Abstract
It is unknown if the somatic mutations in chronic myeloproliferative neoplasms (MPNs), JAK2V617F and Calreticulin, are associated with oxidative stress, or impaired mitochondrial defense against reactive oxygen species. In the Danish General Suburban Population Study (GESUS), including 116 JAK2V617F-mutated, 8 CALR-mutated, and 3310 mutation-negative participants without overt MPN, and in a study of 39 patients with myelofibrosis, the most advances type of MPNs, and 179 matched controls, we compared the urinary concentration of oxidized nucleosides – 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-oxo-7,8-dihydroguanosine (8-oxoGuo) – as markers of oxidative stress. In GESUS, we performed Mendelian randomization analyses, using the Ala16Val single nucleotide polymorphism in the superoxide dismutase2 (SOD2) gene. In the multivariate analyses in GESUS, the 8-oxodG and 8-oxoGuo concentration were 13% (95%CI: 6–21%, p < 0.001) and 6% (95%CI: 0.4–11%, p = 0.035) higher in mutation-positive than in mutation-negative participants, respectively. Each SOD2 T allele was associated with an odds ratio of being mutation-positive of 1.69 (95%CI: 1.12–2.55, p = 0.013) through 8-oxodG. The 8-oxodG and 8-oxoGuo concentrations were 77% (95%CI: 49–110%, p < 0.001) and 105% (95%CI: 80–133%, p < 0.001) higher in myelofibrosis patients than in controls, respectively. In conclusion, an impaired mitochondrial antioxidative defense, that is causatively associated with markers of oxidative stress, may contribute to the development of mutations associated with MPNs.
Keywords: Oxidized nucleosides, 8-oxodG, 8-oxoGuo, Oxidative stress, JAK2V617F, CALR, Myeloproliferative neoplasms
Abbreviations: MPNs, Philadelphia-negative chronic myeloproliferative neoplasms; GESUS, Danish General Suburban Population Study; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine; SOD2, superoxide dismutase2; ROS, reactive oxygen species; MnSOD, mitochondrial manganese superoxide dismutase; ET, Essential thrombocythemia; PV, polycythemia vera; MF, myelofibrosis; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; T2DM, type 2 diabetes mellitus
1. Introduction
Increased oxidative stress has been reported in patients with Philadelphia-negative chronic myeloproliferative neoplasms (MPNs) [[1], [2], [3], [4], [5]]. Furthermore, a murine model suggests that the JAK2V617F mutation leads to an MPN phenotype along with increased oxidative stress [2]. However, it is unclear if oxidative stress also plays a role in the development of MPNs.
Oxidative stress may be defined as excess reactive oxygen species (ROS) production exceeding the capacity of scavenging mechanisms. ROS encompass superoxide, hydrogen peroxide, and hydroxyl radicals [6]. Mitochondrial manganese superoxide dismutase (MnSOD) catalyzes the dismutation of superoxide radicals into hydrogen peroxide. Hydrogen peroxide is converted to H2O and O2 by catalase or glutathione peroxidase or to hydroxyl radicals through the Fenton reaction [6]. Hydroxyl radicals can cause oxidative damage to DNA and RNA, which can be cytotoxic or mutagenic and lead to clonal evolution, as shown in chronic myeloid leukemia and acute myeloid leukemia [6,7]. MnSOD is encoded by the superoxide dismutase (SOD2) gene, and a genetic dimorphism encodes for either valine (Val, T-allele) or alanine (Ala, C-allele) in the mitochondrial targeting sequence, which directs the protein to its location in the mitochondrial matrix [8]. The Val variant partially arrests the precursor in the mitochondrial inner membrane resulting in decreased formation of the active MnSOD in the matrix. The Ala variant results in 30–40% increased activity of the enzyme compared with the Val variant [8]. The SOD2 polymorphism is associated with several cancer types [9]. Oxidative stress may be measured as total amount of hydrogen peroxides and total antioxidant capacity in blood; upregulation of genes involved in oxidative stress and downregulation of antioxidative defense genes; or, as ROS induced modifications like urinary markers of oxidative nucleoside lesions resulting from ROS modifying guanine which results in mismatched nucleotide pairing [10,11]. In the current study, we measured the urinary excretion of oxidized nucleosides, namely 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-oxo-7,8-dihydroguanosine (8-oxoGuo), as markers of oxidative stress.
The MPNs are chronic myeloid cancers arising from a transformation in a hematopoietic stem cell, leading to increased proliferation of the myeloid cell lines and hyperplasia in one or more lineages. MPNs are classified into essential thrombocythemia (ET), polycythemia vera (PV), and primary- or secondary myelofibrosis (MF) [12]. MF is the most advanced of the MPNs. Three key driver mutations have been found in patients with MPNs: JAK2V617F, CALR mutations and myeloproliferative leukemia virus oncogene mutations [13]; JAK2V617F and CALR are the most frequent, and they are practically restricted to MPNs. The mutations variably activate the cytokine/receptor/JAK2 pathways and their downstream signaling through the three homodimeric receptors: erythropoietin receptor, thrombopoietin receptor, and granulocyte colony stimulating receptor. The downstream signaling from these receptors results in proliferation of the erythroid lineage, the megakaryocytic lineage, and the granulocytic lineage, respectively [13]. Prior studies have shown that in patients with MF, several genes associated with increased oxidative stress are upregulated and antioxidative defense genes are downregulated, and the total amount of hydrogen peroxides is elevated whereas the total antioxidant capacity is decreased [1,4]. Likewise, in JAK2V617F knock-in mice, catalase expression is downregulated, and the DNA oxidation product 8-oxo-guanine is elevated [2].
In the Danish General Suburban Population Study (GESUS), 3.2% of the participants carry the JAK2V617F mutation or the CALR mutations with no prior MPN diagnosis [14]. Elevated levels of 8-oxodG have been reported in several cancers, but the association between these markers and the JAK2V617F- or CALR mutations have never been investigated [9]. Therefore, we compare the concentration of 8-oxodG and 8-oxoGuo in participants with JAK2V617F or CALR mutations with that of mutation-negative participants from GESUS. Moreover, we compared 8-oxodG and 8-oxoGuo in MF patients with matched controls and the mutated participants from GESUS. In GESUS, we also performed a Mendelian randomization analysis, using the Ala16Val (rs4880 C to T) single nucleotide polymorphism in the SOD2 gene, to investigate if oxidative stress may cause the somatic mutations associated with MPNs.
2. Subjects and methods
2.1. Study populations
2.1.1. The Danish General Suburban Population Study
GESUS is a cross-sectional study of the adult population in a suburban municipality in Denmark [15]. Participants were included in 2010–2013. The study has been described in detail elsewhere [16]. The study was approved by the Regional Ethical Committee and the Danish Data Protection Agency. The principles of the Declaration of Helsinki were abided by, and participants gave written informed consent.
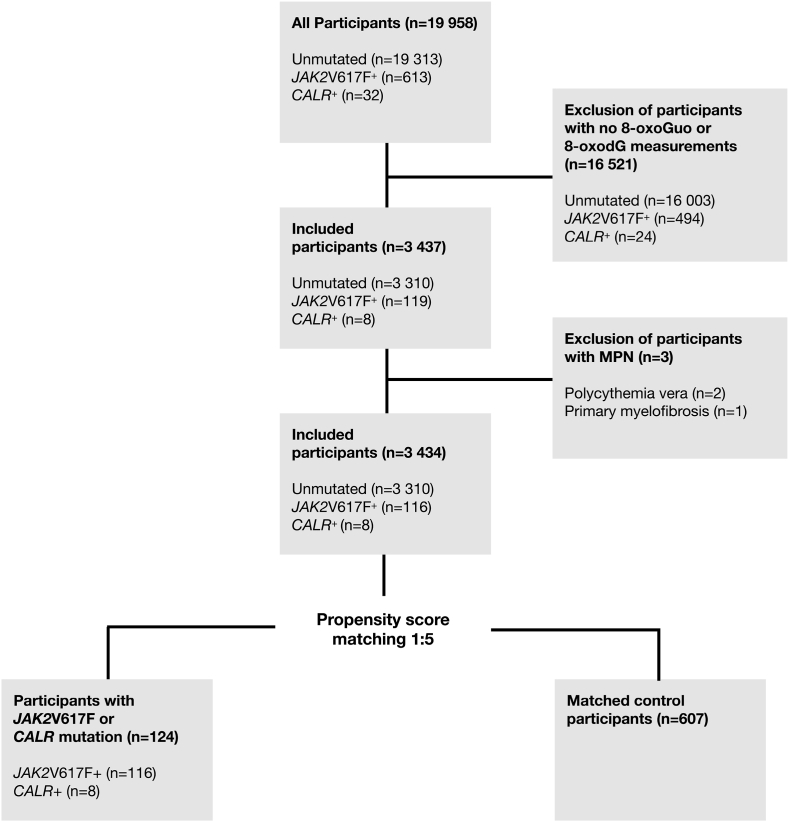
Measurements of JAK2V617F and CALR mutations were carried out in 19,958 participants; 613 had the JAK2V617F mutation, and 32 had the CALR mutation (Fig. 1); 16,521 participants without an 8-oxodG or 8-oxoGuo measurement were excluded (JAK2V617F+: n = 494; CALR+: n = 24; mutation-negative = 16,003). Three participants had a prior MPN diagnosis and were excluded (JAK2V617F+: n = 2; CALR+: n = 1; mutation-negative = 0). In total, we included 3434 (JAK2V617F+: n = 116; CALR+: n = 8; mutation-negative = 3310) participants with information on JAK2V617F- and CALR genotype and oxidation status. We performed a sensitivity analyses, excluding the patients with a mutated allele burden above 2%, to exclude patients with a possible unidentified MPN.
Fig. 1.
Flow chart of participants from The Danish General Subrban Population Study included in the cross-sectional study.
8-oxodG. 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGuo. 8-oxo-7,8-dihydroguanosine.
2.1.2. Myelofibrosis patients
We included 39 patients with primary- or secondary MF. These patients were also included in a previously described study on the impact of ruxolitinib treatment on oxidative stress [17]. The patients were eligible for the current study if they had at least one measurement of urinary 8-oxoGuo and 8-oxodG. If the patient had more than one measurement, we used the first measurement for the current analysis. There were no exclusion criteria. As controls, we used 179 propensity-score matched mutation-negative participants from GESUS.
2.2. Laboratory measurements
In GESUS, JAK2V617F and CALR type 1 and 2 were detected by a pooled multiplex droplet digital PCR assay, as previously described [14]. The sensitivity of the assays was calculated to 0.009% for JAK2V617F and 0.01% for CALR type 1 and 2. In MF patients, the JAK2V617F and CALR alleles were quantified using a high-sensitivity real-time qPCR assay on whole-blood [18,19]. The presence of the MPL mutation was not routinely investigated.
Non-fasting blood samples for GESUS participants were drawn at 15:30–21:00 o'clock and kept at 4 °C until biochemical analysis the next day. Plasma-creatinine and plasma high-sensitivity C-reactive protein (hsCRP) were measured on Cobas-6000 (Roche Diagnostics). Complete blood count was measured using EDTA whole blood on a Sysmex XE-5000 (Sysmex Corporation). The estimated glomerular filtration rate (eGFR) was based on plasma-creatinine and calculated using the Modification of Diet in Renal Disease Equation. Elevated hematological parameters used in this study were defined as the upper reference level of the assay used: hematocrit >0.49/0.48 (women and men), hemoglobin >16.4/15.9 g/dL, platelet count >450 × 109/L, and leucocyte count >11 × 109/L.
Spot urine samples from the general population and MF patients were collected and stored at −80 °C. Urinary 8-oxoGuo and 8-oxodG concentrations were measured using ultra-performance liquid chromatography-tandem mass spectrometry on an Acquity UPL I-class system (Waters) and Xevo TQ-S triple quadrupole mass spectrometer (Waters). The measurements were adjusted for urinary creatinine concentration and reported in nmol/mmol creatinine [20]. Analyses on GESUS participants and MF patients were performed in the same laboratory.
2.3. Comorbidity data
In GESUS, comorbidity data were collected using a self-administered questionnaire. Smoking habits were categorized as current smoker, former smoker, and never smoker. Hypertension was defined by the use of antihypertensive drugs. Type 2 diabetes mellitus (T2DM) was self-reported. A history of ischemic disease was defined as previous acute myocardial infarction, coronary heart disease, or stroke. For MF patients, comorbidity data were collected from medical records.
2.4. Statistical analyses
The statistical software R.3.2.3, Rstudio 1.0.136, and Stata SE 14.0 were used. In the GESUS population, the most frequent missing data were smoking status (5%), eGFR (3%), and information regarding ischemic diseases and T2DM (1%). Missing covariate data were imputed using multiple imputation with the MICE R package.
Distributions of 8-oxodG and 8-oxoGuo were visually assessed and transformed using the natural logarithm to achieve normal distributions (supplemental Fig. S1). Estimates of differences in the log-transformed values were transformed back using the exponential function to give relative differences between groups with 95% confidence intervals.
To compare 8-oxodG and 8-oxoGuo between participants with or without mutation in the GESUS population, we used two approaches. First, we used the entire GESUS population and performed univariate and multivariate linear regressions. For the multivariate analyses, we used the following variables, all shown to affect oxidative stress, as possible confounders: age, sex, BMI, smoking status, eGFR, hypertension, ischemic disease, and T2DM. Possible confounders were chosen before analyses. Secondly, we performed propensity-score matching in a 1:5 ratio using the “nearest neighbor matching” method with a caliper of 0.2. The results from the matching procedure are shown in Supplemental Fig. S2–3. After matching, the cohort included 116 JAK2V617F positive participants, 8 CALR positive participants, and 587 mutation-negative participants (Fig. 1). We then compared values of 8-oxodG and 8-oxoGuo using univariate and multivariate linear regression models. The multivariate analysis included the variables used in the propensity score match.
To produce a comparable control group for the 39 MF patients, we also used propensity-score matching in a 1:5 ratio including 179 controls. The following variables were available from the myelofibrosis patients and used in the propensity score regression model: age, sex, hypertension, ischemic disease, and T2DM. The results from the matching procedure can be seen in Supplemental Fig. S4–5.
The Mendelian randomization analysis was performed using instrumental variable (IV) analyses in Stata SE 14.0. The numerator was the log-odds β regression coefficient for the association of the SOD2 Ala16Val (rs4880 C to T) single nucleotide polymorphism with the JAK2V617F and CALR mutations. The denominator was the linear β regression coefficient for the association of SOD2 Ala16Val with 8-oxodG or 8-oxoGuo per SD increase in these biomarkers. The standardization of 8-oxodG or 8-oxoGuo was done by inverse rank normalization. Analyses were adjusted for age and sex as the assumption in the Mendelian randomization analysis is that the genetic estimate is unconfounded due to the random assortment of alleles [21]. The strength of SOD2 to predict 8-oxodG and 8-oxoGuo was calculated as F = β [2]exposure/SE [2]exposure, (SE = standard error) and was 6.4 for 8-oxodG but only 1.2 for 8-oxoGuo per T-allele.
Summary statistics were presented as frequency and percentages for categorical data and median and interquartile range for numeric data and compared using ANOVA. The concentrations of 8-oxodG or 8-oxoGuo were presented in jitterplots with the median values. Associations between the oxidative markers and the JAK2V617F allele burden were investigated using linear regression. Statistical significance was defined as p-values <0.05.
3. Results
3.1. The Danish General Suburban Population Study (GESUS)
Characteristics of the GESUS cohort stratified by mutational status are shown in Table 1. Participants with JAK2V617F were older and had lower eGFR than mutation-negative participants. Participants with JAK2V617F also had an increased proportion of ischemic disease, hypertension, and elevated platelet count compared with mutation-negative participants.
Table 1.
Characteristics of the included participants from the Danish General Suburban Population Study stratified by mutational status.
| Unmutated (n = 3310) | CALR mutated (n = 8) | JAK2V617F mutated (n = 116) | p-value | |
|---|---|---|---|---|
| Age (years), median [IQR] | 55 [44, 64] | 67 [55, 71] | 58 [49, 67] | 0.003 |
| Sex (male), n (%) | 1357 (41) | 4 (50) | 54 (47) | 0.299 |
| BMI, median [IQR] | 26.02 [23, 29] | 28 [26, 30] | 27 [24, 29] | 0.070 |
| Smoking status, n (%) | 0.098 | |||
| Never | 1439 (44) | 3 (37.5) | 63 (54.3) | |
| Former | 1307 (40) | 4 (50.0) | 33 (28.4) | |
| Current | 564 (17) | 1 (12.5) | 20 (17.2) | |
| Ischemic disease, n (%) | 185 (6) | 1 (13) | 14 (12) | 0.010 |
| T2DM, n (%) | 136 (4) | 0 (0) | 7 (6) | 0.347 |
| Hypertension, n (%) | 809 (24) | 4 (50.0) | 52 (44.8) | <0.001 |
| Prior cancer, n (%) | 232 (7) | 1 (12.5) | 9 (7.8) | 0.551 |
| eGFR (ml/min/1.73 m2), median [IQR] | 81 [71, 91] | 69 [61, 84] | 78 [68, 87] | 0.019 |
| hsCRP (mg/l), median [IQR] | 1.30 [0.60, 2.80] | 2.20 [0.97, 4.32] | 1.40 [0.70, 3.18] | 0.277 |
| Hemoglobin (g/dL), median [IQR] | 14.01 [13.2, 14.8] | 14.2 [13.1, 14.8] | 14.0 [13.0, 15.1] | 0.561 |
| Elevated hemoglobin, n (%) | 69 (2.1) | 0 (0.0) | 3 (2.6) | 0.595 |
| Hematocrit, median [IQR] | 0.43 [0.41, 0.45] | 0.44 [0.40, 0.46] | 0.43 [0.41, 0.46] | 0.377 |
| Elevated hematocrit, n (%) | 61 (1.8) | 0 (0.0) | 3 (2.6) | 0.543 |
| WBC (x 109/l), median [IQR] | 7.00 [6.0, 8.2] | 7.8 [6.5, 8.2] | 7.5 [6.3, 8.6] | 0.060 |
| Elevated WBC, n (%) | 100 (3.0) | 0 (0.0) | 5 (4.3) | 0.447 |
| Platelet count (x 109/l), median [IQR] | 246.00 [213, 283] | 270 [219, 335] | 250 [215, 312] | 0.143 |
| Elevated platelet count (x 109/l), n (%) | 8 (0.2) | 1 (12.5) | 5 (4.3) | <0.001 |
| Elevated myeloid cell parameters, n (%) | 186 (5.6) | 1 (12.5) | 12 (10.3) | 0.073 |
| 8-oxodG (nmol/mmol creatinine), median [IQR] | 1.72 [1.35, 2.16] | 1.98 [1.89, 2.55] | 1.86 [1.53, 2.36] | 0.003 |
| 8-oxoGuo (nmol/mmol creatinine), median [IQR] | 2.26 [1.84, 2.80] | 3.11 [2.38, 3.28] | 2.37 [2.03, 2.86] | 0.022 |
p-values derived from ANOVA analyses.
T2DM, type 2 diabetes mellitus; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; WBC, white blood cell count; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine.
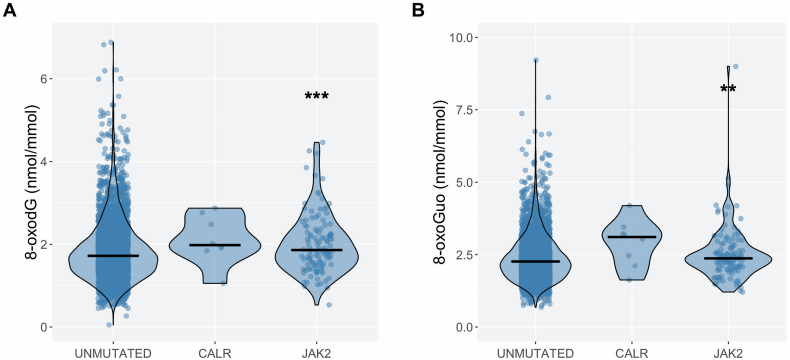
In the univariate analyses, urinary 8-oxodG concentrations were 14% (95%CI: 6–22%, p < 0.001) higher and urinary 8-oxoGuo concentrations were 9% (95%CI: 3–16%, p = 0.002) higher in mutation-positive participants than in mutation-negative participants. Urinary 8-oxodG concentrations were 13% (95%CI: 5–22%, p < 0.001) higher (Fig. 2A) and urinary 8-oxoGuo concentrations were 9% (95%CI: 2.4–16%, p = 0.006) higher in JAK2V617F-positive participants than in mutation-negative participants (Fig. 2B). No statistically significant differences were observed between CALR-positive participants and mutation-negative participants (8-oxodG: 18% higher, 95%CI -9–55%. 8-oxoGuo: 23% higher, 95%CI -2–54%).
Fig. 2.
Violin plots with jitter and median values of urinary 8-oxodG (A) and 8-oxoGuo (B) levels in unmutated (n = 3310), CALR mutated (n = 8), and JAK2V617F mutated participants (n = 116) compared with mutation-negative participants as the reference group.
** p-value <0.01.
*** p-value <0.001.
8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine; JAK2, JAK2V617F mutated participants; CALR, CALR mutated participants.
In the multivariate analyses, adjusting for age, sex, BMI, smoking status, ischemic disease, and T2DM, urinary 8-oxodG concentrations were 13% (95%CI: 6–21%, p < 0.001) higher and urinary 8-oxoGuo concentrations were 6% (95%CI: 0.4–11%, p = 0.035) higher in mutation-positive than in mutation-negative participants. The JAK2V617F-positive participants had 13% (95%CI: 5–21%, p < 0.001) higher concentrations of urinary 8-oxodG than mutation-negative participants, while the urinary 8-oxoGuo concentrations were not significantly different between the groups (β-coef: 5%, 95%CI: 0–11%, p = 0.069). In these analyses, higher age, female sex, higher BMI, current or former smoking, and higher eGFR were associated with higher 8-oxodG and 8-oxoGuo concentrations, and T2DM was associated with higher 8-oxoGuo concentrations. When we excluded participants with elevated cell counts (JAK2V617F+: n = 14, mutation-negative: n = 186), similar differences were observed between JAK2V617F-positive participants and mutation-negative participants (8-oxodG: β-coef: 13%, 95%CI: 5–22%, p < 0.001; 8-oxoGuo: β-coef: 5%, 95%CI: 0–11%, p = 0.070). In sensitivity analyses, excluding participants with a mutated allele burden above 2% (n = 15) we observed similar results (8-oxodG: β-coef: 14%, 95%CI: 6–22%, p < 0.001; 8-oxoGuo: β-coef: 6%, 95%CI: 0–12%, p = 0.049).
Characteristics of participants included in the propensity-score matched analysis are shown in Supplemental Table S1. In the propensity-score matched analysis, urinary 8-oxodG concentrations were 16% (95%CI: 7–24%, p < 0.001) higher and urinary 8-oxoGuo concentrations were 7% (95%CI: 1–13%, p = 0.019) higher in JAK2V617F-positive participants than in mutation-negative participants.
Fourteen JAK2V617F-positive and one CALR-positive participant had increased hemoglobin, hematocrit, white blood cell count, or platelet count. We observed no differences in 8-oxodG or 8-oxoGuo concentrations between JAK2V617F-positive participants with elevated cell count and those without (p > 0.05) (Supplemental Fig. S6).
We investigated the correlation between the 8-oxodG and 8-oxoGuo. There was a significant positive correlation between the two markers in all participants and in the JAK2V617F-positive participants (p < 0.001) (Supplemental Fig. S7). We also investigated the relationship between the concentrations of 8-oxodG and 8-oxoGuo and the JAK2V617F-allele burden (Supplemental Fig. S8). No association was observed.
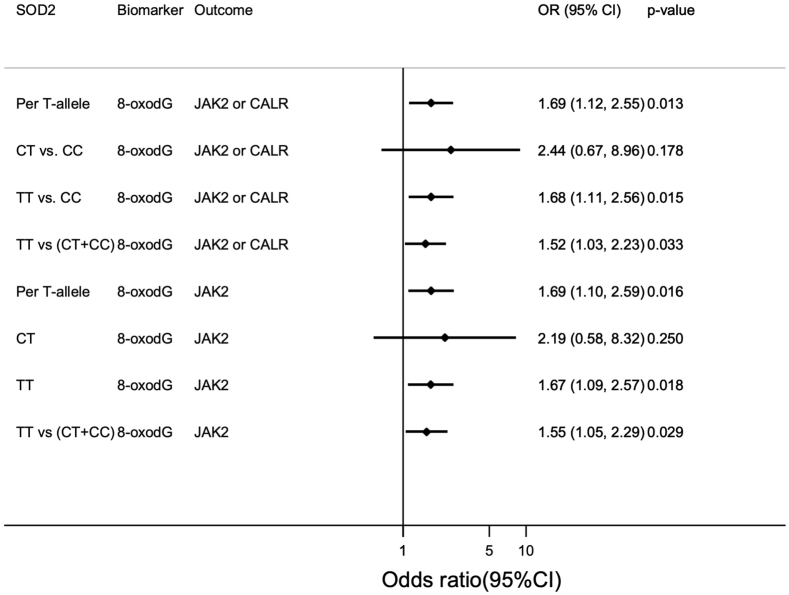
In the Mendelian randomization analysis, the SOD2 T allele (i.e. Val) was associated with 0.058 nmol/mmol creatinine increased 8-oxodG (SE: 0.023, p = 0.011) but not with 8-oxoGuo (β-coef: 0.029 nmol/mmol creatinine, SE: 0.027, p = 0.269) (Supplemental Table S2). Each SOD2 T allele in the trend-analysis was associated with an odds ratio of 1.72 (95%CI: 1.12–2.63, p = 0.013) for being mutation-positive for each 0.1 nmol/mmol increase in 8-oxodG (Fig. 3). TT in the recessive model (vs. CC) and in the dominant model (vs. CC + CT) was also associated with being mutation-positive through 8-oxodG and with similar odds ratio. CT was not associated with being mutation-positive (Fig. 3). Results for JAK2V617F alone were similar to those described for mutation-positive above (Fig. 3). SOD2 was not associated with allele burden (Supplemental Table S3).
Fig. 3.
Mendelian Randomization analysis. The age- and sex adjusted odds ratio (OR) for either the JAK2V617F or the CALR mutation, or just JAK2V617F, for each T-risk-allele (i.e. the Val allele) of SOD2 (reference is the C-allele. i.e. the Ala allele). The OR are shown per 0.1 nmol 8-oxodG/mmol creatinine increment. 8-oxodG were scaled to SD by inverse rank normalization.
3.2. Patients with primary or secondary myelofibrosis
Characteristics of the MF patients included in the study are shown in Table 2. Of 39 patients, 9 (23%) had post-ET or post-PV myelofibrosis, and the median time since MPN diagnosis was almost four years. At the time of analysis, 52% and 64% of the patients received ruxolitinib and darbepoetin-α, respectively; 23% were transfusion dependent. The JAK2V617F-mutation was present in 72%, and the CALR-mutation in 13%.
Table 2.
Characteristics of myelofibrosis patients.
| Number of patients | 39 |
| Age (years), median [IQR] | 71 [64.75] |
| Sex (male), n (%) | 23 (59) |
| Secondary myelofibrosis, n (%) | 9 (23) |
| Post-polycythemia vera, n | 7 |
| Post-essential thrombocythemia, n | 2 |
| Time since diagnosis (years), median [IQR] | 3.95 [0.97.9.96] |
| Treatment | |
| Ruxolitinib, n (%) | 21 (54) |
| Interferon 2α, n (%) | 3 (8) |
| Hydroxyurea, n (%) | 2 (5) |
| Prednisone, n (%) | 1 (3) |
| Darbepoetinα, n (%) | 24 (62) |
| Hemoglobin (g/dL), median [IQR] | 6.7 [6.2, 7.5] |
| White blood cell count (x 109/l), median [IQR] | 8.1 [4.7, 12.6] |
| Neutrophil count (x 109/l), median [IQR] | 4.6 [2.7, 9.1] |
| Platelet count (x 109/l), median [IQR] | 211 [112, 269] |
| Lactate dehydrogenase (U/l), median [IQR] | 440 [341, 650] |
| Transfusion dependent, n (%) | 9 (23) |
| Mutation, n (%) | |
| CALR | 5 (13) |
| JAK2V617F | 28 (72) |
| Double-negative | 4 (10) |
| Unknown | 2 (5) |
| JAK2V617F allele burden, median [IQR] | 57.5 [30.8.75.0] |
| Hypertension, n (%) | 12 (30) |
| Type 2 diabetes mellitus, n (%) | 4 (10) |
| Thromboembolic events, n (%) | 5 (13) |
| 8-oxodG (nmol/mmol creatinine), median [IQR] | 3.21 [2.06, 5.21] |
| 8-oxoGuo (nmol/mmol creatinine), median [IQR] | 4.86 [3.17, 9.30] |
8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine.
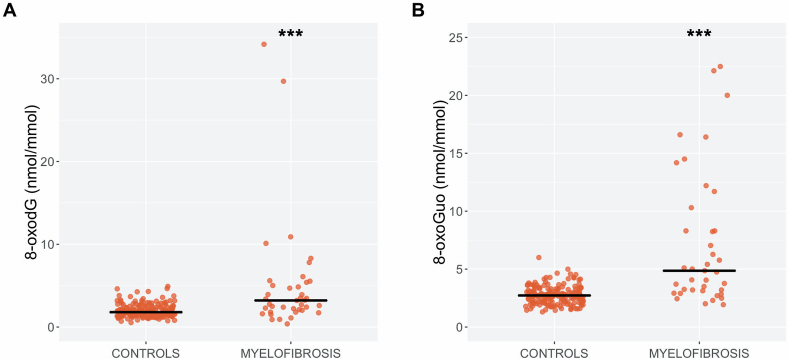
In the univariate analyses, urinary 8-oxodG concentrations were 78% (95%CI: 50–112%, p < 0.001) higher (Fig. 4A) and urinary 8-oxoGuo concentrations were 109% (95%CI: 82–139%, p < 0.001) higher in MF patients than in controls (Fig. 4B). In the multivariate analyses, urinary 8-oxodG concentrations were 77% (95%CI: 49–110%, p < 0.001) higher and urinary 8-oxoGuo concentrations were 105% (95%CI: 80–133%, p < 0.001) higher in MF patients than in the controls. Again, no correlation between the concentrations of urinary oxidative stress markers and the JAK2V617F allele burden was observed (supplemental Fig. S9). When we compared MF patients with the mutated GESUS participants we also found increased levels of the oxidative stress markers in both the univariate (8-oxodG: β-coef: 69%, 95%CI: 38–107%, p < 0.001; 8-oxoGuo: β-coef: 123%, 95%CI: 86–167%, p < 0.001) and the multivariate analyses (8-oxodG: β-coef: 35%, 95%CI: 3–76%, p = 0.031; 8-oxoGuo: β-coef: 75%, 95%CI: 38–123%, p < 0.001). Similar to the GESUS participants, we found a significant correlation between 8-oxodG and 8-oxoGuo (p < 0.001) in MF patients (Supplemental Fig. S7).
Fig. 4.
Jitter and median values of urinary 8-oxodG (A) and 8-oxoGuo (B) levels in myelofibrosis patients (n = 39) and matched controls from the general population (n = 179) as the reference group.
8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine*** p-value <0.001.
4. Discussion
In GESUS, oxidized nucleosides were higher in JAK2V617F- or CALR-positive participants than in mutation-negative participants. Each SOD2 T allele was associated with a 1.7-fold susceptibility to being mutation-positive through higher 8-oxodG urinary excretion, but SOD2 was not associated with oxidized 8-oxoGuo or JAK2V617F allele burden. However, JAK2V617F- or CALR-positive participants had higher levels of 8-oxoGuo than unmutated participants. 8-oxodG and 8-oxoGuo were higher in patients with MF than in controls. These data suggest that oxidative stress may lead to the JAK2V617F mutation ultimately leading to MPNs, and that oxidative stress is a feature of advanced MPNs. This is the first study to investigate oxidative stress in individuals with MPN driver mutations, but without the disease, and it is the first study to present data suggesting that oxidative stress could be involved in the early development of MPNs.
Cross-sectional studies are prone to confounding and reverse causation. However, through Mendelian randomization in GESUS, we were able to provide evidence of the causal direction from an impaired mitochondrial antioxidative defense to increased susceptibility to the JAK2V617F or CALR mutations, which are most frequently observed in MPNs. Thus, the somatic mutations are downstream from the oxidative stress. A previous study also found that polymorphisms associated with lower antioxidative defense, i.e., catalase and glutathione S-transferase, may be associated with MPNs [22]. Conversely, the study did not find an association of SOD2 with MPNs, but the study was approximately five times smaller than our study. Oxidative stress is associated with DNA damage and genotoxic effects could explain the association observed in our study [23,24]. Moreover, oxidative stress may promote cancer development by increased angiogenesis and tumor cell proliferation through inflammatory pathways [23,24]. Indeed, chronic inflammation could be an important factor in the development and progression of MPNs [25]. Although the exact source of urinary 8-oxodG is uncertain, 8-oxodG could be a marker of oxidative DNA damage [10,26]. The association of 8-oxodG with JAK2V617F, which is a G to T mutation, observed in our study is supported by a previous finding that GC to TA is the most frequent mutation induced by oxidative DNA damage [27].
In a sensitivity analysis in GESUS, we demonstrated that even in participants without elevated blood cell counts, the associations between being mutation-positive and higher 8-oxodG excretion were still significant. Likewise, when we excluded participants with a mutated allele burden above 2% the results were similar. Furthermore, we found no association between 8-oxodG and the JAK2V617F allele burden. This could be explained if the oxidative stress preceded the JAK2V617F mutation, and the subsequent clonal expansion of JAK2V617F mutated cells did not cause measurable oxidative stress in individuals without overt MPN, and if the subsequent rate of clonal expansion was not dependent on oxidative stress. Thus, oxidative stress may not be directly associated with the clonal expansion, but oxidative stress may be more pronounced in individuals were the JAK2V617F mutation has developed as a result of the increased oxidative stress.
In our study we found markedly higher excretion of 8-oxodG and 8-oxoGuo in patients with MF compared with controls. Similarly, several prior experimental studies and murine models have shown increased oxidative stress in MPNs. Thus, a PV-like phenotype observed in JAK2V617F knock-in mice was associated with increased ROS accumulation in myeloid stem cells, and treatment with N-acetyl-l-cysteine (NAC), an antioxidant, alleviated the PV-like phenotype and reduced oxidative DNA damage [2]. We did not observe an association between the JAK2V617F allele burden and oxidized nucleosides. This suggest that the MPN phenotype and not necessarily the JAK2V617F mutation itself causes increased oxidative stress. The same interpretation could be used in the murine study. In another murine JAK2V617F knock-in model, treatment with NAC reduced the risk of thrombotic death; however, they did not observe increased levels of ROS when stimulating murine or MPN patient leucocytes in-vitro [28]. Hurtado-Nedelec et al. found that neutrophils from JAK2V617F mutated MPN patients contained higher concentrations of ROS than cells from unmutated patients and healthy controls [5]. The activity of NADPH oxidase, a major source of ROS from neutrophils, was thought to account for some of this difference. Vener et al. found that patients with MF had significantly higher ROS concentrations and lowered antioxidant capacity than healthy controls, which was more pronounced in patients with grade 2–3 fibrosis than in patients with grade 0–1 fibrosis [4]. However, they did not observe a difference between JAK2V617F mutated and nonmutated patients. We previously investigated the effect of JAK1/2 inhibition with ruxolitinib in patients with MF, and found no reductions in 8-oxodG or 8-oxoGuo, implying that JAK2 signaling may not directly induce oxidative stress [17]. Other readily available agents with antioxidative effects, like NAC or statins, could be appropriate treatment options in patients with MPNs [16].
Unlike previous studies which investigated oxidative stress in patients with overt MPNs, our study investigated the association of oxidative stress in individuals from the general population with no prior MPNs and with low-burden JAK2V617F- or CALR mutation, most likely reflecting a very early disease stage [1,4,17,22]. Our study addresses the hypothesis of oxidative stress causing MPN development by predisposing to the JAK2V617F, and possibly the CALR, mutations. Although we do not know how many of these individuals will develop MPN over time. Another Danish general population study found that 48 of 63 patients with JAK2V617F with an allele burden above 0.8% developed MPNs [29]. Only eight participants carried the CALR mutation in GESUS. We observed similar median values for 8-oxodG and 8-oxoGuo in CALR-positive participants and in JAK2V617F-positive participants, but the number of CALR-positive participants was too small to draw any conclusions.
In conclusion, these findings support that impaired mitochondrial antioxidative defense against ROS is associated with increased oxidative stress, which may cause JAK2V617F- and CALR mutations, ultimately leading to MPNs. MF patients had evidence of more oxidative stress than controls. These data suggest that oxidative stress plays a pathogenetic role in the early development of MPNs.
Funding
ALS was supported by a grant from Region Zealand (13-000849).
The Danish General Suburban Population Study was funded by the Region Zealand Foundation, National Headache Foundation, Naestved commune, Johan and Lise Boserup Foundation, TrygFonden, Johnson Family Foundation, Region Zealand, Naestved Hospital, The National Board of Health, and the Local Government Denmark Foundation.
Declaration of competing interest
None of the authors have conflicts of interest to disclose.
Acknowledgements
ALS and CE designed the study, analyzed- and interpreted the data, drafted the paper. HCH, MEB, SC, VS, LK, HEP performed research and interpreted data. CHN interpreted data. All authors critically revised the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101895.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hasselbalch H.C., Thomassen M., Riley C.H., Kjaer L., Larsen T.S., Jensen M.K. Whole blood transcriptional profiling reveals deregulation of oxidative and antioxidative defence genes in myelofibrosis and related neoplasms. Potential implications of downregulation of Nrf2 for genomic instability and disease progression. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marty C., Lacout C., Droin N., Le Couedic J.P., Ribrag V., Solary E. A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression. Leukemia. 2013 Nov;27(11):2187–2195. doi: 10.1038/leu.2013.102. [DOI] [PubMed] [Google Scholar]
- 3.Walz C., Crowley B.J., Hudon H.E., Gramlich J.L., Neuberg D.S., Podar K. Activated Jak2 with the V617F point mutation promotes G1/S phase transition. J. Biol. Chem. 2006 Jun 30;281(26):18177–18183. doi: 10.1074/jbc.M600064200. [DOI] [PubMed] [Google Scholar]
- 4.Vener C., Novembrino C., Catena F.B., Fracchiolla N.S., Gianelli U., Savi F. Oxidative stress is increased in primary and post-polycythemia vera myelofibrosis. Exp. Hematol. 2010 Nov;38(11):1058–1065. doi: 10.1016/j.exphem.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Hurtado-Nedelec M., Csillag-Grange M.J., Boussetta T., Belambri S.A., Fay M., Cassinat B. Increased reactive oxygen species production and p47phox phosphorylation in neutrophils from myeloproliferative disorders patients with JAK2 (V617F) mutation. Haematologica. 2013 Oct;98(10):1517–1524. doi: 10.3324/haematol.2012.082560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hole P.S., Darley R.L., Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood. 2011;117(22):5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- 7.Nieborowska-Skorska M., Kopinski P.K., Ray R., Hoser G., Ngaba D., Flis S. Rac2-MRC-cIII-generated ROS cause genomic instability in chronic myeloid leukemia stem cells and primitive progenitors. Blood. 2012 May 3;119(18):4253–4263. doi: 10.1182/blood-2011-10-385658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao L., St Clair D.K. Regulation of superoxide dismutase genes: implications in disease. Free Radic. Biol. Med. 2009 Aug 15;47(4):344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaunig J.E., Kamendulis L.M., Hocevar B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010 Jan;38(1):96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 10.Frijhoff J., Winyard P.G., Zarkovic N., Davies S.S., Stocker R., Cheng D. Clinical relevance of biomarkers of oxidative stress. Antioxidants Redox Signal. 2015 Nov 10;23(14):1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriksen T., Hillestrom P.R., Poulsen H.E., Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2'-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic. Biol. Med. 2009 Sep 1;47(5):629–635. doi: 10.1016/j.freeradbiomed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Spivak J.L. Myeloproliferative neoplasms. N. Engl. J. Med. 2017 Aug 31;377(9):895–896. doi: 10.1056/NEJMc1708485. [DOI] [PubMed] [Google Scholar]
- 13.Vainchenker W., Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017 Feb 9;129(6):667–679. doi: 10.1182/blood-2016-10-695940. [DOI] [PubMed] [Google Scholar]
- 14.Cordua S., Kjaer L., Skov V., Pallisgaard N., Hasselbalch H.C., Ellervik C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019 Aug 1;134(5):469–479. doi: 10.1182/blood.2019001113. [DOI] [PubMed] [Google Scholar]
- 15.Heltberg A., Andersen J.S., Sandholdt H., Siersma V., Kragstrup J., Ellervik C. Predictors of undiagnosed prevalent type 2 diabetes - the Danish general suburban population study. Prim. Care Diabetes. 2018 Feb;12(1):13–22. doi: 10.1016/j.pcd.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen A.L., Hasselbalch H.C., Nielsen C.H., Poulsen H.E., Ellervik C. Statin treatment, oxidative stress and inflammation in a Danish population. Redox Biol. 2019 Feb;21:101088. doi: 10.1016/j.redox.2018.101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjorn M.E., Brimnes M.K., Gudbrandsdottir S., Andersen C.L., Poulsen H.E., Henriksen T. Ruxolitinib treatment reduces monocytic superoxide radical formation without affecting hydrogen peroxide formation or systemic oxidative nucleoside damage in myelofibrosis. Leuk. Lymphoma. 2019 Feb 20:1–9. doi: 10.1080/10428194.2019.1579323. [DOI] [PubMed] [Google Scholar]
- 18.Larsen T.S., Pallisgaard N., Moller M.B., Hasselbalch H.C. Quantitative assessment of the JAK2 V617F allele burden: equivalent levels in peripheral blood and bone marrow. Leukemia : Off. J. Leukemia Soc. Am., Leukemia Res. Fund, UK. 2008 Jan;22(1):194–195. doi: 10.1038/sj.leu.2404861. [DOI] [PubMed] [Google Scholar]
- 19.Kjaer L., Cordua S., Holmstrom M.O., Thomassen M., Kruse T.A., Pallisgaard N. Differential dynamics of CALR mutant allele burden in myeloproliferative neoplasms during interferon alfa treatment. PloS One. 2016;11(10) doi: 10.1371/journal.pone.0165336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen S.T., Andersen J.T., Nielsen T.K., Cejvanovic V., Petersen K.M., Henriksen T. Simvastatin and oxidative stress in humans: a randomized, double-blinded, placebo-controlled clinical trial. Redox Biol. 2016 Oct;9:32–38. doi: 10.1016/j.redox.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies N.M., Holmes M.V., Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018 Jul 12;362 doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trifa A.P., Banescu C., Dima D., Bojan A.S., Tevet M., Moldovan V.G. Among a panel of polymorphisms in genes related to oxidative stress, CAT-262 C>T, GPX1 Pro198Leu and GSTP1 Ile105Val influence the risk of developing BCR-ABL negative myeloproliferative neoplasms. Hematology. 2016 Oct;21(9):520–525. doi: 10.1080/10245332.2016.1163889. [DOI] [PubMed] [Google Scholar]
- 23.Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative stress in cancer. Canc. Cell. 2020 Aug 10;38(2):167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 2010 Dec 1;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koschmieder S., Mughal T.I., Hasselbalch H.C., Barosi G., Valent P., Kiladjian J.J. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia. 2016 May;30(5):1018–1024. doi: 10.1038/leu.2016.12. [DOI] [PubMed] [Google Scholar]
- 26.Deng X.S., Tuo J., Poulsen H.E., Loft S. Prevention of oxidative DNA damage in rats by brussels sprouts. Free Radic. Res. 1998 Mar;28(3):323–333. doi: 10.3109/10715769809069284. [DOI] [PubMed] [Google Scholar]
- 27.Arai T., Kelly V.P., Minowa O., Noda T., Nishimura S. High accumulation of oxidative DNA damage, 8-hydroxyguanine, in Mmh/Ogg1 deficient mice by chronic oxidative stress. Carcinogenesis. 2002 Dec;23(12):2005–2010. doi: 10.1093/carcin/23.12.2005. [DOI] [PubMed] [Google Scholar]
- 28.Craver B.M., Ramanathan G., Hoang S., Chang X., Mendez Luque L.F., Brooks S. N-acetylcysteine inhibits thrombosis in a murine model of myeloproliferative neoplasm. Blood Adv. 2020 Jan 28;4(2):312–321. doi: 10.1182/bloodadvances.2019000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen C., Bojesen S.E., Nordestgaard B.G., Kofoed K.F., Birgens H.S. JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica. 2014 Sep;99(9):1448–1455. doi: 10.3324/haematol.2014.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.