Abstract
Background
As Asians are more vulnerable to febrile neutropenia (FN) than Caucasians, evaluations of FN incidence and risk factors in Asians are important for the appropriate use of primary pegfilgrastim (PEG-G).
Patients and methods
Japanese breast cancer patients receiving standard adjuvant chemotherapies were prospectively enrolled in multicenter institutions from August 2015 to July 2017. FN was evaluated from 2 treatment policies: true FN (T-FN): ≥37.5 °C, grade 4 neutropenia, mandatory hospital visit (visiting); surrogate FN (S-FN): ≥37.5 °C, oral antibiotic, no mandatory visit (non-visiting). PEG-G was used at the physicians’ discretion. The primary endpoint was FN incidence during all cycles. Multivariate logistic regression analysis was performed to identify T-FN risk factors.
Results
Of 1005 enrolled patients, 980 women treated with FEC, E(A)C, and TC were analyzed. The FN incidence proportions in all patients were 22.5%, 27.5%, and 33.9% for FEC, E(A)C, and TC, respectively. Those of T-FN were 27.7%, 22.4%, and 36.6%; those of S-FN were 17.3%, 32.4%, and 31.5% with more frequent primary PEG-G usage. The relative dose intensity (RDI) of the 3 regimens was ≥0.85 in both groups. In the analysis of risk factors, TC (odds ratio = 2.67), age ≥ 65 years (2.24), and pretreatment absolute neutrophil count (ANC)/1000 μl (0.8) remained significant.
Conclusions
FN incidences were above 20% in the 3 regimens, with TC showing the highest. RDI was maintained at a high level in both visiting and non-visiting groups. Patient-related risk factors were age and pretreatment ANC.
Keywords: Breast cancer, Febrile neutropenia, Adjuvant chemotherapy, Risk factors, Prospective study
Highlights
-
•
This study compared febrile neutropenia (FN) incidences of 3 breast cancer regimens.
-
•
FN incidences were >20% in the 3 regimens (FEC, E(A)C; TC); TC showed the highest.
-
•
The relative dose intensities in visiting and non-visiting groups were at high level.
-
•
Age and pretreatment absolute neutrophil count were found as significant FN factors.
1. Introduction
Febrile neutropenia (FN) is a major hematologic side effect associated with chemotherapy, and results in dose reduction and delayed treatment [1]. Both conditions are directly linked to a reduction in relative dose intensity (RDI), which impedes cure of patients with early breast cancer [2,3]. In addition, FN is a potentially life-threatening toxicity similarly to sepsis, and may require emergency admission and hospitalization [4,5]. For patients with a high risk of FN, primary pegfilgrastim (PEG-G) prophylaxis is recommended in clinical practice guidelines [[6], [7], [8]]. A meta-analysis showed that primary PEG-G support improved the overall survival of breast cancer patients with FN undergoing intensified chemotherapy [9]. However, primary PEG-G is associated with a higher risk for secondary malignancies in solid tumor or malignant lymphoma treatment [9]. The toxicities and also costs associated with PEG-G deserve a careful and systematic study. Therefore, identifying the risk factors and predictors of FN is clinically important to determine the appropriate indication of primary PEG-G.
The incidence of FN is associated with the myelosuppressive effect of chemotherapy regimens and individual patient risk factors. Regimens are categorized into 3 groups according to the risk of FN. Primary PEG-G is recommended for patients when using high-risk regimens with an FN incidence of >20%, whereas primary PEG-G is required for patients with risk factors for moderate-risk regimens with an FN incidence of 10%–20% [[6], [7], [8]].
As for patient-related risk factors of FN, advanced age (>65 years) is listed in all guidelines [[6], [7], [8]]. Except for age, slightly different factors appear in various guidelines. Advanced disease, previous chemotherapy or radiation therapy, pre-existing neutropenia, bone marrow involvement with the tumor, infection, open wounds, poor performance or nutritional status, poor renal function, liver dysfunction, and multiple comorbid conditions are listed as risk factors of FN [[6], [7], [8]].
Ethnic origin may be related to the incidence of FN, with Asians reported to be more vulnerable to FN than Caucasians [[10], [11], [12], [13]]. In a study comparing hematologic toxicity during neoadjuvant and adjuvant FEC treatments in breast cancer patients of 4 races, Asians had a significantly higher rate of grade 3 hematologic toxicity than Caucasians, African-Americans, and Hispanics [12]. This ethnic difference is partly due to differences in physical constitutions, such as the activity of metabolic enzymes [12]. Another possible reason is that calculation of the therapeutic dose practically tends to be based on the ideal weight and not on the actual weight in obese patients, who are less common in Asians [14]. However, only a few studies have examined the incidences and risk factors of FN in Asian patients with breast cancer [12,13]. Examination of these incidences and risk factors may help clarify the indication of primary PEG-G based on race.
For the prevention or early treatment of FN, prophylactic or therapeutic oral antibiotic administration is an alternative to primary PEG-G prophylaxis. In daily clinical practice, some physicians prescribe antibiotics and instruct patients to take these drugs if they have a fever, instead of using primary PEG-G or performing blood tests at the time the nadir is expected. With the current empirical prescription of oral antibiotics, it is practically important to know whether this method is safe in the short and long terms, and in terms of excessive use of antibiotics for prophylaxis.
These backgrounds indicate the importance of evaluating FN in breast cancer patients in Asian countries. The purpose of this cohort study is to evaluate the incidences and risk factors of FN in Japanese breast cancer patients who received 3 mainstream adjuvant regimens according to 2 treatment policies for FN.
2. Patients and methods
2.1. Study design
A multicenter, prospective observational study was performed in female patients who were diagnosed with clinical stages I to III breast ductal or lobular carcinoma and were scheduled for adjuvant or neoadjuvant chemotherapy using the following 6 regimens [15]: (1) 4 cycles of 5-fluorouracil 500 mg/m2 + epirubicin 100 mg/m2 + cyclophosphamide 500 mg/m2 (FEC), (2) 4 cycles of epirubicin 90 mg/m2 (doxorubicin 60 mg/m2) + cyclophosphamide 600 mg/m2 (E(A)C), (3) 4 cycles of docetaxel 60–100 mg/m2 (DOC), (4) 6 cycles of docetaxel 75 mg/m2 + doxorubicin 50 mg/m2 + cyclophosphamide 500 mg/m2 (TAC), (5) 4 cycles of docetaxel 75 mg/m2 + cyclophosphamide 600 mg/m2 (TC), and (6) 6 cycles of docetaxel 75 mg/m2 + carboplatin AUC6 + trastuzumab 8 mg/kg (TCbH). There was no provision regarding the administration of PEG-G, and it was used at the discretion of physicians. Early monitoring after the study initiation showed that only few patients received the TAC, TCbH, and DOC regimens, and identifying the incidence of FN for these regimens would be difficult. Therefore, the protocol was revised in July 2016 to limit the regimens that would be evaluated to FEC, E(A)C, and TC.
Patients were consecutively enrolled from August 2015 to July 2017 from 44 institutions. The number of patients varied from 1 to 98 in various institutions. Written informed consent was obtained from all patients. The study was approved by the protocol review committee of the Comprehensive Support Project for Oncological Research of Breast Cancer on February 10, 2015 and by the institutional review board of Tokyo Medical University on April 28, 2015 (No. 3044). This trial was registered at the UMIN Clinical Trials Registry as UMIN 000017857.
2.2. Baseline assessment
After enrollment, the following information was collected as baseline data: date of birth; site of breast cancer (left or right); pathological stage; status of lymph node metastasis; expression status of estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor type 2 (HER2); nuclear grade; histological grade; performance status (PS); previous treatment for breast cancer (endocrine, radiation and surgical treatments); comorbidity (renal disorder, liver disorder, neutropenia before treatment); and height and weight.
2.3. Data collection during treatment
The following items were investigated on day 1 of each treatment cycle: PS; type and dose of chemotherapeutic agent; and date of administration. Body temperature, laboratory values (white blood cell count, neutrophil count), and adverse event (AE)s were investigated at regular visits (or on admission) for chemotherapy and at unscheduled visits for fever and AEs. Information on the administration of primary PEG-G or therapeutic granulocyte colony-stimulating factor (G-CSF) and antibiotics was investigated in each treatment cycle.
2.4. Patient diary
During the chemotherapy period, patients measured their body temperature in the morning and evening, and when they felt a fever, and recorded the data in a diary.
2.5. Evaluation and definition of FN
Preliminary survey prior to this study revealed that each institution had different policies for responding to a fever in patients undergoing chemotherapy. These policies were broadly divided into 2 types. Some facilities instructed patients to visit the hospital if they developed a fever and to undergo a blood test for FN diagnosis. Other facilities asked patients to immediately take a pre-specified antibiotic if they developed a fever during chemotherapy without visiting the hospital, and only to visit if their fever persisted.
The present study used an observational design based on daily clinical practice. Thus, if patients developed an axillary temperature ≥37.5 °C, 2 procedures were used to manage the fever: (1) undergo a hematologic test with a hospital visit (visiting group) or (2) take a prescribed oral antibiotic without a hospital visit (non-visiting group).
We defined FN in 2 ways in accordance with the above-mentioned procedures (visiting and non-visiting groups) for managing fever during chemotherapy, namely, True-FN (T-FN) and Surrogate-FN (S-FN). T-FN was defined as an axillary temperature ≥37.5 °C and an absolute neutrophil count (ANC) of <500/μl (visiting group). S-FN was defined as an axillary temperature ≥37.5 °C and oral antibiotic/antipyretic intake according to the instructions of each hospital (non-visiting group). Each facility was required to clarify which policy was used to manage FN at the time of the study.
2.6. Endpoints
The primary endpoint was the incidence of FN for the whole 4 cycles of treatment. The patient was used as an analysis unit. The secondary endpoints were (1) FN incidence in the 1st cycle, (2) grade 4 neutropenia, (3) hospitalization, (4) use of therapeutic G-CSF, (5) use of therapeutic antibiotics, (6) AEs due to FN, (7) RDI, and (8) serious AEs.
2.7. Statistical analysis
In the analysis of the primary endpoint, the incidence proportions of FN for each regimen were estimated. To evaluate the difference between the 2 treatment policies, we calculated 3 types of proportions: (1) number of T-FN and S-FN cases/total number of patients in the entire population, (2) number of T-FN cases/number of patients in the visiting group, and (3) number of S-FN cases/number of patients in the non-visiting group.
In the analyses of the secondary endpoints, the endpoints were summarized by proportions and were compared between the visiting and non-visiting groups in each regimen. RDI was calculated as the number and proportion of patients in which 85% or more of the standard dose could be administered. The data show the proportion of cases with an RDI of 85% or higher for each cycle and for all cycles. Grade 4 neutropenia was summarized only in the visiting group.
To evaluate the risk factors of FN, we predefined regimens, age, PS, stage, history of chemotherapy or radiotherapy, infectious wounds, open wounds, ANC, and renal and liver dysfunctions as possible risk factors using the following categorization: age: < 65 vs ≥ 65 years; PS: 0 vs ≤ 1; stage: I or II vs III; prior chemotherapy: positive vs negative; radiotherapy: positive vs negative; infectious wound: positive vs negative; open wound: positive vs negative; ANC: < 1500 vs ≥ 1500/μl; renal dysfunction: Cr < 1.5 vs ≥ 1.5; and liver dysfunction: AST/ALT or total bilirubin >3 or 1.5 times the institutional normal range. Risk factors were evaluated in the visiting group. Multiple logistic regression analysis was used to assess the risk factors of T-FN, and the odds ratio (OR) and 95% confidence interval (CI) were calculated using T-FN in the visiting group as the outcome measure.
The target sample size was calculated based on interval estimation, as described in detail previously [15]. From the clinical perspective, the admissible 95% CIs were the estimated incidence proportion ±5%. If the true incidence proportion is 10% or 20%, the required number of cases was 165 or 264 to achieve an admissible 95% CI. In this sample size calculation, the incidence proportions in all the enrolled patients were considered based on the speculation that these incidence proportions were similar between the visiting and non-visiting groups. Considering that the number of patients who use TAC, TCbH, and DOC is not very large, we set the target sample size to 1000 for the 6 regimens. Initially, 200 patients were planned for the 3 major regimens of E(A)C, FEC, TC and DOC to achieve 95% CI. For TAC and TCbH, 50 patients were planned for these regimens in consideration of feasibility rather than 95% CI. However, early monitoring after the study initiation showed that only few patients received these regimens, and identifying the incidence of FN for these regimens would be difficult. Thus, the protocol was revised in July 2016 to limit the regimens to be evaluated to FEC, E(A)C, and TC with 330 cases each.
3. Results
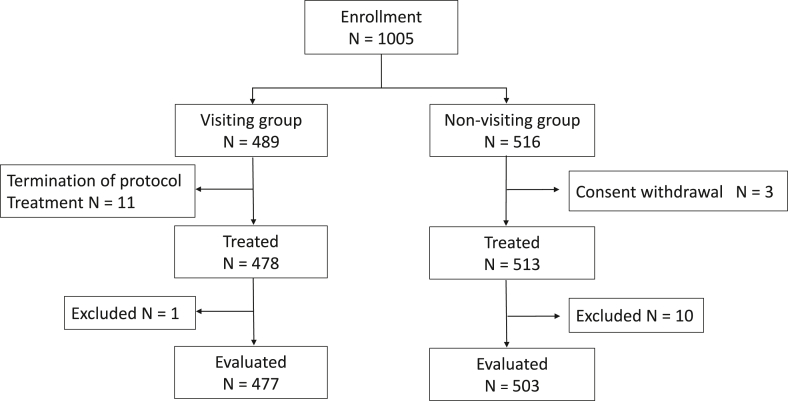
From August 2015 to July 2017, a total of 1005 patients with stages I to III breast cancer were consecutively enrolled in the present study. Of these 1005 patients, 489 patients were registered in the visiting group. Among these 489 patients, 11 were excluded because of chemotherapy interruption and 1 was excluded owing to being lost to follow-up. The reasons for chemotherapy interruption in the 11 patients were refusal of study enrollment in 4 patients, refusal of chemotherapy in 2 patients, change of hospital in 3 patients, change of treatment regimen in 1 patient, and detection of metastatic disease in 1 patient. A total of 516 patients were registered in the non-visiting group. Among these 516 patients, 3 were excluded because of consent withdrawal and 10 were excluded owing to being lost to follow-up. Finally, 980 patients, namely, 477 (48.7%) in the visiting group and 503 (51.3%) in the non-visiting group, were analyzed in the present study (Fig. 1).
Fig. 1.
Flow diagram of patient enrollment and selection. In this study, 1005 patients with stages I to III breast cancer were consecutively enrolled from August 2015 to July 2017. Of these 1005 patients, 489 patients were registered in the visiting group. Among these 489 patients, 11 were excluded because of chemotherapy interruption and 1 was excluded owing to being lost to follow-up. A total of 516 patients were registered in the non-visiting group. Among these 516 patients, 3 were excluded because of consent withdrawal and 10 were excluded owing to being lost to follow-up. Finally, 980 patients, namely, 477 (48.7%) in the visiting group and 503 (51.3%) in the non-visiting group, were analyzed in the present study.
The patient demographics and background factors of the regimens are shown in Table 1. The chemotherapy regimens administered in the visiting and non-visiting groups were FEC in 170 (36%) and 168 (33%), E(A)C in 165 (35%) and 170 (34%), and TC in 142 (30%) and 165 (33%), respectively. The TC regimen was mainly used for patients with earlier stages and ER-positive disease at the adjuvant treatment setting in contrast to the FEC and E(A)C regimens. The FEC regimen tended to be given to younger patients at the neoadjuvant treatment setting. The proportion of primary PEG-G was higher in the patients receiving the TC regimen than in the patients receiving the FEC or E(A)C regimen, and in the non-visiting group than in the visiting group (Table 1, Fig. 2, and Supplementary Table 1). Very few patients had a history of radiation therapy, current wound infections, open wounds, renal dysfunction, and hepatic dysfunction, which were possible risk factors of FN.
Table 1.
Patient demographics and background factors of regimens.
| FEC |
E(A)C |
TC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All patients (N = 338) | Visiting (N = 170) | Non-visiting (N = 168) | All patients (N = 335) | Visiting (N = 165 | Non-visiting (N = 170) | All patients (N = 307) | Visiting (N = 142) | Non-visiting (N = 165) | |
| Age; Years | |||||||||
| Median (range) | 50.5 (22–78) |
50 (25–78) |
52 (22–74) |
54 (27–77) |
55 (27–76) |
53 (30–77) |
53 (30–80) |
52 (30–80) |
53 (31–76) |
| <65 | 292 (86.4%) |
150 (88.2%) |
142 (84.5%) |
251 (74.9%) |
120 (72.7%) |
131 (77.1%) |
236 (76.9%) |
111 (78.2%) |
125 (75.8%) |
| ≥65 | 46 (13.6%) |
20 (11.8%) |
26 (15.5%) |
84 (25.1%) |
45 (27.3%) |
39 (22.9%) |
71 (23.1%) |
31 (21.8%) |
40 (24.2%) |
| ECOG PS | |||||||||
| 0 | 337 (99.7%) |
169 (99.4%) |
168 (100%) |
334 (99.7%) |
164 (99.4%) |
170 (100%) |
305 (99.3%) |
142 (100%) |
163 (98.8%) |
| 1 | 1 (0.3%) |
1 (0.6%) |
0 (0%) |
1 (0.3%) |
1 (0.6%) |
0 (0%) |
2 (0.7%) |
0 (0%) |
2 (1.2%) |
| Stage | |||||||||
| I | 35 (10.4%) |
10 (5.9%) |
25 (14.9%) |
82 (24.5%) |
47 (28.5%) |
35 (20.6%) |
115 (37.5%) |
46 (32.4%) |
69 (41.8%) |
| IIA | 99 (29.3%) |
51 (30%) |
48 (28.6%) |
97 (29.0%) |
48 (29.1%) |
49 (28.8%) |
116 (37.8%) |
57 (40.1%) |
59 (35.8%) |
| IIB | 106 (31.4%) |
55 (32.4%) |
51 (30.4%) |
80 (23.8%) |
36 (21.8%) |
44 (25.9%) |
64 (20.8%) |
32 (22.5%) |
32 (19.4%) |
| IIIA | 41 (12.1%) |
24 (14.1%) |
17 (10.1%) |
39 (11.6%) |
19 (11.5%) |
20 (11.8%) |
7 (2.3%) |
4 (2.8%) |
3 (1.8%) |
| IIIB | 21 (6.2%) |
7 (4.1%) |
14 (8.3%) |
15 (4.5%) |
10 (6.1%) |
5 (2.9%) |
5 (1.6%) |
3 (2.1%) |
2 (1.2%) |
| IIIC | 36 (10.6%) |
23 (13.5%) |
13 (7.7%) |
22 (6.6%) |
5 (3.0%) |
17 (10.0%) |
0 (0%) |
0 (0%) |
0 (0%) |
| Status of estrogen receptor | |||||||||
| Positive | 204 (60.4%) |
112 (65.9%) |
92 (54.8%) |
207 (61.8%) |
102 (61.8%) |
105 (61.8%) |
251 (81.8%) |
123 (86.6%) |
128 (77.6%) |
| Negative | 134 (39.6%) |
58 (34.1%) |
76 (45.2%) |
128 (38.2%) |
63 (38.2%) |
65 (38.2%) |
56 (18.2%) |
19 (13.4%) |
37 (22.4%) |
| Status of HER2 | |||||||||
| 0、1+ | 166 (49.1%) |
84 (49.4%) |
82 (48.8%) |
162 (48.4%) |
66 (40.0%) |
96 (56.5%) |
199 (64.8%) |
104 (73.2%) |
95 (57.6%) |
| 2+/FISH(−) | 42 (12.4%) |
20 (11.8%) |
22 (13.1%) |
44 (13.1%) |
28 (17.0%) |
16 (9.4%) |
48 (15.6%) |
20 (14.1%) |
28 (17.0%) |
| 3+/FISH(−) | 129 (38.2%) |
66 (38.8%) |
63 (37.5%) |
128 (38.2%) |
71 (43.0%) |
57 (33.5%) |
60 (19.5%) |
18 (12.7%) |
42 (25.4%) |
| Unknown | 1 (0.3%) |
0 (0%) |
1 (0.6%) |
1 (0.3%) |
0 (0%) |
1 (0.6%) |
0 (0%) |
0 (0%) |
0 (0%) |
| Treatment setting | |||||||||
| Neoadjuvant | 237 (70.1%) |
122 (71.8%) |
115 (68.5%) |
145 (43.3%) |
83 (50.3%) |
62 (46.5%) |
24 (7.8%) |
6 (4.2%) |
18 (10.9%) |
| Adjuvant | 101 (29.9%) |
48 (28.2%) |
53 (31.5%) |
190 (56.7%) |
82 (49.7%) |
108 (63.5%) |
283 (92.2%) |
136 (95.8%) |
147 (89.1%) |
| Use of PEG-G for 1st cycle | |||||||||
| Yes | 73 (21.6%) |
8 (4.7%) |
65 (38.7%) |
23 (6.9%) |
3 (1.8%) |
20 (11.8%) |
106 (34.5%) |
20 (14.1%) |
86 (52.1%) |
| No | 265 (78.4%) |
162 (95.3%) |
103 (61.3%) |
312 (93.1%) |
162 (98.2%) |
150 (88.2%) |
201 (65.5%) |
122 (85.9%) |
79 (47.9%) |
| Absolute neutropenia count | |||||||||
| Median (range) | 3126.5 (1360–8215) |
3311 (1360–8215) |
3047.5 (1364–7740) |
3198 (638–9314) |
3030 (1092–6578) |
3322 (638–9314) |
3485 (1023–19,890) |
3261 (1453–19,890) |
3570 (1023–18,110) |
FEC, fluorouracil + epirubicin + cyclophosphamide.
E(A)C, epirubicin (doxorubicin) + cyclophosphamide.
TC, docetaxel + cyclophosphamide.
FISH, Fluorescence in situ hybridization.
Fig. 2.
Proportions of FN incidence and primary PEG-G during each treatment cycle. In all the regimens, the FN proportions (solid lines) were the highest in the 1st cycle and decreased after the second cycle in response to the increasing usage of primary PEG-G (dotted lines). The proportions of primary PEG-G (dotted lines) were higher in the non-visiting group (triangles) than in the visiting group (circles) in the FEC and TC regimens, with the highest at more than 50% in the non-visiting group for the TC regimen.
3.1. Incidence of febrile neutropenia
The estimated incidence proportions and 95% CIs of FN during 4 cycles of treatment were 22.5% (18.1%–27.3%) for FEC, 27.5% (22.8%–32.6%) for E(A)C, and 33.9% (28.6%–39.5%) for TC in the entire study population; 27.7% (21.1%–35.0%) for FEC, 22.4% (16.3%–29.6%) for E(A)C, and 36.6% (28.7%–45.1%) for TC in the visiting group; and 17.3% (11.8%–23.8%) for FEC, 32.4% (25.4%–39.9%) for E(A)C, and 31.5% (24.5%–39.2%) for TC in the non-visiting group (Table 2). These results did not consider whether PEG-G was administered.
Table 2.
Incidence proportions of FN during all courses of chemotherapy.
| All patients |
T-FN(Visiting Group) |
S-FN(Non-Visiting Group) |
||||
|---|---|---|---|---|---|---|
| N | Proportion (95%Cl) | N | Proportion (95%Cl) | N | Proportion (95%Cl) | |
| FEC | 338 | 22.5% (18.1%–27.3%) |
170 | 27.7% (21.1%–35.0%) |
168 | 17.3% (11.8%–23.8%) |
| E(A)C | 335 | 27.5% (22.8%–32.6%) |
165 | 22.4% (16.3%–29.6%) |
170 | 32.4% (25.4%–39.9%) |
| TC | 307 | 33.9% (28.6%–39.5%) |
142 | 36.6% (28.7%–45.1%) |
165 | 31.5% (24.5%–39.2%) F |
FEC, fluorouracil + epirubicin + cyclophosphamide.
E(A)C, epirubicin (doxorubicin) + cyclophosphamide.
TC, docetaxel + cyclophosphamide.
The incidence proportion of T-FN was approximately 10% higher than that of S-FN with the FEC regimen, whereas that of S-FN was approximately 10% higher with the E(A)C regimen, with no consistent trend. The proportions of FN incidence and PEG-G during each treatment cycle are shown in Fig. 2 and Supplementary Table 1. The Incidence proportion of FN in the 1st cycle was the highest at approximately 20%, and decreased to 3%–8% after the second cycle. The proportion of primary PEG-G in the 1st cycle was higher in the non-visiting group at 20%–30%, with the highest at 52% for the TC regimen. The PEG-G rate was gradually increased after the 2nd cycle in the visiting group.
3.2. Secondary endpoints
The results of the analysis of the secondary endpoints are summarized in Table 3. Hospitalization occurred most often in the E(A)C regimen in the visiting group at 10.6%. Grade 3 or 4 AEs were found more frequently in the visiting group than in the non-visiting group (30.1% vs 16.5% in FEC, 42.0% vs 29.2% in TC). Therapeutic antibiotics were used for these regimens more often in the visiting group (46.1% vs 37.6% in FEC, 56.9% vs 46.2% in TC). In addition, the rate of therapeutic G-CSF usage was higher in the visiting group in all the 3 regimens. FN-related severe AEs were rarely found at only 0.2% for stomatitis and 0.1% for sore throat. In all the 3 regimens, the percentage of cases with an RDI ≥0.85 was high at approximately 90% in both groups.
Table 3.
Results of analysis of secondary endpoints.
| FEC |
E(A)C |
TC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All patients | Visiting | Non-visiting | All patients | Visiting | Non-visiting | All patients | Visiting | Non-visiting | |
| FN incidence in the first course | 62 (18.3%) | 42 (24.7%) | 20 (11.9%) | 64 (19.1%) | 27 (16.4%) | 37 (21.8%) | 66 (21.5%) | 36 (25.4%) | 30 (18.2%) |
| Grade 4 neutropenia | ー | 46 (27.1%) | ー | ー | 42 (25.5%) | ー | ー | 30 (21.1%) | ー |
| Hospitalization | 21 (6.5%) | 12 (7.3%) | 9 (5.7%) | 24 (7.4%) | 17 (10.6%) | 7 (4.2%) | 22 (7.7%) | 11 (8.2%) | 11 (7.2%) |
| Use of therapeutic G-CSF (all cycles) | 52 (16.2%) | 43 (26.2%) | 9 (5.7%) | 55 (16.9%) | 42 (26.3%) | 13 (7.8%) | 62 (21.5%) | 49 (35.3%) | 13 (8.7%) |
| Use of therapeutic antibiotics (all cycles) | 135 (41.9%) | 76 (46.1%) | 59 (37.6%) | 143 (44.0%) | 60 (37.7%) | 83 (50.0%) | 150 (51.2%) | 78 (56.9%) | 72 (46.2%) |
| Grade 3 or 4 adverse events | 76 (23.5%) | 50 (30.1%) | 26 (16.5%) | 82 (24.9%) | 39 (24.4%) | 43 (25.4%) | 103 (35.3%) | 58 (42.0%) | 45 (29.2%) |
| FN-related adverse events (grade 3 or above, all cycles) | |||||||||
| Stomatitis | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.65%) | 2 (1.41%) | 0 (0%) |
| sore throat | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) | 1 (0.61%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Relative dose intensitya | 307 (90.8%) | 155 (91.2%) | 152 (90.5%) | 311 (92.8%) | 150 (90.9%) | 161 (94.7%) | 272 (88.9%) | 129 (90.9%) | 143 (87.2%) |
| RDI (≥0.85) | |||||||||
| 1st cycle | 337 (99.7%) | 170 (100%) | 167 (99.4%) | 332 (99.1%) | 162 (98.2%) | 170 (100%) | 305 (99.7%) | 142 (100%) | 163 (99.4%) |
| 2nd cycle | 326 (98.2%) | 165 (98.8%) | 161 (97.6%) | 321 (97.0%) | 154 (94.5%) | 167 (99.4%) | 291 (98.3%) | 134 (97.1%) | 157 (99.4%) |
| 3rd cycle | 317 (97.5%) | 159 (98.2%) | 158 (96.9%) | 312 (95.1%) | 150 (93.2%) | 162 (97.0%) | 281 (96.9%) | 130 (96.3%) | 151 (97.4%) |
| 4th cycle | 309 (96.9%) | 157 (97.5%) | 152 (96.2%) | 305 (93.9%) | 145 (90.6%) | 160 (97.0%) | 273 (97.2%) | 129 (96.3%) | 144 (98.0%) |
FEC, fluorouracil + epirubicin + cyclophosphamide E(A)C, epirubicin (doxorubicin) + cyclophosphamide TC, docetaxel + cyclophosphamide.
G-CSF, granulocyte colony-stimulating factor RDI, relative dose intensity.
For all 4 courses ≥ 0.85.
3.3. Risk factors
Risk factors were evaluated in the visiting group. In the logistic regression analysis, we reconsidered which possible risk factors were used because few patients had a low PS, prior chemotherapy, radiation therapy, infected wounds, open wounds, renal dysfunction, and hepatic dysfunction. We used regimen (FEC vs E(A)C vs TC), age (<65 vs ≥ 65), ANC, stage (I vs II vs III), treatment setting (adjuvant vs neoadjuvant), primary PEG-G administration (yes vs no) in the logistic regression models for exploratory evaluation of risk factors. As the ANC of most patients was >1500/μl, ANC was used as a continuous variable. Risk factors were evaluated in the visiting group, with T-FN used for event data.
Logistic regression analysis revealed that the TC regimen (OR = 2.62, 95% CI: 1.34–5.12), age ≥ 65 (OR = 2.24, 95% CI: 1.34–3.75), and ANC/1000 μl (OR = 0.8, 95% CI: 0.67–0.95) were significant risk factors of FN. Adjuvant treatment setting (OR = 0.51, 95% CI: 0.29–0.88) and primary PEG-G administration (OR = 0.04, 95% CI: 0.01–0.34) were significant protective factors against FN (Table 4).
Table 4.
Multivariate analysis for exploring the risk factors of FN.
| Parameter | Odds ratio | 95% CI | p -value | |
|---|---|---|---|---|
| Regimen | TC | 2.67 | 1.36–5.26 | 0.005 |
| E(A)C | 0.66 | 0.38–1.14 | 0.137 | |
| FEC | ref | |||
| Age | >65 | 2.24 | 1.34–3.75 | 0.002 |
| Neutrophils (continuous) | /1000 μL | 0.8 | 0.67–0.95 | 0.012 |
| Stage | III | 0.98 | 0.48–1.99 | 0.961 |
| II | 0.82 | 0.47–1.44 | 0.492 | |
| I | ref | |||
| Treatment setting | Adjuvant | 0.51 | 0.29–0.88 | 0.017 |
| Neoadjuvant | ref | |||
| Primary PEG-G | Yes | 0.04 | 0.01–0.34 | 0.003 |
| No | ref |
FEC, fluorouracil + epirubicin + cyclophosphamide.
E(A)C, epirubicin (doxorubicin) + cyclophosphamide.
TC, docetaxel + cyclophosphamide.
PEG-G, pegfilgrastim.
To search for risk factors as a decision tool for PEG-G usage, another multivariate analysis was conducted in the patients without PEG-G at the 1st cycle (Supplementary Table 3). The results were not significantly different from those shown in Table 4.
4. Discussion
This study set out to evaluate the incidences and risk factors of FN in breast cancer patients who received 3 adjuvant regimens according to 2 treatment policies for FN. To our knowledge, this is the first prospective study directly comparing FN incidences between 3 mainstream regimens. The FN proportions of these 3 regimens were above 20%. The FN proportion of TC could be higher than 40% because the T-FN proportion of TC was 36.6% when primary PEG-G was used at the 1st cycle in 14.1% of the patients (Table 2, Supplementary Table 1). The T-FN proportions of FEC and E(A)C were 27.7% and 22.4% when primary PEG-G was respectively used at the 1st cycle in 4.7% and 1.8% of the patients, which are very low patient rates particularly for the latter (Table 2, Supplementary Table 1). Although the patient characteristics were different in the 3 regimens (Table 1), it is clear that TC is the regimen that most frequently induces the development of FN. In all the regimens, the FN proportions decreased at the 2nd cycle with the increasing usage of PEG-G (Fig. 2) or antibiotics (data not shown) in the second and subsequent cycles, which is defined as secondary prophylaxis in response to the low neutrophil counts or FN-related events observed in the first cycle. In previous reports in which FN without primary PEG-G at the 1st cycle was investigated in Caucasian countries, the FN proportions in E(A)C ranged from 5.4% to 10.0% [[16], [17], [18], [19], [20], [21]]. The comparison of these with 22.4% in E(A)C of this study revealed that an ethnic difference may exist and that the Japanese population may be vulnerable to FN.
In the analysis of secondary endpoints, FN-related events were generally well managed with a few severe events, low rates of hospitalization, and no fatal cases. It is not reasonable to compare these FN-related events between the non-visiting and visiting groups because of many differences in their baseline characteristics. However, grade 3 or 4 AEs and hospitalization more frequently occurred in the visiting group than in the non-visiting group. Possibly related to these increased events, therapeutic G-CSF and antibiotics were used more frequently in the visiting group (Table 3). These trends were preserved in limited cases with primary PEG-G not used at the 1st cycle (data not shown). Importantly, the RDIs in all the 3 regimens were maintained at sufficiently high levels in both groups (Table 3).
As for FN risk factors, we analyzed the entire cohort with PEG-G as one of the confounding factors. As for patient-related FN risk factors, advanced age and low pretreatment ANC remained significant in the multivariate analysis (Table 4). We also performed another multivariate analysis in the patients without PEG-G used at the 1st cycle (Supplementary Table 3). It is possible that the baseline characteristics were different between the patients with and without PEG-G at the 1st cycle. However, the results of the other multivariate analysis were not significantly different from the results of the multivariate analysis shown in Table 4.
Notably, age has been identified as a risk factor of FN in any guidelines [[6], [7], [8]]. The age of 65 years has been used as a threshold established from analyses including hematologic malignancies in Caucasian countries [22,23]. By comparing the FN proportion of E(A)C in Caucasians with that in Asians in this study, it clearly suggests that an ethnic difference in the vulnerability to FN may exist. Thus, it is necessary to determine the threshold of the age for FN in Japanese patients.
The guidelines of The American Society of Clinical Oncology and National Comprehensive Cancer Network have listed pre-existing neutropenia as a risk factor of FN. However, the threshold has not been fixed for prophylaxis. The present prospective study revealed a significant association of pretreatment ANC with FN (Table 4). The categorical analysis on ANC showed a consistent increase in the odds ratio on FN (Supplementary Table 2). Thus, the pretreatment ANC could be a reliable marker for FN development. Previously, there were retrospective studies identifying ANC as a predictive factor of FN [24,25]. These studies investigated 741 patients administered with FEC retrospectively [24] and validated their findings with another set of 263 patients administered with TAC [25]. The combination of ANC and absolute lymphocyte count was reported to be valuable in predicting neutropenic events including FN, in which the association between ANC and FN appears to be most significant [24]. However, the threshold of ANC was not investigated in these studies. Thus, plans are underway for a further study to establish the ANC threshold for predicting FN.
There are several limitations in this study. First, in the visiting group, 47 of the 477 cases (9.8%) did not visit hospitals for a blood test and 12 cases (2.5%) did a blood test 3 days after having a fever. No regulation was established for antibiotic usage. These might have underestimated the FN frequency in the visiting group. Second, the neoadjuvant setting remained as one of the risk factors of FN. It may be unreasonable to make a comparison between neoadjuvant chemotherapy and adjuvant chemotherapy because these settings were diametrically related with the patient characteristics and regimens. The relationship of the neoadjuvant setting with FN may be biased owing to multicollinearity in the multivariate analysis. Third, the managements of FN in the visiting and non-visiting groups were dependent on the institutions, which may result in different characteristics between these 2 groups.
5. Conclusion
The FN proportions in the FEC, E(A)C, and TC regimens were above 20%, with the TC regimen showing the highest proportion. However, regarding the present status, the RDI was maintained at a high level and FN-related events were all well managed in both the visiting and non-visiting groups. Thus, it remains clinically meaningful to select patients with a high risk of FN with the adequate use of primary PEG-G. Age and pretreatment ANC were found to be patient-related risk factors of FN development. The thresholds of these 2 factors, which might be different from the current guidelines, warrant further investigation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: TI has received honoraria from AstraZeneca, Eisai, Daiichi Sankyo, Pfizer, Taiho, Lilly, Nippon Kayaku and Takeda. His institution has received research grants from Taiho, Eisai, Daiichi Sankyo, Nippon Kayaku, and Takeda. KT has received honoraria from AstraZeneca, Eisai, Daiichi Sankyo, Pfizer, Taiho, Lilly, Nippon Kayaku, Chugai, and Takeda. His institution has received research grants from AstraZeneca, Chugai, Taiho, Eisai, Nippon Kayaku, MSD, and Takeda. HM has received honoraria from AstraZeneca, Eisai, Daiichi Sankyo, Pfizer, Taiho, and Takeda. His institution has received research grants from the Japanese government, Eisai, Daiichi Sankyo, Nippon Kayaku, and Pfizer. All remaining authors have declared no conflicts of interest associated with this study.
Acknowledgement
We greatly appreciate Dr. Edward Barroga (http://orcid.org/0000-0002-8920-2607), Medical Editor and Professor of Academic Writing at St. Luke’s International University, Tokyo, Japan for reviewing and editing the article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.01.005.
Author contributions
Conceptualization of study: T. Ishikawa, H. Mukai. Conception and design of study: T. Ishikawa, K. Narui, N. Suganuma, H. Kaise, K. Tsugawa, H. Mukai. Funding acquisition: T. Aihara, H. Mukai. Project administration: T. Aihara, H. Mukai. Analysis of data: T. Ishikawa, K. Sakamaki, K. Narui. Statistical analysis: K. Sakamaki. Contribution of patient accrual: T. Ishikawa, K. Narui, H. Nishimura, T. Sangai, K. Tamaki, Y. Hasegawa, K. Watanabe, N. Suganuma, S. Michishita, S. Sugae, K. Tsugawa. Manuscript writing (original draft, interpretation, and revisions): T. Ishikawa, K. Sakamaki, K. Narui. Manuscript writing (review/revisions for intellectual content): N. Taira, H. Mukai.
Final approval
All authors.
Ethics approval and consent to participate
The study was approved by the protocol review committee of the Comprehensive Support Project for Oncological Research of Breast Cancer on February 10, 2015 and by the institutional review board of Tokyo Medical University on April 28, 2015 (No. 3044). This trial was registered at the UMIN Clinical Trials Registry as UMIN 000017857. The study was performed in accordance with the Declaration of Helsinki. The manuscript is in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. Informed consent was obtained and the privacy rights of the subjects was always observed.
Funding
This research was funded by Kyowa Kirin. All decisions concerning the planning, implementation, and publication of this study were made by the executive committee of CSPOR-BC.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Weycker D., Li X., Barron R. Risk of chemotherapy-induced febrile neutropenia with early discontinuation of pegfilgrastim prophylaxis in US clinical practice. Support Care Canc. 2016;24(6):2481–2490. doi: 10.1007/s00520-015-3039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyman G.H., Kuderer N.M., Djulbegovic B. Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am J Med. 2002;112(5):406–411. doi: 10.1016/s0002-9343(02)01036-7. [DOI] [PubMed] [Google Scholar]
- 3.Kuderer N.M., Dale D.C., Crawford J. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 4.Kuderer N.M., Dale D.C., Crawford J. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 5.Lyman G.H., Kuderer N.M. Epidemiology of febrile neutropenia. Support Canc Ther. 2003;1(1):23–35. doi: 10.3816/SCT.2003.n.002. [DOI] [PubMed] [Google Scholar]
- 6.Smith T.J., Bohlke K., Lyman G.H. American society of clinical Oncology. Recommendations for the use of WBC growth factors: American society of clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 7.Aapro M.S., Bohlius J., Cameron D.A. Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 2011. 2010;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Fort Washington, philadelphia, USA. NCCN clinical practice guidelines in Oncology. Version 1Myeloid Growth Factors. 2017 [Google Scholar]
- 9.Lyman G.H., Yau L., Nakov R. Overall survival and risk of second malignancies with cancer chemotherapy and G-CSF support. Ann Oncol. 2018;29(9):1903–1910. doi: 10.1093/annonc/mdy311. [DOI] [PubMed] [Google Scholar]
- 10.Gandara D.R., Kawaguchi T., Crowley J. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27(21):3540–3546. doi: 10.1200/JCO.2008.20.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yano R., Konno A., Watanabe K. Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol. 2013;18(1):96–104. doi: 10.1007/s10147-011-0349-5. [DOI] [PubMed] [Google Scholar]
- 12.Han H.S., Reis I.M., Zhao W. Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur J Canc. 2011;47(17):2537–2545. doi: 10.1016/j.ejca.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Swain S.M., Im Y.H., Im S.A. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncol. 2014;19(7):693–770. doi: 10.1634/theoncologist.2014-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field K.M., Kosmider S., Jefford M. Chemotherapy dosing strategies in the obese, elderly, and thin patient: results of a nationwide survey. J Oncol Pract. 2008;4(3):108–113. doi: 10.1200/JOP.0832001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa T., Sakamaki K., Narui K. For Comprehensive Support Project for Oncological Research of Breast Cancer. Prospective cohort study of febrile neutropenia in breast cancer patients with neoadjuvant and adjuvant chemotherapy: CSPOR-BC FN study. Jpn J Clin Oncol. 2016;46(7):692–695. doi: 10.1093/jjco/hyw045. [DOI] [PubMed] [Google Scholar]
- 16.Mackey J.R., Pieńkowski T., Crown J. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann Oncol. 2016;27(6):1041–1047. doi: 10.1093/annonc/mdw098. [DOI] [PubMed] [Google Scholar]
- 17.Martín M., Ruiz Simón A., Ruiz Borrego M. Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: results from the GEICAM/2003-10 Study. J Clin Oncol. 2015;33(32):3788–3795. doi: 10.1200/JCO.2015.61.9510. [DOI] [PubMed] [Google Scholar]
- 18.Ju Blohmer, Schmid P., Hilfrich J. Epirubicin and cyclophosphamide versus epirubicin and docetaxel as first-line therapy for women with metastatic breast cancer: final results of a randomised phase III trial. Ann Oncol. 2010;21(7):1430–1435. doi: 10.1093/annonc/mdp585. [DOI] [PubMed] [Google Scholar]
- 19.Gradishar W.J., Wedam S.B., Jahanzeb M. Neoadjuvant docetaxel followed by adjuvant doxorubicin and cyclophosphamide in patients with stage III breast cancer. Ann Oncol. 2005;16(8):1297–1304. doi: 10.1093/annonc/mdi254. [DOI] [PubMed] [Google Scholar]
- 20.Nabholtz J.M., Falkson C., Campos D. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol. 2003;21(6):968–975. doi: 10.1200/JCO.2003.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Biganzoli L., Cufer T., Bruning P. Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: the European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. J Clin Oncol. 2002;20(14):3114–3121. doi: 10.1200/JCO.2002.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Do T., Medhekar R., Bhat R. The risk of febrile neutropenia and need for G-CSF primary prophylaxis with the docetaxel and cyclophosphamide regimen in early-stage breast cancer patients: a meta-analysis. Breast Canc Res Treat. 2015;153(3):591–597. doi: 10.1007/s10549-015-3531-z. [DOI] [PubMed] [Google Scholar]
- 23.Lyman G.H., Morrison V.A., Dale D.C. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44(12):2069–2076. doi: 10.1080/1042819031000119262. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins P., Freeman S. Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer. Ann Oncol. 2009;20(1):34–40. doi: 10.1093/annonc/mdn560. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins P., Scaife J., Freeman S. Validation of a predictive model that identifies patients at high risk of developing febrile neutropaenia following chemotherapy for breast cancer. Ann Oncol. 2012;23(7):1766–1771. doi: 10.1093/annonc/mdr493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.