Abstract
Systemic parameters and its relationship with Somatic Cell Count (SCC) in dairy cows were evaluated. Cows that presented subclinical mastitis (SMC; n = 16) and healthy cows (HC; n = 6) were selected and identified by the California Mastitis Test and SCC in milk. SCC results were logarithmically transformed into somatic cell score (SCS). HC presented SCS 〈 4.32 while SMC presented SCS 〉 4.32. Milk and blood samples were collected in three days: D1 (first day of sampling), D2 (48 h after D1) and D3 (7 days after D1), to determine White Blood Cells (WBC), albumin, total protein, total bilirubin and malondialdehyde (MDA). Results were expressed as mean ± standard deviation. It was considered significant at P < 0.05. The data of SCS on D1, D2 and D3 in SMC were: 6.8 ± 1.7, 6.4 ± 1.8, and 6.3 ± 2.0, respectively. In SMC the MDA (nmolL−1) were: D1 - 9.3 ± 2.6, D2 - 8.6 ± 2.4, and D3 - 11.5 ± 3.5. The MDA on D3 in SMC (11.5 ± 3.5) were increase when compared to HC (6.0 ± 1.3) (P < 0.001). No significant difference was found in WBC, TP, ALB, and TB between groups. It was observed a positive correlation between MDA-SCS (ρ = 0.4) and between WBC-SCS (ρ = 0.3) in the SMC group. It was concluded that the systemic repercussion damage in the mammary gland promoted by subclinical mastitis in dairy cows can be assessed using the MDA and WBC biomarkers.
Keywords: Inflammation, Subclinical mastitis cows, Lipid peroxidation, Somatic cell count
Introduction
The mastitis is one of the most important inflammatory processes in ruminants, regarding the losses associated with the decrease in milk production, culling, costs with drugs and decrease in the quality of dairy products (Bradley, 2002). This pathology manifests itself in two ways according to the presentation of clinical signs, which can be clinical mastitis, that shows evident signs of changes in milk and/or udder; and subclinical mastitis, that can only be detected through laboratory tests (Lakshmi & Jayavardhanan, 2016).
Mastitis is an inflammatory condition of the mammary gland that can be caused by chemical, physical or traumatic accidents as well as by microorganisms, usually bacteria, which results in tissue damage (Bae et al., 2017). This tissue presents several defense mechanisms that composes the local immunity, which maintain homeostasis. However, when microorganisms manage to overcome these barriers, cellular interaction occurs, and soluble inflammatory mediators are released in response to the aggression, consequently causing an increase in vascular permeability that favors the leukocyte migration from blood to the mammary gland, contributing to inflammatory exacerbation (Sordillo, 2018).
In recent years, several indicators or biomarkers have been studied in order to diagnose mastitis. Among them, we highlight the measurement of pH, levels of enzymes, proteins, peptides, milk components, and the somatic cell count (SCC), as well as molecular tests, genomics, and proteomic analyses, which can be used alone or combined (Chakraborty et al., 2019). However, most of these methods are not yet widely used, being difficult to access, requiring specialized labor, in addition to having a high cost (Sharifi et al., 2018; Singhal, Kumar, Kanaujia & Virdi, 2015; Viguier, Arora, Gilmartin, Welbeck & O'Kennedy, 2009). However, the combination of diagnostic methods becomes more accurate for the diagnosis of mastitis, with the use of more economically accessible methods and applicable to the field for an earlier, faster and more accurate diagnosis of this disease (Dasohari, Somasani, Nagaraj & Gopala, 2018; Ferronatto et al., 2018).
Among these biomarkers it can be included the malondialdehyde (MDA) which is formed as a product of the peroxidation of unsaturated fatty acids and is often used as a marker of lipid peroxidation (Turk et al., 2017), as well as the antioxidants bilirubin and albumin that protect the oxidation of structural molecules or specialized functions of the body (Andrei et al., 2009). SCC in bovine milk is used as a well-established indicator of inflammation of the mammary gland that is highly correlated with the presence of a mammary infection, in addition to being used as a criterion for the selection of dairy animals that are less susceptible to mastitis (Rainard, Foucras, Boichard & Rupp, 2018). SCC has shown to be an excellent marker of subclinical mastitis, and its use is essential to indicate reduction in milk production (Kaşikçi, Çetin, Bingöl & Gündüz, 2012). Thus, the objective of the present study was to evaluate the parameters of systemic inflammatory response, oxidant-antioxidant status, and its relationship with SCC in dairy cows.
Material and methods
Location, animals, and diet
The study was carried out from August to September 2018 on a dairy farm located in the city of Aquiraz, State of Ceará, latitude 03°54′05″ South and longitude 38°23′28″ West, Brazil. The climate is classified as semi-humid tropical, with average rainfall of 1532 mm/year concentrated from February to May. On the farm there were 34 lactating cows, but only twenty-two lactating crossbred cows (Girolando) met the inclusion criteria for the experimental protocol. The animals weighed on average 480 kg, raised in a semi-intensive system and milked twice a day in an automated system. Before milking, the cows were cooled by fans and sprinklers, and immediately afterwards they were subjected to the feeding management, which consisted of silage, barley and concentrated ration (based on corn, soybean, cotton cake and commercial mineral/vitamin supplement) according to the milk production.
Protocol
The experimental protocol was approved by the Ethics Committee for Use of Animals - CEUA/UECE under the number 4150920/2017. Cows with subclinical mastitis (SMC) and healthy cows (HC) were selected and identified by the California Mastitis Test (CMT) and the Somatic Cell Count (SCC) in milk. Two protocols with the same animals were evaluated: 1) Analysis of systemic and local parameters in cows with subclinical mastitis (SMC) on three different days, D1 (first day of sampling), D2 (48 h after D1), and D3 (7 days after D1) (Fig. 1); and 2) Evaluation and comparison of the systemic and local parameters of groups SMC and HC on D3 (Fig. 2). SCC results were logarithmically transformed into somatic cell score (SCS). For the SMC group (n = 16) cows with positive CMT and SCS > 4.32 were included, while for the HC group (n = 6) cows with negative CMT and SCS < 4.32 were included (Shook, 1982; Venturini et al., 2009). Blood and milk samples were collected on D1, D2 and D3 in animals of the both SMC and HC groups for the assessment of systemic parameters and local inflammation of the mammary gland.
Fig. 1.
Protocol 1.
Fig. 2.
Protocol 2.
Milk sampling and SCC analyses
Milk sampling was performed in the afternoon milking, just after the initial milking procedures, pre-dipping and individual drying of the teats, with subsequent asepsis of the mammary quarters, using 70% alcohol. Initially, the dark-bottom mug test was performed and for those udder quarters that were negative in this test, the first milk jets were discarded and then the CMT was performed according to the methodology proposed by Esslemont and Kossaibati (2002). To perform the SCC, 50 mL of milk were collected in a sterile tube from each udder quarter before milking. In a maximum period of three hours, at the farm facilities, the SCC test was performed on the fresh milk samples per individual quarter, using the Lactoscan SCC® device through the cell count fluorescent microscopy technique. The samples were homogenized, and 100 μL aliquots were taken and added to a tube containing Sofia Green dye (Lactoscan SCC®). Then this mixture was homogenized and an 8 μL aliquot was taken, which was added to the lactochip chamber for reading. The results were expressed in cells/mL.
Blood sampling and systemic parameters analysis
Blood samples were obtained by coccygeal venipuncture in 10 mL vacuum tubes without anticoagulant and with EDTA anticoagulant, keeping them at room temperature. After collection, the tubes with EDTA were transported to the laboratory for hematological assessment in order to perform the white blood cell count (WBC, x10³ μL) using the automation device (Mindray BC-2800Vet®). Blood samples collected without anticoagulant were centrifuged at 3000 xG for 15 min to obtain the serum, which was aliquoted and stored at −80 °C to perform the biochemical parameters. Serum levels of total bilirubin (TB, mg/dL), total protein (TP, g/dL) and albumin (ALB, g/dL) were measured according specific methodologies from the commercial kits (Interkit®) in a spectrophotometer.
Reference values
For the serum parameters, the reference values for the bovine species were considered, WBC: 4 - 12 (x103/µL), ALB: 2.7 - 3.8 (g/dL), globulin: 3.0 - 5.2 (G, g/dL), TP: 6.6 - 7.5 (g/dL), TB: 0.01 - 0.5 (mg/dL), and A/G: 1 - 1.02 (Jain, 1993; Kaneko, Harvey & Bruss, 2008).
Assessment of systemic lipidic peroxidation
The MDA levels were quantified by using a solution of 1.2% sodium thiobarbiturate (TBA), according to the methodology proposed by Draper and Hadley (1990). Initially, 250 µL of the serum were extracted and incubated in a water bath at 37 °C for 1 hour, followed by the addition of 400 µL of 35% perchloric acid to precipitate the proteins. The mixture was centrifuged at 14,000 xG for 10 min and 600 µL of the supernatant was added to 200 µL of a 1.2% sodium thiobarbiturate solution (TBARS). The mixture was put in the water bath and heated to 95 °C for 30 min. After cooled, the absorbance was measured at 535 nm. The results obtained were expressed in nmol/mL of serum.
Statistical methods
The statistical package GraphPad Prism® was used. The Shapiro Wilk test was performed to verify the normality of the data. The SCC results were logarithmically transformed into somatic cell score (SCS) through the equation SCS = log2 (SCC/100) + 3 (Shook, 1982). All results were expressed as mean ± standard deviation. For the analysis of SMC between the days (D1, D2, D3) the ANOVA for repeated measures with posthoc Tukey was used for the parametric data of SCS, WBC, TP, MDA, globulin, albumin/globulin, ALB and TB, and when necessary the Paired test t was used. For the assessment of nonparametric data of MDA between D1, D2 e D3 it was used the Friedman test with posthoc Dunn`s. For the evaluation between groups SMC and HC it was used the unpaired t-test with correction of Welch`s for the parametric data of SCS, WBC, MDA, TP, globulin, albumin/globulin, ALB and TB. The correlation between the parameters was determined by the Pearson's and Spearman's test. The results were considered significant at P < 0.05.
Results
The selected cows with subclinical mastitis (SMC) showed the following mean values and standard deviation of SCS on the days analyzed: D1- 6.8 ± 1.7, D2- 6.4 ± 1.8 and D3- 6.3 ± 2.0. The SCS of HC presented mean value and standard deviation of 3.3 ± 0.53. Table 1 shows the results of SCC and systemic inflammatory parameters in cows with subclinical mastitis, whose samples were collected on different days (D1, D2 and D3). There was no significant difference in the levels of WBC, TP, G, MDA and A/G ratio between the days analyzed in SMC. The average A/G ratio was below the reference values for the species. TB values at three days analyzed were in accordance with the reference values for species.
Table 1.
SCS and systemic inflammatory parameters of subclinical mastitis cows.
| Sampling days | |||
|---|---|---|---|
| D1 | D2 | D3 | |
| Parameters | Χ ± SD (Min.-Max.) | Χ ± SD (Min.-Max.) | Χ ± SD (Min.-Max.) |
| Somatic Cell Score (SCS) | 6.8 ± 1.7 | 6.4 ± 1.8 | 6.3 ± 2.0 |
| (4.7 – 9.6) | (4.5 – 9.3) | (4.4 – 9.6) | |
| White Blood Cells (x10³ / μL) | 13.6 ± 3.8 | 12.4 ± 3.7 | 14.3 ± 6.3 |
| (7.5 – 20.9) | (7.1 – 20.0) | (6.1 – 26.7) | |
| Serum total proteins (g/dL) | 6.9 ± 0.7 | 7.0 ± 0.9 | 7.1 ± 0.7 |
| (5.6 – 8.3) | (5.3 – 9.3) | (6.2 – 9.0) | |
| Serum MDA (nmol/mL) | 9.3 ± 2.6 | 8.6 ± 2.4 | 11.5 ± 3.5 |
| (9.2 – 14.7) | (8.5 – 15.4) | (8.1 – 16.5) | |
| Globulin (g/dL) | 3.9 ± 0.7 | 3.9 ± 1.0 | 4.0 ± 0.8 |
| (3.0 – 5.7) | (2.4 – 6.7) | (3.1 – 6.0) | |
| A/G ratio | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 |
| (0.4 –1.0) | (0.4 – 1.4) | (0.5 – 1.1) | |
| Albumin (g/dL) | 3.0 ± 0.3 | 3.0 ± 0.3 | 3.1 ± 0.1 |
| (2.3 – 3.3) | (2.6 – 3.4) | (2.7 – 3.4) | |
| Total Bilirubin (mg/dL) | 0.5 ± 0.3a | 0.2 ± 0.2b | 0.3 ± 0.1ab |
| (0.3 – 1.0) | (0.3 – 0.6) | (0.2 – 0.7) | |
Milk sample were collected on different days: D1 = First sampling; D2 = Second sampling; D3 = Third sampling. Min. = minimum values; Max. = maximum values; SCS = log2 (SCC/ 100) + 3. Different lowercase letters in the same row represent a significant difference by the Paired test t (P<0.05).
Table 2 shows the results of SCS and systemic inflammatory parameters on subclinical mastitis cows (SMC) on D3 compared to healthy cows (HC). There was a significant difference (P < 0.001) in the measurement of MDA between the HC and SMC groups. The mean values of WBC in the SMC group were above the reference values for the species, however there was no significant difference when compared to HC. No significant difference was found in the measurement of WBC, TP, ALB, and TB between groups, and the mean values of these parameters were within the reference values for the species studied.
Table 2.
SCS and systemic inflammatory parameters of subclinical mastitis cows (SMC) in comparison to healthy cows (HC).
| Dairy cows | ||
|---|---|---|
| HC (n = 6) | SMC (n = 16) | |
| Parameters | Χ ± SD (Min. – Max.) | Χ ± SD (Min. – Max.) |
| Somatic Cell Score (SCS) | 3.3 ± 0.5 a | 6.3 ± 2.0 b |
| (2.4– 3.9) | (4.4– 9.6) | |
| White blood cells (WBCx10³/μL) | 10.5 ± 3.6 | 14.3 ± 6.3 |
| (6.1 – 14.7) | (6.1 – 26.7) | |
| Serum TP (g/dL) | 6.8 ± 0.9 | 7.1 ± 0.7 |
| (5.3 – 7.9) | (6.2 – 9.0) | |
| Serum MDA (nmol/mL) | 6.0 ± 1.3a | 11.5 ± 3.5b |
| (4.7 – 8.3) | (8.1 – 16.5) | |
| Globulin (g/dL) | 3.8 ± 0.8 | 4.0 ± 0.8 |
| (2.2 – 4.7) | (3.1 – 6.0) | |
| A/G ratio | 0.7 ± 0.03 | 0.8 ± 0.1 |
| (0.7 – 0.8) | (0.5 – 1.1) | |
| Albumin (g/dL) | 3.0 ± 0.1 | 3.1 ± 0.1 |
| (2.8 – 3.2) | (2.7 – 3.4) | |
| TB (mg/dL) | 0.3 ± 0.09 | 0.3 ± 0.1 |
| (0.2 – 0.4) | (0.2 – 0.7) | |
HC = healthy cows; SMC = subclinical mastitis cows. Min. = minimum values; Max. = maximum values; SCS = log2 (SCC/ 100) + 3. Different lowercase letters in the same row represent a significant difference by the Mann-Whitney test and unpaired t-test (P<0.001).
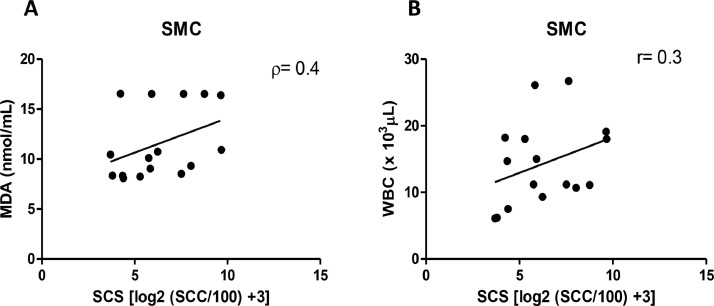
It was observed a positive correlation between MDA-SCS (ρ = 0.4; Fig. 3A) and between WBC-SCS (r = 0.3; Fig. 3B) on SMC.
Fig. 3.
(A) Correlation between MDA and SCS in dairy cows with subclinical mastitis. (B)Correlation between WBC and SCS in dairy cows with subclinical mastitis. SMC = cows with subclinical mastitis. ρ = Spearman's correlation; r=Pearson's correlation.
Discussion
For this study, the cows considered to have subclinical mastitis were those whose milk samples tested positive for CMT and SCC (Venturini et al., 2009). Subclinical mastitis has no clinical signs; however, it does show an increase in SCC in milk. This illness is also associated with the release of free radicals, the increase in total oxidative capacity and decrease in antioxidant capacity (Ellah, 2013). Initially, an individual screening of the udder quarters of each cow used in the study was performed, through the CMT. CMT test has great practical relevance in the research and prevention of mastitis, since it is a quick and practical method most often used for monitoring and evaluating dairy herds (Godden, Royster, Timmerman, Rapnicki & Green, 2017; Leslie, Jansen & Lim, 2002; Oliveira et al., 2020), it might present false-positive or false-negative results (Brito, Caldeira, Verneque & Paiva e Brito, 1997). Thus, it is importance to use it in conjunction with SCC for a safer diagnosis in the herd, as they have great association and dependence (Barbosa, Benedetti, Ribeiro & Guimarães, 2002), but also because SCC has been used and accepted internationally for many years (Harmon, 1998; Milkpoint, 2006). SCC is an important marker of udder health by quantifying the somatic cells present in milk, consisting mainly of desquamated epithelial cells, macrophages and neutrophils (Akers et al., 2011; Alhussien & Dang, 2018; Gokceoglu, Yarim, Gultiken & Yarim, 2020).
Mastitis is an inflammation of the mammary gland that has serious consequences on milk production and causes illness to the animal. The local inflammatory response may have systemic repercussions involving the production of mediators by the liver and damage to cell membranes. The inflammatory process involves increasing the local vascular permeability that allows the influx of plasma proteins and the migration of circulating leukocytes to the target site, in an attempt to overcome the infection. The focus of our work is the subclinical form, which is more difficult to diagnose. Therefore, we decided to evaluate some mediators that are already widely used in the clinical evaluation of systemic inflammation. Associated with these, mediators of the oxidant–antioxidant system were included, as the MDA, a parameter that assesses lipid peroxidation, and non-enzymatic antioxidants, such as Albumin and Bilirubin.
In this study, circulating leukocytes were increased and above the reference values for the species, in cows with subclinical mastitis, but this increase was not significant The increase in circulating leukocytes is expected in infectious processes, including mastitis, since there is a demand for the infected udder (Kumar & Mukherjee, 2013). It has been show that even under healthy conditions of daily handling or milking, leukocytes are present in the mammary gland (Sordillo, 2018). Increased SCC and influx of PMN are seen in cows with subclinical mastitis (Alhussien et al., 2016; Molinari, Blagitz, Della Libera, Batista & Souza, 2018).
In this work, no changes were found in the serum levels of TP, globulins and ALB in cows with subclinical mastitis. Albumin is an acute-phase systemic protein that migrates to inflamed tissues through the increased vascular permeability where it exerts various physiological functions, including antioxidant, and is considered an immune-inflammatory biomarker (Gradel et al., 2018; Osorio et al., 2014; Roche, Rondeau, Singh, Tarnus & Bourdon, 2008). What was observed is that it did not change on the evaluated days in SMC and it did not differ from the levels in HC. It has been reported that the detection of significant changes in this protein is only observed after a period of at least one month, due to its low rate of synthesis and degradation(González, Conceição, Siqueira, & La Rosa, 2000).
An increase in MDA level was observed in SMC. This data can be caused by the tissue damage arising from mammary inflammation. The lipid peroxidation is a molecular mechanism involved in the oxidative damage of cell structures and in the toxicity process that leads to cell death, which is caused by the high generation of reactive oxygen and nitrogen species through the destruction of lipids in the cell membrane (Mohamed-Ibrahim, 2015; Repetto, Semprine & Boveris, 2012). To reduce oxidative damage the antioxidant system comes into action including various mediators. In this study ALB and TB were used as antioxidants, however, there have no alterations on levels of these biomarkers. The antioxidants were synthesized by body, have the purpose of maintaining the integrity of organs and tissues (Neužil & Stocker, 1993; Schneider & Oliveira, 2004). Andrei et al. (2009) observed that an increase in MDA and a decrease in the concentration of antioxidants in cows with subclinical mastitis. A reduction in oxidative stress in bovines with mastitis was observed by increasing the consumption of dietary antioxidants (Sharma, Verma, Rahal, Kumar & Nigam, 2016). The early diagnosis of subclinical mastitis through the use of the relation between MDA and enzymatic antioxidants (SOD, GPx) has been reported (Abdel-Hamied & Mahmoud, 2020; Mahapatra, Panigrahi, Patra, Rout & Ganguly, 2018; Yang et al., 2011; Zigo et al., 2019).
It was observed a positive correlation between MDA-SCS and between WBC-SCS in the SMC. These data showed the existence of systemic changes in SMC associated to SCS in mammary gland. Thus, our data demonstrated that MDA is an excellent marker in subclinical mastitis and that when associated with SCC it provides the repercussion of systemic damage. However, it should be noted that some markers do not have the same performance in subclinical mastitis. Future studies, it would be highly important to carry out microbiological analyses to identify the etiological agents in order to verify the influence on the parameters evaluated. The search for different biomarkers in the diagnosis of mastitis should be encouraged, aiming at alternative ways to avoid productive and economic losses in animals of high zootechnical value.
Conclusion
It was concluded that the systemic repercussion mammary gland damage promoted by subclinical mastitis in dairy cows can be assessed using the MDA and WBC biomarkers. Thus, lipid peroxidation becomes an important tool to be evaluated in mastitis in dairy cows. The analysis of blood parameters associated with milk analysis, contributed to the diagnosis of subclinical mastitis in the evaluated herd, thus providing additional information to the diagnosis of this disease in regions where laboratory analyses of milk used routinely for this purpose are not available.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
To the Financier of Studies and Projects (FINEP) for the promotion and financing of the equipment used in the study; To the National Council for Scientific and Technological Development (CNPq) for providing with scholarship to the first author; To the Coordination for the Improvement of Higher Education Personnel (CAPES) for providing elements for bibliographic research; To the State University of Ceará (UECE) for providing infrastructure to the development of the study.
References
- Abdel-Hamied E., Mahmoud M.M. Antioxidants profile, oxidative stress status, leukogram and selected biochemical indicators in dairy cows affected with mastitis. Journal Animal Health Production. 2020;8:183–188. doi: 10.17582/journal.jahp/2020/8.4.183.188. [DOI] [Google Scholar]
- Akers R.M., Nickerson S.C. Mastitis and its impact on structure and function in the ruminant mammary gland. Journal of Mammary Gland Biology and Neoplasia. 2011;16:275–289. doi: 10.1007/s10911-011-9231-3. [DOI] [PubMed] [Google Scholar]
- Alhussien M.N., Manjari P., Mohammed S., Sheikh A.A., Reddi S., Dixit S., Dang A.K. Incidence of mastitis and activity of milk neutrophils in Tharparkar cows reared under semi-arid conditions. Tropical Animal Health and Production. 2016;48:1291–1295. doi: 10.1007/s11250-016-1068-8. [DOI] [PubMed] [Google Scholar]
- Alhussien M.N., Dang A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Veterinary World. 2018;11:562–577. doi: 10.14202/vetworld.2018.562-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei S., Pintea A., Bunea A., Groza I., Bogdan L., Cuipe S. Non-Enzymatic Antioxidants Concentration and Lipids Peroxidation Level in Milk From Cows with Subclinical Mastitis. Bulletin UASVM CN, 2009;66:196–201. 10.15835/buasvmcn-vm:66:1:3864. [Google Scholar]
- Bae H., Jeong C.H., Cheng W.N., Hong K., Seo H.G., Han S.G. Oxidative stress-induced inflammatory responses and effects of N-acetylcysteine in bovine mammary alveolar cells. Journal of Dairy Research. 2017;84:418–425. doi: 10.1017/s002202991700067x. [DOI] [PubMed] [Google Scholar]
- Barbosa C.P., Benedetti E., Ribeiro S.C.A., Guimarães D.C. Relação entre contagem de células somáticas (CCS) e os resultados do California mastitis test (CMT), no diagnóstico de mastite bovina. Bioscience Journal. 2002;8:93–102. [Google Scholar]
- Bradley A.J. Bovine mastitis: An evolving disease. Veterinary Journal. 2002;164:116–128. doi: 10.1053/tvjl.2002.0724. [DOI] [PubMed] [Google Scholar]
- Brito J.R.F., Caldeira G.A.V., Verneque R.S., Paiva e Brito M.A.V. Sensibilidade e especificidade do California Mastitis Test como recurso diagnóstico da mastite subclínica em relação à Contagem de Células Somáticas. Pesquisa Veterinária Brasileira. 1997;7:49–53. doi: 10.1590/S0100-736X1997000200002. [DOI] [Google Scholar]
- Chakraborty S., Dhama K., Tiwari R., Yatoo M.I., Khurana S.K., Khandia R. Technological interventions and advances in the diagnosis of intramammary infections in animals with emphasis on bovine population –a review. Veterinary Quarterly. 2019;39:76–94. doi: 10.1080/01652176.2019.1642546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasohari A., Somasani A., Nagaraj P., Gopala R.A. Comparative studies for efficacy of different diagnostic tests of sub-clinical mastitis in cows. The Pharma Innovation Journal. 2018;7:149–152. [Google Scholar]
- Draper H.H., Hadley M. Malondialdehyde determination as index of lipid Peroxidation. Methods in Enzymology. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- Ellah M.R. Role of Free Radicals and Antioxidants in Mastitis. Advanced Veterinary Research. 2013;3:1–7. [Google Scholar]
- Esslemont D., Kossaibati M. Mastitis: How to get out of the dark ages. The Veterinary Journal. 2002;164:85–86. doi: 10.1053/tvjl.2002.0742. [DOI] [PubMed] [Google Scholar]
- Ferronatto J.A., Ferronatto T.C., Schneider M., Pessoa L.P., Blagitz M.G., Heinemann M.B., Souza F.N. Diagnosing mastitis in early lactation: Use of Somaticell®, California mastitis test and somatic cell count. Italian Journal of Animal Science. 2018;17:723–729. doi: 10.1080/1828051X.2018.1426394. [DOI] [Google Scholar]
- Godden S.M., Royster E., Timmerman J., Rapnicki P., Green H. Evaluation of an automated milk leukocyte differential test and the California Mastitis Test for detecting intramammary infection in early- and late-lactation quarters and cows. Journal of Dairy Science. 2017;100:6527–6544. doi: 10.3168/jds.2017-12548. [DOI] [PubMed] [Google Scholar]
- Gokceoglu A., Yarim G.F., Gultiken N., Yarim M. High epidermal growth factor concentration associated with somatic cell count in milk of cows with subclinical mastitis. Medycyna Weterynaryjna. 2020;76:354–357. doi: 10.21521/mw.6396. [DOI] [Google Scholar]
- González F.H.D., Conceição T., Siqueira A.S., La Rosa V. Variações sangüíneas de uréia, creatinina, albumina e fósforo em bovinos de corte no rio grande do sul. A hora veterinária. 2000;20:59–62. [Google Scholar]
- Gradel K.O., Vinholt P.J., Magnussen B., Pedersen C., Jensen T.G., Kolmos H.J. Hypoalbuminaemia as a marker of trans-capillary leakage in community-acquired bacteraemia patients. Epidemiology and Infection. 2018;146:648–655. doi: 10.1017/S0950268818000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon R.J. Poster session presentation at the 37th national mastitis council regional meeting. Madison; Bellevue: 1998. Somatic cell counts: Myths vs reality. [Google Scholar]
- Jain N.C. Lea & Febiger; Philadelphia: 1993. Essentials of veterinary hematology. [Google Scholar]
- Kaneko J., Harvey J., Bruss M. 6th ed. Academic Press; Philadelphia: 2008. Clinical biochemistry of domestic animals; p. 928. [Google Scholar]
- Kaşikçi G., Çetin Ö., Bingöl E.B., Gündüz M.C. Relations between electrical conductivity, somatic cell count, California mastitis test and some quality parameters in the diagnosis of subclinical mastitis in dairy cows. Turkish Journal of Veterinary and Animal Sciences. 2012;36:49–55. doi: 10.3906/vet-1103-4. [DOI] [Google Scholar]
- Kumar U., Mukherjee R. Dynamics of milk leukocytes in response to a biological response modifier during bovine subclinical mastitis. Research in Veterinary Science. 2013;95:352–357. doi: 10.1016/j.rvsc.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Lakshmi K., Jayavardhanan K. Screening of milk samples for sub-clinical and clinical mastitis by using CMT and SCC. Journal of Medical Science and Clinical Research. 2016;04:10853–10855. doi: 10.18535/jmscr/v4i6.27. [DOI] [Google Scholar]
- Leslie, K.E., .Jansen, J.T., .& Lim, G.H. (.2002,. May). Opportunities and implications for improved onfarm cowside diagnostics. DeLaval hygiene symposium and R&D center inaugural, Missouri, USA.
- Mahapatra A., Panigrahi S., Patra R.C., Rout M., Ganguly S. A Study on Bovine Mastitis Related Oxidative Stress along with Therapeutic Regimen. International Journal of Current Microbiology and Applied Sciences. 2018;7:147–160. doi: 10.20546/ijcmas.2018.701.027. [DOI] [Google Scholar]
- Mohamed Ibrahim H.M. Cytokine Response and Oxidative Stress Status in Dairy Cows with Acute Clinical Mastitis. Journal of Dairy, Veterinary & Animal Research. 2015;3:1–6. doi: 10.15406/jdvar.2016.03.00064. [DOI] [Google Scholar]
- Molinari P., Blagitz M.G., Della Libera A.M.M.P., Batista C.F., Souza F.N. Intracellular reactive oxygen species production and phagocytosis of Staphylococcus aureus by milk neutrophils as tool to diagnose mastitis and identify susceptible dairy cows. Journal of NeuroInterventional Surgery. 2018;10:480–484. doi: 10.1590/1678-5150-pvb-4704. [DOI] [Google Scholar]
- Neužil J., Stocker R. Bilirubin attenuates radical-mediated damage to serum albumin. FEBS Letters. 1993;331:281–284. doi: 10.1016/0014-5793(93)80353-v. 10.1016/0014-5793(93)80353-V. [DOI] [PubMed] [Google Scholar]
- Oliveira P.V.C., Lima Neto E.S., Lucena N.M., Abrantes M.R., Silva J.B.A., Azevedo Neto C.O., Medeiros D.A.S. Avaliação da qualidade do leite cru e prevalência de mastite no município de Mossoró-RN. Brazilian Journal of Development. 2020;6:64027–64042. doi: 10.34117/bjdv6n8-728. [DOI] [Google Scholar]
- Osorio J.S., Trevisi E., Ji P., Drackley J.K., Luchini D., Bertoni G. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. Journal of Dairy Science. 2014;97:7437–7450. doi: 10.3168/jds.2013-7679. [DOI] [PubMed] [Google Scholar]
- Rainard P., Foucras G., Boichard D.& ., Rupp R. Invited review: Low milk somatic cell count and susceptibility to mastitis. Journal of Dairy Science. 2018;101:6703–6714. doi: 10.3168/jds.2018-14593. [DOI] [PubMed] [Google Scholar]
- Repetto, M., Semprine, J., & Boveris, A. (2012). Lipid Peroxidation: Chemical Mechanism, Biological implications and analytical determination, In: Lipid Peroxidation, Angel Catala, IntechOpen (Chapter 1).
- Roche M., Rondeau P., Singh N.R., Tarnus E., Bourdon E. The antioxidant properties of serum albumin. FEBS Letters. 2008;582:1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- Milkpoint. (2006). O uso da CCS em diferentes países. Retrieved from https://www.milkpoint.com.br/colunas/marco-veiga-dos-santos/o-uso-da-ccs-em-diferentes-paises-parte-1-32730n.aspx. Acessed January 15, 2020.
- Schneider C.D., Oliveira A. Radicais livres de O2 e exercício: Mecanismos de formação e adaptação ao treino. Revista Brasileira de Medicina do Esporte. 2004;10:308–316. doi: 10.1590/S1517-86922004000400008. [DOI] [Google Scholar]
- Sharifi S., Pakdel A., Ebrahimi M., Reecy J.M., Farsani S.F., Ebrahimie E. Integration of machine learning and meta-analysisidentifies the transcriptomic bio-signature of mastitis disease in cattle. PloS one. 2018;13 doi: 10.1371/journal.pone.0191227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma L., Verma A.K., Rahal A., Kumar A., Nigam R. Relationship Between Serum Biomarkers and Oxidative Stress in Dairy Cattle and Buffaloes with Clinical and Sub-clinical Mastitis. Biotechnology (Reading, Mass.) 2016;15:96–100. doi: 10.3923/biotech.2016.96.100. [DOI] [Google Scholar]
- Shook, G.E. (.1982,. January). Approaches to summarizing somatic cell count which improve interpretability. Poster session presentation at the National Mastitis Council21, Washington.
- Singhal N., Kumar M., Kanaujia P.K., Virdi J.S. MALDI-TOF mass spectrometry:ANemerging technology for microbial identification and diagnosis. Frontiers in Microbiology. 2015;6:791–807. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo L.M. Mammary Gland Immunobiology and Resistance to Mastitis. Veterinary Clinics of North America: Food Animal Practice. 2018;34:507–523. doi: 10.1016/j.cvfa.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Turk R. The role of oxidative stress and inflammatory response in the pathogenesis of mastitis in dairy cows. Mljekarstvo. 2017;67:91–101. doi: 10.15567/mljekarstvo.2017.0201. [DOI] [Google Scholar]
- Venturini, T., Api, I., Restelato, R., Paixão, S.J., .Ziech, M.F., .& Montagner, M.M. (.2009). Ocorrência de mastite sub-clínica em vacas das raças holandês e jersey. Poster session presentation at the anais seminário: Sistemas de produção agropecuária, Curitiba.
- Viguier C., Arora S., Gilmartin N., Welbeck K., O'Kennedy R. Mastitis detection: Current trends and future perspectives. Trends in Biotechnology. 2009;27:486–493. doi: 10.1016/j.tibtech.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Yang F.L., Li X.S., He B.X., Yang X.L., Li G.H., Liu P. Malondialdehyde level and some enzymatic activities in subclinical mastitis milk. African Journal of Biotechnology. 2011;10:5534–5538. doi: 10.5455/javar.2015.b48. [DOI] [Google Scholar]
- Zigo F., Elecko J., Vasil M., Ondrasovicova S., Farkasova Z., Malova J. The occurrence of mastitis and its effect on the milk malondialdehyde concentrations and blood enzymatic antioxidants in dairy cows. Veterinarni Medicina. 2019;64:423–432. doi: 10.17221/67/2019-VETMED. [DOI] [Google Scholar]