Abstract
Background
Herpes zoster may significantly impact quality of life (QoL) in older adults. The recombinant zoster vaccine (RZV) is efficacious in adults aged ≥50 and older and is associated with increased reactogenicity compared to placebo. We report here on the impact of reactogenicity of the second RZV dose on the QoL and physical functioning (PF) of vaccine recipients, and summarize findings following both doses.
Method
In this single-arm study, 401 adults aged ≥50 and older were enrolled to receive two RZV doses 2 months apart. Change in mean Short Form Survey-36 (SF-36) PF and EuroQol-5 Dimension (EQ-5D) scores, reactogenicity, safety, productivity loss, and healthcare resource utilization were evaluated.
Results
In total, 391 (97.5%) participants received dose 2. Post-dose 2, the most common solicited local symptoms were injection site pain (75.1%), erythema (22.4%), and swelling (13.9%), and the most common systemic symptoms were fatigue (46.3%), headache (37.5%), and myalgia (32.9%). Grade 3 solicited (local and systemic) adverse events were reported by 61 (15.6%) participants and were associated with a transient clinically significant decrease in SF-36 PF score on Days 1–2 post-dose 2 that recovered by Day 3. Overall, no clinically important reduction in mean SF-36 PF scores was observed from baseline to post-dose 2 (mean change −0.4), and no quality-adjusted-life-year loss was recorded.
Conclusions
Overall, QoL and PF of RZV vaccinees were not affected by vaccine-related reactogenicity. A transient reduction was observed in the first 2 days after RZV vaccination in individuals with Grade 3 adverse events. No safety concerns were identified.
Keywords: Physical function, Physical activity, Pain, Zoster vaccine
Herpes zoster (HZ) consists in a vesicular dermatomal rash associated with pain resulting from the reactivation of latent varicella zoster virus (VZV) (1). The increased risk of HZ in older adults correlates with a decline in VZV-specific T cell-mediated immunity associated with immunosenescence and the presence of some chronic medical conditions and immune-suppressive illnesses or therapies (2). HZ and its complications significantly impact patients’ quality of life (QoL) and activities of daily living (ADL), as well as the life of their partners and caregivers (3–6).
Two types of vaccines are licensed to prevent HZ and associated complications in adults ≥50 years of age, single-dose live-attenuated HZ vaccines and a two-dose adjuvanted recombinant zoster vaccine (Shingrix, RZV; GSK). RZV demonstrated efficacy >90% in preventing HZ in older adults (7,8) and proved cost-effective from both a payer and societal perspective (9,10). The estimated vaccine efficacy in reducing HZ burden of illness and HZ burden of interference was greater than 90% in both the ZOE-50 and the pooled ZOE-70 analyses (11). Pivotal phase III trials conducted in approximately 30,000 adults aged 50 years or older (randomized 1:1 to receive either RZV or placebo) reported Grade 3 solicited or unsolicited events (preventing normal daily activities) for 17.0% (ZOE-50) and 11.9% (ZOE-70) of RZV recipients, after the two-dose schedule (7,8).
The primary objective of the present study was to assess the impact of RZV-related reactogenicity on the QoL of recipients by evaluating the change in Short Form Survey-36 (SF-36) Physical Functioning (PF) scale score from pre- to post-each RZV dose. Secondary objectives included RZV-related changes in (i) SF-36 PF single item scores, (ii) SF-36 role physical scores, (iii) Quality-Adjusted Life Year (QALY), (iv) healthcare resource utilization, (v) work loss by participants and non-dedicated caregivers, (vi) extra work for dedicated caregivers, and (vii) occurrence, intensity, causality and duration of adverse events (AEs). Results of this study will assist Health Care Professionals in informing their patients about the potential impact of vaccine reactogenicity on their QoL, which may affect patients’ decision to receive RZV.
We recently reported the QoL and daily functioning of participants in this study following the first RZV dose. Overall, no clinically significant negative impact was observed, though, as expected, a transient clinically important decrease in daily SF-36 PF score (affecting activities such as walking, carrying groceries, climbing stairs) was recorded on Days 1–2 post-first dose for participants experiencing Grade 3 solicited AEs (12). Here, we present the QoL and PF results, along with safety findings, following the second vaccine dose and provide an overview of the impact of RZV reactogenicity on participants’ daily physical activities after receiving both doses.
A summary of the research, clinical relevance, and the impact on the patient population are displayed in Supplementary Figure S1.
Method
Study Design and Participants
This was a phase III, single-arm, open-label clinical trial conducted in 13 centers in the United States from 16 January 2017 to 24 May 2018 (NCT02979639). Participants were men and women aged ≥50 and older at enrollment, who provided written informed consent before the study start. Each participant was to receive two 0.5 mL intramuscular doses of RZV on Day 0 and Day 60. Details of study inclusion/exclusion criteria, the ethical statement, and vaccine composition are described in the primary publication (12).
Reactogenicity and Safety Assessments
Systemic AEs (fatigue, fever, gastrointestinal symptoms, headache, myalgia, shivering) were recorded prior to vaccination on Days −7 and 0. For 7 days following each vaccination (Days 1–7 post-dose 1 and 61–67 post-dose 2), participants recorded solicited local (injection site pain, redness, swelling) and systemic AEs on diary cards. Unsolicited AEs were recorded for 30 days following each vaccination. Serious adverse events (SAEs) and potential immune-mediated diseases (pIMDs) were recorded over the entire study period from the receipt of the first dose until 12 months following administration of the second dose. The intensity of all AEs was graded from 1 to 3, where a Grade 3 event was defined as diameter >100 mm (injection site redness and swelling), temperature >39.0°C (fever), and “preventing normal activity” (all other events). Supplementary Table S1 summarizes the intensity scale used for AEs grading. All solicited local symptoms were considered as causally related to vaccination; the causality of all other AEs was assessed by the investigator.
QoL Assessments
Two health surveys, SF-36 (13) and EuroQol-5 Dimension (EQ-5D) (14), utilized during this trial have been described elsewhere (12). Briefly, the SF-36 comprises 36 questions, with answers aggregated to generate eight scales with values ranging from 0 to 100 are derived for PF, Role Physical, Bodily Pain, Systemic Health, Vitality, Social Function, Role Emotional, and Mental Health. Higher scores represent greater QoL; mean change from pre-vaccination of 3.3 in PF score was considered to be clinically relevant based on the minimal clinical importance threshold defined by Angst et al. (15). EQ-5D defines health in terms of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. These five items are converted to provide a single index utility score, where a higher score represents a better QoL. Both questionnaires were completed at the study center at Day −7 (7 days pre-dose 1), Day 0 (before receiving dose 1), Day 7 (7 days post-dose 1), Day 60 (before receiving dose 2), and Day 67 (7 days post-dose 2). All participants also completed the PF component of the SF-36 and the entire EQ-5D questionnaire at home on a daily basis for 6 days post-dose 1 and post-dose 2. For dose 2 analysis, baseline values for individual participants were calculated as the mean of the Day −7, Day 0, and Day 60 assessment scores. The mean post-dose 2 scores were defined as the mean of the seven assessment scores recorded from Days 61–67. The changes from pre- to post-dose 2 were calculated on an individual basis, as the difference between the mean post-dose 2 score and baseline score. The participants thereby served as their own control (ie, by using each participants’ own pre-vaccination values).
Healthcare Resource Utilization and Missed Time From Work
Daily diary cards captured self-reported healthcare resource utilization associated with reactogenicity, including medication for related AEs and medically attended visits and consultations as a result of reactogenicity. On Day 7 post-vaccination, participants self-reported vaccine-related lost productivity, which included missed time from work by employed participants and non-dedicated caregivers and extra work expended by dedicated caregivers.
Statistical Analyses
A sample size of 360 participants was considered necessary to generate an estimate of the mean PF scores change with a precision of 2.2 (SD of 21.2). Assuming a non-evaluable proportion of approximately 10% of participants, a total of 400 individuals were planned to be enrolled in the study. Reactogenicity and safety assessment was performed on the total vaccinated cohort (TVC), which included all vaccinated participants. The percentage of participants with at least one solicited (local and systemic) and unsolicited AE, SAE, and pIMD was calculated with exact 95% confidence interval (CI). The QoL analysis was also carried out on the TVC. The eight SF-36 scales and the EQ-5D utility scores were presented by age category and overall. The SF-36 PF scores were also presented by gender, frailty status (categorized as non-frail, pre-frail, and frail), AE type, and reactogenicity grade. The SF-36 PF individual items were presented over time by reactogenicity grade in a post hoc analysis and the change from baseline for each item was presented by frailty status also in a post hoc analysis. The EQ-5D QALY loss was analyzed by age, frailty status, AE type, and reactogenicity grade. The EQ-5D utility scores were assessed overall and in post hoc analyses by reactogenicity grade. Details of the development of the frailty assessment are presented elsewhere (16). The QALY loss was estimated using the area-under-the-curve method, where area under the curve was calculated using the trapezoidal rule (17). All statistical analyses were performed using SAS version 9.2.
Results
Participants
Of the 401 participants enrolled and vaccinated with dose 1, 391 received the second RZV dose (97.5%); 389 (99.5%) of them returned their symptom sheet by Day 67. Completion of the SF-36 and EQ-5D questionnaires was high (98.2% from Day 61 to 66, and 74.4% on Day 67). Participants had a mean age of 64.6 years, 82.8% were Caucasian and 58.6% were female (12).
Reactogenicity and Safety
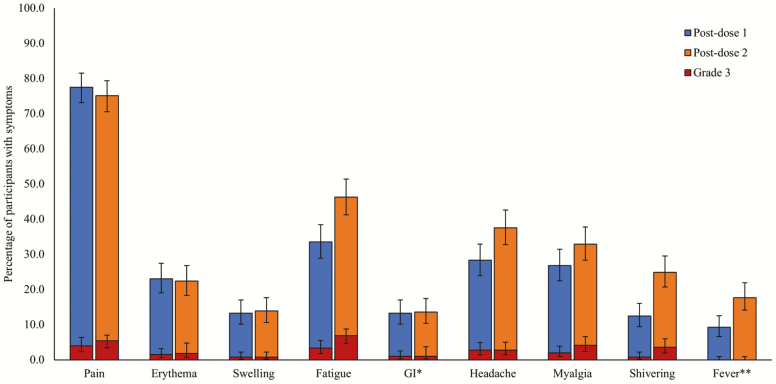
Within the 7 days follow-up period post-dose 2, the majority of participants reported at least one solicited local AE (injection site pain: 75.1%; erythema: 22.4%; swelling: 13.9%). Solicited local AEs of Grade 3 intensity were reported by 5.4% (injection site pain), 1.8% (erythema), and 0.8% (swelling) of participants (Figure 1). The most common solicited systemic AEs post-dose 2 were fatigue (46.3%), headache (37.5%), and myalgia (32.9%); Grade 3 intensity was reported by 6.9% (fatigue), 2.8% (headache), and 4.1% (myalgia) of vaccine recipients (Figure 1). Grade 3 solicited (local and systemic) AEs were reported by 38 participants post-dose 1 and 61 participants post-dose 2. The frequency of Grade 3 AEs post-dose 2 was higher for participants who experienced at least one Grade 3 event after the first dose than for those who experienced AEs at lower intensity after the first dose (Supplementary Table S2).
Figure 1.
Percentage of participants with solicited local and systemic AEs over the 7-day follow-up period post-dose 1 and post-dose 2 (total vaccinated cohort). AE, adverse event; *nausea, vomiting, diarrhea and/or abdominal pain; **≥37.5°C; # RZV Dose 1 administered on Day 0, Dose 2 administered on Day 60. Error bars represent 95% confidence intervals.
Overall, 127 (31.7%) participants reported at least one unsolicited AE during the 30-day post-dose 2 follow-up period, among whom 25 (6.2%) reported Grade 3 unsolicited AEs. A total of 21 SAEs were reported by 14 (3.5%) participants over the entire study period (Supplementary Table S3). Of those, 17 were resolved before the study end, two resulted in death (adenocarcinoma of the duodenum and unknown cause), and two were considered not resolved at study end (cardiomyopathy and myelodysplastic syndrome). None of the SAEs were considered causally related to vaccination as per investigator judgment. SAEs and pIMDs by age group and frailty status are presented in Supplementary Table S3. Three pIMDs were reported by two (0.5%) participants over the entire study period (two episodes of gout for one subject and facial paralysis (Bell’s Palsy) for the other subject). None were assessed as causally related to vaccination as per investigator judgment.
Quality of Life
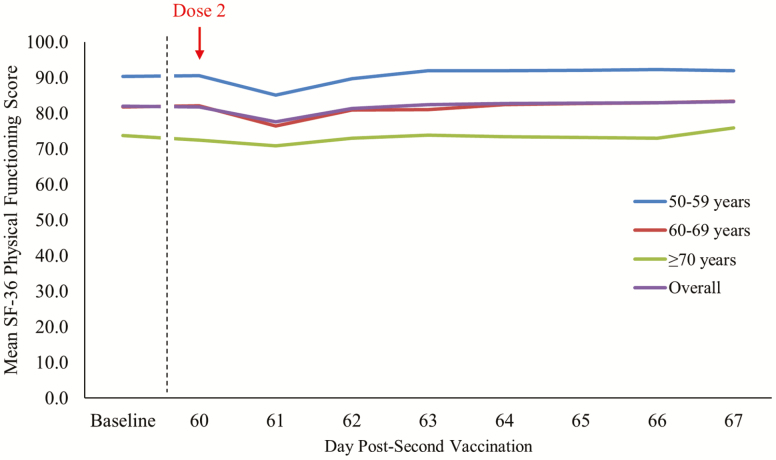
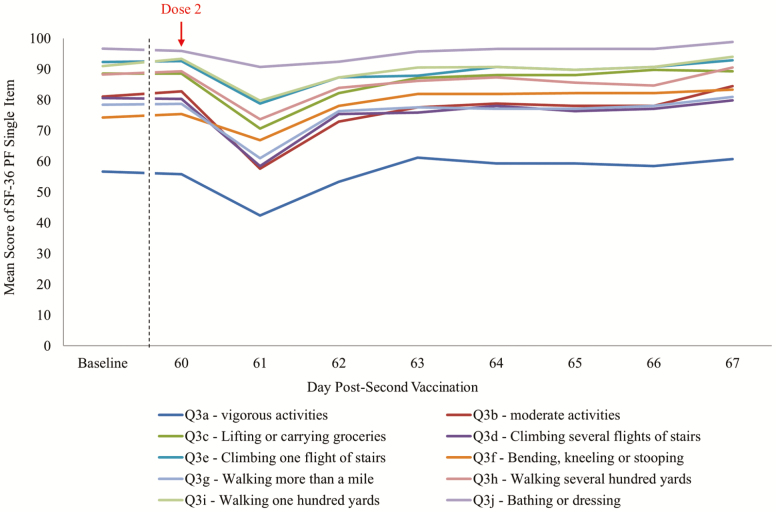
An overall mean change of −0.4 in SF-36 PF score from baseline to post-second RZV dose was observed (Figure 2 and Table 1). By age category, the mean change was +0.3 (from 90.3 to 90.6) in the 50–59 years age group, −0.5 (from 81.8 to 81.3) in the 60–69 years age group, and −0.9 (from 73.7 to 73.2) in the ≥70 years age group (Table 1). For participants experiencing Grade 3 solicited AEs, a point reduction of 2.4 in mean SF-36 PF score from baseline to post-second vaccination was recorded (Table 1), with a transient decrease of approximately 15 points from Day 60 (83.4) to Day 61 (68.0) that recovered by Day 63 (82.2) (Table 2). The mean changes in individual SF-36 PF items from baseline to Day 67 ranged from −3.7 (“climbing several flights of stairs”) to +3.9 (“bending or kneeling”). The most impacted individual SF-36 PF items amongst participants experiencing Grade 3 solicited AEs on Day 61 were Question 3b (moderate activities, eg, moving a table, pushing a vacuum cleaner, bowling) and Question 3d (climbing several flights of stairs) (Figure 3). Supplementary Table S4 shows the mean changes in the different SF-36 scales from pre- to post-second vaccination, ranging from −2.0 (“bodily pain”) to +0.8 (“mental health”). The mean SF-36 role physical score decreased by −1.8 points from baseline to post-dose 2.
Figure 2.
Mean Short Form Survey-36 (SF-36) Physical Functioning scale score at baseline and over the 7-day follow-up period post-dose 2 by age and overall (total vaccinated cohort). Baseline is calculated as the mean of Day −7, Day 0 (pre-dose 1), and Day 60 (pre-dose 2).
Table 1.
Mean SF-36 PF Scale Scores at Baseline and Post-Dose 2 (Total Vaccinated Cohort)
| Baseline | Days 1–7 Post-Dose 2 | Mean Change From Baseline to Post-Dose 2 | SE of the Mean Change | ||||
|---|---|---|---|---|---|---|---|
| N | Mean Score | N | Mean Score | ||||
| Overall | 391 | 82.0 | 389 | 81.8 | −0.4 | 0.53 | |
| Age category (y) | 50–59 | 132 | 90.3 | 131 | 90.6 | 0.3 | 0.79 |
| 60–69 | 130 | 81.8 | 130 | 81.3 | −0.5 | 0.85 | |
| ≥70 | 129 | 73.7 | 128 | 73.2 | −0.9 | 1.08 | |
| Reactogenicity grade | 0 | 63 | 78.5 | 61 | 82.5 | 3.2 | 1.35 |
| 1 and 2 | 267 | 82.6 | 267 | 81.9 | −0.7 | 0.65 | |
| 3 | 61 | 82.8 | 61 | 80.4 | −2.4 | 1.01 | |
| Adverse event type | None | 59 | 79.0 | 57 | 82.1 | 2.2 | 1.24 |
| Local | 302 | 82.7 | 302 | 81.9 | −0.8 | 0.58 | |
| Systemic | 245 | 83.5 | 245 | 82.3 | −1.2 | 0.60 | |
| Gender | Male | 161 | 80.8 | 159 | 81.6 | 0.4 | 0.89 |
| Female | 230 | 82.8 | 230 | 81.9 | −0.9 | 0.64 | |
| Frailty status | Non-Frail | 229 | 91.4 | 228 | 91.4 | 0.0 | 0.60 |
| Pre-Frail | 141 | 72.4 | 140 | 71.9 | −0.9 | 1.04 | |
| Frail | 21 | 43.7 | 21 | 43.7 | 0.0 | 2.39 |
Note: N = total number of participants; PF = physical functioning; SE = standard errors; ; SF-36 = Short Form Survey-36; high score represents high level of functioning/quality of life. Baseline is calculated as the mean of the Day −7, Day 0 (pre-dose 1) and Day 60 (pre-dose 2). Reactogenicity grading: 0 (none/normal); 1 (mild); 2 (moderate); 3 (severe; prevents normal activity); for swelling/redness; greatest surface diameter 0 (<20 mm); 1 (≥20 mm–≤50 mm); 2 (>50 mm–≤100 mm); 3 (>100 mm); for temperature: 0 (<37.5°C); 1 (37.5°C–38.0°C); 2 (38.1°C–39.0°C); 3 (>39.0°C).
Table 2.
Mean SF-36 PF Scale Scores Pre- and Post-Dose 2 by Day and Maximum Reactogenicity Grade (Total Vaccinated Cohort)
| Day | Grade 0 | Grade 1 or 2 | Grade 3 | |||
|---|---|---|---|---|---|---|
| N | Mean Score | N | Mean Score | N | Mean Score | |
| Baseline | 63 | 78.5 | 267 | 82.6 | 61 | 82.8 |
| Day −7 pre-dose 1 | 63 | 75.6 | 267 | 82.0 | 61 | 82.6 |
| Day 0 pre-dose 1 | 63 | 80.4 | 267 | 84.0 | 61 | 82.5 |
| Day 60 pre-dose 2 | 63 | 79.6 | 266 | 81.9 | 61 | 83.4 |
| Post-dose 2 (mean) | 61 | 82.5 | 267 | 81.9 | 61 | 80.4 |
| Day 61 | 61 | 83.9 | 265 | 78.2 | 59 | 68.0 |
| Day 62 | 61 | 82.6 | 265 | 81.5 | 59 | 78.9 |
| Day 63 | 61 | 82.4 | 265 | 82.5 | 58 | 82.2 |
| Day 64 | 61 | 82.0 | 265 | 82.8 | 59 | 82.8 |
| Day 65 | 61 | 82.1 | 265 | 83.1 | 59 | 82.3 |
| Day 66 | 60 | 81.8 | 265 | 83.2 | 59 | 82.6 |
| Day 67a | 44 | 83.2 | 205 | 82.9 | 42 | 85.5 |
Notes: N = total number of participants; PF = physical functioning; SF-36 = Short Form Survey-36; high score represents high level of functioning/quality of life (maximum is 100). Baseline is calculated as the mean of Day −7, Day 0 (pre-dose 1), and Day 60 (pre-dose 2).
aVisit at Day 67: defined as 7-days post-dose 2; not all participants returned for the site visit exactly on Day 67. Reactogenicity grading: 0 (none/normal); 1 (mild); 2 (moderate); 3 (severe; prevents normal activity); for swelling/redness; greatest surface diameter 0 (<20 mm); 1 (≥20 mm–≤50 mm); 2(>50 mm–≤100 mm); 3 (>100 mm); for temperature: 0 (<37.5°C); 1 (37.5°C–38.0°C); 2 (38.1°C–39.0°C); 3 (>39.0°C).
Figure 3.
Mean Short Form Survey-36 (SF-36) Physical Functioning single items from baseline to Day 7 post-dose 2 for participants with Grade 3 reactogenicity only (total vaccinated cohort). Baseline is calculated as the mean of Day −7, Day 0 (pre-dose 1), and Day 60 (pre-dose 2).
EQ-5D: QALY
No QALY loss was observed overall, by age, by gender, or by frailty status over the 7-day follow-up period post-dose 2 (mean overall change observed: +0.1128). For participants experiencing Grade 3 solicited AEs, a decrease in mean EQ-5D utility scores was observed on Day 61, but these had returned to pre-vaccination values by Day 63 (Supplementary Figure 2). The decrease from baseline in EQ-5D utility scores in the 61 participants (15.6%) with Grade 3 solicited AEs was 0.0767 on Day 61 and 0.0365 on Day 62; the decrease from baseline in EQ-5D utility score on Day 61 for the 267 participants (68.3%) with Grade 1 or 2 solicited AEs was 0.0386. The overall estimated QALY loss per vaccine recipient post-dose 2 (sum of (daily utility loss × % with utility loss)/365) was estimated as 0.00012.
Medically Attended Reactogenicity
Five participants sought medical attention for reactogenicity symptoms during the 7-day follow-up period post-dose 2. Four participants sought medical attention by telephone call (two participants in both 60–69 years and ≥70 years age groups) and one participant in the 60–69 years age group visited a general practitioner. Of the participants who sought medical attention, two participants were categorized as non-frail, and 3 were pre-frail. Overall, 37 (9.5%) participants took medication at least once in response to reactogenicity during the 7-day post-dose 2 period.
Participant Work Loss or Additional Caregiver Workload Due to Vaccination
Of the 186 participants who were working, 13 (7.0%) participants reported work loss due to reactogenicity post-dose 2, with a median duration of 1 day (mean duration: 1.1, SD: 0.4); seven of these 13 participants experienced at least one Grade 3 and six experienced at least one Grade 1 or 2 solicited AE on Day 60 or 61. None of the six dedicated caregivers (all for participants aged ≥70 and older) reported any extra work burden following the second RZV dose administration to study participants.
Discussion
In this study, we examined both the frequency of AEs following vaccination and the impact of reactogenicity on the QoL of study participants. Very high second-dose compliance by participants was observed, and nearly all returned their symptom sheets. The pattern of reactogenicity was similar to that reported after the first RZV vaccination (12) and is also in line with the reactogenicity profile observed in the two large pivotal efficacy studies, that enrolled a much larger population than the current study, thus providing more robust results with respect to the frequency of AEs (18). Consistently across studies, injection site pain was the most frequently reported solicited local AE and fatigue, headache, and myalgia were the most frequent solicited systemic AEs after each dose (18,19). Frequency of all-grade solicited systemic AEs tended to be higher after dose 2 compared to dose 1.
Overall, no reductions of more than 3.3 (threshold of minimal clinically important difference (15)) in the mean SF-36 PF scores were observed post-each dose. Overall mean change of SF-36 PF score was +1.9 from pre-vaccination to post-dose 1 (12) and -0.4 from baseline to post-dose 2. A transient but clinically meaningful decrease in mean daily SF-36 PF score from baseline to post-second vaccination was recorded for participants reporting Grade 3 solicited AEs. A similar trend was also observed post-dose 1 (12). Considering the definition of Grade 3 (preventing normal daily activities), this observation was not unexpected. Changes in other SF-36 scales from baseline to post-dose 2 were small, suggesting a minimal impact of RZV vaccination on PF and other QoL parameters.
Overall, no QALY loss associated with RZV vaccination was observed, although transient QALY daily losses were observed in participants with Grade 3 reactogenicity. The overall estimated QALY loss per vaccine recipient was 0.000064 post-dose 1 (12) and 0.00012 post-dose 2. The higher QALY loss post-dose 2 versus post-dose 1 is consistent with the incidence of Grade 3 reactogenicity after the first and second doses in this study. In comparison with the results presented here, a recent study reported that reactogenicity after an adjuvanted monovalent influenza A vaccine resulted in a QALY loss of 0.00011 (20), similar to that observed in the present study. By contrast, it has been previously reported that QALY losses due to HZ, including postherpetic neuralgia (PHN), ranged from 0.021 to 0.22 (21,22).
Five of 391 recipients receiving the second vaccine dose sought medical attention for reactogenicity and 13 reported work loss. In comparison, three out of 401 participants sought medical attention and five reported missing work due to reactogenicity symptoms following the first dose (12). No non-dedicated caregivers missed work and no extra work for dedicated caregivers was recorded after either dose. Previous studies indicated that most affected employees undergo work loss or impaired productiveness due to HZ and PHN symptoms with an average duration of work loss ranging between 27 and 116 hours per employed person (23–25); caregivers have also reported lost work due to an HZ episode (26,27). Taken together with the QALY data reported above, these data demonstrate that the benefits of vaccination in preventing disease greatly outweigh any impact of reactogenicity.
The limitations of this study include the open study design, lack of a control group, and a “response shift” phenomenon (28), as increases in mean SF-36 PF scores were predominantly observed in participants with Grade 0 reactogenicity. In the absence of data on clinically meaningful change of PF scores following vaccination, the threshold of 3.3 determined in patients with osteoarthritis was used in this study. One of the strengths of the study is the thorough evaluations of the pre-vaccination period, that is, allowing for a more precise definition of baseline (mean of the Day −7, Day 0, and Day 60 assessment scores). Other strengths of the study are the high compliance (97.5%) receiving the second dose, the high proportion (99.5%) of participants returning the diary cards completed by Day 67 and the high compliance in returning QoL surveys (98.2% of the participants from Day 61 to Day 66, and 74.4% of the participants on Day 67).
Conclusions
Overall, the QoL and PF of adults ≥50 years were not affected by vaccine-related reactogenicity experienced after RZV dose 1 and dose 2; as expected, a transient, clinically meaningful impact was observed in adults who reported Grade 3 solicited AEs. No safety concerns were reported in this study. The observed safety and reactogenicity are consistent with the previously published profile of RZV. The results of the study suggest that the benefits of preventing HZ, acute HZ episode-associated pain, and PHN through vaccination outweigh the minimal and transient loss in PF and QoL caused by vaccine-related reactogenicity.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants, investigators and study teams involved in this trial, as well as (all affiliated to GSK) Lotte Dehing, Kanika Dey, Laurence Fissette, Wendy Fitzgerald, Céline Maréchal, Laurel Rivera, and Elisa Turriani. The authors also thank Modis for editorial assistance and manuscript coordination, on behalf of GSK. Botond Nagy provided medical writing support and Quentin Deraedt coordinated the manuscript development and provided editorial support. Trademark:Shingrix is a registered trademark owned by or licensed to GlaxoSmithKline Biologicals SA. Data sharing statement: The study protocol and a summary of the study results are available at https://www.gsk-studyregister.com/study/5730 and at clinicaltrials.gov. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Funding
This work was funded by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study and covered all costs associated with developing and publishing this manuscript. K.E.S. was also supported by the National Institute on Aging, Duke Pepper Older Americans Independence Center P30AG028716. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions
Conceived and designed the study: K.E.S., C.H., K.G., L.O., and D.C.; Collected the data: K.E.S., M.J.L., M.C., W.W., and C.H.; Performed the study: K.E.S., M.J.L., M.C., W.W., A.S., and D.C.; Analyzed the data: K.E.S., M.J.L., S.M., M.R., W.W., C.H., K.G., A.S., L.O., and D.C.
Conflict of Interest
K.E.S. reports grants from the GSK group of companies (GSK) during the study. M.J.L. reports grants and fees for Advisory Board from GSK during the study, as well as grants and fees for Advisory Board and speaker at international meetings from Merck outside the submitted work. M.J.L. is serving as an Advisory Board member for GSK, Curevo, and Merck. M.C. reports no potential conflict of interest. S.M. is a freelance consultant for GSK. M.R., W.W., C.H., K.G., A.S., and D.C. are employees and L.O. former employee of GSK. W.W., A.S., L.O., and D.C. own GSK stock options or (restricted) shares. L.O. is employee of CureVac AG and is inventor on a patent owned by GSK and relevant to RZV.
References
- 1. Cohen JI. Herpes zoster. N Engl J Med. 2013;369:1766–1767. doi: 10.1056/NEJMc1310369m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population-based case-control study. BMJ. 2014;348:g2911. doi: 10.1136/bmj.g2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gater A, Uhart M, McCool R, Préaud E. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health. 2015;15:193. doi: 10.1186/s12889-015-1514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meyers JL, Madhwani S, Rausch D, Candrilli SD, Krishnarajah G, Yan S. Analysis of real-world health care costs among immunocompetent patients aged 50 years or older with herpes zoster in the United States. Hum Vaccin Immunother. 2017;13:1861–1872. doi: 10.1080/21645515.2017.1324373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmader KE, Sloane R, Pieper C, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–496. doi: 10.1097/AJP.0b013e318065b6c9 [DOI] [PubMed] [Google Scholar]
- 6. Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–356. doi: 10.1016/j.jpain.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 7. Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group . Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800 [DOI] [PubMed] [Google Scholar]
- 8. Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184 [DOI] [PubMed] [Google Scholar]
- 9. Prosser LA, ed. Economic Evaluation of Vaccination for Prevention of Herpes Zoster and Related Complications. Atlanta, GA: Advisory Committee on Immunization Practices (ACIP), Stephen B. Thacker CDC Library collection; 2017. [Google Scholar]
- 10. Curran D, Patterson B, Varghese L, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36:5037–5045. doi: 10.1016/j.vaccine.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 11. Curran D, Oostvogels L, Heineman T, et al. ; ZOE-50/70 Study Group . Quality of life impact of an adjuvanted recombinant zoster vaccine in adults aged 50 years and older. J Gerontol A Biol Sci Med Sci. 2019;74:1231–1238. doi: 10.1093/gerona/gly150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmader KE, Levin MJ, Grupping K, et al. The impact of reactogenicity after the first dose of recombinant zoster vaccine on the physical functioning and quality of life of older adults: an open-label, Phase III trial. J Gerontol A Biol Sci Med Sci. 2019;74:1217–1224. doi: 10.1093/gerona/gly218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ware JE, Kosinski M. SF-36 Physical & Mental Health Summary Scales: A Manual for Users of Version 1 (2001). [Google Scholar]
- 14. Kind P. The EuroQL instrument: an index of health-related quality of life. In: Spliker B, ed. Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd ed. Philadelphia, PA: Lippincott-Raven Publishers; 1996:191–201. [Google Scholar]
- 15. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–391. doi: [DOI] [PubMed] [Google Scholar]
- 16. Curran D, Andrew MK, Levin MJ, et al. Evaluation of two frailty indices, with practical application in a vaccine clinical trial. Hum Vaccin Immunother 2019;15(12);2960–2968. doi: 10.1080/21645515.2019.1622974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeh ST, ed. Using Trapezoidal Rule for the Area Under a Curve Calculation. SUGI 27 Proceedings2002. http://www2.sas.com/proceedings/sugi27/p229-27.pdf. Accessed February 12, 2018.
- 18. López-Fauqued M, Campora L, Delannois F, et al. ; ZOE-50/70 Study Group . Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine. 2019;37:2482–2493. doi: 10.1016/j.vaccine.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 19. Colindres R, Wascotte V, Brecx A, et al. 2780. Reactogenicity profile of adjuvanted recombinant Zoster vaccine after Dose 2 according to the intensity of the same event experienced after Dose 1. Open Forum Infectious Diseases 2019;6:S981–S982. doi: 10.1093/ofid/ofz360.2457 [DOI] [Google Scholar]
- 20. Standaert B, Dort T, Linden J, et al. Usability of daily SF36 questionnaires to capture the QALD variation experienced after vaccination with AS03A-adjuvanted monovalent influenza A (H5N1) vaccine in a safety and tolerability study. Health Qual Life Outcomes. 2019;17:80. doi: 10.1186/s12955-019-1147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore L, Remy V, Martin M, Beillat M, McGuire A. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc. 2010;8:7. doi: 10.1186/1478-7547-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine. 2007;25:8326–8337. doi: 10.1016/j.vaccine.2007.09.066 [DOI] [PubMed] [Google Scholar]
- 23. Drolet M, Levin MJ, Schmader KE, et al. Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine. 2012;30:2047–2050. doi: 10.1016/j.vaccine.2012.01.045 [DOI] [PubMed] [Google Scholar]
- 24. Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ. 2011;14:639–645. doi: 10.3111/13696998.2011.607482 [DOI] [PubMed] [Google Scholar]
- 25. White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. PharmacoEcon. 2009;27:781–792. doi: 10.2165/11317560-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 26. Matthews S, De Maria A, Passamonti M, et al. The economic burden and impact on quality of life of Herpes Zoster and Postherpetic Neuralgia in individuals aged 50 years or older in Italy. Open Forum Infect Dis. 2019;6:ofz007. doi: 10.1093/ofid/ofz007m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakamura H, Mizukami A, Adachi K, et al. Economic burden of Herpes Zoster and Post-Herpetic Neuralgia in adults 60 years of age or older: results from a prospective, physician practice-based cohort study in Kushiro, Japan. Drugs Real World Outcomes. 2017;4:187–198. doi: 10.1007/s40801-017-0119-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ring L, Höfer S, Heuston F, Harris D, O’Boyle CA. Health Qual Life Outcomes. 2005;3:55. doi: 10.1186/1477-7525-3-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.