Summary
T cells play a key role in adaptive immunity. Defects in specific T cell receptors or signaling proteins can alter their frequency and activation status, which may be associated with immune disease or cancer. Monitoring of T cell frequency and function in genetically modified mice or murine models of disease is therefore of high interest. Here, we provide a detailed protocol to analyze regulatory T cells, T cell activation, and cytokine production in thymus, spleen, or blood via flow cytometry.
For complete details on the use and execution of this protocol, please refer to Demeyer et al. (2020).
Subject areas: Flow cytometry/mass cytometry, Immunology
Graphical abstract
Highlights
-
•
A protocol for the collection of blood, thymus, and spleen from mice
-
•
Flow cytometry allows staining of different T cell populations
-
•
Details for intracellular cytokine staining of T cells
-
•
Full details for both flow cytometry panels and gating strategies
T cells play a key role in adaptive immunity. Defects in specific T cell receptors or signaling proteins can alter their frequency and activation status, which may be associated with immune disease or cancer. Monitoring of T cell frequency and function in genetically modified mice or murine models of disease is therefore of high interest. Here, we provide a detailed protocol to analyze regulatory T cells, T cell activation, and cytokine production in thymus, spleen, or blood via flow cytometry.
Before you begin
Mice
Before starting with this protocol, ensure that you have sufficient amounts of age- and sex-matched control and genetically modified mice from the appropriate mouse strain. We recommend using mice older than 8-weeks for T cell studies (Jackson et al., 2017). To guarantee a pronounced statistical significance, we suggest using a minimum amount of 5 animals per group.
Prepare flow cytometric panels
Timing: 0.5–1 h
-
1.
Set up the flow panels to study T cells in thymus, spleen, and blood of mice and ensure that you have sufficient amounts of flow cytometry antibodies for the number of samples you want to analyze.
Prepare reagents
Timing: 1–4 h, can be done the days prior to the day of the protocol
-
2.
Make sure you have all reagents mentioned under Materials and equipment.
Prepare materials for blood and lymphoid tissue collection / processing
Timing: 0.5–1 h, can be done the day prior to the day of the protocol
-
3.Assure that you have all the necessary materials for blood and lymphoid tissue collection/processing.
-
a.Prepare blood collection tubes by adding 50 μL EDTA (500 mM) in 1.5 mL Eppendorfs.
-
b.Prepare collection tubes for the spleen by adding 3 mL T cell culture medium in 15 mL tubes.
-
c.Prepare collection tubes for the thymus by adding 1 mL T cell culture medium in 1.5 mL Eppendorfs.
-
d.Prepare 15 mL tubes containing 5 mL of ACK lysis buffer for blood samples.
-
e.Pre-label all the required 15 and 50 mL tubes needed to make single-cell suspensions.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD3e PE-Cy5 (145-2C11) | TONBO Biosciences | Cat# 55-0031; RRID: AB_2621815 |
| Anti-mouse CD4 APC-eFluor 780 (RM4-5) | eBioscience | Cat# 47-0042-82; RRID: AB_1272183 |
| Anti-mouse CD4 BUV395 (GK1.5) | BD | Cat# 563790; RRID: AB_2738426 |
| Anti-mouse CD8a PE-Cy7 (53-6.7) | eBioscience | Cat# 25-0081-82; RRID: AB_469584 |
| Anti-mouse CD8a FITC (53-6.7) | BD | Cat# 553030; RRID: AB_394568 |
| Anti-mouse CD16/32 purified (Mouse BD FC Block) | BD | Cat# 553142; RRID: AB_394657 |
| Anti-mouse CD25 BUV395 (PC61) | BD | Cat# 564022; RRID: AB_2722574 |
| Anti-mouse CD44 Alexa Fluor 700 | eBioscience | Cat# 12-0441-81; RRID: AB_465663 |
| Anti-mouse CD44 PE (IM7) | BioLegend | Cat# 103012; RRID: AB_312963 |
| Anti-mouse CD45R/B220 BUV496 (RA3-6B2) | BD | Cat# 612950; RRID: AB_2722578 |
| Anti-mouse CD62L PE (MEL-14) | eBioscience | Cat# 12-0621-82: RRID: AB_465721 |
| Anti-mouse CD62L eFluor 450 (MEL-14) | eBioscience | Cat# 48-0621-80; RRID: AB_1963591 |
| Anti-mouse CD120b BV421 (TR75-89) | BD | Cat# 564088; RRID: AB_2738585 |
| Anti-mouse CD152 PE-eFluor 610 (UC10-4B9) | eBioscience | Cat# 61-1522-82; RRID: AB_2574580 |
| Anti-mouse FoxP3 APC (FJK-16s) | eBioscience | Cat# 17-5773-82; RRID: AB_469456 |
| Anti-mouse IFN gamma PE-Cy7 (XMG1.2) | eBioscience | Cat# 25-7311-82; RRID: AB_469680 |
| Anti-mouse IL-2 APC-Cy7 (JES6-5H4) | BD | Cat# 560547; RRID: AB_1727544 |
| Anti-mouse IL-4 APC (11B11) | eBioscience | Cat# 17-7041-81; RRID: AB_469493 |
| Anti-mouse IL-17 PerCP-Cyanine5.5 (eBio17B7) | eBioscience | Cat# 45-7177-80; RRID: AB_925754 |
| Anti-mouse TNF Alexa Fluor 700 (MP6-XT22) | BD | Cat# 558000; RRID: AB_396980 |
| Chemicals, peptides, and recombinant proteins | ||
| 2-Mercaptoethanol (50 mM) | Gibco | Cat# 31350010 |
| ACK lysis buffer | Gibco | Cat# A1049201 |
| Brefeldin A | Sigma-Aldrich | Cat# B7651 |
| 1× D-PBS liquid w/o Ca and Mg | Gibco | Cat# 14190-094 |
| Dimethyl sulfoxide Hybri-Max (DMSO) | Sigma-Aldrich | Cat# D2650 |
| EDTA disodium salt dihydrate 99.0-101.0%, AnalaR NORMAPUR ACS, Reag. Ph. Eur. analytical reagent | VWR | Cat# 20302.293 |
| Ethanol absolute, EMSURE ACS, ISO, Reag. Ph. Eur. for analysis, Supelco | VWR | Cat# 1.00983.1011 |
| Fetal bovine serum | Bodinco | n/a |
| Ionomycin calcium salt (from Streptomyces conglobatus) | Sigma-Aldrich | Cat# 407952 |
| L-Glutamine (200mM) | Lonza | Cat# BE17-605F |
| Penicillin-streptomycin solution stabilized | Sigma-Aldrich | Cat# P4333 |
| Phorbol 12-myristate 13-acetate (PMA) | Sigma-Aldrich | Cat# P8139 |
| RPMI 1640 w/o NaHCO3, w/ L-glutamine | Gibco | Cat# 52400-025 |
| Sodium pyruvate solution (100 mM) | Sigma-Aldrich | Cat# S8636 |
| Trypan blue | Sigma-Aldrich | Cat# 1.11732 |
| Critical commercial assays | ||
| BD Horizon Brilliant Stain Buffer | BD | Cat# 566349 |
| Fixable Viability Dye eFluor 506 | eBioscience | Cat# 65-0866-14 |
| Fixation/Permeabilization Solution Kit | BD | Cat# 554714 |
| FoxP3/Transcription Factor Staining Buffer Set | eBioscience | Cat# 00-5523-00 |
| UltraComp eBeads Compensation Beads | Invitrogen | Cat# 01-2222-42 |
| Experimental models: organisms/strains | ||
| Mouse: C57BL/6 | SPF-IRC animal house facility LA2400526 | n/a |
| Software and algorithms | ||
| BD FACSDiva Software v8.0 | BD | https://www.bdbiosciences.com/cn/instruments/research/software/flow-cytometry-acquisition/bd-facsdiva-software/m/111112/resourcestools |
| FlowJo v10.7.0 | Treestar | https://www.flowjo.com |
| Other | ||
| 15 mL centrifuge tubes | Corning | Cat# 430791 |
| 50 mL centrifuge tubes | Corning | Cat# CLS430897 |
| 96-well polypropylene storage microplates | Nunc | Cat# 249944 |
| BD LSRFortessa 5 laser flow cytometer | BD | n/a |
| Cell culture CO2 incubator | Thermo Scientific | Cat# 3311 |
| Cell strainers 70 μm white | Falcon | Cat# 352350 |
| Centrifuge | Thermo Scientific | Multifuge 3SR+ |
| Clustertubes 1.2 mL for FACS | Costar | Cat# COS4401 |
| Curved forceps (Dumont #7b Medical Forceps-Inox Standard Tip) | Fine science tools | Cat# 11270-20 |
| Gosselin Petri Dish 100 × 15 mm, 3 Vents, Aseptic | Corning | Cat# SB93-101 |
| Injection needles, 26G × 1/2 inch | HSW FINE-JECT | n/a |
| Cell counting chamber without clamps, dark lined, Bürker | MARIENFELD | Cat# 0640210 |
| SafeSeal tube 1.5 mL | SARSTEDT AG & Co. KG | Cat# 72.706 |
| Stericup Quick Release-GP sterile vacuum filtration system | Millipore | Cat# S2GPU05RE |
| Straight forceps with blunt points L120 mm | Surpodo | Cat# A11623 |
| Student Fine scissors straight 11.5 cm | Fine Science Tools | Cat# 91460-11 |
| Syringe 2 mL (3 mL) luer | Norm-Ject | Cat# 4020-000V0 |
| Tissue culture plate, 48 well, flat bottom with low evaporation lid | Falcon | Cat# 353078 |
Materials and equipment
T cell culture medium
To make 500 mL of T cell culture medium, dissolve 5 mL 200 mM L-glutamine (final concentration 2 mM), 5 mL Penicillin-streptomycin, 1 mL 50 mM 2-mercaptoethanol (final concentration 0.1 mM), 1 mL 100 mM sodium pyruvate solution (final concentration 0.2 mM) and 50 mL of heat inactivated Fetal Bovine Serum (final concentration 10%) in 438 mL of RPMI 1640 and filter the culture media using a GP sterile vacuum filtration system. Store at 4°C for up to 1 month.
Fixable viability dye
Prepare a working solution of the Fixable Viability Dye eFluor 506 by adding 450 μL of 1× D-PBS to the vial (final concentration 1:10). Store at −80°C in 50 μL aliquots for up to 6 months.
Azide
To make 10 mL of Azide, dissolve 2 g Azide in 10 mL of ultra-pure water (final concentration 20% w/v). Store at 4°C for up to 1 year.
EDTA (500 mM)
To make 500 mM EDTA, dissolve 186.12 g EDTA disodium dihydrate in 1,000 mL ultra-pure water and adjust the buffer's pH to 8 by adding 5 M NaOH. Store at 4°C for up to 6 months.
FACS buffer
To prepare 1 L of FACS buffer, dissolve 1 g BSA (final concentration 0.1%), 4 mL 500 mM EDTA (final concentration 2 mM) and 1 mL 20% Azide (final concentration 0.02%) in 900 mL 1× D-PBS and adjust the volume to 1,000 mL with 1× D-PBS. Store at 4°C for up to 6 months.
Fc blocking buffer
To make Fc blocking buffer for 1 sample, add 1 μL of anti-CD16/32 antibody (Key resources table) into 99 μL 1× D-PBS. ALWAYS prepare freshly. Store at 4°C for up to 24 h.
PMA
Prepare a PMA stock solution by dissolving 5 mg PMA in 5 mL DMSO (final concentration 1 mg/mL) under aseptic conditions. Store at −20°C in 50 μL aliquots for up to 1 year.
Ionomycin
Prepare an ionomycin stock solution by dissolving 1 mg ionomycin in 1.338 mL 1× D-PBS (final concentration 747 μg/mL) under aseptic conditions. Store at 4°C in 20 μL aliquots for up to 1 year.
Brefeldin A (BFA)
Prepare a BFA stock solution by adding 250 μL of DMSO to the vial (final concentration 20 μg/μL) under aseptic conditions. Store at −20°C in 15 μL aliquots for up to 1 year.
70% Ethanol
To make 500 mL 70% ethanol, mix 350 mL of absolute ethanol with 150 mL of tap water. Store at 20°C–25°C.
0.1% Trypan blue
Prepare a 0.1% trypan blue stock solution by dissolving 50 mg trypan blue powder in 50 mL 1× D-PBS under aseptic conditions. Store at 20°C–25°C in 1.5 mL aliquots.
Step-by-step method details
Sampling
Timing: 10 min/mouse
This step contains information on collecting blood, thymus, and spleen from mice to study T cells.
-
1.
Euthanize the mouse by CO2 inhalation.
-
2.
Place the animal on its back on a dissection mat, pin the paws on the mat and wet the fur by spraying with 70% ethanol.
-
3.
Make an incision in the abdominal area above the urethral opening, without penetrating the abdominal wall, and extend the incision until the neck with a pair of straight scissors. Extent also the incision to the hind legs and pin the loose skin on the mat.
-
4.
To study circulating T cells, collect blood by cardiac puncture. Penetrate the diaphragm from the xiphoid with a pair of straight scissors and delicately cut the ribs on each side up to the clavicle. Then lift the ribs with a pair of straight forceps with blunt points, pin them to the mat and insert a 26G × 1/2 inch needle attached to a 1 mL syringe in the lower left ventricle part of the heart to collect up to 500 μL of blood. Finally, transfer the blood to a 1.5 mL blood collection tube containing 50 μL EDTA (500 mM), invert the tube 5 times, to avoid blood clotting, and store the sample on ice.
Note: For long-term in vivo monitoring of lymphocytes in mice, one can obtain circulating lymphocytes via submandibular vein puncture instead of cardiac puncture.
-
5.
Next, isolate the thymus by disconnecting the connective tissue surrounding the thymus and gently remove the thymic lobes using a pair of curved forceps. Place the thymus into a 1.5 mL collection tube containing 1 mL of T cell culture medium and store it on ice.
CRITICAL: While cutting the ribs and during the cardiac puncture avoid cutting the thoracic blood vessels, since this will lead to blood leakage and contamination of the thoracic cavity with circulating lymphocytes. If there is blood leakage, the thymus needs to be rinsed in 1× D-PBS before placing the thymus in the collection tube.
-
6.
Finally, isolate the spleen by making a 1.5 cm incision in the peritoneum and gently pull the spleen to the surface. Clean up the spleen by removing the pancreatic tissue attached to the spleen with a pair of straight scissors and transfer the spleen with a pair of straight, blunt pointed forceps to a 15 mL collection tube containing 3 mL of T cell culture medium.
Processing of samples
Timing: 1.5–2 h
This step details how to prepare single-cell suspensions from spleen, thymus, and blood. Online one can find more info concerning this procedure: https://www.youtube.com/watch?v=N0jftyYqM38 and https://www.jove.com/v/61008/flow-cytometry-analysis-immune-cell-subsets-within-murine-spleen-bone.
-
7.Spleen
-
a.Pour the spleen with T cell culture medium into a 10 mm bacterial Petri dish.
 CRITICAL: For maintaining aseptic conditions, work under a fume hood.
CRITICAL: For maintaining aseptic conditions, work under a fume hood. -
b.While holding the spleen in the Petri dish with a pair of straight forceps with blunt points, flush out the splenocytes by injecting T cell culture medium (collected from the Petri dish) in the spleen with a 3 mL syringe (26G × 1/2 inch needle).
-
c.Smash the spleen by using the plunger of the 3 mL syringe and a pair of straight blunt pointed forceps.
-
d.Transfer the medium containing the smashed spleen to a 70 μm cell strainer on top of a 50 mL tube.
-
e.Use the plunger again to smash the remaining spleen pieces on the cell strainer.
-
f.Rinse the Petri dish with 3 mL of T cell culture medium to get all remaining spleen pieces and transfer them to the 70 μm cell strainer.
-
g.Smash the remaining spleen pieces.
-
h.Rinse the 70 μm cell strainer with 3 mL of T cell culture medium, discard the cell strainer, and place the sample immediately on ice.
-
i.Centrifuge the sample at 400 × g for 4 min at 4°C.Note: To decrease experimental time if you are working alone, we advise to start preparing thymic single-cell suspensions during the centrifugation steps of splenocytes isolation.
-
j.Discard supernatant using a vacuum pump and add 2 mL of ACK lysis buffer to the 50 mL tube, close the tube, and resuspend the splenocytes by pulse vortexing. Incubate for 3 min at 20°C–25°C.
-
k.Add 6 mL of T cell culture medium to stop the lysis of red blood cells, place the 50 mL tube on ice and centrifuge the sample at 400 × g for 4 min at 4°C.
-
l.Discard the supernatant and resuspend the splenocytes with 3 mL of T cell culture medium and keep on ice until stimulation/staining.
-
m.Count splenocytes with a counting chamber (1:100 dilution in 0.1% trypan blue).
-
a.
-
8.Thymus
-
a.Transfer the thymus to a 70 μm cell strainer on top of a 50 mL tube using a clean pair of straight forceps with blunt points.
-
b.Smash the thymus on the cell strainer using the plunger of a new 3 mL syringe and rinse the cell strainer with 3 mL of T cell culture medium.
-
c.Smash the remaining thymus pieces, rinse the cell strainer once more with 3 mL of T cell culture medium and place the sample on ice.
-
d.Centrifuge the sample at 400 × g for 4 min at 4°C.
-
e.Discard the supernatant and resuspend the thymocytes with 3 mL of T cell culture medium and keep on ice until staining.
-
f.Count thymocytes with a counting chamber (1:100 dilution in 0.1% trypan blue).
-
a.
-
9.Blood
-
a.Transfer the blood sample to a 15 mL tube containing 5 mL of ACK lysis buffer, close tube, vortex shortly (5 s) and incubate at RT for 3 min.
-
b.Put the tubes back on ice and add 8 mL cold T cell culture medium to stop red blood cells lysis.
-
c.Centrifuge the sample at 400 × g for 4 min at 4°C.
-
d.Discard the supernatant and resuspend the cells with 200 μL of T cell culture medium. Keep on ice until staining.
-
a.
Note: Most of the time, this experiment is labor-intensive. Therefore, we recommend having ≥ two people working together to prepare single-cell suspensions (preferably one researcher per tissue).
Note: Instead of T cell culture medium, one can use 1× D-PBS for the rinsing steps and to stop the ACK lysis step.
Stimulation of splenocytes for intracellular cytokine staining (ICS)
Timing: 4.5 h
This step details how to stimulate splenic single-cell suspensions with PMA/ionomycin/BFA to assess the intracellular cytokine production of T cells.
-
10.Stimulation of splenocytes
-
a.Prepare a 10× mix of PMA/ionomycin/BFA by adding 0.5 μL PMA, 6.66 μL ionomycin and 5 μL BFA to 987.84 μL T cell culture medium (concentration 500 ng/mL, 5,000 ng/mL and 100 μg/mL, respectively). The volume needed is 20 μL/sample.
-
b.Prepare a 10× mix with only BFA by adding 0.5 μL BFA to 99.5 μL T cell culture medium (concentration 10 μg/mL). The volume needed is 20 μL/sample.Note: After use, immediately place the stock solution of ionomycin at 4°C and the stock solutions of PMA and BFA at −20°C.
-
c.Transfer 1.5 million splenocytes to a well of a 48-well flat-bottom culture plate. Adjust the volume in the well at 180 μL by adding T cell culture medium.
-
d.Add 20 μL of the PMA/ionomycin/BFA mix (final concentration for T cell stimulation is 50 ng/mL, 500 ng/mL, 10 μg/mL, respectively) to all samples, with the exception of the negative control sample to which 20 μL of the BFA only mix (final concentration 10 μg/mL) is added.
-
e.Place the culture plate in a 37°C, 5% CO2 cell culture incubator for 4 h.
-
f.After 4 h, place the culture plate on ice until intracellular cytokine staining.
 CRITICAL: For maintaining aseptic conditions, work under a fume hood.
CRITICAL: For maintaining aseptic conditions, work under a fume hood.
-
a.
Table 7.
Antibody mix for the extracellular staining of PMA/ionomycin-stimulated splenic T cells
| Fluorophore | Marker | Final dilution | Volume for 1 sample |
|---|---|---|---|
| eFluor 506 | Fixable Viability Dye | 1/200 | 0.5 μL |
| CD16/32 (Mouse BD Fc Block) | 1/100 | 1 μL | |
| PE-Cy5 | CD3e | 1/200 | 0.5 μL |
| BUV395 | CD4 | 1/200 | 0.5 μL |
| FITC | CD8a | 1/200 | 0.5 μL |
| PE | CD44 | 1/200 | 0.5 μL |
| BUV496 | CD45R/B220 | 1/400 | 0.25 μL |
| Sum of antibodies | 3.75 μL | ||
| 1× D-PBS | 46.25 μL | ||
| BD Horizon Brilliant Stain Buffer | 1/2 | 50 μL | |
| Final staining volume | 100 μL | ||
Table 8.
Antibody mix for the intracellular staining of PMA/ionomycin-stimulated splenic T cells
| Fluorophore | Marker | Final dilution | Volume for 1 sample |
|---|---|---|---|
| PE-Cy7 | IFN-γ | 1/160 | 0.63 μL |
| APC-Cy7 | IL-2 | 1/200 | 0.5 μL |
| APC | IL-4 | 1/100 | 1 μL |
| PerCP-Cyanine5.5 | IL-17 | 1/160 | 0.63 μL |
| Alexa Fluor 700 | TNF | 1/100 | 1 μL |
| Sum of antibodies | 3.76 μL | ||
| 1× Permeabilization Buffer from BD Cytofix/Cytoperm kit | 96.24 μL | ||
| Final staining volume | 100 μL | ||
Flow cytometry antibody staining of T cells in blood, thymus, and spleen
Timing: 5–6 h
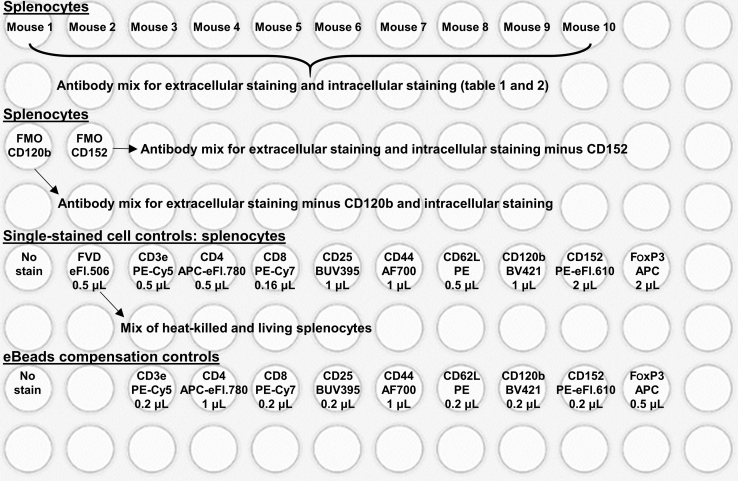
This step details the staining of splenic, thymic and blood single-cell suspensions with fluorophore-conjugated anti-mouse antibodies (see Tables 1, 2, 3, 4, 5, 6, 7, and 8) to study T cells in spleen, thymus, and blood. Single-stained cell control samples are needed to set the PMT voltage for each parameter and single-stained eBeads (UltraComp eBeads Compensation beads) controls are needed for compensation, except for the Fixable Viability Dye for which cells will be used. Figure 1 shows an example of a plate layout to stain for T cell activation and Treg cell functionality in ten mouse spleens.
CRITICAL: All antibodies and buffers should be kept on ice while preparing antibody mixes.
Note:Tables 1, 2, 3, 4, 5, 6, 7, and 8 provide antibody volumes to stain one sample (final staining volume 100 μL). To stain more than one sample, prepare an excess of two samples by multiplying the indicated volumes by the number of samples + 2.
Note: To aid with the discrimination of certain gates, one can set up fluorescent minus one (FMO) controls, where the marker of interest is omitted from the staining mix. In this protocol we recommend the use of FMO controls for CD152 (CTLA4) and the CD120b (TNFR2) antibodies to aid the gating for CTLA4+ and TNFR2+ Treg cells. However, the use of FMO controls can be extended to other markers, depending on the experience of the researcher.
-
11.Before you begin with the antibody staining, freshly prepare the following:
-
a.eBeads mix: add 7 drops from the stock vial to a 15 mL tube containing 7 mL 1× D-PBS (1 drop/mL). Vortex thoroughly and keep on ice. 200 μL of eBeads mix is required per anti-mouse antibody.
-
b.Antibody mixes for the extracellular staining of cells with fluorophore-conjugated anti-mouse antibodies in the appropriately labeled 1.5 mL Eppendorfs, by following the volumes indicated in Tables 1, 3, 5, and 7.
-
i.Start by adding 1× D-PBS to a 1.5 mL Eppendorf.Note: The volume of 1× D-PBS for one sample is based on the following formula: volume of 1× D-PBS = 100 μL (final staining volume/sample) - sum of antibodies - volume of BD Horizon Brilliant Stain Buffer.
-
ii.Continue by adding BD Horizon Brilliant Stain Buffer (at a dilution of 1/2 of the final staining volume).
 CRITICAL: BD Horizon Brilliant Stain Buffer is required when more than one BD Horizon Brilliant dye-conjugated antibody is used in the same flow panel to prevent the non-specific polymer interactions which can lead to data appearing under-compensated. In this experiment, BD Horizon Brilliant Stain Buffer is used only in the antibody mixes of Tables 1 and 7.
CRITICAL: BD Horizon Brilliant Stain Buffer is required when more than one BD Horizon Brilliant dye-conjugated antibody is used in the same flow panel to prevent the non-specific polymer interactions which can lead to data appearing under-compensated. In this experiment, BD Horizon Brilliant Stain Buffer is used only in the antibody mixes of Tables 1 and 7. -
iii.Continue by adding fluorophore-conjugated anti-mouse antibodies and the Fixable Viability Dye.
-
iv.Once finished, vortex for 5 s.
-
v.Store mixes in the fridge at 4°C.Note: The antibody mixes can be made the day before, but the Fixable Viability Dye has to be added just before adding the staining mix to the samples.Alternatives: We use Fixable Viability Dye eFluor 506 because its emission spectrum is compatible with our antibody staining panels. Alternatives, with similar emission properties, are the Zombie Aqua Fixable Viability Kit from BioLegend (cat# 23101) and the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit by Invitrogen (cat# L34957). For the panel described in Tables 1 and 2, the LIVE/DEAD Fixable Green Dead Cell Stain Kit by Invitrogen (cat# L23101), with different emission properties, can be used instead of Fixable Viability Dye eFluor 506. These alternative viability dyes have not been tested and need further optimization.
 CRITICAL: Since this experiment is labor-intensive, we recommend starting with the staining of T cells in splenic, thymic and blood single-cell suspensions during the 4 h incubation period of PMA/ionomycin/BFA-stimulated splenocytes.
CRITICAL: Since this experiment is labor-intensive, we recommend starting with the staining of T cells in splenic, thymic and blood single-cell suspensions during the 4 h incubation period of PMA/ionomycin/BFA-stimulated splenocytes. CRITICAL: Since some surface markers like CD62L are temperature sensitive, always keep the 96-well v-bottom polypropylene plate on ice during the staining procedure and use a centrifuge set at 4°C.
CRITICAL: Since some surface markers like CD62L are temperature sensitive, always keep the 96-well v-bottom polypropylene plate on ice during the staining procedure and use a centrifuge set at 4°C. CRITICAL: All incubation steps should be done in the dark.
CRITICAL: All incubation steps should be done in the dark.
-
i.
-
a.
-
12.Staining for T cell activation and Treg cell functionality in the spleen of mice (see Figure 1 for a layout of a 96-well plate to stain splenocytes from ten mice).
-
a.Transfer 1.5 million splenocytes into a well of a 96-well polypropylene v-bottom plate. Keep the plate on ice.
-
b.Add an unstained cell control and 10 single-stained cell controls by transferring 1.5 million splenocytes into 11 wells of the 96-well v-bottom plate.Note: For the CD152 FMO and the CD120b FMO you need two additional wells with 1.5 million cells.
-
c.Heat-kill 1.5 million splenocytes for 1 min at 95°C to generate a positive cell control for dead cells.
-
d.Place heat-killed cells on ice for 1 min. Mix heat-killed cells with 1.5 million splenocytes of a single-stained cell control sample. Use this sample as a single-stained cell control for the Fixable Viability Dye.
-
e.Centrifuge the plate at 400 × g for 4 min at 4°C.
-
f.Discard the supernatant and resuspend cell pellets with 200 μL 1× D-PBS.
-
g.Centrifuge the plate at 400 × g for 4 min at 4°C.
-
h.Discard the supernatant and resuspend cell pellets with 100 μL of extracellular staining antibody mix (see Table 1).Note: Resuspend the CD152 FMO control in 100 μL of antibody mix for the extracellular staining. Be aware, do not add anti-CD120b antibody in the extracellular staining antibody mix for the CD120b FMO.
-
i.Resuspend unstained and single-stained cell controls with 100 μL of Fc blocking buffer (see Materials and equipment) and add x μL of the relevant extracellular staining antibody to the proper sample. Do not add antibody to the single-stained cell controls used for the detection of intracellular FoxP3 and CD152.Note: In this case x μL is calculated based on the antibody's final dilution, as indicated in Table 1.
-
j.Add 200 μL of eBeads mix to ten wells of the 96-well v-bottom plate and add 0.5 μL of the corresponding extracellular antibody, except for Alexa Fluor 700 and for APC-eFluor780: 1 μL and for PE, PE-tandem, and BV dyes: 0.2 μL.Note: In this protocol, we use eBeads as compensation controls. We are aware that some labs prefer single-stained cell for compensation controls in multicolor flow cytometry experiments. However, for proper compensation one needs a distinct negative and positive population. This will be challenging for markers expressed on a low number of cells, such as FoxP3 and some intracellular cytokines. Therefore, we use eBeads that have a robust signal regardless of the fluorophore-conjugated antibody that is bound. These eBeads consist of two populations of beads: one that captures any mouse, rat, or hamster antibody and one that does not react with antibodies. Adding a fluorophore-conjugated antibody will result in a negative and positive peak, generating a bimodal distribution that can be used to perform compensation. Most of the fluorophore-conjugated antibodies produce an optimal bimodal distribution at average concentrations. However, we have determined that for very bright fluorophores, such as PE, PE-tandem, and BV dyes, less antibody should be used to keep the positive peak on scale. In contrast, we have observed that for dim fluorophores, such as AF700, APC-Cy7, and APC-eFluor780, more antibody is needed to achieve an optimal separation of the two peaks.
 CRITICAL: Since eBeads precipitate rapidly, vortex eBeads mix thoroughly while adding them to the 96-well v-bottom plate.
CRITICAL: Since eBeads precipitate rapidly, vortex eBeads mix thoroughly while adding them to the 96-well v-bottom plate. -
k.Mix samples and eBeads by pipetting up and down and incubate on ice for 20 min in the dark.Note: During the incubation of samples with the antibody mix for extracellular staining, prepare all required buffers to detect intracellular FoxP3 and CTLA4 expression in T cells, as described in the datasheet of the eBioscience Foxp3/Transcription Factor Staining Buffer Set (https://www.thermofisher.com/document-connect/document-connect.html?url=https%3A%2F%2Fassets.thermofisher.com%2FTFS-Assets%2FLSG%2Fmanuals%2F00-5523.pdf&title=VGVjaG5pY2FsIERhdGEgU2hlZXQ6IEZveHAzIC8gVHJhbnNjcmlwdGlvbiBGYWN0b3IgU3RhaW5pbmcgQnVmZmVyIFNldA).
-
l.Add 100 μL 1× D-PBS to each sample, beads excluded, and centrifuge at 400 × g for 4 min at 4°C.
-
m.Discard the supernatant and resuspend samples and eBeads with 100 μL fixation buffer.
-
n.Incubate samples on ice for 30 min in the dark.
-
o.Prepare the antibody mix for the intracellular staining (see Table 2) in an appropriately labeled 1.5 mL Eppendorf and store in the fridge at 4°C.
-
i.Start by adding 1× Permeabilization buffer.
-
ii.Continue by adding fluorophore-conjugated anti-mouse antibodies.
-
i.
-
p.Centrifuge plate at 400 × g for 4 min at 4°C.
-
q.Discard the supernatant and resuspend cell pellets with 100 μL of intracellular staining antibody mix.Note: Resuspend the CD120b FMO control in 100 μL of antibody mix for intracellular staining. Be aware, do not add anti-CD152 antibody in the intracellular staining antibody mix for the CD152 FMO.
-
r.Resuspend unstained and single-stained cell controls with 100 μL 1× Permeabilization buffer and add x μL of the relevant intracellular staining antibody to the proper sample.Note: In this case x μL is calculated based on the antibody's final dilution, as indicated in Table 2.
-
s.Resuspend eBeads with 200 μL 1× Permeabilization buffer and add 0.5 μL of the corresponding intracellular antibody to the beads, except for PE-tandem dyes: 0.2 μL.
-
t.Incubate samples on ice for 30 min in the dark.
-
u.Add 100 μL 1× Permeabilization buffer to each sample (not to the eBeads) and centrifuge at 400 × g for 4 min at 4°C.
-
v.Discard the supernatant and resuspend cell pellets and eBeads with 200 μL FACS buffer (see Materials and equipment).
-
w.Transfer samples to pre-labeled 1.2 mL FACS tubes and store samples in the fridge at 4°C until detection.
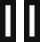 Pause point: Since the cells are fixed, one can collect the data the following day.
Pause point: Since the cells are fixed, one can collect the data the following day.
-
a.
-
13.Staining of T cells and Treg cells in thymic and blood single-cell suspensions
-
a.Transfer 1–1.5 million thymocytes and 100 μL of the blood single-cell suspension containing ∼ 0.2–1 million white blood cells into two distinct 96-well polypropylene v-bottom plates (pre-labeled as thymus and blood plate). Keep both plates on ice.
-
b.Set up an unstained cell control and four single-stained cell controls by transferring 1.5 million thymocytes to the thymus-labeled 96-well v-bottom plate.
-
c.Set up an unstained cell control and seven single-stained cell controls by transferring 50 μL of the blood single-cell suspension (a pool of the blood samples can be used) to the blood-labeled 96-well v-bottom plate.
-
d.Set up single-stained cell controls for the Fixable Viability Dye (1:1 mix of alive and heat-killed cells) in both plates as described in steps 12c and d.
- e.
-
a.
Pause point: Since the cells are fixed, one can collect the data the following day.
-
14.Intracellular cytokine staining of PMA/ionomycin/BFA-stimulated splenic T cells
-
a.Transfer all samples from the 48-well flat-bottom culture plate to a 96-well polypropylene v-bottom plate and keep the plate on ice.Note: To detach all splenocytes from the bottom of the 48-well culture plate and increase cell yield, loosen the cells by pipetting up and down a couple of times (clockwise).
-
b.Generate a single-stained cell control for the Fixable Viability Dye by heat-killing 100 μL of a single-stained cell control sample as described in steps 12c and d.
-
c.Centrifuge the plate at 400 × g for 4 min at 4°C.
-
d.Discard the supernatant, resuspend cell pellets with 200 μL 1× D-PBS and follow the same procedure of extracellular staining with antibodies as described in steps 12g–l. Always refer to Table 7 for the appropriate extracellular antibody mix for the staining of cells.Note: During the incubation of samples with the antibody mix for extracellular staining, prepare all required buffers to detect intracellular cytokine production in T cells, as described in the datasheet of the BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (https://www.bdbiosciences.com/ds/pm/others/554714_554715_555028_Book_Website.pdf).
-
e.Discard the supernatant and resuspend samples and eBeads with 100 μL fixation buffer.
-
f.Incubate samples on ice for 30 min in the dark.
-
g.Prepare the intracellular cytokine antibody staining as described in step 12o and store in the fridge at 4°C (see Table 8).
-
h.Centrifuge plate at 400 × g for 4 min at 4°C.
-
i.Discard the supernatant and resuspend non- and PMA/ionomycin-stimulated splenocytes with 100 μL of intracellular staining antibody mix.
-
j.Resuspend unstained and single-stained cell controls with 100 μL 1× Permeabilization buffer and add x μL of the relevant intracellular staining antibody to the proper sample.Note: In this case x μL is calculated based on the antibody's final dilution, as indicated in Table 8.
-
k.Resuspend eBeads with 200 μL 1× Permeabilization buffer and add 0.5 μL of the corresponding intracellular antibody to the beads, except for Alexa Fluor 700 and APC-eFluor780: 1 μL and for PE, PE-tandem, and BV dyes: 0.2 μL.
-
l.Incubate samples on ice for 30 min in the dark.
-
m.Add 100 μL 1× Permeabilization buffer to each sample (not to the eBeads) and centrifuge at 400 × g for 4 min at 4°C.
-
n.Discard the supernatant and resuspend samples and eBeads with 200 μL FACS buffer (see Materials and equipment).
-
o.Transfer samples in pre-labeled 1.2 mL FACS tubes and store samples in the fridge at 4°C until detection.
 Pause point: Since the cells are fixed, one can collect the data the following day.
Pause point: Since the cells are fixed, one can collect the data the following day.
-
a.
Table 1.
Antibody mix for the extracellular staining of splenic T cells
| Fluorophore | Marker | Final dilution | Volume for 1 sample |
|---|---|---|---|
| eFluor 506 | Fixable viability dye | 1/200 | 0.5 μL |
| CD16/32 (mouse BD Fc block) | 1/100 | 1 μL | |
| PE-Cy5 | CD3e | 1/200 | 0.5 μL |
| APC-eFluor 780 | CD4 | 1/200 | 0.5 μL |
| PE-Cy7 | CD8a | 1/600 | 0.16 μL |
| BUV395 | CD25 | 1/100 | 1 μL |
| Alexa Fluor 700 | CD44 | 1/100 | 1 μL |
| PE | CD62L | 1/200 | 0.5 μL |
| BV421 | CD120b | 1/100 | 1 μL |
| Sum of antibodies | 6.16 μL | ||
| 1× D-PBS | 43.84 μL | ||
| BD Horizon Brilliant Stain Buffer | 1/2 | 50 μL | |
| Final staining volume | 100 μL | ||
Table 2.
Antibody mix for the intracellular staining of splenic T cells
| Fluorophore | Marker | Final dilution | Volume for 1 sample |
|---|---|---|---|
| PE-eFluor 610 | CD152 | 1/50 | 2 μL |
| APC | FoxP3 | 1/50 | 2 μL |
| Sum of antibodies | 4 μL | ||
| 1× Permeabilization Buffer from FoxP3/Transcription Factor Staining Buffer Set | 96 μL | ||
| Final staining volume | 100 μL | ||
Table 3.
Antibody mix for the extracellular staining of thymic T cells
| Fluorophore | Marker | Final dilution | Volume for 1 sample |
|---|---|---|---|
| eFluor 506 | Fixable Viability Dye | 1/200 | 0.5 μL |
| CD16/32 (Mouse BD Fc Block) | 1/100 | 1 μL | |
| APC-eFluor 780 | CD4 | 1/200 | 0.5 μL |
| PE-Cy7 | CD8a | 1/600 | 0.16 μL |
| Sum of antibodies | 2.16 μL | ||
| 1× D-PBS | 97.84 μL | ||
| Final staining volume | 100 μL | ||
Table 4.
Antibody mix for the intracellular staining of thymic T cells
| Fluorophore | Marker | Final dilution | Volume for 1 sample |
|---|---|---|---|
| APC | FoxP3 | 1/50 | 2 μL |
| Sum of antibodies | 2 μL | ||
| 1× Permeabilization Buffer from FoxP3/Transcription Factor Staining Buffer Set | 98 μL | ||
| Final staining volume | 100 μL | ||
Table 5.
Antibody mix for the extracellular staining of T cells in the blood of mice
| Fluorophore | Marker | Final dilution | Volume for 1 sample |
|---|---|---|---|
| eFluor 506 | Fixable Viability Dye | 1/200 | 0.5 μL |
| CD16/32 (Mouse BD Fc Block) | 1/100 | 1 μL | |
| PE-Cy5 | CD3e | 1/200 | 0.5 μL |
| APC-eFluor 780 | CD4 | 1/200 | 0.5 μL |
| PE-Cy7 | CD8a | 1/200 | 0.5 μL |
| Alexa Fluor 700 | CD44 | 1/200 | 0.5 μL |
| eFluor 450 | CD62L | 1/200 | 0.5 μL |
| Sum of antibodies | 4 μL | ||
| 1× D-PBS | 96 μL | ||
| Final staining volume | 100 μL | ||
Table 6.
Antibody mix for the intracellular staining of T cells in the blood of mice
| Fluorophore | Marker | Final dilution | Volume for 1 sample |
|---|---|---|---|
| APC | FoxP3 | 1/50 | 2 μL |
| Sum of antibodies | 2 μL | ||
| 1× Permeabilization Buffer from FoxP3/Transcription Factor Staining Buffer Set | 98 μL | ||
| Final staining volume | 100 μL | ||
Figure 1.
Layout of a 96-well v-bottom plate setup for the staining of splenic T cell activation and Treg cell functionality of ten mice
Splenocytes are stained with the antibody mix for the extracellular staining and the intracellular staining as indicated in Tables 1 and 2 and described in step 12, single-stained cell controls and eBeads compensation controls are stained with the indicated fluorophore-conjugated anti-mouse antibodies, based on Tables 1 and 2. FMO, fluorescence minus one; FVD, fixable viability dye.
Data collection
Timing: 3–4 h
This step details how to collect flow data using a five laser BD LSRFortessa cytometer and analyze them using the FlowJo software.
Alternatives: The staining of T cells and Tregs in thymus and blood (Tables 3, 4, 5, and 6) can also be acquired using a three laser BD LSR II cytometer or a three or four laser BD Fortessa cytometer.
-
15.
Acquire data with a BD LSRFortessa 5 laser flow cytometer within 24 h after staining at a flow rate of 8000 events/s or less. Use unstained and single-stained cell controls to set suitable PMT voltages. For the FSC and SSC, voltages are set so that the cells can be discriminated from the debris, which should be present in the lower left of a FSC-A versus SSC-A plot (see Figures 2, 3, 4, and 5). For the other parameters the CS&T baseline target voltages are a good starting point to set PMT voltages. If a positive population of a single-stained cell control is not on scale, lower the PMT voltage to have the positive population on scale. For auto-compensation, use the eBeads control samples and the heat-killed cell sample stained with Fixable Viability Dye eFluor 506. Once the PMT voltages are set and the auto-compensation is done, acquire at least 0.5 million living cells per tube.
Note: If the researcher is not familiar with setting up PMT voltages and performing auto- compensation, we advise to get assistance from a more experienced researcher.
-
16.
The acquired data can be analyzed with FlowJo software using linear scales for FSC-A, SSC-A and FCS-H, and biexponential scales for the other markers.
Figure 2.
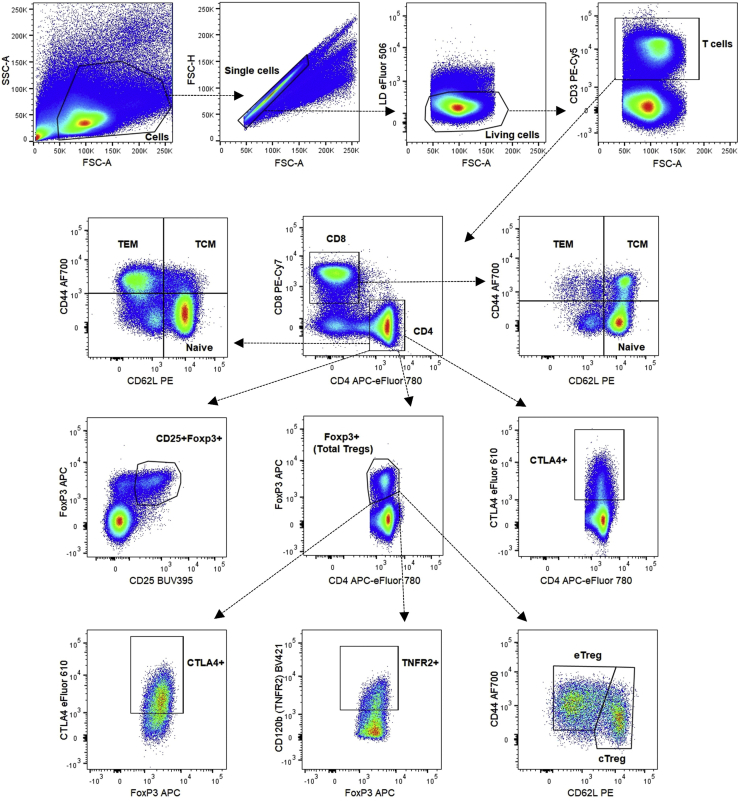
Gating strategy for T cell activation in spleen
CD4+ and CD8+ naive (CD44−CD62L+), TEM (CD44+CD62L−) and TCM (CD44+CD62L+) cells, CTLA4+CD4+ T cells, total Treg cells (FoxP3+) and CD25+FoxP3+ Treg cells, Treg functionality markers (TNFR2+ or CTLA4+), cTregs (CD44loCD62Lhi), and eTregs (CD44hiCD62Llo).
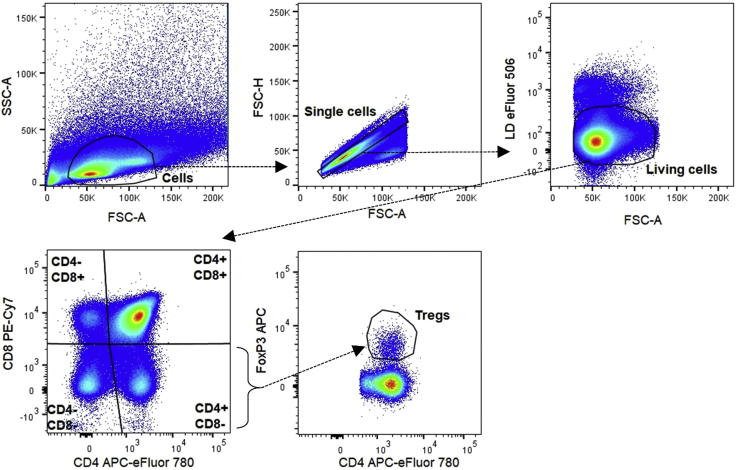
Figure 3.
Gating strategy for CD4−CD8− (DN), CD4+CD8+ (DP), CD4+CD8− (SP), CD4−CD8+ (SP) thymocytes, and FoxP3+CD4+CD8− thymic Tregs
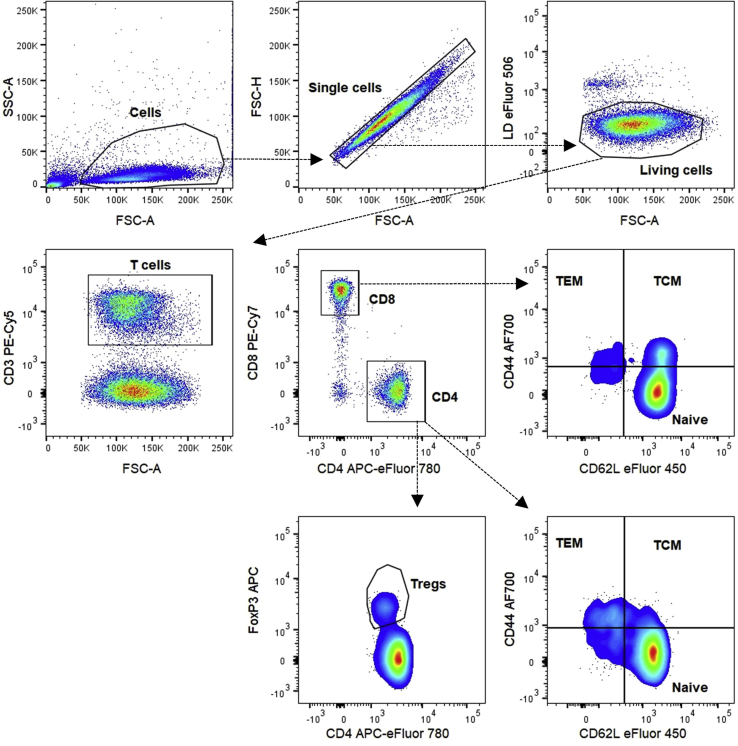
Figure 4.
Gating strategy for T cell activation and Treg cells in blood
CD4+ and CD8+ naive (CD44−CD62L+), TEM (CD44+CD62L−) and TCM (CD44+CD62L+) cells and Treg cells (FoxP3+CD4+). Smooth pseudocolor plots are used to facilitate the gating.
Figure 5.
Gating strategy for cytokine production by T cells
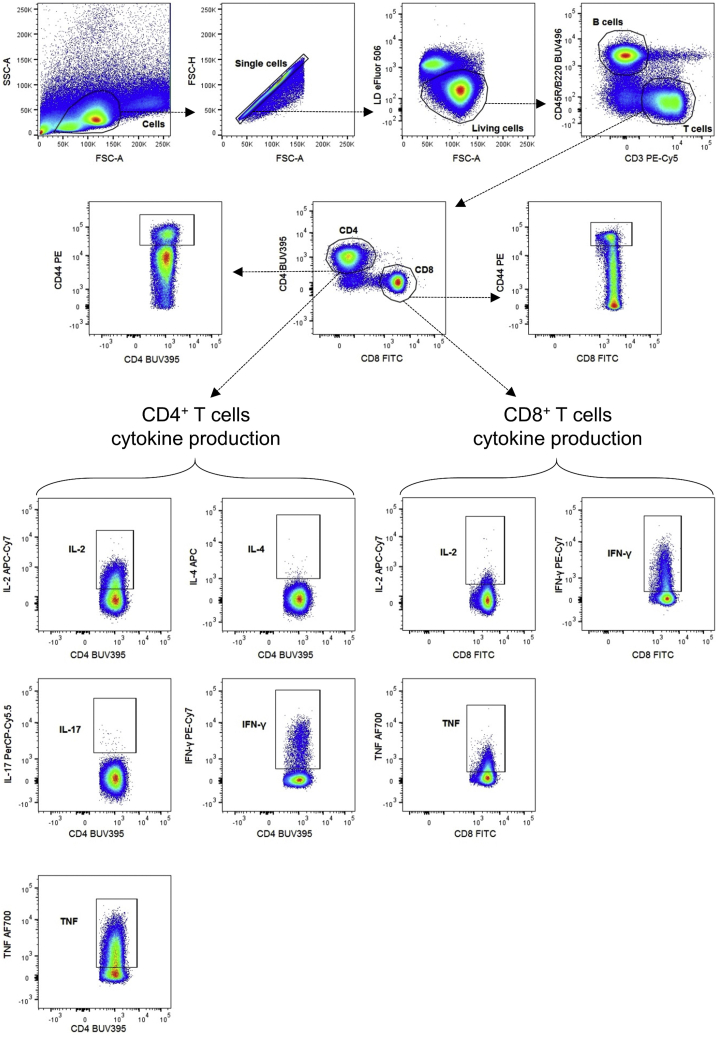
Activated CD4+ and CD8+ T cells are indicated by their CD44 expression. CD4+ T cells expressing IL-2, IL-4, IL-17, IFN-γ, and TNF. CD8+ T cells expressing IL-2, IFN-γ, and TNF.
Expected outcomes
This protocol describes gating strategies to study T cells in mouse thymus, spleen, and blood. We defined the different T cell populations based on extracellular and intracellular markers.
For all panels we first create a cell gate based on SSC-A and FSC-A parameters. We continue by gating single cells by using the FSC-H and FSC-A parameters. Next, we use eFluor 506 Fixable Viability Dye to separate dead from alive cells (Figures 2, 3, 4, and 5). To distinguish CD4−CD8− (DN), CD4+CD8+ (DP) and CD4+CD8− or CD4−CD8+ (SP) thymocytes, we look at CD4 and CD8a expression on living cells (Figure 3). We did not include a CD3e antibody, since not all DN thymocytes express CD3e on their surface and gating for CD3e prior to CD4 and CD8a gating would result in loss of some DN thymocytes. On the living cell population, we can also gate for T cells by using an anti-CD3e antibody (Figures 2 and 4) or T and B cells by using anti-CD3e and anti-CD45R/B220 antibodies (Figure 5). T cells are further divided in CD4+ and CD8+ T cell populations based on the expression of CD4 and CD8a, respectively (Figures 2, 4, and 5). To define the activation status of splenic and circulating CD4+ T and CD8+ T cells, we use anti-CD44 and anti-CD62L antibodies, resulting in plots with CD44−CD62L+ naive, CD44+CD62L− effector memory (TEM) and CD44+CD62L+ central memory (TCM) cells (Figures 2 and 4).
Thymic and circulating Treg cells are defined as FoxP3+CD4+ T cells (Figures 3 and 4), while splenic Treg cells are also more specifically defined as FoxP3+CD25+CD4+ T cells (Figure 2). The splenic FoxP3+ Treg cells are then further divided in CD44loCD62Lhi naive central Treg cells (cTregs) and CD44hiCD62Llo effector Treg cells (eTregs) (Di Pilato et al., 2019; Yang et al., 2019). Finally, with the help of FMO controls and the CTLA4 expression on CD4+ T cells, we can gate for the expression of TNFR2 (CD120b) and CTLA4 (CD152), which are functionality markers for FoxP3+CD4+ Treg cells (Figure 2) (Chen et al., 2008; Klocke et al, 2017).
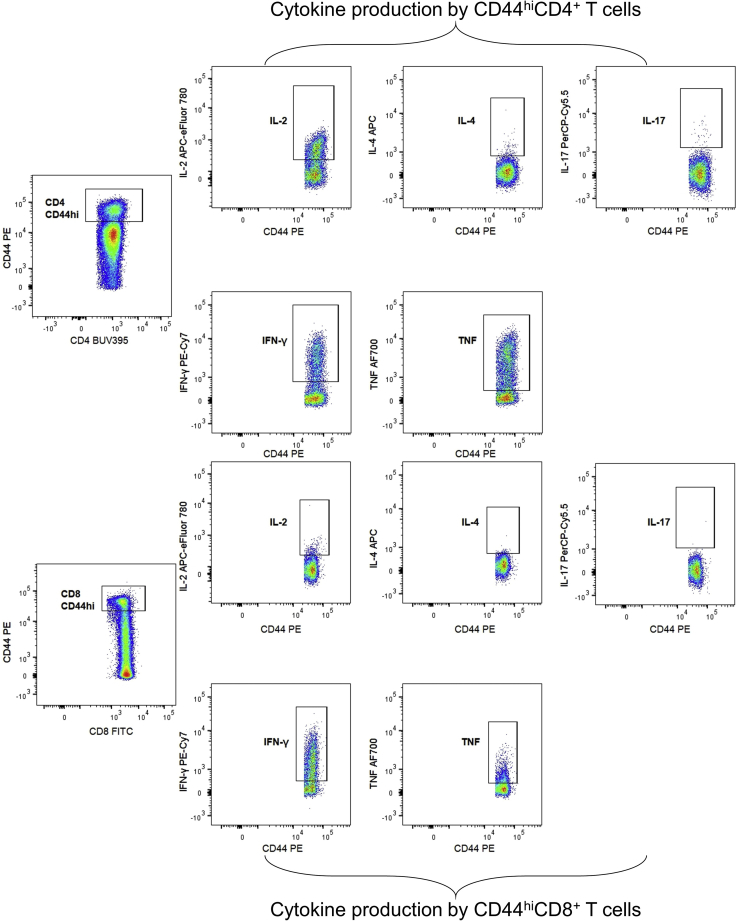
In addition, we developed a gating strategy to study T helper and cytotoxic T cell populations in mouse spleen, based on the intracellular expression of cytokines (Figure 5). PMA/ionomycin/BFA-stimulated cells will produce cytokines, but these cytokines cannot be secreted since BFA is used. By using the BFA only negative control, we can determine PMA/ionomycin-induced IFN-γ (Th1), IL-4 (Th2), IL-17 (Th17), IL-2 and TNF producing CD4+ T cells. Similarly, we determined cytotoxic CD8+ T cells that are subdivided into three categories based on IL-2, IFN-γ and TNF production. We have also included the CD44 marker to be able to distinguish activated CD44hiCD4+ and CD44hiCD8+ T cells. These CD44hi cells can be submitted to a similar gating strategy for cytokine expression (Figure 6).
Note: Stimulation of T cells with PMA/ionomycin (for 4 h) can cause some downregulation of surface CD3 and CD4 expression, which can complicate the gating of CD3+ and CD4+ T cells. However, this problem can be solved by using the negative control (only BFA-treated splenocytes) to set the required gates.
Figure 6.
Gating strategy for cytokine production by activated T cells
Activated CD44hiCD4+ and CD44hiCD8+ T cells expressing IL-2, IL-4, IL-17, IFN-γ, and TNF.
Limitations
This protocol provides the basics to study thymic, splenic, and circulating T cells in mice. The protocol can also be used to analyze T cell activation, Treg functionality, and cytokine production of T cells from lymph nodes. To obtain single-cell suspensions of these lymph nodes, one can use the isolation protocol for thymus, but for the last step one has to adjust the volume of T cell culture medium according to the number of lymph nodes. However, the protocol has not been optimized for the analysis of T cells in non-lymphoid tissues, which requires a more labor-intensive method to isolate immune cells, such as enzymatic digestion of the tissue and density gradient centrifugation.
Though this is a very comprehensive protocol concerning T cells, it does not provide any data concerning the early stages of thymocyte development. Early stages of thymocyte development are well defined by the surface expression of CD44 and CD25 on CD4−CD8− T cells. Therefore, the inclusion of Alexa Fluor 700-conjugated anti-CD44 and BUV395-conjugated anti-CD25 antibodies in the thymocyte flow panel (see Table 2) would solve this issue. In addition, the presence of CD25 in this panel would allow for the separation of mature CD25+FoxP3+ Tregs from CD25+ Treg and FoxP3lo Treg progenitors in the thymus (Owen et al., 2019). Furthermore, the protocol does not provide any information regarding follicular T helper cells and γδ-T cells in mouse spleen.
For a more comprehensive analysis of circulating and peripheral immune cells using flow cytometry, we recommend referring to "Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition)" (Cossarizza et al., 2019).
Troubleshooting
Problem 1
Contamination of blood single-cell suspensions with red blood cells after ACK lysis step.
Potential solution
The potential reason for this problem might be that the ACK lysis buffer was not effective enough. A second ACK lysis step can be included in which the pellet is dissolved in 3 mL of ACK lysis buffer for 3 min and the other steps of the protocol are repeated. However, fixation of samples with fixation buffers provided with the intracellular staining sets should overcome this problem.
Problem 2
Small numbers of PMA/Ionomycin/BFA-stimulated cells after transfer of cells from the 48-well flat-bottom culture plate to the 96-well polypropylene v-bottom plate.
Potential solution
The potential reason for this problem is the sticking of stimulated cells to the bottom of the 48-well flat-bottom culture plate. Therefore, take the time to loosen all cells by pipetting up and down in a clockwise fashion. After cell transfer, one can inspect the plate to see if cells still remain in the culture plate. If this is the case, add 200 μl 1× D-PBS to the wells and start detaching the cells in the same fashion as before. These cells can then be added to the pellet obtained after the first centrifugation step and then the subsequent steps of the protocol can be followed.
Problem 3
No positive staining of some extracellular markers by flow cytometry.
Potential solution
Several reasons may result in this problem.
1) antibody was not added. Ensure adding all the antibodies to the antibody mixes the next time you repeat the experiment; 2) use of a different antibody clone. Using a different antibody clone than the one indicated in our protocol may lead to incompatibility with the fixation step. Therefore, we recommend using the antibody clones mentioned in our protocol. However, if you want to use another clone, please check clone's compatibility with fixation buffer in one of the following links: https://www.thermofisher.com/be/en/home/life-science/cell-analysis/cell-analysis-learning-center/cell-analysis-resource-library/ebioscience-resources/antibody-fixation-considerations.html and https://www.biolegend.com/en-us/fixation; 3) inappropriate PMT voltages. The use of unstained and single-stained cell controls will help to detect the appropriate PMT voltages and overcome this problem; 4) do a titration of the antibody.
Problem 4
Weak or undetectable intracellular staining of cytokines in PMA/ionomycin/BFA-treated cells.
Potential solution
There are several possible reasons for this problem.
1) not adding BFA in the PMA/ionomycin mix. Ensure that you add BFA in the stimulation mix and perform a new experiment; 2) lower efficiency of BFA due to repeated freeze/thaw of BFA, which can affect its inhibitory efficiency. Avoid using BFA aliquots that have been subjected to freeze/thaw for more than three times; 3) insufficient production of cytokines by PMA/ionomycin/BFA-stimulated cells. To overcome this problem, culture the PMA/ionomycin/BFA-stimulated cells for 6 h instead of 4 h. Be aware that culturing cells with BFA for more than 24 h can negatively influence the cell viability; 4) insufficient binding of fluorophore-conjugated anti-cytokine antibodies with their targets, due to the accidental use of the 1× Permeabilization buffer of the FoxP3/Transcription Factor Staining Buffer. Ensure that the fixation, the permeabilization and the intracellular cytokine staining of cells are performed using reagents of the BD Cytofix/Cytoperm Fixation/Permeabilization Kit.
Problem 5
Undetectable intracellular FoxP3 expression in CD4+ T cells.
Potential solution
The following reason might cause this problem.
Insufficient binding of the anti-FoxP3 antibody to its nuclear target due to the accidental use of the BD Cytofix/Cytoperm Fixation/Permeabilization Kit instead of the FoxP3/Transcription Factor Staining Buffer Set during the intracellular staining procedure. In this case, the nucleus will remain intact and the anti-FoxP3 antibody will not bind to FoxP3. To solve this problem, ensure that you always use the fixation and permeabilization buffers of the FoxP3/Transcription Factor Staining Buffer Set during the intracellular FoxP3 detection.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Rudi Beyaert (rudi.beyaert@irc.vib-ugent.be).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate or analyze any datasets.
Acknowledgments
We would like to thank the VIB Flow Core and the VIB Bioimaging Core for training, support, and access to the instrument park. The IRC Transgenic core facility and mouse facility are acknowledged for generation and caretaking of transgenic mice. Research in the authors' laboratory is supported by grants from the Fund for Scientific Research Flanders (FWO), Belgium; Belgian Foundation Against Cancer, Belgium; Ghent University Concerted Research Actions (GOA), Belgium and the VIB Grand Challenges Program (VIB-GC01-C01), Belgium.
Author contributions
Conceptualization, A.D. and R.B.; Methodology, A.D.; Investigation, A.D. and I.S.; Writing –Original Draft, A.D., I.S.; Writing – Review & Editing, A.D. and R.B.; Funding Acquisition, R.B.; Supervision, A.D. and R.B.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Ioannis Skordos, Email: ioannis.skordos@irc.vib-ugent.be.
Rudi Beyaert, Email: rudi.beyaert@irc.vib-ugent.be.
References
- Chen X., Subleski J.J., Kopf H., Howard O.M.Z., Männel D.N., Oppenheim J.J. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4 + CD25 + FoxP3 + T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J. Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossarizza A., Chang H.D., Radbruch A., Acs A., Adam D., Adam-Klages S., Agace W.W., Aghaeepour N., Akdis M., Allez M. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition) Eur. J. Immunol. 2019;49:1457–1973. doi: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer A., Driege Y., Skordos I., Coudenys J., Lemeire K., Elewaut D., Staal J., Beyaert R. Long-term MALT1 inhibition in adult mice without severe systemic autoimmunity. iScience. 2020;23:101557. doi: 10.1016/j.isci.2020.101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pilato M., Kim E.Y., Cadilha B.L., Prüßmann J.N., Nasrallah M.N., Seruggia D., Usmani S.M., Misale S., Zappulli V., Carrizosa E. Targeting the CBM complex causes Treg cells to prime tumours for immune checkpoint therapy. Nature. 2019;570:112–116. doi: 10.1038/s41586-019-1215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.J., Andrews N., Ball D., Bellantuono I., Gray J., Hachoumi L., Holmes A., Latcham J., Petrie A., Potter P. Does age matter? the impact of rodent age on study outcomes. Lab. Anim. 2017;51:160–169. doi: 10.1177/0023677216653984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke K., Holmdahl R., Wing K. CTLA-4 expressed by FOXP3+ regulatory T cells prevents inflammatory tissue attack and not T-cell priming in arthritis. Immunology. 2017;152:125–137. doi: 10.1111/imm.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D.L., Mahmud S.A., Sjaastad L.E., Williams J.B., Spanier J.A., Simeonov D.R., Ruscher R., Huang W., Proekt I., Miller C.N. Thymic regulatory T cells arise via two distinct developmental programs. Nat. Immunol. 2019;20:195–205. doi: 10.1038/s41590-018-0289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Zhao X., Lin X. Bcl10 is required for the development and suppressive function of Foxp3+ regulatory T cells. Cell. Mol. Immunol. 2019;1:1–13. doi: 10.1038/s41423-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate or analyze any datasets.