Abstract
A simple method is required to screen for sarcopenia in patients with chronic liver disease. In the present study, the value of the existing SARC-F questionnaire as well as calculated body muscle mass (CBMM) approaches were assessed for screening of sarcopenia. A total of 482 patients with chronic liver disease underwent CBMM, grip strength (GS) and SARC-F score assessments. Cross-sectional computed tomography images of the third lumbar vertebrae were analyzed to determine the skeletal muscle (SM) mass in 303 patients. Cutoff CBMM values for sarcopenia were <27.903 in females and <39.731 in males. The cutoff SARC-F score for sarcopenia was ≥4 points. Sarcopenia was diagnosed using the criteria described in the Japan Society of Hepatology. GS was moderately correlated with SARC-F score (females, R=-0.578; males, -0.453) and CBMM (females, R=0.497; males, 0.548). The SM index was moderately correlated with CBMM for both sexes (females, R=0.546; males, 0.612), but not with SARC-F score in females (females, R=-0.132; males, -0.246). The area under the curve (AUC) for CBMM against sarcopenia (0.85964) was significantly larger than that for SARC-F score (0.72013) amongst males (P=0.03577) but not females. The AUCs for a modified SARC-F questionnaire (encompassing the SARC-F questionnaire, CBMM, sex and age; mSARC-F) against sarcopenia were 0.864 in males and 0.78185 in females. As a screening method, SARC-F is less useful than CBMM. However, the AUC for mSARC-F is greater than SARC-F and CBMM.
Keywords: CBMM, chronic liver disease, grip strength, SARC-F, sarcopenia, skeletal muscle mass
Introduction
Sarcopenia is a harmful condition in patients with chronic liver disease (1). The definition of sarcopenia varies based on the criteria used. The European Working Group on Sarcopenia in Older People (EWGSOP) defined sarcopenia as encompassing a low handgrip strength (GS), slow walking speed and low skeletal muscle (SM) mass in 2010(2). The International Working Group on Sarcopenia suggested criteria similar to the criteria defined by the EWGSOP, but with a different walking speed in 2011(3). The criteria for Asian people of small builds was developed by the Asian Working Group for Sarcopenia in 2014(4). In 2019, the EWGSOP revised its criteria (5) to include an algorithm for case-finding, diagnosing and quantifying the severity of sarcopenia, and a simple questionnaire (SARC-F) (6). In 2020, the AWGS also revised its criteria to include use of the calf circumference (CC), SARC-F, or SARC-F and CC together for identifying cases in primary health care settings (7). SARC-F has been suggested to be a possible rapid diagnostic test for diagnosing sarcopenia and includes only five areas of consideration: Strength, assistance with working, rising from a chair, climbing stairs and falls (6). Several reports have previously described the potential of SARC-F for screening of sarcopenia in patients with chronic liver disease (8). In our previous study, it was reported that the calculated body muscle mass (CBMM) is a useful screening marker for discerning low SM mass and sarcopenia in chronic liver disease (9). CBMM was calculated using body weight in kg, serum creatinine (Cr) and serum cystatin C (CysC), and the approximated body muscle mass was measured using dual-energy X-ray absorptiometry in both derivation and validation cohorts (10).
The Japan Society of Hepatology (JSH) decided to establish its own criteria for the assessment of sarcopenia in liver disease in 2015 due to a high number of patients with liver disease and sarcopenia (11). Based on the JSH criteria, if the GS is <26 kg in men or <18 kg in women, muscle volume should be evaluated using computed tomography (CT) or bioelectrical impedance analysis. The JSH criteria was used for diagnosing sarcopenia in the present study. Hiraoka et al (12) reported the value of the finger-ring test as an effective screening method for predicting early-stage muscle atrophy in patients with chronic liver disease. Additionally, Hirota et al (13) reported that the liver frailty index predicted muscle atrophy with high sensitivity, even in patients with normal GS. As the value of SARC-F and CBMM have both been evaluated in assessing liver disease, the abilities of these indices in screening for sarcopenia according to the JSH criteria was assessed in the present study. Additionally, a simple diagnostic tool for screening sarcopenia in chronic liver disease was established.
Patients and methods
Patients
A series of 482 patients with chronic liver disease were admitted to Nagasaki Harbor Medical Center between October 2019 and April 2020. In the outpatient department, patients were evaluated for the cause of their liver disease (for example, hepatitis C virus, hepatitis B virus, autoimmune hepatitis, primary biliary cholangitis and other causes); degree of liver damage [using the Child-Pugh score (14), albumin-bilirubin score (15), model for end-stage liver disease (16) and fibrosis-4(17)]; renal function [measuring, serum Cr, CysC, Cr-glomerular filtration rate (GFR) and CysC-GFR]; body mass index (kg/m2); GS (kg); and SARC-F score (Table I). Diabetes mellitus status was evaluated based on the patients' history and prescribed medication at recruitment in the present study. Of the 482 patients, 273 were screened for hepatocellular carcinoma using CT. Informed consent was obtained from each patient included in the study, and the patients were guaranteed the option to leave the study at any point. The study protocol conformed to the Ethical Guidelines of the 1975 Declaration of Helsinki, and was approved by the Human Research Ethics Committee of Nagasaki Harbor Medical Center (approval no. H30-031).
Table I.
Clinicopathological characteristics of the patients.
| A, All cases, n=482 | |
|---|---|
| Characteristic | Number/mean (SD) |
| Sex, female/male | |
| Female | 281 |
| Male | 201 |
| Age, years | 66.29 (14.3) |
| Height, m | 1.584 (0.096) |
| Body weight, kg | 60.003 (14.142) |
| BMI, kg/m2 | 23.79 (4.6) |
| Liver disease | |
| AIH | 24 |
| AL | 31 |
| HBV | 97 |
| HCV | 18 |
| Complicated malignancy disease | |
| CCC | 1 |
| HCC | 12 |
| Gastric cancer | 2 |
| Pancreatic cancer | 5 |
| RCC | 1 |
| Diabetes mellitus | 80 |
| Total bilirubin, mg/dla | 1.068 (3.589) |
| Albumin, g/dlb | 4.324 (3.145) |
| Prothrombin time, %c | 102.65 (17.8) |
| Prothrombin time, INRd | 1.007 (0.159) |
| Hepatic encephalopathyi | |
| 1 | 477 |
| 2 | 5 |
| 3 | 0 |
| Ascitesi | |
| 1 | 467 |
| 2 | 14 |
| 3 | 1 |
| Cr, mg/le | 0.93 (0.996) |
| Cr-eGFR, ml/min/1.73 m2 | 67.51 (20.636) |
| CysC, mg/f | 1.188 (0.875) |
| CysC-eGFR, ml/min/1.73 m2 | 68.05 (25.731) |
| Platelets, x104/µlg | 18.66 (7.1) |
| AST, U/lh | 42.317 (57.25) |
| AL, U/l | 38.8 (52.6) |
| CPS | 5.158 (0.642) |
| CPG | |
| A | 463 |
| B | 17 |
| C | 2 |
| MELD | 7.574 (2.428) |
| FIB-4 | 2.901 (2.597) |
| ALBI | -2.923 (2.671) |
| ALBIG | 370/103/9 |
| 1 | |
| 2 | |
| 3 | |
| GS, kg | 19.76 (9.57) |
| GS low/normal | |
| Low | 285 |
| Normal | 197 |
| Sarcopenia Index | 77.02 (30.7) |
| CBMM | 35.54 (8.39) |
| Sarcopenia/normal, CBMM | 168/310 |
| deGFR | -0.109 (18.55) |
| SARC-F | 1.589 (2.05) |
| Sarcopenia/normal, SARC-F | 85/397 |
| B, Patients who underwent an evaluation of body composition, n=273 | |
| Factors | Number/mean (SD) |
| SM, cm2 | 104.4 (27.08) |
| IMAT, cm2 | 7.356 (0.445) |
| VAT, cm2 | 112.86 (88.68) |
| SAT, cm2 | 129.92 (82.32) |
| MA, HU | 30.21 (7.491) |
| SMI, cm2/m2 | 41.42 (8.167) |
| Low SMI/normal | 120/153 |
| Sarcopenia | 96 |
aTotal bilirubin normal range, 0.3-1.2;
balbumin normal range, 3.8-5.2;
cprothrombin time (%), normal range 70-130 and INR normal range, 0.85-1.15;
dCr normal range males, 0.61-1.04 and females 0.47-0.79;
eCysC normal range males, 0.63-0.95 and females 0.56-0.87;
fplatelets normal range males, 13.1-36.2 and females, 13-36.9;
gAST, 10-40;
hALT, 5-40.
iAscites and hepatic encephalopathy grades: 1, absent; 2 controllable; and 3, uncontrollable. BMI, body mass index; AIH, autoimmune hepatitis; AL, ; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; Cr, creatinine; eGFRP, estimated glomerular filtration rate; CysC, cystatic C; AST, aspartate aminotransferase; AL, alcoholic liver disease; CPS, Child-Pugh score; CPG, CPS grade; MELD, The model for end-stage liver disease; FIB-4, fibrosis -4; ALBI, albumin bilirubin index; ALBIG, ALBI grade; GS, grip strength; CBMM, calculated body muscle mass; deGFR, difference in eGFPR; SM, skeletal muscle; IMAT, intramuscular adipose tissue; VAT, visceral adipose tissue; SAT, subcutaneous; MA, muscle attenuation; HU, Housefield units; SMI, SM index; CCC, cholangiocellular carcinoma; HCC, hepatocellular carcinoma; RCC, renal cell carcinoma; SD, standard deviation.
Measurements
Laboratory and anthropometric measurement data were obtained for each patient during the initial hospital visit as standard procedure. Laboratory examinations included the assessment of total bilirubin (mg/dl), albumin (mg/dl), alanine aminotransferase (U/l), aspartate aminotransferase (U/l), platelet counts (104/µl), prothrombin time (percentage), Cr (mg/dl) and CysC (mg/l). Cr- and CysC-based estimated GFRs (eGFRs) (ml/min/1.73 m2) in females and males were calculated using the Japanese Society of Nephrology for Japanese patients equation guidelines (18). Chronic kidney disease (CKD) was staged based on the levels of Cr-based eGFRs in ml/min/1.73 m2 (18). The difference in GFR was calculated as follows (19): Cr-based eGFR - CysC-based eGFR. The sarcopenia index was calculated as follows (20): Cr/CysC x 100. CBMM was calculated as follows (10): CBMM = [body weight (kg) x Cr]/[(K x body weight (kg) x CysC) + Cr], where K=0.00675 for men and K=0.01006 for women. Cutoff CBMM values for sarcopenia were 27.903 in females and 39.731 in males (9). The cutoff SARC-F score for sarcopenia was ≥4 points (6).
GS was measured using a dynamometer (Smedlay Dynamo Meter; TTM) with participants standing in an erect position with both arms at their sides. The maximum results of two tests were used for further analysis. Using the JSH criteria, female patients with a maximum GS <18 kg and male patients with a maximum GS <26 kg were categorized as the low GS group (11).
CT analysis of body composition
Cross-sectional CT images of the third lumbar vertebrae were analyzed using Slice-O-Matic version 5.0 (Tomovision) to determine the SM mass in 273 patients. Muscle areas of interest included the psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques and rectus abdominis. Tissue Hounsfield unit (HU) thresholds ranging from -29 to 150 HU for SMs (21) were used. The SMs were normalized for height using m2 and expressed as cm2/m2 to determine the SM index (SMI). Patients with an SMI <39 cm2/m2 for women and <42 cm2/m2 for men were categorized into the low SMI group. Sarcopenia was diagnosed as low GS and low SMI based on the JSH guidelines for sarcopenia (11).
Statistical analysis
Data were analyzed using StatFlex version 6.0 (Artech Co., Ltd.) and are presented as mean ± standard deviation. Multivariate analyses was performed using logistic regression analyses. Correlations were evaluated based on Pearson's correlation coefficient (R). Receiver operating characteristic (ROC) curve analyses were used to evaluate associations between groups and factors, with the cutoff points being equal values for sensitivity and specificity. P<0.05 was considered to indicate a statistically significant difference.
Results
Correlation between SARC-F, CBMM and SMI
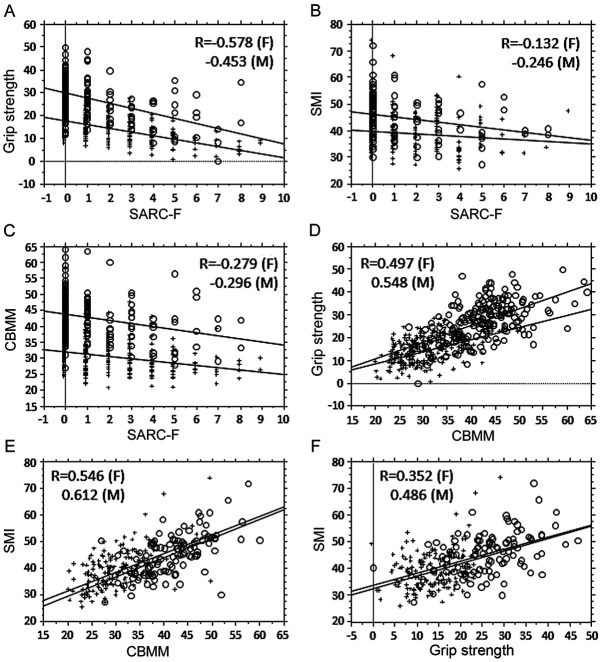
First, the relationship between SARC-F, CBMM, SMI and GS was evaluated (Fig. 1). GS was moderately correlated with SARC-F and CBMM (Fig. 1A and 1D), whereas SMI was moderately correlated with CBMM for both sexes (Fig. 1E), but not with SARC-F in females (Fig. 1B). The association between CBMM and SARC-F was weak (Fig. 1C). Second, the ROC curves in relation to SARC-F, CBMM and sarcopenia were analyzed (Fig. 2). The AUC for CBMM against low GS was larger than that for SARC-F, but the difference was not significant (Fig. 2A and E). Separately, the AUCs for CBMM against low SMI were significantly larger than those for SARC-F for both sexes (Fig. 2B and F), whereas that for CBMM against sarcopenia was significantly larger than that for SARC-F in males (Fig. 2C), but not so amongst females (Fig. 2G). The discrimination efficacy of CBMM for sarcopenia was higher than that of SARC-F in males.
Figure 1.
Relationship between SARC-F score, CBMM and muscle factors. (A) Correlation analysis between SARC-F and GS. Females, R=-0.578, P<0.0001; males, R=-0.453, P<0.0001. Regression line for female GS based on SARC-F=17.51-1.567x SARC-F; regression line for male GS based on SARC-F=29.567-2.193x SARC-F. Females, R2=0.334; males R2=0.205. (B) Correlation analysis between SARC-F and SMI. Females, R=-0.132, P=0.0983; males R=-0.246, P=0.0076. Regression line for female SMI based on SARC-F=39.781-0.491x SARC-F; regression line for male SMI based on SARC-F=46.27-0.986x SARC-F. Females, R2=0.018; males R2=0.06. (C) Correlation analysis between SARC-F and CBMM. Females, R=-0.279, P<0.0001; males R=-0.296, P<0.0001. Regression line for female CBMM based on SARC-F=31.92-0.704x SARC-F; regression line for male CBMM based on SARC-F=43.636-0.961x SARC-F. Females, R2=0.078; males, R2=0.071. (D) Correlation analysis between CBMM and GS. Females, R=0.497, P<0.0001; males, R=0.548, P<0.0001. Regression line for female GS based on CBMM=-1.843+0.533x CBMM; regression line for male GS based on CBMM=-3.798+0.73x CBMM. Females, R2=0.247; males, R2=0.3. (E) Correlation analysis between CBMM and SMI. Females, R=0.546, P<0.0001; males, R=0.612, P<0.0001. Regression line for female SMI based on CBMM=17.438+0.704x CBMM; regression line for male SMI based on CBMM=15.052+0.728x CBMM. Females, R2=0.298; males, R2=0.375 in males. (F) Correlation analysis between SMI and GS. Females, R=0.352, P<0.0001; males, R=0.486, P<0.0001. Regression line for female SMI based on GS=32.251+0.465x GS; regression line for male SMI based on GS=33.396+0.455x GS. Females, R2=0.131; males, R2=0.236. O, males; +, females. CBMM, calculated body muscle mass; GS, grip strength; SMI, skeletal muscle index; F, females; M, males.
Figure 2.
Receiver operating characteristic curve analysis of CBMM, SARC-F score and sarcopenia. (A) Association between low GS with CBMM or SARC-F in males. (B) Association between low SMI with CBMM or SARC-F in males. (C) Association between sarcopenia with CBMM or SARC-F in males. The mSARC-F score was calculated as follows: mSARC-F = SARC-F + CBMM (sarcopenia, 4 points; not sarcopenia, 0 points) + sex (female, 2 points; male, 0 points) + age (≥65 years, 1 point; <65 years, 0 points). (D) Association between sarcopenia and mSARC-F in males. (E) Association between low GS with CBMM or SARC-F in females. (F) Association between low SMI with CBMM or SARC-F in females. (G) Association between sarcopenia with CBMM or SARC-F in females. (H) Association between sarcopenia and mSARC-F in females. (I and J) Association between low GS and mSARC-F in males and females, respectively. (K and L) Association between low SMI and mSARC-F in males and females, respectively. The x-axis is the sensitivity and the y-axis is the specificity; P-values represent comparisons between the AUCs of CBMM and SARC-F in each panel. The fine line refers to CBMM and the bold line refers to SARC-F. AUC, area under the curve; CBMM, calculated body muscle mass; GS, grip strength; skeletal muscle index.
Analysis of factors contributing to sarcopenia and the modified SARC-F
To establish an optimized sarcopenia screening method, the factors that contribute to sarcopenia were evaluated (Table II). In the univariate logistic regression analysis, age (≥65 years), sex (male), albumin-bilirubin score (2-3 points), fibrosis-4 score (<3.25 points), CKD (stages 3-5), SARC-F (≥4 points) and CBMM (low) were significant contributors to the presence of sarcopenia. In the multivariate analysis, age, sex, SARC-F score and CBMM were found to contribute to sarcopenia. As a result, the SARC-F questionnaire was modified (mSARC-F questionnaire) as follows: mSARC-F=SARC-F score + CBMM (sarcopenia, 4 points; not sarcopenia, 0 points) + sex (female, 2 points; male, 0 points) + age (≥65 years, 1 point; <65 years, 0 points). Weighted points for CBMM, SARC-F and older age were decided based on the odds ratio, and 2 points was assigned for female sex. AUCs for mSARC-F against sarcopenia were 0.864 in males (Fig. 2D) and 0.78185 in females (Fig. 2H). When the cutoff mSARC-F for sarcopenia was set to 4 points, the sensitivity and specificity were 0.76923 and 0.68 in males, and 0.8333 and 0.79286 in females, respectively (Table III). AUC for mSARC-F against low GS was 0.79161 in males (Fig. 2I) and 0.7663 in females (Fig. 2J), and that against low SM was 0.7963 in males (Fig. 2K) and 0.62763 in females (Fig. 2L).
Table II.
Factors contributing to sarcopenia.
| Univariate | Multi-variate | |||||
|---|---|---|---|---|---|---|
| Characteristics | P-value | OR | 95% CI | P-value | OR | 95% CI |
| Age ≥65 years | <0.0001c | 6.647 | 3.593-12.3 | 0.0006c | 3.461 | 1.702-7.04 |
| Male sex | 0.0007c | 0.427 | 0.261-0.696 | 0.0012b | 0.375 | 0.208-0.678 |
| High SARC-F score | <0.0001c | 6.157 | 3.364-11.267 | 0.0032b | 2.913 | 1.43-5.936 |
| Low CBMM | <0.0001 | 6.738 | 4.034-11.253 | <0.0001c | 5.113 | 2.854-9.161 |
| ALBIG 2/3 | 0.0173a | 1.897 | 1.12-3.213 | 0.2657 | 1.471 | 0.746-2.902 |
| FIB-4 >3.25 | 0.0004c | 0.41 | 0.251-0.669 | 0.5768 | 0.836 | 0.446-1.567 |
| CKD 3/4/5 | 0.0161a | 1.827 | 1.118-2.985 | 0.281 | 1.403 | 0.758-2.598 |
aP<0.05,
bP<0.01,
cP<0.001. CBMM, calculated body muscle mass; ALBIG, albumin bilirubin index grade; FIB-4, fibrosis 4; CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval.
Table III.
Screening using SARC-F, CBMM, and mSARC-F.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Factor | SARC-F | CBMMa | mSARC-F | SARC-F | CBMMa | mSARC-F |
| Sarcopenia | ||||||
| Sensitivity | 0.15116 | 0.58333 | 0.76923 | 0.17085 | 0.52261 | 0.83333 |
| Specificity | 0.98261 | 0.85187 | 0.68 | 0.97561 | 0.97653 | 0.79286 |
| Low skeletal muscle index | ||||||
| Sensitivity | 0.2093 | 0.71429 | 0.7381 | 0.14286 | 0.57143 | 0.7013 |
| Specificity | 0.9589 | 0.73611 | 0.75 | 0.9 | 0.74684 | 0.61538 |
| Low grip strength | ||||||
| Sensitivity | 0.15116 | 0.58333 | 0.58333 | 0.17085 | 0.51759 | 0.60606 |
| Specificity | 0.96522 | 0.85088 | 0.82456 | 0.97561 | 0.87654 | 0.82716 |
aCutoff values include ≥4 points for SARC- F and mSARC-F in both sexes, and <39 in males <28 in females.
Discussion
When compared with CBMM, the SARC-F showed high specificity but reduced sensitivity for screening of sarcopenia. It is hypothesized that the reasons for the reduced sensitivity include the fact that SARC-F is related to GS but not to muscle mass. As a screening method, the SARC-F questionnaire is less useful than CBMM. However, when SARC-F was modified to encompass CBMM, age and sex (mSARC-F), the AUC of mSARC-F was greater than that of SARC-F and CBMM.
Previous reports have described that SARC-F is a marker of muscle strength (22) and exhibits low sensitivity for sarcopenia (22-24). SARC-F combined with CC or finger-ring testing has been assessed in patients with chronic liver disease as a method of sarcopenia screening (22-25). Additionally, it has been reported that SARC-F is an inadequate screening method for community-dwelling older adults, but a useful screening method in selected populations, such as adults in hospital (26). Interestingly, SARC-F appears suitable for detecting individuals at risk of adverse outcomes from sarcopenia (27), whereas its use alone showed sarcopenia was independently associated with the risk of mortality compared with combination of SARC-F and CC (28). Based on previous reports, it is hypothesized that SARC-F score is more suitable as a marker of disease severity rather than a screening method in patients with sarcopenia.
Separately, CBMM appeared to be a suitable screening method for sarcopenia based on the results of the present study. In our previous study, it was shown that the AUC for CBMM against sarcopenia was 0.78504 in females and 0.85067 in males (9), in agreement with the results of the present study. Since the study population was different between the previous and present study, the efficacy of CBMM for sarcopenia screening has been validated by both. The nature of the association of SMI with CBMM and SARC-F was different. Since CBMM was associated with GS and SMI, CBMM is a better screening tool for sarcopenia than SARC-F. CBMM is simple and minimally invasive to use, where low levels are indicative of sarcopenia in patients with liver disease.
In the present study, sex and age also affected the rate of sarcopenia, and a difference between the sexes was also found in our previous study as well (9). Age is a well-established factor for sarcopenia (29). According to the multivariate analysis encompassing SARC-F, CBMM, sex and age into the mSARC-F questionnaire for sarcopenia screening, the AUCs for mSARC-F against sarcopenia were greater than the AUCs for CBMM. However, the AUC for mSARC-F amongst females was less than that for males. Thus, screening methods in females should be evaluated independently from males.
The present study has some limitations that include the small number of patients with advanced liver disease or CKD, since CBMM is based on Cr and CysC. It is suggested that CBMM is preferable as the screening method, as SARC-F shows less sensitivity and has a lower AUC value for sarcopenia. However, the newly established mSARC-F may be a useful method for screening sarcopenia. SARC-F may instead be better as a marker of the severity of sarcopenia.
In conclusion, CBMM is a more useful sarcopenia screening method than SARC-F, and the newly developed mSARC-F may exhibit better screening ability than both CBMM and mSARC-F.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
TI wrote the paper, analyzed the data and designed the study. HM, SM, YM, MY, SY, MK, YN, TH, HY, RU, OM, YK, KK, NT and KN collected the data. All authors read and approved the final manuscript. TI and HM confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Informed consent was obtained from each patient included in the study, and the patients were guaranteed the option to leave the study at any point. The study protocol conformed to the Ethical Guidelines of the 1975 Declaration of Helsinki, and was approved by the Human Research Ethics Committee of Nagasaki Harbor Medical Center (approval no. H30-031).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, Feshtali S, van Ooijen PMA, Polak WG, Porte RJ, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J Hepatol. 2018;68:707–714. doi: 10.1016/j.jhep.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. European Working Group on Sarcopenia in Older People: Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2009;12:249–256. doi: 10.1016/j.jamda.2011.01.003. IWG on Sarcopenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2: Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malmstrom TK, Morley JE. Sarcopenia: the target population. J Frailty Aging. 2013;2:55–56. doi: 10.14283/jfa.2013.8. [DOI] [PubMed] [Google Scholar]
- 7.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Ida S, Kojima Y, Hamaoka S, Urawa N, Araki J, Kaneko R, Murata K. Validity of Japanese version of SARC-F questionnaire in patients with chronic liver disease. J Gastroenterol Hepatol. 2019;34:947–953. doi: 10.1111/jgh.14449. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, Koike M, Honda T, Yajima H, Uehara R, et al. Calculated body muscle mass as a useful screening marker for low skeleton muscle mass and sarcopenia in chronic liver disease. Hepatol Res. 2020;50:704–714. doi: 10.1111/hepr.13492. [DOI] [PubMed] [Google Scholar]
- 10.Kim SW, Jung HW, Kim CH, Kim KI, Chin HJ, Lee H. A new equation to estimate muscle mass from creatinine and cystatin C. PLoS One. 2016;11(e0148495) doi: 10.1371/journal.pone.0148495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka A, Izumoto H, Ueki H, Yoshino T, Aibiki T, Okudaira T, Yamago H, Suga Y, Iwasaki R, Tomida H, et al. Easy surveillance of muscle volume decline in chronic liver disease patients using finger-circle (yubi-wakka) test. J Cachexia Sarcopenia Muscle. 2019;10:347–354. doi: 10.1002/jcsm.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirota K, Kawaguchi T, Koya S, Nagamatsu A, Tomita M, Hashida R, Nakano D, Niizeki T, Matsuse H, Shiba N, et al. Clinical utility of the Liver Frailty Index for predicting muscle atrophy in chronic liver disease patients with hepatocellular carcinoma. Hepatol Res. 2020;50:330–341. doi: 10.1111/hepr.13453. [DOI] [PubMed] [Google Scholar]
- 14.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 15.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamath PS, Wiesner RH, Malinchoc M, Kremer W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 17.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. Collaborators developing the Japanese equation for estimated GFR. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, Koike M, Takahashi Y, Honda T, Yajima H, et al. Indices calculated by serum creatinine and cystatin C as predictors of liver damage, muscle strength and sarcopenia in liver disease. Biomed Rep. 2020;12:89–98. doi: 10.3892/br.2020.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashani KB, Frazee EN, Kukrálová L, Sarvottam K, Herasevich V, Young PM, Kashyap R, Lieske JC. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med. 2017;45:e23–e29. doi: 10.1097/CCM.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa-Silva TG, Menezes AMB, Bielemann RM, Malmstrom TK, Gonzalez MC. Grupo de Estudos em Composição Corporal e Nutrição (COCONUT): Enhancing SARC-F: Improving sarcopenia screening in the clinical practice. J Am Med Dir Assoc. 2016;17:1136–1141. doi: 10.1016/j.jamda.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Yang M, Hu X, Xie L, Zhang L, Zhou J, Lin J, Wang Y, Li Y, Han Z, Zhang D, et al. Screening sarcopenia in community-dwelling older adults: SARC-F vs SARC-F combined with calf circumference (SARC-CalF) J Am Med Dir Assoc. 2018;19:277.e1–277.e8. doi: 10.1016/j.jamda.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Ida S, Murata K, Nakadachi D, Ishihara Y, Imataka K, Uchida A, Monguchi K, Kaneko R, Fujiwara R, Takahashi H. Development of a Japanese version of the SARC-F for diabetic patients: An examination of reliability and validity. Aging Clin Exp Res. 2017;29:935–942. doi: 10.1007/s40520-016-0668-5. Erratum in: Aging Clin Exp Res 32: 2113, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiraoka A, Nagamatsu K, Izumoto H, Yoshino T, Adachi T, Tsuruta M, Aibiki T, Okudaira T, Yamago H, Suga Y, et al. SARC-F combined with a simple tool for assessment of muscle abnormalities in outpatients with chronic liver disease. Hepatol Res. 2020;50:502–511. doi: 10.1111/hepr.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kera T, Kawai H, Hirano H, Kojima M, Watanabe Y, Motokawa K, Fujiwara Y, Osuka Y, Kojima N, Kim H, et al. Limitations of SARC-F in the diagnosis of sarcopenia in community-dwelling older adults. Arch Gerontol Geriatr. 2020;87(103959) doi: 10.1016/j.archger.2019.103959. [DOI] [PubMed] [Google Scholar]
- 27.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Jiang J, Zeng Y, Tang H. Sarcopenia for predicting mortality among elderly nursing home residents: SARC-F versus SARC-CalF. Medicine (Baltimore) 2019;98(e14546) doi: 10.1097/MD.0000000000014546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulou SK. Sarcopenia: a contemporary health problem among older adult populations. Nutrients. 2020;12(1293) doi: 10.3390/nu12051293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.