Abstract
Dirofilaria immitis and Brugia pahangi are vector-borne parasites found in dogs and cats, including Thailand. In order to evaluate the effects of season and environmental parameters on the prevalence of these parasites, this retrospective study was conducted in 2019. A total of 79,506 canine blood samples were examined. B. pahangi was found in 0.55% of samples (438/79,506; 95% CI 0.50–0.61) while D. immitis was detected in 0.43% (345/79,506; 95% CI 0.39–0.48). One-way ANOVA found no effect of seasonal conditions on prevalence. For B. pahangi, the parameters rainfall, relative humidity and sunshine hours showed associations with p ≤ 0.20 and were included in multiple logistic regressions resulting in adjusted odds ratios of 0.53, 1.31 and 0.55, respectively. For D. immitis, only average temperature showed p ≤ 0.20, resulting in an odds ratio of 0.42. In conclusion, Thailand has environmental parameters that do not change very much during the year, so they might not affect the prevalence of two filarial nematodes. However, the threat of B. pahangi and D. immitis should not be ignored, especially in subtropical regions where their vectors are abundant. Both owners and veterinarians should be concerned about filarial prevention and control of D. immitis and B. pahangi.
Subject terms: Parasite biology, Parasite development, Infectious diseases
Introduction
Dirofilaria immitis and Brugia pahangi are vector-borne parasites in dogs and cats that have zoonotic potential and are common in tropical, subtropical and some temperate regions of the world, including Thailand1. D. immitis is well known as a causative agent of heartworm disease in dogs and cats2,3. Microfilariae of the dog heartworm D. immitis present a subperiodicity without clear nocturnal or diurnal peaks, where a wave pattern is apparent but microfilaria do not completely disappear from the peripheral blood. However, the physiological periodicity is unknown4. It is also known as an occasional cause of pulmonary dirofilariasis in humans5. Lymphatic filariasis, a neglected tropical disease that is caused by filarial nematodes in the genus Brugia, affects approximately 80 countries around the world, particularly in dogs, cats and humans6.
Mosquitoes in the genera Mansonia, Armigeres and Aedes are potential vectors of dirofilariasis and lymphatic filariasis7. The tropical atmosphere is the most suitable for these mosquito vectors to survive. Increasing global temperatures and humidity are advantageous to the spread of mosquitoes and are also enhancing the effectiveness of pathogen transmission through mosquito-borne diseases such as dengue, malaria and lymphatic filariasis8. For instance, there is some evidence indicating that the prevalence of mosquito-borne disease in South America and South-East Asia relates to the El-Niño phenomenon. The relationship between El-Niño and the increasing risk of these diseases can be attributed to the rise in global temperature9–11.
Adults of D. immitis reside in the pulmonary artery and can induce endothelial damage. Some cases may develop into canine eosinophilic pulmonary granulomatosis caused by the infiltration of eosinophils12. The severity of heartworm disease depends on the number of adult worms, the duration of infection and the host immune response. Adult heartworms can release vasoactive substances that result in vasoconstriction and hypoxia, which lead to pulmonary hypertension, and antigens may pass through to the lung causing eosinophilic pneumonitis13. Chronic infection leads to retrograde migration of adults to the right atrium and vena cava causing deflection of the tricuspid valve, resulting in clinical signs of right-sided heart failure14. B. pahangi infection can manifest in four ways: (1) no clinical signs with no microfilaremia; (2) no clinical signs with microfilaremia; (3) acute short duration lymph node enlargement and/or limb oedema with microfilaremia; and (4) chronic limb oedema without microfilaremia15. Experimentally infected dogs showed abscesses in the adipose connective tissue around the popliteal node and nerve-cell tumours near the sciatic nerve. In one dog, lymphatic ducts were dilated distal to the popliteal node16. Dogs demonstrated a range of clinical signs, including episodic lymphadenopathy, lymphangitis, and limb oedema similar to the clinical signs reported in humans17.
Only a few studies performed in South-East Asian countries have reported the incidence and the population of animals affected by dirofilariasis and lymphatic filariasis18–20, and no studies have been designed to monitor the environmental factors affecting the distribution of these diseases. This study evaluated the association between seasonal and environmental factors related to the prevalence of D. immitis and B. pahangi infections in domestic dogs in Bangkok, Thailand and its vicinity.
Results
Brugia pahangi was found in 0.55% (438/79,506; 95% CI 0.50–0.61) and D. immitis was detected in 0.43% (345/79,506; 95% CI 0.39–0.48) of 79,506 samples tested during January to December 2019. The monthly detection rate of B. pahangi and D. immitis is shown in Table 1. The prevalence of B. pahangi infection was higher than that of D. immitis in all months.
Table 1.
Prevalence of B. pahangi and D. immitis positive rate during January–December 2019.
| Months | B. pahangi (%) | 95% CI | D. immitis (%) | 95% CI | N |
|---|---|---|---|---|---|
| January | 0.42 | 0.28–0.60 | 0.11 | 0.25–0.55 | 6925 |
| February | 0.64 | 0.46–0.86 | 0.16 | 0.33–0.68 | 6448 |
| March | 0.69 | 0.51–0.91 | 0.11 | 0.22–0.51 | 6960 |
| April | 0.52 | 0.35–0.74 | 0.10 | 0.22–0.54 | 5965 |
| May | 0.53 | 0.37–0.73 | 0.17 | 0.35–0.70 | 6777 |
| June | 0.50 | 0.35–0.69 | 0.08 | 0.22–0.51 | 7002 |
| July | 0.53 | 0.37–0.72 | 0.15 | 0.28–0.59 | 7219 |
| August | 0.48 | 0.33–0.67 | 0.14 | 0.32–0.66 | 7057 |
| September | 0.57 | 0.41–0.78 | 0.18 | 0.40–0.76 | 7000 |
| October | 0.39 | 0.26–0.57 | 0.16 | 0.36–0.71 | 6838 |
| November | 0.69 | 0.49–0.93 | 0.15 | 0.33–0.71 | 5826 |
| December | 0.71 | 0.51–0.97 | 0.12 | 0.27–0.54 | 5489 |
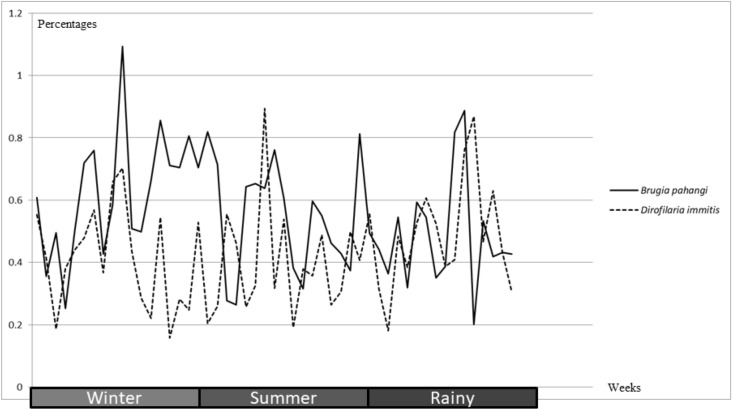
The prevalence by season is shown in Fig. 1. The year was divided into three seasons; winter (November, December, January and February), summer (March, April, May and June) and the rainy season (July, August, September and October). The association between season and prevalence was not significant for either B. pahangi (p = 0.23) or D. immitis (p = 0.09).
Figure 1.
Line chart demonstrated positive rates of B. pahangi and D. immitis by seasons.
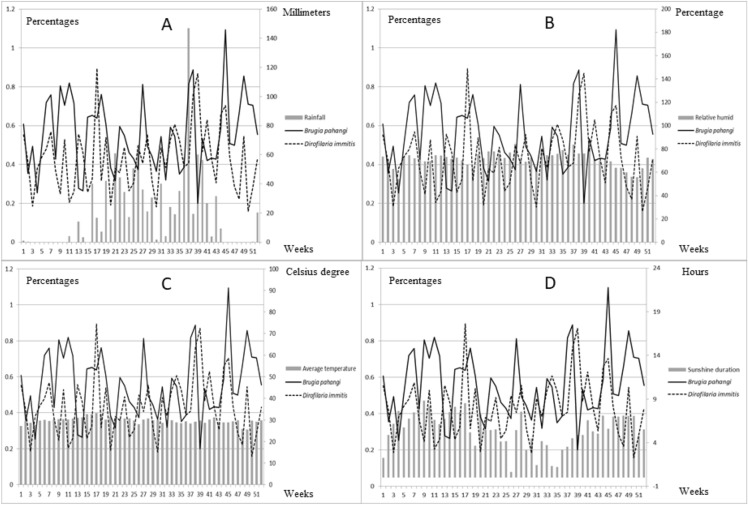
The environmental parameters related to season, including rainfall, relative humidity, average temperature and sunshine duration, were selected based on vector and parasite biology. These parameters along with infection rates of B. pahangi and D. immitis are shown in Fig. 2.
Figure 2.
Line chart (B. pahangi and D. immitis) revealed positive of each filarial by week and bar chart demonstrate parameters including rainfall (A), relative humidity (B), average temperature (C) and sunshine duration (D).
Environmental factors, including rainfall, relative humidity, average temperature and sunshine duration, were analysed to reveal associations between infection rates and each factor using Pearson’s correlation. The results revealed no significant relation for any parameter, as shown in Table 2; hence, no parameter had a significant effect and linear regression was not performed.
Table 2.
Showed the correlation of environmental parameters between B. pahangi and D. immitis using Pearson’s correlation.
| Parameters | B. pahangi | D. immitis | ||||
|---|---|---|---|---|---|---|
| r value | 95% CI | p value | r value | 95% CI | p value | |
| Rainfall | − 0.11 | − 0.37–0.17 | 0.43 | 0.15 | − 0.13–0.41 | 0.27 |
| Sunshine duration | 0.19 | − 0.09–0.44 | 0.19 | − 0.11 | − 0.38–0.17 | 0.43 |
| Relative humidity | − 0.15 | − 0.41–0.13 | 0.30 | 0.19 | − 0.09–0.44 | 0.19 |
| Average temperature | − 0.07 | − 0.35–0.20 | 0.59 | − 0.06 | − 0.33–0.22 | 0.69 |
Since continuous data analysis using Pearson’s correlation could not describe the association, categorical data analysis was performed by transforming the data using the average of each parameter as the cutoff: 20.38 mm rainfall, 29.78 °C average temperature, 5.52 h sunshine duration and 71.35% relative humidity, and the prevalence of B. pahangi 0.55% and D. immitis 0.43%. Categorical analyses including crude odds ratio and 95% CI are shown in Tables 3 and 4. The parameters showing p ≤ 0.20 were included in multiple logistic regression, which was used to calculate the adjusted odds ratio.
Table 3.
Univariates and multivariable logistic regression demonstrate association between B. pahangi infection and environment parameters.
| Parameters | Crude odds ratio | 95% CI | p value | Adjusted odd ratio |
|---|---|---|---|---|
| Rainfall | 0.41 | 0.12–1.34 | 0.14* | 0.53 |
| Sunshine duration | 2.07 | 0.67–6.38 | 0.20* | 1.31 |
| Relative humidity | 0.44 | 0.14–1.39 | 0.16* | 0.55 |
| Average temperature | 1.09 | 0.36–3.29 | 0.88 | ND |
ND not determined, *p > 0.20.
Table 4.
Univariates and multivariable logistic regression demonstrate association between D. immitis infection and environment parameters.
| Parameters | Crude odds ratio | 95% CI | p value | Adjusted odd ratio |
|---|---|---|---|---|
| Rainfall | 1.26 | 0.40–3.93 | 0.69 | ND |
| Sunshine duration | 1.48 | 0.49–4.48 | 0.48 | ND |
| Relative humidity | 1.56 | 0.58–1 | 0.45 | ND |
| Average temperature | 0.42 | 0.14–1.28 | 0.12 | ND |
ND not determined, *p > 0.20.
The results revealed that only average temperature showed an association with D. immitis infection (p ≤ 0.20). However, three parameters were included in the logistic regression in order to estimate associations between environmental parameters and B. pahangi infection. The multicollinearity between relative humidity and rainfall was checked using the Chi-square test and the results revealed no multicollinearity between these parameters. Unfortunately, no parameter was significant using logistic regression. The adjusted odds ratio (OR) for each parameter associated with B. pahangi infection was as follows: rainfall 0.53, sunshine duration 1.31 and relative humidity 0.55.
Discussion
The current study presents the prevalence of B. pahangi and D. immitis circulating in owned dogs in Bangkok and its vicinity in 2019. A large number of the samples were collected from diseased dogs, which may have influenced the prevalence recorded herein. On the other hand, no antigen detection studies were carried out to detect amicrofilaemic animals, and only those that had detectable microfilariae in blood were counted. A limitation of this study was that samples were obtained from a clinical laboratory, and thus we do not have any details about the infected dogs. In this study, the monthly prevalence of the two filarial nematodes shown in Table 1 revealed that B. pahangi was more common than D. immitis. According to veterinarians, owners protect their pets from heartworm disease by using commercial products comprising extra-label ivermectin and its derivatives to control ticks, and these measures have been reducing the prevalence of D. immitis. In 2003, Nithiuthai reported that 10.2% of samples contained microfilaria of D. immitis (n = 83,476) in Bangkok during 1999–200121. Unfortunately, given the lack of a prevention programme for B. pahangi, there is no medication to prevent B. pahangi infection; one of the important outcomes of this study was that the prevalence of canine lymphatic filariasis is higher than canine heartworm disease.
Our analysis showed that prevalence was not influenced by the season. Interestingly, D. immitis was more prevalent than B. pahangi in weeks 17, 34 and 41. Thailand is in South-East Asia, which has a tropical savanna climate under the influence of the South Asian monsoon system. Some environmental parameters vary little throughout the year, including average temperature and humidity, thus making it difficult to associate these parameters with filarial nematode infection. There were no significant correlations between infection rate and these environmental parameters. However, sunshine duration seemed to show some correlation with B. pahangi infection rates (p = 0.19), as did relative humidity with that of D. immitis (p = 0.19). It was assumed that the longer daylight during summer affects some endocrine mechanisms in the dog, which may stimulate the female filarial worms to produce greater numbers of microfilariae4. Univariate analysis of B. pahangi prevalence revealed that three parameters showed p-values of ≤ 0.20: rainfall, relative humidity and sunshine duration, and so these were included in the multivariable logistic regression. Unfortunately, no parameter was significant in this regression; however, sunshine duration showed a positive adjusted OR of 1.31, which related to correlation. Vectors of most importance for filarial nematodes include Aedes spp. Culex spp. and Anopheles spp. and these are considered as potential vectors for D. immitis22,23, whereas Armigeres spp. and Manosonia spp. are considered as vectors for B. pahangi 24. The OR-values of rainfall associated with B. pahangi and D. immitis prevalence were interesting: that for B. pahangi was 0.41, indicating that the rains are a protective factor, by washing floating water plants out of swamp ponds and leading to a lack of suitable places for the life cycle of Mansonia spp.25, one of its potential vectors. On the other hand, the OR-value for D. immitis was 1.26, indicating that rain is a risk factor, due to clean water being suitable for Aedes spp.23, one of its potential vectors.
Brugia pahangi and D. immitis are not the only two filarial nematodes reported in Thailand; other filaria include Brugia malayi26 and Dirofilaria repens27. In this study, D. repens was found in one sample in December. Microfilaria of D. repens can be differentiated morphologically as they have two nuclei in the cephalic space. Molecular diagnosis is an alternative method for identification of D. immitis and D. repens.
Conclusions
In conclusion, environmental parameters in Thailand do not change much during the year, so they might not affect the prevalence of B. pahangi and D. immitis. The prevalence of these two filarial nematodes should not be ignored, and owners and veterinarians should be educated in the prevention and control of filarial nematodes in order to decrease the prevalence of these neglected canine vector-borne diseases.
Methods
Study design and sample collection
This retrospective study was conducted between January and December 2019. The results of blood examination were provided by the Vet Central Lab, which collected samples from private veterinary clinics and animal hospitals around Bangkok and its vicinity. All environmental parameters, including rainfall, relative humidity, average temperature and sunshine duration were obtained from the information service of the Thai Meteorological Department, Ministry of Digital Economy and Society. The data were the average from four stations in Bangkok: the Queen Sirikit National Convention Center, Bangkok Port, Thai Meteorological Department Bang Na and Don Mueang International Airport.
Blood examination and parasite identification
A total of 79,506 EDTA-anticoagulated blood samples were collected from owned dogs and submitted to the Vet Central Lab. Buffy-coat thin blood smears were performed and stained with Wright-Giemsa stain. The positive microfilariae were examined by light microscopy. Unsheathed and sheathed microfilaria were tested for acid phosphatase activity to identify species as D. immitis28 and B. pahangi28, respectively.
Statistical analysis
The prevalence of filarial worms was demonstrated using descriptive statistics with a 95% confidence interval (95% CI). The association between filarial worm infection and environmental parameters was analysed as continuous data using Pearson’s correlation. Since one week was considered as a replicate, each parameter was represented as the average per week, except rainfall, which combined all data to represent one week. If these results were not acceptable, the infection rate and other continuous data were transformed using the average of each parameter or infection rate as the cutoff and analysed using the crude odds ratio at 95% CI. The parameters showing p ≤ 0.20 were checked for multicollinearity and included in multiple logistic regression. Statistical analysis used R software version 3.5.3.
Acknowledgements
This study was supported by Chulalongkorn University (CU_GR_63_42_31_03). The authors would like to thank all staff at the Vet Central Lab who contributed the retrospective data and the Thai Meteorological Department who provided the environmental parameters included in this study.
Author contributions
W.J., P.P. and P.T. prepared the main manuscript text, figures and tables. P.K. and S.C. were responsible for laboratory results. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balbo T, Abate O. Histochemical differentiation of microfilariae of Dirofilaria immitisDirofilaria repens and Dipetalonema sp. Parassitologia. 1972;14:239–244. [Google Scholar]
- 2.Roncalli R, Yamane Y, Nagata T. Prevalence of Dirofilaria immitis in cats in Japan. Vet. Parasitol. 1998;75:81–89. doi: 10.1016/S0304-4017(97)00194-5. [DOI] [PubMed] [Google Scholar]
- 3.Miller M, Atkins C, Stemme K, Robertson-Plouch C, Guerrero J. Prevalence of exposure to Dirofilaria immitis in cats in multiple areas of the United States. Vet. Ther.. 2000;1:169–175. [PubMed] [Google Scholar]
- 4.Lovis L, Grandjean M, Overney L, Seewald W, Sager HJVP. Seasonality and circadian variation of microfilaremia in dogs experimentally infected with Dirofilaria immitis. Vet. Parasitol. 2017;243:235–241. doi: 10.1016/j.vetpar.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Pampiglione S, Rivasi F, Gustinelli A. Dirofilarial human cases in the Old World, attributed to Dirofilaria immitis: a critical analysis. Histopathology. 2009;54:192–204. doi: 10.1111/j.1365-2559.2008.03197_a.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RM, May RM. Population dynamics of human helminth infections: Control by chemotherapy. Nature. 1982;297:557–563. doi: 10.1038/297557a0. [DOI] [PubMed] [Google Scholar]
- 7.Edeson J, Wharton R, Laing A. A preliminary account of the transmission, maintenance and laboratory vectors of Brugia pahangi. Trans. R. Soc. Trop. Med. Hyg. 1960;54:439–449. doi: 10.1016/0035-9203(60)90089-4. [DOI] [PubMed] [Google Scholar]
- 8.Gubler DJ, et al. Climate variability and change in the United States: Potential impacts on vector-and rodent-borne diseases. Environ. Health Perspect. 2001;109:223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouma M, et al. Predicting high-risk years for malaria in Colombia using parameters of El Niño Southern Oscillation. Trop. Med. Int. Health. 1997;2:1122–1127. doi: 10.1046/j.1365-3156.1997.d01-210.x. [DOI] [PubMed] [Google Scholar]
- 10.Bouma MJ, Dye C. Cycles of malaria associated with El Niño in Venezuela. JAMA. 1997;278:1772–1774. doi: 10.1001/jama.1997.03550210070041. [DOI] [PubMed] [Google Scholar]
- 11.Bouma MJ, van der Kaay HJ. The EI Niño Southern oscillation and the historic malaria epidemics on the Indian subcontinent and Sri Lanka: An early warning system for future epidemics? Trop. Med. Int. Health. 1996;1:86–96. doi: 10.1046/j.1365-3156.1996.d01-7.x. [DOI] [PubMed] [Google Scholar]
- 12.Abbott DEE, Allen AL. Canine eosinophilic pulmonary granulomatosis: Case report and literature review. J. Vet. Diagn. Investig. 2020;32:329–335. doi: 10.1177/1040638720907659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoch H, Strickland K. Canine and feline dirofilariasis: Life cycle, pathophysiology, and diagnosis. Compendium. 2008;30:133. [PubMed] [Google Scholar]
- 14.Strickland KN. Canine and feline caval syndrome. Clin. Tech. Small Anim. Pract. 1998;13:88–95. doi: 10.1016/S1096-2867(98)80012-1. [DOI] [PubMed] [Google Scholar]
- 15.Snowden K, Hammerberg B. Dynamics of immune responses related to clinical status in Brugia pahangi-infected dogs. Am. J. Trop. Med. Hyg. 1987;37:143–151. doi: 10.4269/ajtmh.1987.37.143. [DOI] [PubMed] [Google Scholar]
- 16.Schacher JF, Sahyoun PF. A chronological study of the histopathology of filarial disease in cats and dogs caused by Brugia pahangi (Buckley and Edeson, 1956) Trans. R. Soc. Trop. Med. Hyg. 1967;61:234–243. doi: 10.1016/0035-9203(67)90162-9. [DOI] [PubMed] [Google Scholar]
- 17.Snowden KF, Hammerberg B. The lymphatic pathology of chronic Brugia pahangi infection in the dog. Trans. R. Soc. Trop. Med. Hyg. 1989;83:670–678. doi: 10.1016/0035-9203(89)90394-5. [DOI] [PubMed] [Google Scholar]
- 18.Gubler D. The global pandemic of dengue/dengue haemorrhagic fever: Current status and prospects for the future. Ann. Acad. Med. Singapore. 1998;27:227. [PubMed] [Google Scholar]
- 19.Pedersen EM, Stolk WA, Laney SJ, Michael E. The role of monitoring mosquito infection in the global programme to eliminate lymphatic filariasis. Trends Parasitol. 2009;25:319–327. doi: 10.1016/j.pt.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Fischer P, et al. PCR-based detection and identification of the filarial parasite Brugia timori from Alor Island, Indonesia. Ann. Trop. Med. Parasitol. 2002;96:809–821. doi: 10.1179/000349802125002239. [DOI] [PubMed] [Google Scholar]
- 21.Nithiuthai, S. Risk of Canine Heartworm Infection in Thailand. in Proceeding of the WSAVA 2003 Congress, Bangkok, October. 24–27.
- 22.Labarthe N, Serrão ML, Melo YF, Oliveira SJD, Lourenço-de-Oliveira R. Potential vectors of Dirofilaria immitis (Leidy, 1856) in Itacoatiara, oceanic region of Niterói municipality, State of Rio de Janeiro Brazil. Memórias do Instituto Oswaldo Cruz. 1998;93:425–432. doi: 10.1590/S0074-02761998000400001. [DOI] [PubMed] [Google Scholar]
- 23.Ledesma N, Harrington L. Mosquito vectors of dog heartworm in the United States: Vector status and factors influencing transmission efficiency. Topics Compan. Anim. Med. 2011;26:178–185. doi: 10.1053/j.tcam.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Beckett EB, Macdonald W. The distribution of larvae of Brugia malayi and Brugia pahangi in the flight muscle fibres of Aedes aegypti and Mansonia uniformis. Parasitology. 1970;61:211–218. doi: 10.1017/S0031182000041032. [DOI] [PubMed] [Google Scholar]
- 25.Wharton RH. The biology of Mansonia mosquitoes in relation to the transmission of filariasis in Malaya. Bull Inst Med Res Kuala Lumpur. 1962;11:1–114. [PubMed] [Google Scholar]
- 26.Sarasombath PT, et al. First study of topical selamectin efficacy for treating cats naturally infected with Brugia malayi and Brugia pahangi under field conditions. Parasitol. Res. 2019;118:1289–1297. doi: 10.1007/s00436-019-06248-3. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz E, et al. High genetic diversity in the Dirofilaria repens species complex revealed by mitochondrial genomes of feline microfilaria samples from Narathiwat Thailand. Trans. Emerg. Dis. 2019;66:389–399. doi: 10.1111/tbed.13033. [DOI] [PubMed] [Google Scholar]
- 28.Chungpivat S, Taweethavonsawat P. The differentiation of microfilariae in dogs and cats using Giemsa's staining and the detection of acid phosphatase activity. J. Thai. Vet. Prac. 2008;20:47–55. [Google Scholar]