Abstract
We evaluated the impacts of lifestyle behaviors, namely smoking, alcohol consumption, and physical activity, on the development of new-onset AF in patients with DM. Using the Korean Nationwide database, we identified subjects diagnosed with type 2 DM and without previous history of AF between 2009 and 2012. Self-reported lifestyle behaviors were analyzed. Among 2,551,036 included subjects, AF was newly diagnosed in 73,988 patients (median follow-up 7.1 years). Both ex-smokers (hazard ratio [HR] 1.05, 95% confidence interval [CI] 1.02–1.07) and current smokers (HR 1.06, 95% CI 1.03–1.08) demonstrated a higher risk of AF than never smokers. Patients with moderate (15–29 g/day) (HR 1.12, 95% CI 1.09–1.15) and heavy (≥ 30 g/day) (HR 1.24, 95% CI 1.21–1.28) alcohol consumption exhibited an increased risk of AF, while subjects with mild alcohol consumption (< 15 g/day) (HR 1.01, 95% CI 0.99–1.03) had an AF risk similar to that of non-drinkers. Patients who engaged in moderate-to-vigorous physical activity showed a lower risk of AF (HR 0.93, 95% CI 0.91–0.94) than those who did not. This study suggests that smoking, alcohol consumption, and physical activity are associated with new-onset AF in patients with DM, and lifestyle management might reduce the risk of AF in this population.
Subject terms: Cardiology, Epidemiology
Introduction
Diabetes mellitus (DM) is a highly prevalent disease, and its global burden has increased tremendously during the past decades1. DM is one of the primary risk factors of cardiovascular diseases and is associated with an increased risk of cardiovascular mortality2,3. For this reason, various attempts have been made to enhance outcomes in patients with DM, and the importance of preventing and managing cardiovascular complications has been emphasized in clinical guidelines4. Indeed, the US Food and Drug Administration made a guide to evaluate the cardiovascular effect in novel anti-diabetic therapies, and several studies demonstrated that some anti-diabetic drugs exert cardiovascular benefit5–7. The major cardiovascular complication assessed in these studies was the risk of atherosclerotic cardiovascular disease (ASCVD), which was defined as coronary artery disease, cerebrovascular disease, and peripheral arterial disease.
Atrial fibrillation (AF) is the most common arrhythmia seen in clinical practice and imposes a substantial burden on society8–10. Patients with AF are known to have a higher risk of stroke, heart failure, and mortality11. Therefore, there is a demand for better risk stratification and tailored treatment for AF to further improve clinical outcomes in these patients. Hypertension, DM, and obesity are well-known risk factors of AF, and pre-hypertension, impaired fasting glucose, and abdominal obesity are known to be related to AF development12. A previous meta-analysis demonstrated that DM is associated with an increased risk of new-onset AF13, but few studies have investigated independent risk factors of AF in patients with DM14,15. Furthermore, there is a paucity of information regarding the association between DM and new-onset AF in current clinical guidelines4,16.
Though many drugs have shown significant efficacy in controlling hyperglycemia, lifestyle management still serves a pivotal role in diabetes care17. Smoking, alcohol consumption, and physical activity are considered traditional and important components of lifestyle management17. Previous studies have proved that these factors are associated with cardiovascular complications related to ASCVD in patients with DM18–20. However, few studies have investigated whether these factors are also related to AF.
In this study, we aimed to investigate the prognostic value of lifestyle behaviors including smoking, alcohol consumption, and physical activity in predicting new-onset AF among patients with DM. To explore this comprehensively, we analyzed nationwide epidemiologic data from the Korean National Health Insurance Service (NHIS) database.
Results
Clinical characteristics of the study population
Among 2,551,036 DM patients without a previous diagnosis of AF, 73,988 (2.9%) were newly diagnosed with AF during the median follow-up period of 7.1 years. The median follow-up of patients with AF was 3.8 years, while that of those without AF was 7.3 years. Table 1 presents the clinical characteristics of the study population and Table 2 presents the characteristics of patients according to new-onset AF during follow-up. In brief, the incidence of AF during follow-up was 4.09 per 1000 PY. Patients with AF were older, and a higher proportion of this population had a history of hypertension, hyperlipidemia, chronic kidney disease, or anti-diabetic treatment. In regards to lifestyle behaviors, patients with AF exhibited a lower prevalence of smoking and drinking. On the contrary, a higher proportion of patients without AF engaged in MVPA.
Table 1.
Baseline characteristics of overall subjects.
| Total population (n = 2,551,036) | |
|---|---|
| Demographics | |
| Age (years) | 57.7 ± 11.9 |
| < 50 | 634,286 (24.9) |
| 50–59 | 754.438 (29.6) |
| 60–69 | 683,925 (26.8) |
| ≥ 70 | 478,387 (18.8) |
| Sex | |
| Male | 1,527,141 (59.9) |
| Female | 1,023,895 (40.1) |
| Low-income level | 682,550 (26.8) |
| Medical history | |
| Hypertension | 1,450,253 (56.9) |
| Hyperlipidemia | 1,085,129 (42.5) |
| Chronic kidney disease | 281,683 (11.0) |
| Pharmacologic therapy for diabetes | |
| Insulin | 207,123 (8.1) |
| Oral antidiabetics | 1,519,139 (59.6) |
| Physical exam | |
| BMI (kg/m2) | |
| < 18.5 | 40,338 (1.6) |
| 18.5–22.9 | 640,812 (25.1) |
| 23–24.9 | 642,830 (25.2) |
| ≥ 25 | 1,227,056 (48.1) |
| Systolic blood pressure (mmHg) | 129.1 ± 15.7 |
| Diastolic blood pressure (mmHg) | 79.1 ± 10.1 |
| Laboratory findings | |
| Total cholesterol (mg/dL) | 197.0 ± 42.0 |
| HDL-cholesterol (mg/dL) | 51.4 ± 16.8 |
| LDL-cholesterol (mg/dL) | 111.6 ± 37.7 |
| Glomerular filtration rate (mL/min/1.73 m2) | 85.2 ± 35.6 |
| Fasting glucose (mg/dL) | 14.1 ± 43.6 |
| Lifestyle | |
| Smoking | |
| Non | 1,427,015 (55.9) |
| Ex | 467,444 (18.3) |
| Current | 656,577 (25.7) |
| Alcohol consumption | |
| Non | 1,462,406 (57.3) |
| Mild (< 15 g/day) | 546,618 (21.4) |
| Moderate (15–29 g/day) | 286,117 (11.2) |
| Heavy (≥ 30 g/day) | 255,895 (10.0) |
| Exercise | |
| Moderate-to-vigorous physical activitya | |
| No | 1,332,830 (52.3) |
| Yes | 1,218,206 (47.8) |
AF atrial fibrillation, BMI body mass index, DM diabetes mellitus, HDL high-density lipoprotein, LDL low-density lipoprotein.
aModerate intensity at least 30 min/day or vigorous-intensity at least 20 min/day.
Table 2.
Baseline characteristics of DM patients according to new-onset AF during the follow-up.
| No new-onset AF (n = 2,477,048) | New-onset AF (n = 73,998) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 57.5 ± 11.9 | 65.6 ± 10.3 | < 0.001 |
| < 50 | 629,214 (25.4) | 5,072 (6.9) | < 0.001 |
| 50–59 | 740,221 (29.9) | 14,217 (19.2) | |
| 60–69 | 659,278 (26.6) | 24,647 (33.3) | |
| ≥ 70 | 448,335 (18.1) | 30,052 (40.6) | |
| Sex | < 0.647 | ||
| Male | 1,482,789 (59.9) | 44,352 (59.9) | |
| Female | 994,259 (40.1) | 29,636 (40.1) | |
| Low-income level | 663,009 (26.8) | 16,621 (22.5) | < 0.001 |
| Medical history | |||
| Hypertension | 1,396,079 (56.4) | 54,174 (73.2) | < 0.001 |
| Hyperlipidemia | 1,053,295 (42.5) | 31,834 (43.0) | < 0.006 |
| Chronic kidney disease | 266,663 (10.8) | 15,020 (20.3) | < 0.001 |
| Pharmacologic therapy for diabetes | |||
| Insulin | 197,338 (8.0) | 9,785 (13.2) | < 0.001 |
| Oral antidiabetics | 1,467,936 (59.3) | 51,203 (69.2) | < 0.001 |
| Physical exam | |||
| BMI (kg/m2) | < 0.001 | ||
| < 18.5 | 38,931 (1.6) | 1,407 (1.9) | |
| 18.5–22.9 | 622,420 (25.1) | 18,392 (24.9) | |
| 23–24.9 | 625,004 (25.2) | 17,826 (24.1) | |
| ≥ 25 | 1,190,693 (48.1) | 36,363 (49.2) | |
| Systolic blood pressure (mmHg) | 129.0 ± 15.6 | 131.2 ± 16.7 | < 0.001 |
| Diastolic blood pressure (mmHg) | 79.1 ± 10.1 | 79.1 ± 10.6 | 0.705 |
| Laboratory findings | |||
| Total cholesterol (mg/dL) | 197.2 ± 42.0 | 190.5 ± 41.7 | < 0.001 |
| HDL-cholesterol (mg/dL) | 51.4 ± 16.8 | 51.1 ± 18.4 | < 0.001 |
| LDL-cholesterol (mg/dL) | 111.8 ± 37.7 | 107.9 ± 37.2 | < 0.001 |
| Glomerular filtration rate (mL/min/1.73 m2) | 85.4 ± 35.6 | 78.9 ± 36.2 | < 0.001 |
| Fasting glucose (mg/dL) | 144.2 ± 43.6 | 140.3 ± 44.5 | < 0.001 |
| Lifestyle | |||
| Smoking | < 0.001 | ||
| Non | 1,382,957 (55.8) | 44,058 (59.6) | |
| Ex | 452,530 (18.3) | 14,914 (20.2) | |
| Current | 641,561 (25.9) | 15,016 (20.3) | |
| Alcohol consumption | |||
| Non | 1,415,519 (55.8) | 46,887 (63.4) | |
| Mild (< 15 g/day) | 533,609 (21.5) | 13,009 (17.6) | |
| Moderate (15–29 g/day) | 279,172 (11.3) | 6,945 (9.4) | |
| Heavy (≥ 30 g/day) | 248,748 (10.0) | 7,147 (9.7) | |
| Exercise | |||
| Moderate-to-vigorous physical activitya | < 0.001 | ||
| No | 1,289,397 (52.1) | 43,433 (58.7) | |
| Yes | 1,187,651 (48.0) | 30,555 (41.3) | |
AF atrial fibrillation, BMI body mass index, DM diabetes mellitus, HDL high-density lipoprotein, LDL low-density lipoprotein.
aModerate intensity at least 30 min/day or vigorous-intensity at least 20 min/day.
Clinical outcomes according to smoking, alcohol consumption, and physical activity
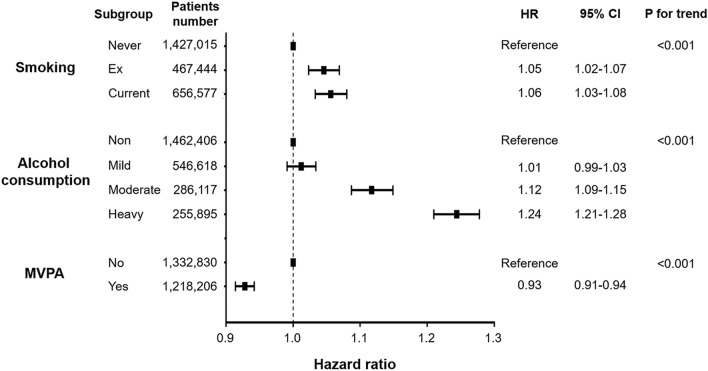
The associations between lifestyle behaviors and new-onset AF are demonstrated in Fig. 1 and Supplementary Table 1. With respect to tobacco smoking, the IRs of AF in never smokers, ex-smokers, and current smokers were 4.33, 4.53, and 3.26 per 1,000 PY, respectively. In the multivariate analyses, however, both ex-smokers (HR 1.05, 95% CI 1.02–1.07; model 3) and current smokers (HR 1.06, 95% CI 1.03–1.08) had a higher risk of AF than never smokers. The cumulative dose of smoking was stratified by the number of pack-years, and AF risk exhibited a positive correlation with cumulative smoking dose (Supplementary Fig. 1 and Supplementary Table 2).
Figure 1.
Clinical outcomes according to smoking, alcohol consumption, and physical activity. New-onset AF risk according to lifestyle behaviors in patients with DM. Data regarding lifestyle behaviors were collected during health check-ups as a self-reported questionnaire. AF atrial fibrillation, CI confidence interval, DM diabetes mellitus, HR hazard ratio, MVPA moderate-to-vigorous physical activity.
With regards to alcohol consumption, the IRs of AF in non-, mild, moderate, and heavy drinkers were 4.50, 3.38, 3.45, and 3.97 per 1000 PY, respectively. After adjusting for covariates using model 3, mild drinkers (HR 1.01, 95% CI 0.99–1.03) had an AF risk similar to that of non-drinkers. However, moderate (HR 1.12, 95% CI 1.09–1.15) and heavy drinkers (HR 1.24, 95% CI 1.21–1.28) had a substantially higher risk of AF than non-drinkers. Subgroup analyses according to average daily alcohol consumption and weekly drinking frequency are presented in Supplementary Fig. 2 and Supplementary Table 3. A J-curve association was identified between alcohol consumption and new-onset AF.
With respect to physical activity, the IRs of AF in patients who engaged in MVPA and those who did not were 4.61 and 3.53 per 1000 PY, respectively, and patients who undertook MVPA exhibited a lower risk of AF in the multivariate analyses (HR 0.93, 95% CI 0.91–0.94; model 3). The individual associations between vigorous- and moderate-intensity physical activity and AF risk were also analyzed (Supplementary Fig. 3 and Supplementary Table 4).
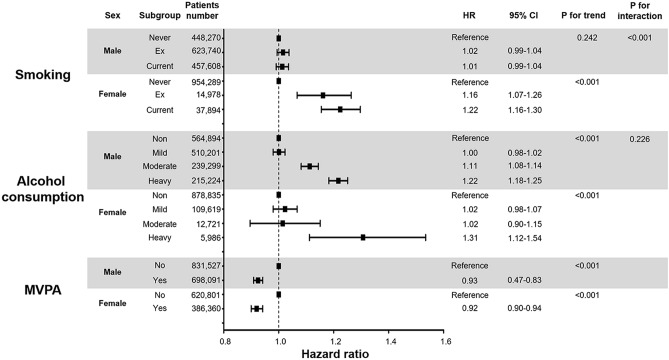
Subgroup analyses according to sex
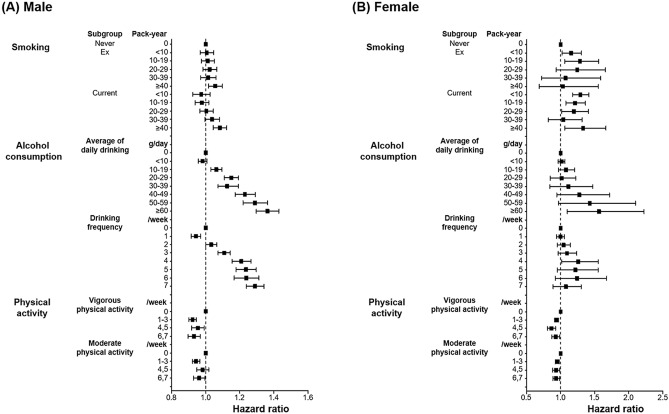
We divided the patients into males and females and investigated the associations between lifestyle behaviors and new-onset AF (Fig. 2 and Supplementary Table 5). Intriguingly, a significant association was observed between sex and smoking (p for interaction < 0.001). Female ex-smokers (HR 1.18, 95% CI 1.08–1.29) and current smokers (HR 1.23, 95% CI 1.16–1.31) demonstrated a substantially higher risk of AF than never smokers. Male ex-smokers (HR 1.03, 95% CI 1.00–1.05), but not current smokers (HR 1.02, 95% CI 1.00–1.05), had an increased risk of AF compared to never smokers. Only males who currently or previously smoked more than 40 pack-years had a higher risk of AF. The subgroup analyses of the cumulative smoking dose are presented in Fig. 3 and Supplementary Table 6.
Figure 2.
Relationship between lifestyle behaviors and new-onset AF according to sex. Subgroup analyses were performed to investigate the associations between smoking (A), alcohol consumption (B), and physical activity (C) and new-onset AF according to sex. Data regarding lifestyle behaviors were collected during health check-ups as a self-reported questionnaire. AF atrial fibrillation.
Figure 3.
Subgroup analyses of the associations between lifestyle behaviors and new-onset AF. Subgroup analyses according to male (A) and female (B) are presented. Detailed stratification were additionally performed according to lifestyle behaviors. Smoking was categorized by pack-years, alcohol consumption by average daily alcohol consumption and weekly drinking frequency, and physical activity by the number of moderate- and vigorous-intensity exercise sessions per week. Data regarding lifestyle behaviors were collected during health check-ups as a self-reported questionnaire.
Conversely, no significant interaction was identified between alcohol consumption and new-onset AF (p for interaction = 0.485). With respect to male subjects, moderate (HR 1.12, 95% CI 1.09–1.15) and heavy drinkers (HR 1.24, 95% CI 1.21–1.28) had a higher risk of AF, and mild drinkers (HR 1.01, 95% CI 0.98–1.03) had an AF risk similar to that of non-drinkers. Regarding female subjects, mild (HR 1.03, 95% CI 0.98–1.08) and moderate drinkers (HR 1.02, 95% CI 0.90–1.15) had a similar AF risk, while heavy drinkers (HR 1.28, 95% CI 1.09–1.51) had a higher AF risk. Figure 3 and Supplementary Table 7 present additional analyses based on more sophisticated stratification methods. In male subjects, a J-shaped association was observed between alcohol consumption and AF risk, but no such association was observed in female subjects.
In both sexes, MVPA was associated with a lower risk of AF (HR 0.93, 95% CI 0.91–0.95 for males; HR 0.93, 95% CI 0.90–0.95 for females). No significant interaction was identified between sex and physical activity (p for interaction = 0.212). The amount of vigorous and moderate exercise showed a linear correlation with AF risk in both sexes (p for trend < 0.01 in both sexes, Fig. 3 and Supplementary Table 8).
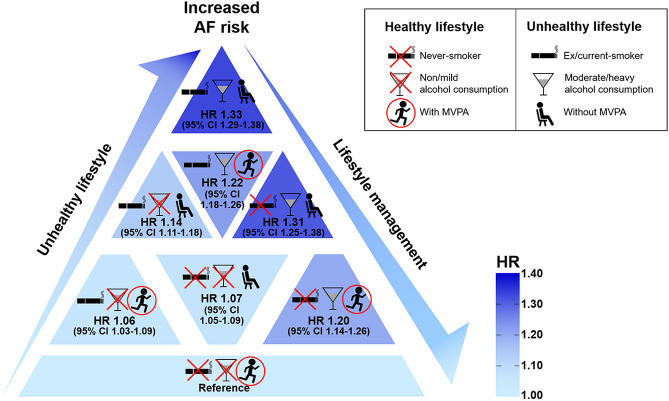
Lifestyle behaviors and AF in patients with DM
Based on tobacco smoking, alcohol consumption, and physical activity, we classified patients into eight groups and analyzed AF risk (Fig. 4 and Supplementary Table 9). Among the entire study population, 542,271 (21.3%) subjects were never smokers, non-/mild drinkers, and engaged in MVPA. When these patients were regarded as the reference group, all other groups showed a higher risk of AF.
Figure 4.
Associations between lifestyle behaviors and AF risk. The percentage of patients and HRs for adjusted AF risk stratified by lifestyle behaviors are presented. Patients who were never smokers, non-/mild drinkers, and engaged in MVPA were considered the reference group. Data regarding lifestyle behaviors were collected during health check-ups as a self-reported questionnaire. AF atrial fibrillation, HR hazard ratio, MVPA moderate-to-vigorous physical activity.
Discussion
In this study, we comprehensively investigated the associations between lifestyle behaviors and newly developed AF in patients with DM. The main findings of our study were as follows: (1) both ex-smokers and current smokers have a higher risk of AF than never smokers; (2) moderate/heavy drinkers have a higher risk of new-onset AF than non-drinkers, but mild drinkers have a similar risk; (3) patients who engage in MVPA have a lower risk of AF than those who do not; and (4) in the subgroup analyses, a significant interaction was observed between smoking and sex, but not between alcohol consumption or physical activity and sex.
We revealed that DM patients with a history of smoking had a higher risk of AF, which correlates with previous studies that identified smoking as an independent risk factor of AF in other populations21,22. Interestingly, there was a significant interaction between sex and smoking for AF incidence (Fig. 2); AF risk was higher in female subjects with a history of smoking than in male subjects with a history of smoking. Indeed, females are known to be more vulnerable to the detrimental cardiovascular effects of smoking such as stroke and myocardial infarction23,24. The biological mechanisms underlying this phenomenon remain unknown, but the anti-estrogen and early menopause-inducing effects of smoking may be involved25.
Previous studies reported a J-shaped association between alcohol consumption and clinical outcomes20,26,27, although whether abstainer bias and the sick quitter phenomenon contribute to this association remains controversial. In this study, mild drinkers showed a similar AF risk, while moderate and heavy drinkers showed a higher AF risk compared to non-drinkers. When subgroup analysis was conducted according to weekly drinking frequency, patients who drank 1 day per week (HR 0.96, 95% CI 0.94–0.99) exhibited a significantly lower risk of AF compared to non-drinkers (Supplementary Table 3), which was consistent with a previous report26. Although we did not explore the biological mechanisms underlying this observation, several possible explanations exist. Small quantities of alcohol have been shown to have an anti-inflammatory effect, improve insulin sensitivity, and reduce postprandial glucose excursions in patients with type 2 DM28,29, all of which have been reported to be associated with the development of AF.
Given that physical activity has been shown to control blood glucose, manage body weight, and reduce cardiovascular mortality, it is considered an important component of DM management17,30. In addition to improving metabolic status, physical activity also maintains arterial elasticity, which is protective against AF31. However, as vigorous-intensity exercise can induce left atrial enlargement, inflammation of the atrium, and an increase in parasympathetic tone, the association between physical activity and AF remains controversial32,33. In this study, we found that both vigorous and moderate-intensity physical activity are associated with a lower risk of new-onset AF in DM patients.
Type 2 DM is prevalent worldwide, and its social burden has substantially surged1. Owing to its pathologic contribution to ASCVD, many attempts have been made to improve clinical outcomes in patients with DM by preventing and managing ASCVD4. The incidence of AF, a common and critical arrhythmia, is thought to be increased in patients with DM13, but few studies have explored the risk factors of AF in these individuals. In this nationwide study, we demonstrated that lifestyle behaviors, including smoking, alcohol consumption, and physical activity, are significantly associated with the development of AF. Therefore, further efforts to manage these factors in DM patients should be made in the future to manage AF risk in these individuals.
There are several limitations in this study. First, this study was an observational study based on a nationwide claims database. Unmeasured confounders, including the HbA1c level, could not be adjusted. Although we have adjusted age and DM duration in Cox regression analysis, the age difference between AF and non-AF groups were significant, so unmeasured confounders related to age could also influence the results. However, given that randomized trials of smoking and alcohol consumption are unlikely due to ethical issues, carefully managed observational studies could provide clinical implications. Second, our comprehensive investigation mainly dealt with epidemiologic data, and the biological mechanisms underlying the associations between lifestyle behaviors and AF risk were not investigated. Third, because we only analyzed East Asians, the study findings cannot be directly extrapolated to other populations of different ethnicities. Finally, as we did not include type 1 DM patients in this study, the results obtained were not applicable to patients with type 1 DM.
In this large nationwide cohort study, we found that smoking, alcohol consumption, and physical activity were strongly associated with new-onset AF in patients with DM. When subgroup analyses were conducted according to sex, a significant interaction was identified between smoking and sex, but no interaction was observed between alcohol consumption or physical activity and sex. These results suggest that lifestyle management may have valuable therapeutic implications in patients with DM. In addition, in further studies of anti-diabetic therapies, the risk of new-onset AF should be evaluated in addition to that of ASCVD.
Materials and methods
Data source and study population
This study used data obtained from the NHIS database. In 2000, the NHIS was established as the single insurer for the entire Korean population. The NHIS also provides regular health check-up programs for the public, and adults are recommended to undergo a check-up at least once every 2 years. Information on the NHIS has been published elsewhere34. The NHIS database is available to researchers, and anonymized data are supplied if an official review committee has approved the study protocol. This study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. E-1908-027-1052).
There are various data subsets of the NHIS database, including the qualification, claim, health check-up, and death information databases35. Together, these databases comprise comprehensive information about demographics, disease diagnosis (according to the 10th revision of the International Classification of Diseases [ICD-10] codes), laboratory exams and imaging studies, medical treatments, and hospitalization.
We screened 2,671,490 DM patients older than 30 years between 2009 and 2012. From this initial population, we excluded 63,015 subjects whose data on lifestyle behaviors was not available. In addition, we excluded further 57,439 individuals who had a previous history of AF. Therefore, 2,551,036 subjects were ultimately included and followed up to 2017.
Definitions of diabetes mellitus, lifestyle, and other covariates
Patients with type 2 DM were defined as follows: (1) patients with claims for the ICD-10 code for type 2 DM (I11-I14) and claims for anti-diabetic drug prescriptions (sulfonylureas, metformin, meglitinides, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, α-glucosidase inhibitors, or insulin) or (2) patients who underwent a fasting glucose level test (≥ 126 mg/dL) during a health check-up and were newly diagnosed with type 2 DM (ICD-10 code I11-I14)36.
Self-reported data on smoking, alcohol consumption and physical activity were collected during health check-ups. With respect to tobacco smoking, patients were stratified into never-smokers (smoked fewer than 100 cigarettes during their life), ex-smokers, and current smokers37. Cumulative smoking was quantified in pack-years. With respect to alcohol consumption, average alcohol intake per day (g/day) and drinking frequency per week were analyzed. The patients were categorized into non-, mild (< 15 g/day), moderate (15–29 g/day), and heavy (≥ 30 g/day) drinkers38. With respect to physical activity, the number of sessions of moderate- and vigorous-intensity physical activity per week (/week) undertaken by each patient was surveyed39. Moderate-intensity exercise for at least 30 min/day and vigorous-intensity exercise for at least 20 min/day were combined as moderate-to-vigorous physical activity (MVPA).
The definitions of other covariates described in our previous reports are summarized in Supplementary Table 1040,41.
The study endpoint
The study endpoint was newly diagnosed atrial fibrillation (AF, ICD-10 code I48.0–48.4, I48.9) during the admission or more than two times in the outpatient clinic36. After the index day of health check-up, subjects were followed for incident AF or until the end of the study period (December 2017), whichever came first.
Statistical analysis
Data are presented as numbers and frequencies for categorical variables and mean ± standard deviation for continuous variables. For comparisons between groups, the chi-square test (or Fisher’s exact test when expected cell counts were < 5 for a 2 × 2 table) was used for categorical variables, and an unpaired Student’s t-test was used for continuous variables. The annual event incidence rates (IR) were calculated as the number of events per 1000 person-years (PY). Multivariate Cox proportional hazard regression models were used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for the associations between lifestyle behaviors and newly developed AF. The models used adjusted for a number of covariates: model 1 adjusted for age and sex; model 2 additionally adjusted for income level, body mass index, a previous history of hypertension and hyperlipidemia, DM duration, and a history of past anti-diabetic medication; and model 3 additionally adjusted for the other lifestyle behaviors (smoking, alcohol consumption, and MVPA). Subgroup analyses were conducted according to sex using Cox models. Two-sided P values < 0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
Supplementary Information
Author contributions
All authors contributed to the conception, design, data acquisition, analysis, or interpretation of data for the work. C.S.P., K.-D.H., and E.-K.C. drafted the manuscript and the rest of authors critically revised it. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, the Ministry of Food and Drug Safety) (Project Number: 202013B14), and by the Korea National Research Foundation funded by the Ministry of Education, Science and Technology (Grant 2020R1F1A106740).
Competing interests
Eue-Keun Choi reports research grants from Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daiichi-Sankyo, Samjinpharm, Sanofi-Aventis, Seers Technology, Skylabs, and Yuhan. The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chan Soon Park and Kyung-Do Han.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84307-5.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors, C et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors, C et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes, A 10. Cardiovascular disease and risk management: Standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S103–S123. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 5.Hennekens, C. H. et al. Academic perspectives on the United States Food and Drug Administration’s guidance for industry on diabetes mellitus. Contemp. Clin. Trials31, 411–413 (2010). [DOI] [PubMed]
- 6.Marso SP, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Schnabel RB, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int. J. Cardiol. 2017;236:226–231. doi: 10.1016/j.ijcard.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Lee SR, Choi EK, Han K, Cha MJ, Oh S. Prevalence of non-valvular atrial fibrillation based on geographical distribution and socioeconomic status in the entire Korean Population. Korean Circ. J. 2018;48:622–634. doi: 10.4070/kcj.2017.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee E, et al. Mortality and causes of death in patients with atrial fibrillation: A nationwide population-based study. PLoS ONE. 2018;13:e0209687. doi: 10.1371/journal.pone.0209687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joung B. Risk FACTOR management for atrial fibrillation. Korean Circ. J. 2019;49:794–807. doi: 10.4070/kcj.2019.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case–control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am. J. Cardiol. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J. Am. Coll. Cardiol. 2012;60:1421–1428. doi: 10.1016/j.jacc.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso A, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: The Look AHEAD randomized trial. Am. Heart. J. 2015;170:770–777.e775. doi: 10.1016/j.ahj.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies MJ, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes, A Lifestyle management: Standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S46–S60. doi: 10.2337/dc19-S005. [DOI] [PubMed] [Google Scholar]
- 18.Sliwinska-Mosson M, Milnerowicz H. The impact of smoking on the development of diabetes and its complications. Diab. Vasc. Dis. Res. 2017;14:265–276. doi: 10.1177/1479164117701876. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann. Intern. Med. 2001;134:96–105. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- 20.O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: The razor-sharp double-edged sword. J. Am. Coll. Cardiol. 2007;50:1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, et al. Association between smoking habits and the first-time appearance of atrial fibrillation in Japanese patients: Evidence from the Shinken Database. J. Cardiol. 2015;66:73–79. doi: 10.1016/j.jjcc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain AM, et al. Smoking and incidence of atrial fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011;8:1160–1166. doi: 10.1016/j.hrthm.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Sex, smoking, and risk for subarachnoid hemorrhage. Stroke. 2016;47:1975–1981. doi: 10.1161/STROKEAHA.116.012957. [DOI] [PubMed] [Google Scholar]
- 24.Bjorck L, Rosengren A, Wallentin L, Stenestrand U. Smoking in relation to ST-segment elevation acute myocardial infarction: Findings from the Register of Information and Knowledge about Swedish Heart Intensive Care Admissions. Heart. 2009;95:1006–1011. doi: 10.1136/hrt.2008.153064. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, et al. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause. 2012;19:126–132. doi: 10.1097/gme.0b013e318224f9ac. [DOI] [PubMed] [Google Scholar]
- 26.Xi B, et al. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in US Adults. J. Am. Coll. Cardiol. 2017;70:913–922. doi: 10.1016/j.jacc.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 27.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenfield JR, Samaras K, Hayward CS, Chisholm DJ, Campbell LV. Beneficial postprandial effect of a small amount of alcohol on diabetes and cardiovascular risk factors: Modification by insulin resistance. J. Clin. Endocrinol. Metab. 2005;90:661–672. doi: 10.1210/jc.2004-1511. [DOI] [PubMed] [Google Scholar]
- 29.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 30.Turner BC, Jenkins E, Kerr D, Sherwin RS, Cavan DA. The effect of evening alcohol consumption on next-morning glucose control in type 1 diabetes. Diabetes Care. 2001;24:1888–1893. doi: 10.2337/diacare.24.11.1888. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, et al. Regular exercise-induced increased number and activity of circulating endothelial progenitor cells attenuates age-related decline in arterial elasticity in healthy men. Int. J. Cardiol. 2013;165:247–254. doi: 10.1016/j.ijcard.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 32.Ofman P, et al. Regular physical activity and risk of atrial fibrillation: A systematic review and meta-analysis. Circ. Arrhythm Electrophysiol. 2013;6:252–256. doi: 10.1161/CIRCEP.113.000147. [DOI] [PubMed] [Google Scholar]
- 33.Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Atrial fibrillation is associated with different levels of physical activity levels at different ages in men. Heart. 2014;100:1037–1042. doi: 10.1136/heartjnl-2013-305304. [DOI] [PubMed] [Google Scholar]
- 34.Seong SC, et al. Data resource profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song SO, et al. Background and data configuration process of a nationwide population-based study using the Korean National Health Insurance System. Diabetes Metab. J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi EK. Cardiovascular research using the Korean National Health Information Database. Korean Circ. J. 2020;50:754–772. doi: 10.4070/kcj.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali FRM, Al-Shawaf M, Wang TW, King BAUS. Adults' attitudes toward lowering nicotine levels in cigarettes. Am. J. Prev. Med. 2019;57:403–407. doi: 10.1016/j.amepre.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Mahabir S, et al. Low to moderate alcohol consumption on serum vitamin D and other indicators of bone health in postmenopausal women in a controlled feeding study. Eur. J. Clin. Nutr. 2014;68:1267–1270. doi: 10.1038/ejcn.2014.191. [DOI] [PubMed] [Google Scholar]
- 39.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in US adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 40.Lee SR, et al. Direct oral anticoagulants in patients with atrial fibrillation and liver disease. J. Am. Coll. Cardiol. 2019;73:3295–3308. doi: 10.1016/j.jacc.2019.04.052. [DOI] [PubMed] [Google Scholar]
- 41.Park CS, et al. Association between adult height, myocardial infarction, heart failure, stroke and death: A Korean Nationwide Population-Based Study. Int. J. Epidemiol. 2018;47:289–298. doi: 10.1093/ije/dyx175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.