Abstract
Increasing evidence points towards the role of mitochondrial functioning, energy metabolism, and oxidative stress in migraine. However not all previous research has been conclusive and some mitochondrial function/oxidative stress markers have not yet been examined. To this end, alpha-lipoic acid (ALA), total thiols, total plasma antioxidant capacity (TAC), lipid peroxide (PerOx), oxidised LDL (oxLDL), HbA1c and lactate were determined in the serum of 32 higher frequency episodic migraineurs (5–14 migraine days/ months, 19 with aura, 28 females) in this cross-sectional study. The majority of patients had abnormally low ALA and lactate levels (87.5% and 78.1%, respectively). 46.9% of the patients had abnormally high PerOx values, while for thiols and TAC over one third of patients had abnormally low values (31.2% and 37.5%, respectively). 21.9% of patients had abnormally low HbA1c and none had an HbA1c level above 5.6%. oxLDL was normal in all but one patient. This study provides further evidence for a role of oxidative stress and altered metabolism in migraine pathophysiology, which might represent a suitable therapeutic target. ALA, being too low in almost 90% of patients, might represent a potential biomarker for migraine. Further research is needed to replicate these results, in particular a comparison with a control group.
This study is part of the trial registration: ClinicalTrials.gov: NCT03132233, registered on 27.04.2017, https://clinicaltrials.gov/ct2/show/NCT03132233.
Subject terms: Migraine, Diagnostic markers, Endocrine system and metabolic diseases
Introduction
Migraine is a complex, common and debilitating neurological disorder1 and yet its primary pathogenic mechanisms are not completely understood. Despite being referred to as a “hypoglycemic headache” in 1935 already2, the focus of clinical and basic research has shifted towards (neuro-)vasculature, cerebral excitability and neurotransmission for several decades. In recent years, metabolism and mitochondrial (dys-)function have regained interest and various lines of evidence—much of it clinical—are suggesting that migraine is—at least partially—an energy deficit syndrome of the brain.
For example, magnetic resonance spectroscopy (MRS) studies in migraine consistently show abnormalities of mitochondrial oxidative phosphorylation (OXPHOS), such as hypometabolism or decreased ATP levels3–13. These findings are supported by early studies showing metabolic changes induced by fasting, glucose or insulin administration, which can even trigger migraine attacks in susceptible patients14–20.
Further support for a link to energy metabolism and/or mitochondrial functioning comes from the migraine preventative effect of several nutraceuticals21, such as riboflavin at high dose (200–400 mg/ day)22–28; coenzyme Q10 (400 mgcapsulesor300 mgliquidsuspension)29–34, magnesium35 and alpha-lipoic acid (ALA; 600 mg)36–38. Dietary approaches, such as a ketogenic diet (KD), which promotes the hepatic production of an alternative energy substrate for the brain and to some extent mimics the state of fasting, have been shown to be migraine protective39–44 (see45 for potential mechanism of ketosis in migraine). Moreover, elevated plasma lactate and pyruvate levels have been reported, but mostly in severely affected patients, such as migrainous stroke46,47.
Reactive oxygen species (ROS), such as hydroxyl radicals, hydrogen peroxide and superoxide radical anions, are produced as by-products of normal metabolic processes, such as electron transport in mitochondria, host defense or enzymatic reactions48. In healthy organisms, antioxidant defense systems protect the cells and tissues against these species49,50. When the generation of ROS exceeds the body’s antioxidant capacity, oxidative stress, i.e. damage to cellular constituents, such as proteins, lipids, DNA and sugars, occurs48,50. Oxidative stress could be the common denominator of most migraine trigger and aggravating factors51. While for some of the more “metabolic” triggers, such as fasting /skipping a meal, physical exercise, stress and relaxation thereafter a direct link to energy homeostasis seems obvious, most of the seemingly unrelated triggers, such as ovarian hormone changes, weather changes, alcohol, strong odors, intense light and loud noises, also have a potential common denominator: changes in mitochondrial metabolism and/or oxidative stress (see reviews51,52 for further details). Mechanistically, transient receptor potential (TRP) channels, expressed in meningeal nociceptive nerve terminals, can be activated by oxidative, nitrosative and electrophilic stress53,54, thereby providing a mechanism by which known migraine trigger factors that increase oxidative stress could lead to migraine pain.
Increased oxidative/nitrosative stress and/or decreased anti-oxidant capacity have directly been found in migraine patients55–68, however the results were not always consistent. Of all biomarkers examined, superoxide dismutase activity seemed to be the one consistently reduced in migraine patients, also interictally69. The inconsistent results for the other markers could be due to differences in methodology, patient selection and variations depending on the migraine cycle. Regarding the latter, nitrosative and oxidative stress68 and nitric oxide69 were significantly elevated during migraine attacks, but not interictally. Whether those metabolic abnormalities observed are primary or secondary to the disease remains to be determined.
The aim of this study was to analyse peripheral markers of mitochondrial functioning/energy metabolism that have either not previously been looked at (to the best of our knowledge) or previously produced inconsistent results, in order to further decipher the metabolic face of migraine, with a particular focus on oxidative stress markers. Through the measurement of ALA, thiols, total plasma antioxidant capacity (TAC), lipid peroxide (PerOx), oxidised LDL (oxLDL), HbA1c and lactate in the serum of 32 medium to high frequency migraineurs the present study aimed to provide peripheral markers of mitochondrial functioning / energy metabolism that could easily be analysed by most practitioners to potentially assist with individualised treatment. Taking into account previous findings, we hypothesised that the three-month average plasma glucose concentration (Hba1c) would be reduced, the oxidative stress markers ALA, thiols and TAC would be reduced, PerPx and oxLDL would be increased and that there would be no difference in lactate levels in our episodic migraine patient population.
Materials and methods
Patients
After receiving ethical approval from the local ethics committee (Ethikkommission Nordwest-und Zentralschweiz (EKNZ), PB 2016-00497), written informed consent was obtained from each patient. We recruited patients from the Neurology Out-patient Departments at the University Hospitals of Basel, Bern, Zurich, the University of Basel, using internet announcements and by advertising in local busses and trains. Patients were part of the MigraKet trial70 (ClinicalTrials.gov Identifier: NCT03132233). The diagnosis of migraine was made by a trained neurologist based on the criteria according to the International Headache Society71. A German version of the migraine disability assessment (MIDAS) was used to assess migraine related disability72. All experiments and methods were performed in accordance with relevant guidelines and regulations (EKNZ, PB 2016-00497).
Inclusion criteria
Patients were included, if they were previously diagnosed with migraine (with or without aura) in accordance with the ICHD-3 (International Classification of Headache Disorders version 3 Beta) Classification criteria71, were between the ages of 18 and 65 years, experienced between 5 and 14 migraine days per month (over the last 4 months), had an age of onset of migraine less than 50 years old and had not changed the type, dosage or frequency of any prophylactic medication (exclusive of medications taken for acute relief of migraine symptoms) for at least 3 months prior to study onset.
Exclusion criteria
Patients were excluded, if they had a history of any significant neurological, psychiatric or other medical condition or a known history of suspected secondary headache, if they were taking simple analgesics or non-steroidal anti-inflammatory drugs (NSAIDs) more than 14 days per 4 weeks or triptans on more than 10 days per 4 weeks for headaches or other body pain or any prescription opioids, if they had a previous diagnosis of medication overuse headache, which has reverted to episodic migraine within the last 6 months or met ICHD-3 Beta Classification criteria71 for chronic migraine (> 15 headache days per month), if they had had a surgery for migraine prevention, if they had received botulinum toxin injections within the last 6 months or if they were pregnant.
Laboratory procedures
The following mitochondrial function markers were examined (normative values from local laboratory (Ganzimmun Diagnostic AG, Mainz, Germany or University Hospital Basel, Basel Switzerland) indicated in brackets):
Total anti-oxidative capacity (TAC; > 280 μmol/l)
Oxidated LDL (OxLDL; < 235 ng/ml)
Alpha-lipoic acid (ALA; > 0.52 μg/l)
Lipid-peroxide (PerOx; < 180 μmol/l)
Thiols (complete; > 55 μmol/l)
HbA1c (4.8–5.9%)
Lactate (1.1–2.0 mol/l)
Venous blood samples were drawn from an antebrachial vein following overnight fasting. After 30–60 min at room temperature the serum was separated from the rest of the blood by centrifugation at 1300G for 10 min. Aliquots of serum were stored at − 80 °C. One aliquot contained 0.3 ml serum. Three aliquots per patient were sent for analysis. Blood samples for HbA1C and lactate were not stored, but immediately sent at room temperature to the inhouse laboratory for immediate analysis.
Total antioxidant capacity
TAC was measured using an ImAnOx‐assay (Ganzimmun Diagnostic AG, Mainz, Germany) (inter-assay variation: 2.43%; intra‐assay variation: 2.33%). This photometric test reflects the sum of all antioxidant components by measuring hydrogen peroxide (H2O2) degradation by the serum antioxidants. Please refer to73 for further details.
Peroxides
Serum total peroxide concentrations were determined photometrically by the peroxide concentration assay (Ganzimmun Diagnostic AG, Mainz, Germany) (inter-assay variation: 3.5–3.6%; intra‐assay variation: 2.0–3.6%), which is based on the reaction of horseradish peroxidase with plasma peroxides using tetramethylbenzidine as a chromogen substrate (450-nm wavelength). Please refer to74 for further details.
Oxidised LDL
The measurement of serum oxidised LDL was performed using a sandwich ELISA method (ox-LDL ELISA kit, Ganzimmun Diagnostic AG, Mainz, Germany) (inter-assay variation: 9–11%; intra‐assay variation: 3.9–5.7%). No antioxidants were added to the plasma samples before collection. Please refer to75 for further details.
Thiols
Thiols were determined in serum using the ImmuChrom HPLC assay (Ganzimmun Diagnostic AG, Mainz, Germany) (inter-assay variation: 5.5–5.9%; intra‐assay variation: 5.2–5.8%), where thiols present in serum proteins are precipitated with 80% saturated ammonium sulfate. Please refer to76 for further details.
Alpha-lipoic acid
ALA was determined in serum using the HPLC method (Ganzimmun Diagnostic AG, Mainz, Germany). The standards and the solutions were sourced from Merck KGaA. In brief, 100 µl of the serum sample was diluted in 1.9 ml acetone. The sample was mixed thoroughly for 5 s. After that, the sample was centrifuged for 10 min at 3500 U/min. 800 µl of the supernatant was evaporated under air at 45 °C for 10 min. The dry residue was dissolved in 400 µl 30/70 0.1% acetic acid/ acetone mix. The sample was mixed thoroughly for 5 s. 300 µl of the solution was transferred in a HPLC vial. A calibration curve in empty serum was prepared with five different standard concentrations. The highest standard was 200 µg/l and the lowest 12.5 µg/l. The preparation of the standard was equal to the sample preparation. The concentration of ALA was determent by LC–MS/MS with a Varian 320 in negative mode. For the HPLC method an Atlantis T3, 3 µm, 150 × 2.1 mm column from Waters GmbH was used. The isocratic gradient was 30% 0.1% acetic acid and 70% acetone with a flowrate of 0.3 ml/min and an injection volume of 40 µl. The runtime was 4 min with a retention time of ALA at 2.1 min.
Glycosylated hemoglobin (HbA1c) and lactate
HbA1c and lactate were analysed using the Cobas 8000 c502 (Roche Diagnostics) at the laboratory of the University Hospital Basel. HbA1c was determined using the HbA1c Turbidimetric Immunoassay (Tina-quant, 3rd generation) from haemolysed full blood EDTA samples. Lactate was analysed by centrifugation at 3004G for 8 min of blood EDTA / fluorid samples, plasma was then separated. Following this, plasma samples were immediately analysed using an enzymatic colour assay.
Statistical analyses
Summary statistics (mean, median, interquartile range, minimum and maximum) and the number and percentage of patients with non-normal values are indicated for each biomarker. Individual measures are both shown separately in the original units and on a common, standardized scale for better comparison. For endpoints with normal ranges (HbA1c and lactate), the original values were scaled such that the minimum and maximum of the normal range correspond to 0 and 1. Values < 0 are thus abnormally low and values > 1 abnormally high. For the other endpoints with a single normal cut-off, the original values were centered, such that the cut-off corresponds to 0, and scaled by dividing by the standard deviation, such that 1 corresponds to one standard deviation. For TAC, ALA and thiols, values < 0 are considered abnormal, while for oxLDL and PerOx, values > 0 are considered abnormal. Boxplots were drawn as follows. The Boxes contain the 25% through 75% quantiles (spanning the interquartile range), the thick horizontal line is the median. Whiskers indicate the most extreme values lying within the box-edge and 1.5 * the interquartile range. All eventual further values (outliers) are plotted as individual points.
Several post-hoc analyses were performed. Correlations of biomarkers and migraine intensity were examined visually and Spearman’s rank correlation coefficient was calculated. As measure of migraine intensity, the number of migraine days and the MIDAS score at baseline were considered. Subgroup comparisons were performed between patients with and without migraine prophylaxis, between patients with and without acute migraine attack at baseline ± 2 days and between MA and MO. Subgroups were tested for a difference using Wilcoxon’s rank sum test (continuous outcomes) and Fisher’s exact test (frequencies).
In accordance with the exploratory nature of the analyses, p values should not be interpreted as confirmative, but can be useful in identifying hypotheses worth of further investigation. In accordance with recent statistical guidelines, the term ‘statistically significant’ is not used (following the strong suggestions made in the ASA Editorial on ‘Moving to a world beyond p < 0.05’77).All analyses were conducted using the statistical software package R78.
Results
Study population
Thirty-two patients were included in the study4,27. The mean age was 34 ± 10.8 years. Twelve patients had migraine without aura (MO) and 20 migraine with aura (MA). Patient characteristics and demographics’ information are shown in Table 1. Eleven patients were using at least one stable migraine prophylaxis (no changes within at least 3 months prior to study onset) (see Table 2 for the migraine preventatives used).
Table 1.
Summary statistics of patient characteristics.
| Variables | All patients |
|---|---|
| Age, mean (SD) | 34 (10.8) |
| Female, N (%) | 28 (87.5) |
| Male, N (%) | 4 (12.5) |
| Height (m), mean (SD) | 1.7 (01.) |
| Weight (kg), mean (SD) | 67.3 (13.9) |
| Migraine days / months, mean (SD) | 8.6 (2.1) |
| Migraine without aura, N (%) | 12 (37.5) |
| Migraine with aura, N (%) | 20 (62.5) |
| MIDAS, mean (SD) | 31 (19.9) |
| Stable migraine prophylaxis, N (%) | 11 (34.4) |
| No migraine prophylaxis, N (%) | 21 (65.6) |
Categorical variables are summarized as frequencies and percentages (%), numerical variables are summarized by mean and one standard deviation (sd).
SD = standard deviation, m = meter, kg = kilogram.
Table 2.
Types and frequencies of migraine prophylactic treatments.
| Type | N |
|---|---|
| Antidepressants | 2 |
| Anticonvulsants | 2 |
| Beta-blockers | 5 |
| Cefaly™ neurostimulator | 1 |
| Calciumantagonists | 2 |
| Magnesium | 7 |
| Riboflavin/Vitamin B2 | 1 |
Note that more than one type of prophylactic drug could be used.
Summary statistics
Summary statistics of all endpoints are given in Table 3. Single observations are visualized in their original units (Fig. 1) and scaled (Figs. 2, 3).
Table 3.
Summary statistics of mitochondrial function markers.
| Marker | Normal range | Minimum | Q1 | Median | Mean | Q3 | Maximum | Non-normal |
|---|---|---|---|---|---|---|---|---|
| ALA | > 0.52 μg/l | 0.13 | 0.24 | 0.29 | 0.72 | 0.41 | 13.25 | 28 (87.5%) |
| TAC | > 280 μmol/l | 264.0 | 276.00 | 284.00 | 286.88 | 295.25 | 348.00 | 12 (37.5%) |
| PerOx | < 180 μmol/l | 6.0 | 75.50 | 166.00 | 283.31 | 306.50 | 1315.00 | 15 (46.9%) |
| Ox-LDL | < 235 ng/ml | 41.3 | 45.10 | 62.15 | 106.35 | 136.38 | 593.90 | 1 (3.1%) |
| Thiols | > 55 μmol/l | 41.0 | 52.75 | 62.50 | 63.62 | 72.25 | 99.00 | 10 (31.2%) |
| HbA1c | 4.8–5.9% | 4.1 | 4.80 | 4.95 | 4.93 | 5.10 | 5.60 | 7 (21.9%) |
| Lactate | 1.1–2.0 mol/l | 0.5 | 0.73 | 0.85 | 1.05 | 1.15 | 2.66 | 25 (78.1%) |
Q1–Q3: interquartile range; non-normal: number (%) of patients with values outside normal range.
ALA = Alpha-lipoic acid; ox-LDL = oxidised LDL; PerOx = total lipid peroxide; TAC = total antioxidant capactity.
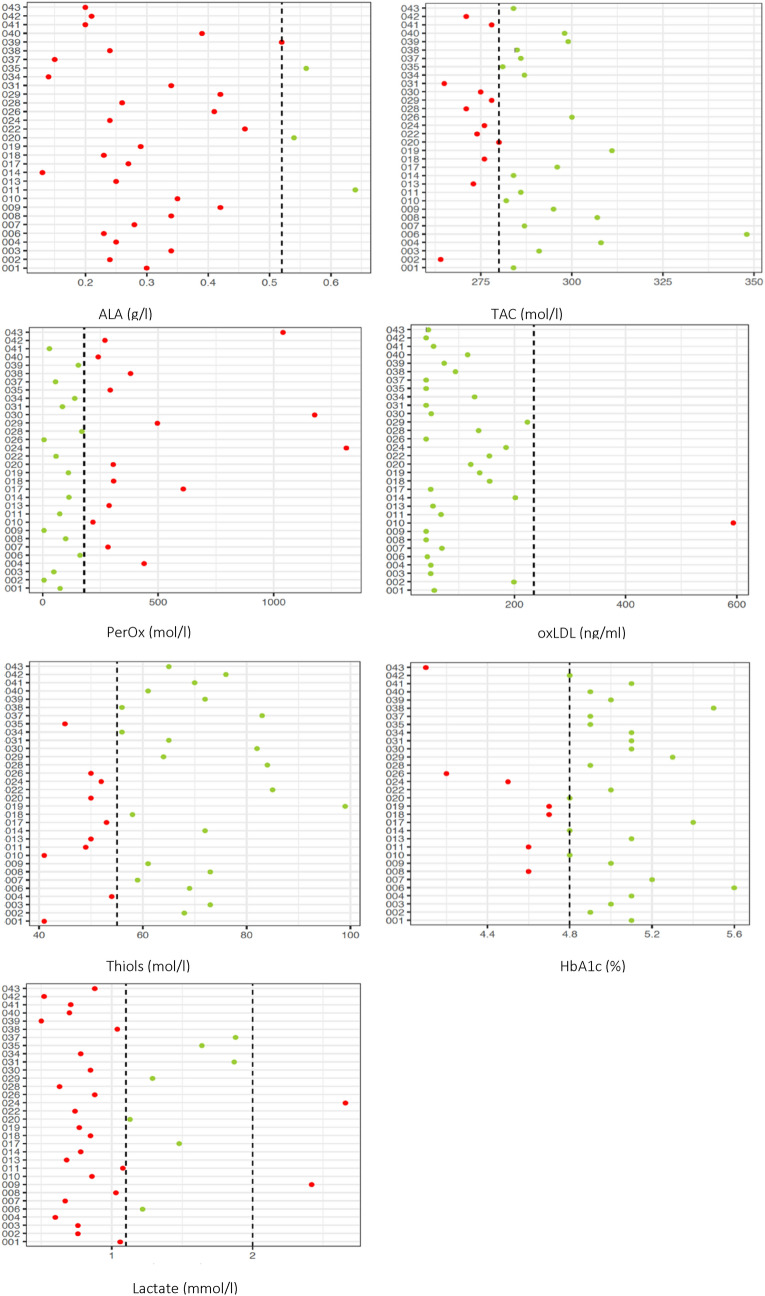
Figure 1.
Baseline values of mitochondrial function markers for each patient. Dashed lines indicate the normal-range cut-off(s). Green dots indicate values within normal range, red dots indicate values outside the normal range. For alpha lipoic acid one very extreme value (13.25) has been removed for better visualisation. ALA = Alpha-lipoic acid; ox-LDL = oxidised LDL; PerOx = total lipid peroxide; TAC = total antioxidant capactity.
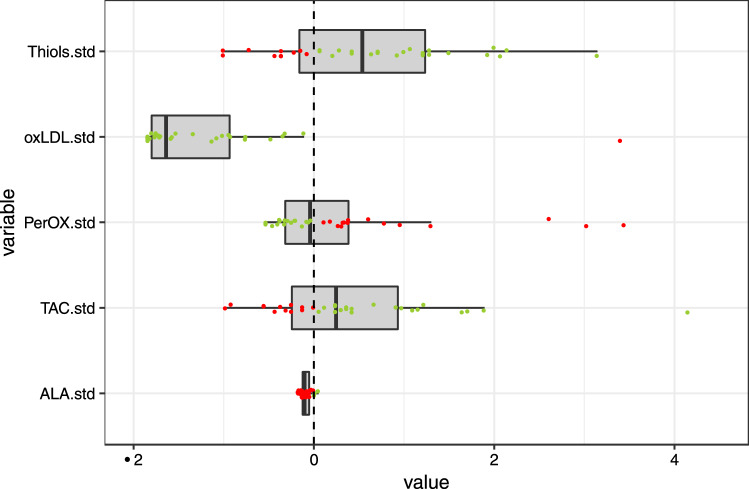
Figure 2.
Standardized (= .std) baseline values of mitochondrial function markers with a single cut-off. Values are standardized such that zero (the dashed line) indicates the normal cut-off, 1 indicates one standard deviation, 2 indicates two standard deviations, etc. Green dots indicate values within normal range, red dots indicate values outside the normal range. For alpha lipoic acid one very extreme value (5.6) has been removed for better visualisation. ALA = Alpha-lipoic acid; ox-LDL = oxidised LDL; PerOx = total lipid peroxide; TAC = total antioxidant capactity.
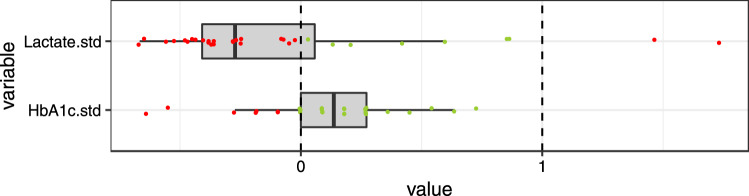
Figure 3.
Standardized (= .std) baseline values of mitochondrial function markers with two cut-offs. Values are standardized such that zero (the dashed line) indicates the lower cut-off of the normal range and 1 indicates the upper cut-off of the normal range. Green dots indicate values within normal range, red dots indicate values outside the normal range. ALA = Alpha-lipoic acid; ox-LDL = oxidised LDL; PerOx = total lipid peroxide; TAC = total antioxidant capactity.
For ALA and lactate, the majority of patients had abnormally low values (28/32 (88%) and 23/32 (72%) respectively). Only two patients’ lactate levels were too high. For one patient, an extremely high level of ALA (13.25) was measured. For PerOx half of the patients (46.9%) had abnormally high values. For thiols and TAC about one third of patients had abnormally low values (31.2% and 37.5%, respectively). For HbA1c about 20% of patients (21.9%) had abnormally low values and no one had an HbA1c above 5.6%. For oxidated LDL, a very high, abnormal level was measured in one patient, while for all other patients, the levels were in the normal range.
Correlations of mitochondrial function biomarkers and migraine severity
We found no indication for a correlation of the 7 mitochondrial function biomarkers with MIDAS score or number of migraine days per month at baseline. Corresponding correlation coefficients and p values are given in supplementary information section 1.1.
Comparison between patients with and without migraine prophylaxis
Summary statistics of absolute levels of the mitochondrial function biomarkers and the frequencies of patients with abnormal values according to migraine prophylaxis are presented in supplementary information section 1.2. Our data provide no evidence for an effect of migraine prophylaxis.
Comparison between patients studied during or outside of an attack
Summary statistics of absolute levels of the mitochondrial function biomarkers and the frequencies of patients with abnormal values according to acute migraine attack at baseline (baseline visit ± 2 days) are presented in supplementary information section 1.3. Most patients presented with acute migraine at baseline; for one patient this information is missing. Our data provide no evidence for any difference between these two groups.
Comparison between patients with and without aura
Summary statistics of absolute levels of the mitochondrial function biomarkers and the frequencies of patients with abnormal values according to aura are presented in supplementary information section 1.4. We found no evidence for differences between patients with or without aura, neither in the absolute values of the biomarkers nor in the proportions of patients with abnormal values.
Discussion
We have shown that apart from oxLDL and HbA1c most other markers of mitochondrial functioning showed abnormalities in a significant proportion (> 30%) of the patients examined.
ALA
To the best of our knowledge ALA levels have not previously been determined in migraine. Almost 90% of patients in this sample had abnormally low values of ALA. ALA, also known as thioctic acid, is an eight-carbon, sulfur-containing compound that functions as a water- and fat-soluble antioxidant79,80. It can directly (by removing reactive species) and indirectly (by chelating transition metal ions) reduce oxidative stress79,80. The human body can synthesize small amounts of ALA79. ALA also plays an important role as co-enzyme in energy metabolism79–81. Furthermore, it is able to regenerate other antioxidants, such as vitamin C and E, CoQ10, it increases intracellular glutathione and activates endogenous antioxidant systems82–84. Apart from its anti-oxidant action, ALA seems to assist weight loss85, increase insulin sensitivity and decrease blood lipids86. All of these mechanisms are probably migraine relevant. Interestingly, ALA supplementation (300–600 mg) per day has been shown to significantly reduce migraine attack frequency, severity and duration36–38, which seems to align with our findings. Further research is needed to see, whether this finding is specific to our medium–high frequency episodic migraine population or a general characteristic of migraine or even a general characteristic of other (neurological) diseases with a mitochondrial/oxidative stress component. Additionally, as ALA measurements are less established and standardised than other markers, these results should be replicated in a larger cohort, with a different laboratory and using a control group. Should this finding be replicated and migraine specific, ALA might represent a potential biomarker.
TAC
Serum (or plasma) concentrations of different antioxidants can be measured separately, but since the measurement of different antioxidant molecules individually is impractical and costly and their antioxidant effects are additive, the total antioxidant capacity of a sample is typically measured, and this is typically referred to as total antioxidant capacity (TAC), total antioxidant status (TAS) or other synonyms, which will be used interchangeably.
Almost 40% of our patients had abnormally low TAC being in line with results of previous research. A study on 75 MO patients demonstrated that the levels of total antioxidants were decreased and the levels of total oxidants and the oxidative stress index were increased55. Another study found TAC to be significantly reduced in migraineurs compared to controls66. TAC levels increased after successful prophylactic treatment compared to the baseline, irrespective of treatment modality (rTMS versus amitriptyline) and the increase correlated with treatment success66.We assume higher TAC with lower migraine severity, less recent oxidative stress exposure, and increased distance to previous and future migraine attack. These assumptions would have to be validated in future research.
PerOx
Lipid peroxidation is the oxidative degradation of lipids via free radical damage of the lipids in cell membranes, polyunsaturated fatty acids in particular. The end products of lipid peroxidation are reactive aldehydes, such as 4-hydroxynonenal (HNE) and malondialdehyde (MDA). Free radicals cause increased accumulation of these lipid peroxidation by-products in the blood. About half of the patients had abnormally high total PerOx levels, being in line with previous research. Several studies have found serum levels of MDAs to be significantly elevated in migraine patients56,67, even in the interictal phase87.
oxLDL
Oxidized low-density lipoprotein (LDL) is a harmful type of cholesterol that is produced when normal LDL cholesterol is damaged by chemical interactions with ROS. All but one patient had normal levels for oxLDL, which is in contrast to the study of Bernecker et al. that found highly significantly elevated levels oxLDL in female migraineurs57. This result could be due to differences in study population, as the migraineurs of the Berecker et al. study tended to have metabolic syndrome and had generally higher BMIs as our migraine patient population.
Thiols
The term “thiol” refers to organic compounds containing sulfur (in form of the functional group –SH, the thiol group). Thiol groups are able to destroy ROS and other free radicals by enzymatic as well as nonenzymatic mechanisms88. Total thiol levels have previously been used to evaluate excess free radical generation, both in physiological and pathological conditions89. Protein thiol levels in serum have been shown to be a direct measure of the in vivo reduction/oxidation (redox) status in humans, because thiols react readily with ROS to form disulfides76. Thiol redox homeostasis plays an important role in neurogenerative diseases90 and in nine other categories of human disorders serum protein thiols have been found to be significantly reduced compared to healthy controls76.
About one third of patients had abnormally low serum thiol levels, but this seems to be in line with previous research. A larger study found significantly reduced thiol levels in 151 migraine patients (74 MO, 77 MA) compared to 70 healthy controls and there was a negative correlation with migraine disability61. A negative correlation between the levels of total thiols and the duration of the headaches has also been demonstrated55. However, others studies found no significant difference in thiol groups between patients and controls, even during attacks68 and one study even found higher total (–SH + – S – S–) & native thiol (–SH) levels in serum of migraineurs, but this did not correlated with disease severity or migraine type63. Recent exposure to oxidative stress, migraine severity, time in the migraine cycle and similar aspects could explain the different results.
HbA1c
HbA1c (glycated hemoglobin) is an indication of the average blood glucose levels over the last two to three months. Just over 20% of patients had abnormally low HbA1c levels and none of them had HbA1c levels that were above 5.6%. To the best of our knowledge HbA1c has rarely been looked at in migraine. One study found no significant difference in HbA1c levels between CM, EM and healthy controls19.
However, magnetic resonance spectroscopy (MRS) studies in migraine have consistently shown abnormalities of mitochondrial oxidative phosphorylation (OXPHOS), such as hypometabolism between3–9 and during migraine attacks10, in the resting brain and in the muscle following exercise3,11,12. A 16% decrease of absolute ATP levels in migraine without aura patients was also demonstrated interictally using 31P-MRS13. These findings are supported by early studies showing that metabolic changes induced by fasting, glucose or insulin administration can trigger migraine attacks; e.g. a 50 g glucose tolerance test (GTT) after a 10-h fast triggered a migraine in 6 out of 10 migraine patients reporting attacks associated with fasting14. Abnormal metabolic responses were also reported in GTT studies14,15 and interictal impaired glucose tolerance and insulin resistance has been reported in various other studies16–20. While only 20% of our migraineurs had abnormally low HbA1c levels, all levels tended to be on the lower side, despite reported higher carbohydrate diets. As HbA1c levels correspond to an average blood sugar measurement, low average values despite probable highs after carbohydrate rich meals could be an indication that there might be lows as well. This would be in line with previous neuroimaging and GTT research results, but it is speculation only and these assumption need to be confirmed by future research.
Lactate
Lactate is typically measured to assess tissue oxygenation, arising from either decreased oxygen delivery or a disorder in oxygen use, both of which lead to increased anaerobic metabolism and increases in lactate levels. In certain types of migraine, especially migrainous stroke, elevated serum lactate and pyruvate levels have previously been reported46,47. In contrast to this, only 2 patients had abnormally high serum lactate levels in our cohort and over 70% of patients serum lactate levels were abnormally low.
While there is little data on serum lactate levels in migraine, data on brain lactate analysed with 1H-MRS have also been shown to vary due to patient selection (see review by Reyngoudt et al. (2012) for details8). Elevated brain lactate levels were found in some studies of MA91,92, but not in MO93–96. Occipital baseline lactate levels were increased in patients with visual auras, but not in those having complex neurological auras. By contrast, during photic stimulation lactate increased significantly in the latter, but not in the former91. Stimulus-induced lactate increases are physiological97 and can be explained by the neuron-astrocyte lactate shuttle98. Hence, their absence in migraine patients, whose neuronal activation is energetically more demanding99, could be considered pathological and might be contributing to an energetic crisis.
To the best of our knowledge, no recent studies have looked at baseline serum lactate levels in episodic migraine patients or subgroups thereof. More research is needed to replicate this finding; in particular a study combining lactate level quantification in the cortex with that of the periphery and with brain energetics seems warranted. We can only speculate as to why lactate levels were predominantly low in the majority of our patients. They all came rested, but fasted overnight to the trial site. Decreased baseline lactate levels might be a sign of increased cerebral lactate consumption and an indicator of an increased cerebral energy demand of the migraine brain, as in addition to ketone bodies, lactate constitutes the only other major alternative brain energy substrate from glucose and is used especially during times of high metabolic demands or hypoglycemia100. A study using 13C-L-lactate and magnetic resonance spectroscopy suggested that the contribution of plasma lactate to brain metabolism can be up to 60%101, which is very similar to ketone bodies. It could also be a sign of decreased lactate synthesis as demonstrated with 1H-MRS91.
In summary, we have shown that apart from oxLDL and HbA1c most other markers of mitochondrial functioning are abnormal in at least > 30% of the patients examined. As oxidative stress is a complex mechanism including different sources of ROS and various pathways, differing results in previous research may at least be partially caused by different oxidative stress parameters examined, e.g. MDA versus HNE, as well as by different study groups investigated, e.g. adults versus children, MA versus MO, females versus males, and differences in migraine severity, recent oxidative stress exposure and the time within the individual migraine cycle, where measurements were taken. Genetic research examining oxidative stress related genes in larger homogenous migraine cohorts could be interesting future research that would hardly be influenced by these factors.
Our data provide no evidence for correlations between any of the seven mitochondrial function / oxidative stress markers and migraine severity. This could be due to our sample population being fairly homogenous or the sample size being too small. In addition, we found no evidence for an effect of migraine prophylaxis. This is not surprising, since patients were still suffering from a substantial number of migraine days/months despite the prophylactic treatment (5–14 days/months), suggesting that the critical migraine pathophysiological mechanisms remained active. Furthermore, no evidence for an effect of a preceding or subsequent migraine attack has been found. This might be due to only 5 patients being migraine attack free within 2 days before and after the venous puncture, making an analysis of the potential impact of an attack difficult. We also found no evidence for a difference between MA and MO patients. For a randomly selected migraine cohort mainly recruited via public advertisements, the number of MA patients was unusually high (62.5%) in our study population. We can only speculate as to why this might be the case. Since participants were part of the 9 months MigraKet intervention trial70, it seems plausible that MA patients might have been more motivated to take place in such a lengthy trial and this led to the observed over-representation.
While we found no correlation between these mitochondrial function/oxidative stress markers and disease severity, differences in methodologies used and patient characteristics, recent oxidative stress exposure and also time in the respective migraine cycle is likely to play a role. Future research examining these markers at different time points during the migraine cycle and in different migraine types would be interesting.
The most important limitation of this study is the absence of a matched control group. While abnormally low levels in 90% of patients in the case of ALA are likely to be of importance, we cannot be sure that PerOx, TAC and thiol level findings would have been significantly different from controls. Future research is needed to replicate these findings in the presence of a control group. Secondly, the sample size was fairly small, in particular with regards to the correlation analyses. In addition, one third of patients was using a migraine prophylaxis. While our data provide no evidence for an effect of migraine prophylaxis, the inclusion of patients who are using a prophylaxis is not ideal.
Conclusion
In conclusion, this study provides further support for metabolic abnormalities in migraine, in particular the role of increase oxidative stress and decreased antioxidant capacity respectively in migraine pathophysiology. The peripheral markers assessed here could easily be examined in most doctor’s offices and might assist personalised migraine treatment that targets oxidative stress and mitochondrial functioning; however, further research is needed to replicate these findings, ideally in the presence of a control group.
Supplementary Information
Abbreviations
- ALA
Alpha-lipoic acid
- ATP
Adenosine triphosphate
- CGRP
Calcitonin gene-related peptide
- CM
Chronic migraineurs
- EM
Episodic migraineurs
- HbA1c
Glycosylated hemoglobin
- ICHD-3
International classification of headache disorders version 3
- IQR
Interquartile range
- KD
Ketogenic diet
- PerOx
Lipid peroxide
- MA
Migraine with aura
- MDA
Malondialdehyde
- MIDAS
Migraine disability assessment
- MO
Migraine without aura
- NSAIDs
Non-steroidal anti-inflammatory drugs
- oxLDL
Oxidised LDL
- H-MRS
Proton magnetic resonance spectroscopy
- H2O2
Hydrogen peroxide
- HNE
4-Hydroxynonenal
- OXPHOS
Oxidative phosphorylation
- ROS
Reactive oxygen species
- SOD2
Superoxide dismutase 2
- TAC
Total plasma antioxidant capacity
- TRP
Transient receptor potential channels
Author contributions
E.C.G. participated in the design of the study and its organisation, conduct and data acquisition, and was responsible for the main composition of the manuscript. N.P. and A.L.O. participated in the conduct of the study. D.R.V. covered all statistical aspects of the study and the manuscript. P.S. and D.F. participated in the study design, its organisation, and edited the manuscript. J.S. provided additional text and citations and in-depth editing of the manuscript. All authors proofread the final manuscript prior to submission.
Funding
This research was funded by the Swiss National Science Foundation (SNF), Grant Number “32003B_173193/1”.
Data availability
For inspection purposes, insight to the original data will be permitted to the members of the appropriate authorities and also for the members of the local ethics committee, EKNZ.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84102-2.
References
- 1.Stovner LJ, Hoff JM, Svalheim S, Gilhus NE. Neurological disorders in the Global Burden of Disease 2010 study. Acta Neurol. Scand. 2014;129(198):1–6. doi: 10.1111/ane.12229. [DOI] [PubMed] [Google Scholar]
- 2.Gray PA, Burtness HI. Hypoglycemic headache*. Endocrinology. 1935;19(5):549–560. doi: 10.1210/endo-19-5-549. [DOI] [Google Scholar]
- 3.Barbiroli B, Montagna P, Cortelli P, Funicello R, Iotti S, Monari L, et al. Abnormal brain and muscle energy metabolism shown by 31P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology. 1992;42(6):1209–1214. doi: 10.1212/WNL.42.6.1209. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Kim S, Suh SI, Koh SB, Park KW, Oh K. Interictal metabolic changes in episodic migraine: A voxel-based FDG-PET study. Cephalalgia. 2010;30(1):53–61. doi: 10.1111/j.1468-2982.2009.01890.x. [DOI] [PubMed] [Google Scholar]
- 5.Lodi R, Montagna P, Soriani S, Iotti S, Arnaldi C, Cortelli P, et al. Deficit of brain and skeletal muscle bioenergetics and low brain magnesium in juvenile migraine: An in vivo 31P magnetic resonance spectroscopy interictal study. Pediatr. Res. 1997;42(6):866–871. doi: 10.1203/00006450-199712000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Lodi R, Iotti S, Cortelli P, Pierangeli G, Cevoli S, Clementi V, et al. Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res. Bull. 2001;54(4):437–441. doi: 10.1016/S0361-9230(01)00440-3. [DOI] [PubMed] [Google Scholar]
- 7.Montagna P, Cortelli P, Monari L, Pierangeli G, Parchi P, Lodi R, et al. 31P-magnetic resonance spectroscopy in migraine without aura. Neurology. 1994;44(4):666–669. doi: 10.1212/WNL.44.4.666. [DOI] [PubMed] [Google Scholar]
- 8.Reyngoudt H, Achten E, Paemeleire K. Magnetic resonance spectroscopy in migraine: What have we learned so far? Cephalalgia Int. J. Headache. 2012;32(11):845–859. doi: 10.1177/0333102412452048. [DOI] [PubMed] [Google Scholar]
- 9.Schulz UG, Blamire AM, Corkill RG, Davies P, Styles P, Rothwell PM. Association between cortical metabolite levels and clinical manifestations of migrainous aura: An MR-spectroscopy study. Brain. 2007;130(Pt 12):3102–3110. doi: 10.1093/brain/awm165. [DOI] [PubMed] [Google Scholar]
- 10.Welch KM, Levine SR, D’Andrea G, Schultz LR, Helpern JA. Preliminary observations on brain energy metabolism in migraine studied by in vivo phosphorus 31 NMR spectroscopy. Neurology. 1989;39(4):538–541. doi: 10.1212/WNL.39.4.538. [DOI] [PubMed] [Google Scholar]
- 11.Lodi R, Kemp GJ, Pierangeli G, Cortelli P, Iotti S, Radda GK, et al. Quantitative analysis of skeletal muscle bioenergetics and proton efflux in migraine and cluster headache. J. Neurol. Sci. 1997;146(1):73–80. doi: 10.1016/S0022-510X(96)00287-0. [DOI] [PubMed] [Google Scholar]
- 12.Barbiroli B, Montagna P, Cortelli P, Martinelli P, Sacquegna T, Zaniol P, et al. Complicated migraine studied by phosphorus magnetic resonance spectroscopy. Cephalalgia. 1990;10(5):263–272. doi: 10.1046/j.1468-2982.1990.1005263.x. [DOI] [PubMed] [Google Scholar]
- 13.Reyngoudt H, Paemeleire K, Descamps B, De Deene Y, Achten E. 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia Int. J. Headache. 2011;31(12):1243–1253. doi: 10.1177/0333102410394675. [DOI] [PubMed] [Google Scholar]
- 14.Hockaday Judith M, Williamson DH, Whitty CWM. Blood-glucose levels and fatty-acid metabolism in migraine related to fasting. Lancet. 1971;297(7710):1153–1156. doi: 10.1016/S0140-6736(71)91662-X. [DOI] [PubMed] [Google Scholar]
- 15.Shaw SW, Johnson RH, Keogh HJ. Metabolic changes during glucose tolerance tests in migraine attacks. J. Neurol. Sci. 1977;33(1–2):51–59. doi: 10.1016/0022-510X(77)90181-2. [DOI] [PubMed] [Google Scholar]
- 16.Dexter JD, Roberts J, Byer JA. The five hour glucose tolerance test and effect of low sucrose diet in migraine. Headache J. Head Face Pain. 1978;18(2):91–94. doi: 10.1111/j.1526-4610.1978.hed1802091.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Li X, Diao Y, Meng S, Xing Y, Zhou H, et al. Are glucose and insulin metabolism and diabetes associated with migraine? A community-based, case-control study. J. Oral Facial Pain Headache. 2017;31(3):240–250. doi: 10.11607/ofph.1843. [DOI] [PubMed] [Google Scholar]
- 18.Rainero I, Limone P, Ferrero M, Valfrè W, Pelissetto C, Rubino E, et al. Insulin sensitivity is impaired in patients with migraine. Cephalalgia. 2005;25(8):593–597. doi: 10.1111/j.1468-2982.2005.00928.x. [DOI] [PubMed] [Google Scholar]
- 19.Fava A, Pirritano D, Consoli D, Plastino M, Casalinuovo F, Cristofaro S, et al. Chronic migraine in women is associated with insulin resistance: A cross-sectional study. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2014;21(2):267–272. doi: 10.1111/ene.12289. [DOI] [PubMed] [Google Scholar]
- 20.Cavestro C, Rosatello A, Micca G, Ravotto M, Marino MP, Asteggiano G, et al. Insulin metabolism is altered in migraineurs: A new pathogenic mechanism for migraine? Headache J. Head Face Pain. 2007;47(10):1436–1442. doi: 10.1111/j.1526-4610.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 21.Shaik MM, Gan SH. Vitamin supplementation as possible prophylactic treatment against migraine with aura and menstrual migraine. Biomed. Res. Int. 2015;2015:469529. doi: 10.1155/2015/469529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhäupl KM, Arnold G. High-dose riboflavin treatment is efficacious in migraine prophylaxis: An open study in a tertiary care centre. Eur. J. Neurol. 2004;11(7):475–477. doi: 10.1111/j.1468-1331.2004.00813.x. [DOI] [PubMed] [Google Scholar]
- 23.Condò M, Posar A, Arbizzani A, Parmeggiani A. Riboflavin prophylaxis in pediatric and adolescent migraine. J. Headache Pain. 2009;10(5):361–365. doi: 10.1007/s10194-009-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaul C, Diener H-C, Danesch U. Migravent® Study Group on behalf of the MS. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain. 2015;16:516. doi: 10.1186/s10194-015-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50(2):466–470. doi: 10.1212/WNL.50.2.466. [DOI] [PubMed] [Google Scholar]
- 26.Rahimdel A, Mellat A, Zeinali A, Jafari E, Ayatollahi P. Comparison between intravenous sodium valproate and subcutaneous sumatriptan for treatment of acute migraine attacks; double-blind randomized clinical trial. Iran. J. Med. Sci. 2014;39(2 Suppl):171–177. [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson DF, Saluja HS. Prophylaxis of migraine headaches with riboflavin: A systematic review. J. Clin. Pharm. Ther. 2017;42(4):394–403. doi: 10.1111/jcpt.12548. [DOI] [PubMed] [Google Scholar]
- 28.Di Lorenzo C, Pierelli F, Coppola G, Grieco GS, Rengo C, Ciccolella M, et al. Mitochondrial DNA haplogroups influence the therapeutic response to riboflavin in migraineurs. Neurology. 2009;72(18):1588–1594. doi: 10.1212/WNL.0b013e3181a41269. [DOI] [PubMed] [Google Scholar]
- 29.Dahri M, Hashemilar M, Asghari-Jafarabadi M, Tarighat-Esfanjani A. Efficacy of coenzyme Q10 for the prevention of migraine in women: A randomized, double-blind, placebo-controlled study. Eur. J. Integr. Med. 2017;16:8–14. doi: 10.1016/j.eujim.2017.10.003. [DOI] [Google Scholar]
- 30.Dahri M, Tarighat-Esfanjani A, Asghari-Jafarabadi M, Hashemilar M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci. 2018;1:1–9. doi: 10.3934/Neuroscience.2018.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Sándor PS, Di Clemente L, Coppola G, Saenger U, Fumal A, Magis D, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: A randomized controlled trial. Neurology. 2005;64(4):713–715. doi: 10.1212/01.WNL.0000151975.03598.ED. [DOI] [PubMed] [Google Scholar]
- 32.Hajihashemi P, Askari G, Khorvash F, Reza Maracy M, Nourian M. The effects of concurrent Coenzyme Q10, L-carnitine supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Cephalalgia. 2019;6:0333102418821661. doi: 10.1177/0333102418821661. [DOI] [PubMed] [Google Scholar]
- 33.Shoeibi A, Olfati N, Soltani Sabi M, Salehi M, Mali S, Akbari OM. Effectiveness of coenzyme Q10 in prophylactic treatment of migraine headache: An open-label, add-on, controlled trial. Acta Neurol. Belg. 2017;117(1):103–109. doi: 10.1007/s13760-016-0697-z. [DOI] [PubMed] [Google Scholar]
- 34.Rozen T, Oshinsky M, Gebeline C, Bradley K, Young W, Shechter A, et al. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia. 2002;22(2):137–141. doi: 10.1046/j.1468-2982.2002.00335.x. [DOI] [PubMed] [Google Scholar]
- 35.Chiu H-Y, Yeh T-H, Huang Y-C, Chen P-Y. Effects of intravenous and oral magnesium on reducing migraine: A meta-analysis of randomized controlled trials. Pain Physician. 2016;19(1):E97–112. [PubMed] [Google Scholar]
- 36.Magis D, Ambrosini A, Sándor P, Jacquy J, Laloux P, Schoenen J. A randomized double-blind placebo-controlled trial of thioctic acid in migraine prophylaxis. Headache. 2007;47(1):52–57. doi: 10.1111/j.1526-4610.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 37.Cavestro C, Bedogni G, Molinari F, Mandrino S, Rota E, Frigeri MC. Alpha-lipoic acid shows promise to improve migraine in patients with insulin resistance: A 6-month exploratory study. J. Med. Food. 2018;21(3):269–273. doi: 10.1089/jmf.2017.0068. [DOI] [PubMed] [Google Scholar]
- 38.Ali AM, Awad TG, Al-Adl NM. Efficacy of combined topiramate/thioctic acid therapy in migraine prophylaxis. Saudi Pharm. J. 2010;18(4):239–243. doi: 10.1016/j.jsps.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strahlman RS. Can ketosis help migraine sufferers? A case report. Headache. 2006;46(1):182. doi: 10.1111/j.1526-4610.2006.00321_5.x. [DOI] [PubMed] [Google Scholar]
- 40.Di Lorenzo C, Currà A, Sirianni G, Coppola G, Bracaglia M, Cardillo A, et al. Diet transiently improves migraine in two twin sisters: Possible role of ketogenesis? Funct. Neurol. 2013;28(4):305–308. [PMC free article] [PubMed] [Google Scholar]
- 41.Maggioni F, Margoni M, Zanchin G. Ketogenic diet in migraine treatment: A brief but ancient history. Cephalalgia Int. J. Headache. 2011;31(10):1150–1151. doi: 10.1177/0333102411412089. [DOI] [PubMed] [Google Scholar]
- 42.Schnabel TG. An experience with a ketogenic dietary in migraine. Ann. Intern. Med. 1928;2(4):341. doi: 10.7326/0003-4819-2-4-341. [DOI] [Google Scholar]
- 43.Di Lorenzo C, Coppola G, Sirianni G, Di Lorenzo G, Bracaglia M, Di Lenola D, et al. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2014;22(1):170–177. doi: 10.1111/ene.12550. [DOI] [PubMed] [Google Scholar]
- 44.Di Lorenzo C, Coppola G, Bracaglia M, Di Lenola D, Evangelista M, Sirianni G, et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: A multimodal evoked potentials study. J. Headache Pain. 2016;17(1):58. doi: 10.1186/s10194-016-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross EC, Klement RJ, Schoenen J, D’Agostino DP, Fischer D. Potential protective mechanisms of ketone bodies in migraine prevention. Nutrients. 2019;11(4):811. doi: 10.3390/nu11040811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagna P, Sacquegna T, Martinelli P, Cortelli P, Bresolin N, Moggio M, et al. Mitochondrial abnormalities in migraine. Preliminary findings. Headache J. Head Face Pain. 1988;28(7):477–480. doi: 10.1111/j.1526-4610.1988.hed2807477.x. [DOI] [PubMed] [Google Scholar]
- 47.Okada H, Araga S, Takeshima T, Nakashima K. Plasma lactic acid and pyruvic acid levels in migraine and tension-type headache. Headache. 1998;38(1):39–42. doi: 10.1046/j.1526-4610.1998.3801039.x. [DOI] [PubMed] [Google Scholar]
- 48.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5(1):9. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogino K, Wang D-H. Biomarkers of oxidative/nitrosative stress: An approach to disease prevention. Acta Med. Okayama. 2007;61(4):181–189. doi: 10.18926/AMO/32871. [DOI] [PubMed] [Google Scholar]
- 50.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borkum JM. Migraine triggers and oxidative stress: A narrative review and synthesis. Headache. 2015;56(1):12–35. doi: 10.1111/head.12725. [DOI] [PubMed] [Google Scholar]
- 52.Gross EC, Lisicki M, Fischer D, Sandor PS, Schoenen J. The metabilic face of migraine. Nat. Neurol. 2019;15(11):627–643. doi: 10.1038/s41582-019-0255-4. [DOI] [PubMed] [Google Scholar]
- 53.Benemei S, Fusi C, Trevisan G, Geppetti P. The TRPA1 channel in migraine mechanism and treatment. Br. J. Pharmacol. 2014;171(10):2552–2567. doi: 10.1111/bph.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxid. Redox Signal. 2014;21(6):971–986. doi: 10.1089/ars.2013.5616. [DOI] [PubMed] [Google Scholar]
- 55.Alp R, Selek S, Alp SI, Taşkin A, Koçyiğit A. Oxidative and antioxidative balance in patients of migraine. Eur. Rev. Med. Pharmacol. Sci. 2010;14(10):877–882. [PubMed] [Google Scholar]
- 56.Aytaç B, Coşkun Ö, Alioğlu B, Durak ZE, Büber S, Tapçi E, et al. Decreased antioxidant status in migraine patients with brain white matter hyperintensities. Neurol. Sci. 2014;35(12):1925–1929. doi: 10.1007/s10072-014-1864-8. [DOI] [PubMed] [Google Scholar]
- 57.Bernecker C, Ragginer C, Fauler G, Horejsi R, Möller R, Zelzer S, et al. Oxidative stress is associated with migraine and migraine-related metabolic risk in females. Eur. J. Neurol. 2011;18(10):1233–1239. doi: 10.1111/j.1468-1331.2011.03414.x. [DOI] [PubMed] [Google Scholar]
- 58.Bolayir E, Celik K, Kugu N, Yilmaz A, Topaktas S, Bakir S. Intraerythrocyte antioxidant enzyme activities in migraine and tension-type headaches. J. Chin. Med. Assoc. 2004;67(6):263–267. [PubMed] [Google Scholar]
- 59.Ciancarelli I, Tozzi-Ciancarelli M, Massimo CD, Marini C, Carolei A. Urinary nitric oxide metabolites and lipid peroxidation by-products in migraine. Cephalalgia. 2003;23(1):39–42. doi: 10.1046/j.1468-2982.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- 60.Ciancarelli I, Tozzi-Ciancarelli M, Spacca G, Massimo CD, Carolei A. Relationship between biofeedback and oxidative stress in patients with chronic migraine. Cephalalgia. 2007;27(10):1136–1141. doi: 10.1111/j.1468-2982.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 61.Eren Y, Dirik E, Neşelioğlu S, Erel Ö. Oxidative stress and decreased thiol level in patients with migraine: Cross-sectional study. Acta Neurol. Belg. 2015;115(4):643–649. doi: 10.1007/s13760-015-0427-y. [DOI] [PubMed] [Google Scholar]
- 62.Geyik S, Altunısık E, Neyal AM, Taysi S. Oxidative stress and DNA damage in patients with migraine. J. Headache Pain. 2016;17(1):10. doi: 10.1186/s10194-016-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gumusyayla S, Vural G, Bektas H, Neselioglu S, Deniz O, Erel O. A novel oxidative stress marker in migraine patients: Dynamic thiol-disulphide homeostasis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2016;37(8):1311–1317. doi: 10.1007/s10072-016-2592-z. [DOI] [PubMed] [Google Scholar]
- 64.Shimomura T, Kowa H, Nakano T, Kitano A, Marukawa H, Urakami K, et al. Platelet superoxide dismutase in migraine and tension-type headache. Cephalalgia. 1994;14(3):215–218. doi: 10.1046/j.1468-2982.1994.014003215.x. [DOI] [PubMed] [Google Scholar]
- 65.Tozzi-Ciancarelli M, De Matteis G, Di Massimo C, Marini C, Ciancarelli I, Carolei A. Oxidative stress and platelet responsiveness in migraine. Cephalalgia. 1997;17(5):580–584. doi: 10.1046/j.1468-2982.1997.1705580.x. [DOI] [PubMed] [Google Scholar]
- 66.Tripathi GM, Kalita J, Misra UK. A study of oxidative stress in migraine with special reference to prophylactic therapy. Int. J. Neurosci. 2018;128(4):318–324. doi: 10.1080/00207454.2017.1374959. [DOI] [PubMed] [Google Scholar]
- 67.Tuncel D, Tolun FI, Gokce M, İmrek S, Ekerbiçer H. Oxidative stress in migraine with and without aura. Biol. Trace Elem. Res. 2008;126(1–3):92–97. doi: 10.1007/s12011-008-8193-9. [DOI] [PubMed] [Google Scholar]
- 68.Yilmaz G, Sürer H, Inan LE, Coskun O, Yücel D. Increased nitrosative and oxidative stress in platelets of migraine patients. Tohoku J. Exp. Med. 2007;211(1):23–30. doi: 10.1620/tjem.211.23. [DOI] [PubMed] [Google Scholar]
- 69.Neri M, Frustaci A, Milic M, Valdiglesias V, Fini M, Bonassi S, et al. A meta-analysis of biomarkers related to oxidative stress and nitric oxide pathway in migraine. Cephalalgia. 2015;35(10):931–937. doi: 10.1177/0333102414564888. [DOI] [PubMed] [Google Scholar]
- 70.Gross E, Putananickal N, Orsini A-L, Schmidt S, Vogt DR, Cichon S, et al. Efficacy and safety of exogenous ketone bodies for preventive treatment of migraine: A study protocol for a single-centred, randomised, placebo-controlled, double-blind crossover trial. Trials. 2019;20(1):61. doi: 10.1186/s13063-018-3120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia Int. J. Headache. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 72.Benz T, Lehmann S, Gantenbein AR, Sandor PS, Stewart WF, Elfering A, et al. Translation, cross-cultural adaptation and reliability of the German version of the migraine disability assessment (MIDAS) questionnaire. Health Qual. Life Outcomes. 2018;16(1):42. doi: 10.1186/s12955-018-0871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stepan H, Heihoff-Klose A, Faber R. Reduced antioxidant capacity in second-trimester pregnancies with pathological uterine perfusion. Ultrasound Obstet. Gynecol. 2004;23(6):579–583. doi: 10.1002/uog.1045. [DOI] [PubMed] [Google Scholar]
- 74.Hildebrandt W, Alexander S, Bärtsch P, Dröge W. Effect of N-acetyl-cysteine on the hypoxic ventilatory response and erythropoietin production: Linkage between plasma thiol redox state and O(2) chemosensitivity. Blood. 2002;99(5):1552–1555. doi: 10.1182/blood.V99.5.1552. [DOI] [PubMed] [Google Scholar]
- 75.Koubaa N, Nakbi A, Smaoui M, Abid N, Chaaba R, Abid M, et al. Hyperhomocysteinemia and elevated ox-LDL in Tunisian type 2 diabetic patients: Role of genetic and dietary factors. Clin. Biochem. 2007;40(13):1007–1014. doi: 10.1016/j.clinbiochem.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 76.Banne AF, Amiri A, Pero RW. Reduced level of serum thiols in patients with a diagnosis of active disease. J. Anti-Aging Med. 2003;6(4):327–334. doi: 10.1089/109454503323028920. [DOI] [PubMed] [Google Scholar]
- 77.Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. Am. Stat. 2019;73(1):1–19. doi: 10.1080/00031305.2019.1583913. [DOI] [Google Scholar]
- 78.Team RC. R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A language and environment for statistical computing. 2018;2013.
- 79.Bast A, Haenen GRMM. Lipoic acid: A multifunctional antioxidant. BioFactors. 2003;17(1–4):207–213. doi: 10.1002/biof.5520170120. [DOI] [PubMed] [Google Scholar]
- 80.Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995;19(2):227–250. doi: 10.1016/0891-5849(95)00017-R. [DOI] [PubMed] [Google Scholar]
- 81.Müller U, Krieglstein J. Prolonged pretreatment with α-lipoic acid protects cultured neurons against hypoxic, glutamate-, or iron-induced injury. J. Cereb. Blood Flow Metab. 1995;15(4):624–630. doi: 10.1038/jcbfm.1995.77. [DOI] [PubMed] [Google Scholar]
- 82.Kim W-J, Kang J-Y, Kwon D-K, Song Y-J, Lee K-H. Effects of α-lipoic acid supplementation on malondialdehyde contents and superoxide dismutase in rat skeletal muscles. Food Sci. Biotechnol. 2011;20(4):1133. doi: 10.1007/s10068-011-0154-y. [DOI] [Google Scholar]
- 83.Packer L. alpha-Lipoic acid: A metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab. Rev. 1998;30(2):245–275. doi: 10.3109/03602539808996311. [DOI] [PubMed] [Google Scholar]
- 84.Packer L, Roy S, Sen CK. Alpha-lipoic acid: A metabolic antioxidant and potential redox modulator of transcription. Adv. Pharmacol. 1997;38:79–101. doi: 10.1016/S1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 85.Huerta AE, Navas-Carretero S, Prieto-Hontoria PL, Martínez JA, Moreno-Aliaga MJ. Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity. 2015;23(2):313–321. doi: 10.1002/oby.20966. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Han P, Wu N, He B, Lu Y, Li S, et al. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity. 2011;19(8):1647–1653. doi: 10.1038/oby.2011.121. [DOI] [PubMed] [Google Scholar]
- 87.Gupta R, Pathak R, Bhatia MS, Banerjee BD. Comparison of oxidative stress among migraineurs, tension-type headache subjects, and a control group. Ann. Indian Acad. Neurol. 2009;12(3):167. doi: 10.4103/0972-2327.56316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cadenas E. Biochemistry of oxygen toxicity. Annu. Rev. Biochem. 1989;58(1):79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 89.Pasaoglu H, Sancak B, Bukan N. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J. Exp. Med. 2004;203(3):211–218. doi: 10.1620/tjem.203.211. [DOI] [PubMed] [Google Scholar]
- 90.McBean GJ, Aslan M, Griffiths HR, Torrão RC. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015;22(5):186–194. doi: 10.1016/j.redox.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sandor PS, Dydak U, Schoenen J, Kollias SS, Hess K, Boesiger P, et al. MR-spectroscopic imaging during visual stimulation in subgroups of migraine with aura. Cephalalgia. 2005;25(7):507–518. doi: 10.1111/j.1468-2982.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe H, Kuwabara T, Ohkubo M, Tsuji S, Yuasa T. Elevation of cerebral lactate detected by localized 1H-magnetic resonance spectroscopy in migraine during the interictal period. Neurology. 1996;47(4):1093–1095. doi: 10.1212/WNL.47.4.1093. [DOI] [PubMed] [Google Scholar]
- 93.Reyngoudt H, Paemeleire K, Dierickx A, Descamps B, Vandemaele P, De Deene Y, et al. Does visual cortex lactate increase following photic stimulation in migraine without aura patients? A functional (1)H-MRS study. J. Headache Pain. 2011;12(3):295–302. doi: 10.1007/s10194-011-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prescot A, Becerra L, Pendse G, Tully S, Jensen E, Hargreaves R, et al. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol. Pain. 2009;30(5):34. doi: 10.1186/1744-8069-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mohamed RE, Aboelsafa AA, Al-Malt AM. Interictal alterations of thalamic metabolic concentration ratios in migraine without aura detected by proton magnetic resonance spectroscopy. Egypt. J. Radiol. Nucl. Med. 2013;44(4):859–870. doi: 10.1016/j.ejrnm.2013.08.004. [DOI] [Google Scholar]
- 96.Becerra L, Veggeberg R, Prescot A, Jensen JE, Renshaw P, Scrivani S, et al. A “complex” of brain metabolites distinguish altered chemistry in the cingulate cortex of episodic migraine patients. Neuroimage Clin. 2016;11:588–594. doi: 10.1016/j.nicl.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 1992;12(4):584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- 98.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos. Trans. R Soc. Lond. B Biol. Sci. 1999;354(1387):1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gantenbein AR, Sandor PS, Fritschy J, Turner R, Goadsby PJ, Kaube H. Sensory information processing may be neuroenergetically more demanding in migraine patients. NeuroReport. 2013;24(4):202–205. doi: 10.1097/WNR.0b013e32835eba81. [DOI] [PubMed] [Google Scholar]
- 100.Riske L, Thomas RK, Baker GB, Dursun SM. Lactate in the brain: An update on its relevance to brain energy, neurons, glia and panic disorder. Ther. Adv. Psychopharmacol. 2017;7(2):85. doi: 10.1177/2045125316675579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J. Neurosci. 2010;30(42):13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For inspection purposes, insight to the original data will be permitted to the members of the appropriate authorities and also for the members of the local ethics committee, EKNZ.