Abstract
Due to the increased production and release of silver nanoparticles (AgNPs) in the environment, the concerns about the possibility of toxicity and oxidative damage to plant ecosystems should be considered. In the present study, the effects of different concentrations of AgNPs (0, 0.5, 1, 2, 3 and 4 g/L) synthesized using the extract of camelina (Camelina sativa) leaves on the growth and the biochemical traits of camelina seedlings were investigated. The results showed that AgNPs significantly increased Ag accumulation in the roots and shoots which decreased the growth and photosynthetic pigments of camelina seedlings. The highest decrease in the height and total dry weight was observed by 53.1 and 61.8% under 4 g/L AgNPs, respectively over control plants. AgNPs application over 2 g/L enhanced the accumulation of proline, malondialdehyde, hydrogen peroxide and methylglyoxal, and up-regulated the activity of antioxidant enzymes (superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase) and glyoxalase (glyoxalase I and II) system which indicates oxidative stress induction in camelina seedlings. Moreover, AgNPs reduced ASA and GSH contents and increased DHA and GSSG contents, hence disrupting the redox balance. These results showed that AgNPs at 4 g/L had the most toxic effects on the camelina growth. Therefore, increasing oxidative stress markers and the activity of antioxidant enzymes and enzymes involved in glyoxalase system indicated the oxidative stress induced by AgNPs treatments over 2 g/L as well as the induction of antioxidant defense systems to combat AgNPs-induced oxidative stress.
Keywords: Silver nanoparticles, Oxidative stress, Glyoxalase system, ASA-GSH cycle, Proline, Camelina sativa L
Introduction
Due to the antimicrobial properties of silver, uses of silver nanoparticles (AgNPs) in the textiles, food packaging, and the detergents industry is increased, and approximately 60 tons AgNPs are annually produced worldwide (Fabrega et al. 2011; Wijnhoven et al. 2009; Bondarenko et al. 2013). The Ag concentration amount in the soil which can induce toxicity in the plants depends on many factors including soil properties (Kolesnikov et al. 2020). However, the accumulation of more than 0.01 mg/kg Ag in the plants induces phytotoxicity which indicates the high toxicity of this metal in the plants (Kabata-Pendias and Pendias 2001). A significant proportion of AgNPs enters to the ecosystems which may cause varying degrees of toxicity to different organisms (Benn et al. 2010). Although AgNPs toxicity in animals and plants was reported, the exact mechanism of AgNPs toxicity remains unknown. Previous reports showed that the main cause of AgNPs-induced toxicity in algae (Oukarroum et al. 2012), animals (Yin et al. 2013; Zhang et al. 2013), and bacteria (Yang et al. 2013) is Ag ions released from the nanoparticles. Ag+ ions released from AgNPs were shown to decline the photosynthetic efficiency in Chlamydomonas reinhardtii and a decrease in the survival of Daphnia magna (Zhao and Wang 2011; Newton et al. 2013) and Escherichia coli (Xiu et al. 2011, 2012). Many studies were done to investigate AgNPs effects on the plant growth and yield. AgNPs were shown to reduce the growth of Pisum sativum (Tripathi et al. 2017) and Oryza sativa (Nair and Chung 2014) and also to induce oxidative stress in Portulaca oleracea medicinal plant (Zare et al. 2020). Lin et al. (2012) indicated that AgNPs negatively affect the germination and early growth of eleven species of common wetland plants. Yin et al. (2011) indicated that AgNPs had more phytotoxic effects on Lolium multiflorum than silver nitrate (AgNO3), while Vishwakarma et al. (2017) showed that AgNO3 had more inhibitory effects on Brassica sp. growth than AgNPs. These results indicate that dissolved Ag+ ions released from AgNps are not the only cause of the toxicity of AgNPs in plants, and more detailed studies are needed to understand the phytotoxic mechanism of AgNPs.
Various reports showed that AgNPs toxicity induces oxidative stress in algae, bacteria, and animals which can be referred to AgNPs-induced oxidative stress in nitrifying bacteria (Choi and Hu 2008), zebrafish embryos (Massarsky et al. 2013), and rat cerebellum granule cells (Yin et al. 2013). However, few studies were conducted on the oxidative stress induced by AgNPs toxicity in higher plants. Zare et al. (2020) indicated that increasing AgNPs concentration enhanced Ag accumulation in the roots and shoots and, as a result, increased hydrogen peroxide (H2O2) and malondialdehyde (MDA) in the purslane plant which indicates oxidative stress induction in the plant. Nair and Chung (2014) showed that AgNPs in a concentration-dependent manner increased H2O2 and MDA content and upregulated the expression level of catalase and ascorbate peroxidase genes in rice. However, there is no systematic study of phytotoxic AgNPs on plant antioxidant defense systems such as the activity of antioxidant enzymes, ascorbic acid-glutathione (ASA-GSH) and the glyoxylate cycle.
In recent years, several studies have been published demonstrating the synthesis of nanoparticles such as silver, gold, sulfur, palladium, selenium, and nickel using plant, bacteria, fungi, and algae extracts (Sudhasree et al. 2014; Sunita et al. 2011; Torres et al. 2012; Najafi et al. 2020). The plant extract due to its phytochemicals such as dihydric phenols, phenols, flavonoids, and terpenoids, as well as enzymes like reductases and hydrogenases reduce the metal salts and synthesizes nanoparticles (Thakkar et al. 2010; Raghunandan et al. 2010). Camelina (Camelina sativa) is one of the most important Brassicaceae family plants which has a high nutritional value for soil fertilizer, livestock feed, and human health (Berti 2016; Pilgeram 2007). Camelina seeds contain from 30 to 49% oil, including eicosenoic acid (14–16%), linoleic acid (16–17%), linolenic acid (36–39%) and oleic acid (13–15%) (Berti 2016). However, the synthesis of AgNPs using camelina extract has not being studied. For the first time, the high-quality synthesis of AgNPs was investigated using the camelina leaf extract. Then, the effect of different concentrations of synthesized AgNPs on the growth and biochemical parameters of the camelina plant, as a model crop, was assessed to evaluate the phytotoxic effects of AgNPs by focusing on the plant antioxidant defense system.
Material and methods
Preparation of AgNPs
To prepare the leaf extract, fresh and healthy leaves of camelina were boiled in 200 ml distilled water for 1 h after washing with distilled water (4 times) and crushing with a pestle mortar. After cooling at room temperature and filtering by Whatman filter paper, the leaf extracts were stored at 4 °C until use.
After preparing an aqueous solution of 0.2 M silver nitrate (AgNO3), 90 ml AgNO3 solution was mixed with 10 ml of camelina leaf extract and incubated in a dark chamber at room temperature. By changing the color of solution from the colorless to brown, Ag reduction was confirmed. The properties of synthesized nanoparticles were examined using a UV–Visible (Perkin-Elmer Lambda 25, USA), FTIR (Bruker Tensor-27), SEM (JEOL JSM-6390 LVSEM), and X-ray diffraction (Panalytical Xpert-PRO 3050/60).
Plant materials and treatments
For the surface sterilization, camelina seeds were washed with distilled water three times after soaking in sodium hypochlorite (2%) for 10 min. Then, the seeds (five seeds per pot) were germinated in pots containing autoclaved sand and soil (2:1), and each pot was reduced to one plant after germination. Seedlings were grown in controlled conditions with 16 h light (250–300 µmol m–2 s–1), 65 ± 5% humidity, and 25/18 °C temperature. After 15 days, the camelina seedlings were exposed to different concentrations of synthesized AgNPs, including zero (control), 0.5, 1, 2, 3 and 4 g/L AgNPs). The pots were fed every alternate day with a half-strength solution of Hoagland containing different concentrations of AgNPs. Sampling was performed 10 days after the beginning of AgNPs treatments and kept at -80 °C to measure biochemical attributes.
Growth parameters
After recording the plant height, the plants were incubated for 72 h at 76° C, and then the total dry weight was measured.
Physiological parameters
Photosynthetic parameters
The extraction of photosynthetic pigments with acetone (80%), the content of chlorophyll (a and b) and carotenoids were measured according to method reported by Lichtenthaler (1987). After 30 min of dark adaptation, Fv/Fm ratio of camelina leaves was measured using a Portable Chlorophyll Fluorometer (Walz, PAM-2100).
The estimation of stress markers
To measure MDA content, fresh camelina leaves were homogenized in thiobarbituric acid (1%) and centrifuged at 15,000 × g for 15 min. The contents of MDA were determined based on the mixture absorbance (supernatant + 1 M KI + 10 mM potassium-phosphate buffer (pH 7.0)) at 390 nm as per Heath and Packer (1968).
To measure the H2O2 content, fresh leaves were extracted using K-P buffer (pH 6.5). After the centrifugation at 12,000 × g for 10 min, a mixture of TiCl4 in H2SO4 (20%) was added to the supernatant. After recording the reading of reaction solution at 410 nm, H2O2 content was calculated according to Yu et al. (2003) utilizing the extinction coefficient of 0.28 µM–1 cm–1.
To measure MG, fresh leaves were homogenized with perchloric acid (5%) and centrifuged at 12,000 × g for 15 min. After decolorization with charcoal and neutralization with saturated sodium carbonate, the supernatants were mixed with N-acetyl-L-cysteine and sodium dihydrogen phosphate and read at 288 nm (Wild et al. 2012).
Silver accumulation
To measure silver accumulation, dried roots and leaves of camellia were digested in HNO3 (60%) at 90 °C for 6 h. Then, silver accumulation in roots and leaves was measured using an ICP-MS (Varian 820-MS, USA).
Proline content
The proline content of camelina leaves was measured according to the method described by Bates et al. (1973) using ninhydrin assay at 520 nm.
Biochemical parameters
Extraction and assay of enzymes
Fresh leaves of camelina were extracted in potassium-phosphate buffer (50 mM, pH 7.0) containing glycerol 10% (w/v), ß-mercaptoethanol (5 mM), ascorbate (1 mM) and KCl (100 mM). The extract was centrifuged at 12,000 × g for 20 min and the supernatant was kept at 4 °C to measure enzyme activity.
The reaction mixture for measuring superoxide dismutase activity (SOD) included supernatant, 2.4 mM xanthine, 0.1 U xanthine oxidase, and potassium-phosphate buffer (50 mM, pH 7.0), 0.1 U catalase, and 2.3 mM NBT.SOD activity was determined using the method described by El-Shabrawi et al. (2010).
The catalase (CAT) enzyme activity was determined calculating the difference in reaction mixture absorbance (enzyme extract, potassium-phosphate buffer (50 mM, pH 7.0) and H2O2 (15 mM)) at 240 nm for 1 min as per the protocol described by Hasanuzzaman and Fujita (2011).
The activity of the ascorbate peroxidase (APX) enzyme was determined by Nakano and Asada (1981) method measuring the absorbance of reading reaction mixture including supernatant, potassium-phosphate buffer (50 mM, pH 7.0), EDTA (0.1 mM), H2O2 (0.1 mM) and ascorbic acid (AsA, 0.5 mM) at 290 nm.
The glutathione reductase (GR) was estimated measuring the absorbance at 390 nm using the method of Foyer and Halliwell (1976). The reaction mixture to measure GR activity included enzyme extract, potassium-phosphate buffer (0.1 M, pH 7.8), NADPH (0.2 mM), glutathione disulfide (1 mM), and EDTA (1 mM).
The glyoxalase I (Gly I) enzyme activity was measured using the method described by Hasanuzzaman and Fujita (2011). The assay mixture contained enzyme extract, potassium-phosphate buffer (100 mM, pH 7.0), MG (3.5 mM), GSH (1.7 mM) and magnesium sulfate (15 mM) and the absorbance was measured at at 240 nm for 1 min.
The glyoxalase II (Gly II) enzyme activity was determined by observing the changes in the absorbance of reaction mixture (enzyme extract, Tris–HCl buffer (100 mM, pH 7.2), S-D-lactoylglutathione (1 mM), and DTNB (0.2 mM)) at 250 nm for 1 min according to the method previously described by Principato et al. (1987).
The determination of ASA and GSH
After extracting the fresh tissue of roots with metaphosphoric (5%) containing EDTA (1 mM) and centrifuging at 12,000 × g for 15 min, the supernatants were used to determine the contents of ASA and GSH. To determine ASA, the supernatants were neutralized by 0.5 M potassium-phosphate buffer (pH 7.0), and the oxidized ASA in the extract was reduced using dithiothreitol (0.1 M). After adding 1 U of ascorbate oxidase and 100 mM potassium-phosphate buffer (pH 7.0), the reduced and total ASA were measured by reading at 265 nm. The content of reduced ASA was determined due to the standard curve of ASA regarding the method of Dutilleul et al. (2003). Oxidized ASA (DHA) was calculated due to the following formula: DHA = total ASA – reduced ASA.
A reaction mixture including the supernatant, NADPH (0.3 mM), DTNB (6 mM), and GR (10 U mL–1) was used to determine the total glutathione (GSH + GSSG). After the reaction mixture incubation (supernatant, triethanolamine (50%, v/v), and 2-vinylpyridine) at 25 °C for 20 min, the GSSG content was determined by reading the absorbance at 412 nm. By subtracting GSSG content from the total glutathione, GSH content was obtained due to the previously described method of Gill et al. (2015).
Statistical analysis
The present study was performed in a completely randomized design with 5 replications. Data analysis was carried out by SPSS 20.0 software, and the means were compared based on Duncan's Multiple Range Test (p < 0.05). All the results are presented as an average ± standard deviation (SD) of the five independent replicates.
Results
Characterization of synthesized silver nanoparticles
FTIR spectroscopy was carried out to identify C. sativa leaf extract's responsible biomolecules for reduction and efficient stabilization of AgNPs. IR spectrum of phytogenic AgNPs shows the intense bands at 3437.15, 2920.23, 1643.35, 1496.76, 1462.04, 1222.87, and 1041.56 cm−1. Also, IR spectrum showed absorption bands of C. sativa leaf extract (with different functional groups) at 3398.57 (OAH, NAH), 2927.94 (OAH, CAH), 1608.63 (NAH), 1516.05 (NAO), 1419.61 (CAC), 1257.05 (CAH) and 1076.28 cm−1 (CAN). The strong absorption band at 1616 cm−1 and 1388.10 are assigned to OAH stretch and C-H (H–bonded) of alcohols or phenols (Fig. 1A).
Fig. 1.
FTIR spectra A, B UV–vis spectra UV–vis spectra – For different time intervals of s1 = 1 h, s2 = 5 h, s3 = 10 h and s4 = 24 h C, SEM micrograph of AgNPs in single (dashed line arrow) and aggregated shape (full line arrow) D and X-ray diffraction (XRD) pattern of greensynthesised AgNPs by extract of Camelina sativa L leaves
UV–Vis spectroscopic analysis of samples at different time intervals is shown in Fig. 1B. The characteristics SPR peak for phytogenic AgNPs were monitored by observing the color change and maximum absorbance in the range from 380 to 600 nm which is the evidence of AgNPs SPR presence. The position and the shape of SPR peak depend on the size, shape, and dielectric constant of solutions. The absorbance maxima peak of the phytogenic AgNPs was observed at 416 nm which confirmed the formation of AgNPs using C. sativa leaf extract. It is observed that the intensity of SPR bands increases as the reaction time progresses.
The morphology of AgNPs is almost spherical with an average size of 41 ± 6 nm in the range of 8–75 nm, and monodispersity due to particle size. Furthermore, Fig. 1C reveals that aggregates of AgNPs are not indirect contact indicating the stabilization by capping agents. These results showed that bio-capped molecules help prevent NPs agglomeration and also enhance the antimicrobial activity (Fig. 1C).
XRD analysis of phytogenic AgNPs for their crystal structures is shown in Fig. 1D. The diffraction intensities of phytogenic AgNPs were recorded in the range of 20–80. Four sharp peaks observed at 38.30, 46.60, 64.80, 77.50, respectively which indexed to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) reflections which confirm the crystalline structure of AgNPs. Moreover, three unassigned peaks in XRD pattern of AgNPs appeared at 28.2, 32.6 and 57.8 which may be related to the phytochemical compounds in the leaf extract as a capping and stabilizing agent.
Zeta potential analysis was used to determine the electric potentials in the slipping plane of nanoparticles. Polydispersity indexes (PDIs) of AgNPs was 0.719. This value confirms particles’ repulsion and increases formulation stability. Green synthesized AgNPs illustrated − 14.8 ± 2.38 mV. Therefore, it was a higher value of zeta potential and stability for green synthesized AgNPs by plant leaf extract. (Fig. 2).
Fig. 2.
Zeta potential of greensynthesised AgNPs by extract of Camelina sativa L. leaves
Growth, photosynthetic pigments and Ag accumulation
This study's results showed that at 0.5 and 1 g/L AgNPs, no significant effect was observed in the plant height; however, 25, 29.5, and 53.2% reduction in plant height was observed at concentrations of 2, 3, and 4 g/L AgNPs, respectively, compared to the control treatment (Fig. 3A). Furthermore, the results showed that the total dry weight of camelina at the concentrations of 1, 2, 3 and 4 g/L AgNPs significantly decreased by 14.8, 31.8, 36.4, and 61.8%, respectively, compared to the untreated plants (Fig. 3B).
Fig. 3.
Effect of different concentrations of AgNPs on height A and total dry weight B of Camelina sativa L. plants. Values (means ± SD, n = 5) followed by the different letter are significant at p < 0.05 (Duncan's Multiple Range Test)
The contents of chlorophyll a and b decreased in a concentration-dependent manner under AgNPs treatments with the highest decrease in the contents of chlorophyll a and b at 4 g/L AgNPs by 47.8 and 65.3%, respectively, compared to the control plants (Table 1). The carotenoid content of camelina leaves started to significantly decrease at 2 g/L AgNPs and reached the lowest carotenoid level at 4 g/L AgNPs (Table 1). Applying AgNPs had a negative effect on chlorophyll fluorescence (Fv/Fm) so that at the concentrations of 1, 2, 3 and 4 g/L AgNPs, Fv/Fm was significantly reduced by 5.7, 22.1, 37.1, and 38.2%, respectively compared to the control treatment (Table 2).
Table 1.
Effect of different concentrations of AgNPs on photosynthetic parameters and contents of proline, malondialdehyde (MDA), hydrogen peroxide and Methylglyoxal (MG) of camelina leaves
| Treatments (g/L) | Chlorophyll a | Chlorophyll b | Carotenoids | Fv/Fm | Proline | MDA | H2O2 | MG |
|---|---|---|---|---|---|---|---|---|
| mg/g fw | µmol/g fw | nmol/g fw | mg/g fw | µmol/g fw | ||||
| Control | 2.62 ± 0.14a | 1.84 ± 0.10a | 0.276 ± 0.011a | 0.682 ± 0.029a | 2.62 ± 0.19f | 3.31 ± 0.15e | 4.70 ± 0.13e | 10.51 ± 0.23e |
| 0.5 AgNP | 2.67 ± 0.15a | 1.89 ± 0.16a | 0.278 ± 0.015a | 0.677 ± 0.020a | 3.18 ± 0.21e | 3.40 ± 0.16e | 4.68 ± 0.20e | 10.42 ± 0.27e |
| 1 AgNP | 2.28 ± 0.12b | 1.46 ± 0.11b | 0.257 ± 0.014a | 0.643 ± 0.023b | 5.94 ± 0.48d | 4.42 ± 0.17d | 5.52 ± 0.18d | 11.49 ± 0.14d |
| 2 AgNP | 1.73 ± 0.10c | 1.12 ± 0.09c | 0.219 ± 0.010b | 0.531 ± 0.022c | 8.69 ± 0.43c | 4.94 ± 0.17c | 7.83 ± 0.33c | 12.16 ± 0.29c |
| 3 AgNP | 1.69 ± 0.15c | 1.19 ± 0.05c | 0.177 ± 0.013c | 0.429 ± 0.024d | 12.61 ± 0.34b | 6.94 ± 0.30b | 9.75 ± 0.38b | 14.72 ± 0.42b |
| 4 AgNP | 1.37 ± 0.15d | 0.64 ± 0.10d | 0.183 ± 0.011c | 0.421 ± 0.017d | 13.45 ± 0.28a | 8.49 ± 0.42a | 11.55 ± 0.29a | 16.39 ± 0.34a |
Values (means ± SD, n = 5) followed by the different letter are significant at p < 0.05 (Duncan's Multiple Range Test)
Table 2.
Effect of different concentrations of AgNPs on the activity of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), glyoxalase I (Gly I) and glyoxalase II (Gly II) enzymes of camelina leaves
| Treatments (g/L) | SOD | CAT | APX | GR | Gly I | Gly II |
|---|---|---|---|---|---|---|
| U/mg Pro | µmol/min mg Pro | |||||
| Control | 98.6 ± 3.42d | 93.6 ± 5.67d | 7.12 ± 0.24e | 3.78 ± 0.23d | 0.196 ± 0.014c | 0.187 ± 0.015c |
| 0.5 AgNP | 100.8 ± 5.93d | 87.5 ± 6.70d | 8.28 ± 0.18d | 4.57 ± 0.28c | 0.193 ± 0.014c | 0.193 ± 0.016c |
| 1 AgNP | 135.0 ± 7.12c | 122.6 ± 6.23c | 12.33 ± 0.27b | 4.32 ± 0.24c | 0.263 ± 0.013b | 0.253 ± 0.017b |
| 2 AgNP | 183.7 ± 8.71b | 172.2 ± 12.5b | 11.54 ± 0.17c | 7.89 ± 0.45b | 0.319 ± 0.018a | 0.307 ± 0.023a |
| 3 AgNP | 234.1 ± 9.41a | 228.2 ± 15.0a | 15.85 ± 0.48a | 11.51 ± 0.36a | 0.347 ± 0.021a | 0.281 ± 0.016ab |
| 4 AgNP | 196.4 ± 13.3b | 132.0 ± 9.41c | 16.16 ± 0.51a | 11.31 ± 0.38a | 0.196 ± 0.025c | 0.206 ± 0.022c |
Values (means ± SD, n = 5) followed by the different letter are significant at p < 0.05 (Duncan's Multiple Range Test)
Increasing AgNPs concentration enhanced Ag accumulation in the roots and shoots of camelina compared to the control treatment, and the highest accumulation of Ag in the roots and shoots observed at 4 g/L AgNPs. At all levels of AgNPs, Ag accumulation at the root was greater than Ag accumulation in the shoot (Fig. 4).
Fig. 4.
Effect of different concentrations of AgNPs on the contents of silver (Ag) in roots A and shoots B of Camelina sativa L. plants. Values (means ± SD, n = 5) followed by the different letter are significant at p < 0.05 (Duncan's Multiple Range Test)
The contents of proline, MDA, H2O2 and MG
The results revealed that AgNPs treatments increased the proline content in the leaves of camelina plant in a dose-dependent manner over the control plants and the highest increase was observed under the highest AgNPs level (4 g/L) (Table 1). The MDA content of leaves at 1 g/L AgNPs started to rise significantly and reached the maximum at 4 g/L AgNPs (Table 1). Increasing AgNPs concentration caused an enhancing trend in H2O2 production in camelina leaves compared to the control treatment so that the highest accumulation of H2O2 was observed at 4 g/L AgNPs (Table 1). It was also found that at 1, 2, 3 and 4 g/L AgNPs, the MG content significantly increased by 9.3, 15.7, 40.1 and 56%, respectively, compared to the control plants (Table 1).
The activity of antioxidant enzymes
The activity of SOD and CAT enzymes of camelina leaves was not significantly changed under 0.5 g/L AgNPs exposure. However, an increasing trend in the activity of SOD and CAT enzymes was observed up to 3 g/L AgNPs. SOD and CAT enzymes' highest activity was observed at 3 g/L AgNPs by 138 and 144% enhancement, respectively, compared to the control ones. The activity of both SOD and CAT enzymes decreased at 4 g/L AgNPs compared to 3 g/L AgNPs, although the activity of both enzymes was higher in 4 g/L AgNPs-treated plants than the control plants (Table 2).
The activity of APX and GR enzymes increased in a dose-dependent manner under AgNPs treatments and reached the maximum level of enzyme activity at 4 g/L AgNPs. The highest activity of APX and GR enzymes recorded at 4 and 3 g/L AgNPs by 127 and 205% increase, respectively, compared to the control treatment (Table 2).
This study's results showed that Gly I enzyme activity in camelina leaves began to rise at 1 g/L AgNPs and reached the highest level of activity at 3 g/L AgNPs. However, at 4 g/L AgNPs, Gly I enzyme activity showed a significant decrease compared to 3 g/L AgNPs (Fig. 4A). There was no significant difference in Gly II activity at 0.5 and 4 g/L AgNPs compared to the untreated plants. However, Gly II enzyme activity significantly enhanced at 1, 2 and 3 g/L AgNPs by 35.3, 64.2 and 50.3%, respectively compared to non-AgNPs treatment (Table 2).
The contents of ASA and GSH
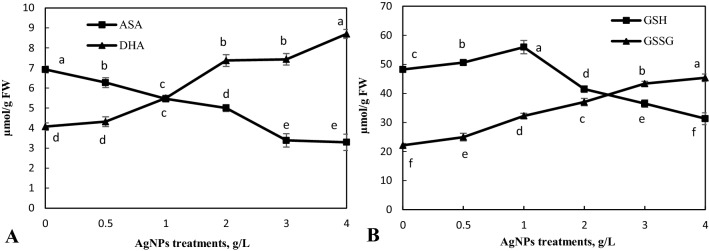
A decreasing trend was observed in ASA content by increasing AgNPs concentration which the lowest content of ASA recorded at 4 g/L AgNPs by 52.5% decrease compared to the control treatment (Fig. 5). In contrast to ASA content, DHA content at 1 g/L AgNPs began to significantly increase and reached the maximum level of DHA at 4 g/L AgNPs (Fig. 5). The results showed that the GSH content at concentrations of 0.5 and 1 g/L AgNPs increased by 4.9 and 16%, respectively, and at 2, 3 and 4 g/L AgNPs reduced by 14, 24.3 and 35%, respectively, compared to the untreated plants (Fig. 5). Applying AgNPs significantly increased GSSG content in all levels of AgNPs and the highest GSSG content was observed at 4 g/L AgNPs by 105% compared to the control plants (Fig. 5). A decreasing trend was observed in both ASA/DHA and GSH/GSSG ratios in camelina leaves by increasing AgNPs concentration. The lowest ASA/DHA and GSH/GSSG ratios were observed at 4 g/L AgNPs (Fig. 6).
Fig. 5.

Effect of different concentrations of AgNPs on the contents of ascorbic acid (ASA), Oxidized ASA (DHA), glutathione (GSH) and Oxidized glutathione (GSSG) of Camelina sativa L. plants. Values (means ± SD, n = 5) followed by the different letter are significant at p < 0.05 (Duncan's Multiple Range Test)
Fig. 6.

Effect of different concentrations of AgNPs on the ratios of ASA/DHA and GSH/GSSG of Camelina sativa L. plants. Values (means ± SD, n = 5) followed by the different letter are significant at p < 0.05 (Duncan's Multiple Range Test)
Discussion
The effect of different concentrations of AgNPs synthesized using camelina plant extract on the growth and physiological characteristics of camelina was evaluated. The results showed that applying AgNPs in a concentration-dependent manner reduced camelina growth (height and biomass). Similar results were reported from the negative effects of AnNPs on the growth of Portulaca oleracea L. (Zare et al. 2020) and Pisum sativum L. (Tripathi et al. 2017). Nair and Chung (2014) indicated that AgNPs in high concentrations induce oxidative stress; as a result, reduce the growth and biomass production in rice plants. Zare et al. (2020) showed that AgNPs at the concentrations of 20, 40, and 80 mg/L inhibited plant growth by reducing the uptake of macro and microelements and thereby disrupting ionic homeostasis. Decreased chlorophyll synthesis was reported under heavy metal toxicity (Xing et al. 2010; Kanounboule et al. 2009; Gerami et al. 2018; Ghorbani et al. 2020). This study's results showed that increasing the concentration of AgNPs reduced the content of photosynthetic pigments (Chl a, Chl b and carotenoids) and chlorophyll fluorescence (Fv/Fm) which is similar to the results reported by Jiang et al. (2012) and Tripathi et al. (2017). Decreased photosynthetic pigments may be due to increased lipid peroxidation of thylakoid membranes induced by oxidative stress under the phytotoxicity of AgNPs (Ma et al. 2013). Jiang et al. (2014) showed that AgNPs-induced oxidative stress caused ultrastructural changes in chloroplasts (fewer intergranular thylakoids and large starch grains). Nair and Chung (2014) indicated that AgNPs reduce carotenoids content which due to the role of carotenoids in quenching excess ROS can exacerbate damage to the photosynthetic apparatus under AgNPs toxicity. Therefore, the reduction in plant growth induced by AgNPs could be due to the negative effects of AgNPs on the contents of photosynthetic pigments and photosynthetic apparatus.
The results showed that Ag accumulation increased in a concentration-dependent manner in the roots and shoots of camelina under AgNPs treatments. Similar results were reported from Ag accumulation in Portulaca oleracea (Zare et al. 2020) and Pisum sativum (Tripathi et al. 2017) plants treated with AgNPs. Jiang et al. (2012) indicated that one of Ag accumulation mechanisms under AgNPs treatment could be the dissolution of AgNPs at the root surface and the uptake of released Ag ions by the root. Tripathi et al. (2017) showed that increasing Ag accumulation in the plant induces oxidative stress and thus reduces plant growth. Therefore, the decrease in camelina plant growth at high concentrations of AgNPs can be due to increased Ag accumulation; consequently, increased phytotoxic effects of Ag on the plant.
As a stress adjustor, proline protects plants against heavy metal toxicity by retaining water balance, scavenging radicals, and chelating heavy metals (Mallick 2004; Ghorbani et al. 2018a). Applying AgNPs increased the proline content due to the results reported by Khan et al. (2019) and Anna et al. (2018). Proline content increase in high concentrations of AgNPs indicates stress induction by AgNPs which the osmolyte can protect proteins from denaturation under stress conditions (Kishor et al. 2005).
Heavy metals produce ROS and induce oxidative stress, causing damage to various parts of the cell, especially membrane lipids, resulting in reduced plant growth. Thes AgNPs application in a concentration-dependent manner increased the contents of MDA, H2O2, and MG which indicates the phytotoxic effects of AgNPs on camelina plants. Increasing the content of MDA, H2O2 and MG indicates oxidative stress induction at high concentrations of AgNPs which can be one of the main reasons for reduced plant growth under AgNPs treatments. Similar results of increasing the MDA and H2O2 contents and thus inducing oxidative stress in purslane (Zare et al. 2020), pearl millet (Khan et al. 2019) and wheat (Anna et al. 2018) plants treated with nanoparticles were previously reported. Zare et al. (2020) indicated that oxidative stress induction under AgNPs treatment could be the result of increasing Ag uptake by the plant and its translocation to the shoots. Plants have an effective antioxidant defense system to reduce the toxic effects of ROS (Ghorbani et al. 2019). The enzyme SOD, by scavenging the anion superoxide, decreases radical hydroxyl production; consequently, reduces damage to biomacromolecules (Ghorbani et al. 2018b). Increasing AgNPs concentration enhanced the activity of SOD, CAT, GR and APX enzymes in camelina leaves which is due to the results previously reported by Zare et al. (2020) and Jiang et al. (2014). Nair and Chung (2014) showed that AgNPs increased the expression level of APXa, APXb, CATa, CATb, CATc and SOD (FSD1 and MSD1) genes in rice. Decreased activity of SOD and CAT enzymes at 4 g/L AgNPs indicates that APX and GR enzymes are more effective to alleviate AgNPs-induced oxidative stress. Khan et al. (2019) also indicated that AgNPs, by interacting with proteins such as antioxidant enzymes, can change their configuration and thus reduce their activity. Increasing glyoxalase system activity is critical for MG detoxification; consequently, increasing plant tolerance to environmental stresses (Yadav et al. 2005). Increased expression of Gly I and Gly II genes was shown to increase plant tolerance to abiotic stresses (Singla-Pareek et al. 2008; Saxena et al. 2011). The results showed that applying AgNPs enhanced the activity of both Gly I and Gly II enzymes. Increased activity of Gly I and Gly II enzymes under the phytotoxicity of heavy metals was previously reported by Mostofa et al. (2014) and Rahman et al. (2015). Decreased activity of Gly I and Gly II enzymes in high concentrations of AgNPs and increased accumulation of MG indicate insufficient detoxification of MG under the AgNPs toxicity which can play a significant role to reduce plant growth.
GSH and ASA, as non-enzymatic antioxidants, play an important role to alleviate the oxidative stress induced by abiotic stresses (Mahmood et al. 2010; Ghorbani et al. 2018b). The results showed that AgNPs reduced the contents of ASA and GSH and increased the contents of DHA and GSSG. Similar results were reported from the reduction of ASA and GSH contents and the ratios of ASA/DHA and GSH/GSSG in Solanum tuberosum L. treated with silver nanoparticles (Bagherzadeh Homaee and Ehsanpour 2016). Since ASA acts as a substrate for APX enzyme in H2O2 detoxification (Nath et al. 2014), ASA content reduction under AgNPs treatment may be due to increased APX activity. Due to the increase in ROS level under the AgNPs toxicity, the decline in GSH content and GSH/GSSG ratio can be due to the direct interaction of GSH with ROS (Bagherzadeh Homaee and Ehsanpour 2016). Due to the binding of Ag to the sulfhydryl groups, the decline in GSH content can also be due to GSH inactivation resulting from Ag binding.
Conclusion
Eco-friendly and reliable methods for the synthesis of metal nanoparticles is one of the essential needs in the field of nanotechnology. We have shown that in an eco-friendly, quick, and low-cost method, metal nanoparticles can be synthesized using camellia leaf extract which prevents the presence of toxic wastes and solvents. The results showed that applying AgNPs over 2 g/L negatively affected height, biomass production and photosynthetic pigments of camelina seedlings. These results also demonstrated that AgNPs at a concentration of 4 g/L had the most toxic effects on the camelina growth. The increased accumulation of MDA, H2O2 and MG, as well as increased activity of antioxidant enzymes and glyoxalase system enzymes indicated the oxidative stress induced by AgNPs as well as the induction of antioxidant defense systems to combat the AgNPs-induced oxidative stress. The results also indicate the risk of nanoparticles containing Ag on plant ecosystems and crops which can be useful to control the toxicity of nanoparticles for other crops.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anna B, Barbara K, Magdalena O. How the surface properties affect the nanocytotoxicity of silver? study of the influence of three types of nanosilver on two wheat varieties. Acta Physiol Plant. 2018;40:31. [Google Scholar]
- Bagherzadeh Homaee M, Ehsanpour AA. Silver nanoparticles and silver ions: oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic Environ Biotechnol. 2016;57:544–553. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P. The release of nanosilver from consumer products used in the home. J Environ Qual. 2010;39:1875–1882. doi: 10.2134/jeq2009.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti M. Camelina uses, genetics, genomics, production, and management. Ind Crops Prod. 2016;94:690–710. [Google Scholar]
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol. 2013;87:1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi O, Hu Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol. 2008;42:4583–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma. 2010;245(1):85–96. doi: 10.1007/s00709-010-0144-6. [DOI] [PubMed] [Google Scholar]
- Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ Intl. 2011;37:517–531. doi: 10.1016/j.envint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133(1):21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Gerami M, Ghorbani A, Karimi S. Role of salicylic acid pretreatment in alleviating cadmium-induced toxicity in Salvia officinalis L. Iranian J Plant Biol. 2018;10(1):81–95. [Google Scholar]
- Ghorbani A, Ghasemi Omran VO, Razavi SM, Pirdashti H, Ranjbar M. Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep. 2019;38:1151–1163. doi: 10.1007/s00299-019-02434-w. [DOI] [PubMed] [Google Scholar]
- Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H. Piriformospora indica inoculation alleviates the adverse effect of NaCl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.) Plant Biol. 2018;20:729–736. doi: 10.1111/plb.12717. [DOI] [PubMed] [Google Scholar]
- Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H. Piriformospora indica alleviates salinity by boosting redox poise and antioxidative potential of tomato. Russ J Plant Physiol. 2018;65:898–907. [Google Scholar]
- Ghorbani A, Tafteh M, Roudbari N, Pishkar L, Zhang W, Wu C. Piriformospora indica augments arsenic tolerance in rice (Oryza sativa) by immobilizing arsenic in roots and improving iron translocation to shoots. Ecotoxicol Environ Saf. 2020;209:111793. doi: 10.1016/j.ecoenv.2020.111793. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Fujita M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res. 2011;143(3):1758–1776. doi: 10.1007/s12011-011-8998-9. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys, 125(1):189–198 [DOI] [PubMed]
- Jiang HS, Li M, Chang FY, Li W, Yin LY. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem. 2012;31(8):1880–1886. doi: 10.1002/etc.1899. [DOI] [PubMed] [Google Scholar]
- Jiang HS, Qiu XN, Li GB, Li W, Yin LY. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ Toxicol Chem. 2014;33(6):1398–1405. doi: 10.1002/etc.2577. [DOI] [PubMed] [Google Scholar]
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants. Washington, DC: CRC Press; 2001. [Google Scholar]
- Kanounboule M, Vicente J, Nabais C, Prasad M, Freitas H. Ecophysiological tolerance of duckweeds exposed to copper. Aquat Toxicol. 2009;91:1–9. doi: 10.1016/j.aquatox.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Khan I, Raza MA, Khalid M, Awan SA, Raja NI, Zhang X, Min S, Wu BC, Hassan MJ, Huang L (2019) Physiological and biochemical responses of pearl millet (Pennisetum glaucum L.) seedlings exposed to silver nitrate (AgNO3) and silver nanoparticles (AgNPs). Int J Environ Res Public Health 16(13):2261. [DOI] [PMC free article] [PubMed]
- Kishor PK, Sangam S, Amrutha R, Laxmi PS, Naidu K, Rao K, Rao S, Reddy K, Theriappan P, Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr Sci. 2005;88:424–438. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol. 1987;148:350–382. [Google Scholar]
- Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP. Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustain Chem Eng. 2013;1:768–778. [Google Scholar]
- Mahmood Q, Ahmad R, Kwak SS, Rashid A, Anjum NA. Ascorbate and glutathione: protectors of plants in oxidative stress. In: Anjum NA, Chan MT, Umar S, editors. Ascorbate-glutathione pathway and stress tolerance in plants. Dordrecht, The Netherlands: Springer; 2010. pp. 209–229. [Google Scholar]
- Mallick N. Copper-induced oxidative stress in the chlorophycean microalga Chlorella vulgaris: response of the antioxidant system. J Plant Physiol. 2004;161:591–597. doi: 10.1078/0176-1617-01230. [DOI] [PubMed] [Google Scholar]
- Massarsky A, Dupuis L, Taylor J, Eisa-Beygi S, Strek L, Trudeau VL, Moon TW. Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere. 2013;92:59–66. doi: 10.1016/j.chemosphere.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Mostofa MG, Seraj ZI, Fujita M (2014) Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251(6):1373–1386. [DOI] [PubMed]
- Nair PM, Chung IM. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere. 2014;112:105–113. doi: 10.1016/j.chemosphere.2014.03.056. [DOI] [PubMed] [Google Scholar]
- Najafi S, Razavi SM, Khoshkam M, Asadi A. Effects of green synthesis of sulfur nanoparticles from Cinnamomum zeylanicum barks on physiological and biochemical factors of Lettuce (Lactuca sativa) Physiol Mol Biol Plants. 2020;26(5):1055–1066. doi: 10.1007/s12298-020-00793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. [Google Scholar]
- Nath S, Panda P, Mishra S, Dey M, Choudhury S, Sahoo L, Panda SK. Arsenic stress in rice: redox consequences and regulation by iron. Plant Physiol Biochem. 2014;80:203–210. doi: 10.1016/j.plaphy.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Newton KM, Puppala HL, Kitchens CL, Colvin VL, Klaine SJ. Silver nanoparticle toxicity to Daphnia magna is a function of dissolved silver concentration. Environ Toxicol Chem. 2013;32:2356–2364. doi: 10.1002/etc.2300. [DOI] [PubMed] [Google Scholar]
- Oukarroum A, Bras S, Perreault F, Popovic R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotox Environ Safe. 2012;78:80–85. doi: 10.1016/j.ecoenv.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Pilgeram AL. Camelina sativa, a montana omega-3 and fuel crop. In: Janick J, Whipkey A, editors. Issues in new crops and new uses. Alexandria: ASHS Press; 2007. pp. 129–131. [Google Scholar]
- Principato GB, Rosi G, Talesa V, Govannini E, Uolila L. Purification and characterization of two forms of glyoxalase II from the liver and brain of wistar rats. Biochim Biophys Acta Protein Struct Molec Enzym. 1987;911(3):349–355. doi: 10.1016/0167-4838(87)90076-8. [DOI] [PubMed] [Google Scholar]
- Raghunandan D, Bedre MD, Basavaraja S, Sawle B, Manjunath S, Venkataraman A. Rapid synthesis of irregular shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution. Colloids Surf B Biointerfaces. 2010;79:235–240. doi: 10.1016/j.colsurfb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Rahman A, Mostofa MG, Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2015) Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed Res Int 2015 [DOI] [PMC free article] [PubMed]
- Saxena M, Deb Roy S, Singla-Pareek SL, Sopory SK, Bhalla-Sarin N. Overexpression of the glyoxalase II gene leads to enhanced salinity tolerance in Brassica juncea. Open Plant Sci J. 2011;5:23–28. [Google Scholar]
- Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK. Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res. 2008;17(2):171–180. doi: 10.1007/s11248-007-9082-2. [DOI] [PubMed] [Google Scholar]
- Sudhasree S, Shakila Banu A, Brindha P, Kurian GA. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol Environ Chem. 2014;96:743–754. [Google Scholar]
- Sunita RB, Veera RG, Tushar KG, Robert VT, Sudarshan KL. Gold, silver, and palladium nanoparticle/nano-agglomerate generation, collection, and characterization. J Nanopart Res. 2011;13:6591–6601. [Google Scholar]
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6:257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Torres SK, Campos VL, Leon CG, Rodr´ıguez-Llamazares SM, Rojas SM, Gonzalez M, Smith C, Mondaca MA (2012) Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J Nanopart Res 14:1236
- Tripathi DK, Singh S, Singh S, Srivastava PK, Singh VP, Singh S, Prasad SM, Singh PK, Dubey NK, Pandey AC, Chauhan DK. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem. 2017;110:167–177. doi: 10.1016/j.plaphy.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Vishwakarma K, Shweta UN, Singh J, Liu S, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S. Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front Plant Sci. 2017;8:1501. doi: 10.3389/fpls.2017.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van De Meent D. Nano-silver-a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3:109–138. [Google Scholar]
- Wild R, Ooi L, Srikanth V, Mu¨nch G, A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-L-cysteine assay. Anal Bioanal Chem. 2012;403(9):2577–2581. doi: 10.1007/s00216-012-6086-4. [DOI] [PubMed] [Google Scholar]
- Xing W, Huang WM, Liu GH. Effect of excess iron and copper on physiology of aquatic plant Spirodela polyrrhiza (L.) Schleid. Environ Toxicol. 2010;25:103–112. doi: 10.1002/tox.20480. [DOI] [PubMed] [Google Scholar]
- Xiu ZM, Ma J, Alvarez PJ. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol. 2011;45:9003–9008. doi: 10.1021/es201918f. [DOI] [PubMed] [Google Scholar]
- Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12:4271–4275. doi: 10.1021/nl301934w. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK. Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants. 2005;11(1):1–11. [Google Scholar]
- Yang Y, Wang J, Xiu Z, Alvarez PJ. Impacts of silver nanoparticles on cellular and transcriptional activity of nitrogen-cycling bacteria. Environ Toxicol Chem. 2013;32:1488–1494. doi: 10.1002/etc.2230. [DOI] [PubMed] [Google Scholar]
- Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES. More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol. 2011;45:2360–2367. doi: 10.1021/es103995x. [DOI] [PubMed] [Google Scholar]
- Yin N, Liu Q, Liu J, He B, Cui L, Li Z, Yun Z, Qu G, Liu S, Zhou Q, Jiang G. Silver nanoparticle exposure attenuates the viability of rat cerebellum granule cells through apoptosis coupled to oxidative stress. Small. 2013;9:1831–1841. doi: 10.1002/smll.201202732. [DOI] [PubMed] [Google Scholar]
- Yu CW, Murphy TM, Lin CH. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol. 2003;30(9):955–963. doi: 10.1071/FP03091. [DOI] [PubMed] [Google Scholar]
- Zare Z, Pishkar L, Iranbakhsh A, Talei D. Physiological and molecular effects of silver nanoparticles exposure on purslane (Portulaca oleracea L.) Russ J Plant Physiol. 2020;67:521–528. [Google Scholar]
- Zhang Y, Ferguson SA, Watanabe F, Jones Y, Xu Y, Biris AS, Hussain S, Ali SF. Silver nanoparticles decrease body weight and locomotor activity in adult male rats. Small. 2013;9:1715–1720. doi: 10.1002/smll.201201548. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Jiang YN, He ZY, Ma M. Cadmium accumulation and oxidative burst in garlic (Allium sativum) J Plant Physiol. 2005;162:977. doi: 10.1016/j.jplph.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Zhao CM, Wang WX. Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem. 2011;30:885–892. doi: 10.1002/etc.451. [DOI] [PubMed] [Google Scholar]