi MK, Hansson I, Bjälkebring P. Up and about: older adults’ well-being during the COVID-19 pandemic in a swedish longitudinal study. J. Gerontol. B. 2020;76:e84. doi: 10.1093/geronb/gbaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López J, et al. Psychological well-being among older adults during the COVID-19 outbreak: A comparative study of the young–old and the old–old adults. Int. Psychogeriatr. 2020;32:1365–1370. doi: 10.1017/S1041610220000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Tilburg TG, Steinmetz S, Stolte E, van der Roest H, de Vries D. Loneliness and mental health during the COVID-19 pandemic: A study among Dutch older adults. J. Gerontol. B. 2020;1:111. doi: 10.1093/geronb/gbaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hikichi H, et al. Increased risk of dementia in the aftermath of the 2011 Great East Japan Earthquake and Tsunami. Proc. Natl. Acad. Sci. USA. 2016;113:E6911–E6918. doi: 10.1073/pnas.1607793113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rafiey H, et al. Are older people more vulnerable to long-term impacts of disasters? Clin. Interv. Aging. 2016;11:1791–1795. doi: 10.2147/CIA.S122122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen T, Slagsvold B. The age and subjective well-being paradox revisited: A multidimensional perspective. Nor. Epidemiol. 2012;22:2. doi: 10.2188/jea.JE20110116. [DOI] [Google Scholar]
- 18.Hikichi H, et al. Social capital and cognitive decline in the aftermath of a natural disaster: A natural experiment from the 2011 Great East Japan Earthquake and Tsunami. Lancet Planet. Health. 2017;1:e105–e113. doi: 10.1016/S2542-5196(17)30041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darlington RB. Multiple regression in psychological research and practice. Psychol. Bull. 1968;69:161–182. doi: 10.1037/h0025471. [DOI] [PubMed] [Google Scholar]
- 20.Paulhus DL, Robins RW, Trzesniewski KH, Tracy JL. Two replicable suppressor situations in personality research. Multivar. Behav. Res. 2004;39:301–326. doi: 10.1207/s15327906mbr3902_7. [DOI] [PubMed] [Google Scholar]
- 21.Maglione JE, et al. Depressive symptoms and subjective and objective sleep in community-dwelling older women. J. Am. Geriatr. Soc. 2012;60:635–643. doi: 10.1111/j.1532-5415.2012.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone C, Wachholtz A. The relationship of anxiety and depression to subjective well-being in a mainland Chinese sample. J. Relig. Health. 2017;57:266–278. doi: 10.1007/s10943-017-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen. Hosp. Psychiatry. 2009;31:306–315. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Curr. Dir. Psychol. Sci. 1995;4:151–156. doi: 10.1111/1467-8721.ep11512261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swift HJ, et al. Revisiting the paradox of well-being: The importance of national context. J. Gerontol. B. 2014;69:920–929. doi: 10.1093/geronb/gbu011. [DOI] [PubMed] [Google Scholar]
- 26.STATBEL. Structuur van de bevolking. https://statbel.fgov.be/nl/themas/bevolking/structuur-van-de-bevolking (2020).
- 27.Sato H, Kawahara J. Selective bias in retrospective self-reports of negative mood states. Anxiety Stress Coping. 2011;24:359–367. doi: 10.1080/10615806.2010.543132. [DOI] [PubMed] [Google Scholar]
- 28.Burmester B, Leathem J, Merrick P. Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol. Rev. 2016;26:376–393. doi: 10.1007/s11065-016-9332-2. [DOI] [PubMed] [Google Scholar]
- 29.van der Ham IJM, van der Kuil MNA, Claessen MHG. Quality of self-reported cognition: Effects of age and gender on spatial navigation self-reports. Aging Ment. Health. 2020;1:1–6. doi: 10.1080/13607863.2020.1742658. [DOI] [PubMed] [Google Scholar]
- 30.Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5:e256. doi: 10.1016/S2468-2667(20)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian F, et al. Psychological symptoms of ordinary Chinese citizens based on SCL-90 during the level I emergency response to COVID-19. Psychiatry Res. 2020;288:112992. doi: 10.1016/j.psychres.2020.112992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czaja SJ. The role of technology in supporting social engagement among older adults. Public Policy Aging Rep. 2017;27:145–148. doi: 10.1093/ppar/prx034. [DOI] [Google Scholar]
- 33.Masi CM, Chen HY, Hawkley LC, Cacioppo JT. A meta-analysis of interventions to reduce loneliness. Pers. Soc. Psychol. Rev. 2011;15:219–266. doi: 10.1177/1088868310377394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chong TWH, Curran E, Ames D, Lautenschlager NT, Castle DJ. Mental health of older adults during the COVID-19 pandemic: Lessons from history to guide our future. Int. Psychoger. 2020;32:1249–1250. doi: 10.1017/S1041610220001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qualtrics Version May-June 2020. Copyright 2019 Qualtrics. Qualtrics and all other Qualtrics product or service names are registered trademarks or trademarks of Qualtrics, Provo, UT, USA. https://www.qualtrics.com.
- 36.Broadbent DE, Cooper PF, Fitzgerald P, Parkes LR. The cognitive failures questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 37.Merckelbach H, Muris P, Nijman HLI, de Jong PJ. Self-reported cognitive failures and neurotic symptomatology. Pers. Individ. Differ. 1996;20:715–724. doi: 10.1016/0191-8869(96)00024-4. [DOI] [Google Scholar]
- 38.Rast P, Zimprich D, Van Boxtel M, Jolles J. Factor structure and measurement invariance of the cognitive failures questionnaire across the adult life span. Assessment. 2009;16:145–158. doi: 10.1177/1073191108324440. [DOI] [PubMed] [Google Scholar]
- 39.Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. 1986;5:165–173. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- 40.Bleeker JAC, Frohn-de Winter ML, Cornelissen E. Dutch translation of the Geriatric Depression Scale. J. Affect. Disord. 1985;33:77–82. [Google Scholar]
- 41.de Craen AJM, Heeren TJ, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int. J. Geriatr. Psychiatry. 2003;18:63–66. doi: 10.1002/gps.773. [DOI] [PubMed] [Google Scholar]
- 42.International Wellbeing Group . Personal wellbeing index. 5. Melbourne: Australian Centre on Quality of Life, Deakin University; 2013. [Google Scholar]
- 43.Van Beuningen, J. & de Jonge, T. The Personal Wellbeing Index. Construct validity for the Netherlands. Discussion paper. (CBS: Den Haag/Heerlen, 2011).
- 44.Forjaz MJ, et al. Measurement properties of the community wellbeing index in older adults. Qual. Life Res. 2011;20:733–743. doi: 10.1007/s11136-010-9794-2. [DOI] [PubMed] [Google Scholar]
- 45.Lubben J, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community–dwelling older adult populations. Gerontologist. 2006;46:503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 46.Smith BW, et al. The brief resilience scale: Assessing the ability to bounce back. Int. J. Behav. Med. 2008;15:194–200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 47.Soer R, Bieleman HJA, Schreurs KMG. Measurement properties and implications of the Brief Resilience Scale in healthy workers. J. Occup. Health. 2019;61:242–250. doi: 10.1002/1348-9585.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.da Silva-Sauer L, et al. Brief Resilience Scale (BRS) Portuguese Version: validity and metrics for the older adult population. Aging Ment. Health. 2020;1:1–10. doi: 10.1080/13607863.2020.1753015. [DOI] [PubMed] [Google Scholar]
- 49.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale: L. Erlbaum Associates; 1988. [Google Scholar]
- 50.Sheather S. A Modern Approach to Regression with R. New York: Springer; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized, raw data that support the findings of this study are available in the Open Science Framework (OSF, https://osf.io/re7sm/) with the identifier “https://doi.org/10.17605/OSF.IO/RE7SM”.
The SPSS code used to generate results that are reported in the paper is available upon request.