Abstract
The advent of antiretroviral therapy almost 25 years ago has transformed HIV-1 infection into a manageable chronic condition, albeit still incurable. The inability of the treatment regimen to eliminate latently infected cells that harbor the virus in an epigenetically silent state poses a major hurdle. Current cure approaches are focused on a “shock and kill” strategy that uses latency-reversing agents to chemically reverse the proviral quiescence in latently infected cells, followed by immune-mediated clearance of reactivated cells. To date, hundreds of compounds have been investigated for viral reactivation, yet none has resulted in a functional cure. The insufficiency of these latency-reversing agents (LRAs) alone indicates a critical need for additional, alternate approaches such as genetic manipulation. Long non-coding RNAs (lncRNAs) are an emerging class of regulatory RNAs with functional roles in many cellular processes, including epigenetic modulation. A number of lncRNAs have already been implicated to play important roles in HIV-1 latency and, as such, pharmacological modulation of lncRNAs constitutes a rational alternative approach in HIV-1 cure research. In this review, we discuss the current state of knowledge of the role of lncRNAs in HIV-1 infection and explore the scope for a lncRNA-mediated genetic approach within the shock and kill strategy of HIV-1 cure.
Keywords: HIV-1, lncRNA, latency reversing agent, shock and kill, viral reservoir
Graphical abstract
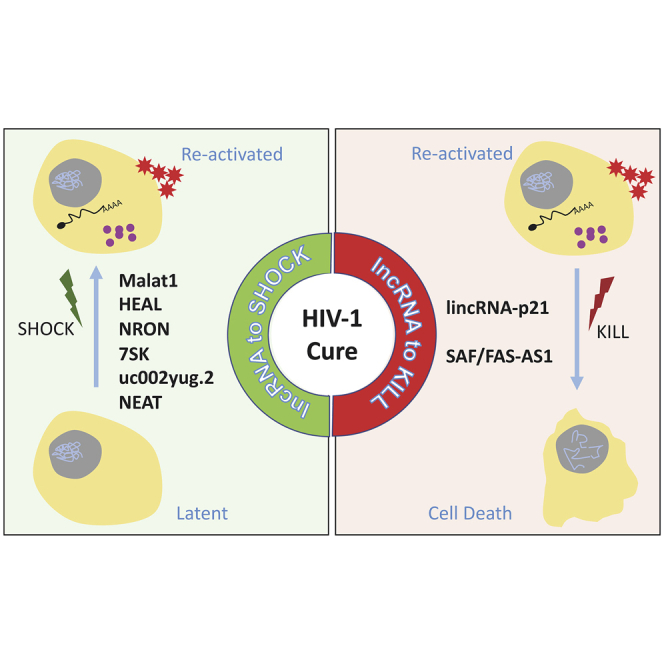
A cure for HIV-1 remains a formidable challenge in biomedical research. Recent evidence indicates critical roles for lncRNAs in HIV-1 infection. This review summarizes the current knowledge of the roles of lncRNAs in HIV-1 latency and explores the potential of lncRNAs as therapeutic targets for an HIV-1 cure.
Main text
Transcription of the mammalian genome is emerging to be far more extensive and complex than previously appreciated.1, 2, 3, 4 More than 80% of the genomic DNA is transcribed into RNA; however, only a small fraction of the transcribed RNA (~2%) is translated into proteins.5 The remaining vast majority of the transcribed RNAs that lack a clear protein-coding potential are loosely categorized into different groups of non-coding RNAs (ncRNAs). Based on length of the RNA transcript, ncRNAs are broadly classified into two groups, that is, “short” (<200 nt) or “long” (>200 nt) ncRNAs.6 Despite lacking translational ability, these ncRNAs are proving to be key regulators in a myriad of pathways with implications in both health and disease sequelae, thereby challenging the years-old dogma that regarded RNA as a mere transient intermediate between the more stable DNA, which preserves genetic codes, and the functionally versatile, catalytic proteins.7,8
For more than a decade, various short ncRNAs (e.g., microRNAs [miRNAs], PIWI-interacting RNAs [piRNAs]) have been extensively investigated for their roles in HIV-1 infection;9, 10, 11, 12, 13, 14, 15 however, our understanding of the significance of long ncRNAs (lncRNAs) during HIV-1 infection is still evolving, albeit expeditiously. Recent technological advances in genomic research have accelerated our growing appreciation of the biological impact of lncRNAs on various cellular pathways and have identified these lncRNA transcripts as an appealing, new class of target molecules for therapeutic intervention.16, 17, 18, 19 Four decades after the discovery of HIV-1 as the causative agent for AIDS, we are still searching for a prophylactic or therapeutic cure for the disease, which necessitates exploration beyond the conventional targets of treatment. In this review, we discuss the current knowledge of the role of lncRNAs in HIV-1 infection and their potential as targets of significance to HIV-1 cure research.
lncRNAs: “junks” turned “gems”
lncRNAs are a heterogeneous group of >200-nt-long RNA molecules that are transcribed by RNA polymerase II and are often capped, spliced, and polyadenylated in manners similar to mRNAs.20 Although widely expressed throughout the body, transcription of lncRNAs is tightly regulated and often specific to the cell and tissue or even to the developmental stage of the organism.21 Perhaps unsurprisingly, there is relatively low cross-species sequence conservation among these RNA transcripts. Based on the position and transcriptional direction relative to their proximal protein-coding genes, lncRNAs are broadly categorized into subgroups of intergenic, intronic, or antisense lncRNAs.22 However, this classification of lncRNAs bears little relevance to how they function. Although lacking a protein-coding potential, lncRNAs contain modular domains that, either by sequence complementarity or formation of higher-order secondary structures, can interact with DNA, RNA, and proteins.23 These nucleic acid- or protein-interacting domains can act as either signals, decoys, guides, or scaffolds to exert their regulatory functions at epigenetic, transcriptional, or post-transcriptional levels.24 The multi-modal functional capabilities of lncRNAs are reflected in the growing evidence of their regulatory roles in a variety of physiological and pathological processes such as cell differentiation, immunity, and cancer.25, 26, 27, 28, 29
Viral latency and persistence: a roadblock to HIV-1 cure
The remarkable success of combination anti-retroviral therapy (cART) in blocking viral replication and new cellular infection has transformed HIV-1 from a fatal disease into a manageable chronic condition. However, the inability of cART to eliminate pre-existing virus-infected cells precludes a complete cure. A small but stable population of latently infected cells that are established very early in infection is the major impediment for eradication of HIV-1.30, 31, 32, 33 Cells of both lymphoid and myeloid lineage can serve as sanctuaries for HIV-1 persistence during cART and, consequently, a source of viral resurgence upon treatment interruption or failure.34, 35, 36, 37 Resting memory CD4+ T cells and tissue macrophages are both long-lived and self-sustaining through homeostatic proliferation, which enables them to endure through years of effective therapy. Silent or minimally active viral transcription and protein production can further promote survival and persistence of infected cells through evasion of viral cytopathy and the host immune surveillance.38, 39, 40
Activated CD4+ T cells, the main target of HIV-1 infection, support robust viral replication and production, but they succumb to viral cytopathic death within days of infection.41 However, a small subset of these cells, following a normal path to establish immunological memory, differentiate into a resting memory state and consequently establish one of the best characterized niches for HIV-1 latency.42 The establishment and maintenance of HIV-1 latency, which is conventionally regarded as a post-integration, transcriptionally silent state, represent a multifaceted process involving epigenetic, transcriptional, and post-transcriptional regulations.43,44 The site of integration of the viral DNA into the host genome is a contributing factor in the determination of whether the provirus is transcribed or retained in a silent state.45 However, in latently infected cells from cART-suppressed individuals, proviruses have also been mapped to actively transcribing genomic regions,46 suggesting that maintenance of HIV-1 latency involves further active interference of viral transcription irrespective of the site of proviral integration. The first step of blockade is at the level of viral transcription initiation. Transition of infected, activated CD4+ T cells to a quiescent memory state leads to sequestration of host transcription factors (nuclear factor κB [NF-κB], nuclear factor of activated T cells [NFAT]) away from the HIV-1 core promoter in the nucleus and out into the cell cytoplasm, preventing formation of the trans-activating response (TAR) element.47 Additionally, viral transcription is prematurely stalled proximal to the transcription start site through inactivation of the positive transcription elongation factor b (P-TEFb)-containing super elongation complex, leading to inhibition of transcription progression and generation of functional HIV-1 transcripts.48 This transcriptional silencing is further augmented by a negative feedback mechanism mediated by the resulting reduction in viral trans-activator protein, Tat, which is a strong cofactor for HIV-1 transcription elongation.49 In the absence of the viral proteins, Tat and Rev, export of unspliced viral RNAs to cytoplasm is also halted.50,51 In addition to transcriptional suppression, maintenance of HIV-1 latency also involves epigenetic silencing, including chromatin remodeling into suppressive structures, DNA deacetylation/methylation, and recruitment of polycomb repressive complex 2 (PRC2), which further limits access of necessary transcription factors to the provirus.52, 53, 54
Apart from CD4+ T lymphocytes, cells of myeloid lineage such as microglia and other tissue macrophages are also known to support HIV-1 replication and harbor the virus, independent of T cells, for long periods even under suppressive cART.55, 56, 57, 58, 59, 60 While latency by transcriptional silencing in CD4+ T cells is well characterized, the molecular mechanisms of HIV-1 persistence in myeloid cells is considerably less defined.61,62 However, emerging evidence suggests that, similar to CD4+ T cells, HIV-1-infected tissue macrophages from cART-suppressed individuals may require reactivation for infectious virus production.63 Nonetheless, both memory CD4+ T cells and macrophages that remain either latently or persistently infected through cART need to be eradicated for a cure of HIV-1.
Current state of “shock and kill”: an approach to purge HIV-1
Current strategies for eradication of latently infected cells are predominantly centered around the shock and kill approach that entails induced reactivation and resumption of viral transcription, translation, and virion production and elimination of reactivated cells by either virus-induced cytopathic death or immune-mediated clearance. Reactivation of the latent provirus is achieved through different latency-reversing agents (LRAs) that target various host factors to promote viral transcription.64,65 These LRAs are broadly categorized into five classes (Table 1) depending on the host factors upon which they act: (1) epigenetic modifiers: inhibitors of histone deacetylase (HDAC), histone methyl transferase (HMT), and bromodomain, activator of P-TEFb; (2) NF-κB/protein kinase C agonists: prostratin derivatives, ingenols, second mitochondria-derived activator of caspase (SMAC) mimetics; (3) Toll-like receptor (TLR) agonists: stimulators of TLR7, TLR9; (4) extracellular stimulators: cytokines, immune checkpoint blockers; and (5) unclassified miscellaneous: disulfiram, ixazomib.
Table 1.
Classes of latency-reversing agents (LRAs)
| Class | Mechanisms of action | Prominent compounds64,65 |
|---|---|---|
| Epigenetic modifiers | HDAC inhibitor | vorinostat (SAHA), valproic acid (VPA), panobinostat, romidepsin |
| HMT inhibitor | AZ391, BIX-01294, UNC-0638 | |
| bromodomain inhibitor | JQ1, OTX-15, PFI-1 | |
| P-TEFb activator | chalcone, Amt-87 | |
| NF-κB stimulators | PKC agonist | bryostatin-1, ingenols, prostratin |
| SMAC mimetic | CAPE, MGD-486, pyrimethamine | |
| TLR agonists | TLRs 1–9 | imiquimod, Pam2CSK4, CL413, R-848, GS-986, 3M-002, MGN1703 |
| Extracellular stimulators | immune checkpoint blocker | nivolumab (anti-PD-1), ipilimumab (anti-CTLA4) |
| cytokine | TNF-α, ALT-803 (IL-15 agonist) | |
| Miscellaneous | proteasome inhibitor | ixazomib |
| PTEN dysregulator | disulfiram | |
| PI3K agonist | oxoglaucine | |
| SRC agonist | MCB-613 |
IL-15, interleukin-15; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; PTEN, phosphatase and tensin homolog; TNF-α, tumor necrosis factor α.
While reactivation of the latent virus with various LRAs has been extensively studied, the “kill” aspect has received relatively less attention. Killing of the reactivated cells is mostly relegated to both viral cytopathy and host anti-viral immunity, which rely heavily on LRA-mediated reactivation, resulting in levels of viral replication robust enough to induce apoptosis or an immune response effective enough to detect and lyse the newly reactivated cells. The inherent challenges of this approach are evident in many clinical studies, where the LRAs were able to reactivate some level of viral transcription but failed to reduce proviral DNA levels or eliminate the reactivated cells.66, 67, 68, 69, 70 More recently, a few studies are starting to explore additional approaches to enhance killing by either inducing apoptosis or potentiating the host immune response toward the reactivated cells.71, 72, 73, 74 The inadequacy of the current LRAs alone in reactivating all replication-competent, latently infected cells, coupled with a dearth of kill strategies, warrants identification of new molecular targets and development of novel approaches to achieve complete viral clearance.
lncRNA: a new player in the HIV-1 arena
Successful reactivation of HIV-1 latency and elimination of the reservoir cells require a comprehensive understanding of the intricate interactions between the various viral and host factors that lead to establishment and maintenance of this pseudo-steady-state of transcriptional silence. A number of recent studies have added a new layer of complexity by revealing important roles of lncRNAs (Table 2) in both regulation of HIV-1 latency as well as longevity of persistently infected cells.
Table 2.
Summary of lncRNAs with roles in HIV-1 infection
| Potential role in HIV-1 therapy | lncRNA | Class of lncRNA | Genomic location | Biological function | Role in HIV-1 infection | Reference |
|---|---|---|---|---|---|---|
| “Shock” | Malat1 | intergenic | 11q13.1 | epigenetic regulation, alternate splicing | promotes viral reactivation by binding to EZH2 and preventing PCR2 translocation to the HIV-1 LTR region | 75,76 |
| HEAL/ linc02574-201 | intergenic | 1p35.3 | epigenetic regulation | promotes viral reactivation by recruitment of histone acetyltransferase p300 to the HIV-1 promoter region | 77 | |
| NRON | antisense | 9q33.3 | transcriptional regulation | promotes viral latency by sequestration of NFAT within the cytoplasm | 78,79 | |
| 7SK | intergenic | 6p12.2 | transcriptional regulation | promotes viral latency by inactivation of P-TEFb | 80, 81, 82, 83 | |
| uc002yug.2 | intergenic | 21q22.12 | transcriptional regulation | promotes viral reactivation by inhibition of the transcription repressor RUNX1 | 84,85 | |
| NEAT1 | intergenic | 11q13.1 | post-transcriptional regulation | promotes viral latency by inhibition of nuclear export of viral mRNAs | 86 | |
| “Kill” | lincRNA-p21 | intergenic | 6p21.2 | transcriptional regulation | promotes viral persistence by inhibition of DNA DSB-induced cell death | 87 |
| SAF | antisense | 10q23.31 | post-transcriptional regulation | promotes viral persistence by inhibition of pro-apoptotic caspases | 88 |
lncRNAs regulate HIV-1 replication and latency: added punch to “shock”
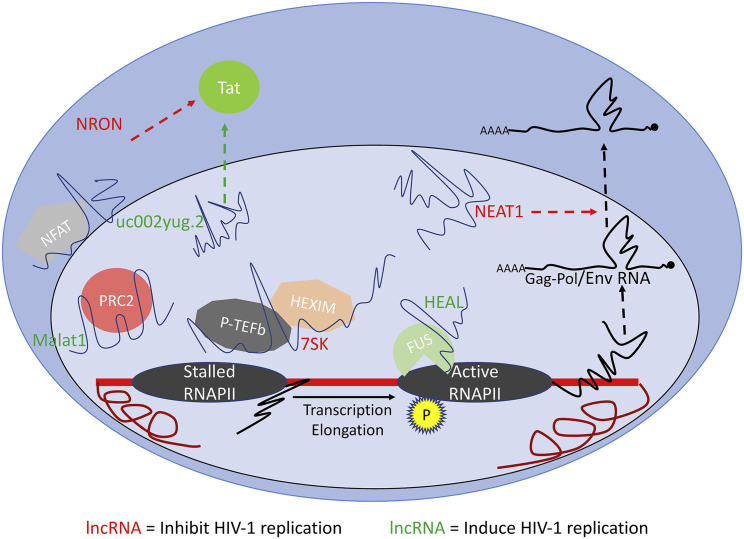
Changes in expression levels of a number of lncRNAs are observed during HIV-1 infection, and several of these lncRNAs have been shown to be integral components in the regulation of various stages of the viral replication cycle (Figure 1).
Figure 1.
Roles of lncRNAs in HIV-1 replication and latency
An overview of lncRNAs with known functions in different stages of HIV-1 replication. lncRNAs in red inhibit viral replication/promote latency and lncRNAs in green induce viral replication/promote reactivation.
Malat1 (metastasis-associated lung adenocarcinoma transcript 1) is an ~8-kb highly conserved, intergenic, nuclear-enriched lncRNA that was initially identified as a biomarker for cancer prognosis, but subsequently its expression in non-cancer cells was also observed.89 Over the years, a number of different functional roles of this lncRNA have been described, including modulation of alternative splicing of cellular precursor (pre-)mRNAs and epigenetic regulation of HIV-1 gene expression.90 Malat1 interacts with the two subunits of the polycomb repressive complex 2 (PRC2), that is, the enhancer of zeste 2 (EZH2) and the suppressor of zeste 12 (SUZ12). Binding of EZH2 by Malat1 prevents PRC2 translocation to the HIV-1 promoter region and its subsequent H3K27 trimethylation, which leads to release of viral transcriptional repression and thereby reactivation from latency.75 Notably, expression of Malat1 is significantly reduced in cART-treated individuals, and treatment of latently infected cells with LRAs induces Malat1 expression.75,76 Moreover, experimental overexpression of Malat1 increases HIV-1 replication in CD4+ T cells, indicating a potential application in reactivation of latent HIV-1 infections.
HEAL or linc02574-201 is a 441-bp intergenic lncRNA with a recently identified function in epigenetic regulation of HIV-1 transcription.77 Upon HIV-1 infection, its expression is upregulated in both CD4+ T cells and macrophages in vitro as well as in peripheral blood mononuclear cells (PBMCs) from HIV-1-infected individuals. The lncRNA HEAL, along with the RNA-binding protein FUS, recruits the histone acetyltransferase p300 to the HIV-1 promoter, which enhances H3K27ac modification of the viral DNA and thereby facilitates its progressive transcription.
Another lncRNA with a demonstrated role in regulation of HIV-1 replication is NRON (non-coding repressor of NFAT). This lncRNA inhibits HIV-1 transcription initiation by spatiotemporal isolation of the transcription factor NFAT in the cytoplasm.78 NRON serves as a scaffold and, along with the calmodulin-binding protein IQGAP1, the nuclear importer KPNB, and the inhibitory kinase LRRK2, sequesters the phosphorylated, inactive form of NFAT in the cytoplasm. Expression of the lncRNA NRON follows a dynamic pattern during active HIV-1 replication in CD4+ T cells. Early in the viral life cycle, Nef inhibits NRON, thereby releasing NFAT for nuclear translocation and activation of viral transcription. However, at the late stage of infection, the viral protein Vpu induces expression of NRON in order to shift the balance from viral transcription to viral assembly and budding. Furthermore, NRON can also regulate HIV-1 latency in a NFAT-independent manner by promoting degradation of the viral trans-activator of transcription, Tat.79 The lncRNA co-recruits Tat with CUL4B and PSMD11, two components of the ubiquitin/proteasome system, and thereby facilitates proteasomal degradation of the viral protein. Expression of NRON is elevated in resting CD4+ T cells, and small interfering RNA (siRNA)-mediated knockdown of NRON in latently infected cells reactivates HIV-1 replication.
Human 7SK, an ~330-nt small nuclear RNA (snRNA), is a prominent player in regulation of HIV-1 transcription.80,81 It is an abundant snRNA that acts as a structural scaffold for assembly of the 7SK small nuclear ribonucleoprotein (snRNP) complex. One of the proteins that 7SK RNA binds to is the elongation factor P-TEFb, a heterodimer of the catalytic subunit CDK9 and the regulatory subunit cyclin T.82 The catalytic function of CDK9 is essential for phosphorylation of the stalled RNA polymerase II and subsequent progression of HIV-1 transcription elongation. The 7SK snRNA co-recruits the inhibitory HEXIM proteins that render P-TEFb catalytically inactive by inhibiting the kinase activity of CDK9. Upon stimulation with the viral protein Tat, P-TEFb is displaced from the 7SK snRNP complex, and that leads to activation and transfer of P-TEFb to the HIV-1 promoter region for successful elongation of the viral transcript. The facts that cyclin T/CDK9 function is downregulated during HIV-1 latency in resting CD4+ T cells,83 and that 7SK snRNA plays a significant role in their sequestration and repression of catalytic functions, bolster the potential of this lncRNA as a means of modulating the establishment and maintenance of HIV-1 latency.
The lncRNA uc002yug.2 is an ~2.5-kb lncRNA that has been shown to reactivate HIV-1 latency and promote viral replication through inhibition of the transcription repressor RUNX1.84 It is known that RUNX represses HIV-1 transcription by binding to the HIV-1 long terminal repeat (LTR), and its inhibition leads to viral reactivation in latently infected CD4+ T cells.91 The lncRNA uc002yug.2 induces alternative splicing of RUNX1, resulting in production of a shorter isoform, RUNX1a, which is unable to bind to the HIV-1 promoter region and thereby relieves the transcriptional repression.85 It further promotes viral replication by increasing expression of Tat protein.84 Expression of uc002yug.2 is also significantly downregulated in individuals receiving cART compared to untreated HIV-1-infected individuals, and overexpression of this lncRNA reactivates viral replication in latently infected resting memory CD4+ T cells.84 These observations suggest that this lncRNA may represent a target for therapeutic intervention.
The lncRNA NEAT1 (nuclear enriched abundant transcript 1) regulates viral replication at a post-transcriptional stage.86 It is a nuclear lncRNA that interacts with the NONO protein and is essential for formation and maintenance of the paraspeckle bodies within the nucleus.92 NEAT1 inhibits Rev-dependent nuclear-to-cytoplasm export of HIV-1 gag/pol and env mRNAs through retention of these instability element (INS)-containing transcripts within the paraspeckle subnuclear structure. Downregulation of NEAT1 leads to an enhancement of HIV-1 replication.86 Given the suboptimal reactivation with LRAs, a boost in the post-transcriptional level could further enhance viral replication following latency reversal.
lncRNAs regulate survival of HIV-1-infected cells: a bait for “kill”
Survival of latently or persistently infected CD4+ T cells and macrophages for a prolonged period even following effective cART is a major hurdle that we need to overcome to achieve complete clearance of HIV-1. Limited viral replication in latently or persistently infected cells undoubtedly favors evasion from virus-induced cytolysis. Not surprisingly, programmed cell death is another pathway that is also actively regulated during HIV-1 infection and persistence.93 As such, the different cellular factors in the apoptotic pathways have long been considered as targets for killing of reactivated cells.72,94,95 Recent discoveries of the role of different lncRNAs in cell death or survival pathways have highlighted the potential utility of manipulating such lncRNAs to specifically induce cell death in the persistently HIV-1-infected or newly reactivated cells.
An essential step in HIV-1 infection is integration of proviral DNA into the host genome, which results in double-strand breaks (DSBs) in the host genome. Mammalian cells are highly sensitive to such DNA damage that is either repaired quickly or it sets off a sequence of events leading to activation of the master-regulator p53 and ultimately apoptosis of the cells. However, HIV-1 has evolved to induce DSBs yet mitigate the downstream cascade of pro-apoptotic pathways through modulation of a pro-apoptotic intergenic lncRNA named lincRNA-p21, specifically in macrophages.87 In healthy cells, HDM2-mediated ubiquitination of the transcription factor p53 and cytoplasmic sequestration of its co-factor hnRNP-K by ERK2 prevents p53 activation and transcription of pro-apoptotic genes. However, in case of an unrepaired DSB, hnRNP-K translocates to the nucleus and along with activated p53 initiates transcription of lincRNA-p21. Subsequently, lincRNA-p21, in complex with hnRNP-K, represses transcription of pro-survival genes and thereby promotes apoptotic cell death. In HIV-1-infected macrophages, the pro-apoptotic lncRNA lincRNA-p21 is suppressed via several mechanisms. Early in infection, HIV-1 induces mitogen-activated protein kinase kinase 1 (MAP2K1)/extracellular signal-regulated kinase 2 (ERK2)-mediated phosphorylation of hnRNP-K, leading to its retention in the cytoplasm, which prevents its subsequent association with lincRNA-p21 in the nucleus. Furthermore, HIV-1 infection of macrophages promotes degradation of lincRNA-p21 by favoring nuclear accumulation of the RNA-binding protein HuR, which in turns drives recruitment of let-7/Ago2 to lncRNA and renders it unstable. Inhibition of MAP2K1/ERK2 function results in restoration of lincRNA-p21/hn-RNP-K interaction, leading to induction of apoptosis in HIV-1-infected macrophages.
SAF or FAS-AS1 is an ~1.5-kb antisense, intronic lncRNA that plays an important role in survival of persistently HIV-1-infected macrophages. Ectopic expression of SAF in HeLa cells promotes alternate splicing of the FAS gene, leading to an increase in a splice variant lacking the transmembrane domain and resulting in production of the soluble form of the protein. This results in protection of the SAF-expressing cells from Fas ligand-driven induction of apoptosis. HIV-1 induces expression of SAF in virus-infected monocyte-derived macrophages in vitro, and the level of this lncRNA is also elevated in HIV-1-positive lung alveolar macrophages isolated from chronically infected individuals.88 Furthermore, siRNA-mediated inhibition of the lncRNA SAF leads to induction of apoptosis exclusively in HIV-1-infected macrophages, leaving the uninfected bystander cells unaffected.88 The fine degree of specificity achieved through inhibition of this lncRNA is a strong argument for its therapeutic potential.
Approaches for therapeutic targeting of lncRNAs
The mode of action of lncRNAs in regulation of HIV-1 latency is diverse and can be either activating or inhibitory in nature. Therefore, strategies for modulating these target RNA molecules need to be tailored for the specific lncRNA. A number of approaches are currently being evaluated to manipulate lncRNA functions in various diseases such as cancer.96
Inhibition of latency-inducing lncRNAs can be achieved by post-transcriptional degradation of the lncRNA with siRNA, short hairpin RNA (shRNA), or anti-sense oligonucleotide (ASO). Regulation of an RNA target with either siRNA, shRNA, or ASO is the most clinically advanced route. Furthermore, new technologies, including chemical modifications of siRNA and ASO (such as 2′-methoxy, 2′-fluoro, or locked nucleic acid), have significantly improved their stability and efficacy, while reducing the off-target effects.97 This can also be achieved by inhibition of lncRNA transcription by promoter blockade or gene editing. A number of studies have used CRISPR-Cas-based gene editing to silence specific lncRNAs in cells lines, indicating the feasibility and potential of this approach.98,99 However, many ethical and technical challenges remain to be addressed before these gene-editing approaches can be effectively translated into clinical therapies. Another approach is impairment of lncRNA functions through steric hindrance. An important mode for exerting function of lncRNAs is through formation of secondary structures to bind with nucleic acids or proteins. Inhibitors that can prevent lncRNA secondary structure formation or block interactions with their cognate DNA/RNA or protein molecules are promising candidates for modulating lncRNA functions. An array of small molecules, nanobodies, aptamers, and RNA decoys are being evaluated for this purpose.100
Most of the current therapeutic approaches are focused on inhibition of the pathogenic lncRNA. However, expression of lncRNAs that support latency reversal would require further enhancement. Upregulation of such favorable lncRNAs can be achieved by delivery of the lncRNA by synthetic RNA. Recent advancements in delivery vehicles for synthetic RNAs through liposomes or nanoparticles have proved to be very promising. This can also be achieved by delivery of the lncRNA by vectors. Viral vectors such as modified adeno-associated virus (AAV) and herpes virus have long been used to deliver genetic material into cells, and utilization of such vectors for delivery of lncRNAs is a feasible approach.
HIV-1 latently infected cells predominantly reside within tissue spaces such as brain and lungs. Therefore, the ability of a method to reach the target site in the body needs to be considered when evaluating its suitability. Innovative modification to ASOs and siRNAs, including cell-penetrating peptides (CPPs), glycosylation (Gal-NP), and nano-ligand carriers (NLCs), are being explored to improve their penetrance of the blood-brain barrier, tissue specificity, and cellular uptake, while reducing toxicity.101, 102, 103, 104 Currently, almost 50 lncRNA-related clinical trials (https://www.clinicaltrials.gov) are ongoing worldwide for various diseases such as diabetes, cardiovascular disease, and cancer. Although mostly in their early stages, challenges and outcomes from these trials will be invaluable in informing and improving the future efforts of targeting lncRNAs.
Future perspective: therapeutic potential and challenges of lncRNAs in HIV-1 cure
Expression of various lncRNAs is dysregulated in HIV-1-infected cells compared to normal cells, and ex vivo manipulation of these lncRNAs has proven to be effective in controlling the fate of the viral infection. These characteristics illustrate the strong appeal of lncRNAs as targets for HIV-1 therapy. However, before such potentials can be realized, candidate lncRNAs identified through in vitro and ex vivo studies need to be carefully validated for their effectiveness as targets through in vivo models of HIV-1/simian immunodeficiency virus (SIV) infection and therapeutic interventions. One of the biggest challenges of lncRNA research is the lack of sequence conservation across species. This prevents a straightforward exchange and confirmation of experimental results between human and primate models of HIV-1 infection and poses an additional hurdle of cross-species validation. However, genetically engineered humanized mouse models can be utilized to circumvent and somewhat alleviate this challenge. Additionally, no specific markers of HIV-1 latently infected cells have yet been identified, which makes it challenging to deliver the lncRNA-targeted therapies specifically to latently infected cells. However, the off-target effects could be minimized by rigorous identification of candidate lncRNAs whose aberrant expressions are rather specific to the pathogenic cells. In fact, the feasibility of such cell-specific targeting was demonstrated by inhibition of the lncRNA SAF, which is upregulated in HIV-1-infected macrophages. Downregulation of the lncRNA SAF with siRNA led to induction of apoptosis in virus-infected cells only, while leaving the bystander cells unaffected.88 Given the complexity of HIV-1 latency, an optimal reactivation and clearance of latently infected cells will probably require a multipronged strategy. As such, any candidate lncRNA target identified for HIV-1 therapy will need to be evaluated for its synergy with other shock and kill approaches. Although currently in a nascent stage, nonetheless recent advances and success of nucleic acid-based therapeutics in diseases such as cancer have afforded an excellent foundation for exploring lncRNAs as druggable targets for HIV-1. This comes at an opportune time in the HIV research field to expand and further explore lncRNAs to fully exploit the potential of targeting lncRNAs for a cure for HIV-1.
Acknowledgments
This work was supported by a Cornell Center for Immunology Seed Grant to S.B. and by NIH, United States grants R01-AI155319 and R33-AI136097 to D.G.R.
Author contributions
Conceptualization, S.B.; writing – original draft, S.B.; writing – review and editing, S.B. and D.G.R.; funding acquisition, S.B and D.G.R. Both authors have read and agreed to the final manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark M.B., Amaral P.P., Schlesinger F.J., Dinger M.E., Taft R.J., Rinn J.L., Ponting C.P., Stadler P.F., Morris K.V., Morillon A. The reality of pervasive transcription. PLoS Biol. 2011;9:e1000625. doi: 10.1371/journal.pbio.1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carninci P., Kasukawa T., Katayama S., Gough J., Frith M.C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C., FANTOM Consortium. RIKEN Genome Exploration Research Group and Genome Science Group (Genome Network Project Core Group) The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 4.Mercer T.R., Gerhardt D.J., Dinger M.E., Crawford J., Trapnell C., Jeddeloh J.A., Mattick J.S., Rinn J.L. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat. Biotechnol. 2011;30:99–104. doi: 10.1038/nbt.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hangauer M.J., Vaughn I.W., McManus M.T. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013;9:e1003569. doi: 10.1371/journal.pgen.1003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapranov P., Cheng J., Dike S., Nix D.A., Duttagupta R., Willingham A.T., Stadler P.F., Hertel J., Hackermüller J., Hofacker I.L. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 7.Morris K.V., Mattick J.S. The rise of regulatory RNA. Nat. Rev. Genet. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercer T.R., Dinger M.E., Mattick J.S. Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 9.Sun B., Yang R., Mallardo M. Roles of microRNAs in HIV-1 replication and latency. MicroRNA. 2016;5:120–123. doi: 10.2174/2211536605666160829123118. [DOI] [PubMed] [Google Scholar]
- 10.Swaminathan G., Navas-Martín S., Martín-García J. MicroRNAs and HIV-1 infection: antiviral activities and beyond. J. Mol. Biol. 2014;426:1178–1197. doi: 10.1016/j.jmb.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Swaminathan S., Murray D.D., Kelleher A.D. The role of microRNAs in HIV-1 pathogenesis and therapy. AIDS. 2012;26:1325–1334. doi: 10.1097/QAD.0b013e328352adca. [DOI] [PubMed] [Google Scholar]
- 12.Swaminathan S., Murray D.D., Kelleher A.D. miRNAs and HIV: unforeseen determinants of host-pathogen interaction. Immunol. Rev. 2013;254:265–280. doi: 10.1111/imr.12077. [DOI] [PubMed] [Google Scholar]
- 13.Sisk J.M., Witwer K.W., Tarwater P.M., Clements J.E. SIV replication is directly downregulated by four antiviral miRNAs. Retrovirology. 2013;10:95. doi: 10.1186/1742-4690-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klase Z., Houzet L., Jeang K.T. MicroRNAs and HIV-1: complex interactions. J. Biol. Chem. 2012;287:40884–40890. doi: 10.1074/jbc.R112.415448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang K., Rice A.P. Mini ways to stop a virus: microRNAs and HIV-1 replication. Future Virol. 2011;6:209–221. doi: 10.2217/fvl.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z., Gerstein M., Snyder M. RNA-seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark M.B., Mercer T.R., Bussotti G., Leonardi T., Haynes K.R., Crawford J., Brunck M.E., Cao K.A., Thomas G.P., Chen W.Y. Quantitative gene profiling of long noncoding RNAs with targeted RNA sequencing. Nat. Methods. 2015;12:339–342. doi: 10.1038/nmeth.3321. [DOI] [PubMed] [Google Scholar]
- 18.Matsui M., Corey D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017;16:167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts T.C., Wood M.J. Therapeutic targeting of non-coding RNAs. Essays Biochem. 2013;54:127–145. doi: 10.1042/bse0540127. [DOI] [PubMed] [Google Scholar]
- 20.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washietl S., Kellis M., Garber M. Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals. Genome Res. 2014;24:616–628. doi: 10.1101/gr.165035.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattick J.S., Rinn J.L. Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol. 2015;22:5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]
- 23.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn R.A., Chang H.Y. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14:752–761. doi: 10.1016/j.stem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald K.A., Caffrey D.R. Long noncoding RNAs in innate and adaptive immunity. Curr. Opin. Immunol. 2014;26:140–146. doi: 10.1016/j.coi.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y.G., Satpathy A.T., Chang H.Y. Gene regulation in the immune system by long noncoding RNAs. Nat. Immunol. 2017;18:962–972. doi: 10.1038/ni.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heward J.A., Lindsay M.A. Long non-coding RNAs in the regulation of the immune response. Trends Immunol. 2014;35:408–419. doi: 10.1016/j.it.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai M.C., Spitale R.C., Chang H.Y. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun T.W., Engel D., Berrey M.M., Shea T., Corey L., Fauci A.S. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colby D.J., Trautmann L., Pinyakorn S., Leyre L., Pagliuzza A., Kroon E., Rolland M., Takata H., Buranapraditkun S., Intasan J., RV411 study group Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat. Med. 2018;24:923–926. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitney J.B., Hill A.L., Sanisetty S., Penaloza-MacMaster P., Liu J., Shetty M., Parenteau L., Cabral C., Shields J., Blackmore S. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haase A.T. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 34.Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T., Smith K., Lisziewicz J., Lori F., Flexner C. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 35.Siliciano J.D., Kajdas J., Finzi D., Quinn T.C., Chadwick K., Margolick J.B., Kovacs C., Gange S.J., Siliciano R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 36.Wong M.E., Jaworowski A., Hearps A.C. The HIV reservoir in monocytes and macrophages. Front. Immunol. 2019;10:1435. doi: 10.3389/fimmu.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson M. HIV persistence in macrophages. Nat. Med. 2017;23:538–539. doi: 10.1038/nm.4337. [DOI] [PubMed] [Google Scholar]
- 38.Chomont N., El-Far M., Ancuta P., Trautmann L., Procopio F.A., Yassine-Diab B., Boucher G., Boulassel M.R., Ghattas G., Brenchley J.M. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sieweke M.H., Allen J.E. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho D.D., Neumann A.U., Perelson A.S., Chen W., Leonard J.M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 42.Shan L., Deng K., Gao H., Xing S., Capoferri A.A., Durand C.M., Rabi S.A., Laird G.M., Kim M., Hosmane N.N. Transcriptional reprogramming during effector-to-memory transition renders CD4+ T cells permissive for latent HIV-1 infection. Immunity. 2017;47:766–775.e3. doi: 10.1016/j.immuni.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruelas D.S., Greene W.C. An integrated overview of HIV-1 latency. Cell. 2013;155:519–529. doi: 10.1016/j.cell.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mbonye U., Karn J. The molecular basis for human immunodeficiency virus latency. Annu. Rev. Virol. 2017;4:261–285. doi: 10.1146/annurev-virology-101416-041646. [DOI] [PubMed] [Google Scholar]
- 45.Jordan A., Defechereux P., Verdin E. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 2001;20:1726–1738. doi: 10.1093/emboj/20.7.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han Y., Lassen K., Monie D., Sedaghat A.R., Shimoji S., Liu X., Pierson T.C., Margolick J.B., Siliciano R.F., Siliciano J.D. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J. Virol. 2004;78:6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coiras M., López-Huertas M.R., Rullas J., Mittelbrunn M., Alcamí J. Basal shuttle of NF-κB/IκBα in resting T lymphocytes regulates HIV-1 LTR dependent expression. Retrovirology. 2007;4:56. doi: 10.1186/1742-4690-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Q., Yik J.H. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol. Mol. Biol. Rev. 2006;70:646–659. doi: 10.1128/MMBR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karn J. The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Curr. Opin. HIV AIDS. 2011;6:4–11. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pomerantz R.J., Trono D., Feinberg M.B., Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 51.Malim M.H., Cullen B.R. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell. 1991;65:241–248. doi: 10.1016/0092-8674(91)90158-u. [DOI] [PubMed] [Google Scholar]
- 52.Rafati H., Parra M., Hakre S., Moshkin Y., Verdin E., Mahmoudi T. Repressive LTR nucleosome positioning by the BAF complex is required for HIV latency. PLoS Biol. 2011;9:e1001206. doi: 10.1371/journal.pbio.1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ylisastigui L., Archin N.M., Lehrman G., Bosch R.J., Margolis D.M. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 54.Kauder S.E., Bosque A., Lindqvist A., Planelles V., Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5:e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jambo K.C., Banda D.H., Kankwatira A.M., Sukumar N., Allain T.J., Heyderman R.S., Russell D.G., Mwandumba H.C. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014;7:1116–1126. doi: 10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan C.A., Watkins B.A., Kufta C., Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J. Virol. 1991;65:736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joseph S.B., Arrildt K.T., Sturdevant C.B., Swanstrom R. HIV-1 target cells in the CNS. J. Neurovirol. 2015;21:276–289. doi: 10.1007/s13365-014-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DiNapoli S.R., Ortiz A.M., Wu F., Matsuda K., Twigg H.L., 3rd, Hirsch V.M., Knox K., Brenchley J.M. Tissue-resident macrophages can contain replication-competent virus in antiretroviral-naive, SIV-infected Asian macaques. JCI Insight. 2017;2:e91214. doi: 10.1172/jci.insight.91214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Honeycutt J.B., Wahl A., Baker C., Spagnuolo R.A., Foster J., Zakharova O., Wietgrefe S., Caro-Vegas C., Madden V., Sharpe G. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Invest. 2016;126:1353–1366. doi: 10.1172/JCI84456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Micci L., Alvarez X., Iriele R.I., Ortiz A.M., Ryan E.S., McGary C.S., Deleage C., McAtee B.B., He T., Apetrei C. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog. 2014;10:e1004467. doi: 10.1371/journal.ppat.1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graziano F., Aimola G., Forlani G., Turrini F., Accolla R.S., Vicenzi E., Poli G. Reversible human immunodeficiency virus type-1 latency in primary human monocyte-derived macrophages induced by sustained M1 polarization. Sci. Rep. 2018;8:14249. doi: 10.1038/s41598-018-32451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gama L., Abreu C., Shirk E.N., Queen S.E., Beck S.E., Metcalf Pate K.A., Bullock B.T., Zink M.C., Mankowski J.L., Clements J.E. SIV latency in macrophages in the CNS. Curr. Top. Microbiol. Immunol. 2018;417:111–130. doi: 10.1007/82_2018_89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ganor Y., Real F., Sennepin A., Dutertre C.A., Prevedel L., Xu L., Tudor D., Charmeteau B., Couedel-Courteille A., Marion S. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat. Microbiol. 2019;4:633–644. doi: 10.1038/s41564-018-0335-z. [DOI] [PubMed] [Google Scholar]
- 64.Ait-Ammar A., Kula A., Darcis G., Verdikt R., De Wit S., Gautier V., Mallon P.W.G., Marcello A., Rohr O., Van Lint C. Current status of latency reversing agents facing the heterogeneity of HIV-1 cellular and tissue reservoirs. Front. Microbiol. 2020;10:3060. doi: 10.3389/fmicb.2019.03060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abner E., Jordan A. HIV “shock and kill” therapy: in need of revision. Antiviral Res. 2019;166:19–34. doi: 10.1016/j.antiviral.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Rasmussen T.A., Lewin S.R. Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents? Curr. Opin. HIV AIDS. 2016;11:394–401. doi: 10.1097/COH.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 67.Archin N.M., Liberty A.L., Kashuba A.D., Choudhary S.K., Kuruc J.D., Crooks A.M., Parker D.C., Anderson E.M., Kearney M.F., Strain M.C. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elliott J.H., Wightman F., Solomon A., Ghneim K., Ahlers J., Cameron M.J., Smith M.Z., Spelman T., McMahon J., Velayudham P. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elliott J.H., McMahon J.H., Chang C.C., Lee S.A., Hartogensis W., Bumpus N., Savic R., Roney J., Hoh R., Solomon A. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV. 2015;2:e520–e529. doi: 10.1016/S2352-3018(15)00226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rasmussen T.A., Tolstrup M., Brinkmann C.R., Olesen R., Erikstrup C., Solomon A., Winckelmann A., Palmer S., Dinarello C., Buzon M. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 71.Cummins N.W., Sainski A.M., Dai H., Natesampillai S., Pang Y.P., Bren G.D., de Araujo Correia M.C.M., Sampath R., Rizza S.A., O’Brien D. Prime, shock, and kill: priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J. Virol. 2016;90:4032–4048. doi: 10.1128/JVI.03179-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li P., Kaiser P., Lampiris H.W., Kim P., Yukl S.A., Havlir D.V., Greene W.C., Wong J.K. Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat. Med. 2016;22:807–811. doi: 10.1038/nm.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desimio M.G., Giuliani E., Ferraro A.S., Adorno G., Doria M. In vitro exposure to prostratin but not bryostatin-1 improves natural killer cell functions including killing of CD4+ T cells harboring reactivated human immunodeficiency virus. Front. Immunol. 2018;9:1514. doi: 10.3389/fimmu.2018.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borducchi E.N., Liu J., Nkolola J.P., Cadena A.M., Yu W.H., Fischinger S., Broge T., Abbink P., Mercado N.B., Chandrashekar A. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. 2018;563:360–364. doi: 10.1038/s41586-018-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu D., Sun W.W., Li L., Ma L., Sun L., Jin X., Li T., Hou W., Wang J.H. Long noncoding RNA MALAT1 releases epigenetic silencing of HIV-1 replication by displacing the polycomb repressive complex 2 from binding to the LTR promoter. Nucleic Acids Res. 2019;47:3013–3027. doi: 10.1093/nar/gkz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin C., Peng X., Xie T., Lu X., Liu F., Wu H., Yang Z., Wang J., Cheng L., Wu N. Detection of the long noncoding RNAs nuclear-enriched autosomal transcript 1 (NEAT1) and metastasis associated lung adenocarcinoma transcript 1 in the peripheral blood of HIV-1-infected patients. HIV Med. 2016;17:68–72. doi: 10.1111/hiv.12276. [DOI] [PubMed] [Google Scholar]
- 77.Chao T.C., Zhang Q., Li Z., Tiwari S.K., Qin Y., Yau E., Sanchez A., Singh G., Chang K., Kaul M. The long noncoding RNA HEAL regulates HIV-1 replication through epigenetic regulation of the HIV-1 promoter. MBio. 2019;10:e02016-19. doi: 10.1128/mBio.02016-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Imam H., Bano A.S., Patel P., Holla P., Jameel S. The lncRNA NRON modulates HIV-1 replication in a NFAT-dependent manner and is differentially regulated by early and late viral proteins. Sci. Rep. 2015;5:8639. doi: 10.1038/srep08639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J., Chen C., Ma X., Geng G., Liu B., Zhang Y., Zhang S., Zhong F., Liu C., Yin Y. Long noncoding RNA NRON contributes to HIV-1 latency by specifically inducing tat protein degradation. Nat. Commun. 2016;7:11730. doi: 10.1038/ncomms11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Contreras X., Barboric M., Lenasi T., Peterlin B.M. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog. 2007;3:1459–1469. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eilebrecht S., Benecke B.J., Benecke A.G. Latent HIV-1 TAR regulates 7SK-responsive P-TEFb target genes and targets cellular immune responses in the absence of Tat. Genomics Proteomics Bioinformatics. 2017;15:313–323. doi: 10.1016/j.gpb.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen V.T., Kiss T., Michels A.A., Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature. 2001;414:322–325. doi: 10.1038/35104581. [DOI] [PubMed] [Google Scholar]
- 83.Budhiraja S., Famiglietti M., Bosque A., Planelles V., Rice A.P. Cyclin T1 and CDK9 T-loop phosphorylation are downregulated during establishment of HIV-1 latency in primary resting memory CD4+ T cells. J. Virol. 2013;87:1211–1220. doi: 10.1128/JVI.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huan C., Li Z., Ning S., Wang H., Yu X.F., Zhang W. Long noncoding RNA uc002yug.2 activates HIV-1 latency through regulation of mRNA levels of various RUNX1 isoforms and increased Tat expression. J. Virol. 2018;92:e01844-17. doi: 10.1128/JVI.01844-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu H., Zheng J., Deng J., Zhang L., Li N., Li W., Li F., Lu J., Zhou Y. lincRNA-uc002yug.2 involves in alternative splicing of RUNX1 and serves as a predictor for esophageal cancer and prognosis. Oncogene. 2015;34:4723–4734. doi: 10.1038/onc.2014.400. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Q., Chen C.Y., Yedavalli V.S., Jeang K.T. NEAT1 long noncoding RNA and paraspeckle bodies modulate HIV-1 posttranscriptional expression. MBio. 2013;4:e00596-12. doi: 10.1128/mBio.00596-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barichievy S., Naidoo J., Boullé M., Scholefield J., Parihar S.P., Coussens A.K., Brombacher F., Sigal A., Mhlanga M.M. Viral apoptosis evasion via the MAPK pathway by use of a host long noncoding RNA. Front. Cell. Infect. Microbiol. 2018;8:263. doi: 10.3389/fcimb.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boliar S., Gludish D.W., Jambo K.C., Kamng’ona R., Mvaya L., Mwandumba H.C., Russell D.G. Inhibition of the lncRNA SAF drives activation of apoptotic effector caspases in HIV-1-infected human macrophages. Proc. Natl. Acad. Sci. USA. 2019;116:7431–7438. doi: 10.1073/pnas.1818662116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao M., Wang S., Li Q., Ji Q., Guo P., Liu X. MALAT1: a long non-coding RNA highly associated with human cancers. Oncol. Lett. 2018;16:19–26. doi: 10.3892/ol.2018.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun Q., Hao Q., Prasanth K.V. Nuclear long noncoding RNAs: key regulators of gene expression. Trends Genet. 2018;34:142–157. doi: 10.1016/j.tig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klase Z., Yedavalli V.S., Houzet L., Perkins M., Maldarelli F., Brenchley J., Strebel K., Liu P., Jeang K.T. Activation of HIV-1 from latent infection via synergy of RUNX1 inhibitor Ro5-3335 and SAHA. PLoS Pathog. 2014;10:e1003997. doi: 10.1371/journal.ppat.1003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamazaki T., Souquere S., Chujo T., Kobelke S., Chong Y.S., Fox A.H., Bond C.S., Nakagawa S., Pierron G., Hirose T. Functional domains of NEAT1 architectural lncRNA induce paraspeckle assembly through phase separation. Mol. Cell. 2018;70:1038–1053.e7. doi: 10.1016/j.molcel.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 93.Timilsina U., Gaur R. Modulation of apoptosis and viral latency—an axis to be well understood for successful cure of human immunodeficiency virus. J. Gen. Virol. 2016;97:813–824. doi: 10.1099/jgv.0.000402. [DOI] [PubMed] [Google Scholar]
- 94.Badley A.D., Sainski A., Wightman F., Lewin S.R. Altering cell death pathways as an approach to cure HIV infection. Cell Death Dis. 2013;4:e718. doi: 10.1038/cddis.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chandrasekar A.P., Cummins N.W., Badley A.D. The role of the BCL-2 family of proteins in HIV-1 pathogenesis and persistence. Clin. Microbiol. Rev. 2019;33:e00107-19. doi: 10.1128/CMR.00107-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arun G., Diermeier S.D., Spector D.L. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol. Med. 2018;24:257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Slaby O., Laga R., Sedlacek O. Therapeutic targeting of non-coding RNAs in cancer. Biochem. J. 2017;474:4219–4251. doi: 10.1042/BCJ20170079. [DOI] [PubMed] [Google Scholar]
- 98.Liu S.J., Horlbeck M.A., Cho S.W., Birk H.S., Malatesta M., He D., Attenello F.J., Villalta J.E., Cho M.Y., Chen Y. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 2017;355:aah7111. doi: 10.1126/science.aah7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu S., Li W., Liu J., Chen C.H., Liao Q., Xu P., Xu H., Xiao T., Cao Z., Peng J. Genome-scale deletion screening of human long non-coding RNAs using a paired-guide RNA CRISPR-Cas9 library. Nat. Biotechnol. 2016;34:1279–1286. doi: 10.1038/nbt.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gutschner T., Richtig G., Haemmerle M., Pichler M. From biomarkers to therapeutic targets-the promises and perils of long non-coding RNAs in cancer. Cancer Metastasis Rev. 2018;37:83–105. doi: 10.1007/s10555-017-9718-5. [DOI] [PubMed] [Google Scholar]
- 101.Kuwahara H., Yokota T. Delivery of siRNA into the blood-brain barrier: recent advances and future perspective. Ther. Deliv. 2012;3:417–420. doi: 10.4155/tde.12.22. [DOI] [PubMed] [Google Scholar]
- 102.Zhou Y., Zhu F., Liu Y., Zheng M., Wang Y., Zhang D., Anraku Y., Zou Y., Li J., Wu H. Blood-brain barrier-penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 2020;6:eabc7031. doi: 10.1126/sciadv.abc7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zou L.L., Ma J.L., Wang T., Yang T.B., Liu C.B. Cell-penetrating peptide-mediated therapeutic molecule delivery into the central nervous system. Curr. Neuropharmacol. 2013;11:197–208. doi: 10.2174/1570159X11311020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu L.P., Ahmadvand D., Su J., Hall A., Tan X., Farhangrazi Z.S., Moghimi S.M. Crossing the blood-brain-barrier with nanoligand drug carriers self-assembled from a phage display peptide. Nat. Commun. 2019;10:4635. doi: 10.1038/s41467-019-12554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]