Abstract
Vigna mungo (L.) Hepper commonly known as blackgram is an important legume crop with good quality dietary proteins and vitamins. Low production of blackgram in the chromium rich soil of Odisha is a serious concern against its demand. Chromium (VI) was tested on V. mungo var. B3-8-8 at 100, 150, 200, 250 and 300 µM concentration on growth, anti-oxidative enzymes and chromium content at 15, 30 and 45 d of treatments. Seed germination and growth decreased with increase dose and duration. Cr uptake induced oxidative burst with significant increase of osmolytes was observed in cell at lower doses but failed to adjust homeostasis at higher dose. Increase of GPX and SOD and decrease of CAT was observed as dose dependent. Increased protein content was detected in < 200 µM Cr concentration whereas, significant decrease of protein was noted thereafter. Down regulation of proteins (29.2 kDa and 32.6 kDa) was observed at > 250 µM of Cr. Total Cr uptake was greater in root than in shoot which might be due to poor translocation of heavy metal or detoxification. Thus, blackgram was able to maintain homeostasis at lower concentrations of Cr by activating the cascade of enzymes following cellular detoxification mechanism.
Supplementary Information
The online version of this article contains supplementary material available at (10.1007/s12298-021-00941-3)
Keywords: Antioxidant enzymes, Chromium accumulation, Hyrdoponic culture, Metal induced protein, ROS scavenging
Introduction
Blackgram [Vigna mungo (L.) Hepper], popularly called as ‘urad bean’ in Indian households, is the third important leguminous pulse in India and is probably the best source of easily digestible good quality protein (26% protein) and vitamins (A, B1 and B3). This crop is generally cultivated after paddy harvest in the rain-fed agriculture to enrich the soil through biological nitrogen fixation (Kannaiyan 1999). Blackgram is an essential component and its complementary relationship with the vital amino acids (arginine, isoleucine, leucine, lysine, phenylalanine, valine) improves greatly the biological value of diet with wheat or rice. It is an annual crop native to central Asia, extensively cultivated in India and also in Australia, Thailand and several countries in Asia and South Pacific (Poehlman 1991). Being resistant to drought, this crop is fit for dry land cultivation and mostly used for intercropping (Tawfik and Steiner 2011). Lack of suitable varieties and genotypes with adaptation against heavy metal contaminated soil, salinity and drought limits production of this crop (Srivastav et al. 2011).
Toxicity of heavy metal on quality and production of agriculturally important economic crops create challenges for farming efficiency and poses serious concern (Bishehkolaei et al. 2011). Being an inorganic chemicals, heavy metals are of > 20 atomic mass and density is > 5 g cm−3. Cytotoxicity, mutagenicity and genotoxicity of heavy metals on all living organisms are pronounced and owing to their non-biodegradability and persistence causing human health hazard (Mudgal et al. 2010). Higher levels of heavy metals are associated with the increased production of reactive oxygen species (ROS) such as hydroxyl free radicals (OH−) or non-free radical species such as singlet oxygen (1O2), superoxide free radicals (O2−), and hydrogen peroxide (H2O2). Chromium (Cr) is highly toxic out of the hazardous metal elements common in soil and absorbed by the plant resulting in anomalies in plant metabolism. Contamination of the soil by Cr, mostly available as oxides, hydroxides and sulfates, thus becomes a principal agony for its toxicity level in plants that trusts on its valency i.e. Cr (VI) being more mobile and toxic than the trivalent form (Tokunaga et al. 2003; Saminathan 2013). Chromium is quickly oxidized to its hexavalent form in acidic soils in the existence of manganese (Mn) that affect growth and development of plant adversely (Ali et al. 2011). Cr(VI) is a priority pollutant than its trivalent counterpart and more toxic for the entire biota (Oliveira 2012).
However, accumulation of Cr generally found in roots which translocated to the aerial parts very little (Paiva et al. 2009; Sundaramoorthy et al. 2010). Mostly roots has 100-fold greater concentration of Cr content than in the shoots (Zayed et al. 1998). The factors which dictate its uptake include Cr concentration, valencies and pH, salinity of the medium (Babula et al. 2008). Once Cr enters the plants, it relies on sulfur and iron channels for translocation to the shoots. Inhibited germination of seed, root growth drop, chlorosis mediated low photosynthetic rates (Anjum et al. 2016), reduced biomass and protein inactivation (Mota et al. 2015) are reported in plants under Cr toxicity (Sharma et al. 1995; Babu et al. 2014). The Cr toxicity on soil microorganisms (Xie et al. 2016), and plant growth promoting rhizobacteria (Saif and Khan 2018; Khanna et al. 2019) on wheat (Adrees et al. 2015) and chickpea (Kaur and Nayyar 2013; Imtiaz et al. 2016) have been reported time to time. Chromium stress induced antioxidative response to glutathione metabolism have been detailed in Pisum sativum (Duhan 2012) and in Vigna radiata (Shanker et al. 2004). Recently, Sharma et al. (2020) reviewed the impacts of Cr bioaccumulation in plants. Relatively, very scanty reports are available on tolerance capacity and mechanism of Cr in blackgram. Thus, it becomes essential to evaluate the important physiological parameters and antioxidant enzyme levels of blackgram to establish a relationship between extent of cellular damage, maintenance of homeostasis and the possible role of enzymes in conferring tolerance to V. mungo. A considerable amount of work has been carried out with respect to Cr (VI) toxicity but our knowledge is very much restricted on exact mechanisms of Cr (VI) stress tolerance in blackgram and a relationship is yet to be established between maintenance of normal plant metabolism and the levels of antioxidative enzymes (Malar et al. 2014). The current investigation was conducted to examine the effects of varying hexavalent chromium concentrations on seed germination, biochemical changes along with alterations in the protein profile and probable changes in the antioxidative enzyme levels on a high yielding popular blackgram variety [Vigna mungo (L.) Hepper] var. B3-8-8 under hydroponic culture in the seedling stage for sustainable agricultural practice of this valuable legume crop in Cr contaminated mine area of Odisha, India.
Materials and methods
Seed sterilization and experimental plan
Blackgram seed [Vigna mungo (L.) Hepper] var. B3-8–8 was obtained from the Orissa University of Agriculture and Technology, Bhubaneswar, Odisha. Seeds uniform in size and weight were sterilized superficially with absolute alcohol for 5 min followed by 0.2% mercuric chloride (HgCl2) treatment for 25 min followed by 2–3 washes in double distilled water and subsequently soaked in distilled water for overnight. Potassium dichromate (K2Cr2O7) having the molecular weight of 294.185 g mole−1 was chosen as the Cr (VI) source. Different concentrations of chromium (100, 150, 200, 250 and 300 µM) were prepared from 1 mM freshly prepared stock solution in modified Hoagland’s (Hoagland and Arnon 1950) solution after Epstein (1972) as described by Taiz and Zeiger (2002) and were used for experiments. The experiments were replicated thrice and the mean data of each parameter was recorded. The surface sterilized seeds of blackgram were placed in sterilized petridishes overnight containing filter paper soaked with double distilled water uniformly. Next day the seeds were shifted to thermocole cups containing Hoagland’s nutrient solution containing appropriate concentrations of chromium that were equipped with an air bubbler. Control plants were maintained and treated in Hoagland’s solution. The control as well as treated plants were collected for different Cr concentrations after 15, 30 and 45 days of experimentations.
Seed germination
For the estimation of the germination percentage, the emergence of radical was considered as the deciding factor. For the estimation of shoot and root lengths, plants collected from different concentrations were measured in different days of treatment. For estimation of fresh weight, whole plants were weighed after thorough washing with tap water followed by wiping in blotting paper. Estimation of dry weight was done by covering whole plants with aluminum foil and dried until their weight remained constant.
Estimation of total Cr content
Total Cr content was evaluated in 15 d, 30 d and 45 d treated samples by Inductively Coupled Plasma—Optical Emission Spectrometry (ICP-OES, Perkin Elmer Avio 200, USA) after extraction by the method of Davies et al. (2002). Briefly, harvested roots and shoots were oven dried (65 °C, 48 h) and 0.5 g of ground samples were digested with concentrated HNO3 and H2O2 in a micro digester. Digested solutions were filtered and analyzed for total Cr concentrations in ICP-OES and the data was calculated in terms of mg kg−1 d.w.
Biochemical analysis
Estimation of photosynthetic pigments
The estimation of chlorophyll (Chl) and carotenoid contents were undertaken using Arnon’s method (Arnon 1949). The absorbance of the supernatant was measured at 663 nm, 645 nm and 480 nm using an UV–VIS spectrophotometer for Chl a, Chl b and carotenoids, respectively.
Estimation of carbohydrate and reducing sugar
The total carbohydrate content was estimated following the anthrone reagent method of DuBois et al. (1956) and the absorbance was read at 630 nm. The reducing sugar was measured following the procedure described by Nelson-Somogyi (Nelson 1944; Somogyi 1952) and the O.D. was checked at 525 nm.
Estimation of proline and polyphenol content
For the estimation of proline, Bates' method (Bates et al. 1973) was followed and absorbance was recorded spectrophometrically at 520 nm. The content of polyphenol was estimated following a modified method of Singleton and Rossi (1965). The values were plotted in a standard curve (prepared with gallic acid solution) and the total phenolic content was calculated as mg of gallic acid equivalents per g of fresh weight (mg GAE g−1 f.w.).
Enzyme extraction and assay for enzyme activity
The extraction of leaf tissues was done following the method of Gossett et al. (1994). The supernatants obtained from the centrifuged (for 10 min at 10,000 × g) homogenate were collected and utilised for the assays of CAT, GPX and SOD.
Catalase (EC 1.11.1.6)
Catalase (CAT) activity was determined spectrophotometrically by the methodology of Patterson et al. (1984). The reaction mixture was prepared with enzyme extract equivalent to 20 µg of protein, 50 mM L−1 potassium phosphate buffer (pH 7.0) and 10.5 mM L−1 H2O2 (Miyagawa et al. 2000). The reaction was carried for 2 min at 25 °C and the enzyme activity was calculated by the linear rate of decrease in absorbance at 240 nm.
Guaiacol peroxidase (EC 1.11.17)
Guaiacol peroxidase (GPX) activity was estimated at 25 °C by the procedure of Tatiana et al. (1999). The 2 ml reaction mixture contained enzyme extract equal to 5 µg protein, 50 mM L−1 potassium phosphate buffer (pH 7.0), 2 mM L−1 H2O2 and 2.7 mM L−1 guaiacol. Formation of tetraguaiacol was measured at 470 nm after adding the enzyme extract.
Superoxide dismutase (EC 1.15.1.1)
For assay of superoxide dismutase (SOD), extraction and estimation was carried out according to the method of Beyer and Fridovich (1987) and absorbance at 560 nm was measured.
Activity staining of enzymes
Native polyacrylamide gel electrophoresis (PAGE) was carried out in Laemmli (1970) buffer system at 4 °C for all the enzymes. Samples were stirred with equal volumes 10% glycerol (v/v), 0.25% bromophenol blue prior to loading onto the wells. The gel was run at a constant current of 35 mA at 4 °C in a Bio-Rad protein IIxi electrophoresis apparatus (Parida et al. 2004).
Catalase (CAT)
A 7.5% polyacrylamide gel having 0.5% soluble starch was made for staining of CAT activity by the staining process of Thorup et al. (1961). The gel showing negative bands of CAT appearing on the green background was photographed.
Guaiacol peroxidase (GPX)
Polyacrylamide gel (7.5%) was used to visualize the activity of GPX by the staining procedure of Birecka and Garraway (1975). After the bands were stained sufficiently, photographs were taken.
Superoxide dismutase (SOD)
12% polyacrylamide gels were prepared for visualization of SOD activity by the staining method of Beauchamp and Fridovich (1971). Negative bands of SOD appeared on a purple background.
Estimation of protein
Protein content
Protein contents were estimated according to Lowry’s method, using standard solution made with Bovine Serum Albumin (BSA) (Lowry et al. 1951) and O.D. measured at 660 nm.
Leaf protein profile
0.5 g leaf samples were taken from plants of different treatments, rinsed thoroughly in distilled water and homogenized at 4 °C with 2 ml of an extraction buffer and the supernatants were loaded onto 12.5% SDS-PAGE gels (Parida et al. 2004). Gels were made according to Laemmli (1970). Electrophoresis was performed in a Bio-Rad Mini-PROTEAN Tetra Cell at 35 mA for 4 h. 0.25% Coomassie Brilliant Blue (CBB R-250, Sigma) was used to stain the gels for 2 h and de-stained till blue bands were prominent before being photographed.
Statistical analysis
The data represents mean and standard deviation of three replications, each from three consecutive experiments. Two way analysis of variance (ANOVA) and Duncan’s multiple range test (DMRT) were used to analyze the Cr stress effects on V. mungo and p ≤ 0.05 was set as the statistical significance (Sokal and Rohlf 1995).
Results
Seed germination and growth of plants
Germination percentage decreased gradually with increasing Cr concentrations. A significant decrease of seed germination of 50.70% was found at 300 µM against control (99.54%). Germination was recorded 89.32% till 200 µM which dropped to 73.11% in 250 µM (Fig. 1a–f). The root length of blackgram seedlings showed a significant decrease with increasing chromium concentrations (Supplementary Table 1). The root length of 30 d control seedlings was found to be 22.73 cm which showed a significant reduction to 11.56 cm in 100 µM which further reduced to 9.56 cm in 300 µM treatment of Cr. Also the 45 d control seedlings was found to be 25.08 cm against their 300 µM treated counterparts showing a significant decrease of 10.99 cm. On the other hand, secondary rooting was increased along with increasing chromium concentrations. The shoot length of seedlings showed a gradual decline with increasing Cr concentrations (Supplementary Table 1, Fig. 1g). The shoot lengths of the control and treated seedlings did not show much variation on the 15 d treatment whereas a significant reduction occurred on subsequent days of exposure (29.66 cm in control to 20.33 cm in 300 µM plants on 30 d; 32.17 cm in control to 23.67 cm in 300 µM treated plants on 45 d). The fresh and dry weights of blackgram seedlings decreased with increasing concentration of Cr treatment (Supplementary Table 1).
Fig. 1.
Seed germination of V. mungo var. B3-8–8 in different concentration of Cr in petridish (a–f). Seedling growth of V. mungo var. B3-8–8 plants in different concentrations of Cr in hydroponic culture (g)
Chromium contents in shoot and root
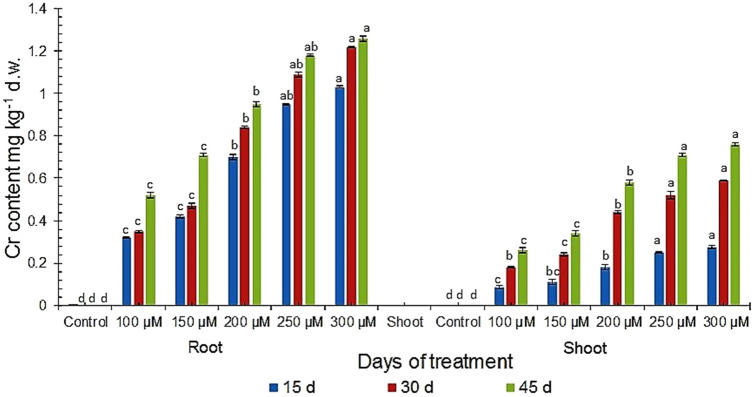
The content of Cr in roots was found much higher as compared to shoots at all the studied concentrations after all days of treatment which varied from 0.32 mg g−1 to 1.26 mg g−1 in roots of 100 µM to 300 µM, and from 0.07 mg g−1 to 0.76 mg g−1 in shoots of 100 µM to 300 µM respectively (Fig. 2). The roots as well as shoots of 300 µM 45 d Cr-treated plants showed 2.42 times and 2.92 times increase in accumulation of Cr compared to 100 µM plants respectively. The shoots also showed substantial increase in Cr accumulation ranging from 1.29 to 3.23 fold increase at 15 d, 1.33 to 3.21 times at 30 d and 1.31 to 2.92 times at 45 d as compared to 100 µM treated plants (Fig. 2). The root: shoot content of Cr gradually became less from 15 to 30 d in treated plants in 200 µM and then became stable at the 45 d plants containing 250 µM and 300 µM Cr treatment. The root: shoot ratio found in our study is in accordance to those of Rai et al. (2004) in Ocimum tenuiflorum. However, in contrast to the study of Karuppanapandian and Manoharan (2008), compared to the Cr provided in the medium, a relatively low amount of Cr is taken up by the root and a still lesser amount is transferred to the shoots which may be indicative of the high tolerance level of the plant growing under acute Cr stress.
Fig. 2.
Total Cr content in root and shoot of V. mungo var. B3-8–8 under varying concentrations of Cr at different days of treatments
Biochemical analysis
Pigment content
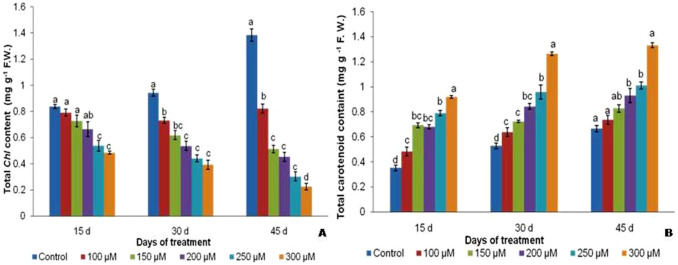
Chl a, b as well as total chlorophyll (Chl) contents were observed decreased with increase in the concentrations of Cr (Supplementary Table 2, Fig. 3a). The total Chl of 300 µM chromium treated plants showed substantial decrease from 2.12 mg g−1 on 15 d to 1.71 mg g−1 on 45 d against control plants which increased from 3.37 mg g−1 on 15 d to 4.67 mg g−1 f.w. on 45 d treated plant (Fig. 3a). The ratio of Chl a:b also decreased remarkably with increasing days of exposure and concentration of Cr (Supplementary Table 2). However, the carotenoid contents showed significantly increase from 1.9 mg g−1 in untreated plants to 6.9 mg g−1 in 100 µM treated 45 d old plants (Fig. 3b).
Fig. 3.
Total chlorophyll (a) and carotenoid content (b) in V. mungo var. B3-8–8 under varying concentrations of Cr respectively
Carbohydrate and reducing sugar
The carbohydrate content of seedlings showed a decreasing trend with increasing chromium concentrations on subsequent days of exposure. Control plants showed 31.05 mg g−1 carbohydrate content whereas it decreased to 16.41 mg g−1 in 100 µM plants on the 45 d treated plant (Fig. 4a). The Cr-treated plants showed decreased contents of reducing sugars at high chromium concentrations. The amount of reducing sugar in 200 µM treated plants decreased from 12.72 mg g−1 to 9.88 mg g−1 in 250 µM plants on 45 d (Fig. 4b).
Fig. 4.
Total carbohydrate (a) and reducing sugar (b) in V. mungo var. B3-8–8 under varying concentrations of Cr respectively
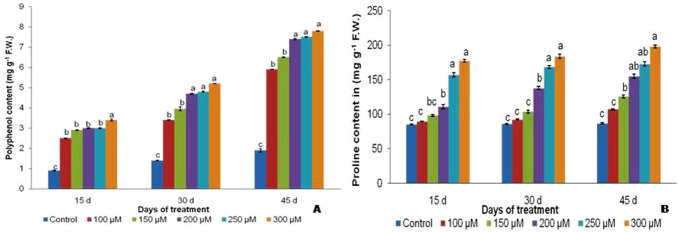
Total polyphenol and proline
The total polyphenol content was found to increase from 1.9 mg g−1 to 6.9 mg g−1 in control and 100 µM treated 45 d plants was marked (Fig. 5a). Proline content increased with increasing chromium concentrations along with increasing days of exposure. In 30 d treated plants, proline content increased from 103.95 mg g−1 f.w. in 150 µM to 137.96 mg g−1 f.w. in 200 µM plants (Fig. 5b). Similar trends were observed on the 45 d treated plants with proline contents of 107.1, 125.9, 155.3 mg g−1 in 100 µM, 150 µM and 200 µM, respectively.
Fig. 5.
Total polyphenol (a) and proline content (b) in V. mungo var. B3-8–8 under varying concentrations of Cr respectively
Antioxidative enzymes
Quantitative assay of CAT, GPX, SOD
The CAT concentrations decreased at all concentrations of Cr whereas the GPX and SOD activity got arrested only at higher concentrations of Cr. This was observed substantially on the 45 d showing an increase of 143.7 mg protein−1 min−1 in 250 µM plants to 165.2 mg protein−1 min−1 in 300 µM plants in case of GPX and 14.5 mg protein−1 on the 15 d to 18.8 mg−1 protein on the 45 d in case of SOD. On the 45 d, the concentrations of control plants were 17.8 mg protein min−1 which decreased from 12.5 mg protein min−1 in 150 µM treated plants to 8.9 mg protein min−1 in 300 µM treated plants (Supplementary Table 3).
Activity staining of enzymes
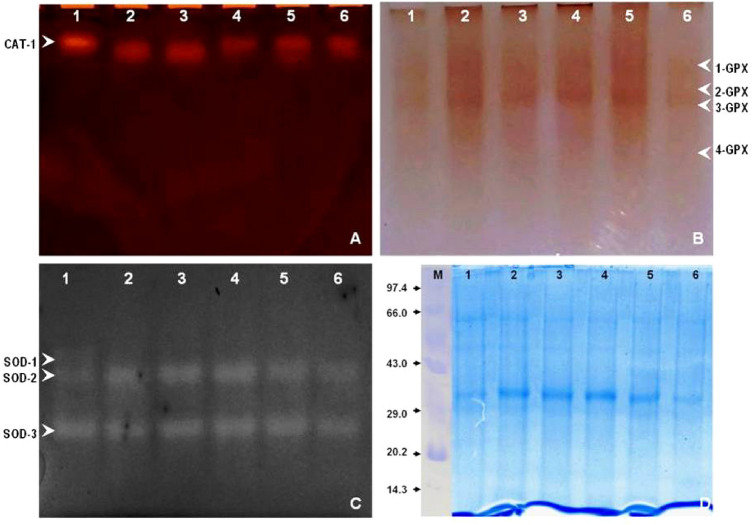
It was found from the native gel analysis of the enzyme activities that the activity of CAT decreased at all concentrations of Cr treatment whereas the enzyme activities of GPX and SOD increased upto a concentration of 200 µM Cr, after which it decreased upto 300 µM Cr. The native gel of catalase (CAT-1) showed decrement with an increase in Cr concentrations (Fig. 6a). All the four isoforms of GPX (GPX-1, -2, -3, -4) showed enhanced activity upto 200 µM Cr after which it declined (Fig. 6b). Similar trends were observed for those of SOD-2 and-3 (Fig. 6).
Fig. 6.
Effect of Cr on the CAT (a), GPX (b), SOD (c) and protein profile (d) of the leaves of V. mungo var. B3-8–8 after 45 days of treatment respectively
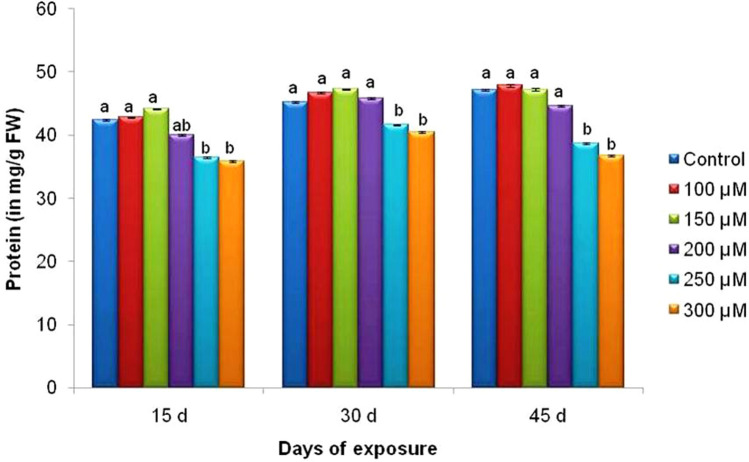
Changes in protein content and profile
Protein content of the leaves found to increase upto a concentration of 150 µM Cr after which it dropped upto 300 µM concentration in V. mungo plants (Fig. 7). The protein content varied from 44.17 mg g−1 in 150 µM plants to 35.9 mg g−1 f.w.in 15 d treated plants, from 47.29 in 150 µM plants to 40.57 mg g−1 f.w. in 30 d treated plants and 47.86 in 100 µM plants to 36.85 mg g−1 f.w. in 45 d treated plants. As visualized on SDS-PAGE, three bands of molecular weight 29.2, 32.6 and 47.1 kDa, were changed markedly (Fig. 6d). The intensity of nearly all the bands at the higher concentrations decreased whereas in lower concentrations i.e. upto 200 µM Cr, it increased significantly. The intensity of the bands of 32.6 kDa and 29.2 kDa decreased at concentrations above 200 µM Cr (Fig. 6d).
Fig. 7.
Effect of Cr on the protein content of the leaves of V. mungo var. B3-8–8 at different days of treatment
Discussion
Seed germination and plant growth
The induction of hexavalent chromium stress in the nutrient solution reduced the seed germination. The significant decrease in germination percentage from 95.23% in control to 75.45% at 300 µM may be due to the accumulation of Cr in the seed at germination stage that leads to upset in enzyme activity (Ganesh et al. 2006). Higher ROS generation due to Cr treatment might have enhanced breakdown of reserved nutrients in seeds which leads to changes in cell membrane properties (Shafiq et al. 2008). Zeid (2001) and DalCorso (2012) detailed a decline in α and β-amylase activities as well as activation of proteases under Cr treatment, which account for hampering germination due to the reduced supply of sugar to the plumule and radical. The significant reduction of seedling length from 29.66 cm in control to 20.33 cm in 300 µM 30 d treated plants and 32.17 cm in control to 23.67 cm in 300 µM 45 d treated plants during Cr stress could be attributed to the reduced water potential, obstructed absorption of nutrients leading to secondary stress (John et al. 2009). Also reduced seedling growth particularly of root growth under heavy metal treatment can be attributed to the reduced number of meristematic cells in root tips as compared to the cotyledons and shoot apex. Under Cr stress, the activities of hydrolytic enzymes are affected depriving the radical and plumule of food and ultimately causing reduction in seedling growth. Hexavalent chromium concentration even causes chromosomal aberrations in roots of blackgram (Sundaramoorthy et al. 2009) that triggered c-mitosis of the root cells leading to very stunted root growth. Chromium toxicity alters functioning of the root cells resulting in decreased uptake of nutrients uptake and low water mobility. Increasing accumulation of Cr in the root region and low uptake from roots to other aerial tissues and inhibition of shoot growth as reported in our study has also been in accordance to earlier report (Diwan et al. 2010). Reduced plant growth under Cr toxicity as evident in our result is in accordance with the reports of inducing changes of cell membrane, leaf chlorosis, root cell damage, content of pigments, misbalance in water potential, mineral uptake, transpiration and nitrogen assimilation, alteration of different enzyme activities leading to reactive oxygen species (ROS) mediated disruption in the redox balance in plants (Eleftheriou et al. 2015; Anjum et al. 2017). Plants contain efficient antioxidant system during Cr stress but at higher concentrations breaks the metabolic defense system in plant and there is jeopardization in the activities of antioxidants leading to reduction in growth of plant.
Chromium uptake and transport
The result of chromium content in 45 d old plant showed 2.42 times increase of Cr in root and 2.92 times increase in shoot at 300 µM concentration. Chromium content increased 3.21 times in root against 3.11 timesin shoot at 100 µM concentration. Significant increase of chromium content was noted from 200 µM dose onward both in shoot and root with a higher accumulation in root: shoot ratio found at 100 µM and 150 µM concentrations (Fig. 2). Although, Cr concentrations of most plants growing in mine soil are 0.02–0.2 mg kg−1 but, Prosopis seedling tolerate higher Cr accumulation in its roots and aerial parts upto 700–1000 mg kg−1 assisting phytoremediation, in association with Glomus deserticola (Arias et al. 2011) or with Bacillus sp. (Ramírez et al. 2019) or with arbuscular mycorrhizal fungi (Stambulska et al. 2018). Since, the experiments were conducted in hydroponics without any amelioration of soil microbes, thus accumulation obtained is purely relies on the basis of cellular metabolic process of Cr uptake and transport related enzyme mechanisms rather than iron (Fe) and sulfur (S) channels mediated comparative active transport mechanism operated in soil-grown plant. Although, Bacillus isolated from Dalbergia odori (Lu et al. 2017), Prosopis juliflora (Abdelmoteleb et al. 2017), Medicago (Chinnaswamy et al. 2018) roots are not the nodule forming bacteria but are probable natural endopyte which resists Cr uptake from rhizosphere and promote plant development via IAA synthesis (Sethuraman and Balasubramanian 2010). Neutralization of the chromium (VI) toxicity via Cr (VI) reduction to Cr (III), Cr (VI) efflux pumps, activations of enzymes involved in ROS detoxification, and DNA repair (Viti et al. 2014) might be some of the mechanism activated by the endophyte.
However, uptake of heavy metals depends on specific carriers (Shahid et al. 2017; Sharma et al. 2020) although uptake of Cr (III) in plants occurs through passive transport and Cr (VI) moves through the root plasma membrane actively involving transporters of phosphorus or sulfur because of their structural resemblance with hexavalent Cr (De Oliveira et al. 2016; Shahid et al. 2017). So, translocation of chromium in the roots might be mediated by Fe and S channels guiding competitions between Fe and Cr (Cary et al. 1977; Mallick et al. 2010). The Cr mobility is low compared to the other heavy metals; its concentration in the roots is dependent on valency state of Cr ions. Thus, a higher attention of Cr was found in cytoplasm, cell wall and intercellular space of Iris pseudacorus of root tissue than in the shoots (Caldelas et at. 2012) which may be attributed to the Cr sequestration in the vacuoles of the root cells and is considered as a protective mechanism (Mangabeira et al. 2011). The Cr (VI) is converted to its trivalent form in plant tissue which has the affinity to bind with the cell walls, thus, hinder the further transport of Cr to shoot. However, metal transporter genes of ATP binding cassette (ABC) superfamily, heavy metal ATPase (HMA), cation diffusion facilitator (CDF) and ZRT, IRT-like protein (ZIP) have been reported for Pb, Cd, Zn and As (Kim et al. 2007; Gustin et al. 2009) but information on role of transporter families for Cr translocation is missing in plants.
Effect on pigments and osmolytes
Chl a, Chl b and total Chl significantly decreased under increasing chromium concentrations. A significant reduction of 1.57 times Chl content was recorded in 250 µM at 15 d which became 1.7 times at 100 µM at 30 d and further decreased to ~ 7.0 times at 300 µM at 45 d compared with their respective controls. The carotenoid content increased up 2.4, 2.6 and 2.1 fold at 300 µM concentration at 15 d, 30 d, and 45 d respectively at increased dose and time of treatment (Fig. 3b). Upset in photosynthesis might be due to thyllakoid damage and decline in shoot growth as reported in Crambe sativa and Eruca sativa (Hu et al. 2015) also corresponds to our result. These phenomena of heavy metal stress on photosynthetic pingment also observed in earlier reports of chlorosis in young leaves, degradation of Chl pigment, thyllakoid membrane structure loosening and changes in photosynthetic apparatus as reported in salt stress (Parida et al. 2004). Chlorophyll a:b ratio also significantly decreased in 250 and 300 µM Cr treatment (Supplementary Table 2). Significant reductions in Chl a:b ratio were seen in 250 µM plants (1.47 on 15 d–1.135 on 30 d–0.83 on the 45 d) and 300 µM plants (1.41 on 15 d–1.05 on 30 d–0.79 on the 45 d). Heavy metals like Cr might translocate to plant parts and accumulate in cytosol and detoxification might require priming of microorganism in seedling stage which is out of the scope in this study. Higher chromium content in soils more than commonly available Cr concentration (5–120 mg kg−1) showed poor crop growth (Kabata-Pendias 2010). Augmentation of carotenoid with increasing dose of Cr and day of exposure with decreased Chl content might be due to the activation of carotenoid biosynthesis pathway and to synthesize more accessory pigment to compensate the Chl loss in higher chromium dose for adjustment of photosynthetic loss of the plant.
A significant decrease of carbohydrates found 1.3, 1.69 and 2.08 times in 15d, 30 and 45d respectively. Carbohydrate content decreased from 25.05 mg g−1 f.w. in untreated plants to 16.41 mg g−1 f.w. in 100 µM to 14.89 mg g−1 f.w. in 150 µM plants on 45 d (Fig. 4a). In the present study, the carbohydrate accumulation and distribution in plants are influenced by a high metal concentration. Reducing sugars decreased from 12.39 mg g−1 f.w. in 150 µM to 10.66 mg g−1 f.w. in 200 µM plants on 15 d; 16.03 mg g−1 f.w. in 150 µM to 12.72 mg g−1 f.w. in 200 µM plants on 45 d (Fig. 4b). Our results are in accordance with the reported findings in different plants (Wang and Nil 2000). Consumption of soluble sugars in large amounts in order to sustain the vital physiological functions like photosynthesis and respiration is the reason for the low soluble carbohydrate content. Proline contents increased from 110.91 mg g−1 f.w. in 200 µM to 157.16 mg g−1 f.w. in 250 µM plants on 15 d and 168.69 mg g−1 f.w. in 250 µM to 184.17 mg g−1 f.w. in 300 µM plants on the 30 d (Fig. 5a). The abiotic stresses i.e. drought, extreme temperatures and salinity results in accumulation of proline in different species of plant. Apart from its function and as an important constituent of cell wall proteins, proline mediates osmotic behaviour, stabilizes sub-cellular structures, scavenges free radicals and also acts as a oxidation/reduction potential buffer during environmental stresses (Molinari et al. 2007). Besides, polyphenol content increased drastically from ~ 3.4 times in 300 µM treated 15 d exposed plant which further significantly amplified to ~ 4.2 times in 300 µM treatment till 45 d of treatment. This sharp increase of polyphenol in increasing Cr dose might be one of the mechanism of stress tolerance by inducing different osmoprotectants in the cytoplasm to combat oxidative stress enabling blackgram plant to survive even in stunted growth (Fig. 5b).
Effect on anioxidative enzymes
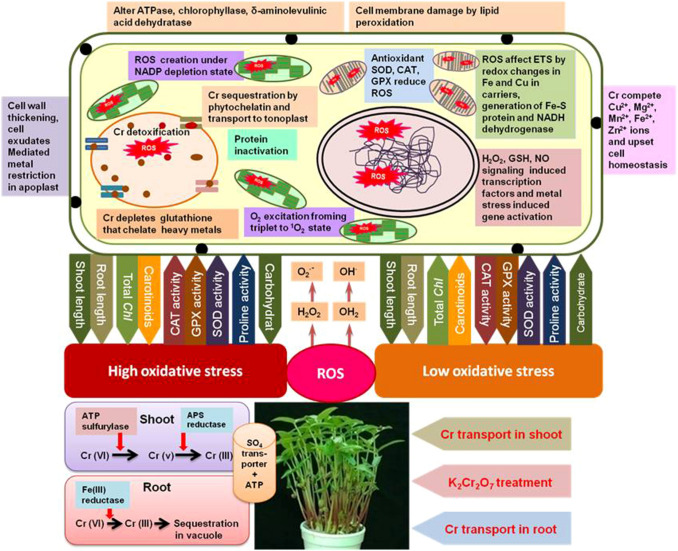
Antioxidative enzymes activity changes dramatically in Vigna mungo treated with Cr (VI) which lead to ROS mediated oxidative stress in subcellular compartments. A schematic diagram of Cr uptake, oxidative burst, ROS generation and its effect on cellular function is depicted in Fig. 8. Catalase activity decreased ~ 1.33 times at 15 d treat which reduced further ~ 1.88 times at 45 d treatment at 250 µM chromium. A significant decrease of CAT activity also found at 300 µM chromium too and the activity loss was varied from ~ 1.36 times at 15 d and ~ 2.0 times at 45 d treatment which is in according to the reports on barley seedlings where decreased CAT activity in oxidative injuries was recorded (Zancan et al. 2008). But, in contrast, enhanced APX, CAT, GPX and SOD activities under UV-B radiation was also reported earlier (Kondo and Kawashima 2000; Mishra et al. 2009). It has already been shown that plants attenuate the harmful effects of oxidative stress by activation of their structured antioxidant protection system that renders effective shield against free radicals and ROS. But oxidative injury takes place under severe conditions of stress when the level of ROS exceeds the operational antioxidant machinery of plants. The reports of Feierabend et al. (1992), supports the H2O2 accumulation linked inactivation of CAT which is in accordance with our findings. Mild oxidative stress reduce CAT activity induced by Cr in pea plant before reaching to the threshold value (Stambulska et al. 2018). Under, comparatively higher dose of Cr ROS levels might not return to their initial range as in control plant and got stabilize (Lushchak 2012). CAT consists of an iron-porphyrin ring, thus, reduction of activity points that chromium has the prospective to act together with iron in metabolic pool and it offers a crucial part in ROS removal by dismuting H2O2 into O2 (Mhamdi et al. 2010). Nevertheless, drastic increase in ROS was noticed (Ali et al. 2015; Sharma et al. 2019; Fahad et al. 2019) with increased malondialdehyde (MDA) contents under Cr toxicity (Adrees et al. 2015). Cr has tendency to bind and employ GSH, the reduced form of glutathione and its derivatives, which remarkably ameliorates the effects of ROS (Lee et al. 2003).
Fig. 8.
A schematic diagram of Cr uptake, oxidative burst, ROS generation and its effect on cellular function in blackgram
Guiacol peroxidase (GPX) activity significantly increased from ~ 1.05 to ~ 1.19 times in 250 µM Cr at upto 45 day of treatment which is further increased from ~ 1.08 to ~ 1.37 times increase at 300 µM Cr at 45 d of treatment. Superoxide dismutase (SOD) also increased like GPX activity which was dose dependent with range of ~ 1.38 fold at 15 d which increased to ~ 1.66 times at 45 d in 300 µM. The overall activity found significant at 45 d of all concentrations of Cr treatments on V. mungo as observed (Fig. 2). Significant hyperactivity of GPX and SOD was reported in Cr stressed Ocimum tenuiflorum leaf (Rai et al. 2004). In general, increase of SOD and GPX activity could be due to the production of increased free radicals at the arrival of a verge level of oxidative stress which beats the resistance parameters of the species under heavy metal stress. Out of the antioxidant enzymes, GPX2 and SOD1 have decreased activity and increase CAT activity in increased Cr concentration might be related to oxidative stress management of the plants. SOD serves as a initial line of control against various stresses that are catalyzed by superoxide ion dismutation in apoplast, chloroplast, cytosol, mitochondria and peroxisomes and finally help to liberate oxygen from hydrogen peroxide (Gill and Tuteja 2010; Shahzad et al. 2018). Cr (VI) escalated H2O2 leads to the lipid peroxidation; triggering GPX and SOD activities in contrast to decreased peroxidase (POD), SOD and CAT in Cr (III) exposure (Tang et al. 2014). Cr toxicity has inhibition of enzyme activities on glutathione reductase, ascorbate peroxidase, GPX and POD (Ali et al. 2015). Thus in plants, balancing between ROS generation and both enzyme and non-enzyme antioxidants is a stress mitigation mechanism maintaining metabolic homeostasis (Wu et al. 2017; Shahzad et al. 2018). Moreover, NADPH oxidase, present in plasma membrane, also leads to oxidative stress as they are linked with Cr (Weyemi and Dupuy 2012; Potocký et al. 2012; Pourrut et al. 2013). When Cr is present, NADPH oxidases consumes more cytosolic NADPH producing free radical O2−, which become rapidly converted to H2O2 by SOD enzyme (Pourrut et al. 2008). Enhanced production of ROS in plants under Cr toxicity heads towards oxidative burst by damaging DNA, lipids, pigments, proteins and enhances lipid peroxidation process (Ullah et al. 2019).
Effect on proteins
Protein contents also decreased at the higher concentrations of Cr (44.17 mg g-1 f.w. in 150 µM to 40.03 mg g-1 f.w. in 250 µM plants on the 15 d). The enhanced rate of denaturation of protein might be the reason for the reduced contents of protein in leaf (Vajpayee et al. 2002) and root (Tripathi and Gautam 2007). Slatni et al. (2010) reported increasing rate of protein denaturation along with the denaturation of subsisting protein into amino acid subunits as the main cause of decreased protein amounts, which is in agreement with our results. Higher Cr contents in roots of treated plants at all concentrations indicate the absence of a suitable mechanism for its transport. The sequestration of Cr in the vacuoles of the root cells could be due to the poor translocation of Cr to the shoots; which in turn detoxifies it by some intrinsic mechanism or it may be possible that there is unavailability of a suitable transporter in the root cells to facilitate Cr transport to the shoots. Three bands of molecular weight 29.2, 32.6 and 47.1 kDa, were changed markedly as visualized in SDS gel (Fig. 6d). Significant down regulation of nearly all the bands at the higher concentrations were found above 200 µM Cr. The intensity of ~ 29.2 kDa and ~ 32.6 kDa decreased at higher concentrations at 200 µM, 250 µM, and 300 µM of chromium (Fig. 6d). The up and down-regulation of some of the proteins could be interesting to find out molecular mechanism of chromium. Redox-inactive metals have the tendency to make covalent association with the protein thiol groups as these metals have the tendency of sharing the electrons. Interaction of Cr with the catalytic site of proteins might deactivate enzymes leading to the alteration of enzymatic activities (Gupta et al. 2010). Moreover, disturbance of cations disturbs cellular ROS equilibrium by interacting with enzyme binding sites (Shahzad et al. 2016).
Conclusion
Our study deduces that increased exposure to chromium brought a remarkable reduction in germination and growth in blackgram (Vigna mungo var. B3-8-8). The roots were more severely affected with a marked decrease in their lengths as compared to shoot. The reduction in the total chlorophyll carbohydrates, proteins and reducing sugars contents confirm that Cr adversely affects the concerned plant. On the contrary, an increase in the concentrations of carotenoids might be compensated with the decrease of Chl b reducing PSII activity. A substantial increase in free amino acids, proline and total polyphenols in higher concentration (i.e. 250 and 300 µM) indicate that chromium induces severe oxidative stress in blackgram which is managed by up-regulation of secondary metabolites for maintaining the cellular equillibrium. The increase in the levels of the antioxidative enzymes also indicate that they have a very appreciable role in scavenging mechanisms that plants opted during stressful conditions. The accumulation levels of Cr indicate that blackgram var. B3-8-8 can accumulate moderate amount of Cr in root and translocate insignificant amount to shoot, thus can be recommended for growing safely in surrounding areas of chromium mine with Cr-contaminated sites.
Supplementary Information
Acknowledgements
The financial assistance received from DST INSPIRE Fellowship (DST/INSPIRE/03/2017/002373; Inspire Fellow No. IF180328) to A. Rath, and the facility used in the Department of Botany, Utkal University developed under UGC-SAP DRS-III and DST-FIST Programme, Government of India are gratefully acknowledged. We acknowledge the support of Central Instrumentation Centre, Orissa University and Agriculture and Technology, Bhubaneswar for use of Inductively Coupled Plasma—Optical Emission Spectrometry (ICP-OES, Perkin Elmer Avio 200, USA) for Cr data analysis on payment basis.
Compliance with ethical standards
Conflict of interest
We declare that, the manuscript is prepared and approved by us and we do not have any conflict of interest in submitting this paper to this journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayushee Rath, Email: 212ayushee@gmail.com.
Anath Bandhu Das, Email: abdas.uubot@gmail.com, Email: a_b_das@hotmail.com.
References
- Abdelmoteleb A, Troncoso R, Gonzalez T, González D. Antifungical activity of autochthonous Bacillus subtilis isolated from Prosopis juliflora against phytopathogenic fungi. Mycobiol. 2017;45:385–391. doi: 10.5941/MYCO.2017.45.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrees M, Ali S, Iqbal M, Bharwana SA, Siddiqi Z, Farid M, Ali Q, Saeed R, Rizwan M. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol Environ Saf. 2015;122:1–8. doi: 10.1016/j.ecoenv.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Ali S, Bai P, Zeng F. The ecotoxicological and interactive effects of chromium and aluminum on growth, oxidative damage and antioxidant enzymes on two barley genotypes differing in Al tolerance. Environ Exp Bot. 2011;70:185–191. [Google Scholar]
- Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Poll Res. 2015;22:10601–10609. doi: 10.1007/s11356-015-4271-7. [DOI] [PubMed] [Google Scholar]
- Anjum SA, Ashraf U, Khan I, Tanveer M, Saleem MF, Wang L. Aluminum and chromium toxicity in maize: implications for agronomic attributes, net photosynthesis, physio-biochemical oscillations, and metal accumulation in different plant parts. Water, Air Soil Pollut. 2016;227:326–330. [Google Scholar]
- Anjum SA, Ashraf U, Khan I, Tanveer M, Shahid M, Shakoor A, Wang L. Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere. 2017;27:262–273. [Google Scholar]
- Arias A, Peralta-Videa JR, Ellzey JT, Viveros MN, Ren M, Mokgalaka NS, Castillo-Michel H, Gardea-Torresdey JL. Plant growth and metal distribution in tissues of Prosopis juliflora-velutina grown on chromium-contaminated soil in the presence of Glomus desertícola. Environ Sci Technol. 2011;44:7272–7279. doi: 10.1021/es1008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu TN, Varaprasad D, Bindu YH, Kumari MK, Dakshayani L, Reddy MC, Chandrasekhar T. Impact of heavy metals (Cr, Pb and Sn) on in vitro seed germination and seedling growth of green gram (Vigna radiata (L.) R. Wilczek) Curr Trends Biotechnol Pharm. 2014;8:160–165. [Google Scholar]
- Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R. Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett. 2008;6:189–213. [Google Scholar]
- Bates LS, Waldran RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–208. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assay applicable to polyacrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Birecka H, Garraway MO. Corn leaf isoperoxide reaction to mechanical injury and infection with Helminthosporium maydis. Plant Physiol. 1975;61:561–566. doi: 10.1104/pp.61.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishehkolaei R, Fahimi H, Saadatmand S, Nejadsattari T, Lahouti M, Yazdi FT. Ultrastructural localisation of chromium in Ocimum basilicum. Turk J Bot. 2011;35:261–268. [Google Scholar]
- Caldelas C, Bort J, Febrero A. Ultrastructure and subcellular distribution of Cr in Iris pseudacorus L. using TEM and X-ray microanalysis. Cell Biol Toxicol. 2012;28:57–68. doi: 10.1007/s10565-011-9205-7. [DOI] [PubMed] [Google Scholar]
- Cary EE, Allaway WH, Olson OE. Control of chromium concentrations in food plants. 1. Absorption and translocation of chromium by plants. J Agric Food Chem. 1977;25:300–304. doi: 10.1021/jf60210a048. [DOI] [PubMed] [Google Scholar]
- Chinnaswamy A, Coba de la Peña T, Stoll A, de la Peña Rojo D, Bravo J, Rincón A, Lucas MM, Pueyo JJ. A nodule endophytic Bacillus megaterium strain isolated from Medicago polymorpha enhances growth, promotes nodulation by Ensifer medicae and alleviates salt stress in alfalfa plants. Ann Appl Biol. 2018;172:295–308. doi: 10.1111/aab.12420. [DOI] [Google Scholar]
- DalCorso G. Heavy metal toxicity in plants. In: Furini A, editor. Plants and heavy metals. Springer, Dordrecht: Springer Briefs in Molecular Science; 2012. pp. 1–25. [Google Scholar]
- Davies FT, Puryear JD, Newton RJ, Egilla JN, Grossi JAS. Mycorrhizal fungi increase chromium uptake by sunflower plants: influence on tissue mineral concentration, growth and gas exchange. J Plant Nutr. 2002;25:2389–2407. [Google Scholar]
- De Oliveira LM, Gress J, De J, Rathinasabapathi B, Marchi G, Chen Y, Ma LQ. Sulfate and chromate increased each other’s uptake and translocation in As-hyperaccumulator Pteris vittata. Chemosphere. 2016;147:36–43. doi: 10.1016/j.chemosphere.2015.12.088. [DOI] [PubMed] [Google Scholar]
- Diwan H, Khan I, Ahmad A, Iqbal M. Induction of phytochelatins band antioxidant defense system in Brassica juncea and Vigna radiata in response to chromium treatments. Plant Growth Regul. 2010;61:97–107. [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Duhan JS. Chromium stress on peroxidase, ascorbate peroxidase and acid invertase in pea (Pisum sativum L.) seedling. Int J Biotechnol Mol Biol Res. 2012;3:15–21. [Google Scholar]
- Eleftheriou E, Adamakis I-D, Panteris E, Fatsiou M. Chromium-induced ultrastructural changes and oxidative stress in roots of Arabidopsis thaliana. Int J Mol Sci. 2015;16:15852–15871. doi: 10.3390/ijms160715852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Mineral nutrition of plants: principles and perspectives. London: John Wiley and Sons; 1972. p. 412. [Google Scholar]
- Fahad S, Rehman A, Shahzad B, Tanveer M, Saud S, Kamran M, Ihtisham M, Khan SU, Turan V, Ur Rahman MH. Rice responses and tolerance to metal/metalloid toxicity. In: Hasanuzzaman M, Fujita M, Nahar K, Biswas JK, editors. Advances in rice research for abiotic stress tolerance. Cambridge, UK: Woodhead Publishing; 2019. pp. 299–312. [Google Scholar]
- Feierabend J, Schaan C, Hertwig B. Photoinactivation of catalase occurs under both high and low temperature stress conditions and accompanies photoinhibition of photosystem II. J Plant Physiol. 1992;110:1554–1561. doi: 10.1104/pp.100.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh KS, Sundaramoorthy P, Chidambaram ALA. Chromium toxicity effect on black gram, soybean and paddy. Poll Res. 2006;25:257–261. [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gossett DR, Millholon EP, Lucas MC. Antioxidant response to NaCl stress in salt tolerant and salt sensitive cultivars of cotton. Crop Sci. 1994;34:706–714. [Google Scholar]
- Gupta D, Huang H, Yang X, Razafindrabe B, Inouhe M. The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J Hazard Mater. 2010;177:437–444. doi: 10.1016/j.jhazmat.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J Cell Mol Biol. 2009;57:1116–1127. doi: 10.1111/j.1365-313X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon HI. The water-culture method for growing plants without soil. Calif Exp Agric Station Circ. 1950;347:1–32. [Google Scholar]
- Hu J, Deng Z, Wang B, Zhi Y, Pei B, Zhang G, Luo M, Huang B, Wu W, Huang B. Influence of heavy metals on seed germination and early seedling growth in Crambe abyssinica, a potential industrial oil crop for phytoremediation. American J Plant Sci. 2015;6:150–156. [Google Scholar]
- Imtiaz M, Mushtaq MA, Rizwan MS, Arif MS, Yousaf B, Ashraf M, Shuanglian X, Rizwan M, Mehmood S, Tu S. Comparison of antioxidant enzyme activities and DNA damage in chickpea (Cicer arietinum L.) genotypes exposed to vanadium. Environ Sci Pollut Res. 2016;23:19787–19796. doi: 10.1007/s11356-016-7192-1. [DOI] [PubMed] [Google Scholar]
- John RP, Ahmad K, Gadgil S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int J Plant Prod. 2009;3:65–76. [Google Scholar]
- Kabata-Pendias A. Trace elements in soils and plants. 4. Boca Raton: CRC Press; 2010. [Google Scholar]
- Kannaiyan S. Bioresearches technology for sustainable agriculture. New Delhi: Associated Publishing Company; 1999. p. 422. [Google Scholar]
- Karuppanapandian T, Manoharan K. Uptake and translocation of tri-and hexavalent chromium and their effects on black gram (Vigna mungo L. Hepper cv. Co4) roots. J Plant Biol. 2008;51:192–201. [Google Scholar]
- Kaur N, Nayyar H. Heavy metal toxicity to food legumes: effects, antioxidative defense and tolerance mechanisms. J Food Legumes. 2013;26:1–18. [Google Scholar]
- Khanna K, Jamwal VL, Sharma A, Gandhi SG, Ohri P, Bhardwaj R, Al-Huqail AA, Siddiqui MH, Ali HM, Ahmad P. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere. 2019;230:628–639. doi: 10.1016/j.chemosphere.2019.05.072. [DOI] [PubMed] [Google Scholar]
- Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J Cell Mol Biol. 2007;50:207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
- Kondo N, Kawashima M. Enhancement of the tolerance to oxidative stress in cucumber (Cucumis sativus L.) seedlings by UV-B irradiation: possible involvement of phenolic compounds and antioxidative enzymes. J Plant Res. 2000;113:311–317. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S, Moon JS, Ko T-S, Petros D, Goldsbrough PB, Korban SS. Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol. 2003;131:656–663. doi: 10.1104/pp.014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with Folin Phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu J, Yang F, Wang S, Ma H, Liang J, Chen Y. Co-existence of rhizobia and diverse non-rhizobial bacteria in the rhizosphere and nodules of Dalbergia odorifera seedlings inoculated with Bradyrhizobium elkanii, Rhizobium multihospitium–like and Burkholderia pyrrocinia–like strains. Front Microbiol. 2017;8:2255. doi: 10.3389/fmicb.2017.02255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012;2012:1–26. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malar S, Sahi SV, Favas P, Perumal V. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)] Bot Stud. 2014 doi: 10.1186/s40529-014-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick S, Sinam G, Kumar MR, Sinha S. Interactive effects of Cr and Fe treatments on plants growth, nutrition and oxidative status in Zea mays L. Ecotoxicol Environ Saf. 2010;73:987–995. doi: 10.1016/j.ecoenv.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Mangabeira PA, Ferreira AS, de Almeida A-AF, Fernandes VF, Lucena E, Souza VL, dos Santos Júnior AJ, Oliveira AH, Grenier-Loustalot MF, Barbier F. Compartmentalization and ultrastructural alterations induced by chromium in aquatic macrophytes. Biometals. 2011;24:1017–1026. doi: 10.1007/s10534-011-9459-9. [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou J-P. Arabidopsis Glutathione Reductase1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 2010;153:1144–1160. doi: 10.1104/pp.110.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Srivastava G, Prasad SM. Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Scientia Hort. 2009;120:373–378. [Google Scholar]
- Miyagawa Y, Tamori M, Shigeoka S. Evaluation of the defense system in chloroplasts to photooxidative stress caused by paraquat using transgenic tobacco plants expressing catalase from Escherichia coli. Plant Cell Physiol. 2000;41:311–320. doi: 10.1093/pcp/41.3.311. [DOI] [PubMed] [Google Scholar]
- Molinari HBC, Marur CJ, Daros E, de Campos MKF, de Carvalho J, Filho JCB, Pereira LFP, Vieira LGE. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant. 2007;130:218–229. [Google Scholar]
- Mota R, Pereira SB, Meazzini M, Fernandes R, Santos A, Evans CA, De Philippis R, Wright PC, Tamagnini P. Effects of heavy metals on Cyanothece sp. CCY0110 growth, extracellular polymeric substances (EPS) production, ultrastructure and protein profiles. J Proteom. 2015;120:75–94. doi: 10.1016/j.jprot.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Mudgal V, Madaan N, Mudgal A. Heavy metals in plants: phytoremediation: plants used to remediate heavy metal pollution. Agric Biol J North Am. 2010;1:40–45. [Google Scholar]
- Nelson N. A photometric adaption of the Somogyi’s methods for the determination of glucose. J Biol Chem. 1944;153:375–379. [Google Scholar]
- Oliveira H. Chromium as an environmental pollutant: insights on induced plant toxicity. J Bot. 2012;2012:1–8. doi: 10.1155/2012/375843. [DOI] [Google Scholar]
- Paiva LB, de Oliveira JG, Azevedo RA, Ribeiro DR, da Silva MG, Vitoria AP. Ecophysiological responses of water hyacinth exposed to Cr3+ and Cr6+ Environ Exp Bot. 2009;65:403–409. [Google Scholar]
- Parida AK, Das AB, Mohanty P. Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isoforms of some antioxidative enzymes. J Plant Physiol. 2004;161:531–542. doi: 10.1078/0176-1617-01084. [DOI] [PubMed] [Google Scholar]
- Patterson BD, Pyne LA, Chen Y, Graham D. An inhibitor of catalase induced by cold-chilling-sensitive plants. Plant Physiol. 1984;76:1014–1018. doi: 10.1104/pp.76.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlman JM. The Mung bean. New Delhi: Oxford and IBH Publishing Co.; 1991. [Google Scholar]
- Potocký M, Pejchar P, Gutkowska M, Jiménez-Quesada MJ, Potocká A, de Dios Alché J, Kost B, Žárský V. NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J Plant Physiol. 2012;169:1654–1663. doi: 10.1016/j.jplph.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Pourrut B, Perchet G, Silvestre J, Cecchi M, Guiresse M, Pinelli E. Potential role of NADPH-oxidase in early steps of lead-induced oxidative burst in Vicia faba roots. J Plant Physiol. 2008;165(6):571–579. doi: 10.1016/j.jplph.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Pourrut B, Shahid M, Douay F, Dumat C, Pinelli E. Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In: Gupta D, Corpas f, Palma J, editors. Heavy metal stress in plants. Berlin, Heidelberg: Springer; 2013. [Google Scholar]
- Rai V, Vajpayee P, Singh SN, Mehrotra S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci. 2004;167:1159–1169. [Google Scholar]
- Ramírez V, Baez A, López P, Bustillos R, Villalobos MÁ, Carreño R, Contreras JL, Muñoz Rojas J, Fuentes LE, Martínez J, Munive JA. Chromium hyper-tolerant Bacillus sp. MH778713 assists phytoremediation of heavy metals by Mesquite trees (Prosopis laevigata) Front Microbiol. 2019 doi: 10.3389/fmicb.2019.01833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif S, Khan MS. Assessment of toxic impact of metals on proline, antioxidant enzymes, and biological characteristics of Pseudomonas aeruginosa inoculated Cicer arietinum grown in chromium and nickel-stressed sandy clay loam soils. Environ Monit Assess. 2018;190:290. doi: 10.1007/s10661-018-6652-0. [DOI] [PubMed] [Google Scholar]
- Saminathan B. Effect of chromium studies on germination and biochemical content of black gram. Int J Adv Res. 2013;1:216–222. [Google Scholar]
- Sethuraman P, Balasubramanian N. Removal of Cr (VI) from aqueous solution using Bacillus subtilis, Pseudomonas aeruginosa and Enterobacter cloacae. Int J Eng Sci. 2010;2:1811–1825. doi: 10.3390/ma8125461. [DOI] [Google Scholar]
- Shafiq M, Iqbal MZ, Mohammad A. Effect of lead and cadmium on germination and seedling growth of Leucaena leucocephala. J Applied Sci Environ Manag. 2008;12:61–66. [Google Scholar]
- Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere. 2017;178:513–533. doi: 10.1016/j.chemosphere.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Shahzad B, Tanveer M, Hassan W, Shah AN, Anjum SA, Cheema SA, Ali I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities: a review. Plant Physiol Biochem. 2016;107:104–115. doi: 10.1016/j.plaphy.2016.05.034. [DOI] [PubMed] [Google Scholar]
- Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, Sharma A, Song H, Ur Rehman S, Zhaorong D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol Environ Saf. 2018;147:935–944. doi: 10.1016/j.ecoenv.2017.09.066. [DOI] [PubMed] [Google Scholar]
- Shanker AK, Djanaguiraman M, Sudhagar R, Chandrashekar C, Pathmanabhan G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R. Wilczek. cv CO4) roots. Plant Sci. 2004;166:1035–1043. [Google Scholar]
- Sharma DC, Chatterjee C, Sharma CP. Chromium accumulation and its effects on wheat (Triticum aestivum L. cv HD 2204) metabolism. Plant Sci. 1995;111:145–151. [Google Scholar]
- Sharma A, Kumar V, Shahzad B, Ramakrishnan M, Sidhu GPS, Bali AS, Handa N, Kapoor D, Yadav P, Khanna K. Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul. 2019;38:1–23. [Google Scholar]
- Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D. Chromium bioaccumulation and its impacts on plants: an overview. Plants. 2020;9:1–100. doi: 10.3390/plants9010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi A. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. American J Env Vitic. 1965;16:144–158. [Google Scholar]
- Slatni T, Vigani G, Salah IB, Kouas S, Dell’Orto M, Gouia H. Metabolic changes of iron uptake in nitrogen fixing common bean nodules during iron deficiency. Plant Sci. 2010;181:151–158. doi: 10.1016/j.plantsci.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3. New York: W. H. Freeman and Company; 1995. pp. 321–356. [Google Scholar]
- Somogyi M. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- Srivastav A, Stoss J, Hamre K. A Study on enrichment of the rotifer Brachionus “Cayman” with iodine and selected vitamins. Aquaculture. 2011;319:430–438. [Google Scholar]
- Stambulska UY, Bayliak MM, Lushchak VI. Chromium (VI) toxicity in legume plants: modulation effects of rhizobial symbiosis. BioMed Res Int. 2018;3:1–13. doi: 10.1155/2018/8031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramoorthy P, Baskaran L, Chidambaram ALA, Sankar G. Growth and physiological activity of green gram (Vigna radiata L.) under effluent stress. Iran J Environ Health Sci Engg. 2009;6:17–22. [Google Scholar]
- Sundaramoorthy P, Chidambaram A, Ganesh KS, Unnikannan P, Baskaran L. Chromium stress in paddy: (i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. CR Biol. 2010;333:597–607. doi: 10.1016/j.crvi.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. Sunderland, Massachusetts: Sinaeur Associates Inc; 2002. [Google Scholar]
- Tang M, Mao D, Xu L, Li D, Song S, Chen C. Integrated analysis of miRNA and mRNA expression profiles in response to Cd exposure in rice seedlings. BMC Genom. 2014;15:835. doi: 10.1186/1471-2164-15-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatiana Z, Yamashiat K, Matsumoto H. Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber roots. Plant Cell Physiol. 1999;40:273–280. [Google Scholar]
- Tawfik AB, Steiner AL. The role of soil ice in land-atmosphere coupling over the United States: a soil moisture-precipitation winter feedback mechanism. J Geophysical Res. 2011;116:4402–4410. [Google Scholar]
- Thorup OA, Strole WB, Leavell BS. A method for the localization of catalase on starch gels. J Lab Clin Med. 1961;58:122–128. [PubMed] [Google Scholar]
- Tokunaga TK, Wan J, Hazen TC. Distribution of chromium contamination and microbial activity in soil aggregates. J Environ Qual. 2003;32:541–549. doi: 10.2134/jeq2003.5410. [DOI] [PubMed] [Google Scholar]
- Tripathi AK, Gautam M. Biochemical parameters of plants as indicators of air pollution. J Env Biol. 2007;28:127–132. [PubMed] [Google Scholar]
- Ullah A, Shahzad B, Tanveer M, Nadeem F, Sharma A, Lee DJ, Rehman A. Abiotic stress tolerance in plants through pre-sowing seed treatments with mineral elements and growth regulators. In: Hasanuzzaman M, Fotopoulos V, editors. Priming and pretreatment of seeds and seedlings: implication in plant stress tolerance and enhancing productivity in crop plants. Singapore: Springer; 2019. pp. 427–445. [Google Scholar]
- Vajpayee P, Rai UN, Ali MB, Tripathi RD, Yadav V, Sinha S, Singh SN. Chromium induced physiologic changes in Vallisneria spiralis L. and its role in phytoremediation of tannery effluent. Bull Environ Contam Toxicol. 2002;67:267–272. doi: 10.1007/s001280117. [DOI] [PubMed] [Google Scholar]
- Viti C, Marchi E, Decorosi F, Giovannetti L. Molecular mechanisms of Cr (VI) resistance in bacteria and fungi. FEMS Microbiol Rev. 2014;38:633–659. doi: 10.1111/1574-6976.12051. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nil N. Changes in chlorophyll, ribulose biphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J Hort Sci Biotech. 2000;75:623–627. [Google Scholar]
- Weyemi U, Dupuy C. The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat Res/Rev Mutat Res. 2012;751:77–81. doi: 10.1016/j.mrrev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Wu H, Tito N, Giraldo JP. Anionic Cerium Oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano. 2017;11:11283–11297. doi: 10.1021/acsnano.7b05723. [DOI] [PubMed] [Google Scholar]
- Xie Y, Fan J, Zhu W, Amombo E, Lou Y, Chen L, Fu J. Effect of heavy metals pollution on soil microbial diversity and bermuda grass genetic variation. Front Plant Sci. 2016;7:755. doi: 10.1016/S0016-7061(03)00083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zancan S, Suglia I, La Rocca N, Ghisi R. Effect of UV-B radiation on antioxidant parameters of iron-deficient barley plants. Environ Exp Bot. 2008;63:71–79. [Google Scholar]
- Zayed A, Lytle CM, Qian JH, Terry N. Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta. 1998;206:293–299. [Google Scholar]
- Zeid IM. Responses of Phaseolus vulgaris to chromium and cobalt treatments. Biol Plant. 2001;44:111–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.