Abstract
Bioleaching is one of the well-known methods of metal recovery with Environmental benefits. This process has been extensively used for combating improper waste management issues along with metal reclamation. The aim of this study is to bioleach spent petroleum refinery catalyst at variant pulp densities (PD) (5, 10 and 15%) using microorganisms in acidic pH (1.5–1.6) and mesophilic temperature (30–35 °C). The study includes leaching yields of metals like nickel, molybdenum, copper and aluminum. The three bioleaching experiments with different pulp densities yielded a maximum of more than 90% nickel, 73% copper, 87% molybdenum and 24% aluminum. The results are validated 5, 10, and 15% pulp density and the result is validated with pH, Redox potential, microbial population, sulphate concentration and ferrous iron, concentration. The time saving due to faster nickel dissolution using iron and sulphur oxidizing microorganisms would be economical for the bioleaching process.
Keywords: Bioleaching, Mesophilic chemolithotrophs, Pulp density, Dissolution
Introduction
During the production of the full range of transportation fuels, petroleum refining catalysts are extensively used to increase the efficiency of the refining process by facilitating hydrocarbon transformations (Silvy 2004). After several cycles of reuse and regeneration the catalyst loses its activity due to structural changes by thermal degradation, phase transformation and fouling of active surfaces with coke, sulphur, vanadium and nickel. The lifespan of a petroleum refinery catalyst varies from 3 months to 6 years (Dufresne 2007; Asghari and Mousavi 2014). This end-of-life petroleum catalyst is referred to as ‘spent catalyst’. This material is eventually dumped off as one of the most hazardous petroleum refinery solid waste corresponding to 4% of the total refinery wastes and is estimated in the range of 150,000–170,000 tons/ year (Akcil et al. 2015; Asghari and Mousavi 2014; Ferreira et al. 2016; Marafi and Stanislaus 2008). Spent catalysts are generally rich in metal values and their usual composition is 7–20% V + Ni, 1–14% Mo, 0.2–3% Co and 17–40% Al (Asghari et al. 2013; Marafi and Stanislaus 2008). By exploiting this spent catalyst, its rich metallic values can be recovered sustainably and therefore prevent the environmental impact caused by improper disposal of this material. Recovering these valuable metals will also cater for the rising global demand of critical metals in alloy manufacturing industries. Hydrometallurgical process is one of the conventional methods that can be used to recover the metal values of this type of material containing either metal sulphides or metal oxides in its composition. This process of metal recovery can either be performed through a chemical extraction process or biological leaching (Banisadi et al. 2019). Earlier investigation has shown the effect of chemical leaching process parameters such as temperature, time, pressure, average particle size and agitation speed on nickel extraction from spent catalyst where about 94% of nickel was recovered in just 4 h (Banisadi et al. 2019; Khalid and Athraa 2017), which shows the extraction of Ni in very short retention time. Li et al. (2017) have applied mechano-chemical approach to transform the molybdenum sulphide and vanadium sulphide content of spent hydrodesulphurization (HDS) catalyst into the corresponding molybdenates and vanadates by co-grinding the catalyst with sodium carbonate and oxidants, which has led to achieving good recoveries of not only molybdenum and vanadium but also nickel and cobalt (Li et al. 2017; Banisadi et al. 2019). Though the conventional methods are less time consuming, they involve high operational cost, high energy inputs, use of harmful acid and alkali and toxic gases are emitted. Bioleaching is also important for the built environment. This process can not only be applied for conservation sciences via biological approaches but also causes some biodeterioration of stone materials (Yang et al. 2019; Liu et al. 2020; Gadd 2017). Bioleaching is a clean and green process associated with low process cost, higher efficiency for low-grade ores and environmental feasibility. Both heterotrophic and autotrophic microorganisms are being used for metal recovery from spent catalyst. Autotrophic microorganisms like chemolithotrophic acidophilic archaea can be considered over heterotrophs as they save sterilization cost by growing at low pH (1–2) at which no other microorganism can sustain (Srichandan et al. 2012). Bioleaching of spent petroleum catalyst by bacteria of genera Acidithiobacillus have been reported to have good leaching efficiencies with a maximum recovery of 99.8% Ni, 64.5% Mo, 90% V (Asghari et al. 2013; Beolchini et al. 2010). Researchers have also investigated the efficacy of bioleaching with Acidithiobacillus ferrooxidans on the mobility of critical metals by evaluating the different chemical fractions/forms of Al, Ni, Mo and V and the changes they undergo during bioleaching (Pathak et al. 2014, 2018). Assessments using iron and sulphur oxidizing mesophiles revealed no effect of particle size (45 μ—> 212 μ) on Ni leaching yield, depicting easy liberation of Ni from the matrix via grinding (Srichandan et al. 2013). The dissolution of Ni and V has been observed to be a fast process suggesting that the rate of reaction becomes slower after 1–2 days, thus a period of 7 days has been chosen by several researchers for an effective leaching (Kim et al. 2010; Mishra et al. 2008; Mishra et al. 2008; Pradhan et al. 2009, 2010a, b). Electrochemical bioleaching by coupling of mineral sulfide through galvanic interaction or as electrocatalyst with low energy consumption and occurring with high redox potential minerals (pyrite), carbonic material, or electrocatalytic ions (Ag) resulting in the dissolution of chalcopyrite. Another approach is electrolytic bioreactors with controlled electron transfer implying the regulation of the ratio of Fe2+ to Fe3+ ion with a redox potential leading to less passivation effects promoting microbial activity (Tanne and Schippers 2017; Liu et al. 2015). Redox mediator plays an important role in extracellular electron transfer in bioleaching environments where microbial electrocatalysis explains the mechanism of charge transport and electrochemical reactions occurring between microorganism-electrode interfaces resulting in the leaching of metals (Liu et al. 2018). Study conducted on copper recovery from chalcopyrite stated that the copper recovery can be improved by electrochemical bioleaching in control of redox potential between 400 and 425 mV as the precipitation of iron oxy-hydroxides on the surface of chalcopyrite acting as a diffusion barrier preventing copper dissolution from chalcopyrite can be improved due to electrochemical reduction of chalcopyrite (Ahmadi et al. 2010). Several studies on different parameters like pulp density, temperature, particle size, kinetics on bioleaching of spent catalyst using thermophilic archaea in a medium with no iron supplements have been reported with good leaching yields (Srichandan et al. 2012, 2014). Extremely thermophilic archaea surviving above 70 °C, which are acidophilic by nature are well known to mobilize base metals, precious metals and strategic metals from mineral ores (Straub et al. 2018). The feasibility of the bioleaching process with higher pulp density (50 g/L) of HDS catalyst using Acidithiobacillus thiooxidans FG01 has resulted in good recovery of Mo, Al and V (Ferreira et al. 2016). Reviewing the work done so far, it has been found that fewer studies have been conducted on bioleaching of different pulp densities of spent petroleum catalyst in an iron free medium using mesophilic microorganisms which were previously grown in 9 K medium supplemented with 4.5 g/L iron and 2 mM tetrathionate. Therefore, the objective of the present study is to investigate the bioleaching efficiencies of iron and sulphur oxidizing chemolithotrophs in an iron free medium using different pulp densities of spent petroleum refinery catalyst along with the insight on the nickel recovery.
Materials and methods
Petroleum refinery spent catalyst
The spent refinery catalyst utilized in the present study was collected from Indian Oil Corporation Limited, Mathura, Uttar Pradesh, India. The spent catalysts as received were 6 mm size and was further crushed on a roll crusher for size reduction below 1 mm followed by ball milling to reduce the size below 100 microns. Particle size distribution analysis of the ground spent catalyst showed 80% passing below 100 microns. To have an efficient leaching process, a particle size of below 100 μm was aimed at since the inbound metals in this size range are well-liberated from the material matrix and therefore exposed to the lixiviant attack. Ground samples were thoroughly mixed and divided by coning and quartering method to ensure homogeneity of the feed material for all the bioleaching experiments. The chemical composition of the spent petroleum catalyst was analyzed by an X-Ray Fluorescence analyzer (XRF).
Microbial consortium
The microbial culture or inoculum used in the bioleaching experiments were collected from the laboratory culture of Luleå University of Technology (Luleå, Sweden). The microbial culture was a mixed culture of chemolithotrophic acidophilic mesophile consisting of iron-oxidizing, sulphur oxidizing and both iron and sulphur oxidizing microorganisms as revealed from the Q-PCR analysis (Bioclear B.V., Netherlands). The microbial consortia comprised of dominating Acidithiobacillus ferrooxidans (Fe & S-oxidizers), Leptospirillum ferriphilum (Fe-oxidizer) followed by Acidithiobacillus caldus (S-oxidizer), and with approximately the same amount of Acidithiobacillus thiooxidans (S-oxidizer), Sulfobacillus sp. (Fe-oxidizer) and Ferroplasma sp. (Archaeal species, Fe-oxidizers) (Gahan et al. 2009, 2010; Srichandan et al. 2013). The mixed microbial culture used in the experiment was grown in 0 K medium [(NH4)2SO4, 3.0 g/L; KCl, 0.1 g/L; K2HPO4, 0.5 g/L; MgSO4. 7H2O, 0.5 g/L; Ca(NO3)2.4H2O, 0.01 g/L], supplemented with 22.4 g/L ferrous sulphate heptahydrate (FeSO4.7H2O) as an iron source and 0.605 g/L (2 mM) of potassium tetrathionate (K2S4O6) as a sulfur source and sub-cultured repeatedly prior to its use in the bioleaching experiment. The culture was grown in a 2 L baffled glass reactor, with a working volume of 1L (v/v). As the microbial culture was a mesophile, the temperature for their growth was maintained at 35 ± 2 °C using a hot plate placed under the bioreactor with a set temperature (Franzmann et al. 2005). The pH of the growth medium and culture was maintained at a value of 1.50 ± 0.05 by adding 2 M/5 M H2SO4 whenever required for providing an acidic environment for luxuriant growth of the microorganisms (Plumb et al. 2008). The microbial growth analysis was carried out by regular measurement of pH, Redox potential, Ferrous (Fe2+) ion and Ferric (Fe3+) ion concentrations, total iron concentration, sulphate concentration and viable cell count. Once all the parameters of growth analysis were stable, the microbial culture was full grown as there was no substrate available for further oxidation. The residual ferrous iron concentration was found to be zero or negligible, Redox potential reached 700 mV approximately. Repeated sub-culturing of the microorganisms was conducted to activate the substrate oxidizing potential and shortening the growth phase for a better activity in the bioleaching experiments.
Bioleaching experiments
Batch bioleaching of the spent catalyst was conducted in 250 mL Erlenmeyer flasks in an orbital shaking incubator at a temperature of 35 °C. The rotation speed of the bioleaching flasks was maintained at 180 rpm to ensure homogenous mixing of the bioleaching pulp. The working volume for all of the bioleaching experiments was 100 mL, which was composed of 90 mL of the growth medium and 10 mL of the full-grown microbial culture. The growth media used in all three experiments were both 0 K medium. The varying pulp density used in all the three bioleaching experiment was 5, 10 and 15% (w/v), and the size fractions of the feed material were below 100 microns. A control experiment was conducted without microbial culture keeping all other conditions were kept intact, but the dissolution of the metal values from the spent catalyst was found to be negligible. Triplicate experiments were conducted and the average value was considered for all the parameters studied. All the bioleaching experiments were pH controlled by adding either 2 M or 5 M H2SO4 whenever a pH level of 1.5 was required just to provide luxuriant growth condition as well as luxuriant growth of microbes. The redox potential of the bioleaching pulp was measured regularly to follow the growth profile of the microorganisms. The viable planktonic microbial cell (freely motile cells in the bioleaching solution) population was quantified at regular intervals to ensure the presence of viable cells in the bioleaching system. The planktonic cells provided the information of the active cells involved in the bioleaching solution to ensure the viability of the microbial population in the bioleaching system. The leaching study in all the batch bioleaching experiments with 5, 10, and 15% pulp density was carried out from the nickel concentration in the bioleaching solution during the course of the experiment. The bioleaching experiments continued until the pH and redox potential stabilized. After the completion of each experiment, the bioleaching pulp was filtered by vacuum filtration using Whatman filter paper 42 with a pore size of 2.5 μm and diameter of 4.25 cm. The filtered cake was washed with a measured volume of distilled water and acidified to pH 1.5 with H2SO4 to avoid the precipitation of metal ions from the solution into the residue. The total volume of leach liquor, including the wash water, was measured before the assay. The washed bioleaching residue was dried in a hot air oven at 50 °C for several days until no further change in weight was observed. The chemical composition of the dry bioleach residues were analyzed using XRF, whereas the mineralogy of the residues was characterized by XRD. The leaching yield of the bioleaching experiments with 5, 10, and 15% pulp density was calculated from the elemental analysis of the feed material and bioleach residues, taking feed weight and bioleach residue weight into consideration using the formula provided in Eqs. 1–3.
| 1 |
| 2 |
| 3 |
Mf = metal content in the feed; Mbr = metal content in bioleached residue; fw = feed weight; Brw = Bioleach residue weight; M% = Metal percentage; LY (%) = Leaching Yield percentage.
Analytical and Instrumentation techniques
The pH measurements of the bioleaching solution during the experiments were carried out by a pH meter (Model: Eutech PC 450). Calibration of the pH meter was done regularly with a three-point calibration using the standard buffers of pH 1.68, 4.0 and 7.0 and slope value ranged between 95 and 100. The oxidation–reduction potential (ORP) of the bioleaching solution was measured by an ORP meter having a platinum electrode with an Ag/AgCl reference electrode. The Fe (II) concentration in the bioleaching experiment was estimated by a titrimetric method using cerium sulphate with 1, 10-Phenanthroline as an indicator (Sundkvist et al. 2008). The Fe (Total) concentration of the bioleaching solution was measured by Atomic Absorption Spectrophotometer (AAS) (Thermo Scientific iCE 3000 SERIES), whereas the Fe3+ ion concentration of the bioleaching solution was calculated by subtracting the concentration of Fe2+ ion from the Fe (Total). The concentration of Ni in the bioleaching solution was measured by AAS. A turbidimetric method by visible spectrophotometric method (420 nm) was used to determine the sulphate ion concentration of the bioleaching solution as per the procedure described in American Public Health Association, 1975 (APHA) (Kolmert et al. 2000). The population dynamics study of the microorganisms was carried out by counting planktonic viable microbial cells by a bright-field microscope at 100X magnification on a Neubauer hemocytometer. The elemental composition of the feed and residues were carried out by XRF, whereas the mineralogy was determined by Powder XRD. Prior to XRD examination, the samples were pulverized to ensure homogeneity of the sample, and the diffraction patterns were measured at angles between 10° and 90°, the step size was 0.02 angle/sec. The crystalline phases were identified by using the joint committee for powder diffraction standards (JCPDS) file. The surface morphology along with the mineralogy of the feed and the bioleached residue was studied using SEM–EDX [Nova Nano FE-SEM 450 (FEI)].
Results and discussion
Characterization of spent petroleum catalyst
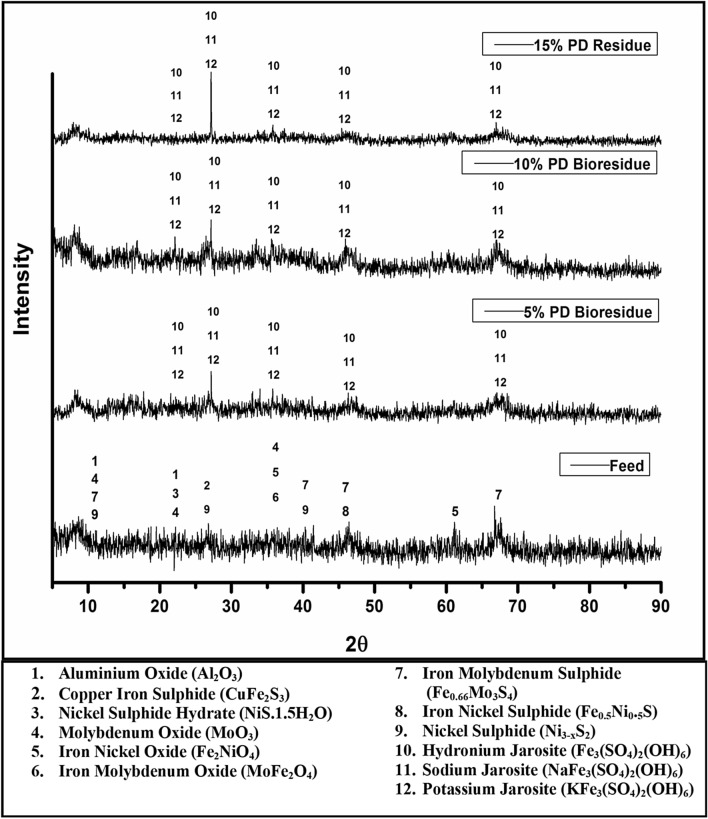
An X-Ray Fluorescence analyzer (XRF) was used to analyze the elemental composition of the ground petroleum refinery spent catalyst and the results are presented in Table 1. The analysis showed that aluminium (43.8%), molybdenum (32%), sulphur (11%) and nickel (5.16%) were the major elements, and phosphorus (3.59%) along with silica (2.25%) was in minor constituents while iron (0.98%), potassium (0.49%), calcium (0.37%), chromium (0.036%) and copper (0.02%) were present in trace level (Table 1). Figure 1 presents the X-Ray diffractometer (XRD) results of the mineralogical composition of the spent material. The sample was composed of the following mineral constituents—aluminium oxide (Al2O3), nickel sulphide hydrate (NiS.1.5H2O), molybdenum oxide (MoO3), iron-nickel oxide (Fe2NiO4), iron molybdenum oxide (MoFeO4), iron molybdenum sulphide (Fe0.66Mo3S4), iron-nickel sulphide (Fe0.5Ni0.5S), nickel sulphide (Ni3-xS2) and copper-iron sulphide (CuFe2S3) (Fig. 1). A Scanning Electron Microscopy coupled with Energy Dispersive X-Ray Spectroscopy (SEM–EDX) was performed to reveal the arrangements of different elements and their complexes in the feed material. The sample was mounted on a stub with the assistance of a carbon tape. To avoid charging of the sample surface during scanning it was gold coated in a sputter. The data of SEM mapping image and EDX profile is published and therefore not shown (Nagar et al. 2019). It clearly reveals that nickel (Ni) and molybdenum (Mo) are present as both sulphide and oxide phases, which were also confirmed by the XRD analysis of the sample (Fig. 1).
Table 1.
Elemental composition of the feed material Petroleum refinery spent catalyst
| Feed Material (Petroleum refinery spent catalyst) | % (w/w) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Mo | S | Ni | P | Si | Fe | K | Ca | Cr | Cu | |
| 43.8 | 32.0 | 11.0 | 5.16 | 3.59 | 2.25 | 0.98 | 0.49 | 0.37 | 0.04 | 0.02 | |
Fig. 1.
X-Ray Diffractogram of petroleum refinery spent catalyst and bioleach residues with 5, 10 and 15% pulp density (PD)
pH profile during the bioleaching experiment
The pH of all the experiments with 5, 10 and 15% pulp density increased initially from 1.5 to a range of 1.7–2.4 during the first 2 days of the experiments (Fig. 2). The possible reason for an increase in pH value may be due to the proton-consuming metal oxides in the spent petroleum catalyst (Eq. 4). As the pH increased, it was important to control the pH at 1.5 by the addition of 5 M H2SO4 to avoid precipiation of ferric iron, the primary oxidant in the bioleaching process.
| 4 |
where MeO stands for metal oxides present in the spent petroleum catalyst.
Fig. 2.
Change in pH and cumulative acid addition during bioleaching experiments (a 5% PD; b 10% PD; c 15% PD)
The two major oxidants during the bioleaching process are ferric ion and protons. It is noteworthy that no ferrous was supplemented along with media, the necessity of ferrous ion for biooxidation by the iron-oxidizing microorganisms was fulfilled by the iron present in the feed. Oxidized ferrous in the form of 0.9 g/L ferric ion present in 20% inoculum initializes the metal sulfide dissolution. The reduced iron from inoculum and the feed is reoxidized by the bacteria to ferric ion which further attacks on the metal sulfides (Eqs. 5 and 6). The oxidation–reduction cycle goes on until all the metal sulfide in the pulp is oxidized.
| 5 |
| 6 |
The ferrous ion oxidation is also a proton (H+) consuming process (Eq. 5). Therefore, apart from proton consumption by metal oxides, the increase in pH is a resultant of ferrous biooxidation as well. Acid soluble metal sulfides like nickel sulfide follow polysulphide pathway in the presence of iron and sulfur-oxidizing microorganisms (Eqs. 5–8). Moreover, the sulfur species () generated by the oxidation of metal sulfides are further oxidized to sulphate by the sulfur-oxidizing bacteria resulting in acid production which in turn helps in controlling the pH at 1.5 (Eqs. 7 and 8).
| 7 |
| 8 |
The above-mentioned statement can be justified by the fall in pH at the fourth day of the experiment to 1.5, 1.6, 1.5 in 5, 10, 15% pulp density, respectively, indicating the acclimatization of sulfur oxidizers to the stressed environment leading to acid compensation.
The highest amount of acid consumption (kg/ton of spent catalyst) was in 15% followed by 10% and then 5% (Fig. 2 and Table 3). Acid consumption in 15% pulp density experiment was higher than in 10 and 5% pulp density due to the higher feed concentration (more presence of acid consuming metal oxides). In contrast, the concentration of sulphate measured during the experiment was the lowest (Fig. 3). The sulphate concentration in the 0 h or starting point of the experiments were sulphate10.09, 14.29, 15.20 g/L at 5% P.D., 10% P.D. and 15% P.D. respectively, while the final sulphate concentration at the end of the experiment was 15.78, 29.11, 34.89 g/L at 5% P.D., 10% P.D. and 15% P.D., respectively (Fig. 3). Total sulphate concentration represents the sulphate from biological production as well as externally added 5 M H2SO4. The experimental data suggest that the less sulphate concentration in 5% pulp density may be due more jarosite precipitation in higher pulp densities leading to higher acid production as per Eq. 9 (Gahan et al. 2009).
| 9 |
Table 3.
Summary of the batch bioleaching of petroleum refinery spent catalyst with 5, 10 and 15% pulp density (P.D.) experimental results
| 5% P.D | 10% P.D | 15% P.D | |
|---|---|---|---|
| Feed spent catalyst weight, g | 5.0 | 10.0 | 15.0 |
| Bioleach residue, g | 4.21 | 5.96 | 11.0 |
| Weight loss, % | 15.8 | 40.4 | 26.7 |
| Acid consumption, kg Conc. H2SO4 per ton spent catalyst | 252.6 | 287.7 | 348.2 |
Fig. 3.

Sulphate concentration profile of the bioleaching experiments with 5, 10 and 15% PD
[X+ = K+, Na+, H3O+, ……..]
According to the above reaction, jarosite formation is a proton-producing reaction. The highest intensity peak for jarosite in XRD graph (Fig. 1) was observed in PD (15%) followed by PD (10%). The requirement of acid in these two bioleaching experiments (PD 15% and 10%) during the dissolution of metal oxides and acid-consuming gangue minerals was compensated by the proton production during jarosite formation. Whereas, the 5% pulp density experiment acid demand was compensated by the addition of 5 M H2SO4. Therefore, 5% PD consumed the highest amount of acid. Moreover, instead of higher jarosite precipitation, 15% PD experiment consumed more acid than 10% because of the higher pulp density.
Changes in redox potential
The reduction and oxidation of ferric/ferrous couple during the course of bioleaching experiments govern the redox potential. There are several other redox couples in the bioleaching system but their impact is small. The redox values on the day of feed addition were ~ 400 mV for 5 and 10% pulp density and ~ 340 mV for 15% pulp density experiments (Fig. 4). There is an initial steep decrease in the redox potential after the first day of feed addition suggesting that the oxidation of metal occurs by the collective action of ferric ions and protons. The redox pattern in 5 and 15% pulp density experiments implies a less role of ferric/ferrous coupling in the metal dissolution process during which the redox values fluctuated between the range ~ 300–340 mV signifying that the dissolution is mainly governed by protons generated by the activity of sulphur oxidizers and 5 M H2SO4 with a lesser role of biooxidation of ferrous. In contrast, the nickel dissolution profile of 10% pulp density experiment supports the role of microbially oxidized ferric ion in nickel leaching, where maximum nickel is leached up to the 5th day of the experiment at which pH, redox and ferrous profile also suggest the occurrence of biooxidation (Figs. 2, 4, 5 and 7). Thereafter, a small increase in redox and a decrease in ferrous ion concentration were noted on the 10th day in 5% pulp density; a minute increase in redox with an increase in pH was observed on the 11th and 14th day in 10% pulp density; a slight increase in ferrous ion concentration with a decrease in pH and redox on 10th day and a small increase in redox and pH with decrease in ferrous ion concentration on 13th day suggest as low microbial ferrous ion oxidation rate. Another reason for less participation of ferric ion in the bioleaching process may be due to iron precipitation which is evident from the XRD results (Fig. 1) of the bioleached residues. XRD reveals that potassium and hydronium jarosite was prominent in all three bioleach residues. A further decrease in the ferrous ion concentration after 10th day in 5% pulp density and 15% pulp density, and 8th day in 10% pulp density experiments strengthen the observation of iron precipitation as consistent low values of redox potential also deny any major ferrous/ferric redox phenomena. The aforesaid discussion follows the role of redox mediator playing the role in extracellular electron transfer (EET) which drives a series of microbial electro-catalytic reactions. This also explains the way charge transport and electrochemical reactions occur in a biooxidation process at the microorganism-electrode interface. There occurs a wide range of redox reactions via mediators explaining functional mechanisms in EET carrying out a series of microbial electro-catalytic reactions (Liu et al. 2018).
Fig. 4.

Redox Potential profiles of the bioleaching experiments with 5, 10 and 15% PD
Fig. 5.

Fe (II) concentration profile of the bioleaching experiments with 5, 10 and 15% PD
Fig. 7.

Ni concentration (g/L) in bioleaching solution as a function of time
Changes in viable planktonic cell count
The viable cell count was done to monitor the cell population dynamics which positively correlates with microbial activity during the bioleaching experiments. The planktonic cells were comparatively assessed for the three increasing pulp densities. It is important to note that the higher pulp densities may not only result in the formation of thick slurry leading to inefficient gas transfer necessary for the bioleaching and microbial growth (Rawlings et al. 2003). All the three pulp densities i.e., 5, 10, and 15% showed a remarkably similar microbial cell count which remained between 106 and 107 cells/mL (Fig. 6). The results ensure that the microbial growth remained good and unaffected at higher pulp densities. The initial decrease in the cell counts from 8.35 × 108 cells/mL (from 5 × diluted inoculums) to 106 cells/mL in all pulp densities was due to stress on the microbial population due to the sudden addition of feed. This sudden fall in cell count has been reported earlier also (Gahan et al. 2009), but soon the bacterial population seem to get acclimatized to the bioleaching conditions and contributed to metal dissolution in all the experiments.
Fig. 6.

Viable planktonic cell count profile of the bioleaching experiments with 5, 10 and 15% PD
Nickel bioleaching study
The bioleached liquor samples withdrawn at various intervals of the experiment were analyzed by AAS for nickel concentration profile with respect to the time (Fig. 7). In the experiment with 5% pulp density, nickel leached very fast and was due to the ferric ion and protons present in the inoculum. In the experiment with 15% pulp density showed relatively higher acid consumption during the initial days, resulting in Ni dissolution due to proton leaching (Fig. 7). On the contrary, the experiment with 10% pulp density nickel dissolution increased slowly with time due to biogenic ferric ions and proton-mediated leaching. The Ni content in the bioleaching slurry decreased with time for all three experiments with varying pulp densities, only after maximal leaching of nickel (Fig. 7). Moreover, the variation in Ni concentration trend during the bioleaching experiments irrespective of varying pulp density was due to the difference in jarosite precicpitation during the experiments. The jarosite formation was in the order 15% PD > 10% PD > 5% PD. Jarosite formation is a normal phenomenon in batch bioleaching, which influences the bioprocess dynamics as well as Ni leaching. The results of Ni precipitation show a strong aversion towards iron precipitation. The decrease in Ni concentration after 100 h of the experiments may be due to co-precipitation of Ni and Cu together with jarosite as reported by Yang et al. (2011). In addition, it was also observed that the total soluble Cu and Ni ions slowly decreased together with decreasing total iron concentrations, which implicated the co-precipitation of ferric ion with Cu and Ni as jarosite. However, few reports suggest propositions on Cu and Ni ions incorporation into the active sites of the jarosite structure or encapsulation within jarosite aggregate or absorption into the jarosite surface (Acero et al. 2006; Gräfe et al. 2008; van Hille et al. 2010; Welch et al. 2007, 2008; Yang et al. 2011). Studies on the inclusion of metal ions with jarosites suggest the order of Fe3+ > Cu2+ > Zn2+ > Co2+ > Ni2+ during jarosite precipitation (Dutrizac and Chenn 2004). The jarosite formers such as NH4+, K+ and Na+ ion present in the 0 K growth medium used in the bioleaching experiment might have catalyzed the Ni jarosite precipitation.
Bioleaching yields with different pulp densities
The leaching yield of metals was calculated based on metal concentration analysis in the feed and the bioleached residues. The percentage of metal recovered during the bioleaching tests was calculated and presented in Figs. 8 and 9 and Table 2. The results indicate that, at 5, 10 and 15% pulp density, the leaching yields for nickel were 91.96, 93.91 and 91.09%; for copper were 20.17, 73.29 and 58.49%; for molybdenum were 87.61, 86.72 and 47.52%, respectively (Fig. 9). In the experiment with 5% pulp density, the aluminum and chromium were concentrated by 0.73 and 60%, respectively, whereas in 10 and 15% pulp density experiments their recoveries were 24.8, 100%, and 21.5 and 27.67%, respectively. The leaching behavior of chromium in the three bioleaching systems was found to be similar to Ni and Cu. Similar result with chromium (Cr) has also been presented by Baron et al. (1996) where solids incorporating Fe3+ and Cr6+ have been reported to form under acidic conditions (Baron et al. 1996; Lee and Hering 2005). Other elements such as P, Ca, Si and Ti remain concentrated in all the bioleach residues (Fig. 8). As observed, Ni concentration in the bioleaching solution bioleach decreased with time along with iron in the solution strongly suggests co-precipitation of Ni, Cr and Cu along with the jarosite (as discussed in Sect. 3.5). The XRD and SEM–EDS analyses also reveal that jarosites were the major mineralogical phases present in the bioleach residues which were caused by ferric precipitation (Figs. 1 and 10). The XRF results also indicate that there were an increased amount of jarosite formers like K+, Na+, H3O+, etc. in the bioleached residues (Fig. 1 and Table 1). Despite of precipitation, Ni was absent in the XRD/SEM–EDS analysis of the bioleached residues due to their detection limit constraints. However, the residue analysis by XRF clearly showed the presence of Ni and if the Ni content based on the residue weight is calculated, the presence of 7–8% of residual Ni in the residue can be still noted which is further clearly indicated from the leaching yield (%) (Fig. 9). The weight loss percentage of bioleach residues is also in agreement with the leaching results where the highest weight loss was observed in 10% pulp density experiment (due to the highest metal leaching) losing most of its weight (40.4%) followed by 15% and 5% pulp densities (Table 3). The differences in weight loss might be due to incomplete oxidation as well as the relatively dissimilar dissolution of several other mineralogical phases present in the spent petroleum catalyst fed at variable pulp densities.
Fig. 8.
Bar graph comparing the mass (g) of elements (a Major, Minor & b Trace) in feed (spent catalyst) and bioresidues. A(a)—Comparing mass of major and minor elements in 5 g feed and bioleached residue. A(b)—Comparing mass of trace elements in 5 g feed and bioleached residue. B(a)—Comparing mass of major and minor elements in 10 g feed and bioleached residue. B(b)—Comparing mass of trace elements in 10 g feed and bioleached residue. C(a)—Comparing mass of major and minor elements in 15 g feed and bioleached residue. C(b)—Comparing mass of trace elements in 15 g feed and bioleached residue
Fig. 9.

Leaching yield % of metals in all the bioleaching experiments with 5, 10 and 15% PD calculated on the basis of residue analysis
Table 2.
Elemental composition of the bioleaching residue from petroleum refinery spent catalyst with 5, 10 and 15% pulp density (P.D.)
| Batch Bioleach residue | % (w/w) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Mo | S | Ni | P | Si | Fe | K | Ca | Cr | Cu | |
| 5% P.D | 52.4 | 4.71 | 13.1 | 0.493 | 4.99 | 18.1 | 1.88 | 2.46 | 0.653 | 0.0689 | 0.0201 |
| 10% P.D | 55.3 | 7.13 | 11.5 | 0.527 | 4.64 | 15 | 2.09 | 2.16 | 0.564 | N.A.* | 0.0095 |
| 15% P.D | 46.9 | 22.9 | 10.4 | 0.627 | 3.27 | 11.3 | 1.33 | 1.94 | 0.566 | 0.0357 | 0.012 |
*N.A. not analyzed
Fig. 10.
EDX analysis of a spent petroleum catalyst, b 5% PD bioleach residue, c 10% PD bioleach residue and d 15% PD bioleach residue
Conclusions
The effect of varying pulp density (5, 10, and 15%) of spent petroleum catalyst on the bioleaching efficiency was studied based on process feasibility, leaching yields of metal values. The rapid nickel dissolution suggests successful recovery of nickel with much lesser time compared to other metals with 90% Ni recovery in all three experiments with varying pulp density. The dissolution of Cu, Mo, and Al varied with varying pulp density. The pH, redox, ferrous ion, viable cell count and nickel concentration profiles supported the metal recovery trend and acid consumption profile. Influence of jarosite precipitation during bioleaching of Ni was clearly observed during the study. The bioleach residues were detected with jarosite precipitates with metal ions lke Ni, Cu and Cr which co-precipitated along with the jarosite. It can be concluded that 5% and 10% pulp density of spent catalyst was found to be the most suitable pulp density. It is important to mention here that a further scale-up of all the experiments at higher pulp densities would be promising to improve the operational parameters of the bioleaching process.
Acknowledgements
The authors are thankful to the Department of Science and Technology, Govt. India for funding the research from DST-SERB for Young Scientist YSS/2014/000895 and the DST-Inspire Fellowship for the work. UGC and CURAJ for the fellowship are thankfully acknowledged. Authors like to thank Indian Oil Corporation Limited (IOCL) Refinery, Mathura, Uttar Pradesh, India for providing the spent catalyst samples for the research work. Authors are grateful to Dr. Ranjan Kumar Dwari, Mineral Processing Department, CSIR-IMMT for his coordination and support for the work. Authors would like to thanks Dr. Prakash Chandra Sahoo, IOCL, Faridabad for his coordination and support for the work. Analytical work support from MRC, MNIT, Jaipur, Rajasthan, India is also acknowledged for SEM studies work. Authors would finally thank Department of Physics, CURAJ for the support for XRD analysis of the samples.
Authors’ contribution
NN—carried out the work in the laboratory and preparation of the manuscript. HG—partially carried out the work in the laboratory and preparation of the manuscript. NS—partially carried out the work in the laboratory and preparation of the manuscript. SAA—Preparation of the manuscript and language correction and Scientific inputs. CSG—conceived the idea, planning of the work, preparation of the manuscript and funding of the work from his own project.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Neha Nagar, Email: nagar.neha15@gmail.com.
Himanshi Garg, Email: himanshiagg.11@gmail.com.
Neha Sharma, Email: nehasharma96500@gmail.com.
Samuel Ayowole Awe, Email: samawe2003@gmail.com.
Chandra Sekhar Gahan, Email: gahancsbiometal@gmail.com.
References
- Acero P, Ayora C, Torrentó C, Nieto J-M. The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim Cosmochim Acta. 2006;70:4130–4139. [Google Scholar]
- Ahmadi A, Schaffie M, Manafi Z, Ranjbar M. Electrochemical bioleaching of high grade chalcopyrite flotation concentrates in a stirred bioreactor. Hydrometallurgy. 2010;104(1):99–105. [Google Scholar]
- Akcil A, Vegliò F, Ferella F, Okudan MD, Tuncuk A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015;45:420–433. doi: 10.1016/j.wasman.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Asghari I, Mousavi SM, Amiri F, Tavassoli S. Bioleaching of spent refinery catalysts: a review. J Ind Eng Chem. 2013;19:1069–1081. [Google Scholar]
- Asghari I, Mousavi SM. Effects of key parameters in recycling of metals from petroleum refinery waste catalysts in bioleaching process. Rev Environ Sci Bio. 2014;13:139–161. [Google Scholar]
- Baniasadi M, Farzane V, Bahaloo-Horeh N, Mousavi SM, Farnaud S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: a review. J Ind Eng Chem. 2019;76:75–90. [Google Scholar]
- Baron D, Palmer CD, Stanley JT. Identification of two iron—chromate precipitates in a Cr (VI)-contaminated soil. Environ Sci Technol. 1996;30:964–968. [Google Scholar]
- Beolchini F, Fonti V, Ferella F, Vegliò F. Metal recovery from spent refinery catalysts by means of biotechnological strategies. J Hazard Mater. 2010;178:529–534. doi: 10.1016/j.jhazmat.2010.01.114. [DOI] [PubMed] [Google Scholar]
- Dufresne P. Hydroprocessing catalysts regeneration and recycling. Appl Catal A Gen. 2007;322:67–75. [Google Scholar]
- Dutrizac JE, Chen TT. Factors affecting the incorporation of Cobalt and Nickel in jarosite-type compounds. Can Metall Q. 2004;43:305–319. [Google Scholar]
- Ferreira PF, Sérvulo EFC, Ferreira DM, Oliveira FJS. Assessment of metal recovery from raw spent hydrodesulfurization catalyst through bioleaching and chemical leaching. Brazilian J Pet Gas. 2016;9:137. [Google Scholar]
- Franzmann PD, Haddad CM, Hawkes RB, Robertson WJ, Plumb JJ. Effects of temperature on the rates of iron and sulfur oxidation by selected bioleaching Bacteria and Archaea: application of the Ratkowsky equation. Miner Eng. 2005;18:1304–1314. [Google Scholar]
- Gadd GM. Geomicrobiology of the built environment. Nat Microbiol. 2017;2:16275. doi: 10.1038/nmicrobiol.2016.275. [DOI] [PubMed] [Google Scholar]
- Gahan CS, Sundkvist J, Sandström Å. Use of mesalime and electric arc furnace (EAF) dust as neutralising agents in biooxidation and their effects on gold recovery in subsequent cyanidation. Miner Eng. 2010;23(9):731–738. [Google Scholar]
- Gahan CS, Sundkvist J-E, Sandström Å. A study on the toxic effects of chloride on the biooxidation efficiency of pyrite. J Hazard Mater. 2009;172:1273–1281. doi: 10.1016/j.jhazmat.2009.07.133. [DOI] [PubMed] [Google Scholar]
- Gräfe M, Beattie DA, Smith E, Skinner WM, Singh B. Copper and arsenate co-sorption at the mineral–water interfaces of goethite and jarosite. J Colloid Interface Sci. 2008;322:399–413. doi: 10.1016/j.jcis.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Khalid M, Athraa B. Experimental study on factors affecting the recovery of nickel from spent catalyst. J Powder Met Min. 2017;6:1. [Google Scholar]
- Kim D-J, Pradhan D, Ahn J-G, Lee S-W. Enhancement of metals dissolution from spent refinery catalysts using adapted bacteria culture—effects of pH and Fe (II) Hydrometallurgy. 2010;103:136–143. [Google Scholar]
- Kolmert Å, Wikström P, Hallberg KB. A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J Microbiol Methods. 2000;41:179–184. doi: 10.1016/s0167-7012(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Lee G, Hering JG. Oxidative dissolution of chromium (III) hydroxide at pH 9, 3, and 2 with product inhibition at pH 2. Environ Sci Technol. 2005;39:4921–4928. doi: 10.1021/es048073w. [DOI] [PubMed] [Google Scholar]
- Li Z, Chen M, Zhang Q, Liu X, Saito F. Mechanochemical processing of molybdenum and vanadium sulfides for metal recovery from spent catalysts wastes. Waste Manag. 2017;60:734–738. doi: 10.1016/j.wasman.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Liu W, Yang HY, Song Y, Tong LL. Catalytic effects of activated carbon and surfactants on bioleaching of cobalt ore. Hydrometallurgy. 2015;152:69–75. [Google Scholar]
- Liu X, Koestler RJ, Warscheid T, Katayama Y, Gu JD. Microbial deterioration and sustainable conservation of stone monuments and buildings. Nat Sustain. 2020;3:991–1004. [Google Scholar]
- Liu X, Shi L, Gu JD. Microbial electrocatalysis: redox mediators responsible for extracellular electron transfer. Biotechnol Adv. 2018;36(7):1815–1827. doi: 10.1016/j.biotechadv.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Marafi M, Stanislaus A. Spent catalyst waste management: a review: part I—developments in hydroprocessing catalyst waste reduction and use. Resour Conserv Recycl. 2008;52:859–873. [Google Scholar]
- Mishra D, Kim DJ, Ralph DE, Ahn JG, Rhee YH. Bioleaching of spent hydro-processing catalyst using acidophilic bacteria and its kinetics aspect. J Hazard Mater. 2008;152:1082–1091. doi: 10.1016/j.jhazmat.2007.07.083. [DOI] [PubMed] [Google Scholar]
- Nagar N, Garg H, Gahan CS. Integrated Bio-Pyro-Hydro-Metallurgical approach to recover metal values from petroleum refinery spent catalyst. Biocatal Agric Biotechnol. 2019;20:101252. [Google Scholar]
- Pathak A, Healy MG, Morrison L. Changes in the fractionation profile of Al, Ni, and Mo during bioleaching of spent hydroprocessing catalysts with Acidithiobacillus ferrooxidans. J Environ Sci Health Part A. 2018;53:1006–1014. doi: 10.1080/10934529.2018.1471033. [DOI] [PubMed] [Google Scholar]
- Pathak A, Srichandan H, Kim D-J. Fractionation behavior of metals (Al, Ni, V, and Mo) during bioleaching and chemical leaching of spent petroleum refinery catalyst. Water Air Soil Pollut. 2014;225:1893. [Google Scholar]
- Plumb JJ, Muddle R, Franzmann PD. Effect of pH on rates of iron and sulfur oxidation by bioleaching organisms. Miner Eng. 2008;21:76–82. [Google Scholar]
- Pradhan D, Kim D-J, Ahn J-G, Chaudhury GR, Lee S-W. Kinetics and statistical behavior of metals dissolution from spent petroleum catalyst using acidophilic iron oxidizing bacteria. J Ind Eng Chem. 2010;16:866–871. [Google Scholar]
- Pradhan D, Mishra D, Kim DJ, Ahn JG, Chaudhury GR, Lee SW. Bioleaching kinetics and multivariate analysis of spent petroleum catalyst dissolution using two acidophiles. J Hazard Mater. 2010;175:267–273. doi: 10.1016/j.jhazmat.2009.09.159. [DOI] [PubMed] [Google Scholar]
- Pradhan D, Mishra D, Kim DJ, Chaudhury GR, Lee SW. Dissolution kinetics of spent petroleum catalyst using two different acidophiles. Hydrometallurgy. 2009;99:157–162. [Google Scholar]
- Rawlings DE, Dew D, du Plessis C. Biomineralization of metal-containing ores and concentrates. Trends Biotechnol. 2003;21:38–44. doi: 10.1016/s0167-7799(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Silvy RP. Future trends in the refining catalyst market. Appl Catal A Gen. 2004;261:247–252. [Google Scholar]
- Srichandan H, Gahan CS, Kim D-J, Lee S-W. Bioleaching of spent catalyst using moderate thermophiles with different pulp densities and varying size fractions without Fe supplemented growth medium. Int J Chem Biol Eng. 2012;6:22–28. [Google Scholar]
- Srichandan H, Kim D-J, Gahan CS, Singh S, Lee S-W. Bench-scale batch bioleaching of spent petroleum catalyst using mesophilic iron and sulfur oxidizing acidophiles. Korean J Chem Eng. 2013;30:1076–1082. [Google Scholar]
- Srichandan H, Singh S, Pathak A, Kim D-J, Lee S-W, Heyes G. Bioleaching of metals from spent refinery petroleum catalyst using moderately thermophilic bacteria: effect of particle size. J Environ Sci Health Part A. 2014;49:807–818. doi: 10.1080/10934529.2014.882211. [DOI] [PubMed] [Google Scholar]
- Straub CT, Counts JA, Nguyen DMN, Wu CH, Zeldes BM, Crosby JR, Conway JM, Otten JK, Lipscomb GL, Schut GJ, Adams MWW, Kelly RM. Biotechnology of extremely thermophilic archaea. FEMS Microbiol Rev. 2018;42(5):543–578. doi: 10.1093/femsre/fuy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist J, Gahan CS, Sandström Å. Modeling of ferrous iron oxidation by a Leptospirillum ferrooxidans-dominated chemostat culture. Biotechnol Bioeng. 2008;99:378–389. doi: 10.1002/bit.21563. [DOI] [PubMed] [Google Scholar]
- Tanne CK, Schippers A (2017) Electrochemical applications in metal bioleaching. In: Harnisch F, Holtmann D (eds) Bioelectrosynthesis. Advances in Biochemical Engineering/Biotechnology, 167. p 327–359. [DOI] [PubMed]
- van Hille RP, van Zyl AW, Spurr NRL, Harrison STL. Investigating heap bioleaching: effect of feed iron concentration on bioleaching performance. Miner Eng. 2010;23:518–525. [Google Scholar]
- Welch SA, Christy AG, Kirste D, Beavis SG, Beavis F. Jarosite dissolution I—trace cation flux in acid sulfate soils. Chem Geol. 2007;245:183–197. [Google Scholar]
- Welch SA, Kirste D, Christy AG, Beavis FR, Beavis SG. Jarosite dissolution II—reaction kinetics, stoichiometry and acid flux. Chem Geol. 2008;254:73–86. [Google Scholar]
- Yang C, Qin W, Lai S, Wang J, Zhang Y, Jiao F, Ren L, Zhuang T, Chang Z. Bioleaching of a low grade nickel–copper–cobalt sulfide ore. Hydrometallurgy. 2011;106:32–37. [Google Scholar]
- Yang Y, Ferrier J, Csetenyi L, Gadd GM. Direct and indirect bioleaching of cobalt from low grade laterite and pyritic ores by Aspergillus niger. Geomicrobiol J. 2019;36(10):940–949. [Google Scholar]