Abstract
Poliovirus importations into polio-free countries represent a major concern during the final phases of global eradication of wild polioviruses (WPVs). We extend dynamic transmission models to demonstrate the dynamics of population immunity out through 2020 for three countries that only used inactivated poliovirus vaccine (IPV) for routine immunization: the US, Israel, and The Netherlands. For each country, we explore the vulnerability to re-established transmission following an importation for each poliovirus serotype, including the impact of immunization choices following the serotype 1 WPV importation that occurred in 2013 in Israel. As population immunity declines below the threshold required to prevent transmission, countries become at risk for re-established transmission. Although importations represent stochastic events that countries cannot fully control because people cross borders and polioviruses mainly cause asymptomatic infections, countries can ensure that any importations die out. Our results suggest that the general US population will remain above the threshold for transmission through 2020. In contrast, Israel became vulnerable to re-established transmission of importations of live polioviruses by the late 2000s. In Israel, the recent WPV importation and outbreak response use of bivalent oral poliovirus vaccine (bOPV) eliminated the vulnerability to an importation of poliovirus serotypes 1 and 3 for several years, but not serotype 2. The Netherlands experienced a serotype 1 WPV outbreak in 1992–1993 and became vulnerable to re-established transmission in religious communities with low vaccine acceptance around the year 2000, although the general population remains well-protected from widespread transmission. All countries should invest in active management of population immunity to avoid the potential circulation of imported live polioviruses. IPV-using countries may wish to consider prevention opportunities and/or ensure preparedness for response. Countries currently using a sequential IPV/OPV schedule should continue to use all licensed OPV serotypes until global OPV cessation to minimize vulnerability to circulation of imported polioviruses.
Keywords: polio, eradication, population immunity, vaccine
INTRODUCTION
The risk of infectious agents crossing international borders motivates global disease coordination and management efforts, including the Global Polio Eradication Initiative.[1] As long as wild polioviruses (WPVs) circulate anywhere, they pose some risk of importation (i.e., crossing the border) into all countries. Not surprisingly, once countries succeed in stopping endemic (i.e., indigenous) WPV transmission (i.e., national elimination) and become “polio free,” their concerns about WPVs primarily turn to potential importations. Detection of an importation typically depends on the Global Polio Laboratory Network finding paralytic cases, and consequently WPV importations that do not result in identified paralytic cases go unnoticed. Notable exceptions occurred with the detection of asymptomatic WPV serotype 1 (WPV1) transmission in 2013 by the extensive Israeli environmental surveillance system, which allowed Israel to respond to the circulation and successfully prevent cases,[2–4] and similar detection and response to the same WPV1 in Egypt.[5]
Recently, the World Health Organization focused on importations as a primary concern for the polio endgame and established temporary recommendations for international travel immunization to reduce the international spread of poliovirus.[6] While efforts to increase the immunity of individual international travelers may reduce the number of importation events, this approach does not eliminate the risk altogether and focuses only on the nationally less-controllable part of the risk of re-established transmission. In addition to the importation event (e.g., WPV entering the population), the risk of re-established transmission of an imported WPV depends on the vulnerability of the population receiving the imported virus to sustain transmission, which depends on its population immunity to poliovirus transmission.[7] Thus, while countries cannot easily control all of the border crossings that may lead to importation events,[8, 9] particularly for a disease that primarily spreads asymptomatically, national immunization decisions determine population immunity to transmission and thus the overall national risk of re-established transmission of imported polioviruses.[7]
Population immunity to transmission represents the aggregation of the immunity of all individuals within a population, and it changes over time with demographic changes (i.e., births of immunologically-naïve individuals, deaths of immune individuals, and immigration) and factors that impact individual immunity (i.e., immunization, infection, and waning of antibodies).[7] Models of population immunity must consider all dynamic inputs, and also account for the different types of immunological protection provided by oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV).[7] As a live, attenuated virus, OPV causes infections in vaccine recipients who can spread their infections to effectively immunize contacts or boost their immunity, providing benefits beyond the vaccine recipient. However, OPV comes with a small risk of vaccine-associated paralytic polio (VAPP),[10] and OPV-using populations with low immunity levels can support sustained transmission of OPV-related viruses that evolve to become circulating vaccine-derived polioviruses (cVDPVs), which behave like WPVs.[10, 11] For serotype 2, cVDPVs now represent the primary importation risk given the absence of any serotype 2 WPV since 2000.[8] In contrast to OPV, IPV provides protection only to vaccine recipients and it does not come with VAPP or cVDPV risks. However, IPV does not protect as well as OPV against asymptomatic intestinal infections or fecal-oral transmission.[12, 13] After successful immunization with IPV or recovery from an infection with a live poliovirus (LPV, i.e., WPV, cVPDV, OPV, or OPV-related virus) of a specific serotype, individuals benefit from permanent homotypic protection from paralysis, but they can get re-infected and participate asymptomatically in homotypic transmission to some degree.[12–15]
Figure 1 summarizes the number of calendar years that countries reported one or more WPV or cVDPV cases during 2000–2014 and demonstrates ongoing national challenges associated with maintaining high population immunity. Social disruptions appear to represent a significant risk factor (e.g., Syria, Iraq), which suggests that areas with social unrest (e.g., Somalia, Pakistan, and more recently, Ukraine, Guinea, Liberia, Sierra Leone) may warrant particular attention. Full protection from paralytic polio requires immunity for all three poliovirus serotypes. Both IPV and trivalent OPV (tOPV) currently used for routine immunization (RI) contain all three serotypes, but countries can use bivalent OPV (bOPV, containing serotypes 1 and 3) and monovalent OPV (mOPV) formulations for supplemental immunization activities (SIAs).[8, 16] Immunization choices imply trade-offs,[16] and current discussions about the polio endgame lead to questions about the dynamics of coordinated global OPV cessation and the role of IPV with respect to managing population immunity.[17–20] Current plans include globally-coordinated cessation of serotype-2 containing OPV (i.e., OPV2 cessation) first, followed by globally-coordinated OPV cessation of serotypes 1 and 3 (i.e., OPV1&3 cessation).[21] The GPEI identified 6 criteria as prerequisites to OPV2 cessation[21], and we highlighted the importance of assuring high enough population immunity at the time of OPV2 using sufficient tOPV SIAs as an additional prerequisite to the safe withdrawal of OPV2.[16]
Figure 1.
Number of calendar years during 2000–2014 that each country reported at least one paralytic polio cases caused by a WPV or cVDPV indicating insufficient population immunity to stop or prevent transmission, (a) including years with endemic circulation and (b) including only years with circulation of cVDPVs* or imported WPV after becoming polio-free. Based on the summary table for 2000–2012 data[8] with updated data for 2013 and 2014[1]
* Includes years with no WPV circulation with imported or domestic cVDPVs for the following countries (number of calendar years with cVDPV but no WPV): Cambodia (2), China (1), Dominican Republic (2), Ethiopia (2), Haiti (2), Kenya (1), Madagascar (3), Mozambique (1), Myanmar (2), Niger (1), Philippines (1), Somalia (4), South Sudan (1), and Yemen (3)
Models characterizing the dynamics of poliovirus transmission and population immunity demonstrate the importance of maintaining high population immunity to achieve WPV eradication and successfully stop OPV use.[15, 17–20, 22, 23] Prior modeling emphasizes that OPV-using countries must keep their population immunity sufficiently above the threshold required to prevent transmission in order to prevent cVDPV emergences prior to and after OPV cessation.[17–20] Thus, OPV-using countries should use tOPV with sufficiently high coverage (i.e., RI with SIAs as needed) up until the point of OPV2 cessation, at which point they should switch to bOPV and again maintain high coverage to ensure high population immunity until OPV1&3 cessation.[16–20] Countries should recognize that their vaccine choices will also affect their probabilities of undetected LPV circulation after apparent interruption of transmission.[24] The prior models focused on OPV-using countries.[16–20] However, with all countries at risk for importations from any circulating WPVs or cVDPVs,[8] we recognize the importance of considering national vulnerability to re-established transmission following a LPV importation into IPV-only using countries.
METHODS
We extend our prior modeling[4, 15, 17–20, 22–24] to characterize vulnerability to re-established transmission and options that IPV-only using countries may consider to reduce or eliminate their vulnerability (see appendix). Briefly, the model tracks the population in different immunity states as a result of births, deaths, immigration, immunization, infection, and waning. We developed generic model inputs for human immunological responses to polioviruses and poliovirus transmission characteristics by serotype that remain constant across all modeled situations (i.e., immunity state inputs for susceptibility, infectiousness, and duration of the latent and infectious periods, kinetics of waning immunity and OPV virus evolution (i.e., to become cVDPVs following sufficient sustained transmission), and relative poliovirus transmissibility and paralysis-to-infection ratios by serotype) based on an extensive expert review and elicitation process.[12, 13, 15] We calibrated the model inputs across ten diverse epidemiological situations (i.e., geographic areas with different conditions and experiences with WPVs and cVDPVs), which used situation-specific appropriate inputs for population, historical RI and SIA vaccination, basic reproductive number (R0), seasonality, and relative proportion of overall (i.e., fecal-oral and oropharyngeal) transmissions that occur via the oropharyngeal route (poro). The calibration process focused on ensuring that the model inputs yielded behavior and estimates consistent with the actual reported WPV and/or cVDPV incidence by age, the actual apparent timing of WPV die-out (where appropriate), the absence or emergence of cVDPVs, and available data on secondary OPV transmission and children missed by SIAs. [4, 15, 17–20, 22–24] The model tracks viral transmission, including asymptomatic infections in individuals with prior immunity, and explicitly recognizes that relative susceptibility to infection and relative infectiousness over time determine the relative potential contribution to transmission for individuals in each immunity state.[7, 15] Aggregating the proportions of individuals in each immunity state, their potential contribution to transmission, and considering the mixing properties for different age groups and subpopulations in the model, we characterize population immunity to poliovirus transmission by computing the age-and-subpopulation-mixing-adjusted effective immune proportion (EIPM).[20] We also characterize the seasonally-varying immunity threshold EIP*=(1- 1/R0) above which infections eventually die out.[20]
US Model
Our prior analyses for the US suggested that while WPV or cVDPV importations into a pocket of under-vaccinated individuals might lead to limited transmission,[25] they would not likely lead to re-established transmission in the general population.[22] Our current model explicitly accounts for both fecal-oral and oropharyngeal transmission, which IPV use affects differentially.[15] Given the evidence that IPV protects well against participation in oropharyngeal transmission, but not as well against participation in fecal-oral transmission,[12, 13] we explore the reference case (RC) of continued IPV-only immunization and one different assumption about the proportion of transmissions via the oropharyngeal route (poro), which determines the relative importance of oropharyngeal transmission on population immunity without changing the overall R0 assumption.[15]
Israel Model
For Israel, our prior analysis explored the historical transmission of WPVs and the impacts of the 2013 WPV1 importation and immunization response on population immunity for serotype 1 through 2015.[4] The model divides the Israeli population geographically into the Southern district and the rest of Israel and socially into Jews and non-Jews for each geographic area (i.e., 4 preferentially-mixing subpopulations). Most of the transmission of the 2013 WPV1 occurred in the Southern district, particularly among the non-Jews,[4] but some limited transmission also occurred in the rest of Israel. Based on the epidemiology of the outbreak that focused on Bedouin communities with below-average hygiene standards, we characterize a somewhat higher R0 (i.e., of 6 vs. 5 elsewhere in Israel) and lower poro (i.e., 0.6 vs. 0.7 elsewhere) for the non-Jewish subpopulation in the Southern district.[2, 26] The RC includes the recent immunization response to the actual 2013 signal of a WPV1 detected by the Israeli environmental surveillance system.[4] We extend the model through 2020 and for all 3 serotypes. We consider retrospectively several hypothetical importations of WPV1, WPV3, or cVDPV2 with high and low seasonality and not outbreak response to explore the timing of vulnerability to re-established transmission. With some countries that currently use IPV/OPV sequential schedules possibly considering use of IPV-only, we explore the counterfactual of Israel historically not switching to IPV-only RI (i.e., continued sequential IPV/OPV). We also modeled prospective options with hypothetical introductions of WPV1, WPV3, or cVDPV2, for which we assumed the RC includes continued use of 2 bOPV doses in RI through the end of the time horizon. We considered the reality that Israel could stop using bOPV at any time (i.e., 2 bOPV doses from 2014 with cessation on indicated date).[4] We also considered the impact of Israel using tOPV instead of bOPV in RI starting on January 1, 2015 (i.e., switch to tOPV in 2015 with OPV cessation on indicated date) to demonstrate the impact of vaccine choices.
The Netherlands Model
The Netherlands model includes two subpopulations (i.e., the orthodox reformed communities of about 300,000 people at the time of the 1992–1993 outbreak, and the general Dutch population).[15] For the general population, we assumed RI coverage with 3 or more IPV doses decreased from 97% 1994[15] to about 95% from 2003 forward.[27] For the orthodox reformed communities, we use a best estimate of 40% relative coverage compared to the general population, and given uncertainty we consider a range of 20%−60% from 1994 forward.
RESULTS
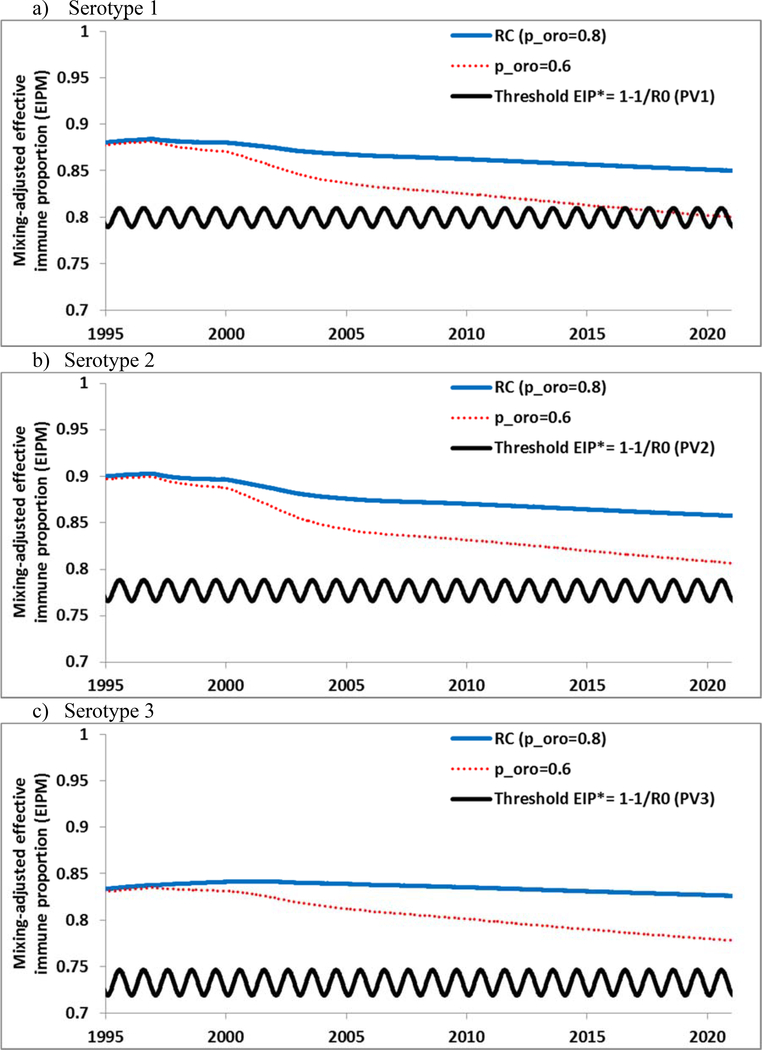
Figure 2 shows the US population immunity (i.e., EIPM) and the threshold (i.e., EIP*) for each serotype from 1995 through 2020 for the RC. For each serotype, EIPM>EIP*, which suggests no vulnerability to re-established poliovirus transmission in the US general population, even after a long period of IPV-only RI, similar to prior studies.[22, 25] Thus, although real heterogeneity in the US implies some risk of limited localized transmission of imported LPVs within some subpopulations with much lower than average RI coverage (e.g., rural clusters of religious groups that object to vaccination or more urban upper-income communities that refuse vaccination based on personal beliefs),[22, 25] re-established transmission in the general population appears unlikely based on projected coverage. Figure 2 further highlights that sustained high population immunity provided by IPV depends on the realistic assumption that poliovirus transmission in the US primarily occurs via oropharyngeal contact.[12–15] If we unrealistically increase the relative importance of fecal-oral transmission (i.e., decrease poro from 0.8 in the RC to 0.6), then population immunity would drop below the threshold within the next few years for serotype 1 and creep towards the threshold for serotypes 2 and 3 (for which we assume lower values for R0[15] and thus lower thresholds). This decline in population immunity would occur despite continued high RI coverage and no change in the assumed absolute transmissibility of polioviruses (i.e., same R0), and it relates to the limited impact of IPV on fecal-oral transmission. While we believe hygiene and sanitation levels remain high in most places in the US, Figure 2 suggests that any clusters of people living in sub-optimal hygiene conditions in the US could see some spread despite high RI coverage with IPV. Similarly, populations using IPV-only with more fecal-oral transmission in other countries may become vulnerable to re-established transmission of WPVs or cVDPVs more quickly over time, even with sustained high RI coverage.
Figure 2.
Population immunity in the US general population by serotype for the RC compared to the threshold (EIP*) from 1995 forward, and for hypothetically lower proportion of transmissions via the oropharyngeal route (poro=0.6).
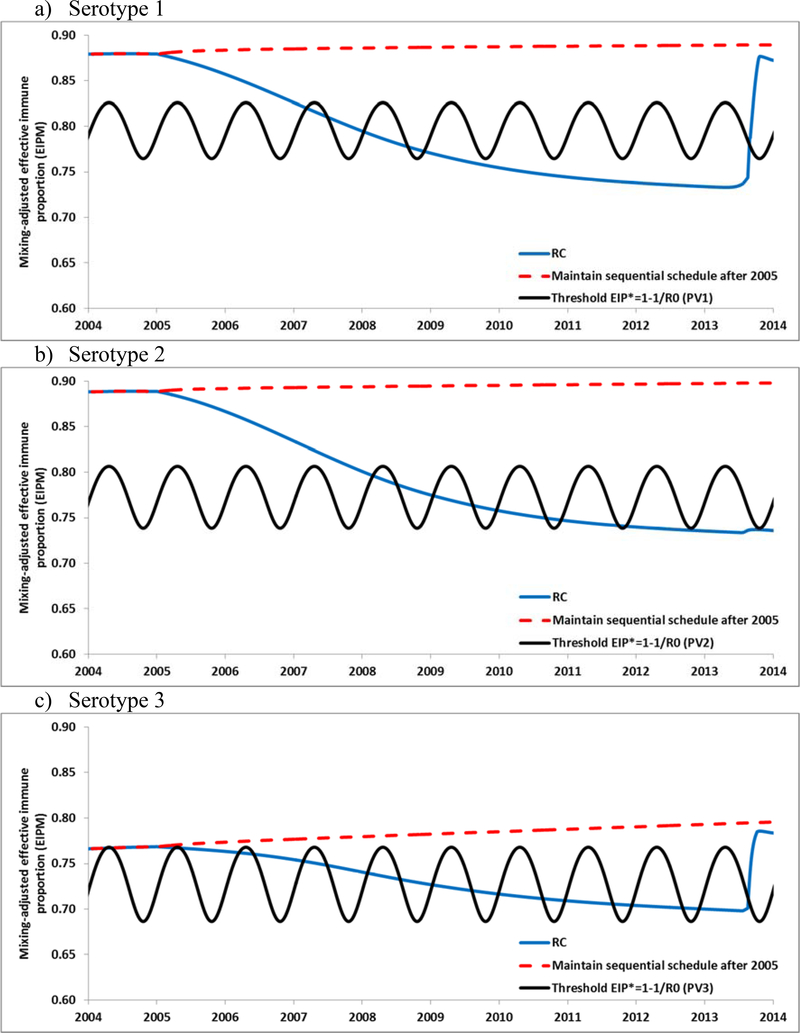
South Israel provides an example of conditions conducive to re-established transmission. Figure 3 shows how population immunity changed for the RC in Israel for each serotype for 2005–2014 and suggests that extension of the IPV/OPV sequential schedule would have maintained population immunity high enough to prevent Israel from becoming vulnerable to re-established transmission following any WPV or cVDPV importations. As shown in Figures 3a–3c, the population immunity for the RC for each serotype starts to decrease and drops to a level consistently below the EIP*, although this occurs at different times for each serotype, with increases in population immunity for serotypes 1 and 3 starting in 2013 due to the WPV1 importation and subsequent bOPV use. Figure 3 suggests that population immunity dropped below the thresholds and exposed Israel to the possibility of limited, low-level transmission in the high season as early as 2007, 2008, and 2006, for serotypes 1, 2, and 3, respectively.
Figure 3.
Population immunity in Israel by serotype for the RC compared to the threshold (EIP*) and if Israel hypothetically had maintained an IPV/OPV sequential schedule.
Table 1 shows how many of the 4 subpopulations in the Israel model participated in transmission and the overall transmission behavior in the model following hypothetical introductions of imported WPV1, WPV3, and cVDPV2. In the context of the results shown in Figure 3, the results in the top of Table 1 suggest that the development of outbreaks and re-established transmission requires an extended period of population immunity below the threshold, with re-established transmission in the general population shown in Table 1 (top) possible in Israel for importations as early as 2009, 2012, and 2010 for serotypes 1, 2, and 3, respectively.
Table 1.
Vulnerability to re-established transmission in Israel following a hypothetical importation of WPV1, WPV3, or cVDPV2 between 2008–2012 (retrospective) and between 2015–2020 (prospective) for different immunization scenarios and resulting transmission behavior of the imported virus within the 4 modeled subpopulations in the absence of further outbreak response measures.
| Timing of introduction | Immunization assumptions scenario | Number of subpopulations affected (out of 4) after virus introduction indicated | Transmission behavior of introduced virus | ||||
|---|---|---|---|---|---|---|---|
| WPV1 | cVDPV2 | WPV3 | WPV1 | cVDPV2 | WPV3 | ||
| Retrospective scenarios | |||||||
| Feb 9, 2008 | Reference case (RC) | 4 | 1 | 1 | Low level in 2008, re-established in 2009 | Die-out in 2008 | Die-out in 2008 |
| Feb 9, 2009 | Reference case | 4 | 1 | 2 | Re-established in 2009 | Low level in 2009, re-established in 2010 | Low level in 2009, re-established in 2010 |
| Feb 9, 2010 | Reference case | 4 | 1 | 3 | Re-established in 2010 | Re-established in 2010 | Low level in 2010, re-established in 2011 |
| Feb 9, 2011 | Reference case | 4 | 2 | 3 | Re-established in 2011 | Re-established in 2011 | Low level in 2011, re-established in 2012 |
| Aug 7, 2011 | Reference case | 4 | 3 | 1 | Low level in 2011, re-established in 2012 | Low level in 2011, re-established in 2012 | Die-out in 2011 |
| Feb 9, 2012 | Reference case | 4 | 3 | 3 | Re-established in 2012 | Re-established in 2012 | Low level in 2012, re-established in 2013 |
| Aug 7, 2012 | Reference case | 4 | 4 | 1 | Low level in 2012, re-established in 2013 | Low level in 2012, re-established in 2013 | Die-out in 2012 |
| Any time | Continued sequential IPV/OPV | 0 | 0 | 0 | No spread | No spread | No spread |
| Prospective scenarios | |||||||
| Nov 1, 2015 | Reference case | 0 | 4 | 0 | No spread | Low level in 2016, re-established in 2017 | No spread |
| Feb 9, 2017` | 2 bOPV doses from 2014 with bOPV cessation in Oct 2015 | 1 | 4 | 1 | Die-out in 2017 | Low level in 2017, re-established in 2018 | Die-out in 2017 |
| Feb 9, 2019 | 2 bOPV doses from 2014 with bOPV cessation in Oct 2015 | 3 | 4 | 1 | Low level in 2019, re-established in 2020 | Low level in 2019, re-established in 2020 | Die-out in 2019 |
| Nov 1, 2015 | Switch to tOPV in Jan 2015 | 0 | 0 | 0 | No spread | No spread | No spread |
| Feb 9, 2017 | Switch to tOPV in Jan 2015 with tOPV cessation in Apr 2016 | 1 | 1 | 1 | Die-out in 2017 | Die-out in 2017 | Die-out in 2017 |
| Feb 9, 2019 | Switch to tOPV in Jan 2015 with tOPV cessation in Apr 2016 | 1 | 1 | 1 | Low level in 2019, re-established in 2021* | Low level in 2019, re-established in 2021* | Die-out in 2019 |
| Feb 9, 2019 | Switch to tOPV in Jan 2015, switch back to bOPV in Apr 2016 with OPV cessation in Apr 2019 | 1 | 1 | 1 | Die-out in 2019 | Low level in 2019, re-established in 2021* | Die-out in 2019 |
Note:
Possible re-establishment in 2021 (outside the analytical time frame) based on observed trend
Abbreviations: bOPV, bivalent types 1 and 3 oral poliovirus vaccine; IPV, inactivated poliovirus vaccine; PV1,2,3, poliovirus type 1, 2, or 3, respectively; tOPV, trivalent oral poliovirus vaccine
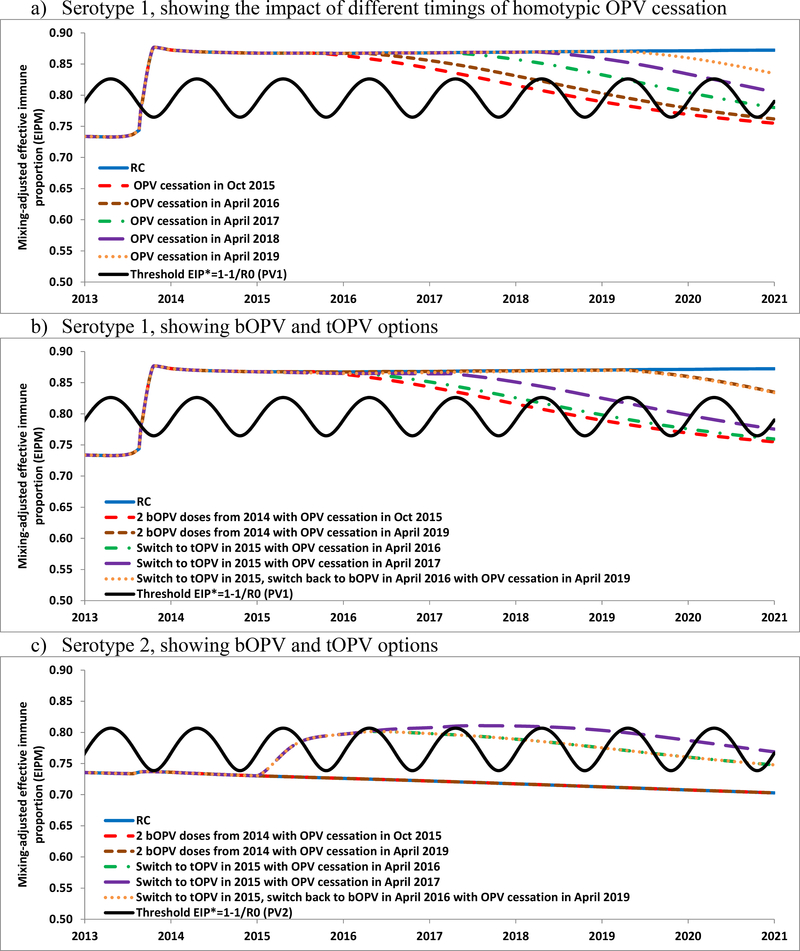
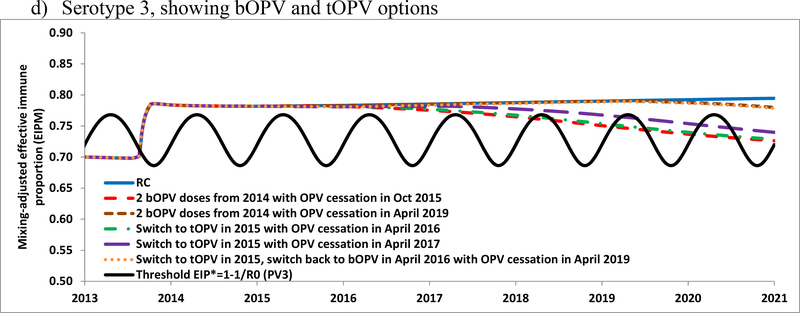
Figure 4a shows the population immunity for serotype 1 for the RC and for several alternative prospective dates for stopping serotype 1 OPV use, which demonstrates that population immunity begins declining as soon as use of OPV stops, even with high IPV coverage. Figures 4b, 4c, and 4d show the population immunity of the RC modeled prospectively for serotypes 1, 2, and 3, respectively, along with several options for homotypic OPV cessation dates and considering the switch from bOPV to tOPV in January 2015 until the homotypic OPV cessation date indicated. Table 1 (bottom) indicates the degree of spread of imported WPV1, WPV3, or cVDPV2 for different prospective hypothetical introduction times during 2015–2020. The model suggests that continued OPV use would prevent any importation of the included OPV serotypes (i.e., all serotypes for tOPV, serotypes 1 and 3 for bOPV) from spreading. For the RC, as shown in the retrospective analysis (Table 1, top) a cVDPV2 imported after 2012 leads to widespread transmission, so the prospective introduction in November 2015 takes off. If Israel decided to use tOPV instead of bOPV from January 2015 until global OPV2 cessation, then the model suggests this would prevent re-established serotype 2 transmission associated with a November 2015 importation. The model results also suggest that Israel might again become vulnerable to a WPV1 importation 3 years after discontinued bOPV use (i.e., the February 2017 importation dies-out the same year whereas the February 2019 importation spreads and leads to sustained transmission in the following year). Similarly, discontinued bOPV use would allow transmission of a WPV3 importation 4 years after discontinued bOPV use.
Figure 4.
Population immunity and the impacts of using tOPV or bOPV in RI after 2014 in Israel
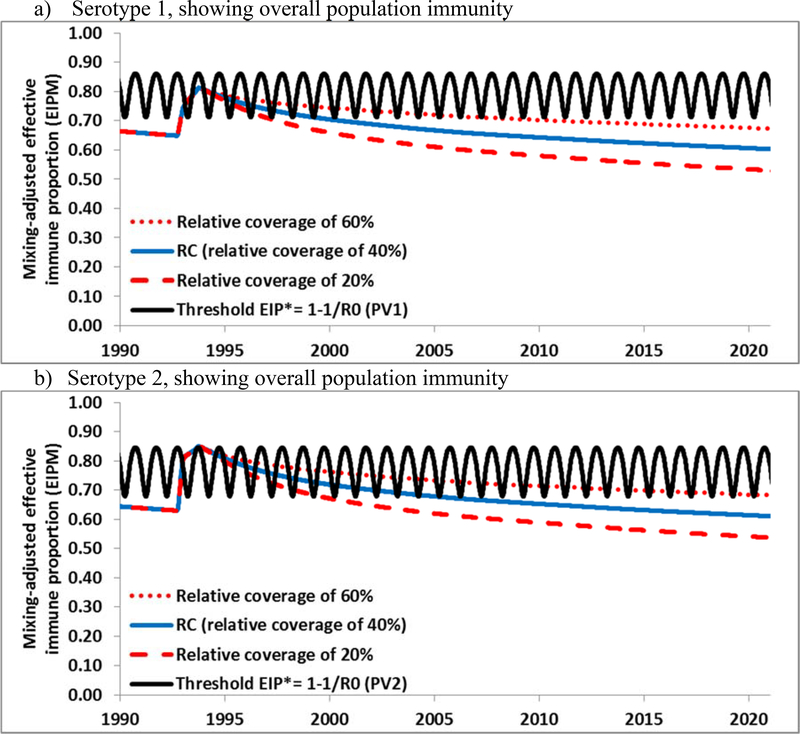
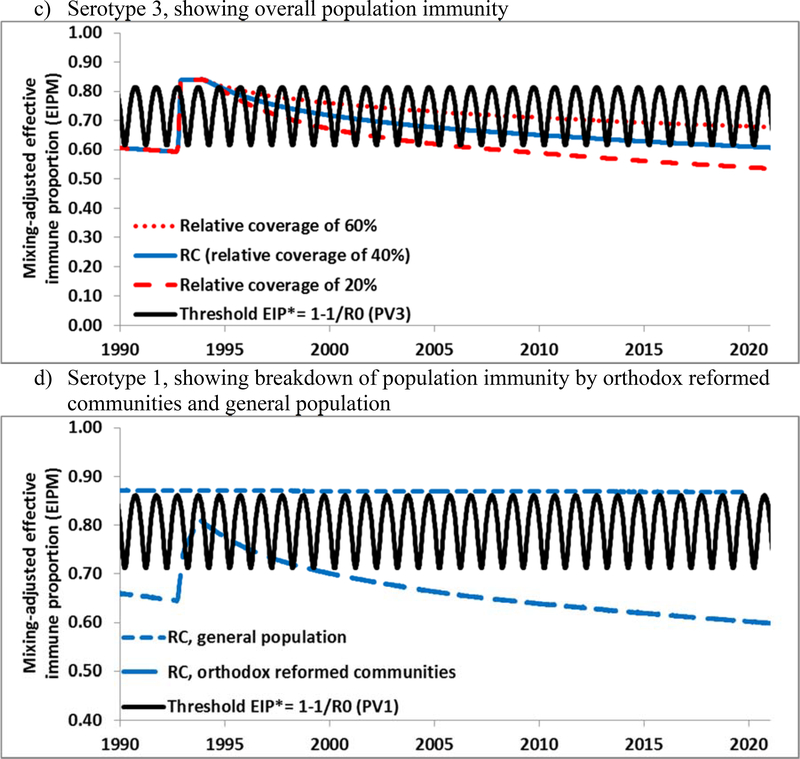
Figure 5 shows the expected population immunity in The Netherlands through 2020. Unlike the US model, we explicitly characterized a subpopulation of orthodox reformed communities with known low vaccine acceptance, because these communities represent a significant proportion of the population and they historically experienced poliovirus outbreaks in 1978[28] and 1992–3[29] and a measles outbreak as recently as 2013.[30] Unlike the under-vaccinated Amish communities in the US, the Dutch orthodox reformed communities remain in relatively close geographic proximity in addition to their social clustering.[31] Also unlike the US, which used OPV-only for RI for several decades before switching to an IPV/OPV sequential schedule for a few years and then to IPV-only RI in 2000,[32] The Netherlands used an IPV-only RI schedule since it introduced poliovirus vaccination in the 1950s.[33], which implies lower population immunity to transmission in the Dutch general population. The presence of a preferentially-mixing subpopulation with very low RI coverage implies that a WPV or cVDPV of any serotype can circulate in this subpopulation and therefore in The Netherlands. Figures 5a–b show that for serotypes 1 and 2, which have not circulated widely since at least 1978, population immunity appears well below the threshold as a result of the low coverage in the orthodox reformed communities and absence of natural immunity from an outbreak. This implies that a WPV or cVDPV of these serotypes could establish widespread transmission if introduced into the orthodox reformed communities. For serotype 3, population immunity remains somewhat higher due to the WPV3 outbreak in these communities in 1992–1993, but it also decreases below the threshold at a rate that depends on the subpopulation coverage assumptions (Figure 5c). Figure 5d provides the breakdown of population immunity for the general population, which remains above the threshold, and for the subpopulation, which does not (similar breakdowns for the other two serotypes not shown). Figure 5d suggests that although a WPV or cVDPV introduced in The Netherlands could circulate in the orthodox reformed communities, the general population remains well-protected and would most likely not sustain extensive re-established transmission (similar to the US, Figure 2). Although the orthodox communities may benefit to some extent from high population immunity in the general population, viruses may or may not take off depending on chance and the timing and place of introduction.[25] Our results overall suggest high vulnerability to a WPV or cVDPV introduction in clusters of under-vaccinated people in IPV-using high-income countries.
Figure 5.
Overall population immunity in The Netherlands by serotype for the RC compared to the threshold (EIP*) from 1990 forward for all 3 serotypes for different assumptions (i.e., 20%, 40%, and 60%) of relative coverage in the orthodox reformed communities (compared to the general population) and for type 1 the breakdown of population immunity for the orthodox reformed communities and general population separately.
DISCUSSION
Maintaining high population immunity to poliovirus transmission should represent a priority for all countries due to the importation risk of WPVs or cVDPVs. Most OPV-using countries probably face a more significant risk of creating a domestic cVDPV prior to or after OPV cessation than from importation of a cVDPV, and for these countries we emphasize that national risks of cVDPV creation alone should motivate efforts to maintain high population immunity throughout the polio endgame.[4, 15, 17–20, 22, 23] However, IPV-only using countries should recognize that they still face importation risks and consider opportunities to decrease their risks, including establishing sensitive environmental surveillance and ensuring access to OPV for any needed outbreak response.[34, 35] With successful cessation of WPV1 circulation in Israel confirmed, Israel would likely benefit from switching from the use of bOPV to tOPV for its two RI OPV doses starting in early 2015 until the time of coordinated global OPV2 cessation to maximize its serotype 2 population immunity. However, such a strategy could prove challenging from a policy perspective, because this would introduce serotype 2 LPV into Israel in the absence of an already circulating cVDPV2. Using tOPV now would provide Israel with some insurance such that any importations of cVDPV2s from other countries, which Israel cannot fully control, will not re-establish transmission and necessitate another outbreak response. However, Israel could also decide to accept the low risk of a cVDPV2 importation and focus on preparedness instead of prevention. Prior to introducing tOPV in RI, Israeli policy makers may want to consider further modeling of the actual options, which may include SIAs or expanded target ages in RI not addressed here.
Our results suggest that countries using or considering an IPV/OPV sequential schedule should continue to use the global formulation of OPV with all serotypes allowable up until the time of coordinated OPV cessation by serotype occurs (e.g., tOPV now, bOPV after OPV2 cessation). Countries should recognize that the adoption of an IPV-only immunization schedule may make some populations vulnerable to re-established transmission following LPV importations, even with relatively high coverage, particularly in the context of hygiene and sanitation conditions that favor fecal-oral transmission. Similarly, substitution of IPV for OPV doses in RI may lead to reductions in overall population immunity and countries should consider this as they manage their risks in the polio endgame.[36] Countries considering a switch from IPV/OPV use to IPV-only to eliminate VAPP should weigh the expected small reduction in VAPP cases[37] against the risk of outbreaks of imported WPV or cVDPV in the context of any under-vaccinated subpopulations or subpopulation that may support fecal-oral poliovirus transmission. All countries should recognize the importance of not “demonizing” OPV when making changes in national immunization policy in case OPV becomes needed for outbreak response during the endgame.
The introduction of imported viruses into clusters of susceptible individuals represents a high risk, and countries should explore opportunities to potentially increase population immunity in these groups to the extent possible and monitor these groups for indications of transmission throughout the endgame. Focusing on prevention and risk management will represent an important strategy to successfully achieve WPV eradication and successful OPV cessation, but it may imply greater than currently anticipated demands for tOPV in the short term. Failing to understand and manage population immunity in all countries will most likely continue to delay polio eradication goals, increase the needs for vaccine and other resources for outbreak response, and increase overall costs.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Bill and Melinda Gates Foundation for providing a contract to Kid Risk, Inc. to support completion of this work under Work Order 4533–25298. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Bill and Melinda Gates Foundation.
Footnotes
CONFLICTS: None
REFERENCES
- [1].World Health Organization. Global Polio Eradication Initiative. 2014. [PMC free article] [PubMed] [Google Scholar]
- [2].Anis E, Kopel E, Singer S, Kaliner E, Moerman L, Moran-Gilad J, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveillance. 2013;18:pii=20586. [DOI] [PubMed] [Google Scholar]
- [3].Shulman LM, Mendelson E, Anis E, Bassal R, Gdalevich M, Hindiyeh M, et al. Laboratory challenges in response to silent introduction and sustained transmission of wild type 1 poliovirus into Israel in 2013. J Infect Dis. 2014;210:S304–14. [DOI] [PubMed] [Google Scholar]
- [4].Kalkowska DA, Duintjer Tebbens RJ, Grotto I, Shulman LM, Anis E, Wassilak SGF, et al. Modeling options to manage type 1 wild poliovirus imported into Israel in 2013. J Infect Dis. 2014;2014; doi: 0.1093/infdis/jiu674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization. Poliovirus detected from environmental samples in Egypt. Geneva: WHO; 11 February 2013. Available from: http://wwwwhoint/csr/don/2013_02_11/en/ Accessed 27 October 2014. 2013. [Google Scholar]
- [6].World Health Organization. Temporary recommendations to reduce international spread of poliovirus, Available at: http://www.polioeradication.org/Infectedcountries/PolioEmergency.aspx, accessed 3 June 2014.
- [7].Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SGF, Cochi SL. Modeling population immunity to support efforts to end the transmission of live polioviruses. Risk Anal. 2013;33:647–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SG, Kim J-H, Cochi SL. Pre-eradication vaccine policy options for poliovirus infection and disease control. Risk Anal. 2013;33:516–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mach O, Tangermann RH, Wassilak SGF, Singh S, Sutter RW. Outbreaks of paralytic poliomyelitis during 1996–2012: The changing epidemiology of a disease in the final stages of eradication. J Infect Dis. 2014;210:S347–52. [DOI] [PubMed] [Google Scholar]
- [10].Duintjer Tebbens R, Pallansch M, Kew O, Cáceres V, Jafari H, Cochi S, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 2006;26:1471–505. [DOI] [PubMed] [Google Scholar]
- [11].Duintjer Tebbens RJ, Pallansch MA, Kim J-H, Burns CC, Kew OM, Oberste MS, et al. Review: Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Anal. 2013;23:680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, et al. Expert review on poliovirus immunity and transmission. Risk analysis : an official publication of the Society for Risk Analysis. 2013;33:544–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, et al. Review and assessment of poliovirus immunity and transmission: Synthesis of knowledge gaps and identification of research needs. Risk Anal. 2013;33:606–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Sutter RW, Thompson KM. A dynamic model of poliomyelitis outbreaks: Learning from the past to help inform the future. Am J Epidemiol. 2005;162:358–72. [DOI] [PubMed] [Google Scholar]
- [15].Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SG, Cochi SL, Thompson KM. Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Anal. 2013;23:703–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thompson KM, Duintjer Tebbens RJ. Current polio global eradication and control policy options: Perspectives from modeling and prerequisites for OPV cessation. Exp Rev Vaccines. 2012;11:449–59. [DOI] [PubMed] [Google Scholar]
- [17].Thompson KM, Duintjer Tebbens RJ. Modeling the dynamics of oral poliovirus vaccine cessation. J Infect Dis. 2014;210:475–84. [DOI] [PubMed] [Google Scholar]
- [18].Duintjer Tebbens RJ, Thompson KM. Modeling the potential role of inactivated poliovirus vaccine to manage the risks of oral poliovirus vaccine cessation. J Infect Dis. 2014;210:485–97. [DOI] [PubMed] [Google Scholar]
- [19].Kalkowska DA, Duintjer Tebbens RJ, Thompson KM. Modeling strategies to increase population immunity and prevent poliovirus transmission in two high-risk areas in northern India. J Infect Dis. 2014;210:398–411. [DOI] [PubMed] [Google Scholar]
- [20].Kalkowska DA, Duintjer Tebbens RJ, Thompson KM. Modeling strategies to increase population immunity and prevent poliovirus transmission in the high-risk area of northwest Nigeria. J Infect Dis. 2014;210:412–23. [DOI] [PubMed] [Google Scholar]
- [21].World Health Organization. Global Polio Eradication Initiative: Polio Eradication and Endgame Strategic Plan (2013–2018). Geneva: 2013. [Google Scholar]
- [22].Thompson KM, Wallace GS, Duintjer Tebbens RJ, Smith PH, Barskey AE, Pallansch MA, et al. Trends in the risk of U.S. polio outbreaks and poliovirus vaccine availability for response. Public Health Rep. 2012;127:23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Duintjer Tebbens RJ, Kalkowska DA, Wassilak SG, Pallansch MA, Cochi SL, Thompson KM. The potential impact of expanding target age groups for polio immunization campaigns. BMC Infect Dis. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kalkowska DA, Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, Thompson KM. Modeling undetected live poliovirus circulation after apparent interruption of transmission: Implications for surveillance and vaccination. BMC Infect Dis. 2015;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kisjes KH, Duintjer Tebbens RJ, Wallace GS, Pallansch MA, Cochi SL, Wassilak SGF, et al. Individual-based modeling of potential poliovirus transmission in connected religious communities in North America with low uptake of vaccination. J Infect Dis. 2014;210:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shulman LM, Martin J, Sofer D, Burns CC, Manor Y, Hindiyeh M, et al. Genetic analysis and characterization of wild poliovirus type 1 during sustained transmission in a population with >95% vaccine coverage, Israel 2013. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014; 10.1093/cid/ciu1136. [DOI] [PubMed] [Google Scholar]
- [27].van Lier EA, Oomen PJ, Mulder M, Conyn-van Spaendonck MAE, Drijfhout IH, de Hoogh PAAM, et al. Vaccinatiegraad Rijksvaccinatieprogramma Nederland: Verslagjaar 2013 (in Dutch). Rijksinstituut voor Volksgezondheid en Milieu, RIVM Rapport 150202001. 2013. [Google Scholar]
- [28].Schaap GJP, Bijkerk H, Coutinho RA, Kapsenberg JG, Vanwezel AL. The spread of wild poliovirus in the well-vaccinated Netherlands in connection with the 1978 epidemic. Prog Med Virol. 1984;29:124–40. [PubMed] [Google Scholar]
- [29].Oostvogel PM, Rumke HC, Conyn-Van Spaendonck MA, van der Avoort HG, Leeuwenburg J, van Loon AM. Poliovirus circulation among schoolchildren during the early phase of the 1992–1993 poliomyelitis outbreak in The Netherlands. J Infect Dis. 2001;184:1451–5. [DOI] [PubMed] [Google Scholar]
- [30].Knol M, Urbanus A, Swart E, Mollema L, Ruijs W, van Binnendijk R, et al. Large ongoing measles outbreak in a religious community in the Netherlands since May 2013. Euro Surveill. 2013;18:pii=20580. [DOI] [PubMed] [Google Scholar]
- [31].Ruijs WL, Hautvast JL, van der Velden K, de Vos S, Knippenberg H, Hulscher ME. Religious subgroups influencing vaccination coverage in the Dutch Bible belt: An ecological study. BMC public health. 2011;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thompson KM, Duintjer Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 2006;26:1423–40. [DOI] [PubMed] [Google Scholar]
- [33].Oostvogel P, van Wijngaarden J, van der Avoort HG, Mulders MN, Conyn-van Spaendonck MA, Rümke H, et al. Poliomyelitis outbreak in an unvaccinated community in the Netherlands, 1992–3. The Lancet. 1994;344:665–70. [DOI] [PubMed] [Google Scholar]
- [34].Thompson KM, Duintjer Tebbens RJ. The case for cooperation in managing and maintaining the end of poliomyelitis: Stockpile needs and coordinated OPV cessation. Medscape J Med. 2008;10:190. [PMC free article] [PubMed] [Google Scholar]
- [35].Jenkins PC, Modlin JF. Decision analysis in planning for a polio outbreak in the United States. Pediatrics. 2006;118:611–8. [DOI] [PubMed] [Google Scholar]
- [36].Thompson KM, Duintjer Tebbens RJ. National choices related to inactivated poliovirus vaccine, innovation, and the end game of global polio eradication. Exp Rev Vaccines. 2014;13:221–34. [DOI] [PubMed] [Google Scholar]
- [37].Alexander LN, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, et al. Vaccine policy changes and epidemiology of poliomyelitis in the United States. J Am Med Assoc. 2004;292:1696–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.