Abstract
Modern agricultural efforts are now in search of an efficient, eco-friendly and sustainable approach for enhanced crop production. Nearly 50–60% of seeds lost occurs due to improper technical handling. Seed deterioration manifests itself as reduction in the rate of germination and growth with increased susceptibility to biotic and abiotic stresses. Furthermore, seed ageing is another economic and scientific issue that is associated with an array of internal (structural, physiological and genetic) and external (storage temperature and relative humidity) factors. Reactive oxygen species (ROS) are believed to be a key player in ageing phenomenon. However, hydrated storage, or ROS blockers are a few of the conventionally used methods to minimize the ageing process. Recently, exogenous applications of different inorganic nanoparticles (metal and metal oxide) are suggested to revitalize and revive aged seeds. Owing to their special properties of nano-size with high surface area they easily penetrate the seed coat. Exposure of nanoparticles has been suggested to neutralize the excess of ROS to a level that initiates hormonal signaling to support early emergence of radicles from the seeds. Nanotechnology has been well explored to enhance the crops nutritional quality, livestock productivity, plant protection from various stressors and in enhancement of seed quality via nanopesticides and nanofertilizers. Aiming at sustainable agriculture practices with fewer inputs, maximum benefits, ecologically safe and compatible technique the nanotechnology is an efficient approach to counteract problems of seed ageing incurring during storage, which is relatively less explored and unresolved conventionally, in general.
Keyword: Ageing, Deteriorative reactions, Nanoparticles, Oxidative stress, ROS
Introduction
Seeds represent reproductive stage in the life cycle of plants. It is used as major planting material for the production of next season crop; therefore, high yield and, production of viable and vigorous seeds are necessary. Followed by harvesting, crop seeds are stored under ambient conditions for few weeks to years, depending upon requirement. Germination is the very first step to determine seeds viability and vigor, consequently growth in the soil for successful crop establishment (Panda and Mondal 2020). Seed vigor is an important determinant for rapid and homogeneous radicle emergence. Longevity determines the vigor index (VI) that depends upon seeds physiology, genetic makeup and pace of deterioration that prevails during storage (Zinsmeister et al. 2020). Additionally, numerous other factors regulating vigor of seeds, directly or indirectly, includes environmental temperature, relative humidity (RH), moisture and oil content, pathogen attack, mechanical damage, storage time, and gaseous exchange (Solberg et al. 2020).
Generation and accretion of reactive oxygen species (ROS) has widely been known as the key issues leading to seed deterioration during ambient storage (Chandra et al. 2018; Kurek et al. 2019). Inequity in growth hormones and enzymes, impaired metabolism, disturbed cellular membranes and cytoplasmic glassy state during storage is caused by over-produced ROS. These physiological aspects are chiefly associated with seed water content and storage environment. In metabolically active seeds, alteration in mitochondrial respiration leads to accumulation of ROS. This ROS has popularly been known to damage cellular macromolecules. However, efficient elimination of ROS is dependent on expression and effective operation of various non-enzymatic and enzymatic antioxidants (Chandra et al. 2018).
Recently, engineered nanoparticles (NPs) have been accomplished great attention worldwide, in various sectors including agriculture due to their beneficial impacts on plant growth, development and stress resistance. Applications of several metal based NPs are suggested to revitalize and revive aged seeds. Nanoparticles have been shown to promote seed quality by altering deteriorative physiological processes through ROS scavenging (Acharya et al. 2019; Kumar et al. 2020a, b). Application of NP in the agricultural field is a safe and feasible approach as it can promote plants efficiency to take up more nutrients and water, and to endure harsh climatic conditions, which results in better yield (Younis et al. 2019). Nanoparticles can be synthesized following various procedures; chemical, electrochemical, photochemical, γ-radiation, laser ablation and green chemistry route (De Souza et al. 2019) However, the biological route of NP synthesis is most preferred one now-a-days, as it is eco-friendly, non-toxic, biocompatible and economical too (Khan et al. 2020). A number of positive and negative impacts of NPs were observed on plants, which depend on their shape, size, concentration, surface coating, dose, duration of exposure, and plant species (Sharma et al. 2019). This review discussed the insights of ageing phenomenon and, roles of metal and metal oxide NPs as revitalizing agents for aged seeds.
Seed ageing
Ageing is a natural time dependent phenomenon and seed acquire deterioration under long-term storage. Mbofung et al. (2012) studied the effects of storage conditions (temperature and RH) on vigor and viability of Glycine max in view to suggest suitable environment that would reduce the pace of deterioration. Their findings revealed that the seeds can be stored for many seasons if the storage temperature and RH were maintained at 10 °C, and below 40% respectively. After determining storage conditions for Cajanus cajan, Jaganathan and Liu (2014) hypothesized that maintenance of low level of seed moisture content (MC) is more important for extended storage life than the low temperature. Seed deterioration could be defined as an inevitable practice which cannot be inverted. Only, the rate of damage can be slowed down by regulating storage environment (Solberg et al. 2020). Studies have shown that the high storage temperature and RH fasten the rate of deterioration multifold. Moreover, MC plays an important role in seed deterioration. Seeds with dynamic MC are damaged faster than the static ones. Thus, the magnitudes of variation in temperature and RH along with period of storage are crucial regulatory factors to determine seed deterioration (Wawrzyniak et al. 2020).
Researchers have popularly employed accelerated ageing (AA) tests to evaluate physiological potential of seeds of various species. This test provided consistent information regarding mechanism(s) involved in seed deterioration. It is based on accelerated deterioration of seeds following their brief exposure to comparatively higher temperature and RH (Somasundaram and Bhaskaran 2017). These conditions deteriorate weak seeds more rapidly than the vigorous ones, revealing a differential impact on viability. Similarly, the controlled deterioration (CD) of seed is another practice of vigor testing for quality control purposes. In CD test, MC of seed is adjusted to its initial level, before exposing to higher temperatures and RH (Damir and Mavi 2008). Unlike the AA test, seed MC in a CD test is kept constant (18–24%).
Indicators of seed ageing
Physiological indicators
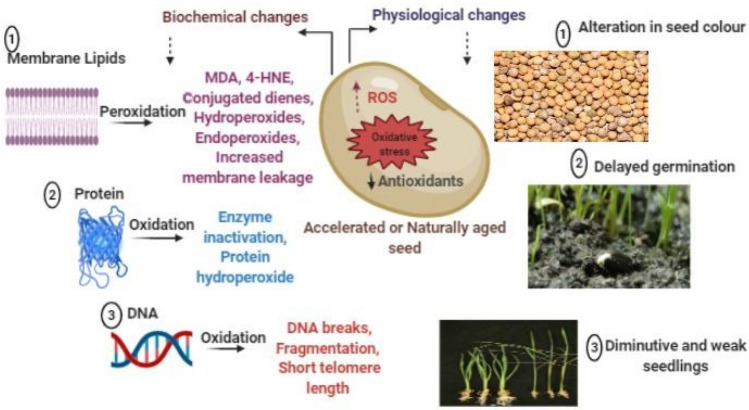
Correlation between ageing and physiological aspects of seeds such as germination percentage (%G) and germination index (GI), shoot–root length, MC, and VI were determined and found that these are the primarily affected parameters by ageing phenomena. It includes metabolic inequity, failure of cytoplasmic state and disturbed membrane structure. Changes in seed color and density, alteration in structure of cotyledons and increased hardness of grains are some of the indicators of ageing (Yin et al. 2015) (Fig. 1). Seed moisture level and storage environments are the major determinants of these physiological defects. Germination performance of a seed is characterized mainly by three factors; onset of germination, speed of germination, and extent or capacity of germination. Germination parameters are useful in estimating the switch over from seed to seedling. Reduction in the rate of germination is the first hallmark of ageing phenomenon (Fu et al. 2015).
Fig. 1.
Ageing-induced physiological and biochemical alterations in seed (4-HNE- 4-hydroxy-2-nonenal, MDA- Malondialdehyde, ROS- Reactive oxygen species)
Electrolyte leakage (EL), an indicator of membrane damage, has been widely opted for evaluation of vigor, and intensity of ageing. It is an indirect but dependable determinant of membrane integrity. However, seed integrity, genotype, size, MC, soaking period and incubation temperature affects EL (Prado et al. 2019). Aged seeds with disorganized membrane exude more ions, sugars and other metabolites. From such seeds, more amount of EL is permitted due to absence/ineffective repair mechanisms (Khan et al. 2020). Cell membranes are particularly rich in lipids, majorly PUFAs (polyunsaturated fatty acids), which are prime targets of ROS thus susceptible to peroxidation reactions (Fig. 1). The ROS reacts with membrane lipids and cause peroxidation, which can induce irreparable damage in membrane permeability and plasticity, ultimately leading to loss of cellular integrity. Lipid peroxidation could be a predominant damaging process in ageing. Changes in membrane lipids as well as storage lipids, especially an increase in the peroxidation products have been reported with natural and AA (Oenel et al. 2017).
Biochemical indicators
Reactive oxygen species
Free radicals/ROS are chemical species with one or more unpaired electrons. During normal cellular metabolism, ROS are produced as by-products and play significant signaling role in germination and dormancy alleviation. Many types of ROS are known, among those superoxide (O2•−), hydrogen peroxide (H2O2) and hydroxyl (·OH) radicals are potentially cytotoxic (Kurek et al. 2019; Wawrzyniak et al. 2020). Over accumulation of ROS leads to oxidative stress situation, which is detrimental to viability during long term storage of seeds (Fig. 1). Thus, the efficient antioxidant system is a pre-requisite mechanism to encounter seed deterioration reactions. Over accumulation of ROS in AA seed of Acrocomia aculeata has been reported to be an outcome of lowered antioxidant potential (Barreto and Garcia 2017).
Lipid peroxidation
Biochemical alterations associated with seed ageing include impairment in cellular macromolecules. Among these, membrane lipids, particularly, PUFAs are sensitive to ROS, and their peroxidation is a major reason for loss of seed quality, vigor and viability under ambient storage (Chandra and Keshavkant 2018). Hydroperoxides and endoperoxides are formed due to the PUFA oxidation, along with several reactive intermediates. In addition to ROS, lipoxygenase is also involved with lipid catabolism by hydroperoxidation of the cis–cis-1,4-pentadiene structures, and releases lipid hydroperoxide (LOOH). The LOOH thus formed is further degraded into several other aldehydic reactive products viz.; 4-hydroxy-2-nonenal (4-HNE), malondialdehyde (MDA) and conjugated dienes, which can decrease seed viability (Anjum et al. 2015). Levels of MDA, lipid peroxides and oxidized triacylglycerols have been shown to increase in aged seeds. Among various lipid peroxidized products, the 4-HNE is highly toxic, as it further attacks over proteins, nucleic acids, and disturb mitochondrial respiration leading to loss of viability (Fig. 1).
Protein oxidation
Reactive oxygen species also alter protein synthesis and enzyme activity, consequently cause inactivation, hydrolysis and post-translational modifications of proteins. The ROS oxidizes proteins by directly attacking specific amino acids to cause carbonylation, glutathionylation and nitrosylation, while indirectly by conjugating with lipid peroxidized products like MDA (Parkhey et al. 2014). Protein peroxidized products are highly reactive and unstable. They easily attack over nucleophilic groups of proteins resulting in irreversible structural, functional and chemical modifications (Fig. 1). Accumulation of protein hydroperoxide, a well identified derivative of protein oxidation, has been determined during AA of Cicer arietinum (Keshavkant et al. 2013).
Along with direct attack of ROS, lipid peroxidized product 4-HNE also forms adducts with proteins by two pathways; either directly reacting with lysine, cysteine or histidine residues following Michael reaction or involving lysine side-chain residues leading to formation of pyrrole adducts. Alterations in amino acids of proteins by lipid peroxidation product MDA can result in formation of MDA-lysine and carboxymethyl-lysine adducts, through non-enzymatic reactions. Non-enzymatic events such as Maillard reaction lead to a series of complex reactions, consequently protein aggregation (Chandra et al. 2020b). This reaction contributes to seed ageing by chemical alterations of functional proteins and, metabolic capability to limit ROS and repair mechanisms during germination. Ageing-induced deterioration increases protein oxidation and, causing hindrance in functions of proteins and enzymes (Wawrzyniak et al. 2020). Dehydrogenases enzymes are directly related to loss of seed viability. The decrease in the activity of mitochondrial dehydrogenase has also been seen during seed ageing (Mahakham et al. 2017).
DNA damage
Researchers have shown that seed ageing is intimately clubbed with chromosomal aberration, alteration in telomere length, DNA methylation/damage, and abnormal gene expression (Chandra et al. 2018). Chromosomal aberration in aged seeds comprises fragmentation, fusion, bridge and ring formation, and alteration in nuclear size (Fig. 1). Ageing associated DNA alterations have been illustrated through DNA profiling of differentially aged Glycine max and Carthamus tinctorius (Vijay et al. 2009). Alterations in chromosomes can affect the gene expression of enzymes involved with seed germination and seedling emergence. A negative correlation has been observed between telomere length and ageing phenomena in seeds of Triticum aestivum (Bucholc and Buchowicz 1992). The ROS abstracts hydrogen atom from sugar moieties of DNA, consequently leads to strand breakage and fragmentation of DNA which are highly correlated with seed ageing (Demidchik 2015). Mira et al. (2020) studied the instability in nucleic acid content of deteriorated seed with the help of random amplified polymorphic DNA, and methylation-sensitive amplification polymorphism molecular markers.
Plant defense mechanisms
Seeds have various antioxidative defense strategies to fight against oxidative stress by scavenging ROS before they attack cellular components (Barreto and Garcia 2017). These antioxidants include various enzymes like superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (POD), glutathione reductase (GR), ascorbate peroxidase (APX) and glutathione-S-transferase (GST), and non-enzymatic members such as ascorbate, glutathione, NADPH, α-tocopherol and phenolic compounds (Xia et al. 2015). In addition, few other enzymes viz.; dehydroascorbate reductase, monodehydroascorbate reductase and glutathione reductase are also required, in sufficient concentrations, for regeneration of different antioxidants (Vellosillo et al. 2010). Superoxide dismutase is first line of defense. It catalyzes conversion of two molecules of O2•− into molecular oxygen and H2O2. Further, CAT, and POD are implicated in removal of H2O2. Another enzyme, GR also controls endogenous H2O2 accumulation by an oxidation–reduction of glutathione and ascorbate. Further, APX reduces H2O2 into water, using ascorbate as an electron donor (Mittler 2017).
Nanoparticle in revival of ageing
Bulk materials when collapsed to nano size can exhibit dynamic behavior from their microscale forms because of surface area and volume effects. Nanomaterials possess more surface area to volume ratio compared to their complex forms, consequently have high surface energy. Increased surface energy of a substance is found to be associated with enhanced velocity of chemical reaction (Aslani et al. 2014). Nanoparticles are referred as colloidal particulate system that ranges from 10 to 100 nm in size in at least two of their dimensions. Nanomaterials are categorized into diverse classes: (a) carbon based nanomaterials such as carbon nanomaterials, carbon nanotubes, graphene, fullerene, etc., (b) inorganic metal [aluminum (Al), cobalt (Co), copper (Cu), gold (Au), iron (Fe), silver (Ag), titanium (Ti), zinc (Zn), etc.], and (c) metal oxide (Al2O3-aluminium oxide, CeO2-cerric oxide, CuO-copper oxide, NiO-nickle oxide, SiO2-silicon dioxide, TiO2-titanium dioxide, ZnO-zinc oxide, Fe3O4-iron oxide, etc.) NPs. Recently, a variety of NPs has been used either as a pretreatment solution or dry dressing for early emergence of radicles, improvement in seed quality, reduction in pathogen infection during storage, improvement in seedling growth, and mitigation of environmental stress factors (Verma et al. 2018, 2019). These are reported to enhance the biological and catalytic activities by inducing chemical reactivity, which is required to improve seed quality (Mahakham et al. 2017; Chandrasekaran et al. 2020). During pretreatment of seeds, either suspensions or emulsions and organic nanopowders are used for improving germination and growth responses. Effects of different NPs applied on seeds for ameliorations of ageing associated damages are discussed below:
Silver nanoparticles
Silver nanoparticles (AgNPs) are the most popular and highly commercialized among all the NPs. In a study, decline in %G, shoot–root length and VI of Capsicum annum due to AA were improved drastically by treatment of AgNPs (1000 mgkg−1) (Kumar et al. 2020b) (Table 1A). Several researchers compared the efficiencies of chemically and biosynthesized NPs, and concluded that the green manufactured nanomaterials are relatively more suitable due to their biocompatibility and stability offered by phytochemicals (Katiyar et al. 2020; Khan et al. 2020). Mahakham et al. (2017) applied different concentrations; 10 and 20 ppm of phytosynthesized AgNPs on aged seeds of Oryza sativa, which significantly improved the germination by 1.12 and 1.15 folds respectively compared to those recorded with non-treated ones (Table 1A). Likewise, Psophocarpus tetragonolobus seed recorded an enhancement in germination by 1.5 folds, and VI by 3.1 folds after treatment with phytochemically fabricated AgNPs (50 mgL−1) (Kumar et al. 2020a). Study of green and chemically synthesized AgNPs for amelioration of ageing associated damages in Cicer arietinum exhibited 1.6–1.75 folds increase in %G with 2% concentration (Khan et al. 2020).
Table 1.
Implication of different nanoparticles for reversal of ageing associated damages in plants
| S. No. | Species | Ageing/storage treatments | Nanoparticles | Fold change in %G after NP treatment | Key implications | References | ||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | Shape | Concentration | ||||||
| (A) Silver nanoparticles | ||||||||
| 1 | Cicer arietinum |
Artificial ageing (40 °C for 24 h) |
15–60 | – |
Green NP 2% Chemical NP 2% |
1.75 1.66 |
Improved seed health with upliftment in physiological parameters and MSI along with up-regulation in enzymatic antioxidants, and proline | Khan et al. 2020 |
| 2 | Psophocarpus tetragonolobus |
Natural storage (3 Months) |
15.4–20.98 | Oval |
10 mg 50 mg 100 mg 250 mg |
1.31 1.53 1.33 1.32 |
Enhancement in germinability, antioxidant enzymes and biochemical activity, with genetic stability | Kumar et al. 2020a |
| 3 | Allium cepa |
Natural storage (10 Years) |
19–37 | Spherical and ellipsoidal | 31.3 ppm |
1.5 (6th day) 1.08 (21st day) |
Improved seedling emergence, growth parameters and higher chlorophyll content | Acharya et al. 2019 |
| 4 | Glycine max |
Natural storage (10 Months) |
– | – |
500 ppm 1000 ppm 1500 ppm |
1.13 1.16 1.06 |
Maintenance of seed quality with high vigor and viability, lower EC | Dangi et al. 2019 |
| 5 | Capsicum annum | Natural ageing | 85 | Needle |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1.01 1.09 1.03 |
Enhanced %G, root and shoot length, VI, along with minimal pathogen infection | Kumar et al. 2020b |
| 6 | Allium cepa |
Natural storage (10 Months) |
50–100 | Spherical |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 1500 mgkg−1 |
1.08 1.15 1.11 1.1 |
Promoted germination and vigor of seeds | Anandraj et al. 2018 |
| 7 | Allium cepa |
Natural storage (9 Months) |
50–100 | Spherical |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 1500 mgkg−1 |
1.08 1.15 1.11 1.1 |
Augmented germination and VI | Anandaraj and Natarajan 2017 |
| 8 | Oryza sativa |
Natural ageing (3 years) |
6–36 | Spherical and ellipsoidal |
10 mgL−1 20 mgL−1 |
1.12 1.15 |
Improved germination and vigor performance with up-regulation in gene expressions of α-amylase, aquaporins, and antioxidants | Mahakham et al. 2017 |
| 9 | Arachis hypogaea |
Natural storage (12 Months) |
40 | Spherical |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1.04 1.30 1.38 |
Maintenance of seed qualities during storage | Shyla and Natarajan 2016 |
| 10 | Zea mays | Natural storage | < 50 | – | 1250 mgkg−1 | 1.15 | Increased germination percentage with high protein and carbohydrate contents | Maithreyee and Gowda 2015 |
| 11 | Arachis hypogaea |
Natural storage (13 Months) |
100 | Cylindrical |
500 mgkg−1 750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1.01 1.12 1.25 1.32 |
Improvements in seed %G, VI, root and shoot length | Shyla and Natarajan 2014 |
| (B) Gold nanoparticles | ||||||||
| 1 | Allium cepa |
Natural storage (10 Years) |
30–113 | Spherical | 5.4 ppm | 1.68 | Enhanced seed germination, seedling growth and chlorophyll content | Acharya et al. 2019 |
| 2 | Zea mays |
Natural storage (10 years) |
10–30 | Spherical |
5 ppm 10 ppm 15 ppm |
1.92 1.84 1.69 |
Improved physiological and biochemical properties of seedlings | Mahakham et al. 2016 |
| (C) Titanium dioxide nanoparticles | ||||||||
| 1 | Capsicum annum | Natural ageing | 100 | Cylindrical |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1 1.03 1.01 |
Enhanced germination, shoot length and VI | Kumar et al. 2020b |
| 2 | Zea mays | Natural storage | 100 | – |
200 mgkg−1 400 mgkg−1 600 mgkg−1 800 mgkg−1 |
1.25 1.25 1.18 1.14 |
Lower EC with higher dehydrogenase and peroxidase activity | Vijayalakshmi et al. 2018b |
| 3 | Arachis hypogaea |
Natural storage (12 months) |
100 | Cylindrical |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1.02 1.33 1.19 |
Enhancement in germination, VI and CAT activity of aged seeds | Shyla and Natarajan 2016 |
| 4 | Arachis hypogaea |
Natural ageing (13 months) |
85–100 | Spherical |
500 mgkg−1 750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1 1.09 1.29 1.21 |
Improved germination, root and shoot length along with vigor | Shyla and Natarajan 2014 |
| 5 | Spinacia oleracea |
Natural ageing (3 years) |
– | – |
0.25% 0.5% 1.0% 1.5% 2.0% 2.5% 4.0% 6.0% |
1.06 1.09 1.17 1.42 1.52 1.75 1.64 −1.22 |
Improved growth potential and photosynthetic rate | Zheng et al. 2005 |
| (D) Zinc/Zinc oxide nanoparticles | ||||||||
| Zinc nanoparticles | ||||||||
| 1 | Capsicum annum | Natural ageing | 35–40 | Spherical |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1.06 1.13 1.10 |
Increased germination parameters, EC and antioxidant system | Kumar et al. 2020b |
| 2 | Allium cepa |
Natural storage (6 months) |
16 | Rod |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 1500 mgkg−1 |
1.06 1.2 1.16 1.1 |
Enhanced germination, seed vigor and physiological parameters | Anandraj et al. 2018 |
| 3 | Allium cepa |
Natural ageing (6 months) |
50–80 | Rod |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 1500 mgkg−1 |
1.06 1.2 1.16 1.1 |
Enhanced germination, shoot and root length with VI | Anandaraj and Natarajan 2017 |
| 4 | Lycopersicon esculentum | Accelerated ageing | – | Rod |
400 mgkg 600 mgkg 800 mgkg 1000 mgkg |
1.07 1.10 1.10 1.07 |
Improvement in germination and VI | Tamilkumar et al. 2016 |
| 5 | Zea mays | Natural storage | < 50 | – | 1500 mgkg−1 | 1.12 | Boosted seed quality and biochemical activity | Maithreyee and Gowda 2015 |
| 6 | Arachis hypogaea |
Natural ageing (13 months) |
35–40 | Rod |
500 mgkg−1 750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1.05 1.14 1.36 1.29 |
Improved VI with germination, root and shoot length | Shyla and Natarajan 2014 |
| Zinc oxide nanoparticles | ||||||||
| 7 | Arachis hypogaea | Natural storage | 25 | – |
50 ppm 100 ppm 250 ppm 500 ppm 750 ppm 1000 ppm |
1.12 1.19 1.21 1.23 1.33 1.27 |
Promoted seed germination, root and shoot length, and VI | Harish et al. 2019 |
| 8 | Cajanus cajan |
Natural storage (10 months) |
– | – |
500 ppm 750 ppm |
1.26 1.29 |
Lowered insect infestation with higher germination and VI | Korishettar et al. 2017 |
| 9 | Arachis hypogaea |
Natural storage (12 months) |
35–45 | Rod |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 |
1.04 1.38 1.33 |
Enhanced seed germination, VI and CAT activity | Shyla and Natarajan 2016 |
| (E) Iron/Iron oxide nanoparticles | ||||||||
| Iron nanoparticles | ||||||||
| 1 | Glycine max |
Natural storage (10 months) |
– | – |
500 ppm 1000 ppm 1500 ppm |
1.22 1.18 1.09 |
Augmentation in seed germination, root and shoot length, seedling growth, dry weight, VI and MSI | Dangi et al. 2019 |
| 2 | Arachis hypogaea | Natural storage | – | – |
50 ppm 100 ppm 250 ppm 500 ppm 1000 ppm 2000 ppm |
1.11 1.13 1.18 1.16 1.15 1.11 |
Improvement in germination, VI and seed viability | Harish et al. 2019 |
| 3 | Cajanus cajan |
Natural ageing (10 months) |
– | – |
250 ppm 500 ppm |
1.24 1.27 |
Increased germination, seedling length, field emergence, dry weight, α-amylase and dehydrogenase activities | Korishettar et al. 2017 |
| Iron oxide nanoparticles | ||||||||
| 4 | Glycine max | Natural storage | < 50 | – | 1250 mgkg−1 | 1.15 | Improved seedling VI | Maithreyee and Gowda 2015 |
| (F) Silica nanoparticles | ||||||||
| 1 | Oryza sativa |
Natural storage (1 month) |
93.14 | Spherical |
25 mgL−1 50 mgL−1 75 mgL−1 100 mgL−1 125 mgL−1 150 mgL−1 175 mgL−1 200 mgL−1 |
1 1 1.01 1.04 1.11 1.07 1.06 1.05 |
Improved physiological performance and minimized pathogen infection | Patil et al. 2018 |
| 2 | Glycine max | Accelerated ageing (41 °C for 24–48 h) | – | – |
40 ppm 60 ppm |
– | Increased germination rate, and reduced the time lapse of germination | Gandomani and Omidi 2017 |
| (G) Copper oxide nanoparticles | ||||||||
| 1 | Allium cepa |
Natural storage (6 month) |
80–140 | Crystalline |
750 mgkg−1 1000 mgkg−1 1250 mgkg−1 1500 mgkg−1 |
1 1.1 1.1 1.01 |
Improved germination and physiological traits | Anandraj and Natarajan 2017 |
| 2 | Zea mays | Natural storage | < 50 | – | 1500 mgkg−1 | 1.13 | Increased amylase activity consequently germinability | Maithreyee and Gowda 2015 |
| (H) Calcium carbonate nanoparticles | ||||||||
| 1 | Arachis hypogaea | Natural storage | – | – |
50 ppm 100 ppm 250 ppm 500 ppm 1000 ppm 2000 ppm |
1.12 1.17 1.15 1.10 1.09 1.07 |
Improved germination and physiological parameters | Harish et al. 2019 |
| (I) Nanoformulated materials | ||||||||
| Organic nanoparticle | ||||||||
| 1 | Solanum lycopersicum | Accelerated ageing |
Annona muricata Leaf, Trigonella foenum-graecum Seed, Curcuma longa rhizome |
100–400 | – |
0.10% 0.25% 0.5% 1% |
Improvement in seed qualities | Vijayalakshmi et al. 2018a |
| 2 | Cajanus cajan |
Accelerated ageing (40 °C for 3 days) |
Sargassum myricocystum, Caulerpa racemose, Gracilaria edulis |
– | – | 1% | Enhancement in seed quality and germinability | Ambika and Sujatha 2016 |
| Nanoemulsion | ||||||||
| 3 | Allium cepa |
Natural storage (10 years) |
Curcuma longa Citrus limon |
141.3 139.8 |
– | – | Increased germination, plant height and leaf number | Acharya et al. 2019 |
| (J) Chitosan nanoparticles | ||||||||
| 1 | Capsicum annum | Accelerated ageing | – | Cubic with sharp edges |
20 ppm 100 ppm |
1.19 1.30 |
Lowered the pathogen infection | Chookhongkha et al. 2012 |
*%G: germination percentage; CAT: catalase; EC: electrical conductivity; NP: nanoparticle; MSI: membrane stability index; VI: vigor index
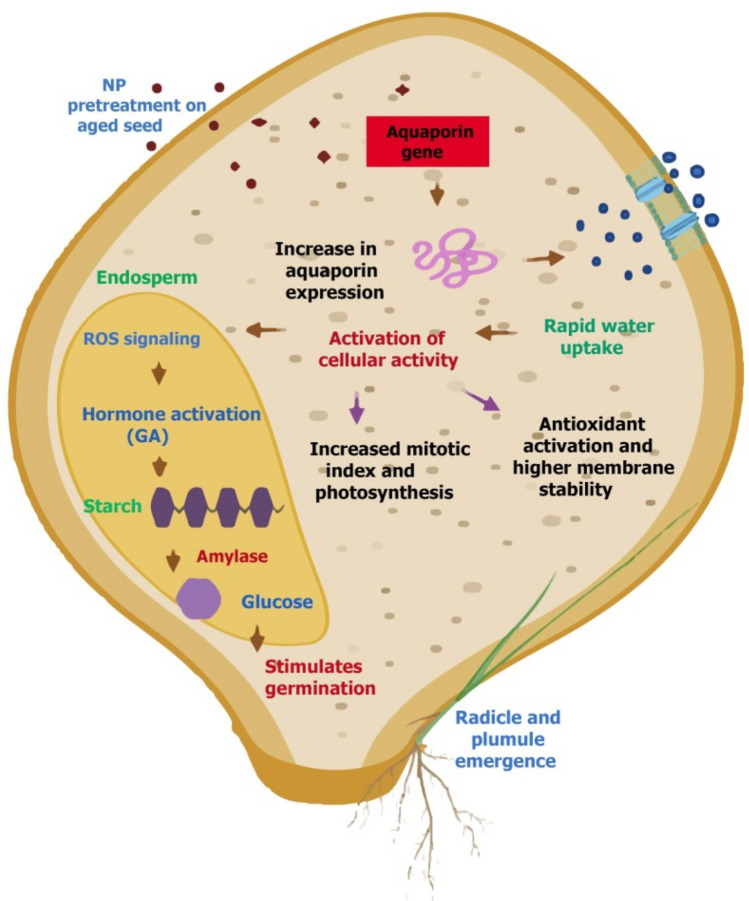
Silver NPs have also been used as coating material to Arachis hypogaea (Shyla and Natarajan 2014), Allium cepa (Anandraj and Natarajan 2017; Anandraj et al. 2018) and Glycine max (Dangi et al. 2019) to increase their storability. Coating with AgNP maintained the germinablity of seeds without affecting their vigor and emergence rate. Coating of AgNPs showed 1.3, 1.15, 1.15, 1.02 folds enhancement in %G of Arachis hypogaea, Allium cepa, and Glycine max seeds at 1250 mgKg−1, 1000 mgKg−1, 1000 mgKg−1, and 1000 ppm respectively (Table 1A). Improved germination in nanoprimed seeds has been positively correlated with faster water uptake consequently early activation of cellular/metabolic processes hence growth. Better uptake of water in aged seeds has been directly linked with up-regulation in aquaporins genes (Fig. 2). Aquaporins act as membrane channels to facilitate uptake of water, carbon dioxide, nutrients, ROS and maintaining water homeostasis. Water uptake transforms the dry seed to a turgid state followed by cell expansion and growth of embryonic axis. Reactive oxygen species act as signaling molecule in germinating seeds (Fig. 2). Coordinated hormonal (GA and ethylene) signaling is also associated with water uptake, regulation of turgor pressure, endosperm weakening, and cell wall loosening during radicle emergence and seedling establishment. Water influx is a crucial factor for expansion of cell wall and exerts adequate pressure to rupture seed coat resulting in protrusion of radicles (Hoai et al. 2020). With water imbibition, H2O2 activates GA in view to induce α-amylase activity for utilization of starch reserves. Aged Zea mays seeds showed an abrupt enhancement in α-amylase activity after treatment with AgNP (Maithreyee and Gowda 2015). This α-amylase hydrolyzes starch reserves by breaking at α-1–4 linked glucose polymers, and releases shorter fragments of carbohydrate, which plays an active role in maintenance of respiratory metabolism in germinating seeds and growing seedlings till the time photosynthesis gets capable to support growth (Fig. 2). It has also been shown that the hydrolysis of starch increases total soluble sugars within the seeds which are primarily required for energy generation to support speedy germination and seedling growth (Mahakham et al. 2017).
Fig. 2.
Schematic diagram representing nanoparticle mediated revitalization events in aged seed (GA-Gibberellic acid, NP- Nanoparticle)
Generation/accumulation of ROS is a hallmark of AgNP on treated models, however; level of ROS within the seeds is under the strict control of antioxidants. As membrane lipids are the prime targets of attack by ROS in aged seeds, it results membrane disintegration and reduces the membrane stability index (MSI). Such an event increases EL from the cell. An increase in the MSI was observed on exogenous application of AgNPs in AA seeds of Cicer arietinum (Khan et al. 2020) (Table 1A). Nanoparticles regulate the levels of ROS thereby reducing the rate of lipid peroxidation and enhance the MSI. Nanoprimed seeds possess more robust antioxidant system, thus aid the seed to overcome ageing phenomenon leading to better seedling growth. Nanoparticle would induce the oxidation–reduction reaction in germinating seeds resulting in quenching of generated free radicals. The reason of successful scavenging of free radicals was attributed to donation of extra electrons in aged seeds (Shyla and Natarajan 2014). Down-regulation in gene expressions of various antioxidants (SOD, CAT, GST, APX) is widespread phenomena in aged seeds. High storage temperature inactivates and thus negatively affects the levels of antioxidants within the seed and accelerates ageing. Pretreatment of aged seeds with NPs elevates the expression levels of these enzymes which suppresses the toxic level of ROS below damaging concentration and abet in repairing ageing associated damages in cells (Khan et al. 2020). Among various antioxidants, CAT plays role in recovery of vigor of aged seeds. In a study, 10 ppm AgNP pretreated aged seeds showed 71% increase in the CAT activity which coincided with early radicle protrusion. Proline is an important stress marker in abiotic stress conditions and seed ageing. It serves as an osmoregulator, protein stabilizer and ROS scavenger. Artificial ageing-induced decline in the proline content due to down-regulation of expression of pyrroline-5-carboxylate synthetase (proline synthesizing enzyme) in Cicer arietinum was further enhanced (8.8–34%) by application of green and chemically synthesized AgNPs (Khan et al. 2020) (Table 1A).
During prolonged storage, seeds suffer molecular damages such as DNA breaks, and reduced DNA synthesis resulting into chromosomal aberration and, variations in chromosomal structure and number (Chandra et al. 2018). Younis et al. (2019) assessed the effects of AgNPs on seven year old Vicia faba seeds (Table 1A). Their results suggested that the application of different concentrations (10, 50 and 100 ppm) of AgNP-induced considerable effect on mitotic indices in comparison to control. Mitotic index reflects frequency of cell division and coincided with higher root growth in treated samples. Germination and growth of aged seeds were due to simultaneous support of different processes such as DNA repair, gene transcription and translation, and various other metabolic processes (Varier et al. 2010). Pretreatment of aged seeds with AgNP significantly reduced the frequency of chromosomal abnormalities. Inter Simple Sequence Repeats analysis of AgNP treated stored seeds of Psophocarpus tetragonolobus did not exhibited any toxicity symptoms, rather showed phytostimulatory effects on germination and antioxidants (APX, POD, CAT, SOD) (Kumar et al. 2020a) (Table 1A).
Gold nanoparticles
Comparatively limited research has been carried out on application of AuNPs in agriculture sector. Gold NP has huge potential to improve quality of aged seeds, because it's inert property makes this material less toxic and highly biocompatible towards biological systems compared to other metallic NPs (Wang et al. 2015). Aged seeds are characterized by reduced %G and slower seedling establishment due to decreased activity of plasma membrane H+-ATPase. Priming with optimum concentration of AuNP might loosen the walls of seed coat and help in the process of radicle protrusion. Gold NPs aids ageing revival by improving radicle emergence index and shortening germination time. This exhibited significant (1.9 fold) improvement on germination of naturally aged Zea mays seeds as compared to control (Mahakham et al. 2016) (Table 1B). Pretreatment of these seeds with AuNPs caused cell wall loosening and triggered early metabolic events inside the cells. Additionally, this treatment opened many small pores in seed coat which increased water absorption. Alongside, AuNPs entered inside the seeds and induced ROS generation at moderate level, which is an essential factor for activation of cell metabolism required in pre-germinative processes (Paparella et al. 2015).
Phytosynthesized AuNPs have been shown to induce relatively better impact on traits like rate of germination and radicle length than those posed by their chemical manufactured ones. Differences in physiochemical properties such as size, coating, shape, surface chemistry, and crystalline facets present on the biosynthesized AuNPs are some of the suggested reasons for their better performance (Mahakham et al. 2016). Likewise, Acharya et al. (2019) noted comparatively early seedling emergence from ten years old Allium cepa seeds after treatment with biosynthesized AuNPs (5.4 ppm), which may be due to the modulation in antioxidant enzymes (Table 1B). Peroxidase (POX) is a primary antioxidant enzyme involved in the conversion of H2O2 into water, which was seen to be increased in AuNP primed seeds as compared to hydroprimed ones. Increased POX activity coincided with the rapid seed germination due to faster water imbibitions (Mahakham et al. 2017). Additionally, AuNP treatment also resulted in higher vegetative growth, number, length and area of leaves, chlorophyll thereby photosynthesis, and bulb yield (23.9%) from those seedlings which were emerged out of non-treated seeds (Acharya et al. 2019).
Titanium/Titanium dioxide nanoparticles
Among others, TiO2NP is a major engineered material that is popularly utilized in reactivation of aged seeds. It is considered as a beneficial element for nitrogen fixation (Carvajal and Alcaraz 1998). Pretreatment of seeds with TiO2NPs before sowing could significantly reverse poor effects of ageing. Its treatment appreciably increased the %G and VI of aged Spinacia oleracea seeds by 1.75 and 3.91 folds respectively at 2.5% concentration (Zheng et al. 2005) (Table 1C). Moreover, penetration of seed capsules by TiO2NPs facilitates water and dioxygen uptake into cells resulting in better metabolism and early germination. Compared to control, a 1.2 fold enhancement in %G was observed in aged seeds of Arachis hypogaea after the treatment of 500 ppm TiO2NPs (Harish et al. 2019) (Table 1C). Recently, Kumar et al. (2020b) assessed the comparative impacts of AgNPs, ZnONPs and TiO2NPs in improving AA Capsicum annum seeds (Table 1C). Nanoparticles of TiO2 showed strong potential in enhancing %G (1.7 folds) and VI (2 folds) than those reflected by bulk material of it at 1000 mgKg−1. Assessments of %G and seedling vigor of aged Zea mays seeds were done after application of 200 mgKg−1 TiO2NP, which exhibited 1.25 folds increase in %G with lower EC, high dehydrogenase and POX activity (Vijayalakshmi et al. 2018b) (Table 1C). Shyla and Natarajan (2014, 2016) also used TiO2NP for quality improvement of Arachis hypogaea seeds. Titanium NPs have been reported to improve the rate of germination by inducing ROS generation to meet the required quantity that leads this process, repaired cellular membranes thereby improved stability, and boosted the antioxidant system (Hoai et al. 2020). Specifically, TiO2NPs have been observed to be involved with stimulation of photosynthetic reactions like enhanced activity of RuBisCo, and a higher level of carbon fixation resulting in increased biomass of plants (Zheng et al. 2005; Xu et al. 2019).
Zinc/Zinc Oxide nanoparticles
Zinc (Zn) is an essential micronutrient for plant growth and development. It is involved in various enzymatic oxidation–reduction reactions, and metabolic processes. Despite its role in maintaining functions of important enzymes, Zn also plays a vital role in promoting the viability of aged seeds. Seed storage potential of Cajanus cajan has been extended for ten months by the coating of 500 and 750 ppm ZnNPs (Korishettar et al. 2017) (Table 1D). Studies suggested that the nanoscale ZnO gets easily absorbed by plant systems as compared to zinc sulphate or any other form of it. Nano sized ZnO actively penetrates seeds through cracks present on their coat (Kumar et al. 2020b). The ZnONP has been reported to be used as pre-treatment agent to promote seed germination, seedling growth, and abiotic stress tolerance. In a study, treatment of Capsicum annum seeds with 1000 ppm ZnONP has been shown to increase the rate of germination (1.13 folds), shoot–root length (1.17–1.36 folds), and VI (1.49 folds) considerably (Kumar et al. 2020b) (Table 1D). Prasad et al. (2012) reported that higher concentration of Zn within the seeds positively affects health status of seedlings growing out of these. It donates electrons to scavenge ROS generated in the aged seeds thus enhance seedling VI thereby faster emergence of radicles. Zinc oxide nano rods enhanced the physiological and biochemical properties resulting in improved vigor and viability of aged Vigna mungo seeds (Senthilkumar 2011). Treatment of ZnONPs could reduce the extent of deterioration in aged seeds of Lycopercion esculentum with 1.9 folds increase in %G and VI at 1000 mgKg−1 concentration (Tamilkumar et al. 2016). Likewise, 1.2 and 1.16 fold increase in germination was observed at 1000 mgKg−1 ZnONP in Allium cepa (Anandaraj et al. 2018) and Arachis hypogaea (Shyla and Natarajan 2016) seeds respectively (Table 1D). Higher %G of ZnONP treated seeds could also be attributed due to increased auxin production, as Zn plays a crucial role in tryptophan synthesis, a precursor of indole acetic acid (IAA) (Anandraj and Natarajan 2017). Auxin signaling pathway has an essential role in the initiation of lateral-root formation. Zinc can increase the activities of enzymes involved in the transformation of proteins into amino acids, starch into simple sugars and, fat into a source of providing abundant nutrients and energy for enhanced germination (Adhikari et al. 2016).
Treatment of ZnONP showed lower EC which can be attributed due to improved membrane integrity compared to non-treated seeds. A significant (1.09 fold) decline in EC was recorded in Arachis hypogaea after treatment with 1000 mgKg−1 ZnONP (Shyla and Natarajan 2016). Zinc oxide NPs quench the ROS through induction of various antioxidants and maintains cellular integrity (Chandrasekaran et al. 2020). Oxygen released during the quenching of ROS is further used in the respiration process which is beneficial for seed germination and seedling growth. Pretreatment of aged Zea mays seeds with ZnONP improved both the physiological and biochemical attributes (Maithreyee and Gowda 2015). Additionally, significant enhancement in the VI of aged Arachis hypogaea seeds was observed after treatment of 1000 ppm ZnONP (Harish et al. 2019).
Iron/Iron dioxide nanoparticle
Iron plays an important role in various physiological, biochemical, and metabolic processes like photosynthesis, respiration, nitrogen fixation, etc., thus a prerequisite for plant growth and development. Ghafari and Razmjoo (2013) have been used FeNP for quality improvement of Triticum aestivum seed. Studies showed that foliar application of FeONP (2 gL−1) improved the yield and quality of grains, foliar chlorophyll, and carotenoid contents, and POX, CAT and APX activities. Sheykhbaglou et al. (2018) used Fe3O4NP for enhancement of nutritional quality of Glycine max. Foliar spraying of 0.75 gL−1 of nano FeO leads to 1.21–1.28 folds enhancement in protein and lipid respectively, compared to control. Iron NP has also been successfully used for the storage and quality enhancement of aged seeds. About 1.18 folds germination enhancement was observed in aged seeds of Arachis hypogaea compared to control, after treatment of 250 ppm FeONP (Harish et al. 2019) (Table 1E). Iron oxide NPs also enhanced the length of root and shoot with higher VI. Coating of seeds with polymer of FeNPs (500 ppm) maintained the quality of Cajanus cajan seeds even after ten months of storage due to capability of scavenging the free radicals generated during storage, thus maintained the vigor (Korishettar et al. 2017) (Table 1E). Dangi et al. (2019) studied the storability of Glycine max seeds after FeNP treatment (Table 1E). Iron NP of 500 ppm has been seen to improve pace of germination, shoot–root length, seedling VI, and lowered EC compared to control seeds. Reduction in EC is possibly due to repair of the cell membrane.
Silicon dioxide nanoparticle
Silicon dioxide NPs have also been used to enhance plant growth and development. In recent studies effect of SiNP on Cicer arientinum under Al toxicity was assessed. Results showed that the SiNP was efficient in providing tolerance by compensating the cellular redox balance via enhancement of antioxidants gene expressions (Chandra et al. 2020a). Siddiqui and Al-Whaibi (2014) demonstrated that the application of SiO2NPs significantly enhanced the characteristics of seed germination in Solanum lycopersicum. Similarly, Gandomani and Omidi (2017) studied the effect of SiO2NP in improving germination of AA Glycine max seeds (Table 1F). Silica dioxide NPs had significant effect on traits like %G, germination rate, seedling number and fresh weight, root to shoot ratio, relative water content and chlorophyll. Pretreatment with 60 ppm SiO2NP increased the germination by 20% and reduced the time required to lead this process. Silica NP showed concentration dependant response; 125 ppm concentration has been improved germination quality parameters in Oryza sativa (Patil et al. 2018) (Table 1F). Silica NPs enhanced the germination by 1.11 folds, shoot–root length by 1.22–1.44 folds, seedling length by 1.28 folds, and dry weight by 1.05 folds, with higher VI I and II (1.42 and 1.18 folds) compared to control. Higher vigor was seen to reduce the level of infection by seed born fungi to control samples. Improvement in seed quality parameters might be due to faster imbibition of water resulting in activation of essential metabolic reactions to enhance the germination. Higher concentration (150 to 200 ppm) of SiO2NP had toxic effect on seed quality parameters.
Copper/Copper oxide nanoparticle
Copper is an important structural component of various enzymes and regulatory proteins associated with mitochondrial respiration, photosynthetic electron transport, oxidative stress responses, hormone signaling, and cell wall metabolism (Solymosi and Bertrand 2012). Gautam et al. (2016) studied the influence of CuONP on growth, chlorophyll content, and germination of Glycine max. A positive change in total chlorophyll by 1.2 folds was observed at 200 ppm concentration of CuONP compared to control. Moreover, higher concentrations had an inhibitory effect on germination. Anandraj and Natarajan (2017) compared the effect of CuONP with other NPs at concentration of 750, 1000, 1250 and 1500 mgKg−1 (Table 1G). Copper oxide NPs (1250 mgKg−1) enhanced the germination (1.1 folds) and VI (1.15 folds) compared to control. Moreover, Maithreyee and Gowda (2015) used CuONP to enhance the quality of aged Zea mays (Table 1G). They recorded the highest germination (1.13 folds), shoot and root length (1.47–1.27 folds), seedling length (1.35 folds), dry weight (1.54 folds) and, VI I and II (1.58 and 1.81 folds) respectively at 1500 mgKg−1 concentration. Seeds treated with CuONP had highest α-amylase activity (1440 mg maltose liberated h−1 mg−1 of protein) compared to control, thus enhanced the biochemical activity and %G.
Calcium carbonate nanoparticle
Calcium is an essential plant nutrient, required for the structural maintenance of cell wall and membrane. Calcium carbonate (CaCO3) is widely used for the neutralization of acidic soil. Yugandhar and Savithramma (2013) studied the effect of CaCO3NP on the germination of Vigna mungo seeds. Under the influence of CaCO3NP an enhancement in germination (1.04 folds) was observed compared to control. Nanoparticles positively affected the seedling VI along with shoot and root length. In a study, different concentrations (50, 100, 250, 500, 1000 and 2000 ppm) of CaCO3NP were tested for quality improvement of Arachis hypogaea seeds (Harish et al. 2019) (Table 1H). Calcium carbonate NPs (100 ppm) increased the germination (1.17 folds) and VI (1.4 folds). Enhancement in germination attributes was an outcome of enhanced biochemical activities in seeds. Activities of α-amylase, and antioxidants (CAT, SOD and POX) were significantly higher in treated seeds compared to un-treated ones (Harish 2017).
Nanoformulations
Nanoemulsion (NE) is the emulsion of nano sized (20 to 200 nm) materials (Wang et al. 2007). Nanotechnology has been utilized in the formulation of emulsions with a purpose to refine their function and stability. Acharya et al. (2019) utilized NEs of Curcuma longa oil and Citrus limon for the quality enhancement of Allium cepa seeds (Table 1I). Internalization of NE within the seeds was confirmed by assessment of Ar-turmerone and fatty acid methyl esters, respectively. Owning to NE interanalization, seed showed enhanced germination, faster radicle emergence, growth and yield compared to unprimed and hydroprimed controls. Polyphenols present in plant extract readily react with cellular ROS and cause loosening of cell walls, thereby growth.
Organic NPs have been used for the amelioration of ageing in Solanum lycopersicum seeds (Vijayalakshmi et al. 2018a) (Table 1I). Three different organic solutions of powders of Annona muricata leaf, Trigonella foenum-graecum seed, and Curcuma longa rhizome, with a range of concentrations (0.10, 0.25, 0.5 and 1%) were used to study their impacts on aged seeds. Organic compounds are rich in polyphenolics and flavonoids which possess high antioxidant activity. Higher antioxidant activities quench the excess free radicals generating in deteriorating seed and thus enhance rate of germination. Enhancement in seed quality was observed by treatment of 0.25% Trigonella foenum-graecum nano powder. Ambika and Sujatha (2016) studied the effects of nano powders of Sargassum myricocystum, Caulerpa racemosa, and Gracilatia edulis on physiological quality and enzyme activity of ageing Cajanus cajan seed during storage (Table 1I). Among these, Sargassum myricocystum (1%) nano powder-induced maximum improvement in %G, pace of germination, seedling length, seed vigor, α-amylase and POX activity. The promotary effect exhibited by this on seed quality is due to the presence of antioxidants, growth promoting substances, metabolic enhancers and, micro and macro elements. Nano powders promote water uptake in seeds thereby utilization of stored starch reserves for energy generation, leading to germination.
Chitosan nanoparticle
Chitosan is a proven second most abundant bio-functional material that induces many biological responses in plants. Chemically chitosan is a polycationic polymer of β-1,4 linked-glucosamine, which is known to be antifungal. It is a well accepted nanomaterial owing to its non-toxicity, versatility, biocompatibility, excellent physiochemical properties and biodegradability (Divya and Jisha 2018). Li et al. (2019) showed a positive effect of chitosan NP (ChNP) on seed germination and seedling growth of Triticum aestivum. Low concentration of ChNP (5 μgmL−1) induced the auxin-related gene expression and, enhanced IAA biosynthesis and transport, while reduced the activity of IAA oxidase, resulting in an abrupt increase of IAA concentration in Triticum aestivum shoots and roots. Divya et al. (2019) applied the ChNP as elicitor of germination in Oryza sativa. Chitosan NP was found to induce the activities of enzymes responsible for the conversion of stored complexes in simpler forms for successful seedling establishment of Zea mays (Saharan et al. 2016). Stored seeds are prone to fungal infections, and ChNP has been used as an effective material to overcome this problem. Chookhongkha et al. (2012) compared the effects of ChNP and chitosan solution on fungal growth and quality control of AA Capsicum annum seeds and observed that the ChNP acts as an antifungal agent thereby control deterioration (Table 1J).
Conclusions and future perspectives
Seeds exhibit high genetic variability, thus believed to be the best alternative for conservation of natural genetic diversity, compared to somatic tissues. However, the longevity of seeds is crucial for sustainable agricultural practices. Decline in viability due to ageing poses a significant challenge to seed storage phenomenon. Various studies have documented the potential physiological, biochemical, and genetic markers which can be used to assess the degree of ageing/quality of seeds. Ageing considerably affects genome stability, reduces efficiency of repair mechanisms and, diminishes seed vigor and viability. Therefore, immense study is required to know the exact mechanisms that regulate ageing phenomenon, and to understand the genetic basis of it. Exogenous application of metal NPs on aged seeds has recently been demonstrated to revive the damaging reactions by stimulating the repair mechanisms. Nanoparticle triggers seed machinery to enter into the germination phase and promote faster radicle emergence. It improves the vigor of aged seeds by maintaining the cellular redox homeostasis. Seed germination is triggered by ROS signaling, and H2O2 is identified as a profound signaling molecule that stimulates the onset of early germination of aged seeds. Reversal of ageing in seeds is regulated by stimulated α-amylase activity which controls germination by hydrolyzing stored carbohydrates. Treatment of NP has prolonged effect on germinated seedlings. It promotes root-shoot elongation by increasing mitotic index along with biomass.
Since nanotechnology is assuring betterment in agricultural practices, an enormous consideration should be taken into account to make sure that it cannot be tinkering with the environment. Use of NPs must be within nature threshold limits. Newer goals should be set to look into the depth of whole germination process controlled by NPs. Analysis of whole spectrum of growth in terms of germination of aged seeds is required. Limited studies have been done in this area, thus robustness of technology relies on how easily it can be translatable to fields with reproducible results. Studies are also required to be focused on cost-effective syntheses of NPs for bulk application. Large scale application must be free from any sort of stoichiometric defect. Focus must be drawn on uptake, transport and accumulation of NPs for prolonged effect. Significant attention is required to observe the interaction and signaling pathways. Each type of NP has some special properties, hence; in depth studies are required in context to revival of ageing in seeds. Lastly, with the increase in global population we cannot afford to waste our stored foods/seeds, therefore; nanotechnology imposed improvement in seed quality has been proven to be boon for solving this problem.
Availability of data and material
Not Applicable.
Acknowledgements
The authors would like to thank Pt. Ravishankar Shukla University, Raipur, for awarding Research Fellowship (No. 1528/Finance-Scholarship/2020, dated 20.05.2020) to Rasleen Kaur. The authors would also like to thank Pt. Ravishankar Shukla University, Raipur and University Grants Commission, New Delhi, for awarding fellowship to Jipsi Chandra under Research Fellowship (No. 79/8/Fin.Sch/2014, dated 16.04.14) and National Fellowship for students of Other Backward Classes (F./2016-17/NFO-2015-17-OBC-CHH-27902) respectively.
Author contributions
S. Keshavkant conceptualized the topic, and finalized the manuscript draft. Rasleen Kaur and Jipsi Chandra gathered information and drafted the manuscript. All the authors read and approved the final manuscript.
Funding
The authors would like to thank Pt. Ravishankar Shukla University, Raipur, for awarding Research Fellowship (No. 1528/Finance-Scholarship/2020, dated 20.05.2020) to Rasleen Kaur. The authors would also like to thank Pt. Ravishankar Shukla University, Raipur, and University Grants Commission, New Delhi, for awarding fellowship to Jipsi Chandra under Research Fellowship (No. 79/8/Fin.Sch/2014, dated 16.04.14), and National Fellowship for students of Other Backward Classes (F./2016–17/NFO-2015–17-OBC-CHH-27902) respectively.
Compliance with ethical standards
Conflict of interest
Authors have no conflict of interest.
Ethics approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acharya P, Jayaprakasha GK, Crosby KM, Jifon JL, Patil BS (2019) Green-synthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L.). ACS Sustain Chem Eng 7(17): 14580–14590. 10.1021/acssuschemeng.9b02180
- Adhikari T, Kundu S, Rao AS. Zinc delivery to plants through seed coating with nano-zinc oxide particles. J Plant Nutr. 2016;39(1):136–146. doi: 10.1080/01904167.2015.1087562. [DOI] [Google Scholar]
- Ambika S, Sujatha K. Organic seed treatment with seaweed nano powders on physiological quality and enzyme activities in aged seeds of pigeon pea. Bioscan. 2016;11(1):353–357. [Google Scholar]
- Anandaraj K, Ilakkiya R, Natarajan N (2018) Customizing zinc oxide (ZnO) and silver (Ag) nanoparticles for seed quality enhancement in onion (Allium cepa (Linn) cv. CO (On) 5. Int J Curr Microbiol App Sci 7(11):1522–1530. 10.20546/ijcmas.2018.711.175
- Anandaraj K, Natarajan N (2017) Effect of nanoparticles for seed quality enhancement in onion [Allium cepa (Linn) cv. CO (On)] 5. Int J Curr Microbiol App Sci 6:3714–3724. 10.20546/ijcmas.2017.611.435
- Anjum NA, Sofo A, Scopa A, Roychoudhury A, Gill SS, Iqbal M, Lukatkin AS, Pereira E, Duarte AC, Ahmad I. Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res. 2015;22(6):4099–4121. doi: 10.1007/s11356-014-3917-1. [DOI] [PubMed] [Google Scholar]
- Aslani F, Bagheri S, Julkapli NM, Juraimi AS, Hashemi FSG, Baghdadi A. Effects of engineered nanomaterials on plants growth: an overview. Sci World J. 2014;28:948. doi: 10.1155/2014/641759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto LC, Garcia QS. Accelerated ageing and subsequent imbibitions affect seed viability and the efficiency of antioxidant system in macaw palm seeds. Acta Physiol Plant. 2017;39(3):72. doi: 10.1007/s11738-017-2367-z. [DOI] [Google Scholar]
- Bucholc M, Buchowicz J. Synthesis of extrachromosomal DNA and telomere-related sequences in germinating wheat embryos. Seed Sci Res. 1992;2(3):141–146. doi: 10.1017/S0960258500001264. [DOI] [Google Scholar]
- Carvajal M, Alcaraz CF. Why titanium is a beneficial element for plants. J Plant Nutr. 1998;21(4):655–664. doi: 10.1080/01904169809365433. [DOI] [Google Scholar]
- Chandra J, Chauhan R, Korram J, Satnami ML, Keshavkant S. Silica nanoparticle minimizes aluminium imposed injuries by impeding cytotoxic agents and over expressing protective genes in Cicer arietinum. Sci Hortic. 2020;260:108885. doi: 10.1016/j.scienta.2019.108885. [DOI] [Google Scholar]
- Chandra J, Dubey M, Keshavkant S (2020b) Influence of protein damage and proteasome gene expression on the longevity of recalcitrant Madhuca latifolia Roxb. seeds. Botany 98(3):173–183. 10.1139/cjb-2019-0130
- Chandra J, Keshavkant S. Desiccation-induced ROS accumulation and lipid catabolism in recalcitrant Madhuca latifolia seeds. Physiol Mol Biol Plants. 2018;24(1):75–87. doi: 10.1007/s12298-017-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J, Parkhey S, Keshavkant S. Ageing-regulated changes in genetic integrity of two recalcitrant seeded species having contrasting longevity. Trees. 2018;32(1):109–123. doi: 10.1007/s00468-017-1615-6. [DOI] [Google Scholar]
- Chandrasekaran U, Luo X, Wang Q, Shu K. Are there unidentified factors involved in the germination of nanoprimed seeds? Front Plant Sci. 2020;11:832. doi: 10.3389/fpls.2020.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chookhongkha N, Sopondilok T, Photchanachai S (2012) Effect of chitosan and chitosan nanoparticles on fungal growth and chilli seed quality. In I international conference on postharvest pest and disease management in exporting horticultural crops-PPDM2012 973, pp. 231–237
- Damir I, Mavi K (2008) Controlled deterioration and accelerated ageing test to estimate the relative storage potential of curcurbit seed lots. Hortscience 43(5):1544–1548. 10.21273/HORTSCI.43.5.1544
- Dangi S, Biradarpatil NK, Deshpande VK, Hunje R, Mogali S (2019) Effect of seed treatment with nanoparticles on seed storability of soybean. Int J Curr Microbiol App Sci 8(11):2535–2545. 10.20546/ijcmas.2019.811.293
- De Souza TAJ, Souza LRR, Franchi LP. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol Environ Saf. 2019;171:691–700. doi: 10.1016/j.ecoenv.2018.12.095. [DOI] [PubMed] [Google Scholar]
- Demidchik V. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot. 2015;109:212–228. doi: 10.1016/j.envexpbot.2014.06.021. [DOI] [Google Scholar]
- Divya K, Jisha MS. Chitosan nanoparticles preparation and applications. Environ Chem Lett. 2018;16(1):101–112. doi: 10.1007/s10311-017-0670-y. [DOI] [Google Scholar]
- Divya K, Vijayan S, Nair SJ, Jisha MS. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int J Biol Macromol. 2019;124:1053–1059. doi: 10.1016/j.ijbiomac.2018.11.185. [DOI] [PubMed] [Google Scholar]
- Fu YB, Ahmed Z, Diederichsen A. Towards a better monitoring of seed ageing under ex-situ seed conservation. Conserv Physiol. 2015;3(1):1–16. doi: 10.1093/conphys/cov026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandomani VM, Omidi H (2017) The effect of nano-particle silicon dioxide (SiO2) on improving soybean seed germination under accelerated aging conditions. SST 6(1):193–203. 10.22034/ijsst.2017.113683
- Gautam S, Misra P, Shukla PK, Ramteke PW. Effect of copper oxide nanoparticle on the germination, growth and chlorophyll in soybean (Glycine max L.) Vegetos. 2016;29:157–160. doi: 10.5958/2229-4473.2016.00050.1. [DOI] [Google Scholar]
- Ghafari H, Razmjoo J. Effect of foliar application of nano-iron oxidase, iron chelate and iron sulphate rates on yield and quality of wheat. Intl J Agron Plant Prod. 2013;4(11):2997–3003. [Google Scholar]
- Harish M (2017) Influence of seed treatment with nanoparticles on morpho physiological and biochemical changes in groundnut (Arachis hypogaea L.). Doctoral dissertation, University of Agricultural Sciences GKVK, Bengaluru
- Harish MS, Gowda R, Nethra N (2019) Standardization of nano particles for enhancing groundnut seed quality Cv. ICGV-91114. Int J Pharmacogn Phytochem 8(1):2208–2212
- Hoai PT, Tyerman SD, Schnell N, Tucker M, McGaughey SA, Qiu J, Groszmann M, Byrt CS. Deciphering aquaporin regulation and roles in seed biology. J Exp Bot. 2020;71(6):1763–1773. doi: 10.1093/jxb/erz555. [DOI] [PubMed] [Google Scholar]
- Jaganathan GK, Liu B (2014) Traditional method of storing Pigeonpea (Cajanus cajan L.) seeds using red soil. Res J Recent Sci 3(10):48–52
- Katiyar P, Yadu B, Korram J, Satnami ML, Kumar M, Keshavkant S. Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J Environ Sci (China) 2020;92:18–27. doi: 10.1016/j.jes.2020.02.013. [DOI] [PubMed] [Google Scholar]
- Keshavkant S, Sahu B, Parkhey S. Artificial ageing induced metabolic changes in Cicer arietinum seeds: ROS catabolism, lipid peroxidation, protein carbonylation, nucleic acid integrity and antioxidants. Germany: Lambert Academic Publishing; 2013. [Google Scholar]
- Khan J, Chandra J, Xalxo R, Korram J, Satnami ML, Keshavkant S. Amelioration of ageing associated alterations and oxidative inequity in seeds of Cicer arietinum by silver nanoparticles. J Plant Growth Regul. 2020 doi: 10.1007/s00344-020-10193-2. [DOI] [Google Scholar]
- Korishettar P, Vasudevan SN, Shakuntala NM, Doddagoudar SR, Hiregoudar S, Kisan B (2017) Influence of seed polymer coating with Zn and Fe nanoparticles on storage potential of pigeonpea seeds under ambient conditions. J Appl Nat Sci 9(1):186–191. 10.31018/jans.v9i1.1169
- Kumar GD, Raja K, Natarajan N, Govindaraju K, Subramanian KS (2020b) Invigouration treatment of metal and metal oxide nanoparticles for improving the seed quality of aged chilli seeds (Capsicum annum L.). Mater Chem Phys 242:122492. 10.1016/j.matchemphys.2019.122492
- Kumar VK, Muthukrishnan S, Rajalakshmi R (2020a) Phytostimulatory effect of phytochemical fabricated nanosilver (AgNPs) on Psophocarpus tetragonolobus (L.) DC. seed germination: An insight from antioxidative enzyme activities and genetic similarity studies. Curr Plant Biol 23:100158. 10.1016/j.cpb.2020.100158
- Kurek K, Plitta-Michalak B, Ratajczak E. Reactive oxygen species as potential drivers of the seed aging process. Plants. 2019;8(6):174. doi: 10.3390/plants8060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, He J, Xie H, Wang W, Bose SK, Sun Y, Hu J, Yin H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.) Int J Biol Macromol. 2019;126:91–100. doi: 10.1016/j.ijbiomac.2018.12.118. [DOI] [PubMed] [Google Scholar]
- Mahakham W, Sarmah AK, Maensiri S, Theerakulpisut P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci Rep. 2017;7(1):1–21. doi: 10.1038/s41598-017-08669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahakham W, Theerakulpisut P, Maensiri S, Phumying S, Sarmah AK. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci Total Environ. 2016;573:1089–1102. doi: 10.1016/j.scitotenv.2016.08.120. [DOI] [PubMed] [Google Scholar]
- Maithreyee MN, Gowda R. Influence of nanoparticles in enhancing seed quality of aged seeds. Mysore J Agric Sci. 2015;49(2):310–313. [Google Scholar]
- Mbofung GCY, Goggi AS, Leandro LFS, Mullen RE. Effects of storage temperature and relative humidity on viability and vigor of treated soybean seeds. Crop Sci. 2012;53(3):1086–1095. doi: 10.2135/cropsci2012.09.0530. [DOI] [Google Scholar]
- Mira S, Pirredda M, MartínSánchez M, Marchessi JE, Martín C. DNA methylation and integrity in aged seeds and regenerated plants. Seed Sci Res. 2020 doi: 10.1017/S0960258520000021. [DOI] [Google Scholar]
- Mittler R. ROS are good. Trends Plant Sci. 2017;22(1):11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Oenel A, Fekete A, Krischke M, Faul SC, Gresser G, Havaux M, Mueller MJ, Berger S. Enzymatic and non-enzymatic mechanisms contribute to lipid oxidation during seed aging. Plant Cell Physiol. 2017;58(5):925–933. doi: 10.1093/pcp/pcx036. [DOI] [PubMed] [Google Scholar]
- Panda D, Mondal S. Seed enhancement for sustainable agriculture: an overview of recent trends. Plant Arch. 2020;20(1):2320–2332. [Google Scholar]
- Paparella S, Araujo SS, Rossi G, Wijayasinghe M. Seed priming: state of the art and new perspectives. Plant Cell Rep. 2015;34(8):1281–1293. doi: 10.1007/s00299-015-1784-y. [DOI] [PubMed] [Google Scholar]
- Parkhey S, Naithani SC, Keshavkant S. Protein metabolism during natural ageing in desiccating recalcitrant seeds of Shorea robusta. Acta Physiol Plant. 2014;36(7):1649–1659. doi: 10.1007/s11738-014-1540-x. [DOI] [Google Scholar]
- Patil NB, Sharanagouda H, Doddagoudar SR, Ramachandra CT, Ramappa KT (2018) Effect of rice husk silica nanoparticles on rice (Oryza sativa L.) seed quality. Int J Curr Microbiol App Sci 7(12):3232–3244. 10.20546/ijcmas.2018.712.374
- Prado JP, Krzyzanowski FC, Martins CC, Vieira RD. Physiological potential of soybean seeds and its relationship to electrical conductivity. J Seed Sci. 2019;41(4):407–415. doi: 10.1590/2317-1545v41n4214988. [DOI] [Google Scholar]
- Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad TS, Sajanlal PR, Pradeep T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr. 2012;35(6):905–927. doi: 10.1080/01904167.2012.663443. [DOI] [Google Scholar]
- Saharan V, Pal A. Chitosan based nanomaterials in plant growth and protection. New Delhi India: Springer; 2016. [Google Scholar]
- Senthilkumar S (2011) Customizing nanoparticle for the maintenance of seed vigor and viability in black gram (Vigna mungo) cv. VBN 4. M.Sc. Thesis, Tamil Nadu Agricultural University, Coimbatore
- Sharma D, Kanchi S, Bisetty K. Biogenic synthesis of nanoparticles: a review. Arab J Chem. 2019;12(8):3576–3600. doi: 10.1016/j.arabjc.2015.11.002. [DOI] [Google Scholar]
- Sheykhbaglou R, Sedghi M, Fathi-Achachlouie B. The effect of ferrous nano-oxide particles on physiological traits and nutritional compounds of soybean (Glycine max L.) seed. An Acad Bras Cienc. 2018;90(1):485–494. doi: 10.1590/0001-3765201820160251. [DOI] [PubMed] [Google Scholar]
- Shyla KK, Natarajan N. Customizing zinc oxide, silver and titanium dioxide nanoparticles for enhancing groundnut seed quality. Indian J Sci Technol. 2014;7(9):1376–1381. doi: 10.17485/ijst/2014/v7i9.29. [DOI] [Google Scholar]
- Shyla KK, Natarajan N (2016) Synthesis of inorganic nanoparticles for the enhancement of seed quality in groundnut cv. VRI-2. Adv Res J Crop Improv 7(1):32–39. 10.15740/HAS/ARJCI/7.1/32-39
- Siddiqui MH, Al-Whaibi MH. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.) Saudi J Biol Sci. 2014;21(1):13–17. doi: 10.1016/j.sjbs.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg SO, Yndgaard F, Andreasen C, Von Bothmer R, Loskutov IG, Asdal A. Long-term storage and longevity of orthodox seeds: A systematic review. Front Plant Sci. 2020;11:1007. doi: 10.3389/fpls.2020.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymosi K, Bertrand M. Soil metals, chloroplasts, and secure crop production: a review. Agron Sustain Dev. 2012;32(1):245–272. doi: 10.1007/s13593-011-0019-z. [DOI] [Google Scholar]
- Somasundaram G, Bhaskaran M. Standardization of accelerated ageing duration for screening of rice genotypes for seed longevity. Int J Agric Sci. 2017;7:397–404. [Google Scholar]
- Tamilkumar P, Sivaji M, Vinoth R, Kumar SS, Natarajen N. Customizing zinc oxide nanoparticles for extending seed vigour and viability in tomato (Lycopersicon esculentum Mill) Int J Agric Sci. 2016;12:186–190. doi: 10.5740/HAS/IJAS/12.2/000-000. [DOI] [Google Scholar]
- Varier A, Vari AK, Dadlani M. The subcellular basis of seed priming. Curr Sci. 2010;99(4):450–456. [Google Scholar]
- Vellosillo T, Vicente J, Kulasekaran S, Hamberg M, Castresana C. Emerging complexity in reactive oxygen species production and signaling during the response of plants to pathogens. Plant Physiol. 2010;154(2):444–448. doi: 10.1104/pp.110.16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SK, Das AK, Gantait S, Kumar V, Gurel E. Applications of carbon nanomaterials in the plant system: a perspective view on the pros and cons. Sci Total Environ. 2019;667:485–499. doi: 10.1016/j.scitotenv.2019.02.409. [DOI] [PubMed] [Google Scholar]
- Verma SK, Das AK, Patel MK, Shah A, Kumar V, Gantait S. Engineered nanomaterials for plant growth and development: a perspective analysis. Sci Total Environ. 2018;630:1413–1435. doi: 10.1016/j.scitotenv.2018.02.313. [DOI] [PubMed] [Google Scholar]
- Vijay D, Dadlani M, Kumar PA, Panguluri SK. Molecular marker analysis of differentially aged seeds of soybean and safflower. Plant Mol Biol Rep. 2009;27(3):282–291. doi: 10.1007/s11105-008-0085-9. [DOI] [Google Scholar]
- Vijayalakshmi V, Ramamoorthy K, Natarajan N. Amelioration of aged tomato seeds through nano sized organic particles. J Pharmacogn Phytochem. 2018;7:402–406. [Google Scholar]
- Vijayalakshmi V, Ramamoorthy K, Natarajan N. TiO2 Nano particles on extending seed vigour and viability of naturally aged maize (Zea mays L.) Seeds. Int J Pharmacogn Phytochem. 2018;7(1):2221–2224. [Google Scholar]
- Wang L, Li X, Zhang G, Dong J, Eastoe J. Oil-in water nanoemulsions for pesticide formulations. J Colloid Interface Sci. 2007;314:230–235. doi: 10.1016/j.jcis.2007.04.079. [DOI] [PubMed] [Google Scholar]
- Wang T, JiaoY CQ, Yu X. Gold nanoparticles: synthesis and biological applications. Nano Life. 2015;5(03):1542007. doi: 10.1142/S1793984415420076. [DOI] [Google Scholar]
- Wawrzyniak MK, Kalemba EM, Ratajczak E, Chmielarz P. Oxidation processes related to seed storage and seedling growth of Malus sylvestris, Prunus avium and Prunus padus. PLoS ONE. 2020;15(6):e0234510. doi: 10.1371/journal.pone.0234510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia F, Chen L, Sun Y, Mao P. Relationships between ultrastructure of embryo cells and biochemical variations during ageing of oat (Avena sativa L.) seeds with different moisture content. Acta Physiol Plant. 2015;37(4):89–100. doi: 10.1007/s11738-015-1825-8. [DOI] [Google Scholar]
- Xu ML, Zhu YG, Gu KH, Zhu JG, Yin Y, Ji R, Du WC, Guo HY. Transcriptome reveals the rice response to elevated free air CO2 concentration and TiO2 nanoparticles. Environ Sci Technol. 2019;53(20):11714–11724. doi: 10.1021/acs.est.9b02182. [DOI] [PubMed] [Google Scholar]
- Yin X, He D, Gupta R, Yang P. Physiological and proteomic analyses on artificially aged Brassica napus seed. Front Plant Sci. 2015;6:112. doi: 10.3389/fpls.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis ME, Abdel-Aziz HMM, Heikal YM. Nanopriming technology enhances vigor and mitotic index of aged Vicia faba seeds using chemically synthesized silver nanoparticles. S Afr J Bot. 2019;125:393–401. doi: 10.1016/j.sajb.2019.08.018. [DOI] [Google Scholar]
- Yughandar P, Savithramma N. Green synthesis of calcium carbonate nanoparticle and their effect on seed germination and seedling growth of vigna mungo (L.) Hepper. Intl J Adv Res. 2013;1(8):89–103. [Google Scholar]
- Zheng L, Hong F, Lu S, Liu C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res. 2005;104(1):83–91. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]
- Zinsmeister J, Leprince O, Buitink J. Molecular and environmental factors regulating seed longevity. Biochem J. 2020;477(2):305–323. doi: 10.1042/BCJ20190165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.