Highlight
-
•
Neoadjuvant chemoradiation (NCRT) followed by surgery was regarded as the standard treatment of locally advanced esophageal cancer. However, studies have reported that almost 31%−50% of esophageal cancer patients still have local recurrence and or distant metastasis after NCRT and surgery. At present, there are few reports on the risk stratification of patients with esophageal carcinoma after NCRT and surgery. The valuable effect of adjuvant chemotherapy in esophageal squamous cell carcinoma (ESCC) patients underwent NCRT followed by surgery remains controversial. There is also no consensus that whether patients need adjuvant chemotherapy. Reasonable risk stratification is therefore required that helps postoperatively surveillance and classify patients suitable for adjuvant chemotherapy. There remains, however, no reliable forecasting system for ESCC patients after NCRT and surgery. Based on the current status, we carry out a noval risk stratification to predict survival, recurrence and classify patients at high risk that may benefit from adjuvant therapy according to clinicophological factors
Keywords: Neoadjuvant chemoradiation, Esophageal squamous cell carcinoma, Risk stratification, Recurrence, Adujvant treatment
Abbreviations: ESCC, esophageal squamous cell carcinoma; NCRT, neoadjuvant chemoradiation; OS, overall survival; DFS, disease-free survival; pCR, pathological complete respond; PNI, perineural invasion; LVI, lymph vessel invasion; KPS, Karnofsky Performance Status; GTV, gross tumor volume; CTV, clinical target volume; PTV, planning target volume; 2DRT, two-dimensional conformal radiation therapy; 3DRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; LN, lymph node; HR, hazard ratio; CI, confidence interval
Abstract
Objective
Nowadays, there were few studies reporting the risk stratification of patients with esophageal squamous cell carcinoma (ESCC) after neoadjuvant chemoradiation (NCRT) and surgery. We aimed to establish a simple risk stratification to help postoperative detection and adjuvant treatment.
Methods
We included 146 patients with locally advanced ESCC who received NCRT followed by esophagectomy. The impacts of clinicopathological factors on overall survival (OS) and disease-free survival (DFS) were analyzed. The recurrence site, time, and frequency were recorded as well.
Results
The median follow-up was 53 months. The pathological complete respond (pCR) group demonstrated better 5-year OS and DFS (78.6% and 77.0%) than the non-pCR group (44.8% and 35.2%, all P < 0.005). Multivariate analysis for the non-pCR group revealed perineural invasion (PNI) (HR:2.296, P = 0.013) and ypTNM stage (I/II vs III/IV) (HR:1.972, P = 0.046) were considered as independent unfavorable factors affecting OS, while PNI (HR:1.866, P = 0.045) and lymph vessel invasion (LVI) (HR:3.370, P < 0.001) were considered as independent adverse factors for DFS. Based on clinicopathological factors (including pCR, ypTNM stage, PNI, LVI), patients were divided into the low-risk (pCR), mediate-risk (non-pCR without PNI, LVI, stage III/IV), high-risk (non-pCR with one factor of PNI, LVI or stage III/IV (n = 45)), highest risk (non-pCR with two or more factors of PNI, LVI or stage III/IV) groups. The corresponding 5-year OS rates were 78.6%, 60.4%, 49.6%, 18.6%, respectively (P < 0.005) and 5-year DFS rates were 77.0%, 46.9%, 41.1%, 12.1%, respectively (P < 0.005). Adjuvant chemotherapy may improve survival in high or highest risk groups of patients with low prognostic nutritional index (< 49).
Conclusions
A novel risk stratification based on clinicopathological factors may be conducive to postoperative surveillance and guide adjuvant chemotherapy.
Introduction
Esophageal cancer was the sixth leading cause of cancer death in the world. It is reported that more than 572,000 are newly diagnosed per year, causing more than 508,000 deaths worldwide [1]. Surgery remains the primary treatment modality for early esophageal cancer with a 5 year survival rate of up to 80%. However, for locally advanced esophagus, the 5-year survival rate of surgery alone was less than 30% [1,2].
A well-known prospective randomized controlled, multi-center study, CROSS, comparing neoadjuvant chemoradiotherapy with surgery alone, which included 366 patients with locally advanced esophageal cancer, showed a median survival time of 49.4 months in the neoadjuvant group, which was considerably higher than the 24.0 months in the surgery group alone (P = 0.003) [3]. Additionally, the NEOCRTEC5010 trial also showed that the combinations of NCRT and surgery may improve survival rates in locally advanced ESCC compared with those of surgery alone [4].
After the publication of the CROSS trial, NCRT followed by surgery is regarded as the standard treatment for locally advanced esophageal cancer [3]. Compared with surgery alone, neoadjuvant chemoradiation and surgical resection can not only significantly increase the tumor local resection rate and reduce distant metastasis, but also improve the long-term survival in locally advanced esophageal cancer [3,5]. However, some studies have reported that almost 31%−50% of esophageal cancer patients still have local recurrence and/or distant metastasis after NCRT and surgery [6,7]. It remained unclear whether adjuvant therapy after neoadjuvant chemoradiotherapy could improve patients’ prognosis by reducing cancer recurrence.
At present, there are few reports on the risk stratification of patients with esophageal carcinoma after NCRT and surgery [8,9]. Reasonable risk stratification is therefore required that helps postoperatively surveillance and classify patients suitable for adjuvant chemotherapy. There remains, however, no reliable forecasting system for ESCC patients after NCRT and surgery. Based on the current status, we aim to carry out risk stratification to predict survival, recurrence and classify patients at high-risk that may benefit from adjuvant therapy according to clinicophological factors.
Materials and methods
Patients selection
Patient with esophageal cancer who underwent preoperative NCRT was retrospectively analyzed from January 2009 to December 2019 at Fujian Provincial Cancer Hospital. The inclusive criteria were as follows: (1) histologically confirmed locally advanced squamous cell carcinoma of the thoracic esophagus; (2) neither history of malignancy nor second primary tumor; (3) complete resection; (4) age 18–70 years; (5) KPS ≥70; (6) absence of severe organic disease. All patients were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system.The current study was approved by the ethics committee of Fujian Medical University Cancer Hospital, Fuzhou, China (YKT2020–017–01).
Treatment
The median dose of neoadjuvant radiotherapy was 40 (36 to 50.4) Gy, 1.8 to 20 Gy per fraction, 5 days per week. 70 patients received intensity-modulated radiotherapy (IMRT) and 8 received three-dimensional conformal radiotherapy (3DRT). The patient was placed in the supine position, fixed with a vacuum bag or styrofoam, and simulated by CT scan for positioning. The gross tumor volume (GTV) was determined by the barium swallow, contrast-enhanced CT, MRI or PET-CT, which included both the primary tumor and the metastatic lymph nodes. The clinical target volume (CTV) included subclinical lesions (GTV extending 0.5–1.0 cm axially and 3 cm longitudinally) and the corresponding mediastinal lymphatic drainage area. The planning target volume (PTV) was defined as the CTV plus 0.5 cm in all directions for CTV considering organ movement and plaque errors. 68 received two-dimensional conventional radiotherapy (2DRT) which used anterior and posterior opposing techniques. The length of the irradiated field included the tumor and a proximal and distal margin of 3 cm and a 0.5–1.0 cm radial margin around tumor. For upper thoracic, double supraclavicular lymphatic drainage areas were included. The chemotherapy regimen for all patients was platinum-based in combination with two chemotherapeutic agents [1]. Paclitaxel 135 mg/m2 D1 or docetaxel 75 mg/m2 D1 + cisplatin or nedaplatin 75 mg/m2 D2 [2] 5-fluorouracil (5-FU) 700–1000 mg/m2 D1–2 + cisplatin 75 mg/m2 D2 were administered every 3 weeks. Surgery was conducted 4 to 8 weeks after neoadjuvant chemoradiation. The surgical approach was consisted of an esophagectomy with three field lymph node dissection.
Follow-up
All patients were followed up until death or the last follow-up. The median follow-up period was 53 months (range 2–154 months). Follow-up inspections were conducted regularly every 3 months in the first year, every 6 months for the next 2 years, and once a year later. Routine review items included physical examination, blood routine, biochemistry, tumor markers, chest CT and esophageal barium.
Endpoint definition
The endpoints of this study included overall survival (OS) and disease-free survival (DFS). The OS referred to the calculation of the time from the end of the operation to death from any cause or the last follow-up. The DFS referred to the time from the end of surgery to the first recurrence or death of the disease. Recurrences included local recurrence and distant recurrence. Local recurrence was defined as the recurrence of the primary tumor site or locoregional lymph nodes. Recurrence of lymph nodes in the abdominal trunk or supraclavicular area was considered to regional lymph node recurrence, which were also belonged to local recurrence. Distant recurrences were defined as non-regional lymph node recurrence or systemic metastasis.
Statistical analysis
All statistical calculations were analyzed using SPSS (version 25.0, IBM, Armonk, NY, USA). When comparing categorical data, Chi-square or Fisher's exact test is used. The Mann-Whitney U test was used when comparing continuous variables. The OS and DFS were calculated using the Kaplan-Meier method, and then the difference was compared by log-rank test. In univariate analysis, all factors with P value <0.10 were entered into multivariate cox regression analysis to determine independent prognostic factors. All statistical analyses were two-sided analysis, and significance is defined as P < 0.05.
Results
Patient characteristics
Patients’ characteristics were shown in Table 1. A total of 146 patients with ESCC who underwent surgery after NCRT were analyzed. The included patients were mainly male (128 [87.7%]), and the median age was 57 years (range, 38–70 years). The locations of the primary tumor were 40/85/21 in the upper/middle/lower thorax. Most patients had cT3 (65.8%) or cN1 (47.9%) disease. According to the AJCC-8th, 19 (13.0%), 79 (54.1%), and 48 (32.9%) patients had diseases of stage II, III, and IV, respectively. The median number of total removed lymph nodes was 25 (range: 3–75). A total of 87 (59.6%) patients had no lymph node metastases (ypN0), while 69 (41.4%) patients had positive nodes (ypN1–N3). There were 28 (19.2%) patients with LVI and 20 (13.7%) patients with PNI. Besides, there were 64 (43.8%) patients received adjuvant chemotherapy.
Table 1.
Patients’ characteristics of 146 ESCC patients and patients’ clinicopathological characteristics according to pCR.
| Characteristic | Total (n = 146),% | pCR (n = 42),% | Non-pCR (n = 104),% | P |
|---|---|---|---|---|
| Age (yrs) | 0.992 | |||
| ≤55 | 59(40.4) | 17(40.5) | 42(40.4) | |
| >55 | 87(59.6) | 25(59.5) | 62(59.6) | |
| Sex | 0.039 | |||
| Male | 128(87.7) | 33(78.6) | 95(91.3) | |
| Female | 18(12.3) | 9(21.4) | 9(8.7) | |
| KPS | 0.717 | |||
| Median | 80(70–90) | 80(70–90) | 80(70–90) | |
| Smoking | 0.231 | |||
| Yes | 74(50.7) | 18(42.9) | 56(53.8) | |
| No | 72(49.3) | 24(57.1) | 48(46.2) | |
| Prognostic nutrition index | 0.404 | |||
| <49 | 74(50.7) | 19(45.2) | 55(52.9) | |
| ≥49 | 72(49.3) | 23(54.8) | 49(47.1) | |
| Tumor location | 0.189 | |||
| Upper | 40(27.4) | 16(38.1) | 24(23.1) | |
| Middle | 85(58.2) | 21(50.0) | 64(61.5) | |
| Distal | 21(14.4) | 5(11.9) | 16(15.4) | |
| Primary tumor length | 0.837 | |||
| <7cm | 68(46.6) | 19(45.2) | 49(47.1) | |
| ≥7cm | 78(53.4) | 23(54.8) | 55(52.9) | |
| Clinical T stage | 0.217 | |||
| T2 | 3(2.1) | 1(2.4) | 2(1.9) | |
| T3 | 96(65.8) | 32(76.2) | 64(61.5) | |
| T4 | 47(32.2) | 9(21.4) | 38(36.5) | |
| Clinical N stage | 0.859 | |||
| N0 | 27(18.5) | 9(21.4) | 18(17.3) | |
| N1 | 70(47.9) | 21(50.0) | 49(47.1) | |
| N2 | 45(30.8) | 11(26.2) | 34(32.7) | |
| N3 | 4(2.7) | 1(2.4) | 3(2.9) | |
| Clinical TNM stage | 0.208 | |||
| II | 19(13.0) | 8(19.0) | 11(10.6) | |
| III | 79(54.1) | 24(57.1) | 55(52.9) | |
| IV | 48(32.9) | 10(23.8) | 38(36.5) | |
| Radiation dose (Gy) | 0.030 | |||
| <40 | 35(24.0) | 5(11.9) | 30(28.8) | |
| ≥40 | 111(76.0) | 37(88.1) | 74(71.2) | |
| Radiotherapy modality | 0.497 | |||
| 2DRT | 68(46.6) | 22(52.4) | 46(44.2) | |
| 3DRT | 8(5.5) | 3(7.1) | 5(4.8) | |
| IMRT | 70(47.9) | 17(40.5) | 53(51.0) | |
| Chemotherapy cycle | 0.552 | |||
| 1 | 47(32.2) | 12(28.6) | 35(33.7) | |
| ≥2 | 99(67.8) | 30(71.4) | 69(66.3) | |
| yp T stage | – | |||
| T0 | 50(34.2) | 42(100) | 8(7.7) | |
| T1 | 12(8.2) | 0 | 12(11.5) | |
| T2 | 24(16.4) | 0 | 24(23.1) | |
| T3 | 47(32.2) | 0 | 47(45.2) | |
| T4 | 13(8.9) | 0 | 13(12.5) | |
| yp N stage | – | |||
| N0 | 87(59.6) | 42(100) | 45(43.3) | |
| N1 | 36(24.7) | 0 | 36(24.7) | |
| N2 | 14(9.6) | 0 | 14(13.5) | |
| N3 | 9(6.2) | 0 | 9(8.7) | |
| yp TNM stage | – | |||
| I | 61(41.8) | 42(100) | 19(18.3) | |
| II | 20(13.7) | 0 | 20(19.2) | |
| III | 48(32.9) | 0 | 48(46.2) | |
| IV | 17(11.6) | 0 | 17(16.3) | |
| Number of LN examined | 0.895 | |||
| Median | 25(3–75) | 24(6–48) | 25(3–75) | |
| Lymph vessel invasion | <0.001 | |||
| Yes | 28(19.2) | 1(2.4) | 27(26.0) | |
| No | 118(80.8) | 41(97.6) | 77(74.0) | |
| Perineural invasion | 0.001 | |||
| Yes | 20(13.7) | 0 | 20(19.2) | |
| No | 126(86.3) | 42(100) | 84(80.8) | |
| Adjvant chemotherapy | 0.003 | |||
| Yes | 64(43.8) | 10(23.8) | 54(51.9) | |
| No | 82(56.2) | 32(76.2) | 50(48.1) |
KPS, Karnofsky performance status; 2DRT, two-dimensional conformal radiation therapy; 3DRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; LN, lymph node.
Pathological complete response and survival
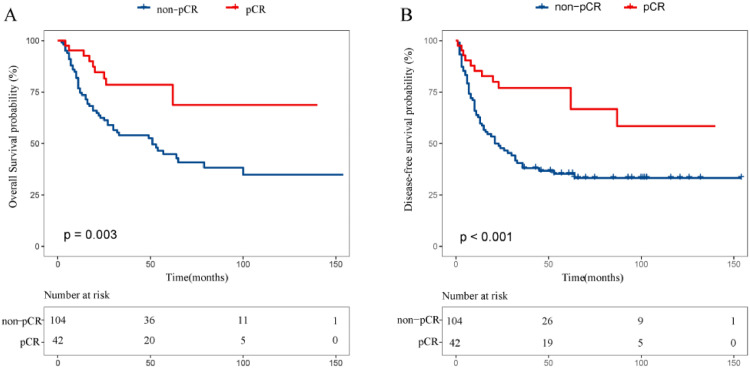
As shown in Table 1, of 146 patients, 42 had obtained pathological complete response (pCR)(n = 42) and the rests were non-pCR (n = 104). The pCR is related to radiation dose, gender and LVI. Patients with the radiation dose greater than 40 Gy had a higher pCR rate (P = 0.03), and female patients were more likely to achieve pCR (P = 0.039). In addition, in the non-pCR group, there were more patients with LVI (P < 0.001) and PNI (P = 0.001). As shown in Fig. 1, there was a significant difference in overall survival (OS) and disease-free survival (DFS) between pCR and non-pCR. The 5-year OS and DFS rates were 78.6% and 77.0% in the pCR in comparison to 44.8% and 35.2% in the non-pCR (P < 0.005 for all; Fig. 1).
Fig. 1.
Comparison of overall (A), disease-free survival (B) between pCR group and non-pCR.
Univariate and multivariate survival analysis in non-pCR group
Factors that affected OS and DFS among non-pCR patients were assessed by univariate and multivariate analyses. In univariate analysis, ypTNM stage (P = 0.021), LVI (P = 0.006), and PNI (P = 0.009) were significantly associated with overall survival (OS) (Table 2) and disease-free survival (DFS) (Table 3). In multivariate analysis, PNI (HR:2.296, P = 0.013) and ypTNM stage (I/II vs III/IV) (HR:1.972, P = 0.046) were considered as independent unfavorable prognostic factors affecting OS, while PNI (HR:1.866, P = 0.045) and LVI (HR:3.370, p < 0.001) were considered as independent adverse prognostic factors for DFS.
Table 2.
Predictors of Overall Survival in ESCC patients with Non-pCR to neoadjuvant chemoradiotherapy.
| Clinicopathologic parameters | Untivariate Analysis | Mutivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age (yrs) | ||||
| ≤55 VS >55 | 0.881(0.513–1.151) | 0.647 | ||
| Sex | ||||
| Female VS Male | 1.596(0.497–5.123) | 0.432 | ||
| Smoking | ||||
| No VS Yes | 1.156(0.666–2.009) | 0.606 | ||
| Prognostic nutritional index | ||||
| <49 VS ≥49 | 1.318(0.768–2.261) | 0.317 | ||
| Tumor location | ||||
| Upper VS Middle | 1.477(0.729–2.991) | 0.279 | ||
| Upper VS Distal | 2.023(0.821–4.986) | 0.126 | ||
| Primary tumor length | ||||
| <7 cm VS ≥7cm | 1.031(0.601–1.768) | 0.911 | ||
| Clinical T stage | ||||
| T2 VS T3 | 0.409(0.055–3.038) | 0.382 | ||
| T2 VS T4 | 0.433(0.057–3.284) | 0.418 | ||
| Clinical N stage | ||||
| N0 VS N+ | 1.519(0.683–3.379) | 0.306 | ||
| Clinical TNM stage | ||||
| II VS III | 1.046(0.406–2.699) | 0.925 | ||
| II VS IV | 1.075(0.398–2.903) | 0.887 | ||
| yp T stage | ||||
| T0–2 VS T3–4 | 1.586(0.912–2.759) | 0.102 | ||
| yp N stage | ||||
| N0 VS N+ | 1.400(0.803–2.441) | 0.236 | ||
| yp TNM stage | ||||
| I/II VS III/VI | 2.203(1.111–3.683) | 0.021 | 1.872(1.012–3.463) | 0.046 |
| Radiation dose (Gy) | ||||
| <40 VS ≥40 | 0.919(0.510–1.656) | 0.779 | ||
| Radiotherapy modality | ||||
| 2DTR VS 3DRT/IMRT | 1.377(0.792–2.394) | 0.256 | ||
| Chemotherapy cycle | ||||
| 1 VS ≥2 | 0.775(0.446–1.347) | 0.366 | ||
| Number of LN examined | ||||
| <25 VS ≥25 | 0.994(0.578–1.708) | 0.981 | ||
| Lymph vessel invasion | ||||
| No VS Yes | 2.279(1.260–4.122) | 0.006 | 1.821(0.985–3.368) | 0.056 |
| Perineural invasion | ||||
| No VS Yes | 2.362(1.245–4.481) | 0.009 | 2.296(1.195–4.412) | 0.013 |
| Adjuvant chemotherapy | ||||
| No VS Yes | 1.086(0.633–1.863) | 0.765 | ||
HR, hazard ratio; CI, confidence interval; 2DRT, two-dimensional conformal radiation therapy; 3DRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; LN, lymph node.
Table 3.
Predictors of disease-free survival in ESCC patients with Non-pCR to neoadjuvant chemoradiotherapy.
| Clinicopathologic Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (y) | ||||
| ≤55 VS >55 | 0.945(0.570–1.566) | 0.826 | ||
| Sex | ||||
| Female VS Male | 1.611(0.584–4.441) | 0.357 | ||
| Smoking | ||||
| No VS Yes | 1.000(0.605–1.653) | 1.000 | ||
| Prognostic nutritional index | ||||
| <49 VS ≥49 | 1.179(0.716–1.940) | 0.518 | ||
| Tumor location | ||||
| Upper VS Middle | 1.172(0.633–2.170) | 0.613 | ||
| Upper VS Distal | 1.769(0.801–3.904) | 0.158 | ||
| Primary tumor length | ||||
| <7 cm VS ≥7cm | 1.087(0.660–1.790) | 0.743 | ||
| Clinical T stage | ||||
| T2 VS T3 | 0.427(0.057–3.187) | 0.427 | ||
| T2 VS T4 | 0.527(0.070–3.975) | 0.534 | ||
| Clinical N stage | ||||
| N0 VS N+ | 1.440(0.709–2.922) | 0.313 | ||
| Clinical TNM stage | ||||
| II VS III | 1.074(0.450–2.564) | 0.872 | ||
| II VS IV | 1.237(0.503–3.039) | 0.643 | ||
| yp T stage | ||||
| T0–2 VS T3–4 | 1.516(0.908–2.531) | 0.112 | ||
| yp T stage | ||||
| N0 VS N+ | 1.230(0.742–2.041) | 0.422 | ||
| yp TNM stage | ||||
| I/II VS III/VI | 1.607(0.946–2.727) | 0.079 | 1.328(0.803–2.197) | 2.269 |
| Radiation dose (Gy) | ||||
| <40 VS ≥40 | 0.834(0.5489–1.421) | 0.504 | ||
| Radiotherapy modality | ||||
| 2DTR VS 3DRT/IMRT | 1.377(0.792–2.394) | 0.256 | ||
| Chemotherapy cycle | ||||
| 1 VS ≥2 | 0.977(0.580–1.645) | 0.929 | ||
| Number of LN examined | ||||
| <25 VS ≥25 | 0.829(0.504–1.364) | 0.460 | ||
| Lymph vessel invasion | ||||
| No VS Yes | 3.312(1.909–5.746) | <0.001 | 3.370(1.916–5.927) | <0.001 |
| Perineural invasion | ||||
| No VS Yes | 2.011(1.103–3.665) | 0.023 | 1.866(1.015–3.433) | 0.045 |
| Adjuvant chemotherapy | ||||
| No VS Yes | 0.945(0.574–1.557) | 0.825 | ||
Prognostic factors for risk stratification
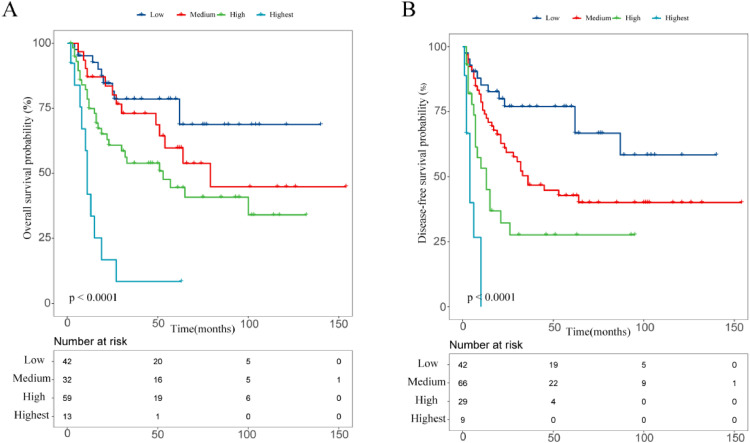
From Fig. 1, pCR was found to be a significant favorable prognostic factor for both OS and DFS. 5-year OS and DFS rates in the pCR were higher than in the non-pCR (78.6% vs 44.8%, 77.0% vs 35.2%, P < 0.005 for all). In addition, PNI and ypTNM stage were considered as independent prognostic factors affecting OS, while PNI and LVI were considered as independent prognostic factors for DFS. Therefore, non-pCR, ypTNM stage III/IV, PNI and LVI were considered as significant adverse prognostic factors for developing risk stratification. Based on these risk factors, we then classified patients into 4 categories: low-risk group: pCR (n = 42); medium-risk group: non-pCR without PNI, LVI, stage III/IV (n = 29); high-risk group: non-pCR with one factor of PNI, LVI or stage III/IV (n = 45); highest-risk group: non-pCR with two or more factors of PNI, LVI or stage III/IV (n = 30), with corresponding 5-year OS rates of 78.6%, 60.4%, 49.6%, 18.6%, respectively (P < 0.005; Fig. 2) and 5-year DFS rates of 77.0%, 46.9%, 41.1%, 12.1%, respectively (P < 0.005; Fig. 2).
Fig. 2.
Comparison of overall (A), disease-free survival (B) in different risk categories.
Recurrence site in different risk groups
The recurrence sites in various risk categories were summarized in Table 4. For the high or highest risk groups, tumor recurrence emerged in 42.7% (32/75) of patients, including 27 (36%) loco-regional recurrences and 20 (26.7%) distant recurrences. For the low or medium risk groups, tumor recurrence decreased dramatically with 13 (18.3%) loco-regional recurrences and 15 (21.1) distant recurrences. The most common sites of regional recurrence were regional lymph node recurrence, including cervical, mediastinal and abdominal lymph nodes and the most frequent sites of distant metastases were lung, liver, and bone. The high or highest risk groups had significantly higher regional lymph node recurrence rates compared to the low or medium risk groups (P = 0.003). Mediastinal lymph node relapses occurred in 20.0% versus 5.6% (P = 0.015), and abdominal lymph node relapses occurred in 13.3% versus 2.8% (P = 0.035) respectively, between the high or highest risk and low or medium risk groups. The distant rates of recurrence were comparable among the groups at high or highest risk (26.7%) and the groups with low or medium risk (21.1%, P = 0.434).
Table 4.
Recurrence site in different risk categories.
| Recurrence Site | low and medium | high and highest | HR (95% CI) | P |
|---|---|---|---|---|
| (n = 71),% | (n = 75),% | |||
| Loco-regional or distant recurrence | 20(28.2) | 32(42.7) | 1.898(0.951–3.785) | 0.069 |
| Loco-regional recurrence | 13(18.3) | 27(36.0) | 2.510(1.169–5.389) | 0.018 |
| Anastomosis | 4(5.6) | 3(4.0) | 0.698(0.151–3.234) | 0.646 |
| Regional lymph node | 9(12.7) | 26(34.7) | 3.655(1.569–8.514) | 0.003 |
| Cervical | 5(7.0) | 12(16.0) | 2.514(0.838–7.545) | 0.100 |
| Mediastinum | 4(5.6) | 15(20.0) | 4.187(1.317–13.313) | 0.015 |
| Abdominal | 2(2.8) | 10(13.3) | 5.308(1.120–25.140) | 0.035 |
| Distant recurrence | 15(21.1) | 20(26.7) | 1.358(0.631–2.920) | 0.434 |
| Liver | 5(7.0) | 5(6.7) | 0.943(0.261–3.406) | 0.943 |
| Lung | 8(11.3) | 10(13.3) | 1.212(0.449–3.268) | 0.705 |
| Bone | 6(8.5) | 9(12.0) | 1.477(0.498–4.386) | 0.482 |
| Others | 2(2.8) | 3(4.0) | 1.437(0.233–8.867) | 0.696 |
Recurrence time and frequency in different risk categories
In the current research, 36% (52/146) of patients had tumor recurrence. The medians for recurrence in different risk groups were 8 months (range, 1–23 mon) for the low-risk group, 23 months (range, 3–45 mon) for the medium-risk group, 9 months (range, 2–36 mon) for the high-risk group and 5 months (range, 1–21 mo) for the highest-risk group for patients who developed recurrences. In high or highest risk groups, there was a trend in the recurrence period of patients earlier than in low- or medium-risk groups. As shown in Table 5, for the low-risk group only 9 patients (21.4%) experienced recurrences, and most of the recurrences occurred within 24 months of operation. For the medium-risk group, 11 patients (38.0%) developed recurrences, 64% of recurrences occurred 12–36 months following surgery. Recurrences were commonly reported in high- and highest-risk groups, 16 (36%) were high-risk patients and 16 (53%) were at the highest risks, with a majority of recurrences in both groups occurring for 12 months after surgery, in particular 3 months after surgery.
Table 5.
Recurrence time in different risk categories.
| Recurrence Time | Low risk (n = 42),% | Medium risk (n = 29),% | High risk (n = 45),% | Highest risk (n = 30),% |
|---|---|---|---|---|
| 0–3 months | 2(4.8) | 2(6.9) | 4(8.9) | 6(20) |
| 3–6 months | 2(4.8) | 0(0) | 2(4.4) | 3(10) |
| 6–12 months | 2(4.8) | 1(3.4) | 4(8.9) | 4(13.3) |
| 12–24 months | 3(7.1) | 3(10.3) | 3(6.7) | 3(10) |
| 24–36 months | 0(0) | 4(13.8) | 3(6.7) | 0(0) |
| 36–60 months | 0(0) | 1(3.4) | 0(0) | 0(0) |
Adjuvant chemotherapy
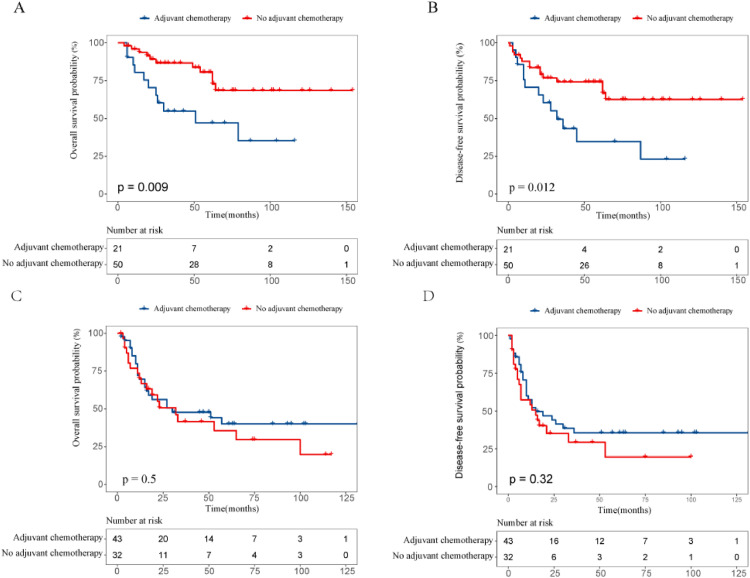
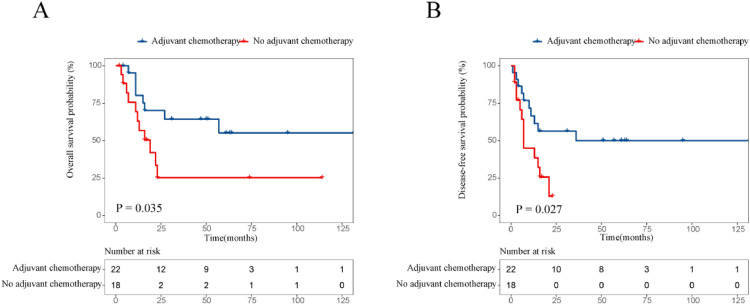
The survival effect of adjuvant chemotherapy in various risk groups of ESCC patients receiving NCRT followed by esophagectomy has also been measured. 21 patients were treated for adjuvant chemotherapy for low- or medium-risk groups, while 50 were observed. No better OS (P = 0.009; Fig. 3A) and DFS (P = 0.012; Fig. 3B) than those without treatment were seen in patients who had adjuvant chemotherapy. In the high or highest risk groups, 43 received adjuvant chemotherapy had better OS and DFS compared with 32 did not receive, but no substantial difference was observed. The 5-year OS and DFS rates were 40.1% and 35.6% in patients receiving adjuvant chemotherapy and 35.6 and 19.6% in those receiving observation only (P = 0.5, P = 0.32, respectively; Fig. 3C, Fig. 3D). In the subgroup analysis, we found that adjuvant chemotherapy related to increase survivals with the 3-year OS and DFS rates, 64.3% and 50% respectively, compared to 25.2% and 12.8% for without adjuvant chemotherapy when patients’ prognostic nutritional index was less than 49 (P = 0.035 for OS, and P = 0.027 for DFS; Fig. 4A, Fig. 4B).
Fig. 3.
Kaplan–Meier overall and disease-free survival curves for ESCC patients after NCRT and surgery with or without adjuvant chemotherapy for low- and medidum-risk groups (A, B). Kaplan–Meier overall and disease-free survival curves for ESCC patients after NCRT and surgery with or without adjuvant chemotherapy for high- and highest-risk groups (C, D).
Fig. 4.
Comparison of overall (A), disease-free survival (B) for patient after NCRT and surgery with low prognostic nutritional index (<49) in high- or highest-risk groups.
Discussion
In our research, the prognostic importance of clinicopathological factors for patients with ESCC was evaluated retrospectively. Compared to non-pCR patients, pCR patients had significantly better OS and DFS. Furthermore, multivariate Cox analysis showed that in the case of non-pCR the PNI and ypTNM stage were regarded as independent prognostic factors that affect the OS, PNI and LVI as independent forecast factors for DFS. Therefore, the risk stratification was established to focuse on clinicopathological variables, including pCR, ypTNM, PNI and LVI.
The pCR was defined as no histologic evidence of tumor in the surgical specimen, which was a favorable prognostic factor in terms of improving survival and reducing recurrence rate [4,6,10]. Donahue et al. included 162 patients in the study demonstrated that patients with pCR have significantly better long-term survival with 5-year survival for overall in complete, near complete, and partial response patients 34%, 55%, and 27%, respectively (P<0.013) [11]. Similarly, Patients achieving pCR had 5-year overall survival of 52% compared with 38% in partial pathological respond and 19% in non-respond (NR) (P < 0.001)(Meredith et al.,2010) [12]. Our research showed that 5-year OS and DFS rates of pCR patients were 78.6% and 77.0%,respectively. Hence, patients with pCR could be classified as a low-risk group.
The PNI was the process of neoplastic invasion of the nerves in a variety of tumors, which was also an important factor influencing the pathological characteristics and prognosis of malignant tumors, presenting a low survival rate and poor prognosis [13]. Several studies have evaluated the significance of PNI in esophageal cancer. For example, Lagarde et al. including 396 patients with esophageal cancer who underwent esophagectomy after neoadjuvant therapy, concluded that the presence of PNI has an adverse impact for patients with lower survival time [14]. Cheng-Che Tu et al. also demonstrated PNI (HR: 2.226, P = 0.019) as unfavorable prognostic factors affecting overall survival [15]. In our study, we demonstrated that PNI was considered as an independent prognostic factor in esophageal squamous cell carcinoma for both OS and DFS.
The LVI was generally considered an indicator of adverse prognosis which was defined as the presence of tumor cells within arterial, venous, or lymphatic vessels [16,17]. Previous studies had shown that LVI predicts poor outcomes in patients with primarily resected esophageal cancer [18,19]. For example, Lagarde and Brucher et al. proposed that LVI was an indicator of adverse prognosis [14,18]. Furthermore, Chen et al. reported that the presence of LVI was related to lymph node metastasis. They also concluded LVI was independently associated with shorter OS in ESCC patients receiving NCRT. The findings of our research were similar to this result [16].
Based on clinicopathological factors (including pCR, ypTNM stage, PNI, LVI), patients were divided into the low-risk (pCR), medium-risk (non-pCR without PNI, LVI, stage III/IV), high-risk (non-pCR with one factor of PNI, LVI or stage III/IV (n = 45)), highest risk (non-pCR with two or more factors of PNI, LVI or stage III/IV) groups. The corresponding 5-year OS rates were 78.6%, 60.4%, 49.6%, 18.6%, respectively (P < 0.005) and 5-year DFS rates were 77.0%, 46.9%, 41.1%, 12.1%, respectively (P < 0.005). Furthermore, we analyzed the recurrence patterns according to risk stratification. The high or highest risk groups had considerably higher incidence of regional lymph recurrence rates compared to the low or medium-risk groups (P = 0.018). Nevertheless, the distant recurrence rate was nevertheless similar between these groups (P = 0.434). The most common sites of loco-regional recurrence were regional lymph node recurrence and the most frequent sites for distant metastases were lung, liver, and bone. Therefore, a strict surveillance strategy was required to focus on common recurrence sites. Moreover, we had to pay attention to potential distant metastasis regardless of low- or high-risk groups through inspections.
Our study also found that the regularity of recurrence time in different risk groups. At high-or highest risk patients, due to the higher risk of recurrence and earlier incidence, most of them occur within 2 years after surgery, especially within 3 months after surgery. Therefore, we recommend that patients with high-and highest risk groups should be closely followed up for 2 years, especially pay attention to closing follow-up within 3 months after surgery. For low- and medium-risk patients, the recurrence risk time was primarily concentrated after 1-year post-surgery, and hence it was necessary to strengthen close follow-up after 1-year post-surgery.
The valuable effect of adjuvant chemotherapy in ESCC patients underwent NCRT followed by surgery remains controversial. There was also no consensus that whether patients need adjuvant chemotherapy. Only a few studies had assessed the role of adjuvant therapy in trimodal treatment. Due to poorly tolerated post-operative chemotherapy or chemoradiation in patients with oesophageal cancer, only a few studies had assessed the role of adjuvant therapy in trimodal treatment. A large National Cancer Database study by Mokdad et al. examined the effects of adjuvant chemotherapy for gastroesophageal cancer, and revealed that patients treated with adjuvant chemotherapy following trimodality treatment had better OS compared with those who had not [20]. But most of the patients included in this study had adenocarcinoma. In our study, we merged clinicopathological variables to establish a predictive risk stratification for ESCC. For low- and medium-risk patients, adjuvant chemotherapy not only did not bring survival benefits to the patients, but reduces the prognosis of patients. One explanation reason was that the side effects of chemotherapy outweigh the survival benefit. For high-risk groups, adjuvant chemotherapy may improve the prognosis, however, no substantial difference was found between with and without adjuvant chemotherapy in terms of OS (P = 0.499) and DFS (P = 0.322). In the subgroup analysis of high- and highest-risk groups, we found that adjuvant chemotherapy was correlated with improved survivals with the 3-year OS and DFS rates, 64.3% and 50% respectively, compared to 25.2% and 12.8% for without adjuvant chemotherapy when patients’ prognostic nutritional index was low (<49) before NCRT (P = 0.035 for OS, and P = 0.027 for DFS).
The prognostic nutritional index (PNI) was determined by the serum albumin level (g/L) 5 × the absolute lymphocyte count. Low PNI meant hypoalbuminemia and low lymphocyte count, indicating the body's immunosuppressive state, providing favorable conditions for tumor recurrence and metastasis [21], [22], [23]. The lower PNI, the greater the possibility of recurrence and metastasis. Adjuvant chemotherapy was a vital therapy that may eliminate residual cancer cells and reduce the risk of recurrence and metastasis. In our research, we found that the prognosis of patients who had lower PNI (<49), higher risks of postoperative clinicopathological factors and no adjuvant chemotherapy was much poor, and most of theses patients died within two years after surgery. Hence, we recommend aggressive nutritional intervention and adjuvant chemotherapy for these people. However, evidence is still limited. The potential benefits of adjuvant chemotherapy in patients who received NCRT still need to be investigated in prospective studies.
This study includes a variety of limitations. It was a retrospective analysis with a small number of patients and a follow-up period, and the risk group stratification was not externally validated. Furthermore, as in any review study, it is difficult to rule out selection bias or disagreement between the criteria of the reviewers.
Conclusions
A novel risk stratification based on pCR, ypTNM, PNI, and LVI was effective for predicting survival and pattern of recurrence, that may be conducive to postoperative surveillance and guiding treatment. For those high-risk or highest-risk patients with low prognostic nutrition index, adjuvant chemotherapy may confer benefit survival. Prospective studies are required to validate clinical significance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This work was supported by the Joint Funds for the innovation of science and technology, Fujian province (Grant number: 2018Y9111).
Ethics approval and consent to participate
The current study was approved by the ethics committee of Fujian Medical University Cancer Hospital, Fuzhou, China (YKT2020–017–01). All patients provided written informed consent prior to treatment, and all the information was anonymized prior to analysis.
Authors’ contributions statement
JCL, YHW and MQL participated in the design of the study, carried out the clinical data analysis and drafted the manuscript; YHW and ZPW participated in the data collection; YHW, MQL and ZPW contribute with the clinical data analysis and involved in revising the manuscript; All authors read and approved the final manuscript.
Contributor Information
Ya-hua Wu, Email: wuyahua6@163.com.
Ming-qiang Lin, Email: mingqianglin1995@126.com.
Zhi-ping Wang, Email: 707770685@qq.com.
Jian-cheng Li, Email: jianchengli_jack@126.com.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Herskovic A., Russell W., Liptay M., Fidler M.J., Al-Sarraf M. Esophageal carcinoma advances in treatment results for locally advanced disease: review. Ann. Oncol. 2012;23(5):1095–1103. doi: 10.1093/annonc/mdr433. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P., Hulshof M.C., van Lanschot J.J., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Yang H., Liu H., Chen Y., Zhu C., Fang W., Yu Z. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J. Clin. Oncol.. 2018;36(27):2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjoquist K.M., Burmeister B.H., Smithers B.M., Zalcberg J.R., Simes R.J., Barbour A. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 6.Oppedijk V., van der Gaast A., van Lanschot J.J., van Hagen P., van Os R., van Rij C.M. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J. Clin. Oncol. 2014;32(5):385–391. doi: 10.1200/JCO.2013.51.2186. [DOI] [PubMed] [Google Scholar]
- 7.Robb W.B., Messager M., Dahan L., Mornex F., Maillard E., D'Journo X.B. Patterns of recurrence in early-stage oesophageal cancer after chemoradiotherapy and surgery compared with surgery alone. Br. J. Surg. 2016;103(1):117–125. doi: 10.1002/bjs.9959. [DOI] [PubMed] [Google Scholar]
- 8.Dixit S., Tilston M., Peter W.M. Risk stratification for recurrence in patients with esophageal and junctional carcinoma treated with neoadjuvant chemotherapy and surgery. Med. Oncol.. 2010;27(2):242–248. doi: 10.1007/s12032-009-9199-7. [DOI] [PubMed] [Google Scholar]
- 9.Xi M., Hallemeier C.L., Merrell K.W., Liao Z., Murphy M.A.B., Ho L. Recurrence risk stratification after preoperative chemoradiation of esophageal adenocarcinoma. Ann. Surg. 2018;268(2):289–295. doi: 10.1097/SLA.0000000000002352. [DOI] [PubMed] [Google Scholar]
- 10.Barbetta A., Sihag S., Nobel T., Hsu M., Tan K.S., Bains M. Patterns and risk of recurrence in patients with esophageal cancer with a pathologic complete response after chemoradiotherapy followed by surgery. J. Thorac Cardiovasc Surg. 2018 doi: 10.1016/j.jtcvs.2018.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donahue J.M., Nichols F.C., Li Z., Schomas D.A., Allen M.S., Cassivi S.D. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann. Thorac Surg.. 2009;87(2):392–398. doi: 10.1016/j.athoracsur.2008.11.001. discussion 8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meredith K.L., Weber J.M., Turaga K.K., Siegel E.M., McLoughlin J., Hoffe S. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann. Surg. Oncol. 2010;17(4):1159–1167. doi: 10.1245/s10434-009-0862-1. [DOI] [PubMed] [Google Scholar]
- 13.C S.H., Z B.Y., Z B., Z C.Z., S L.Q., F. Y.J. Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am .J. Cancer Res. 2019;91:1–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Lagarde S.M., Phillips A.W., Navidi M., Disep B., Immanuel A., Griffin S.M. The presence of lymphovascular and perineural infiltration after neoadjuvant therapy and oesophagectomy identifies patients at high risk for recurrence. Br. J. Cancer. 2015;113(10):1427–1433. doi: 10.1038/bjc.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu C.C., Hsu P.K., Chien L.I., Liu W.C., Huang C.S., Hsieh C.C. Prognostic histological factors in patients with esophageal squamous cell carcinoma after preoperative chemoradiation followed by surgery. BMC Cancer. 2017;17(1):62. doi: 10.1186/s12885-017-3063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W.H., Huang Y.L., Chao Y.K., Yeh C.J., Chang H.K., Tseng C.K. Prognostic significance of lymphovascular invasion in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Ann. Surg. Oncol. 2015;22(1):338–343. doi: 10.1245/s10434-014-3881-5. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y.M., Yang Y.S., Hu W.P., Wang W.P., Yuan Y., Chen L.Q. Prognostic value of lymphovascular invasion in patients with esophageal squamous cell carcinoma. Ann. Transl. Med. 2019;7(12):256. doi: 10.21037/atm.2019.05.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brücher B.L., Stein H.J., Werner M., Siewert J.R. Lymphatic vessel invasion is an independent prognostic factor in patients with a primary resected tumor with esophageal squamous cell carcinoma. Cancer. 2001;92:2228–2233. doi: 10.1002/1097-0142(20011015)92:8<2228::aid-cncr1567>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.von Rahden B.H., Stein H.J., Feith M., Becker K., Siewert J.R. Lymphatic vessel invasion as a prognostic factor in patients with primary resected adenocarcinomas of the esophagogastric junction. J. Clin. Oncol. 2005;23(4):874–879. doi: 10.1200/JCO.2005.12.151. [DOI] [PubMed] [Google Scholar]
- 20.Mokdad A.A., Yopp A.C., Polanco P.M., Mansour J.C., Reznik S.I., Heitjan D.F. Adjuvant chemotherapy vs postoperative observation following preoperative chemoradiotherapy and resection in gastroesophageal cancer: a propensity score-matched analysis. JAMA Oncol. 2018;4(1):31–38. doi: 10.1001/jamaoncol.2017.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okadome K., Baba Y., Yagi T., Kiyozumi Y., Ishimoto T., Iwatsuki M. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700. doi: 10.1097/SLA.0000000000002985. [DOI] [PubMed] [Google Scholar]
- 22.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Shang X., Ren P., Gong L., Ahmed A., Ma Z. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J. Cell Physiol. 2019;234(2):1794–1802. doi: 10.1002/jcp.27052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.