Abstract
Introduction
The incubation of airway epithelia cells at low temperatures is a common in vitro experimental approach used in the field of cystic fibrosis (CF) research to thermo-stabilise F508del-CFTR and increase its functional expression. Given that the airway epithelium includes numerous ion transporters other than CFTR, we hypothesised that there was an impact of low temperature incubation on CFTR-independent ionoregulatory mechanisms in airway epithelia derived from individuals with and without CF.
Methods
After differentiation at the air–liquid interface, nasal epithelia were incubated at either 37°C or 29°C (low temperature) for 48 hours prior to analysis in an Ussing chamber.
Results
While F508del-CFTR activity was increased after low temperature incubation, activity of CFTR in non-CF epithelia was unchanged. Importantly, cultures incubated at 29°C demonstrated decreased transepithelial potential difference (TEPD) and short-circuit currents (Isc) at baseline. The predominant factor contributing to the reduced baseline TEPD and Isc in 29°C cultures was the reduced activity of the epithelial sodium channel (ENaC), evidenced by a reduced responsiveness to amiloride. This effect was observed in cells derived from both non-CF and CF donors.
Discussion
Significant transcriptional downregulation of ENaC subunits β and γ were observed, which may partially explain the decreased ENaC activity. We speculate that low temperature incubation may be a useful experimental paradigm to reduce ENaC activity in in vitro epithelial cultures.
Keywords: airway epithelium, cystic fibrosis, lung physiology
Key messages.
Given the wide use of low temperature incubation of airway epithelial cells to experimentally correct mutant cystic fibrosis transmembrane conductance regulator (CFTR), the current investigation sought to evaluate the impact of low temperature on the activities of other epithelial ion transporters of the airway epithelium.
It appears that low temperature treatment downregulates the epithelial sodium channel (ENaC) activity and expression through a mechanism that is not dependent on CFTR, and that the reduced ENaC function at low temperature has a direct impact on baseline transepithelial membrane potential.
Future studies seeking to use low temperature incubation to experimentally correct mutant CFTR should consider the impact low temperature incubation may have on other ion transporters as well as on epithelial function more broadly, including impacts on transepithelial potential difference, airway surface hydration and mucociliary transport.
Introduction
The conducting and respiratory airway epithelia of the nose, mouth, trachea, bronchi and alveoli process inhaled air for gas exchange while protecting from potentially harmful inhaled particles and pathogens. Key to both the respiratory and protective functions of airway epithelia is maintaining hydromineral balance of the periciliary fluid compartment of the airway surface liquid (ASL), a thin layer of fluid that serves to trap and flush out debris and pathogens.1 ASL hydration is maintained by the osmotic movement of water driven by ion transport across the epithelium. Three of the most prominent ion transporters on the apical side of the airway epithelium are: (i) epithelial Na+ channel (ENaC), which transports Na+ from the ASL into the cell; (ii) cystic fibrosis transmembrane conductance regulator (CFTR), a Cl− channel that transports Cl− out of the cell to the ASL and (iii) Ca2+-activated Cl− channels (CaCCs), which also transport Cl− out to the ASL.2
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the CFTR gene resulting in a deterioration of the ASL and an overaccumulation of mucus in the airways leading to respiratory dysfunction and chronic lung disease.3 The most common CFTR mutation, F508del,4 results in misfolding and retention of the mutant CFTR protein in the endoplasmic reticulum, leading to the premature degradation of F508del-CFTR by proteasomes and an inadequate amount of F508del-CFTR at the cell surface.5 6 The misfolding of F508del-CFTR is temperature-sensitive and can be partially rescued by incubation at low temperature.7–9 Thus, a widely used experimental methodology to correct F508del-CFTR folding in vitro is to thermo-stabilise F508del-CFTR using low temperature incubation (at ~27°C–29°C rather than 37°C) of cells harbouring F508del-CFTR. Low temperature correction of F508del-CFTR is also an important experimental tool in translational research on CF as it has served as a positive control treatment in studies screening for and investigating the efficacy of novel pharmacological correctors of F508del-CFTR.10–12
In CF research, ENaC has also been widely investigated for its abundance on the airway epithelium and its prominent contribution to ion and fluid homeostasis on the airway surface.2 The balance of ENaC-mediated ion absorption and CFTR-mediated ion secretion largely determines proper ASL homeostasis.13 CFTR and ENaC interact on the apical membrane of the airway epithelium,14 both through direct and indirect means. In non-CF epithelia, membrane-expressed CFTR directly interacts with and downregulates the function of ENaC. Likewise, ENaC activity is increased in CF epithelia, which have low membrane expression of CFTR.14 This relationship between ENaC and CFTR is so crucial that it has been suggested that ENaC should be a target in treating CF.15 16 For example, it has been posited that pharmacologically reduced ENaC activity on the apical surface could lead to increased periciliary fluid and enhanced lung function in CF airways.13 This paradigm is perhaps most conspicuous in murine models of CF, of which models overexpressing ENaC in the airway more closely exhibit pulmonary symptoms of CF than Cftr -/- models.17
Given the use of low temperature incubation experiments to investigate F508del-CFTR and airway epithelial ion transport, the current study sought to evaluate the impact of low temperature on the activities of other epithelial ion transporters of the airway epithelium. In this study, we exposed patient-derived non-CF and CF (F508del homozygous) nasal epithelial cells to low temperature. We performed electrophysiological analyses to examine the effect of low temperature incubation on the activities of amiloride-sensitive ENaC, forskolin-activated CFTR and ATP-responsive CaCCs. This report provides further characterisation of the impact of low temperature on the electrophysiological properties and ion channel functions of cultured airway epithelia.
Materials and methods
Primary human nasal epithelial cell expansion and maintenance
Primary nasal cells were obtained from three to four individuals with and without CF by nasal brushing and cultured as previously described.18–21 Cells were expanded on irradiated National Institutes of Health (NIH)-registered 3T3 feeder layers with F-media (73% complete Dulbecco’s Modified Eagle’s Medium (DMEM) (89% DMEM, 9% fetal bovine serum, 1% L-glutamine, 1% penicillin/streptomycin), 0.1% hydrocortisone/EGF mix, 0.00086% cholera toxin, 1.6% adenine, 0.1% insulin, 25% Ham’s F-12) containing 10 µM Y-27632 Rho kinase inhibitor. For differentiation at air–liquid interface (ALI), expanded cells were seeded on type 1 bovine collagen-coated (3 mg/mL) cell culture permeable polyester transwell inserts (Corning) at a density of 250 000 cells/cm2 in PneumaCult-Ex Plus (STEMCELL Technologies). Apical media was removed and basal media was replaced by PneumaCult-ALI media (STEMCELL Technologies) after 2 days of expansion. The basal media was changed every 2–3 days with fresh PneumaCult-ALI media. Cells were well-differentiated and analysed after 21–28 days at ALI. For low temperature incubation, cells were either held at 37°C (control) or transferred to 29°C for 48 hours prior to analysis in the Ussing chamber (four replicates per group for each individual donor).
Electrophysiological measurement by Ussing chamber
Electrophysiological measurements were performed in an Ussing chamber (Physiological Instruments) under open-circuit conditions with continuous measurement of transepithelial potential difference (TEPD). Monolayers were intermittently voltage-clamped to 0 mV for measurement of short-circuit current (Isc). During periods of short-circuiting, epithelia were pulsed (200 ms of ±5 mV) and measurements of transepithelial electrical resistance (TEER) were obtained. Cells were placed between apical and basolateral compartments filled with Ringer’s solution (120 mM NaCl, 10 mM D-glucose, 3.3 mM KH2PO4, 0.83 mM K2HPO4, 1.2 mM MgCl2, 1.2 mM CaCl2, saturated with 95% O2/5% CO2, pH 7.4 at 37°C). After mounting in the Ussing chamber, solutions were continuously gassed with 5% CO2 and 95% O2. Acute treatments of the monolayers in the Ussing chamber consisted of apical 100 µM amiloride (Alfa Aesar), apical/basal 20 µM forskolin (Fsk; Tocris)/100 µM 3-isobutyl-1-methylxanthine (IBMX; Sigma), apical 1 µM VX-770 (Selleck Chemicals), apical 10 µM CFTR(inh)-172 (CFTR Chemical Compound Distribution Program) and apical 100 µM ATP (Sigma). For non-CF cultures, CFTR was potentiated with VX-770 then activated by Fsk/IBMX. For CF cultures, CFTR was activated by Fsk/IBMX then potentiated with VX-770. After Ussing chamber analysis, monolayers were immediately frozen and stored at −80°C.
Gene expression analysis
Total RNA was isolated from frozen cells using the TRI-zol method following manufacturer’s protocol (Molecular Research Center) and quantified and assessed for purity using a NanoDrop spectrophotometer (ND-1000; NanoDrop Technologies). First-strand complementary DNA (cDNA) was synthesised using a reverse transcription kit following the manufacturer’s protocol (M-MLV Reverse Transcriptase; Invitrogen). Real-time quantitative PCR (RT-qPCR) was performed using a StepOnePlus system (Applied Biosystems) and carried out in 10 μL reactions containing 10 ng cDNA, 150 nM forward and reverse primers and 1X SYBRselect master mix, following the manufacturer’s protocol (ThermoFisher). The thermal profile of the reactions was 2 min at 50°C, 2 min at 95°C (holding and activation), 40 cycles of 15 s at 95°C, 30 min at 60°C, 30 s at 72°C (cycling), then finally a ramp from 60°C to 95°C (melt curve analysis) was used to confirm a single product in each reaction. Relative mRNA expression of SCNN1A, SCNN1B and SCNN1G (the genes corresponding to α, β and γ subunits of ENaC, respectively) was calculated using the comparative method (2-ΔΔCT) using α-tubulin (ATUBA1A) as a reference gene. Nucleotide sequences of primer pairs used for analysis were as follows: SCNN1A forward, 5’-AGGGGAACAAGCGTGAGGA-3’; SCNN1A reverse, 5’-GGTGGAACTCGATCAGGGC-3’; SCNN1B forward, 5’-CCCGGCTACACGTACAAGG-3’; SCNN1B reverse, 5’-CCCTCACAGATGATGCGCTT-3’; SCNN1G forward, 5’-GCACCCGGAGAGAAGATCAAA-3’; SCNN1G reverse, 5’-TACCACCGCATCAGCTCTTTA-3’.
Western blot analysis
Frozen monolayers were washed with ice-cold phosphate-buffered saline and lysed with 1X Laemmli buffer. Equal amounts of protein (20 μg) were loaded for SDS-PAGE, then transferred on to nitrocellulose membrane (GE-Amersham Biosciences). Blots were subsequently blocked with 5% Blotto (non-fat dry milk; Sigma) in tris-buffered saline with 0.1% Tween-20 (TBS-T) for 1 hour at room temperature (RT), followed by overnight incubation with primary antibodies: ENaC (1:1000 dilution; Invitrogen, Cat. No. 920A); actin (1:3000 dilution; Cell Signaling Technology, Cat. No. 12262). After primary incubation, blots were washed with TBS-T then incubated in HRP-conjugated secondary antibodies (1:3000 dilution; Cell Signaling Technology, Cat. No. 7074) for 1 hour at RT, then washed again before chemiluminescent detection (ECL western blotting substrate; Pierce, Cat. No. 32106). ENaC α-subunit band densities were normalised to β-actin and quantified using ImageJ software (NIH).
Calculations and statistical analysis
To control for donor-to-donor variation, data were normalised to the donor-matched 37°C control. Statistical analyses were carried out on normalised replicates from each donor (n=16–34 replicates from three to four donors) by either one-way or two-way analysis of variance with Tukey’s multiple comparisons test, unpaired t-test or linear regression as described in figure legends. All data are presented as mean±SD. Figure assembly and all statistical analyses were completed in Prism V.6.0 (GraphPad Software).
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Non-CF and CF primary nasal epithelial cell cultures formed visibly ciliated and electrically tight monolayers, ranging 93–358 Ω/cm2 at baseline. All monolayers exhibited an immediate response and subsequent stabilisation after exposure to the test compounds amiloride, VX-770, Fsk/IBMX, CFTR(inh)-172 and ATP (figure 1A, B). Non-CF and CF epithelia had significantly different baseline TEPD (non-CF: −6.3±2.3 mV; CF: −10.5±2.5 mV), amiloride-responsive ΔTEPD (non-CF: 5.9±2.8 mV; CF: 13.6±2.5 mV) and VX-770/Fsk/IBMX-responsive ΔTEPD (non-CF: −3.8±1.8 mV; CF: −0.92±0.29 mV) (figure 1C–E).
Figure 1.
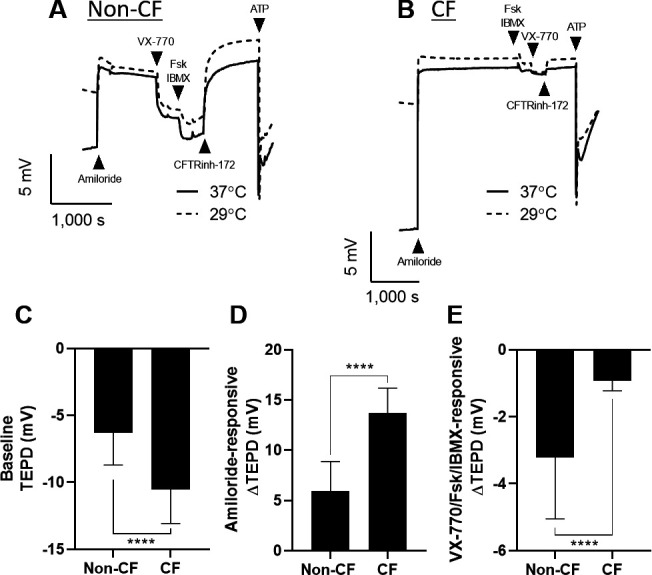
Electrophysiological properties of nasal airway epithelia. (A) Traces of transepithelial potential difference (TEPD) across non-cystic fibrosis (non-CF) (A) and CF (F508del/F508del) (B) primary nasal epithelial cells after 48 hours incubation at low temperature (29°C) and normal temperature (37°C) were obtained by analysis in an Ussing chamber. Baseline TEPD (C), amiloride-responsive ΔTEPD (D) and VX-770/Fsk/IBMX-mediated ΔTEPD (E) in non-CF and CF epithelia were quantified. Data are presented as mean±SD (n=16–34 replicates, from three individual donors for each group). Brackets and asterisks indicate significant differences (t-test; ****p<0.0001).
We first sought to compare the effects of low temperature (29°C) incubation on baseline values (prior to administration of test compounds) for TEPD, Isc and TEER between non-CF and CF nasal epithelial cell cultures. We observed a significant reduction in baseline TEPD and Isc after 29°C incubation compared with the control temperature (37°C), and this effect was consistent in both non-CF and CF epithelial cell cultures (figure 2A, B). Low temperature-mediated decreases in baseline TEPD and Isc were greater in CF cells (TEPD was reduced 82%±14%; Isc was reduced in 80%±17%) compared with non-CF cells (TEPD was reduced 60%±13%; Isc was reduced in 67%±13%). Low temperature incubation had only modest influences on TEER, slightly increasing TEER in non-CF cells and having no effect in CF cells (figure 2C).
Figure 2.

Baseline transepithelial properties. Effects of 48 hours treatment with normal (37°C) and low temperature (29°C) on baseline transepithelial potential difference (TEPD) (A), short-circuit currents (Isc) (B) and transepithelial electrical resistance (TEER) (C) across non-cystic fibrosis (non-CF) and CF (F508del/F508del) primary nasal epithelial cells were quantified. Values were normalised to donor-matched 37°C values. Data are presented as mean±SD (n=16–34 replicates, from three individual non-CF and three individual CF donors). Brackets and asterisks indicate significant differences (two-way analysis of variance; Tukey’s post hoc; **p<0.01; ****p<0.0001).
The acute application of amiloride, a potent inhibitor of ENaC, had large and temperature-dependent effects on TEPD and Isc across both non-CF and CF epithelia. The magnitude of amiloride-responsive ΔTEPD and ΔIsc were significantly reduced at 29°C compared with 37°C control epithelia (figure 3A, B). In non-CF epithelia at 29°C, amiloride-responsive ΔTEPD and ΔIsc were decreased to 47%±22% and 59%±21%, respectively, compared with 37°C controls. In CF epithelia at 29°C, amiloride-responsive ΔTEPD and ΔIsc were decreased to 62%±9% and 59%±13%, respectively. In non-CF epithelia, CFTR activation by VX-770 and Fsk/IBMX was not different between 29°C and 37°C treatments (figure 3C, D). However, in CF epithelia, CFTR activation by Fsk/IBMX and VX-770 resulted in a significant increase in ΔTEPD at 29°C compared with the 37°C control (figure 3C). Low temperature incubation had no detectable effect on CFTR-activated ΔIsc in either non-CF or CF epithelia (figure 3D). It is important to note that the effect of temperature correction on F508del-CFTR is very moderate when assayed under symmetrical Cl− conditions; more dramatic increases in F508del-CFTR activity after low-temperature incubation are observed in the presence of a Cl− gradient.18 Although the acute addition of ATP elicited a strong response in Cl− conductance in both non-CF and CF epithelia (figure 1), no significant effect of low temperature incubation on the ATP response was detected in either non-CF or CF epithelia (figure 3E).
Figure 3.
Transepithelial response to amiloride, cystic fibrosis transmembrane conductance regulator (CFTR) activation and ATP stimulation. Treatment with normal (37°C) and low temperature (29°C) for 48 hours affects amiloride (100 μM)-sensitive Δtransepithelial potential difference (TEPD) (A) and Δshort-circuit currents (Isc) (B) in non-cystic fibrosis (non-CF) and CF (F508del/F508del) epithelia. CFTR-mediated changes in TEPD (C) and Isc (D) responded to low temperature in CF (F508del/F508del) but not non-CF primary nasal epithelial cells. Values for CFTR activation represent total changes in response to VX-770 and Fsk/IBMX application. ATP-responsive ΔTEPD (E) in non-CF and CF primary nasal epithelial cells remained unchanged. Data are presented as mean±SD (n=16–34 replicates, from three individual non-CF and three individual CF donors). Brackets and asterisks indicate significant differences (two-way analysis of variance; Tukey’s post hoc; **p<0.01; ***p<0.001; ****p<0.0001).
For non-CF and CF epithelia at both incubation temperatures, baseline TEPD was significantly correlated with ENaC-mediated ΔTEPD (figure 4A). The results of linear regression were as follows: 37°C non-CF: m=−0.7796, r2=0.9191, p<0.0001; 29°C non-CF: m=−0.5133, r2=0.9441, p<0.0001; 37°C CF: m=−0.9911, r2=0.9535, p<0.0001; 29°C CF: m=−1.060, r2=0.8251, p<0.0001. The influence of ENaC on baseline TEPD was greater in CF epithelia than in non-CF epithelia; the slopes of the lines were significantly different (p<0.0001). In non-CF epithelia, the influence of ENaC on baseline TEPD was greater at 37°C compared with 29°C (p=0.0007). Baseline TEPD was only significantly associated with CFTR activity in non-CF cells (figure 4B): 37°C non-CF: m=1.053, r2=0.6530, p=0.0002; 29°C non-CF: m=0.6273, r2=0.5941, p=0.0005. After the addition of amiloride in non-CF epithelia, significant differences in TEPD and Isc (but not TEER) remained between 37°C and 29°C treatments (figure 4C–E).
Figure 4.
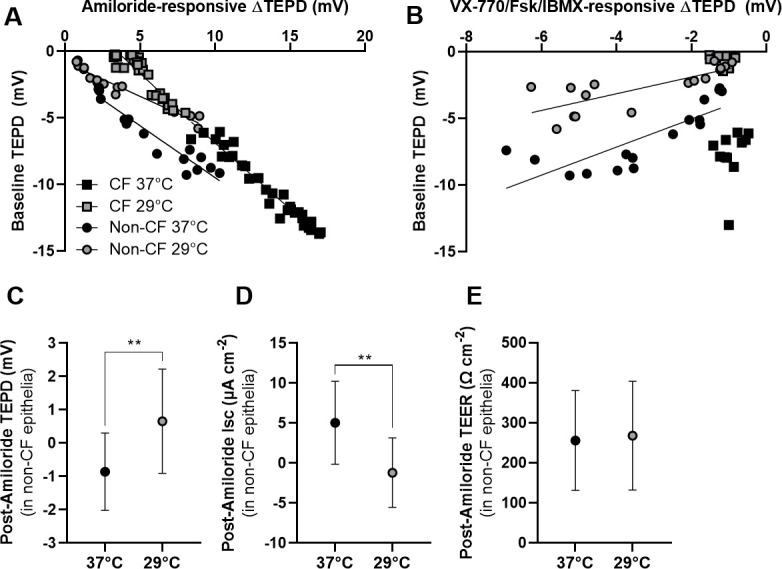
Relationship between ion transporter activities and baseline epithelial properties. The effect of 48 hours treatment with normal (37°C) and low (29°C) temperature on transepithelial potential difference (TEPD) across non-cystic fibrosis (non-CF) and CF (F508del/F508del) primary nasal epithelial cells was analysed. Correlations between baseline TEPD and amiloride (100 μM)-sensitive ΔTEPD (A) or ΔTEPD from cystic fibrosis transmembrane conductance regulator (CFTR) activation (B) are presented. Values for CFTR activation represent total changes in response to VX-770 (1 µM) and Fsk/IBMX (20 µM/100 µM) application. (C–E) Absolute values for TEPD, short-circuit currents (Isc) and transepithelial electrical resistance (TEER) in non-CF epithelial cells at normal (37°C) and low (29°C) temperature after the acute addition of amiloride were quantified. Data in panels A and B are presented as individual replicates (n=12–34 replicates, from three individual non-CF and three individual CF donors) and analysed using linear regression. Data in panels C–E are presented as mean±SD. Brackets and asterisks indicate significant differences (t-test; **p<0.01).
Lastly, we hypothesised that the effect of low temperature incubation on ENaC activity was explained by reduced ENaC expression. RT-qPCR analysis (figure 5A–C) revealed that SCNN1B and SCNN1G transcript expression were significantly reduced at 29°C compared with 37°C (over 50% reduction each). This effect of temperature was observed in both non-CF and CF epithelia. Interestingly, SCNN1A transcript expression was not significantly downregulated at 29°C. Further analysis of SCNN1A by western blot analysis indicated that low temperature incubation resulted in a modest (~25%) but significant decrease in levels of ENaC α-subunit—the 97 kDa band corresponds to the glycosylated, thus active, form of ENaC α-subunit.22 23 This downregulatory effect on glycosylated ENaC α-subunit protein expression was observed in both CF and non-CF cells (figure 5D, E).
Figure 5.

Transcript and protein expression of epithelial sodium channel (ENaC) subunits. (A–C) Transcript expression of ENaC subunits α, β and γ (SCNN1A, SCNN1B and SCNN1G, respectively) are presented. Transcript expression data were calculated using the comparative method (2-ΔΔCT) with α-tubulin (ATUBA1A) serving as the reference gene. Transcript levels are presented as relative to 37°C (set to 1) (n=4 replicates). (D) Western blot analyses demonstrate the expression of ENaC α-subunit in cystic fibrosis (CF) and non-CF after 48 hours treatment with normal (37°C) and low temperature (29°C). β-Actin was used as a loading control. (B) Quantification of ENaC protein band densities are presented (n=5–6 replicates from two donors). Data are presented as mean±SD. Asterisks indicate significant differences (unpaired t-test; *p<0.05; **p<0.01, ****p<0.0001).
Discussion
The balance between ENaC and CFTR on the airway epithelium is critical in maintaining airway surface ion and fluid homeostasis. In the present study, we demonstrated that low temperature treatment of airway epithelial cells in vitro, which is an experimental approach often used to modulate F508del-CFTR, also reduced ENaC activity in both non-CF and CF airway epithelia. We further demonstrated that ENaC activity is highly correlated to baseline TEPD, implying that ENaC is critical in producing the lumen-negative TEPD present in non-CF and CF airway epithelia. Protein-level and transcript-level data indicate that temperature-sensitive changes in ENaC activity may be attributed to the downregulation of ENaC expression.
Low temperature incubation is a widely used experimental tool in vitro to enhance folding and trafficking of F508del-CFTR.8 10–12 24–27 Fewer studies have sought to identify the impact of experimental temperature manipulation on other epithelial ion transport processes. In CF bronchial epithelial (CFBE) cells, low temperature incubation increased CFTR surface expression and channel activity, and resulted in lower activity of another Cl− channel, the outwardly rectifying Cl− channel.9 In the present investigation, low temperature incubation resulted in an expected increase in F508del-CFTR activity after incubation at 29°C compared with 37°C, a decrease in ENaC activity and no effect on the ATP response, which is traditionally viewed as an indicator of CaCC activity. Thus, the impact of low temperature, which could affect many protein regulatory processes, such as protein synthesis, proteolytic activation or protein turnover, appears to be protein-specific rather than general. Importantly, the impact of low temperature incubation on ENaC activity was substantial and will be the primary topic of the discussion that follows.
In the 37°C controls for our study, ENaC activity was greater in CF than non-CF airway epithelia (figure 1D). The upregulation of ENaC activity in CF cultures is firmly established.13 14 28–36 In both epithelial and non-epithelial cells, CFTR membrane expression and activity is negatively associated with ENaC expression or ENaC-mediated Na+ transport.14 28–34 The reciprocal balance of the activities of ENaC and CFTR on the airway epithelium may aid in maintaining proper osmoregulation of periciliary fluid in non-CF epithelia, but likely exacerbates the dysregulation of ASL homeostasis caused by mutations in CFTR.35 When CFTR membrane expression on airway epithelia decreases due to improper folding and membrane-trafficking (such as in the case of F508del-CFTR), ENaC functional expression is enhanced,36 leading to decreased ASL volume, thus worsening mucociliary clearance.13
The mechanism by which CFTR and ENaC interact is not well understood. Investigations into the CFTR interacting proteome (ie, the CFTR ‘interactome’) using co-immunoprecipitation (co-IP) of endogenously expressed CFTR did not find any interaction between ENaC and wild-type-CFTR or F508del-CFTR at 37°C or F508del-CFTR at 30°C.37 38 Conversely, studies using systems exogenously overexpressing CFTR and ENaC have shown a direct interaction between CFTR and ENaC α, β and γ subunits in co-IP and fluorescence energy transfer (FRET) experiments.28 39 40 Interestingly, low-temperature (27°C) incubation is required for F508del-CFTR to exhibit direct interaction (co-IP) and physical proximity (FRET) with ENaC,39 indicating that the interaction between CFTR and ENaC likely takes place only once the proteins have trafficked to the plasma membrane. Neither incubation at 37°C nor 27°C resulted in any such interaction between ENaC and the Cl− channel CLC-1,39 although ENaC is known to interact with another CLC family member, CLC-2.41 From these previous studies, it appears that the functional relationship between CFTR and ENaC is likely occurring through direct and indirect mechanisms, which both require future investigation. It appears that CFTR can physically interact with ENaC on the plasma membrane to reduce ENaC function in cell lines exogenously expressing CFTR and ENaC, and that the defective membrane-trafficking of F508del-CFTR prevents direct interaction with ENaC. However, the lack of detection of ENaC in the interactome of wild-type-CFTR or F508del-CFTR in cell lines endogenously expressing CFTR indicates that indirect functional interaction between CFTR and ENaC, facilitated through intermediary or accessory proteins, is likely a predominant mode of interaction between CFTR and ENaC.28 34 42
In the present study, low temperature incubation of F508del-CFTR monolayers resulted in increased CFTR activity and decreased ENaC activity (figure 3A and C). This finding agrees with the well-known effects of low temperature in modulating F508del-CFTR membrane presence.7–9 A novel aspect of the present study is that we assessed the influence of low temperature in non-CF in addition to CF primary-derived epithelial cells. In CFBE41o- cells, which exogenously express CFTR, low temperature (29°C) incubation reduced wild-type-CFTR activity.25 In contrast, in the present study we found no change in CFTR activity in non-CF epithelia at 29°C (figure 3C). The difference between the exogenous expression of CFTR in CFBE41o- cells and the endogenous expression of CFTR in our primary-derived nasal epithelial cells may account for this difference in the CFTR-regulatory response at low temperature.
An important finding in the present study was that in both non-CF and CF epithelial cells, low temperature incubation reduced apparent ENaC activity (figure 3A). Furthermore, the reduction in ENaC activity substantially altered baseline transepithelial electrical properties such as membrane potential (figure 4A). The strong relationship between ENaC activity and baseline TEPD demonstrates the important influence of ENaC function on hydromineral balance on the airway epithelium. ENaC activity appeared to have a greater influence on baseline TEPD in CF than non-CF epithelia, which is likely due to lower CFTR activity in CF epithelia. Furthermore, in CF epithelia, the relationship between ENaC activity and baseline TEPD was similar regardless of temperature incubation (figure 4A). From these data, it appears that low temperature treatment affects ENaC function through a mechanism that is not necessarily dependent on CFTR, and that the reduced ENaC function at low temperature has a direct impact on baseline transepithelial membrane potential.
The cellular/molecular mechanisms responsible for the downregulation of ENaC activity at low temperature are not clear. Dysregulation of ENaC channel gating, membrane localization and surface residency time are all known pathologies for several human diseases.43 Along with reduced CFTR function, hyperactivity of ENaC-mediated Na+ absorption exacerbates mucous dehydration and worsens symptoms of CF.44 Hyperactivation of ENaC as a contributing factor of CF lung disease specifically is thought to be the result of increased ENaC open probability (Po) and/or increased ENaC surface expression. It has been shown in Xenopus oocytes that CFTR activation decreases the ENaC Po without affecting its surface expression.45 Increased ENaC Po can occur by proteolytic cleavage of an extracellular loop by various proteases.46 Increased ENaC surface expression can be regulated by cAMP/PKA inhibition of NEDD4-mediated ubiquitination and proteasomal degradation of ENaC.46
The extent to which altered Po and/or surface expression is the cause of the reduced ENaC activity that we observed at 29°C is only partially answered in the present study. Direct comparisons of the results in the present study to the literature are difficult. Although many studies have used low temperature incubation to experimentally enhance F508del-CFTR, we could not identify any studies describing the effect of low temperature incubation on the ENaC activity. It was shown in CFBE41o- cells that low temperature (29°C) incubation increased TEER independently of CFTR membrane presence or activity,24 although the impact of low temperature incubation on ENaC expression or activity was not disclosed. In the present study, we similarly demonstrate an increase in TEER after low temperature incubation (figure 1C) and attribute this change primarily to a change in ENaC activity. Experimentally applied acute low temperature treatment has been shown to increase ENaC Po.47 48 It is possible that prolonged exposure to low temperature (such as in the present study) results in sustained increases in ENaC Po, thus eliciting a regulatory response by the cells to decrease ENaC activity.
Our RT-qPCR and western blot analyses demonstrated that transcript and/or protein expression of all three ENaC subunits (α, β and γ) were downregulated. As ENaC activity requires all three subunits to be present, the downregulation of any one of the ENaC subunits could explain the observed decreases in ENaC activity. Our results indicate differential regulation of ENaC subunits; transcription of SCNN1B and SCNN1G was strongly downregulated at low temperature whereas SCNN1A was not (figure 5A–C). Despite no observed reduction at the transcript level, protein expression of the ENaC α subunit was still reduced (figure 5E). Given this finding, we surmise that protein expression of β and γ subunits, which were strongly downregulated at the transcript level, were likely reduced. Thus, our results indicate that it is possible that downregulation of ENaC transcript and protein synthesis at low temperature can fully explain the downregulation in ENaC activity. It is important to note that both microarray12 and proteomic27 analyses have revealed that many cellular functions are affected by low temperature incubation. Therefore, there could be other cellular mechanisms responding to low temperature incubation that may be affecting ENaC activity. Given that, in the present study, differences in the TEPD and Isc of non-CF epithelia were observed between 37°C and 29°C treatments after the addition of amiloride (figure 4C–E), we think it is likely that low temperature incubation affects other processes related to epithelial ion transport in addition to the downregulatory effect low temperature incubation has on ENaC activity.
In conclusion, we have reported that ENaC activity in primary-derived airway epithelia is substantially reduced after low temperature incubation. This effect of low temperature appears to be specifically acting on some ion transport mechanisms, namely F508del-CFTR and ENaC, but not others, such as CaCCs. Importantly, the reduction in ENaC activity and expression after low temperature incubation was observed in both non-CF and CF cultures indicating that this effect may occur through a CFTR-independent mechanism. Future studies seeking to use low temperature incubation to experimentally correct mutant CFTR should consider the impact low temperature incubation may have on other ion transporters as well as on epithelial function more broadly, including impacts on TEPD, ASL hydration and mucociliary transport. Lastly, for investigators interested in studying ENaC function, we speculate that low temperature incubation may be a useful experimental paradigm to induce specific downregulation of ENaC activity in order to study the ENaC dysfunction in various disease and model systems.
Acknowledgments
The authors would like to thank Dr Robert Bridges, the Rosalind Franklin University of Medicine and the Cystic Fibrosis Foundation for the contribution of CFTRinh-172 through the CFTR Chemical Compound Distribution Programme.
Footnotes
SY and CAS contributed equally.
Contributors: SY, CAS and PEB conceived of and designed the research and performed experiments; SY, CAS and PEB analysed data and prepared the figures; SY and CAS produced the original manuscript draft; SY, CAS, PLZ and PEB revised and approved the final version of manuscript; PLZ and PEB facilitated the research.
Funding: This research was supported by the Cystic Fibrosis Foundation (ZEITLI2010 to PLZ and BRATCH16I0 to PEB), the Eugene F. and Easton M. Crawford Charitable Lead Unitrust (to CAS and PEB), the Gilead Sciences Research Scholars Programme in Cystic Fibrosis (to PEB) and the Department of Paediatrics at National Jewish Health.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Institutional Review Board of National Jewish Health: HS-2832.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article.
References
- 1.Boucher R. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch - Eur J Physiol 2003;445:495–8. 10.1007/s00424-002-0955-1 [DOI] [PubMed] [Google Scholar]
- 2.Bartoszewski R, Matalon S, Collawn JF. Ion channels of the lung and their role in disease pathogenesis. Am J Physiol Lung Cell Mol Physiol 2017;313:L859–72. 10.1152/ajplung.00285.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992–2001. 10.1056/NEJMra043184 [DOI] [PubMed] [Google Scholar]
- 4.Kerem B, Rommens J, Buchanan J, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073–80. 10.1126/science.2570460 [DOI] [PubMed] [Google Scholar]
- 5.Cheng SH, Gregory RJ, Marshall J, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990;63:827–34. 10.1016/0092-8674(90)90148-8 [DOI] [PubMed] [Google Scholar]
- 6.Jensen TJ, Loo MA, Pind S, et al. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 1995;83:129–35. 10.1016/0092-8674(95)90241-4 [DOI] [PubMed] [Google Scholar]
- 7.Denning GM, Anderson MP, Amara JF, et al. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 1992;358:761–4. 10.1038/358761a0 [DOI] [PubMed] [Google Scholar]
- 8.Rennolds J, Boyaka PN, Bellis SL, et al. Low temperature induces the delivery of mature and immature CFTR to the plasma membrane. Biochem Biophys Res Commun 2008;366:1025–9. 10.1016/j.bbrc.2007.12.065 [DOI] [PubMed] [Google Scholar]
- 9.Egan ME, Schwiebert EM, Guggino WB. Differential expression of ORCC and CFTR induced by low temperature in CF airway epithelial cells. Am J Physiol 1995;268:C243–51. 10.1152/ajpcell.1995.268.1.C243 [DOI] [PubMed] [Google Scholar]
- 10.Farinha CM, King-Underwood J, Sousa M, et al. Revertants, low temperature, and correctors reveal the mechanism of F508del-CFTR rescue by VX-809 and suggest multiple agents for full correction. Chem Biol 2013;20:943–55. 10.1016/j.chembiol.2013.06.004 10.1016/j.chembiol.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Sondo E, Tomati V, Caci E, et al. Rescue of the mutant CFTR chloride channel by pharmacological correctors and low temperature analyzed by gene expression profiling. Am J Physiol Cell Physiol 2011;301:C872–85. 10.1152/ajpcell.00507.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Ciciriello F, Anjos SM, et al. Ouabain mimics low temperature rescue of F508del-CFTR in cystic fibrosis epithelial cells. Front Pharmacol 2012;3:1–15. 10.3389/fphar.2012.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol 2013;591:4377–87. 10.1113/jphysiol.2012.240861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunzelmann K, Schreiber R, Nitschke R, et al. Control of epithelial Na+ conductance by the cystic fibrosis transmembrane conductance regulator. Pflugers Arch - Eur J Physiol 2000;440:193–201. 10.1007/s004240000255 [DOI] [PubMed] [Google Scholar]
- 15.Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst 2009;5:123–7. 10.1039/B810471A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collawn JF, Lazrak A, Bebok Z, et al. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 2012;302:L1141–6. 10.1152/ajplung.00036.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z, Duerr J, Johannesson B, et al. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros 2011;10 Suppl 2:S172–82. 10.1016/S1569-1993(11)60021-0 [DOI] [PubMed] [Google Scholar]
- 18.Bratcher PE, Yadav S, Shaughnessy CA, et al. Effect of apical chloride concentration on the measurement of responses to CFTR modulation in airway epithelia cultured from nasal brushings. Physiol Rep 2020;8:e14603. 10.14814/phy2.14603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chioccioli M, Feriani L, Kotar J, et al. Phenotyping ciliary dynamics and coordination in response to CFTR-modulators in cystic fibrosis respiratory epithelial cells. Nat Commun 2019;10:1763. 10.1038/s41467-019-09798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldfarbmuren KC, Jackson ND, Sajuthi SP, et al. Dissecting the cellular specificity of smoking effects and reconstructing lineages in the human airway epithelium. Nat Commun 2020;11. 10.1038/s41467-020-16239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds SD, Rios C, Wesolowska-Andersen A, et al. Airway progenitor clone formation is enhanced by Y-27632–Dependent changes in the transcriptome. Am J Respir Cell Mol Biol 2016;55:323–36. 10.1165/rcmb.2015-0274MA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashlan OB, Kinlough CL, Myerburg MM, et al. N -linked glycans are required on epithelial Na + channel subunits for maturation and surface expression. Am J Physiol Renal Physiol 2018;314:F483–92. 10.1152/ajprenal.00195.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shabbir W, Tzotzos S, Bedak M, et al. Glycosylation-Dependent activation of epithelial sodium channel by solnatide. Biochem Pharmacol 2015;98:740–53. 10.1016/j.bcp.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 24.LeSimple P, Liao J, Robert R, et al. Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers. J Physiol 2010;588:1195–209. 10.1113/jphysiol.2009.182246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Froux L, Coraux C, Sage E, et al. Short-Term consequences of F508del-CFTR thermal instability on CFTR-dependent transepithelial currents in human airway epithelial cells. Sci Rep 2019;9:1–12. 10.1038/s41598-019-50066-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurkuvenaite A, Chen L, Bartoszewski R, et al. Functional stability of rescued ΔF508 cystic fibrosis transmembrane conductance regulator in airway epithelial cells. Am J Respir Cell Mol Biol 2010;42:363–72. 10.1165/rcmb.2008-0434OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes-Alves P, Neves S, Coelho AV, et al. Low temperature restoring effect on F508del-CFTR misprocessing: a proteomic approach. J Proteomics 2009;73:218–30. 10.1016/j.jprot.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 28.Gentzsch M, Dang H, Dang Y. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na+ channel. Journal of Biological Chemistry 2010;285:32227–32. 10.1074/jbc.M110.155259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ismailov II, Awayda MS, Jovov B, et al. Regulation of epithelial sodium channels by the cystic fibrosis transmembrane conductance regulator. Journal of Biological Chemistry 1996;271:4725–32. 10.1074/jbc.271.9.4725 [DOI] [PubMed] [Google Scholar]
- 30.König J, Schreiber R, Voelcker T, et al. The cystic fibrosis transmembrane conductance regulator (CFTR) inhibits ENaC through an increase in the intracellular Cl − concentration. EMBO Rep 2001;2:1047–51. 10.1093/embo-reports/kve232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mall M, Bleich M, Greger R, et al. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest 1998;102:15–21. 10.1172/JCI2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mall M, Bleich M, Kuehr J, et al. CFTR-mediated inhibition of epithelial Na + conductance in human colon is defective in cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 1999;277:G709–16. 10.1152/ajpgi.1999.277.3.G709 [DOI] [PubMed] [Google Scholar]
- 33.Yan W, Samaha FF, Ramkumar M, et al. Cystic fibrosis transmembrane conductance regulator differentially regulates human and mouse epithelial sodium channels in Xenopus oocytes. J Biol Chem 2004;279:23183–92. 10.1074/jbc.M402373200 [DOI] [PubMed] [Google Scholar]
- 34.Stutts M, Canessa C, Olsen J, et al. Cftr as a cAMP-dependent regulator of sodium channels. Science 1995;269:847–50. 10.1126/science.7543698 [DOI] [PubMed] [Google Scholar]
- 35.Matsui H, Grubb BR, Tarran R, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998;95:1005–15. 10.1016/S0092-8674(00)81724-9 [DOI] [PubMed] [Google Scholar]
- 36.Rubenstein RC, Lockwood SR, Lide E, et al. Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2011;300:L88–101. 10.1152/ajplung.00142.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Venable J, LaPointe P, et al. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell 2006;127:803–15. 10.1016/j.cell.2006.09.043 [DOI] [PubMed] [Google Scholar]
- 38.Pankow S, Bamberger C, Calzolari D, et al. ∆F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature 2015;528:510–6. 10.1038/nature15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qadri YJ, Cormet-Boyaka E, Rooj AK, et al. Low temperature and chemical rescue affect molecular proximity of ΔF508-cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium channel (ENaC). Journal of Biological Chemistry 2012;287:16781–90. 10.1074/jbc.M111.332031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berdiev BK, Cormet-Boyaka E, Tousson A, et al. Molecular proximity of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel assessed by fluorescence resonance energy transfer. J Biol Chem 2007;282:36481–8. 10.1074/jbc.M708089200 [DOI] [PubMed] [Google Scholar]
- 41.Henry KR, Lee S, Walker D, et al. Direct interactions between ENaC gamma subunit and CLCN2 in cystic fibrosis epithelial cells. Physiol Rep 2015;3:e12264–10. 10.14814/phy2.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem 1997;272:14037–40. 10.1074/jbc.272.22.14037 [DOI] [PubMed] [Google Scholar]
- 43.Butterworth MB. Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Biochim Biophys Acta 2010;1802:1166–77. 10.1016/j.bbadis.2010.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarran R, Grubb BR, Parsons D, et al. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell 2001;8:149–58. 10.1016/s1097-2765(01)00286-6 [DOI] [PubMed] [Google Scholar]
- 45.Konstas A-A, Koch J-P, Korbmacher C. cAMP-Dependent activation of CFTR inhibits the epithelial sodium channel (ENaC) without affecting its surface expression. Pflugers Arch - Eur J Physiol 2003;445:513–21. 10.1007/s00424-002-0957-z [DOI] [PubMed] [Google Scholar]
- 46.Moore PJ, Tarran R. The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis lung disease. Expert Opin Ther Targets 2018;22:687–701. 10.1080/14728222.2018.1501361 [DOI] [PubMed] [Google Scholar]
- 47.Askwith CC, Benson CJ, Welsh MJ, et al. Deg/Enac ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci U S A 2001;98:6459–63. 10.1073/pnas.111155398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chraïbi A, Horisberger J-D. Dual effect of temperature on the human epithelial Na+ channel. Pflugers Arch 2003;447:316–20. 10.1007/s00424-003-1178-9 [DOI] [PubMed] [Google Scholar]



