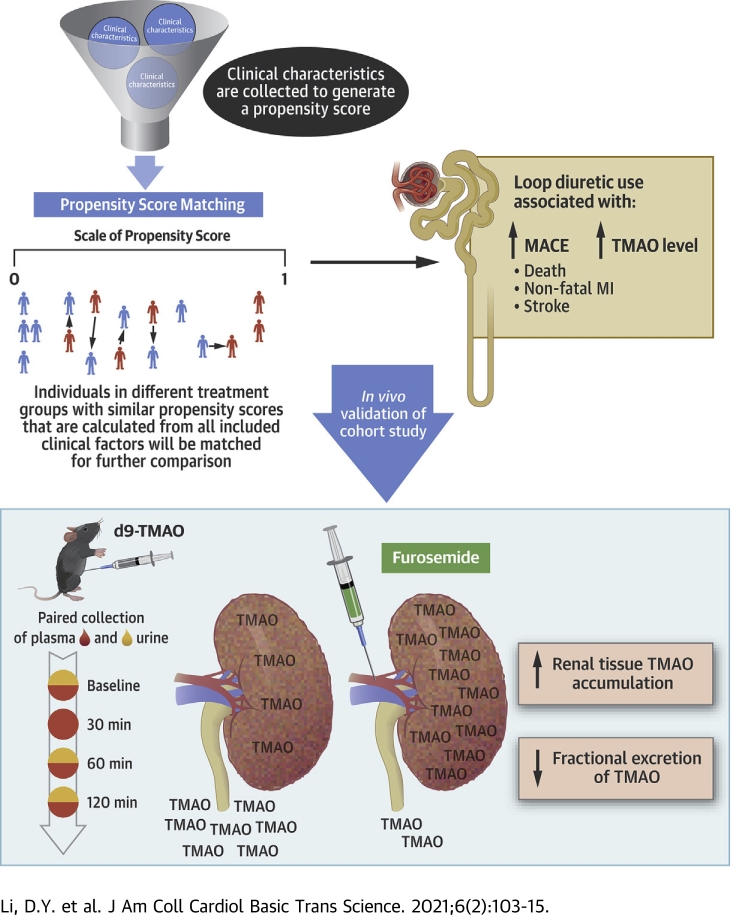
Visual Abstract
Key Words: cardiorenal, intestinal microbiota, loop diuretic, prognosis, TMAO
Abbreviations and Acronyms: CI, confidence interval; HR, hazard ratio; IP, intraperitoneal; MACE, major adverse cardiac event(s); MI, myocardial infarction; TMAO, trimethylamine N-oxide
Highlights
-
•
Uremic retention solutes predominantly eliminate through the kidneys largely via specific efflux channels in the proximal renal tubules.
-
•
For the first time, we demonstrated in vivo that renal tubular excretion of TMAO can be inhibited by concomitant loop diuretic administration via competition at the level of renal transporters.
-
•
We further observed accumulation of TMAO in the renal parenchyma, which implied differential distributions of TMAO across various tissues and/or systems as a consequence of efflux channel control.
-
•
Poorer outcomes in patients who receive long-term loop diuretic agents may therefore be associated with metabolic perturbations, such as retention of metabolites like TMAO, beyond impaired glomerular filtration.
Summary
This study demonstrates, for the first time, that renal tubular excretion of trimethylamine N-oxide (TMAO) is inhibited by concomitant loop diuretic administration. The observed marked accumulation in the renal parenchyma, and to lesser extent, plasma, implies differential distributions of TMAO across various tissues and/or systems as a consequence of efflux channel control. A better understanding of TMAO renal clearance and its potential interactions with current and future therapies in patients with heart failure are warranted.
Trimethylamine N-oxide (TMAO) is a gut microbiota metabolite that has been implicated, in recent years, as a marker for poor prognosis in a growing list of diseases (1, 2, 3, 4). Observational studies have linked elevated levels of TMAO to adverse prognosis in patients with heart failure and patients with chronic kidney disease (2,3). These associations are corroborated by experimental evidence from animal models that have demonstrated that increased plasma TMAO directly contributes to cardiac dysfunction, tubular injury, and kidney fibrosis (3,5,6). To date, studies have largely focused on how alterations of gut microbiota, dietary differences, and increased intestinal permeability in diseased states can contribute to increased TMAO production (7). However, despite being a predominant kidney (>95%) excreted metabolite, few investigations have been initiated toward understanding the mechanisms of TMAO elimination (8,9).
Epidemiological studies have suggested that up to 40% to 50% of those with heart failure also have comorbid chronic kidney disease. The presence of dysfunction in either the heart or the kidney can initiate or aggravate the accelerated failure of the other. This cardiorenal pathology is linked to common pathways in altered hemodynamics, fluid status, neurohormonal response, and metabolic regulation (10, 11, 12). In the treatment of fluid overload, the use of diuretics, especially loop diuretics, has been a cornerstone of contemporary therapy (13). Although vital for symptomatic relief of vascular congestion, it is believed that long-term loop diuretic use results in worsening renal function and increased mortality (14, 15, 16). However, the mechanisms that lead to these worse outcomes are still unclear.
Large-scale genetic studies of TMAO accumulation have thus far yielded few answers on specific molecular targets of TMAO, which are blurred by the significant environmental factors that contribute to TMAO variability, including a major role of the gut microbiome (17). Therefore, alternative methods are necessary to tackle this question. Because of the dependence of TMAO excretion on an appropriately functioning kidney, we reasoned that medications such as loop diuretics, which significantly alter renal physiology, could also influence TMAO elimination. Furthermore, the mechanisms that mediate the metabolic effects of loop diuretic agents are poorly understood. Therefore, in this study, we examined the treatment effect of loop diuretic agents on plasma TMAO levels and their relationships to adverse cardiovascular outcomes.
Methods
Human cohort selection
This was a single-center prospective cohort study approved by the Cleveland Clinic Institutional Review Board, and all participants provided written informed consent. We enrolled sequential consenting patients who underwent elective coronary angiographic evaluation at the Cleveland Clinic between 2001 and 2007, as previously described (4). Major adverse cardiac events (MACEs) were defined as death, nonfatal myocardial infarction (MI), or stroke and were prospectively tracked over 3 years for all participants through medical chart review and adjudicated by follow-up contact and the Social Security Death Index. Fasting blood samples were obtained from all participants before intravenous contrast dye administration during elective diagnostic coronary angiography during cardiac catheterization.
Animal study
To test the effects of furosemide treatment on TMAO renal excretion in vivo, we tested the rate of stable isotope d9-TMAO (Cambridge Isotope Lab, Tewksbury, Massachusetts) excretion after intraperitoneal (IP) injection. Female C57BL/6J mice (16 to 20 weeks old) were purchased from the Division of Laboratory Animal Medicine of the University of California Los Angeles facilities. Baseline plasma by saphenous vein draw and first urine void were collected after a 3-h fast in the morning. To collect urine, the mouse was grasped by the scruff and immediately held over a cellophane membrane to prevent loss of urine; bladder massage was applied, and urine collected into microcentrifuge tubes. After first void, mice were placed into individual cages with hydrophobic sand bedding (Braintree Scientific, Braintree, Massachusetts) to avoid urine loss between time points. Thirty min after baseline collection, a single dose of 25 mg/kg furosemide (prototypic loop diuretic) diluted in normal saline was injected IP; for control mice, the same volume of normal saline was used. Furosemide dosing was extrapolated based on Food and Drug Administration guidance documents for conversion of animal dose to a human equivalent dose based on normalization to body surface area (18). We used the equation: human equivalent dose mg/kg = (animal dose mg/kg) × (weight animal [kg]/weight human [kg])(1–0.67). The exponent for body surface area (0.67) was a pre-determined constant to account for differences in metabolic rates when converting doses between animals and humans. Therefore, using average mice and human weights, this equated to 25 mg/kg × (0.025 kg/70 kg)(0.33) = approximately 1.8 mg/kg of furosemide. Therefore, for an average 70-kg individual, our mice dose was equal to 130 mg of furosemide, which was at the upper end of typical dosing for this medication. One hour after baseline collection, 70 μmol/kg d9-TMAO diluted in normal saline was injected IP. This d9-TMAO dose was chosen to generate blood levels of TMAO previously reported to be at approximately the highest unbound plasma level in patients with CKD or patients with end-stage renal disease (19). Subsequently, paired plasma and urine collection, as previously described, was performed at 30 min and at 1 and 2 h. To adjust for the rapid change in plasma TMAO and obtain a best estimate of fractional excretion, the urine from the 30-min and 1-h collection was combined, and the plasma measured at 30 min and 1 h was averaged. This procedure was developed based on laboratory preliminary data after multiple TMAO doses in mice. Samples were immediately stored on ice after collection, spun down, and acidified to a concentration of 0.1% formic acid before (to also permit trimethylamine [TMA] analysis) storage at −80 °C in gas-tight cryovials. Animal experimental protocols were approved by the Division of Laboratory Animal Medicine of the University of California Los Angeles.
Preparation of kidney tissue homogenates
At the end of the furosemide treatment study (2 h post-stable isotope d9-TMAO injection), mice were anesthetized and euthanized via cervical dislocation. Mice were then immediately perfused via the left ventricle with 50-ml phosphate-buffered saline. The kidneys were then dissected, the capsule removed, and frozen in liquid nitrogen before storage at −80 °C. Tissue homogenization was performed using a tissue grinder and diluted in a 1:10 ratio with ice cold 10-mM HEPES, pH 7.4. Samples were spun down, and aliquots were taken from the supernatant solution. d9-TMAO concentrations were measured via stable isotope dilution liquid chromatography/mass spectrometry/mass spectrometry using electrospray ionization in positive-ion mode with [13C3]TMAO (IsoSciences, Ambler, Pennsylvania) used as internal standard. d9-TMAO and [13C3]TMAO were monitored using multiple reaction monitoring of precursor and characteristic product ions: m/z 85→66 and 79→61, separately. Protein concentration was determined via bicinchoninic acid (BCA) assay (Thermo Fisher Waltham, Massachusetts).
Laboratory testing in human and mouse samples
Methodology for human sample acquisition and mass spectrometry was previously described (4). Briefly, fasting blood samples were collected at the time of cardiac catheterization before administration of medications. An aliquot of 24-h urine was also obtained. Sample processing was performed at 0°C to 4°C and stored at −80°C. Measurements of human metabolite creatinine, TMAO, trimethylamine, choline, betaine, and their d9-isotopologues (as internal standards) were quantified using a stable isotope dilution assay and high-performance liquid chromatography with online electrospray ionization tandem mass spectrometry on an AB SCIEX QTRAP 5500 mass spectrometer (AB SCIEX, Framingham, Massachusetts) using electrospray ionization in positive-ion mode, as previously described (4). Furthermore, additional assays for brain natriuretic peptide, high-sensitivity C-reactive protein, cystatin C, fasting lipid panel, insulin, glucose, and serum creatinine were measured using the Architect ci8200 platform (Abbott Laboratories, Abbott Park, Illinois).
For murine experiments, d9-TMAO, rather than the natural abundance TMAO, was measured. Therefore, internal standards in these experiments consisted of d3-creatinine (Cambridge Isotope Lab, Tewksbury Massachusetts), [13C3]TMAO, and d4-choline (Cambridge Isotope Lab). TMAO, d9-TMAO, choline, d9-choline, betaine, and d9-betaine were monitored using multiple reaction monitoring of precursor and characteristic product ions: m/z 76→58 for TMAO; m/z 85→66 for d9-TMAO; m/z 104→60 for choline; m/z 113→69 for d9-choline; m/z 118→59 for betaine; m/z 127→68 for d9-betaine; m/z 114→44 for creatinine; m/z 117→47 for d3-creatinine; m/z 108→60 for d4-choline; and m/z 79→61 for [13C3]TMAO.
Statistical analysis
Descriptive statistics were used to characterize the population as a whole: data were reported as mean ± SD and as frequencies (percentages) for categorical variables. For the human study, we used a propensity score matching model to match patients based on their propensity to receive loop diuretic treatment (20). We calculated the probability (propensity) of the patient being on loop diuretic treatment at their entry into the propensity cohort using multivariate logistic regression. This regression analysis included covariates (Table 1) for demographics, history of comorbidities, baseline laboratory measures, and baseline medications. These covariates consisted of traditional cardiovascular disease risk factors, as well as literature-supported covariates suggested to affect plasma TMAO (14,15,21). The estimated glomerular filtration rate was calculated according to the Chronic Kidney Disease Epidemiology Collaboration creatinine and cystatin C formula (22). Missing data were addressed by 10-fold imputation, with the highest percentage of missing data being cystatin C at 8.4% (missing data are reported in Supplemental Table S1). After calculation of the log-odds (logit) from the propensity regression analysis, patient matching was performed by R package MatchIt 3.0.1 (R Foundation, Vienna, Austria) using 1-to-1 nearest-neighbor matching without replacement. We used a caliper width of 0.2 of the SD of the logit of the propensity score, which was shown to be optimal in a range of settings (23). By this strategy, 678 matched pairs were successfully created from the 844 patients (80%) reported to be on loop diuretic agents at baseline. The 2 matched cohorts could then be compared based on loop diuretic usage in the adjusted analysis.
Table 1.
GeneBank Patients Stratified on Loop Diuretic Use Before and After Matching
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| No Loop Diuretic (N = 3,163) | Loop Diuretic (n = 844) | SMD | No Loop Diuretic (n = 678) | Loop Diuretic (n = 678) | SMD | |
| Demographics | ||||||
| Age at blood draw (yrs) | 61.94 ± 10.83 | 66.68 ± 10.46 | 0.445 | 65.58 ± 10.31 | 66.14 ± 10.56 | 0.054 |
| Male | 2,149 (67.9) | 433 (51.3) | 0.344 | 377 (55.6) | 367 (54.1) | 0.030 |
| BMI | 29.24 ± 5.73 | 31.17 ± 7.38 | 0.291 | 30.81 ± 7.09 | 30.95 ± 6.99 | 0.020 |
| Father: White | 3,021 (95.8) | 777 (92.1) | 0.158 | 627 (92.9) | 627 (92.5) | 0.016 |
| Father: Black | 104 (3.3) | 58 (6.9) | 0.163 | 39 (5.8) | 42 (6.2) | 0.018 |
| Father: American Indian | 12 (0.4) | 8 (0.9) | 0.070 | 8 (1.2) | 8 (1.2) | <0.001 |
| Mother: White | 3,027 (95.9) | 782 (92.7) | 0.139 | 634 (93.6) | 632 (93.2) | 0.017 |
| Mother: Black | 103 (3.3) | 58 (6.9) | 0.165 | 39 (5.8) | 42 (6.2) | 0.018 |
| Mother: American Indian | 12 (0.4) | 3 (0.4) | 0.004 | 4 (0.6) | 3 (0.4) | 0.021 |
| History of comorbidities | ||||||
| CAD | 2,281 (72.6) | 623 (74.1) | 0.033 | 495 (74.1) | 501 (74.1) | <0.001 |
| Canadian angina class | 0.203 | 0.058 | ||||
| 0 | 64 (2.1) | 12 (1.5) | 14 (2.2) | 10 (1.5) | ||
| I | 814 (27.2) | 289 (35.5) | 211 (32.6) | 223 (34.3) | ||
| II | 803 (26.8) | 170 (20.9) | 140 (21.6) | 138 (21.2) | ||
| III | 411 (13.7) | 115 (14.1) | 91 (14.0) | 92 (14.2) | ||
| IV | 905 (30.2) | 229 (28.1) | 192 (29.6) | 187 (28.8) | ||
| CHF | 296 (9.6) | 424 (51.5) | 1.021 | 247 (37.1) | 274 (41.3) | 0.087 |
| Arrhythmia | 829 (27.4) | 272 (33.1) | 0.124 | 194 (29.7) | 216 (32.9) | 0.069 |
| Hypertension | 2,154 (69.1) | 676 (81.2) | 0.284 | 527 (79.4) | 535 (80.1) | 0.018 |
| Stroke | 168 (5.5) | 81 (9.9) | 0.164 | 60 (9.3) | 58 (8.8) | 0.019 |
| Diabetes mellitus | 550 (17.5) | 283 (33.8) | 0.380 | 198 (29.4) | 209 (31.1) | 0.038 |
| Current dialysis | 26 (0.8) | 10 (1.2) | 0.037 | 11 (1.6) | 9 (1.3) | 0.024 |
| Smoking | 2,058 (65.1) | 551 (65.3) | 0.005 | 440 (64.9) | 454 (67.0) | 0.044 |
| Cancer | 522 (18.0) | 170 (21.6) | 0.089 | 132 (21.9) | 137 (21.6) | 0.007 |
| Clinical measures | ||||||
| Systolic BP | 134.42 ± 20.82 | 133.02 ± 22.11 | 0.065 | 134.49 ± 21.00 | 133.84 ± 22.16 | 0.030 |
| Diastolic BP | 75.52 ± 11.49 | 71.66 ± 12.86 | 0.317 | 72.98 ± 11.79 | 72.33 ± 12.48 | 0.053 |
| Cholesterol | 167.20 ± 38.85 | 165.32 ± 42.90 | 0.046 | 166.45 ± 42.26 | 164.59 ± 41.51 | 0.044 |
| Creatinine | 0.92 ± 0.55 | 1.10 ± 0.76 | 0.274 | 1.05 ± 0.75 | 1.07 ± 0.80 | 0.027 |
| eGFR | 84.39 ± 19.85 | 69.13 ± 22.57 | 0.718 | 73.75 ± 21.29 | 71.92 ± 22.38 | 0.084 |
| Sodium | 139.74 ± 2.68 | 139.26 ± 3.37 | 0.158 | 139.25 ± 3.04 | 139.28 ± 3.39 | 0.007 |
| BNP | 185.60 ± 415.56 | 485.54 ± 916.12 | 0.422 | 372.05 ± 777.78 | 414.23 ± 593.14 | 0.061 |
| CRP | 6.11 ± 14.88 | 9.71 ± 18.05 | 0.217 | 8.32 ± 19.80 | 9.05 ± 16.36 | 0.040 |
| Cystatin C | 0.98 ± 0.46 | 1.14 ± 0.57 | 0.307 | 1.08 ± 0.58 | 1.11 ± 0.52 | 0.057 |
| Choline | 10.04 ± 3.60 | 12.10 ± 4.44 | 0.508 | 11.44 ± 4.47 | 11.89 ± 4.49 | 0.099 |
| Betaine | 42.60 ± 15.36 | 45.52 ± 18.52 | 0.171 | 43.96 ± 16.61 | 45.68 ± 19.10 | 0.097 |
| Insulin glucose ratio | 0.09 ± 0.12 | 0.12 ± 0.22 | 0.149 | 0.11 ± 0.22 | 0.11 ± 0.17 | 0.013 |
| Medications | ||||||
| ACEi or ARB | 1,445 (45.7) | 563 (66.7) | 0.434 | 422 (62.2) | 431 (63.6) | 0.027 |
| CCB | 591 (18.7) | 197 (23.3) | 0.114 | 150 (22.1) | 160 (23.6) | 0.035 |
| Beta blocker | 1,958 (61.9) | 574 (68.0) | 0.128 | 462 (68.1) | 454 (67.0) | 0.025 |
| Thiazide diuretic | 412 (13.0) | 89 (10.5) | 0.077 | 74 (10.9) | 80 (11.8) | 0.028 |
| Nitrate | 931 (29.4) | 356 (42.2) | 0.268 | 270 (39.8) | 278 (41.0) | 0.024 |
| Statin | 1,916 (60.6) | 499 (59.1) | 0.030 | 405 (59.7) | 395 (58.3) | 0.030 |
| Other cholesterol lowering | 404 (12.8) | 118 (14.0) | 0.036 | 97 (14.3) | 90 (13.3) | 0.030 |
| Aspirin | 2,412 (76.3) | 547 (64.8) | 0.253 | 460 (67.8) | 451 (66.5) | 0.028 |
| Oral diabetic medication | 358 (11.3) | 178 (21.1) | 0.268 | 124 (18.3) | 134 (19.8) | 0.038 |
| Insulin medication | 154 (4.9) | 124 (14.7) | 0.335 | 81 (11.9) | 92 (13.6) | 0.049 |
Values are mean ± SD or n (%).
ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; BNP = brain natriuretic peptide; BP = blood pressure; CAD = coronary artery disease; CCB = calcium channel blocker; CHF = congestive heart failure; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate.
The efficacy of propensity score models is best assessed by estimating between-group post-match absolute standardized differences of baseline characteristics (23). Absolute standardized differences directly quantify bias in the means (or proportions) of covariates across the 2 treatments or exposure groups. A difference of 0% indicates no residual bias, and values <10% are considered inconsequential. Therefore, we assessed the effectiveness of our propensity score model by estimating absolute standardized differences, visually presented as a love plot. The association between the use of loop diuretic agents and outcomes was illustrated using Kaplan–Meier curves in the unmatched and matched populations. Differences in survival for the unmatched cohort was assessed by a log-rank test, whereas the matched cohort was assessed using robust variance estimators as described by Austin et al. (23). Subsequent Cox proportional hazard regression analysis took into account the matching of paired subjects with similar propensity scores and was reported as a hazard ratio (HR) with 95% confidence intervals (CIs). Plasma TMAO level differences in the unmatched cohort were analyzed by unpaired Student’s t-test, whereas the matched cohort was compared by paired Student’s t-test (23). Comparison of goodness of fit between statistical models was assessed by likelihood ratio tests. Normality was assessed by visualization of data before analysis, with the median and quartiles of continuous data reported in Supplemental Table S1. Sensitivity analyses was performed using Rosenbaum bounds to address the impact of an unknown covariate that would be necessary to make our hypothesis unlikely (24).
For animal experiments, 2-way analysis of variance with repeated measures was performed to assess the furosemide treatment effect over time. Student's t-test was used to determine significant differences of variables between mice at relevant time points or for tissue TMAO level comparisons. A 2-tailed p value <0.05 was considered significant. All analyses were performed using Prism (Graphpad, La Jolla, California) or R (version 3.2.3, R Foundation).
Results
Baseline characteristics
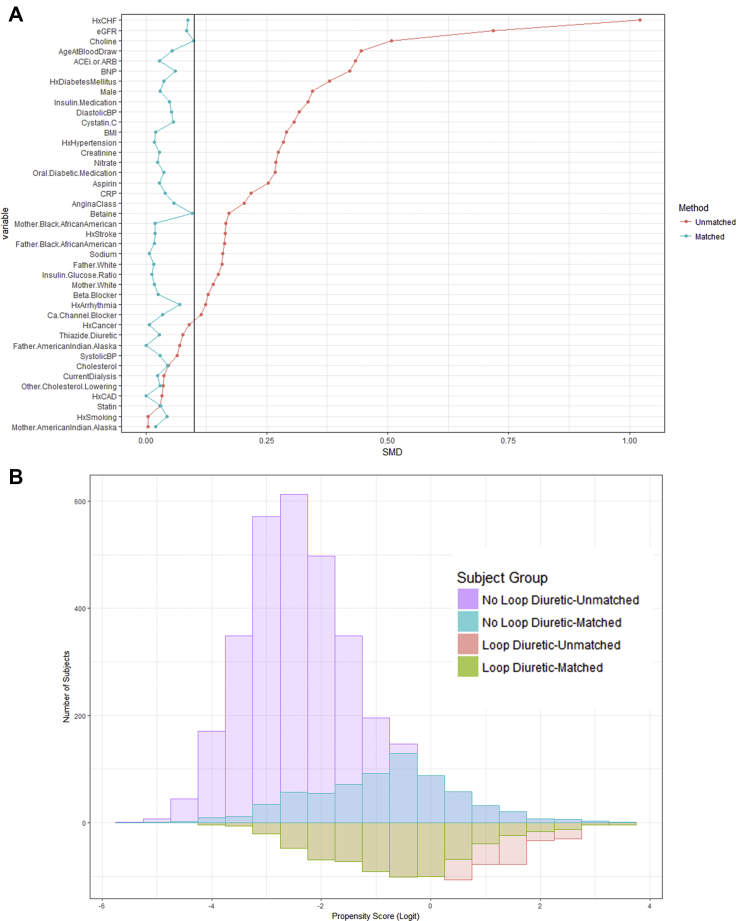
Loop diuretic use data were available in the entire cohort of 4,007 patients with measured plasma TMAO levels. Of the total cohort at baseline, 844 patients were using a loop diuretic. Baseline statistics showed that those taking loop diuretic agents had a worsened cardiovascular disease profile, with an increased history of congestive heart failure, elevated brain natriuretic peptide, and a decreased estimated glomerular filtration rate more frequently with other medical comorbidities and greater use of medications, such as nitrates, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and diabetic medications (Figure 1A).
Figure 1.
Propensity Score Matching Metrics
(A) Love plot showing standardized mean differences (SMD) before and after matching reveals equal matching with all matching covariates having SMD <0.1. (B) Distribution of propensity scores before and after matching shows that an even distribution of cases (loop diuretic use) were selected. ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI = body mass index; BNP = brain natriuretic peptide; BP = blood pressure; Ca = calcium; CAD = coronary artery disease; CHF = coronary heart failure; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate; Hx = history of.
To control for confounding by indication, we used a propensity score model to adjust for important variables that might have affected both mortality and plasma TMAO levels. Propensity score matching on loop diuretic use resulted in 678 of the 844 loop diuretic−treated subjects to be matched to an untreated individual with a similar propensity score. Approximately 20% of unmatched subjects were excluded because no appropriate untreated control subjects could be identified within the specified caliper width. All variables applied to generate the propensity score demonstrated standardized mean differences of <0.1 after matching on loop diuretic use and visualization of propensity score distribution, which showed equal matching across a complete spectrum of cases (Figure 1B). Table 1 shows the descriptive statistics of subjects stratified on loop diuretic use before and after matching.
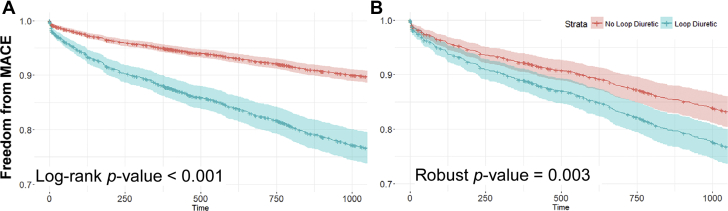
Loop diuretic use is associated with increased MACEs
During follow-up over 3 years (mean follow-up: 30 months; median: 3 years), 269 patients experienced our composite endpoint for MACEs. In the unmatched cohort model, patients who received loop diuretic agents had a significantly greater incidence of the primary endpoint compared with those without loop diuretic use (Figure 2A). After propensity score match, the greater incidence of MACEs remained in the patients who used loop diuretic agents (Figure 2B). Cox proportional hazards analysis of the matched cohort showed that loop diuretic use was associated with MACEs (HR: 1.44; 95% CI: 1.13 to 1.83; p = 0.002). This association remained after adjusting for TMAO in model 2 (HR: 1.36; 95% CI: 1.06 to 1.73; p = 0.012). Moreover, within model 2, TMAO was observed to be independently and significantly associated with MACEs (HR: 1.47; 95% CI: 1.29 to 1.64; p < 0.001). The TMAO-adjusted model showed improved fit compared with stratification by loop diuretic alone, as demonstrated by a comparison through a likelihood ratio test (model 2 vs. model 1: p < 0.001). These lines of evidence were consistent with previous findings of both loop diuretic use and elevated TMAO being prognostic for worsened clinical outcomes (1,4,15,16).
Figure 2.
Kaplan-Meier Curves Showing Freedom From MACE
(A) Before (p < 0.001) and (B) after propensity score matching (p = 0.003) for loop diuretic use with the shaded areas representing a 95% confidence interval. Loop diuretic use remained associated with worse survival after matching for known covariates suggesting effects beyond confounding by indication. MACE = major adverse cardiac event(s).
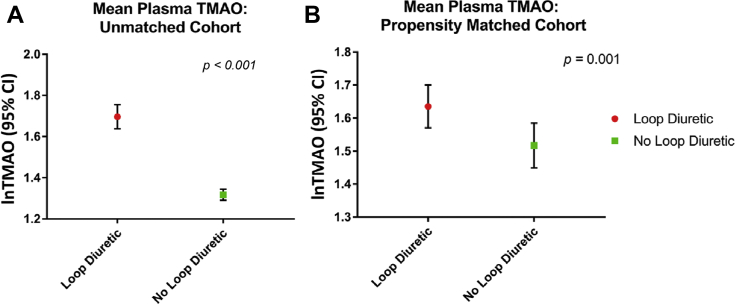
TMAO was elevated in patients using loop diuretics
Because the finding that TMAO was independently prognostic for MACEs in patients on loop diuretics, we sought to understand the relationship between TMAO and loop diuretic use. We examined the difference in baseline plasma TMAO between subjects who used loop diuretics. Before matching, mean plasma TMAO was significantly increased in the loop diuretic cohort (5.5 μM vs. 3.7 μM; p < 0.001) (Figure 3A). After propensity matching that included dietary precursors, this difference remained significant (5.2 μM vs. 4.5 μM; p = 0.001) (Figure 3B).
Figure 3.
Difference in Baseline Plasma TMAO Levels Between Loop Diuretic Use Groups
(A) Unmatched and (B) matched cohorts shows that loop diuretic agents are associated with increased plasma trimethylamine N-oxide (TMAO) levels. CI = confidence interval.
Sensitivity analysis
A propensity score−matched study is an excellent method to match the covariates used. However, this method was unable to account for any unobserved covariates. To address the sensitivity of our clinical model, we used Rosenbaum bounds, as previously described, to assess the necessary impact of an unknown covariate to alter our outcomes (23). In the survival analysis, an unobserved covariate would need to increase the odds of receiving loop diuretic agents by 22% and be strongly associated with MACEs to render the clinical outcomes between treatment groups insignificant. In the plasma TMAO analysis, an unobserved covariate would need to increase the odds of receiving loop diuretic agents by 17% and be strongly associated with TMAO to render the plasma differences as insignificant.
Loop diuretic administration abolishes tubular TMAO secretion and results in plasma and kidney TMAO accumulation
We inferred that TMAO, a kidney excreted metabolite, was likely subject to alterations by medications that affect the function of the kidney. To clarify the associations between TMAO accumulation and loop diuretic use from our propensity-matched cohort, we examined the in vivo accumulation of TMAO after IP injection.
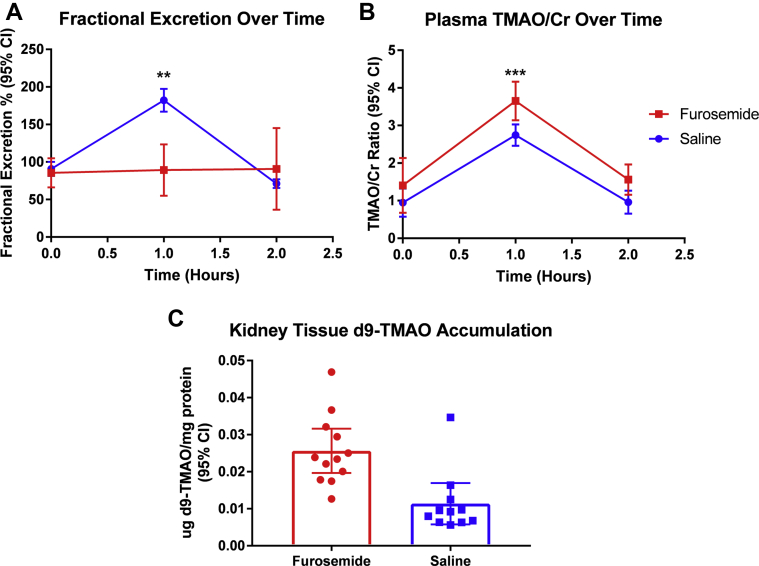
After delivery of d9-TMAO to furosemide-treated mice, the fractional excretion (at 1 h: 89 ± 34% vs. 182 ± 51%; treatment effect; p = 0.004) (Figure 4A) was significantly lower in the furosemide-treated group (n = 13) compared with control mice that received saline (n = 11), whereas a corresponding increase was observed in in TMAO/creatine ratios (d9-TMAO/creatinine ratio at 1 h: 3.7 ± 0.51 vs. 2.7 ± 0.45; treatment effect: p < 0.001) (Figure 4B) at 1 h post−d9-TMAO injection. Our measurements of d9-TMAO showed, for the first time in a mammalian model, that TMAO was secreted and that this secretion was strongly attenuated after furosemide treatment.
Figure 4.
Comparison of d9-TMAO Excretion and Accumulation Between Furosemide (n = 13) and Saline-Treated Mice (n = 11)
(A) Fractional excretion of d9-TMAO is impaired in furosemide-treated mice; repeated measures analysis of variance treatment effect (p = 0.004). (B) Plasma TMAO level normalized to plasma creatinine is increased after furosemide treatment; repeated measures analysis of variance treatment effect (p < 0.001). (C) d9-TMAO is significantly increased in the renal tissue of furosemide treated mice; Student’s t-test (p < 0.001). ∗∗p ≤ 0.01; and ∗∗∗p ≤ 0.001. Cr = creatine; other abbreviations as in Figure 3.
Because TMAO secretion was impaired, we wanted to assess if this impairment occurred as a result of altered TMAO uptake or as efflux by the kidney. Kidneys were harvested from d9-TMAO−injected mice, thoroughly perfused, homogenized, and the concentration of remaining d9-TMAO within the parenchyma measured. We observed that, compared with saline-injected control subjects, furosemide-treated mice had an approximately 25-fold increased intra-renal accumulation of d9-TMAO after adjusting for total kidney protein levels (0.23 ± 0.009 μg d9-TMAO/mg protein vs. 0.009 ± 0.008 μg d9-TMAO/mg protein; p < 0.001) (Figure 4C). These data suggested that furosemide ultimately blocked the secretion of TMAO into renal tubules, which resulted in the accumulation of TMAO in both the kidney and plasma.
Discussion
Historically, the action of loop diuretic agents is believed to primarily target the sodium, potassium, and chloride symporters in the thick ascending limb after transporter-mediated delivery to the tubules to induce diuresis. However, our experimental evidence showed differences in fractional excretion, as well as plasma TMAO to creatinine ratios, which suggested that the increased levels of TMAO observed go beyond simply diuresis and hemoconcentration. We observed a clear relationship between loop diuretic use, elevated TMAO levels, and adverse clinical outcomes (myocardial infarction, stroke, and death). We then tested the causality of this observation and observed that administration of furosemide abolished fractional excretion of TMAO and incited an immediate rise in plasma TMAO levels even after adjusting for plasma creatinine. We also observed that furosemide administration directly caused accumulation of TMAO in the parenchymal tissue of the kidney. The observation of plasma and intrarenal accumulation of d9-TMAO suggested that furosemide impaired the efflux of TMAO at the level of the kidney. Through these observations, we described a mechanistic characterization of the loop diuretic medication as a novel mediator of TMAO plasma variability and proposed TMAO accumulation to be a modifiable contributor toward the detrimental effects of long-term loop diuretic use. These observations highlighted the need to further investigate the interactions between acute or long-term use of loop diuretic drugs (commonly in the setting of heart failure) with accumulation of circulating and tissue content of uremic toxins, as well as their clinical implications.
There continues to be a conundrum in the decision to use loop diuretics, because despite the immediate symptomatic relief attained for volume overload, the long-term clinical outcomes of these medications are not well understood. Retrospective studies on loop diuretic use have been inconclusive due to the potential for confounding by indication. However, previous propensity score−matched studies, which targeted this issue, suggested that the effect of long-term loop diuretic use on outcomes might be drug specific, beyond the fact that sick patients wee more likely to be on loop diuretic agents (14,15,21). Similar to previous findings, the hazard reported in our study in the loop diuretic cohort was closely related to that published by previous studies (14,15). However, our observations of TMAO accumulation through loop diuretic use suggested a novel modifiable mechanism that contributed to the observed adverse patient outcomes. This added to processes (e.g., worsened renal function or neurohormonal activation) previously suggested to mediate the detrimental effects of long-term loop diuretic use (15,16). It also suggested that use of a microbial choline TMA lyase inhibitor, which suppresses TMA (and hence, TMAO) generation, and in animal models, attenuates atherosclerosis and thrombosis, might be of interest as a companion agent to pair with loop diuretic use (25,26).
TMAO is a metabolite that is generated from a meta-organismal pathway initiated through nutritional precursors (e.g., phosphatidylcholine and carnitine) and is dependent on gut microbiota metabolism (1,27). Transient increases of TMAO can be observed when individuals ingest foods high in TMAO, such as certain fish or precursors that generate TMAO (9), which suggests that renal excretion of TMAO is a major regulator of TMAO levels (4). Recent studies demonstrated that administration of TMAO in heart failure models worsened cardiac function, whereas removal or inhibition of TMAO production resulted in improved cardiac function (5,6). In addition, we previously demonstrated that long-term TMAO feeding in mice resulted in cystatin C elevation, increased tubulin, and accompanied increases in renal fibrosis (3). Similarly, several human clinical studies supported the association between high levels of TMAO and incident accelerated decline in renal function (28, 29, 30, 31, 32). In this study, we used a stable isotope to track the movement of TMAO after injection, which eliminated concerns of intraindividual variability due to differences in microbiota production of TMAO. As demonstrated by the fractional excretion observed in mice injected with d9-TMAO, a strong secretory component was required to control the excretion of TMAO in the presence of supraphysiological concentrations. This suggested that, with appropriate kidney function, transient increases of TMAO might not have a clear negative impact on health. TMAO accumulation due to chronically impaired renal clearance could potentially contribute to a feed-forward cycle of injury.
Our findings might help explain the poorer outcomes observed with long-term loop diuretic use—a finding that could never be explained because of the lack of definitive data showing renal damage in acute aggressive diuresis with loop diuretics. Studies also further supported the conclusion that TMAO serves as a mediator of deleterious mechanisms. Ahmad et al. (33) demonstrated that aggressive diuresis in patients with acute heart failure did not result in increased tubular injury markers (33). In previous observational studies of TMAO, the highest levels of TMAO were observed in patients with elevated cystatin C and/or end-stage renal disease, which suggested a mechanistic relationship between TMAO and worsening renal function (3). Furthermore, it was clear from our animal experiments that acute aggressive diuresis would immediately raise plasma TMAO levels. Because TMAO was also suggested to be prognostic for poor outcomes in patients with acute heart failure, these findings together suggested that TMAO could serve as an early marker for the harmful effects of diuresis (34).
Which transporters mediate TMAO excretion?
A recent clinical trial for AST-120 adsorption of uremic toxins in chronic kidney disease showed no significant difference in the outcome (35). However, it has since been observed that AST-120, despite decreasing a range of uremic toxin levels, also increases toxins such as plasma TMAO levels in 5/6 nephrectomized mice compared with control mice, perhaps counteracting the decrease of other uremic toxins (36). Because of the importance of renal excretion for TMAO clearance from the body, it is important to better understand the transporters involved in this process and the potential drug−metabolite interactions in this complex patient disease cohort.
Because of our finding that furosemide was capable of inhibiting TMAO secretion, this served as a reasonable starting point to work backwards and to identify transporters that might mediate this process. Secretion and reabsorption mechanisms for TMAO were reported in fish, whereas TMAO was also believed to be passively secreted in chicken (37,38). Our data added to these findings and suggested that an active renal secretory mechanism for TMAO elimination exists in mammals. A recent study identified OAT3, incidentally a major apical uptake transporter of furosemide, as a potential contributor to TMAO renal excretion (39). In this study, both OAT3 knockout, as well as a “chemical” double knockout by introducing OAT1/3 inhibitor probenecid, resulted in increased levels of plasma TMAO. Similarly, efflux transporters for furosemide, such as ABCG2, ABCC2, and ABCC4, were also identified as candidates for TMAO transport (40). Our in vivo experiments with furosemide provided further evidence for this set of uptake and efflux transporters in an excretion model and directly quantified the impact of this inhibition on TMAO renal excretion in a mammalian system. We also provided direct evidence from isotope-labeled TMAO administration regarding the accumulation of TMAO levels in the renal parenchyma with concomitant loop diuretic administration. It is therefore conceivable that other uremic toxins may share similar interactions with loop diuretic agents at the level of renal clearance. With recent validation of the prognostic value of TMAO by the BIOSTAT-CHF (A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure) cohort, which might be independent of guideline-based pharmacological treatment (41), the potential contributions of loop diuretic and impaired clearance of uremic toxins might warrant further investigations.
Study limitations
Our clinical analyses were carried out on a nonrandomized cohort. Therefore, association findings might be biased by unmeasured confounders or severity of disease burden despite extensive matching to adjust for these biases. However, it was difficult to ascertain if any unmeasured variable could be completely unrelated to any of the covariates used in our propensity score analysis. Our clinical findings were in line with propensity score−matched studies in other independent cohorts (14,15). We also included additional continuous covariates not present in previous propensity score studies investigating the loop diuretic relationship with clinical outcomes. These covariates included those that controlled for kidney function (cystatin C), inflammation (C-reactive protein), diabetes (insulin/glucose ratio), and TMAO-related metabolites (choline, betaine).In this cohort, we did not have the clinical data to perform additional stratification on loop diuretic dose or type. However, based on general institutional practices, furosemide likely predominated as the treatment of choice. Moreover, because this study cohort was focused on coronary outcomes, additional data on New York Heart Association functional heart failure class, left ventricular ejection fraction, mineralocorticoid receptor antagonist use, and follow-up for heart failure hospitalization were unavailable.
Although our clinical association between loop diuretic use and elevated TMAO was confirmed in vivo, it is also important to note that the detailed molecular mechanisms of TMAO excretion remain inconsistent in published reports. In a recent report, it was suggested that the organic osmolyte function of TMAO induced diuresis when given at supraphysiologic levels, which led to improved heart failure outcomes. The investigators reported a primary diuretic effect in TMAO-fed rats (42). Interestingly, all the TMAO-fed rats (a different animal model compared with most TMAO studies) in this study also exhibited decreased plasma sodium with increased plasma renin, which is typically seen in worsened heart failure physiology. This raised the question if acute diuresis (whereas the control group had no method for additional diuresis) only provided short-term symptom relief while still retaining harmful uremic toxins that might have worsened outcomes if the control also received equivalent volume removal. At the molecular level, other studies on uremic toxin retention with OAT3 did not find TMAO levels to change (43). Although our measurements of d9-TMAO suggested that TMAO was secreted and that this secretion was strongly attenuated after furosemide treatment with adjustment for glomerular filtration with plasma creatinine, we could not exclude that the accumulation of d9-TMAO in the kidney might have been secondary to dehydration, with a resultant increase in the concentration of filtered d9-TMAO in the tubules secondary to increased resorption of water in the distal tubule. During the preparation of this paper, a study was published that also characterized the positive association between furosemide and elevated TMAO in a limited number of patients (n = 19 furosemide users) (44). Using a backward multiple linear regression analysis, the investigators also identified loop diuretic agents and the estimated glomerular filtration rate to be associated with elevated TMAO. However, they did not observe TMAO increases with the use of OAT3 inhibitor probenecid. Further rigorous investigations into mechanisms of TMAO transport require confirmation of functional transporter expression, use of appropriate controls, and demonstration of clinically relevant pharmacokinetics.
Conclusions
Loop diuretic use is associated with poor outcomes. Loop diuretic use also shows independent association with elevated plasma TMAO, and animal model studies suggest that competition at the level of renal transporters between furosemide, a prototypic loop diuretic, and TMAO contributes to elevated plasma and renal tissue TMAO levels. Both TMAO and loop diuretic use are independently associated with the overall poor prognosis.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: We observed a clear relationship between loop diuretic use, elevated TMAO levels, and adverse clinical outcomes (MI, stroke, and death), despite careful propensity matching.
TRANSLATIONAL OUTLOOK: The observation of plasma and intrarenal accumulation of d9-TMAO suggests that furosemide impairs the efflux of TMAO at the level of the kidney. Therefore, TMAO accumulation may be a modifiable contributor toward the detrimental effects of long-term loop diuretic use.
Funding Support And Author Disclosures
Dr. Li was supported as a 2016-2017 Fellow of the Sarnoff Cardiovascular Research Foundation. Drs. Hazen and Tang were supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (R01DK106000, R01HL103866, and R01HL126827). Dr. Hazen was supported by a grant from the National Institutes of Health (1P01HL-147823). Dr. Wang was supported by a grant from the National Institutes of Health (R01HL130819). Dr. Lusis was supported by a grant from the National Institutes of Health (R01HL144651). Drs. Hazen and Lusis were supported in part by grants from the Leducq Fondation (17CVD01). Drs. Wang and Hazen are co-inventors on pending and issued patents held by the Cleveland Clinic related to cardiovascular diagnostics and therapeutics, and have received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, a fully owned subsidiary of Quest Diagnostics, and Procter and Gamble. Dr. Hazen has been a paid consultant for Procter and Gamble; and has received research funding from Pfizer Inc., and Roche Diagnostics. Dr. Tang has been a consultant for Sequana Medical Inc and Owkin Inc., unrelated to the contents of this paper. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Wang Z., Klipfell E., Bennett B.J. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang W.H., Wang Z., Fan Y. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang W.H., Wang Z., Kennedy D.J. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang W.H., Wang Z., Levison B.S. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K., Zheng X., Feng M., Li D., Zhang H. Gut microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front Physiol. 2017;8:139. doi: 10.3389/fphys.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organ C.L., Otsuka H., Bhushan S. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagatomo Y., Tang W.H. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail. 2015;21:973–980. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith J.L., Wishnok J.S., Deen W.M. Metabolism and excretion of methylamines in rats. Toxicol Appl Pharmacol. 1994;125:296–308. doi: 10.1006/taap.1994.1076. [DOI] [PubMed] [Google Scholar]
- 9.Svensson B.G., Akesson B., Nilsson A., Paulsson K. Urinary excretion of methylamines in men with varying intake of fish from the Baltic Sea. J Toxicol Environ Health. 1994;41:411–420. doi: 10.1080/15287399409531853. [DOI] [PubMed] [Google Scholar]
- 10.Ezekowitz J., McAlister F.A., Humphries K.H. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–1592. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 11.Hillege H.L., Nitsch D., Pfeffer M.A. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 12.McAlister F.A., Ezekowitz J., Tonelli M., Armstrong P.W. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 13.Schefold J.C., Filippatos G., Hasenfuss G., Anker S.D., von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12:610–623. doi: 10.1038/nrneph.2016.113. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A., Young J.B., Love T.E., Levesque R., Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol. 2008;125:246–253. doi: 10.1016/j.ijcard.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damman K., Kjekshus J., Wikstrand J. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2016;18:328–336. doi: 10.1002/ejhf.462. [DOI] [PubMed] [Google Scholar]
- 16.Testani J.M., Cappola T.P., Brensinger C.M., Shannon R.P., Kimmel S.E. Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58:375–382. doi: 10.1016/j.jacc.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartiala J., Bennett B.J., Tang W.H. Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler Thromb Vasc Biol. 2014;34:1307–1313. doi: 10.1161/ATVBAHA.114.303252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research (CDER) Oprs Alert. 2005;7 [Google Scholar]
- 19.Cheung K.W.K., Hsueh C.H., Zhao P. The effect of uremic solutes on the organic cation transporter 2. J Pharm Sci. 2017;106:2551–2557. doi: 10.1016/j.xphs.2017.04.076. [DOI] [PubMed] [Google Scholar]
- 20.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshaghian S., Horwich T.B., Fonarow G.C. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 22.Inker L.A., Schmid C.H., Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin P.C. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum P.R., Rubin D.B. Assessing sensitivity to an unobserved binary covariate in an observational study with binary outcome. J Royal Statist Soc Series B (Methodological) 1983;45:212–218. [Google Scholar]
- 25.Wang Z., Roberts A.B., Buffa J.A. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts A.B., Gu X., Buffa J.A. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–1417. doi: 10.1038/s41591-018-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koeth R.A., Wang Z., Levison B.S. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stubbs J.R., House J.A., Ocque A.J. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–313. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafi T., Powe N.R., Meyer T.W. Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol. 2017;28:321–331. doi: 10.1681/ASN.2016030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manor O., Zubair N., Conomos M.P. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 2018;24:935–946. doi: 10.1016/j.celrep.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 31.Li D.Y., Tang W.H.W. Contributory role of gut microbiota and their metabolites toward cardiovascular complications in chronic kidney disease. Semin Nephrol. 2018;38:193–205. doi: 10.1016/j.semnephrol.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee E.P., Clish C.B., Ghorbani A. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–1338. doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad T., Jackson K., Rao V.S. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation. 2018;137:2016–2028. doi: 10.1161/CIRCULATIONAHA.117.030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T., Heaney L.M., Bhandari S.S., Jones D.J., Ng L.L. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016;102:841–848. doi: 10.1136/heartjnl-2015-308826. [DOI] [PubMed] [Google Scholar]
- 35.Schulman G., Berl T., Beck G.J. Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol. 2015;26:1732–1746. doi: 10.1681/ASN.2014010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiyama Y., Takeuchi Y., Kikuchi K. A metabolomic approach to clarifying the effect of AST-120 on 5/6 nephrectomized rats by capillary electrophoresis with mass spectrometry (CE-MS) Toxins (Basel) 2012;4:1309–1322. doi: 10.3390/toxins4111309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen J.J., Krupp M.A., Chidsey C.A., 3rd Renal conservation of trimethylamine oxide by the spiny dogfish, Squalus acanthias. Am J Physiol. 1958;194:229–235. doi: 10.1152/ajplegacy.1958.194.2.229. [DOI] [PubMed] [Google Scholar]
- 38.Acara M., Camiolo S., Rennick B. Renal N-oxidation of trimethylamine in the chicken during tubular excretion. Drug Metab Dispos. 1977;5:82–90. [PubMed] [Google Scholar]
- 39.Wu W., Bush K.T., Nigam S.K. Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci Rep. 2017;7:4939. doi: 10.1038/s41598-017-04949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teft W.A., Morse B.L., Leake B.F. Identification and characterization of trimethylamine-N-oxide uptake and efflux transporters. Mol Pharm. 2017;14:310–318. doi: 10.1021/acs.molpharmaceut.6b00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki T., Yazaki Y., Voors A. Association with outcomes and response to treatment of trimethylamine N-oxide in heart failure: results from BIOSTAT-CHF. Eur J Heart Fail. 2019;21:877–886. doi: 10.1002/ejhf.1338. [DOI] [PubMed] [Google Scholar]
- 42.Gawrys-Kopczynska M., Konop M., Maksymiuk K. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. Elife. 2020;9 doi: 10.7554/eLife.57028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsueh C.H., Yoshida K., Zhao P. Identification and quantitative assessment of uremic solutes as inhibitors of renal organic anion transporters, OAT1 and OAT3. Mol Pharm. 2016;13:3130–3140. doi: 10.1021/acs.molpharmaceut.6b00332. [DOI] [PubMed] [Google Scholar]
- 44.Latkovskis G., Makarova E., Mazule M. Loop diuretics decrease the renal elimination rate and increase the plasma levels of trimethylamine-N-oxide. Br J Clin Pharmacol. 2018;84:2634–2644. doi: 10.1111/bcp.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.