Abstract
In the present scenario of climate change with constantly increasing CO2 concentration, there is a risk of altered crop performance in terms of growth, yield, grain nutritional value and seed quality. Therefore, an experiment was conducted in open top chamber (OTCs) during 2017–18 and 2018–19 to assess the effect of elevated atmospheric carbondioxide (e[CO2]) (600 ppm) on chickpea (cv. JG 14) crop growth, biomass accumulation, physiological function, seed yield and its quality in terms of germination and vigour. The e[CO2] treatment increased the plant height, leaf and stem biomass over ambient CO2 (a[CO2]) treatment. The e[CO2] increased seed yield by 11–18% which was attributed to an increase in the number of pods (6–10%) and seeds plant−1 (8–9%) over a[CO2]. However, e[CO2] reduced the seed protein (7%), total phenol (13%) and thiobarbituric acid reactive substances (12%) and increased the starch (21%) and water uptake rate as compared to seeds harvested from a[CO2] environment. Exposing chickpea plant to e[CO2] treatment had no impact on germination and vigour of the harvested seeds. Also, the physical attributes, total soluble sugar and antioxidant enzymes activities of harvested seeds were comparable in a[CO2] and e[CO2] treatment. Hence, the experimental findings depict that e[CO2] upto 600 ppm could add to the growth and productivity of chickpea in a sub-tropical climate with an implication on its nutritional quality of the produce.
Keywords: Biomass, Chickpea, Elevated carbondioxide, Germination, Open top chambers, Protein, Vigour, Yield
Introduction
World population is estimated to reach about 9 billion by 2050 and to feed them agricultural production needs to be increased further (Godfray et al. 2010). Increasing agricultural production in resource depleted condition is already challenging and effects of changing climatic conditions are making it even more difficult. Atmospheric CO2 concentrations ([CO2]) have steadily increased from 280 ppm to currently exceeding 400 ppm due to rapidly growing industrialization and human activities (Canadell et al. 2007). Climate models predict an increase in atmospheric [CO2] to reach 700 ppm by the year 2100 (IPCC 2014). Increase in the [CO2] and an associated increase in temperature is expected to cause significant impact on agricultural production and food security (Streck 2005). Most of the studies on effect of e[CO2], have focused on plant growth and biomass, physiology, yield and grain quality (nutrient content, milling properties etc.) (Saha et al. 2013, 2015a; Rai et al. 2016; Singh et al. 2017). Elevated [CO2] resulted in an increase in photosynthesis leading to improved plant growth, biomass and yield (Rai et al. 2016; Ainsworth and Long 2005). Based on the available literature, the effect of e[CO2] on seed quality is found to be variable (Hampton et al. 2013). Germination of seeds harvested from plant exposed to e[CO2] either increased (Ziska and Bunce 1993; Edwards et al. 2001), decreased (Saha et al. 2015b; Lamichaney et al. 2019) or remain unchanged (Thomas et al. 2009; Thinh et al. 2017). Due to these effects, understanding of plant responses to e[CO2] have become increasingly important.
Chickpea (Cicer arietinum L.) is the most important food legume of Indian sub-continent contributing 68% share in the global chickpea production (FAO 2014). The comprehensive report on the effect of exposure of chickpea plant to e[CO2] on its growth, physiology, yield is limited especially on the quality of the seed harvested with respect to its planting value. Therefore, an experiment was conducted with the objectives (1) to study the effect of e[CO2] on crop growth, biomass accumulation and photosynthesis at different crop stages; (2) to understand the effect of e[CO2] on yield and yield attributes; and (3) to study the effect of growing mother plant at e[CO2] on quality of the seed produced.
Materials and methods
Experiment site
The experiment was conducted during the winter season (November–March) of 2017–18 and 2018–19 in open top chambers (OTCs) facility available at the experimental farm of ICAR-Indian Institute of Pulses Research, Kanpur, Uttar Pradesh, India (26° 27′ N latitude and 80° 14′ E longitude and approximately 152 m above mean sea level). The experimental soil is sandy loam in texture and belongs to the order Fluvisol. The climate of the study site was sub-tropical humid with an average rainfall of 720 mm. The site received 8.2 mm and 27.8 mm rainfall during crop growth period of 2017–18 and 2018–19, respectively. The average maximum temperature, minimum temperature, relative humidity and sunshine hours during the crop season were 28.74 °C, 16.64 °C, 84.36%, 7.31 h for the year 2017–18 and the corresponding values for the year 2018–19 were and 29.77 °C, 13.81 °C, 72.31% and 5.77 h, respectively.
Crop management
The plot inside the OTCs was manually tilled and properly leveled before sowing. Chickpea (cv. JG 14) was sown during the first fortnight of November in rows separated by 30 cm. About 15 days after emergence, plant populations were maintained at approximately one seedling at every 10 cm, maintaining about 30–35 plants/m2. The recommended fertilizer rate of nitrogen, phosphorus and potassium at 20, 40 and 60 kg ha−1 was applied as basal dose at the time of plot preparation. The crop was irrigated twice at 50–55 and 80–85 days after sowing (DAS). Two hand weeding were performed at 25 and 45 DAS to keep the plots weed free. Need based plant protection measures were taken to raise a healthy crop.
Treatment detail
Two different CO2 concentrations [ambient (379–423 ppm) and e[CO2] (566–630 ppm)] were used as treatment to evaluate the effect of e[CO2] on plant growth, physiology, yield and seed quality. The aluminium framed square shaped open top chambers (4 m × 4 m × 4 m) made up of polycarbonate sheets having minimum 80% light transmittance were installed in the field. The top end of the OTCs was kept open to maintain the natural condition as far as possible. To reduce the dilution of CO2, at a height of 3.5 m a frustum of 0.5 m was provided. Inside the OTC, the desired level of [CO2] was maintained from 9:00 am to 5:00 pm throughout the crop growth period i.e. from emergence to harvest. Carbondioxide with purity of 99.7% supplied by M/S Murari Gas, Kanpur, was released from a commercial cylinder mounted with a dura series regulator of ESAB, India (CM/L-5006845), via solenoid valve and PV tubes to an air compressor, where it is mixed with air and is supplied to OTC. Carbondioxide sensor (Non Dispersive Infrared, Topac, USA) placed at the centre of the OTC senses the level of CO2 and maintains at desired level with the help of computer–based control system (SCADA software, Genesis Technology, India). The [CO2] inside the OTCs was recorded by Data loggers (TC–800) at every 1 min interval and the data was stored in WINLOG program. During the crop season, the a[CO2] varied between 385 and 422 ppm in 2017–18 and from 379 to 429.7 ppm in 2018–19. Likewise, e[CO2] varied between 575.4 and 625.3 ppm and 565.6–629.9 ppm in 2017–18 and 2018–19, respectively (Fig. 1). Each [CO2] treatment was replicated twice.
Fig. 1.
Variation in CO2 concentration and air temperature inside ambient and elevated Open Top Chambers during 2017–18 and 2018–19
Plant growth measurements
At branching, flowering and podding stages, three plants were randomly sampled from each OTC for measuring plant height and root length. The plants were carefully sampled to remove the entire root and cleaned with gentle running water to remove soil. The root, stem, leaves and pod (podding stage) were separated and dried in an oven at 65 ± 2 °C for 3 days for dry matter measurements.
Measurements of gaseous exchange
Light saturated (1500 µmole m−2 s−1) rate of photosynthesis, stomatal conductance, transpiration rate, internal CO2 concentration were measured at 20 °C using infra-red gas analyzers (IRGA) portable photosynthetic system (Model LI-COR-6400, USA) on the third leaf from top on five randomly selected plants from each OTC at flowering (~ 60 days after emergence). The ratio of photosynthetic rate to stomatal conductance and photosynthetic rate to transpiration rate was used to calculate intrinsic and instantaneous water use efficiency. Measurements were taken in between 10.00 am to 12.00 noon, in the plants pre-exposed to naturally lit OTC.
Biochemical assay of leaves
The biochemical assay of leaves was done at 30, 60 and 90 days after emergence. The chickpea leaves (third leaf from the top of the plant) collected from a[CO2] and e[CO2] environments were weighed and subjected to biochemical analysis. Both biological as well as technical replicates were taken for analysis.
Antioxidant enzymes activity
For the assay of antioxidant enzymes, 0.1 g of chickpea leaves were homogenized in 50 mM sodium phosphate buffer (pH 7.0) and centrifuged at 16,000 rpm for 30 min. The supernatant thus obtained was used for enzyme assays of super oxide dismutase (SOD), peroxidase (POX) and glutathione reductase (GR). The SOD activity was quantified based on its ability to inhibit the photochemical reduction of nitro-blue tetrazolium following the protocol of Beauchamp and Fridovich (1971) and expressed as U min−1 mg−1 of protein. POX activity was determined by the rate of formation of oxidized guaiacol following the protocol of Castillo et al. (1984). The activity of GR was measured as per the methodology given by Smith et al (1988).
Total phenols
The total phenols were estimated using the method given by Xu and Chang (2007). Briefly, 0.1 g of chickpea leaves was extracted twice with 70% ethanol for 3 h. The supernatant obtained after centrifugation was collected in amber bottles and used for estimation of phenols. Under the alkaline conditions provided by sodium carbonate the phenols reduce the Folin–Ciocalteau reagent (FCR) and the absorbance was measured at 765 nm.
Malondialdehyde content
For the estimation of malondialdehyde content, 1 g of chickpea leaves were homogenized in 10 ml of distilled water and centrifuged at 16,000 rpm for 30 min. The resulted supernatant was used for subsequent estimation of malondialdehyde content as per the method given by Ohkawa et al. (1979).
Total soluble sugar (TSS) and starch
To estimate the TSS, 0.1 g chickpea leaves were homogenized in 5 ml of 80% ethanol and supernatant was collected after centrifugation. The extraction was repeated and supernatants were pooled to be evaporated on a water bath at 80 °C. Then, 10 ml of water was added to dissolve the sugars and 1 ml aliquot from each replication was taken for subsequent analysis using phenol–sulfuric acid method (Dubois et al. 1956). Total starch was estimated using the Anthrone method (Loewus 1952). A conversion factor of 0.9 was used to convert glucose to starch.
Seed yield and its components
At maturity, ten plants were randomly sampled from each OTC to measure the number of pods plant−1 and number of seeds plant−1. The yield of five inner rows of 2 m length was considered for estimation of seed yield and converted into kg ha−1. The seeds from each OTC were bulked and sun–dried till ∼12% seed moisture content was achieved. A sample of one-hundred seeds in three replications were manually counted and weighed for determination of 100–seed weight.
Seed quality parameters
Seed physical and physiological characteristics
Seed length, width and thickness were measured using digital Vernier caliper (accuracy of ± 0.02 mm) from a sample of 30 seeds. For seed volume determination, 30 seeds were allowed to fall in a measuring cylinder containing 50 ml of water and the volume was determined based on the displacement of water. Ratio of seed weight to its volume was used to determine seed density. For determining water uptake rate, pre weighed 50 seeds were soaked in 250 ml distilled water which was incubated in dark at 20 °C. After, 1, 2, 3, 4, 5, 6, 12 and 24 h of soaking the seeds were removed, surface dried by blotted paper and weighed. The rate of water uptake was calculated based on the increase in seed weight and expressed as percentage.
Laboratory germination
One hundred pure seeds in four replications were placed between moist germination papers which were incubated in dark for 7 days at 20 °C. The samples were examined every day and de-ionized water was added as and when required. On 8th day of incubation, seedlings and seeds were grouped into normal and abnormal seedlings; hard, dead and freshly un–germinated seeds respectively (ISTA 2015). The percentage of normal seedlings represents the final germination percentage. The root and shoot lengths of ten randomly selected seedlings were measured which were dried in an oven at 80 °C for 24 h to determine the seedling dry weight. Seedling vigour index I and II was calculated following the method described by Abdul Baki and Anderson (1973).
Seed vigour (accelerated ageing and storage studies)
Accelerated ageing test (AAT) of chickpea seeds was performed as per International Seed Testing Association (ISTA 2015). The seeds harvested from ambient and e[CO2] environments from the first year of experiment (2017–18) were used for storage study. The dried seeds were placed in air tight container and kept at room temperature in Seed Technology Laboratory of Indian Institute of Pulses Research, Kanpur for 12 months. After every 4 months interval, the seeds were tested for germination as described above.
Biochemical assay of chickpea seeds
Flour was prepared from approximately 3–4 g of chickpea seeds using a coffee grinder. 0.2 g flour from each sample was analyzed for biochemical assays in triplicate. Similar procedure as described previously in biochemical analysis of leaf was followed for the estimation of POX, GR, SOD activities, total phenol content, 2-Thiobarbituric Acid Reactive Substances (TBARS) content, TSS and total starch content of seeds. For total protein estimation, total nitrogen content (%) in seeds was analysed by Kjeldahl method which was then multiplied by a factor of 6.38 (Kjeldahl 1883).
Statistical analysis
Significance of differences between the treatments was calculated using t test. SPSS software 16.0 was used to analyze all the data. The arcsine transformation was performed to normalize the percentage data before statistical analysis.
Results
Crop growth parameters
Results showed that e[CO2] had positive influence on chickpea crop growth in a sub-tropical environment. The e[CO2] treatment increased plant height by 4–8% and 8–29% at flowering and podding stages. However, the effect of e[CO2] on root length was non-significant, except at flowering stage during 2018–19, where a higher root length was recorded in e[CO2] treatment (Table 1).
Table 1.
Plant height, root length, leaf, stem, root, pod, total plant dry weight of chickpea at different growth stages as influenced by ambient and elevated, e[CO2]
| Year | Parameter | Treatment | Branching | Flowering | Podding |
|---|---|---|---|---|---|
| 2017–18 | Plant height (cm) | Ambient | 32.01 | 42.5 | 56.8 |
| Elevated | 31.0 | 44.0 | 62.8 | ||
| P value | ns | 0.007 | 0.025 | ||
| Root length (cm) | Ambient | 11.1 | 13.5 | 15.0 | |
| Elevated | 11.6 | 15.2 | 14.9 | ||
| P value | ns | ns | ns | ||
| Leaf dry weight (g) | Ambient | 0.5 | 2.2 | 5.2 | |
| Elevated | 0.6 | 2.9 | 6.1 | ||
| P value | ns | 0.001 | < 0.001 | ||
| Stem dry weight (g) | Ambient | 0.3 | 2.3 | 5.6 | |
| Elevated | 0.4 | 3.1 | 6.4 | ||
| P value | ns | < 0.001 | < 0.001 | ||
| Root dry weight (g) | Ambient | 0.1 | 0.5 | 0.7 | |
| Elevated | 0.2 | 0.5 | 0.7 | ||
| P value | ns | ns | ns | ||
| Pod dry weight (g) | Ambient | - | - | 2.1 | |
| Elevated | - | - | 2.5 | ||
| P value | - | - | 0.002 | ||
| Total plant dry weight (g) | Ambient | 1.02 | 5.0 | 13.6 | |
| Elevated | 1.17 | 6.4 | 15.6 | ||
| P value | ns | < 0.001 | < 0.001 | ||
| 2018–19 | Plant height (cm) | Ambient | 39.6 | 53.1 | 63.4 |
| Elevated | 42.1 | 57.2 | 81.8 | ||
| P value | ns | 0.032 | 0.002 | ||
| Root length (cm) | Ambient | 13.3 | 14.2 | 19.0 | |
| Elevated | 13.1 | 18.3 | 17.0 | ||
| P value | ns | 0.008 | ns | ||
| Leaf dry weight (g) | Ambient | 0.6 | 4.0 | 6.3 | |
| Elevated | 0.7 | 4.4 | 7.7 | ||
| P value | Ns | 0.005 | < 0.001 | ||
| Stem dry weight (g) | Ambient | 0.5 | 3.5 | 8.3 | |
| Elevated | 0.6 | 5.2 | 9.5 | ||
| P value | ns | 0.003 | 0.013 | ||
| Root dry weight (g) | Ambient | 0.15 | 0.4 | 1.2 | |
| Elevated | 0.14 | 0.6 | 1.2 | ||
| P value | ns | 0.007 | ns | ||
| Pod dry weight (g) | Ambient | - | - | 3.7 | |
| Elevated | - | - | 4.4 | ||
| P value | - | - | 0.029 | ||
| Total plant dry weight (g) | Ambient | 1.29 | 8.0 | 19.6 | |
| Elevated | 1.46 | 10.2 | 22.8 | ||
| P value | ns | 0.002 | 0.005 |
Number of observations (n) = 2 replication X 3 sub samples from each carbondioxide treatment
ns = P > 0.05 according to t test (two tailed)
At branching stage, the growth parameters were comparable in a[CO2] and e[CO2] environments. Stem dry weight recorded an increase of 33–48% and 13–15% at flowering and podding stage respectively (Table 1). Root dry weight did not vary due to increase in [CO2] as compared to a[CO2] at all the stages except for flowering stage in 2018–19. Leaf dry weight recorded an increase of 31 and 10% at flowering and podding stages respectively during 2017–18 whereas 17 and 21% at similar stages during 2018–19 (Table 1). Pod dry weight showed significant increase (19%) upon [CO2] elevation as compared to a[CO2] in both the years (Table 1). In e[CO2] condition, total plant dry weight increased significantly by 28–29% and 15–17% at flowering and podding stages respectively, during 2017–18 and 2018–19 (Table 1).
Yield and yield attributes
In this study, carbon dioxide enrichment had a positive influence on seed yield of chickpea (Table 2). The number of pods plant−1 and number of seeds plant−1 were increased by 8–10% and 8–9% respectively in e[CO2] treatment over a[CO2]. However, there was no differences in 100-seed weight of plants grown in e[CO2] and a[CO2] environments. Significant (P < 0.05) increment of about 11–18% (P < 0.05) was recorded in seed yield due to e[CO2] (Table 2). The interaction of year x treatment was non-significant for yield and yield attributing parameters.
Table 2.
Seed yield and its attributes of chickpea grown under ambient use a[CO2] and elevated CO2 during 2017–18 and 2018–19
| Year | Treatment | Number of pods plant−1 | Number of seeds plant−1 | 100-seed weight (g) | Seed yield (kg ha−1) |
|---|---|---|---|---|---|
| 2017–18 | Ambient | 28.5 | 31.4 | 27.2 | 2052.9 |
| Elevated | 30.9 | 34.0 | 28.1 | 2282.9 | |
| % change | 8.4 | 8.3 | 3.3 | 11.2 | |
| P value | 0.03 | 0.02 | ns | 0.03 | |
| 2018–19 | Ambient | 29.9 | 33.0 | 26.3 | 2354.8 |
| Elevated | 32.8 | 36.0 | 26.5 | 2768.7 | |
| % change | 9.7 | 9.1 | 0.8 | 17.6 | |
| P value | 0.001 | 0.01 | ns | 0.009 | |
| Year | ns | 0.03 | ns | 0.009 | |
| Treatment | 0.02 | 0.009 | ns | 0.03 | |
| Year x Treatment | ns | ns | ns | ns | |
Physiological and biochemical parameters of leaf
Elevated [CO2] increased the SOD activity in leaf at both branching (95%) and flowering (120%) stages (P < 0.01). When compared with the a[CO2] conditions, a higher leaf POX activity (132%) was observed in e[CO2] treatment. While the GR activity was decreased marginally (14%) at podding stage under e[CO2] treatment (Fig. 2). Elevated [CO2] increased the leaf phenol, total soluble sugar and starch content at both branching and flowering stages, while at the podding stage, all these parameters were measured higher in a[CO2] plants (Fig. 3).
Fig. 2.
Activities of superoxide dismutase (SOD), peroxidase and glutathione reductase of chickpea leaves at branching, flowering and podding stages at a[CO2] and e[CO2]. Different lowercase letters represents significant difference at P < 0.05 following t-test method
Fig. 3.
Phenol, total soluble sugar (TSS), 2-Thiobarbituric Acid Reactive Substances (TBARS) and starch content of chickpea leaves at branching, flowering and podding stages at a[CO2] and e[CO2]. Different lowercase letters represent significant difference at P < 0.05 following t-test method
Plants grown under e[CO2] had a higher rate of photosynthesis (4%) and water use efficiency [higher intrinsic water use efficiency (19%) and instantaneous water use efficiency (15%)] as compared to plants grown under a[CO2]. In contrast, plants grown under a[CO2] environment had higher stomatal conductance (14%), internal CO2 concentration (5%) and transpiration rate (9%) over plants at e[CO2] (Table 3).
Table 3.
Physiological parameters of chickpea plants grown in a[CO2] and e[CO2]
| Treatment | Photosynthetic rate (μmol CO2 m–2 s–1) | Stomatal conductance (mol m–2 s–1) | Internal CO2 concentration (ppm) | Transpiration rate (mmol m–2 s–1) | Intrinsic water use efficiency (μmol CO2 m–2 s–1/mol m–2 s–1) | Instantaneous water use efficiency (μmol CO2 m–2 s–1/mmol m–2 s–1) |
|---|---|---|---|---|---|---|
| Ambient | 17.75 | 0.44 | 305.35 | 5.31 | 40.25 | 3.35 |
| Elevated | 18.41 | 0.38 | 291.24 | 4.79 | 48.12 | 3.84 |
| P value | 0.053 | 0.018 | 0.009 | 0.006 | 0.002 | 0.03 |
Data was recorded at 60 days after emergence
Seed quality attributes
Exposing chickpea plants to e[CO2] did not affect the physical attributes (volume, density, length, width and thickness) of seeds harvested (Table 4). Exposing mother plant to e[CO2] had no influence on germination capacity of the seeds (normal seedlings) produced as seeds from both the environments recorded similar germination percentage. Likewise, the percentage of abnormal seedlings, root and shoot lengths, seedling dry weight and vigour indices of seeds did not differ with [CO2] treatments (Table 5). Upon accelerated ageing test (AAT), the germination was reduced in seeds harvested from both the a[CO2] (13–15%) and e[CO2] (9–13%) environments. However, after AAT, the seed germinability was comparable a[CO2] and e[CO2]. Also, the abnormal seedlings, root and shoot lengths, seedling dry weight and vigour indices of seeds did not differ with [CO2] treatments (Table 6). No significant differences were observed in the percentage of normal seedlings, abnormal seedlings, seedling length, seedling dry weight, vigour index I and vigour index II of seeds produced under a[CO2] and e[CO2] after one year of storage (Fig. 4).
Table 4.
Effect of elevated [CO2] on physical attributes of chickpea seeds
| CO2 concentration | Seed length (mm) | Seed width (mm) | Seed thickness (mm) | Seed volume (ml/100 seeds) |
Seed density (g/ml) |
|---|---|---|---|---|---|
| Ambient | 9.69 | 6.65 | 6.62 | 11.33 | 1.25 |
| Elevated | 9.82 | 6.78 | 6.59 | 12.00 | 1.28 |
| P value | ns | ns | ns | ns | ns |
ns not significant
Table 5.
Effect of elevated [CO2] on fresh seed germination of chickpea
| Year | CO2 concentration | Normal seedlings (%) | Abnormal seedlings (%) | Root length (cm) | Shoot length (cm) | Seedling length (cm) | Seedling dry weight (g) | Vigour index I | Vigour index II |
|---|---|---|---|---|---|---|---|---|---|
| 2017–18 | Ambient | 86.67 | 13.33 | 12.95 | 5.50 | 18.45 | 0.29 | 1599.00 | 24.99 |
| Elevated | 87.33 | 12.67 | 10.55 | 5.90 | 16.45 | 0.28 | 1436.63 | 24.72 | |
| ns | ns | ns | ns | ns | ns | ns | ns | ||
| 2018–19 | Ambient | 81.00 | 16.00 | 12.14 | 5.97 | 18.11 | 0.24 | 1466.91 | 19.60 |
| Elevated | 80.00 | 20.00 | 12.47 | 6.08 | 18.55 | 0.22 | 1484.00 | 17.60 | |
| ns | ns | ns | ns | ns | ns | ns | ns |
ns not significant
Table 6.
Effect of elevated [CO2] on seed vigour of chickpea as determined by accelerated ageing test
| Year | CO2 concentration | Normal seedlings (%) | Abnormal seedlings (%) | Root length (cm) | Shoot length (cm) | Seedling length (cm) | Seedling dry weight (g) | Vigour index I | Vigour index II |
|---|---|---|---|---|---|---|---|---|---|
| 2017–18 | Ambient | 75.00 | 23.00 | 10.10 | 4.07 | 14.17 | 0.20 | 1067.43 | 15.14 |
| Elevated | 76.00 | 22.00 | 9.63 | 4.10 | 13.73 | 0.21 | 1043.48 | 15.88 | |
| ns | ns | ns | ns | ns | ns | ns | ns | ||
| 2018–19 | Ambient | 69.00 | 30.00 | 12.22 | 4.75 | 16.97 | 0.16 | 1170.93 | 16.69 |
| Elevated | 73.00 | 27.00 | 11.85 | 4.15 | 16.00 | 0.17 | 1168.00 | 16.06 | |
| ns | ns | ns | ns | ns | ns | ns | ns |
ns not significant
Fig. 4.
Storage studies of seeds produced at a[CO2] and e[CO2]
Variation observed in the activity of POX, GR, SOD and total soluble sugar content in the seeds was not significant (Fig. 5). However, the seeds harvested from e[CO2] had a lower phenol (13%), 2-Thiobarbituric Acid Reactive Substances (12%) and protein (7%) and a higher starch (21%) content (Fig. 5). Seeds produced at e[CO2] had higher water uptake rate (during 3 h to 12 h of imbibition) over the seeds produced from a[CO2]; however, the total water uptake was comparable after 24 h of imbibition (Fig. 6).
Fig. 5.
Activities of peroxidase, glutathione reductase, superoxide dismutase (SOD) and content of phenol, 2-Thiobarbituric Acid Reactive Substances (TBARS), total soluble sugar (TSS), starch and protein of chickpea seed produced at a[CO2] and e[CO2]. Different lower case letter represents significant difference at P < 0.05 level of significance following t test
Fig. 6.
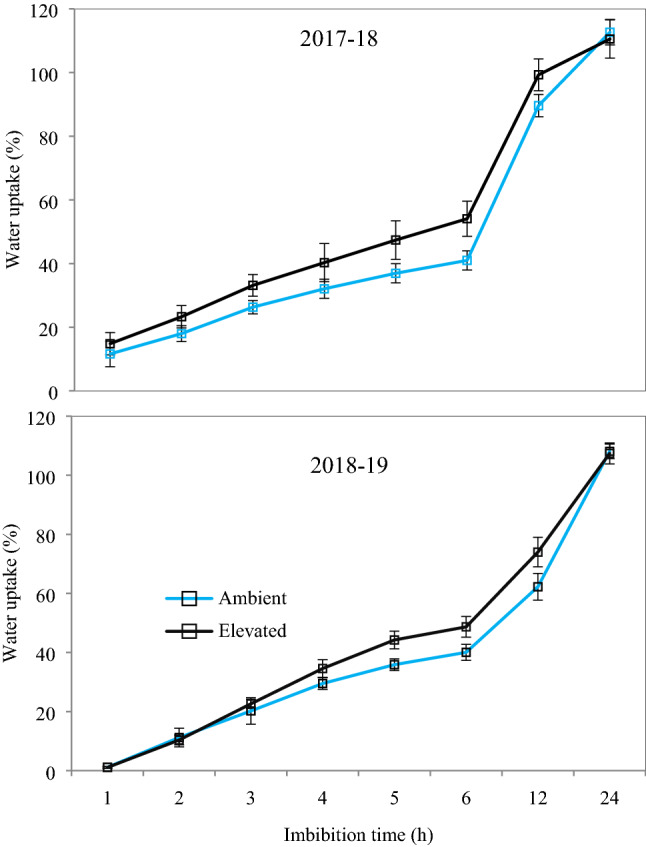
Water uptake (%) of chickpea seeds produced under a[CO2] and e[CO2] during 2017–18 and 2018–19. The error bar represents the standard error of means
Discussion
The growth and yield response of chickpea to e[CO2] was found to be positively influenced as the plants grown in e[CO2] was taller with higher biomass and yield as compared to the plants in a[CO2]. Plant height up to branching stage was similar in e[CO2] and a[CO2] treatments, however, at flowering and podding stages e[CO2] recorded significantly higher plant height. Our findings corroborated with the findings of Saha et al. (2013) and Singh et al (2017), who also reported an increase in chickpea plant height upon exposure to e[CO2]. Higher biomass accumulation upon e[CO2] exposure may be attributed to enhanced photosynthetic rate as a result of increase in leaf area and leaf number (Mishra and Agrawal 2014). Elevated [CO2] increases photosynthetic rate of almost all C3 species, provided nutrients are not a limiting factor (Long and Drake 1992). Legumes are expected to have a especial capability of responding to e[CO2] with increased photosynthesis and growth due to their ability to fix atmospheric nitrogen (Rogers et al. 2009).
In our experiment, the stomatal conductance, internal [CO2] and transpiration rate was low under e[CO2], while the photosynthetic rate and water use efficiency was high under e[CO2]. Low internal [CO2] under e[CO2] may be due to the greater affinity of CO2 to bind with ribulose biphosphate carboxylase-oxygenase (Rubisco) enzyme which led to higher photosynthetic rates. The current concentration of atmospheric CO2 is not sufficient to completely saturate Rubisco in C3 species; therefore, an increased availability of CO2 increases carboxylation and photosynthetic rates (Bowes 1993). The result indicated that prolonged exposure to e[CO2] perhaps modified the carboxylation efficiency of Rubisco due to change in the CO2:O2 ratio of inter cellular leaf micro environment.
The increase in seed yield with increase in atmospheric [CO2] was significant, which was attributed to an increase in number of pods plant−1 and number of seeds plant−1. Chickpea, being a leguminous crop, is able to fix atmospheric N2 to a form that can be easily taken up by the plants. In e[CO2] condition, chickpea plant may translocate extra Carbon to the nodules as an energy source as a result gets maximum benefit of e[CO2]. An increase in yield of chickpea due to e[CO2] has been reported earlier (Chakrabarti et al. 2020; Rai et al. 2016; Singh et al. 2017). Chakrabarti et al (2020) reported the yield of chickpea (cv BGD 72) to increase by 21 to 22% with no effect on seed N content due to its atmospheric N fixing ability, which otherwise decreased in wheat seed. Previous reports shows that legumes are more responsive to e[CO2] as compare to other plant species (Rogers et al. 2009).
Among the inputs required for improving productions, quality seed is the most important factor which alone has the potential of improving productivity by 15–20% (Pal et al. 2016). Physical and genetic purity, ability of seed to germinate and produce a healthy plant even in unfavourable condition, high seed vigour, storability behavior, seed health, its colour, weight, shape and size etc. are the quality attributes of a quality seed (Maity and Chakrabarty 2013; Maity and Pramanik 2013; Maity et al. 2016). Since, seed production is a sequential and long process, its quality depends on the condition to which the mother plant is exposed to during the entire growing period, the methods of harvesting and threshing followed, seed handling during processing and environmental condition during storage (Lamichaney et al. 2017, 2018). In the present investigation, exposing mother plant to e[CO2] upto 600 ppm did not influence any of the seed physical quality traits like 100-seed weight, seed length, width, thickness, density and volume. This was in agreement with the previous reports by Saha et al (2015b) in chickpea and Lamichaney et al. (2019) in rice. Seed germination and vigour (AAT and storage studies) was not influenced by e[CO2]. Previous reports revealed a variable effect of e[CO2] on seed quality in terms of germination and vigour. Studies on rice (Chen et al. 2015), Bromus species (Steinger et al. 2000), kidney bean (Thomas et al. 2009), Pinus taeda (Way et al. 2010), wheat (Sanhewe et al. 1996) and yam (Thinh et al. 2017) showed no effect of exposing mother plant to e[CO2] on per cent seed germination. In contrast, seeds of rice and chickpea, produced at e[CO2] had lower germination than seeds produced at a[CO2] (Saha et al. 2015b; Lamichaney et al. 2019). However, seeds of Plantago, Trifolium, Poa, Lolium and alfalfa harvested from plants grown at e[CO2] had higher germination than seeds harvested from plants grown at a[CO2] (Ziska and Bunce 1993; Edwards et al. 2001; Wulf and Alexander 1985). Saha et al (2015b) reported negative effect of e[CO2] of 580 ppm on seed germination of chickpea, whereby the germination was reduced by about 45–47% which was mainly attributed to decrease in hydrolytic enzymes like protease and seed N content. Such variable result may be due to varietal differences or due to differences in chickpea type as Saha et al (2015b) used kabuli chickpea (cv. Pusa 1105) as opposed to desi (cv. JG 14) in present investigation. Kabuli and desi chickpea are two distinct market type of chickpea, kabuli types is characterized by large seeds with unpigmented and thin seed coat, while desi types are usually small seeded with pigmented seed coat. Pigmented desi and unpigmented kabuli types are known to differ in seed vigour (Lamichaney et al. 2016, 2017) which may have attributed to such contrasting result. However, [CO2] above 610 ppm reduced vigour of rice seeds which was attributed to reduced nitrogen content, availability and translocation of substrate (Lamichaney et al. 2019). Ziska and Bunce (1993) reported an increase in germination due to e[CO2], which was attributed to e[CO2] mediated increase in ethylene production as ethylene is known to promote seed germination. In the present investigation, the germination of seeds harvested from a[CO2] and e[CO2] did not differ even after one year of storage period. To the best of our knowledge, this is the first report on the long term storage studies of seeds produced from CO2 enriched environment.
This study demonstrated that e[CO2] has a negative effect on seed phenol content (Fig. 5). Margna et al. (1989) attributed decrease in availability of nitrogen mediated lesser availability of amino acids such as L-phenylalanine, precursor molecule for biosynthesis of phenols as a reason for decrease in phenol content of seed produced at e[CO2] condition. Goufo et al. (2014) have reported reduction in the content of phenylalanine in the grain produced at e[CO2], which might have limited its access for phenol biosynthesis. Elevated [CO2] enabled increase in starch concentration is attributed to the ability of sinks to assimilate photosynthates in leaves (Wang et al. 2013). A previous study on reduction of seed protein content upon [CO2] enrichment is attributed to the dilution effect caused by an increase in carbohydrates accumulation (Wu et al. 2004). Reduction in seed protein content under e[CO2] is quite expected because e[CO2] is reported to reduce the cellular nitrogen pool and thereby the protein content. Chickpea plant although fixes atmospheric N, reduction in protein content suggests its shortfall. Our findings corroborated with the findings of Saha et al (2015b), who also reported reduction in seed protein with increase in seed starch of chickpea at e[CO2]. Reduction in protein content in the seeds produced under e[CO2] may be explained by the observations of Fangmeier et al. (1999), who suggested that e[CO2] decreased protein concentrations in photosynthetic tissues is largely due to the carbohydrate dependent decrease in expression of Rubisco (Ainsworth and Long 2005). The decrease in Rubisco can decrease the seed protein concentration because the N supply to seeds is mainly from translocation of catabolized proteins in senescing photosynthetic tissues (Fangmeier et al. 1999). Another important observation which is well reported is that the decrease in protein content is less in nodulated plants as compared to the non-nodulated plants. This is because the legume nodules serve as sink for excess photo-assimilates that are generated under e[CO2], preventing the increase in leaf hexose flux responsible for down regulation of Rubisco (Moore et al. 1999).
Conclusions
Exposure of chickpea plant to 600 ppm [CO2] increased the plant biomass and yield which is attributed to the greater availability of photosynthates. However, the seeds harvested from e[CO2] had low protein content. Thus, e[CO2] could increase the productivity of chickpea, helping to overcome the problem of food security, but reduction in seed protein content upon e[CO2] exposure may hamper in achieving nutritional security. The result suggested no effect of e[CO2] mediated changes in chickpea crop physiology on the germination and vigour of seeds produced, which is expected have no impact on chickpea seed industry.
Acknowledgements
Authors are thankful to the Division of Crop Protection, ICAR-Indian Institute of Pulses Research for providing the Open Top Chamber facility.
Compliance with ethical standards
Conflict of interest
The authors report no any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdul-Baki AA, Anderson JD. Vigour determination in soybean seed by multiple criteria. Crop Sci. 1973;13:630–633. [Google Scholar]
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bowes G. Facing the inevitable: plant and increasing atmospheric CO2. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:309–332. [Google Scholar]
- Canadell JG, Le Quere C, Raupach MR, Field CB, Buitenhuis ET, Ciais P, Conway TJ, Gillett NP, Houghton RA, Marland G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. PNAS. 2007;104(47):18866–18870. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo FJ, Penel C, Greppin H. Peroxidase release induced by ozone in Sedum album leaves. Plant Physiol. 1984;74:846–851. doi: 10.1104/pp.74.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B, Singh SD, Bhatia A, Kumar V, Harit RC. yield and nitrogen uptake in wheat and chickpea grown under elevated carbon dioxide level. Natl Acad Sci Lett. 2020;43:109–113. [Google Scholar]
- Chen C, Jiang Q, Ziska LH, Zhu J, Liu G, Zhang J, Ni K, Seneweera S, Zhu C. Seed vigor of contrasting rice cultivars in response to elevated carbon dioxide. Field Crops Res. 2015;178:63–68. [Google Scholar]
- DuBois M, Gilles K, Hamilton J, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. [Google Scholar]
- Edwards GR, Clark H, Newton PCD. The effects of elevated CO2 on seed production and seedling recruitment in a sheep-grazed pasture. Oecologia. 2001;127:383–394. doi: 10.1007/s004420000602. [DOI] [PubMed] [Google Scholar]
- Fangmeier A, De Temmerman L, Mortensen L, Kemp K, Burke J, Mitchell R, van Oijen M, Weigel HJ. Effects of nutrients on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European multiple-site experiment ‘ESPACE-wheat’. Eur J Agron. 1999;10:215–229. [Google Scholar]
- Food and Agriculture Organization . FAOSTAT Database. Rome: Food and Agriculture Organization of the United Nations; 2014. [Google Scholar]
- Godfray HC, Crute IR, Haddad L, Lawrence D, Muir JF, Nisbett N, Pretty J, Robinson S, Toulmin C, Whiteley R. The future of the global food system. Philos Trans R Soc Lond B Biol Sci. 2010;365(1554):2769–2777. doi: 10.1098/rstb.2010.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goufo P, Pereira J, Figueiredo N, Oliveira MBP, Carranca C, Rosa EA, Trindade H. Effect of elevated carbon dioxide (CO2) on phenolic acids, flavonoids, tocopherols, tocotrienols, γ-oryzanol and antioxidant capacities of rice (Oryza sativa L.) J Cereal Sci. 2014;59(1):1524. [Google Scholar]
- Hampton JG, Boelt B, Rolston MP, Chastain TG. Effects of elevated CO2 and temperature on seed quality. J Agric Sci. 2013;151(2):154–162. doi: 10.1017/S0021859612000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (2014) Climate change impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge university press, Cambridge.
- ISTA . International Rules for Seed Testing. Bassersdorf: International Seed Testing Association; 2015. [Google Scholar]
- Kjeldahl J. New method for the determination of nitrogen in organic substances. Z Anal Chem. 1883;22(1):366–383. [Google Scholar]
- Lamichaney A, Katiyar PK, Laxmi V, Pratap A. Variation in pre-harvest sprouting tolerance and fresh seed germination in mungbean (Vigna radiata L) genotypes. Plant Genet Resour. 2018;16(5):437–445. [Google Scholar]
- Lamichaney A, Katiyar PK, Natarajan S, Sripathy KV. Relationship among some seed characters, laboratory germination and field emergence in chickpea (Cicer arietinum L.) genotypes differing in testa colour. J Food Leg. 2016;29(1):29–32. [Google Scholar]
- Lamichaney A, Kudekallu S, Kamble U, Sarangapany N, Katiyar PK, Bohra A. Differences in seed vigour traits between desi (pigmented) and kabuli (non–pigmented) ecotypes of chickpea (Cicer arietinum) and its association with field emergence. J Environ Biol. 2017;38(5):735–742. [Google Scholar]
- Lamichaney A, Swain DK, Biswal P, Kumar V, Singh NP, Hazra KK. Elevated atmospheric carbon–dioxide affects seed vigour of rice (Oryza sativa L.) Environ Exp Bot. 2019;157:171–176. [Google Scholar]
- Loewus FA. Improvement in the anthrone assay for determination of carbohydrates. Anal Chem. 1952;24:219. [Google Scholar]
- Long SP, Drake BG. Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations. In: Baker NR, Thomas H, editors. Crop photosynthesis: Spatial and temporal determinants. Amsterdam: Elsevier Science Publishers BV; 1992. pp. 69–103. [Google Scholar]
- Maity A, Chakrabarty SK. Effect of environmental factors on hybrid seed quality of Indian mustard (Brassica juncea) Afr J Agric Res. 2013;8(48):6213–6219. [Google Scholar]
- Maity A, Pramanik P. Climate change and seed quality; an alarming issue in crop husbandary. Curr Sci. 2013;105(10):1136–1138. [Google Scholar]
- Maity A, Vijay D, Mukherjee A, Lamichaney A. Potential impacts of climate change on quality seed production: a perspective of hill agriculture. In: Bisht J, Meena V, Mishra P, Pattanayak A, editors. Conservation agriculture. Singapore: Springer; 2016. pp. 459–485. [Google Scholar]
- Margna U, Vainjlrv T, Laanest L. Different L-phenylalanine pools available for the biosynthesis of phenolics in buckwheat seedling tissues. Phytochemistry. 1989;28:469–475. [Google Scholar]
- Mishra AK, Agrawal SB. Cultivar specific response of CO2 fertilization on two tropical mung bean (Vigna radiata L.) cultivars: ROS generation, antioxidant status, physiology, growth, yield and seed quality. J Agron Crop Sci. 2014;200(4):273–289. [Google Scholar]
- Moore BD, Cheng SH, Sims D, Seemann JR. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 1999;22:567–582. [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pal G, Channanamchery R, Singh RK, Kethineni UB, Ram H, Prasad SR. An economic analysis of pigeonpea seed production technology and its adoption behavior: Indian context. Sci World J. 2016;2016:1–7. doi: 10.1155/2016/7973638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai P, Chaturvedi AK, Shah D, Pal M. Impact of elevated CO2 on high temperature induced effects in grain yield of chickpea (Cicer arietinum) Indian J Agric Sci. 2016;86(3):414–417. [Google Scholar]
- Rogers A, Ainsworth EA, Leakey AD. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol. 2009;151(3):1009–1016. doi: 10.1104/pp.109.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chakraborty D, Sehgal VK, Pal M. Potential impact of rising atmospheric CO2 on quality of grains in chickpea (Cicer arietinum L.) Food Chem. 2015;187:431–436. doi: 10.1016/j.foodchem.2015.04.116. [DOI] [PubMed] [Google Scholar]
- Saha S, Chakraborty D, Sehgal VK, Pal M. Rising atmospheric CO2: potential impacts on chickpea seed quality. Agric Ecosyst Environ. 2015;203:140–146. [Google Scholar]
- Saha S, Sehgal VK, Chakraborty D, Pal M. Growth behavior of kabuli chickpea under elevated atmospheric CO2. J Agric Phys. 2013;13(1):55–61. [Google Scholar]
- Sanhewe AJ, Ellis RH, Hong TD, Wheeler TR, Batts GR, Hadley P, Morrison JIL. The effect of temperature and CO2 on seed quality development in wheat (Triticum aestivum L.) J Exp Bot. 1996;47:631–637. [Google Scholar]
- Singh RN, Mukherjee J, Sehgal VK, Bhatia A, Krishnan P, Das DK, Kumar V, Harit R. Effect of elevated ozone, carbon dioxide and their interaction on growth, biomass and water use efficiency of chickpea (Cicer arietinum L) J Agrometeorol. 2017;19(4):301–305. [Google Scholar]
- Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5′- dithiobis (2-nitrobenzoic acid) Anal Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Steinger T, Gall R, Schmid B. Maternal and direct effects of elevated CO2 on seed provisioning, germination and seedling growth in Bromus erectus. Oecologia. 2000;123:475–480. doi: 10.1007/s004420000342. [DOI] [PubMed] [Google Scholar]
- Streck NA. Climate change and agroecosystems: the effect of elevated atmospheric carbondioxide and temperature on crop growth, development and yield. Ciencia Rural Santa Maria. 2005;35(3):730–740. [Google Scholar]
- Thinh NC, Kumagai E, Shimono H, Kawasaki M. Effects of elevated CO2 concentration on bulbil germination and early seedling growth in Chinese yam under different air temperatures. Plant Prod Sci. 2017;20(3):313–322. [Google Scholar]
- Thomas JMG, Prasad PVV, Boote KJ, Allen LH. Seed composition, seedling emergence and early seedling vigour of red kidney bean seed produced at elevated temperature and carbon dioxide. J Agron Crop Sci. 2009;195:148–156. [Google Scholar]
- Wang L, Feng ZZ, Schjoerring JK. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): A meta-analytic test of current hypotheses. Agric Ecosyst Environ. 2013;178:57–63. [Google Scholar]
- Way DA, Ladeau SL, Mccarthy HR, Clark JS, Oren R, Finzi AC, Jackson RB. Greater seed production in elevated CO2 is not accompanied by reduced seed quality in Pinus taeda L. Glob Chang Biol. 2010;16:1046–1056. [Google Scholar]
- Wu DX, Wang GX, Bai YF, Liao JX. Effects of elevated CO2 concentration on growth, water use, yield and grain quality of wheat under two soil water levels. Agric Ecosyst Environ. 2004;104:493–507. [Google Scholar]
- Wulf RD, Alexander HM. Intraspecific variation in the response to CO2 enrichment in seeds and seedlings of Plantago lanceolata. Oecologia. 1985;66:458–460. doi: 10.1007/BF00378315. [DOI] [PubMed] [Google Scholar]
- Xu B, Chang S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72:159–166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Ziska LH, Bunce JA. The influence of elevated CO2 and temperature on seed germination and emergence from soil. Field Crops Res. 1993;34:147–157. [Google Scholar]







