Abstract
Uraemic Cardiomyopathy (UC) is recognised as an intricate and multifactorial disease which portends a significant burden in patients with End-Stage Renal Disease (ESRD). The cardiovascular morbidity and mortality associated with UC is significant and can be associated with the development of arrythmias, cardiac failure and sudden cardiac death (SCD). The pathophysiology of UC involves a complex interplay of traditional implicative factors such as haemodynamic overload and circulating uraemic toxins as well as our evolving understanding of the Chronic Kidney Disease-Mineral Bone Disease pathway. There is an instrumental role for multi-modality imaging in the diagnostic process; including transthoracic echocardiography and cardiac magnetic resonance imaging in identifying the hallmarks of left ventricular hypertrophy and myocardial fibrosis that characterise UC. The appropriate utilisation of the aforementioned diagnostics in the ESRD population may help guide therapeutic approaches, such as pharmacotherapy including beta-blockers and aldosterone-antagonists as well as haemodialysis and renal transplantation. Despite this, there remains limitations in effective therapeutic interventions for UC and ongoing research on a cellular level is vital in establishing further therapies.
Keywords: Uraemic cardiomyopathy, left ventricular hypertrophy, FGF 23, klotho, end-stage renal disease, chronic kidney disease-bone mineral disease
Introduction
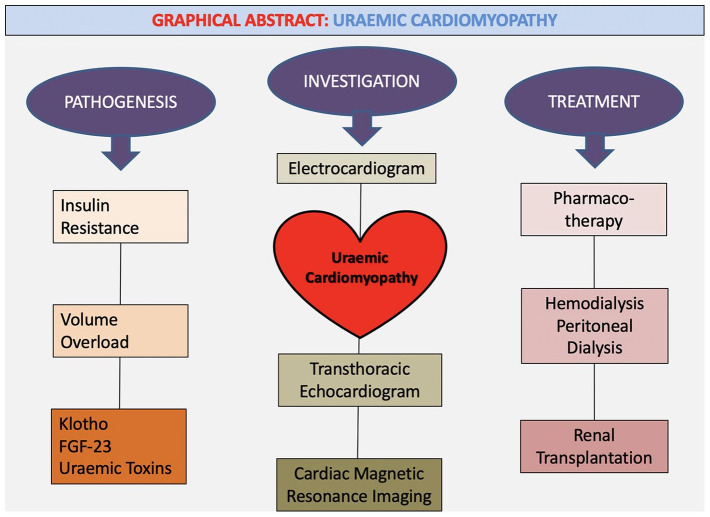
Uraemic Cardiomyopathy (UC) is classically characterised by diastolic dysfunction in association with left ventricular hypertrophy (LVH) and myocardial fibrosis in patients with chronic kidney disease (CKD).1 Patients with underlying end-stage renal disease (ESRD) and resultant myocardial remodelling often have associated high levels of cardiovascular morbidity and mortality.2,3 Whilst the pathophysiology of UC is traditionally multifactorial, there is emerging research in this area which may help expand therapeutic options for this patient population.4,5 There is growing evidence to suggest that cardiovascular death among these patients are increasingly secondary to LVH and its sequela of congestive cardiac failure (CCF), rather than purely atherosclerotic heart disease, highlighting the mechanistics behind this entity.3,6-8 Appropriate screening for these patients requires a multimodality imaging approach with transthoracic echocardiogram (TTE) and Cardiac Magnetic Resonance Imaging (CMRI) with current interventions centred around appropriate cardiac-specific pharmacotherapy, haemodialysis as well as renal transplantation3 See Graphical Abstract
Epidemiology
The burden of cardiovascular disease (CVD) in patients with ESRD is significant, with mortality from CVD approximately 15 to 30 times higher than the general population.9 The phenotypic hallmark of LVH which characterises UC is found in over 70% of patients with ESRD.10 In patients with ESRD on dialysis, sudden cardiac death (SCD), usually indicative of an underlying cardiomyopathy, accounts for roughly 40% of all deaths.5 There are predictions that the number of patients receiving dialysis for ESRD in the United States (US) will increase from a total of 320,000 in 2003 to 2 million by 2030, illustrating both the current and future burden of disease.3,10
Pathophysiology
The pathogenesis of UC remains complex and multifaceted with many implicative factors. These factors include anaemia, haemodynamic overload, hypertension, alterations in mineral metabolism, endothelial dysfunction, insulin resistance and cardiotonic steroids as well as several circulating uraemic toxins.1,5 See Table 1. However recently there has been an emergence of the pathophysiological role of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) in UC with hyperphosphatemia, high FGF-23 levels and reduced serum levels of Klotho integral in the process of abnormal myocardial remodelling and resultant cardiac sequelae.1,2,11
Table 1.
| Traditional factors | Uraemic factors |
|---|---|
| Diabetes Hypertension Hyperlipidemia Older age Physical inactivity Smoking |
Uraemic toxins Indoxil sulfate P-cresyl sulfate (pCS) β2-microglobulin Homocysteine CKD-MBD Anaemia Inflammation Cardiotonic steroids Endothelial dysfunction |
UC may manifest as a result of pressure overload, volume overload and a systemic uremic state.3 Left ventricular (LV) pressure overload may occur as a result of hypertension, arteriosclerosis and aortic stenosis whilst LV volume overload can occur in the setting of haemodynamic overload and anaemia.3,15 LV pressure overload mediates hypertrophy through increasing of LV wall thickness with minimal change in chamber size whereas LV volume overload results in increased chamber size but regular LV wall thickness.15 Whilst LV hypertrophy (LVH) initially is a compensatory adaptative response, continual LV overload leads to cardiomyocyte death.3 This acute or chronic loss of cardiomyocytes will eventually lead to systolic heart failure.16 This process with increased passive stiffness and fibrosis will eventually lead to the development of diastolic dysfunction, a characteristic hallmark of UC.16 It is also increasingly being recognised that the degree of myocardial fibrosis correlates strongly with the development of arrythmias and sudden cardiac death.17
The uraemic state also contributes to LVH through the accumulation of substances such as endothelin, parathyroid hormone (PTH), tumour necrosis factor alpha (TNF- α), interleukin 1 alpha (IL- 1α) and IL-6.18,19 Another example of such hypertrophic substances are endogenous cardiotonic steroids classed as cardenolides (oubain and digoxin) and bufadienolides (marinobufagenin and proscillaridin A).20 These substances interact with the α-subunit of the Na, K-ATPase transmembrane protein on the surface of cardiomyocytes, and whilst their exact mechanism is unknown, they are thought to be vital in the molecular pathogenesis of UC.3,21-23
Oxidative stress and inflammation, and endothelial dysfunction
Endothelial dysfunction represents a characteristic finding in patients with ESRD who have high rates of heart failure and cardiovascular mortality.24 Intricately intertwined with this is the role of oxidative stress and inflammation, often seen in CKD.25 For instance, CKD often results in a persistent inflammatory state, which may result in endothelial dysfunction and worsen atherosclerosis. Such inflammatory effects may also have other detrimental effects, such as vascular calcification, erythropoietin (EPO) resistance, increased hepcidin and decreased iron absorption.25 In turn, anaemia itself contributes to cardiac dysfunction, evident in the CKD population.26
Soluble α-Klotho and Vitamin D also help maintain endothelial integrity, with their declining concentrations in ESRD contributing to the dysfunction of the endothelial lining.27 The subsequent increase in circulating FGF-23 and phosphate further damage the endothelial lining.27 The underlying renal dysfunction also leads to an excess of inflammatory mediators and uraemic toxins that inhibit the recovery of the damaged endothelial lining.27 There is evidence to suggest that this impaired endothelial function correlates with abnormal left ventricular structure and function as well as cardiovascular mortality in CKD.27,28 Endothelial dysfunction can be further analysed through microvascular or macrovascular endothelial dysfunction. Macrovascular endothelial dysfunction does not have a significant association with increased LV mass in ESRD.24 Contrastingly, microvascular endothelial dysfunction has been shown to be associated with LV diastolic dysfunction, RV systolic dysfunction and RV diastolic dysfunction in ESRD.24 The clinical significance of this is still unknown, with the lack of an established causal relationship between endothelial function and cardiac function, however it illustrates the need for trials of novel endothelial therapies to determine the possibility of regression of LVH and cardiac failure in patients with ESRD.24
Carnitine deficiency
One postulated contributing factor for uraemic cardiomyopathy is the role of carnitine, which plays an important part in myocardial energy metabolism, given its role in beta-oxidation of fatty acids. Carnitine deficiency is often observed in uraemic patients, particularly those on haemodialysis, given that it can be lost through dialytic membranes.29 While some studies have shown that cardiac hypertrophy may develop without alteration in myocardial metabolism in renal dysfunction,30 there is a growing amount of evidence that suggests a positive role for carnitine supplementation in terms of improving cardiac function, exemplified by a case report by Kaneko et al whereby a haemodialysis patient’s cardiac dysfunction improved following the administration of L-carnitine.31
Insulin resistance
Insulin resistance, which may commonly occur in patients with CKD, represents an independent risk factor for cardiac disease in CKD.31-37 One explanation to the increased prevalence of insulin resistance in CKD is the interruption in the intracellular insulin pathway that occurs because of increased angiotensin II, inflammation, metabolic acidosis and uremic toxins.37 The phosphoinositide-3 kinase (PI3K)-Akt pathway is of particular interest in the development of LVH, myocardial fibrosis, cellular apoptosis and metabolic dysfunction with insulin resistance conferring maladaptive alterations within this pathway.38 Akt1 and Akt2 are also predominantly found in the heart, with imbalance of these substances resulting in a cardiac phenotype similar to the uraemic heart.38,39 In insulin-resistant states, an Akt2 defect produces a compensatory hyperinsulinemia and upregulation of Akt1 signalling, producing a cardiac hypertrophy associated with fibrosis.39 This is particularly important as there are therapies which have been shown to target the Akt pathway. Rapamycin, a mTOR target downstream of Akt, has shown to reduce cardiac hypertrophy and fibrosis in uraemic mice whereas Glitazones have been shown to reduce cardiac hypertrophy in mice.40,41 Thus, through our understanding of insulin resistance and its alterations in the Akt pathway, we can understand its cardiac effects and devise potential therapeutic options.
CKD-MBD
The role of CKD-MBD in UC is now increasingly being recognised following advancements in dialysis and resultant correction of uraemia-related abnormalities.42-44 In order to fully appreciate the role of CKD-MDB in the development of cardiomyopathy, it is important to analyse the role of parathyroid hormone (PTH), phosphate, FGF-23 and Klotho.5 Current literature states the role of high PTH levels and its correlation with LV mass and degree of diastolic dysfunction, with these effects reversible post treatment of hyperparathyroidism.5,45-47 Vitamin D deficiency, especially in the setting of secondary hyperparathyroidism has been associated with LV dysfunction and increased risk of cardiac events, including CCF.48 Phosphate plays a central role in the CKD-MDB and phosphate toxicity can contribute to cardiovascular mortality.5,43 Hyperphosphatemia has been directly associated with increased LV mass and diastolic dysfunction.49-51 It can promote LVH, potentially through changes in arterial stiffness or by directly acting on the myocardium.49-52
FGF-23
There is now increasing data to support the contributions of FGF-23 and Klotho to uraemic cardiomyopathy in a coadjutant manner.11 FGF-23 regulates phosphate and Vitamin D metabolism and binds FGF receptor/Klotho co-receptor complexes, with the net result of stimulating phosphate excretion, inhibiting PTH secretion and decreasing active Vitamin D.11,53 FGF-23 has been shown to directly induce hypertrophic growth of the cardiac myocytes, with this process requiring the presence of FGR-4.54 FGF-23 can activate FGR-4 in cardiac myocytes, resulting in stimulation of the phospholipase Cγ (PLCγ)/calcineurin / nuclear target of activated T cells (NFAT) signalling pathway and subsequent cardiac hypertrophy in a blood-pressure and αKlotho independent manner.5,11,54 Although significant exploration of the role of FGF-23 and FGR-4 has taken place in rat models, there is still support for its role in humans, if not direct causal evidence.39 A recent retrospective study has shown that in children with ESRD, FGR4 expression levels have been associated with cardiac hypertrophy, with another study showing that lowering serum FGF-23 levels in CKD patients is associated with reduced cardiovascular events and mortality.55,56 There is also increasing evidence that FGF-23 stimulates cardiac fibrosis through the TGF-β and β-catenin pathways via activation of FGR-4, which has been demonstrated in mice models.19,57 Thus, it is evident that increased FGF-23 levels contribute to UC, with myocardial FGR4 representing a promising therapeutic target.5,11
Klotho
Klotho acts as a co-receptor for FGF-23 and helps mediate its’ role in the regulation of phosphate haemostasis.11 Klotho exists in a deficient state in CKD.5 The Klotho gene encodes the αKlotho protein which is expressed in tissues and organs including the kidneys and parathyroid glands.58 Although the mechanism by which αKlotho acts is poorly understood and the myocardium does not express αKlotho, it is known that FGF23 does not seem to induce myocardial hypertrophy in the presence of normal levels of soluble αKlotho.59-62 There is some evidence of a mechanism of αKlotho inhibiting TRPC 6, a calcium permeable cation channel that helps modulate cardiac remodelling and induces cardiac hypertrophy through the calcineurin/NFAT pathway.11,63 Xie et al also demonstrated in a mice model, that soluble Klotho protects against UC independent of FGF-23 and phosphate.60,61 In patients with ESRD, as the kidney is the main source of systemic αKlotho, it may represent the primary alteration in CKD-MBD.5
Thus, the pathogenesis of UC and its complexities are evident. There is a significant role of CKD-MBD in UC with the evolving understanding of elevated FGF-23 and αKlotho deficiency in particular pivotal to the development of appropriate targeted therapy in the future.
Diagnostics
As UC is a heterogeneous disease process, the accurate diagnosis incorporates the use of both imaging and non-imaging modalities. The non-imaging diagnostic tools include laboratory investigations and electrocardiography (ECG), whereas imaging tools involve a multi-modality approach with non-invasive investigations such as transthoracic echocardiography (TTE), cardiac CT and cardiac magnetic resonance imaging (CMRI). Furthermore, invasive diagnostics, whilst rarely performed, involve myocardial biopsy with histological assessment. These tools continue to evolve in the 21st century, providing additional prognostic information and guidance on selected therapeutics.
Electrocardiography
Bedside ECG is the most readily available test for rapid assessment of presence of LVH in this patient demographic. UC is known to ubiquitously manifest with signs of LVH, as fulfilled by Sokolov-Lyon criteria on ECG.64 In early stages of the disease process, LVH manifests as an eccentric hypertrophy with a subsequent concentric hypertrophy and progressive worsening of CKD.64 Other important ECG findings in UC include the presence of Q waves, dynamic ST segment changes, prolonged QRS duration, tachycardia and left and right atrial enlargement as shown by Shafi et al.65 These findings may assist in prompt diagnosis and quantification of burden of UC, allowing for directed pharmacotherapy.
Electrocardiography may also have a role in SCD and mortality stratification in patients with ESRD on dialysis.66 There is association between the ECG parameters of QT interval, spatial QRS-T angle, signal averaged ECG, heart rate variability and T-wave alternans with increased rates of mortality and SCD in patients on haemodialysis, however it is unclear if these are independent risk factors or whether they predispose to structural cardiac disease such as LVH that may predispose patients to SCD.66
Echocardiography
Cardiac remodelling is a fundamental process that occurs in UC, affecting all chambers through intricate mechanisms involving pressure and volume overload. TTE is a non-invasive and readily accessible imaging modality that provides detailed geometric and functional assessment of the cardiac structures throughout systole and diastole.67 Its utility also lies in that we know echocardiographic cardiovascular disease is already present in a high proportion of patients starting ESRD therapy and are independent mortality factors.68 In a trial of approximately 600 patients on HD without symptomatic cardiac disease or cardiac dilatation, there was progression of LVH, with LVMI (LV Mass Index) 114 g/m2 at baseline increasing to 128 g/m2 at 96 weeks.69 LVH also portends significant all-cause and CV mortality with (HR 2.9) and (HR 2.7) respectively.70 In addition to presence of LVH as determined by LVMI, there is also evidence of direct impairment in LV systolic function, and this correlates with cardiovascular mortality as shown by Kramann et al.71 However there has been conflicting data on whether patients undergoing renal replacement therapy have progression of LVH and LV systolic dysfunction, with Shi et al demonstrating the maintenance of stable LV structure and systolic function in a cohort of 40 patients on peritoneal dialysis (PD).72 Furthermore, peak global longitudinal strain is also an independent risk factor for cardiovascular mortality and circumferential early diastolic strain rate is an independent risk factor for all-cause mortality.71 Other studies have shown similar findings, as assessed by 2-dimensional speckle tracking echocardiography (2D-STE), demonstrating impaired LV longitudinal strain in patients with CKD when compared to controls (P < .001), highlighting significant subclinical systolic dysfunction.72 These findings highlight the importance of echocardiography and speckle-tracking echocardiography for sensitive assessment of LV systolic function in this population group for diagnosis and prognostication.
The severity of UC also parallels grades of diastolic dysfunction (elevated E/e’, P < .001), highlighting a direct correlation between renal failure, volume overload and impaired relaxation.73 LA indices are a surrogate for diastolic impairment in many cardiac pathologies. Patel et al. have shown that an elevated LA volume indexed (LAVI) correlates with poor survival in patients on haemodialysis, and that LAVI and LVF portend similar correlations with death on multivariate analysis.74 In addition, Zapolski et al have shown evidence of reverse atrial remodelling in patients post renal transplantation with significant reductions in LAVI from a mean of 34.63 to 27.57 after 3 years.75 These findings highlight the significant association of left atrial dysfunction and prognosis in these patients. In essence, a correction of the underlying pathology and subsequent haemodynamics results in reversal of LA myocyte dysfunction.
However, despite its immense utility, there are limitations to TTE in UC. A meta-analysis of 73 studies by Badve et al in 2016, which investigated whether LVM could be used as a surrogate end point for all-cause and cardiovascular mortality in CKD, particularly in the context of pharmacologic or nonpharmacologic interventions, suggested that there was no consistent association between intervention-induced LVM change and mortality.76 Therefore, while TTE has a role in assessment of cardiac function, clinicians should be cognisant of its limitations within such contexts.
Cardiac MRI
The role of cardiac MRI in quantification of myocardial fibrosis in UC is a growing field of expertise, specifically as the extent of fibrosis is a strong biomarker for cardiovascular death.67 This fibrotic process can be demonstrated non-invasively on CMR by T1 mapping; a technique that quantifies the relaxation time of protons on inversion recovery prepared images (T1 times) by using analytical expression of image-based signal intensities.77 T1 relaxation times increase with interstitial expansion due to oedema, infarction, infiltration and fibrosis.10 Patients on haemodialysis have been shown to have higher global T1 (ms) (1171 vs 1154), LV mass indexed (g/m2) (69.8 vs 55), LVH (%) (42.4 vs 3.6) and a lower peak GLS (%) (−17.7 vs 21.8) when compared to healthy controls.78 In the group of patients on haemodialysis, the peak GLS significantly correlated with LV mass indices (R = 0.426) as well as galectin-3, a biomarker of cardiac fibrosis.78 This highlights the presence of findings consistent with myocardial fibrosis on CMRI and a potential relationship with structural and functional abnormalities.78 In perspective, Charytan et al showed 12% increase in myocardial fibrosis in stage 3 to 4 CKD patients and a 77% increase in stage 5 CKD when compared to healthy controls.79 In addition to myocardial fibrosis, myocardial oedema as demonstrated by dynamic changes in T1 and T2 mapping values with volume-removal on HD can help differentiate between UC and other hypertrophic phenotypes.80 Kotecha et al demonstrated both native T1 and T2 values reduce significantly post HD with these changes suggestive of reduction in myocardial water content rather than regression of LVH.81 These findings elucidate the importance of this underlying fibrotic process and myocardial oedema as a key mechanism in the pathophysiology of UC.
Myocardial biopsy
Myocardial biopsy is an invasive diagnostic modality that may be of use in undifferentiated pathologies or for guidance of appropriate treatment. Studies on myocardial biopsy show that many subjects with ESRD have myocardial appearances resembling the dilated phase of hypertrophic cardiomyopathy (HCM) with severe myocyte hypertrophy and myocyte disarray.82 In severe cases, there is evidence of diffuse myocardial fibrosis (DMF) and replacement fibrosis with significant increases in volume of extracellular matrix.83 Whilst the identification of myocardial fibrosis has historically been done with myocardial biopsy, its role is limited by sampling error in addition to significant morbidity and mortality associated with the procedure.17 Hence myocardial biopsy could be cautiously considered in conjunction with cardiac MRI to assist quantification of fibrosis and in the diagnosis of UC where imaging modalities alone are indefinite.
Management
The management of UC is multi-faceted and involves a comprehensive approach in a multi-disciplinary (cardiologist, nephrologist, dialysis team) setting with a complete understanding of the underlying pathophysiological disease states (see Table 2). Specifically, overactivity of the underlying sympathetic nervous system (SNS) and renin-angiotensin activating system (RAAS) require directed treatment approach.84
Table 2.
Treatment of uraemic cardiomyopathy.
Pharmacological
There are several derived pharmacological treatment approaches for management and reversal of this disease process, which includes beta-blockade, angiotensin receptor blockers, mineralocorticoid antagonists and HMG-CoA reductase inhibitors.84
Carvedilol has been shown to improve symptoms, LV End-Diastolic Pressure (LVEDP), LV End-Systolic Volume (LVESV) (74-62 ml/m2) and LVEF (26.3%-34.8%) along with a reduction in all-cause cause mortality and hospital admissions in patients on haemodialysis with dilated cardiomyopathy.85
The role of renin-angiotensin system (RAS) blockade in patients with CKD with and without diabetic nephropathy is well known. RAS blockade was associated with a decreased risk of heart failure in patients with diabetic nephropathy (0.78, 95%CI 0.66-0.92, P = .03) and without diabetic nephropathy (0.74, 95%CI 0.58-0.95, P = .02) when compared to placebo in addition to a reduction in the risk of myocardial infarction and total CV outcomes.86 Spironolactone, when used in patients with stage II-III CKD has been shown to reduce LV mass (−14 ± 13 g vs +3 ± 11 g) and arterial stiffness when compared to placebo.87 However, within the haemodialysis population, there appears to be a more limited benefit with Spironolactone; for instance, a study by Hammer et al suggested that in comparison to placebo, Spironolactone did not improve left ventricular mass index in the haemodialysis population,88 and this was also supported by a study by Charytan et al which compared Spironolactone to placebo in the haemodialysis population, and they too did not find an improvement in cardiovascular function or structure in the Spironolactone group.89 There has also been significant concern with Angiotensin Converting Enzyme-Inhibitors (ACE-I), Angiotensin Receptor Blockers (ARB) and Spironolactone causing hyperkalaemia, which is not limited to the population with ESRD.1
The SHARP trial has shown that the addition of simvastatin plus ezetimibe in patients with CKD has resulted in a reduction of first major atherosclerotic events.90 However, it is worth noting that the 4D and AURORA investigators found that in the haemodialysis population, statins, in comparison to placebo, did not reduce the risk of cardiovascular events in the AURORA study and did not improve the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and stroke in the 4D study.91,92 On the other hand, in the renal transplant population, the ALERT study, which compared Fluvastatin to placebo, suggested an improvement in cardiac deaths and non-fatal myocardial infarction in those treated with Fluvastatin; however, it is worth nothing that Fluvastatin did not generally reduce rates of mortality or coronary intervention procedures.93 Therefore, the beneficial role of statins may be dependent on the context of the population managed.
The role of Vitamin D is also increasingly being explored, with a study by McGonigle et al demonstrating that the addition of Vitamin D in patients on HD resulted in a significant reduction in PTH, increase in LV fibre fraction shortening and an increase in mean velocity of LV fibre shortening. These findings highlight the importance of targeted pharmacotherapy in this population group.94
The role of erythropoietin in treatment of anaemia in CKD and its effect of mortality has been a controversial area but is best exemplified by several studies. The CHOIR investigation explored whether EPO treatment to a higher haemoglobin (Hb) target of 13.5 grams (g) per decilitre (dL) compared to Hb target of 11.3 g/dL would be beneficial, and found that this higher target was not associated with additional improvement in quality of life and in fact was associated with increased harm.95 The CREATE investigators investigated the role of EPO in CKD stage 3 and 4 in early and complete correction of anaemia versus partial correction of anaemia, and found that cardiovascular events were not reduced in those with early and complete correction.96 This was supported by the TREAT investigators, who found that the use of darbepoetin alfa in CKD patients not undergoing dialysis with co-morbid type 2 diabetes and moderate anaemia to a target of 13 g/dL, compared to placebo (albeit with rescue darbepoetin alfa when the Hb was less than 9 g/dL), resulted in an increased risk of stroke and did not confer a survival benefit.97 Therefore, while correction of anaemia with EPO may be required in such populations, clinicians need to be cognisant of its potential adverse effects.
Intertwined with the administration of EPO in CKD patients, the PIVOTAL investigators explored the role of high dose versus lose dose intravenous iron supplementation in CKD patients recently initiated on haemodialysis with ferritin concentrations less than 400 μg/L and a transferrin saturation of less than 30% who were receiving an EPO agent. They found that the high dose supplemental group had fewer major adverse cardiovascular events and lower risk of death.98 Interestingly, this high dose regimen group also required fewer blood transfusions and lower doses of EPO, which as mentioned previously could potentially be associated with adverse effects.
Dialysis
An important treatment for UC in the setting of ESRD is haemodialysis (HD). There is evidence to suggest it is associated with a reduction in LVH, and reversal of systolic dysfunction (LVEF change (%) 31–50).99,100 Short daily HD (~2 hours daily) in comparison to conventional HD has also been shown to reduce LVH (LVMI change: 120.1 ± 60.4 g/m(2)) and antihypertensive use.101 Therefore, frequent dialysis with close maintenance of euvolemia largely potentiates reverse cardiac remodelling. Similarly, nocturnal home HD has also shown to reduce LVMI by 22% compared to a 6% progression on conventional HD in a cohort of patients with ESRD associated cardiomyopathy.102 However, despite many studies that have shown HD can potentiate and reverse some of the clinical sequelae of UC, there is conflicting data from other studies. In 2015, Foley et al demonstrated in a population of 596 HD patients with no symptomatic cardiac disease or dilatation that there was progressive concentric LVH and hyperkinesis.72 This suggests the interplay of other factors such as hypertension that may influence the role of HD on UC. There is data to suggest that PD may portend increased mortality in patients with UC which increases with the length of follow up and has increased requirement of anti-hypertensives.3,103 Important complications to monitor for with HD include dialysis-induced myocardial stunning, resulting in further hypotension, which may compound the underlying cardiomyopathy.104
Renal transplantation
Renal transplantation can partially reverse the underlying disease state and confers a significant survival advantage compared to the other treatment modalities described. Dzemidzić et al have shown that renal transplantation dramatically reduces the proportion of patients with LVH from 67% to 37% with correlations in improved creatinine clearance and in reduction of the serum creatinine values; as well as values of parathyroid hormone.105 Additionally, Wali et al have shown that 70% of patients with a baseline LVEF <40% prior to renal transplantation, recovered at follow-up at 6 months with LVEF >50% on ventriculography-gated blood pool. Normalization of LVEF was associated with improvement in NYHA class and was the only significant factor associated with reduced hazard for CCF hospitalization and death (RR 0.90).106 Native T1 measurements, a method of assessment of myocardial fibrosis in CMRI without gadolinium contrast, was explored by Contti et al where it was found that 6 months following renal transplantation, the native myocardial T1 time decreased, suggestive of regression of reactive fibrosis.107 These findings demonstrate significant reverse cardiac remodelling as a result of renal transplantation in this high-risk population group.
Conclusion
UC remains a complex and multifaceted disease with high associated morbidity and mortality. Amongst existing therapies, haemodialysis and kidney transplantation are instrumental in halting disease progression.3 The role of non-invasive and invasive diagnostic tools alongside our growing understanding of the pathophysiology of UC remains vital in illuminating the path for future medical and surgical therapies. Thus, a structured approach which utilises existing therapies in addition to ongoing research on a cellular level may represent the best opportunity of reducing the burden of UC.
Footnotes
Funding:The author(s) received no financial support for the research, authorship and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Author Contributions: All authors contributed significantly to the final manuscript.
CREDIT Author Statement: Kartheek Garikapati
• Conceptualisation
• Visualisation
• Writing original draft
• Project administration
Daniel Goh
• Writing, Reviewing and Editing
• Formal analysis
Shaun Khanna
• Formal analysis
• Writing original draft
• Resources
Krishna Echampati
• Writing, Reviewing and Editing
• Supervision
ORCID iDs: Kartheek Garikapati  https://orcid.org/0000-0001-8226-7021
https://orcid.org/0000-0001-8226-7021
Shaun Khanna  https://orcid.org/0000-0002-6741-1096
https://orcid.org/0000-0002-6741-1096
References
- 1. Wang X, Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol. 2019;15(3):159-175. [DOI] [PubMed] [Google Scholar]
- 2. Roberts MA, Polkinghorne KR, McDonald SP, Ierino FL. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis. 2011;58(1):64-72. [DOI] [PubMed] [Google Scholar]
- 3. Alhaj E, Alhaj N, Rahman I, Niazi TO, Berkowitz R, Klapholz M. Uremic cardiomyopathy: an underdiagnosed disease. Congest Heart Failure. 2013;19(4):E40-E50. [DOI] [PubMed] [Google Scholar]
- 4. Grollier G, Hurault DLB, Bonnet H, Scanu P, Potier J. So-called uremic heart diseases. Arch Mal Coeur Vaiss. 1990;83(3):401. [PubMed] [Google Scholar]
- 5. de Albuquerque Suassuna PG, Sanders-Pinheiro H, de Paula RB. Uremic cardiomyopathy: a new piece in the chronic kidney disease-mineral and bone disorder puzzle. Front Med. 2018;5:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rostand SG, Gretes JC, Kirk KA, Rutsky EA, Andreoli TE. Ischemic heart disease in patients with uremia undergoing maintenance hemodialysis. Kidney Int. 1979;16(5):600-611. [DOI] [PubMed] [Google Scholar]
- 7. Rostand SG, Kirk KA, Rutsky EA. Dialysis-associated ischemic heart disease: insights from coronary angiography. Kidney Int. 1984;25(4):653-659. [DOI] [PubMed] [Google Scholar]
- 8. Clyne N, Lins L-E, Pehrsson SK. Occurrence and significance of heart disease in uraemia: an autopsy study. Scand J Urol Nephrol. 1986;20(4):307-311. [DOI] [PubMed] [Google Scholar]
- 9. Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10(7):1606-1615. [DOI] [PubMed] [Google Scholar]
- 10. Radhakrishnan A, Pickup LC, Price AM, Law JP, Edwards NC, Steeds RP, et al. Coronary microvascular dysfunction: a key step in the development of uraemic cardiomyopathy? Heart. 2019;105(17):1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grabner A, Faul C. The role of FGF23 and klotho in uremic cardiomyopathy. Curr Opin Nephrol Hypertens. 2016;25(4):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lisowska-Myjak B. Uremic toxins and their effects on multiple organ systems. Nephron Clin Pract. 2014;128:303-311 [DOI] [PubMed] [Google Scholar]
- 13. Vlagopoulos PT, Sarnak MJ. Traditional and nontraditional cardiovascular risk factors in chronic kidney disease. Med Clin North Am. 2005;89:587-661 [DOI] [PubMed] [Google Scholar]
- 14. Foley RN, Parfrey PS. Cardiac disease in chronic uremia: clinical outcome and risk factors. Adv Ren Replac Ther. 1997;1:234-248. [DOI] [PubMed] [Google Scholar]
- 15. Brønnum H, Kalluri R. Cardiac fibrosis: cellular and molecular determinants. In: Muscle. Elsevier Inc.; 2012:389-404. [Google Scholar]
- 16. Heinzel FR, Hohendanner F, Jin G, Sedej S, Edelmann F. Myocardial hypertrophy and its role in heart failure with preserved ejection fraction. J Appl Physiol. 2015;119(10):1233-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham-Brown MP, Patel A, Stensel DJ, March DS, Marsh A-M, McAdam J, et al. Imaging of myocardial fibrosis in patients with end-stage renal disease: current limitations and future possibilities. BioMed Res Int. 2017;2017:5453606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winchester JF, Audia PF, eds. Unresolved Issues in Dialysis: Extracorporeal Strategies for the Removal of Middle Molecules. Seminars in Dialysis. Wiley Online Library; 2006. [DOI] [PubMed] [Google Scholar]
- 19. Lekawanvijit S. Cardiotoxicity of uremic toxins: a driver of cardiorenal syndrome. Toxins. 2018;10(9):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61(1):9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lingrel JB, Van Huysse J, O’Brien W, Jewell-Motz E, Askew R, Schultheis P. Structure-function studies of the Na, K-ATPase. Kidney Int Suppl. 1994;44:S32-S39. [PubMed] [Google Scholar]
- 22. Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Coordinated shifts in Na/K-ATPase isoforms and their endogenous ligands during cardiac hypertrophy and failure in NaCl-sensitive hypertension. J Hypertens. 2004;22(2):389-397. [DOI] [PubMed] [Google Scholar]
- 23. Kennedy D, Malhotra D, Shapiro J. Molecular insights into uremic cardiomyopathy: cardiotonic steroids and Na/K ATPase signaling. Cell Mol Biol. 2006;52(8):3-14. [PubMed] [Google Scholar]
- 24. Dubin RF, Guajardo I, Ayer A, et al. Associations of macro- and microvascular endothelial dysfunction with subclinical ventricular dysfunction in end-stage renal disease. Hypertension. 2016;68(4):913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhandari S. Risk factors and metabolic mechanisms in the pathogenesis of uraemic cardiac disease. Front Biosci. 2011;16:1364-1387. [DOI] [PubMed] [Google Scholar]
- 26. Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28(1):53-61. [DOI] [PubMed] [Google Scholar]
- 27. Hordijk P, Vervloet M. Most exposed: the endothelium in chronic kidney disease. Nephrol Dial Transplant. 2019;35(9):1478-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ioannou K, Stel VS, Dounousi E, Jager KJ, Papagianni A, Pappas K, et al. Inflammation, endothelial dysfunction and increased left ventricular mass in chronic kidney disease (CKD) patients: a longitudinal study. PLoS One. 2015;10(9):e0138461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellinghieri G, Santoro D, Calvani M, Mallamace A, Savica V. Carnitine and hemodialysis. Am J Kidney Dis. 2003;41(3):S116-S120. [DOI] [PubMed] [Google Scholar]
- 30. Reddy V, Bhandari S, Seymour A-ML. Myocardial function, energy provision, and carnitine deficiency in experimental uremia. J Am Soc Nephrol. 2007;18(1):84-92. [DOI] [PubMed] [Google Scholar]
- 31. Kaneko M, Fukasawa H, Ishibuchi K, Niwa H, Yasuda H, Furuya R. L-carnitine improved the cardiac function via the effect on myocardial fatty acid metabolism in a hemodialysis patient. Intern Med. 2019;57(24):3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shinohara K, Shoji T, Emoto M, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13(7):1894-1900. [DOI] [PubMed] [Google Scholar]
- 33. Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16(4):1091-1098. [DOI] [PubMed] [Google Scholar]
- 34. Dogra G, Irish A, Chan D, Watts G. Resistance, inflammation, and blood pressure determine vascular dysfunction in CKD. Am J Kidney Dis. 2006;48(6):926-934. [DOI] [PubMed] [Google Scholar]
- 35. Nishimura M, Murase M, Hashimoto T, et al. Insulin resistance and impaired myocardial fatty acid metabolism in dialysis patients with normal coronary arteries. Kidney Int. 2006;69(3):553-539. [DOI] [PubMed] [Google Scholar]
- 36. Takenaka T, Kanno Y, Ohno Y, Suzuki H. Key role of insulin resistance in vascular injury among hemodialysis patients. Metabolism. 2007;56(2):153-159. [DOI] [PubMed] [Google Scholar]
- 37. Thomas SS, Zhang L, Mitch WE. Molecular mechanisms of insulin resistance in chronic kidney disease. Kidney Int. 2015;88(6):1233-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Semple D, Smith K, Bhandari S, Seymour A-ML. Uremic cardiomyopathy and insulin resistance: a critical role for akt? J Am Soc Nephrol. 2011;22(2):207-215. [DOI] [PubMed] [Google Scholar]
- 39. Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38(1):63-71. [DOI] [PubMed] [Google Scholar]
- 40. Kato MF, Shibata R, Obata K, et al. Pioglitazone attenuates cardiac hypertrophy in rats with salt-sensitive hypertension: role of activation of AMP-activated protein kinase and inhibition of Akt. J Hypertens. 2008;26(8):1669-1676. [DOI] [PubMed] [Google Scholar]
- 41. Siedlecki AM, Jin X, Muslin AJ. Uremic cardiac hypertrophy is reversed by rapamycin but not by lowering of blood pressure. Kidney Int. 2009;75(8):800-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol. 2016;11(6):1088-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Otani-Takei N, Masuda T, Akimoto T, et al. Association between serum soluble klotho levels and mortality in chronic hemodialysis patients. Int J Endocrinol. 2015;2015:406269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. London GM, Fabiani F, Marchais SJ, et al. Uremic cardiomyopathy: an inadequate left ventricular hypertrophy. Kidney Int. 1987;31(4):973-980. [DOI] [PubMed] [Google Scholar]
- 46. Walker M, Fleischer J, Di Tullio M, et al. Cardiac structure and diastolic function in mild primary hyperparathyroidism. J Clin Endocrinol Metabol. 2010;95(5):2172-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Segall L, Nistor I, Covic A. Heart failure in patients with chronic kidney disease: a systematic integrative review. BioMed Res Int. 2014;2014:937398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang AY-M, Lam CW-K, Sanderson JE, et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr. 2008;87(6):1631-1638. [DOI] [PubMed] [Google Scholar]
- 49. Stróżecki P, Adamowicz A, Nartowicz E, Odrowąż-Sypniewska G, Włodarczyk Z, Manitius J. Parathormon, calcium, phosphorus, and left ventricular structure and function in normotensive hemodialysis patients. Renal Failure. 2001;23(1):115-126. [DOI] [PubMed] [Google Scholar]
- 50. Chue CD, Edwards NC, Moody WE, Steeds RP, Townend JN, Ferro CJ. Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: a cardiac magnetic resonance study. Heart. 2012;98(3):219-224. [DOI] [PubMed] [Google Scholar]
- 51. Galetta F, Cupisti A, Franzoni F, et al. Left ventricular function and calcium phosphate plasma levels in uraemic patients. J Int Med. 2005;258(4):378-384. [DOI] [PubMed] [Google Scholar]
- 52. Achinger SG, Ayus JC. Left ventricular hypertrophy: is hyperphosphatemia among dialysis patients a risk factor? J Am Soc Nephrol. 2006;17(12 suppl 3):S255-S261. [DOI] [PubMed] [Google Scholar]
- 53. Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118(12):3820-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grabner A, Amaral AP, Schramm K, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metabol. 2015;22(6):1020-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leifheit-Nestler M, Große Siemer R, Flasbart K, et al. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant. 2016;31(7):1088-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moe SM, Chertow GM, Parfrey PS, et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation. 2015;132(1):27-39. [DOI] [PubMed] [Google Scholar]
- 57. Hao H, Li X, Li Q, et al. FGF23 promotes myocardial fibrosis in mice through activation of β-catenin. Oncotarget. 2016;7(40):64649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu MC, Kuro-o M, Moe OW, eds. Renal and Extrarenal Actions of Klotho. Seminars in Nephrology. Elsevier; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hu MC, Shi M, Cho HJ, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26(6):1290-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xie J, Yoon J, An S-W, Kuro-o M, Huang C-L. Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. 2015;26(5):1150-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kuwahara K, Wang Y, McAnally J, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116(12):3114-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stewart GA, Gansevoort R, Mark PB, et al. Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int. 2005;67(1):217-226. [DOI] [PubMed] [Google Scholar]
- 65. Saleem M, Anjum R, Abdullah W, Shafi T. ECG abnormalities in patients with chronic kidney disease. J Ayub Med Coll: JAMC. 2017;29:61-64. [PubMed] [Google Scholar]
- 66. Waks JW, Tereshchenko LG, Parekh RS. Electrocardiographic predictors of mortality and sudden cardiac death in patients with end stage renal disease on hemodialysis. J Electrocardiol. 2016;49(6):848-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McIntyre CW, John SG, Jefferies HJ. Advances in the Cardiovascular Assessment of Patients with Chronic Kidney Disease. Oxford University Press; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(1):186-192. [DOI] [PubMed] [Google Scholar]
- 69. Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol. 2010;5(5):805-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36(2):286-290. [DOI] [PubMed] [Google Scholar]
- 71. Kramann R, Erpenbeck J, Schneider RK, et al. Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J Am Soc Nephrol. 2014;25(10):2351-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shi Q, Zhu J, Feng S, Shen H, Chen J, Song K. Nonparallel progression of left ventricular structure and function in long-term peritoneal dialysis patients. Cardiorenal Med. 2017;7(3):198-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hassanin N, Alkemary A. Early detection of subclinical uremic cardiomyopathy using two-dimensional speckle tracking echocardiography. Echocardiography. 2016;33(4):527-536. [DOI] [PubMed] [Google Scholar]
- 74. Patel RK, Jardine AG, Mark PB, et al. Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis. 2010;55(6):1088-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zapolski T, Furmaga J, Wysokiński AP, Wysocka A, Rudzki S, Jaroszyński A. The atrial uremic cardiomyopathy regression in patients after kidney transplantation–the prospective echocardiographic study. BMC Nephrol. 2019;20(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Badve SV, Palmer SC, Strippoli GF, et al. The validity of left ventricular mass as a surrogate end point for all-cause and cardiovascular mortality outcomes in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(4):554-563. [DOI] [PubMed] [Google Scholar]
- 77. Mark P, Johnston N, Groenning B, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69(10):1839-1845. [DOI] [PubMed] [Google Scholar]
- 78. Rutherford E, Talle MA, Mangion K, et al. Defining myocardial tissue abnormalities in end-stage renal failure with cardiac magnetic resonance imaging using native T1 mapping. Kidney Int. 2016;90(4):845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Charytan DM, Padera R, Helfand AM, et al. Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol. 2014;176(1):99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arcari L, Hinojar R, Engel J, et al. Native T1 and T2 provide distinctive signatures in hypertrophic cardiac conditions–comparison of uremic, hypertensive and hypertrophic cardiomyopathy. Int J Cardiol. 2020;306:102-108. [DOI] [PubMed] [Google Scholar]
- 81. Kotecha T, Martinez-Naharro A, Yoowannakul S, et al. Acute changes in cardiac structural and tissue characterisation parameters following haemodialysis measured using cardiovascular magnetic resonance. Sci Rep. 2019;9(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Aoki J, Ikari Y, Nakajima H, et al. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int. 2005;67(1):333-340. [DOI] [PubMed] [Google Scholar]
- 83. Chiu DYY, Sinha S, Kalra PA, Green D. Sudden cardiac death in haemodialysis patients: preventative options. Nephrology. 2014;19(12):740-749. [DOI] [PubMed] [Google Scholar]
- 84. Hawwa N, Schreiber MJ, Tang WW. Pharmacologic management of chronic reno-cardiac syndrome. Curr Heart Failure Rep. 2013;10(1):54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cice G, Ferrara L, Di Benedetto A, et al. Dilated cardiomyopathy in dialysis patients—beneficial effects of carvedilol: a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2001;37(2):407-411. [DOI] [PubMed] [Google Scholar]
- 86. Balamuthusamy S, Srinivasan L, Verma M, et al. Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: a meta-analysis. Am Heart J. 2008;155(5):791-805. [DOI] [PubMed] [Google Scholar]
- 87. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54(6):505-512. [DOI] [PubMed] [Google Scholar]
- 88. Hammer F, Malzahn U, Donhauser J, et al. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int. 2019;95(4):983-991. [DOI] [PubMed] [Google Scholar]
- 89. Charytan DM, Himmelfarb J, Ikizler TA, et al. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int. 2019;95(4):973-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238-248. [DOI] [PubMed] [Google Scholar]
- 92. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395-1407. [DOI] [PubMed] [Google Scholar]
- 93. Holdaas H, Fellström B, Jardine AG, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361(9374):2024-2031. [DOI] [PubMed] [Google Scholar]
- 94. McGonigle R, Fowler M, Timmis A, Weston M, Parsons V. Uremic cardiomyopathy: potential role of vitamin D and parathyroid hormone. Nephron. 1984;36(2):94-100. [DOI] [PubMed] [Google Scholar]
- 95. Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085-2098. [DOI] [PubMed] [Google Scholar]
- 96. Drüeke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071-2084. [DOI] [PubMed] [Google Scholar]
- 97. Pfeffer MA, Burdmann EA, Chen C-Y, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019-2032. [DOI] [PubMed] [Google Scholar]
- 98. MacDougall IC, White C, Anker SD, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380(5):447-458. [DOI] [PubMed] [Google Scholar]
- 99. Zoccali C, Mallamaci F, Benedetto FA, et al. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12(7):1508-1515. [DOI] [PubMed] [Google Scholar]
- 100. Adhyapak SM, Iyengar SS. Characteristics of a subset of patients with reversible systolic dysfunction in chronic kidney disease. Congest Heart Failure. 2011;17(3):120-126. [DOI] [PubMed] [Google Scholar]
- 101. Fagugli RM, Reboldi G, Quintaliani G, et al. Short daily hemodialysis: blood pressure control and left ventricular mass reduction in hypertensive hemodialysis patients. Am J Kidney Dis. 2001;38(2):371-376. [DOI] [PubMed] [Google Scholar]
- 102. Pauly RP, Chan CT, eds. Cardiovascular and Survival Paradoxes in Dialysis Patients: Reversing the Risk Factor Paradox: Is Daily Nocturnal Hemodialysis the Solution? Seminars in Dialysis. Wiley Online Library; 2007. [DOI] [PubMed] [Google Scholar]
- 103. Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B. Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int. 2003;64(3):1071-1079. [DOI] [PubMed] [Google Scholar]
- 104. Breidthardt T, McIntyre CW. Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Rev Cardiovasc Med. 2011;12(1):13-20. [DOI] [PubMed] [Google Scholar]
- 105. Džemidžić J, Rašić S, Saračević A, et al. Predictors of left ventricular remodelling in kidney transplant recipents in the first posttransplant year. Bosnian J Basic Med Sci. 2010;10(Suppl 1):S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wali RK, Wang GS, Gottlieb SS, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005;45(7):1051-1060. [DOI] [PubMed] [Google Scholar]
- 107. Contti MM, Barbosa MF, Mauricio ADCV, et al. Kidney transplantation is associated with reduced myocardial fibrosis. A cardiovascular magnetic resonance study with native T1 mapping. J Cardiovasc Magn Reson. 2019;21(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]