Visual Abstract
Key Words: anakinra, colchicine, IL-1α, IL-1β, IL-1 trap, pericarditis, NLRP3 inflammasome
Abbreviations and Acronyms: IL, interleukin; NLRP3inh, NLRP3 inflammasome inhibitor
Highlights
-
•
Acute pericarditis is characterized by an intense inflammatory response involving the pericardium. Although mostly benign in its clinical course, 30% of patients may experience complications (recurrence, treatment failure, cardiac tamponade).
-
•
The pathogenesis of pericarditis is poorly understood. The scarcity of animal models might justify the limited understanding of this syndrome and the lack of targeted therapies.
-
•
Acute pericarditis is believed to represent a stereotypical response to an acute injury of the pericardium. The NLRP3 inflammasome, through its main product, IL-1β, could play a central role in the clinical manifestations.
-
•
A mouse model of acute pericarditis was developed through the intrapericardial injection of zymosan A, leading to the classical features of the inflamed pericardium: pericardial effusion, pericardial thickening, and increased expression of the NLRP3 inflammasome. By inhibiting the NLRP3 inflammasome or IL-1β, the pericardial effusion and thickening and the NLRP3 inflammasome expression were greatly reduced compared with vehicle.
-
•
Treatment with IL-1 trap, neutralizing both IL-1β and IL-1α, produced a powerful effect on pericardial inflammation in the experimental pericarditis model.
Summary
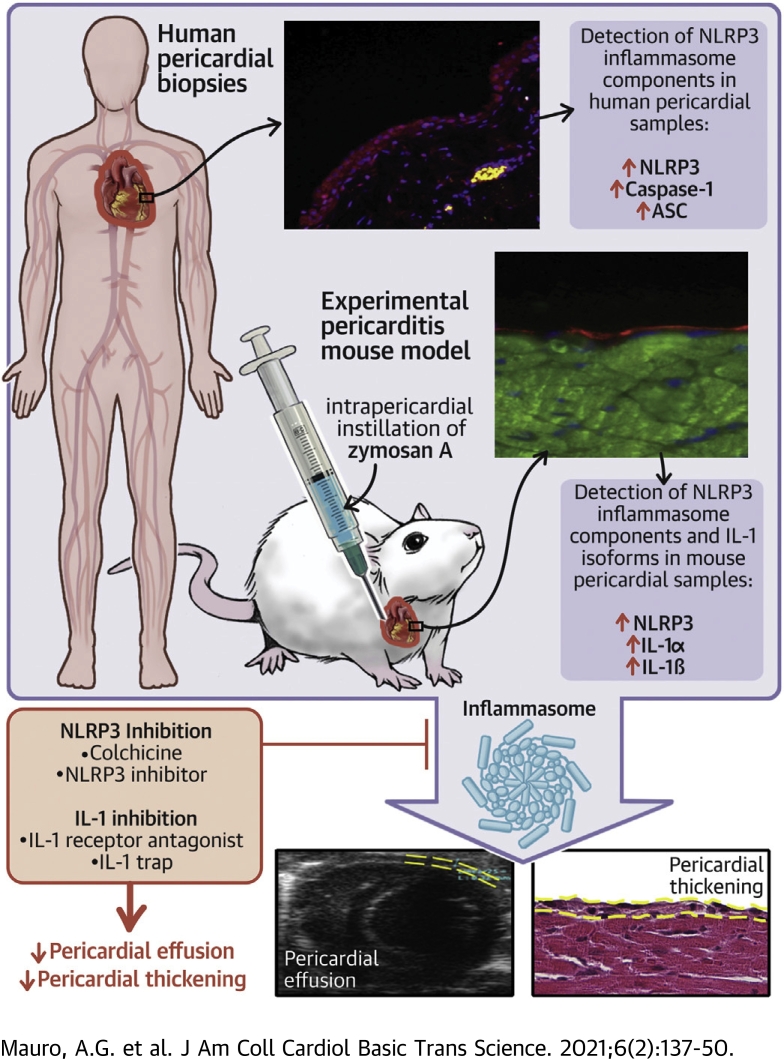
Human samples of patients with chronic pericarditis and appropriate control subjects were stained for the inflammasome components. A mouse model of pericarditis was developed through the intrapericardial injection of zymosan A. Different inflammasome blockers were tested in the mouse model. Patients with pericarditis presented an intensification of the inflammasome activation compared with control subjects. The experimental model showed the pathological features of pericarditis. Among inflammasome blockers, NLRP3 inflammasome inhibitor, anakinra, and interleukin-1 trap were found to significantly improve pericardial alterations. Colchicine partially improved the pericardial inflammation. An intense activation of the inflammasome in pericarditis was demonstrated both in humans and in mice.
Acute pericarditis is a clinical syndrome characterized by an intense inflammatory response involving the pericardium, generally occurring in healthy individuals as an isolated entity or as a manifestation of a systemic disease (1). Acute pericarditis was reported in 27.7 cases per 100,000 person-years in an Italian urban area, being responsible for 0.1% of all hospital admissions and 5% of emergency room admissions for chest pain (2).
Although its pathogenesis is not completely understood (3), it has been hypothesized that acute pericarditis represents a stereotypical response to an acute injury of the mesothelial cells of the pericardium (4). The trigger, an “irritant,” such as a virus or cellular debris following a viral infection, may activate the NLRP3 (NACHT, leucine-rich repeat, and pyrin domain-containing protein 3) inflammasome, a macromolecular intracellular complex evolved to sense stress or injury and trigger a local or systemic inflammatory response through the release of proinflammatory cytokines, such as interleukin (IL)-1β (5,6). The NLRP3 inflammasome is formed by the oligomerization of: 1) a sensor, namely NLRP3, which is a member of the NLR (NOD-like receptor) proteins; 2) a scaffold protein, ASC (apoptosis-associated speck-like protein containing a COOH-terminus caspase activation and recruitment domain); and 3) an effector, caspase-1, whose activation is strictly required for the activity of the inflammasome as it cleaves pro–IL-1β to the active IL-1β (6). IL-1α is another isoform of the IL-1 family that is released during cellular injury, functioning as an alarmin, and triggering the same inflammatory signaling than IL-1β (7,8). Given the limited knowledge on its pathophysiology, there are limited treatment options for acute pericarditis (9), mostly consisting of nonsteroidal anti-inflammatory drugs and colchicine, with the latter recently being recognized as an NLRP3 inhibitor and very effective both in acute and recurrent pericarditis (10). Recent data also suggest beneficial effects of IL-1 blockade with anakinra (4,11). However, none of these treatments has been approved by the U.S. Food and Drug Administration for the treatment of pericarditis in humans. The clinical effect of these drugs is hypothesized to be an indirect evidence of the involvement of the NLRP3 inflammasome signaling in the pathophysiology of pericarditis. However, to date no direct demonstration of the presence of the NLRP3 inflammasome in the pericardium during an acute episode of pericarditis has been provided. The dearth of pathology studies in patients and of animal models of acute pericarditis are likely to explain the limited understanding of this syndrome and the lack of targeted therapies, which are urgently needed to reduce the disease morbidity.
The current study aimed to: 1) detect the presence of the NLRP3 inflammasome in human pericarditis samples; 2) develop an innovative mouse model of acute pericarditis secondary to NRLP3 inflammasome activation; and 3) evaluate whether an inhibitory strategy against the NLRP3 inflammasome itself or cytokines involved in the activity of the inflammasome (i.e., IL-1β as well as IL-1α) could be beneficial as a future treatment in patients with pericarditis.
Methods
Human pericardial samples
The Pathology Service of the University of Trieste (Trieste, Italy) provided human samples of patients with chronic pericarditis experiencing an acute flare and showing a constrictive evolution who needed pericardiectomy (n = 6, cases) and pericardial samples from autopsies of patients who died for causes not related to pericardial syndromes (n = 5, controls). The study was approved by the Ethical Committee of the Azienda Sanitaria-Universitaria Integrata Trieste, Trieste, Italy. Patients gave their consent before entering the study.
Experimental mouse model of acute pericarditis
Adult male outbred CD1 (8 to 12 weeks of age) mice were supplied by Envigo (Frederick, Maryland). The experiments were conducted under the Guidelines of Laboratory Animals for Biomedical Research published by the National Institutes of Health (No. 85-23, revised 2011). The study protocol was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
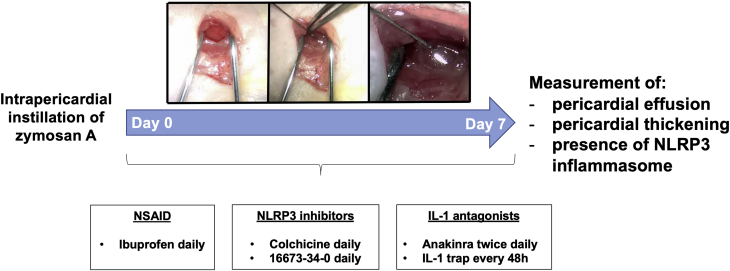
Prior to surgery, mice were put under general anesthesia using intraperitoneal injection of pentobarbital sodium (50 to 70 mg/kg) and shaved on the lateral left side of the thorax. Mice were then orotracheally intubated and ventilated using a rodent ventilator (Minivent, Harvard Apparatus, Holliston, Massachusetts). The heart was accessed with the animal lying recumbent in the right lateral decubitus position by making a ventral to dorsal incision following the curve of the rib cage approximately in the region of the third and fourth rib (Figure 1). Muscle layers were then bluntly dissected to expose the interosseous space between the third and fourth rib, granting access to the thoracic cavity. Finally, 1 mg of zymosan A (Sigma-Aldrich, St. Louis, Missouri) dissolved in 50 μl of sterile NaCl 0.9% was injected under direct visualization into the pericardial space by carefully lifting the pericardial sac with curved-tip forceps, using a 30-gauge needle, until a complete distribution of the solution into the pericardium was observed (Figure 1). Zymosan A dose was chosen based on previous experiments conducted by our group on a model of peritonitis induced by the intraperitoneal injection of zymosan A (1 mg/mouse) (12). The muscle layers and skin were then brought together and closed with 5.0 sutures. Zymosan A is a cell wall extract from the yeast Saccharomyces cerevisiae and a known agonist of the TLR-2 (toll-like receptor-2). Zymosan A leads to the activation of the NLRP3 inflammasome (13) and was already used in mouse models of peritonitis and arthritis (14,15).
Figure 1.
Schematic Representation of the Experimental Mouse Model of Acute Pericarditis
Under general anesthesia, 1 mg of zymosan A (in 50 μl of sterile NaCl 0.9%) was injected in the pericardial sac of the mouse on day 0. Sham control procedures were performed injecting an equal volume of sterile NaCl 0.9% in the pericardial sac. Selected drugs were administered after the surgery was completed until day 7. On day 7, mice underwent echocardiography and were euthanized. IL = interleukin; NLRP3 = NACHT, leucine-rich repeat, and pyrin domain-containing protein 3; NSAID = nonsteroidal anti-inflammatory drug.
Sham procedures were performed, in which mice underwent the same surgical procedure, injecting an equal volume of sterile NaCl 0.9% in the pericardial space (n = 4 to 6).
Treatment of mice following induction of acute pericarditis
Following the recovery after surgery, mice (6 to 8 per group) were randomly allocated to 1 of 6 different treatments performed by intraperitoneal injection (over a period of 1 week): 1) ibuprofen (100 mg/kg/day; Sigma-Aldrich); 2) colchicine (100 μg/kg/day; Enzo Life Sciences, Farmingdale, New York); 3) an NLRP3 inflammasome inhibitor (NLRP3inh) (16673-34-0; 100 mg/kg/day) (16, 17, 18); 4) anakinra, recombinant human IL-1 receptor antagonist (100 mg/kg twice daily; Swedish Orphan Biovitrum, Stockholm, Sweden); 5) recombinant murine IL-1 trap, (1, 5, and 30 mg/kg/day every 48 h; Regeneron Pharmaceuticals, Tarrytown, New York), a fusion protein mouse homologue of rilonacept, which combines the ectodomains of the IL-1R1 (IL-1 type 1 receptor) and IL-1RAcP (IL-1 receptor accessory protein) to bind and block IL-1α and IL-1β, as previously tested by our group (19); or 6) matching volume of vehicle (NaCl 0.9%). All drugs were administered after surgery and then once daily with the exception of anakinra, given twice daily, and IL-1 trap, given once every 48 h.
All tested doses of anakinra, NLRP3inh, and ibuprofen were determined after a thorough review of the previous literature and validated in our laboratory (16,17,20, 21, 22). IL-1 trap was administered at 3 different doses (1, 5, and 30 mg/kg) also previously validated in our laboratory (19). A dose-response evaluation of colchicine was tested in 3 different concentrations: 100, 500, and 1,000 μg/kg, with the 500- and 1,000-μg/kg doses resulting in toxicity showing a 100% mortality rate.
Echocardiography
Transthoracic echocardiography was performed prior to sacrifice at day 7 under general anesthesia with pentobarbital (50 to 70 mg/kg) using the Vevo770 imaging system (VisualSonics, Toronto, Ontario, Canada) equipped with a 30-MHz probe. The left ventricle was visualized in the parasternal short axis view at the midventricular level in the 2-dimensional mode echocardiography. The image was optimized for the anterior wall and was zoomed to visualize the anterior pericardial structures. After optimization of the image, a B-mode and M-mode echocardiogram was acquired for optimal spatial-temporal resolution and for measurements of the pericardial effusion, defined as the echo-free space between the 2 layers of the pericardium. We, however, considered images acquired through B-mode, as they were found to be clearer in all subsets of animals.
Two investigators blinded to group allocation measured maximal pericardial effusion, expressed as the average among 3 different assessments.
Assessment of pericardial thickness
Hearts were collected and fixed in 10% formalin for 48 h, dehydrated, and embedded in paraffin. The 5-μm-thick transverse sections were deparaffinized, rehydrated, and stained with standard hematoxylin and eosin protocol (Sigma-Aldrich). Two investigators blinded to group allocation measured the thickness of the visceral pericardial layers with the aid of a computer morphometry analysis software (Image Pro Plus 6.0, Media Cybernetics, Silver Spring, Maryland).
Detection of the NLRP3 inflammasome components in pericardial specimens
Immunofluorescence staining
ASC represents the scaffold of the NLRP3 inflammasome. Increased staining for ASC together with the formation of dense areas of aggregation provide evidence for the oligomerization of the inflammasome macromolecular structure (23,24).
In human pericardial samples, ASC expression was evaluated on 3-μm transverse-sectioned hearts using an immunofluorescence technique, as previously described (25,26). Antigen epitopes were retrieved with 5 mM citrate buffer pH 6.0 at 95°C. Slides were incubated overnight at 4°C with a primary antibody against human ASC (1:250; Sigma-Aldrich). Conjugated anti-rabbit Alexa Fluor 594 (1:100; Invitrogen, Carlsbad, California) was used as a secondary antibody for 4 h at room temperature.
Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (1:100) for 5 min. Finally, slides were covered after adding SlowFade Antifade (Invitrogen). The images (400× magnification) were acquired through Olympus IX70 microscope and analyzed by cellSens software (Olympus Life Science, Waltham, Massachusetts).
For murine samples, ASC, IL-1α, and IL-1β expression was evaluated on 5-μm transversal sections using a primary antibody against murine ASC (1:100; Sigma-Aldrich), IL-α (1:100; R&D Systems, Minneapolis, Minnesota), and IL1-β (1:25; Sigma-Aldrich), respectively. Conjugated anti-rabbit Alexa Fluor 594 (1:100; Invitrogen) was used as a secondary antibody for 4 h at room temperature. Additionally, heart slides were incubated with a primary antibody against cardiac α-actin (1:200; Sigma-Aldrich) revealed by a secondary antibody, conjugated anti-mouse Alexa Fluor 488 (1:100; Invitrogen), to identify the myocardial cell and easily identify the pericardium, not positive for the cardiac α-actin.
Quantifications were accomplished for all samples after consensus by 2 different investigators blinded to treatment allocation using a semi-quantitative scale ranging from 0 to 3 adapted from Abbate et al. (27) (Supplemental Table 1).
Immunohistochemistry staining
Staining for NLRP3 and cleaved caspase-1 was performed using immunohistochemistry. After antigen retrieval, endogenous peroxidases were inactivated by a solution of 3% hydrogen peroxide for 15 min. A primary antibody raised against mature, active caspase-1 (cleaved Asp210, 1:50; Thermo Fisher Scientific, Waltham, Massachusetts) or NLRP3 (1:100; Novus Biologicals, Centennial, Colorado) was incubated overnight at 4°C. An anti-rabbit IgG conjugated to the horseradish peroxidase secondary antibody was incubated for 2 h at room temperature, followed by incubation with VECTOR NovaRED Peroxidase Substrate (1:100; Vector Laboratories, Burlingame, California) and counterstaining with hematoxylin. Finally, slides were dehydrated and coverslipped with Cytoseal XYL (Thermo Fisher Scientific). Images were acquired using a Zeiss Axio Imager A.1 microscope (Zeiss, Oberkochen, Germany) using 10× objective for immunohistochemistry. Quantification was performed as previously mentioned (Supplemental Table 1).
Real-time polymerase chain reaction
RNA was extracted from the parietal pericardium through affinity columns (ReliaPrep RNA Miniprep Systems, Promega, Madison, Wisconsin) and converted to complementary DNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, California). Real-time polymerase chain reaction was done using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), with a CFX384 Touch Real-Time PCR Detection System (Bio-Rad). Gene expression was carried for the following messenger RNAs: Il-1α, Il-1β, and Nlrp3. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used as a reference gene.
Statistical analysis
All data are expressed as mean ± SEM. Analysis of variance was used to analyze differences across treatment groups, followed by Student’s t test for multiple pairwise comparisons. Discrete variables between groups were evaluated using the chi-square test. Pearson's correlation coefficients were calculated to evaluate the correlation between pericardial effusion and pericardial thickness. A 2-sided p value <0.050 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM, Armonk, New York) and GraphPad Prism version 8.2 for Windows (GraphPad Software, San Diego, California).
Results
The NLRP3 inflammasome in human pericarditis
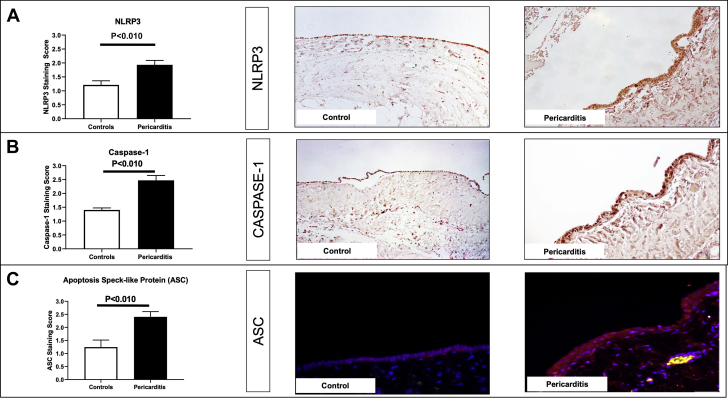
The expression of the NLRP3 inflammasome components was detected in all pericardial samples of patients with chronic pericarditis experiencing an acute flare. In particular, NLRP3 and caspase-1 expression were found upregulated in the human pericardial samples (Figures 2A and 2B), with a significant increased expression compared with the control subjects (NLRP3: 1.9 ± 0.15 vs. 1.21 ± 0.1; p = 0.009; caspase-1: 2.5 ± 0.2 vs. 1.4 ± 0.09; p < 0.001). As readouts of inflammasome activation, ASC aggregates were measured by immunofluorescence staining. ASC was found significantly increased in pericarditis cases versus controls (Figure 2C): 2.4 ± 0.2 versus 1.1 ± 0.3 (p = 0.006). Hence, patients with chronic pericarditis presented an intensification of the NLRP3 inflammasome expression and activation compared with control subjects.
Figure 2.
Upregulation of NLRP3 Inflammasome Components in Human Pericardial Specimens of Patients Affected by Pericarditis
(A) Immunohistochemistry reveals upregulation of the NLRP3 protein following pericarditis in human samples (original magnification 40×). (B) Caspase-1 upregulation detected by immunohistochemical staining (original magnification 40×). C) Immunofluorescence staining of ASC (apoptosis-associated speck-like protein containing a COOH-terminus caspase activation and recruitment domain) (red) showing aggregates in the pericardium. Counterstaining of the nuclei achieved with DAPI (4′,6-diamidino-2-phenylindole) (blue) (original magnification 40×). Abbreviations as in Figure 1.
Induction of acute pericarditis by zymosan A in the mouse
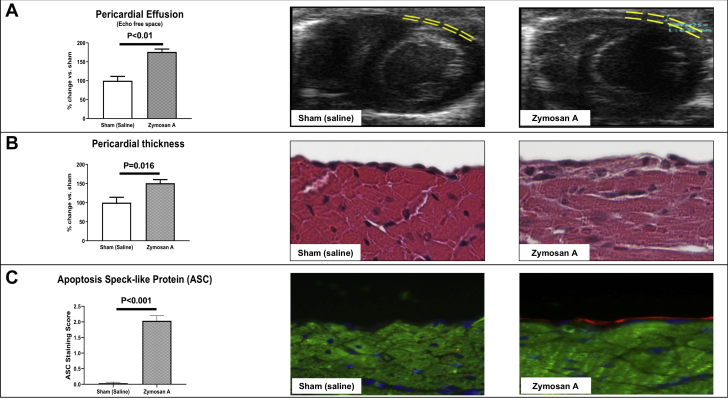
Based on literature, zymosan A was shown to induce NLRP3 inflammasome activation (12, 13, 14, 15), and here we used it to develop a mouse model of acute pericarditis. In order to assess induction of pericarditis, we evaluated 3 parameters reflecting inflammation of the pericardium: presence of pericardial effusion, pericardial thickness, and ASC expression. Seven days after the intrapericardial injection of zymosan A, mice developed a significantly larger pericardial effusion (+83%) compared with sham (p < 0.001) (Figure 3A, Supplemental Figures 1C and 1D). This finding was found already after 3 days from surgery, with zymosan A–treated mice showing a larger effusion compared with sham mice (p < 0.001 in B-mode and p = 0.004 in M-mode) (Supplemental Figures 1A and 1B). A morphometrical analysis on hematoxylin and eosin–stained heart sections was carried out at day 7 and revealed a significant increase (45%) in the visceral pericardial thickness compared with sham-operated mice (p = 0.016) (Figure 3B). No fibrinous deposits were observed at a macroscopic or microscopic evaluation at the time of harvesting. Positive correlations between pericardial effusion (assessed through echocardiography) and pericardial thickness (assessed through histology) were found (Supplemental Figure 2). At day 7, mice treated with zymosan A showed a 60-fold increase expression of ASC compared with sham mice indicating activation of the NLRP3 inflammasome (p < 0.001) (Figure 3C).
Figure 3.
Assessment of Pericarditis in Mice
(A) Pericardial effusion was measured at 7 days by transthoracic echocardiography as echo-free space between the 2 layers of the pericardium. Instillation of zymosan A produces a significant increase in pericardial fluid compared with sham control subjects injected with saline. (B) Pericardial thickening was measured at day 7 by histological assessment of hematoxylin and eosin–stained heart slides. Zymosan A increased significantly the thickness of the visceral pericardial layer compared with sham (original magnification 40×). (C) Immunofluorescence staining for ASC (red) and cardiac α-actin (green) in the pericardium of mice with experimental pericarditis. DAPI was used to counterstain the nuclei (blue) (original magnification 40×). Abbreviations as in Figure 2.
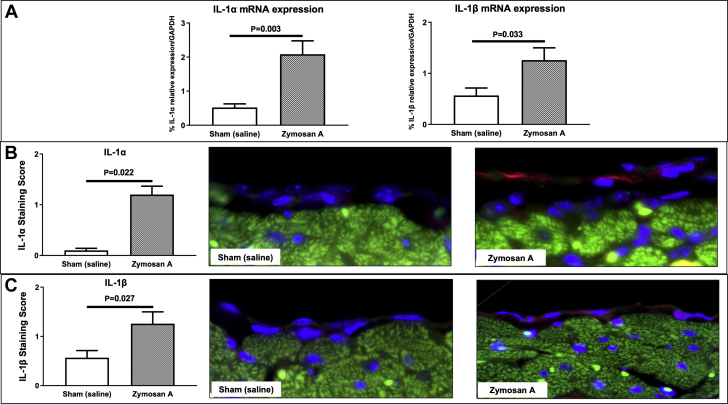
Recent findings highlighted the possible role of both IL-α and IL1-β in driving the clinical symptoms (11,28). For this reason, we investigated the presence of these 2 cytokines in our animal model of acute pericarditis. A subset of mice, including a group injected with zymosan A and one group with sham mice, were sacrificed 3 days after surgery to perform molecular biological analysis and immunofluorescence for IL-α and IL1-β. A greater expression of IL-α and IL1-β was found both at a transcriptional (Figure 4A) and translational level (Figures 4B and 4C) in mice treated with zymosan A compared with sham-operated mice.
Figure 4.
Assessment of IL-1α and IL-1β in Mice
(A) IL-1α and IL-1β messenger RNA (mRNA) relative expression in the parietal pericardium was measured at 3 days by real-time polymerase chain reaction. Mice treated with intrapericardial instillation of zymosan A showed a significant increase in mRNA expression of both IL-1α and IL-1β compared with sham control subjects injected with saline. (B) Pericardial expression of IL-1α was measured at day 3 by immunofluorescence staining. Zymosan A–treated mice had a higher expression of IL-1α compared with sham. IL-1α positive cells are stained in red, while DAPI was used to counterstain the nuclei (blue). The myocardium appears green by natural autofluorescence. Original magnification 40×. (C) Immunofluorescence staining for IL-1β (red) in the pericardium of mice with experimental pericarditis demonstrated a higher expression of this cytokine in zymosan A–treated mice compared with sham control subjects. IL-1β is stained in red, while DAPI was used to counterstain the nuclei (blue). The myocardium appears green by natural autofluorescence. Original magnification 40×. Abbreviations as in Figures 1 and 2.
Cardiac function was preserved in mice injected with zymosan A and in sham mice both at 3 and 7 days.
In sum, the mouse model of acute pericarditis replicates several common features mediated by the local inflammation of the pericardium as observed in patients with pericarditis.
Effects of ibuprofen in experimental pericarditis
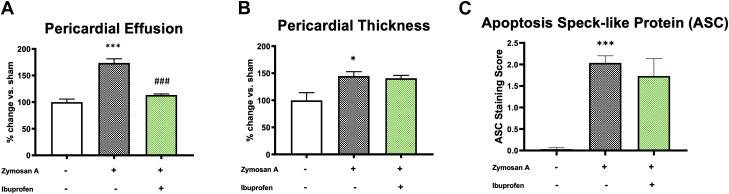
Ibuprofen, a COX-2 (cyclooxygenase-2) inhibitor, is currently used as a first-line therapy along with colchicine in patients with acute pericarditis (1). Treatment with ibuprofen in mice with acute pericarditis reduced pericardial effusion compared with vehicle-treated mice by 42% (p < 0.001) (Figure 5A). In contrast, ibuprofen failed to prevent pericardial thickening (Figure 5B) and ASC expression (Figure 5C).
Figure 5.
Effects of Ibuprofen Treatment in the Experimental Model of Pericarditis in Mice
(A) Pericardial effusion was measured at 7 days following daily treatment with ibuprofen (100 mg/kg). Daily injection with ibuprofen significantly reduced fluid accumulation. (B) Treatment with ibuprofen did not reduce pericardial thickness. (C) ASC (apoptosis-associated speck-like protein containing a COOH-terminus caspase activation and recruitment domain) aggregates were not reduced by treatment with ibuprofen. ∗p < 0.05, ∗∗∗p < 0.001 vs. sham-treated mice; ###p < 0.001 vs. vehicle-treated mice.
Effects of NLRP3 inflammasome inhibition in experimental pericarditis
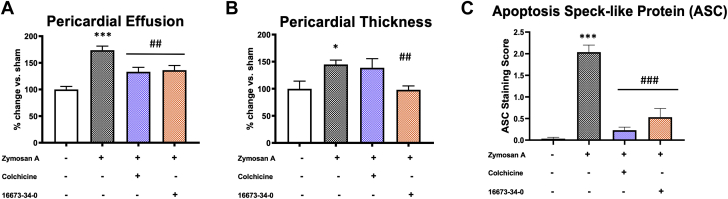
In order to block the NLRP3 inflammasome activity, we used colchicine, recently recognized as an NLRP3 inhibitor and largely used both in acute and recurrent pericarditis (10) as well as the NLRP3inh (16673-34-0), generated here at Virginia Commonwealth University (16) and previously tested by our group in experimental myocardial ischemia (17,18). Colchicine and the NLRP3inh 16673-34-0 significantly reduced pericardial effusion at day 7 by 28% and 46%, respectively (p < 0.010 for both) (Figure 6A). Colchicine was ineffective in reducing pericardial thickness, whereas the NLRP3inh 16673-34-0 significantly reduced the pericardial thickening by 32% (p = 0.003) (Figure 6B). ASC aggregation was reduced at day 7 both by colchicine (–93% vs. vehicle-treated mice; p < 0.001) and the NLRP3inh (–78% vs. vehicle-treated mice; p < 0.001) (Figure 6C).
Figure 6.
Inhibition of the NLRP3 Inflammasome in Mice With Acute Pericarditis
(A) Pericardial effusion measured at 7 days following daily treatment with colchicine (100 μg/kg) and NLRP3 inhibitor 16673-34-0 (100 mg/kg). Colchicine and 16673-34-0 significantly reduced pericardial effusion in mice with pericarditis. (B) Treatment with the NLRP3 inhibitor 16673-34-0, but not colchicine, showed a significant reduction of the pericardial thickness following acute pericarditis in mice compared with the vehicle-treated group. (C) ASC aggregates in the pericardium of mice with acute pericarditis were significantly reduced by colchicine and 16673-34-0. ∗p < 0.05, ∗∗∗p < 0.001 vs. sham-treated mice; ##p < 0.01, ###p < 0.001 vs. vehicle-treated mice. Abbreviations as in Figures 1 and 2.
Effects of IL-1 blockade in experimental pericarditis
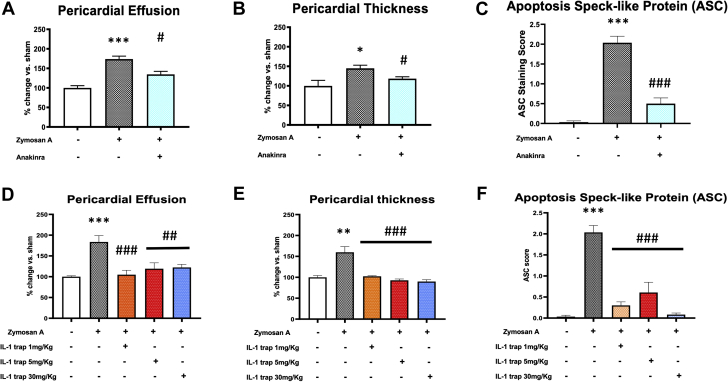
IL-1 blockade was evaluated using 2 different drugs: anakinra, an IL-1 receptor antagonist, proposed as treatment for recurrent pericarditis, and IL-1 trap, a cytokine trap that prevents soluble IL-1α and IL-1β from binding to their receptor, IL-1R1. Treatment with anakinra twice daily attenuated pericardial effusion by 13% compared with the vehicle group (p < 0.050) (Figure 7A). Similarly, IL-1 trap given every 48 h reduced pericardial effusion by 43%, 35%, and 33% at all 3 doses tested, respectively, compared with the vehicle-treated group (p < 0.010 for all comparison) (Figure 7D). Anakinra reduced pericardial thickening by 20% (p < 0.050 vs. vehicle) (Figure 7B). Similarly, the IL-1 trap consistently reduced pericardial thickening (–36%, –42%, and –44%, respectively; p < 0.001 for all) (Figure 7E). Finally, the formation of the inflammasome, measured as ASC aggregates, was significantly reduced by anakinra (–75% vs. vehicle; p < 0.001) (Figure 7C) and by all of the 3 doses of IL-1 trap (–85% for 1 mg/kg, –69% for 5 mg/kg, and –96% for 30 mg/kg; p < 0.001 for all) (Figure 7F).
Figure 7.
IL-1 Blockade in Experimental Acute Pericarditis
Pericardial effusion, thickness, and formation of ASC aggregates were measured 7 days after twice daily treatment with anakinra (100 mg/kg) or IL-1 trap. Anakinra significantly reduced (A) pericardial fluid accumulation, (B) thickening of the pericardium, and (C) ASC aggregation following acute pericarditis in mice. A dose response of IL-1 trap was tested in mice subjected to experimental pericarditis. Treatment with IL-1 trap every 48 h significantly reduced (D) pericardial effusion, (E) pericardial thickness, and (F) inflammasome formation with each dose tested. ∗p < 0.05, ∗∗∗p < 0.001 vs. sham-treated mice; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. vehicle-treated mice. Abbreviations as in Figures 1 and 2.
Supplemental Table 2 summarizes main findings of the different tested drugs with regard to the efficacy in reducing pericardial effusion, pericardial thickness, and ASC expression.
Discussion
In the present study, we were able to describe for the first time the presence of NLRP3 inflammasome expression in human pericardial specimens from patients with pericarditis. We also demonstrated that the phenotype of acute pericarditis—thickening of the pericardium, presence of an effusion, and inflammation associated with NLRP3 expression—can be reproduced in a mouse model with the instillation of zymosan A within the pericardial space. The expression of IL-1α and IL-1β in the pericardium of mice with acute pericarditis was also demonstrated with a higher expression compared with sham mice. Finally, different treatments for pericarditis have been tested and results are summarized in Supplemental Table 2.
Zymosan A is a TLR-2 agonist and can induce NLRP3 inflammasome pathway activation (12,13,17,29). Our mouse model showed that the NLRP3 inflammasome promotes the inflammatory changes occurring in acute pericarditis (i.e., pericardial effusion and pericardial thickening). As supportive evidence, drugs known to inhibit the NLRP3 inflammasome pathway (i.e., colchicine, NLRP3inh 16673-34-0, anakinra, and IL-1 trap) were found to reduce all or most of the above-mentioned inflammatory effects.
Other models of pericarditis were proposed in the past, using irritating agents capable to activate the NLRP3 inflammasome, although there was no knowledge about it at the time of publication. Pagé et al. (30) used hydrated magnesium silicate (talc) dusted on the pericardium of dogs. Now we know that silicate crystals can induce the NLRP3 inflammasome activation (24). The intrapericardial injection of heat-killed staphylococci and Freund’s adjuvant in sheep, as performed by Leak et al. (31), showed the different stages of the pericardial inflammation, allowing to evaluate the inflammatory cell infiltration, the production of fibrin, and the formation of pericardial adhesions until the final resolution with fibrosis. The bacterial products of the Freund’s adjuvant (containing aluminum) are canonical stimuli for the NLPR3 inflammasome activation (32), resulting again in an inflammasome-dependent model of acute pericarditis. Recently, a mouse model mimicking pericardial inflammation was performed with the intrapericardial injection of blood, minocycline, picibanil, and talc through a single-shot injection (33). Compared with others, our model has obvious advantages. In fact, the use of large animals, such as sheep or dogs, can better mimic what happens in humans, but also has clear disadvantages, primarily related to the complexity and the costs associated with their housing and handling. We therefore believe that the reproduction of the pathophysiological alterations of acute pericarditis in the mouse may represent an easy and widely replicable model of pericarditis. The model of acute pericarditis reflecting the modifications of the acute pericardial inflammation may, however, not be adequate to evaluate long-term effects of pericardial inflammation, as occurs in chronic pericarditis. However, we are interested in tackling the acute phase of the disease presenting with disabling symptoms, such as chest pain, and therapies should be targeted to block the detrimental effects of the acute phase of the disease in order to prevent recurrences (3). Moreover, the use of genetically modified mice offers the advantage to study new pathways involved in the pathophysiology of pericarditis.
Although generally self-limiting, pericarditis can be refractory to standard therapies and/or recur in approximately 30% of cases (34,35). Despite this, its pathophysiology is still poorly understood, and current therapeutic strategies are limited and not always effective in avoiding recurrences. Indeed, no drug is approved by the U.S. Food and Drug Administration for the treatment of acute pericarditis. Currently, nonsteroidal anti-inflammatory drugs are commonly used as a first-line treatment, but despite being generally effective in controlling symptoms, they did not show a change in the disease course and are associated with a significant recurrence rate after discontinuation of treatment (1). Accordingly, we found that ibuprofen treatment affects some of the features of acute pericarditis in a mouse model. Indeed, ibuprofen reduced pericardial effusion but did not prevent the formation of the inflammasome nor the pericardial thickening. This effect was somewhat expected given its downstream effect on COX-2 with respect to the NLRP3 inflammasome (4).
The limited therapeutic armamentarium is in part due to the scarcity of animal models replicating the pathophysiological modifications occurring in acute pericarditis. With this regard, our mouse model of pericarditis with zymosan A mimics the acute injury occurring into the pericardium through which the NLRP3 inflammasome can be activated. Indeed, the NLRP3 inflammasome activation was only indirectly hypothesized until now, but not yet demonstrated. In fact, the positive results with anakinra coming from the AIRTRIP (Anakinra—Treatment of Recurrent Idiopathic Pericarditis) trial (11), and others (36) strongly supported the hypothesis that the NLRP3 inflammasome importantly contributes to the clinical symptoms of the pericardial syndrome. In addition, we now demonstrated that the NLRP3 inflammasome is expressed by pericardial cells and activates during an acute flare of pericarditis leading to the release of IL-1α and IL-1β, that explain the typical inflammatory symptoms complained by patients. Release of IL-1α from dying cells may also trigger an inflammatory response in pericarditis (6,8). Although IL-1 has been extensively studied in cardiovascular (i.e., acute myocardial infarction and heart failure) (37, 38, 39) and rheumatic diseases (i.e., rheumatoid arthritis, gout, and adult onset Still’s disease) (40), very little is known about its role in pericarditis (3). In this manuscript, we reported for the first time the presence and activation of the NLRP3 inflammasome in human samples of chronic pericarditis. This finding was corroborated by the concurrent presence of all of the 3 components of the inflammasome at the same time (ASC, NLRP3, and caspase-1) (6,23,24). We were also able to demonstrate an increased expression of IL-1α and IL-1β in the pericardium of mice treated with zymosan A compared with sham mice. This suggests that both these cytokines are strongly involved in the pathogenesis of acute pericarditis, as suggested by a recent report in which canakinumab failed to improve steroid-dependent idiopathic recurrent pericarditis in 2 children (28). In addition, blocking IL-1 using both anakinra and IL-1 trap in mice with acute pericarditis was highly effective in decreasing pericardial effusion and thickness as well as blunting the NLRP3 inflammatory response. Anakinra required a high and frequent dosing, consistent with its mechanism of action as a receptor antagonist, whereas the IL-1 trap was effective across a wide range of dosing and less frequent administrations, functioning as an entrapment for circulating IL-1β and IL-1α. In this regard, the double-blind placebo-controlled randomized-withdrawal phase 3 trial testing Interleukin-1 trap rilonacept in patients with multiple episode of recurrent pericarditis (NCT03737110), rilonacept significantly led to rapid resolution of recurrent pericarditis episodes and to a significantly lower risk of pericarditis recurrence than placebo (41).
Colchicine is now considered among first-line treatments for both acute and recurrent pericarditis based on different clinical trials showing its efficacy (1). The main mechanism of colchicine is not completely elucidated. Colchicine relies on the inhibition of microtubule polymerization and prevents neutrophil migration (42). Colchicine has been shown to interfere with the formation of the macromolecular structure of the NLRP3 inflammasome, preventing the association of its components and IL-1β release (10,43,44). In our study, colchicine, at the dose tested, significantly reduced the pericardial effusion and ASC aggregation in the zymosan A pericarditis model, while it failed to prevent the pericardial thickening. However, the effects of higher doses of colchicine could not be tested due to the toxicity of the drug. The use of the NLRP3inh was effective in preventing inflammasome formation, reducing pericardial effusion, and reducing pericardial thickening. This is of great interest because although colchicine is effective, a significant proportion of patients are refractory or cannot tolerate colchicine and require additional immunomodulating drugs. The role of the NLRP3inh is consistent with the clinical efficacy of IL-1 blockers in preliminary case reports (36,45, 46, 47, 48, 49, 50, 51).
However, this study is not without limitations. Human samples of pericarditis came from patients with long-term, chronic pericarditis needing pericardiectomy, and this may represent a selection bias. Unfortunately, pericardiectomy is neither routinely indicated in acute pericarditis nor ethically feasible but reserved only for those cases presenting with a constrictive evolution. The available pericardial specimens were limited in number and additional samples may be needed to further confirm and expand our results. The small surgical procedures require specialized skills, a learning curve, and are time-consuming. The mouse model presents clear challenges in the assessment of symptoms and behavioral effects. Additionally, the surgery in the mouse demands the use of narcotics for pain control, which means that chest pain, a common symptom in acute pericarditis, cannot be adequately assessed. Pericarditis is often considered related to a viral infection or an immune response to the virus, and therefore this model may not recapitulate all the upstream events leading to the inflammatory response. This animal model was conceived to explore the pathophysiological events occurring in acute pericarditis, and not to explore long-term effects as in chronic pericarditis. Chronic intrapericardial injection of zymosan A is not feasible as it necessitates anesthesia and surgery at every time and should be repeated at least every 7 days, thus possibly jeopardizing long-term survival of mice. In addition, the persistent stress induced on the pericardium by the needle may create local inflammation, that could represent a confounder in the analysis of results. As the model was designed with a translational perspective in mind, we chose to use pharmacological inhibitors and chose not to study genetically modified (i.e., NLRP3, IL-1, or IL-1R knockout) mice in this study. A systematic characterization of the cellular infiltrate in the pericardium was not performed at this time. A post hoc adjustment, such as Dunnett's test, was not considered for multiple pairwise comparisons, therefore potentially increasing the risk of an inflated type I error. Despite its limitations, this model may represent a first step to explore novel pathophysiological mechanisms and therapeutics in the future.
Conclusions
We demonstrated for the first time the expression and the activation of the NLRP3 inflammasome both in humans and in mice with pericarditis. Additionally, the instillation of zymosan A within the murine pericardium reproduces, through an NLRP3 inflammasome–dependent mechanism, the phenotype of acute pericarditis, which is responsive to colchicine, NLRP3inh, anakinra, and IL-1 trap. Using a small rodent model brings many advantages, including limited cost, ease of use, and the ability to manipulate genetic expression. Through this option, it will be possible to plan the development of new therapeutic approaches targeting NLRP3-driven pathways for further mechanistic and translational studies. The study also shows powerful inhibition of the inflammatory response in this pericarditis model with inhibitors of IL-1.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Acute pericarditis is generally a benign, self-limiting disease, although poor response to standard therapies or recurrences may occur in up to 30% of patients. To date, first-line treatments include colchicine, aspirin, and nonsteroidal anti-inflammatory drugs; however, anakinra, a recombinant IL-1 receptor antagonist, was shown to be effective in treating patients with recurrences and resistant to first-line treatments. This shed some light on the pathogenesis of pericarditis, which is not completely understood yet. Our results provide a strong support to the previously hypothesized role of the NLRP3 inflammasome in determining the clinical manifestations of pericarditis (chest pain, increase in inflammatory markers, pericardial effusion). We have shown that an increased expression of the inflammasome is present within the pericardium of patients with pericarditis compared with normal control subjects. Additionally, we created a mouse model of acute pericarditis and tested common drugs currently used to treat patients with pericarditis (e.g., ibuprofen, colchicine, anakinra, and also a direct inhibitor of the NLRP3 inflammasome and a recombinant murine IL-1 trap blocking IL-1α and IL-1β, the equivalent of rilonacept). All inflammasome blockers (i.e., NLRP3 inhibitor, anakinra, and IL-1 trap) were found to significantly improve pericardial alterations, suggesting that inhibition of the NLRP3 pathway may represent a valid therapeutic approach for the treatment of pericarditis.
TRANSLATIONAL OUTLOOK: The use of an animal model is of help to unravel the pathophysiological mechanisms of pericarditis. Our results recognize the importance of the NLRP3 inflammasome in the pathogenesis of pericarditis, thus allowing the development of targeted therapies in the future. In particular, the challenge is represented by the direct inhibition of the inflammasome in order to translate the encouraging results shown in the mouse model of pericarditis to humans. Indeed, the direct blockade of the NLRP3 inflammasome appears promising, as it occurs upstream of IL-1, so that other inflammasome products, such as IL-18 and IL-1α, might be inhibited. Future studies on this topic are warranted to prove this hypothesis and develop targeted therapeutic strategies.
Funding Support And Author Disclosures
This investigator-initiated study was supported by funding from Kiniksa Pharmaceuticals Ltd. Dr. Abbate has served as a consultant for AstraZeneca, Janssen, Kiniksa Pharmaceuticals, Merck, Novartis, Olatec, and Serpin Pharma. Dr. Toldo has received research support from Kiniksa, Serpin Pharma, and Olatec. Drs. Bonaventura and Vecchié have received travel grant support from Kiniksa Pharmaceuticals to attend the 2019 American Heart Association Scientific Sessions (Philadelphia, Pennsylvania). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Annalisa D’Andrea, Emmanuelle Hugentobler, and Moses Njenga (Kiniksa Pharmaceuticals) for critically reviewing the paper. The authors thank Regeneron Pharmaceuticals for supplying the IL-1 trap.
Footnotes
Part of the data have been presented as a poster during the American Heart Association Scientific Sessions in November 2019 in Philadelphia, Pennsylvania.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Adler Y., Charron P., Imazio M. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imazio M., Gaita F., LeWinter M. Evaluation and treatment of pericarditis: a systematic review. JAMA. 2015;314:1498–1506. doi: 10.1001/jama.2015.12763. [DOI] [PubMed] [Google Scholar]
- 3.Bonaventura A., Montecucco F. Inflammation and pericarditis: are neutrophils actors behind the scenes? J Cell Physiol. 2019;234:5390–5398. doi: 10.1002/jcp.27436. [DOI] [PubMed] [Google Scholar]
- 4.Buckley L.F., Viscusi M.M., Van Tassell B.W., Abbate A. Interleukin-1 blockade for the treatment of pericarditis. Eur Heart J Cardiovasc Pharmacother. 2018;4:46–53. doi: 10.1093/ehjcvp/pvx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 6.Mauro A.G., Bonaventura A., Mezzaroma E., Quader M., Toldo S. NLRP3 Inflammasome in Acute Myocardial Infarction. J Cardiovasc Pharmacol. 2019;74:175–187. doi: 10.1097/FJC.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 8.Bertheloot D., Latz E. HMGB1, IL-1alpha, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14:43–64. doi: 10.1038/cmi.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiabrando J.G., Bonaventura A., Vecchie A. Management of acute and recurrent pericarditis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:76–92. doi: 10.1016/j.jacc.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Bayes-Genis A., Adler Y., de Luna A.B., Imazio M. Colchicine in pericarditis. Eur Heart J. 2017;38:1706–1709. doi: 10.1093/eurheartj/ehx246. [DOI] [PubMed] [Google Scholar]
- 11.Brucato A., Imazio M., Gattorno M. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA. 2016;316:1906–1912. doi: 10.1001/jama.2016.15826. [DOI] [PubMed] [Google Scholar]
- 12.Toldo S., Das A., Mezzaroma E. Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ Cardiovasc Genet. 2014;7:311–320. doi: 10.1161/CIRCGENETICS.113.000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar H., Kumagai Y., Tsuchida T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol. 2009;183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 14.Monroe L.L., Armstrong M.G., Zhang X. Zymosan-induced peritonitis: effects on cardiac function, temperature regulation, translocation of bacteria, and role of dectin-1. Shock. 2016;46:723–730. doi: 10.1097/SHK.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti C., Swartzwelter B., Koenders M.I. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res Ther. 2018;20:169. doi: 10.1186/s13075-018-1664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchetti C., Chojnacki J., Toldo S. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63:316–322. doi: 10.1097/FJC.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti C., Toldo S., Chojnacki J. Pharmacologic inhibition of the NLRP3 inflammasome preserves cardiac function after ischemic and nonischemic injury in the mouse. J Cardiovasc Pharmacol. 2015;66:1–8. doi: 10.1097/FJC.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toldo S., Marchetti C., Mauro A.G. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol. 2016;209:215–220. doi: 10.1016/j.ijcard.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 19.Van Tassell B.W., Varma A., Salloum F.N. Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;55:117–122. doi: 10.1097/FJC.0b013e3181c87e53. [DOI] [PubMed] [Google Scholar]
- 20.Smilde B.J., Woudstra L., Fong Hing G. Colchicine aggravates coxsackievirus B3 infection in mice. Int J Cardiol. 2016;216:58–65. doi: 10.1016/j.ijcard.2016.04.144. [DOI] [PubMed] [Google Scholar]
- 21.Wolach B., Gotfried M., Jedeikin A., Lishner M., Brossi A., Ravid M. Colchicine analogues: effect on amyloidogenesis in a murine model and, in vitro, on polymorphonuclear leukocytes. Eur J Clin Invest. 1992;22:630–634. doi: 10.1111/j.1365-2362.1992.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 22.Toldo S., Mezzaroma E., O'Brien L. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1025–H1031. doi: 10.1152/ajpheart.00795.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masumoto J., Taniguchi S., Ayukawa K. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 24.Toldo S., Mezzaroma E., Mauro A.G., Salloum F., Van Tassell B.W., Abbate A. The inflammasome in myocardial injury and cardiac remodeling. Antioxid Redox Signal. 2015;22:1146–1161. doi: 10.1089/ars.2014.5989. [DOI] [PubMed] [Google Scholar]
- 25.Mezzaroma E., Toldo S., Farkas D. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kron J., Mauro A.G., Bonaventura A. Inflammasome formation in granulomas in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbate A., Santini D., Biondi-Zoccai G.G. Cyclo-oxygenase-2 (COX-2) expression at the site of recent myocardial infarction: friend or foe? Heart. 2004;90:440–443. doi: 10.1136/hrt.2003.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Signa S., D'Alessandro M., Consolini R. Failure of anti Interleukin-1 beta monoclonal antibody in the treatment of recurrent pericarditis in two children. Pediatr Rheumatol Online J. 2020;18:51. doi: 10.1186/s12969-020-00438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato M., Sano H., Iwaki D. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 30.Pagé P.L., Plumb V.J., Okumura K., Waldo A.L. A new animal model of atrial flutter. J Am Coll Cardiol. 1986;8:872–879. doi: 10.1016/s0735-1097(86)80429-6. [DOI] [PubMed] [Google Scholar]
- 31.Leak L.V., Ferrans V.J., Cohen S.R., Eidbo E.E., Jones M. Animal model of acute pericarditis and its progression to pericardial fibrosis and adhesions: ultrastructural studies. Am J Anat. 1987;180:373–390. doi: 10.1002/aja.1001800408. [DOI] [PubMed] [Google Scholar]
- 32.Franchi L., Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima A., Sakaue T., Okazaki M. A simple mouse model of pericardial adhesions. J Cardiothorac Surg. 2019;14:124. doi: 10.1186/s13019-019-0940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imazio M., Spodick D.H., Brucato A., Trinchero R., Adler Y. Controversial issues in the management of pericardial diseases. Circulation. 2010;121:916–928. doi: 10.1161/CIRCULATIONAHA.108.844753. [DOI] [PubMed] [Google Scholar]
- 35.Vecchie A., Chiabrando J.G., Dell M.S. Clinical presentation and outcomes of acute pericarditis in a large urban hospital in the United States of America. Chest. 2020;158:2556–2557. doi: 10.1016/j.chest.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wohlford G.F., Buckley L.F., Vecchie A. Acute effects of interleukin-1 blockade using anakinra in patients with acute pericarditis. J Cardiovasc Pharmacol. 2020;76:50–52. doi: 10.1097/FJC.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 37.Abbate A., Toldo S., Marchetti C., Kron J., Van Tassell B.W., Dinarello C.A. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126:1260–1280. doi: 10.1161/CIRCRESAHA.120.315937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbate A., Dinarello C.A. Anti-inflammatory therapies in acute coronary syndromes: is IL-1 blockade a solution? Eur Heart J. 2015;36:337–339. doi: 10.1093/eurheartj/ehu369. [DOI] [PubMed] [Google Scholar]
- 39.Buckley L.F., Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39:2063–2069. doi: 10.1093/eurheartj/ehy128. [DOI] [PubMed] [Google Scholar]
- 40.Dinarello C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019;15:610–632. doi: 10.1038/s41584-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 41.Klein A.L., Imazio M., Cremer P. RHAPSODY Investigators. Phase 3 Trial of Interleukin-1 Trap Rilonacept in recurrent pericarditis. N Engl J Med. 2021;384:31–41. doi: 10.1056/NEJMoa2027892. [DOI] [PubMed] [Google Scholar]
- 42.Paschke S., Weidner A.F., Paust T., Marti O., Beil M., Ben-Chetrit E. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J Leukoc Biol. 2013;94:1091–1096. doi: 10.1189/jlb.1012510. [DOI] [PubMed] [Google Scholar]
- 43.Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 44.Marques-da-Silva C., Chaves M.M., Castro N.G., Coutinho-Silva R., Guimaraes M.Z. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: implications for its therapeutic action. Br J Pharmacol. 2011;163:912–926. doi: 10.1111/j.1476-5381.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camacho-Lovillo M., Mendez-Santos A. Successful treatment of idiopathic recurrent pericarditis with interleukin-1 receptor antagonist (anakinra) Pediatr Cardiol. 2013;34:1293–1294. doi: 10.1007/s00246-013-0663-y. [DOI] [PubMed] [Google Scholar]
- 46.D'Elia E., Brucato A., Pedrotti P. Successful treatment of subacute constrictive pericarditis with interleukin-1beta receptor antagonist (anakinra) Clin Exp Rheumatol. 2015;33:294–295. [PubMed] [Google Scholar]
- 47.Lazaros G., Vasileiou P., Danias P. Effusive-constrictive pericarditis successfully treated with anakinra. Clin Exp Rheumatol. 2015;33:945. [PubMed] [Google Scholar]
- 48.Picco P., Brisca G., Traverso F., Loy A., Gattorno M., Martini A. Successful treatment of idiopathic recurrent pericarditis in children with interleukin-1beta receptor antagonist (anakinra): an unrecognized autoinflammatory disease? Arthritis Rheum. 2009;60:264–268. doi: 10.1002/art.24174. [DOI] [PubMed] [Google Scholar]
- 49.Scardapane A., Brucato A., Chiarelli F., Breda L. Efficacy of an interleukin-1beta receptor antagonist (anakinra) in idiopathic recurrent pericarditis. Pediatr Cardiol. 2013;34:1989–1991. doi: 10.1007/s00246-012-0532-0. [DOI] [PubMed] [Google Scholar]
- 50.Schatz A., Trankle C., Yassen A. Resolution of pericardial constriction with Anakinra in a patient with effusive-constrictive pericarditis secondary to rheumatoid arthritis. Int J Cardiol. 2016;223:215–216. doi: 10.1016/j.ijcard.2016.08.131. [DOI] [PubMed] [Google Scholar]
- 51.Vassilopoulos D., Lazaros G., Tsioufis C., Vasileiou P., Stefanadis C., Pectasides D. Successful treatment of adult patients with idiopathic recurrent pericarditis with an interleukin-1 receptor antagonist (anakinra) Int J Cardiol. 2012;160:66–68. doi: 10.1016/j.ijcard.2012.05.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.