Abstract
Over the years, an increasing proportion of metastatic prostate cancer patients has been found to experience an initial bone flare phenomenon under both standard therapies (androgen deprivation therapy, chemotherapy, radiotherapy, abiraterone, enzalutamide) and novel agents (immunotherapy, bone-targeting radioisotopes). The underlying biological mechanisms of the flare phenomenon are still elusive and need further clarification, particularly in relation to different types of treatment and their treatment response assessment. Flare phenomenon is often underestimated and, in some cases, can negatively affect clinical outcome. In cases with suspected bone flare, the treatment should be continued for a minimum of 12 more weeks before further decisions about efficacy can be taken. Physicians and patients should be aware of this effect to avoid unwarranted anxiety and inadequate early discontinuation of treatment. This review aims at highlighting new evidence on flare phenomenon arising after the introduction of new drugs extending across the biochemical, radiographic and clinical spectrum of the disease.
Keywords: bone metastasis, castration-resistant prostate cancer, flare phenomenon, imaging, systemic treatment
Introduction
Prostate cancer (PC) is the most common malignancy in men, accounting for about one quarter of new cancer diagnoses worldwide. Despite several improvements in early detection and management of PC patients, it remains the second leading cause of cancer-related mortality.1,2 Prostate tumor is characterized by a high propensity to metastasize to bone (i.e. osteotropism). Bone metastases are diagnosed in approximately 80% of advanced PC patients.3,4
The bone flare phenomenon consists of an initial apparent deterioration of certain lesions or the detection of novel lesions on the images.5 Some studies have addressed bone flare phenomenon in PC patients at various tumor stages and treated with different standard therapies such as androgen-deprivation therapy with luteinizing hormone-releasing hormone (LH-RH) analogs and standard chemotherapeutic agents such as docetaxel and cabazitaxel.6–8 However, no consensus exists on how exactly to interpret this phenomenon and what impact it could have on patient outcome. In the past 10 years, new effective systemic agents for patients with bone metastatic PC have been introduced showing relevant clinical activity at different PC stages, including, second-generation hormonal treatments (i.e. abiraterone,9,10 enzalutamide),11,12 radionuclides e.g. radium-223,13 177-lutetium14 and novel targeted agents, such as poly Adenosine diphosphate ribose (ADP)-ribose polymerase (PARP) inhibitors,15 immunotherapy,16 and AKT (a serine/threonine-specific protein kinase) inhibitor.17 An adequate evaluation of treatment response to these different drugs is sometimes challenging, often as a consequence of the bone flare phenomenon.
In this review, we investigated the biological mechanisms underlying bone flare and its significance in terms of clinical impact focusing on these new different treatments. We discuss the incidence of flare phenomenon in novel imaging techniques and we debate the impact of this event on survival outcomes.
Pathogenesis of bone flare
After successful therapy for metastatic disease, the healing processes of new bone formation can cause an initial increase in tracer uptake (akin to callus formation), and scans carried out during this phase are likely to show increased intensity and number of hot spots. Following 6 months of treatment, bone scan appearances might improve, as the increased production of immature new bone slows down and, accordingly, the bone-seeking radiotracer uptake gradually falls. This ‘deterioration’ followed by subsequent ‘improvement’ in bone scintigraphy (BS) appearances after successful therapy has been named flare response.5,18 In addition, clinical and preclinical evidence has shown a direct role of abiraterone on anti-resorptive and anabolic activity in the bone microenvironment that could be responsible for bone flare, independently from the modulation of prostate specific antigen (PSA) and other factors levels.19
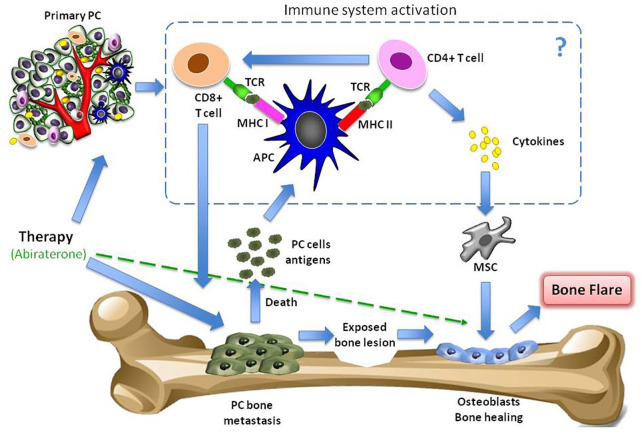
The destruction of tumor cells could elicit a tumor-specific immune response, specifically a T-cell reaction, which could, in turn, favor tumor cell lysis. In addition, the tumor microenvironment may be involved in the flare reaction as it may contribute to recruit and activate immune cells with the release of pro-inflammatory cytokines, initiating a reciprocal interaction with PC cells (Figure 1).20
Figure 1.
Pathogenetic mechanisms of flare phenomenon. A therapy for metastatic disease may lead to the healing processes of new bone formation and cause an initial increase in tracer uptake (akin to callus formation). This ‘deterioration’ followed by subsequent ‘improvement’ in the bone scan appearances after successful therapy is defined as flare response. In addition, abiraterone could have a direct anti-resorptive and anabolic activity in the bone microenvironment. Finally, the destruction of tumor cells could elicit a tumor-specific immune response, specifically T-cell reaction, which could, in turn, favor tumor cell lysis. In addition, the tumor microenvironment may be involved in the flare reaction as it may contribute to recruit and activate immune cells with the release of pro-inflammatory cytokines, initiating a reciprocal interaction with prostate tumor cells and, perhaps, facilitating an action of mesenchymal stem cells in bone healing.
APC, antigen presenting cell; MHC, major histocompatibility complex; MSC, mesenchymal stem cell; PC, prostate cancer; TCR, T-cell receptor.
Bone flare and imaging
The incidence of bone flare is still controversial. It was initially demonstrated in the hormone-sensitive PC population ranging from 6% to 23% of cases at least 2–18 weeks after treatment initiation.21–24 A prospective study21 on 22 PC patients with skeletal metastases starting first-line hormone therapy demonstrated an incidence of bone flare in up to 41% on repeated BS at 6 weeks from androgen deprivation therapy (ADT) start. In addition, the frequency of bone flare has also been explored in patients with negative staging scans but considered at high risk for skeletal metastases and in patients with equivocal baseline BS abnormalities, reporting its occurrence in 11% and 20% of cases, respectively. In this case, a possible mechanism of flare phenomenon may be explained by the fact that occult lesions need time to become visible on BS and computed tomography (CT) images, suggesting that the ‘flare’ phenomenon could amplify the scintigraphic signal and improve the sensitivity and specificity towards pre-existing occult lesions. Therefore, an earlier identification of bone flare could improve the diagnostic accuracy of BS and potentially lead to changes in the management of PC patients.
Appropriate interpretation of PC bone metastases imaging presents several challenges. The conventional X-ray survey is not indicated for bone metastases because lesions are usually small-sized and lately become sclerotic. Bone scintigraphy is the mainstay for bone lesion detection, but it is not suitable for the evaluation of treatment response, due to the scintigraphic flare phenomenon subsequent to a favorable response to treatment. Bone flare phenomenon was well described on bone scans; a study25 revealed the appearance of new or worsening bone sclerosis at 3-month CT assessment in three of 67 castration-resistant prostate cancer (CRPC) patients undergoing systemic treatment. The 3-month CT scan showed a rise in number, size, or density of sclerotic lesions although there was an improvement in PSA and soft tissue lesions. The role of whole-body magnetic resonance imaging (MRI) (WB-MRI) with the use of a combination of different sequences, including T1-weighted, short-TI inversion recovery (STIR), and diffusion weighted imaging (DWI), was investigated and showed high diagnostic accuracy in the detection of bone disease.26 However, bone flare can also appear on MRI because of red marrow reconversion due to anemia, chemotherapy and bone marrow-stimulating drugs such as Granulocyte colony-stimulating factor (G-CSF) and erythropoietin.27 Newer techniques, including Positron Emission Tomography (PET) with different radiotracers, hold promise for PC lesion detection and response assessment. Several studies explored the potential occurrence of PET flare caused by a rise in blood flow due to an inflammatory response, elevated turnover of hydroxyapatite in the new bone laid down as part of the healing process, or increased vascular permeability.28
As we move into an era of next generation imaging (e.g. WB-MRI, PET using radiopharmaceuticals, prostate-specific membrane antigen-PET labeled with several radiotracers, fluciclovine-PET), these advanced techniques, providing better sensitivity and specificity, should be incorporated into prospective clinical trial to clarify their role in the detection of bone metastasis burden.
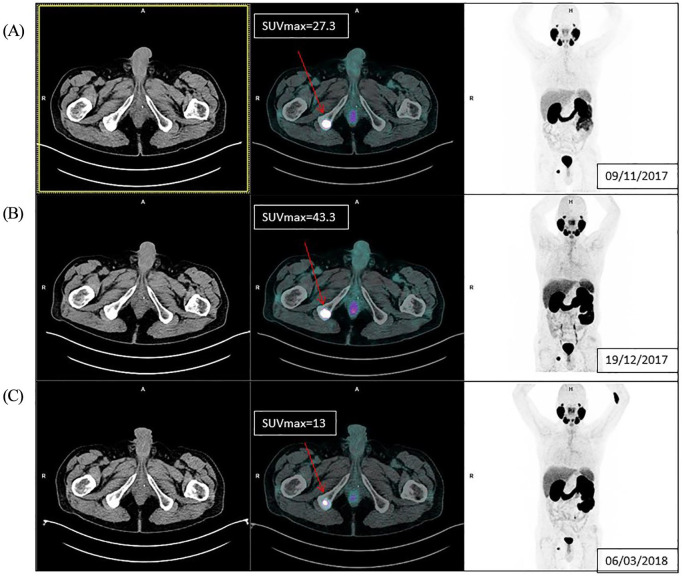
Due to the evolution of nuclear medicine, novel hybrid imaging and emerging radiopharmaceutical agents will open new questions in the diagnostic and theranostic field in the detection of recurrent and metastatic PC. Recently, bone flare phenomenon has also been observed in PC patients by using PSMA-PET, as shown in Figure 2.
Figure 2.
Example of bone flare on PSMA-PET. A 61-year-old metastatic castration-resistant prostate cancer patient in treatment with enzalutamide from November 2017. Picture (A) PSMA-PET at baseline showed a right ischiatic bone metastasis Standardized Uptake Value ([SUV] = 27.3); (B) PSMA-PET at week 6 from starting enzalutamide treatment showed a rise in PSMA uptake (SUV = 43.3), but the patient had a PSA decrease >50% from baseline;. (C) PSMA-PET at week 17 presented a relevant decrease in PSMA uptake (SUV = 13).
PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PSMA-PET, prostate-specific membrane antigen–positron emission tomography.
In 2018, the occurrence of this phenomenon in PSMA-PET imaging was evaluated by Aggarwal et al.29 A prospective, single-institution study designed to evaluate changes in 68Ga-PSMA-11 PET uptake at initiation of androgen receptor (AR)-targeted therapy including a total of eight patients (four metastatic hormone-sensitive patients treated with ADT and four metastatic CRPC (mCRPC) patients in treatment with enzalutamide plus ADT) was conducted. The study reported that an increase in PSMA-PET tracer uptake was observed in seven out of eight patients who subsequently declined prostate-specific antigen (PSA) of >50% compared to nadir. Overall, 49% of metastatic lesions exhibited a flare effect, with a higher incidence in metastatic hormone-sensitive patients and with more variable patterns of uptake in the mCRPC setting. According to the authors’ conclusions, at the beginning of AR-directed treatment flare phenomenon was variably detected on PSMA-PET and was not associated with disease progression.
In 2019, a study30 including 26 patients in treatment with enzalutamide or abiraterone suggested that, after a median follow-up of 3 months, PSMA expression modifications on PET/CT were strongly associated with response to treatment. In that study patients were retrospectively classified as PSMA responders (decrease in uptake and/or number of metastases) or PSMA non-responders (new lesions or increase/stable uptake of lesions). According to the results, no PSMA expression flare phenomenon was detected. However, a flare phenomenon of short duration or early presentation could not be excluded.
Recently, the ADTPSMA2 trial31 studied the role of 18F-PSMA-PET and bone flare in 35 patients with de novo metastatic hormone-sensitive PC to determine a potential predictive value. Heterogeneous bone flare in PSMA expression 2–3 weeks after beginning ADT was observed. The authors’ hypothesis was that metastatic lesions presenting PSMA flare respond differently to ADT and have a different outcome to those without PSMA flare.
The role of fluorine 18-fluorodeoxyglucose-PET in flare phenomenon detection was investigated by Weisman et al.32 in a study that included 33 mCRPC patients receiving an androgen receptor inhibitor (abiraterone acetate or orteronel or enzalutamide): 18F-sodium fluoride (NAF) PET was performed at baseline, week 6 and week 12 of therapy and PSA at baseline and week 8. Flare detection was identified in 61% of patients receiving CYP17A1 inhibitors (abiraterone, orteronel). According to multivariable analysis, higher SUVmean at week 6 and a decline in PSA at week 8 were independent predictors of prolonged progression-free survival (PFS) hazard ratio (HR) = 0.57, p = 0.02, HR = 1.97, p = 0.03). Moreover, that study reported no evidence of flare in patients receiving enzalutamide, as previously observed.
Bone flare related to AR-directed therapies
The flare phenomenon had mainly been investigated in CRPC patients treated with second-generation hormonal drugs such as abiraterone and enzalutamide as PSA surge phenomenon.33–35 Hormone therapy-related bone flare has been shown in a study36 on CRPC patients treated with abiraterone progressing after docetaxel therapy. The authors concluded that early18F-fluorocholine (FCH)-PET/CT could predict clinical outcome better than serum PSA response. Interestingly, that study observed the FCH-PET/CT bone flare effect in nearly 10% of patients, defined as the combination of progressive disease on FCH-PET/CT at first follow-up with a decrease in PSA level of ⩾50% and no evidence of progression disease on the CT scan at 3 months. Similar results for CRPC patients treated with abiraterone were observed by Messiou et al.25 They showed that of the 39 patients who had baseline, 3 and 6-month CT scans, eight (20.5%) presented with 22 new sclerotic lesions or sclerotic lesions increasing in size or density on the 3-month CT scan discordant to PSA/RECIST response. Out of the eight patients with imaging/biochemical discordance, three (7.7%) retained partial response or stable disease at follow-up by PSA and RECIST criteria and were considered patients with bone flare response. In a subanalysis of the COU-AA-302 study involving a blinded central radiology review, Morris et al.37 showed an incidence of bone flare in 15% (166 of 1088 patients) of the total study population. Ryan et al.5 assessed the bone flare phenomenon in a multicenter study of 33 chemotherapy-naive CRPC patients treated with abiraterone. In that study, BS flare was defined as the combination, after 3 months of treatment, of ‘disease progression’ in the context of a 50% or more decline in PSA level, with scan improvement or stability 3 months later. Scintigraphic flare was reported in 11 of 23 (48%) evaluable patients or 11 of 33 (33%) enrolled patients. The large proportion of patients with bone flare observed in that study should occur because BS flare was firstly prospectively defined.
In contrast to the studies evaluating bone flare related to abiraterone, one study demonstrated the incidence of bone flare in two of 40 (5%) CRPC patients (20 chemotherapy-treated and 20 chemotherapy-naive patients).38 On the contrary, De Giorgi et al.39 did not find FCH-PET/CT bone flare in metastatic CRPC patients treated with enzalutamide. However, some limitations of that study40 could also explain the absence of bone flare: (a) the small sample size (36 patients receiving enzalutamide and 42 receiving abiraterone); (b) the difference in median time from baseline to follow-up FCH-PET/CT of 2 weeks (7 weeks for enzalutamide versus 5 weeks for abiraterone); and (c) the heavily pretreated patient population treated with enzalutamide (33% had received at least three lines of therapy for CRPC compared with only 3% in the study with abiraterone). Other potential explanations of the higher incidence of flare in patients treated with abiraterone compared to those treated with enzalutamide could derive from biological reasons, such as a failure or delay of the production of D4, 3-keto-abi (D4A)40 a metabolite of abiraterone with potent CYP17A1 inhibition activity AR antagonistic activity similar to enzalutamide.
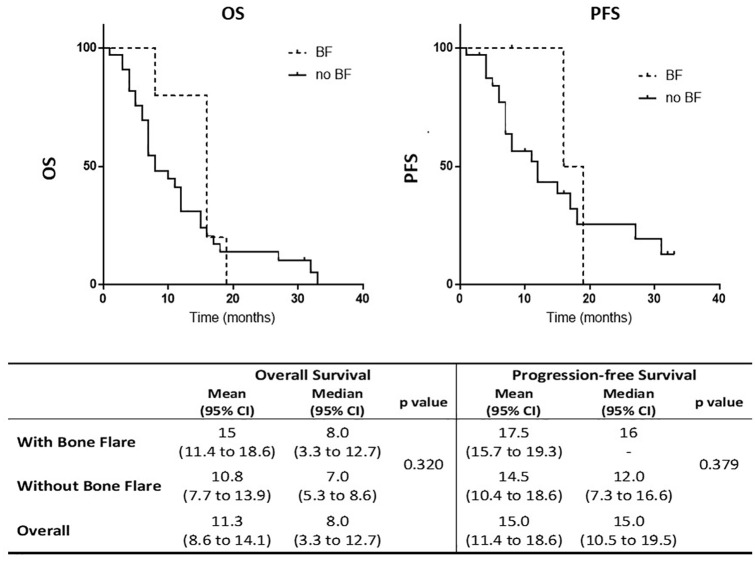
Recently, we performed an update on the bone flare phenomenon associated with abiraterone treatment in our institute.39 We observed bone flare related to abiraterone treatment by the evidence of the mismatch highlighted between the PSA reduction or stability and the detection of an increased uptake of bone lesions on FCH-PET/CT after 6 and 7 weeks, respectively. Moreover, we evaluated the presence of bone flare in 102 CRPC post-docetaxel patients treated with abiraterone included in our previous study33 with un updated median follow-up from 16.8 months to 32.1 months. Kaplan–Meier curves depicting overall survival (OS) and progression-free survival (PFS) according to the presence of bone flare (bone flare versus no bone flare) showed no impact of bone flare on survival (Figure 3). A logistic regression analysis of different parameters in patients with bone flare versus patients without bone flare showed no significant factor associated with the phenomenon of bone flare, except for serum levels of chromogranin A that were reported to be significantly higher in patients with bone flare compared to those without bone flare. However, the number of patients is too small to draw conclusions.
Figure 3.
Survival curves in bone flare: Kaplan–Meier curves depicting OS and PFS in CRPC patients treated with abiraterone according to the presence of bone flare (BF) (BF versus no BF). BF, bone flare; CRPC, castration-resistant prostate cancer; OS, overall survival; PFS, progression-free survival.
A prospective phase-2 study41 on 60 CRPC patients revealed a subcellular shift of AR from the nucleus to cytoplasm associated with an increased testosterone concentration within 8 weeks of enzalutamide treatment without reporting clinical manifestations. This study provided the first evidence in humans that enzalutamide can suppress AR signaling while inducing an adaptive feedback, which is not responsible for the flare phenomenon.
Recently, Armstrong et al. performed a secondary analysis42 of the PREVAIL and AFFIRM randomized clinical trials to study the association between new bone lesion detection on BS follow-up and enzalutamide response in men with mCRPC, respectively, in pre or post-docetaxel settings. A total of 1672 patients was included in this post-hoc analysis. In the former study, early (at week 9) and late (at week 17) healing of bone lesions was observed in 27.5% of patients in treatment with enzalutamide; in the latter study, early (at 13 weeks) and late (at 25 weeks) flares were observed in 18.1% of men with PSA response at any time and/or partial or stable disease in soft tissue according to RECIST v1.1. In the PREVAIL trial, median OS, radiographic progression-free survival (rPFS) and time to PSA progression were equivalent to those patients without flare phenomenon. Conversely, pre-treated patient in the AFFIRM study had a worse median OS compared to patients not showing new bone lesions at post-treatment scan, but the median rPFS and time to PSA progression were not reduced.
Bone flare related to therapy with radionuclides
The ‘flare’ phenomenon is evident in about 10% of patients undergoing radiometabolic therapy of bone metastases, using both osteomimetic agents, such as strontium-89, and radiopharmaceuticals based on bone-seeking radionuclides labelled with beta-emitting isotopes, such as 153samarium or 186rhenium.43
In the recent past, radium-223 chloride, an alpha-emitting radioisotope targeting areas of osteoblastic metastatic disease, was also characterized by the presence of flare phenomenon. A phase-1 dose-escalation and safety study44 conducted on 25 CRPC men demonstrated a transient increase in bone pain in about one-fifth of patients (seven out of 25) during the first week of treatment. A subsequent case report45 on a CRPC patient with symptomatic bone disease treated with radium-223 confirmed an initial flare in pain and PSA, followed by stable improvement in pain, alkaline phosphatase, and BS. A retrospective study46 of 29 patients with metastatic CRPC who received radium-223 showed that an increasing PSA level during radium treatment is not an uncommon phenomenon (10.3% of cases), especially in heavily pretreated patients. In these cases, PSA surge is strongly associated with a major incidence of pain flare (52%), but no correlation has been observed with radiographic changes and overall response to treatment. Another study on 130 patients receiving radium-223 post-docetaxel confirmed a transient increase in bone metastases-related pain in 27% of cases with a low incidence (6%) of radiological bone flare.47
In 2018, Castello et al.48 investigated the role of PSA surge in 168 patients treated with radium-223 dichloride (223RaCl2) therapy. They reported flare phenomenon in 11.9% of patients presenting with PSA decrease and 23.8% patients with subsequent PSA decrease but not below the baseline. The group with flare phenomenon presented with a median PFS of 20.8 and median OS of 23.9 months, similar to patients that presented with an early decrease of PSA levels. Thus, the survival in the flare group did not differ to patients with no flare.
Prostate-specific membrane antigen (PSMA) is a receptor on the surface of PC cells. New small molecule ligands with high-binding affinity for the PSMA receptor have allowed high quality, highly specific PET imaging, in addition to the development of targeted radionuclide therapy for PC patients, including, in particular, lutetium 177 (Lu) labelled PSMA peptides.49
So far, few data about bone flare and 177-Lu radiolabeled anti-PSMA are available; probably because many trials are still ongoing. A phase II trial of 14 CRPC patients treated with 177Lu radiolabeled anti-PSMA revealed on BS at week 12 only one (7.1%) flare response.50
More recently, a retrospective trial showed that PSA surge at 6 weeks in 166 mCRPC patients treated with 177-Lu-PSMA radionuclides are uncommon, involving fewer than 1% of patients. In this study PSA decreasing values at 6 weeks correlated with survival benefit.51
Currently, the diagnostic and theranostic role of 68Ga-PSMA-PET labeled with the beta emitter 177Lu-PSMA and alpha-emitter actinium-225 PSMA (225Ac-PSMA) holds promise, although the experience is still limited. Future studies could investigate largely the flare phenomenon in this contest.
Table 1 summarizes the main studies showing bone flare phenomenon in patients affected with advanced prostate cancer treated with different treatments.5,21–23,25,29,36,37,42,46,47,52–54
Table 1.
Main studies showing bone flare phenomenon in advanced prostate cancer patients.
| Study | Treatment | Study design | Type of imaging | No. of patients | Worsened bone scan at 3rd month | Bone flare | Pain flare | PSA flare |
|---|---|---|---|---|---|---|---|---|
| Pollen23 | ADT ± CHT | Prospective | BS | 33 | 9% (3/33) | 6% (2/33) | n/a | n/a |
| Johns22 | Leuprolide | Prospective | BS | 26 | 19% (5/26) | n/a | n/a | |
| Cook21 | Leuprolide | Prospective | BS | 22 | 0/22 | 41% (9/22) | n/a | n/a |
| Ryan5 | Abiraterone | Prospective | BS/ CT/MRI | 23 | 52% (12/23) | 48% (11/23) | 24% | n/a |
| Messiou25 | CYP17 inhibitor | Retrospective | CT | 39 | 21% (8/39) | 8% (3/39) | n/a | n/a |
| De Giorgi36 | Abiraterone | Retrospective | FCH PET/CT | 43 | 29% (12/42) | 10% (4/42) | n/a | n/a |
| Morris37 | Abiraterone | Retrospective | BS/ CT/MRI | 1088 | 15% (166/1088) at week 8 | 2.5% (27/1088) at week 12 | n/a | n/a |
| Modi46 | Radium-223 | Retrospective | BS | 29 | n/a | 21% (6/29) | 52% | 10% |
| Keizman47 | Radium-223 ± abiraterone or enzalutamide | Retrospective | BS or CT | 113 | 26% (29/113) | 20% (23/113) | 27% | n/a |
| Aggarwal29 | Enzalutamide | Prospective | PSMA PET | 8# | n/a | 6/8 (75%) | n/a | n/a |
| Isensee52 | Radium-223 | Retrospective | BS | 19 | 21% (4/19) | 15.8% (3/19) | n/a | n/a |
| Kadomoto53 | Abiraterone or enzalutamide | Retrospective | BS | 31 | 45% (14/31) | 26% (8/31) | n/a | n/a |
| De Laroche54 | Abiraterone | Prospective | SPECT-CT | 19 | 26% (5/19) | 21% (4/19) | n/a | n/a |
| Armstrong42 | Enzalutamide | Post hoc retrospective | BS | 872* 800** | 20%* (177/872) 9%** (73/800) | 27.5%* at week 9 and 13 18.1%** at weeks 17 and 25 | n/a | n/a |
Chemotherapy-naive patients enrolled in the PREVAIL trial.
Chemotherapy-treated patients enrolled in the AFFIRM trial.
#Four castration-sensitive prostate cancer + four castration-resistant prostate cancer patients.
ADT, androgen deprivation therapy; BS, bone scan; CHT, chemotherapy; CT, computed tomography; FCH PET/CT; 18F-fluorocholine positron emission tomography/computed tomography; MRI, magnetic resonance imaging; n/a, not available; SPECT, single photon emission computed tomography.
Bone flare related to immunotherapy
The association between flare event and immunotherapy (e.g. anti PD-1 antibodies) has not been well described yet and is very limited in PC. Only one case55 of pseudoprogression during immunotherapy was described in a metastatic CRPC patient treated with pembrolizumab. A PSMA-PET was performed after about 2 months of therapy and another one month later showing new bone lesions; conversely the PSA level decreased. Pseudoprogression associated with immunotherapy is a well known event in solid tumor; the evidence is still very limited in PC, thus we should pay attention to this phenomenon in order to avoid premature drug discontinuation and misinterpretation.
Potential factors associated with bone flare
In this review we have explored the possible correlation among different types of flare phenomenon. The association between bone flare and PSA flare is uncommon; in a retrospective series on 43 patients36 both FCH-PET/CT bone flare and PSA flare were reported in only one out of four patients. Another work suggested a change in the serum alkaline phosphatase (ALP) level in CRPC patients with bone metastases as an independent predictive factor for PSA flare.56 Bone flare phenomenon and skeletal metastases are often characterized by increased levels of biochemical bone markers corresponding to the extent degree of skeletal involvement in CRPC patients. Consequently, total ALP, one of the serum bone formation markers, correlates with the extent of bone metastasis and survival. Initial changes in ALP could be a useful biomarker to differentiate PSA flare from early PSA progression during docetaxel chemotherapy in CRPC patients with bone metastasis.54 However, ALP did not change in patients experiencing bone flare, as shown by Ryan et al.5 The recent post-analysis42 of the PREVAIL and AFFIRM trials showed that patients responding to enzalutamide (with and without bone flare) had a rise in ALP at week 13 and a subsequent decrease compared with men with progressive disease. In addition, Modi et al.46 showed no statistically significant relationship between PSA changes and pain flare. Fifteen out of 29 patients experienced pain flare-up during radium-223 therapy, and all patients with pain flare had an initial increase in PSA, two of these patients ultimately experienced PSA declines. The poor relationship between bone flare, pain flare and PSA surge highlights the complexity of this phenomenon that includes several interconnected etiopathogenetic factors with the different mechanisms of action of available drugs for CRPC patients.
Moreover, radiation therapy is effective in the treatment of symptomatic bone metastasis in solid tumors, including PC. About 30–40% patients undergoing radiotherapy presented with acute pain flare. The worsening of pain was described during the first 10 days after spine Stereotactic Body Radiotherapy (SBRT) in steroid-naive patients.57 A study has demonstrated that dexamethasone is useful in the prophylaxis to avoid pain flare.58
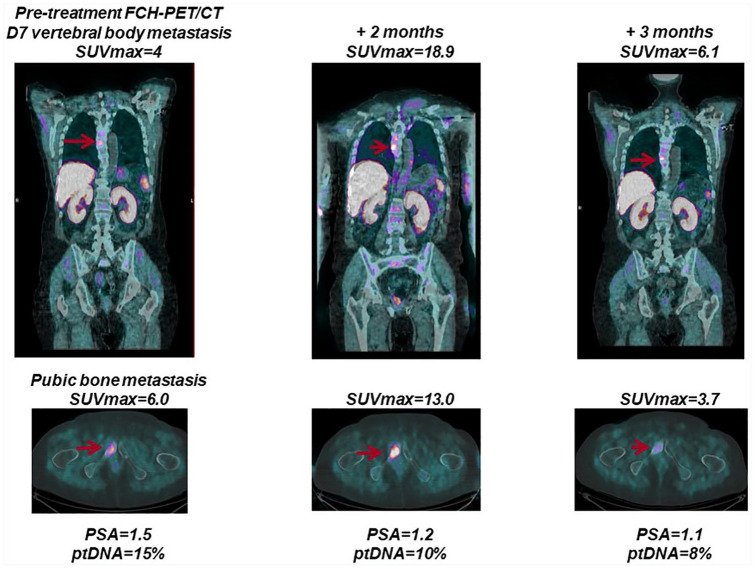
More recently, we have investigated the role of plasma tumor DNA (ptDNA) levels and clinical outcomes in 43 metastatic CRPC patients treated with abiraterone acetate. Using liquid biopsies, blood samples were collected before starting therapy, during treatment and at disease progression and analyzed by next generation sequencing (NGS). We examined the correlation between plasma tumor DNA fraction, PSA levels and radiographic imaging (Figure 4). Interestingly, after 3 months of abiraterone, a ptDNA fraction increase was associated with PSA level increase and worse outcome. Patients that presented with PSA surge or bone flare on imaging did not have a rise in ptDNA and subsequent cancer progression. ptDNA assessment was associated with radiological progression or response at any decline in PSA. Future studies could support the integration of ptDNA into composite biomarker tests for early assessment of treatment benefit.59
Figure 4.
Association of bone flare and plasma tumor DNA (ptDNA) changes with treatment response. A patient with metastatic castration-resistant prostate cancer (mCRPC) receiving abiraterone was characterized by an early increase in the intensity of bone lesions in the context of treatment response and was associated with a PSA response and ptDNA level reduction at about 2 months of treatment. mCRPC, metastatic castration-resistant prostate cancer; PSA, prostate-specific antigen; ptDNA, plasma tumor DNA.
Conclusion and future perspectives
Monitoring of bone involvement is an important clinical issue in prostate cancer patients, mainly due to a strong association between bone metastases and morbidity and to the proper interpretation of response findings to treatment. It is known that individual patients may have a mixed therapeutic response, with, for example, improvement in soft tissue disease but with progression of bone lesions or with some bone metastases responding and others progressing. In addition, currently, there is a plethora of new drugs, with a different toxicity profile and often extremely costly; therefore, it is important to recognize rapidly whether there is a ‘true’ or a ‘pseudo’ disease progression, and this is more evident in patients entered into clinical trials with fixed protocols, often requiring a radiographic evaluation ‘early’ after the beginning of treatment. Consequently, the use of additional imaging techniques in clinical practice along with PSA evaluation may help improve early prediction of outcome and monitor response to therapy in metastatic CRPC patients, optimizing the use of this high-cost treatment.
The Prostate Cancer Working Group 2 (PCWG2) criteria60 formally considered the problem of bone flare, including the recommendation to carry out a first follow-up BS at 12 or more weeks after treatment start. In addition, PCWG2 criteria defined progression in bone when a minimum of two new lesions are observed and confirmed on a second scan performed at least 6 weeks later. Interestingly, the PCWG361 accurately considered the likelihood of the bone flare phenomenon and suggested an increase in the scanning interval to 16 weeks rather than 8 weeks. The PCWG3 recognized that the early detection of novel sites of disease on a first follow-up BS could represent a flare of a pre-existing subclinical metastatic lesion or a real transition from a non-metastatic to a metastatic state. The problem of bone flare could lead to the introduction of additional primary endpoints as shown by the COU-AA-302 phase-III randomized, double-blind, placebo controlled study37 comparing abiraterone acetate plus prednisone with placebo plus prednisone in asymptomatic or mildly symptomatic men with chemotherapy-naive metastatic CRPC. That study also considered rPFS as a primary endpoint with radiological progression defined as ⩾2 new lesions on a 8-week BS plus two additional lesions on a confirmatory scan, or ⩾2 new confirmed lesions on any scan at ⩾12 weeks after random assignment, and/or progression in nodes or viscera on cross-sectional imaging, or death. This requirement (i.e. 2+2) was designed to prevent misinterpreting healing bone from a successful therapy (flare phenomenon) and new lesions. The study concluded that rPFS was highly consistent and highly associated with OS, providing initial prospective evidence on the clinical utility of further primary endpoints in metastatic CRPC trials all considering the challenge of bone flare.
The use of novel imaging techniques and/or new tracers could improve the assessment of treatment response. Recently, PET/CT imaging using PSMA ligands has gained attention as a promising new radiotracer in patients with metastatic CRPC. Many studies have demonstrated a higher diagnostic efficacy of PSMA ligand PET/CT compared to conventional imaging including PET with other tracers (e.g. 18F-choline, 11C-choline).62,63 The new tracer PSMA has the advantage of high specificity, independence of PSA level and low non-specific tracer uptake in surrounding tissue. To date, functional and in vitro studies have shown that ADT can result in a rise of PSMA expression.64 There are few data in the literature describing the flare phenomenon associated with PSMA PET/CT for the evaluation of treatment response, and future works are warranted.
In the coming years, a more thorough study on the pathogenetic mechanisms and clinical and biological tumor characteristics could lead to a better understanding of the different aspects involved in the ‘flare phenomenon’ in CRPC patients. To date, the flare findings highlight a need for closer communication among clinicians with different medical specialties (oncology, urology, radiotherapy, radiology, and nuclear medicine). It is very important for an adequate treatment of CRPC patients that physicians are aware of this phenomenon in order not to exclude patients prematurely from potentially beneficial chemotherapy or other treatments, assuming that progression has occurred. In addition, clinicians should inform their CRPC patients about the challenge of PSA increasing early after the start of therapy to prevent unnecessary pressure and anxiety. Although the physiopathology of clinical flare/PSA surge is not completely clear, future clinical trials need to consider this phenomenon in their study designs, particularly in view of combined and sequential therapies and the introduction of novel drugs designed to act on specific molecular pathways. Moreover, mandating a central review of radiological imaging whenever possible is recommended, in order to avoid institutional or regional bias. It appears mandatory that the flare phenomenon consequent on different treatments should be recognized to avoid premature discontinuation of effective therapy based on a potentially erroneous interpretation.
Well designed and larger prospective studies better to characterize the clinical significance of flare phenomenon are warranted, especially with the introduction of survival-prolonging drugs for CRPC, and novel molecular and functional imaging. Surely, the optimal management of bone flare phenomenon in advanced prostate cancer patients should require a multidisciplinary team, integrating expertise in systemic treatments, radiation therapy, nuclear medicine, orthopedic surgery, radiology and supportive care for the effective treatment of metastatic bone disease. Finally, translational studies are also required in order to understand the supporting biological mechanisms involving tumor and its microenvironment better.
Footnotes
Conflict of interest statement: VC has received speaker honoraria or travel support from Astellas, Janssen-Cilag and Sanofi-Aventis, and has received consulting fee from Bayer and Janssen-Cilag. PM has/had a consultant/advisory role for BMS, Roche, Genentech, MSD, Novartis, Amgen, Merck Serono, Pierre Fabre, and Incyte. UDG has served as consultant/advisory board member for Astellas, Bayer, BMS, Ipsen, Janssen, Merck, Pfizer and Sanofi; has received travel support from BMS, Ipsen, Janssen and Pfizer; and has received research funding from AstraZeneca, Roche and Sanofi (Inst). No potential conflicts of interest were disclosed by the other authors.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ugo De Giorgi  https://orcid.org/0000-0001-7520-2908
https://orcid.org/0000-0001-7520-2908
Contributor Information
Vincenza Conteduca, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Via Piero Maroncelli 40, Meldola (FC), Emilia-Romagna 47014, Italy.
Giulia Poti, Department of Clinical and Molecular Medicine, University “La Sapienza”, Rome, Lazio, Italy.
Paola Caroli, Department of Nuclear Medicine, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Meldola, Emilia-Romagna, Italy.
Sabino Russi, Laboratory of Preclinical and Translational Research, IRCCS-CROB, Referral Cancer Center of Basilicata (CROB), Rionero in Vulture (PZ), Italy.
Nicole Brighi, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Meldola, Emilia-Romagna, Italy.
Cristian Lolli, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Meldola, Emilia-Romagna, Italy.
Giuseppe Schepisi, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Meldola, Emilia-Romagna, Italy.
Antonino Romeo, Department of Radiotherapy,Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Meldola, Italy.
Federica Matteucci, Department of Nuclear Medicine, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Meldola, Emilia-Romagna, Italy.
Giovanni Paganelli, Department of Nuclear Medicine, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Meldola, Emilia-Romagna, Italy.
Paolo Marchetti, Department of Clinical and Molecular Medicine, University “La Sapienza”, Rome, Lazio, Italy.
Ugo De Giorgi, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori “Dino Amadori” (IRST), IRCCS, Meldola, Emilia-Romagna, Italy.
References
- 1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49: 1374–1403. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathology 2000; 31: 578–583. [DOI] [PubMed] [Google Scholar]
- 4. Cooper CR, Chay CH, Gendernalik JD, et al. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer 2003; 97: 739–747. [DOI] [PubMed] [Google Scholar]
- 5. Ryan CJ, Shah S, Efstathiou E, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res 2011; 17: 4854–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tannock IF, De Wit R, Berry WR; et al. TAX Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 7. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 8. Heidenreich A, Bastian PJ, Bellmunt J, et al. European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014; 65: 467–479. [DOI] [PubMed] [Google Scholar]
- 9. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryan CJ, Smith MR, De Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 13. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- 14. Fendler WP, Rahbar K, Herrmann K, et al. 177Lu-PSMA radioligand therapy for prostate cancer. J Nucl Med 2017; 58: 1196–1200. [DOI] [PubMed] [Google Scholar]
- 15. Hussain M, Mateo J, Fizazi K, et al. PROfound: phase III study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene alterations. Ann Oncol 2019; 30: v881–v882. [Google Scholar]
- 16. Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: facts and hopes. Clin Cancer Res 2017; 23: 6764–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US National Library of Medicine. Clinicaltrials.gov 2020. https://clinicaltrials.gov/ct2/show/NCT03072238 (accessed 5 January 2020).
- 18. Coleman RE, Mashiter G, Whitaker KB, et al. Bone scan flare predicts successful systemic therapy for bone metastases. Soc Nucl Med 1988; 29: 1354–1359. [PubMed] [Google Scholar]
- 19. Iuliani M, Pantano F, Buttigliero C, et al. Biological and clinical effects of abiraterone on anti-resorptive and anabolic activity in bone microenvironment. Oncotarget 2015; 6: 12520–12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 21. Cook GJ, Venkitaraman R, Sohaib AS, et al. The diagnostic utility of the flare phenomenon on bone scintigraphy in staging prostate cancer. Eur J Nucl Med Mol Imaging 2011; 38: 7–13. [DOI] [PubMed] [Google Scholar]
- 22. Johns WD, Garnick MB, Kaplan WD. Leuprolide therapy for prostate cancer. An association with scintigraphic “flare” on bone scan. Clin Nucl Med 1990; 15: 485–487. [DOI] [PubMed] [Google Scholar]
- 23. Pollen JJ, Witztum KF, Ashburn WL. The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. AJR Am J Roentgenol 1984; 142: 773–776. [DOI] [PubMed] [Google Scholar]
- 24. Shimizu N, Masuda H, Yamanaka H, et al. Fluorodeoxyglucose positron emission tomography scan of prostate cancer bone metastases with flare reaction after endocrine therapy. J Urol 1999; 161: 608–609. [PubMed] [Google Scholar]
- 25. Messiou C, Cook G, Reid AH, et al. The CT flare response of metastatic bone disease in prostate cancer. Acta Radiol 2011; 52: 557–561. [DOI] [PubMed] [Google Scholar]
- 26. Larbi A, Omoumi P, Pasoglou V, et al. Whole-body MRI to assess bone involvement in prostate cancer and multiple myeloma: comparison of the diagnostic accuracies of the T1, short tau inversion recovery (STIR), and high b-values diffusion-weighted imaging (DWI) sequences. Eur Radiol 2019; 8: 4503–4513. [DOI] [PubMed] [Google Scholar]
- 27. Yu YS, Li WH, Li MH, et al. False-positive diagnosis of disease progression by magnetic resonance imaging for response assessment in prostate cancer with bone metastases: a case report and review of the pitfalls of images in the literature. Oncol Lett 2015; 10: 3585–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Messiou C, Cook G, de Souza NM. Imaging metastatic bone disease from carcinoma of the prostate. Br J Cancer 2009; 101: 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aggarwal R, Wei X, Kim W, et al. Heterogeous flare in prostate-specific membrane antigen positron emission tomography tracer with initiation of androgen pathway blockade in metastatic prostate cancer. Eur Urol Oncol 2018; 1: 78–82. [DOI] [PubMed] [Google Scholar]
- 30. Plouznikoff N, Artigas C, Sideris S, et al. Evaluation of PSMA expression changes on PET/CT before and after initiation of novel antiandrogen drugs (enzalutamide or abiraterone) in metastatic castration-resistant prostate cancer patients. Ann Nucl Med 2019; 33: 945–954. [DOI] [PubMed] [Google Scholar]
- 31. US National Library of Medicine. ClinicalTrials.gov 2020. https://clinicaltrials.gov/ct2/show/NCT03876912 (accessed 5 January 2020).
- 32. Weisman AJ, Harmon SA, Perk TG, et al. Quantification of bone flare on 18F-NaF PET/CT in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2019; 22: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burgio SL, Conteduca V, Rudnas B, et al. PSA flare with abiraterone in patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer 2015; 13: 39–43. [DOI] [PubMed] [Google Scholar]
- 34. Conteduca V, Caffo O, Lolli C, et al. Long-term clinical impact of PSA surge in castration-resistant prostate cancer patients treated with abiraterone. Prostate 2017; 77: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 35. Ueda Y, Matsubara N, Tabata KI, et al. Prostate-specific antigen flare phenomenon induced by abiraterone acetate in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer 2016; 15: 320–325. [DOI] [PubMed] [Google Scholar]
- 36. De Giorgi U, Caroli P, Burgio SL, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget 2014; 5: 12448–12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morris MJ, Molina A, Small EJ, et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol 2015; 33: 1356–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoshi SH, Numahata K, Bilim V, et al. ALP and bone scan flare following enzalutamide treatment of castration resistant prostate cancer (CRPC) with bone metastasis. Eur Urol 2015; 14: 676. [Google Scholar]
- 39. De Giorgi U, Caroli P, Scarpi E, et al. (18)F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging 2015; 42: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 40. Li Z, Bishop AC, Alyamani M, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature 2015; 523: 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 2015; 67: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Armstrong AJ, Al-Adhami M, Lin P, et al. Association between new unconfirmed bone lesions and outcomes in men with metastatic castration-resistant prostate cancer treated with enzalutamide: secondary analysis of the PREVAIL and AFFIRM randomized clinical trials. JAMA Oncol 2020; 6: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bodei L, Lam M, Chiesa C, et al. EANM procedure guideline for treatment of refractory metastatic bone pain. Eur J Nucl Med Mol Imaging 2008; 35: 1934–1940. [DOI] [PubMed] [Google Scholar]
- 44. Nilsson S, Larsen RH, Fosså SD, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005; 11: 4451–4459. [DOI] [PubMed] [Google Scholar]
- 45. McNamara MA, George DJ. Pain, PSA flare, and bone scan response in a patient with metastatic castration-resistant prostate cancer treated with radium-223, a case report. BMC Cancer 2015; 15: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Modi D, Hwang C, Mamdani H, et al. Radium-223 in heavily pretreated metastatic castrate-resistant prostate cancer. Clin Genitourin Cancer 2016; 14: 373–380; e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keizman D, Fosboel MO, Reichegger H, et al. Imaging response during therapy with radium-223 for castration-resistant prostate cancer with bone metastases – analysis of an international multicenter database. Prostate Cancer Prostatic Dis 2017; 20: 289–293. [DOI] [PubMed] [Google Scholar]
- 48. Castello A, Macapinlac HA, Lopci E, et al. Prostate-specific antigen flare induced by 223 RaCl 2 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2018; 45: 2256–2263. [DOI] [PubMed] [Google Scholar]
- 49. Matteucci F, Mezzenga E, Caroli P, et al. Reduction of (68)Ga-PSMA renal uptake with mannitol infusion: preliminary results. Eur J Nucl Med Mol Imaging 2017; 44: 2189–2194. [DOI] [PubMed] [Google Scholar]
- 50. Morris MJ, Milowsky MI, Pandit-Taskar N, et al. Phase 2 trial of 177Lutetium (177Lu) radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody (mAb) J591 (177Lu-J591) in patients (pts) with metastatic androgen-independent prostate cancer (AIPC). J Clin Oncol 2006; 24: 4613–4613. [Google Scholar]
- 51. Gafita A, Heck M, Rauscher I, et al. Early prostate-specific antigen changes and clinical outcome following 177Lu-PSMA radionuclide treatment in patients with metastatic castration-resistant prostate cancer. J Nucl Med 2020; 61: 1476–1483. [DOI] [PubMed] [Google Scholar]
- 52. Isensee G, Péporté A, Müller J, et al. Is there a flare phenomenon on bone scintigraphy in men with advanced prostate cancer treated with radium-223? Clin Genitourin Cancer 2018; 16: 349–354. [DOI] [PubMed] [Google Scholar]
- 53. Kadomoto S, Yaegashi H, Nakashima K, et al. Quantification of bone metastasis of castration-resistant prostate cancer after enzalutamide and abiraterone acetate using bone scan index on bone scintigraphy. Anticancer Res 2019; 39: 2553–2559. [DOI] [PubMed] [Google Scholar]
- 54. de Laroche R, Bourhis D, Robin P, et al. Feasibility study and preliminary results of prognostic value of bone SPECT-CT quantitative indices for the response assessment of bone metastatic prostate carcinoma to abiraterone. Front Med (Lausanne) 2020; 6: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Costa LB, Queiroz MA, Barbosa FD, et al. Pseudoprogression on PSMA PET imaging on a mCRPC patient under anti-PD1 treatment. Eur J Nucl Med Mol Imaging 2019; 46: 1576–1577. [DOI] [PubMed] [Google Scholar]
- 56. Han KS, Hong SJ. Serum alkaline phosphatase differentiates prostate-specific antigen flare from early disease progression after docetaxel chemotherapy in castration-resistant prostate cancer with bone metastasis. J Cancer Res Clin Oncol 2014; 140: 1769–1776. [DOI] [PubMed] [Google Scholar]
- 57. Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol 2017; 7: 4–12. [DOI] [PubMed] [Google Scholar]
- 58. Chow E, Meyer RM, Ding K, et al. Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a double-blind, randomised placebo-controlled, phase 3 trial. Lancet Oncol 2015; 16: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 59. Conteduca V, Wetterskog D, Scarpi E, et al. Plasma tumour DNA as an early indicator of treatment response in metastatic castration-resistant prostate cancer. Br J Cancer 2020; 123: 982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scher HI, Eisenberger M, D’Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 2004; 22: 537–556. [DOI] [PubMed] [Google Scholar]
- 61. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016; 34: 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bluemel C, Krebs M, Polat B, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-choline-PET/CT. Clin Nucl Med 2016; 41: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schwenck J, Rempp H, Reischl G, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging 2017; 44: 92–101. [DOI] [PubMed] [Google Scholar]
- 64. Ettala O, Malaspina S, Tuokkola T, et al. Prospective study on the effect of short-term androgen deprivation therapy on PSMA uptake evaluated with 68Ga-PSMA-11 PET/MRI in men with treatment-naïve prostate cancer. Eur J Nucl Med Mol Imaging 2020; 47: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]