Abstract
We report a case of cardiac injury in a 46-year-old man affected by COVID-19. The patient presented with shortness of breath and fever. ECG revealed sinus tachycardia with ventricular extrasystoles and T-wave inversion in anterior leads. Troponin T and N-terminal pro B-type natriuretic peptide were elevated. Transthoracic echocardiography showed severely reduced systolic function with an estimated left ventricle ejection fraction of 30%. A nasopharingeal swab was positive for SARS-CoV-2. On day 6, 11 days after onset of symptoms, the patient deteriorated clinically with new chest pain and type 1 respiratory failure. Treatment with colchicine 0.5 mg 8-hourly resulted in rapid clinical resolution. This case report highlights how cardiac injury can dominate the clinical picture in COVID-19 infection. The role of colchicine therapy should be further studied to determine its usefulness in reducing myocardial and possibly lung parenchymal inflammatory responses.
Keywords: COVID-19, drugs and medicines, cardiovascular medicine
Background
Cardiac involvement is increasingly recognised as a complication of SARS-CoV-2 infection.1 Since the pandemic started, more and more SARS-CoV-2 related cardiac injury cases have been reported and several anecdotal reports of myocarditis in patients infected with COVID-19 have emerged in the literature,2–4 but the characteristic features and range of clinical presentations remain poorly defined.
The diagnosis of cardiac injury several days after initiation of COVID-19 symptoms may be indicative of myocardial damage caused by viral infection. The current report describes a case of cardiac injury in a patient affected by COVID-19 successfully treated with colchicine.
Case presentation
A 46-year-old obese man with medical history of essential hypertension and bicuspid aortic valve with a normal left ventricle ejection fraction (LVEF) of 55% 6 months before admission, presented to the emergency department with moderate shortness of breath and fever, following a 5-day history of flu-like symptoms. On admission, he was tachycardic (heart rate: 110 beats/min), hypotensive (blood pressure: 97/66 mm Hg), feverish (37.5°C), tachypnoeic and auscultation revealed bilateral crackles. His oxygen saturations remained poor despite oxygen therapy (95% with a fractional inspired oxygen of 40%).
Blood tests showed a C reactive protein (CRP) of 5 mg/L (normal range: <3 mg/L), elevated N-terminal pro B-type natriuretic peptide 1134 ng/L (normal range: <97 ng/L) and elevated troponin T level 38 ng/L (normal range: <14 ng/L). Twelve lead electrocardiogram demonstrated sinus tachycardia, T-wave inversion in the anterior leads and frequent ventricular extrasystoles (1:3). Chest radiography revealed cardiomegaly and bilateral air space opacity peripherally in both mid zones, which was considered suggestive of COVID-19 and/or pulmonary oedema (figure 1). A diagnosis of COVID-19 was later confirmed on real time PCR of a nasopharingeal swab. A bedside transthoracic echocardiography revealed severely reduced systolic function, with an estimated LVEF of 30%. Treatment was commenced with furosemide and bisoprolol for cardiac injury with left ventricular failure associated with COVID-19 infection, alongside antibiotic therapy with oral doxycycline for possible superadded respiratory bacterial infection. On day 6 (11 days after onset of symptoms), the patient deteriorated clinically with a new onset of continuous central thoracic chest pain, ongoing fevers and worsening dyspnoea with tachypnoea, type 1 respiratory failure and increasing oxygen requirements. His CRP rose to 43 mg/L and troponin to 60 ng/L but D dimers remained normal. In view of his clinical deterioration, treatment with oral colchicine 0.5 mg 8-hourly was instigated. No specific antiviral treatment for SARS-CoV-2, none of the forms of steroid therapy and no immunotherapy were administered to the patient, as no treatment guidelines were available at the time of this case report.
Figure 1.
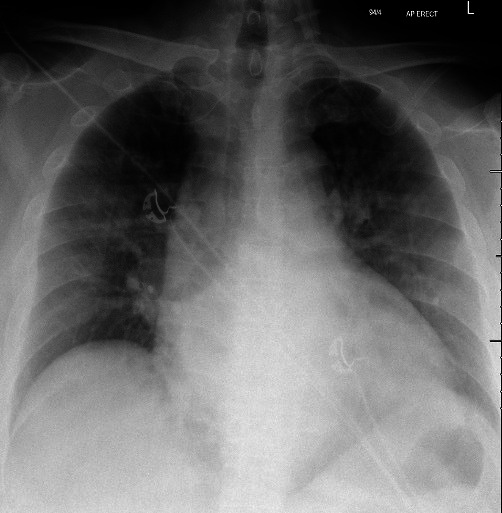
Cardiomegaly and bilateral air space opacity peripherally in both mid zones, suggestive of COVID-19 and/or pulmonary oedema.
Outcome and follow-up
Our patient improved rapidly following initiation of colchicine. Following 48 hours of colchicine initiation, his chest pain resolved, his dyspnoea improved and his oxygen requirements progressively decreased.
Serial laboratory indices are shown in figure 2. Cardiac MRI on day 10 post-admission, confirmed a dilated left ventricle and a persistent severely reduced systolic function (LVEF 30%), although the patient did not unfortunately tolerate full cardiac MRI. On day 12, the patient was no longer hypoxic (saturation of 97% on room air for >48 hours) and was discharged home.
Figure 2.
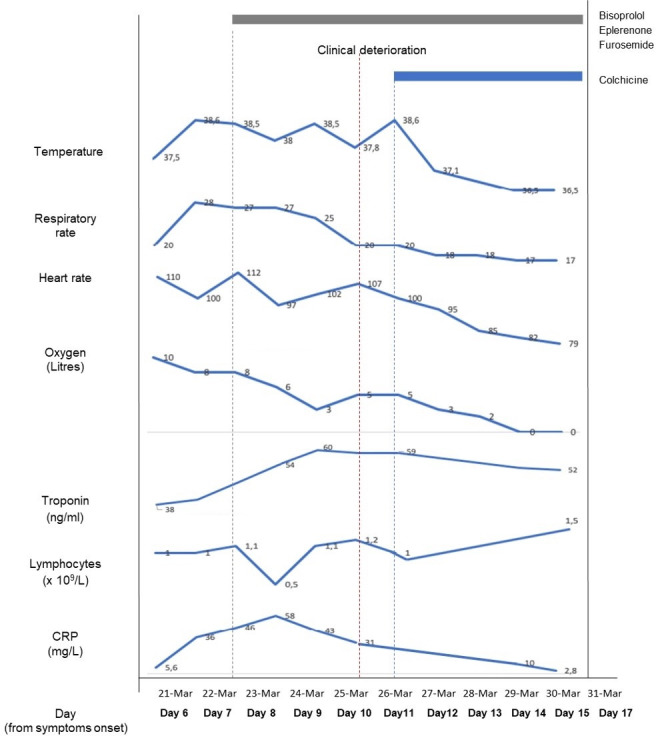
Graph illustrating the clinical course of COVID-19 associated myocarditis case reported, showing changes in clinical evolution and lymphocyte count, CRP and troponin. CRP, C reactive protein.
Despite full resolution of symptoms on discharge, persistent systolic dysfunction with a low LVEF was shown on post-discharge echocardiography. To date, the patient continues to be in cardiac follow-up.
Discussion
In COVID-19, there is a good reason to suppose that cardiac injury is a direct effect of the viral infection itself.5 6 SARS-CoV-2 virus, a novel enveloped RNA beta-coronavirus enters into cells by binding of the spike protein of the virus to the ACE 2.6 Since ACE2 is expressed in many different organs, including the myocardium, it is possible that SARS-CoV-2 could infect cardiac myocytes and induce myocarditis.5 More recently, in an autopsy series of patients affected of acute myocarditis and infected with COVID-19, SARS-CoV-2 RNA was detected in interstitial cytopathic macrophages invading the myocardial tissue.7
Clinical presentation of acute myocarditis is widely variable, ranging from asymptomatic to cardiogenic shock.8 In our case, the cardiac presentation occurred in the second week of the illness with a classical picture of dyspnoea, reduced LVEF, sustained ventricular arrhythmias and a mildly raised troponin. Strikingly, in our patient, this did not occur in the context of a cytokine storm (CRP never exceeded 60 mg/L) or widespread thrombotic events (D dimer was not elevated). Thus, this case appears to demonstrate a very tissue-tropic presentation of COVID-19 with predominantly cardiac disease in the absence of widespread other-organ involvement. A clinical diagnosis of likely COVID-19-related acute myocarditis was made; however, the diagnosis was not confirmed, as the patient did not tolerate cardiac MRI and cardiac biopsy was not performed.
In our patient, treatment for heart failure was started on admission and colchicine was added in view of poor control of symptoms in keeping with acute myocarditis. Colchicine is an anti-inflammatory agent, its main working mechanism is based on the inhibition of microtubule polymerization, interleukins 1 and 6, granulocyte macrophage colony stimulating factor and the nucleotide-binding oligomerisation leucine-rich repeat and pyrin domain (NLRP3) inflammasome.9–11 Colchicine has been shown to be effective in the treatment of acute myocarditis caused by different viruses.12 13 In the particular case of COVID-19, the blockage of inflammatory cytokines and NLRP3 inflammasome may be key in the management and treatment of patients with COVID-19.14 New data suggests that colchicine–tubulin complex may block viral entry and replication in COVID-19 infection, as microtubules are involved in the transport and assembly of spike proteins into virions during the viral replication cycle of SARS-CoV-2.15 In addition, colchicine acts as an anti-fibrotic and endothelial protective agent,16 which has been recently shown to be effective for the treatment of acute pericarditis, postpericardiotomy syndrome and postmyocardial infarction.17–19 Caution should be taken before initiating colchicine, due to frequent side effects such as gastrointestinal disturbances and potential drug interactions with cytochrome P450 3A4 inhibitors.18 More importantly, colchicine has a narrow therapeutic-toxicity window, which has been associated with acute systemic toxicity and high mortality rates if the dose exceeds 0.5 mg/kg.20 This was a main concern for clinicians to abandon temporarily the use of the drug until low-dose colchicine was shown to be safe and effective.21
This case report highlights the risk of acute cardiac injury in COVID-19 infection and raises awareness about the importance of baseline and follow-up cardiac examinations as appropriate in patients with COVID-19. The role of colchicine therapy should be further studied to determine its usefulness in reducing myocardial and possibly lung parenchymal inflammatory response.
Learning points.
Cardiac injury several days after initiation of COVID-19 symptoms may be indicative of myocardial damage caused by viral infection.
Baseline and follow-up cardiac investigations are recommended to detect COVID-19-related cardiac pathology.
The role of colchicine in patients with cardiac injury associated with SARS-CoV-2 should be further studied.
Footnotes
Contributors: VA-V, LH, LA and LB participated in the management of the patient. VA-V and LH reviewed the literature and VA-V drafted the manuscript. All the authors were involved in the production of the final edit and have approved the manuscript submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Zheng Y-Y, Ma Y-T, Zhang J-Y, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–60. 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819–24. 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim I-C, Kim JY, Kim HA, et al. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J 2020;41:1859. 10.1093/eurheartj/ehaa288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng J-H, Liu Y-X, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection 2020;48:773–7. 10.1007/s15010-020-01424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oudit GY, Kassiri Z, Jiang C, et al. Sars-Coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39:618–25. 10.1111/j.1365-2362.2009.02153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escher F, Pietsch H, Aleshcheva G, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail 2020;7:2440–7. 10.1002/ehf2.12805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper LT. Myocarditis. N Engl J Med 2009;360:1526–38. 10.1056/NEJMra0800028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocco G, Chu DCC, Pandolfi S. Colchicine in clinical medicine. A guide for internists. Eur J Intern Med 2010;21:503–8. 10.1016/j.ejim.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 10.Leung YY, Yao Hui LL, Kraus VB. Colchicine--Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015;45:341–50. 10.1016/j.semarthrit.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabbani AB, Parikh RV, Rafique AM. Colchicine for the Treatment of Myocardial Injury in Patients With Coronavirus Disease 2019 (COVID-19)-An Old Drug With New Life? JAMA Netw Open 2020;3:e2013556. 10.1001/jamanetworkopen.2020.13556 [DOI] [PubMed] [Google Scholar]
- 12.Gultekin N, Kucukates E. Microtubule inhibition therapy by colchicine in severe myocarditis especially caused by Epstein-Barr and cytomegalovirus co-infection during a two-year period: a novel therapeutic approach. J Pak Med Assoc 2014;64:1420–3. [PubMed] [Google Scholar]
- 13.Al-Zakhari R, Upadhya G, Galligan S, et al. The myth of colchicine in treating myopericarditis: case report and literature review. Cureus 2020;12:e8933. 10.7759/cureus.8933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020;20:355–62. 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlesinger N, Firestein BL, Brunetti L. Colchicine in COVID-19: an old drug, new use. Curr Pharmacol Rep 2020:1–9. 10.1007/s40495-020-00225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deftereos S, Giannopoulos G, Papoutsidakis N, et al. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol 2013;62:1817–25. 10.1016/j.jacc.2013.08.726 [DOI] [PubMed] [Google Scholar]
- 17.Imazio M, Brucato A, Cemin R, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013;369:1522–8. 10.1056/NEJMoa1208536 [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos C, Patoulias D, Teperikidis E, et al. Colchicine as a potential therapeutic agent against cardiovascular complications of COVID-19: an exploratory review. SN Compr Clin Med 2020:1419–29. 10.1007/s42399-020-00421-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tardif J-C, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–505. 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein Y, Aks SE, Hutson JR, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol 2010;48:407–14. 10.3109/15563650.2010.495348 [DOI] [PubMed] [Google Scholar]
- 21.Terkeltaub RA, Furst DE, Bennett K, et al. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum 2010;62:1060–8. 10.1002/art.27327 [DOI] [PubMed] [Google Scholar]


