Abstract
The companion dog has recently been promoted as powerful translational model of aging. However, while dogs share environments with their human owners and develop many of the same age-related morbidities, little is known about the underlying mechanisms that drive their health and longevity. In addition, dogs have a well described phenotypic pattern in which small dogs live significantly longer than large dogs, such that weight can be used as a crude proxy for longevity. To investigate this pattern, we completed a small lipidomics study on 41 dogs in the Birmingham, Alabama, United States, area to determine individual circulating lipids that were associated with age and body weight. We discovered that sphingomyelins were significantly higher in large, short-lived dogs, independent of age, and triglycerides were higher in older dogs of all sizes. Our results point towards physiological differences that may explain a portion of the variation in longevity seen in companion dogs.
Keywords: Body mass, Lipidomics, Longevity, Sphingomyelins, Triglycerides
Human life expectancy is increasing rapidly across the globe such that most death and disability are now a consequence of aging. Therefore, understanding the molecular mechanisms that underlie aging has come to the forefront of biomedical research. However, commonly studied laboratory animals (eg, worms, flies, and mice) do not naturally develop many of the age-associated pathologies seen in humans. On the other hand, companion dogs do show many similar age-associated pathologies seen in humans. Consequently, companion dogs have recently been promoted as informative translational models of aging and longevity (1), and furthermore, dogs share the human environment. These features have led to a flurry of studies on the health and mortality in the dog (2,3). While veterinarians have studied canine aging, diseases, and mortality for decades (4,5), it is only recently that scientists have sought to unravel the molecular mechanisms that contribute to canine aging.
In addition to their strong potential as a translational model of human aging, dogs show a striking pattern in which smaller individuals live significantly longer than larger dogs, and this pattern may also yield additional clues as to fundamental mechanisms that underlie their aging rate. Consequently, body size can be used as a rough proxy for dog longevity. This size-longevity tradeoff may at least be partially explained by size-related differences in GH/IGF-I signaling (6,7), and recent work out of our lab and others hypothesizes that the tryptophan metabolic network (8) and mitochondrial metabolism (9,10) may also contribute to this size-related variation in life span. However, at this point all putative mechanisms seeking to explain longevity variation in dogs remain at best informed speculations.
We have recently focused our attention on lipids as possible contributing mediators of aging and longevity (11). It is now well established that there are thousands of distinct lipids in the mammalian body serving 3 major functions: (i) triglycerides are involved in energy storage and mobilization; (ii) glycerophospholipids form the major structural elements of all cell membranes, impacting their propensity for budding, fission, and fusion; and (iii) fragments of structural lipids can act in signal transduction and cellular recognition (12). Early lipid-focused aging studies emphasized fatty acids, particularly polyunsaturated fatty acids (PUFAs) because they are located at the mitochondrial membrane and are prone to propagate lipid peroxidation leading to excessive production of reactive oxygen species (11,13). Small, short-lived mammals were found to possess membranes rich in omega-3 (also termed n-3) PUFAs, whereas larger, longer-lived mammals have more omega-6 (n-6) PUFAs and fewer n-3s [(11), but see (14)]. Interestingly, small long-lived rodents including naked mole rats (15) and Ames dwarf mice (16) were all found to be low in n-3s. It has been pointed out that there may be an association between high levels of n-3 fatty acids and a comparatively short life but the magnitude varies according to the tissues and datasets used (17).
With the emergence of modern chromatography coupled to mass spectrometry, more detailed inspection of lipid profiling has become possible, including the exact identification of all 8 categories of lipids (18). The field of lipidomics, a subcategory of metabolomics, arose as the study of the structure and function of the complete set of lipids in a sample. We present here one of the first studies to investigate how lipid compositions in dog plasma samples vary with age and body weight. Our results point towards a potential role of lipid metabolism in the regulation of aging in the dog, as well as suggest physiological differences that may partially explain the differences in longevity seen in large and small dogs.
Methods
Dog Samples
Blood plasma was collected from dogs residing in the Birmingham, Alabama, United States, area as described previously (8). Briefly, 2–5 mL of whole blood was collected into ethylenediamine tetraacetic acid tubes from dogs that had been fasted 4–6 hours. Tubes were spun at 4°C for 10 minutes, and plasma was removed and frozen at −80°C. When ready for processing, plasma samples were shipped on dry ice. Collection of dog samples was approved under UAB IACUC protocol 21121.
Lipidomics
A modified methyl-tert-butyl ether method (19) was used to extract lipids from the dog plasma samples. Isotopically labeled internal standards were added to the samples to ensure semi-quantitative analysis. Liquid chromatography–mass spectrometry was performed using the Vanquish UHPLC system (Thermo Fisher Scientific) combined with an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific). The lipid species were separated by reverse phase chromatography using an Accucore C18, 2.6 µm, 150 × 2 mm (Thermo Fisher Scientific) analytical column. The mass spectrometer with an inclusion list, polarity switching, CID ddMS2 for phospholipids, and HCD ddMS2 for glycerolipids was employed to ensure sensitive and comprehensive measurements. The data provided information on the lipid species from more than 12 major lipid classes as well as on the fatty acid composition. Several software tools were used for data analysis including TraceFinder (ThermoFisher Scientific) and Lipid Data Analyzer (TU Graz).
Statistical Analyses
Statistical analyses were completed in the program R 3.5.2 (www.r-project.org). Lipid data was log-transformed and centered and scaled, as previously described (8). We were first interested in determining which individual lipids were associated with either age or weight across all samples. To this end, we ran a general linear model looking at the effects of age and weight on lipid concentration. We also ran the models controlling for the effects of sex, sterilization status, and body condition score. To control for the effects of multiple comparisons, we applied a false discovery rate correction at α = .05. We then looked at how ratios of n-3 to n-6 lipids were associated with age and weight, using the same general linear model. Lastly, we ran unsupervised principal components analysis (PCA) to determine how the entire lipidome was associated with age and weight. Age and weight classes were set as follows: young (<5 years), middle (5–10 years), and old (>10 years); small (<9.1 kg), medium (9.1–22.7 kg), and large (>22.7 kg).
Results
Our final dataset consisted of 205 lipids from 41 dog samples. Samples were slightly male-biased (25 vs 16). Of the 35 dogs for which surgical sterilization status was recorded, 54.4% were sterilized. Average age of all dogs was 4.7 years (one dog was missing age data) while average weight was 25.3 kg. Demographic information for the 41 dogs is shown in Supplementary Table S1. The majority of all lipids (93%) were present in all samples, and every lipid was present in at least 71% of individuals.
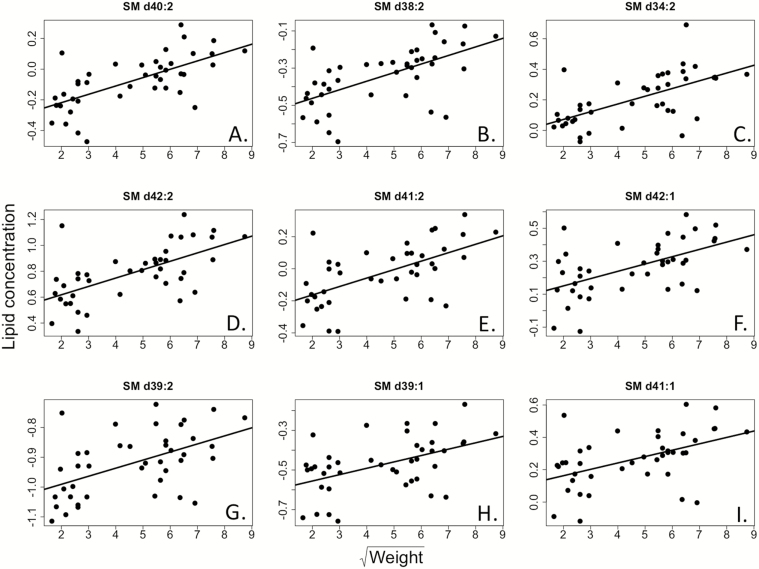
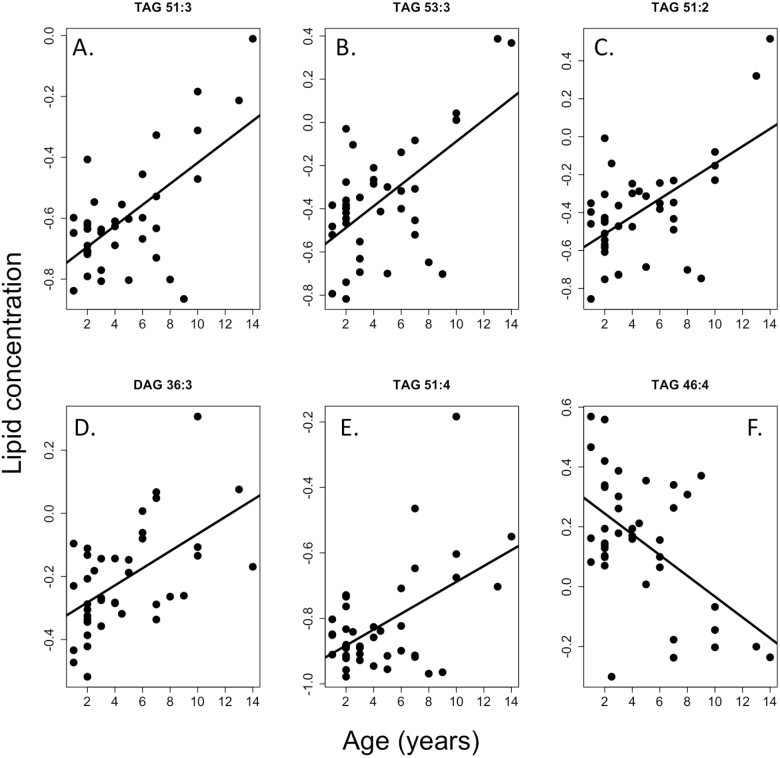
Our linear model suggested a strong association of weight (51 lipids) and age (34 lipids) on individual plasma lipid concentrations (Supplementary Table S2). Overall 12 of 25 (48%) sphingomyelins, a major lipid category involved in cell membranes and in cell signaling via lipid rafts in plasma (20), were positively associated with weight (Figure 1; Supplementary Table S2). No sphingomyelins showed a significant negative association with body weight. The largest age effects were seen with regards to di- and triglycerides. Fourteen of 46 di- or triglycerides were associated with age; the majority (79%) of which were positively associated (Figure 2). In addition, 8 triglycerides were associated with body weight, all in the negative direction, suggesting, small, long-lived dogs have naturally higher triglyceride levels. No n-3 or n-6 fatty acids nor their ratios were associated with weight or age (Supplementary Tables S2 and S3). Similarly, no lipids were significantly associated with sex, body condition score, or sterilization status.
Figure 1.
Sphingomyelins are positively associated with body weight in the companion dog. Panels (A–I) indicate nine different sphingomyelins that all increase with weight. Weight is presented in kilograms while lipid concentration is shown in relative units after data processing. Note weight is square-rooted for visualization purposes.
Figure 2.
Tri- and diglycerides are associated with age in the companion dog. The majority are positively associated (A–E) with one negative association (F). Lipid concentration is presented in relative units.
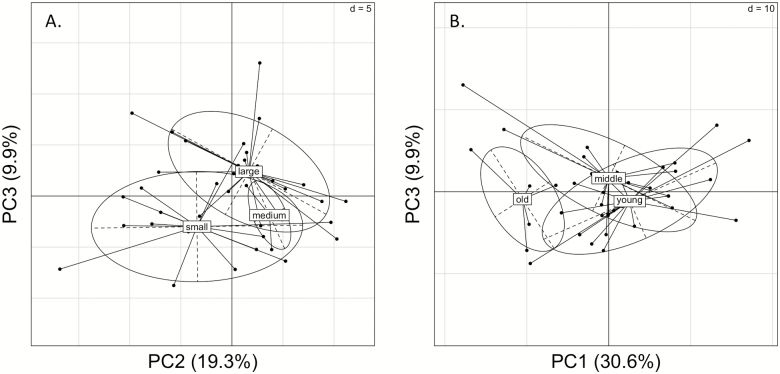
Our PCA suggests that the entire blood plasma lipidome is associated with weight and age (Figure 3). While more individual lipids were significantly associated with weight than age, age was a stronger factor influencing the entire lipidome, as PC1 was significantly different between the 3 age class groups (p = .0029 by one-way analysis of variance); a similar result was found if age was modeled as a continuous variable (p = .0034 by linear regression).
Figure 3.
Principal components analysis (PCA) of blood plasma lipidome, specified by weight class (A) and age class (B). Weight class groups were significantly different across PC2 (p = .024) and PC3 (p = .0005), as determined by a one-way analysis of variance (ANOVA). Age groups were significantly different across PC1 (p = .003) as determined by one-way ANOVA.
Discussion
Here, we have presented one of the first studies on lipid metabolism in companion dogs, and their associations with age and body weight (a proxy for canine longevity). We found a relatively large proportion of the circulating lipidome was associated with weight (24.9%) and age (16.6%). There were much higher percentages than our previous results on the non-lipid blood metabolome (8), suggesting that the lipidome may be more associated with longevity than the entire metabolome. However, whether any effects are causative requires further study.
The largest lipidomic changes associated with weight were seen in the sphingomyelins, which were all significantly lower in small, long-lived dogs. Sphingomyelins compose a large portion of cell membranes, and thus help determine the physical properties of the lipid bilayer. Though their cellular origination is still unknown, the fact that they were found in circulating blood emphasizes their known role in cell signaling. Recent research suggests that sphingomyelins also play large roles in other biological processes, including aging. For example, sphingomyelins have been implicated in multiple age-related diseases, and ceramides, the precursors of sphingomyelins increase substantially with age [reviewed in Cutler and Mattson (21)]. In addition, low levels of circulating sphingolipids, including ceramides and sphingomyelins, are found in centenarians, suggesting low levels are indicative of “healthy aging” (22), and high sphingomyelins have been proposed as a biomarker of Alzheimer’s disease (23). This ties nicely into another aspect of “healthy aging,” reducing the bioactive lipid mediators known for promoting systemic chronic inflammation. Further investigation of bioactive sphingolipids including ceramide, ceramide-1 phosphate and sphingosine-1 phosphate in the pathogenesis of inflammatory diseases has recently been promoted in biomedical research (24,25). However, the direct causative roles of these sphingolipids and sphingomyelins in disease pathogenesis are still unknown. As chronic inflammation maybe a driving factor of aging and disease (26), determining the exact role of proportional changes of circulating bioactive lipids and sphingomyelins in the susceptibility to age-related chronic inflammatory pathologies such as atherosclerosis, cardiovascular disease, type II diabetes, and cancer seems a useful avenue of future research. Importantly, these relationships will have to be addressed both within and between species. Again, long- and short-lived dogs provide useful models due to the similarity of their age-related pathologies to humans (1).
Interestingly, a growing body of evidence has suggested an association between insulin/IGF-I signaling and ceramides and sphingomyelins. That is, these lipid moieties directly interact with the IGF-1 receptor as well as AKT, and so may be involved in the modulation of insulin signaling [reviewed in Jesko et al. (27)]. As IGF-I signaling is one of the most plausible mechanisms contributing to the size-longevity tradeoffs in dogs (7), our results support the intriguing hypothesis that sphingomyelins interacting with the IGF-I receptor could potentially influence size and life span. Previous research in dogs has suggested that sphingomyelin levels differ between breeds; however, the effects of age and weight themselves were not addressed (28). In addition, decreases in growth hormone activity leading to decreases in circulating IGF-I is arguably the most robust genetic manipulation to increase life span. However, the effects of downregulated IGF-I on lipid metabolism have only recently been investigated. We proposed recently (29) that growth hormone-deficient mice, such as the Ames dwarf mouse, have altered lipid storage patterns and metabolism, and this was shown comprehensively when Ames dwarf mice were found to have significant changes in the adipose tissue metabolome (30).
Our group has previously published that tryptophan metabolism was significantly different between large and small dogs (8). We therefore were interested in discovering if these 2 pathways might be intertwined as the samples were run here were from the same dogs as our previous work. Interestingly, to our knowledge, there does not appear to be a strong literature on the association between these two pathways. While both tryptophan metabolism and sphingomyelin biology have been individually linked to aging in multiple species, we fail to find studies that link them together. For instance, research on mouse livers has shown that caloric restriction leads to significant alterations in tryptophan metabolites, however, no changes in sphingolipids (31). Therefore, we believe this is an interesting area of research on which to follow up. Potentially there is an unknown relationship between the pathways that may be worth exploring further with the exceptional tools available in model organisms. However, we would also not be surprised if either there is no functional relationship between sphingomyelins and tryptophan, or the changes we see may related to other physiological processes. No matter which hypothesis is correct, discovering the role of tryptophan metabolism and circulating sphingomyelins will greatly increase our understanding of physiological aging across species.
We also discovered tri- and diglycerides were significantly associated with age, and to a lesser extent size. Di- and triglycerides are commonly thought of as storage lipids, as they make up a large proportion of the fat droplets in adipose tissue. Age-related increases in triglycerides have been reported in multiple species, including humans (32), though some evidence in men also suggests triglycerides may decline after 60 years of age. In humans, these increases have been found to have negative effects, influencing the development of numerous age-related pathologies, including type 2 diabetes (33) and cardiovascular disease (34). In dogs, previous research has also found an increase in triglycerides with age (35), though there is variation depending on the specific type of triglyceride. Interestingly, we found several triglycerides were higher in small, long-lived dogs. This is somewhat unexpected as high triglycerides are commonly associated with negative phenotypic outcomes; however, we have no information on the trajectory of these triglycerides with age. The results do suggest that specific triglycerides may play previously unappreciated roles in health and longevity.
Generally, the correlation between increasing adiposity and aging is well accepted in humans as well as laboratory rodents and is frequently observed in aging cats and dogs as well (36). This is often referred to as “adipaging” and most likely drives inflammation in companion animals as well (37,38). In addition, obesity was recently shown to lead to decreased life span in dogs (39). Within our study, the majority of dogs were either normal to overweight, though body condition score, a measure of obesity in the dog, was not associated with any circulating lipids in our study.
Our PCA found a fairly strong separation of the lipidome by age and weight, in contrast to our previous metabolomic results (8). Similar to our individual lipid level analysis, the overall lipidome, at least in blood plasma, appears to be more tightly associated with size and age than the metabolome. Potentially, variation in individual lipids is contributing a larger proportion to the size-longevity tradeoff seen in dogs, or at least they are affected as a byproduct to a larger degree.
We failed to find a significant association of sex and sterilization status in the circulating lipidome, which is surprising as sterilization status especially is associated with life span and cause of death (40). However, these results are in agreement with our previous study on the dog metabolome (8). In addition, sex differences in aging in the dog appear to be much more minor than in other mammalian species (41). Here, we were only investigating the blood plasma lipidome, and potentially, sex and sterilization differences would become more apparent when analyzing other tissues. We do believe sex and sterilization status most likely play a strong physiological role in the dog, but further studies are required to determine the nuances of hormonal effects in companion animals.
Caveats
While we find striking differences in blood plasma lipid levels in large and small dogs, our study is not without its caveats and biases, many of which have been explained in our previous metabolomics work (8). These include the inability to control for diet timing, diet composition, and total dietary intake. In addition, the sample size used in this study is quite small (only 41 dogs), as such our results might be applicable to this specific population in Birmingham, Alabama, United States, and not others around the world. However, it is surprising that we find so many lipids that are associated with age and weight in this small sample, considering the varied environments, diets, and other external factors in which our dogs no doubt differ, suggesting that our results may represent general changes that occur in dogs. Concurrently, as a consequence of our small sample size, we were not able to determine any breed specific effect, nor control for the effects of breed. As lipid profiles have previously been shown to be different across breeds (28), this lack of breed information in our statistical model may be biasing our results.
Conclusions
Overall, we find the blood plasma lipidome contains signatures of both age and longevity (body mass) in the companion dog. The higher levels of sphingomyelins in small dogs points towards physiological differences that might underlie a portion of the large size-longevity tradeoff seen in dogs and adds to the growing body of literature on aging and longevity in the dog. Future experimental studies are now needed to determine if these lipids play direct roles in aging or longevity and whether age-related changes in circulating lipids might serve as potential biomarkers.
Supplementary Material
Acknowledgments
We would like to thank the dogs and their owners for providing samples for this study. We are grateful for the suggested revisions from the 2 anonymous reviewers who helped to improve the manuscript.
Funding
This work was supported by a Glenn/AFAR Postdoctoral Fellowship to J.M.H., the National Institutes of Health (K99AG059920 to J.M.H. and P30AG050886 to S.N.A.), and the Austrian Science Foundation (P26246B16 to T.G.V.).
Conflict of Interest
None declared.
Author Contributions
J.M.H., T.G.V., and S.N.A. designed the study. J.V.K. collected the blood samples. K.K. ran the lipidomics samples. J.M.H. analyzed the data and wrote the first draft of the manuscript. All authors commented and approved the final manuscript.
References
- 1. Hoffman JM, Creevy KE, Franks A, O’Neill DG, Promislow DEL. The companion dog as a model for human aging and mortality. Aging Cell. 2018;17:e12737. doi: 10.1111/acel.12737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleming JM, Creevy KE, Promislow DE. Mortality in North American dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J Vet Intern Med. 2011;25:187–198. doi: 10.1111/j.1939-1676.2011.0695.x [DOI] [PubMed] [Google Scholar]
- 3. O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of owned dogs in England. Vet J. 2013;198:638–643. doi: 10.1016/j.tvjl.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 4. Bonnett BN, Egenvall A. Age patterns of disease and death in insured Swedish dogs, cats and horses. J Comp Pathol. 2010;142(Suppl. 1):S33–S38. doi: 10.1016/j.jcpa.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 5. Comfort A. Longevity and mortality in dogs of four breeds. J Gerontol. 1960;15:126–129. doi: 10.1093/geronj/15.2.126 [DOI] [PubMed] [Google Scholar]
- 6. Favier RP, Mol JA, Kooistra HS, Rijnberk A. Large body size in the dog is associated with transient GH excess at a young age. J Endocrinol. 2001;170:479–484. doi: 10.1677/joe.0.1700479 [DOI] [PubMed] [Google Scholar]
- 7. Greer KA, Hughes LM, Masternak MM. Connecting serum IGF-1, body size, and age in the domestic dog. Age (Dordr). 2011;33:475–483. doi: 10.1007/s11357-010-9182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffman JM, Kiklevich JV, Austad M, et al. Tryptophan metabolism is differently regulated between large and small dogs. Geroscience. 2020;42:881–896. doi: 10.1007/s11357-019-00114-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jimenez AG, Winward J, Beattie U, Cipolli W. Cellular metabolism and oxidative stress as a possible determinant for longevity in small breed and large breed dogs. PLoS One. 2018;13:e0195832. doi: 10.1371/journal.pone.0195832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicholatos JW, Robinette TM, Tata SVP, et al. Cellular energetics and mitochondrial uncoupling in canine aging. Geroscience. 2019;41:229–242. doi: 10.1007/s11357-019-00062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hulbert AJ. Life, death and membrane bilayers. J Exp Biol. 2003;206:2303–2311. doi: 10.1242/jeb.00399 [DOI] [PubMed] [Google Scholar]
- 12. van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2 [DOI] [PubMed] [Google Scholar]
- 14. Valencak TG, Ruf T. N-3 polyunsaturated fatty acids impair lifespan but have no role for metabolism. Aging Cell. 2007;6:15–25. doi: 10.1111/j.1474-9726.2006.00257.x [DOI] [PubMed] [Google Scholar]
- 15. Hulbert AJ, Faulks SC, Buffenstein R. Oxidation-resistant membrane phospholipids can explain longevity differences among the longest-living rodents and similarly-sized mice. J Gerontol A Biol Sci Med Sci. 2006;61:1009–1018. doi: 10.1093/gerona/61.10.1009 [DOI] [PubMed] [Google Scholar]
- 16. Valencak TG, Ruf T. Phospholipid composition and longevity: lessons from Ames dwarf mice. Age (Dordr). 2013;35:2303–2313. doi: 10.1007/s11357-013-9533-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valencak TG, Azzu V. Making heads or tails of mitochondrial membranes in longevity and aging: a role for comparative studies. Longev Healthspan. 2014;3:3. doi: 10.1186/2046-2395-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35(Web Server issue):W606–W612. doi: 10.1093/nar/gkm324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chakraborty M, Jiang XC. Sphingomyelin and its role in cellular signaling. In: Capelluto D, ed. Lipid-mediated Protein Signaling. Advances in Experimental Medicine and Biology. Springer; 2013. [DOI] [PubMed] [Google Scholar]
- 21. Cutler RG, Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mech Ageing Dev. 2001;122:895–908. doi: 10.1016/s0047-6374(01)00246-9 [DOI] [PubMed] [Google Scholar]
- 22. Jové M, Naudí A, Gambini J, et al. A stress-resistant lipidomic signature confers extreme longevity to humans. J Gerontol A Biol Sci Med Sci. 2017;72:30–37. doi: 10.1093/gerona/glw048 [DOI] [PubMed] [Google Scholar]
- 23. Koal T, Klavins K, Seppi D, Kemmler G, Humpel C. Sphingomyelin SM(d18:1/18:0) is significantly enhanced in cerebrospinal fluid samples dichotomized by pathological amyloid-β42, tau, and phospho-tau-181 levels. J Alzheimers Dis. 2015;44:1193–1201. doi: 10.3233/JAD-142319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009;158:982–993. doi: 10.1111/j.1476-5381.2009.00281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jęśko H, Stępień A, Lukiw WJ, Strosznajder RP. The cross-talk between sphingolipids and insulin-like growth factor signaling: significance for aging and neurodegeneration. Mol Neurobiol. 2019;56:3501–3521. doi: 10.1007/s12035-018-1286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lloyd AJ, Beckmann M, Wilson T, Tailliart K, Allaway D, Draper J. Ultra high performance liquid chromatography-high resolution mass spectrometry plasma lipidomics can distinguish between canine breeds despite uncontrolled environmental variability and non-standardized diets. Metabolomics. 2017;13:15. doi: 10.1007/s11306-016-1152-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valencak TG, Spenlingwimmer T, Nimphy R, Reinisch I, Hoffman JM, Prokesch A. Challenging a “cushy” life: potential roles of thermogenesis and adipose tissue adaptations in delayed aging of ames and Snell dwarf mice. Metabolites. 2020;10:176. doi: 10.3390/metabo10050176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darcy J, Fang Y, McFadden S, et al. Integrated metabolomics reveals altered lipid metabolism in adipose tissue in a model of extreme longevity. Geroscience. 2020. doi: 10.1007/s11357-020-00221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jové M, Naudí A, Ramírez-Núñez O, et al. Caloric restriction reveals a metabolomic and lipidomic signature in liver of male mice. Aging Cell. 2014;13:828–837. doi: 10.1111/acel.12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heiss G, Tamir I, Davis CE, et al. Lipoprotein-cholesterol distributions in selected North American populations: the lipid research clinics program prevalence study. Circulation. 1980;61:302–315. doi: 10.1161/01.cir.61.2.302 [DOI] [PubMed] [Google Scholar]
- 33. Tirosh A, Shai I, Bitzur R, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. 2008;31:2032–2037. doi: 10.2337/dc08-0825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 35. Kawasumi K, Kashiwado N, Okada Y, et al. Age effects on plasma cholesterol and triglyceride profiles and metabolite concentrations in dogs. BMC Vet Res. 2014;10:57. doi: 10.1186/1746-6148-10-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. German AJ. The growing problem of obesity in dogs and cats. J Nutr. 2006;136:1940S–1946S. doi: 10.1093/jn/136.7.1940S [DOI] [PubMed] [Google Scholar]
- 37. Perez LM, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Lucia A, Galvez BG. “Adipaging”: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J Physiol. 2016;594:3187–3207. doi: 10.1113/JP271691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. German AJ, Ryan VH, German AC, Wood IS, Trayhurn P. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J. 2010;185:4–9. doi: 10.1016/j.tvjl.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 39. Salt C, Morris PJ, Wilson D, Lund EM, German AJ. Association between life span and body condition in neutered client-owned dogs. J Vet Intern Med. 2019;33:89–99. doi: 10.1111/jvim.15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoffman JM, Creevy KE, Promislow DE. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS One. 2013;8:e61082. doi: 10.1371/journal.pone.0061082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffman JM, O’Neill DG, Creevy KE, Austad SN. Do female dogs age differently than male dogs? J Gerontol A Biol Sci Med Sci. 2017;73:150–156. doi: 10.1093/gerona/glx061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.