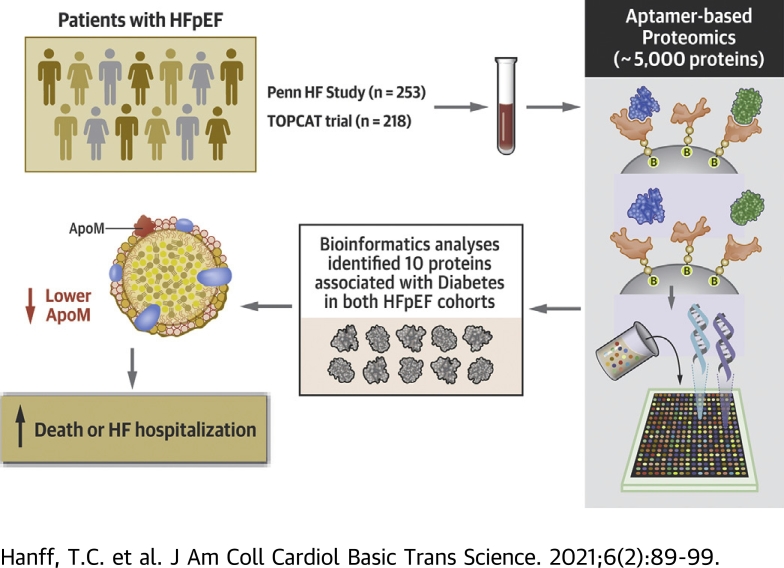
Visual Abstract
Key Words: apolipoprotein M, diabetes, heart failure, mediation analysis, proteomics
Abbreviations and Acronyms: ApoM, apolipoprotein M; CILP2, cartilage intermediate layer protein 2; CI, confidence interval; DM, diabetes mellitus; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; LASSO, least absolute shrinkage and selection operator
Highlights
-
•
DM is a significant risk factor for major adverse cardiovascular events in patients with HFpEF.
-
•
Patients with diabetes with HFpEF have a distinct proteome compared with patients without diabetes with HFpEF.
-
•
Proteomics analysis identified higher levels of alpha-1-microglobulin/bikunin precursor protein in patients with diabetes with HFpEF and lower levels of CILP2 and Apo M.
-
•
Lower Apo M levels mediate most of the association between diabetes and major adverse cardiovascular events in HFpEF.
Summary
Diabetes mellitus (DM) is associated with a higher risk of heart failure hospitalization and mortality in patients with heart failure with preserved ejection fraction (HFpEF). Using SomaScan assays and proteomics analysis of plasma from participants in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) trial and the Penn Heart Failure Study, this study identified 10 proteins with significantly different expression in patients with HFpEF and DM. Of these, apolipoprotein M was found to mediate 72% (95% CI: 36% to 100%; p < 0.001) of the association between DM and the risk of cardiovascular death, aborted cardiac arrest, and heart failure hospitalization.
Heart failure with preserved ejection fraction (HFpEF) affects 6.5 million patients in the United States each year, leading to high mortality rates and poor quality of life (1,2). Heterogeneous pathophysiology in HFpEF has impeded the discovery of therapies that, although ineffective in the broader population with HFpEF, could improve outcomes in select HFpEF subgroups. This pathophysiological heterogeneity is believed to have contributed to the absence of survival benefit in several large negative trials, including several trials of antihypertensive and neurohormonal agents (3,4). A refined understanding of phenotypic differences that mediate outcomes in high-risk HFpEF subgroups could aid clinical decision-making and potentially accelerate drug discovery via targeted trial enrollment.
Diabetes mellitus (DM) frequently coexists with HFpEF and is associated with a worse prognosis (5). Approximately 45% of patients with HFpEF have diabetes, more than in those with heart failure with reduced ejection fraction (6), and its concurrence in HFpEF is increasing (7). In an analysis of HFpEF phenogroups, DM was seen frequently in a distinct subset of patients who also had a greater incidence of chronic kidney disease, obesity, renin activation, and systemic inflammation, all of which might indicate a common pathophysiology related to metabolic dysregulation (8). Patients in this phenogroup also had the worst prognosis compared with other HFpEF subtypes, corroborating longitudinal analyses in HFpEF that showed higher rates of cardiovascular death and heart failure hospitalization in patients with diabetes (3,9). Moreover, DM is frequently observed as an antecedent to HFpEF, raising the possibility that the development and progression of HFpEF in patients with diabetes may partially be a sequelae of insulin resistance or metabolic dysregulation.
Several mechanisms have been proposed to explain the adverse interaction between DM and HFpEF. In general, patients with DM tend to have excess renin and angiotensin activation and upregulated sodium-glucose transporter 2 activity, which could exacerbate volume overload in HFpEF (10). In addition, DM and HFpEF are both associated with increased systemic inflammation (11), which could exacerbate endothelial dysfunction and cardiac extracellular matrix remodeling. Glycated end-products from hyperglycemia also lead to microvascular dysfunction and decreased nitric oxide bioavailability in the vascular endothelium, which are processes implicated in HFpEF pathogenesis (12). Lastly, insulin resistance is associated with increased free fatty acid use by the myocardium, which over time may lead to the accumulation of toxic lipid intermediates that impair myocyte mitochondrial function (12). Each of these mechanisms could contribute to HFpEF risk on their own or strengthen the association between HFpEF and the poor outcomes mediated by other conditions such as hypertension, obesity, and advanced age. Nevertheless, the specific mechanisms mediating the increased risk of adverse outcomes associated with DM in HFpEF are unknown.
Advances in peripheral blood analytical techniques and proteomics analysis now permit the simultaneous characterization of thousands of peripheral blood biomarkers, which may help clarify the relative importance of divergent biological pathways in HFpEF with DM. This strategy has been used previously in patients with DM and HFrEF (13). In this study, we sought to: 1) characterize key proteomic differences between patients with and without diabetes in 2 independent HFpEF cohorts; and 2) use mediation analysis to determine the extent to which these proteomic differences mediate the association between DM and adverse HFpEF outcomes.
Methods
Data source and study population
Data for this study were obtained from 2 independent cohorts, the PHFS (Penn Heart Failure Study) and the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial) trials (4). TOPCAT was a randomized, double-blinded, international trial of spironolactone therapy versus placebo that enrolled 3,445 patients with HFpEF from 2006 to 2012. Key inclusion criteria were age 50 years or older, left ventricular ejection fraction ≥45%, and at least 1 sign and 1 symptom of heart failure. Clinical data from the parent trial used for this study are available to researchers through the National Institutes of Health BioLINCC. The PHFS was a prospective cohort study of patients referred to specialty heart failure centers at the University of Pennsylvania (Philadelphia, Pennsylvania), Case Western Reserve University (Cleveland, Ohio), or the University of Wisconsin (Madison, Wisconsin) (14). In PHFS, HFpEF was defined by a left ventricular ejection fraction ≥50%. For this study, PHFS served as the index cohort for the initial proteomic quantification, and TOPCAT was used for proteomic validation and mediation analysis. All study participants provided written informed consent, and all participating institutions received approval from local institutional review boards.
Baseline medical history and biomarkers were collected before randomization and study drug administration in TOPCAT or following the initial intake visit at participating centers in PHFS. In PHFS, plasma samples for proteomic quantification were available from 253 of 269 participants with HFpEF (94%). In TOPCAT, frozen blood plasma samples were available from the National Institutes of Health for proteomic quantification in 218 subjects. This constituted only a subset of the 3,445 trial participants (6.3%), all of whom were enrolled in the United States and Canada.
The presence or absence of DM at baseline was documented in each cohort by medical history. We compared clinical differences between patients with and without DM at baseline within each cohort using the unpaired Student's t-test for normally distributed variables, the Wilcoxon rank-sum test for non-normal variables, and the chi-square test for categorical variables. We examined the possibility of selection bias in TOPCAT by comparing baseline characteristics in patients with and without available plasma samples. Continuous variables are represented as mean ± SD or median with 25th and 75th percentiles (quartile 1, quartile 3), where appropriate. Statistical significance for clinical variables was defined as a 2-tailed p value < 0.05.
Plasma protein quantification
All plasma samples were analyzed using the SomaScan assay version 4, (SomaLogic Inc., Boulder, Colorado), which is a multiplexed, modified aptamer-based binding-assay. The SomaScan assay uses slow-off-rate modified aptamer (SOMAmer) reagents, which are chemically modified nucleotides, to bind and quantify target proteins in relative fluorescent units directly proportional to the amount of target protein in the sample. Assay details have been previously described (15). This assay includes 4,979 modified aptamer reagents to 4,776 unique protein targets.
Proteomic feature selection
Proteins were selected for mediation analysis using a 3-stage procedure. First, the univariate association of DM with each protein was analyzed separately in the index and validation cohorts, following Box-Cox transformation and standardization into a Z-score (16). A Benjamini-Yekutieli false discovery rate cutoff of 0.05 defined significance within each cohort to control for multiple comparisons (17). Next, proteins with overlapping significance in both cohorts were selected for further analysis in the validation cohort using adaptive least absolute shrinkage and selection operator (LASSO) regression. This allowed dimension reduction down to a set of proteins that each contributed independent explanatory power for the presence of DM (18,19). The LASSO regression procedure was performed in a model that included only proteins and a second model that incorporated clinical covariates, including age, sex, race/ethnicity, body mass index, smoking history, hyperlipidemia, and statin use. Finally, we were interested in proteins selected by LASSO that potentially mediated the association between DM and adverse outcomes. For this, we modeled the time to a composite of cardiovascular death, aborted cardiac arrest, or heart failure hospitalization using accelerated failure time models based on the Weibull distribution. We derived unadjusted models and models adjusted for age, sex, race/ethnicity, body mass index, smoking history, hyperlipidemia, statins, and insulin. Non-cardiovascular death was treated as a competing risk. Proteins that satisfied each selection step were selected for mediation analysis.
Mediation analysis
The goal of mediation analysis is to quantify the proportion of an association between an exposure (e.g., diabetes) and an outcome (e.g., the time to event) that is mediated by a proposed intermediary variable (e.g., a protein). Such analyses suggest mediating effects and causal pathways at a population level that can inform experimental approaches for confirmation. The total effect of DM on the outcome can be decomposed into the pure indirect effect mediated by a protein and the total direct effect of DM that is independent of the intermediary protein (20). Mediation effects were modeled using an accelerated failure time model for the outcome and linear regression for the mediation. All models were adjusted for the same covariates as described previously. Analyses were performed using Stata 16.0 for Mac (StataCorp LLC., College Station, Texas).
Results
Baseline clinical characteristics
In PHFS, 253 participants had plasma samples available for protein quantification. This constituted 94% of all participants with HFpEF, 75 of whom (30%) had DM at baseline. In TOPCAT, 218 subjects (6.3%) had available plasma samples, 103 of whom (47%) had DM. Differences in baseline characteristics between participants in TOPCAT with and without available samples are listed in Supplemental Table S1. All TOPCAT samples were from participants enrolled in North America. Participants with available samples were more likely to have diabetes (47.2% vs. 31.5%), to be older (72 years vs. 69 years), and to be male (56% vs. 48%). The distribution of reported ethnicities was comparable.
The baseline characteristic of the sampled populations from PHFS and TOPCAT are listed in Table 1, stratified by the presence or absence of DM. Participants with diabetes in PHFS were older compared with participants without diabetes (65.1 years vs. 59.3 years), less likely to be men (37.3% vs. 56.7%), more likely to be black (36% vs. 21.9%), and had a higher estimated glomerular filtration rate (71 ml/min vs. 67 ml/min). Conversely, participants with diabetes in TOPCAT were younger (69 years vs. 76 years), had a lower estimated glomerular filtration rate (58 ml/min vs. 67 ml/min), and had no statistically significant difference in sex or ethnicity. Participants with diabetes in both cohorts had a higher body mass index and a higher frequency of hyperlipidemia, statin use, and hyperlipidemia. Insulin therapy was present in 44% of PHFS participants and 41.7% of TOPCAT participants.
Table 1.
Baseline Demographic and Clinical Variables in PHFS and TOPCAT
| PHFS (n = 253) |
TOPCAT (n = 218) |
|||||
|---|---|---|---|---|---|---|
| Not Diabetic (n = 178) | Diabetic (n = 75) | p Value | Not Diabetic (n = 115) | Diabetic (n = p103) | p Value | |
| Demographics | ||||||
| Age (yrs) | 59 (47−72) | 65 (59−74) | 0.002 | 76 (67−82) | 69 (61−77) | <0.001 |
| Male | 101 (57) | 28 (37) | 0.005 | 59 (51) | 63 (61) | 0.14 |
| Ethnicity | 0.038 | 0.10 | ||||
| White | 131 (74) | 43 (57) | 103 (90) | 85 (83) | ||
| Black | 39 (22) | 27 (36) | 9 (8) | 17 (17) | ||
| Other | 8 (5) | 5 (7) | 3 (3) | 1 (1) | ||
| Medical history | ||||||
| CABG | 17 (10) | 15 (20) | 0.022 | 27 (24) | 31 (30) | 0.27 |
| PCI | 28 (16) | 20 (27) | 0.043 | 21 (18) | 35 (34) | 0.008 |
| Hypertension | 109 (61) | 66 (88) | <0.001 | 106 (92) | 100 (97) | 0.11 |
| Hyperlipidemia | 84 (47) | 54 (72) | <0.001 | 82 (71) | 86 (84) | 0.033 |
| Atrial fibrillation | 65 (37) | 20 (27) | 0.13 | 67 (58) | 41 (40) | 0.007 |
| Active smoker | 12 (7) | 3 (4) | 0.40 | 7 (6) | 6 (6) | 0.94 |
| Former smoker | 81 (46) | 46 (61) | 0.021 | 60 (52) | 67 (65) | 0.054 |
| Medications | ||||||
| Beta-blocker | 118 (66) | 54 (72) | 0.37 | 92 (80) | 89 (86) | 0.21 |
| CCB | 49 (28) | 23 (31) | 0.61 | 43 (37) | 42 (41) | 0.61 |
| ACEi or ARB | 100 (56) | 54 (72) | 0.019 | 82 (71) | 82 (80) | 0.16 |
| Statin | 75 (42) | 48 (64) | 0.001 | 75 (65) | 86 (84) | 0.002 |
| Insulin % | 0 (0) | 33 (44) | <0.001 | 0 (0) | 43 (42) | <0.001 |
| Vitals and labs | ||||||
| BMI (kg/m2) | 29 (25−34) | 34 (29−41) | <0.001 | 31 (27−34) | 36 (32−42) | <0.001 |
| SBP (mm Hg) | 123 (110−137) | 131 (117−150) | 0.001 | 122 (110−132) | 128 (118−137) | 0.044 |
| DBP (mm Hg) | 70 (64−81) | 72 (65−79) | 0.85 | 70 (62−78) | 70 (61−78) | 0.83 |
| GFR (ml/min) | 67 (51−81) | 71 (66−82) | 0.004 | 67 (57−78) | 58 (45−74) | 0.011 |
| BNP (pg/ml) | 93 (27−326) | 83 (33−241) | 0.94 | 461 (174−864) | 506 (265−1,311) | 0.21 |
Values are or median (25th to 75th percentiles) or n (%).
ACE = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; BNP = brain natriuretic peptide; CABG = coronary artery bypass graft; CCB = calcium channel blocker; COPD = chronic obstructive pulmonary disease; DBP = diastolic blood pressure; GFR = glomerular filtration rate; PCI = percutaneous coronary intervention; PHFS = Penn Heart Failure Study; SBP = systolic blood pressure; TOPCAT = Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial.
Differential protein expression in participants with diabetes
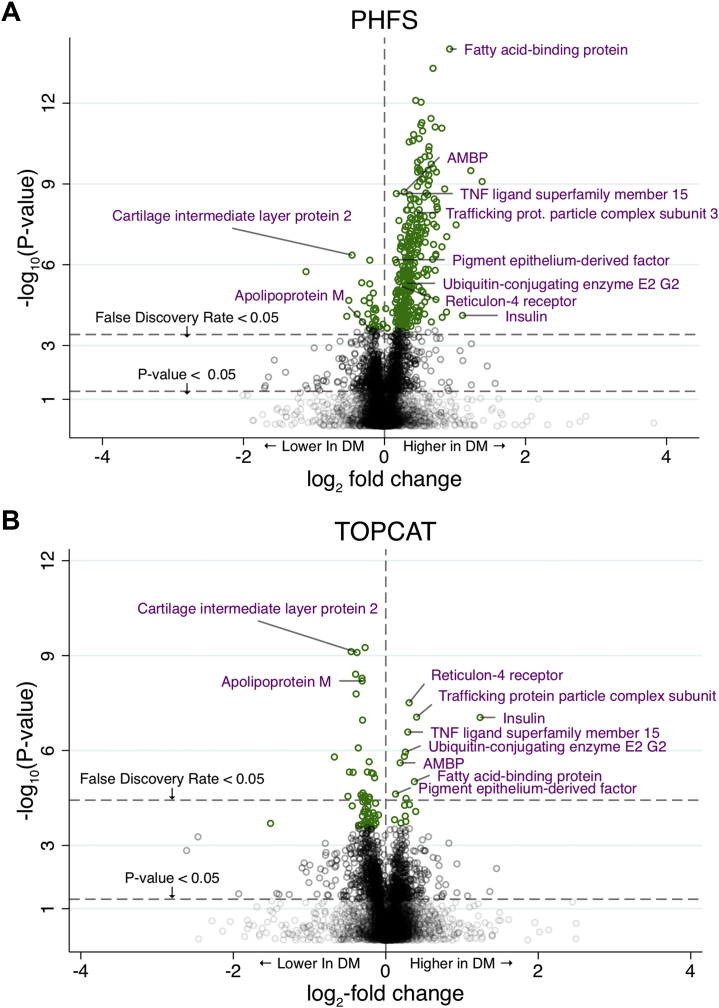
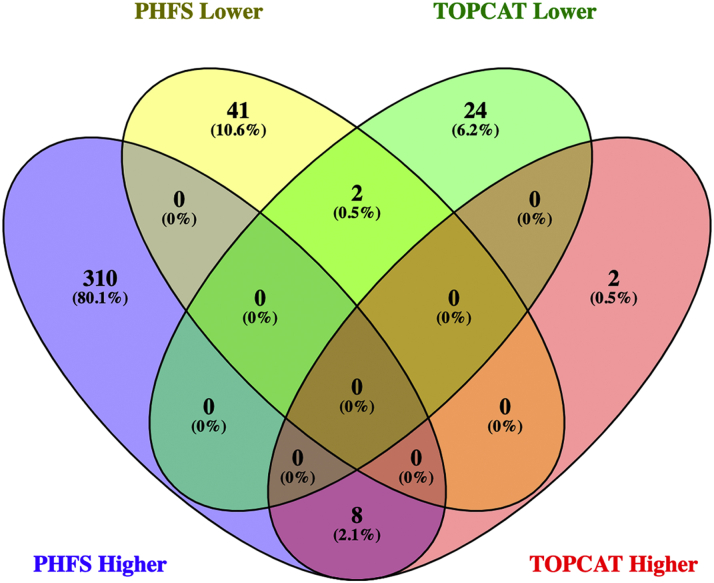
In univariate models, 361 proteins had significantly different expression between participants with or without diabetes in PHFS after correction for multiple comparisons, of which 318 were upregulated and 43 were downregulated in participants with diabetes (Figure 1A). In TOPCAT, 36 proteins had significantly different expression, of which 10 were upregulated and 26 were downregulated (Figure 1B). This resulted in 10 proteins that were significantly associated with DM in both cohorts with the same direction of change (Figure 2). Table 2 shows the univariate associations of these proteins with DM. These proteins included fatty acid-binding protein, alpha-1-microglobulin/bikunin precursor, trafficking protein particle complex subunit 3, pigment epithelium-derived factor, tumor necrosis factor ligand superfamily member 15, ubiquitin-conjugating enzyme E2 G2, reticulon-4 receptor, insulin, cartilage intermediate layer protein 2 (CILP2), and apolipoprotein M (ApoM). Using adaptive LASSO regression, 6 of the 10 proteins were identified as those that contributed independent information for the presence of DM before adjustment for clinical covariates, and 3 proteins were retained by LASSO regression after adjustment for clinical covariates (Table 3). Proteins retained after covariate adjustment included the alpha-1-microglobulin/bikunin precursor protein, which is higher in DM, as well as ApoM and CILP2, which are both lower in DM.
Figure 1.
Proteins Associated With Diabetes at Baseline in 2 Independent HFpEF Cohorts
Volcano plots showing the strength (log2-fold change) and significance (-log10(P-value)) of univariate associations between proteins and diabetes. Shared proteins between the Penn Heart Failure Study (PHFS) and TOPCAT are labeled. HFpEF = heart failure with preserved ejection fraction; PHFS = Penn Heart Failure Study; TOPCAT=Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial.
Figure 2.
Number of Overlapping Proteins in TOPCAT and the Penn Heart Failure Study
PHFS = Penn Heart Failure Study; TOPCAT = Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial.
Table 2.
Proteins Associated With DM in 2 Independent HFpEF Cohorts, Unadjusted
| UNIPROT ID |
Increased in DM |
PHFS (n = 253) |
TOPCAT (n = 218) |
||
|---|---|---|---|---|---|
| Protein Name | Log 2-Fold Change | p Value | Log 2-Fold Change | p Value | |
| P05413 | Fatty acid-binding protein | 0.92 | 9.8 × 10−15 | 0.38 | 9.6 × 10−6 |
| P02760 | Alpha-1-microglobulin/bikunin precursor | 0.28 | 2.0 × 10−9 | 0.19 | 2.4 × 10−6 |
| O43617 | Trafficking protein particle complex subunit 3 | 0.46 | 1.2 × 10−8 | 0.40 | 8.8 × 10−8 |
| P36955 | Pigment epithelium-derived factor | 0.15 | 6.5 × 10−7 | 0.13 | 2.4 × 10−5 |
| O95150 | Tumor necrosis factor ligand superfamily member 15 | 0.17 | 2.3 × 10−9 | 0.29 | 2.6 × 10−7 |
| P60604 | Ubiquitin-conjugating enzyme E2 G2 | 0.32 | 4.9 × 10−6 | 0.26 | 1.1 × 10−6 |
| Q9BZR6 | Reticulon-4 receptor | 0.25 | 6.5 × 10−6 | 0.31 | 3.1 × 10−8 |
| P01308 | Insulin | 1.11 | 7.6 × 10−5 | 1.24 | 9.0 × 10−8 |
| Decreased in DM | |||||
| Q8IUL8 | Cartilage intermediate layer protein 2 | −0.46 | 4.0 × 10−7 | −0.38 | 8.0 × 10−10 |
| O95445 | Apolipoprotein M | −0.21 | 2.3 × 10−4 | −0.31 | 6.3 × 10−9 |
DM = diabetes mellitus; HFpEF = heart failure with preserved ejection fraction; UNIPROT = Universal Protein.
Table 3.
Proteins Associated With TOPCAT After Adaptive LASSO Selection
| UNIPROT ID |
Proteins Only |
Proteins + Covariates∗ |
|
|---|---|---|---|
| Odds Ratio for DM† | Odds Ratio for DM | ||
| Alpha-1-microglobulin/bikunin precursor | P02760 | — | 2.1 |
| Trafficking protein particle complex subunit 3 | O43617 | 1.4 | — |
| Ubiquitin-conjugating enzyme E2 G2 | P60604 | 1.3 | — |
| Pigment epithelium-derived factor | P36955 | 1.7 | — |
| Reticulon-4 receptor | Q9BZR6 | 1.7 | — |
| Insulin | P01308 | 3.3 | — |
| Cartilage intermediate layer protein 2 | Q8IUL8 | 0.4 | 0.4 |
| Apolipoprotein M | O95445 | 0.5 | 0.6 |
Covariates retained in the least absolute shrinkage and selection operator (LASSO) regression include age, race/ethnicity, body mass index, smoking history, hyperlipidemia, and statin use. — = a protein was not retained by LASSO in that model.
Odds ratio are per SD.
Finally, of these proteins, only ApoM was significantly associated with the time to cardiovascular death, aborted cardiac arrest, or heart failure hospitalization (Table 4). After adjusting for age, race/ethnicity, body mass index, smoking history, hyperlipidemia, statin use, and insulin use in addition to DM and levels of CILP2 and alpha-1-microglobulin/bikunin precursor protein, ApoM remained associated with a 47% reduction in the primary outcome (hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.36 to 0.78; p = 0.001). DM was associated with the primary outcome in unadjusted models that did not include any proteins (unadjusted HR: 2.1; 95% CI: 1.3 to 3.6; p = 0.003), but these effects dissipated upon the inclusion of ApoM. This, together with the fact that participants with diabetes in TOPCAT had lower levels of ApoM (log2-fold change −0.31; p < 0.001), suggested that lower levels of ApoM might mediate some or all of the association between DM and the primary outcome.
Table 4.
Multivariable Association of Diabetes and Feature-Selected Proteins With the Composite Cardiovascular Endpoint
| UNIPROT | Proteins Only |
Proteins + Covariates∗ |
|||
|---|---|---|---|---|---|
| Hazard Ratio† (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | ||
| DM | N/A | 1.32 (0.71−2.45) | 0.38 | 1.00 (0.47−2.15) | 0.99 |
| Alpha-1-microglobulin/bikunin precursor | P02760 | 1.17 (0.84−1.61) | 0.35 | 1.29 (0.90−1.85) | 0.17 |
| Cartilage intermediate layer protein 2 | Q8IUL8 | 0.99 (0.73−1.34) | 0.93 | 1.00 (0.70−1.44) | 0.98 |
| Apolipoprotein M | O95445 | 0.60 (0.42−0.85) | 0.004 | 0.53 (0.36−0.78) | 0.001 |
CI = confidence interval; other abbreviation as in Table 3.
Adjusted for age, race/ethnicity, body mass index, smoking history, hyperlipidemia, statin use, and insulin use.
Hazard ratios are per SD. Non-cardiovascular death was incorporated in the survival model as a competing risk.
Mediation analysis results
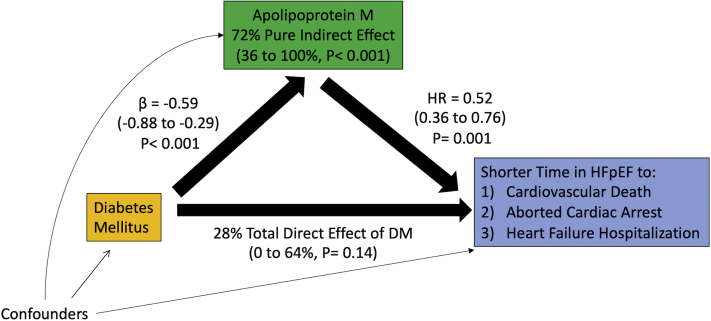
Mediation analysis confirmed that ApoM mediated nearly three-quarters of the association between DM and the primary outcome as a pure indirect effect (72%; 95% CI: 36% to 100%; p < 0.001), which refers to the excess risk of the outcome directly attributable to an intermediary variable. Conversely, the total direct effect of DM after controlling for ApoM and other covariates was insignificant (28%; 95% CI: 0% to 64%; p = 0.14) (Table 5, Figure 3).
Table 5.
Estimated Mediation Effects of ApoM on the Association Between DM and the Composite Cardiovascular Endpoint
| Mediation Term |
Percent of Total Excess Risk (95% CI) | p Value | |
|---|---|---|---|
| Total excess relative risk of DM∗ | −0.63 (−1.03 to −0.23)† | 1 | 0.002 |
| Total direct effect of DM | −0.17 (−0.48 to 0.13) | 28% (0% to 64%) | 0.14 |
| Pure indirect effect of ApoM | −0.45 (−0.70 to −0.21) | 72% (36% to 100%) | 0.001 |
The total excess relative risk of DM can be decomposed into a total direct effect of DM (that which is independent of apolipoprotein M [ApoM]) and a pure indirect effect (that which is directly mediated by ApoM).
Estimates of excess risk are presented along with 95% CIs.
Figure 3.
Mediation Pathway Diagram of Apolipoprotein M and Diabetes Mellitus in HFpEF
Diabetes mellitus (DM) is associated with lower ApoM (0.59 SDs lower), whereas higher ApoM is protective against the composite outcome (hazard ratio [HR] 0.52 per SD). The indirect pathway through ApoM mediates 72% (95% CI: 36% to 100%) of the association between DM and the composite outcome. The total direct effect of DM on the outcome was insignificant after removing the effect of ApoM. Models are adjusted for sex, race/ethnicity, body mass index, smoking history, hyperlipidemia, statin use, and insulin use.
Discussion
DM is a HFpEF comorbidity associated with adverse clinical outcomes. We used an aptamer-based proteomics approach and mediation analysis to evaluate proteins that mediated the association between DM and adverse HFpEF outcomes. This approach revealed several interesting and novel findings.
First, proteomic analysis identified 10 proteins that were strongly associated with DM across 2 independent HFpEF cohorts, 3 of which were independently associated with the presence of DM after LASSO selection and adjustment for each of the other proteins and baseline comorbidities. These proteins were ApoM and CILP2, which were both lower in participants with diabetes, as well as alpha-1-microglobulin/bikunin precursor, which was higher in participants with diabetes. These results were consistent with other proteomic and transcriptomic analysis in patients with DM without HFpEF (21). Several other proteins were significantly different in DM in only 1 of the 2 cohorts, which could reflect differences in baseline comorbidities between the 2 cohorts or phenotyopic heterogeneity in HFpEF. Consequently, the simultaneous significance of the selected proteins across both cohorts provided additional evidence of their consistent effect in HFpEF and DM2.
Next, we confirmed that DM was associated with a shorter time to cardiovascular death, aborted cardiac arrest, or heart failure hospitalization, which was previously reported in TOPCAT and other cohorts (4,5,12). Of the 3 proteins independently associated with DM, only ApoM appeared to directly mediate the association between DM and HFpEF outcomes. Using formal mediation analysis, we confirmed that ApoM mediated nearly three-quarters of the association between DM and the time to death, aborted cardiac arrest, or heart failure hospitalization (72%; 95% CI: 36% to 100%; p < 0.001), whereas the direct effect of DM itself was insignificant (28%; 95% CI: 0% to 64%; p = 0.14).
HFpEF is a conglomeration of multiple complex, inter-related cardiovascular and systemic impairments that combine to produce symptomatic heart failure (22). Subgroups in HFpEF with distinct pathophysiological characteristics require deeper phenotyping to match patients with appropriate therapies and investigate novel therapeutic interventions. DM is highly prevalent in HFpEF and is associated with worse outcomes, although the exact mechanism by which it exacerbates HFpEF outcomes is unknown. Our results suggested a potential causal pathway linking DM to decreased ApoM levels as an essential mediator of outcomes.
ApoM is a protein that is primarily secreted by the liver and bound to high-density lipoprotein in the plasma. It exerts pleiotropic anti-inflammatory, antioxidant, and anti-atherogenic effects (23). The main function of ApoM is that it binds sphingosine-1-phosphate, a bioactive sphingolipid that is involved in endothelial barrier function, inflammation, and apoptosis (24). The association between ApoM and adverse HFpEF outcomes has been previously identified, including analyses from the PHFS cohort and TOPCAT trial using both SomaScan and enzyme-linked immunosorbent assay (25). However, those analyses began by specifically interrogating the role of ApoM in heart failure as an a priori hypothesis based on a biologically plausible relationship. The present analysis arrived at a key role for ApoM using a fundamentally different agnostic approach, in which we first searched for proteins associated with DM, giving equal initial consideration to approximately 5,000 proteins, regardless of potential associations with outcomes. In addition, this analysis extended previous observations to show that ApoM was important not only as an independent risk factor for HFpEF outcomes, but also as a potential mediator of the association between DM and HFpEF outcomes. Because of the high prevalence of DM in HFpEF (upwards of 50%), the population attributable risk of ApoM in HFpEF is high, and there might be prognostic usefulness to screening for ApoM in patients with HFpEF and DM.
External validation of the direction of the proposed causal pathway between DM and low ApoM is critical because mediators can behave similarly to confounders in observational studies. In this regard, there are several lines of evidence that suggest that DM is upstream of decreased ApoM, corroborating the biological plausibility of ApoM as a downstream mediator of HFpEF outcomes in patients with diabetes. Levels of ApoM were markedly decreased in hyperglycemic mouse models of DM (26). A similar effect was observed in humans, in whom type 2 diabetes and insulin resistance in non-HFpEF populations were shown to be associated with reduced serum ApoM (27). Lastly, Mendelian randomization studies suggested that reduced ApoM did not lead to the development of DM, attenuating a hypothesis that the causal pathway operates in the reverse direction from ApoM to DM (28). Nevertheless, whether ApoM is causally related to outcomes in HFpEF remains unknown. Similarly, whether administration of exogenous ApoM or other therapeutic approaches aimed at increasing ApoM levels can ameliorate the risk of adverse outcomes in patients with diabetes with HFpEF remains to be determined.
Study limitations
This study included global proteomic quantification across 2 independent cohorts, which increased the probability that the observed protein associations with DM were generalizable and not due to random chance. Use of the false discovery rate minimized the overall type I error rate, but some meaningful proteomic differences could be excluded that would be detected with a larger sample size. We eliminated potentially redundant or confounded biomarkers through the use of adaptive LASSO regression, which increased the likelihood that the remaining set of proteins carried useful independent information about the DM proteome.
A limitation of this analysis was that biomarkers were only measured after the development of both DM and HFpEF. Taken alone, this can cloud the temporal relationship of these conditions because we could not be certain whether DM led to the observed proteomic differences in HFpEF or vice versa. However, with regard to the primary finding of ApoM, previous animal and human studies suggested that decreased ApoM might be downstream of DM, as discussed previously (26,28,29). Only a subset of participants in the TOPCAT cohort had plasma samples available for de novo proteomic quantification. This raised the possibility of selection bias, evidenced by some observed differences between participants with and without available samples, including a higher prevalence of DM in sampled participants (Supplemental Table S1). This provided further rationale for our use of a second cohort to cross-validate proteome changes. However, a previous association between ApoM and adverse HFpEF outcomes was already identified in TOPCAT and PHFS (25). Although the results of the present analysis arrived at the role for ApoM using an agnostic proteomic approach starting with approximately 5,000 candidate proteins, further validation of an association between DM and ApoM in a third cohort would make this even more robust. Lastly, the definition of DM in this study was binary, which might mask gradient effects proportional to diabetes severity. To partially account for this, we adjusted for insulin treatment at baseline. In addition, a portion of the participants labeled as having DM might have had type 1 rather than type 2 DM, which likely had different levels of important plasma proteins. Because of the high prevalence of type 2 DM in HFpEF, we expected the proportion of participants with type 2 DM to be small.
Conclusions
Using a high-throughput proteomic strategy, we identified several proteins related to lipid metabolism, inflammation, and oxidative stress that are differentially expressed in patients with diabetes with HFpEF. A particularly important protein in diabetic HFpEF was ApoM, a high-density lipoprotein−associated protein that mediates endothelial protective, anti-inflammatory, and anti-apoptotic effects. Using mediation analysis, we demonstrated that 72% of the association between DM and shorter time to death, aborted cardiac arrest, or heart failure hospitalization was mediated by decreased ApoM. Thus, ApoM may be a useful prognostic screening tool in patients with HFpEF and DM, and therapies that lead to increased ApoM in DM HFpEF could have therapeutic efficacy, which warrants further study.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: HFpEF is a conglomeration of multiple complex, inter-related cardiovascular and systemic impairments that combine to produce symptomatic heart failure. Comorbidities in HFpEF may contribute significantly to the risk of major adverse cardiovascular events. In particular, DM is associated with worse outcomes in HFpEF. Our results suggested that most of the association between DM adverse cardiovascular events in HFpEF is mediated through lower levels of Apo M, which is a protein secreted by the liver that exerts pleiotropic anti-inflammatory, antioxidant, and anti-atherogenic effects.
TRANSLATIONAL OUTLOOK: Several pre-clinical mouse models and transcriptomic analyses have shown an association between DM and reduced Apo M levels, which would promote inflammation, oxidative stress, and atherogenic effects. This study extended these results to patients with HFpEF and DM, using an unbiased proteomics approach that identified a unique role for Apo M as a potential mediator of the association between DM and major adverse cardiovascular events. Future investigation of a role for exogenous Apo M administration is warranted in patients with HFpEF and DM.
Funding Support and Author Disclosures
Dr. Hanff is supported by the National Institutes of Health (grant HL-00789). Dr. Cohen is supported by K23-HL133843. Dr. Javaheri is supported by K08-HL138262. Dr. Zamani is supported by grant K23-HL-13055. Dr. Chirinos is supported by a research grant from Bristol-Myers Squibb and by grants R01-HL 121510- 01A1, R61-HL-146390, R01-AG058969, 1R01-HL104106, P01-HL094307, R03-HL146874-01, and R56-HL136730. Dr. Javaheri has been named co-inventor on the patent application for the use of fusion protein nanodiscs for the treatment of heart failure. Drs. Zhao, Walsh, Maranville, Wang, Adam, Ramirez-Valle, Schafer, Seiffert, Gordon, and Cvijic own stock in Bristol-Myers Squibb. Dr. Cappola has received research funding from Bristol-Myers Squibb. Dr. Zamani has consulted for Vyaire. Dr. Chirinos has been a consultant for Sanifit, Microsoft, Fukuda-Denshi, Bristol-Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck, Bayer, JNJ, and Edwards Life Sciences; and received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb, and Microsoft, and has been named as inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of heart failure and preserved ejection fraction. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
This paper was prepared using TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial) research materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. Results and conclusions do not necessarily reflect the opinions or views of the TOPCAT Trial or the National Heart, Lung, and Blood Institute.
Footnotes
Dwight Towler, MD, served as Guest Editor for this paper. Michael R. Bristow, PhD, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart disease and stroke statistics’2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S., Pfeffer M.A., Swedberg K. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B., Pfeffer M.A., Assmann S.F. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 5.Lindman B.R. The diabetic heart failure with preserved ejection fraction phenotype: is it real and is it worth targeting therapeutically? Circulation. 2017;135:736–740. doi: 10.1161/CIRCULATIONAHA.116.025957. [DOI] [PubMed] [Google Scholar]
- 6.Mentz R.J., Kelly J.P., Von Lueder T.G. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echouffo-Tcheugui J.B., Xu H., DeVore A.D. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get With The Guidelines–Heart Failure registry. Am Heart J. 2016;182:9–20. doi: 10.1016/j.ahj.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J.B., Schrauben S.J., Zhao L. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. J Am Coll Cardiol HF. 2020;8:172–184. doi: 10.1016/j.jchf.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McHugh K., DeVore A.D., Wu J. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:602–611. doi: 10.1016/j.jacc.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Scheen A.J. Cardiovascular effects of new oral glucose-lowering agents DPP-4 and SGLT-2 inhibitors. Circ Res. 2018;122:1439–1459. doi: 10.1161/CIRCRESAHA.117.311588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah S.J., Kitzman D.W., Borlaug B.A. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dei Cas A., Khan S.S., Butler J. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. J Am Coll Cardiol HF. 2015;3:136–145. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Tromp J., Voors A.A., Sharma A. Distinct pathological pathways in patients with heart failure and diabetes. J Am Coll Cardiol HF. 2020;8:234–242. doi: 10.1016/j.jchf.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Basuray A., French B., Ky B. Heart failure with recovered ejection fraction clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–2387. doi: 10.1161/CIRCULATIONAHA.113.006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold L., Ayers D., Bertino J. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Box G.E.P., Cox D.R. An analysis of transformations. J R Stat Soc Ser B. 1964;26:211–252. [Google Scholar]
- 17.Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- 18.Hilario M., Kalousis A. Approaches to dimensionality reduction in proteomic biomarker studies. Br Bioinform. 2008;9:102–118. doi: 10.1093/bib/bbn005. [DOI] [PubMed] [Google Scholar]
- 19.Zou H. The adaptive Lasso and its oracle properties. J Am Stat Assoc. 2006;101:1418–1429. [Google Scholar]
- 20.Vanderweele T.J. A three-way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiology. 2013;24:224–232. doi: 10.1097/EDE.0b013e318281a64e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gudmundsdottir V., Zaghlool S.B., Emilsson V. Circulating protein signatures and causal candidates for type 2 diabetes. Diabetes. 2020;69:1843–1853. doi: 10.2337/db19-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borlaug B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 23.Christoffersen C., Jauhiainen M., Moser M. Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J Biol Chem. 2008;283:1839–1847. doi: 10.1074/jbc.M704576200. [DOI] [PubMed] [Google Scholar]
- 24.Sattler K., Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res. 2009;82:201–211. doi: 10.1093/cvr/cvp070. [DOI] [PubMed] [Google Scholar]
- 25.Chirinos J.A., Zhao L., Jia Y. Reduced apolipoprotein M and adverse outcomes across the spectrum of human heart failure. Circulation. 2020;141:1463–1476. doi: 10.1161/CIRCULATIONAHA.119.045323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Jiang B., Luo G., Nilsson-Ehle P., Xu N. Hyperglycemia down-regulates apolipoprotein M expression in vivo and in vitro. Biochim Biophys Acta Mol Cell Biol Lipids. 2007;1771:879–882. doi: 10.1016/j.bbalip.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y.W., Zheng L., Wang Q. Characteristics of apolipoprotein M and its relation to atherosclerosis and diabetes. Biochim Biophys Acta Mol Cell Biol Lipids. 2010;1801:100–105. doi: 10.1016/j.bbalip.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Hajny S., Christoffersen M., Dalila N., Nielsen L., Tybjærg-Hansen A., Christoffersen C. Apolipoprotein M and risk of type 2 diabetes. J Clin Endocrinol Metab. 2020;105:dgaa433. doi: 10.1210/clinem/dgaa433. [DOI] [PubMed] [Google Scholar]
- 29.Xu N., Nilsson-Ehle P., Ahrén B. Suppression of apolipoprotein M expression and secretion in alloxan-diabetic mouse: partial reversal by insulin. Biochem Biophys Res Commun. 2006;342:1174–1177. doi: 10.1016/j.bbrc.2006.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.