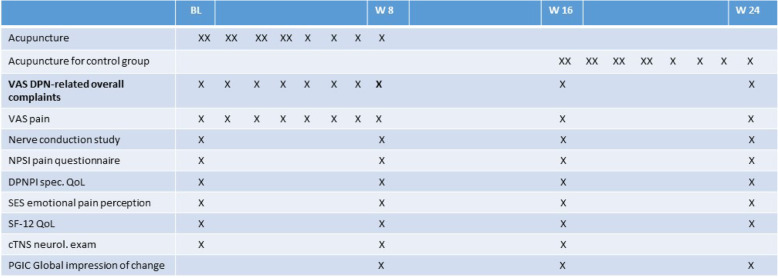
Table 1.
Participant timeline {13}. Time schedule, design, and outcome parameters of the ACUDPN study. BL, baseline; W8, W16, etc., week 8, week 16, etc. after inclusion in the study. Outcome measures: VAS pain and VAS overall DPN-related complaints, German versions of the Neuropathic Pain Symptom Inventory (NPSI), Diabetic Peripheral Neuropathic Pain Impact (DPNPI) measure, the emotional pain perception scale (SES), the SF-12 QoL, the Patient Global Impression of Change (PGIC), and neurological exam with clinical Total Neuropathy Score (cTNS)