Summary
In genetic and pharmacological models of neurodevelopmental disorders, and human data, neural activity is altered within the developing neocortical network. This commonality begs the question of whether early enhancement in excitation might be a common driver, across etiologies, of characteristic behaviors. We tested this concept by chemogenetically driving cortical pyramidal neurons during postnatal days 4–14. Hyperexcitation of Emx1-, but not dopamine transporter-, parvalbumin-, or Dlx5/6-expressing neurons, led to decreased social interaction and increased grooming activity in adult animals. In vivo optogenetic interrogation in adults revealed decreased baseline but increased stimulus-evoked firing rates of pyramidal neurons and impaired recruitment of inhibitory neurons. Slice recordings in adults from prefrontal cortex layer 5 pyramidal neurons revealed decreased intrinsic excitability and increased synaptic E/I ratio. Together these results support the prediction that enhanced pyramidal firing during development, in otherwise normal cortex, can selectively drive altered adult circuit function and maladaptive changes in behavior.
Subject areas: Behavioral Neuroscience, Developmental Neuroscience, Cellular Neuroscience
Graphical abstract
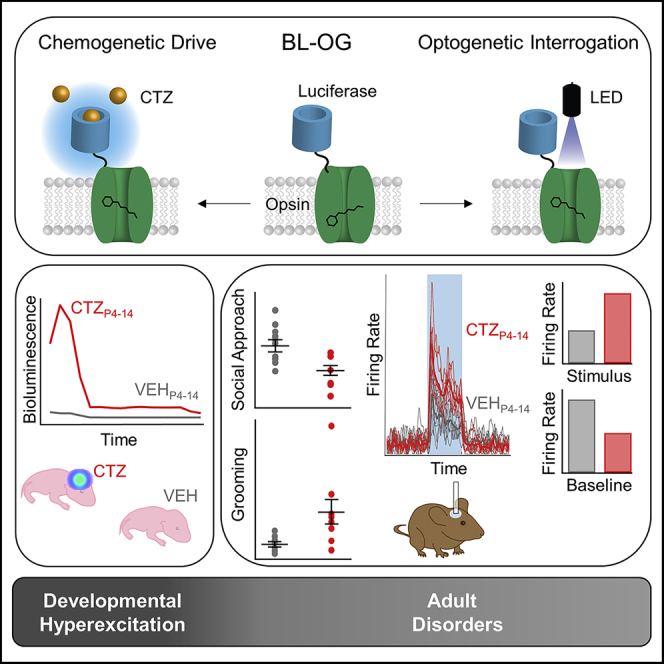
Highlights
-
•
BL-OG allows chemogenetic activation and optogenetic interrogation in the same animal
-
•
Developmental hyperexcitation in normal mice leads to neurodevelopmental disorders
-
•
In these mice adult neurons show reduced baseline activity and increased excitability
-
•
Reduced activity-triggered coherence and altered oscillations in cortex and striatum
Behavioral Neuroscience; Developmental Neuroscience; Cellular Neuroscience
Introduction
Internally generated neural activity during brain development is critically involved in the configuration of connections. In this context, experience-dependent activity builds further synapse formation, elimination, and rearrangements during development and throughout life (Andreae and Burrone, 2014; Golshani et al., 2009; Katz and Shatz, 1996; Penn and Shatz, 1999). Presumably, alterations in intrinsic excitability, and concomitant changes in internally and externally dependent driven activity, underlie neurological and psychiatric disorders. Consistent with this view, a wealth of studies in adult animals have implicated altered neocortical excitatory-inhibitory (E/I) balance in the etiology of autism spectrum disorder (ASD) and other neurodevelopmental disorders, with a net shift toward excitation (Nelson and Valakh, 2015; Rubenstein and Merzenich, 2003; Sohal and Rubenstein, 2019). Recent work analyzing multiple mouse models of ASD has found that net decrease in inhibitory transmission can be relative, as excitatory and inhibitory synapses are both weakened (Antoine et al., 2019). Although acute changes in adult E/I balance can induce and reduce ASD phenotypes (Selimbeyoglu et al., 2017; Yizhar et al., 2011), whether these changes can result from early life alterations to the excitability of pyramidal neurons, or whether this shift has different mechanistic origins, is not known.
To directly address whether early life alterations in pyramidal activity can induce adult phenotypes and related circuit changes, we systematically enhanced pan-neocortical pyramidal activity levels in early development in healthy mice using non-invasive BioLuminescent-OptoGenetic (BL-OG)-mediated chemogenetics (Berglund et al., 2013, 2016; Moore and Berglund, 2020). By activating Emx1-positive neurons, we found that developmental pyramidal over-drive in otherwise healthy subjects selectively led to decreased social interaction and increased grooming activity in adult animals, two key symptoms of ASD. In vivo, both prefrontal neural activity and functional markers of cortico-striatal connectivity were impaired in over-excited Emx1-positive mice, and ex vivo slice recordings revealed alterations to both intrinsic excitability and synaptic E/I ratio in L5 prefrontal cortex pyramidal neurons. These data show that brief and selective increases in pyramidal activity over a limited developmental window can generate key behavioral and neurophysiological signatures of ASD. As such, they implicate aberrant neocortical pyramidal activity during early development as a potential common driver of ASD-like phenotypes.
Results
Developmental hyperexcitation of Emx1 pyramidal neurons is distinctive in causing behavioral phenotypes
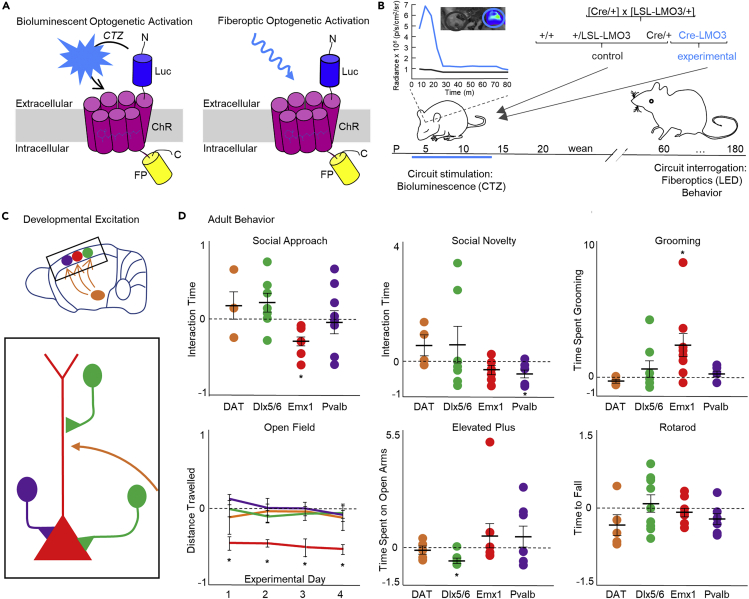
To stimulate genetically targeted neural circuits, we employed bioluminescent optogenetics (BL-OG) (Berglund et al., 2013, 2016; Gomez-Ramirez et al., 2020; Park et al., 2020; Song et al., 2018; Yu et al., 2019; Zenchak et al., 2020). This approach has the distinct dual advantage of enabling both non-invasive chemogenetic manipulation and temporally selective optogenetic control with the same molecular construct. In the BL-OG approach, an optogenetic element can be activated either by light emitted from a tethered luciferase oxidizing a small molecule substrate or by light emitted from physical sources, such as LEDs or lasers (Figure 1A). To activate brain-wide genetically defined populations in a distinct period in early development, we generated a conditional mouse line expressing the excitatory luminopsin 3 (LMO3) in a Cre-dependent manner (Figure S1). Crossing the lox-stop-lox (LSL)-LMO3 line to Cre driver mouse lines enabled hyperexcitation of defined neural populations (Figure 1B).
Figure 1.
Developmental hyperexcitation of Emx1 pyramidal neurons is distinctive in causing behavioral phenotypes
(A) Schematics of a luminopsin (Luc, luciferase, is tethered to ChR, channelrhodopsin; FP, fluorescent protein). Application of the small molecule substrate coelenterazine (CTZ) results in production of photons and bioluminescent optogenetic activation of the nearby opsin (left). The same molecule is accessible to stimulation by a physical light source for standard fiberoptic optogenetic activation (right).
(B) Experimental design. Heterozygous Cre driver mice (Cre/+) were mated with heterozygous conditional (lox-stop-lox) luminopsin-3 mice (LSL-LMO3/+), generating three groups of control mice and one group of experimental mice expressing LMO3 in cells specified by the Cre driver (Cre-LMO3). All pups of a litter were injected once a day intraperitoneally with CTZ postnatal days 4–14. Inset shows representative example of IVIS imaging of Emx1-LMO3 positive and negative pup and ROIs plotted over time. See also Figures S1 and S2.
(C) Schematics of circuits targeted for developmental hyperexcitation. Color codes are used consistently for C and D: red—Emx1, green—Dlx5/6, purple—Pvalb, orange—DAT.
(D) Adult behavior of developmentally hyperexcited mice. Each group of Cre (DAT, Dlx5/6, Emx1, Pvalb)-LMO3 mice is normalized to their non-LMO3 expressing controls. Bars represent mean ± SEM.
See also Figure S3. N = 5-9 per group. ∗p < .05, see also Table S1.
We induced hyperexcitation in developing mouse pups once a day during post-natal days 4–14, capturing the window of significant outgrowth and development of key cortical circuits (Romand et al., 2011; Sasaki et al., 2015). BL-OG has been used to both activate and silence neurons in adult mice and rats, using various routes of administration of CTZ (Berglund et al., 2016; Gomez-Ramirez et al., 2020; Park et al., 2020; Tung et al., 2015; Yu et al., 2019). To confirm the efficacy of BL-OG in pups, we tested for bioluminescence emission and neural activity after intraperitoneal CTZ administration. Bioluminescence emission occurred within 10–15 min and declined over the following hour in Emx1-LMO3 expressing, but not in non-expressing, pups (Figure 1B inset). Neural activity followed a similar timeline when we recorded spikes from putative layer 5 medial prefrontal cortex (mPFC) pyramidal neurons in vivo in P12–P14 pups (Figure S2A). The results in pups regarding onset of activity changes due to CTZ and duration of the effect resemble those obtained in adult animals (Berglund et al., 2016), including the correspondence of bioluminescence increases with higher firing rates (Gomez-Ramirez et al., 2020). We also recorded in slices from P7 Emx1-LMO3 pups, where brief bath application of CTZ depolarized the membrane potential and increased the firing response to current stimulation (Figure S2B). Taken together, our results demonstrate the suitability of using a conditional BL-OG system for exploring the effects of developmental hyperexcitation.
In developing pups, the effect of selective hyperexcitation was examined in multiple neural populations (Figure 1C). We employed the Emx1-Cre line to enhance pan-neocortical pyramidal activity (Gorski et al., 2002), Pvalb-Cre to target inhibitory neurons of the cortex (Hippenmeyer et al., 2005), and Dlx5/6-Cre to target GABAergic neurons of the forebrain (Monory et al., 2006). In complement to targeting these cell types focally creating E/I balance in cortex, we also targeted the dopaminergic system using the DAT-Cre mouse (Backman et al., 2006).
Stimulation of selected neural populations was limited to the postnatal period P4–14, after which the animals received no further CTZ application. To test the prediction that behavioral phenotypes will emerge from over-excitation of distinct neural populations during development, we assayed selected behaviors associated with neurodevelopmental disorders (Silverman et al., 2010), including social interaction, stereotypic behavior, and anxiety (Figure 1D). Selective developmental BL-OG activation of pyramidal neurons, but not interneurons or dopaminergic systems, generated key ASD phenotypes of reduced social behavior and increased compulsivity, as well as hypo-locomotion. Developmentally hyperexcited Emx1-LMO3 mice showed significantly reduced interaction times during the social approach test in a three-chamber setting (Figures 1D and S3). Social interaction was measured as time spent by the test animal actually interacting within a 1-inch radius with the stationary mouse, with Emx1-LMO3 showing significantly reduced time spent interacting (students t test: t(18) = 3.07, p = 0.0067). To disambiguate changes in social preference from those in overall exploration or activity, we conducted a two-way ANOVA test with dependent variable of time spent in a chamber and independent variables of chamber type (empty or with stationary mouse) and genotype (control or experimental). We found a significant interaction of chamber by genotype (F(2,54) = 7.26, p = 0.0016). Further post-hoc analysis revealed Emx1-LMO3 mice spent significantly less time in the chamber with the stationary mouse compared with controls (Tukey's post-hoc: p < 0.05). No significant main effect was found for chamber, indicating no bias was found for any one chamber across groups. In the social novelty test, Emx1-LMO3 mice showed no significant differences from controls on time spent interacting with the novel mouse (students t test: t(18) = 1.97, p = 0.0650). Pvalb-LMO3 mice demonstrate over 50% reduced time spent interacting with the novel mouse compared with non-expressing littermates (students t test: t(21) = 2.19, p = 0.0398) but showed no differences from controls in social approach. DAT-LMO3 and Dlx6a-LMO3 mice did not show significant differences versus littermate controls during either the social approach test or the social novelty test (see also Figure S3 and Table S1). Emx1-LMO3 mice were the only group to exhibit evidence of repetitive behaviors, showing significantly increased time spent grooming by 250% compared with non-expressing littermates (student's t test: t(18) = −2.92, p = 0.0091; Figures 1D and S3). Emx1-LMO3 mice again were the only cohort to demonstrate altered exploration in open field, displaying significantly reduced movements over time (two-way repeated measures ANOVA—main effect between groups: F(1,14) = 11.56, p = 0.0043; Figures 1D and S3). These behavioral effects were selective to pyramidal activation: interneuron drive, for example in the Dlx5/6 group, had opposing effects. Further, the pyramidal activation had selective behavioral effects: developmental hyperexcitation of Emx1 neurons did not result in changes in anxiety for which imbalances in excitatory glutamatergic and inhibitory GABAergic circuits have been implicated (Depino et al., 2008). Indeed, developmental hyperexcitation of GABAergic Dlx5/6 neurons caused mice to display evidence of increased anxiety. These mice spent significantly reduced time on the open arms during the elevated plus maze compared with non-expressing littermates (log-transformed data, student's t test: t(23) = 2.52, p = 0.0096; Figures 1D and S3). To test whether these interventions affected basic coordination, we employed the rotarod, a commonly used metric of basic learning and coordination. No significant differences on time to fall were observed in any of the developmentally treated mice compared with non-expressing littermates (Figures 1D and S3).
Developmental pyramidal hyperexcitation leads to decreased cortico-striatal communication
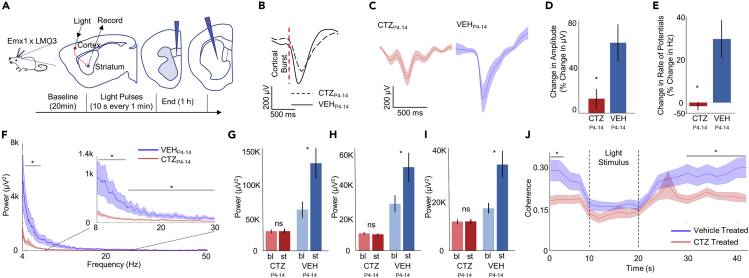
Studies in humans and mouse models directly indicate that cortico-striatal communication is altered in neurodevelopmental disorders with basal ganglia association, such as Tourette and obsessive-compulsive disorders. Also, many ASD models show altered connectivity between the cortex and the striatum (Martella et al., 2018; Nagarajan et al., 2018; Peca et al., 2011; Rothwell et al., 2014; Shofty et al., 2019; Wang et al., 2016). To probe cortico-striatal communication in the relay of endogenous and controlled neocortical events, we measured the impact of naturally occurring neocortical bursts and induced optogenetic bursts on striatal evoked potential responses. Recordings were carried out in adult Emx1-LMO3 mice treated during postnatal days 4–14 with CTZ (CTZP4-14 mice) or with vehicle (VEHP4-14 mice). Use of LMOs enables optogenetic access to the same Emx1 pyramidal neurons initially hyperexcited chemogenetically during development. We placed laminar electrodes in the medial prefrontal cortex (layer 5) and the dorsal striatum (Figure 2A). After 20 min of baseline, mice received acute stimulation to the prefrontal cortex through light pulses of 10s separated by 1-min intervals. To identify changes in striatal responses between baseline and light stimulus periods, event-related local field potentials (erLFP) were captured after optogenetic-stimulus-induced bursts of cortical activity (Figures 2B–2E, 5 mice per group, 35 trials). Spike-triggered LFPs provide a measure of cortical input, allowing indirect measure of change in effective communication (Syed et al., 2011). CTZP4-14 mice showed significantly reduced amplitude during baseline conditions (ANOVA: F(3,11752) = 51.28, p < 0.0001; Bonferroni post-hoc: p < .001; Figures 2B and 2C), suggesting weaker descending excitatory drive. Similarly, CTZP4-14 mice showed no significant differences in the amplitude of erLFPs evoked by light stimulus versus the baseline (Bonferroni post-hoc: p = 0.913; Figure 2D). VEHP4-14 mice, by contrast, showed significantly larger amplitude responses to light stimulation (Bonferroni post-hoc: p < 0.001; Figure 2D). Frequency of LFPs during baseline was not significantly different between groups; however, CTZP4-14 mice showed a generalized decline in frequency power during the light stimulus, whereas the VEHP4-14 group displayed an increase (Mann-Whitney: z −1.964, p = 0.0496; Figure 2E). Ongoing population activity was also different between the groups during optical stimulation, as CTZP4-14 mice showed significantly lower power in the striatum (Theta: F(3,108) = 3.19, p = 0.0265; Alpha: F(3,108) = 3.48, p = 0.0184; Beta: F(3,108) = 3.40, p = 0.0204; Figure 2F). This decrease in the Beta and Gamma bands in striatum is notable, given the robust increase in their expression during optogenetic drive of cortical pyramidal neurons (see Figure 3), supporting the suggestion of a functional decrease in connection between these key forebrain structures. Baseline- and optical-stimulation-associated power spectra revealed no significant change among CTZP4-14 mice during light stimulus in either the Theta, Alpha, or Beta frequency ranges, indicating no increased response to incoming neocortical signals (Bonferroni post-hoc: p = 1.0; Figures 2G–2I). VEHP4-14 mice, by contrast, showed significantly elevated power during light stimulus compared with baseline in all three frequency ranges (Bonferroni post-hoc, Theta: p = 0.050; Alpha: p = 0.036; Beta: p = 0.042). Based on these several indicators of decreased input connectivity, we also directly measured signal coherence (Kramer, 2013). CTZP4-14 mice exhibited significantly lower coherence before and after light stimulus (Interaction of Time x Group: F(20,1128) = 1.87, p = 0.0117; Figure 2J), consistent with a broad decrease in coupling efficiency between the two structures. Coherence in both groups declined during the light stimulus to near chance levels. Although based on indirect measurements, our findings fit well into results from prior studies that have shown that cortico-striatal communication in ASD models transitions across development, with early enhancement and adult degradation (Peixoto et al., 2016, 2019; Peca et al., 2011; Wang et al., 2016).
Figure 2.
Developmental pyramidal hyperexcitation leads to decreased cortico-striatal communication
(A) Schematic of experimental setup. Laminar probes were inserted in the prelimbic area of the medial prefrontal cortex and the striatum.
(B) Striatal event-related local field potentials (erLFPs) time locked to cortical action potential bursts (2 spikes in 10 ms) for adult Emx1-LMO3 mice developmentally stimulated (CTZP4-14, N = 5) and controls (VEHP4-14, N = 5).
(C) erLFPs during optogenetic stimulation: CTZP4-14 mice (red) demonstrate smaller negative deflections compared with VEHP4-14 mice (blue). Shaded area represents mean ± SEM.
(D) Amplitude changes of erLFPs during optogenetic stimulus normalized to baseline before stimulus. Bars represent mean ± SEM.
(E) Frequency changes among erLFPs during optogenetic stimulus normalized to baseline before stimulus. Bars represent mean ± SEM.
(F) Power spectra of striatal neurons during light stimulus for CTZP4-14 (red)- and VEHP4-14 (blue)-treated groups. Shaded area represents mean ± SEM.
(G–I) Average power spectra for the Theta (G), Alpha (H), or Beta (I) range during baseline (bl) and stimulus (st) conditions for both CTZP4-14- and VEHP4-14-treated groups. Bars represent mean ± SEM.
(J) Coherence between cortex and striatum for both CTZP4-14 (red)- and VEHP4-14 (blue)-treated mice before, during and after optogenetic stimulus. Shaded area represents mean ± SEM. ∗p < .05, see also Table S1.
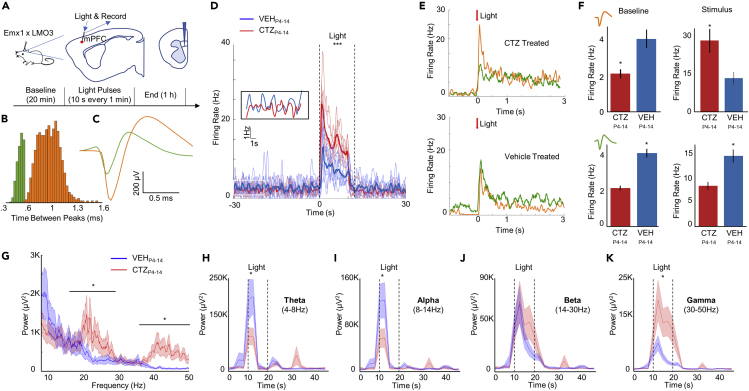
Figure 3.
Developmental pyramidal hyperexcitation leads to decreased baseline firing, enhanced stimulus-evoked firing, and decreased output connectivity in pyramidal neurons
(A) Schematic of experimental setup. Laminar probes were inserted in the prelimbic area of the medial prefrontal cortex.
(B) Waveforms were sorted based on time between peaks. Histogram of these peak to peak times shows a cluster of inhibitory neurons (green) and pyramidal neurons (orange).
(C) Traces of waveforms from putative inhibitory (green) and pyramidal neurons (orange).
(D) The group effects of optogenetic stimulation: CTZP4-14-treated mice show lower baseline levels (inset) and a consistently larger response to stimulation compared with VEHP4-14-treated mice, N = 5 per group.
(E) Light-dependent responses by pyramidal and interneuron populations: CTZP4-14 mice (upper panel) show far greater pyramidal neuron response (orange trace) to light stimulation, whereas VEHP4-14 mice (lower panel) show greater interneuron response (green trace) to light stimulation.
(F) Average firing rate for each group by neuron type and by time point (baseline or stimulus). Bars show mean ± SEM (upper panel—pyramidal neurons, lower panel—inhibitory neurons).
(G) Time-independent power-spectra of cortical neuron LFP during light stimulus. Shaded area represents mean ± SEM.
(H–K) Power over time before, during and after light stimulus for Theta (H), Alpha (I), Beta (J), and Gamma (K) frequency ranges. Shaded area represents mean ± SEM. ∗p < .05, see also Table S1.
Developmental pyramidal hyperexcitation leads to decreased baseline firing, enhanced stimulus-evoked firing, and decreased output connectivity in pyramidal neurons
Alterations in ASD-associated proteins can create profound decreases in homeostatic plasticity, directly implicating such dynamics in evolution of the disorder (Tatavarty et al., 2020). Such changes may manifest through changes in intrinsic excitability and/or synaptic communication. To directly test the impact of developmental hyperexcitation of pyramidal neurons, we measured neural activity in mPFC of adult Emx1-LMO3 animals developmentally treated with CTZ (CTZP4-14 mice) or with vehicle (VEHP4-14 mice).
Recordings of cortical activity were conducted using a laminar probe traversing all layers of the cortex (Figure 3A). Spikes sorting identified putative fast-spiking interneuron and pyramidal neuron subtypes (Figures 3B and 3C). In agreement with the basic prediction of a tonic decrease in network-level excitability, we found a lower baseline firing rate among putative pyramidal neurons in CTZP4-14 mice (Figure 3D inset). Interestingly, these same cells consistently showed a larger increase in firing rate in response to direct optogenetic drive compared with VEHP4-14 mice (two-way repeated measures ANOVA, main effect between groups: F(1,6) = 6.86, p = 0.0396; Figure 3D). No effect was found by trial number, indicating the light stimulus did not produce differential effects depending on when the trial occurred (main effect for trial: F(6,36) = 1.38, p = 0.2494). No significant differences were found in the maximum firing rate between groups (main effect between groups: F(1,6) = 1.30, p = 0.2977), demonstrating that VEHP4-14 mice do reach a similar firing rate later in the light stimulation. Average firing rates after acute light stimulus between pyramidal neurons and interneurons revealed lower rates for interneurons relative to the increased rates for pyramidal neurons in CTZP4-14 mice, whereas interneurons showed a slightly higher response than pyramidal neurons in VEHP4-14 mice (Figure 3E). During baseline, CTZP4-14 mice showed significantly reduced activity among pyramidal neurons in the cortex compared with VEHP4-14 mice (Students t test: t(7) = −6.082, p = 0.0005; Figure 3F, upper panel). During light stimulus, by contrast, CTZP4-14 mice displayed significantly increased pyramidal firing rates compared with VEHP4-14 mice (t(7) = 2.541, p = 0.0386). For interneurons, firing rates were significantly reduced in CTZP4-14 mice at baseline (Students t test: t(7) = 2.526, p = 0.0395) and, different from pyramidal neurons, were also recruited less effectively by light stimulation (Students t test: t(7) = −2.41, p = 0.0468; Figure 3F, lower panel).
These optogenetic results suggest that pyramidal recruitment of feedback inhibition by fast-spiking interneurons is diminished in CTZP4-14 mice. Several studies have demonstrated that altered E/I dynamics at many different points in the neocortical networks can result in altered neural oscillations (Atallah and Scanziani, 2009; Brunel and Wang, 2003; Carlén et al., 2012). These studies suggest that an altered ratio toward excitation can result in a shift in neural oscillations to higher power in the lower Gamma range (30–80 Hz). To assess whether oscillatory dynamics are altered in CTZP4-14 mice, we analyzed power spectra of the LFP signal (Figure 3G). During light stimulus, CTZP4-14 mice showed significantly greater power in the Beta and the Gamma range (Student's t test: t(6) = 2.517, p = 0.0455; t(6) = 2.732, p = 0.0341), suggesting these mice may experience altered excitation/inhibition balance. Given the elevated power in the Beta and Gamma range among CTZP4-14 mice, we assessed how these oscillations change over time. Fast Fourier transforms performed before, during, and after light stimulation revealed a burst-like increase in the Theta and Alpha frequency ranges (two-way repeated measures—interaction of time x group: Theta: F(20,1128) = 3.21, p < 0.0001; Alpha: F(20,1128) = 3.49, p < 0.0001; Figures 3H and 3I). The differences occur within the first few seconds of light stimulation (Bonferroni post-hoc: Theta: p = 0.0203; Alpha: p = 0.0302), with the power returning to baseline levels roughly half-way through the light stimulus. In the Beta and Gamma range, power increases were sustained for the length of light stimulation (Figures 3J and 3K). VEHP4-14 mice and CTZP4-14 mice exhibit different responses to light stimulation. In lower frequencies, the burst-like response was damped in CTZP4-14 mice (two-way repeated measures—interaction of time x group: Theta: F(20,1128) = 3.21, p < 0.0001; Alpha: F(20,1128) = 3.49, p < 0.0001; Figures 3H and 3I). Although a main effect of differences in the Beta power was observed (Figure 3G), putatively due to an evolving difference with sustained stimulation (Figure 3J), no significant differences were found between individual time points (F(20,1128) = 0.78, p = 0.7392). In the Gamma frequency range, CTZP4-14 mice showed significantly higher power compared with VEHP4-14 mice (two-way repeated measures—interaction of time x group: F(20,1128) = 5.40, p < 0.0001; Figure 3K).
Developmental pyramidal hyperexcitation produces enduring alterations in intrinsic excitability and synaptic E/I ratio of L5 prefrontal cortex pyramidal neurons
To elaborate on cellular and synaptic mechanisms to explain the observed effects of developmental hyperexcitation on neural activity in prefrontal cortex in vivo, we recorded from Emx1-LMO3 positive layer 5 (L5) pyramidal neurons in prefrontal cortex slices from young adult (P30–40) animals that received either CTZP4-14 or VEHP4-14 (Figure 4A).
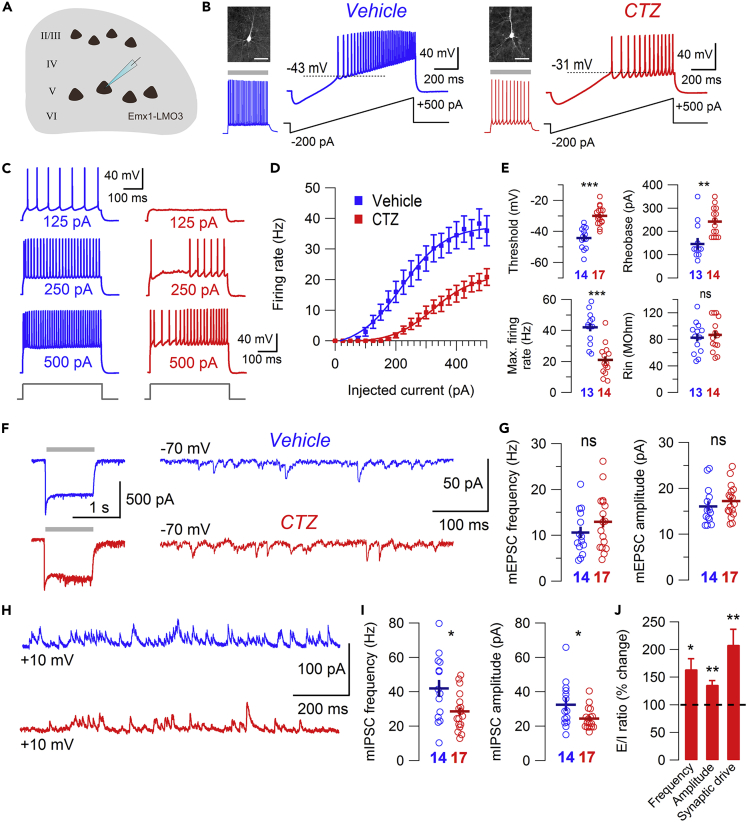
Figure 4.
Developmental pyramidal hyperexcitation produces enduring alterations in intrinsic excitability and synaptic E/I ratio of L5 prefrontal cortex pyramidal neurons
(A) Schematic of mouse prefrontal cortex brain slice featuring LMO3 pyramidal neurons.
(B) Firing response of biocytin-filled L5 prefrontal cortex pyramidal neurons to depolarizing current ramps (dotted line indicates threshold) in VEHP4-14 (blue) and CTZP4-14 (red) groups (scale bars: 100 μm). LMO3 expression confirmed with blue light stimulation.
(C) Example traces of firing response to depolarizing square current injections of increasing magnitude.
(D) Frequency-current relationship of L5 pyramidal neurons. Bars represent mean ± SEM.
(E) Summary graphs showing effect of developmental hyperexcitation on firing threshold, rheobase, maximum firing rate, and input resistance. Bars represent mean ± SEM.
(F) Example traces of mEPSCs recorded from LMO3 positive L5 pyramidal neurons (LMO3 expression confirmed with blue light stimulus at −70 mV, left).
(G) Summary graphs showing effect of developmental hyperexcitation on mEPSC frequency and amplitude. Bars represent mean ± SEM.
(H) Example traces of mIPSCs recorded from LMO3 positive L5 pyramidal neurons.
(I) Summary graphs showing effect of developmental hyperexcitation on mIPSC frequency and amplitude. Bars represent mean ± SEM.
(J) Summary graph showing effect of developmental hyperexcitation on E/I ratio of mPSC frequency, amplitude, and synaptic drive. Bars represent mean ± SEM. ∗p < 0.05, ∗∗pP < 0.01, ∗∗∗p < 0.001. See also Table S1.
We first examined the intrinsic firing properties of L5 pyramidal neurons in the presence of synaptic blockers (D-AP5, CNQX, picrotoxin) and found the firing threshold was significantly increased in CTZP4-14 animals (−30 ± 1.4 mV versus −44.2 ± 1.8 mV, Figures 4B and 4E). In response to depolarizing square current injections of increasing magnitude (Figures 4C and 4D), CTZP4-14 L5 pyramidal neurons displayed an increased rheobase current (242.9 ± 16.5 pA versus 146.2 ± 22.6 pA, Figure 4E) as well as a lower maximum firing frequency (21 ±
2.7 Hz versus 42 ± 2.9 Hz, Figure 4E), resulting in a pronounced right-shift of the frequency-current (f-I) curve. No significant effect on input resistance was observed between groups (82.6 ± 6.6 MΩ versus 86.9 ± 6.6 MΩ, Figure 4E). These results suggest that sustained developmental hyperexcitation of Emx1 positive pyramidal neurons leads to an intrinsically hypoexcitable phenotype in adulthood.
To evaluate the net effect of developmental hyperexcitation on synaptic efficacy onto pyramidal neurons in the prefrontal network, we performed voltage-clamp recordings of miniature excitatory and inhibitory postsynaptic currents (mEPSCs/mIPSCs) from LMO3 positive L5 cells at −70 and +10 mV, respectively, in the presence of tetrodotoxin. We found no significant change in the frequency (13 ± 1.5 Hz versus 10.6 ± 1.3 Hz, Figures 4F and 4G) or amplitude (17.2 ± 0.9 pA versus 16.1 ± 1.1 pA, Figures 4F and 4G) of mEPSCs between groups. In contrast, we found that both the frequency (28.6 ± 2.7 Hz versus 42 ± 5.1 Hz, Figures 4H and 4I) and amplitude (24.5 ± 1.5 pA versus 32.5 ± 3.6 pA, Figures 4H and 4I) of mIPSCs were significantly reduced in CTZP4-14 animals, indicating a net decrease in synaptic efficacy of GABAA-ergic transmission. The respective effects on frequency and amplitude of mEPSCs and mIPSCs between groups resulted in increased E/I ratios in CTZP4-14 mice (frequency: 0.49 ± 0.06 versus 0.3 ± 0.05; amplitude: 0.73 ± 0.04 versus 0.53 ± 0.04; Figure 4J), producing a 2-fold increase in E/I ratio of synaptic drive (0.36 ± 0.05 versus 0.17 ± 0.04, Figure 4J). Together these results show chronic excitation of Emx1 neurons during early development produces lasting changes to intrinsic excitability and functional connectivity of L5 pyramidal neurons of the prefrontal cortex, where a reduction in intrinsic excitability is accompanied by a lowered synaptic efficacy of inhibitory transmission.
Discussion
Here, we tested in healthy animals the impact of early enhancement in neural activity within specific cell types. We found that early hyperexcitation of pyramidal neurons alone, and for a limited time window, is sufficient to create adult neurophysiological and behavioral phenotypes in the absence of other genetic or environmental insults. Moreover, early postnatal interference with neural activity of other distinct populations resulted in different behavioral symptoms, indicating selective effects. In addition to causally linking developmental neural activity with adult behavior, our results provide insights into the dynamics of neural networks responding to aberrant activity patterns during development.
The sufficiency of brief hyperexcitability to derail circuit formation in an otherwise normal brain highlights an underappreciated vulnerability of developing neural circuits where seemingly innocuous events can have lifelong behavioral consequences. This finding may provide an explanation for the large variety of potential etiologies reported for symptoms of ASD and other neurodevelopmental disorders, with infections, autoimmune processes, and environmental causes acting in concert with genetic and epigenetic susceptibilities (Amaral, 2017; Grandjean and Landrigan, 2006; Jones and Van de Water, 2019; Landrigan, 2010; Mawson and Croft, 2019; Roberts et al., 2019; Tartaglione et al., 2019).
An increased E/I ratio in the cortical network, resulting from overexcitation of pyramidal neurons, impaired GABAergic inhibition, or both, has remained central in ASD research (Rubenstein and Merzenich, 2003; Sohal and Rubenstein, 2019). Our data both in vivo and ex vivo show that inappropriate “E/I balance” may be most important to disease development if expressed, even for just a narrow window, early in development, and that imbalance during this window is sufficient in driving a cascade leading to phenotypic behavioral and circuit outcomes in the disorder.
The use of the BL-OG control strategy here uniquely enabled this study. Combined chemogenetic and optogenetic access through the same genetically targeted molecule provided developmental perturbation and adult functional probe of system connectivity and excitability. This approach offers an experimental framework for delineating developmental network disturbances and their consequences in establishing adult neural connections and their resulting behaviors in the general field of neurodevelopmental disorders.
Limitations of the study
In this study, we tested the effects of hyperexcitation over a relatively broad temporal window, P4–14, that covers a range of postnatal developmental events. This time window is sufficient to create the observed behavioral and electrophysiological changes in adults. However, this result does not mean that this particular developmental window will be the only one to create the effects observed. In fact, it will be interesting in future studies both to break up the current 10-day window into smaller time periods, as well as probing different windows, such as P14–21, or even later. The experimental model system we present in this study is uniquely suited to address these questions.
We initially “screened” Emx1-, Pvalb-, DAT-, and Dlx6a-LMO3 mice for behavioral effects of developmental hyperexcitation. We then focused on the Emx1-LMO3 mice as the group presenting with the most significant behavioral changes within the chosen test battery. For this study the other groups served as controls to rule out non-specific effects of developmental hyperexcitation. There are many more genetically defined populations that can be tested utilizing available Cre driver lines and probing the effects on a larger variety of behaviors by exploiting more extensive behavioral tests.
From our behavioral testing we had strong evidence of social deficits, and given the mPFC's known role in social behavior, we chose this area as the first region to probe in more detail in in vivo and ex vivo electrophysiological recordings. The changes observed within the mPFC are not expected to happen in mPFC alone, but rather we anticipate that they will generalize throughout the cortex, which, again, will be interesting to examine in future studies.
We are aware of the limitations of our current cortico-striatal studies. We observed robust changes in striatal activity patterns triggered on the fine-timing of neocortical spikes, a measure commonly interpreted as reflecting direct neocortical input. However, we do not know whether the reduced coupling in the developmentally hyperexcited group is due to reduced excitability of PNs, a decrease in cortico-striatal synaptic efficacy, or both. Given the reduced excitability of PNs we observed in mPFC slices, hypoexcitability of CTZ-treated PNs should contribute to the lower coupling. Definitive answers regarding the effect on synaptic efficacy in striatum can only be obtained by measuring connectivity in slices. Furthermore, spikes might be originating from neurons other than corticostriatal neurons, and the relative proportion of spikes arising from corticostriatal neurons versus other neuron types might differ in the control versus experimental groups. In this scenario, it is possible that measured spike-LFP coupling could go down even though corticostriatal coupling is actually unchanged or even increased. Yet, even if some component of the neocortically triggered signals are transmitted through a third locus (e.g., a thalamic relay), the ultimate logic of using spike-triggered analyses and downstream impact is to measure a change in effective communication, which our data do clearly suggest.
Resource availability
Lead contact
Additional resources related to this study are available upon request from the Lead Contact (Ute Hochgeschwender, hochg1u@cmich.edu).
Materials availability
Plasmids used in this study and their sequences are available at Addgene (LMO3, #114099).
Mouse lines generated in this study are available from The Jackson Laboratory (B6N.129-Gt(ROSA)26Sortm4.1(CAG-sbGLuc/COP3/EYFP)Ute, Stock No: 034853).
Data and code availability
No new code was generated in this study.
The raw datasets supporting the current study are available from the Lead Contact on request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the members of the Bioluminescence Hub (http://www.bioluminescencehub.org/) for helpful discussions. This study was supported by grants from the US National Institutes of Health to U.H. (R21MH101525) and C.I.M. (U01NS099709); the National Science Foundation to U.H. and C.I.M. (NSF NeuroNex 1707352); the W.M. Keck Foundation to U.H. and C.I.M.; the Swedish Research Council to A.B. (2016-06760); and the CMU Office of Research and Graduate Studies to W.E.M. (Graduate Student Research & Creative Endeavors Grant).
Author contributions
Conceptualization, W.E.M., C.I.M., and U.H.; Methodology, W.E.M. and U.H.; Software, W.E.M.; Validation, W.E.M., A.B., C.I.M., and U.H.; Formal Analysis, W.E.M. and A.B.; Investigation, W.E.M., A.B., E.L.C., M.P., A.P., and M.L.W.; Resources, U.H.; Data Curation, W.E.M., and A.B.; Writing—Original Draft, W.E.M., C.I.M., and U.H.; Writing—Review & Editing, W.E.M., A.B., C.I.M., and U.H.; Visualization, W.E.M., A.B., and U.H.; Supervision, U.H. and C.I.M.; Project Administration, U.H.; Funding Acquisition, U.H. and C.I.M.
Declaration of interests
The authors declare no competing interests.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102157.
Contributor Information
Christopher I. Moore, Email: christopher_moore@brown.edu.
Ute Hochgeschwender, Email: hochg1u@cmich.edu.
Supplemental information
References
- Amaral D.G. Examining the Causes of Autism. Cerebrum. 2017;2017 cer-01-17. [PMC free article] [PubMed] [Google Scholar]
- Andreae L.C., Burrone J. The role of neuronal activity and transmitter release on synapse formation. Curr. Opin. Neurobiol. 2014;27:47–52. doi: 10.1016/j.conb.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine M.W., Langberg T., Schnepel P., Feldman D.E. Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron. 2019;101:648–661.e4. doi: 10.1016/j.neuron.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah B.V., Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman C.M., Malik N., Zhang Y., Shan L., Grinberg A., Hoffer B.J., Westphal H., Tomac A.C. Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Berglund K., Birkner E., Augustine G.J., Hochgeschwender U. Light-emitting channelrhodopsins for combined optogenetic and chemical-genetic control of neurons. PLoS One. 2013;8:e59759. doi: 10.1371/journal.pone.0059759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund K., Clissold K., Li H.E., Wen L., Park S.Y., Gleixner J., Klein M.E., Lu D., Barter J.W., Rossi M.A. Luminopsins integrate opto- and chemogenetics by using physical and biological light sources for opsin activation. Proc. Natl. Acad. Sci. U S A. 2016;113:E358–E367. doi: 10.1073/pnas.1510899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N., Wang X.J. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J. Neurophysiol. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- Carlén M., Meletis K., Siegle J.H., Cardin J.A., Futai K., Vierling-Claassen D., Rühlmann C., Jones S.R., Deisseroth K., Sheng M. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depino A.M., Tsetsenis T., Gross C. GABA homeostasis contributes to the developmental programming of anxiety-related behavior. Brain Res. 2008;1210:189–199. doi: 10.1016/j.brainres.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Golshani P., Gonçalves J.T., Khoshkhoo S., Mostany R., Smirnakis S., Portera-Cailliau C. Internally mediated developmental desynchronization of neocortical network activity. J. Neurosci. 2009;29:10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ramirez M., More A.I., Friedman N.G., Hochgeschwender U., Moore C.I. The BioLuminescent-OptoGenetic in vivo response to coelenterazine is proportional, sensitive, and specific in neocortex. J. Neurosci. Res. 2020;98:471–480. doi: 10.1002/jnr.24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J.A., Talley T., Qiu M., Puelles L., Rubenstein J.L.R., Jones K.R. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Landrigan P.J. Developmental neurotoxicity of industrial chemicals. Lancet (London, England) 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S., Vrieseling E., Sigrist M., Portmann T., Laengle C., Ladle D.R., Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.L., Van de Water J. Maternal autoantibody related autism: mechanisms and pathways. Mol. Psychiatry. 2019;24:252–265. doi: 10.1038/s41380-018-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L.C., Shatz C.J. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kramer M.A. The Science of Large Data Sets: Spikes, Fields, and Voxels. Society for Neuroscience); 2013. An introduction to field analysis techniques: the power spectrum and coherence; pp. 18–25. [Google Scholar]
- Landrigan P.J. What causes autism? Exploring the environmental contribution. Curr. Opin. Pediatr. 2010;22:219–225. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- Martella G., Meringolo M., Trobiani L., De Jaco A., Pisani A., Bonsi P. The neurobiological bases of autism spectrum disorders: the R451C-neuroligin 3 mutation hampers the expression of long-term synaptic depression in the dorsal striatum. Eur. J. Neurosci. 2018;47:701–708. doi: 10.1111/ejn.13705. [DOI] [PubMed] [Google Scholar]
- Mawson A.R., Croft A.M. Rubella virus infection, the congenital rubella syndrome, and the link to autism. Int. J. Environ. Res. Public Health. 2019;16:3543. doi: 10.3390/ijerph16193543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K., Massa F., Egertova M., Eder M., Blaudzun H., Westenbroek R., Kelsch W., Jacob W., Marsch R., Ekker M. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C.I., Berglund K. BL-OG: BioLuminescent-OptoGenetics. J. Neurosci. Res. 2020;98:469–470. doi: 10.1002/jnr.24575. [DOI] [PubMed] [Google Scholar]
- Nagarajan N., Jones B.W., West P.J., Marc R.E., Capecchi M.R. Corticostriatal circuit defects in Hoxb8 mutant mice. Mol. Psychiatry. 2018;23:1868–1877. doi: 10.1038/mp.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S.B., Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Song S.H., Palmateer B., Pal A., Petersen E.D., Shall G.P., Welchko R.M., Ibata K., Miyawaki A., Augustine G.J. Novel luciferase–opsin combinations for improved luminopsins. J. Neurosci. Res. 2020;98:410–421. doi: 10.1002/jnr.24152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J., Feliciano C., Ting J.T., Wang W., Wells M.F., Venkatraman T.N., Lascola C.D., Fu Z., Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto R.T., Wang W., Croney D.M., Kozorovitskiy Y., Sabatini B.L. Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B(-/-) mice. Nat. Neurosci. 2016;19:716–724. doi: 10.1038/nn.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto R.T., Chantranupong L., Hakim R., Levasseur J., Wang W., Merchant T., Gorman K., Budnik B., Sabatini B.L. Abnormal striatal development underlies the early onset of behavioral deficits in Shank3B(-/-) mice. Cell Rep. 2019;29:2016–2027.e4. doi: 10.1016/j.celrep.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A.A., Shatz C.J. Brain waves and brain wiring: the role of endogenous and sensory-driven neural activity in development. Pediatr. Res. 1999;45:447–458. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- Roberts J.R., Dawley E.H., Reigart J.R. Children’s low-level pesticide exposure and associations with autism and ADHD: a review. Pediatr. Res. 2019;85:234–241. doi: 10.1038/s41390-018-0200-z. [DOI] [PubMed] [Google Scholar]
- Romand S., Wang Y., Toledo-Rodriguez M., Markram H. Morphological development of thick-tufted layer v pyramidal cells in the rat somatosensory cortex. Front. Neuroanat. 2011;5:5. doi: 10.3389/fnana.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell P.E., Fuccillo M.V., Maxeiner S., Hayton S.J., Gokce O., Lim B.K., Fowler S.C., Malenka R.C., Sudhof T.C. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J.L.R., Merzenich M.M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Aoi H., Oga T., Fujita I., Ichinohe N. Postnatal development of dendritic structure of layer III pyramidal neurons in the medial prefrontal cortex of marmoset. Brain Struct. Funct. 2015;220:3245–3258. doi: 10.1007/s00429-014-0853-2. [DOI] [PubMed] [Google Scholar]
- Selimbeyoglu A., Kim C.K., Inoue M., Lee S.Y., Hong A.S.O., Kauvar I., Ramakrishnan C., Fenno L.E., Davidson T.J., Wright M. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci. Transl. Med. 2017;9:eaah6733. doi: 10.1126/scitranslmed.aah6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shofty B., Bergmann E., Zur G., Asleh J., Bosak N., Kavushansky A., Castellanos F.X., Ben-Sira L., Packer R.J., Vezina G.L. Autism-associated Nf1 deficiency disrupts corticocortical and corticostriatal functional connectivity in human and mouse. Neurobiol. Dis. 2019;130:104479. doi: 10.1016/j.nbd.2019.104479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J.L., Yang M., Lord C., Crawley J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal V.S., Rubenstein J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry. 2019;24:1248–1257. doi: 10.1038/s41380-019-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Yang Q., Lang Y., Wen Z., Xie Z., Zheng D., Yan T., Deng Y., Nakanishi H., Quan Z. Manipulation of hippocampal CA3 firing via luminopsins modulates spatial and episodic short-term memory, especially working memory, but not long-term memory. Neurobiol. Learn. Mem. 2018;155:435–445. doi: 10.1016/j.nlm.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Syed E.C.J., Sharott A., Moll C.K.E., Engel A.K., Kral A. Effect of sensory stimulation in rat barrel cortex, dorsolateral striatum and on corticostriatal functional connectivity. Eur. J. Neurosci. 2011;33:461–470. doi: 10.1111/j.1460-9568.2010.07549.x. [DOI] [PubMed] [Google Scholar]
- Tartaglione A.M., Schiavi S., Calamandrei G., Trezza V. Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder. Neuropharmacology. 2019;159:107477. doi: 10.1016/j.neuropharm.2018.12.024. [DOI] [PubMed] [Google Scholar]
- Tatavarty V., Torrado Pacheco A., Groves Kuhnle C., Lin H., Koundinya P., Miska N.J., Hengen K.B., Wagner F.F., Van Hooser S.D., Turrigiano G.G. Autism-associated Shank3 is essential for homeostatic compensation in rodent V1. Neuron. 2020;106:769–777.e4. doi: 10.1016/j.neuron.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J.K., Gutekunst C.-A., Gross R.E. Inhibitory luminopsins: genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition. Sci. Rep. 2015;5:14366. doi: 10.1038/srep14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Bey A.L., Katz B.M., Badea A., Kim N., David L.K., Duffney L.J., Kumar S., Mague S.D., Hulbert S.W. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat. Commun. 2016;7:11459. doi: 10.1038/ncomms11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O., Fenno L.E., Prigge M., Schneider F., Davidson T.J., O’Shea D.J., Sohal V.S., Goshen I., Finkelstein J., Paz J.T. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.P., Tung J.K., Wei Z.Z., Chen D., Berglund K., Zhong W., Zhang J.Y., Gu X., Song M., Gross R.E. Optochemogenetic stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery after ischemic stroke. J. Neurosci. 2019;39:6571–6594. doi: 10.1523/JNEUROSCI.2010-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenchak J.R., Palmateer B., Dorka N., Brown T.M., Wagner L.M., Medendorp W.E., Petersen E.D., Prakash M., Hochgeschwender U. Bioluminescence-driven optogenetic activation of transplanted neural precursor cells improves motor deficits in a Parkinson’s disease mouse model. J. Neurosci. Res. 2020;98:458–468. doi: 10.1002/jnr.24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new code was generated in this study.
The raw datasets supporting the current study are available from the Lead Contact on request.