Highlights
-
•
PD with mild behavioral impairment revealed deficits in cognitive flexibility.
-
•
Brain activities during a set-shifting task linked with MBI in PD was evaluated.
-
•
PD-MBI revealed reduced activity in the prefrontal and posterior parietal cortices.
-
•
The prefrontal activity was associated with cognitive impairment in PD-MBI.
-
•
High MBI-C score was associated with reduced deactivation in the hippocampus.
Keywords: Mild behavioral impairment, Parkinson’s disease, Set-shifting, fMRI, Neuropsychiatric symptoms, Cognition
Abstract
Mild behavioral impairment (MBI) is a neurobehavioral syndrome characterized by later life emergence of sustained neuropsychiatric symptoms, as an at-risk state for incident cognitive decline and dementia. Prior studies have reported that neuropsychiatric symptoms are associated with cognitive abilities in Parkinson’s disease (PD) patients, and we have recently found a strong correlation between MBI and cognitive performance. However, the underlying neural activity patterns of cognitive performance linked to MBI in PD are unknown. Fifty-nine non-demented PD patients and 26 healthy controls were scanned using fMRI during performance of a modified version of the Wisconsin card sorting task. MBI was evaluated using the MBI-checklist, and PD patients were divided into two groups, PD-MBI and PD-noMBI. Compared to the PD-noMBI group and healthy controls, the PD-MBI group revealed less activation in the prefrontal and posterior parietal cortices, and reduced deactivation in the medial temporal region. These results suggest that in PD, MBI reflects deficits in the frontoparietal control network and the hippocampal memory system.
1. Introduction
Mild behavioral impairment (MBI) is a diagnostic construct for the identification of individuals with an increased risk of developing dementia, which is characterized by later life acquired, sustained and impactful neuropsychiatric symptoms (NPS) of any severity that cannot be better accounted for by other formal medical and psychiatric nosology (Ismail et al., 2016). MBI represents the neurobehavioral axis of pre-dementia risk states, as a complement to the neurocognitive risk axis represented by mild cognitive impairment (MCI). NPS are among the most common non-motor symptoms in Parkinson’s disease (PD) (Aarsland et al., 2009). The frequency of MBI was 84.1% in PD and it showed a tendency to increase with disease progression, when MBI was captured using the neuropsychiatric inventory (NPI) (Baschi et al., 2019). We have recently found that MBI in non-demented PD patients is associated with impairment in all five cognitive domains including attention, executive function, language, memory and visuospatial function, and atrophy in the middle temporal cortex (Yoon et al., 2019). Moreover, PD patients with MBI revealed altered functional corticostriatal connectivity, particularly between the head of the caudate and precuneus, lateral parietal cortex, and anterior cingulate cortex (Lang et al., 2020). Our initial results suggest that MBI might be an important marker to detect cognitive decline and associated functional and structural brain changes in PD.
Impairment of executive functions in PD is common in early disease and constitutes the core feature of dementia in PD (Owen et al., 1992). The Wisconsin card sorting task (WCST) has been widely used in PD to assess executive dysfunction, specifically cognitive flexibility that requires establishing and then shifting response rules, or task-sets (Grant and Berg, 1948). PD patients with NPS including apathy (Pluck and Brown, 2002, Varanese et al., 2011), depression (Starkstein et al., 1989) and impulse control disease (Vitale et al., 2011) revealed impairment on WCST performance compared to those without. The WCST requires a multifactorial cognitive process, such as updating the cognitive set, inhibiting the incorrect cognitive set, consolidating and maintaining the correct cognitive set. The neural processes in various brain regions underlying WCST performance that were identified in previous functional neuroimaging studies seem in line with the complexity of the WCST. Healthy volunteers displayed significant activation in the fronto-striatal loop while performing the WCST. Specifically, the caudate nucleus and prefrontal cortex (PFC) revealed significant activation when planning a set-shift, while significant activation in the putamen and premotor cortex was found when executing a set-shift (Monchi et al., 2004, Monchi et al., 2001). The set-shifting deficits in PD are associated with reduced recruitment the fronto-striatal loops (Monchi et al., 2004), and reduced activity in those fronto-striatal areas were prominent in PD patients with MCI compared to those without (Nagano-Saito et al., 2014). Meta analyses of neuroimaging studies have highlighted the involvement of a distributed frontoparietal network to support attention, working memory, and inhibition during performing the WCST (Buchsbaum et al., 2005, Kim et al., 2012). In healthy volunteers, dopamine depletion reduced the degree of deactivation in the medial PFC, posterior cingulate cortex, and hippocampus as well as the degree of fronto-striatal connectivity when a set-shift was required (Nagano-Saito et al., 2008). Also, a functional connectivity analysis found that the caudate and hippocampus interact indirectly via the medial orbitofrontal area during cognitive set-shifting in healthy controls (HC) (Graham et al., 2009). Those studies of HC support the role of task-negative network or default mode network and hippocampal learning system in the WCST. The WCST would therefore allow us for the evaluation of activity in various brain regions in terms of cognitive function related with MBI in PD.
We focused here on comparing patterns of brain activation during performing two stages of the WCST, planning and executing the set-shift, between PD patients with and without MBI. For this purpose, we divided the PD patients into two groups, PD-noMBI and PD-MBI using MBI checklist (MBI-C) scores. The MBI-C is a simple and efficient MBI case ascertainment tool designed to elicit emergent NPS in accordance with the MBI criteria. Because MBI and cognitive impairment can co-exist in PD (Baschi et al., 2019, Yoon et al., 2019), the patients also performed neuropsychological assessment and divided into two groups, PD-noMCI and PD-MCI, in order to assess the differential effect of cognitive symptoms on activity patterns during WCST compared with MBI in PD patients. Based on impairment in all cognitive function domains in PD-MBI (Baschi et al., 2019, Lang et al., 2020, Yoon et al., 2019) along with activation patterns in distributed brain networks during WCST (Buchsbaum et al., 2005, Graham et al., 2009, Kim et al., 2012, Monchi et al., 2004, Monchi et al., 2001, Nagano-Saito et al., 2008), we hypothesized that the PD-MBI group would reveal impairment in WCST performances and related activation changes in distributed brain regions of fronto-striatal, frontoparietal and default mode networks in both the planning and execution stages of the set-shift compared to the PD-noMBI group.
2. Materials and methods
2.1. Participants
Fifty-nine non-demented PD patients at Hoehn & Yahr II-III and 26 HC were included in this study. Patients were diagnosed by movement disorders neurologists and met the UK Brain Bank criteria for idiopathic PD (Hughes et al., 1992). All PD patients received dopaminergic medication and were responsive to it. No patient was asked to change her/his medication for this study. The severity of motor symptoms was rated using the motor section of the unified PD rating scale (UPDRS-III). Exclusion criteria were alcohol dependency, presence or history of psychiatric disorders diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria, neurological disorders other than PD, cerebrovascular disorders, and general anesthesia in the past 6 months. All participants provided written informed consent according to the declaration of Helsinki and the study was approved by the Conjoint Health Research Ethics Board at the University of Calgary. Group demographic and clinical characteristics are listed in Table 1.
Table 1.
Demographic and clinical characteristics of participants.
| Characteristics | Healthy Control (n = 26) | PD-noMBI (n = 38) | PD-MBI (n = 21) | P-value |
||
|---|---|---|---|---|---|---|
| HC vs PD-noMBI | HC vs PD-MBI | PD-noMBI vs PD-MBI | ||||
| Age, mean ± SD (range), yearsa | 68.6 ± 6.1 (60.1–80.9) | 69.9 ± 6.3 (58.5–80.1) | 70.9 ± 6.6 (57.9–81.3) | 0.816 | 0.533 | 0.826 |
| Female, No. (%)b | 16 (62) | 15 (39) | 5 (24) | 0.204 | 0.037 | 0.263 |
| Education, mean ± SD (range), yearsc | 16.4 ± 2.8 (12–21) | 15.4 ± 2.6 (9–21) | 14.1 ± 3.4 (9–20) | 0.180 | 0.024 | 0.176 |
| Disease duration, mean ± SD (range), yearsd | na | 5.0 ± 3.5 (0.7–12.5) | 5.0 ± 2.7 (1.2–10.2) | na | na | 0.733 |
| LED, mean ± SD (range), mg/dayd | na | 681.1 ± 272.2 (225–1350) | 882.8 ± 478.0 (300–1925) | na | na | 0.198 |
| UPDRS-III, mean ± SD (range)d | na | 15.3 ± 7.8 (4–34) | 21.9 ± 8.4 (6–40) | na | na | 0.003 |
| PD subtype, No. (%)e | na | na | 0.055 | |||
| Tremor | 12 (32) | 1 (5) | ||||
| Mixed | 3 (8) | 3 (14) | ||||
| A/R | 23 (60) | 17 (81) | ||||
| MoCA, mean ± SDa | 27.6 ± 1.8 (23–30) | 26.6 ± 2.4 (20–30) | 23.5 ± 4.4 (16–30) | 0.367 | <0.001 | <0.001 |
| MBI-C, mean ± SD (range)c | ||||||
| Total | 0.0 ± 1.4 (0–7) | 1.6 ± 2.0 (0–7) | 15.1 ± 9.3 (8–44) | <0.001 | <0.001 | <0.001 |
| Drive/Motivation | 0.1 ± 0.4 (0–2) | 0.6 ± 0.9 (0–3) | 3.4 ± 2.9 (0–11) | 0.004 | <0.001 | <0.001 |
| Mood/Anxiety | 0.1 ± 0.4 (0–2) | 0.5 ± 1.0 (0–4) | 4.5 ± 2.3 (1–9) | 0.079 | <0.001 | <0.001 |
| Impulse dyscontrol | 0.2 ± 0.8 (0–4) | 0.4 ± 0.8 (0–4) | 5.0 ± 5.4 (0–21) | 0.072 | <0.001 | <0.001 |
| Social inappropriateness | 0.0 ± 0.0 | 0.1 ± 0.3 (0–1) | 1.5 ± 2.3 (1–10) | 0.134 | <0.001 | <0.001 |
| Abnormal perception/thought | 0.0 ± 0.0 | 0.2 ± 0.7 (0–4) | 0.7 ± 1.0 (0–3) | 0.082 | <0.001 | 0.006 |
HC, healthy controls; PD, Parkinson’s disease; MBI, Mild Behavioral Impairment; PD-noMBI, PD without MBI; PD-MBI, PD with MBI; LED, levodopa equivalent dose; UPDRS-III, motor section of the unified Parkinson’s disease rating scale; MoCA, Montreal cognitive assessment; A/R, akinetic/rigidity; MBI-C, MBI checklist; SD, standard deviation; na, not applicable.
One-way ANOVA with Tukey’s post hoc test.
Chi-square test with Bonferroni correction, p < 0.016 was considered significant (p = 0.05/3).
Kruskal-Wallis test followed by Mann-Whitney U test using Bonferroni correction, p < 0.016 was considered significant (p = 0.05/3).
Mann-Whitney U test.
Motor phenotypes were identified based on the ratio of mean tremor to the mean akinetic/rigid score of UPDRS-III. Patients with a ratio greater than 1.0, <0.80, and between 0.80 and 1.0 were classified into tremor, akinetic/rigid, and mixed types respectively (Schiess et al., 2000). The group difference was evaluated using Chi-square test.
2.2. MBI checklist
The MBI-C was structured to be consistent with the five domains of MBI: impaired drive/motivation, affective/emotional dysregulation, impulse dyscontrol, social inappropriateness, and abnormal thoughts/perception (Ismail et al., 2017). Symptoms should be persistent for at least six months and represent a meaningful change from baseline. Based on a cut-off point of 7.5 (Mallo et al., 2018a, Mallo et al., 2018b, Yoon et al., 2019), we divided the PD patients into two groups, one with high MBI-C scores referred to as PD-MBI, and the other with low MBI-C scores referred to as PD-noMBI. Among 59 PD participants, 21 were categorized as PD-MBI (35.6%). Details of MBI-C are provided in the Supplementary Methods.
2.3. Neuropsychological assessment and MCI criteria
All participants underwent a comprehensive neuropsychological assessment that targeted 5 cognitive domains: attention, executive function, language, memory, and visuospatial function (Details in Supplementary Methods) (Litvan et al., 2012). The performance on the neuropsychological tests was transformed into a Z-score based on the age-, sex- and education-adjusted normative performance data. All the neuropsychological evaluations were administered before and within two weeks of the imaging session.
We determined MCI status in PD patients according to the level II criteria of Movement Disorder Society Task Force (Litvan et al., 2012), requiring the following: (i) objective evidence of cognitive decline: performance 1.5 standard deviations below standardized mean on at least 2 tests within a domain or across different cognitive domains; (ii) subjective complaint of cognitive decline by the patient or accompanying person; (iii) absence of significant decline in daily living activities (based on clinical observations of the referring neurologists and neuropsychologist); and (iv) no dementia as diagnosed by the evaluating neuropsychologist based on the Movement Disorder Society Task Force guidelines (Emre, 2003). HC were also assessed for MCI using the same criteria, and only participants without MCI participated the subsequent imaging session. The PD-MBI group had more patients with MCI than the PD-noMBI group (χ2 = 4.01, p = 0.045). Eleven PD patients out of 21 PD-MBI patients (52.4%) and 10 PD patients out of 38 PD-noMBI patients (26.3%) were classified as having MCI.
2.4. MRI acquisition
All participants were scanned using the GE DISCOVERY MR750 3.0-T MRI scanner at the Seaman family imaging centre at the University of Calgary. The session contained the following sequences: high-resolution T1-weighted 3D inversion recovery prepared fast spoiled gradient recalled (IR-FSPGR) sequence (repetition time = 7.176 ms, echo time = 2.252 ms, flip angle = 100°, acquisition matrix = 256 × 256, voxel size = 1 × 1 × 1 mm3, 172 slices), three series of BOLD T2*-weighted fMRI of 8 min 30 s each during which participants performed the WCST (repetition time = 2900 ms, echo time = 30 ms, flip angle = 90°, acquisition matrix = 96 × 96, voxel size = 2.5 × 2.5 × 3 mm3, 48 slices).
2.5. Cognitive task during fMRI
A computerized version of the WCST described in Monchi et al. (Monchi et al., 2004) was administered using Presentation software version 17.2 (Neurobehavioral Systems, Inc). At each trial of the task, participants were asked to match a new test card to one of the four fixed reference cards based either on the color, shape, or the number of the stimuli in each reference card. Participants had to find the rule for classification using the feedback that followed each trial. A bright screen indicated a correct classification, and a dark screen indicated an incorrect classification. The feedback was presented for 2.3 s. The length of each matching period depended on the participant’s response time, but the trial ended if the participant did not answer within 8.0 s. Six consecutive correct matching responses were required before a change in classification rule occurred. Each fMRI run contained blocks of each of the 3 trial dimensions (color, shape, number) presented in pseudorandom order. To evaluate the pattern of activation during the different stages of the WCST, four experimental periods were defined as follows: (1) receiving negative feedback (RNF): the period that starts immediately after an incorrect selection is made and ends when the next card is presented, indicating that a set-shift is required and must be planned; (2) matching after negative feedback (MNF): the period that starts from the moment new test card is presented after negative feedback and ends when the participant complete the choice, indicating the execution of a set-shift; (3) receiving positive feedback (RPF): the period starts immediately after an correct selection is made and ends when the next card is presented, requiring that the current matching criterion must be maintained on the next trial; (4) matching after positive feedback (MPF): the period that starts from the moment new test card is presented after positive feedback and ends when the participant complete the choice, requiring selection using the same rule as in the previous trial.
The responses were scored according to Heaton’s criteria (Heaton, 1981) with one exception, the first wrong classification following a change in rule was not scored as an error because the participant could not yet have received feedback indicating that the previously correct rule was now incorrect. If cards sorted according to the previously correct rule after the first classification following a change in rule, the incorrect responses were referred to as perseverative errors. Other incorrect responses that do not match the principle for perseverative errors were scored as non-perseverative errors.
2.6. fMRI data analysis
2.6.1. Preprocessing and whole brain analysis
Preprocessing and analysis of the task-based fMRI data were performed using FSL FEAT (fMRI Expert Analysis Tool version 6.00). Image preprocessing details are found in Supplementary Methods. A general linear model (GLM) was employed at three levels of analyses. At the first level, the following two contrasts were computed individually for each run of each participant: (1) RNF vs. RPF: reflecting the planning of the set-shift and (2) MNF vs. MPF: reflecting the execution of the set-shift. The hemodynamic response function was modeled with a gamma-function and its temporal derivatives and six motion parameters were included as covariates of no interest. At the second level, a fixed effects analysis combined three runs of each participant. At the group level analysis, individual subjects’ activation maps for each of the two contrasts were entered into mixed-effects GLM models. Because age and medication may alter vascular function (Tsvetanov et al., 2015) and UPDRS-III scores revealed difference between PD groups, age was included as a covariate of no interest for all group comparisons and UPDRS-III and levodopa equivalent daily dose (LEDD) were included as additional covariates for comparisons between PD groups. Correlation analyses between BOLD data and MBI-C total score were performed in all PD patients, adjusting for age, UPDRS-III and LEDD. In subsequent analyses, we aimed to assess the brain activity pattern independent from cognitive impairment in PD. For this purpose, we repeated the comparison between PD groups and correlation analyses with MBI-C after adding MoCA score as a covariate of no interest and performed 2-way analysis of covariance (ANCOVA) to estimate main effects of MBI and MCI, and their interaction. In all imaging analyses, a cluster-forming threshold of Z > 2.58 and a cluster-corrected significance of p < 0.05 were applied.
2.6.2. ROI analysis
To complement the whole-brain analyses, we also performed a ROI analysis on the key regions for performing the WCST, the caudate and ventrolateral PFC for the contrast of RNF vs. RPF, putamen and premotor cortex for the contrast of MNF vs. MPF, and hippocampus for both contrasts in each hemisphere, based on previous functional neuroimaging studies of the WCST (Graham et al., 2009, Monchi et al., 2004, Nagano-Saito et al., 2016, Nagano-Saito et al., 2014). Details of the ROIs are found in the Supplementary Methods. The BOLD changes in ROIs were analyzed in the same manner as whole-brain analyses using mixed-effect GLM, 2-way ANCOVA and Spearman’s partial correlations using SPSS 25.0, with Bonferroni correction to correct for multiple comparisons across ROIs (p = 0.05/6 ROIs; p < 0.008).
We investigated whether the percent signal change estimates in significant clusters showing group differences or correlations with MBI-C scores correlated with error rates on the WCST. The correlation analyses were performed using Spearman’s partial correlations while controlling for age, UPDRS-III, LEDD as appropriate using SPSS 25.0, with Bonferroni correction to correct for multiple comparisons across ROIs (p = 0.05/4 ROIs; p < 0.0125).
3. Results
3.1. Behavioral results during fMRI
HC completed an average of 77.5 experimental WCST trials (Color, 25.8; Number, 25.6; shape, 26.1) and 28.8 control trials during the three runs. The PD-noMBI group completed an average 75.2 experimental trials (Color, 23.6; Number, 24.2; Shape, 27.4) and 21.9 control trials. The PDMBI group completed an average 75.1 experimental trials (Color, 22.8; Number, 18.7; Shape, 33.7), and 13.5 control trials.
Mixed-effects GLM models with age, UPDRS-III and LEDD as covariates of no interest were performed to evaluate the group differences in error rates. Compared with HC, The PD-MBI group made significantly more perseverative errors (F(2,81) = 7.04, p = 0.001), and non-perseverative errors (F(2,81) = 15.04, p < 0.001), but there were no significant group differences between the PD-noMBI group and HC in two types of errors. The PD-MBI group made significantly more non-perseverative errors than the PD-noMBI group (F(1,54) = 5.22, p = 0.026), but there was no significant difference in the average rate of perseverative errors between the two PD groups (F(1,54) = 0.88 , p = 0.352). When we compared the error rates on each experimental dimension, color, number and shape, the PD-noMBI group did not reveal any differences compared with HC in all three dimensions. The PD-MBI group made significantly more perseverative errors in color and shape dimensions and more non-perseverative errors in in all dimensions compared with HC. Compared with PD-noMBI, the PD-MBI group revealed significantly higher rate of non-perseverative error in the shape dimension. Details of all the error rates are presented in the Supplementary Table 1.
The mean reaction time for MNF was significantly longer in the PD-noMBI and PD-MBI groups compared with HC, whereas there was no significant difference between groups in the reaction time for MPF (Supplementary Table 1).
3.2. Whole brain analysis
3.2.1. Planning the set-shift
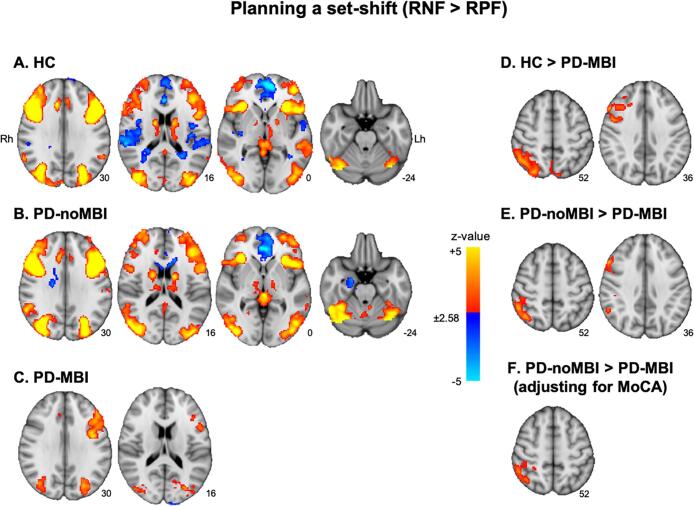
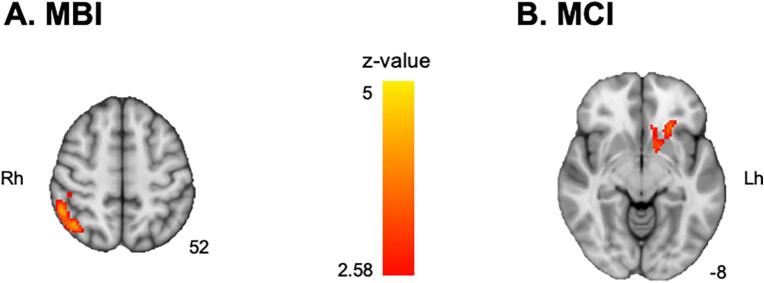
When RNF was compared with RPF (RNF minus RPF), the brain regions showing significant activation were similar between HC and the PD-noMBI group. Both groups revealed significant activation in the bilateral dorsolateral and ventrolateral PFC, caudate, thalamus, posterior parietal cortex, lateral occipital cortex and occipitotemporal gyrus and deactivation in the medial PFC and anterior cingulate cortex. The PD-noMBI group also revealed significant deactivation in the right hippocampus (Fig. 1A, B). On the other hand, the PD-MBI group showed significant activation in the bilateral posterior parietal cortex, dorsolateral PFC, and occipitotemporal cortex only (Fig. 1C). In group comparisons, the PD-MBI group showed significantly less activation in the right dorsolateral PFC and inferior parietal lobule compared with HC, but no significant differences in activation were observed between HC and the PD-noMBI group (Fig. 1D). In the PD groups comparison, the PD-MBI group showed significantly less activation in the right dorsolateral PFC and inferior parietal lobule than in the PD-noMBI group (Fig. 1E; Supplementary Table 2 for Fig. 1A−E). However, after adding MoCA as a covariate of no interest, significantly reduced activation in the PD-MBI group was only found in the right inferior parietal lobule (Fig. 1F; MNI coordinates, x = 52, y = −42, z = 54; z-value = 3.81). The 2-way ANCOVA (MBI × MCI groups) revealed a main effect of MBI on the right inferior parietal lobule (Fig. 2A; x = 42, y = −60, z = 54; z-value = 4.27), and of MCI on the left ventral striatum (Fig. 2B; x = −20, y = −16, z = −14; z-value = 4.59) with no significant interaction. The left ventral striatum showed increased deactivation during planning the set-shift in the PD patients with MCI compared to the PD patients without MCI (Supplementary Fig. 1). There was no significant correlation between activity patterns while planning the set-shift and MBI-C total scores whether or not MoCA added as a covariate of no interest.
Fig. 1.
Brain regions with significant activation in the contrast receiving negative feedback (RNF) vs. receiving positive feedback (RPF), corresponding to planning the set-shift. A) healthy controls (HC), B) Parkinson’s disease patients (PD) without mild behavioral impairment (MBI, PD-noMBI), and C) PD with MBI (PD-MBI). Red-to-yellow color means more activation in the RNF than RPF, and blue-to-light blue color means less activation in the RNF than RPF. D) Brain regions showing significant reduced activity in the PD-MBI group compared with HC and E) compared with PD-noMBI. F) The comparison between the PD-noMBI and PD-MBI groups after controlling for Montreal cognitive assessment (MoCA). The number under each slice is a z-coordinate. Rh, right hemisphere; Lh, left hemisphere. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Brain regions showing significant main effect of A) mild behavioral impairment (MBI) and B) mild cognitive impairment (MCI) in 2-way analysis of covariance. The number under each slice is a z-coordinate. Rh, right hemisphere; Lh, left hemisphere.
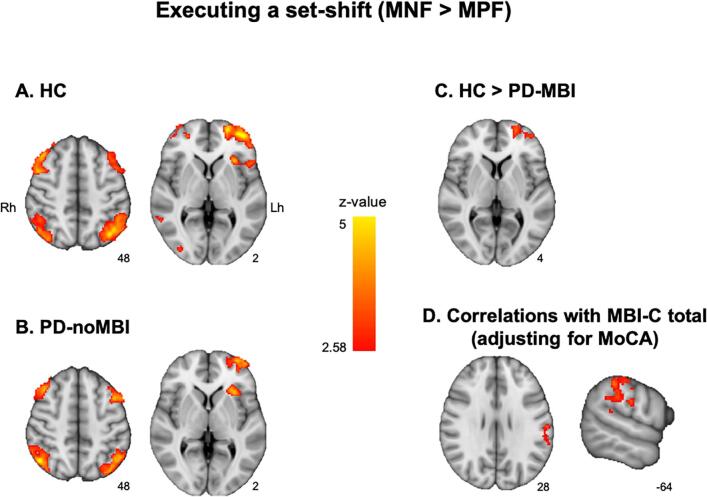
3.2.2. Executing the set-shift
When MNF was compared to MPF (MNF minus MPF), the HC and the PD-noMBI group showed significant brain activation in similar regions. Both groups revealed significant activation in the bilateral dorsolateral PFC, lateral frontopolar and posterior parietal cortex, and lateral occipital cortex (Fig. 3A, B). However, the PD-MBI group did not reveal any significant activation when executing the set-shift. In group comparisons, the activation in the left lateral frontopolar area was significantly less in the PD-MBI group compared to HC (Fig. 3C; Supplementary Table 3 for Fig. 3A−C). No significant difference in brain activation was observed between HC and the PD-noMBI group. In the comparison between the PD-noMBI and PD-MBI groups, there was no significant difference whether or not MoCA was added as a covariate of no interest. The 2-way ANCOVA did not reveal any main effect and interaction. For the correlation analysis between brain activity during executing the set-shift and MBI-C total scores, there was no significant correlation without MoCA as a covariate of no interest, but after adding MoCA as the covariate, the left supramarginal gyrus together with the adjacent postcentral cortex revealed positive correlation with MBI-C total score (Fig. 3D; MNI coordinates, x = −60, y = −36, z = 44; z-value = 3.68).
Fig. 3.
Brain regions with significant activation in the contrast matching after negative feedback (MNF) vs. matching after positive feedback (MPF), corresponding to executing the set-shift. A) healthy controls (HC) and B) Parkinson’s disease patients (PD) without mild behavioral impairment (MBI, PD-noMBI). For the PD with MBI (PD-MBI) group, there was no significant activation. Red-to-yellow color means more activation in the MNF than MPF. C) Brain regions showing significant reduced activity in the PD-MBI group compared with HC. D) Brain regions showing significant correlation with MBI-C total score during executing the set-shift after controlling for Montreal cognitive assessment (MoCA). The number under each slice is z-coordinate for the axial slice and x-coordinate for the sagittal slice. Rh, right hemisphere; Lh, left hemisphere. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
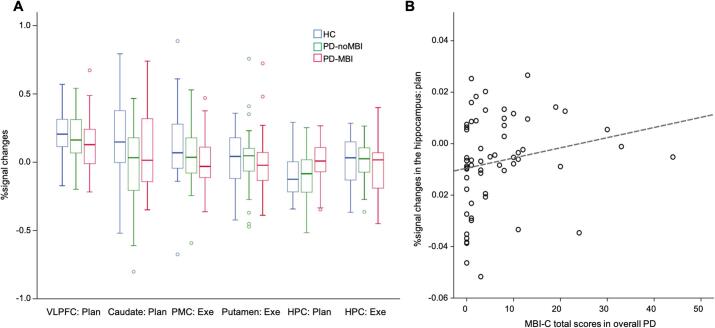
3.3. ROI analysis
Compared to HC, the PD-noMBI and PD-MBI groups did not reveal any group differences in activation in the ventrolateral PFC, caudate, premotor cortex, putamen, and hippocampus while planning or executing the set-shift. In the comparisons between the PD-noMBI and PD-MBI groups, there were no significant group differences in all ROIs whether or not MoCA was added as a covariate of no interest (Fig. 4A). The hippocampus activation during planning the set-shift was greater in the PD-MBI group compared with the PD-noMBI group (F(1,54) = 4.10, p = 0.048; F(1,54) = 6.64, p = 0.013, with MoCA as a covariate of no interest), but it was not significant after Bonferroni correction. For all ROIs, there were no significant main effects of MBI and MCI and interaction of them in the 2-way ANCOVA.
Fig. 4.
Results of ROI analysis. A) Group differences in percent signal changes in each ROI, B) correlations between MBI-C total scores and the hippocampal activity during planning the set-shift in all Parkinson’s disease (PD) patients. HC, healthy controls; PD-noMBI, PD without mild behavioral impairment (MBI); PD-MBI, PD with MBI; VLPFC, ventrolateral prefrontal cortex; PMC premotor cortex; HPC, hippocampus; Plan, planning a set-shift; Exe, executing a set-shift; MBI-C, MBI checklist.
The correlation analyses revealed positive correlation between the hippocampal activation during planning the set-shift with the MBI-C total score in all PD patients (Spearman’s rho = 0.369; p = 0.006; Spearman’s rho = 0.409; p = 0.002, with MoCA as a covariate of no interest; Fig. 4B). There was no significant correlation with the MBI-C total score in other ROIs whether or not MoCA was added as a covariate of no interest.
3.4. Correlations between brain activity and error rates while performing the WCST
The correlations with error rates on the WCST were evaluated in the four significant clusters, right dorsolateral PFC, right inferior parietal lobule and the hippocampus in the RNF versus RPF contrast, and the left frontopolar area in the MNF versus MPF contrast. When averaging the three groups together, the activity in the right inferior parietal lobule while planning the set-shift showed significant negative correlations with both perseverative error rates (inferior parietal lobule, Spearman’s rho = -0.314, p = 0.004) and non-perseverative error rates (rho = -0.322, p = 0.003). The activity in the dorsolateral PFC revealed significant a negative correlation with rates of non-perseverative error (rho = -0.361, p = 0.001) in all participants. When we evaluated correlations between the error rates on the WCST and the four significant clusters for each group, HC, PD-noMBI, and PD-MBI, there were no significant correlations between them (Supplementary Fig. 2).
4. Discussion
In the current study, we evaluated the effect of MBI on brain activation during a set-shifting task in PD patients. PD patients were categorized into two groups, PD-noMBI and PD-MBI using MBI-C scores and their BOLD activation was compared during planning and executing the set-shift.
While planning the set-shift, both the HC and PD-noMBI group had significantly increased activity in the dorsolateral and ventrolateral PFC, posterior parietal cortex, caudate nucleus, and thalamus, in line with previous results reported in HC and PD without MCI (Monchi et al., 2004, Monchi et al., 2001, Nagano-Saito et al., 2014). However, the PD-MBI group revealed a lack of brain activation in those regions including the ventrolateral and dorsolateral PFC and caudate nucleus, similarly to what was reported previously in PD patients with MCI (Nagano-Saito et al., 2014).
In the group comparisons, the PD-MBI group revealed significantly less activation in the right dorsolateral PFC and inferior parietal lobule compared to both HC and the PD-noMBI group. Functional neuroimaging studies have identified distributed frontal and parietal regions including dorsolateral PFC, ventrolateral PFC and posterior parietal cortex often work together to support adaptive behavioral control across a broad range of cognitive demands. Therefore, these brain areas are often referred to as the frontoparietal control network (Cocchi et al., 2013, Duncan, 2010). In patients with PD, activities in the prefrontal and posterior parietal areas were decreased in patients with MCI compared to those without MCI (Nagano-Saito et al., 2014), and correlated significantly with accuracy of WCST (Nagano-Saito et al., 2016). Cognitive flexibility, a core component of cognitive control, is thought to be reflected by the perseverative errors on the WCST (Lange et al., 2016a). In a event related potential study, perseverative errors on the WCST in PD was correlated with the fronto-central P3a whose generation likely involves prefrontal cortical areas and the sustained parietal positivity that likely reflects the activation of fronto-parietal brain networks (Lange et al., 2016b). We found higher rates of perseverative errors in the PD-MBI group compared with HC, and the activities in the right dorsolateral frontal and inferior parietal cortices during planning the set-shift were correlated with rates of perseverative error in all participants. These results suggest that MBI in PD is associated with impaired cognitive flexibility likely due to dysfunction in the frontoparietal control network.
Moreover, previous studies have emphasized that the frontoparietal control network is a flexible hub, meaning it has a high degree of connectivity with other brain networks across the brain and can rapidly modify functional connections according to the current goals (Cole et al., 2010). Recent studies have reported alterations of this hub across a range of NPS (Cole et al., 2014). Specifically, functional connectivity at rest was decreased within the frontoparietal control network in late-life depression (Alexopoulos et al., 2012). Also, depressive symptoms severity in adults who had not been diagnosed with depression negatively correlated with between-network global connectivity of the frontoparietal network (Schultz et al., 2019). In individuals with social anxiety disorders, functional connectivity in the frontoparietal network showed disrupted and the connectivity correlated with their symptom severity (Liao et al., 2010). In light of these previous studies, our current results suggest that the decreased activation in the dorsolateral PFC and inferior parietal cortex during planning the set-shift in the PD-MBI group reflects disruptions of cognitive control functions associated with their NPS.
In the present study, because MBI associated with impairment in cognitive function, such as significantly low MoCA score and higher proportion of MCI in the PD-MBI group, we tried to evaluate the effect of MBI on brain activation during performing WCST independently from cognitive function. The reduced activity in the right dorsolateral PFC in the PD-MBI group was not found in the analysis with adjustment of MoCA and in the main effect of MBI of the 2-way ANCOVA, whereas the reduced activity in the right inferior parietal cortex was still significant in both cases. These results suggest that the activation of the dorsolateral PFC during planning the set-shift is related to impaired global cognitive ability in PD patients, whereas the inferior parietal cortex is relatively more associated with their behavioral impairment. In line with this idea, evidence suggests dissociated roles of two brain regions in the frontoparietal network during executive control processes. The posterior parietal cortex is implicated in sustained attention in preparation of upcoming stimuli and the working memory capacity, whereas the PFC is involved in the ability to assess goal-directed attention, and away from goal irrelevant information (Dodds et al., 2011, Li et al., 2017). Further study should be performed in the future to clarify the distinct neural mechanisms between behavioral and cognitive impairments in PD.
In ROI analyses, the hippocampus showed negative percent signal changes while planning the set-shift in HC and the PD-noMBI group, but the deactivation was not present in the PD-MBI group. Moreover, the reduced deactivation in the hippocampus was significantly correlated with the high MBI-C total score. A previous study found that the hippocampal activity during a set-shifting task was relatively decreased during shifting from the old rule and generating new rules compared to maintaining the rule, and the hippocampus interacted with the caudate indirectly via the medial prefrontal and posterior cingulate cortex during the task (Graham et al., 2009). During the WCST, reduced dopamine levels in HC were associated with reduced deactivation in the hippocampus as well as impairment in the fronto-striatal connectivity (Nagano-Saito et al., 2008). The deactivation of the hippocampus during attention-demanding cognitive tasks is thought to reflect the suppression of stimulus-independent thoughts or ruminations (Fox et al., 2005). These previous studies suggest that the deactivation in the hippocampus during planning the set-shift is associated with preventing interference of memory of previous trials with current trials, and that the hippocampal function interacts with the fronto-striatal system. The impaired deactivation of the hippocampus in our PD-MBI group indicates that MBI in PD is associated with hippocampal memory function as well as frontal executive function.
Moreover, the hippocampus is involved in the default mode network, which is functionally defined as being activated during rest and deactivated during attention-demanding cognitive tasks (van den Heuvel and Hulshoff Pol, 2010). While performing a set-shifting task, the more set-shifts were being performed, the more the activity in the fronto-striatal regions was increased, while the default mode network was more deactivated (Provost and Monchi, 2015). Also, previous evidence suggests that the frontoparietal control network negatively regulates activity in the default mode network (Chen et al., 2013). Therefore, together with the reduced activation in the PFC and posterior parietal areas in the PD-MBI group, the less deactivation in the hippocampus could be associated with the disrupted interaction between frontoparietal and default mode networks during planning the set-shift in the PD-MBI group.
While executing the set-shift, the PD-MBI group revealed reduced activation in the left frontopolar area compared to HC. The left frontopolar area was associated with updating process of executive function that refers to the process of constantly monitoring and rapidly adding or deleting information from the working-memory contents (Collette et al., 2005). Similarly, in a previous study using a modified WCST, the left frontopolar cortex was more active in the interference trials in which subjects were required to inhibit proactive interference than in dual-match trials in which both the current selection rule and the previously learned rule led to the same response (Konishi et al., 2005). In line with these previous studies, the higher rates of perseverative and non-perseverative errors in the PD-MBI group compared to HC in the present study may indicate the impaired updating and inhibition processes linked with reduced activation in the frontopolar area during executing the set-shift. These findings, together with reduced activation in the dorsolateral PFC during planning the set-shift, suggest that the MBI in patients with PD has an effect on the multiple prefrontal processes subserving successful performance of cognitive set-shifting.
Interestingly, we found significant positive correlation between MBI-C scores and activation in the left supramarginal gyrus and postcentral cortex during executing the set-shift only after adjusting MoCA. In a previous study, the inferior parietal and postcentral cortices revealed increased activation during set-shift trials in drug-naïve PD patients compared to HC and behaviorally the PD patients did not make more errors compared to HC. This suggests that the increased activity might reflect a compensatory role of the parietal brain areas (Gerrits et al., 2015). Although the PD-MBI group made relatively more errors in the present study, we hypothesize that the PD patients with high MBI-C scores recruited the left parietal cortex during executing the set-shift in order to compensate for the decreased activity in the frontopolar cortex. Moreover, the fact that the correlation was significant only after controlling for MoCA suggests that the compensatory activation of the left parietal area may function properly with normal global cognitive ability only.
It should be noted that the PD-noMBI group did not reveal any different activity compared to HC, which might seem to contradict previous studies where PD patients had significant reduced activation in the frontal and striatal areas when planning and executing a set-shift compared to HC (Monchi et al., 2004, Monchi et al., 2007). This discrepancy likely originates in the fact that those studies did not differentiate between PD patients with and without NPS. Furthermore, previous studies found different activity cross-sectionally and longitudinally in PD with MCI compared to PD without MCI, although there were no comparisons with HC (Nagano-Saito et al., 2016, Nagano-Saito et al., 2014). In this respect, we suggest that changes in brain activity during performing the WCST might be unique to PD with behavioral or cognitive impairment.
There are several limitations to be discussed in the current study. First, we evaluated the brain activation during the ON-dopamine replacement therapy state. Dopamine depletion in healthy participants was associated with reduced activity in front-striatal regions and their connectivity while performing the WCST (Nagano-Saito et al., 2008), and dopamine replacement therapy can normalize some part of aberrant functional connectivity patterns during resting state in PD (Tomasi and Volkow, 2011). These suggest that our results in PD group might be associated with their dopamine levels. However, a previous study identified that the dopamine medication in PD patients helped restoring motor corticostriatal loop but was not associated with the pattern of activity in cognitive corticostriatal loop during performing WCST (Jubault et al., 2009). Also, all analyses in the present study were performed with LEDD as a covariate of no interest. Therefore, the abnormal activity in the PD-MBI group could not be accounted for dopamine medication. Second, in this study, we only focused on MBI-C total score, but individual MBI domains may have different effects on cognitive set-shifting and related brain activation. Further large cohort study will be required to evaluate the associations with individual MBI domains.
In summary, during a cognitive set-shifting task, we found that PD patients with high MBI-C scores revealed deficiency of activation in the prefrontal and posterior parietal cortices and reduced degree of deactivation in the medial temporal region. These results suggest that MBI is associated with impairment in frontoparietal control network and hippocampal memory system as well as frontal executive impairment in PD. Therefore, MBI might be an important marker for cognitive impairment in PD and capture a risk group for incident cognitive decline and dementia in patients with PD, in parallel with its utility in non-PD dementias. The ease of MBI case ascertainment provides promise to improve screening for cognitive risk in patients with PD to help guide subsequent neuropsychological testing and imaging investigations.
CRediT authorship contribution statement
Eun Jin Yoon: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft. Zahinoor Ismail: Conceptualization, Writing - review & editing. Iris Kathol: Investigation. Mekale Kibreab: Investigation. Tracy Hammer: Investigation. Stefan Lang: Investigation. Mehrafarin Ramezani: Investigation. Noémie Auclair-Ouellet: Investigation. Justyna R. Sarna: Investigation, Resources. Davide Martino: Investigation, Resources. Sarah Furtado: Resources. Oury Monchi: Conceptualization, Methodology, Formal analysis, Investigation, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank Daniel Pittman for programing the Wisconsin card sorting task in the Presentation software version 17.2 (Neurobehavioral Systems, Inc).
Funding
This study was funded by a project grant from the Canadian Institutes of Health Research project grant (PJT-1661232), the Tourmaline Oil Chair in Parkinson’s Disease, The Canada Research Chair in non-motor symptoms of Parkinson’s disease to OM, and Natural Sciences and Engineering Research Council (NSERC) CREATE I3T Program and the E. Marie and R. Garey Cameron Postdoctoral Fellowship in Dementia from the Hotchkiss Brain Institute to EJY.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102590.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aarsland D., Marsh L., Schrag A. Neuropsychiatric symptoms in Parkinson's disease. Mov. Disord. 2009;24(15):2175–2186. doi: 10.1002/mds.v24:1510.1002/mds.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos G.S., Hoptman M.J., Kanellopoulos D., Murphy C.F., Lim K.O., Gunning F.M. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschi R., Restivo V., Nicoletti A., Cicero C.E., Luca A., Recca D., Zappia M., Monastero R., Mecocci P. Mild Behavioral impairment in Parkinson's disease: Data from the Parkinson's Disease Cognitive Impairment Study (PACOS) J. Alzheimers Dis. 2019;68(4):1603–1610. doi: 10.3233/JAD-181117. [DOI] [PubMed] [Google Scholar]
- Buchsbaum B.R., Greer S., Chang W.-L., Berman K.F. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum. Brain Mapp. 2005;25(1):35–45. doi: 10.1002/(ISSN)1097-019310.1002/hbm.v25:110.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.C., Oathes D.J., Chang C., Bradley T., Zhou Z.-W., Williams L.M., Glover G.H., Deisseroth K., Etkin A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc. Natl. Acad. Sci. USA. 2013;110(49):19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L., Zalesky A., Fornito A., Mattingley J.B. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn. Sci. 2013;17(10):493–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Pathak S., Schneider W. Identifying the brain's most globally connected regions. Neuroimage. 2010;49(4):3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Repovš G., Anticevic A. The frontoparietal control system: A central role in mental health. Neuroscientist. 2014;20(6):652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F., Van der Linden M., Laureys S., Delfiore G., Degueldre C., Luxen A., Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Hum. Brain Mapp. 2005;25(4):409–423. doi: 10.1002/(ISSN)1097-019310.1002/hbm.v25:410.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds C.M., Morein-Zamir S., Robbins T.W. Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb. Cortex. 2011;21(5):1155–1165. doi: 10.1093/cercor/bhq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Emre M. Dementia associated with Parkinson's disease. Lancet Neurol. 2003;2(4):229–237. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits N.J., van der Werf Y.D., Verhoef K.M., Veltman D.J., Groenewegen H.J., Berendse H.W., van den Heuvel O.A. Compensatory fronto-parietal hyperactivation during set-shifting in unmedicated patients with Parkinson's disease. Neuropsychologia. 2015;68:107–116. doi: 10.1016/j.neuropsychologia.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Graham S., Phua E., Soon C.S., Oh T., Au C., Shuter B., Wang S.-C., Yeh I.B. Role of medial cortical, hippocampal and striatal interactions during cognitive set-shifting. Neuroimage. 2009;45(4):1359–1367. doi: 10.1016/j.neuroimage.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Grant D.A., Berg E.A. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J. Exp. Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Heaton R.K. Psychological Assessment Resources Inc; Odessa, FL: 1981. Wisconsin Card Sorting Test Manual. [Google Scholar]
- Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail Z., Aguera-Ortiz L., Brodaty H., Cieslak A., Cummings J., Fischer C.E., Gauthier S., Geda Y.E., Herrmann N., Kanji J., Lanctot K.L., Miller D.S., Mortby M.E., Onyike C.U., Rosenberg P.B., Smith E.E., Smith G.S., Sultzer D.L., Lyketsos C., Research, N.P.S.P.I.A.o.t.I.S.o.t.A.A.s., Treatment The Mild Behavioral Impairment Checklist (MBI-C): A Rating Scale for Neuropsychiatric Symptoms in Pre-Dementia Populations. J. Alzheimers Dis. 2017;56:929–938. doi: 10.3233/JAD-160979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail Z., Smith E.E., Geda Y., Sultzer D., Brodaty H., Smith G., Aguera-Ortiz L., Sweet R., Miller D., Lyketsos C.G., Area I.N.S.P.I. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12:195–202. doi: 10.1016/j.jalz.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubault T., Monetta L., Strafella A.P., Lafontaine A.-L., Monchi O., Gribble P.L. L-dopa medication in Parkinson's disease restores activity in the motor cortico-striatal loop but does not modify the cognitive network. PLoS One. 2009;4(7):e6154. doi: 10.1371/journal.pone.000615410.1371/journal.pone.0006154.g001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Cilles S.E., Johnson N.F., Gold B.T. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum. Brain Mapp. 2012;33(1):130–142. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S., Chikazoe J., Jimura K., Asari T., Miyashita Y. Neural mechanism in anterior prefrontal cortex for inhibition of prolonged set interference. Proc. Natl. Acad. Sci. USA. 2005;102(35):12584–12588. doi: 10.1073/pnas.0500585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S., Yoon E.J., Kibreab M., Kathol I., Cheetham J., Hammer T., Sarna J., Ismail Z., Monchi O. Mild behavioral impairment in Parkinson's disease is associated with altered corticostriatal connectivity. Neuroimage Clin. 2020;26:102252. doi: 10.1016/j.nicl.2020.102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange F., Kroger B., Steinke A., Seer C., Dengler R., Kopp B. Decomposing card-sorting performance: Effects of working memory load and age-related changes. Neuropsychology. 2016;30:579–590. doi: 10.1037/neu0000271. [DOI] [PubMed] [Google Scholar]
- Lange F., Seer C., Loens S., Wegner F., Schrader C., Dressler D., Dengler R., Kopp B. Neural mechanisms underlying cognitive inflexibility in Parkinson's disease. Neuropsychologia. 2016;93:142–150. doi: 10.1016/j.neuropsychologia.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Li S., Cai Y., Liu J., Li D., Feng Z., Chen C., Xue G. Dissociated roles of the parietal and frontal cortices in the scope and control of attention during visual working memory. Neuroimage. 2017;149:210–219. doi: 10.1016/j.neuroimage.2017.01.061. [DOI] [PubMed] [Google Scholar]
- Liao W., Chen H., Feng Y., Mantini D., Gentili C., Pan Z., Ding J., Duan X., Qiu C., Lui S.u., Gong Q., Zhang W. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 2010;52(4):1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Litvan I., Goldman J.G., Tröster A.I., Schmand B.A., Weintraub D., Petersen R.C., Mollenhauer B., Adler C.H., Marder K., Williams-Gray C.H., Aarsland D., Kulisevsky J., Rodriguez-Oroz M.C., Burn D.J., Barker R.A., Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement disorder society task force guidelines. Mov. Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo S.C., Ismail Z., Pereiro A.X., Facal D., Lojo-Seoane C., Campos-Magdaleno M., Juncos-Rabadán O. Assessing mild behavioral impairment with the mild behavioral impairment checklist in people with subjective cognitive decline. Int. Psychogeriatr. 2018;31(2):231–239. doi: 10.1017/S1041610218000698. [DOI] [PubMed] [Google Scholar]
- Mallo S.C., Ismail Z., Pereiro A.X., Facal D., Lojo-Seoane C., Campos-Magdaleno M., Juncos-Rabadán O., Abbate C. Assessing mild behavioral impairment with the mild behavioral impairment-checklist in people with mild cognitive impairment. J. Alzheimers Dis. 2018;66(1):83–95. doi: 10.3233/JAD-180131. [DOI] [PubMed] [Google Scholar]
- Monchi O., Petrides M., Doyon J., Postuma R.B., Worsley K., Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. J. Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O., Petrides M., Mejia-Constain B., Strafella A.P. Cortical activity in Parkinson's disease during executive processing depends on striatal involvement. Brain. 2007;130:233–244. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O., Petrides M., Petre V., Worsley K., Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J. Neurosci. 2001;21(19):7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A., Al-Azzawi M.S., Hanganu A., Degroot C., Mejia-Constain B., Bedetti C., Lafontaine A.L., Soland V., Chouinard S., Monchi O. Patterns of longitudinal neural activity linked to different cognitive profiles in Parkinson's Disease. Front. Aging Neurosci. 2016;8:275. doi: 10.3389/fnagi.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A., Habak C., Mejía-Constaín B., Degroot C., Monetta L., Jubault T., Bedetti C., Lafontaine A.-L., Chouinard S., Soland V., Ptito A., Strafella A.P., Monchi O. Effect of mild cognitive impairment on the patterns of neural activity in early Parkinson's disease. Neurobiol. Aging. 2014;35(1):223–231. doi: 10.1016/j.neurobiolaging.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A., Leyton M., Monchi O., Goldberg Y.K., He Y., Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J. Neurosci. 2008;28(14):3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN A.M., JAMES M., LEIGH P.N., SUMMERS B.A., MARSDEN C.D., QUINN N.P., LANGE K.W., ROBBINS T.W. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115(6):1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Pluck G.C., Brown R.G. Apathy in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2002;73:636–642. doi: 10.1136/jnnp.73.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost J.-S., Monchi O. Exploration of the dynamics between brain regions associated with the default-mode network and frontostriatal pathway with regards to task familiarity. Eur. J. Neurosci. 2015;41(6):835–844. doi: 10.1111/ejn.12821. [DOI] [PubMed] [Google Scholar]
- Schiess M.C., Zheng H., Soukup V.M., Bonnen J.G., Nauta H.J.W. Parkinson's disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat Disord. 2000;6(2):69–76. doi: 10.1016/S1353-8020(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Schultz D.H., Ito T., Solomyak L.I., Chen R.H., Mill R.D., Anticevic A., Cole M.W. Global connectivity of the fronto-parietal cognitive control network is related to depression symptoms in the general population. Netw. Neurosci. 2019;3(1):107–123. doi: 10.1162/netn_a_00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein S.E., Preziosi T.J., Berthier M.L., Bolduc P.L., Mayberg H.S., Robinson R.G. Depression and cognitive impairment in Parkinson's disease. Brain. 1989;112(Pt 5):1141–1153. doi: 10.1093/brain/112.5.1141. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Association between functional connectivity hubs and brain networks. Cereb. Cortex. 2011;21:2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov K.A., Henson R.N., Tyler L.K., Davis S.W., Shafto M.A., Taylor J.R., Williams N., Cam C., Rowe J.B. The effect of ageing on fMRI: Correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Hum. Brain Mapp. 2015;36:2248–2269. doi: 10.1002/hbm.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Varanese S., Perfetti B., Ghilardi M.F., Di Rocco A., Aleman A. Apathy, but not depression, reflects inefficient cognitive strategies in Parkinson's disease. PLoS One. 2011;6(3):e17846. doi: 10.1371/journal.pone.0017846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale C., Santangelo G., Trojano L., Verde F., Rocco M., Grossi D., Barone P. Comparative neuropsychological profile of pathological gambling, hypersexuality, and compulsive eating in Parkinson's disease. Mov. Disord. 2011;26(5):830–836. doi: 10.1002/mds.23567. [DOI] [PubMed] [Google Scholar]
- Yoon E.J., Ismail Z., Hanganu A., Kibreab M., Hammer T., Cheetham J., Kathol I., Sarna J.R., Martino D., Furtado S., Monchi O. Mild behavioral impairment is linked to worse cognition and brain atrophy in Parkinson disease. Neurology. 2019;93(8):e766–e777. doi: 10.1212/WNL.0000000000007968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.