Abstract
Biofilms are structured microbial communities attached to surfaces, which play a significant role in the persistence of biofoulings in both medical and industrial settings. Bacteria in biofilms are mostly embedded in a complex matrix comprised of extracellular polymeric substances that provide mechanical stability and protection against environmental adversities. Once the biofilm is matured, it becomes extremely difficult to kill bacteria or mechanically remove biofilms from solid surfaces. Therefore, interrupting the bacterial surface sensing mechanism and subsequent initial binding process of bacteria to surfaces is essential to effectively prevent biofilm-associated problems. Noting that the process of bacterial adhesion is influenced by many factors, including material surface properties, this review summarizes recent works dedicated to understanding the influences of surface charge, surface wettability, roughness, topography, stiffness, and combination of properties on bacterial adhesion. This review also highlights other factors that are often neglected in bacterial adhesion studies such as bacterial motility and the effect of hydrodynamic flow. Lastly, the present review features recent innovations in nanotechnology-based antifouling systems to engineer new concepts of antibiofilm surfaces.
Keywords: bacterial adhesion, biofilm formation, material properties, bacterial motility, hydrodynamics, antibiofilm surfaces, bacterial surface sensing
Introduction
Biofilm is a three-dimensional structure formed as a result of microorganism’s surface sensing, initial adhesion to surfaces, followed by subsequent colonization and production of an extracellular polysaccharides matrix (EPS) (Flemming et al., 2016). The development of the biofilm is a sequential process that starts with a loose association of the microorganisms to a surface then converted to strong adhesion. At the final stage of adhesion, the bacterial cell wall is deformed, which reinforces the bacteria’s adhesion toward the surface by positioning the cytoplasmic bacterial molecules closer to the surface. This enables bacteria to interact with surfaces using their bacterial surface molecules in the form of Lifshitz-van der Waals attractive forces (Carniello et al., 2018). Once the microorganisms adhere to a surface, they often aggregate and form microcolonies that maturate over time (An and Friedman, 1998). The structured channels within the biofilm facilitate the exchanges of nutrients and byproducts between the embedded microorganisms and the external environment, which attributes to the microorganisms’ colonization growth and maturation (Flemming et al., 2016). After biofilm maturation, the microorganisms shed and move from the matured biofilm to join another biofilm community or to become a pioneer of a new one (Hall-Stoodley et al., 2004).
A significant feature of the biofilm is that it can form either on a biotic or abiotic surface. Thus, it is deeply associated with a diverse spectrum of industrial biofouling as well as human health problems, such as dental caries, infective endocarditis, cystic fibrosis pneumonia, and peritoneal dialysis catheters infection (Hall-Stoodley et al., 2004). Particularly, 60–70% of all healthcare-associated infections are attributed to biofilm infections in implantable medical devices (Bryers, 2008), thereby it is imperative to develop novel anti-infectious biomaterials. Biofilm formation is essential for microorganisms’ survival since it benefits bacteria; it stimulates bacterial growth and acts as a barrier that protects the embedded microorganisms from environmental challenges and administered antimicrobials (Lebeaux et al., 2014). During the biofilm maturation, the EPS matrix enhances cell adhesion and cohesion that promote both microbial accumulation onto a surface and the development of densely packed cell aggregates, resulting in a highly structured and adherent biofilm. As such, once biofilms are established, it becomes extremely difficult to kill embedded bacteria or mechanically remove biofilms from surfaces. Therefore, interrupting the bacterial surface sensing mechanism and their initial binding process to surfaces is essential to effectively prevent biofilm-related problems.
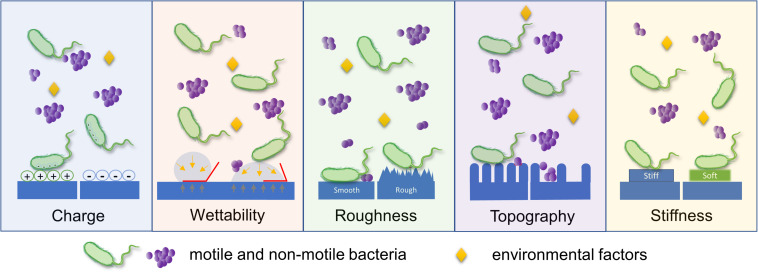
Highlighting that biofilm formation is initiated by bacterial adhesion to a surface, bacterial sensing and responding to surfaces have been widely studied. There are many factors affecting the process of bacterial adhesion to a surface; duration of exposure of bacteria to surfaces, population of inoculated bacteria, bacterial characteristics (e.g., cell wall components, appendages, and motility), and type/richness of nutrients could affect. Surface properties of the substrate, such as surface charge density, wettability, roughness, stiffness, and surface topography are also considered important factors governing initial bacterial adhesion to surfaces (as illustrated in Figure 1) (An and Friedman, 1998; Song et al., 2015; Carniello et al., 2018; Li et al., 2019; Chien et al., 2020). From that aspect, there were many attempts to explain the mechanism of bacterial binding to surfaces using physicochemical approaches such as thermodynamic theory, Lifshitz-van der Waals, and electrostatic-double layer interactions (Carniello et al., 2018). On the other hand, various strategies have been developed to prevent biofilm formation at an early stage, either by engineering anti-adhesive surface properties or introducing antibacterial elements to a surface (Zhang et al., 2018; Chi et al., 2019; Li et al., 2019).
FIGURE 1.
Schematic illustration of various surface parameters that influence bacterial adhesion. Bacterial adhesion is governed by diverse surface properties, including surface charge density, wettability, roughness, topography, and stiffness. This diagram describes the major aspect of bacterial response to a single surface parameter.
In this review, we aim to summarize and discuss the recent publications regarding bacterial sensing and responding to individual/combined surface properties. Furthermore, we introduce other important but not yet sufficiently highlighted parameters in regards to bacterial interaction with surfaces, such as bacterial motility and the effect of hydrodynamic flow. Lastly, we give a quick glance at the nano-science and new anti-adhesion approaches.
Surface Properties
Surface Charge Density
Surface charge density is one of the important surface properties that can determine bacterial adhesion onto surfaces. According to Renner and Weibel (2011), van der Waals force and electrostatic interactions are one of the major forces in bacterial adhesion onto material surfaces. Many have proposed the mechanism by which surface charge affects bacterial initial adhesion. Considering that bacteria usually possess a net negative charge due to carboxyl, amino, and phosphate groups on their cell wall surfaces (Rijnaarts et al., 1999), more adhesion on positively charged surfaces were often observed (Gottenbos et al., 1999; Zhu et al., 2015; Kovacevic et al., 2016; Guo et al., 2018; Oh et al., 2018; Chen et al., 2019). An early study on the effect of surface charge on bacterial adhesion showed that initial adhesion and growth of Pseudomonas aeruginosa on positively charged poly(methacrylates) were facilitated 2-fold compared to negatively charged surfaces (Gottenbos et al., 1999). Since then, similar observations have been reported by testing different bacteria and surfaces. For example, Zhu et al. (2015) demonstrated greater adhesion of Pseudomonas, Escherichia coli, and Staphylococcus aureus on positively charged poly(acrylic acid) (PAA) and poly(diallyldimethylammonium chloride) (PDADMAC) surfaces. Increased bacterial adhesion on positively charged surfaces was also observed for P. aeruginosa on a different type of positively-charged poly(allylamine hydrochloride) (PAH) multilayers compared to negatively charged poly(styrenesulfonate) (PSS) multilayers (Kovacevic et al., 2016). The binding trend of S. aureus and E. coli onto polyelectrolyte multilayers (PEM) was also primarily controlled by surface charge, showing reduced bacterial adhesion on the negatively charged surface (Guo et al., 2018).
Interestingly, some of the studies showed that surface charge density not only affects the initial bacterial attachment but also the subsequent biofilm accumulation on material surfaces (Ueshima et al., 2002; van Merode et al., 2006; Kao et al., 2017; Shen et al., 2020). It’s worth noting, however, that the trends for initial attachment and later biofilm formation were not always coincident. For example, E. coli adhered more to positively charged surfaces, but the high charge density induced lower cell viability and retarded biofilm growth later on (Terada et al., 2012). The structure and strength of biofilms on differently charged surfaces were also varied; E. coli biofilm on negatively charged surfaces was heterogeneous, sparse, mushroom-shaped, and less shear resistant, while that on positively charged surfaces was homogenous, dense, uniform, and greater shear resistant (Terada et al., 2012).
While numerous studies demonstrated that negatively charged surfaces reduce bacterial adhesion to its surface, the uncertainty on the trend between charge and bacterial attachment is further complicated by research that shows mixed results. It has been reported that some bacteria have an ability to overcome electrostatic repulsion despite net negative charge on their surface and they even bind tightly to negatively charged surfaces due to their surface appendages, such as fimbriae (Ueshima et al., 2002). Also, lipopolysaccharide (LPS), a bacterial surface polymer of Gram-negative bacteria (e.g., P. aeruginosa and E. coli), was able to assist their adhesion to negatively charged surfaces (Rzhepishevska et al., 2013). Oppositely, reduced adhesion of Streptococcus mutans to a positively charged surface has been reported (He et al., 2019). Frueh et al. (2014) found that the negative ends of PEM films reduced the attachment of Gram-positive bacteria, and the positive ends reduced the attachment of Gram-negative bacteria. On the other hand, both positively and negatively charged polystyrene plates were found to decrease P. aeruginosa adhesion compared to unmodified plates (Kao et al., 2017). Therefore, further research is needed to investigate to fully understand how surface charge density affects adhesion and biofilm formation of different types of bacteria. Studies regarding surface charge density and its effects on bacterial adhesion and biofilm formation are summarized in Table 1.
TABLE 1.
Influence of surface charge on bacterial adhesion and biofilm growth.
| Microorganism | Surface material | Influence on adhesion/biofilm growth | References |
| E. coli | Polythylene sheets modified by radiation-induced graft polymerization (RIGP) of an epoxy-group containing monomer glycidyl methacrylate (GMA) | Increased bacterial adhesion on positively charged surface, biofilm structure was dense, homogenous and uniform | Terada et al., 2012 |
| Pseudomonas, E. coli, and S. aureus | Positively charged poly(acrylic acid) (PAA) and poly(diallyl dimethyl ammonium chloride) (PDADMAC) | Increased bacterial adhesion on a positively charged surface | Zhu et al., 2015 |
| P. aeruginosa | Poly(allylamine hydrochloride)/sodium poly(4-styrenesulfonate) (PAH/PSS) polyelectrolyte multilayers | Increased bacterial adhesion on a positively charged surface | Kovacevic et al., 2016 |
| S. aureus and E. coli | Gold coated plates with thin thiol layers of 1-octanethiol, 1-decanethiol, 1-octadecanethiol, 16-mercaptohexadecanoic acid, and 2-aminoethanethiol hydrochloride | Increased bacterial adhesion and biofilm thickness on hydrophilic substrates with positive surface charge | Oh et al., 2018 |
| E. coli | Layer-by-layer (LbL) assembly of cationic polyvinylamine (PVAm)/anionic cellulose nanofibril/PVAm | Increased bacterial adhesion and bacterial viability as surface charge increases | Chen et al., 2019 |
| S. aureus and E. coli | Polyethylenimine multilayers | Reduced bacterial adhesion on a negatively charged surface | Guo et al., 2018 |
| S. mutans | Chimaeric peptide-mediated nanocomplexes of carboxymethyl chitosan/amorphous calcium phosphate (CMC/ACP) | Reduced bacterial adhesion on a positively charged surface | He et al., 2019 |
| S. aureus, P. aeruginosa and E. coli | Hydroxide coated titanium alloy (Ti-OH) discs coated with (3-aminopropyl) triethoxysilane, resulting in hydrophobic alkyl chain and positively charged amino group | Reduced bacterial adhesion and subsequent growth on a positively charged surface | Shen et al., 2020 |
| P. aeruginosaa | Positively charged poly(allylamine hydrochloride) (PAH) and negatively charged poly(styrenesulfonate) (PSS) | Reduced bacterial adhesion to both positively and negatively charged plates | Kao et al., 2017 |
| Bacteria in fresh water | Polyelectrolyte multilayers (PEM) on PDMS | Selective reduction of bacterial adhesion to charged surfaces | Frueh et al., 2014 |
Surface Wettability
As the point of contact between bacteria and the bulk of a material, the surface plays a pivotal role in promoting or preventing bacterial adhesion. Surface wettability is a central property that governs the interactions between solid and liquid phases in biological systems. Briefly, the liquid phase “wets” the surface of a solid surface by maximizing its area in contact with the surface. This in turn increases the interaction between the liquid and the solid surface. In general, surfaces with low surface energy and liquids with high surface tension tend to reduce surface wettability. On the other hand, surfaces with high surface energy and liquids with low surface tension tend to increase surface wettability (Song et al., 2019; Jothi Prakash and Prasanth, 2021).
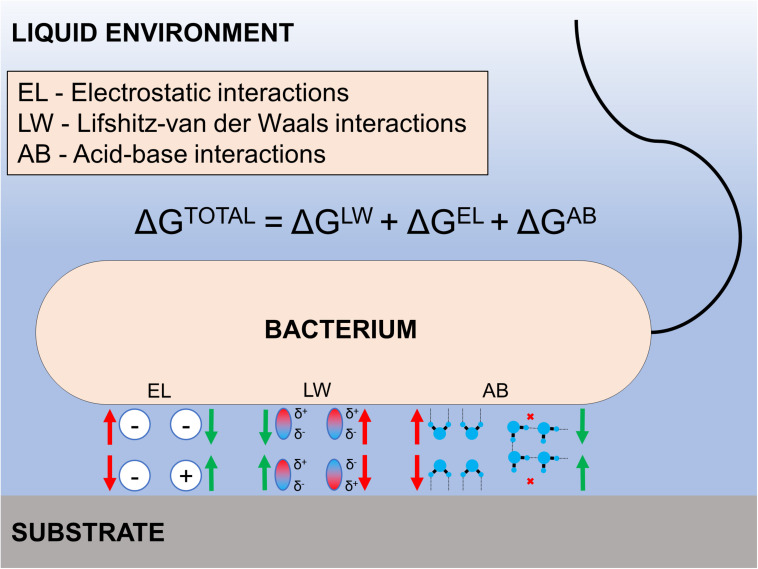
The intrinsic wettability of a surface depends primarily on its surface energy and roughness (Song et al., 2019). Various models have been utilized to explain the relationship between surface energy/interfacial interaction energy and bacterial adhesion. These models stem from Derjaguin-Landau-Verwey-Overbeek (DLVO), extended-DLVO, and thermodynamics-based approaches (Bos et al., 1999). For example, a thermodynamic approach based on the pairwise interplay of surface free energies among surface, fluid, and bacteria was utilized to explain bacterial adhesion phenomena (Rigolin et al., 2019; Bilgili et al., 2020; Ruan et al., 2020). The adhesion energy can be estimated as the sum of the Lifshitz-van der Waals interactions, the electric double layer interactions, and the often-overpowering acid-base interactions. A resultant negative free energy favors bacterial adhesion, while positive free energy may inhibit it. Overall, the key benefit of using a thermodynamic approach is its usefulness in explaining generic like-to-like observations, such as bacteria with hydrophobic cell surfaces favor hydrophobic material surfaces while those with hydrophilic cell surfaces favor hydrophilic material surfaces (Krasowska and Sigler, 2014). Similarly, bacterial adhesion is often suitably described by DLVO and its derivative theories (Hwang et al., 2010, 2012; Carniello et al., 2018; Zhang et al., 2019) since the size of most bacteria (0.5–2 μm) is close to the range of colloidal particles (Hori and Matsumoto, 2010). Briefly, the relative strengths of van der Waals and Coulomb interactions enable bacterial adhesion to surfaces depending on the bacterium-surface distance and ionic strength of the surrounding fluid. In general, higher ionic strengths favor bacterial adhesion (Sheng et al., 2008) while lower ionic strengths present an insurmountable energy barrier that prevents adhesion (Hori and Matsumoto, 2010). Notably, it is critical to consider the polar interaction energy for the aqueous system to fully account for the total interactive energies between bacterium and counter substrate surface (extended DLVO theory; see Figure 2).
FIGURE 2.
Illustration of the extended DLVO theory. The total interaction energy between the bacterium and a substrate is the sum of the electrostatic double layer interactions, Lifshitz-van der Waals interactions, and acid-base interactions. Each energy component can be either attractive or repulsive depending on the surface properties of the bacterium and substrate in aquatic environmental conditions.
However, it is necessary to point out that researchers should be cautious in drawing broad conclusions on the impact of wettability on bacterial adhesion. There are conflicting findings on the initial adhesion of bacteria on surfaces with moderate hydrophobicity and hydrophilicity (Lorenzetti et al., 2015; Mandracci et al., 2015; Wassmann et al., 2017; Yuan et al., 2017; El-Chami et al., 2020; Pajerski et al., 2020; Verhorstert et al., 2020). Nevertheless, engineered materials and surface treatments can result in extreme water contact angles – either superhydrophobic or superhydrophilic surfaces that can limit bacterial adhesion (Moazzam et al., 2016; Qin et al., 2019; Naderizadeh et al., 2020; Ozkan et al., 2020; Montgomerie and Popat, 2021). For example, the adhesion of S. aureus on superhydrophobic surfaces (commercial polyurethane sponges modified by zinc oxide and copper nanoparticles) was severely impaired (Ozkan et al., 2020). Similarly, the adhesion of E. coli on superhydrophilic surfaces (stainless steel plates coated with TiO2) was greatly reduced (Yoon et al., 2014). Overall, low surface energy is the primary means of attaining an anti-wetting surface. However, higher levels of anti-wetting surfaces can only be achieved by the implementation of surface roughness (Song et al., 2019). Studies regarding surface wettability and its effects on bacterial adhesion and biofilm formation are summarized in Table 2.
TABLE 2.
Influence of surface wettability on bacterial adhesion and biofilm growth.
| Microorganism | Surface material | Parameter tested | Influence on adhesion/biofilm growth | References |
| F. nucleatum, P. gingivalis, and S. sanguinis | Aged titanium and zirconium oxide discs | Contact angles and surface free energy | Increased surface free energy values led to increased biofilm adhesion on zirconium oxide surfaces as compared to titanium surfaces | Rigolin et al., 2019 |
| S. mutans and S. mitis | Various commercial bulk-fill composite dental resins | Contact angles and surface free energy | Bacterial adhesion increased with higher surface free energies | Bilgili et al., 2020 |
| Sphingomonas sp. GY2B | Montmorillonite | Ionic strength of surrounding liquid, DLVO components | Adhesion was driven by long-range DLVO forces at low ionic strength and short-range van der Waals and hydrophobic interactions at high ionic strength | Ruan et al., 2020 |
| E. coli | Titanium substrates with TiO2-anatase nanostructured coating | Contact angles | Increased hydrophilicity of treated surfaces reduced bacterial adhesion | Lorenzetti et al., 2015 |
| S. mutans and S. mitis | Silicon-oxygen thin films on dental composite resins | Contact angles | Hydrophilic coated samples reduced S. mitis adhesion. S. mutans adhesion was not affected by wettability | Mandracci et al., 2015 |
| S. epidermidis, S. aureus, P. aeruginosa, and E. coli | Oxygen-plasma treatment of conductive graphene sheets | Contact angles, surface free energy, and DLVO components | Short time of plasma modification led to significant increases in work function, surface free energy, hydrophilicity, and bacterial adhesion | Pajerski et al., 2020 |
| S. aureus and E. coli | Poly-4-hydroxybutyrate (P4HB) and polypropylene | Contact angles | Hydrophilic substrates had lower adhesion than hydrophobic substrates | Verhorstert et al., 2020 |
| E. coli | Polystyrene | Contact angles and surface energy | Moderate hydrophobicity led to the highest bacterial adhesion. Superhydrophilic substrates limited bacterial binding | Yuan et al., 2017 |
| S. sanguinis and S. epidermidis | Titanium and zirconium oxide dental implants | Contact angles | Hydrophobic surfaces favored adhesion of S. epidermidis. S. sanguinis adhesion was not affected by wettability | Wassmann et al., 2017 |
| S. aureus and P. aeruginosa | Bare, polyurethane, and parylene-coated titanium | Contact angles | Higher contact angles for parylene-coated samples in comparison to bare samples led to reduced bacterial adhesion | El-Chami et al., 2020 |
| E. coli | Stainless steel with TiO2 coating | Contact angles | Superhydrophilic surfaces greatly reduced bacterial adhesion | Yoon et al., 2014 |
| E. coli | Polydimethylsiloxane (PDMS) layered with 2-methacryolyl phosphorylcholine (MPC) | Contact angles | Superhydrophilicity of MPC layer significantly lowered bacterial attachment | Qin et al., 2019 |
| P. aeruginosa | Polyvinyl chloride | Contact angles | Superhydrophobic substrates delayed initial bacterial adhesion by up to 24 h | Loo et al., 2012 |
| P. aeruginosa, S. epidermidis, and S. aureus | Silanized aluminum | Contact angles and surface free energy | Superhydrophobic modification significantly reduced bacterial attachment | Moazzam et al., 2016 |
| E. coli and S. aureus | Titania nanoflowers on titanium alloy | Contact angles | Superhydrophobic surfaces significantly reduced bacterial adhesion | Montgomerie and Popat, 2021 |
| E. coli, S. aureus, and P. aeruginosa | Aluminum foil substrates coated with polyfurfuryl alcohol, perfluorinated acrylic copolymer and Silica nanoparticles | Contact angles | Superhydrophobic coatings exhibited very low bacterial adhesion | Naderizadeh et al., 2020 |
| S. aureus | Polyurethane sponges with zinc oxide and copper nanoparticles | Contact angles | Superhydrophobic characteristics reduced bacterial adhesion over 4 days | Ozkan et al., 2020 |
Surface Roughness
The effect of surface roughness on bacterial adhesion and biofilm formation has been extensively investigated. Surface roughness increases the surface area available for bacterial attachment and provides a scaffold for adhesion (Yoda et al., 2014). Additionally, rough surfaces can provide protection for bacteria against shear forces (Bollen et al., 1996), thereby resisting the detachment of bound bacteria. Therefore, the general consensus is that as surface roughness increases, bacterial adhesion and biofilm formation also increases (Xing et al., 2015; Yu et al., 2016; Yao et al., 2020). For instance, the adhesion of Staphylococcus epidermidis, P. aeruginosa, and Ralstonia pickettii on rougher surfaces with larger surface areas were remarkably higher, compared to the smoother surfaces (James et al., 2019). A similar pattern was also observed from oral bacteria (such as Streptococci) exhibiting that bacterial adhesion was significantly enhanced to rougher surfaces, regardless of surface materials (Yu et al., 2016; Nogueira et al., 2017; Abdalla et al., 2020). Furthermore, an in vivo study reported similar results showing that biofilm accumulation increased in proportion to the nanoscale surface roughness of titanium disc samples (ranging from 29 to 214 nm) placed intraorally via a removable splint model (Xing et al., 2015). Furthermore, biofilm accumulation was more pronounced on the irregularly textured titanium disc surfaces (surface roughness of 190 and 214 nm) than the flat and grooved surfaces (<113 nm) (Xing et al., 2015).
Some studies have proposed the idea of a threshold arithmetic average roughness (Ra) of 0.2 μm. For example, an in vitro study claimed that threshold values for the adhesion of S. mutans and Streptococcus sobrinus to composite resin surfaces were estimated between 0.15 and 0.35 μm (Park et al., 2019). However, other studies contain findings that disprove the existence of such a threshold. Bollen et al. (1996) reported that smoothing a surface past the threshold had no significant reductive effect on bacterial adhesion by testing two types of dental implant abutments in vivo (Ra = 0.21 μm and Ra = 0.06 μm) using a split-mouth design over 12 months. Other studies showed increased bacterial adhesion in proportional to surface roughness even in the range lower than the proposed threshold (0.2 μm). As observed by Yu et al. (2016), an increase in surface roughness of zirconia discs from fine (Ra = 11.89 ± 1.68 nm) to coarse (Ra = 23.94 ± 2.52 nm) was positively correlated with adhesion strength and the number of attached bacteria. Furthermore, Yoda et al. (2014) reported that the number of adhered S. epidermidis on various biomaterials was higher in the course group (Ra = 7.2–30.0 nm) compared with the fine group (Ra = 1.8–8.5 nm). Various studies continue to observe different findings with regards to the threshold roughness of Ra = 0.2 μm. Thus, the existence of a threshold roughness is currently debatable.
Although surface roughness is often positively correlated with the degree of bacterial adhesion and biofilm formation, higher surface roughness in some cases resulted in reduced bacterial adhesion (Wu et al., 2018; Matalon et al., 2020). For example, the adhesion of P. aeruginosa and S. aureus on unpolished stainless steel samples (Ra = 172.5 nm) were decreased significantly compared with the electropolished smoother surfaces with average roughness ranging from 45.2 to 84.4 nm (Wu et al., 2018). Moreover, the qualitative images of the tested samples revealed the presence of scattered single bacterial cells attached to the rough stainless steel surface, while clusters of the bacterial cells were observed on the smooth, electropolished surfaces (Wu et al., 2018). This finding illustrates that the nano-rough surfaces can restrain the bacterial adhesion by decreasing the contact area between the bacteria and surface (Wu et al., 2018). In contrast, one study reported that surface roughness affected bacterial adhesion selectively; higher surface roughness increased the adhesion of Streptococcus sanguinis, while there was no difference in S. epidermidis adhesion (Wassmann et al., 2017).
Another study found that surface root-mean-square roughness (Rq) of Titania up to 20 nm was linked with increased bacterial adhesion and biofilm formation (Singh et al., 2011). However, further increasing the roughness to 25 nm resulted in a significant decrease in bacterial adhesion and biofilm formation. This phenomenon was explained as the passivation and flattening effects induced by bovine serum albumin adsorption on the surface of the Titania. Increased protein adsorption to the rougher surface created an intermediary layer between bacteria and Titania, which limited the interaction of bacteria to the nanostructured surface (Singh et al., 2011). Puckett et al. (2010) observed a similar phenomenon when comparing fibronectin adsorption and bacterial adhesion on a nano-rough titanium surface to a smooth, unmodified Ti surface. The nanorough Ti surface exhibited reduced adhesion of S. aureus, S. epidermidis, and P. aeruginosa than those to smooth Ti surface. The authors explained these findings by the effect of the nanometer surface roughness in enhancing the adherence of fibronectin protein, which has been claimed to decrease bacterial attachment (Puckett et al., 2010). They also found that it was possible to reverse the surface ability to repel bacterial adhesion by altering the chemical composition or crystallinity of the nano- featured Ti surfaces. It indicates that bacterial response to surface roughness can be varied by other factors, such as surface priming by proteins and changes in surface chemistry.
Like other surface properties, there are other variables that can affect the bacterial behavior and adherence toward the surface in response to its roughness, including differences in the bacterial species tested and mediums used in experiments (surface priming proteins). Those need to be accounted for precisely evaluating the effect of surface roughness on bacterial adhesion. Studies regarding surface roughness and its effects on bacterial adhesion and biofilm formation are summarized in Table 3.
TABLE 3.
Influence of surface roughness on bacterial adhesion and biofilm growth.
| Microorganism | Surface material | Surface roughness | Influence on adhesion/biofilm growth | References |
| S. epidermidis | Oxidized zirconium-niobium alloy (Oxinium), cobalt-chromium-molybdenum alloy (Co-Cr-Mo), titanium alloy (Ti-6Al-4 V), commercially pure titanium (Cp-Ti) and stainless steel (SUS316L) | Ra: 1.8–8.5 nm (Fine) Ra: 7.2–30.0 nm (Coarse) | Increased bacterial adhesion in proportion to surface roughness | Yoda et al., 2014 |
| Intraoral biofilm | Titanium Zirconium | Ra: 29–214 nm | Increased biofilm accumulation in proportion to surface roughness | Xing et al., 2015 |
| S. mutans | Zirconia | Ra: 11.89 ± 1.68 nm (Fine) Ra: 23.94 ± 2.52 nm (Coarse) | Increased early bacterial adhesion in proportion to surface roughness | Yu et al., 2016 |
| S. mutans | Dental enamel (sound enamel, enamel surface treated with laser only, treated with laser and fluoride varnish) | Sa: 2–3 μm (laser and fluoride varnish) <2 μm (sound enamel, and laser only) | Increased bacterial adhesion on roughened enamel surface treated with laser and fluoride varnish | Nogueira et al., 2017 |
| S. epidermidis, P. aeruginosa, and Ralstonia pickettii | Breast implants materials. [Natrelle® (Smooth), SmoothSilk®/SilkSurface® (Silk), VelvetSurface® (Velvet), Siltex®, and Biocell®] | Not reported. | Increased bacterial adhesion on rougher surfaces | James et al., 2019 |
| S. mutans | Ceramics (Feldspathic ceramic, lithium disilicate glass IPS e.max and zirconia reinforced lithium silicate) (Roughened and polished) | Ra: 4.4–4.78 nm (rough) Ra: 1.65–2.07 nm (smooth) | Increased bacterial adhesion on rougher ceramic material | Abdalla et al., 2020 |
| S. epidermidis | Titanium (smooth and double acid etched) | Ra: around 4,700 nm (double acid etched titanium) | Increased bacterial adhesion on higher roughness surface with double acid-etched titanium | Kunrath et al., 2020a |
| S. epidermidis | Titanium (Ti) and Zirconia (Zr) | Sa: 0.12 ± 0.01 μm and 0.08 ± 0.00 μm (Machined Ti and Zr) Sa: 2.67 ± 0.20 μm and 0.60 ± 0.05 μm (micro-textured Ti and Zr) | Increased initial bacterial adhesion on rougher microtextured Titanium and Zirconia surfaces. | Kunrath et al., 2020b |
| Prevotella intermedia | Ceramics (leucite-based glass ceramic, lithium disilicate-based glass ceramic, glass ceramic based on zirconia-reinforced lithium silicate, and monolithic zirconia) | Ra: 0.67–0.90 μm (control) Ra: 0.76–1.09 μm (glazed) Ra: 1.04–1.50 μm (grinded surface with bur) | Increased bacterial adhesion on rougher grinded ceramics surface | Poole et al., 2020 |
| S. mutans | Enamel surface | Ra: 19.48 ± 3.34 nm (sound enamel) Ra: 150.53 ± 12.54 nm – 341.05 ± 74.14 nm (rougher enamel surface induced by gradual increase in etching time) | Increased adhesion forces for S. mutans to rougher enamel surface | Wang et al., 2020 |
| S. mutans, S. sobrinus | Dental composite resin | Smooth: 0.15 μm Rough: 1.45 and 0.62 μm | Reduced bacterial adhesion on the smooth surface (roughness values of around 0.15 μm) | Park et al., 2019 |
| Lactobacillus rhamnosus | Gold (smooth/nano-rough and micro-rough/sub micro- rough) | Ra: 2.4 nm (smooth/nano-rough) Ra: 7.0 nm (micro-rough/sub micro- rough) | Reduced bacterial adhesion and biofilm density on smoother gold surface. | Grudzień et al., 2020 |
| Porphyromonas gingivalis | Titanium (Ti) (control and laser treated) | Ra: 0.167 μm (controlled, untreated Ti) Ra: 0.142 μm (laser treated Ti) | Reduced bacterial adhesion on the smooth surface treated by laser | Yao et al., 2020 |
| S. sanguinis | Cr-Co base metal discs, zirconia discs, and lithium disilicate discs | Sa: 0.36 ± 0.12 μm, 0.638 ± 0.24 μm, and 1.23 ± 0.42 μm | Increased bacterial accumulation on the smoothest surface (Cr-Co metal). | Matalon et al., 2020 |
| P. aeruginosa and S. aureus | Stainless steel | Ra: 172.5 nm (unpolished) Ra: 84.4–45.2 nm (electropolished) | Reduced bacterial adhesion on the rough surface | Wu et al., 2018 |
| S. sanguinis and S. epidermidis | Zirconia and titanium | Ra: 0.09 ± 0.02 μm and 0.05 ± 0.00 μm (smooth Ti and Zr) Ra: 2.98 ± 0.31 μm and 1.32 ± 0.10 μm (rough Ti and Zr) | Surface roughness did not influence the adhesion of S. epidermidis, while higher surface roughness increased the adhesion of S. sanguinis | Wassmann et al., 2017 |
Ra indicates arithmetical mean height of a line (2D roughness profile) and Sa indicates arithmetical mean height of a surface (3D areal roughness parameter).
Surface Topography
Bacteria are capable of sensing mechanical cues associated with surfaces, such as the topography of surfaces (Cheng et al., 2019). In general, microscale features, in the same order as bacteria, impact their attachment through hydrodynamics whereas nanoscale features impact their attachment through chemical gradients, physicochemical forces, and cell membrane deformation (Cheng et al., 2019). Alterations in surface topography are also known to affect the expression of bacterial adhesins (Rizzello et al., 2012, 2013).
Non-covalent interactions between bacteria and substrate surfaces can be directly influenced by the structural surface features (Lutey et al., 2018). Briefly, surfaces with a characteristic feature of a dimension larger than a single bacterium provide greater available contact area and sheltering for the microorganism, thereby resulting in an enhanced bacterial attachment (Scardino et al., 2008; Helbig et al., 2016). This effect is more pronounced on engineered surfaces that have well-defined features and spacing. It is well documented that microbes tend to preferentially align to valley and pillar structures to maximize attachment area (Perera-Costa et al., 2014; Vasudevan et al., 2014). Oppositely, many surfaces with a smaller feature than a single microorganism exhibited reduced bacterial adhesion (Yang et al., 2015; Helbig et al., 2016; Xu and Siedlecki, 2017; Schwibbert et al., 2019; Tang et al., 2021).
Recently, several natural surfaces of plants and animals have shed light on the new concept of an antifouling strategy. These include lotuses (Barthlott and Neinhuis, 1997; Bhushan et al., 2009), cicadae (Ivanova et al., 2012), sharks (Chien et al., 2020), termites (Watson et al., 2010), geckos (Watson et al., 2015; Li et al., 2017), butterflies (Fang et al., 2007), and dragonfly wings (Bandara et al., 2017). Such surfaces exhibit contaminant-free surfaces due to their unique superhydrophobic physical surface structures and/or some bactericidal activities. Some prominent recent examples are as follows. Chien et al. (2020) have shown that the topography of sharkskin could significantly alter bacterial attachment and biofilm formation. From their study, the analysis of the height profiles highlights the differences in the topography of the sharkskin. Interestingly, the bacterial attachment was substantially increased on the smooth surface over time, while the protruding surface features inhibited further biofilm development on its surface. Similarly, the micro-/nano-structures on gecko skin exhibited superhydrophobic properties that act as an anti-wetting barrier, resulting in extremely low adhesion (Watson et al., 2015). Gecko skin also revealed antibacterial activity against Gram-negative bacteria, such as Porphyromonas gingivalis, and the nanoscale tips on their hair can efficiently kill oral pathogenic bacteria such as S. mutans and P. gingivalis (Li et al., 2017). Similarly, cicada wings (Ivanova et al., 2012) and dragonfly wings (Bandara et al., 2017) were also known to cause mechanical rupture of bacteria via the nanoscale topography of their wing features (see Figure 3A for the effect of dragonfly wing on the bacteria integrity). Another study proposed an intriguing mechanism that these infection-free natural surfaces may facilitate physical rupture of bacteria by stretching their membrane upon adhesion (Pogodin et al., 2013).
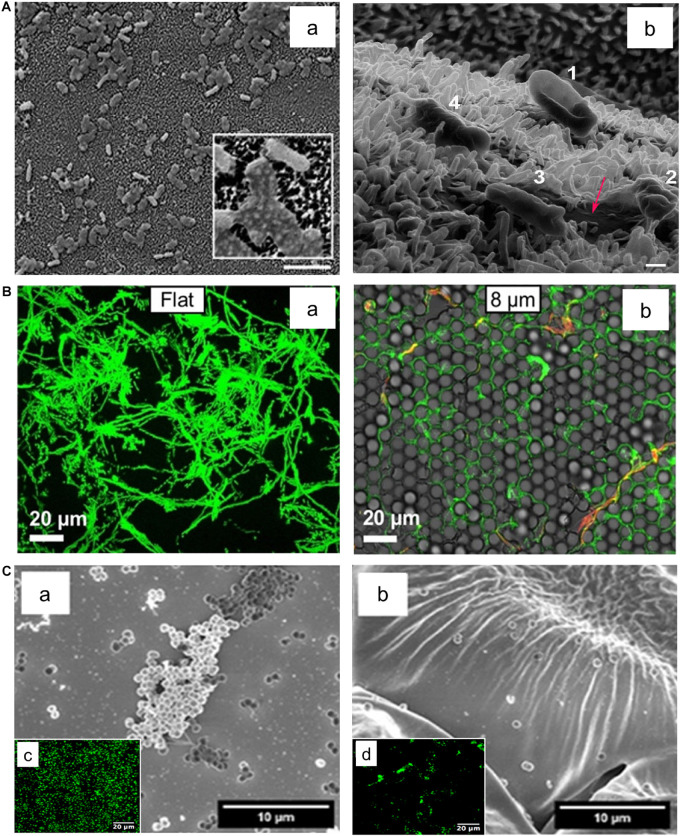
FIGURE 3.
Effect of surface topography on bacterial adhesion. (A) E. coli bacteria attached on textured surface of dragonfly wing (a). The Helium ion microscopy image (b) indicate progressive dying stages of E. coli on the dragonfly wing starting with No. 1 where the cell attached to the surface and its membrane deforms, ending with No. 4 where the cell membrane lost its integrity and cell sank into nanopillars. Scale bar is 200 nm. Adapted with permission from Bandara et al. (2017) Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl Mater Interfaces 9, 6,746–6,760. Copyright © 2017, American Chemical Society. (B) Comparison of P. aeruginosa adhesion on flat (a) and textured surfaces (b). P. aeruginosa appears to cover a smaller surface area on the textured surface with features in 8 μm range in comparison to the flat surface. Adapted with permission from Chang et al. (2018) Surface Topography Hinders Bacterial Surface Motility. ACS Appl Mater Interfaces 10, 9,225–9,234. Copyright © 2018, American Chemical Society. (C) Adherence of S. epidermidis on flat (a) and rose petal-textured (b) surfaces after 2 h incubation. SEM (a,b) and fluorescence microscopy (c,d) images of S. epidermidis showed a decreased amount of bacterial adhesion on the textured surface. Adapted with permission from Cao et al. (2019) Hierarchical Rose Petal Surfaces Delay the Early-Stage Bacterial Biofilm Growth. Langmuir 35, 14,670–14,680. Copyright © 2019, American Chemical Society.
With the aid of various engineering technologies, hierarchical micro- and nanoscale structures with precisely controlled spacing and height of features on surfaces have been used to develop antifouling surfaces via self-cleaning (Patankar, 2004; Cheng and Rodak, 2005; Ma et al., 2011) as well as killing adhered bacteria (Linklater et al., 2017; Watson et al., 2017; Ma et al., 2020). For example, Linklater et al. (2017) used surface geometries in black silicon with linearly increasing heights to show that smaller, densely-packed pillars exhibited the greatest bactericidal effects against both Gram-positive (S. aureus) and Gram-negative (P. aeruginosa) bacteria. Similarly, cones (Perni and Prokopovich, 2013) and hemispheres (Chang et al., 2018) were engineered onto surfaces to control bacterial adhesion (see Figure 3B for the effect of engineered surface topography on bacterial adhesion). Furthermore, there have been attempts to develop bio-inspired biofilm-resistant surfaces by utilizing natural surfaces as templates for those antibiofilm applications. Cao et al. (2019) engineered a rose petal-textured surface utilizing UV-epoxy, which could disrupt the adhesion of S. epidermidis (Figure 3C). Similarly, Ye et al. (2019) patterned cicada wing-like structures on polyether ether ketone (PEEK) substrates and added a top-layer of catkin-like ZnO nano-slices to effectively kill S. aureus. Lastly, Nguyen et al. (2018) used wrinkled gold-coated polystyrene surfaces with nano- and microscale topologies to develop air-water interfacial areas that were unavailable for bacterial attachment for P. aeruginosa and S. aureus. Some studies exhibited distinct anti-adhesion behaviors depending on the type of bacteria (Lutey et al., 2018) or topography structures (Spengler et al., 2019). Studies regarding surface topography and its effects on bacterial adhesion and biofilm formation are summarized in Table 4.
TABLE 4.
Influence of surface topography on bacterial adhesion and biofilm growth.
| Microorganism | Surface material | Scale of surface topography | Influence on adhesion/biofilm growth | References |
| E. coli and S. aureus | Honeycomb-patterned silicon wafers | Microscale | 1 μm patterns displayed a significant decrease in bacterial adhesion and a subsequent antibacterial effect | Yang et al., 2015 |
| S. epidermidis and E. coli | Fuctionalized photoresist on silicon wafers | Microscale | Periodicities in the range of the cell size increased bacterial retention. Smaller periodicities reduced retention | Helbig et al., 2016 |
| S. epidermidis | Polyethylene glycol-grafted, textured polyurethane urea films | Microscale | Texturing reduced bacterial adhesion. Chemical grafting further reduced bacterial adhesion | Xu and Siedlecki, 2017 |
| P. aeruginosa | Norland Optical Adhesive textured on PDMS | Microscale | Texturing reduced bacterial adhesion | Chang et al., 2018 |
| S. epidermidis | Bio-inspired rose petal-textured surfaces (made by UV-epoxy) | Microscale | Texturing reduced bacterial adhesion | Cao et al., 2019 |
| E. coli and S. aureus | Laser-modified polyethylene | Microscale | Adhesion of E. coli was reduced while adhesion of S. aureus was not affected | Schwibbert et al., 2019 |
| E. coli and S. aureus | Sharkskin and its Polymethyl methacrylate (PMMA) replicates | Microscale | Protruding surface features inhibited biofilm development | Chien et al., 2020 |
| S. epidermidis, S. aureus, and P. aeruginosa | Textured fluorinated alkoxyphosphazene surface | Microscale | Patterning led to significant reductions in bacterial adhesion and biofilm formation | Tang et al., 2021 |
| P. gingivalis | Gecko skin | Micro/nano scale | Micro/nanostructures displayed an antibacterial effect | Watson et al., 2015 |
| S. mutans and P. gingivalis | Gecko skin and equivalent acrylic replicates | Micro/nano scale | Micro/nanostructures disrupted normal bacterial adhesion and prevented biofilms by killing bacteria | Li et al., 2017 |
| E. coli and S. aureus | Stainless steel with laser-induced surface structures | Micro/nano scale | E. coli retention was highest when the characteristic dimensions were much larger than the cell size. S. aureus retention was inhibited under the same conditions | Lutey et al., 2018 |
| P. aeruginosa and S. aureus | Gold-coated polystyrene | Micro/nano scale | Topography led to areas unavailable for bacterial attachment | Nguyen et al., 2018 |
| P. aeruginosa and S. aureus | NiTi sheets with laser-ablation and fluorination | Micro/nano scale | Biofilm formation was suppressed along with the substantial killing of colonized bacteria | Ma et al., 2020 |
| E. coli | Dragonfly wing | Nanoscale | Nanostructures displayed an antibacterial effect | Bandara et al., 2017 |
| P. aeruginosa and S. aureus | Black silicon | Nanoscale | Smaller and densely packed pillars exhibited bactericidal activity and a subsequent decrease in attached cells | Linklater et al., 2017 |
| S. aureus | Etched hydrophobized silicon wafers | Nanoscale | Larger nanostructures led to reduced adhesion. Taller nanostructures did not affect adhesion but had a bactericidal effect | Spengler et al., 2019 |
| S. aureus | Cicada wing pattern on polyether ether ketone (PEEK) with zinc oxide coating | Nanoscale | Patterned surfaces led to lower bacterial adhesion, wider antimicrobial range, and longer antibacterial durability | Ye et al., 2019 |
| E. coli and S. aureus | Laser-nanostructured zirconium-based bulk metallic glasses | Nanoscale | Nanostructuring led to a significant reduction in bacterial adhesion | Du et al., 2020 |
Despite the ability to engineer surfaces that passively exhibit antiadhesion properties, it is vital to realize that many strategies for biofilm prevention that are based on surface treatments and microstructure may affect initial bacterial attachment transiently. However, there are a few promising studies that may lead to a broad range of antibiofilm clinical and industrial applications using topographical features. For example, Epstein et al. (2012) were able to prevent P. aeruginosa, S. aureus, and E. coli adhesion over 7 days on slippery liquid-infused porous surfaces. It is worth noting that elucidating the effect of surface topography on bacterial attachment often leads to contradictory conclusions as many studies use roughness as the primary descriptor of surface topography while neglecting compounding factors such as surface chemistry (Cheng et al., 2019). Furthermore, cell stiffness seems to play a major role in promoting or limiting a cell’s ability to adapt to the pattern on a surface (Whitehead and Verran, 2006; Lazzini et al., 2017). Hence, it is important to design studies that measure surface properties from several different aspects to validate existing hypotheses.
Surface Stiffness
Stiffness is another important factor that affects bacterial adhesion and biofilm formation. Young’s modulus, which is defined by the ratio of stress to strain, is a common parameter used to represent stiffness. A low Young’s modulus indicates that the material is softer and more elastic. Previous studies have attempted to test the relationship between bacterial adhesion and surface stiffness. Using poly(ethylene glycol) dimethacrylate (PEGDMA) and agar hydrogels as substrate surfaces and E. coli and S. aureus as model bacteria, Kolewe et al. (2015) found that bacterial adhesion increases with increasing material stiffness regardless of hydrogel chemistry or adhesion mechanism. In fact, studies that use hydrogels, either poly-(ethylene glycol) hydrogels (Kolewe et al., 2015) or agarose hydrogels (Guegan et al., 2014), all demonstrated a positive correlation between adhesion and stiffness. However, studies using polydimethylsiloxane (PDMS) mostly showed a negative correlation between stiffness and adhesion (Song and Ren, 2014; Song F. et al., 2017; Song et al., 2018; Straub et al., 2019), while one study showed an opposite trend (Peng et al., 2019). In Song and Ren’s study, they tested PDMS surfaces that had a range of Young’s modulus between 0.1 and 2.6 MPa, and found that in addition to bacterial adhesion, bacterial cell size is also negatively correlated with stiffness regardless of surface chemistry, roughness, and electrostatic force (Song and Ren, 2014). Interesting results reported by Siddiqui et al. (2019) showed that the retention rate and adhesion strength were higher on softer surfaces when exposed to external shear stress although similar initial bacterial adhesion rates on PDMS were observed. This suggests stiffness-specific interactions between bacteria and PDMS that require further exploration.
Some studies compared the effect of surface stiffness on Gram-positive and -negative bacteria, which exhibited contrasting conclusions. A positive correlation between adhesion and stiffness was observed for Gram-positive S. epidermidis and Gram-negative E. coli on PEM films prepared from PAH and PAA (Lichter et al., 2008). On the other hand, a negative correlation is observed for Gram-negative E. coli on the stiffer photo-cross-linked PEM made from poly(L-lysine) (PLL), a hyaluronan derivative modified with photoreactive vinylbenzyl groups, and softer non-cross-linked PEM films. In contrast, no correlation was observed for Gram-positive Lactococcus lactis on the same material (Saha et al., 2013). Such contrast is likely due to the different range of elastic modulus selected for the material. Lichter’s group chose a range of 1 to 100 MPa, while Saha’s group tested using the surface with an elastic modulus of 30 to 150 kPa. However, both studies attributed the mechanoselectivity of bacteria to their surface appendages like flagella and fimbriae (pili), which are lacking from the non-stiffness-responsive L. Lactis. In addition, Saha et al. (2013) also proposed that the thick and rigid peptidoglycan structure surrounding Gram-positive L. lactis may reduce its mechanoselectivity compared to Gram-negative E. coli that has a thinner and softer peptidoglycan layer.
While many studies have been reported, investigations on the underlying mechanism in this topic are not yet sufficient. Song F. et al. (2017) and Song et al. (2018) determined expressed genes that are involved in the bacterial response to material stiffness during adhesion and biofilm formation of E. coli and P. aeruginosa. Furthermore, they found that high levels of intracellular cyclic demerit guanosine monophosphate (c-di-GMP), a key regulator of biofilm formation, is expressed when P. aeruginosa is bound to softer surfaces (Song et al., 2018). Peng et al. (2019) also observed increased levels of c-di-GMP that reduce bacteria motility and enhance biofilm development when E. coli and Pseudomonas sp. adhered on the PDMS surface with higher stiffness. Another study showed that surface priming of collagen and fibronectin weakens bacterial adhesion significantly on both soft and hard PDMS (Siddiqui et al., 2019). Peng et al. (2019) had taken a different approach to understanding whether bacterial adhesion is affected by the inherent surface chemical properties of PDMS or affected by the material stiffness. PDMS surfaces of varying stiffness were coated with cross-linked PDMS-like polymer film to establish the same surface chemistry. They observed similar adhesion counts across the varying stiffnesses for the coated surfaces. In contrast, the uncoated surfaces showed increased adhesion of E. coli, its fimbriae mutants, P. aeruginosa, and S. epidermidis. These results highlighted how the presence of free polymer chains of uncoated PDMS rather than the material stiffness promotes bacterial adhesion. This was the first study suggesting that the bacterial adhesion to viscoelastic surfaces could be attributed to the available PDMS polymer chain ends by interfacial adhesion (Pan et al., 2020). Studies regarding surface stiffness and its effects on bacterial adhesion and biofilm formation are summarized in Table 5.
TABLE 5.
Influence of surface stiffness on bacterial adhesion.
| Microorganism | Surface material | Young’s Modulus | Influence on adhesion/biofilm growth | References |
| Bacillus and Pseudoalteromonas | Agarose hydrogels | 6.6 and 110 kPa | Reduced bacterial adhesion on softer hydrogels | Guegan et al., 2014 |
| E. coli and S. aureus | Poly(ethylene glycol) dimethacrylate (PEGDMA) and agar hydrogels | Soft: 44.05 – 308.5 kPa. Intermediate: 1495 – 2877 kPa. Stiff: 5152 – 6489 kPa. | Reduced bacterial adhesion on softer hydrogels | Kolewe et al., 2015 |
| S. aureus | poly-N-isopropylmethacrylamide based microgel coatings | Soft: 21 ± 8 kPa. Intermediate: 117 ± 20 kPa. Stiff: 346 ± 125 kPa. | Reduced bacterial adhesion on softer microgel with lower cross-linking density | Keskin et al., 2019 |
| E. coli and Pseudomonas sp. | PDMS | 3.4–278.1 MPa | Reduced bacterial adhesion and levels of c-di-GMP when bound to the softer PDMS | Peng et al., 2019 |
| E. coli and L. lactis | Photo-cross-linked polyelectrolyte films | 30–150 kPa | Increased E. coli adhesion on softer films, no effect for L. lactis adhesion | Saha et al., 2013 |
| S. aureus | Polyacrylamide (PAAm) and poly(ethylene glycol) dimethacrylate (PEGDMA) | 0.017–0.654 kPa | Increased bacterial adhesion on softer hydrogels | Wang et al., 2016 |
| E. coli | PDMS | 0.1–2.6 MPa | Increased bacterial adhesion on softer PDMS | Song F. et al., 2017 |
| E. coli and S. aureus | Poly(ethylene glycol) (PEG) hydrogels | Soft: 20 kPa. Intermediate: 300 kPa, Stiff: 1,000 kPa. | Increased bacterial adhesion and biofilm formation on softer surface | Kolewe et al., 2018 |
| P. aeruginosa | PDMS | 0.1–2.6 MPa | Increased bacterial adhesion and levels of c-di-GMP when bound to the softer PDMS | Song et al., 2018 |
| P. aeruginosa E. coli | PDMS | 4.52 ± 0.09–0.06 ± 0.001 MPa | Increased bacterial adhesion on softer PDMS | Straub et al., 2019 |
| E. coli, P. aeruginosa, and S. epidermidis | PDMS | 64.2–2326.8 kPa | Increased bacterial adhesion on softer PDMS | Pan et al., 2020 |
| E. coli | PDMS | 0.26 ± 0.01 and 124 ± 36 kPa | Similar initial levels of bacterial adhesion on soft and stiff surfaces but increased bacterial adhesion strength on softer surface | Siddiqui et al., 2019 |
As shown, most previous studies did not account for other surface properties (e.g., physicochemical properties) of the tested substrates when attempting to examine the role of material stiffness on bacterial adhesion. However, the density of polymer cross-linking, material hydrophobicity, material viscosity and topography of surface with the same stiffness can also be responsible for the differences in bacterial adhesion. Thus, further research regarding the underlying mechanism of bacterial sensing on the materials’ stiffness while considering intrinsic material properties are required.
Complex Surface Properties
Examining combinations of surface properties may give more significant insights into the mechanics of bacterial adhesion and biofilm formation as surface parameters are intrinsically interdependent. While the dominance of one factor over others is certainly possible, particularly for specifically engineered surfaces, it is often necessary to evaluate several surface parameters simultaneously to validate existing hypotheses.
Surface wettability and roughness/topography are the representative surface properties that correlate with each other. Generally, when the roughness of a surface increases on a hydrophobic surface, small air pockets may become entrapped within the pores and grooves of the surface (as illustrated in Figure 4). Liquids resting on this interface are not able to penetrate the grooves of the surface and are easily removed. This phenomenon, known as the Cassie-Baxter state, is the basis for superhydrophobic surfaces driven by combined surface roughness and wettability (Giacomello et al., 2012). For example, by applying femtosecond laser ablation to generate similar hierarchical structural topography on titanium surfaces, superhydrophobic surfaces (Contact angle; CA: 166 ± 4°) were created to mimic the features of the lotus leaf Nelumbo nucifera (Fadeeva et al., 2011). On initial contact with water, about 50% of the superhydrophobic structured titanium surface area was covered with air pockets trapped in the micro- and nanostructures on the surface. In turn, P. aeruginosa was unable to colonize on the superhydrophobic surface. In contrast, S. aureus successfully colonized on both the polished, hydrophilic surface (CA: 73 ± 3°) and the superhydrophobic surface with more success (Fadeeva et al., 2011). These contradicting results between species in terms of their ability to colonize on superhydrophobic surfaces might be attributed to the physical properties of the bacterial species; S. aureus is a spherical shape, which may require a smaller surface area to bind compared to a rod-shaped bacterium such as P. aeruginosa. This indicates that certain species may overcome the antiadhesive properties of superhydrophobic surfaces stemming from a rough surface. In another study, polyvinyl chloride (PVC) was treated with varying concentrations of ethanol and methanol to create surfaces with increasing roughness and hydrophobicity (Loo et al., 2012). The rougher modified PVC surfaces containing micro- and nanoscale structures with measured roughness of 1,250 nm enhanced the hydrophobicity of modified PVCs. The colonization of P. aeruginosa was retarded on the superhydrophobic surfaces (150 ± 3°), while colonization was promoted on the untreated, smooth PVC surface (80 ± 1°).
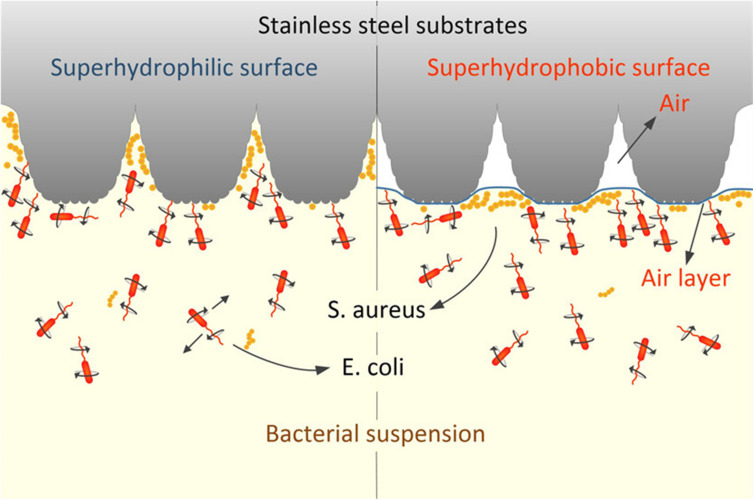
FIGURE 4.
Schematic illustration of bacterial adhesion in response to the superhydrophobic surface. The air entrapment phenomena happened in the presence of increased water contact angle (hydrophobicity), and roughness of the substratum. Reprinted with permission from Pan et al. (2019) Picosecond Laser-Textured Stainless Steel Superhydrophobic Surface with an Antibacterial Adhesion Property. Langmuir 35, 11,414–11,421. Copyright © 2019, American Chemical Society.
As discussed earlier, surface stiffness and topography are the factors interdependently affecting bacterial adhesion. Interestingly, Arias et al. (2020) found that E. coli tend to adhere on PDMS surfaces with variable stiffness in a different pattern when surface topography was introduced to the surface. The wrinkled topography on PDMS surfaces significantly reduced both E. coli adhesion and the overall biofilm biomass, regardless of material stiffness. This result suggests that topographical patterns of surfaces may render surface stiffness negligible regarding bacterial adhesion and biofilm accumulation (Arias et al., 2020). Another example of the interdependence of surface parameters that affect bacterial adhesion can be found in a recent study by Yuan et al. (2017). They demonstrated the preferential binding of E. coli to moderately hydrophobic polystyrene surfaces by accounting for surface energy, surface roughness, surface charge, and entrapped air. In particular, superhydrophilic substrates with large negative surface charge limited bacterial binding. Additionally, the solid area fraction for superhydrophobic surfaces was reduced due to the entrapped air, which in turn lowered bacterial adhesion. This increased their self-cleaning potential and made the weakly attached bacteria susceptible to removal by washing.
In summary, it is important to comprehensively consider several surface parameters to enhance the antifouling activity of surfaces. Often these multiple parameters are not considered comprehensively, thereby resulting in contradicted bacterial binding results. Thus, the effect of merging multiple surface parameters on bacterial-surface interaction requires further investigation.
Environmental Factors
Fluid Dynamics
Hydrodynamic conditions can interfere or enhance bacterial sensing on various surface properties, thereby affecting biofilm architecture, composition, and mechanical strength. Indeed, many infectious biofilms in the human body are formed under dynamic conditions. In the oral cavity, for example, dental plaques are subject to salivary and gingival crevicular fluid flow (Faran Ali and Tanwir, 2012). Furthermore, fluid flow is pertinent in catheter microenvironments, thereby affecting biofilm formation (Weaver et al., 2012; Alves et al., 2020). While hydrodynamic conditions can significantly affect bacterial behavior on surfaces and subsequent biofilm formation, the influence of dynamic conditions on bacterial adhesion and biofilm formation has been often neglected.
According to previous literature, biofilm growth can be facilitated under fluid conditions (Thomen et al., 2017; Liu et al., 2019). A study done by Hou et al. (2018) showed that shear flow enhances biofilm formation by increasing the EPS production and strength of the EPS-matrix in S. aureus. Their study confirmed the previous speculation on pressure-induced EPS production. It has been suggested that the resulting EPS-matrix serves a protective role in allowing biofilm to recover from mechanical challenges induced by pressure and flow, resulting in more resistant and compressible biofilms (Limoli et al., 2015). Biofilms cultured under shear stress also increased the expression of molecules involved in signal transduction and improved oxygenation, favoring bacterial growth. For instance, Rodesney et al. (2017) found that applied shear stress to biofilm resulted in increased levels of c-di-GMP signal in P. aeruginosa, which then promote biofilm development. It has been proposed that fluid shear creates a positive feedback loop, initiating biofilm formation by stimulating EPS production and providing nutrients for growth (Zhu et al., 2020).
Another study regarding the dynamic conditions and the influence of species composition using a parallel plate flow chamber showed that the multi-species biofilms, composed of Streptococcus oralis and Actinomyces naeslundii, became ten times more resistant to compressive forces than single-species biofilms (Paramonova et al., 2009). These findings were confirmed by a study done by Dunsmore et al. (2002). They experimented on the Desulfovibrio species and found differences in the viscoelastic properties of biofilms under varying fluid velocities. High-flow biofilm clusters exhibited higher elastic modulus with a greater propensity to return to original shape after the exertion of strain, while low-flow biofilm was less rigid with irreversible damage to the structure after strain (Dunsmore et al., 2002). Overall, biofilm grown in dynamic conditions tends to be more elastic, more resistant, denser in matrix proteins and EPS as reported elsewhere (Lemos et al., 2015; Araújo Paula et al., 2016).
As indicated, the human mouth is a good example to study the influence of dynamic flow conditions on bacterial adhesion and biofilm formation as there is continuous salivary flow. Various artificial mouth models containing a continuous flow of saliva have been applied while mimicking the temperature, pH, and sucrose supply in the oral cavity. In a recent study done by Santos et al. (2019), they compared biofilm formation in static and semi-dynamic conditions by utilizing McBain saliva. They found that the biofilm viability was significantly lower for the static model than the semi-dynamic counterpart (Santos et al., 2019). Many previous studies confirm the findings that dynamic flow can increase the thickness of biofilm growth, (Paramonova et al., 2009), biofilm density (Lewandowski and Beyenal, 2003), biofilm elasticity, resistance, tolerance to antibiotics (Kostenko et al., 2010), and biofilm rigidity (Stoodley et al., 2001).
Despite previous findings on how dynamic conditions favors biofilm growth and viability, many studies reported contradictory findings (Fink et al., 2015; Hizal et al., 2017; Song W.S. et al., 2017; Oder et al., 2018). A study done by Stepanovic et al. (2001) showed S. epidermidis and S. aureus, tested under dynamic conditions, resulted in a lower degree of biofilm formation. Another study done by Song W.S. et al. (2017) showed that periodontal biofilms composed of Fusobacterium nucleatum and P. gingivalis formed loose biofilms when cultured under dynamic fluid, thereby exhibiting less bacterial count. It suggested that higher than a certain degree of hydrodynamic conditions may cause recirculating eddies that can disrupt cohesive bonds between biofilms and surfaces (Picioreanu et al., 2000). In contrast, other studies have found no significant differences in biofilm production by oral bacterium A. naeslundii (Paramonova et al., 2009) or Actinomyces oris (Ding et al., 2010) under shear stress. Differences in those results could be attributed to the type of bacterial species, type of flow (laminar or turbulent), the magnitude of shear stress applied, and surface properties.
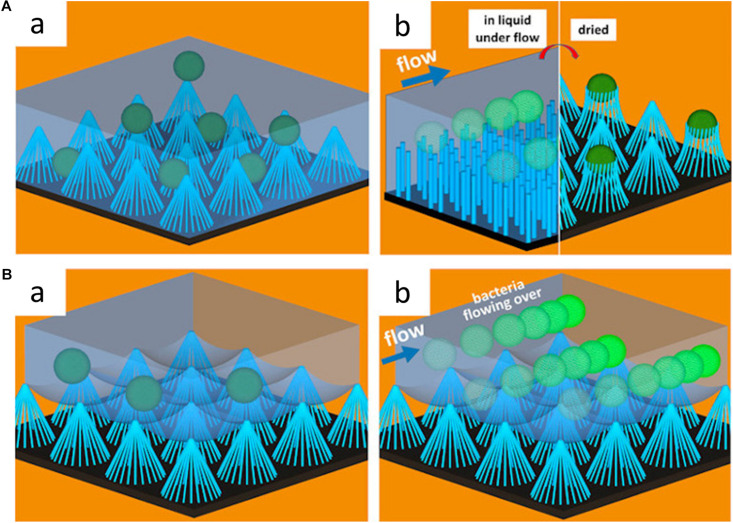
These hydrodynamic conditions can also significantly affect the anti-adhesion properties of a surface. Under flow conditions, for instance, the anti-adhesion properties of superhydrophobic surfaces can be more pronounced. Hizal et al. (2017) compared the number of colony-forming units adhering to sample surfaces with different surface topography and surface hydrophobicity under both static and flow conditions. They found that hydrophobic nanopillared surfaces exhibited improved reduction of S. aureus and E. coli adhesion under flow conditions compared to static conditions (Hizal et al., 2017). The high fraction of trapped air in the hydrophobic layer minimized the contact of bacterial suspension to the surface. The air layer entrapped at the interface between bacteria and nanopillar structure helps floating bacteria at the surface to be easily driven off by flow (see Figure 5). The decreased contact areas resulted in the enhancement of the hydrodynamic detachment force and mitigated bacteria attachment (Hizal et al., 2017). Studies regarding fluid dynamics and its effects on bacterial adhesion and biofilm formation are summarized in Table 6.
FIGURE 5.
Schematic illustration of the effects of surface wettability on bacterial adhesion under dynamic conditions. (A) Schematic illustration of S. aureus adhesion on hydrophilic nanopillar surfaces under static and fluid conditions. In the static condition, bacteria adhere to both the nanopillar tips and troughs, while bacteria adhere to only the nanopillar tips under fluid conditions. Adapted from Hizal et al. (2017). (B) Schematic illustration of S. aureus adhesion on hydrophobic nanopillar surfaces under static and fluid conditions. In the static condition, the bacteria float over the entrapped air layer, and bacteria are swept away by fluid flow. Reprinted with permission from Hizal et al. (2017) Nanoengineered Superhydrophobic Surfaces of Aluminum with Extremely Low Bacterial Adhesivity. ACS Appl Mater Interfaces 9, 12,118–12,129. Copyright© 2017, American Chemical Society.
TABLE 6.
Influence of fluid dynamics biofilm growth and characteristic.
| Microorganism | Flow conditions tested | Influence on biofilm growth and characteristic | References |
| Bacillus cereus | Shear stress | Biofilm density increased with an increase in shear stress, while the biofilm thickness decreased | Lemos et al., 2015 |
| P. aeruginosa | Shear stress | Shear acts as a cue for surface adhesion and activates c-di-GMP signaling, increasing the strength of bacterial adhesion | Rodesney et al., 2017 |
| E. coli | Shear stress | Low shear stress resulted in uniform biofilm growth while high stress resulted in biofilm growth from the edge toward the center of the channel | Thomen et al., 2017 |
| S. aureus | Shear stress | Increased shear stress increases EPS production, resulting in higher biofilm density | Hou et al., 2018 |
| Thalassospira strain | Shear stress | Flow velocity of 1.66 mm/s is the optimal rate for fast and strong biofilm adhesion, while too high shear stress prevented biofilm formation and removed adhered biofilms | Liu et al., 2019 |
| Actinobacillus succinogenes | Shear stress | Enhanced bacterial metabolic activities and biofilm viability under high shear condition | Mokwatlo et al., 2020 |
| E. coli and S. aureus | Shear stress | Higher shear force was required to reduce bacterial adhesion on the hydrophilic surface compared with the hydrophobic surface | Zhu et al., 2020 |
| E. coli | Laminar and turbulent flow | Turbulent flow significantly reduced bacterial adhesion compared with laminar flow | Fink et al., 2015 |
| P. fluorescens | Linear flow | High flow velocities resulted in thinner biofilms with higher cell densities and contents of the matrix/extracellular polysaccharides | Araújo Paula et al., 2016 |
| E. coli | Laminar and turbulent flow | Laminar flow promotes biofilm growth over a 72 h period, while turbulent flow after 48 h causes reduction in biofilm biomass | Oder et al., 2018 |
| S. aureus and E. coli | Static and dynamic | Greater reduction in bacterial adhesion to hydrophobic surfaces under flow conditions | Hizal et al., 2017 |
| F. nucleatum and P. gingivalis | Static and dynamic | Loosely formed biofilm and reduced bacterial count were observed under the dynamic condition | Song W.S. et al., 2017 |
| Biofilm from human saliva | Static and dynamic | Biofilm viability in the static model was lower compared with the semi-dynamic model | Santos et al., 2019 |
As such, fluid flow dynamics can alter not only the bacterial behaviors on surfaces but also the anti-adhesion activities of surfaces. This important yet often overlooked factor can play an important role in understanding the pathogenesis of bacterially-induced infectious diseases and eradicating them. Further exploration of the influence of hydrodynamics on bacterial colonization on surfaces with various surface properties will contribute to developing novel antifouling strategies. Therefore, the impact of flow dynamics needs to be considered when evaluating the effect of surface properties on bacterial adhesion.
Bacterial Motility
Microorganisms can be broadly divided into motile and non-motile bacteria. Since these two have clear differences in the structural and behavioral patterns, their adhesion to the substrate surface could be greatly varied. Unlike non-motile bacteria, motile bacteria have a chemotaxis transducer, a protein that makes bacteria sensitively detect physical and chemical changes in the surrounding environment, and a flagellum, a surface appendage that confers bacteria active locomotion. Using these characteristics, motile bacteria can actively search, sense, and accumulate toward a favorable environment or move away from unfavorable environments. Therefore, the binding mechanism of the non-motile bacteria to substrate surfaces may heavily rely on gravitational sedimentation, while the motile bacteria with locomotion may exhibit different adhesive reactions to the same substrate surface.
Since the diffusion coefficient of motile bacteria is more than 3 times higher than paralyzed cells, it contributes more to the adhesion rate, increasing the chance to encounter the substrate surface in the same volume of fluid, thereby increasing the number of adherent cells per unit area when favorable. The flagella also act as an anchor to firmly attach cells to the substrate by electrostatic attraction. Gram-positive bacteria contain long glycan chains in their membrane which make the body negatively charged and hydrophilic, so they hardly move when attached to the hydrophobic glass surface. In contrast, motile bacterial cells (mostly Gram-negative bacteria) rarely adhere and rather move freely on the hydrophilic glass surface, indicating that hydrophobic surfaces may accommodate motile bacteria more efficiently as reported elsewhere (Jindai et al., 2020). In addition, attachment of E. coli with or without flagellum was compared on PDMS substrate with an array of hexagonal features (designated as “HEX” pattern). The filC mutant (without flagellum) showed a reduced adhesion to the HEX pattern on PDMS (vs. wild type E. coli with flagellum), suggesting that the flagellum of E. coli played a crucial role in the attachment process (Friedlander et al., 2013).
As discussed earlier, electrostatic interaction and the tangential shear force between the cell and the surface are important factors affecting bacterial response to surfaces. It has been suggested that the effect of ionic strength and flow rates on bacterial adhesion could be varied between motile and non-motile bacteria. McClaine and Ford (2002) showed that the rate of adhesion of E. coli motility deficient mutant strain was 4–10-fold lower than that of E. coli wild type strain in a low ionic strength medium. They also investigated the effect of flagella rotation direction on the surface adhesion. In E. coli, counterclockwise flagellar rotation causes runs, and clockwise flagellar rotation causes tumbles (Manson, 2010). Interestingly, bacteria with counterclockwise rotating flagella showed a similar adhesion behavior to E. coli wild type strains, while “Tumble” cells, that rotate only clockwise, adhered at a much lower rate (McClaine and Ford, 2002). This suggests that the rotation of the flagella in the clockwise and counterclockwise directions has an important effect on cell adhesion. Also, de Kerchove and Elimelech (2008) reported that the initial attachment rate of non-motile bacteria decreased with reduced flow rate, rather than ionic strength. In contrast, the binding of motile bacteria increased significantly with increased ionic strength and a reduced flow rate. Such enhanced attachment rate of motile bacteria with increasing ionic strength indicates that electrolytic concentration significantly affects the bacterial motility as the kinetic mechanism of the flagella motor takes place in a complex electrostatic interaction. Therefore, it is necessary to carefully consider the ionic strength and flow rate of the buffer depending on the type of bacteria and its motility when interpreting the effect of surface properties on bacterial adhesion.
As shown in many studies, Gram-negative (mostly motile) bacteria showed higher cell adhesion and growth rates in all stiffness than Gram-positive (non-motile) bacteria. However, some studies showed that bacterial adhesion to surface stiffness tends to be quantitatively similar regardless of bacterial motility. It has been reported that the colony density of non-motile S. epidermidis and motile E. coli increased linearly as the substrate stiffness increased over the range of 1 MPa < E < 100 MPa (Lichter et al., 2008). Bakker et al. (2003) also reported that marine bacteria adhere more to polyurethane (PU) substrates with higher stiffness under certain flow chamber conditions. In addition, Saha et al. (2013) demonstrated that both non-motile L. lactis and motile E. coli had a surface coverage of about 17–31% higher on a harder cross-linking film (150 kPa) compared to a softer non-crossing (30 kPa). However, non-motile L. lactis was found to grow slowly on both films regardless of stiffness, while motile E. coli showed faster growth on soft films. Similarly, Kolewe et al. (2015) showed that the number of adhered cells increased when the stiffness of the hydrogel (PEGDMA and Agar) increased in both motile E. coli and non-motile S. aureus regardless of incubation time in the range of 44.05 kPa < E < 6489 kPa. In another study, Song F. et al. (2017) compared the adhesion of motile E. coli and P. aeruginosa on soft and hard PDMS surfaces. They reported that E. coli exhibited higher motility on the surface of hard PDMS (2.6 MPa) than soft PDMS (0.1 MPa), and the flagellar motor protein motB played an important role in response to PDMS stiffness during initial adhesion (Song F. et al., 2017).
As above, the behavior of motile bacteria contrasts with non-motile bacteria. Bacterial surface appendages such as flagella can play an important role in the adhesion by inducing a more dynamic response of motile bacteria to surface properties than non-motile bacteria. Once bacteria adhere to surfaces, motile bacteria can settle biofilms faster than non-motile bacteria by attracting free bacteria through chemotaxis and quorum sensing (Gutman et al., 2013). Most of the natural and manmade systems are an open ecosystem composed of various microorganisms with different sizes, shapes, cell types, and motility. Even on the same surface, it is possible to induce a contradictory reaction to the initial deposition rate depending on the cell’s motility. Therefore, it is necessary to carefully design the characteristics of the substrate according to the characteristics of the target majority of bacteria.
Implication for Developing New Anti-Fouling Strategies
The antibacterial surfaces can be created by impregnating bactericidal elements in their structure or coating the surface with a durable antimicrobial material. Although these antibacterial surfaces can prevent the formation of biofilm mainly by killing surrounding bacteria, the continuous release of the antibacterial elements to the surrounding environment may lead to bacterial resistance to these specific elements. Also, it may affect the long-term efficiency of these surfaces (Nino-Martinez et al., 2019). To overcome this issue, alternative approaches have been proceeded; developing a surface that (i) can hinder bacterial adhesion at its early stages that eventually alter biofilm formation (Zhang et al., 2018; Chi et al., 2019), (ii) physically disrupt membrane of adhered bacteria (Reed et al., 2019; Wandiyanto et al., 2019), or (iii) exhibits multifunctional antiadhesion/antibacterial properties (Balaure et al., 2016; Borcherding et al., 2019).
Laser-based techniques used to modify surface topography can be a powerful technique for controlling bacterial colonization. As an alternative approach to antibacterial coating, Laser-induced periodic surface structures (LIPSS) was introduced to inhibit bacterial attachment by utilizing ultrashort laser pulses to create nanostructures on most surface materials. One study done using laser structured steel samples were subjected to microbial adhesion tests in a dynamic flow chamber with E. coli and S. aureus. The result showed an anti-adhesion effect for E. coli but not for S. aureus. Differences in adhesion were attributed to the geometry and colonization characteristics of S. aureus (Epperlein et al., 2017). Another study also used femtosecond laser irradiation to produce nano-ripples. They exhibited reduced adhesion of E. coli on nano-ripples and micro-grooves. The deep grooves of the nanostructure were conducive to the rupture of bacteria cells (Luo et al., 2020). To confirm these studies, Siddiquie et al. (2020) used both LIPSS and multiscale structures (MS) to create microtopographies on PDMS and PU elastomers to investigate bacterial retention. They found both LIPSS and MS topographs reduced E. coli adhesion by >89% (Siddiquie et al., 2020). Many recent studies have also confirmed the bactericidal actions of laser-induced topography (Kudryashov et al., 2018; Jalil et al., 2020; Ma et al., 2020).
The combination of mechano- and chemo- bactericidal properties can be a powerful and effective tool to develop hybrid anti-biofilm solutions. Coating of Si nano-ripples with Se, TeO2, Sb2O3, and Ag NPs was able to damage the bacterial DNA by producing reactive oxygen species. The combined chemical toxicity of nanoparticles and mechanical damage by nanoscale structures synergistically increased the antibacterial properties of the surface and effectively reduced S. aureus biofilms formation (Saraeva et al., 2020). Utilizing the knowledge of antibacterial nanoparticles and topography, Qian et al. (2017) prepared a multilayer antibacterial film with hierarchical nanostructures and superhydrophobicity using polydopamine (PDA) and silver nanoparticles. This Ag-deposited surface (SS) film exhibited bifunctional antiadhesion and antibacterial activities against E. coli and S. aureus, which may allow for lower cytotoxicity compared to the surface with only antibacterial components (e.g., silver nanoparticles). They also quantified the biofilm thickness and coverage area; the addition of Ag nanoparticles alone failed to control biofilm thickness after 3 days while the SS successfully prevented the formation of biofilm. The results of this study highlight the synergistic effect of Ag nanoparticles and superhydrophobic surfaces (Qian et al., 2017). Similarly, another study paired bactericidal copper ions with superhydrophobic surfaces. The hybrid approach exhibited both anti-adhesive and bactericidal properties, suggesting short and long-term antibacterial protection against the Synechococcus species. The superhydrophobic and Cu-enriched surfaces exhibited lower bacterial adhesion and higher antibacterial properties than either parameter alone (Ellinas et al., 2017). Using inorganic nanoparticles in combination with antibiotics, a 10,000-fold reduction of bacterial cells was achieved against S. aureus and P. aeruginosa biofilms. This was created using matrix-assisted pulsed laser evaporation to create a thin film consisting of a magnetite/salicylic acid/silica shell with an antibiotic coating (Mihaiescu et al., 2013).
While nanostructured surface-based approaches could open new perspectives for developing antibiofilm surface materials, nanoscience-based surface modifications are still at an early stage of research. Several nanoparticle surface coating solutions have been uncovered but very few hybrid approaches have been developed. Thus, more investigation is needed for these to become promising candidates for the development of novel materials for biofilm inhibition and the exploitation of nanoscience-based technologies.
Concluding Remarks
Bacterial adhesion and biofilm formation is a complex process controlled by the interplay between physicochemical, mechanical, topographical surface properties, bacterial characteristics, and environmental conditions. This review provides a comprehensive briefing and insight into the characterization of parameters that affect bacterial adhesion. Much of the research done to date did not consider the implications of multiple surface parameters, bacterial motility, or surrounding hydrodynamic conditions to the bacterial sensing and binding behavior on surfaces. Importantly, in reality, bare surfaces are covered by conditioning films of organic and inorganic matters before bacteria bind, thereby also affecting bacterial binding behaviors significantly. Therefore, rather than focusing on a single surface parameter and its effect on adhesion, scientific efforts assessing the impact of multiple surface parameters on bacterial adhesion are essential to further advance knowledge. Furthermore, the temperature may play an important role as an environmental factor governing bacterial adhesion; it induces not only the complex biological functions (e.g., gene expression, piliation, cell wall hydrophobicity) but also alters the properties of the substrate surface (e.g., wettability, surface charge density). Therefore, more research reflecting the corresponding environmental conditions, bacterial types, and surface properties are needed to develop efficient and reliable antibiofilm solutions. Nanopatterning of surfaces and their hybrid approach with bactericidal compounds have great prospects to provide more advanced solutions for biofilm-related fouling in medical or industrial settings.
Author Contributions
GH planned and constructed the study. SZ, MB, AD, H-EK, LH, and JH contributed to data acquisition and interpretation. SZ, MB, AD, H-EK, LH, JH, and GH drafted the manuscript and critically revised the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported in part by the National Institutes for Dental and Craniofacial Research (NIDCR) grants DE027970 (GH).
References
- Abdalla M. M., Ali I. A. A., Khan K., Mattheos N., Murbay S., Matinlinna J. P., et al. (2020). The influence of surface roughening and polishing on microbial biofilm development on different ceramic materials. J. Prosthodont. (in press). [DOI] [PubMed] [Google Scholar]
- Alves P., Gomes L. C., Vorobii M., Rodriguez-Emmenegger C., Mergulhao F. J. (2020). The potential advantages of using a poly(HPMA) brush in urinary catheters: effects on biofilm cells and architecture. Colloids Surf. B Biointerf. 191:110976. 10.1016/j.colsurfb.2020.110976 [DOI] [PubMed] [Google Scholar]
- An Y. H., Friedman R. J. (1998). Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 43 338–348. [DOI] [PubMed] [Google Scholar]
- Araújo Paula A., Malheiro J., Machado I., Mergulhão F., Melo L., Simões M. (2016). Influence of flow velocity on the characteristics of pseudomonas Fluorescens Biofilms. J. Environ. Eng. 142:04016031. 10.1061/(asce)ee.1943-7870.0001068 29515898 [DOI] [Google Scholar]
- Arias S. L., Devorkin J., Civantos A., Allain J. P. (2020). Escherichia coli adhesion and biofilm formation on Polydimethylsiloxane are independent of substrate stiffness. Langmuir. 37 16–25. 10.1021/acs.langmuir.0c00130 [DOI] [PubMed] [Google Scholar]
- Bakker D. P., Huijs F. M., de Vries J., Klijnstra J. W., Busscher H. J., van der Mei H. C. (2003). Bacterial deposition to fluoridated and non-fluoridated polyurethane coatings with different elastic modulus and surface tension in a parallel plate and a stagnation point flow chamber. Colloids Surf. B Biointerf. 32 179–190. 10.1016/s0927-7765(03)00159-0 [DOI] [Google Scholar]
- Balaure P. C., Popa R. A., Grumezescu A. M., Voicu G., Radulescu M., Mogoanta L., et al. (2016). Biocompatible hybrid silica nanobiocomposites for the efficient delivery of anti-Staphylococcal drugs. Int. J. Pharm. 510 532–542. 10.1016/j.ijpharm.2016.03.037 [DOI] [PubMed] [Google Scholar]
- Bandara C. D., Singh S., Afara I. O., Wolff A., Tesfamichael T., Ostrikov K., et al. (2017). Bactericidal effects of natural nanotopography of dragonfly wing on Escherichia coli. ACS Appl. Mater. Interf. 9 6746–6760. 10.1021/acsami.6b13666 [DOI] [PubMed] [Google Scholar]
- Barthlott W., Neinhuis C. (1997). Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202 1–8. 10.1007/s004250050096 [DOI] [Google Scholar]
- Bhushan B., Jung Y. C., Niemietz A., Koch K. (2009). Lotus-like biomimetic hierarchical structures developed by the self-assembly of tubular plant waxes. Langmuir 25 1659–1666. 10.1021/la802491k [DOI] [PubMed] [Google Scholar]
- Bilgili D., Dundar A., Barutcugil C., Tayfun D., Ozyurt O. K. (2020). Surface properties and bacterial adhesion of bulk-fill composite resins. J. Dent. 95:103317. 10.1016/j.jdent.2020.103317 [DOI] [PubMed] [Google Scholar]
- Bollen C. M., Papaioanno W., Van Eldere J., Schepers E., Quirynen M., van Steenberghe D. (1996). The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin. Oral Implants Res. 7 201–211. 10.1034/j.1600-0501.1996.070302.x [DOI] [PubMed] [Google Scholar]
- Borcherding K., Marx D., Gatjen L., Bormann N., Wildemann B., Specht U., et al. (2019). Burst release of antibiotics combined with long-term release of silver targeting implant-associated infections: design, characterization and in vitro evaluation of novel implant hybrid surface. Materials 12:3838. 10.3390/ma12233838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R., van der Mei H. C., Busscher H. J. (1999). Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol. Rev. 23 179–230. 10.1016/s0168-6445(99)00004-2 [DOI] [PubMed] [Google Scholar]
- Bryers J. D. (2008). Medical biofilms. Biotechnol. Bioeng. 100 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Jana S., Bowen L., Tan X., Liu H., Rostami N., et al. (2019). Hierarchical rose petal surfaces delay the early-stage bacterial Biofilm growth. Langmuir 35 14670–14680. 10.1021/acs.langmuir.9b02367 [DOI] [PubMed] [Google Scholar]
- Carniello V., Peterson B. W., van der Mei H. C., Busscher H. J. (2018). Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interf. Sci. 261 1–14. 10.1016/j.cis.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Chang Y. R., Weeks E. R., Ducker W. A. (2018). Surface topography hinders bacterial surface motility. ACS Appl. Mater. Interf. 10 9225–9234. 10.1021/acsami.7b16715 [DOI] [PubMed] [Google Scholar]
- Chen C., Petterson T., Illergard J., Ek M., Wagberg L. (2019). Influence of cellulose charge on bacteria adhesion and viability to PVAm/CNF/PVAm-modified cellulose model surfaces. Biomacromolecules 20 2075–2083. 10.1021/acs.biomac.9b00297 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Feng G., Moraru C. I. (2019). Micro- and nanotopography sensitive bacterial attachment mechanisms: a review. Front. Microbiol. 10:191. 10.3389/fmicb.2019.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.-T., Rodak D. E. (2005). Is the lotus leaf superhydrophobic? Appl. Phys. Lett. 86:144101. 10.1063/1.1895487 [DOI] [Google Scholar]
- Chi M., Qi M., Lan A., Wang P., Weir M. D., Melo M. A., et al. (2019). Novel bioactive and therapeutic dental polymeric materials to inhibit periodontal pathogens and Biofilms. Int. J. Mol. Sci. 20:278. 10.3390/ijms20020278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien H. W., Chen X. Y., Tsai W. P., Lee M. (2020). Inhibition of biofilm formation by rough shark skin-patterned surfaces. Colloids Surf. B Biointerf. 186:110738. 10.1016/j.colsurfb.2019.110738 [DOI] [PubMed] [Google Scholar]
- de Kerchove A. J., Elimelech M. (2008). Bacterial swimming motility enhances cell deposition and surface coverage. Environ. Sci. Technol. 42 4371–4377. 10.1021/es703028u [DOI] [PubMed] [Google Scholar]
- Ding A. M., Palmer R. J., Jr., Cisar J. O., Kolenbrander P. E. (2010). Shear-enhanced oral microbial adhesion. Appl. Environ. Microbiol. 76 1294–1297. 10.1128/aem.02083-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C., Wang C., Zhang T., Yi X., Liang J., Wang H. (2020). Reduced bacterial adhesion on zirconium-based bulk metallic glasses by Femtosecond laser Nanostructuring. Proc. Inst. Mech. Eng. H 234 387–397. 10.1177/0954411919898011 [DOI] [PubMed] [Google Scholar]
- Dunsmore B. C., Jacobsen A., Hall-Stoodley L., Bass C. J., Lappin-Scott H. M., Stoodley P. (2002). The influence of fluid shear on the structure and material properties of sulphate-reducing bacterial biofilms. J. Ind. Microbiol. Biotechnol. 29 347–353. 10.1038/sj.jim.7000302 [DOI] [PubMed] [Google Scholar]
- El-Chami M. F., Mayotte J., Bonner M., Holbrook R., Stromberg K., Sohail M. R. (2020). Reduced bacterial adhesion with parylene coating: potential implications for Micra transcatheter pacemakers. J. Cardiovasc. Electrophysiol. 31 712–717. 10.1111/jce.14362 [DOI] [PubMed] [Google Scholar]
- Ellinas K., Kefallinou D., Stamatakis K., Gogolides E., Tserepi A. (2017). Is there a threshold in the antibacterial action of superhydrophobic surfaces? ACS Appl. Mater. Interf. 9 39781–39789. 10.1021/acsami.7b11402 [DOI] [PubMed] [Google Scholar]
- Epperlein N., Menzel F., Schwibbert K., Koter R., Bonse J., Sameith J., et al. (2017). Influence of femtosecond laser produced nanostructures on biofilm growth on steel. Appl. Surf. Sci. 418 420–424. 10.1016/j.apsusc.2017.02.174 [DOI] [Google Scholar]
- Epstein A. K., Wong T. S., Belisle R. A., Boggs E. M., Aizenberg J. (2012). Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. U.S.A. 109 13182–13187. 10.1073/pnas.1201973109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeeva E., Truong V. K., Stiesch M., Chichkov B. N., Crawford R. J., Wang J., et al. (2011). Bacterial retention on superhydrophobic titanium surfaces fabricated by Femtosecond laser ablation. Langmuir 27 3012–3019. 10.1021/la104607g [DOI] [PubMed] [Google Scholar]
- Fang Y., Sun G., Wang T., Cong Q., Ren L. (2007). Hydrophobicity mechanism of non-smooth pattern on surface of butterfly wing. Chin. Sci. Bull. 52 711–716. 10.1007/s11434-007-0120-5 [DOI] [Google Scholar]
- Faran Ali S. M., Tanwir F. (2012). Oral microbial habitat a dynamic entity. J. Oral Biol. Craniofac. Res. 2 181–187. 10.1016/j.jobcr.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R., Oder M., Rangus D., Raspor P., Bohinc K. (2015). Microbial adhesion capacity. Influence of shear and temperature stress. Int. J. Environ. Health Res. 25 656–669. 10.1080/09603123.2015.1007840 [DOI] [PubMed] [Google Scholar]
- Flemming H. C., Wingender J., Szewzyk U., Steinberg P., Rice S. A., Kjelleberg S. (2016). Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14 563–575. 10.1038/nrmicro.2016.94 [DOI] [PubMed] [Google Scholar]
- Friedlander R. S., Vlamakis H., Kim P., Khan M., Kolter R., Aizenberg J. (2013). Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. U.S.A. 110 5624–5629. 10.1073/pnas.1219662110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh J., Gai M., Yang Z., He Q. (2014). Influence of polyelectrolyte multilayer coating on the degree and type of biofouling in freshwater environment. J. Nanosci. Nanotechnol. 14 4341–4350. 10.1166/jnn.2014.8226 [DOI] [PubMed] [Google Scholar]
- Giacomello A., Meloni S., Chinappi M., Casciola C. M. (2012). Cassie-baxter and Wenzel states on a nanostructured surface: phase diagram, metastabilities, and transition mechanism by atomistic free energy calculations. Langmuir 28 10764–10772. 10.1021/la3018453 [DOI] [PubMed] [Google Scholar]
- Gottenbos B., Van Der Mei H. C., Busscher H. J., Grijpma D. W., Feijen J. (1999). Initial adhesion and surface growth of Pseudomonas aeruginosa on negatively and positively charged poly(methacrylates). J. Mater. Sci. Mater. Med. 10 853–855. [DOI] [PubMed] [Google Scholar]
- Grudzien J., Jarosz M., Kaminski K., Kobasa M., Wolski K., Koziel M., et al. (2020). Growth of lactic acid bacteria on gold-influence of surface roughness and chemical composition. Nanomaterials 10:2499. 10.3390/nano10122499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guegan C., Garderes J., Le Pennec G., Gaillard F., Fay F., Linossier I., et al. (2014). Alteration of bacterial adhesion induced by the substrate stiffness. Colloids Surf. B Biointerf. 114 193–200. 10.1016/j.colsurfb.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Guo S., Kwek M. Y., Toh Z. Q., Pranantyo D., Kang E. T., Loh X. J., et al. (2018). Tailoring polyelectrolyte architecture to promote cell growth and inhibit bacterial adhesion. ACS Appl. Mater. Interf. 10 7882–7891. 10.1021/acsami.8b00666 [DOI] [PubMed] [Google Scholar]
- Gutman J., Walker S. L., Freger V., Herzberg M. (2013). Bacterial attachment and viscoelasticity: physicochemical and motility effects analyzed using quartz crystal microbalance with dissipation (QCM-D). Environ. Sci. Technol. 47 398–404. 10.1021/es303394w [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L., Costerton J. W., Stoodley P. (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2 95–108. 10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- He J., Bao Y., Li J., Qiu Z., Liu Y., Zhang X. (2019). Nanocomplexes of carboxymethyl chitosan/amorphous calcium phosphate reduce oral bacteria adherence and biofilm formation on human enamel surface. J. Dent. 80 15–22. 10.1016/j.jdent.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Helbig R., Günther D., Friedrichs J., Rößler F., Lasagni A., Werner C. (2016). The impact of structure dimensions on initial bacterial adhesion. Biomater. Sci. 4 1074–1078. 10.1039/c6bm00078a [DOI] [PubMed] [Google Scholar]
- Hizal F., Rungraeng N., Lee J., Jun S., Busscher H. J., van der Mei H. C., et al. (2017). Nanoengineered superhydrophobic surfaces of aluminum with extremely low bacterial adhesivity. ACS Appl. Mater. Interf. 9 12118–12129. 10.1021/acsami.7b01322 [DOI] [PubMed] [Google Scholar]
- Hori K., Matsumoto S. (2010). Bacterial adhesion: from mechanism to control. Biochem. Eng. J. 48 424–434. 10.1016/j.bej.2009.11.014 [DOI] [Google Scholar]
- Hou J., Veeregowda D. H., van de Belt-Gritter B., Busscher H. J., van der Mei H. C. (2018). Extracellular polymeric matrix production and relaxation under fluid shear and mechanical pressure in Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 84 e1516–e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang G., Ahn I. S., Mhin B. J., Kim J. Y. (2012). Adhesion of nano-sized particles to the surface of bacteria: mechanistic study with the extended DLVO theory. Colloids Surf. B Biointerf. 97 138–144. 10.1016/j.colsurfb.2012.04.031 [DOI] [PubMed] [Google Scholar]
- Hwang G., Lee C.-H., Ahn I.-S., Mhin B. J. (2010). Analysis of the adhesion of Pseudomonas putida NCIB 9816-4 to a silica gel as a model soil using extended DLVO theory. J. Hazard. Mater. 179 983–988. 10.1016/j.jhazmat.2010.03.101 [DOI] [PubMed] [Google Scholar]
- Ivanova E. P., Hasan J., Webb H. K., Truong V. K., Watson G. S., Watson J. A., et al. (2012). Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 8 2489–2494. 10.1002/smll.201200528 [DOI] [PubMed] [Google Scholar]
- Jalil S. A., Akram M., Bhat J. A., Hayes J. J., Singh S. C., ElKabbash M., et al. (2020). Creating superhydrophobic and antibacterial surfaces on gold by femtosecond laser pulses. Appl. Surf. Sci. 506:144952. 10.1016/j.apsusc.2019.144952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G. A., Boegli L., Hancock J., Bowersock L., Parker A., Kinney B. M. (2019). Bacterial adhesion and biofilm formation on textured breast implant shell materials. Aesthetic Plast. Surg. 43 490–497. 10.1007/s00266-018-1234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindai K., Nakade K., Masuda K., Sagawa T., Kojima H., Shimizu T., et al. (2020). Adhesion and bactericidal properties of nanostructured surfaces dependent on bacterial motility. RSC Adv. 10 5673–5680. 10.1039/c9ra08282d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothi Prakash C. G., Prasanth R. (2021). Approaches to design a surface with tunable wettability: a review on surface properties. J. Mater. Sci. 56 108–135. 10.1007/s10853-020-05116-1 [DOI] [Google Scholar]
- Kao W. K., Gagnon P. M., Vogel J. P., Chole R. A. (2017). Surface charge modification decreases Pseudomonas aeruginosa adherence in vitro and bacterial persistence in an in vivo implant model. Laryngoscope 127 1655–1661. 10.1002/lary.26499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin D., Mergel O., van der Mei H. C., Busscher H. J., van Rijn P. (2019). Inhibiting bacterial adhesion by mechanically modulated microgel coatings. Biomacromolecules 20 243–253. 10.1021/acs.biomac.8b01378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolewe K. W., Peyton S. R., Schiffman J. D. (2015). Fewer bacteria adhere to softer hydrogels. ACS Appl. Mater. Interf. 7 19562–19569. 10.1021/acsami.5b04269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolewe K. W., Zhu J., Mako N. R., Nonnenmann S. S., Schiffman J. D. (2018). Bacterial adhesion is affected by the thickness and stiffness of Poly(ethylene glycol) hydrogels. ACS Appl. Mater. Interf. 10 2275–2281. 10.1021/acsami.7b12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenko V., Salek M. M., Sattari P., Martinuzzi R. J. (2010). Staphylococcus aureus biofilm formation and tolerance to antibiotics in response to oscillatory shear stresses of physiological levels. FEMS Immunol. Med. Microbiol. 59 421–431. 10.1111/j.1574-695x.2010.00694.x [DOI] [PubMed] [Google Scholar]
- Kovacevic D., Pratnekar R., Godic Torkar K., Salopek J., Drazic G., Abram A., et al. (2016). Influence of polyelectrolyte multilayer properties on bacterial adhesion capacity. Polymers 8:345. 10.3390/polym8100345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowska A., Sigler K. (2014). How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 4:112. 10.3389/fcimb.2014.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryashov S. I., Nguyen L. V., Kirilenko D. A., Brunkov P. N., Rudenko A. A., Busleev N. I., et al. (2018). Large-scale laser fabrication of antifouling silicon-surface nanosheet arrays via nanoplasmonic ablative self-organization in liquid CS2 tracked by a sulfur dopant. ACS Appl. Nano. Mater. 1 2461–2468. 10.1021/acsanm.8b00392 [DOI] [Google Scholar]
- Kunrath M. F., Dos Santos R. P., de Oliveira S. D., Hubler R., Sesterheim P., Teixeira E. R. (2020a). Osteoblastic cell behavior and early bacterial adhesion on Macro-, Micro-, and nanostructured titanium surfaces for biomedical implant applications. Int. J. Oral Max. Impl. 35 773–781. 10.11607/jomi.8069 [DOI] [PubMed] [Google Scholar]
- Kunrath M. F., Monteiro M. S. G., Gupta S., Hubler R., de Oliveira S. D. (2020b). Influence of titanium and zirconia modified surfaces for rapid healing on adhesion and biofilm formation of Staphylococcus epidermidis. Arch. Oral Biol. 117:104824. 10.1016/j.archoralbio.2020.104824 [DOI] [PubMed] [Google Scholar]
- Lazzini G., Romoli L., Blunt L., Gemini L. (2017). Design and characterization of textured surfaces for applications in the food industry. Surf. Topogr. Metrol. Prop. 5:44005. [Google Scholar]
- Lebeaux D., Ghigo J. M., Beloin C. (2014). Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 78 510–543. 10.1128/mmbr.00013-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos M., Mergulhão F., Melo L., Simões M. (2015). The effect of shear stress on the formation and removal of Bacillus cereus biofilms. Food Bioprod. Process 93 242–248. 10.1016/j.fbp.2014.09.005 [DOI] [Google Scholar]
- Lewandowski Z., Beyenal H. (2003). Biofilm monitoring: a perfect solution in search of a problem. Water Sci. Technol. 47 9–18. 10.2166/wst.2003.0267 [DOI] [PubMed] [Google Scholar]
- Li X., Cheung G. S., Watson G. S., Watson J. A., Lin S., Schwarzkopf L., et al. (2017). Correction: The nanotipped hairs of gecko skin and biotemplated replicas impair and/or kill pathogenic bacteria with high efficiency. Nanoscale 9:464. 10.1039/c6nr90257j [DOI] [PubMed] [Google Scholar]
- Li Y., Yang Y., Li R., Tang X., Guo D., Qing Y., et al. (2019). Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: a review of current techniques. Int. J. Nanomed. 14 7217–7236. 10.2147/ijn.s216175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter J. A., Thompson M. T., Delgadillo M., Nishikawa T., Rubner M. F., Van Vliet K. J. (2008). Substrata mechanical stiffness can regulate adhesion of viable bacteria. Biomacromolecules 9 1571–1578. 10.1021/bm701430y [DOI] [PubMed] [Google Scholar]
- Limoli D. H., Jones C. J., Wozniak D. J. (2015). Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 3:10.1128/microbiolspec.MB-0011-2014. 10.1128/microbiolspec.MB-0011-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linklater D. P., Nguyen H. K. D., Bhadra C. M., Juodkazis S., Ivanova E. P. (2017). Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces. Nanotechnology 28:245301. 10.1088/1361-6528/aa700e [DOI] [PubMed] [Google Scholar]
- Liu N., Skauge T., Landa-Marban D., Hovland B., Thorbjornsen B., Radu F. A., et al. (2019). Microfluidic study of effects of flow velocity and nutrient concentration on biofilm accumulation and adhesive strength in the flowing and no-flowing microchannels. J. Ind. Microbiol. Biotechnol. 46 855–868. 10.1007/s10295-019-02161-x [DOI] [PubMed] [Google Scholar]
- Loo C. Y., Young P. M., Lee W. H., Cavaliere R., Whitchurch C. B., Rohanizadeh R. (2012). Superhydrophobic, nanotextured polyvinyl chloride films for delaying Pseudomonas aeruginosa attachment to intubation tubes and medical plastics. Acta. Biomater. 8 1881–1890. 10.1016/j.actbio.2012.01.015 [DOI] [PubMed] [Google Scholar]
- Lorenzetti M., Dogsa I., Stosicki T., Stopar D., Kalin M., Kobe S., et al. (2015). The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl. Mater. Interf. 7 1644–1651. 10.1021/am507148n [DOI] [PubMed] [Google Scholar]
- Luo X., Yao S., Zhang H., Cai M., Liu W., Pan R., et al. (2020). Biocompatible nano-ripples structured surfaces induced by Femtosecond laser to rebel bacterial colonization and biofilm formation. Opt. Laser Technol. 124:105973. 10.1016/j.optlastec.2019.105973 [DOI] [Google Scholar]
- Lutey A. H. A., Gemini L., Romoli L., Lazzini G., Fuso F., Faucon M., et al. (2018). Towards laser-textured antibacterial surfaces. Sci. Rep. 8:10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Sun Y., Gleichauf K., Lou J., Li Q. (2011). Nanostructure on taro leaves resists fouling by colloids and bacteria under submerged conditions. Langmuir 27 10035–10040. 10.1021/la2010024 [DOI] [PubMed] [Google Scholar]
- Ma Y., Jiang L., Hu J., Liu H., Wang S., Zuo P., et al. (2020). Multifunctional 3D micro-nanostructures fabricated through temporally shaped Femtosecond laser processing for preventing thrombosis and bacterial infection. ACS Appl. Mater. Interf. 12 17155–17166. 10.1021/acsami.9b20766 [DOI] [PubMed] [Google Scholar]
- Mandracci P., Mussano F., Ceruti P., Pirri C. F., Carossa S. (2015). Reduction of bacterial adhesion on dental composite resins by silicon-oxygen thin film coatings. Biomed. Mater. 10:15017. [DOI] [PubMed] [Google Scholar]
- Manson M. D. (2010). Dynamic motors for bacterial flagella. Proc. Natl. Acad. Sci. U.S.A. 107 11151–11152. 10.1073/pnas.1006365107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S., Safadi D., Meirowitz A., Ormianer Z. (2020). The effect of aging on the roughness and bacterial adhesion of lithium Disilicate and Zirconia ceramics. J. Prosthodont. (in press). [DOI] [PubMed] [Google Scholar]
- McClaine J. W., Ford R. M. (2002). Reversal of flagellar rotation is important in initial attachment of Escherichia coli to glass in a dynamic system with high- and low-ionic-strength buffers. Appl. Environ. Microbiol. 68 1280–1289. 10.1128/aem.68.3.1280-1289.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaiescu D. E., Cristescu R., Dorcioman G., Popescu C. E., Nita C., Socol G., et al. (2013). Functionalized magnetite silica thin films fabricated by MAPLE with antibiofilm properties. Biofabrication 5:15007. [DOI] [PubMed] [Google Scholar]
- Moazzam P., Razmjou A., Golabi M., Shokri D., Landarani-Isfahani A. (2016). Investigating the BSA protein adsorption and bacterial adhesion of Al-alloy surfaces after creating a hierarchical (micro/nano) superhydrophobic structure. J. Biomed. Mater. Res. A 104 2220–2233. 10.1002/jbm.a.35751 [DOI] [PubMed] [Google Scholar]
- Mokwatlo S. C., Brink H. G., Nicol W. (2020). Effect of shear on morphology, viability and metabolic activity of succinic acid-producing Actinobacillus succinogenes biofilms. Bioprocess Biosyst. Eng. 43 1253–1263. 10.1007/s00449-020-02322-8 [DOI] [PubMed] [Google Scholar]
- Montgomerie Z., Popat K. C. (2021). Improved hemocompatibility and reduced bacterial adhesion on superhydrophobic titania nanoflower surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 119:111503. 10.1016/j.msec.2020.111503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderizadeh S., Dante S., Picone P., Di Carlo M., Carzino R., Athanassiou A., et al. (2020). Bioresin-based superhydrophobic coatings with reduced bacterial adhesion. J. Colloid Interf. Sci. 574 20–32. 10.1016/j.jcis.2020.04.031 [DOI] [PubMed] [Google Scholar]
- Nguyen D. H. K., Pham V. T. H., Truong V. K., Sbarski I., Wang J., Balcytis A., et al. (2018). Role of topological scale in the differential fouling of Pseudomonas aeruginosa and Staphylococcus aureus bacterial cells on wrinkled gold-coated polystyrene surfaces. Nanoscale 10 5089–5096. 10.1039/c7nr08178b [DOI] [PubMed] [Google Scholar]
- Nino-Martinez N., Salas Orozco M. F., Martinez-Castanon G. A., Torres Mendez F., Ruiz F. (2019). Molecular mechanisms of bacterial resistance to metal and metal oxide nanoparticles. Int. J. Mol. Sci. 20:2808. 10.3390/ijms20112808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira R. D., Silva C. B., Lepri C. P., Palma-Dibb R. G., Geraldo-Martins V. R. (2017). Evaluation of surface roughness and bacterial adhesion on tooth enamel irradiated with high intensity lasers. Braz. Dent. J. 28 24–29. 10.1590/0103-6440201701190 [DOI] [PubMed] [Google Scholar]
- Oder M., Arlic M., Bohinc K., Fink R. (2018). Escherichia coli biofilm formation and dispersion under hydrodynamic conditions on metal surfaces. Int. J. Environ. Health Res. 28 55–63. 10.1080/09603123.2017.1415309 [DOI] [PubMed] [Google Scholar]
- Oh J. K., Yegin Y., Yang F., Zhang M., Li J., Huang S., et al. (2018). The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci. Rep. 8:17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan E., Mondal A., Singha P., Douglass M., Hopkins S. P., Devine R., et al. (2020). Fabrication of bacteria- and blood-repellent superhydrophobic polyurethane sponge materials. ACS Appl. Mater. Interf. 12 51160–51173. [DOI] [PubMed] [Google Scholar]
- Pajerski W., Duch J., Ochonska D., Golda-Cepa M., Brzychczy-Wloch M., Kotarba A. (2020). Bacterial attachment to oxygen-functionalized graphenic surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 113:110972. 10.1016/j.msec.2020.110972 [DOI] [PubMed] [Google Scholar]
- Pan F., Altenried S., Liu M., Hegemann D., Bülbül E., Moeller J., et al. (2020). A nanolayer coating on polydimethylsiloxane surfaces enables a mechanistic study of bacterial adhesion influenced by material surface physicochemistry. Mater. Horiz. 7 93–103. 10.1039/c9mh01191a [DOI] [Google Scholar]
- Pan Q., Cao Y., Xue W., Zhu D., Liu W. (2019). Picosecond laser-textured stainless steel superhydrophobic surface with an antibacterial adhesion property. Langmuir 35 11414–11421. 10.1021/acs.langmuir.9b01333 [DOI] [PubMed] [Google Scholar]
- Paramonova E., Kalmykowa O. J., van der Mei H. C., Busscher H. J., Sharma P. K. (2009). Impact of hydrodynamics on oral biofilm strength. J. Dent. Res. 88 922–926. 10.1177/0022034509344569 [DOI] [PubMed] [Google Scholar]
- Park J. W., An J. S., Lim W. H., Lim B. S., Ahn S. J. (2019). Microbial changes in biofilms on composite resins with different surface roughness: an in vitro study with a multispecies biofilm model. J. Prosthet. Dent. 122 493 e491–493 e498. [DOI] [PubMed] [Google Scholar]
- Patankar N. A. (2004). Mimicking the lotus effect: influence of double roughness structures and slender pillars. Langmuir 20 8209–8213. 10.1021/la048629t [DOI] [PubMed] [Google Scholar]
- Peng Q., Zhou X., Wang Z., Xie Q., Ma C., Zhang G., et al. (2019). Three-dimensional bacterial motions near a surface investigated by digital holographic microscopy: effect of surface stiffness. Langmuir 35 12257–12263. 10.1021/acs.langmuir.9b02103 [DOI] [PubMed] [Google Scholar]
- Perera-Costa D., Bruque J. M., Gonzalez-Martin M. L., Gomez-Garcia A. C., Vadillo-Rodriguez V. (2014). Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 30 4633–4641. 10.1021/la5001057 [DOI] [PubMed] [Google Scholar]
- Perni S., Prokopovich P. (2013). Micropatterning with conical features can control bacterial adhesion on silicone. Soft Matter. 9 1844–1851. 10.1039/c2sm26828k [DOI] [Google Scholar]
- Picioreanu C., Van Loosdrecht M. C. M., Heijnen J. J. (2000). Effect of diffusive and convective substrate transport on biofilm structure formation: a two-dimensional modeling study. Biotechnol. Bioeng. 69 504–515. [DOI] [PubMed] [Google Scholar]
- Pogodin S., Hasan J., Baulin V. A., Webb H. K., Truong V. K., Phong N. H., et al. (2013). Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophy. J. 104 835–840. 10.1016/j.bpj.2012.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole S. F., Pitondo-Silva A., Oliveira-Silva M., Moris I. C. M., Gomes E. A. (2020). Influence of different ceramic materials and surface treatments on the adhesion of Prevotella intermedia. J. Mech. Behav. Biomed. Mater. 111:104010. 10.1016/j.jmbbm.2020.104010 [DOI] [PubMed] [Google Scholar]
- Puckett S. D., Taylor E., Raimondo T., Webster T. J. (2010). The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 31 706–713. 10.1016/j.biomaterials.2009.09.081 [DOI] [PubMed] [Google Scholar]
- Qian H., Li M., Li Z., Lou Y., Huang L., Zhang D., et al. (2017). Mussel-inspired superhydrophobic surfaces with enhanced corrosion resistance and dual-action antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 80 566–577. 10.1016/j.msec.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Qin X. H., Senturk B., Valentin J., Malheiro V., Fortunato G., Ren Q., et al. (2019). Cell-membrane-inspired silicone interfaces that mitigate proinflammatory macrophage activation and bacterial adhesion. Langmuir 35 1882–1894. 10.1021/acs.langmuir.8b02292 [DOI] [PubMed] [Google Scholar]
- Reed J. H., Gonsalves A. E., Román J. K., Oh J., Cha H., Dana C. E., et al. (2019). Ultrascalable multifunctional nanoengineered copper and aluminum for antiadhesion and bactericidal applications. ACS Appl. Bio Mater. 2 2726–2737. 10.1021/acsabm.8b00765 [DOI] [PubMed] [Google Scholar]
- Renner L. D., Weibel D. B. (2011). Physicochemical regulation of biofilm formation. MRS Bull. 36 347–355. 10.1557/mrs.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigolin M. S. M., Barbugli P. A., Jorge J. H., Reis M. R. D., Adabo G. L., Casemiro L. A., et al. (2019). Effect of the aging of titanium and zirconia abutment surfaces on the viability, adhesion, and proliferation of cells and the adhesion of microorganisms. J. Prosthet. Dent. 122 564 e561–564 e510. [DOI] [PubMed] [Google Scholar]
- Rijnaarts H. H. M., Norde W., Lyklema J., Zehnder A. J. B. (1999). DLVO and steric contributions to bacterial deposition in media of different ionic strengths. Colloids Surf. B: Biointerf. 14 179–195. 10.1016/s0927-7765(99)00035-1 [DOI] [Google Scholar]
- Rizzello L., Cingolani R., Pompa P. P. (2013). Nanotechnology tools for antibacterial materials. Nanomedicine 8 807–821. 10.2217/nnm.13.63 [DOI] [PubMed] [Google Scholar]
- Rizzello L., Galeone A., Vecchio G., Brunetti V., Sabella S., Pompa P. P. (2012). Molecular response of Escherichia coli adhering onto nanoscale topography. Nanoscale Res. Lett. 7:575. 10.1186/1556-276x-7-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodesney C. A., Roman B., Dhamani N., Cooley B. J., Katira P., Touhami A., et al. (2017). Mechanosensing of shear by Pseudomonas aeruginosa leads to increased levels of the cyclic-di-GMP signal initiating biofilm development. Proc. Natl. Acad. Sci. U.S.A. 114 5906–5911. 10.1073/pnas.1703255114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan B., Wu P., Liu J., Jiang L., Wang H., Qiao J., et al. (2020). Adhesion of Sphingomonas sp. GY2B onto montmorillonite: a combination study by thermodynamics and the extended DLVO theory. Colloids Surf. B Biointerf. 192:111085. 10.1016/j.colsurfb.2020.111085 [DOI] [PubMed] [Google Scholar]
- Rzhepishevska O., Hakobyan S., Ruhal R., Gautrot J., Barbero D., Ramstedt M. (2013). The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 1 589–602. 10.1039/c3bm00197k [DOI] [PubMed] [Google Scholar]
- Saha N., Monge C., Dulong V., Picart C., Glinel K. (2013). Influence of polyelectrolyte film stiffness on bacterial growth. Biomacromolecules 14 520–528. 10.1021/bm301774a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D., Pires J. G., Braga A. S., Salomao P. M. A., Magalhaes A. C. (2019). Comparison between static and semi-dynamic models for microcosm biofilm formation on dentin. J. Appl. Oral Sci. 27 e20180163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraeva I. N., Tolordava E. R., Nastulyavichus A. A., Ivanova A. K., Kudryashov S. I., Rudenko A. A., et al. (2020). A bacterial misericorde: laser-generated silicon nanorazors with embedded biotoxic nanoparticles combat the formation of durable biofilms. Laser Phys. Lett. 17:025601. 10.1088/1612-202x/ab5fca [DOI] [Google Scholar]
- Scardino A. J., Guenther J., de Nys R. (2008). Attachment point theory revisited: the fouling response to a microtextured matrix. Biofouling 24 45–53. 10.1080/08927010701784391 [DOI] [PubMed] [Google Scholar]
- Schwibbert K., Menzel F., Epperlein N., Bonse J., Kruger J. (2019). Bacterial adhesion on femtosecond laser-modified polyethylene. Materials 12:3107. 10.3390/ma12193107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Gao P., Han S., Kao R. Y. T., Wu S., Liu X., et al. (2020). A tailored positively-charged hydrophobic surface reduces the risk of implant associated infections. Acta. Biomater. 114 421–430. 10.1016/j.actbio.2020.07.040 [DOI] [PubMed] [Google Scholar]
- Sheng X., Ting Y. P., Pehkonen S. O. (2008). The influence of ionic strength, nutrients and pH on bacterial adhesion to metals. J. Colloid Interf. Sci. 321 256–264. 10.1016/j.jcis.2008.02.038 [DOI] [PubMed] [Google Scholar]
- Siddiqui S., Chandrasekaran A., Lin N., Tufenkji N., Moraes C. (2019). Microfluidic shear assay to distinguish between bacterial adhesion and attachment strength on stiffness-tunable silicone substrates. Langmuir 35 8840–8849. 10.1021/acs.langmuir.9b00803 [DOI] [PubMed] [Google Scholar]
- Siddiquie R. Y., Gaddam A., Agrawal A., Dimov S. S., Joshi S. S. (2020). Anti-biofouling properties of Femtosecond laser-induced submicron topographies on elastomeric surfaces. Langmuir 36 5349–5358. 10.1021/acs.langmuir.0c00753 [DOI] [PubMed] [Google Scholar]
- Singh A. V., Vyas V., Patil R., Sharma V., Scopelliti P. E., Bongiorno G., et al. (2011). Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PLoS One 6:e25029. 10.1371/journal.pone.0025029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F., Brasch M. E., Wang H., Henderson J. H., Sauer K., Ren D. (2017). How bacteria respond to material stiffness during attachment: a role of Escherichia coli flagellar motility. ACS Appl. Mater. Interf. 9 22176–22184. 10.1021/acsami.7b04757 [DOI] [PubMed] [Google Scholar]
- Song W. S., Lee J. K., Park S. H., Um H. S., Lee S. Y., Chang B. S. (2017). Comparison of periodontitis-associated oral biofilm formation under dynamic and static conditions. J. Periodont. Implant Sci. 47 219–230. 10.5051/jpis.2017.47.4.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F., Koo H., Ren D. (2015). Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 94 1027–1034. 10.1177/0022034515587690 [DOI] [PubMed] [Google Scholar]
- Song F., Ren D. (2014). Stiffness of cross-linked poly(dimethylsiloxane) affects bacterial adhesion and antibiotic susceptibility of attached cells. Langmuir 30 10354–10362. 10.1021/la502029f [DOI] [PubMed] [Google Scholar]
- Song F., Wang H., Sauer K., Ren D. (2018). Cyclic-di-GMP and oprF Are Involved in the Response of Pseudomonas aeruginosa to Substrate Material Stiffness during Attachment on Polydimethylsiloxane (PDMS). Front. Microbiol. 9:110. 10.3389/fmicb.2018.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K., Lee J., Choi S. O., Kim J. (2019). Interaction of surface energy components between solid and liquid on wettability, and its application to textile anti-wetting finish. Polymers 11:498. 10.3390/polym11030498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler C., Nolle F., Mischo J., Faidt T., Grandthyll S., Thewes N., et al. (2019). Strength of bacterial adhesion on nanostructured surfaces quantified by substrate morphometry. Nanoscale 11 19713–19722. 10.1039/c9nr04375f [DOI] [PubMed] [Google Scholar]
- Stepanovic S., Vukovic D., Jezek P., Pavlovic M., Svabic-Vlahovic M. (2001). Influence of dynamic conditions on biofilm formation by Staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 20 502–504. [DOI] [PubMed] [Google Scholar]
- Stoodley P., Jacobsen A., Dunsmore B. C., Purevdorj B., Wilson S., Lappin-Scott H. M., et al. (2001). The influence of fluid shear and AICI3 on the material properties of Pseudomonas aeruginosa PAO1 and Desulfovibrio sp. EX265 biofilms. Water. Sci. Technol. 43 113–120. 10.2166/wst.2001.0353 [DOI] [PubMed] [Google Scholar]
- Straub H., Bigger C. M., Valentin J., Abt D., Qin X. H., Eberl L., et al. (2019). Bacterial adhesion on soft materials: passive physicochemical interactions or active bacterial mechanosensing? Adv. Healthc. Mater. 8:e1801323. [DOI] [PubMed] [Google Scholar]
- Tang M., Chen C., Zhu J., Allcock H. R., Siedlecki C. A., Xu L. C. (2021). Inhibition of bacterial adhesion and biofilm formation by a textured fluorinated alkoxyphosphazene surface. Bioact. Mater. 6 447–459. 10.1016/j.bioactmat.2020.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada A., Okuyama K., Nishikawa M., Tsuneda S., Hosomi M. (2012). The effect of surface charge property on Escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 109 1745–1754. 10.1002/bit.24429 [DOI] [PubMed] [Google Scholar]
- Thomen P., Robert J., Monmeyran A., Bitbol A. F., Douarche C., Henry N. (2017). Bacterial biofilm under flow: first a physical struggle to stay, then a matter of breathing. PLoS One 12:e0175197. 10.1371/journal.pone.0175197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueshima M., Tanaka S., Nakamura S., Yamashita K. (2002). Manipulation of bacterial adhesion and proliferation by surface charges of electrically polarized hydroxyapatite. J. Biomed. Mater. Res. 60 578–584. 10.1002/jbm.10113 [DOI] [PubMed] [Google Scholar]
- van Merode A. E., van der Mei H. C., Busscher H. J., Krom B. P. (2006). Influence of culture heterogeneity in cell surface charge on adhesion and biofilm formation by Enterococcus faecalis. J. Bacteriol. 188 2421–2426. 10.1128/jb.188.7.2421-2426.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan R., Kennedy A. J., Merritt M., Crocker F. H., Baney R. H. (2014). Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloids Surf. B Biointerf. 117 225–232. 10.1016/j.colsurfb.2014.02.037 [DOI] [PubMed] [Google Scholar]
- Verhorstert K. W. J., Guler Z., de Boer L., Riool M., Roovers J. W. R., Zaat S. A. J. (2020). In vitro bacterial adhesion and biofilm formation on fully absorbable Poly-4-hydroxybutyrate and nonabsorbable polypropylene pelvic floor Implants. ACS Appl. Mater. Interf. 12 53646–53653. 10.1021/acsami.0c14668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandiyanto J. V., Cheeseman S., Truong V. K., Kobaisi M. A., Bizet C., Juodkazis S., et al. (2019). Outsmarting superbugs: bactericidal activity of nanostructured titanium surfaces against methicillin- and gentamicin-resistant Staphylococcus aureus ATCC 33592. J. Mater. Chem. B 7 4424–4431. 10.1039/c9tb00102f [DOI] [Google Scholar]
- Wang R., Deng L., Lei Z., Wu P., Wang Y., Hao L., et al. (2020). Nanoscale adhesion forces of glucosyltransferase B and C genes regulated Streptococcal mutans probed by AFM. Mol. Oral Microbiol. 35 49–55. [DOI] [PubMed] [Google Scholar]
- Wang Y., Guan A., Isayeva I., Vorvolakos K., Das S., Li Z., et al. (2016). Interactions of Staphylococcus aureus with ultrasoft hydrogel biomaterials. Biomaterials 95 74–85. 10.1016/j.biomaterials.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Wassmann T., Kreis S., Behr M., Buergers R. (2017). The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant Dent. 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G. S., Cribb B. W., Watson J. A. (2010). How micro/nanoarchitecture facilitates anti-wetting: an elegant hierarchical design on the termite wing. ACS Nano 4 129–136. 10.1021/nn900869b [DOI] [PubMed] [Google Scholar]
- Watson G. S., Green D. W., Schwarzkopf L., Li X., Cribb B. W., Myhra S., et al. (2015). A gecko skin micro/nano structure - A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 21 109–122. 10.1016/j.actbio.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Watson G. S., Watson J. A., Cribb B. W. (2017). Diversity of cuticular micro- and nanostructures on insects: properties, functions, and potential applications. Annu. Rev. Entomol. 62 185–205. 10.1146/annurev-ento-031616-035020 [DOI] [PubMed] [Google Scholar]
- Weaver W. M., Milisavljevic V., Miller J. F., Di Carlo D. (2012). Fluid flow induces biofilm formation in Staphylococcus epidermidis polysaccharide intracellular adhesin-positive clinical isolates. Appl. Environ. Microbiol. 78 5890–5896. 10.1128/aem.01139-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K. A., Verran J. (2006). The effect of surface topography on the retention of microorganisms. Food Bioprod. Process. 84 253–259. 10.1205/fbp06035 [DOI] [Google Scholar]
- Wu S., Altenried S., Zogg A., Zuber F., Maniura-Weber K., Ren Q. (2018). Role of the surface nanoscale roughness of stainless steel on bacterial adhesion and microcolony formation. ACS Omega 3 6456–6464. 10.1021/acsomega.8b00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing R., Lyngstadaas S. P., Ellingsen J. E., Taxt-Lamolle S., Haugen H. J. (2015). The influence of surface nanoroughness, texture and chemistry of TiZr implant abutment on oral biofilm accumulation. Clin. Oral. Implants Res. 26 649–656. 10.1111/clr.12354 [DOI] [PubMed] [Google Scholar]
- Xu L. C., Siedlecki C. A. (2017). Protein adsorption, platelet adhesion, and bacterial adhesion to polyethylene-glycol-textured polyurethane biomaterial surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 105 668–678. 10.1002/jbm.b.33592 [DOI] [PubMed] [Google Scholar]
- Yang M., Ding Y., Ge X., Leng Y. (2015). Control of bacterial adhesion and growth on honeycomb-like patterned surfaces. Colloids Surf. B Biointerf. 135 549–555. 10.1016/j.colsurfb.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Yao W. L., Lin J. C. Y., Salamanca E., Pan Y. H., Tsai P. Y., Leu S. J., et al. (2020). Er,Cr:YSGG laser performance improves biological response on titanium surfaces. Materials 13:756. 10.3390/ma13030756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Deng J., Chen Y., Yang T., Zhu Y., Wu C., et al. (2019). Cicada and catkin inspired dual biomimetic antibacterial structure for the surface modification of implant material. Biomater. Sci. 7 2826–2832. [DOI] [PubMed] [Google Scholar]
- Yoda I., Koseki H., Tomita M., Shida T., Horiuchi H., Sakoda H., et al. (2014). Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 14:234. 10.1186/s12866-014-0234-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. H., Rungraeng N., Song W., Jun S. (2014). Superhydrophobic and superhydrophilic nanocomposite coatings for preventing Escherichia coli K-12 adhesion on food contact surface. J. Food Eng. 131 135–141. 10.1016/j.jfoodeng.2014.01.031 [DOI] [Google Scholar]
- Yu P., Wang C., Zhou J., Jiang L., Xue J., Li W. (2016). Influence of surface properties on adhesion forces and attachment of Streptococcus mutans to Zirconia in vitro. Biomed. Res. Int. 2016:8901253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Hays M. P., Hardwidge P. R., Kim J. (2017). Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 7 14254–14261. 10.1039/c7ra01571b [DOI] [Google Scholar]
- Zhang K., Baras B., Lynch C. D., Weir M. D., Melo M. A. S., Li Y., et al. (2018). Developing a new generation of therapeutic dental polymers to inhibit oral biofilms and protect teeth. Materials 11:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhou X., Xi H., Sun J., Liang X., Wei J., et al. (2019). Interpretation of adhesion behaviors between bacteria and modified basalt fiber by surface thermodynamics and extended DLVO theory. Colloids Surf. B Biointerf. 177 454–461. 10.1016/j.colsurfb.2019.02.035 [DOI] [PubMed] [Google Scholar]
- Zhu J., Wang M., Zhang H., Yang S., Song K.-Y., Yin R., et al. (2020). Effects of hydrophilicity, adhesion work, and fluid flow on biofilm formation of PDMS in microfluidic systems. ACS Appl. Biol Mater. 3 8386–8394. 10.1021/acsabm.0c00660 [DOI] [PubMed] [Google Scholar]
- Zhu X., Janczewski D., Guo S., Lee S. S., Parra Velandia F. J., Teo S. L., et al. (2015). Polyion multilayers with precise surface charge control for antifouling. ACS Appl. Mater. Interf. 7 852–861. 10.1021/am507371a [DOI] [PubMed] [Google Scholar]