Abstract
Background:
In the Protective Ventilation in Cardiac Surgery (PROVECS) randomized, controlled trial, an open-lung ventilation strategy did not improve postoperative respiratory outcomes after on-pump cardiac surgery. In this prespecified subanalysis, the authors aimed to assess the regional distribution of ventilation and plasma biomarkers of lung epithelial and endothelial injury produced by that strategy.
Methods:
Perioperative open-lung ventilation consisted of recruitment maneuvers, positive end-expiratory pressure (PEEP) = 8 cm H2O, and low-tidal volume ventilation including during cardiopulmonary bypass. Control ventilation strategy was a low-PEEP (2 cm H2O) low-tidal volume approach. Electrical impedance tomography was used serially throughout the perioperative period (n = 56) to compute the dorsal fraction of ventilation (defined as the ratio of dorsal tidal impedance variation to global tidal impedance variation). Lung injury was assessed serially using biomarkers of epithelial (soluble form of the receptor for advanced glycation end-products, sRAGE) and endothelial (angiopoietin-2) lung injury (n = 30).
Results:
Eighty-six patients (age = 64 ± 12 yr; EuroSCORE II = 1.65 ± 1.57%) undergoing elective on-pump cardiac surgery were studied. Induction of general anesthesia was associated with ventral redistribution of tidal volumes and higher dorsal fraction of ventilation in the open-lung than the control strategy (0.38 ± 0.07 vs. 0.30 ± 0.10; P = 0.004). No effect of the open-lung strategy on the dorsal fraction of ventilation was noted at the end of surgery after median sternotomy closure (open-lung = 0.37 ± 0.09 vs. control = 0.34 ± 0.11; P = 0.743) or in extubated patients at postoperative day 2 (open-lung = 0.63 ± 0.18 vs. control = 0.59 ± 0.11; P > 0.999). Open-lung ventilation was associated with increased intraoperative plasma sRAGE (7,677 ± 3,097 pg/ml vs. 6,125 ± 1,400 pg/ml; P = 0.037) and had no effect on angiopoietin-2 (P > 0.999).
Conclusions:
In cardiac surgery patients, open-lung ventilation provided larger dorsal lung ventilation early during surgery without a maintained benefit as compared with controls at the end of surgery and postoperative day 2 and was associated with higher intraoperative plasma concentration of sRAGE suggesting lung overdistension.
DURING mechanical ventilation under general anesthesia, collapsed alveoli have been associated with impaired gas exchange,1 mechanical lung trauma,2 and a biologic injurious response.3 The use of limited tidal volumes, part of a protective ventilation strategy, improves postoperative outcomes4 but may also promote heterogeneities in lung aeration and expose the low and normally aerated lung regions to higher mechanical stress and strain.5,6 The open-lung approach aims to homogenize lung aeration during protective ventilation by using recruitment maneuvers and moderate to high levels of positive end-expiratory pressure (PEEP).7 In previous clinical studies, this ventilation strategy improved pulmonary mechanics,8 reduced atelectasis formation,9 and inhibited the intraoperative inflammatory response.10 However, the systematic use of open-lung strategies during general anesthesia is still controversial11 owing to potential hemodynamic side effects, risk of alveolar overdistension, and uncertain clinical benefits in the normal-lung and obese surgical population.12,13 Specific to cardiac surgery, while patients are at increased risk for pulmonary atelectasis,14,15 median sternotomy may induce significant changes in chest wall mechanics,16 thereby increasing susceptibility to lung overdistension.17,18
In our multicenter trial, the PROtective VEntilation in Cardiac Surgery (PROVECS) trial,19 we evaluated the clinical effect of a perioperative multimodal open-lung approach including a moderate PEEP = 8 cm H2O, recruitment maneuvers, and mechanical ventilation during cardiopulmonary bypass (CPB) on postoperative pulmonary complications. This trial revealed that such a ventilation strategy did not improve clinical outcomes after on-pump cardiac surgery as compared with a strategy of low PEEP = 2 cm H2O and no recruitment maneuvers.
Pulmonary electrical impedance tomography measures the regional distribution of intrathoracic bioimpedance and allows for noninvasive real-time tomographic imaging of regional lung aeration and ventilation at the bedside. It has been validated against computed tomography in critically ill patients undergoing mechanical ventilation20 for identification of processes such as alveolar recruitment and overdistension.21,22 Among proposed lung injury biomarkers, the soluble form of the receptor for advanced glycation end-products (sRAGE) has been advanced as sensitive to epithelial injury in acute respiratory distress syndrome (ARDS)23 and ventilator-induced lung injury,24 and the protein angiopoietin-2 has been studied as a biomarker of endothelial injury in ARDS25 and sepsis.26
Here, we present a physiologic substudy aimed at investigating the effects of the PROVECS experimental strategy on perioperative regional lung ventilation, as determined by pulmonary electrical impedance tomography, and on plasma sRAGE and angiopoietin-2. We hypothesized that open-lung ventilation homogenizes the regional distribution of ventilation and attenuates lung injury during and after on-pump cardiac surgery.
Materials and Methods
Design
This is a preplanned single-center substudy of the pragmatic, nonblinded, parallel-arm, multicenter, randomized, controlled PROVECS clinical trial (clinicaltrials.gov NCT02866578; registered on August 15, 2016; principal investigator: David Lagier; appendix 1).19 An ethical committee (Comité de Protection des Personnes Sud Mediterranée I, Marseille, France) approved the protocol (ID-RCB 2016-A00352–49). Written informed consent was obtained from all enrolled patients. Randomization (1:1) was performed with a computer-generated list, using permuted block design. Local investigators performed the allocation using a web-based, secured system, centralized on an electronic platform (CleanWEB, Telemedicine Technologies S.A.S., France).
Participants
Our inclusion criteria encompassed adult patients scheduled for elective cardiac surgery with general anesthesia, invasive mechanical ventilation, complete median sternotomy, full conventional CPB, aortic cross clamp, and cardioplegia. Patients enrolled in the PROVECS clinical trial in the Marseille, France center were also enrolled in the substudy if pulmonary electrical impedance tomography-trained investigators (D.L. and B.G.) or biologists for the biomarker assessment were available. We excluded patients who presented significant preoperative cardiac or pulmonary dysfunction or undergoing surgical procedures that could substantially confound the primary pulmonary outcomes (appendix 2).
Procedures
Mechanical ventilation was performed using volume-controlled ventilation. Patients were ventilated with low-tidal volumes before and after CPB (6 to 8 ml/kg of predicted body weight). Patients were assigned to one of the two strategies: (1) open-lung ventilation, PEEP = 8 cm H2O from intubation for surgery to extubation in the intensive care unit (ICU), recruitment maneuvers (continuous positive airway pressure maintained at 30 cm H2O for 30 s) were delivered at predefined stages (table 1), and ultra-protective ventilation during CPB (tidal volume = 3 ml/kg of predicted body weight, respiratory rate at 12 cycles/min and fraction of inspired oxygen [Fio2] = 0.4); and (2) control ventilation, PEEP = 2 cm H2O from intubation to extubation, no recruitment maneuvers, and no mechanical ventilation during CPB (table 1). In case of surgical requirements (e.g., reduced visualization of the surgical field), or systolic arterial pressure less than 80 mmHg despite the adequate fluid administration and/or vasoactive drugs, interruption of a recruitment maneuver and transient lung deflation by lowering PEEP levels in 1 cm H2O steps was allowed in both ventilation strategies. In both arms of the study, unplanned recruitment maneuvers and/or increased PEEP levels were permitted as a rescue strategy in case of critical intraoperative hypoxemia (peripheral capillary oxygen saturation less than 92% with Fio2 = 0.8). During sternal sawing, PEEP was temporarily set to 0 cm H2O to prevent pleural injury. Before aortic unclamping, de-airing surgical maneuvers accompanied by manual large breath balloon ventilation were performed in both groups with or without the use of transesophageal echocardiography. Patients were extubated 4 to 6 h after the end of surgery. Perioperative data collection, including baseline comorbidities, was performed by the anesthesiologist in charge of the intraoperative care.
Table 1.
Perioperative Ventilation Protocol in Each of the Two Strategies
| Control Ventilation | Open-lung Ventilation | |
|---|---|---|
| Ventilation before CPB | Tidal volume 6–8 ml/kg of predicted body weight | Tidal volume 6–8 ml/kg of predicted body weight |
| PEEP 2 cm H2O | PEEP 8 cm H2O | |
| RR for ETCO2 35–45 mmHg | RR for ETCO2 35–45 mmHg | |
| Lowest Fio2 for Spo2 greater than 94% | Lowest Fio2 for Spo2 greater than 94% | |
| I:E ratio at 1:2 | I:E ratio at 1:2 | |
| Systematic recruitment maneuvers | No | Yes* |
| Ventilation during CPB | No | Yes |
| Continuous positive airway pressure = 2 cm H2O | Tidal volume 3 ml/kg of predicted body weight | |
| Fio2 40% | PEEP 8 cm H2O | |
| RR 12 cycles per minute | ||
| Fio2 40% | ||
| Ventilation after CPB (including in ICU) | Tidal volume 6–8 ml/kg of predicted body weight | Tidal volume 6–8 ml/kg of predicted body weight |
| PEEP 2 cm H2O | PEEP 8 cm H2O | |
| RR for ETCO2 35–45 mmHg | RR for ETCO2 35–45 mmHg | |
| Lowest Fio2 for Spo2 greater than 94% | Lowest Fio2 for Spo2 greater than 94% | |
| I:E ratio at 1:2 | I:E ratio at 1:2 |
cm H2O, centimeter of water; CPB, cardiopulmonary bypass; ETCO2, end-tidal CO2; Fio2, inspired oxygen fraction, I:E inspiratory time to expiratory time ratio; ICU, intensive care unit; PEEP, positive end-expiratory pressure; RR respiratory rate; Spo2, oxygen saturation as detected by the pulse oximeter.
Continuous airway pressure at 30 cm of water for 30 s. Recruitment maneuvers were planed after orotracheal intubation, at CPB initiation, at aortic unclamping after de-airing maneuvers, at ICU arrival, and after every breathing circuit disconnection.
Pulmonary Electrical Impedance Tomography Measurements
Unblinded investigators performed pulmonary electrical impedance tomography measurements (PulmoVista 500 EIT system, Dräger Medical GmbH, Germany). A 16-electrode belt was placed at the anterior fifth intercostal space after confirming, with pulmonary ultrasound, the presence of lung tissue. In each patient, belt position was maintained at the same dermatome throughout the study by using cutaneous marks outlined with a marking pen. Measurements were performed in a 45° semi-sitting position at four perioperative timepoints: preoperatively in spontaneously breathing awake patients, after intubation at the onset of anesthesia and mechanical ventilation (after the first recruitment maneuver if indicated), at the end of surgery, and at postoperative day 2 in extubated patients (fig. 1). The Dräger EIT Data Analysis Tool software V6.0 (Dräger Medical GmbH, Germany) was used for quantification.
Fig. 1.
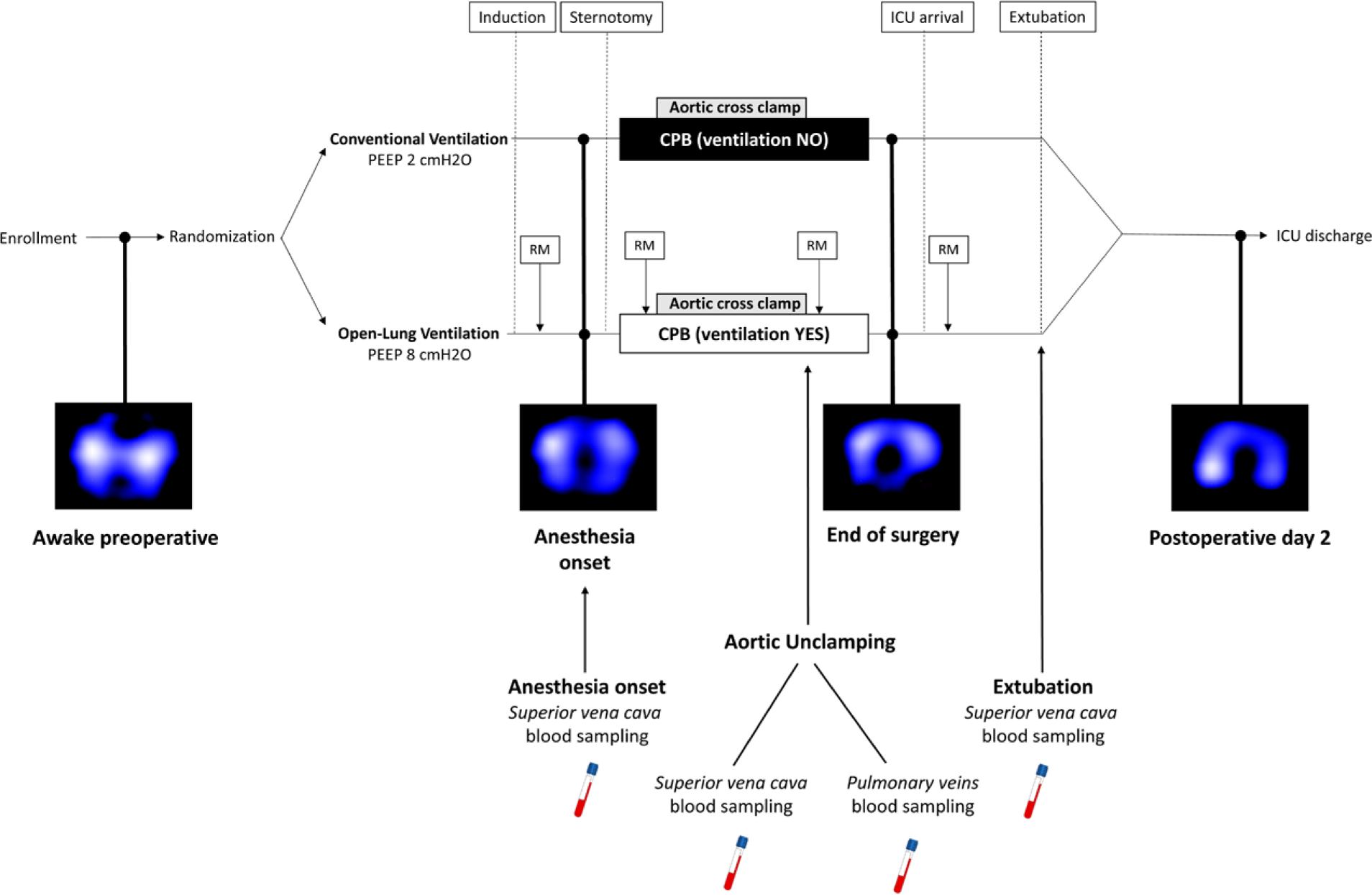
Experimental protocol. CPB, cardiopulmonary bypass; ICU, intensive care unit; PEEP, positive end-expiratory pressure; RM, recruitment maneuver.
Pulmonary Electrical Impedance Tomography–derived Parameters
Tidal impedance variation was quantified as the difference between end-inspiratory and end-expiratory impedances produced by tidal volume ventilation and expressed using arbitrary units specific to the PulmoVista 500 device. Regional tidal impedance variation was assessed in two regions of interest: the ventral nondependent lung (TVventral) and the dorsal dependent lung (TVdorsal). The limit between ventral and dorsal lung regions was automatically displayed by the device as a middle line between 4th and 5th electrode on the right chest and between 12th and 13th electrode on the left chest. Regional distribution of tidal ventilation was analyzed by quantifying the dorsal fraction of ventilation, defined as the ratio between the dorsal and the sum of dorsal and ventral tidal impedance variations and expressed as a decimal between 0 and 1:
Regional lung compliance was measured with pulmonary electrical impedance tomography (CLEIT) as the regional tidal impedance variation divided by the driving pressure and expressed in arbitrary units per cm H2O:
Biomarkers Assays
Blood was sampled from the central venous catheter at three time points: (1) after intubation, (2) at the end of CPB, immediately before aortic unclamping (a simultaneous blood sample was also collected from the pulmonary veins [through a left atrium vent]), and (3) at extubation 4 to 6 h after ICU arrival (fig. 1). Blood samples (4 ml) were drawn into a vacuum tube containing EDTA, immediately centrifuged at 5,000 rpm for 10 min, and serum supernatants frozen at −80°C. The analyses were performed by a biologist blinded to randomization group. sRAGE and angiopoietin-2 concentrations were measured by using commercially available ELISA kits (R&D Systems, USA) according to the manufacturer’s instructions.
Respiratory Function Data Recording
Arterial oxygen tension (Pao2)/Fio2 ratio along with static and dynamic global respiratory system compliance (CRS) were calculated at the beginning and at the end of surgery:
Alveolar arterial O2 gradient was calculated at the end of surgery and at extubation using an online calculator (https://www.mdcalc.com/a-a-o2-gradient, accessed August 3, 2017).
Statistical Analysis
Pulmonary electrical impedance tomography and plasma biomarker investigations were performed in two independent cohorts of the substudy (fig. 2). The power analysis for each cohort was performed before the beginning of the study using two-tailed t test and online software (https://biostatgv.sentiweb.fr, accessed February 1, 2016). Our previous clinical experience and literature data suggest that the dorsal fraction of ventilation is reduced by 50% from awake spontaneous breathing to conditions of mechanical ventilation.27 We expected that open-lung ventilation would limit such reduction of the dorsal fraction of ventilation to 15%. Assuming a common SD of 45% for these percent reductions, we estimated that a minimum of 26 patients in each group would be required for the pulmonary electrical impedance tomography study (α error level of P < 0.05 and a β error level = 0.8). Based on previous measurement of postoperative plasmatic sRAGE in cardiac surgery,28 we expected mean postoperative sRAGE = 2,000 pg/ml in controls and 1,500 pg/ml (25% reduction) in the open-lung ventilation group with a common SD of 480 pg/ml. We estimated that a minimum of 15 patients in each group would be required for the biologic study (α error level of P < 0.05 and a β error level = 0.8). The data analysis plan was written after the data were accessed. Data are presented as the mean and SD or the median and interquartile range for quantitative variables and count (percentages) for qualitative variables. With regard to the D’Agostino and Pearson normality tests, between-group comparisons of quantitative variables were conducted with independent t test or Mann–Whitney test. Chi-square test was used for qualitative variables. Kruskal–Wallis test was performed to evaluate the effect of perioperative measurement time on tidal impedance variation with post hoc Dunn’s multiple comparisons test in case of significant variation. To handle missing values and repeated measures, a mixed-effects model (restricted maximum likelihood) with post hoc Bonferroni’s multiple comparisons test was used for the analyses of the dorsal fraction of ventilation and the biomarkers. The two fixed factors were timepoints of pulmonary electrical impedance tomography recordings or blood sampling (within-subject comparisons) and ventilation strategy (between-subjects comparison). We used Pearson’s test for correlation analysis. P values less than 0.05 were considered significant. Statistical analyses were performed with Prism 8.4.2 (GraphPad Software, Inc., USA).
Fig. 2.
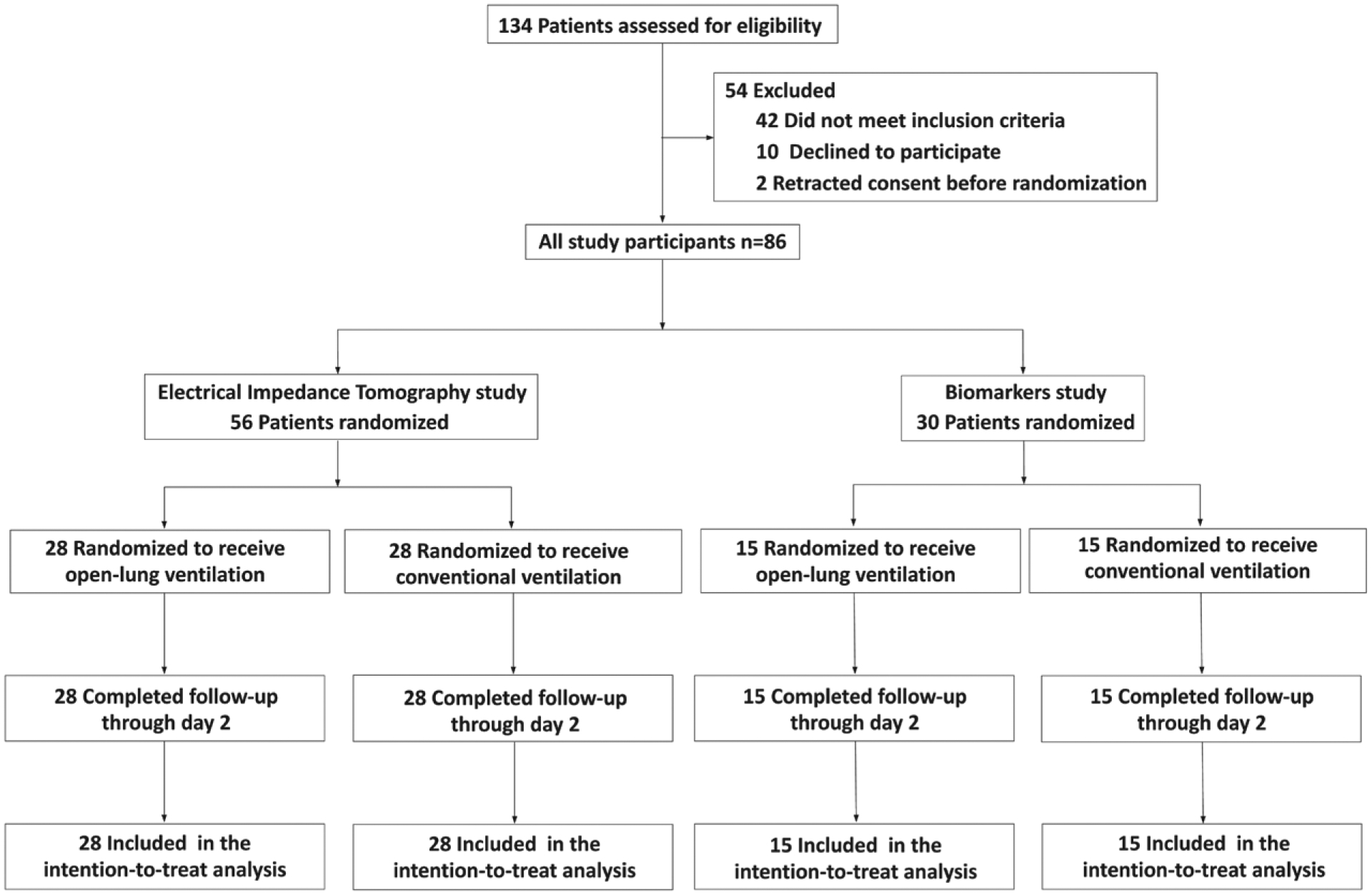
Flow diagram of the trial.
Results
Between August 2017 and June 2018, 86 patients were randomly assigned to one of the two ventilation strategies (43 patients in each group; fig. 2). No clinically important between-group imbalance in age, gender, body mass index, American Society of Anesthesiologists (ASA) score, smoking status, preexisting conditions, and type of surgery was found at baseline (table 2). EuroSCORE II revealed a mostly low-risk population. Tidal volume did not differ between ventilation strategies. PEEP, as shown by maximum, minimum, and mode levels, was significantly higher in patients assigned to the open-lung ventilation strategy than controls. Open-lung ventilation allowed for lower level of Fio2 at the onset of anesthesia and at the end of surgery than that used in the control group. Patients included in the open-lung ventilation group received larger intraoperative volume of colloids (table 3). No severe adverse events occurred during the study period.
Table 2.
Baseline Characteristics
| Electrical Impedance Tomography Cohort | Biomarkers Cohort | |||
|---|---|---|---|---|
| Characteristic | Open-lung Ventilation (n = 28) | Control Ventilation (n = 28) | Open-lung Ventilation (n = 15) | Control Ventilation (n = 15) |
| Age, median (interquartile range), yr | 66 (55–75) | 66 (58–74) | 68 (65–74) | 62 (53–72) |
| Male sex, n (%) | 15 (53.6) | 18 (64.2) | 13 (86.7) | 11 (73.3) |
| Body mass index, mean ± SD, kg/m2 | 25.5 ± 3.7 | 25.5 ± 3.7 | 27.1 ± 3.8 | 25.7 ± 2.8 |
| ASA score, n (%) | ||||
| ≤ 2 | 2 (7.1) | 1 (3.6) | 2 (13.3) | 5 (33.3) |
| 3 | 25 (89.3) | 26 (92.8) | 13 (86.7) | 10 (66.7) |
| 4 | 1 (3.6) | 1 (3.6) | 0 | 0 |
| Smoking status, n (%) | ||||
| Current smoker | 4 (14.3) | 8 (28.6) | 5 (33.3) | 3 (20) |
| Former smoker (weaning less than 6 month) | 2 (7.1) | 2 (7.1) | 1 (6.7) | 0 |
| COPD, n (%)* | 1 (3.6) | 2 (7.1) | 1 (6.7) | 0 |
| Diabetes, n (%) | 2 (7.1) | 5 (17.9) | 5 (33.3) | 3 (20) |
| Hypertension, n (%) | 19 (67.6) | 19 (67.6) | 9 (60) | 4 (26.7) |
| Preoperative left ventricle ejection fraction, mean ± SD, % | 59 ± 8 | 62 ± 8 | 59 ± 8 | 61 ± 9 |
| Type of surgery, n (%) | ||||
| Isolated CABG | 9 (32.1) | 9 (32.1) | 5 (33.3) | 5 (33.3) |
| Isolated valve surgery | 13 (46.4) | 12 (42.9) | 5 (33.3) | 5 (33.3) |
| Other procedures† | 6 (21.4) | 7 (25) | 5 (33.3) | 5 (33.3) |
| Euroscore II, median (interquartile range), % | 1.07 (0.84–1.53) | 1.49 (0.79–2.35) | 2.01 (0.96–2.29) | 0.96 (0.81–1.52) |
ASA, American Society of Anesthesiologists; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease.
Under chronic inhalation therapy.
Thoracic aorta surgeries and combined procedures (valve + CABG, valve + aorta, aorta + CABG).
Table 3.
Intraoperative Procedures
| Variable | Open-lung Ventilation (n = 43) | Control Ventilation (n = 43) | P Value |
|---|---|---|---|
| Tidal volume, mean ± SD, ml/kg of predicted body weight | 6.5 ± 1.0 | 6.8 ± 1.0 | 0.231 |
| Intraoperative PEEP, median (interquartile range), cm H2O | |||
| Minimum level | 4 (0–5) | 2 (0–2) | < 0.001 |
| Maximum level | 8 (8–8) | 2 (2–2) | < 0.001 |
| Mode level* | 6 (6–8) | 2 (2–2) | < 0.001 |
| Recruitment maneuver, n (%)† | < 0.001 | ||
| At least 1 | 43 (100) | 7 (16.3) | |
| At least 2 | 35 (81.4) | 0 | |
| At least 3 | 34 (79.1) | 0 | |
| At least 4 | 29 (67.4) | 0 | |
| More than 4 | 27 (62.7) | 0 | |
| Inspired oxygen fraction | |||
| Anesthesia onset | 0.49 ± 0.07 | 0.53 ± 0.07 | 0.040 |
| End of surgery | 0.48 ± 0.09 | 0.54 ± 0.07 | 0.011 |
| CPB duration, mean ± SD, min | 93 ± 23 | 90 ± 33 | 0.663 |
| Aortic cross clamp duration, mean ± SD, min | 68 ± 21 | 67 ± 28 | 0.837 |
| Mammary artery harvesting, n (%) | 1.0 | ||
| None | 28 (65.1) | 28 (65.1) | |
| Unilateral | 7 (16.3) | 7 (16.3) | |
| Bilateral | 8 (18.6) | 8 (18.6) | |
| Volume of fluids administered, mean ± SD, ml | |||
| Crystalloid | 2000 ± 657 | 1843 ± 500 | 0.223 |
| Colloid | 788 ± 365 | 618 ± 321 | 0.030 |
| Blood products transfusion, n (%) | 3 (7.0) | 4 (9.3) | 0.693 |
Predicted body weight was calculated as 50 + 0.91 × (height in cm − 152.4) for men and 45.5 + 0.91 × (height in cm − 152.4) for women. Data come from n = 49 for inspired oxygen fraction. CPB, cardiopulmonary bypass; PEEP, positive end-expiratory pressure.
Mode PEEP level corresponds to intraoperative PEEP that was applied most of the time after review of ventilation settings trends of the dedicated ventilator screen.
Recruitment maneuver effectively performed: continuous airway pressure at 30 cm of water for 30 s.
Fifty-six patients were studied with pulmonary electrical impedance tomography (fig. 2). Data from 10 of 224 (4.5%) recordings were missing (appendix 3). As compared with awake preoperative measurements, dorsal tidal impedance variation was significantly reduced in both groups at anesthesia onset (P < 0.001), end of surgery (P < 0.001), and postoperative day 2 (P < 0.05; fig. 3A). In contrast, awake preoperative ventral tidal impedance variation was not significantly affected by general anesthesia irrespective of the ventilation strategy (P > 0.999 vs. anesthesia onset and vs. end of surgery; fig. 3B). Accordingly, there was a marked reduction of the dorsal fraction of ventilation at anesthesia onset both with open-lung (0.63 ± 0.14 preoperatively vs. 0.38 ± 0.07 at anesthesia onset; P < 0.001) and control ventilation (0.58 ± 0.15 preoperatively vs. 0.30 ± 0.10 at anesthesia onset; P < 0.001; fig. 4). Extubation and return to spontaneous ventilation were associated with a restoration of the dorsal distribution of ventilation, as shown by increased dorsal fraction of ventilation on postoperative day 2 (0.63 ± 0.18 in the open-lung group and 0.59 ± 0.11 in the control group; P < 0.001 as compared to within-group value at anesthesia onset or end of surgery; fig. 4).
Fig. 3.
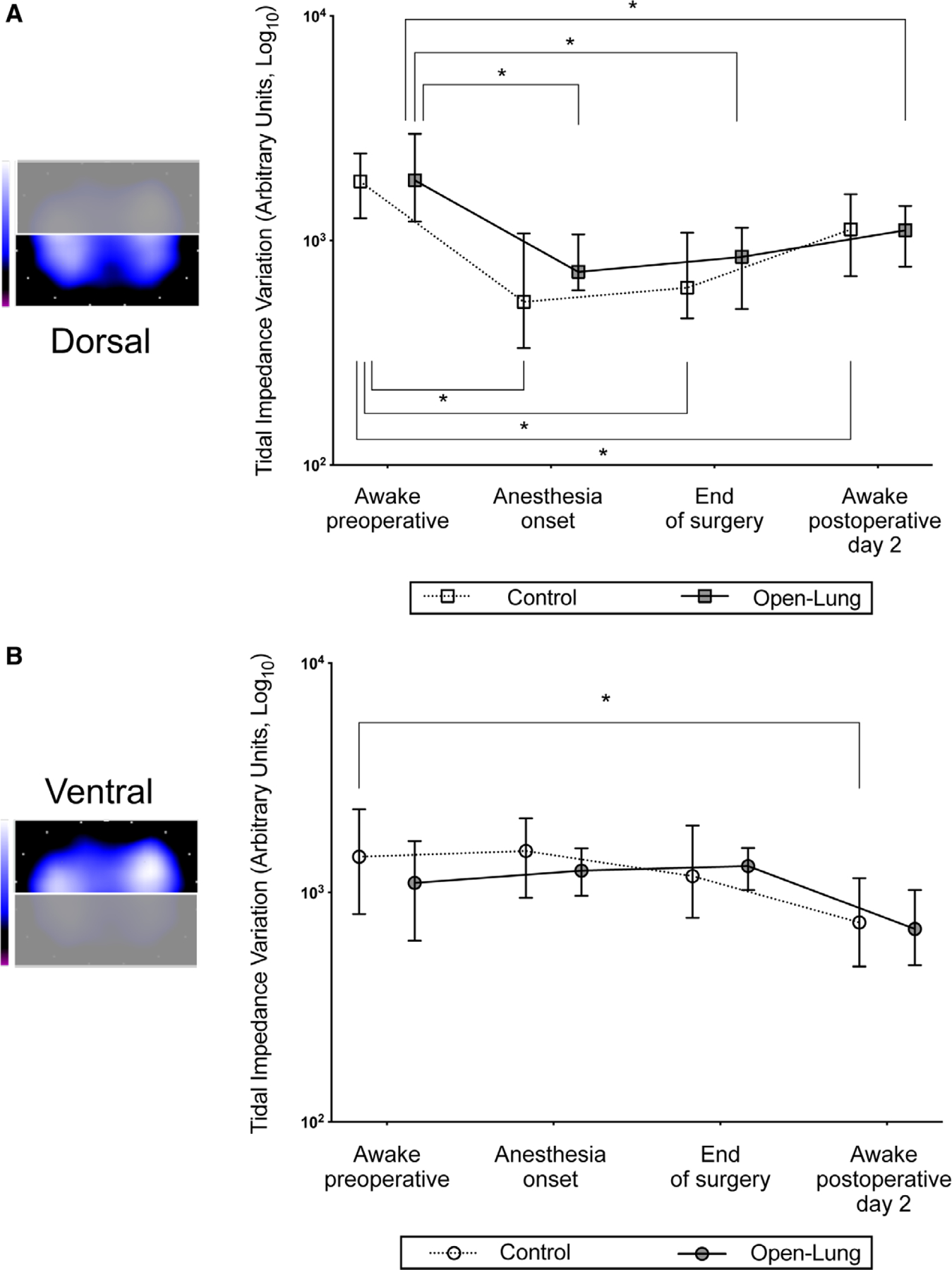
Perioperative changes in tidal impedance variations in the predefined regions of interests: dorsal (A) and ventral (B). Bars indicate the median and interquartile range. *P < 0.05 compared with awake preoperative (within-group comparison).
Fig. 4.
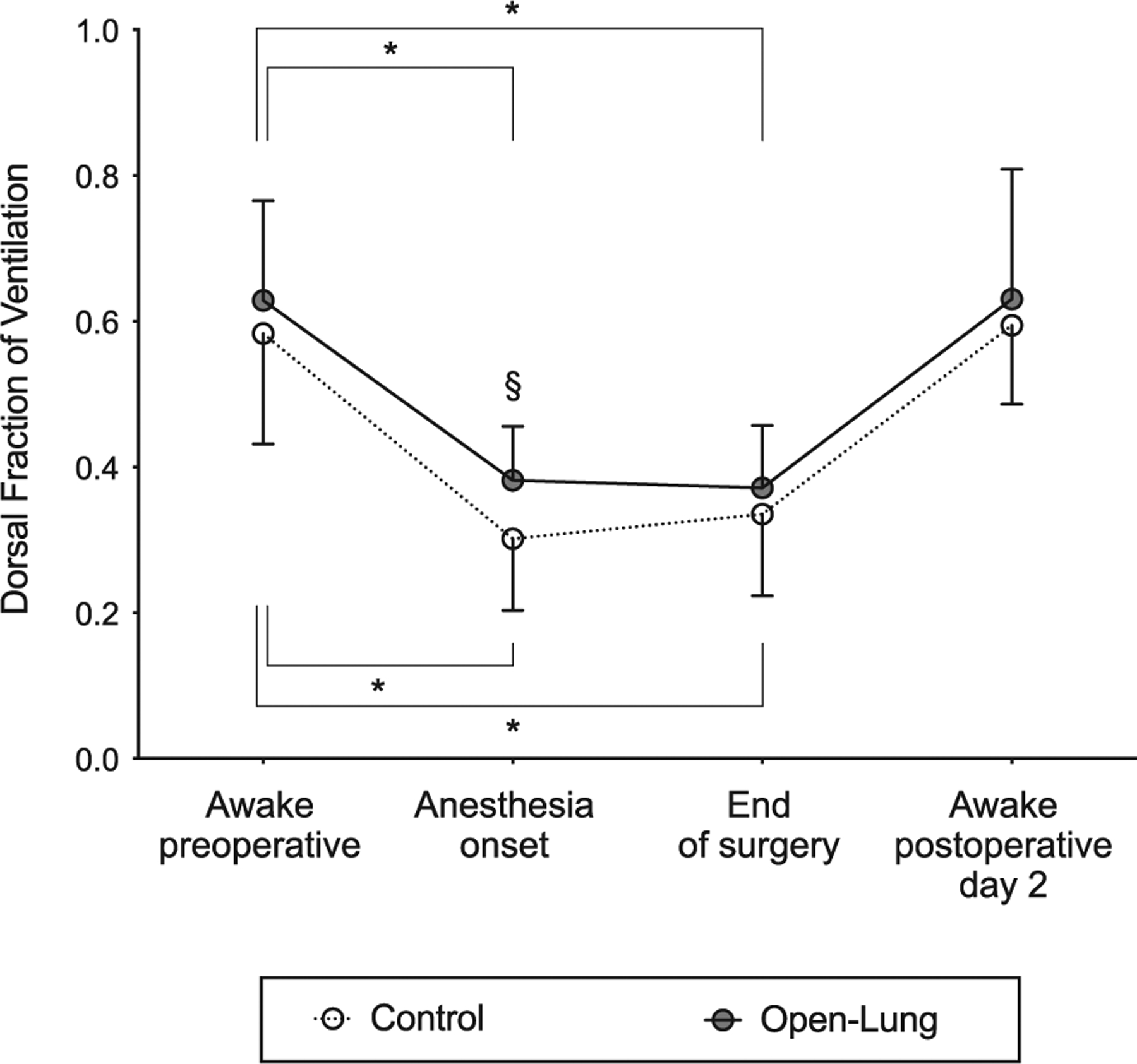
Perioperative changes in dorsal fraction of ventilation. Bars indicate the mean and SD. *P < 0.05 compared with awake preoperative (within-group comparison). §P < 0.05 compared with control ventilation group (between-group comparison).
At the onset of anesthesia, open-lung ventilation enhanced the dorsal distribution of tidal volumes when compared with control strategy, as revealed by significantly larger dorsal fraction of ventilation (open-lung = 0.38 ± 0.07 vs. controls = 0.30 ± 0.10; P = 0.004; figs. 4 and 5). At the onset of anesthesia, open-lung ventilation significantly improved regional compliance of the dorsal lung as measured by pulmonary electrical impedance tomography (open-lung = 123.6 ± 76.6 arbitrary units per cm H2O vs. controls = 73.1 ± 56.6 arbitrary units per cm H2O; P = 0.005; table 4). Open-lung ventilation did not affect the dorsal fraction of ventilation between the anesthesia onset and the end of surgery (0.38 ± 0.07 vs. 0.37 ± 0.09; P > 0.999). Dorsal fraction of ventilation did not significantly differ between ventilation strategies at the end of surgery (open-lung = 0.37 ± 0.09 vs. controls = 0.34 ± 0.11; P = 0.743) or at postoperative day 2 (open-lung = 0.63 ± 0.18 vs. controls = 0.59 ± 0.11; P > 0.999; figs. 4 and 5). Similarly, no significant differences were found between groups for dorsal lung compliance measured at the end of surgery (table 4).
Fig. 5.
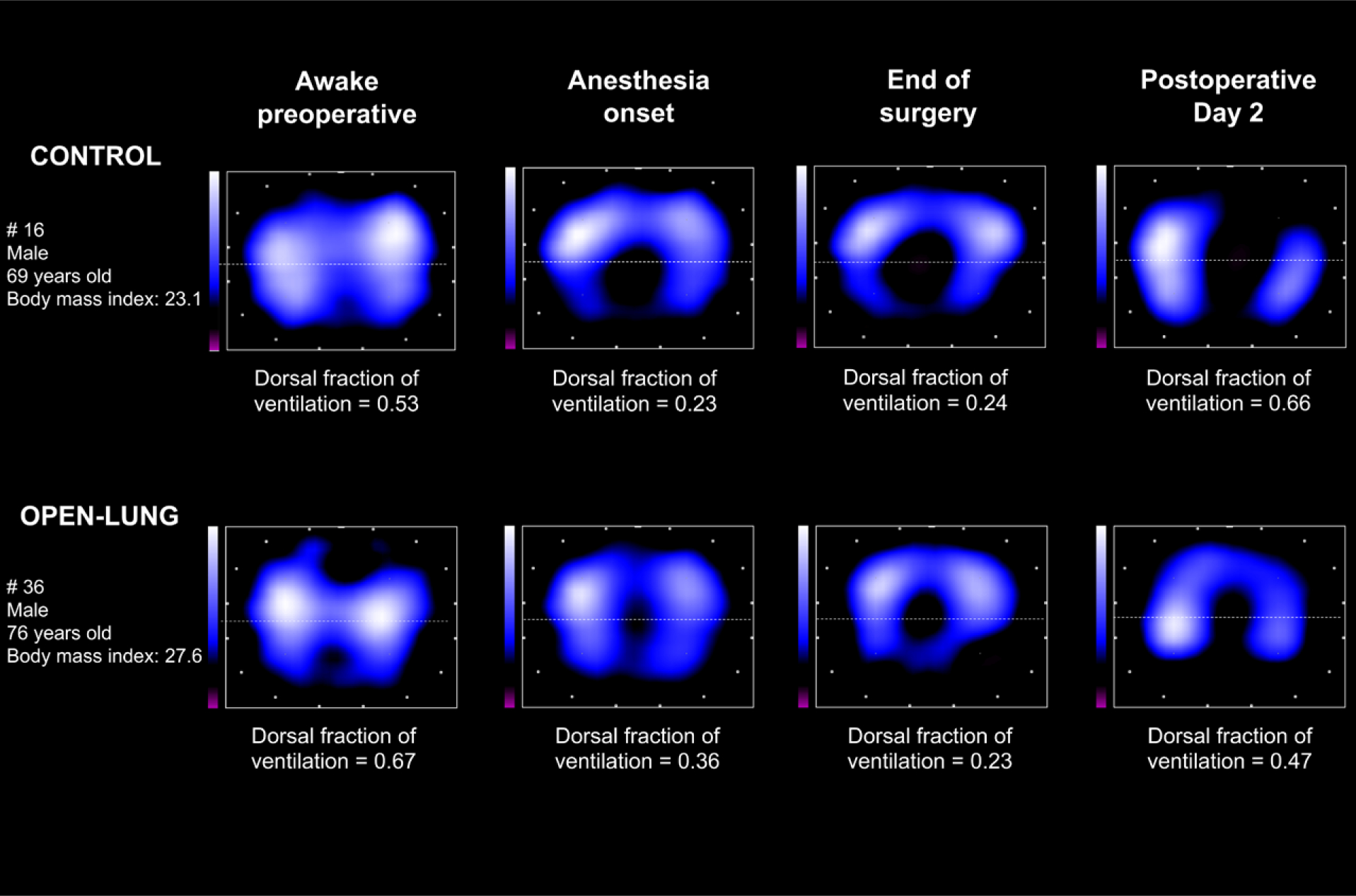
Examples of electrical impedance tomography tidal images of two patients.
Table 4.
Respiratory Mechanics and Blood Oxygenation under Mechanical Ventilation
| Variable | Open-lung Ventilation (n = 43) | Control Ventilation (n = 43) | P Value |
|---|---|---|---|
| Regional lung compliance,* mean ± SD, arbitrary units per cm H2O | |||
| Anesthesia onset | |||
| Ventral | 176.0 ± 73.4 | 163.7 ± 115.6 | 0.639 |
| Dorsal | 123.6 ± 76.6 | 73.1 ± 56.6 | 0.005 |
| End of surgery | |||
| Ventral | 185.9 ± 96.9 | 167.4 ± 121.1 | 0.535 |
| Dorsal | 133.4 ± 111.9 | 89.5 ± 70.3 | 0.088 |
| Respiratory system compliance, mean ± SD, ml per cm H2O | |||
| Anesthesia onset | |||
| Dynamic | 67.1 ± 25.0 | 49.6 ± 24.3 | 0.015 |
| Static | 61.6 ± 16.8 | 45.5 ± 17.3 | 0.002 |
| End of surgery | |||
| Dynamic | 48.3 ± 22.6 | 46.5 ± 29.9 | 0.286 |
| Static | 53.9 ± 13.8 | 49.7 ± 18.7 | 0.119 |
| Pao2/Fio2 ratio, mean ± SD, mmHg | |||
| Anesthesia onset | 441 ± 129 | 311 ± 131 | 0.001 |
| End of surgery | 374 ± 103 | 273 ± 97 | < 0.001 |
| Alveolar arterial gradient, mean ± SD, mmHg | |||
| End of surgery | 166 ± 81 | 172 ± 70 | 0.843 |
| Extubation time | 97 ± 48 | 142 ± 57 | 0.026 |
Measured with pulmonary electrical impedance tomography. Data come from n = 86 for respiratory system compliance and arterial oxygen tension (Pao2)/fraction of inspired oxygen (Fio2) ratio, n = 56 for regional lung compliance, and n = 30 for alveolar arterial gradient.
Static and dynamic respiratory system compliance were significantly increased in the open-lung ventilation group at the onset of anesthesia but not at the end of surgery (table 4). The open-lung ventilation strategy significantly improved blood oxygenation as revealed by increased perioperative Pao2/Fio2 ratios and alveolar arterial gradient (table 4). Pao2/Fio2 ratios moderately correlated with dorsal fraction of ventilation (r = 0.384; 95% CI [0.194 to 0.547]; P < 0.001; fig. 6).
Fig. 6.
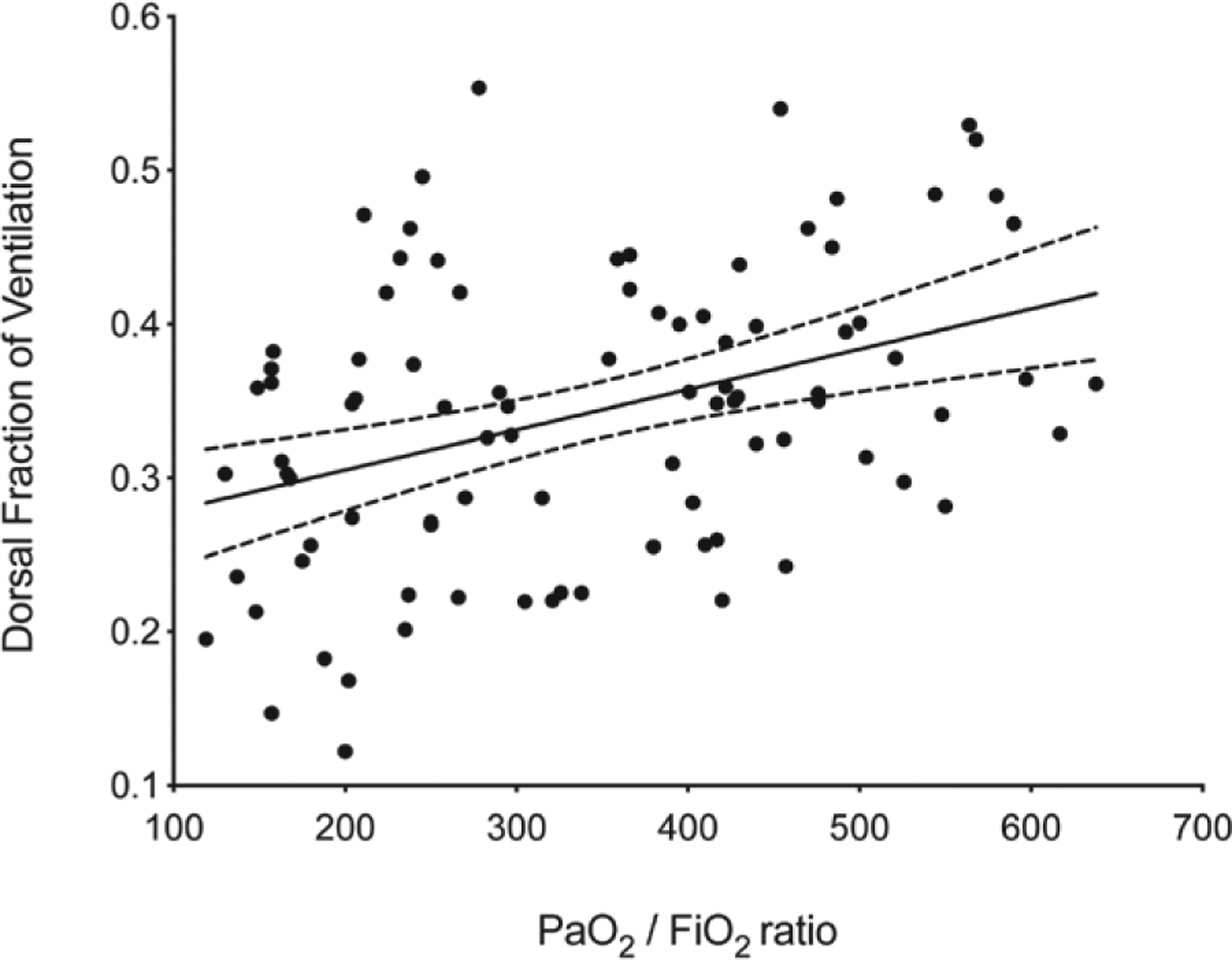
Correlation between dorsal fraction of ventilation and arterial oxygen tension (Pao2)/fraction of inspired oxygen (Fio2) ratios. Error band represents the 95% CI of the linear regression slope (r = 0.384; 95% CI [0.194 to 0.547]; P < 0.001).
Plasma biomarkers of lung injury were assessed at thirty patients (fig. 2). Data from six of 240 (2.5%) blood samples were missing (appendix 3). Both groups presented significant increases in central and pulmonary venous sRAGE concentrations at aortic unclamping as compared with concentration at anesthesia onset (P < 0.001). Intragroup analyses showed higher sRAGE concentration in pulmonary venous than central venous blood at aortic unclamping evidencing a significant pulmonary-to-central sRAGE gradient (P < 0.001 in both groups; fig. 7A). The sRAGE plasma concentration in pulmonary venous blood at aortic unclamping was significantly larger in open-lung than control ventilation (7,677 ± 3,097 pg/ml vs. 6,125 ± 1,400 pg/ml; P = 0.037; fig. 7A). At extubation, sRAGE plasma concentration did not differ from concentration observed at anesthesia onset (P > 0.999 in both groups). Central and pulmonary venous angiopoietin-2 concentrations at aortic unclamping were significantly increased in both groups as compared with concentration at anesthesia onset but did not reveal a significant pulmonary-to-central gradient (P = 0.356 in the control group; P = 0.083 in the open-lung group). Ventilation strategy did not significantly impact perioperative angiopoitein-2 plasma concentrations (P > 0.999 at all timepoints; fig. 7B).
Fig. 7.
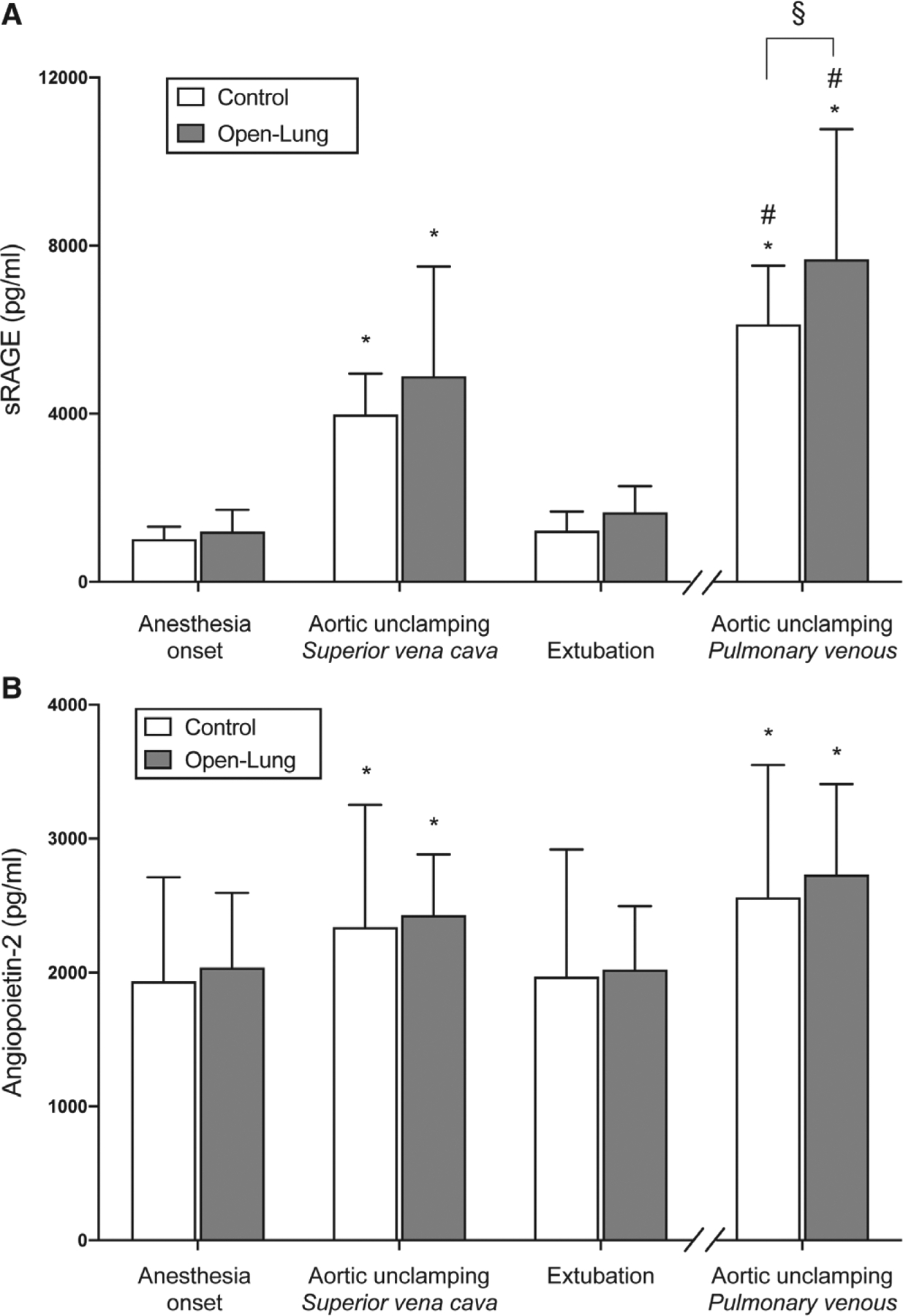
sRAGE (A) and angiopoietin-2 (B) plasma concentration over the perioperative period. Bars indicate the mean and SD. *P < 0.05 compared with anesthesia onset value and #P < 0.05 compared with superior vena cava at aortic unclamping (within-group comparison). §P < 0.05 between-group comparison. sRAGE, soluble form of the receptor for advanced glycation end-products.
Discussion
Open-lung ventilation, as applied to our low-to-moderate risk cardiac surgery patients, produced a significantly larger dorsal tidal ventilation before median sternotomy than that achieved with a low-PEEP strategy. However, that benefit to regional ventilation distribution with the open-lung strategy did not persist to the end of surgery or 2 days later. Additionally, open-lung ventilation was associated with increased intraoperative plasma concentrations of sRAGE specifically in the pulmonary venous blood. These findings suggest that an open-lung strategy does not provide substantial benefits to lung ventilation in cardiac surgery patients and may increase the risk for lung injury.
Previous pulmonary electrical impedance tomography studies have described the beneficial effect of open-lung ventilation strategies in homogenizing the distribution of pulmonary ventilation.29,30 However, most of those studies included ARDS or general surgery patients, and data are scant on the impact of open-lung ventilation in patients undergoing open-chest surgery. In this work, we have confirmed that general anesthesia and loss of spontaneous breathing was associated, in semi-sitting patients, with an immediate ventral redistribution of tidal ventilation and a reduction in dorsal lung ventilation. The combined effect of gravity and changes in chest wall function (i.e., loss of respiratory muscles tone) results in the rapid collapse of the dependent alveoli in response to general anesthesia.31 Preoxygenation with Fio2 = 100% further facilitates such collapse through oxygen absorption in the well-perfused dependent alveoli.32 As expected, open-lung ventilation significantly increased the dorsal distribution of tidal volumes after the application of the first recruitment maneuver. At the onset of anesthesia, higher dorsal lung compliance and increased Pao2/Fio2 ratios strongly suggest an early alveolar recruiting effect. The correlation between the dorsal fraction of ventilation and Pao2/Fio2 ratio confirmed that the distribution of ventilation plays a significant role in blood oxygenation. However, larger Pao2/Fio2 ratios at the end of surgery in those receiving the open-lung strategy, in spite of similar ventilation distribution, suggest that the open-lung strategy had an effect on other determinants of gas exchanges (e.g., lung perfusion).
Our multimodal open-lung ventilation strategy aimed to maximize alveolar recruitment. Although it promoted dorsal ventilation larger than in controls immediately after intubation, the strategy failed to maintain that benefit on dorsal regional ventilation as compared with controls at the end of surgery. Several factors could explain this unexpected result. First, some methodologic aspects have probably mitigated intergroup differences at the end of surgery. Indeed, patients with no specific respiratory risk represented a large fraction of the studied population. Also, the use of a 45° semi-sitting position during measurements likely reduced the susceptibility to dorsal lung collapse. The use of semi-erect position during intubation (head of bed elevated = 30°) and postoperatively in the ICU (head of bed elevated = 45°) is part of usual care in our institution, and we aimed to warrant the comparability between measurements while respecting ICU protocols. Finally, de-airing maneuvers before CPB weaning with application of lung expansion may have produced alveolar recruitment in controls. Second, lungs exposed to CPB-induced lung injury are at high risk for severe lung collapse at the end of surgery.14 Therefore, the lack of benefit of our open-lung strategy as compared with controls at the end of surgery may be secondary to insufficient level of airway pressure to achieve lung recruitment. Consistent with this, a recent trial in cardiac surgery patients has shown benefits to clinical and pulmonary electrical impedance tomography outcomes of an intensive open-lung ventilation strategy using larger PEEP levels than those used in our study (13 to 30 cm H2O). Importantly, this strategy was applied in the immediate postoperative period (i.e., in
closed chest conditions) to hypoxemic patients, who presumably would be more responsive to lung recruitment than our study population.30 Third, median sternotomy inherent to cardiac surgery significantly affects respiratory mechanics by notably increasing respiratory system compliance,16 functional residual capacity of the lung,33 and the volume-expanding effect of PEEP.34 The pleural pressure is a critical determinant of ventilation distribution35 that depends on a complex interaction between the lung, the chest wall, gravity, and the respiratory muscles.36 Sternotomy and retraction of the ribs, with or without opening of the mediastinal parietal pleurae, have been associated with lowered pleural pressures as evaluated by esophageal pressure.37 Under these conditions, alveolar recruitment may have been maximized at PEEP = 2 cm H2O after median sternotomy thus reducing the intergroup differences.
Our biomarker findings revealed that on-pump cardiac surgery was associated with increased circulating sRAGE. Plasma sRAGE has been widely associated with lung injurious processes28,38–40 and recent evidence supports that this biomarker may be associated with alveolar epithelial injury and reduced alveolar fluid clearance.23 Access to pulmonary venous blood allowed us to confirm the lung-specificity of the sRAGE through the observation of significant central-to-pulmonary venous plasma concentration gradients. Moreover, the multiple timepoints analysis revealed that sRAGE plasma clearance was a relatively rapid process after cardiac surgery. Such results have never been reported in humans and add an important step toward the mechanistic validation of sRAGE as a specific biomarker of lung injury.41 Remarkably, open-lung ventilation was associated with higher concentration of sRAGE in the pulmonary venous blood, raising concerns about epithelial lung injury induced by the intervention strategy.40 For instance, sRAGE has previously been associated with alveolar overdistension secondary to the use of high tidal volumes24,42 or specific approaches to recruitment maneuvers.43 In contrast, open-lung ventilation was not associated with significant change on angiopoietin-2, implying lower likelihood of endothelial lung injury. Such a differential epithelial-endothelial injurious profile has been identified in experimental ventilator-induced lung injury studies.44,45 As compared with closed-chest conditions,39 changes in the interaction between the chest wall and the lungs as a result of median sternotomy could have contributed to increased transpulmonary pressures and a greater risk of ventral overdistension.16,34 Indeed, Dechman et al.18 reported that stepwise PEEP increments from 0 to 10 cm H2O continuously increased lung elastance, supposedly as a result of progressive overdistension in cardiac surgery patients, whereas they significantly reduced lung elastance in abdominal surgery patients, suggesting lung recruitment. In our study, the systemic inflammatory response secondary to surgical stress and CPB may have represented a “second-hit” further contributing to lung injury. Reduced plasma concentration of sRAGE at extubation indicate that the injurious lung process was probably limited to the intraoperative period.
Overall, we demonstrate that, in spite of a significant recruiting effect at anesthesia onset, the intraoperative use of an open-lung strategy is limited in the context of cardiac surgery. This occurs either because median sternotomy facilitates lung expansion even at low PEEPs or because an even more robust recruitment strategy is required. Signs of potential lung epithelial injury consistent with alveolar overdistension were already present in the open-lung strategy. Further studies are required to better understand the relation between positive pressure ventilation, regional lung expansion, and median sternotomy.
Limitations
There are several limitations to our study. First, we used fixed pressures for recruitment maneuvers and PEEP. This aimed to provide group separation consistent with some current practices in controls46 and the aim of achieving lung recruitment without compromising hemodynamics and surgical visualization in the open-lung strategy. Use of a PEEP titration method could have identified a PEEP level conducive to more homogeneous ventilation distribution in individual patients. Second, recent sternotomy, chest drainage system, or epicardial pacing may have disturbed end of surgery and postoperative pulmonary electrical impedance tomography measurements. However, this imaging technique has already been used as a reliable tool to evaluate postoperative lung function in cardiac surgery patients.47,48 Third, intervention adjustments for surgical or hemodynamic requirements might have reduced alveolar recruitment efficiency in patients assigned to the open-lung group. However, between-group differences in the use of recruitment maneuvers and levels of PEEP were statistically significant. Fourth, an increase in respiratory system compliance was found at the end of surgery in the parent clinical trial but not in this subgroup analysis. This discrepancy is likely attributable to a lack of statistical power rather than a selection bias as the entry criteria were similar in the parent clinical trial and the present substudy. Fifth, based on our exclusion criteria, the study population mostly represents a low risk cardiac surgery population from both pulmonary and cardiac perspective. The implications for higher-risk patients are still unknown. Finally, plasma concentration of sRAGE can be influenced by extrapulmonary factors such as myocardial ischemia-reperfusion injury.49 However, our groups did not differ with regard to CPB and aortic cross clamp durations.
Conclusions
During cardiac surgery, an open-lung ventilation strategy, using a moderate level of PEEP, recruitment maneuvers, and maintained ventilation during CPB, brought benefits to dorsal lung ventilation only after anesthesia induction as compared with a low–tidal volume low-PEEP strategy. Such benefits were not maintained at the end of surgery or 2 days thereafter. Larger intraoperative sRAGE in the open-lung strategy together with larger pulmonary venous than central venous concentration suggest risk for lung epithelial injury consistent with lung overdistension.
EDITOR’S PERSPECTIVE.
What We Already Know about This Topic
The optimal ventilatory strategy for patients undergoing on-pump cardiac surgery remains controversial
The authors previously reported a randomized, controlled trial comparing an open-lung strategy consisting of low tidal volumes, moderate levels of positive end-expiratory pressure, recruitment maneuvers, and ventilation during cardiopulmonary bypass versus European-based conventional management (low positive end-expiratory pressure, low tidal volumes, no ventilation during bypass) in 488 low-risk patients, which failed to show a difference in the incidence of postoperative pulmonary complications
A prespecified substudy monitored a subset of those patients with electrical impedance tomography to assess dorsal versus ventral distribution of ventilation and serially assessed biomarkers of potential epithelial (soluble form of the receptor for advanced glycation end-products) or endothelial (angiopoietin-2) lung injury
What This Article Tells Us That Is New
After induction, tidal volume was redistributed to ventral regions with a statistically significant higher dorsal fraction of ventilation in the open-lung group
However, this effect was transient with no differences noted at the end of surgery or in extubated patients at postoperative day 2
Significantly higher intraoperative levels of soluble form of the receptor for advanced glycation end-products were noted in the open-lung group, suggestive of epithelial damage from lung overdistention
Research Support
The study was funded by the French Ministry of Health, Paris, France (grant No. PHRCI-15-055). Dr. Vidal Melo was funded by National Institutes of Health-National Heart, Lung, and Blood Institute, Bethesda, Maryland (grant No. R01 HL121228).
Appendix 1. Summary of the PROVECS Clinical Trial Protocol
Design
PROVECS is a prospective, multicenter, randomized, controlled, two-arm trial comparing two perioperative ventilatory strategies in cardiac surgery with cardiopulmonary bypass: (1) experimental strategy: surgeon-controlled open-lung ventilation; (2) control strategy: conventional protective ventilation with low PEEP. Double-blinding is ensured by the general anesthesia in the trial participants and by masking the outcome assessor. Hiding all the intraoperative data (including ventilator settings) on the electronic case report form at the end of surgery ensures the masking of the treatment arm.
Partners
The patients will be recruited in six French adult cardiac surgery departments. The methodologic support will be provided by the Clinical Research Unit (Unité Aide Méthodologique à la Recherche Clinique, Assistance Publique - Hôpitaux de Marseille, France). The study is sponsored by the Assistance Publique - Hôpitaux de Marseille (Project Manager, Patrick Sudour). This work is supported by institutional grants from the French Clinical Research Program 2015 (Program Hospitalier de Recherche Clinique).
Interventions
Experimental Strategy: Surgeon-controlled Open-lung Ventilation
In the experimental open-lung group, recruitment maneuvers (continuous positive airway pressure maintained at 30 cm H2O for 30 s) are systematically implemented at predefined stages in the surgical procedure:
After intubation and invasive arterial line placement
After CPB initiation when targeted blood-flow is reached
Before aortic declamping, after standard balloon de-airing maneuvers
At ICU arrival with the ICU ventilator
-
After each breathing circuit disconnection
PEEP levels in the experimental open-lung group are set at 8 cm H2O from intubation in the operating room to extubation in the ICU. During CPB, ultraprotective ventilation is used with PEEP at 8 cm H2O, very low tidal volumes (3 ml/kg of predicted body weight), a respiratory rate of 12 cycles per minute and Fio2 of 40%. Surgical protocol deviation has been standardized.
Control Strategy: Conventional Protective Ventilation with Low PEEP
No recruitment maneuvers are carried out. The PEEP is set at 2 cm H2O from intubation to extubation. Continuous positive airway pressure is maintained at 2 cm H2O during CPB (Fio2 40%).
Protocol Adjustment
In the experimental strategy group, the recruitment maneuver before and after the CPB can be avoided or interrupted on surgical demand or in the case of systolic arterial pressure less than 80 mmHg despite the adequate use of fluids and/or vasoactive drugs. The recruitment maneuver during CPB can be interrupted on surgical demand, or in case of a severe decrease in venous return with the inability to maintain the blood flow. PEEP levels can be decreased on surgical demand or on the anesthesiologist’s decision in the case of hemodynamic impairment despite the adequate use of fluids and/or vasoactive drugs. In these cases, PEEP will be decreased in stages of 1 cm H2O until correction of the problem. In the conventional strategy group, in the case of intraoperative hypoxemia (Spo2 less than 92% despite Fio2 80%), unplanned recruitment maneuvers and/or increased PEEP are permitted as a rescue strategy at the anesthesiologist’s discretion. Data on deviations from the protocol (including the number of completed recruitment maneuvers and effective intraoperative PEEP levels) will be analyzed.
Surgery
The type of drugs used for the anesthesia, the management of the CPB and fluid, and transfusion strategies are implemented according to local protocols in each recruiting center. Nonetheless, the use of peridural thoracic anesthesia is not permitted. During sternal sawing, PEEP will be temporarily set to 0 cm H2O in both groups to prevent unnecessary pleural opening. Before aortic declamping, de-airing maneuvers with manual balloon ventilation are performed in both groups according to local protocols, with or without the use of transesophageal echocardiography and under surgical guidance.
Follow-up
During transport from the operating room to the ICU, ventilation is operated with a self-inflating balloon or transport ventilator. If the transport ventilator is used, respiratory parameters are set according to the allocated treatment arm. A fast-track extubation protocol, defined by extubations performed before the sixth postoperative hour, is followed in all centers. The postoperative care, including sedation drugs, analgesia, fluid management, respiratory physiotherapy, and the duration of the stay in the ICU, is performed according to local protocols and at the discretion of the physician in charge. The postoperative use of curative noninvasive ventilation or nasal high-flow oxygen therapy is implemented according to local protocols in each recruiting center. Prophylactic use (before any type of respiratory failure) of these techniques is not permitted. New invasive mechanical ventilation will be indicated at the discretion of the ICU physician in charge. The minimal ICU length of stay is 24 h.
Study Endpoints
The primary endpoint, the proportion of postoperative pulmonary complications, is defined as a composite endpoint taking the presence of at least one of the following items during the first seven postoperative days into account. These postoperative pulmonary complications have been defined as follows:
Mild respiratory failure: Spo2 less than 90% or Pao2 less than 60 mmHg after breathing ambient air for 10 min (excluding hypoventilation) and corrected with an oxygen supply of 1 to 3 L/min with a nasal cannula
Moderate respiratory failure: Spo2 less than 90% or Pao2 less than 60 mmHg despite a 3 l/min oxygen supply with a nasal cannula (excluding hypoventilation) and corrected with an oxygen supply from 4 to 10 l/ min with a face mask
Severe respiratory failure: Spo2 less than 90% or Pao2 less than 60 mmHg despite a 10 l/min oxygen supply with a face mask (excluding hypoventilation) and corrected with an oxygen supply greater than 10 L/min with a high-flow face mask or with noninvasive ventilation or with high-flow nasal oxygen therapy or with invasive mechanical ventilation
Fast-track extubation failure associated with hypoxemia: delayed extubation after the first 6 h postoperative, associated with Pao2/Fio2 less than 300
New invasive mechanical ventilation associated with hypoxemia, defined as Pao2/Fio2 less than 300
Bronchospasm: new wheezing, indicating bronchodilator treatment (except preoperative chronic obstructive pulmonary disease or asthma)
Severe tracheobronchial congestion: audible ronchi associated with disturbance in respiratory mechanics
Postextubation respiratory acidosis defined by pH less than or equal to 7.30 and Paco2 greater than 45 mmHg
Suspected pneumonia: new pulmonary infiltrate on a chest x-ray, plus at least two of the following: temperature greater than 38.5°C or less than 35.5°C, leukocytosis or leukopenia (white blood cells greater than 12,000 cells/mm3 or less than 4,000 cells/mm3), purulent secretions, and antibiotic treatment
Confirmed pneumonia: new pulmonary infiltrate on a chest x-ray plus microbiologic documentation (more than 107CFU/mm3 on expectorated sputum, more than 105 CFU/mm3 on trans-tracheal aspiration, or more than 104 CFU/mm3 on bronchoalveolar lavage)
Pleural effusion with need for further postoperative pleural drainage.
Radiological atelectasis: new lung opacity on a chest x-ray with a shift in the mediastinum or ipsilateral hemi-diaphragm
ARDS as defined by the Berlin definition
The secondary clinical endpoints include:
Each preceding postoperative pulmonary complication by postoperative day (POD) 7 analyzed individually
Use of noninvasive ventilation by POD 7
Use of high-flown nasal oxygen therapy by POD 7
Use of new invasive mechanical ventilation by POD 7
- Postoperative extrapulmonary complications analyzed individually by POD 7:
- Systemic inflammatory response syndrome, sepsis, and septic shock
- Postoperative wound infection (sepsis with purulent wound drainage and antibiotic administration)
- Postoperative pericardial tamponade (need for reintervention)
- De novo postoperative atrial fibrillation
- Cardiogenic pulmonary edema (acute hypoxemia with diffuse bilateral pulmonary infiltrate on a chest x-ray, high left atrial pressure on cardiac ultrasound or pulmonary capillary wedged pressure greater than 18 mmHg)
- Acute kidney injury (Kidney Disease Improving Global Outcomes (KDIGO) stage 2 or 3)
- Delirium (disturbed state of consciousness and cognitive dysfunction with or without agitation)
- Adverse events by POD 7:
- Postoperative pneumothorax (need for further postoperative pleural drainage)
- Use of intraoperative or postoperative vasoactive drugs (excluding ephedrine and phenylephrine)
- Use of high-dose inotropes (greater than 8 μg · kg−1 · min−1 of dobutamine or greater than 0.8 μg · kg−1 · min−1 of milrinone)
- Acute postoperative bleeding with need for reintervention before the 12th postoperative hour
Survival in terms of ICU-free days by POD 7
Global mortality by POD 7
Appendix 2. Participants’ Entry Criteria
Patients were eligible for enrollment if they fulfilled all of the inclusion criteria and did not meet one or more of the exclusion criteria.
Inclusion Criteria
Adult patient
Written informed consent
- Elective cardiac surgery with ALL the following characteristics:
- General anesthesia
- Invasive mechanical ventilation
- Conventional cardiopulmonary bypass
- Aortic cross clamp and cardioplegia
- Complete median sternotomy
Exclusion Criteria
- Surgery-related:
- Emergent surgery, including cardiac transplantation, aortic dissection, and active endocarditis surgery
- Left ventricular assist device implantation
- Surgery with circulatory arrest
- Redo surgery
- Patient-related:
- Acute or chronic hypoxemia defined by a pressure of arterial oxygen (Pao2) less than 65 mmHg or a peripheral capillary oxygen saturation (Spo2) less than 95% on ambient air
- Mechanical ventilation in the 7 days before surgery
- Preoperative shock state
- Body mass index (BMI) more than 35 kg/m2
- Obstructive sleep apnea syndrome treated with continuous positive airway pressure
- Preoperative left ventricular ejection fraction less than 40%
- Right ventricular systolic dysfunction (Doppler–derived tricuspid lateral annular systolic velocity less than 10 cm · s−1)
- Systolic pulmonary artery pressure greater than 50 mmHg
- Glomerular filtration rate less than 30 ml · min−1
Appendix 3. Missing Data
EIT Analysis
One of 56 (1.8%) awake preoperative measurement was missing in the open-lung ventilation group owing to artefacts in signal
Zero of 56 (0%) anesthesia onset measurement was missing
One of 56 (1.8%) end of surgery measurements in the open-lung ventilation group was missing owing to artefacts in signal
- Eight of 56 (14.3%) postoperative day 2 measurements were missing (four in the open-lung ventilation group; four in the control group) owing to artefacts in signal
- Total EIT: 10 of 224 (4.5%) missing data.
Biomarkers Analysis
One of 120 (0.8%) sRAGE plasma level (aortic unclamping – pulmonary venous) is missing in a control group patient owing to sampling failure before aortic unclamping and coronary arteries reperfusion
- Five of 120 (4.2%) angiopoietin-2 plasma levels were missing:
- Four out-of-range enzyme-linked immunosorbent assay results at the four timepoints of the same open-lung ventilation group patient
- One plasma level (aortic unclamping – pulmonary venous) is missing in a control group patient owing to sampling failure before aortic unclamping and coronary arteries reperfusion
- Total missing data biomarkers: 6 of 240 (2.5%) missing data.
Footnotes
Competing Interests
The authors declare no competing interests.
Contributor Information
David Lagier, Department of Anesthesiology and Critical Care Medicine, University Hospital Timone, Aix Marseille University, Marseille, France C2VN, Inserm 1263, Inrae 1260, Aix Marseille University, Marseille, France.
Catherine Guidon, Department of Anesthesiology and Critical Care Medicine, University Hospital Timone, Aix Marseille University, Marseille, France
Marcos F. Vidal Melo, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
Marie-Christine Alessi, C2VN, Inserm 1263, Inrae 1260, Aix Marseille University, Marseille, France.
References
- 1.Magnusson L, Zemgulis V, Wicky S, Tydén H, Thelin S, Hedenstierna G: Atelectasis is a major cause of hypoxemia and shunt after cardiopulmonary bypass: An experimental study. Anesthesiology 1997; 87:1153–63 [DOI] [PubMed] [Google Scholar]
- 2.Duggan M, Kavanagh BP: Pulmonary atelectasis: A pathogenic perioperative entity. Anesthesiology 2005; 102:838–54 [DOI] [PubMed] [Google Scholar]
- 3.Chu EK, Whitehead T, Slutsky AS: Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med 2004; 32:168–74 [DOI] [PubMed] [Google Scholar]
- 4.Lellouche F, Dionne S, Simard S, Bussières J, Dagenais F: High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 2012; 116:1072–82 [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS, Ranieri VM: Ventilator-induced lung injury. N Engl J Med 2013; 369:2126–36 [DOI] [PubMed] [Google Scholar]
- 6.Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, Chierichetti M, Coppola S, Conte G, Gatti S, Leopardi O, Masson S, Lombardi L, Lazzerini M, Rampoldi E, Cadringher P, Gattinoni L: Lung stress and strain during mechanical ventilation: Any safe threshold? Am J Respir Crit Care Med 2011; 183:1354–62 [DOI] [PubMed] [Google Scholar]
- 7.Futier E, Marret E, Jaber S: Perioperative positive pressure ventilation: An integrated approach to improve pulmonary care. Anesthesiology 2014; 121:400–8 [DOI] [PubMed] [Google Scholar]
- 8.D’Antini D, Huhle R, Herrmann J, Sulemanji DS, Oto J, Raimondo P, Mirabella L, Hemmes SNT, Schultz MJ, Pelosi P, Kaczka DW, Vidal Melo MF, Gama de Abreu M, Cinnella G; European Society of Anaesthesiology and the PROtective VEntilation Network: Respiratory system mechanics during low versus high positive end-expiratory pressure in open abdominal surgery: A substudy of PROVHILO randomized controlled trial. Anesth Analg 2018; 126:143–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Östberg E, Thorisson A, Enlund M, Zetterström H, Hedenstierna G, Edmark L: Positive end-expiratory pressure alone minimizes atelectasis formation in non-abdominal surgery: A randomized controlled trial. Anesthesiology 2018; 128:1117–24 [DOI] [PubMed] [Google Scholar]
- 10.Reis Miranda D, Gommers D, Struijs A, Dekker R, Mekel J, Feelders R, Lachmann B, Bogers AJ: Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. Eur J Cardiothorac Surg 2005; 28:889–95 [DOI] [PubMed] [Google Scholar]
- 11.Pelosi P, Rocco PRM, Gama de Abreu M: Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Crit Care 2018; 22:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The PROVE Network Investigators, Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ: High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): A multicentre randomised controlled trial. Lancet 2014; 384: 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Writing Committee for the PROVE Network, Bluth T, Serpa Neto A, Schultz MJ, Pelosi P, Gama de Abreu M, Group PC, Bluth T, Bobek I, Canet JC, Cinnella G, de Baerdemaeker L, Gama de Abreu M, Gregoretti C, Hedenstierna G, Hemmes SNT, Hiesmayr M, Hollmann MW, Jaber S, Laffey J, Licker MJ, Markstaller K, Matot I, Mills GH, Mulier JP, Pelosi P, Putensen C, Rossaint R, Schmitt J, Schultz MJ, Senturk M, Serpa Neto A, Severgnini P, Sprung J, Vidal Melo MF, Wrigge H: Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: A randomized clinical trial. JAMA 2019; 321: 2292–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neves FH, Carmona MJ, Auler JO Jr, Rodrigues RR, Rouby JJ, Malbouisson LM: Cardiac compression of lung lower lobes after coronary artery bypass graft with cardiopulmonary bypass. PLoS One 2013; 8:e78643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho AR, Ichinose F, Schettino IA, Hess D, Rojas J, Giannella-Neto A, Agnihotri A, Walker J, MacGillivray TE, Vidal Melo MF: Tidal lung recruitment and exhaled nitric oxide during coronary artery bypass grafting in patients with and without chronic obstructive pulmonary disease. Lung 2011; 189:499–509 [DOI] [PubMed] [Google Scholar]
- 16.Armaganidis A, Diplas D, Floros I, Roussos C: Effect of median sternotomy on respiratory system compliance in humans: Evaluation without sophisticated instrumentation. Interact Cardiovasc Thorac Surg 2009; 8:22–6 [DOI] [PubMed] [Google Scholar]
- 17.Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ: A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 1998; 158(5 Pt 1):1644–55 [DOI] [PubMed] [Google Scholar]
- 18.Dechman GS, Chartrand DA, Ruiz-Neto PP, Bates JH: The effect of changing end-expiratory pressure on respiratory system mechanics in open- and closed-chest anesthetized, paralyzed patients. Anesth Analg 1995; 81:279–86 [DOI] [PubMed] [Google Scholar]
- 19.Lagier D, Fischer F, Fornier W, Huynh TM, Cholley B, Guinard B, Heger B, Quintana G, Villacorta J, Gaillat F, Gomert R, Degirmenci S, Colson P, Lalande M, Benkouiten S, Minh TH, Pozzi M, Collart F, Latremouille C, Vidal Melo MF, Velly LJ, Jaber S, Fellahi JL, Baumstarck K, Guidon C; PROVECS Study Group: Effect of open-lung vs conventional perioperative ventilation strategies on postoperative pulmonary complications after on-pump cardiac surgery: The PROVECS randomized clinical trial. Intensive Care Med 2019; 45:1401–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedenstierna G: Using electric impedance tomography to assess regional ventilation at the bedside. Am J Respir Crit Care Med 2004; 169:777–8 [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T, Piraino T, Lima CAS, Kavanagh BP, Amato MBP, Brochard L: Regional ventilation displayed by electrical impedance tomography as an incentive to decrease positive end-expiratory pressure. Am J Respir Crit Care Med 2019; 200:933–7 [DOI] [PubMed] [Google Scholar]
- 22.Victorino JA, Borges JB, Okamoto VN, Matos GF, Tucci MR, Caramez MP, Tanaka H, Sipmann FS, Santos DC, Barbas CS, Carvalho CR, Amato MB: Imbalances in regional lung ventilation: A validation study on electrical impedance tomography. Am J Respir Crit Care Med 2004; 169:791–800 [DOI] [PubMed] [Google Scholar]
- 23.Jabaudon M, Blondonnet R, Roszyk L, Bouvier D, Audard J, Clairefond G, Fournier M, Marceau G, Déchelotte P, Pereira B, Sapin V, Constantin JM: Soluble receptor for advanced glycation end-products predicts impaired alveolar fluid clearance in acute respiratory distress syndrome. Am J Respir Crit Care Med 2015; 192:191–9 [DOI] [PubMed] [Google Scholar]
- 24.Kuipers MT, Aslami H, Tuinman PR, Tuip-de Boer AM, Jongsma G, van der Sluijs KF, Choi G, Wolthuis EK, Roelofs JJ, Bresser P, Schultz MJ, van der Poll T, Wieland CW: The receptor for advanced glycation end products in ventilator-induced lung injury. Intensive Care Med Exp 2014; 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA; NHLBI ARDS Network: Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 2012; 40:1731–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C: Angiopoietin-2 is increased in severe sepsis: Correlation with inflammatory mediators. Crit Care Med 2007; 35:199–206 [DOI] [PubMed] [Google Scholar]
- 27.Radke OC, Schneider T, Heller AR, Koch T: Spontaneous breathing during general anesthesia prevents the ventral redistribution of ventilation as detected by electrical impedance tomography: A randomized trial. Anesthesiology 2012; 116:1227–34 [DOI] [PubMed] [Google Scholar]
- 28.Uchida T, Ohno N, Asahara M, Yamada Y, Yamaguchi O, Tomita M, Makita K: Soluble isoform of the receptor for advanced glycation end products as a biomarker for postoperative respiratory failure after cardiac surgery. PLoS One 2013; 8:e70200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shono A, Katayama N, Fujihara T, Böhm SH, Waldmann AD, Ugata K, Nikai T, Saito Y: Positive end-expiratory pressure and distribution of ventilation in pneumoperitoneum combined with steep trendelenburg position. Anesthesiology 2020; 132:476–90 [DOI] [PubMed] [Google Scholar]
- 30.Costa Leme A, Hajjar LA, Volpe MS, Fukushima JT, De Santis Santiago RR, Osawa EA, Pinheiro de Almeida J, Gerent AM, Franco RA, Zanetti Feltrim MI, Nozawa E, de Moraes Coimbra VR, de Moraes Ianotti R, Hashizume CS, Kalil Filho R, Auler JO Jr, Jatene FB, Gomes Galas FR, Amato MB: Effect of intensive vs moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: A randomized clinical trial. JAMA 2017; 317:1422–32 [DOI] [PubMed] [Google Scholar]
- 31.Froese AB, Bryan AC: Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974; 41:242–55 [DOI] [PubMed] [Google Scholar]
- 32.Reber A, Engberg G, Wegenius G, Hedenstierna G: Lung aeration. The effect of pre-oxygenation and hyperoxygenation during total intravenous anaesthesia. Anaesthesia 1996; 51:733–7 [DOI] [PubMed] [Google Scholar]
- 33.Jonmarker C, Nordström L, Werner O: Changes in functional residual capacity during cardiac surgery. Br J Anaesth 1986; 58:428–32 [DOI] [PubMed] [Google Scholar]
- 34.Chapin JC, Downs JB, Douglas ME, Murphy EJ, Ruiz BC: Lung expansion, airway pressure transmission, and positive end-expiratory pressure. Arch Surg 1979; 114:1193–7 [DOI] [PubMed] [Google Scholar]
- 35.Lai-Fook SJ, Rodarte JR: Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J Appl Physiol (1985) 1991; 70: 967–78 [DOI] [PubMed] [Google Scholar]
- 36.Loring SH, Topulos GP, Hubmayr RD: Transpulmonary pressure: The importance of precise definitions and limiting assumptions. Am J Respir Crit Care Med 2016; 194:1452–7 [DOI] [PubMed] [Google Scholar]
- 37.Barnas GM, Gilbert TB, Watson RJ, Sequeira AJ, Roitman K, Nooroni RJ: Respiratory mechanics in the open chest: effects of parietal pleurae. Respir Physiol 1996; 104:63–70 [DOI] [PubMed] [Google Scholar]
- 38.Jabaudon M, Berthelin P, Pranal T, Roszyk L, Godet T, Faure JS, Chabanne R, Eisenmann N, Lautrette A, Belville C, Blondonnet R, Cayot S, Gillart T, Pascal J, Skrzypczak Y, Souweine B, Blanchon L, Sapin V, Pereira B, Constantin JM: Receptor for advanced glycation end-products and ARDS prediction: A multicentre observational study. Sci Rep 2018; 8:2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabaudon M, Hamroun N, Roszyk L, Guérin R, Bazin JE, Sapin V, Pereira B, Constantin JM: Effects of a recruitment maneuver on plasma levels of soluble RAGE in patients with diffuse acute respiratory distress syndrome: A prospective randomized crossover study. Intensive Care Med 2015; 41:846–55 [DOI] [PubMed] [Google Scholar]
- 40.Yehya N, Thomas NJ, Meyer NJ, Christie JD, Berg RA, Margulies SS: Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Intensive Care Med 2016; 42:1137–45 [DOI] [PubMed] [Google Scholar]
- 41.Guo WA, Knight PR, Raghavendran K: The receptor for advanced glycation end products and acute lung injury/acute respiratory distress syndrome. Intensive Care Med 2012; 38:1588–98 [DOI] [PubMed] [Google Scholar]
- 42.Jabaudon M, Futier E, Roszyk L, Sapin V, Pereira B, Constantin JM: Association between intraoperative ventilator settings and plasma levels of soluble receptor for advanced glycation end-products in patients without pre-existing lung injury. Respirology 2015; 20:1131–8 [DOI] [PubMed] [Google Scholar]
- 43.Silva PL, Moraes L, Santos RS, Samary C, Ramos MB, Santos CL, Morales MM, Capelozzi VL, Garcia CS, de Abreu MG, Pelosi P, Marini JJ, Rocco PR: Recruitment maneuvers modulate epithelial and endothelial cell response according to acute lung injury etiology. Crit Care Med 2013; 41:e256–65 [DOI] [PubMed] [Google Scholar]
- 44.Riva DR, Oliveira MB, Rzezinski AF, Rangel G, Capelozzi VL, Zin WA, Morales MM, Pelosi P, Rocco PR: Recruitment maneuver in pulmonary and extrapulmonary experimental acute lung injury. Crit Care Med 2008; 36:1900–8 [DOI] [PubMed] [Google Scholar]
- 45.Frank JA, McAuley DF, Gutierrez JA, Daniel BM, Dobbs L, Matthay MA: Differential effects of sustained inflation recruitment maneuvers on alveolar epithelial and lung endothelial injury. Crit Care Med 2005; 33:181–8; discussion 254–5 [DOI] [PubMed] [Google Scholar]
- 46.Fischer MO, Courteille B, Guinot PG, Dupont H, Gérard JL, Hanouz JL, Lorne E; ARCOTHOVA, CARGO Groups: Perioperative ventilatory management in cardiac surgery: A French nationwide survey. Medicine (Baltimore) 2016; 95:e2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF: Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 2011; 107:998–1004 [DOI] [PubMed] [Google Scholar]
- 48.Heinze H, Eichler W, Karsten J, Sedemund-Adib B, Heringlake M, Meier T: Functional residual capacity-guided alveolar recruitment strategy after endotracheal suctioning in cardiac surgery patients. Crit Care Med 2011; 39:1042–9 [DOI] [PubMed] [Google Scholar]
- 49.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, Song F, Qu W, Gomez T, Zou YS, Yan SF, Schmidt AM, Ramasamy R: Receptor for advanced-glycation end products: Key modulator of myocardial ischemic injury. Circulation 2006; 113:1226–34 [DOI] [PubMed] [Google Scholar]


