Abstract
Inflammatory bowel disease (IBD) patients have an increased risk of developing colitis-associated colon cancer (CAC); however, the basis for inflammation-induced genetic damage requisite for neoplasia is unclear. Several studies have shown that IBD patients have signs of increased oxidative damage, which could be a result of genetic and environmental factors such as an excess in oxidant molecules released during chronic inflammation, mitochondrial dysfunction, a failure in antioxidant capacity, or oxidant promoting diets. It has been suggested that chronic oxidative environment in the intestine leads to the DNA lesions that precipitate colon carcinogenesis in IBD patients. Indeed, several preclinical and clinical studies show that different endogenous and exogenous antioxidant molecules are effective at reducing oxidation in the intestine. However, most clinical studies have focused on the short-term effects of antioxidants in IBD patients but not in CAC. This review article examines the role of oxidative DNA damage as a possible precipitating event in CAC in the context of chronic intestinal inflammation and the potential role of exogenous antioxidants to prevent these cancers.
Keywords: Colitis, Inflammatory Bowel Disease, Colorectal Cancer, Antioxidants, DNA Damage
Abbreviations used in this paper: AOM, azoxymethane; AT1, angiotensin II type 1; CAC, colitis-associated colon cancer; CAT, catalase; CRC, colorectal cancer; DSS, dextran sodium sulfate; DUOX2, dual oxidase 2; Gpx, glutathione peroxidase; GST, glutathione-S-transferase; GSTTT1, glutathione-S-transferase theta 1; HFD, high-fat diet; H2O2, hydrogen peroxidase; IBD, inflammatory bowel disease; MMR, mismatch repair; mtROS, mitochondrial ROS; NAC, N-acetylcysteine, NOX, nicotinamide adenine dinucleotide phosphate oxidase; O2•-, superoxide; PRDX, peroxiredoxin, RNI, reactive nitrogen intermediaries; ROS, reactive oxygen species; SOD, superoxide dismutase; UC, ulcerative colitis; vitC, vitamin C; vitE, vitamin E; 8-oxoG, 8-oxo-7,8-dihydro-2′-deoxyguanosine
Summary.
Preclinical studies suggest a role for oxidative molecules in the pathophysiology of colitis-associated cancer. This review analyzes evidence for DNA oxidation as a precipitating event in gastrointestinal cancers and synthesizes an argument for the use of antioxidants as a viable therapeutic treatment to prevent colitis-associated colon cancers.
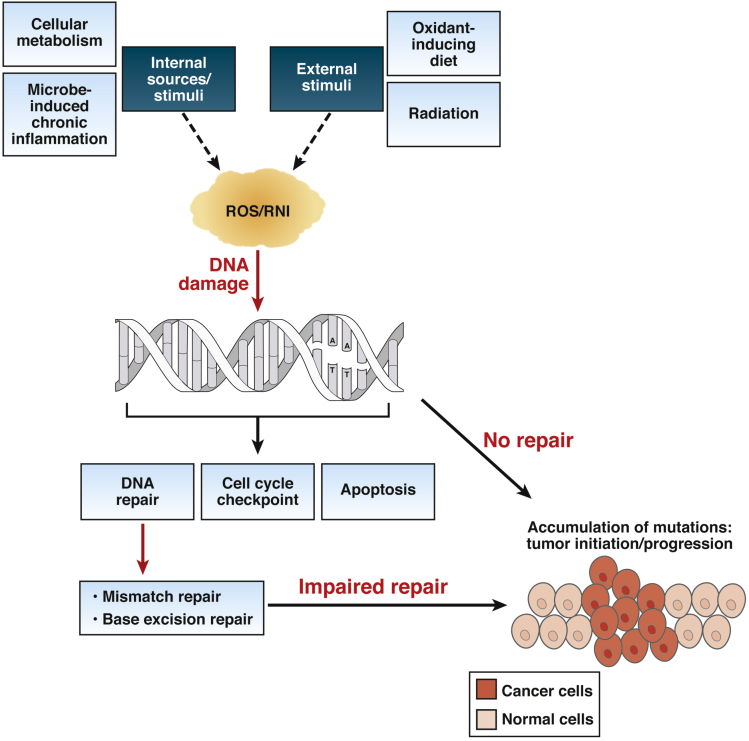
Inflammatory bowel disease (IBD) is a multifactorial chronic inflammatory disorder associated with dysregulation in the interaction between the host’s immune system and the environment within the gastrointestinal tract. Chronic inflammation of the intestinal epithelium is positively associated with cancer development, and although there are several mechanisms by which inflammation could induce epithelial damage, only a few of those point to a direct source of the DNA lesions necessary for cellular transformation and cancer initiation. The oxidant environment created by activated inflammatory cells in the intestinal epithelium has been associated with carcinogenesis.1, 2, 3, 4 Although linked to cancer initiation, reactive oxygen species (ROS) also act as signalling molecules that regulate multiple signaling pathways associated with mitogenesis, immune and stress response, and autophagy and are therefore required to maintain homeostasis.5 In this review, we will discuss the evidence that supports the notion that chronic intestinal oxidation is one of the main factors leading to DNA lesions that promote carcinogenesis in IBD patients (Figure 1). This review will also discuss how antioxidants could be used to suppress tumor development within the inflamed intestinal tissue.
Figure 1.
Unrepaired oxidative DNA damage can lead to cancer-promoting accumulation of mutations. Several stimuli from both endogenous and environmental sources induce increased production of ROS and RNI that can directly produce mutagenic DNA lesions. Oxidative DNA damage is primarily repaired by MMR repair and base excision repair pathways involving the excision of modified bases followed by repairing of the gaps. Oxidative DNA damage could exceed the repair capacity of these DNA repair pathways, or deficiency in these specific DNA repair pathways can lead to the accumulation of oncogenic mutations, precipitating cellular transformation and ultimately tumor initiation and/or progression.
What Is the Source of Oxidative Molecules and DNA Lesions in the Intestinal Epithelium?
There are several sources of oxidative molecules in the intestinal epithelium. Classically activated macrophages, infiltrating neutrophils, and intestinal epithelial cells are all equipped with enzymes that produce ROS and reactive nitrogen intermediaries (RNI) in response to the gut microbiota, specific gut pathogens, or other stimuli. ROS and RNI are normally produced to keep microbes in line and maintain homeostasis within the intestine. For example, the nicotinamide adenine dinucleotide phosphate oxidase (NOX) 2, which produces superoxide (O2•-), is expressed in macrophages, dendritic cells, and neutrophils that infiltrate the lamina propria.6 Intestinal epithelial cells express the O2•- producing enzyme NOX1 and the hydrogen peroxide (H2O2) producing enzyme dual oxidase 2 (DUOX2). Usually O2•- is further converted into the more stable molecule H2O2 by the enzyme superoxide dismutase (SOD). In phagocytes, H2O2 is further transformed into hypochlorous acid, which has antimicrobial properties. In addition, macrophages, epithelial cells, and neutrophils also express the enzyme inducible nitric oxide synthase, which produces the highly diffusible molecule NO•.6,7
Although ROS is necessary for various cellular functions,8, 9, 10, 11, 12 excessive accumulation causes damage to biological molecules. Consequently, tissues are armed with several defense mechanisms including an intricate antioxidant defense system. The antioxidant defense system primarily functions through (1) limiting the excessive production of ROS/RNI, (2) scavenging free radicals, and (3) converting toxic free radicals into less toxic molecules.13 An incompetent or dysregulated antioxidant system is associated with inflammatory diseases. Accordingly, mice deficient in nuclear factor erythroid-2–related factor 2, a master regulator of antioxidant responses in tissue through transcriptional regulation of antioxidant genes, develop colitis and colitis-associated cancer (CAC).14
During chronic inflammation, ROS and RNI surpass the physiological antioxidant detoxifying capacity of cells, leading to the generation of high amounts of oxidant molecules. H2O2 can react with transition metals via Fenton reaction and produce the highly reactive hydroxyl radical (HO•).7 On the other hand, •NO can react with O2•- to generate the highly reactive molecule peroxynitrite (ONOO-).15 When these oxidant molecules are not neutralized by intracellular antioxidants, these agents induce cell membrane damage and cancer-causing DNA lesions.1,3,16 In addition, inflamed colons in IBD patients and mice with colitis have decreased expression of antioxidant enzymes such as glutathione-S-transferase theta 1 (GSTT1). GSTT1 is not only necessary for the detoxification of oxidative free radicals, but it is also necessary to induce goblet cell differentiation and mucin production in response to triggers such as interleukin 22 and H2O2.17 Because mucus produced by goblet cells is the first barrier that protects intestinal cells from the microbiome, this study suggests that GSTT1 deficiency would disrupt the epithelial barrier, generating a positive loop for oxidative epithelial damage.17
Inflammation, Diets, and Oxidative Environment
Several innate immune cells produce O2•-, H2O2, and NO as a defense mechanism. However, some studies suggest that an increase in oxidative molecules can precede inflammation. For example, an inhibitor of the DNA repair enzyme OGG1 (8-oxoguanine DNA glycosylase 1) has been shown to prevent proinflammatory gene expression and cell recruitment in mouse lungs,18 suggesting that repair of oxidative DNA damage induces inflammation. Other studies showed that mitochondrial ROS (mtROS), generated when electrons leak from Complex I and III during oxidative phosphorylation and react with oxygen to form O2•-, can act as a signal-transducing molecule that either activates the NLRP3 inflammasome inducing the production of proinflammatory cytokines19,20 or mediates an increased mitogen-activated protein kinase signaling that induces inflammatory cytokine production after TLR4 activation.21 Furthermore, gene transfer of the mitochondrial antioxidant enzyme GSTT1 into the colon of mice confers protection against colitis,17 suggesting that in some cases, an increase in mtROS could precede chronic intestinal inflammation. However, mtROS can have anti-inflammatory properties as well because it can protect the intestine from inflammation by inducing polarization of alternatively activated macrophages and a reduction in the production of proinflammatory cytokines.22 Overall, these data suggest that mtROS must surpass a physiological threshold to lead to activation of inflammatory pathways in the intestine.
Another factor that can induce an oxidative environment that results in intestinal permeability and inflammation are high-fat diets (HFDs).23,24 Non-esterified long chain saturated fatty acids present in HFD can increase expression of Nos2 and endoplasmic reticulum stress in goblet cells, which in turn triggers a reduction in the production of the mucus barrier and creates a positive feedback loop for inflammation.23 In addition, mice fed a HFD had decreased expression of tight junctions proteins and MUC2 and increased expression of the enzymes NOX1, NOX4, and NOS2, which produce O2•-, H2O2, and NO, respectively.24 However, none of these molecules or their oxidative effects were directly measured. Although the mechanism by which HFD induces the expression of these ROS-producing enzymes is not clear, it is possible that intestinal permeability associated with deficiencies in tight junctions and/or reduced mucus layer lead to the penetration of molecules such as lipopolysaccharide through the epithelial layer of the gastrointestinal tract. This in turn can lead to the induction of ROS-producing enzymes in immune cells. Interestingly, flavonoid anthocyanins could revert HFD-induced intestinal permeabilization and endotoxemia in part by modulating NOX expression and preventing the production of RNI.24 This finding supports the idea that HFD-induced oxidative environment can lead to a positive feedback loop for intestinal permeability and intestinal oxidation. Anthocyanins also showed a promising anti-inflammatory potential in a small trial in ulcerative colitis (UC) patients,21,25 and because HFD is a risk factor for IBD,26 it is possible that anthocyanins could counteract the initial ROS-related processes that precede chronic intestinal inflammation.
Overall, it seems that the source of oxidative damage in the intestine could be dependent or independent of inflammatory cells. Mitochondrial dysfunction and certain diets can mediate an increase in the production of O2•- and NO in the intestinal epithelium,20,21,23, 24, 25,27 which in turn can activate inflammatory pathways and may lead to mutations that perpetuate inflammation and/or initiate cancer. However, in the majority of cases, it is likely that inflammation is itself caused by a response to microbial stimuli that causes an oxidative environment that can precipitate cancer.
How Is Oxidative DNA Damage Repaired?
HO• and ONOO- directly damage DNA via strand breakage or nucleotide oxidation. Guanine is the nucleotide with the highest oxidation potential,15 and oxidized guanine is commonly used to detect oxidative DNA damage. Elevated levels of oxidized guanine indicate that the oxidized environment has superseded the capacity of the cell to repair lesions caused by oxidation and therefore indicates potential oxidative damage.
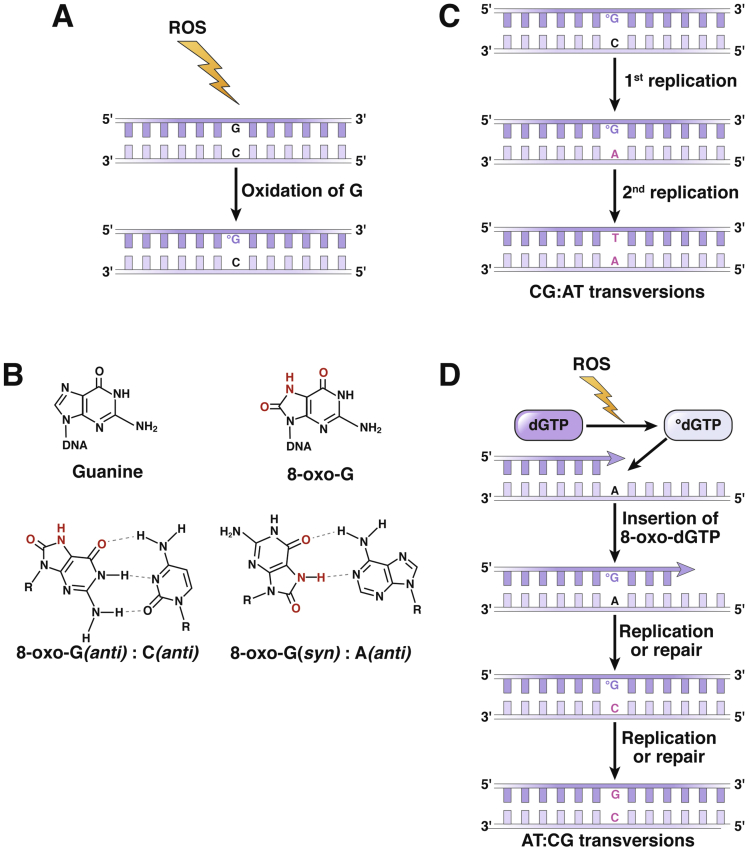
Guanine oxidation leads to the formation of 8-hydroxy-2′-deoyguanosine or 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxoG), which can lead to mutations if not repaired (Figure 2). If 8-oxoG is not removed from the DNA before replication and because 8-oxoG preferentially pairs with an adenine, unrepaired 8-oxoG will ultimately lead to G → T and C → A transversion mutations (Figure 2). Multiple DNA repair systems repair oxidative DNA lesions at different stages of the cell cycle.28, 29, 30, 31 C:8-oxoG pairs in DNA are recognized and repaired by the base excision repair and the nucleotide excision repair systems.29,31 In addition, 8-oxoG nucleotides from the dNTP pool are usually removed by MTH1, and failure to do so can lead to incorporation of this oxidized base into the nascent DNA strand during DNA replication (Figure 2D).29, 30, 31 The mismatch repair (MMR) system has an especially important role in the repair of 8-oxoG lesions in highly proliferative tissues such as the intestinal epithelium because MMR-deficient human and mouse colonic tissue have exceptionally high levels of this DNA lesion.3
Figure 2.
Mutations caused by 8-oxoG. (A) ROS-mediated oxidation of guanine (G) generates C:8-oxoG base pairs that are normally repaired by OGG1-initiated base excision repair. (B) 8-oxoG has base-pairing properties similar to thymine (T); therefore 8-oxoG in DNA during S-phase of the cell cycle leads to preferential insertion of adenine (A) opposite the 8-oxoG instead of cytosine (C) by replicative DNA polymerases. (C) A:8-oxoG mispairs can be recognized and repaired by MMR. However, if this mismatch is left unrepaired, a second round of replication will lead to C:G→A:T transversion mutation in one daughter cell. (D) Deoxyguanosine triphosphate (dGTP) in the nucleotide pool can be oxidized and incorporated into the nascent DNA strand opposite an A during replication, which can be repaired by MMR. However, A:8-oxo-G mispairs also can be processed through inappropriate MUTYH-initiated base excision repair, leading to the formation of C:8-oxo-G pairs, which could be further repaired by OGG1, generating an A:T→C:G transversion mutation. To avoid this, it is believed that cells avoid MUTYH activity during replication, giving preference to the MMR system. Therefore, lack of MMR activity is particularly an issue for highly proliferative tissues under oxidative environment such as gastrointestinal tract.
In the next sections, we discuss the role of endogenous and exogenous antioxidants on IBD and how these agents might prevent cellular transformation in inflamed intestinal tissue.
Examining the Roles of Endogenous Antioxidants in IBD and CAC
The endogenous antioxidant defense system comprises both enzymatic antioxidants such as SOD, glutathione peroxidase (GPx), catalase (CAT), peroxiredoxin (PRDX), and thioredoxin as well as nonenzymatic antioxidants such as glutathione, alpha-lipoic acid, uric acid, melatonin, bilirubin, and ferritin. Multiple studies have found altered expression and/or activities of these antioxidant proteins in IBD and CAC patients, which suggest a role in disease pathology. SOD is a metalloenzyme that catalyzes the reduction of O2•- to H2O2 and O2, whereas CAT catalyzes the detoxification of H2O2 to O2 and H2O. Patients with active Crohn’s disease have increased SOD activity, which returned to control levels at remission.32 However, CAT activity remained permanently inhibited and was independent of disease activity.32 In a different study, a reduction in CAT or total SOD activity was found to be associated with increased risk of colorectal cancer (CRC) and gastric adenocarcinoma, respectively.33
GPx, PRDX, and thioredoxin, the thiol-dependent proteins that catalyze the reduction of H2O2, lipid peroxides, and peroxynitrite, are found to be up-regulated in colonic mucosa of IBD and CRC patients compared with healthy subjects.34, 35, 36, 37, 38, 39, 40 The Gpx isoforms Gpx1 to Gpx4 are expressed in healthy gastrointestinal mucosa; however, their deficiency or genetic variants that affect their functions have been associated with increased mucosal damage and risk of developing CRC.39, 40, 41 Mice deficient in Gpx1, Gpx2, or Gpx3 develop normally but are susceptible to develop IBD and CAC upon Salmonella infection or azoxymethane (AOM)/dextran sodium sulfate (DSS) treatment.42,43 Compartmentalized expression of Gpx1 and Gpx2 is reported along the crypt-villus axis in the intestine; Gpx1 expression is predominantly found in the villus, whereas Gpx2 is localized mainly in the crypt.44 Gpx2 knockout mice have increased apoptosis and mitosis of crypt cells under selenium restriction. However, upon selenium supplementation in Gpx2–/– mice, Gpx1 expression in the crypt increases, partially compensating for Gpx2 loss of expression and protecting from crypt cell apoptosis. This result suggests an overlapping complementary role of Gpx1 and Gpx2 in maintaining intestinal homeostasis.44 Accordingly, Gpx1 and Gpx2 double-knockout mice develop spontaneous colitis, dependent on excessive production of ROS by NOX1 and DUOX2.45, 46, 47
PRDXs are highly reactive peroxidases that account for the reduction of more than 90% of total cellular peroxides, while also crucial for maintaining physiological levels of cellular peroxides for vital cellular functions.48 All mammalian PRDXs, PRDX1–6, are overexpressed in the mucosa of active colitis and CRC patients, and their level in mucosa is positively correlated with disease severity and cancer metastasis.34, 35, 36, 37,49,50 Increased expression of PRDXs in diseased mucosa seems to be a host antioxidant defense response because PRDX4–/– mice have higher disease severity and endoplasmic reticulum stress after DSS treatment.51 Studies suggest a dual role of PRDXs in cancer. PRDXs can either inhibit ROS-induced DNA damage and carcinogenesis or potentiate cancer progression through inhibition of ROS-mediated cell death in cancerous tissues.49,50 However, their role has not been investigated in CAC.
Researchers have examined nonenzymatic endogenous antioxidants and their roles in IBD and CAC. Some of these antioxidants such as glutathione, bilirubin, and uric acid are produced during normal metabolism, whereas melatonin is a hormone that is secreted from enterochromaffin cells in the intestine. Glutathione, the most important intracellular nonenzymatic antioxidant, is the substrate for glutathione-S-transferase (GST) that catalyzes the step of reduced glutathione conjugation with reactive electrophiles in the reduction of peroxides by glutathione peroxidase. The cellular level of glutathione was found to be reduced in intestinal mucosa of IBD patients, whereas reduced mucosal expression and/or activity of GST was also observed in IBD and CAC patients.52,53 Moreover, the serum levels of bilirubin, uric acid, and melatonin are found to be negatively associated with disease severity in IBD patients.54, 55, 56
The above findings not only imply the pathophysiological role of these endogenous antioxidants in IBD and CAC development but also suggest that the altered regulation of their expression in a diseased state is a compensatory response of the host to limit disease progression and severity.
Testing Endogenous Antioxidants in IBD and CAC
Because epidemiologic studies have recognized the association of endogenous antioxidants with IBD and CAC pathophysiology, investigators have tested their therapeutic potential in these diseases. However, the short life span of recombinant enzymatic antioxidants in the gastrointestinal tract remains a barrier for therapeutic evaluation in intestinal diseases. Accordingly, attempts have been made to produce either stable proteins using genetic engineering or transgenic probiotic strains. Supplementation of genetically engineered Lactobacillus fermentum expressing recombinant SOD, hyperthermostable SOD from Thermus thermophilus HB27, or SOD mimics having enhanced stability and activity and ameliorated colitis severity in both mouse and human models.57, 58, 59, 60 In addition, treatment with genetically engineered Lactobacillus casei BL23 or Streptococcus thermophilus CRL807 expressing recombinant CAT or SOD restored endogenous antioxidant pools and reduced disease severity in colitis models.61,62 Remarkably, Ishihara et al57 observed a bell-shaped dose-response of SOD in colitis, demonstrating that a protective effect of SOD at lower doses is through reduction in colonic ROS level and ineffectiveness at higher doses is due to accumulation of H2O2. Accordingly, simultaneous administration of CAT restored the protective effect at higher doses of SOD.57
Recently, multiple studies demonstrate that boosting colonic H2O2 with probiotics can improve mucosal barrier integrity, increase colonization resistance, and suppress inflammatory responses in the colon, whereas exceeding the physiological levels of H2O2 could be detrimental.10, 11, 12 Because SOD converts O2•- to H2O2 and physiological levels of H2O2 are important for gastrointestinal health, it is likely that H2O2 mediates the protective effect of SOD at lower doses in IBD.
Transgenic overexpression of another enzymatic antioxidant thioredoxin in mice led to reduced levels of tumor necrosis factor-α and interferon-γ upon DSS treatment compared with controls, suggesting an anti-inflammatory action of this enzyme.63 Accordingly, administration of recombinant human thioredoxin significantly ameliorated DSS-induced colitis and colonic inflammation in interleukin 10 KO mice.63 Thioredoxin can modulate the DNA binding properties of multiple transcriptional factors to regulate expression of inflammatory mediators.63,64 Overall, these studies report that restoring mucosal enzymatic antioxidants could be protective in IBD.
Nonenzymatic antioxidants as a therapeutic intervention in IBD have also been evaluated. Because IBD patients are depleted of glutathione in their gastrointestinal tracts, Ardite et al65 found that treating colitis-induced mice with glutathione attenuated acute colitis. In addition, ectopic expression of GSTTT1 1 in DSS-treated mice attenuated colitis severity via interleukin 22–dependent restoration of epithelial cell functions.17 Similar to glutathione, supplementing another thiol-containing endogenous antioxidant alpha-lipoic acid also reduced colitis and ileitis in animal models.66,67 Melatonin is another nonenzymatic compound recognized to have enteroprotective activity through its antioxidant and anti-inflammatory action.68 Exogenous administration of melatonin in experimental colitis models improved the disease pathology by reducing inflammation and epithelial damage.69, 70, 71 Melatonin also reduced the levels of oxidative DNA damage in colonic mucosa of IBD patients72; however, the effects on CAC were not evaluated.
Collectively, these studies suggest the therapeutic potential of endogenous antioxidants in IBD. However, few have evaluated the role of these agents in CAC in both preclinical models and in the clinic.
Exogenous Antioxidants as Therapy in IBD and CAC
The current pathophysiological understanding of chronic inflammatory diseases and their association with endogenous antioxidants has encouraged researchers to develop therapeutics for IBD and CAC by using exogenous antioxidants. Exogenous antioxidants are substances that our body cannot produce and therefore must be provided as supplements from natural or synthetic sources. Synthetic antioxidants include compounds with antioxidant activities, precursors or mimics of endogenous antioxidants, and derivatives of amino acids such as propionyl-L-carnitine. Natural antioxidants consist of vitamins, polyphenolic compounds, polyunsaturated fatty acids, and trace metals. Numerous exogenous antioxidants have been investigated for their therapeutic potential in IBD, CAC, and CRC with promising results in preclinical models. However, most of the clinical trials do not validate the preclinical findings. Antioxidant supplementation was generally found to be ineffective or detrimental for cancer in most of the clinical studies73, 74, 75 (Tables 1 and 2), although some of the compounds used as antioxidants in these clinical studies do not have strict ROS-specific effects. Excessive ROS promotes mutagenesis through oxidative DNA damage and can trigger cancer development. However, it also has inhibitory roles on cancer progression through oxidation-induced cytotoxicity in cancer cells. Cytoplasmic ROS levels are significantly higher in cancer cells because of their increased metabolic activity compared with normal cells.76,77 To cope with oxidative damage-induced cytotoxicity, cancer cells depend on various mechanisms including an increase in their antioxidant pool for their survival. Thus, the concept of a negative correlation between antioxidant levels and cancer initiation/progression is now not universally valid,77 and elevating oxidation in tumors by using compounds with pro-oxidant activity is developing as a new chemopreventive therapy in cancer.76 Exogenous antioxidants not only indiscriminately block indispensable physiological redox-mediated cellular functions; these can also prevent cancer cells from oxidation-induced death.77 Indeed, antioxidants such N-acetylcysteine (NAC) or vitamin E (vitE) accelerate tumor progression in mouse models of B-RAF– and K-RAS–induced lung cancer by inactivating p53.78 NAC or vitE also potentiates disease progression in melanoma patients by promoting metastasis dependent on NADPH-generating folate pathway79 or activation of small guanosine triphosphate RHOA.80 However, studies evaluating antioxidants in cancer initiation, particularly in CAC, are scarce.
Table 1.
Summary of Clinical Studies Using Antioxidants in IBD Patients
| Trial characteristic | Population | Subjects | Intervention; duration | Concomitant therapy | Outcome summary | Conclusion | Reference, year |
|---|---|---|---|---|---|---|---|
| Randomized, placebo-controlled pilot study | UC patients; age: 18–70 y | n =37 | Oral NAC (0.8 g/day); 4 weeks | Mesalamine | Clinical remission rate (MTWSI ≤ 2) 63% in treated vs 50% in placebo; clinical response (MTWSI ≥ 2) 66% in treated vs 44% in placebo; reduced interleukin 8 and MCP-1 level; no adverse effect | NAC as combination therapy with mesalamine resulted in clinical improvement in UC patients | 87, 2008 |
| Randomized pilot study | UC patients | n=42 | Intravenous PC-SOD (40 or 80 mg/day); 4 weeks | Immunosuppressants (azathioprine, mercaptopurine) and/or anti-UC agents (mesalazine, salazosulfapyridine) | Decreased Ulcerative Colitis-Disease Activity Index (UC-DAI) in both 40 mg and 80 mg groups; no severe side effects with any of the doses | PC-SOD improved UC more rapidly than previously existing drugs | 58, 2008 |
| Randomized double-blind placebo-controlled | Mild to moderate UC patients; age: 18–75 y | n=121 | Oral tablets of PLC (ST 261; 1 g or 2 g/day); 4 weeks | Aminosalicylates or thiopurine | Clinical/endoscopic response in 75% of patients with 1 g/day and 69% in patients with 2 g/day; remission rates were 55%, 49%, and 35% in PLC (1 g/day), PLC (2 g/day), and placebo groups, respectively. | PLC could be potent treatment modality for mild to moderate UC patients | 89, 2011 |
| Open-label, proof-of-concept pilot study | Mild to moderate IBD patients; age: 16–80 y | N=14 | Oral PLC (ST 261; 2 g/day); 4 weeks | Aminosalicylates, mercaptopurine, or azathioprine | Reduction of Disease Activity Index (DAI) in both UC and CD patients; improvement in Histological Index (HI); no adverse effects | PLC improved endoscopic and histologic activity of mild to moderate UC | 88, 2012 |
| Case-control study | IBD patients; age: 15–34 y | n=219; 111 (UC) + 128 (CD) | VitC from food source, calculated from FFQ collected; 5 y | None | Low risk of UC development with vitC intake | Intake of vitC was negatively associated to UC risk | 96, 2005 |
| Randomized double-blind placebo-controlled | CD patients; age: 38.3 ± 2.9 y (treated); 36.5 ± 1.7 y (placebo) | n=57 | Oral vitC (1000 mg) and vitE (800 IU) daily; 4 weeks | None | Reduction in oxidant burden (measured by breath pentane and ethane output, plasma lipid peroxides, and F2-isoprostane; no change in disease activity | Significant reduction in oxidant burden, but disease activity remained stable in vitC-treated group | 93, 2003 |
| Randomized double-blind placebo-controlled | UC patients; age: 20–45 y | n=150 | Oral vitA (25,000 IU/day); 2 mo | Mesalamine | Decreased DAI and higher clinical response and mucosal healing in vitA group | VitA had positive clinical and endoscopic effects in UC patients | 129, 2018 |
| Open-label study | Mild and moderately active UC patients; age: 21–55 y | n=15 | Enema of α-tocopherol (8000 U/day); 12 weeks | Mesalamine | Decreased average DAI, remission in 64% of patients of treated group | α-tocopherol decreased disease severity in patients with active UC | 92, 2008 |
| Randomized double-blind placebo-controlled multicentric | Patients with quiescent UC; age: 13–65 y | n=89 | Oral curcumin (1 g twice a day); 6 mo | Sulfasalazine or mesalamine | Improved Clinical Activity Index (CAI) and endoscopic index (EI), and suppression in morbidity associated with UC in curcumin group | Curcumin could be a promising and safe medication for maintaining remission in patients with quiescent UC | 130, 2006 |
| Randomized double-blind placebo-controlled | Mild to moderate UC patients; age: 18–70 y | n=70 | Oral curcumin (500 mg capsule 3 times a day); 8 weeks | Salicylates and/or immunomodulators and/or corticosteroids | Significant improvement in Clinical Colitis Activity Index, significantly higher score of quality of life, reduced serum hs-CRP and ESR in curcumin group than placebo | Curcumin supplementation along with traditional drug was associated with improved clinical outcome in mild to moderate UC patients | 131, 2020 |
| Randomized double-blind placebo-controlled | Mild to moderate UC patients; age: 18 y and older | n=56 | Oral curcuminoids nanomicelles (80 mg 3 times a day); 4 weeks | Mesalamine | Decreased SCCAI score in curcuminoid group; reduced frequency of urgent defecation; improved patient’s self-reported well-being | Curcuminoids nanomicelles treatment significantly improved clinical activity of UC patients | 132, 2018 |
| Randomized double-blind placebo-controlled | Mild to moderate UC patients; age: 18–70 y | n=50 | Oral curcumin capsules (1000 mg capsule twice a day); 4 weeks | Mesalamine | Clinical remission in 53.8% and endoscopic remission in 38% of curcumin group compared with 0% in placebo | Addition of curcumin to drug (mesalamine) therapy was superior in inducing clinical and endoscopic remission in UC patients | 133, 2015 |
| Randomized double-blind placebo-controlled pilot study | Patients with mild to moderate distal UC; age: >18 y | n=45 | Enema of NCB-02 (standardized curcumin preparation) ie, equivalent to 140 mg curcumin once daily; 8 weeks | Mesalamine | Significantly better response in NCB-02 compared with placebo in terms of clinical response (92.9% vs 50%), clinical remission (71.4% vs 31.3%), and improvement in endoscopic activity (85.7% vs 50%) | NCB-02 enema improved disease activity in patients with mild to moderate distal UC | 134, 2014 |
| Randomized double-blind placebo-controlled multicentric | Mild to moderate Crohn’s disease patients; age: 21–65 y | n=30 | Theracurmin (a new curcumin derivative with increased absorption rate; 360 mg/day); 12 weeks | Mesalamine (90% of patients), immunomodulators (33.3% of patients), steroids (3.3% of patients), and anti-TNFα (6.7% of patients) | Reduction in clinical disease activity; 40% clinical remission rate and 15% endoscopic remission rate in the Theracurmin group compared with 0% in placebo; better healing of anal lesion with no adverse effect in Theracurmin-treated group | Theracurmin treatment showed significant clinical and endoscopic efficacy with favorable safety profile in mild to moderate Crohn’s disease | 135. 2020 |
| Randomized double-blind placebo-controlled pilot study | Mild to moderate UC patients; age: >18 y | n=56 | Oral resveratrol capsule (500 mg pure trans-resveratrol/day); 6 weeks | — | Decreased disease activity, increased quality of life, increased serum SOD and TAC, and decreased serum MDA in resveratrol group | Supplementation of resveratrol reduced oxidative damage and improved quality of life and disease activity of UC patients | 136, 2016 |
| Randomized double-blind placebo-controlled pilot study | Mild to moderate UC patients; age: >18 y | n=50 | Oral resveratrol capsule (500 mg pure trans-resveratrol/day); 6 weeks | — | Reduction in plasma levels of TNFα and hs-CRP; suppression of NF-kB in peripheral blood mononuclear cells, decrease in clinical colitis activity index score and increase in IBDQ-9 in resveratrol group | Supplementation of resveratrol reduced inflammation and improved quality of life and colitis activity of UC patients | 137, 2015 |
ESR, erythrocyte sedimentation rate; FFQ, food frequency questionnaire; hs-CRP, high sensitivity C-reactive protein; IBDQ-9, inflammatory bowel disease questionnaire-9; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; MTWSI, Modified Truelove-Witts Severity Index; PC-SOD, lecithinized superoxide dismutase; PLC, propionyl-L-carnitine; SCCAIQ, Simple Clinical Colitis Activity Index Questionnaire; SOD, superoxide dismutase; TAC, total antioxidant capacity.
Table 2.
Summary of Clinical Trials Using Antioxidants in Patients Diagnosed With CRC
| Trial characteristic | Population | Subjects | Intervention; duration | Outcome summary | Conclusion | Reference, year |
|---|---|---|---|---|---|---|
| Randomized, double-blind, placebo controlled (ATBC study) | Male smokers of southwestern Finland; age: 50–69 y | n=29,133 | Oral alpha-tocopherol (50 mg/day) or beta-carotene (20 mg/day) or combination of both; 5–8 y | CRC incidence was modestly lower but not significant in alpha-tocopherol group (RR = 0.78; 95% CI, 0.55–1.09); beta-carotene had no effect on CRC incidence (RR = 1.05; 95% CI, 0.75–1.47) | No response on CRC incidence in older male smokers | 138, 2000 |
| Randomized, double-blind, placebo controlled (ATBC study) | Male smokers of southwestern Finland; age: 50–69 y | N=15,538 | Oral alpha-tocopherol (50 mg/day) or beta-carotene (20 mg/day) or combination of both; 6.3 y | Alpha-tocopherol increased the risk of adenoma (RR = 1.66; 95% CI, 1.19–2.32); beta-carotene had no effect on adenoma risk (RR = 0.98; 95% CI, 1.71–1.35) | Negative response; alpha-tocopherol increased the risk of adenoma; however, beta-carotene had no effect on adenoma in older male smokers | 139, 1999 |
| Randomized, controlled clinical trial | Patients post-removal of at least 1 colonic adenoma | n=864 | Oral beta carotene (25 mg/day) or vitC (1 g/day) and vitE (400 mg/day); 4 y | RR for beta carotene was 1.01 (95% CI, 0.85–1.20) and for vitC and E was 1.08 (95% CI, 0.91–1.29) | No response; neither treatment was effective in prevention of any subtype of polyp irrespective of size and location | 114, 1994 |
| Prospective interventional study | Patients previously diagnosed with colorectal adenomas; age: 50–76 y | n= 116 | Oral antioxidants and calcium tablet once daily that contains beta-carotene (15 mg), vitC (150 mg), vitE (75 mg), selenium (101 μg), and calcium (1.6 g); 3 y | No difference was detected in growth of adenomas between treated and placebo groups; significantly lower number of patients free of new adenomas in placebo group compared with treated group | No response on polyp growth; positive response on protection from developing new adenoma | 140, 1998 |
| Randomized, double-blind, placebo-controlled | Patients with history of sporadic colorectal adenoma; age: 30–74 y | n=47 | Oral antioxidant micronutrient cocktail delivering vitE (800 mg), beta-carotene (24 mg), vitC (1 g), selenium (200 μg), riboflavin (7.2 mg), niacin (80 mg), zinc (60 mg), and manganese (5 mg) per day; 4 mo | TNF-α decreased by 37% and cystine decreased by 19% in antioxidants treatment group relative to placebo; interleukin 6 and F2-isoprostane levels decreased in antioxidant-treated nonsmokers but increased in smokers | Positive response only in nonsmoker subjects; an antioxidant micronutrient cocktail decreased the level of oxidants and inflammation only in nonsmokers | 141, 2010 |
| Randomized, controlled study | Patients with colonic polypectomy; mean age: 59.2 y | n=255 | Oral vitamins tablet containing vitC (1 g/day), vitA (30,000 IU/day), and vitE (70 mg/day); ∼5 y | Percentage of recurrence of adenomas was 5.7% in vitamins group compared with 35.9% in untreated group | Positive response; vitamins treatment lowered recurrence rate of colonic adenomas | 142, 1993 |
| Randomized, double-blind trial | Patients post-removal of at least 1 colonic adenoma | n=200 | Oral vitC (400 mg/day) and vitE (400 mg/day); 2 y | Difference in incidence of polyp recurrence was small in treated group compared with placebo (RR = 0.86; 95% CI) | Positive response (small effect); small reduction in rate of polyp recurrence with vitamin supplement | 143, 1988 |
| Randomized, double-blind, placebo-controlled | Patients with advanced colonic adenocarcinoma | n=100 | Oral vitC (10 g/day) as capsule; 2 y | No benefit with high-dose vitC either as disease progression or survival compared with placebo | No response on either overall survival or progression of advanced CRC | 144, 1985 |
| Pilot study | Patients with terminal cancer including colon cancer; age: 32–93 y | n=100 vitC treated and 1000 control subjects | VitC; 10 g/day IV for 10 days followed by 10 g/day oral; ∼ 210 days | Survival was about 4.2 times greater in treated group (∼210 days) compared with control group (∼50 days) | Positive response on overall survival; treatment with vitC increased survival time by about 3 times in terminal cancer patients | 145, 1976 |
| Randomized, double-blind, placebo-controlled | Patients with large bowel adenoma/polyposis coli; age: 20–63 y | n=36 | Oral vitC (3 g/day); ∼2 y | Reduction in both number and area of rectal polyps in vitC group at 9 months of follow-up | Positive response (temporary, only at 9 months of follow-up) on reduction of polyp growth and turnover | 112, 1982 |
| Phase 1 open-label, single-center, dose escalation, and speed-expansion study | Metastatic colorectal cancer (mCRC) or gastric cancer (mGC); age: 18–75 y | n=36 | VitC infusion in dose escalation (0.2–1.5 g/kg) and in speed expansion study (1.5 g/kg) once daily for 3 days in 14-day cycle in combination with mFOLFOX6 or FOLFIRI; 12 cycles | Maximum tolerated dose of vitC not achieved; recommended phase 2 dose of vitC at 1.5 g/kg/day was established; response rate was 58.3%, and disease control rate was 95.8% in treated group | Positive response as combination therapy; favorable safety profile and potential clinical efficacy were observed with combined treatment of vitC and mFOLFOX6/FOLFIRI | 146, 2019 |
| Randomized, placebo-controlled trial, Selenium and vitE Cancer Prevention Trial (SELECT) | SELECT participants who underwent lower endoscopy; age: ≥50 y (African American), ≥55 y (all other men) | N=8094 | Oral selenium (200 μg/day) and vitE (400 IU/day); 7–12 y | RR for adenoma occurrence in selenium group was 0.96 (95% CI, 0.90–1.02) and in vitE group was 1.03 (95% CI, 0.96–1.10) compared with placebo | No response on colorectal adenoma occurrence | 147, 2017 |
| Randomized, placebo-controlled trial | Patients post-removal of at least 1 colorectal adenoma; age: 40–80 y | n=1621 | Selenium (200 μg/day) as selenized yeast in combination with celecoxib (400 mg daily); ∼33 mo | RR of adenoma in selenium group was 1.03 (95% CI, 0.91–1.16) compared with placebo; adenoma recurrence in patients with baseline advanced adenomas was reduced by 18% with selenium | No response on colorectal adenoma formation but showed only modest benefit on adenoma recurrence | 148, 2016 |
| Randomized, placebo-controlled trial | Patients with confirmed recent histories of nonmelanoma skin cancer; age: <80 y | n=1312 | Selenium (200 μg/day) as selenized yeast; 7.9 y | Suggestive but nonsignificant decrease in risk associated with selenium on prevalent adenomas (odds ratio = 0.67; 95% CI, 0.43–1.05); significant reduced risk was observed in subjects with lowest baseline selenium and current smokers | Positive response only in subjects with low baseline selenium or smoking habit | 149, 2006 |
| Randomized double-blind placebo-controlled | Post-polypectomy (colonic) patients; age: 29–83 y | n=411 | One tablet daily composed of 200 μg selenium, 30 mg zinc, 2 mg vitA, 180 mg vitC, and 30 mg vitE; 5 y | A 39% reduction in risk of adenoma recurrence with intervention compared with placebo; similar risk reduction was also observed in small tubular and advanced recurrent adenomas | Positive response on adenoma recurrence | 150, 2013 |
| Randomized, placebo-controlled, prospective trial | Patients post-surgical resection of colon or rectal adenocarcinoma; age: 50–75 y | n=24 | Oral zinc capsules (70 mg/day) in combination with capecitabine or capecitabine with oxaliplatin/5-fluorouracil; 16 weeks | No change in plasma level of vitC, vitE, MDA, or 8-isoprostane but increased SOD activity in zinc-treated group compared with placebo | No response on lipid peroxidation markers but improved SOD activity in zinc-treated group | 151, 2016 |
| Randomized, double-blind, placebo-controlled | Patients with familial adenomatous polyposis; age: 18–85 y | n=44 | Oral curcumin (3000 mg/day); 12 mo | No significant difference in mean polyp number or size was observed between curcumin and placebo-treated groups | No response on polyp number and size in FAP patients | 152, 2018 |
| Randomized, open-labelled, controlled trial | Patients with metastatic colorectal cancer; age: >18 y | n=28 | Oral curcumin C3 complex/d (2 g/day) in combination with FOLFOX; ∼24 weeks | Daily oral supplementation of curcumin to FOLFOX chemotherapy was safe and tolerable; no significant difference between arms for quality of life or neurotoxicity | No response on quality of life, but curcumin could be safe and tolerable adjunct to FOLFOX chemotherapy in patients with metastatic CRC | 153, 2019 |
| Single-center prospective randomized open-labelled | Patients with colonic polypectomy; age: 19–85 y | n=176 | Oral GTE as tablet (0.9 g/day) equivalent to 0.6 g/day of catechin or 0.2 g/day of EGCG; 12 mo | Decreased incidence of metachronous adenoma and number of relapsed adenomas in GTE group | Positive response on metachronous colorectal adenomas | 154, 2018 |
| Pilot study | Patients with colonic polypectomy; age: 20–80 y | n=136 | Oral GTE as tablet (1.5 g/day); 12 mo | Decreased incidence of metachronous adenoma and smaller size of relapsed adenomas in GTE group | Positive response on metachronous colorectal adenoma | 155, 2008 |
| Prospective cohort study | Patients with resected colon cancer or polypectomy; age: median age 74 and 77 for treated and control groups, respectively | n=87 | Oral flavonoid mixture consists of apigenin (20 mg) and epigallocathechin-gallat (20 mg) daily; 4 y | Recurrence rate for neoplasia was 7% in treated group compared with 47% in control group | Positive response with long-term treatment on recurrence rate of colon neoplasia | 156, 2008 |
| Randomized, placebo-controlled trial | Patients with previous adenomatous colonic polyps | n=64 | Oral NAC (800 mg/day) as capsule; 12 weeks | Proliferative index of colonic epithelial cells was reduced in NAC group in comparison with placebo group | Positive response on reducing colonic epithelium hyperproliferation; could be a chemopreventive agent in human colon cancer | 157, 1999 |
| Randomized and controlled | Patients with gastrointestinal cancer undergoing major abdominal surgery | n=33 | NAC (1200 mg/day) through parenteral nutrition starting from 2 days before surgery until fifth post-surgery day; 7 days | Reduced plasma MDA but higher ratio of reduced to oxidized glutathione in NAC group; no change in plasma level of vitA, vitC, or vitE but reduction in urinary nitrate level with NAC treatment | Positive response on reducing oxidant and improving antioxidant parameters in cancer patients undergoing major abdominal surgery | 158, 2015 |
ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CI, confidence interval; EGCG, (-)-epigallocatechin gallate; GTE, green tea extract; MDA, malondialdehyde; RR, relative risk.
Testing Exogenous Antioxidants in IBD
Investigators have examined the protective role of synthetic compounds such as inhibitors of pro-oxidant enzymes and precursor of endogenous antioxidants in IBD. Among several drugs, inhibitors of angiotensin II type 1 (AT1) and hydroxymethylglutaryl coenzyme A reductase are reported to have both antioxidant and anti-inflammatory activities. AT1 increases mitochondrial production of O2•- and H2O2 through NADPH oxidase and inflammation through nuclear factor kappa B. Accordingly, the AT1 antagonist telmisartan was protective in DSS-induced colitis.81 Hydroxymethylglutaryl coenzyme A inhibitors such as simvastatin, rosuvastatin, and pravastatin are primarily lipid-lowering drugs, but they ameliorate disease severity in colitis models by reducing inflammation and inducing endogenous antioxidants such as SOD and glutathione.82,83
As discussed earlier, glutathione is depleted in IBD patients. Administration of NAC, a synthetic precursor of glutathione, in colitis models ameliorated colitis severity,84, 85, 86 but not all studies are in agreement.3 NAC treatment in UC patients resulted in a significant improvement in clinical features and a reduction in serum proinflammatory cytokines.87 Similarly, restoring colonic SOD level by administering lecithinized SOD, a synthetic SOD mimic, improved colitis severity in preclinical and human studies.57,58 Other synthetic antioxidants such as propionyl-L-carnitine, an ester derivative of L-carnitine, improved disease in mild to moderate UC patients.88,89
Natural exogenous antioxidants have also been examined in IBD. VitE is a lipid-soluble vitamin primarily involved in protecting cell membrane from oxidative damage. Supplementation of vitE in preclinical models of colitis ameliorated colitis severity.90,91 However, results from clinical studies are inconclusive.92,93 Vitamin C (vitC) is a water-soluble vitamin that acts as a potent antioxidant because of its ability to donate electrons. Low or high doses of vitC reduce inflammation in animal models94,95; however, not all studies are in agreement.3 Clinical studies using vitC in IBD patients are also inconsistent.93,96
Altogether, the use of exogenous antioxidants to treat IBD needs further work because many studies are preliminary especially in clinical trials, and there are many conflicting findings (Table 1).
Testing Exogenous Antioxidants in CAC
Despite some promising albeit conflicting results with exogenous antioxidants in treating IBD, it is possible that many antioxidants have little to no anti-inflammatory activity and thus may not be useful in treating IBD. On the other hand, exogenous antioxidants could be used to protect from CAC by reducing oxidative DNA damage. However, only a few studies have evaluated antioxidants in preclinical models of CAC.
Long-term administration of NAC reduces oxidative damage (nitrotyrosine and 8-oxoG) in colonic mucosa and protects from CAC development.3,97,98 In addition to its role in cellular redox signaling, peroxynitrite, which is a coupling product of nitric oxide and superoxide, can oxidize DNA and produce DNA lesions. Accordingly, L-NIL, an inducible nitric oxide synthase inhibitor, reduced 8-oxoG levels and colonic polyps in multiple mouse models of CAC, despite having no significant effect on mucosal inflammation.3 In agreement with this study, a derivative of L-NIL (SC-51) reduced inducible nitric oxide synthase and COX-2 activities and lessened the incidence of AOM-induced colonic aberrant crypt foci in rats.99 These findings suggest that limiting nitrosative DNA damage might curb CAC development. Other synthetic compounds such as GL-V9, a flavonoid derivative with strong antioxidant and anti-inflammatory activities, protect against tumorigenesis in a CAC model through NLRP3 inflammasome degradation.100 Statins were found to reduce CAC in IBD patients in one study101 but not in another study.102
Among natural antioxidants, vitC reduces oxidative DNA damage by neutralizing mutagenic ROS and RNI3,103 and protects from inflammation-associated tumorigenesis in different animal models of CAC.3 Paradoxically, a pro-oxidant role for vitC has also been reported at high doses or in presence of transition metals.104 High doses of vitC induce cytotoxicity in cancer cells,105 and it was thus evaluated as a therapeutic agent in CRC patients. However, the results are inconsistent106 (Table 2). Although studies on therapeutic evaluation of curcumin in CAC are limited, some preclinical studies have found a reduction in colonic tumor burden in CAC models.107,108 The effects of resveratrol on CAC have been evaluated in one study that reported a reduction in tumor incidence in AOM/DSS model.109,110
Although there have been numerous trials investigating the effects of antioxidants on disease pathology in IBD patients with mixed results (Table 1), few have used CAC as an endpoint. In light of the findings in preclinical models, serious consideration should be taken to test the role of antioxidants to prevent CAC in IBD patients.
Concluding Thoughts
Several translational studies have shown that antioxidants are effective at reducing both an overt oxidative environment and oxidative DNA lesions in the intestine and other tissues.3,93,95,103 For years these studies have supported the belief that antioxidants can protect DNA from oxidative damage that could precipitate cancer. However, clinical studies that have tested this hypothesis have not reached consistent results.111, 112, 113, 114, 115 One factor that could explain these contradictory results is that antioxidants have been promoted and tested as the panacea for all cancers. Although an excess in oxidative molecules could theoretically induce tumor initiating DNA lesions in any cell, susceptibility to oxidative DNA damage is expected to vary widely in different tissues.78,116,117 Differences in cell proliferation, gene expression, and the cell’s oxidative environment are expected to influence the probability of acquiring oxidative DNA mutations. For example, because oxidative DNA lesions that occur during S-phase of the cell cycle are more likely to result in mutations (Figure 2), highly proliferative tissues such as the intestine are more susceptible to acquire tumor-initiating mutations in oxidative environments. In addition, some tissues such as the intestine are in close contact with microbes and as a result are in a harsh oxidative environment produced by immune cells to keep microbes in check. It is therefore expected that oxidative DNA lesions only promote certain types of cancers.116 Indeed, an analysis of mutational signatures in more than 40 different cancers found that most of colorectal and stomach adenocarcinomas have a ROS mutational signature, whereas other cancers do not.116
The path of genetic mutations that are required for cellular transformation will be different in different tissues and cells. This depends on a number of factors such as the cellular environment and the type of cell being transformed. In the case for CRC and CAC, the cell type that is transformed is similar, but the environments where the cancers arise are different, which might explain the different genetic mutations associated with each of these cancers. For example, whereas mutations that affect the Wnt pathway occur in 85% of sporadic CRCs118 and are considered to be the first step that leads to CRC initiation, CAC tumors first acquire mutations in p53, followed by KRAS mutations.119 p53 is a transcription factor that controls the DNA damage response by inducing cell cycle arrest and apoptosis.120 However, p53 is also involved in other cellular processes such as the antioxidant response, and its down-regulation results in increased DNA oxidation and mutation rates in lymphoma models.120,121 Hence, it is tempting to speculate that inactivation of genes that regulate the antioxidant response is more important for the development of CAC than CRC possibly because of the high oxidative environment of the inflamed gut. This notion is supported by findings that antioxidants only reduced tumorigenesis in CAC models but not in a familial model of CRC (ie, MMR-deficient Lynch syndrome).3 This result could be explained by the fact that most mutations in MMR-deficient cells are due to replication errors and spontaneous cytidine deamination, with only 20% of mutations potentially attributed to oxidative DNA lesions.122 Hence, most mutations that appear in MMR-deficient cells cannot be prevented with antioxidant treatment. In contrast, antioxidants reduced tumorigenesis by 50% in all CAC models tested,3 suggesting that a larger fraction of genetic lesions in inflamed colons is a consequence of oxidative DNA damage. This argument might provide an explanation for the conflicting results in clinical trials that tested antioxidants in CRC and suggests that clinical trials using antioxidants should stratify patients according to genetic susceptibility to acquire oxidative DNA lesions.
Importantly, because ROS are required to maintain homeostasis in the intestinal epithelium, antioxidants should be administered with precaution. Molecules such as O2•- and H2O2 have both proliferative and antiproliferative effects and can regulate cell differentiation, intestinal repair, and antimicrobial defense.8, 9, 10, 11, 12 Therefore, completely shutting off Redox signaling could potentially disrupt homeostasis and cause disease. Indeed, NOX1, NOX2, and DUOX2 deficiencies have been associated with a higher risk of developing pediatric123 and very early onset IBD.124, 125, 126 Furthermore, patients suffering from chronic granulomatous disease, a rare disorder characterized by deficiency in phagocytic NOX function, have a high risk to develop IBD,127,128 suggesting that defective O2•- production can lead to IBD and therefore antioxidant doses should be carefully adjusted for these patients.
In conclusion, both in vitro and in vivo studies suggest the potential role of ROS and RNI in the pathophysiology of IBD and CAC. Epidemiologic studies show altered levels and activity of endogenous antioxidants in IBD and CAC patients. However, further studies are required to confirm their association with disease pathophysiology. There are some promising results from preclinical studies that the use of exogenous compounds (natural or synthetic) with antioxidant activity prevents oxidative DNA damage and CAC. However, this treatment strategy needs to be confirmed in the clinic.
Acknowledgments
The authors thank the Martin lab for their helpful comments.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the Canadian Cancer Society (grant 703185) and Canadian Institute of Health Research (grant PJT-173501).
References
- 1.Frick A., Khare V., Paul G., Lang M., Ferk F., Knasmuller S., Beer A., Oberhuber G., Gasche C. Overt increase of oxidative stress and DNA damage in murine and human colitis and colitis-associated neoplasia. Mol Cancer Res. 2018;16:634–642. doi: 10.1158/1541-7786.MCR-17-0451. [DOI] [PubMed] [Google Scholar]
- 2.Nair J., Gansauge F., Beger H., Dolara P., Winde G., Bartsch H. Increased etheno-DNA adducts in affected tissues of patients suffering from Crohn's disease, ulcerative colitis, and chronic pancreatitis. Antioxidants & Redox Signaling. 2006;8:1003–1010. doi: 10.1089/ars.2006.8.1003. [DOI] [PubMed] [Google Scholar]
- 3.Irrazabal T., Thakur B.K., Kang M., Malaise Y., Streutker C., Wong E.O.Y., Copeland J., Gryfe R., Guttman D.S., Navarre W.W., Martin A. Limiting oxidative DNA damage reduces microbe-induced colitis-associated colorectal cancer. Nat Commun. 2020;11:1802. doi: 10.1038/s41467-020-15549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westbrook A.M., Wei B., Braun J., Schiestl R.H. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009;69:4827–4834. doi: 10.1158/0008-5472.CAN-08-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou D., Shao L., Spitz D.R. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cellular & Molecular Immunology. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myant K.B., Cammareri P., McGhee E.J., Ridgway R.A., Huels D.J., Cordero J.B., Schwitalla S., Kalna G., Ogg E.L., Athineos D., Timpson P., Vidal M., Murray G.I., Greten F.R., Anderson K.I., Sansom O.J. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coant N., Ben Mkaddem S., Pedruzzi E., Guichard C., Treton X., Ducroc R., Freund J.N., Cazals-Hatem D., Bouhnik Y., Woerther P.L., Skurnik D., Grodet A., Fay M., Biard D., Lesuffleur T., Deffert C., Moreau R., Groyer A., Krause K.H., Daniel F., Ogier-Denis E. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus U.G., Hertzberger R., Pircalabioru G.G., Yousefi S.P., Branco Dos Santos F. Pathogen control at the intestinal mucosa: H2O2 to the rescue. Gut Microbes. 2017;8:67–74. doi: 10.1080/19490976.2017.1279378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A.K., Hertzberger R.Y., Knaus U.G. Hydrogen peroxide production by lactobacilli promotes epithelial restitution during colitis. Redox Biology. 2018;16:11–20. doi: 10.1016/j.redox.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews J.D., Reedy A.R., Wu H., Hinrichs B.H., Darby T.M., Addis C., Robinson B.S., Go Y.M., Jones D.P., Jones R.M., Neish A.S. Proteomic analysis of microbial induced redox-dependent intestinal signaling. Redox Biology. 2019;20:526–532. doi: 10.1016/j.redox.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 14.Osburn W.O., Karim B., Dolan P.M., Liu G., Yamamoto M., Huso D.L., Kensler T.W. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer. 2007;121:1883–1891. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 15.Niles J.C., Wishnok J.S., Tannenbaum S.R. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide. 2006;14:109–121. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Ö Canli, Nicolas A.M., Gupta J., Finkelmeier F., Goncharova O., Pesic M., Neumann T., Horst D., Löwer M., Sahin U., Greten F.R. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32:869–883.e5. doi: 10.1016/j.ccell.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.H., Ahn J.B., Kim D.H., Kim S., Ma H.W., Che X., Seo D.H., Kim T.I., Kim W.H., Cheon J.H., Kim S.W. Glutathione S-transferase theta 1 protects against colitis through goblet cell differentiation via interleukin-22. FASEB J. 2020;34:3289–3304. doi: 10.1096/fj.201902421R. [DOI] [PubMed] [Google Scholar]
- 18.Visnes T., Cazares-Korner A., Hao W., Wallner O., Masuyer G., Loseva O., Mortusewicz O., Wiita E., Sarno A., Manoilov A., Astorga-Wells J., Jemth A.S., Pan L., Sanjiv K., Karsten S., Gokturk C., Grube M., Homan E.J., Hanna B.M.F., Paulin C.B.J., Pham T., Rasti A., Berglund U.W., von Nicolai C., Benitez-Buelga C., Koolmeister T., Ivanic D., Iliev P., Scobie M., Krokan H.E., Baranczewski P., Artursson P., Altun M., Jensen A.J., Kalderen C., Ba X., Zubarev R.A., Stenmark P., Boldogh I., Helleday T. Small-molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation. Science. 2018;362:834–839. doi: 10.1126/science.aar8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik E., Dixit V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 21.Bulua A.C., Simon A., Maddipati R., Pelletier M., Park H., Kim K.Y., Sack M.N., Kastner D.L., Siegel R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Formentini L., Santacatterina F., Núñez de Arenas C., Stamatakis K., López-Martínez D., Logan A., Fresno M., Smits R., Murphy M.P., Cuezva J.M. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep. 2017;19:1202–1213. doi: 10.1016/j.celrep.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Gulhane M., Murray L., Lourie R., Tong H., Sheng Y.H., Wang R., Kang A., Schreiber V., Wong K.Y., Magor G., Denman S., Begun J., Florin T.H., Perkins A., Cuív P., McGuckin M.A., Hasnain S.Z. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci Rep. 2016;6:28990. doi: 10.1038/srep28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cremonini E., Daveri E., Mastaloudis A., Adamo A.M., Mills D., Kalanetra K., Hester S.N., Wood S.M., Fraga C.G., Oteiza P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biology. 2019;26:101269. doi: 10.1016/j.redox.2019.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biedermann L., Mwinyi J., Scharl M., Frei P., Zeitz J., Kullak-Ublick G.A., Vavricka S.R., Fried M., Weber A., Humpf H.U., Peschke S., Jetter A., Krammer G., Rogler G. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis: an open pilot study. J Crohns Colitis. 2013;7:271–279. doi: 10.1016/j.crohns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Hou J.K., Abraham B., El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 27.Zhong Z., Umemura A., Sanchez-Lopez E., Liang S., Shalapour S., Wong J., He F., Boassa D., Perkins G., Ali S.R., McGeough M.D., Ellisman M.H., Seki E., Gustafsson A.B., Hoffman H.M., Diaz-Meco M.T., Moscat J., Karin M. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown K.D., Rathi A., Kamath R., Beardsley D.I., Zhan Q., Mannino J.L., Baskaran R. The mismatch repair system is required for S-phase checkpoint activation. Nat Genet. 2003;33:80–84. doi: 10.1038/ng1052. [DOI] [PubMed] [Google Scholar]
- 29.Markkanen E. Not breathing is not an option: how to deal with oxidative DNA damage. DNA Repair. 2017;59:82–105. doi: 10.1016/j.dnarep.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Colussi C., Parlanti E., Degan P., Aquilina G., Barnes D., Macpherson P., Karran P., Crescenzi M., Dogliotti E., Bignami M. The mammalian mismatch repair pathway removes DNA 8-oxodGMP incorporated from the oxidized dNTP pool. Curr Biol. 2002;12:912–918. doi: 10.1016/s0960-9822(02)00863-1. [DOI] [PubMed] [Google Scholar]
- 31.Russo M.T., De Luca G., Degan P., Bignami M. Different DNA repair strategies to combat the threat from 8-oxoguanine. Mutat Res. 2007;614:69–76. doi: 10.1016/j.mrfmmm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Beltran B., Nos P., Dasi F., Iborra M., Bastida G., Martinez M., O'Connor J.E., Saez G., Moret I., Ponce J. Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naive and treated Crohn's disease. Inflamm Bowel Dis. 2010;16:76–86. doi: 10.1002/ibd.21027. [DOI] [PubMed] [Google Scholar]
- 33.Chang D., Hu Z.L., Zhang L., Zhao Y.S., Meng Q.H., Guan Q.B., Zhou J., Pan H.Z. Association of catalase genotype with oxidative stress in the predication of colorectal cancer: modification by epidemiological factors. Biomed Environ Sci. 2012;25:156–162. doi: 10.3967/0895-3988.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Horie K., Mikami T., Yoshida T., Sato Y., Okayasu I. Peroxiredoxin 1 expression in active ulcerative colitis mucosa identified by proteome analysis and involvement of thioredoxin based on immunohistochemistry. Oncology Letters. 2018;15:2364–2372. doi: 10.3892/ol.2017.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer J., Eglinton T.W., Frizelle F.A., Hampton M.B. Peroxiredoxins in colorectal cancer: predictive biomarkers of radiation response and therapeutic targets to increase radiation sensitivity? Antioxidants. 2018;7(10) doi: 10.3390/antiox7100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng L., Wang R., Shang J., Xiong Y., Fu Z. Peroxiredoxin 2 is associated with colorectal cancer progression and poor survival of patients. Oncotarget. 2017;8:15057–15070. doi: 10.18632/oncotarget.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W.S., Huang C.Y., Hsieh M.C., Kuo Y.H., Tung S.Y., Shen C.H., Hsieh Y.Y., Teng C.C., Lee K.C., Lee K.F., Kuo H.C. Expression of PRDX6 correlates with migration and invasiveness of colorectal cancer cells. Cell Physiol Biochem. 2018;51:2616–2630. doi: 10.1159/000495934. [DOI] [PubMed] [Google Scholar]
- 38.Jia J.J., Geng W.S., Wang Z.Q., Chen L., Zeng X.S. The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharmacol. 2019;84:453–470. doi: 10.1007/s00280-019-03869-4. [DOI] [PubMed] [Google Scholar]
- 39.Barrett C.W., Short S.P., Williams C.S. Selenoproteins and oxidative stress-induced inflammatory tumorigenesis in the gut. Cell Mol Life Sci. 2017;74:607–616. doi: 10.1007/s00018-016-2339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Te Velde A.A., Pronk I., de Kort F., Stokkers P.C. Glutathione peroxidase 2 and aquaporin 8 as new markers for colonic inflammation in experimental colitis and inflammatory bowel diseases: an important role for H2O2? Eur J Gastroenterol Hepatol. 2008;20:555–560. doi: 10.1097/MEG.0b013e3282f45751. [DOI] [PubMed] [Google Scholar]
- 41.Meplan C., Hughes D.J., Pardini B., Naccarati A., Soucek P., Vodickova L., Hlavata I., Vrana D., Vodicka P., Hesketh J.E. Genetic variants in selenoprotein genes increase risk of colorectal cancer. Carcinogenesis. 2010;31:1074–1079. doi: 10.1093/carcin/bgq076. [DOI] [PubMed] [Google Scholar]
- 42.Barrett C.W., Ning W., Chen X., Smith J.J., Washington M.K., Hill K.E., Coburn L.A., Peek R.M., Chaturvedi R., Wilson K.T., Burk R.F., Williams C.S. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 2013;73:1245–1255. doi: 10.1158/0008-5472.CAN-12-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steven Esworthy R., Kim B.W., Wang Y., Gao Q., Doroshow J.H., Leto T.L., Chu F.F. The Gdac1 locus modifies spontaneous and Salmonella-induced colitis in mice deficient in either Gpx2 or Gpx1 gene. Free Radic Biol Med. 2013;65:1273–1283. doi: 10.1016/j.freeradbiomed.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Florian S., Krehl S., Loewinger M., Kipp A., Banning A., Esworthy S., Chu F.F., Brigelius-Flohe R. Loss of GPx2 increases apoptosis, mitosis, and GPx1 expression in the intestine of mice. Free Radic Biol Med. 2010;49:1694–1702. doi: 10.1016/j.freeradbiomed.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esworthy R.S., Aranda R., Martin M.G., Doroshow J.H., Binder S.W., Chu F.F. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–G855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- 46.Chu F.F., Esworthy R.S., Doroshow J.H., Grasberger H., Donko A., Leto T.L., Gao Q., Shen B. Deficiency in Duox2 activity alleviates ileitis in GPx1- and GPx2-knockout mice without affecting apoptosis incidence in the crypt epithelium. Redox Biology. 2017;11:144–156. doi: 10.1016/j.redox.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esworthy R.S., Kim B.W., Chow J., Shen B., Doroshow J.H., Chu F.F. Nox1 causes ileocolitis in mice deficient in glutathione peroxidase-1 and -2. Free Radic Biol Med. 2014;68:315–325. doi: 10.1016/j.freeradbiomed.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkins A., Nelson K.J., Parsonage D., Poole L.B., Karplus P.A. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci. 2015;40:435–445. doi: 10.1016/j.tibs.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu W., Fu Z., Wang H., Feng J., Wei J., Guo J. Peroxiredoxin 2 is upregulated in colorectal cancer and contributes to colorectal cancer cells' survival by protecting cells from oxidative stress. Mol Cell Biochem. 2014;387:261–270. doi: 10.1007/s11010-013-1891-4. [DOI] [PubMed] [Google Scholar]
- 50.Nicolussi A., D’Inzeo S., Capalbo C., Giannini G., Coppa A. The role of peroxiredoxins in cancer. Molecular and Clinical Oncology. 2017;6:139–153. doi: 10.3892/mco.2017.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takagi T., Homma T., Fujii J., Shirasawa N., Yoriki H., Hotta Y., Higashimura Y., Mizushima K., Hirai Y., Katada K., Uchiyama K., Naito Y., Itoh Y. Elevated ER stress exacerbates dextran sulfate sodium-induced colitis in PRDX4-knockout mice. Free Radic Biol Med. 2019;134:153–164. doi: 10.1016/j.freeradbiomed.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 52.Sido B., Hack V., Hochlehnert A., Lipps H., Herfarth C., Droge W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485–492. doi: 10.1136/gut.42.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clapper M.L., Szarka C.E. Glutathione S-transferases: biomarkers of cancer risk and chemopreventive response. Chem Biol Interact. 1998;111–112:377–388. doi: 10.1016/s0009-2797(97)00174-9. [DOI] [PubMed] [Google Scholar]
- 54.Zhang M.H., Wang H.G., Shi Y.T., Zhou J.F., Yan W., Ma T.H., Wu S.N., Yang X.Z. Efficacy of serum total bilirubin in predicting the severity of ulcerative colitis: a cross-sectional study. Ann Clin Lab Sci. 2020;50:228–232. [PubMed] [Google Scholar]
- 55.Su Q., Li X., Mo W., Yang Z. Low serum bilirubin, albumin, and uric acid levels in patients with Crohn's disease. Medicine. 2019;98 doi: 10.1097/MD.0000000000015664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terry P.D., Villinger F., Bubenik G.A., Sitaraman S.V. Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research. Inflamm Bowel Dis. 2009;15:134–140. doi: 10.1002/ibd.20527. [DOI] [PubMed] [Google Scholar]
- 57.Ishihara T., Tanaka K., Tasaka Y., Namba T., Suzuki J., Ishihara T., Okamoto S., Hibi T., Takenaga M., Igarashi R., Sato K., Mizushima Y., Mizushima T. Therapeutic effect of lecithinized superoxide dismutase against colitis. J Pharmacol Exp Ther. 2009;328:152–164. doi: 10.1124/jpet.108.144451. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki Y., Matsumoto T., Okamoto S., Hibi T. A lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Colorectal Dis. 2008;10:931–934. doi: 10.1111/j.1463-1318.2008.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou C.L., Zhang J., Liu X.T., Liu H., Zeng X.F., Qiao S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-kappaB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J Appl Microbiol. 2014;116:1621–1631. doi: 10.1111/jam.12461. [DOI] [PubMed] [Google Scholar]
- 60.Sheng Y., Li H., Liu M., Xie B., Wei W., Wu J., Meng F., Wang H.Y., Chen S. A manganese-superoxide dismutase from Thermus thermophilus HB27 suppresses inflammatory responses and alleviates experimentally induced colitis. Inflamm Bowel Dis. 2019;25:1644–1655. doi: 10.1093/ibd/izz097. [DOI] [PubMed] [Google Scholar]
- 61.LeBlanc J.G., del Carmen S., Miyoshi A., Azevedo V., Sesma F., Langella P., Bermudez-Humaran L.G., Watterlot L., Perdigon G., de Moreno de LeBlanc A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn's disease in mice. Journal of Biotechnology. 2011;151:287–293. doi: 10.1016/j.jbiotec.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Del Carmen S., de Moreno de LeBlanc A., Martin R., Chain F., Langella P., Bermudez-Humaran L.G., LeBlanc J.G. Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. Appl Environ Microbiol. 2014;80:869–877. doi: 10.1128/AEM.03296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamaki H., Nakamura H., Nishio A., Nakase H., Ueno S., Uza N., Kido M., Inoue S., Mikami S., Asada M., Kiriya K., Kitamura H., Ohashi S., Fukui T., Kawasaki K., Matsuura M., Ishii Y., Okazaki K., Yodoi J., Chiba T. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131:1110–1121. doi: 10.1053/j.gastro.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 64.Hirota K., Murata M., Sachi Y., Nakamura H., Takeuchi J., Mori K., Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus: a two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 65.Ardite E., Sans M., Panes J., Romero F.J., Pique J.M., Fernandez-Checa J.C. Replenishment of glutathione levels improves mucosal function in experimental acute colitis. Lab Invest. 2000;80:735–744. doi: 10.1038/labinvest.3780077. [DOI] [PubMed] [Google Scholar]
- 66.El-Gowelli H.M., Saad E.I., Abdel-Galil A.G., Ibrahim E.R. Co-administration of alpha-lipoic acid and cyclosporine aggravates colon ulceration of acetic acid-induced ulcerative colitis via facilitation of NO/COX-2/miR-210 cascade. Toxicol Appl Pharmacol. 2015;288:300–312. doi: 10.1016/j.taap.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Moura F.A., de Andrade K.Q., de Araujo O.R., Nunes-Souza V., Santos J.C., Rabelo L.A., Goulart M.O. Colonic and hepatic modulation by lipoic acid and/or N-acetylcysteine supplementation in mild ulcerative colitis induced by dextran sodium sulfate in rats. Oxidative Medicine and Cellular Longevity. 2016;2016:4047362. doi: 10.1155/2016/4047362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirza-Aghazadeh-Attari M., Mohammadzadeh A., Mostavafi S., Mihanfar A., Ghazizadeh S., Sadighparvar S., Gholamzadeh S., Majidinia M., Yousefi B. Melatonin: an important anticancer agent in colorectal cancer. J Cell Physiol. 2020;235:804–817. doi: 10.1002/jcp.29049. [DOI] [PubMed] [Google Scholar]
- 69.Tahan G., Gramignoli R., Marongiu F., Aktolga S., Cetinkaya A., Tahan V., Dorko K. Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig Dis Sci. 2011;56:715–720. doi: 10.1007/s10620-010-1364-5. [DOI] [PubMed] [Google Scholar]
- 70.Iravani S., Eslami P., Dooghaie Moghadam A., Moazzami B., Mehrvar A., Hashemi M.R., Mansour-Ghanaei F., Mansour-Ghanaei A., Majidzadeh A.K. The role of melatonin in colorectal cancer. Journal of Gastrointestinal Cancer. 2019 doi: 10.1007/s12029-019-00336-4. [DOI] [PubMed] [Google Scholar]
- 71.Chojnacki C., Wisniewska-Jarosinska M., Kulig G., Majsterek I., Reiter R.J., Chojnacki J. Evaluation of enterochromaffin cells and melatonin secretion exponents in ulcerative colitis. World J Gastroenterol. 2013;19:3602–3607. doi: 10.3748/wjg.v19.i23.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trivedi P.P., Jena G.B. Melatonin reduces ulcerative colitis-associated local and systemic damage in mice: investigation on possible mechanisms. Dig Dis Sci. 2013;58:3460–3474. doi: 10.1007/s10620-013-2831-6. [DOI] [PubMed] [Google Scholar]
- 73.Myung S.K., Kim Y., Ju W., Choi H.J., Bae W.K. Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol. 2010;21:166–179. doi: 10.1093/annonc/mdp286. [DOI] [PubMed] [Google Scholar]
- 74.Gill J.G., Piskounova E., Morrison S.J. Cancer, oxidative stress, and metastasis. Cold Spring Harb Symp Quant Biol. 2016;81:163–175. doi: 10.1101/sqb.2016.81.030791. [DOI] [PubMed] [Google Scholar]
- 75.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 76.Kohan R., Collin A., Guizzardi S., Tolosa de Talamoni N., Picotto G. Reactive oxygen species in cancer: a paradox between pro- and anti-tumour activities. Cancer Chemother Pharmacol. 2020;86:1–13. doi: 10.1007/s00280-020-04103-2. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q., Pi J., Woods C.G., Andersen M.E. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 2010;244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sayin V.I., Ibrahim M.X., Larsson E., Nilsson J.A., Lindahl P., Bergo M.O. Antioxidants accelerate lung cancer progression in mice. Science Translational Medicine. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 79.Piskounova E., Agathocleous M., Murphy M.M., Hu Z., Huddlestun S.E., Zhao Z., Leitch A.M., Johnson T.M., DeBerardinis R.J., Morrison S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Gal K., Ibrahim M.X., Wiel C., Sayin V.I., Akula M.K., Karlsson C., Dalin M.G., Akyurek L.M., Lindahl P., Nilsson J., Bergo M.O. Antioxidants can increase melanoma metastasis in mice. Science Translational Medicine. 2015;7:308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 81.Saber S., Basuony M., Eldin A.S. Telmisartan ameliorates dextran sodium sulfate-induced colitis in rats by modulating NF-kappaB signalling in the context of PPARgamma agonistic activity. Arch Biochem Biophys. 2019;671:185–195. doi: 10.1016/j.abb.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 82.Peppas S., Piovani D., Peyrin-Biroulet L., Danese S., Bonovas S. Statins and inflammatory bowel disease: where do we stand? European Journal of Internal Medicine. 2020;75:10–14. doi: 10.1016/j.ejim.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 83.Maheshwari R.A., Balaraman R., Sailor G.U., Sen D.B. Protective effect of simvastatin and rosuvastatin on trinitrobenzene sulfonic acid-induced colitis in rats. Indian Journal of Pharmacology. 2015;47:17–21. doi: 10.4103/0253-7613.150311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Q., Hou Y., Yi D., Wang L., Ding B., Chen X., Long M., Liu Y., Wu G. Protective effects of N-acetylcysteine on acetic acid-induced colitis in a porcine model. BMC Gastroenterology. 2013;13:133. doi: 10.1186/1471-230X-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uraz S., Tahan G., Aytekin H., Tahan V. N-acetylcysteine expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic acid-induced colitis in rats. Scand J Clin Lab Invest. 2013;73:61–66. doi: 10.3109/00365513.2012.734859. [DOI] [PubMed] [Google Scholar]
- 86.Kurutas E.B., Cetinkaya A., Bulbuloglu E., Kantarceken B. Effects of antioxidant therapy on leukocyte myeloperoxidase and Cu/Zn-superoxide dismutase and plasma malondialdehyde levels in experimental colitis. Mediators of Inflammation. 2005;2005:390–394. doi: 10.1155/MI.2005.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guijarro L.G., Mate J., Gisbert J.P., Perez-Calle J.L., Marin-Jimenez I., Arriaza E., Olleros T., Delgado M., Castillejo M.S., Prieto-Merino D., Gonzalez Lara V., Pena A.S. N-acetyl-L-cysteine combined with mesalamine in the treatment of ulcerative colitis: randomized, placebo-controlled pilot study. World J Gastroenterol. 2008;14:2851–2857. doi: 10.3748/wjg.14.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merra G., Gasbarrini G., Laterza L., Pizzoferrato M., Poscia A., Scaldaferri F., Arena V., Fiore F., Cittadini A., Sgambato A., Franceschi F., Gasbarrini A. Propionyl-L-carnitine hydrochloride for treatment of mild to moderate colonic inflammatory bowel diseases. World J Gastroenterol. 2012;18:5065–5071. doi: 10.3748/wjg.v18.i36.5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mikhailova T.L., Sishkova E., Poniewierka E., Zhidkov K.P., Bakulin I.G., Kupcinskas L., Lesniakowski K., Grinevich V.B., Malecka-Panas E., Ardizzone S., D'Arienzo A., Valpiani D., Koch M., Denapiene G., Vago G., Fociani P., Zerbi P., Ceracchi M., Camerini R., Gasbarrini G. Randomised clinical trial: the efficacy and safety of propionyl-L-carnitine therapy in patients with ulcerative colitis receiving stable oral treatment. Aliment Pharmacol Ther. 2011;34:1088–1097. doi: 10.1111/j.1365-2036.2011.04844.x. [DOI] [PubMed] [Google Scholar]
- 90.Masri O.A., Chalhoub J.M., Sharara A.I. Role of vitamins in gastrointestinal diseases. World J Gastroenterol. 2015;21:5191–5209. doi: 10.3748/wjg.v21.i17.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tahan G., Aytac E., Aytekin H., Gunduz F., Dogusoy G., Aydin S., Tahan V., Uzun H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 2011;54:333–338. doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mirbagheri S.A., Nezami B.G., Assa S., Hajimahmoodi M. Rectal administration of d-alpha tocopherol for active ulcerative colitis: a preliminary report. World J Gastroenterol. 2008;14:5990–5995. doi: 10.3748/wjg.14.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aghdassi E., Wendland B.E., Steinhart A.H., Wolman S.L., Jeejeebhoy K., Allard J.P. Antioxidant vitamin supplementation in Crohn's disease decreases oxidative stress. a randomized controlled trial. Am J Gastroenterol. 2003;98:348–353. doi: 10.1111/j.1572-0241.2003.07226.x. [DOI] [PubMed] [Google Scholar]
- 94.Kondo K., Hiramoto K., Yamate Y., Goto K., Sekijima H., Ooi K. Ameliorative effect of high-dose vitamin C administration on dextran sulfate sodium-induced colitis mouse model. Biol Pharm Bull. 2019;42:954–959. doi: 10.1248/bpb.b18-00967. [DOI] [PubMed] [Google Scholar]
- 95.Yan H., Wang H., Zhang X., Li X., Yu J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. International Journal of Clinical and Experimental Medicine. 2015;8:20245–20253. [PMC free article] [PubMed] [Google Scholar]
- 96.Sakamoto N., Kono S., Wakai K., Fukuda Y., Satomi M., Shimoyama T., Inaba Y., Miyake Y., Sasaki S., Okamoto K., Kobashi G., Washio M., Yokoyama T., Date C., Tanaka H. Epidemiology Group of the Research Committee on Inflammatory Bowel Disease in Japan. Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis. 2005;11:154–163. doi: 10.1097/00054725-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 97.Seril D.N., Liao J., Ho K.L., Yang C.S., Yang G.Y. Inhibition of chronic ulcerative colitis-associated colorectal adenocarcinoma development in a murine model by N-acetylcysteine. Carcinogenesis. 2002;23:993–1001. doi: 10.1093/carcin/23.6.993. [DOI] [PubMed] [Google Scholar]
- 98.Amrouche-Mekkioui I., Djerdjouri B. N-acetylcysteine improves redox status, mitochondrial dysfunction, mucin-depleted crypts and epithelial hyperplasia in dextran sulfate sodium-induced oxidative colitis in mice. Eur J Pharmacol. 2012;691:209–217. doi: 10.1016/j.ejphar.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 99.Rao C.V., Indranie C., Simi B., Manning P.T., Connor J.R., Reddy B.S. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–170. [PubMed] [Google Scholar]
- 100.Zhao Y., Guo Q., Zhao K., Zhou Y., Li W., Pan C., Qiang L., Li Z., Lu N. Small molecule GL-V9 protects against colitis-associated colorectal cancer by limiting NLRP3 inflammasome through autophagy. Oncoimmunology. 2017;7 doi: 10.1080/2162402X.2017.1375640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ananthakrishnan A.N., Cagan A., Cai T., Gainer V.S., Shaw S.Y., Churchill S., Karlson E.W., Murphy S.N., Liao K.P., Kohane I. Statin use is associated with reduced risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:973–979. doi: 10.1016/j.cgh.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mak J.W.Y., So J., Tang W., Yip T.C.F., Leung W.K., Li M., Lo F.H., Ng K.M., Sze S.F., Leung C.M., Tsang S.W.C., Shan E.H.S., Chan K.H., Lam B.C.Y., Hui A.J., Chow W.H., Chan F.K.L., Ng S.C. Cancer risk and chemoprevention in Chinese inflammatory bowel disease patients: a population-based cohort study. Scand J Gastroenterol. 2020;55:279–286. doi: 10.1080/00365521.2020.1731760. [DOI] [PubMed] [Google Scholar]
- 103.Lutsenko E.A., Carcamo J.M., Golde D.W. Vitamin C prevents DNA mutation induced by oxidative stress. J Biol Chem. 2002;277:16895–16899. doi: 10.1074/jbc.M201151200. [DOI] [PubMed] [Google Scholar]
- 104.Kazmierczak-Baranska J., Boguszewska K., Adamus-Grabicka A., Karwowski B.T. Two faces of vitamin C: antioxidative and pro-oxidative agent. Nutrients. 2020;12 doi: 10.3390/nu12051501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R., Shacter E., Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El Halabi I., Bejjany R., Nasr R., Mukherji D., Temraz S., Nassar F.J., El Darsa H., Shamseddine A. Ascorbic acid in colon cancer: from the basic to the clinical applications. International Journal of Molecular Sciences. 2018;19 doi: 10.3390/ijms19092752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McFadden R.M., Larmonier C.B., Shehab K.W., Midura-Kiela M., Ramalingam R., Harrison C.A., Besselsen D.G., Chase J.H., Caporaso J.G., Jobin C., Ghishan F.K., Kiela P.R. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm Bowel Dis. 2015;21:2483–2494. doi: 10.1097/MIB.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marjaneh R.M., Rahmani F., Hassanian S.M., Rezaei N., Hashemzehi M., Bahrami A., Ariakia F., Fiuji H., Sahebkar A., Avan A., Khazaei M. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J Cell Physiol. 2018;233:6785–6798. doi: 10.1002/jcp.26538. [DOI] [PubMed] [Google Scholar]
- 109.Cui X., Jin Y., Hofseth A.B., Pena E., Habiger J., Chumanevich A., Poudyal D., Nagarkatti M., Nagarkatti P.S., Singh U.P., Hofseth L.J. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prevention Research. 2010;3:549–559. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang J., Zhang Z., Fang A., Wu K., Chen X., Wang G., Mao F. Resveratrol attenuates inflammatory bowel disease in mice by regulating SUMO1. Biol Pharm Bull. 2020;43:450–457. doi: 10.1248/bpb.b19-00786. [DOI] [PubMed] [Google Scholar]
- 111.Bjelakovic G., Nikolova D., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 112.Bussey H.J., DeCosse J.J., Deschner E.E., Eyers A.A., Lesser M.L., Morson B.C., Ritchie S.M., Thomson J.P., Wadsworth J. A randomized trial of ascorbic acid in polyposis coli. Cancer. 1982;50:1434–1439. doi: 10.1002/1097-0142(19821001)50:7<1434::aid-cncr2820500733>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 113.Forman D., Altman D. Vitamins to prevent cancer: supplementary problems. Lancet. 2004;364:1193–1194. doi: 10.1016/S0140-6736(04)17153-5. [DOI] [PubMed] [Google Scholar]
- 114.Greenberg E.R., Baron J.A., Tosteson T.D., Freeman D.H., Jr., Beck G.J., Bond J.H., Colacchio T.A., Coller J.A., Frankl H.D., Haile R.W. A clinical trial of antioxidant vitamins to prevent colorectal adenoma: Polyp Prevention Study Group. N Engl J Med. 1994;331:141–147. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 115.Diakowska D., Lewandowski A., Kopec W., Diakowski W., Chrzanowska T. Oxidative DNA damage and total antioxidant status in serum of patients with esophageal squamous cell carcinoma. Hepatogastroenterology. 2007;54:1701–1704. [PubMed] [Google Scholar]
- 116.Alexandrov L.B., Kim J., Haradhvala N.J., Huang M.N., Tian Ng A.W., Wu Y., Boot A., Covington K.R., Gordenin D.A., Bergstrom E.N., Islam S.M.A., Lopez-Bigas N., Klimczak L.J., McPherson J.R., Morganella S., Sabarinathan R., Wheeler D.A., Mustonen V., Group P.M.S.W., Getz G., Rozen S.G., Stratton M.R., Consortium P. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. doi: 10.1038/s41586-020-1943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diao Q.X., Zhang J.Z., Zhao T., Xue F., Gao F., Ma S.M., Wang Y. Vitamin E promotes breast cancer cell proliferation by reducing ROS production and p53 expression. Eur Rev Med Pharmacol Sci. 2016;20:2710–2717. [PubMed] [Google Scholar]
- 118.Markowitz S.D., Bertagnolli M.M. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beaugerie L., Itzkowitz S.H. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441–1452. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 120.Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harbor Perspectives in Medicine. 2016;6:a026104. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sablina A.A., Budanov A.V., Ilyinskaya G.V., Agapova L.S., Kravchenko J.E., Chumakov P.M. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lang G.I., Parsons L., Gammie A.E. Mutation rates, spectra, and genome-wide distribution of spontaneous mutations in mismatch repair deficient yeast. G3 (Bethesda) 2013;3:1453–1465. doi: 10.1534/g3.113.006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Denson L.A., Jurickova I., Karns R., Shaw K.A., Cutler D.J., Okou D.T., Dodd A., Quinn K., Mondal K., Aronow B.J., Haberman Y., Linn A., Price A., Bezold R., Lake K., Jackson K., Walters T.D., Griffiths A., Baldassano R.N., Noe J.D., Hyams J.S., Crandall W.V., Kirschner B.S., Heyman M.B., Snapper S., Guthery S.L., Dubinsky M.C., Leleiko N.S., Otley A.R., Xavier R.J., Stevens C., Daly M.J., Zwick M.E., Kugathasan S. Clinical and genomic correlates of neutrophil reactive oxygen species production in pediatric patients with Crohn's disease. Gastroenterology. 2018;154:2097–2110. doi: 10.1053/j.gastro.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dhillon S.S., Fattouh R., Elkadri A., Xu W., Murchie R., Walters T., Guo C., Mack D., Huynh H.Q., Baksh S., Silverberg M.S., Griffiths A.M., Snapper S.B., Brumell J.H., Muise A.M. Variants in nicotinamide adenine dinucleotide phosphate oxidase complex components determine susceptibility to very early onset inflammatory bowel disease. Gastroenterology. 2014;147:680–689 e2. doi: 10.1053/j.gastro.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 125.Hayes P., Dhillon S., O'Neill K., Thoeni C., Hui K.Y., Elkadri A., Guo C.H., Kovacic L., Aviello G., Alvarez L.A., Griffiths A.M., Snapper S.B., Brant S.R., Doroshow J.H., Silverberg M.S., Peter I., McGovern D.P., Cho J., Brumell J.H., Uhlig H.H., Bourke B., Muise A.A., Knaus U.G. Defects in NADPH oxidase genes NOX1 and DUOX2 in very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2015;1:489–502. doi: 10.1016/j.jcmgh.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parlato M., Charbit-Henrion F., Hayes P., Tiberti A., Aloi M., Cucchiara S., Begue B., Bras M., Pouliet A., Rakotobe S., Ruemmele F., Knaus U.G., Cerf-Bensussan N. First identification of biallelic inherited DUOX2 inactivating mutations as a cause of very early onset inflammatory bowel disease. Gastroenterology. 2017;153:609–611 e3. doi: 10.1053/j.gastro.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 127.Huang C., De Ravin S.S., Paul A.R., Heller T., Ho N., Wu Datta L., Zerbe C.S., Marciano B.E., Kuhns D.B., Kader H.A., Holland S.M., Malech H.L., Brant S.R., Consortium N.I.G. Genetic risk for inflammatory bowel disease is a determinant of Crohn's disease development in chronic granulomatous disease. Inflamm Bowel Dis. 2016;22:2794–2801. doi: 10.1097/MIB.0000000000000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Angelino G., De Angelis P., Faraci S., Rea F., Romeo E.F., Torroni F., Tambucci R., Claps A., Francalanci P., Chiriaco M., Di Matteo G., Cancrini C., Palma P., D'Argenio P., Dall'Oglio L., Rossi P., Finocchi A. Inflammatory bowel disease in chronic granulomatous disease: an emerging problem over a twenty years' experience. Pediatr Allergy Immunol. 2017;28:801–809. doi: 10.1111/pai.12814. [DOI] [PubMed] [Google Scholar]
- 129.Masnadi Shirazi K., Nikniaz Z., Masnadi Shirazi A., Rohani M. Vitamin A supplementation decreases disease activity index in patients with ulcerative colitis: a randomized controlled clinical trial. Complement Ther Med. 2018;41:215–219. doi: 10.1016/j.ctim.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 130.Hanai H., Iida T., Takeuchi K., Watanabe F., Maruyama Y., Andoh A., Tsujikawa T., Fujiyama Y., Mitsuyama K., Sata M., Yamada M., Iwaoka Y., Kanke K., Hiraishi H., Hirayama K., Arai H., Yoshii S., Uchijima M., Nagata T., Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 131.Sadeghi N., Mansoori A., Shayesteh A., Hashemi S.J. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytother Res. 2020;34:1123–1133. doi: 10.1002/ptr.6581. [DOI] [PubMed] [Google Scholar]
- 132.Masoodi M., Mahdiabadi M.A., Mokhtare M., Agah S., Kashani A.H.F., Rezadoost A.M., Sabzikarian M., Talebi A., Sahebkar A. The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients' self-reported well-being: a randomized double-blind controlled trial. J Cell Biochem. 2018;119:9552–9559. doi: 10.1002/jcb.27273. [DOI] [PubMed] [Google Scholar]
- 133.Lang A., Salomon N., Wu J.C., Kopylov U., Lahat A., Har-Noy O., Ching J.Y., Cheong P.K., Avidan B., Gamus D., Kaimakliotis I., Eliakim R., Ng S.C., Ben-Horin S. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444–1449 e1. doi: 10.1016/j.cgh.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 134.Singla V., Pratap Mouli V., Garg S.K., Rai T., Choudhury B.N., Verma P., Deb R., Tiwari V., Rohatgi S., Dhingra R., Kedia S., Sharma P.K., Makharia G., Ahuja V. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis: a randomized, placebo-controlled, pilot study. J Crohns Colitis. 2014;8:208–214. doi: 10.1016/j.crohns.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 135.Sugimoto K., Ikeya K., Bamba S., Andoh A., Yamasaki H., Mitsuyama K., Nasuno M., Tanaka H., Matsuura A., Kato M., Ishida N., Tamura S., Takano R., Tani S., Osawa S., Nishihira J., Hanai H. Highly bioavailable curcumin derivative ameliorates Crohn's disease symptoms: a randomized, double-blind, multicenter study. J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa097. [DOI] [PubMed] [Google Scholar]
- 136.Samsamikor M., Daryani N.E., Asl P.R., Hekmatdoost A. Resveratrol supplementation and oxidative/anti-oxidative status in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res. 2016;47:304–309. doi: 10.1016/j.arcmed.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 137.Samsami-Kor M., Daryani N.E., Asl P.R., Hekmatdoost A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res. 2015;46:280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 138.Albanes D., Malila N., Taylor P.R., Huttunen J.K., Virtamo J., Edwards B.K., Rautalahti M., Hartman A.M., Barrett M.J., Pietinen P., Hartman T.J., Sipponen P., Lewin K., Teerenhovi L., Hietanen P., Tangrea J.A., Virtanen M., Heinonen O.P. Effects of supplemental alpha-tocopherol and beta-carotene on colorectal cancer: results from a controlled trial (Finland) Cancer Causes Control. 2000;11:197–205. doi: 10.1023/a:1008936214087. [DOI] [PubMed] [Google Scholar]
- 139.Malila N., Virtamo J., Virtanen M., Albanes D., Tangrea J.A., Huttunen J.K. The effect of alpha-tocopherol and beta-carotene supplementation on colorectal adenomas in middle-aged male smokers. Cancer Epidemiol Biomarkers Prev. 1999;8:489–493. [PubMed] [Google Scholar]
- 140.Hofstad B., Almendingen K., Vatn M., Andersen S.N., Owen R.W., Larsen S., Osnes M. Growth and recurrence of colorectal polyps: a double-blind 3-year intervention with calcium and antioxidants. Digestion. 1998;59:148–156. doi: 10.1159/000007480. [DOI] [PubMed] [Google Scholar]
- 141.Hopkins M.H., Fedirko V., Jones D.P., Terry P.D., Bostick R.M. Antioxidant micronutrients and biomarkers of oxidative stress and inflammation in colorectal adenoma patients: results from a randomized, controlled clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19:850–858. doi: 10.1158/1055-9965.EPI-09-1052. [DOI] [PubMed] [Google Scholar]
- 142.Roncucci L., Di Donato P., Carati L., Ferrari A., Perini M., Bertoni G., Bedogni G., Paris B., Svanoni F., Girola M. Antioxidant vitamins or lactulose for the prevention of the recurrence of colorectal adenomas: Colorectal Cancer Study Group of the University of Modena and the Health Care District 16. Dis Colon Rectum. 1993;36:227–234. doi: 10.1007/BF02053502. [DOI] [PubMed] [Google Scholar]
- 143.McKeown-Eyssen G., Holloway C., Jazmaji V., Bright-See E., Dion P., Bruce W.R. A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res. 1988;48:4701–4705. [PubMed] [Google Scholar]
- 144.Moertel C.G., Fleming T.R., Creagan E.T., Rubin J., O'Connell M.J., Ames M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: a randomized double-blind comparison. N Engl J Med. 1985;312:137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 145.Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1976;73:3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang F., He M.M., Wang Z.X., Li S., Jin Y., Ren C., Shi S.M., Bi B.T., Chen S.Z., Lv Z.D., Hu J.J., Wang Z.Q., Wang F.H., Wang D.S., Li Y.H., Xu R.H. Phase I study of high-dose ascorbic acid with mFOLFOX6 or FOLFIRI in patients with metastatic colorectal cancer or gastric cancer. BMC Cancer. 2019;19:460. doi: 10.1186/s12885-019-5696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lance P., Alberts D.S., Thompson P.A., Fales L., Wang F., San Jose J., Jacobs E.T., Goodman P.J., Darke A.K., Yee M., Minasian L., Thompson I.M., Roe D.J. Colorectal adenomas in participants of the SELECT randomized trial of selenium and vitamin E for prostate cancer prevention. Cancer Prevention Research. 2017;10:45–54. doi: 10.1158/1940-6207.CAPR-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thompson P.A., Ashbeck E.L., Roe D.J., Fales L., Buckmeier J., Wang F., Bhattacharyya A., Hsu C.H., Chow H.H., Ahnen D.J., Boland C.R., Heigh R.I., Fay D.E., Hamilton S.R., Jacobs E.T., Martinez M.E., Alberts D.S., Lance P. Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reid M.E., Duffield-Lillico A.J., Sunga A., Fakih M., Alberts D.S., Marshall J.R. Selenium supplementation and colorectal adenomas: an analysis of the nutritional prevention of cancer trial. Int J Cancer. 2006;118:1777–1781. doi: 10.1002/ijc.21529. [DOI] [PubMed] [Google Scholar]
- 150.Bonelli L., Puntoni M., Gatteschi B., Massa P., Missale G., Munizzi F., Turbino L., Villanacci V., De Censi A., Bruzzi P. Antioxidant supplement and long-term reduction of recurrent adenomas of the large bowel: a double-blind randomized trial. J Gastroenterol. 2013;48:698–705. doi: 10.1007/s00535-012-0691-z. [DOI] [PubMed] [Google Scholar]
- 151.Ribeiro S.M., Braga C.B., Peria F.M., Domenici F.A., Martinez E.Z., Feres O., da Rocha J.J., da Cunha S.F. Effect of zinc supplementation on antioxidant defenses and oxidative stress markers in patients undergoing chemotherapy for colorectal cancer: a placebo-controlled, prospective randomized trial. Biol Trace Elem Res. 2016;169:8–16. doi: 10.1007/s12011-015-0396-2. [DOI] [PubMed] [Google Scholar]
- 152.Cruz-Correa M., Hylind L.M., Marrero J.H., Zahurak M.L., Murray-Stewart T., Casero R.A., Jr., Montgomery E.A., Iacobuzio-Donahue C., Brosens L.A., Offerhaus G.J., Umar A., Rodriguez L.M., Giardiello F.M. Efficacy and safety of curcumin in treatment of intestinal adenomas in patients with familial adenomatous polyposis. Gastroenterology. 2018;155:668–673. doi: 10.1053/j.gastro.2018.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Howells L.M., Iwuji C.O.O., Irving G.R.B., Barber S., Walter H., Sidat Z., Griffin-Teall N., Singh R., Foreman N., Patel S.R., Morgan B., Steward W.P., Gescher A., Thomas A.L., Brown K. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149:1133–1139. doi: 10.1093/jn/nxz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shin C.M., Lee D.H., Seo A.Y., Lee H.J., Kim S.B., Son W.C., Kim Y.K., Lee S.J., Park S.H., Kim N., Park Y.S., Yoon H. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: a randomized clinical trial. Clin Nutr. 2018;37:452–458. doi: 10.1016/j.clnu.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 155.Shimizu M., Fukutomi Y., Ninomiya M., Nagura K., Kato T., Araki H., Suganuma M., Fujiki H., Moriwaki H. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020–3025. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]
- 156.Hoensch H., Groh B., Edler L., Kirch W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J Gastroenterol. 2008;14:2187–2193. doi: 10.3748/wjg.14.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Estensen R.D., Levy M., Klopp S.J., Galbraith A.R., Mandel J.S., Blomquist J.A., Wattenberg L.W. N-acetylcysteine suppression of the proliferative index in the colon of patients with previous adenomatous colonic polyps. Cancer Lett. 1999;147:109–114. doi: 10.1016/s0304-3835(99)00281-5. [DOI] [PubMed] [Google Scholar]
- 158.Kuyumcu A., Akyol A., Buyuktuncer Z., Ozmen M.M., Besler H.T. Improved oxidative status in major abdominal surgery patients after N-acetyl cystein supplementation. Nutrition Journal. 2015;14:4. doi: 10.1186/1475-2891-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]