Abstract
Behavioral synchrony during social interactions is foundational for the development of social relationships. Behavioral inhibition (BI), characterized by wariness to social novelty and increased anxiety, may influence how children engage in moment-to-moment behavioral synchrony. EEG-derived frontal Alpha asymmetry and Delta-Beta coupling reflect approach-avoidance behavior and emotion regulation, respectively. We examined the relation between intradyadic behavioral synchrony in energy levels and peer gaze, BI, and EEG measures (N=136, 68 dyads, MeanAge=10.90 years) during unstructured and structured interactions. Energy levels were negatively synchronized when both children exhibited right Alpha asymmetry. If either child exhibited left Alpha asymmetry, the dyad exhibited more positive synchrony. Peer gaze was less synchronized during the unstructured task with left Alpha asymmetry. Greater positive Delta-Beta coupling in BI children was associated with more peer gaze synchrony. Peer gaze was asynchronous when BI children exhibited negative Delta-Beta coupling and their partner exhibited positive coupling.
Keywords: behavioral inhibition, Delta-Beta coupling, frontal EEG Alpha asymmetry, temperament, social behavior
Behavioral synchrony is marked by the spontaneous temporal coordination of actions between people (Mayo & Gordon, 2020). Synchrony can range from positive coordination (i.e., the presence of one action is matched by the dyadic partner) to negative covariation (i.e., the presence of an action is matched by the opposing action), or show no stable relation (i.e., asynchronous action). Behavioral synchrony is a dynamic mechanism underlying both individual instances of social behavior and broader patterns of social development (Wass et al., 2019), with outcomes determined by the directionality, strength, length, and frequency of the interaction, as well as the specific behaviors in question. The current study focuses on interpersonal concordance among children characterized for a temperamental trait, behavioral inhibition, linked to broad profiles of social functioning and distinct trajectories of socioemotional development. In doing so, we also examine the extent to which neural correlates of approach-withdrawal behavior and emotion regulation are associated with dyadic-level variation in concordance.
Behavioral Synchrony and Building Social Relationships
Previous work suggests that coordination in affect (Lobo & Lunkenheimer, 2020), gaze (Farran & Kasari, 1990; Feldman, 2007), and contingent responding during open-ended interactions and goal-oriented tasks (Lunkenheimer et al., 2017) may facilitate positive social interactions and the emergence of adaptive social competencies over time. A large literature has examined the role of parent-child behavioral synchrony, particularly in relation to the development of self-regulation (Davis et al., 2017; Feldman et al., 1999; Lobo & Lunkenheimer, 2020; MacLean et al., 2014). Broadly, positive parent-child synchrony, defined as greater positive correlations in behavior and affect during an interaction, is associated with more effective and efficient self-regulation by young children in the moment and across time (Bornstein, 2013; Lunkenheimer et al., 2011).
As children mature and expand their social networks, interactions with peers afford further opportunities for the development of regulatory processes and social competencies, taking on greater prominence in middle childhood and into adolescence. Indeed, behavioral synchrony during peer interactions is related to the development of empathy (Xavier et al., 2013) and prosocial behaviors (Tunçgenç & Cohen, 2018), both of which support adaptive social functioning. Although behavioral synchrony may be detrimental in negative contexts, such as harsh parenting environments (Lunkenheimer et al., 2017), behavioral synchrony in neutral and positive contexts appears to enhance the internalization of specific social processes (e.g., joint attention, contingent responding, collaboration) that support adaptive socialization and social learning (Davis et al., 2017).
Most current work examines synchrony within established relationship pairs (i.e., parent-child, romantic partners, and friends). However, even strangers exhibit behavioral synchrony during initial social interactions (Vacharkulksemsuk & Fredrickson, 2012). Furthermore, an individual’s reported desire to cultivate a relationship with a stranger is associated with greater synchrony in movement throughout an initial social interaction (Fujiwara et al., 2020). In the context of a novel social interaction, an effort to synchronize with an interaction partner may promote social bonding (Launay et al., 2016) as individuals work to secure social relationships and expand their social networks.
Behavioral Inhibition and Behavioral Synchrony
Systematic individual differences in how children and adults experience social interactions may influence the magnitude and efficacy of behavioral synchrony in a given interaction. Behavioral Inhibition (BI) is a temperament profile characterized by shy, fearful, and hypervigilant responses to novel social situations (Garcia-Coll et al., 1984). Early precursors of behavioral inhibition can be identified in the first months of life by high motor reactivity and negative affect (Fox et al., 2015). Children who retain a profile of behavioral inhibition are more likely to show avoidance and fear of novel toys and strangers as toddlers (Kagan et al., 1987), display shyness and negative affect in novel social situations as young children (Rubin et al., 2002), and have fewer positive interactions with school peers (Bohlin et al., 2005). Behavioral inhibition in early life is associated with more reticence when engaging with novel peers during early childhood (Degnan et al., 2014) as well as greater neural and emotional sensitivity to peer feedback during adolescence (Guyer et al., 2014).
Early emerging patterns in how behaviorally inhibited children process and engage with their social world may become entrenched over time (Guyer & Jarcho, 2018; Jarcho & Guyer, 2018), hindering children’s abilities to even make eye contact or start conversations with a peer in the most extreme cases. Indeed, longitudinal studies demonstrate that children with a history of behavioral inhibition who retain their social reticence into adolescence are seven times more likely to develop social anxiety disorder (Clauss & Blackford, 2012). The distinct social profile reflected in behavioral inhibition may influence the behavior of interaction partners, as they react to and reflect signals from their inhibited partner. Opportunities for behavioral synchrony may be missed if behaviorally inhibited children inadvertently cause confusion for the interaction partner or appear disinterested in interacting. Non-inhibited children may find it difficult to interpret a behaviorally inhibited child’s social cues and find themselves confused about how to proceed. The level of confusion or discord may vary with the specific situation, such as rote or scripted tasks versus open-ended or unstructured interactions. Indeed, recent work indicates that anxiety behaviors are heightened and social skills are deployed less effectively by adolescents during unstructured interactions compared to structured interactions with unfamiliar peers (Glenn et al., 2019).
Much of the literature examining social behavior in behaviorally inhibited children has relied on static scores created by aggregating mean values over fairly wide time epochs (Buss & McDoniel, 2016). However, recent work suggests that capturing the temporal dynamic patterns of behavior with fine-grained time series data can reveal subtle variation in the time-course and functioning of reactive and regulatory mechanisms (Benson et al., 2018; Morales et al., 2018). For children high in behavioral inhibition, difficulty engaging in core aspects of social interactions, such as making eye contact and contingent responding, may inhibit opportunities to establish dyadic behavioral synchrony with peers. Fewer synchronous interactions may lead to difficulties establishing social relationships, thus shaping broader patterns of socioemotional development and anxiety risk. Indeed, restricted or fragile social relationships are independent risk factors for social reticence and social anxiety (Levula et al., 2018; Rubin et al., 2018).
Neural Markers associated with Behavioral Synchrony
Although behavioral inhibition is a strong predictor of future difficulty with social interactions and social anxiety, not all behaviorally inhibited children show worrisome functional impairment (Degnan & Fox, 2007). The extensive behavioral inhibition literature has identified additional risk factors, particularly during middle childhood, that may reinforce reticent tendencies and mark children at greatest risk for difficulties in creating social relationships and building a strong social network. With respect to social behavior, neural markers of individual differences in approach-avoidance motivation, as captured by frontal EEG Alpha asymmetry (Coan & Allen, 2003, 2004), and emotion regulation, captured in Delta-Beta coupling (Knyazev et al., 2006), may be associated with variations in behavioral synchrony in the moment.
Frontal EEG Alpha asymmetry at rest reflects broad motivational patterns that shape how an individual interacts with their environment (Coan & Allen, 2004). For example, right frontal EEG Alpha asymmetry is related to withdrawal tendencies and behaviors, whereas left frontal EEG Alpha asymmetry is related to approach tendencies and behaviors (Coan & Allen, 2003). Naturalistic observations during peer play support this model, showing that children who display more approach behaviors and social competencies also exhibit more left frontal EEG Alpha asymmetry at rest, whereas children who display more withdrawal behaviors exhibit more right frontal EEG Alpha asymmetry (Fox et al., 1995). However, emotional context may additionally influence state changes in underlying biological predispositions (Coan et al., 2006). For example, individuals generally exhibit a shift from greater left frontal EEG Alpha asymmetry to greater right frontal EEG Alpha asymmetry when moving from a low stress to a higher stress experience (Lewis et al., 2007). Additionally, heighted right frontal EEG Alpha asymmetry in the presence of a stressor may be further exacerbated in individuals with anxious predispositions (Cole et al., 2012).
Frontal EEG Alpha asymmetry also modulates the relation between fearful temperament and socioemotional behaviors. For example, infants high in negative affect and reactivity at 4-months who also display right frontal EEG Alpha asymmetry at 9-months exhibit more contemporaneous withdrawal behaviors in novel social situations, whereas infants high in positive affect and left frontal EEG Alpha asymmetry exhibit more approach behaviors (Hane et al., 2008). Additionally, infants exhibiting higher negative affect and more right frontal EEG Alpha asymmetry at 9-months later exhibit more social wariness at 4-years (Henderson et al., 2001). Although the current evidence highlights the ways in which approach-avoidance motivation is associated with higher order profiles of social reticence and withdrawal, it may also be linked to more granular social characteristics, such as moment-to-moment behavioral synchrony throughout a social interaction.
Delta-Beta coupling refers to the synchronized activity of EEG brain oscillations in the Delta and Beta frequency bands (Knyazev et al., 2006), usually measured by the statistical correlation between Delta and Beta relative power. Evolutionary theories of brain oscillations (Knyazev, 2012) postulate that slow-wave activity (e.g., Delta) reflects subcortical systems linked to reward and emotion (Schutter & Knyazev, 2012), while fast-wave activity (e.g., Beta) reflect higher-order cortical systems linked to cognitive control and regulation of subcortical systems (Engel et al., 2001). Positive coupling of Delta and Beta oscillations is thought to reflect cortical networks functioning in a coordinated manner to inhibit or regulate arousal in subcortical networks (Knyazev, 2012). Conversely, decoupling of Delta and Beta oscillations has been linked to synthetic administrations of testosterone as well as high basal testosterone levels, which reflect physiological states of fear suppression and disinhibition (Miskovic & Schmidt, 2009). Taken together, these studies suggest that Delta-Beta coupling may reflect cross-talk between subcortical and cortical networks. The strength and directionality of the coupling may vary with shifts in overt regulatory state or underlying regulatory mechanisms. Thus, the initial data suggest that Delta-Beta coupling can be used as a neural marker of emotion regulation.
While this functional interpretation of Delta-Beta coupling continues to be empirically tested, several studies now indicate that heightened, positive Delta-Beta correlations are associated with social anxiety (De Pascalis et al., 2020; Knyazev et al., 2006; Miskovic et al., 2011). The suggestion is that whereas moderate levels of positive coupling is indicative of flexible and adaptive emotion regulation, over-coupling between these systems reflects the rigid, over-controlling, and ruminating tendencies often seen in socially fearful and avoidant phenotypes (Degnan & Fox, 2007). Behavioral inhibition is also associated with rigid behavioral patterns (Henderson & Wilson, 2017; Pérez-Edgar, 2018), leading researchers to explore potential associations between this temperament profile and Delta-Beta coupling. Early data suggest that heightened positive Delta-Beta coupling is associated with shyness, temperamental risk, and anxiety in children (Anaya et al., 2020; Poole et al., 2020; Poole & Schmidt, 2019, 2020). Novel social encounters require that children process new information regarding the social partner in real time and flexibly shape their own behavior in order to effectively support the dynamic social interaction. Variation in neural correlates of emotion regulation, captured in the dynamic coupling of Delta and Beta power, may therefore influence moment-to-moment behavioral regulation.
The Current Study
In the current study, we examine behavioral synchrony throughout a naturalistic peer interaction during middle childhood. Despite emerging evidence linking frontal EEG Alpha asymmetry and Delta-Beta coupling to socially fearful and inhibited phenotypes (Harrewijn et al., 2016, 2018; Miskovic et al., 2011), to our knowledge no study has tested the link between these neural measures and dyadic states of behavioral synchrony captured during actual social interactions. Previous studies support strong links between behavioral inhibition and poor peer engagement. Dyadic synchrony may represent the active temporal conduit of social engagement, ‘carrying’ social interaction from moment to moment and shaping the broader higher-order patterns typically observed when behaviorally inhibited children interact with their peers.
Our first goal was to characterize dynamic peer social interactions by modeling behavioral synchrony in peer gaze and energy levels as intradyadic processes. These channels were chosen to reflect broad behaviors (i.e., energy level) and micro-level social signals (i.e., peer gaze). They were coded separately at the level of the individual and the temporal match with the partner was then examined analytically after the fact to assess interpersonal synchrony. We paired children high in behavioral inhibition with a non-behaviorally inhibited (BN) peer given the known influence of behavioral inhibition on social interactions (Degnan et al., 2014). Pairs were unfamiliar with one another, allowing us to assess an interaction that should encourage efforts for social bonding and social network expansion (Launay et al., 2016), which have been associated with higher levels of behavioral synchrony (Fujiwara et al., 2020). Furthermore, the dyads engaged in both an unstructured (e.g., Get to know you) and structured (e.g., Cooperative goal-driven task) interaction allowing us to assess if contextual factors altered levels of behavioral synchrony. We hypothesized that intradyadic synchrony would be stronger during the structured interaction, which provided instructions for cooperation and a shared goal, and could potentially scaffold behaviorally inhibited children to engage with their peers.
Our second goal was to test the extent to which individual differences in neural markers of approach-avoidance motivation (frontal EEG Alpha asymmetry) and emotion regulation (Delta-Beta coupling) were associated with each channel of behavioral synchrony. Additionally, we predicted that right frontal EEG Alpha asymmetry, especially in behaviorally inhibited children, would be associated with lower intradyadic synchrony. Finally, based on the link between heightened Delta-Beta coupling and more rigid emotion regulation and behavior, we predicted that stronger, positive Delta-Beta coupling would also be associated with lower intradyadic synchrony.
Method
Data were derived from the baseline visit of a larger project examining temperament, attention, and anxiety in children. The recruitment details and main findings of the project have been reported elsewhere (Liu et al., 2018). The Behavioral Inhibition Questionnaire (BIQ; Bishop et al., 2003) was used to initially screen (N = 706) and classify children as BI if total scores ≥ 119 or social novelty subscale scores ≥ 60, based on prior literature (Broeren & Muris, 2010). Non-inhibited (BN) children scoring below the set cut-offs were recruited as a sex- and age-matched comparison group. A total of 251 children (112 high BI; Mean Age = 10.9 years, SD = 0.98, Female = 136) enrolled in the larger study. From the enrolled sample, 30 families discontinued participation before we collected any variables of interest for the current study. From these remaining families, 148 children completed the peer dyad and provided usable EEG data. During coding, 6 dyads were excluded due to technical issues with the video.
Participants
The final sample in the current study included 136 children (72 Female) for a total of 68 dyads. Each dyad consisted of a BI and BN unfamiliar peer matched on age and parent-reported sex. Paired-sample t-test of continuous BIQ scores, yoked by dyad, indicated that on average, dyad peers were significantly different from each other on their BIQ score (MΔ = 49.22, t(67) = 16.84, p = .001). Within-dyad, individual differences in BIQ and Social Novelty scores peers were +25 points away from each other for most of the dyads (> 75%). On average, dyad peers were 2.9 (SD = 2.5) months apart in age (Range = 4 days – 9.9 months) when they came into the lab and completed the tasks reported in this study. The sample was 77% white, 4.4% African American, 4.4% Latino, 2.9% Asian, 5.1% Biracial, and 6.2% declined to respond. The sample was recruited from Central Pennsylvania and surrounding areas through a university-supported database, direct outreach, and word-of-mouth. Study procedures were approved by the Institutional Review Board of The Pennsylvania State University. Participants and their parents provided written assent and consent and were compensated for their participation.
Measures
EEG Data Collection.
EEG activity was continuously recorded using a 128-channel geodesic sensor net (Electrical Geodesics Inc., Eugene, Oregon) during a four-minute resting-state period (alternating between one-minute eyes open and one-minute eyes closed). EEG signal was sampled at 1000 Hz rate and collected with channels referenced to Cz and re-referenced offline to the average of the left and right mastoids. Eye movements were recorded using electrodes placed at approximately 1 cm above and below the eye (vertical) and at the outer canthi of each eye (horizontal). Impedances were kept below 50 kᘯ. Data were processed offline using Brain Vision Analyzer (Brain Products GmbH, Germany). Data were filtered using a Butterworth filter with a high-pass frequency of 0.1 Hz, a low-pass frequency of 40 Hz, and a 60 Hz notch filter. Bad channels were manually removed from the data of each participant. After visual inspection, ocular artifacts from eye blinks and horizontal eye movements were corrected using the Gratton et al. (1983) method.
Frontal EEG Alpha asymmetry.
Data were segmented into 1-sec epochs using 50% overlap and baseline corrected. Epochs exceeding ± 120 μV, a voltage step of more than 75 μV between sample points, or a maximum voltage difference of less than .50 μV within any 100-ms interval were marked as artifacts and automatically removed. Data were also visually inspected for any remaining artifacts. EEG power was computed using a Fast Fourier Transformation with full spectrum and a Hamming window length of 50%. These spectra were then averaged across epochs within each baseline type (eyes open, eyes closed) for each participant to produce the total power in the alpha range (8–13 Hz). In order to enhance levels of alpha power (Henderson et al., 2001), analyses employed data from the eyes-closed epochs.
We used homologous frontal electrode clusters (F3: 19, 20, 24, 23, 27, 28; F4: 3, 4, 117, 118, 123, 124) conventionally used for asymmetry calculations (Allen et al., 2004). Asymmetry scores were calculated by subtracting the natural logarithm of alpha for the F3 electrodes (left hemisphere) from the corresponding F4 electrodes (right hemisphere). Because alpha power is thought to be inversely related to brain activity (Davidson, 2004), a positive frontal EEG Alpha asymmetry score represents greater left-sided activity (greater right-sided power), whereas a negative score represents greater right-sided activity (greater left-sided power).
Delta-Beta Coupling.
Data were segmented into 1 sec epochs with no overlap. Automatic artifact detection algorithm excluded segments with a voltage step > 30 μV/ms, absolute difference > 150 μV/ms, amplitude < −100 μV or > 100 μV, and low activity < 0.5 μV for any electrode. Data were then transformed using a Fast-Fourier Transformation with a Hamming window length of 50%. We exported second-by-second EEG power for the Delta (1–4 Hz) and Beta (13–25Hz) frequency bands. This method yielded up to 240 segments of Delta and Beta power for individual participants. In line with previous work showing no difference in Delta-Beta coupling across eyes-open and eyes-closed conditions (Anaya et al., 2020), we used continuous segments over the entire task.
Power values across target electrodes (Poole et al., 2020) were averaged to create composites for the Frontal (F3, Fz, F4: 24, 11, 124) and Central (C3, Cz, C4: 36, 129, 104) regions based on the 10–20 System of Electrode Placement. Average Delta-Beta coupling scores for each participant were computed as the correlation between the within-person, repeated Delta and Beta power observations. In line with a recent finding suggesting that social fear and inhibited phenotypes were associated with inter-individual Delta-Beta coupling (i.e., average coupling scores) only at Central regions (Anaya et al., 2020), we focused our analysis on Central region Delta-Beta coupling scores and provided findings from the Frontal region in Supplement S1.
Peer Dyadic Interaction.
The peer-dyad assessment consisted of four, 5-minute tasks always completed in the same order: Get-to-know-you, Speech Preparation, Speech Delivery, and Cooperation. The current study used behavioral data from the Get-to-know-you and Cooperation tasks, which represent unstructured and structured interactions between the dyads, respectively. The other two tasks were completed individually in the presence of the peer, but were not structured to capture variation in social interaction.
During the Get-to-know-you task, children were left in the room with no instructions. During the Cooperation task, children were instructed to work together to complete either a Lego puzzle (Cooperation Lego) or an Etch-a-Sketch pattern (Cooperation Sketch)1. Table 1 provides detailed descriptions for the dyadic tasks included in the present study. Interactions were videotaped and each task was segmented into 15-second epochs for behavioral coding in Datavyu (Datavyu Team, 2014). Children’s behaviors were coded by teams of undergraduate research assistants who were trained by two laboratory managers. Training involved learning and understanding the coding scheme and coding 10 tapes until they reached reliability with the laboratory managers (weighted kappa > 0.75 and intra-class correlation coefficients > 0.85). The remaining dyads were coded individually. Periodic drift reliability training with the laboratory managers was implemented to maintain reliability standards.
Table 1.
Unstructured and Structured Dyadic Tasks
| Dyadic Task | Description |
|---|---|
| Unstructured: Get-to-know-you | Peers were seated at a table facing each other and briefly introduced to each other by the experimenter. The experimenter immediately announced he/she needed to find some paperwork and would be back shortly. Children were left alone to interact freely for 5 minutes. |
| Structured: Cooperation Task | Each Cooperation task lasted 5 minutes once dyads were given instructions. |
| Lego Puzzle: Experimenter gave a Lego car model to the BN peer, and all the necessary pieces to build the same model to the BI peer. The experimenter instructed the children to work together to recreate the car. A cardboard divider was propped on the table so that the BI peer could not see the built model, forcing the peers to work together under the BN peer’s directions. | |
| Etch-a-Sketch: The experimenter provided an Etch-a-Sketch toy and a sketch pattern. Children were instructed to work together to draw the pattern, with each child assigned to control only one knob. | |
Coders rated the child’s energy level, peer gaze, and affect within each of the 15-second epochs. Energy level was defined as the level of arousal displayed by children via their facial expressions and body posture, and was rated on a scale of 0 = Unaroused, 1 = Mildly aroused, and 2 = Highly Aroused. Behaviors rated as Unaroused usually included slouching, placing head down on the table, and minimal to no gestures. Mildly aroused behaviors included sitting upright for the majority of the epoch, moderate gesturing such as raising the eyebrows and nodding. Highly aroused behaviors included animated body language, standing up or walking around, and excessive gesturing. Peer gaze was defined as the duration (in seconds) that a child directly looked at the partner, independent of the partner’s gaze. Coded within each epoch, “looking at the partner” was operationalized as instances when the child looked to the partner’s face, including glances or short gazes. There was no length requirement for how long a look had to be to count as an instance of peer gaze. The code ended any time the child looked away and resumed any time the child gazed at the peer within the 15-second epoch.
Given that affect may be associated with the dyadic behaviors of interest, affect was coded as 0 = Neutral, 1 = Happy, 2 = Angry, and 3 = Sad during the interaction. Happy was defined as any instance during each 15-second epoch that a child genuinely smiled with full rounded cheeks (not a smirk or nervous smiles), and/ or spoke in a louder, rhythmic pitch that was accompanied by laughs and giggles. Angry was defined as any instance that a child furrowed the brow and clenched the jaw, and/or spoke in a harsh, irritated, contemptuous, or protesting tone, and/ or aggressive movements. Sad was defined as any instance that a child frowned and slumped the shoulders, and/ or spoke with a quiet tone that dropped at the end of utterances, and or cried/ whined expressing sadness. Neutral affect was coded when no indicators of happy, angry, or sad emotion were present.
Final reliability was assessed by re-coding 20% of the videos and computing ICCs for each code separately. These analyses indicated excellent agreement for energy level (r = 0.91) and good agreement for peer gaze (r = 0.86) and affect (r = 0.88).
Data Analysis
Epoch-by-epoch assessments of energy level and peer gaze were exported for each participant and matched by dyad ID, such that each independent observation of the BI and the BN peer within the dyad was yoked throughout each segment. Building on the epoch-by-epoch repeated measures of each behavior, each model was estimated using a total of 1,866 observations.
Data analysis was carried out through multilevel models conducted separately for each behavioral code (energy levels and peer gaze) and for each neural measure (frontal EEG Alpha asymmetry and Delta-Beta coupling). Our first step was to characterize energy level and peer gaze intradyadic synchrony between BN and BI peers across Unstructured and Structured interactions. In a second step, we tested the extent to which intradyadic synchrony in peer gaze and energy level changed as a function of BI and BN frontal EEG Alpha asymmetry scores within each dyad. Finally, we tested a similar model to examine whether BI and BN Delta-Beta coupling scores were associated with intradyadic synchrony of peer gaze and energy levels.
Modeling intradyadic synchrony across Unstructured and Structured task
We used three-level multilevel models to examine intradyadic synchrony of peer gaze and energy levels separately, and whether intradyadic synchrony was significantly different across Unstructured and Structured interactions. Specifically, we examined intradyadic synchrony using a model of the form
| (1) |
| (2) |
| (3) |
where up to 21 repeated measures of each behavioral code (peer gaze or energy levels) obtained from a BN and a BI peer in a dyad i at task v during epoch t, BI Peer Gazeivt and BN Peer Gazeivt, were modeled as deviations from person-specific and task-specific means. Deviations were calculated by subtracting each peer’s mean gaze and mean energy level from their individual peer gaze and energy level epoch-series, during each task.
In all models, epoch-specific, person- and task-centered deviations in the BI peer’s gaze or energy levels were always modeled as a function of a 0 intercept (to keep focus on the intradyadic synchrony), a dyad-specific synchrony coefficient b1iv, and a residual. The dyad-specific synchrony coefficient was the BN peer’s epoch-specific, person- and task-centered deviations of gaze and energy levels, yoked to the matching BI peer. To this basic model, we added an interaction term between the synchrony coefficient, b1iv and a binary task variable b2iTaski to examine whether intradyadic synchrony differed from the Unstructured to the Structured task. The interaction coefficient, b3i was modeled as deviations from dyad-specific synchrony between BI and BN peer gaze or energy levels as a function of task category (0=Unstructured; 1=Structured).
| (4) |
We then added between-person variables of mean epochs, parent-reported sex, age, and affect code as covariates to these models. Covariates were trimmed if not significant.
Moderators of Intradyadic Synchrony
In a second and third step, we expanded the multilevel models to examine peer intradyadic synchrony across Unstructured and Structured interactions as a function of between-person variables of frontal EEG Alpha asymmetry and Delta-Beta coupling scores, in separate models for each behavioral code and each neural measure for a total of four separate models. In order to keep the focus on intradyadic synchrony, and because dyad pairs were intentionally matched between a BI and a BN peer, predictor variables were always entered separately for BI and BN peers. In the equation below, we show the complex regression form of the intradyadic synchrony for the peer gaze and frontal EEG Alpha asymmetry model as an example:
| (5) |
Significant effects for intradyadic synchrony during the Structured task were probed with follow-up models where the coefficient γ20Taski was replaced by a binary coefficient, γ20Cooperation Taski in order to compare intradyadic synchrony across Cooperation Lego and Cooperation Sketch within the Structured interactions. Higher-order interactions in all models were tested and trimmed when non-significant. All models were fit using the lme4 and lmer packages in R (Bates et al., 2015; Pinheiro et al., 2020) and statistical significance was set at ɑ = .05. We applied a 200-resampling bootstrap to the models including Frontal Alpha Asymmetry and Delta-Beta Coupling. The bootstrapped results are included in Supplement Tables S1–S4.
Results
Across the sample, average energy levels were significantly higher during the Unstructured (M = 1.23; SD = 0.46) compared to the Structured (M = 1.03; SD = 0.15; p = .001) interaction. Similarly, average peer gaze was significantly higher during the Unstructured (M = 5.06; SD = 3.45) compared to the Structured (M = 1.21; SD = 1.30; p = .001) interaction, and was higher in dyads who completed the Cooperation Lego (M = 1.52; SD = 1.49) compared to the Cooperation Sketch (M = 0.85; SD = 0.94; p = .001) task.
Average energy levels were higher in BN (M = 1.06; SD = 0.16) compared to BI children (M = 0.99; SD = 0.13; p = .017) during the Structured task only, and this difference was significant for both Cooperation Lego and Cooperation Sketch. In contrast, BI and BN peer gaze did not significantly differ for either the Structured (p = .66) or the Unstructured tasks (p = .24). Table 2 includes means, standard deviations, and zero-order correlations between the behavioral codes and our variables of interest.
Table 2.
Descriptive Statistics and Zero-order Correlations Across Behavioral Codes, Behavioral Inhibition, and Neural Measures.
| Descriptives |
Zero-Order Correlations |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALL | BI | BN | ||||||||||
| M (SD) | Skew | Kurtosis | M (SD) | M (SD) | 1. | 2. | 3. | 4. | 5. | 6. | 7. | |
| 1. BIQ | 101.72 (31.22) | −0.11 | −0.66 | 127.31 (18.03) | 78.49 (20.67) | - | ||||||
| 2. Frontal Asymmetry | −0.12 (0.36) | −0.09 | 1.01 | −0.06 (0.34) | −0.21 (0.36) | 0.14 | - | |||||
| 3. Central Delta-Beta Coupling | 0.21 (0.47) | −0.39 | −0.35 | 0.16 (0.51) | 0.33 (0.40) | −0.20 | −0.03 | - | ||||
|
Get to Know You |
||||||||||||
| 4. Energy Level | 1.23 (0.46) | 0.08 | 0.28 | 1.20 (0.47) | 1.29 (0.42) | −0.12 | −0.07 | 0.11 | - | |||
| 5. Peer Gaze | 5.06 (3.45) | 0.34 | −0.91 | 5.28 (3.59) | 5.03 (3.28) | −0.01 | 0.00 | 0.00 | 0.47 | - | ||
|
Cooperation |
||||||||||||
| 6. Energy Level | 1.03 (0.15) | 0.45 | 25.53 | 0.99 (0.13) | 1.06 (0.16) | −0.19 | −0.05 | 0.15 | 0.13 | 0.15 | - | |
| 7. Peer Gaze | 1.21 (1.30) | 2.24 | 6.68 | 1.09 (0.98) | 1.33 (1.53) | −0.13 | 0.12 | 0.05 | −0.02 | 0.15 | 0.19 | - |
Note: Significant between-group differences and correlations (p < .05) designated in bold.
Intradyadic Synchrony Across Structured and Unstructured Tasks
We first examined the extent to which BI and BN peer gaze and energy levels were synchronized, and whether intradyadic synchrony changed as a function of the structure of the tasks. Mean number of epochs and affect were significant covariates in the energy level model, and were therefore included in all energy level analyses. There were no other significant covariates for energy level or peer gaze.
The model for energy levels suggested positive intradyadic synchronization during the Unstructured task, γ10 = 1.19 (SE = 0.34, p = .001), such that when energy levels in the BN peer were high, energy levels in the BI peer were also high. While energy levels were also positively and significantly synchronized during the Structured task, such that high energy levels in the BN peer were associated with high energy levels in the BI peer, γ10 + γ80 = 1.19 + (−0.14) = 1.05, the overall level of synchronization was lower than in the Unstructured task, γ80 = −0.14 (SE = 0.05, p = .007). Interestingly, we noted a high positive skew and kurtosis during the Structured task, driven by the BI peer. We speculate that the BI child may have been more goal-oriented during the task, perhaps as a counterbalance to any underlying discomfort. Of course, direct examination will be needed in the future to test this proposal.
The model for peer gaze also suggested positive synchronization during the Unstructured task, γ10 = 0.25 (SE = 0.04, p = .001), which was in turn significantly stronger during the Structured task, γ40 = 0.10 (SE = 0.01, p = .03), such that longer gaze from the BN peer to the BI partner was generally more strongly associated with longer gaze from the BI partner to the BN peer when dyads were instructed to cooperate on a task.
We probed intradyadic synchrony during the Structured task further to understand whether it may systematically change as a function of cooperation task. This analysis suggested that intradyadic peer gaze, but not energy levels, was significantly less synchronous during Cooperation Lego, γ40 = −0.33 (SE = 0.09, p = .001) compared to the Cooperation Sketch, γ10 + γ40 = 0.50 + (−0.33) = 0.17.
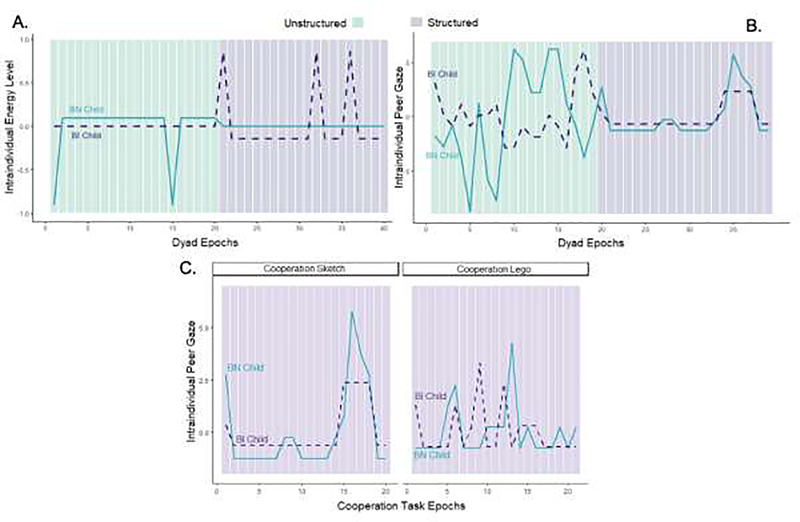
Figure 1 displays raw, epoch-by-epoch intraindividual data from a single dyad to illustrate intradyadic synchrony in energy levels (Panel A) and peer gaze (Panel B) across Structured and Unstructured tasks. Additionally, Panel C illustrates differences in peer gaze synchronization as a function of cooperation task.
Figure 1.
Differences in intradyadic synchrony in energy levels (A) and peer gaze (B) across Unstructured (green) and Structured (purple) tasks for a single dyad and synchrony in peer gaze as a function of Cooperation task type (C).
Intradyadic Synchrony as a Function of Frontal EEG Alpha Asymmetry
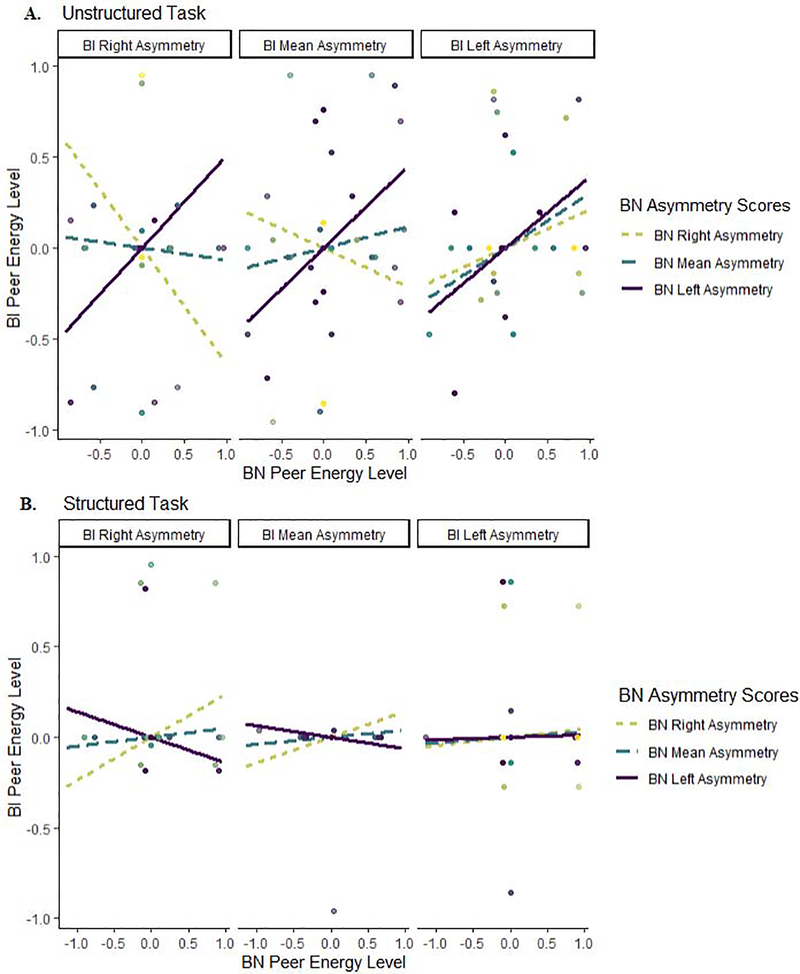
The extent of intradyadic synchrony in energy levels was significantly associated with BN frontal EEG Alpha asymmetry scores during the Unstructured task, γ140 = 0.84 (SE = 0.19, p = .001), suggesting that for dyads in which the BN peer exhibited higher asymmetry values (left asymmetry), intradyadic energy levels were strongly and positively synchronized γ10 + γ140 = 0.95 + 0.84 = 1.79. However, this association was also qualified by an interaction between the BI and BN frontal EEG Alpha asymmetry scores, during the Unstructured task, γ160 = −2.09 (SE = 0.73, p = .004). Simple-slopes analysis (Figure 2a) suggested that in dyads where at least one peer exhibited frontal EEG Alpha asymmetry above +1SD (left asymmetry), regardless of BI status, intradyadic energy levels were always positively synchronized. In contrast, for all other dyadic combinations of mean level or below −1SD (right) frontal EEG Alpha asymmetry, intradyadic energy levels were actually asynchronous and, in some cases, negatively synchronized. That is, higher energy levels in the BN peer were associated with lower energy levels in the BI peer. This relation was not evident in the Structured task (Figure 2b).
Figure 2.
Intradyadic synchrony of energy levels during the (A) Unstructured and (B) Structured task as a function of frontal EEG Alpha asymmetry.
Intradyadic synchrony in peer gaze varied as a function of frontal EEG Alpha asymmetry and BI status. For every unit increase in the BI child’s frontal EEG Alpha asymmetry scores, there was a significant 0.65 decrease in intradyadic synchrony of peer gaze during the Unstructured task only (SE = 0.21, p = .002). No effect was found for the BN child’s frontal EEG Alpha asymmetry scores. This pattern suggests that intradyadic peer gaze was less likely to be synchronized for dyads where the BI peer exhibited left frontal EEG Alpha asymmetry, γ10 + γ70 = 0.29 + (−0.65) = −0.36, compared to all other dyads. This interaction did not differ between dyads as a function of which Cooperation task they completed.
Intradyadic Synchrony as a Function of Delta-Beta Coupling
Central region Delta-Beta coupling scores were not significantly associated with intradyadic synchrony of energy levels (p’s > .23).
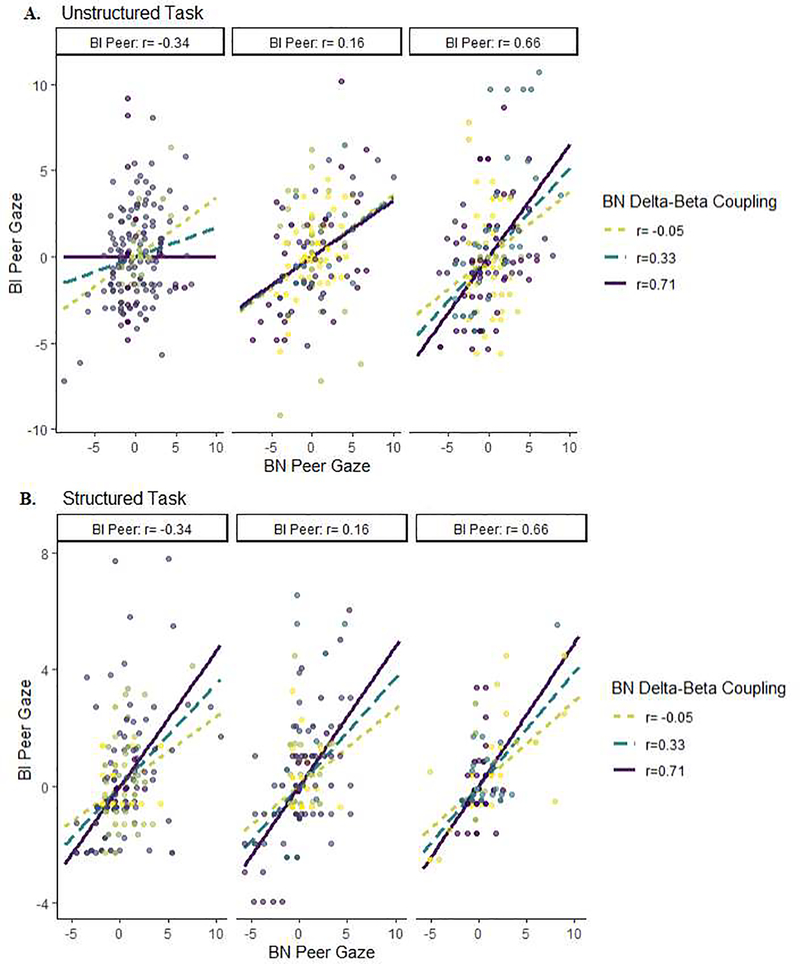
In contrast, intradyadic synchrony in peer gaze significantly varied as a function of the interaction between BI and BN Delta-Beta coupling scores during the Unstructured task, γ120 = 0.95 (SE = 0.41, p = .022). Simple-slopes analysis (Figure 3a) suggested that for dyads in which the BI peer exhibited Delta-Beta coupling scores at mean levels or above +1SD (moderate and heightened, positive Delta-Beta correlation), higher gaze in the BN peer was generally associated with higher gaze in the BI peer regardless of the BN peer’s coupling score. In contrast, for dyads where the BI peer exhibited Delta-Beta coupling scores below −1SD (negative Delta-Beta correlation), intradyadic peer gaze was asynchronous when the BN partner exhibited heightened, positive Delta-Beta coupling scores. The relation was not evident in the Structured task (Figure 3b).
Figure 3.
Intradyadic synchrony of peer gaze as a function of Central region Delta-Beta coupling during the (A) Unstructured and (B) Structured tasks (presented at −1 SD, Mean, and +1 SD for the BI and BN groups).
Discussion
Dyadic behavioral synchrony between peers may play an important role in facilitating social interactions critical for socioemotional development (Harrist & Waugh, 2002; Tunçgenç & Cohen, 2018; Xavier et al., 2013). Social interactions serve as the dynamic conduits for adaptive socialization and social learning (Lunkenheimer et al., 2020). In the context of behavioral inhibition, in particular, positive peer interactions lessen levels of social withdrawal and may buffer the child from risk for social anxiety (Rubin et al., 2018). However, only recently have researchers employed moment-by-moment analyses of dyadic synchrony to capture social behavior, rather than relying on aggregated mean scores over larger windows of time (Hollenstein et al., 2013). In addition, few studies have then examined the moderating role of multiple neural markers already known to shape socioemotional trajectories in children at temperamental risk for anxiety in a single sample of children (Anaya et al., 2020; Hane et al., 2008; Harrewijn et al., 2016; Henderson et al., 2001; Poole & Schmidt, 2020).
In the current study, our first goal was to capture dyadic synchrony in peer gaze and energy levels measured during a novel social encounter with an unfamiliar peer in both unstructured and structured contexts. In doing so, we characterized the dynamic and intradyadic nature of peer social engagement between behaviorally inhibited children and their non-inhibited peers. Our second goal was to examine the extent to which moment-to-moment dyadic synchrony was associated with individual differences in EEG-derived correlates of approach-motivation and regulation. Our findings suggest that this approach can capture subtle variation in social dynamics across contexts and individuals.
First, we note that energy levels and peer gaze were greater in the unstructured task versus the cooperation tasks. This pattern reflects recent data suggesting that individuals display relatively less peer gaze synchrony with a social partner than usually predicted, particularly when engaged in an active task (MacNeill et al., in press). Synchrony patterns were also sensitive to the specific context of interaction since levels of peer gaze and energy did not carry overs across tasks. Thus, our data join a growing body of work noting that traditional social tasks that rely on the presentation of relatively static, computer-based stimuli may lack the ecological validity needed to capture active social behavior (Fu & Pérez-Edgar, 2019).
Although the emergence of behavioral synchrony is anticipated for interactions with unfamiliar individuals (Fujiwara et al., 2020; Vacharkulksemsuk & Fredrickson, 2012), individual differences may influence the ability to smoothly enter into a synchronized state. For example, recent work suggests that children on the autism spectrum often have difficulty engaging in spontaneous dyadic coupling (Kellerman et al., 2020). The level of difficulty, in turn, is associated with symptom severity (Zampella et al., 2020). Similarly, variation in dyadic synchrony mediates the association between temperamental irritability and externalizing behavior (Quiñones- Camacho et al., 2019). Here, our focus was on behavioral inhibition. As noted, children high in behavioral inhibition are particularly sensitive to social novelty, have more tenuous peer relationships, and are more likely to show elevated levels of social withdrawal and social anxiety (Degnan & Fox, 2007).
As such, we looked to see if variation in behavioral inhibition was also associated with group-level differences in the social dyad. When examining mean scores, we noted fewer behavioral inhibition-linked differences than expected. That is, while behaviorally inhibited children displayed less energy than their non-inhibited peers, this was only evident in the structured task. With respect to gaze, we found no differences between the two groups of children. These null findings may reflect the fact that mean-level aggregates can wash away variability in behavior that differs intradyadically or across time (Benson et al., 2018; Morales et al., 2018). In addition, our sample of children was generally healthy and while, mildly stressful, the study tasks were in line with many daily social activities, particularly in an academic setting. Thus, the encounter may not have reached the level of novelty or stress needed to reveal broad differences in behavior.
In our second set of analyses, we noted that the pattern of social behavior was subtly different when examining dyadic synchrony, rather than mean level comparisons. We found significant positive dyadic synchrony for both energy and peer gaze in the unstructured and structured tasks. However, the relative balance differed in that synchrony for energy level was greater in the unstructured task, while peer gaze synchrony was relatively stronger in the structured task. This may reflect the fact that the structured task required cooperation to reach a concrete shared goal, which likely required that dyads “check in” with each other to assess performance. In contrast, the unstructured task was more directly linked to the presence and identity of the peer, without the distraction of a shared concrete task. Synchronizing energy levels may act as an unconscious tool by which unfamiliar peers become socially aligned, supporting more effective social engagement (Criss et al., 2003; Simony et al., 2016). The presence of one behaviorally inhibited child in each dyad may have depressed levels of synchronization in peer eye gaze across the study as behavioral inhibition is often operationally defined by low levels of direct social gaze (Bishop et al., 2003; Fox et al., 2015). Because behavioral inhibition status was perfectly yoked in the dyads (BI with BN), we could not directly assess the impact of temperament on synchronization.
Our third set of analyses examined whether EEG-derived markers previously associated with behavioral inhibition, social behavior, and anxiety risk (Hane et al., 2008; Harrewijn et al., 2016; Henderson et al., 2001; Poole & Schmidt, 2020) were associated with patterns of behavioral synchrony during the social dyad. Decades of work has linked right frontal EEG Alpha asymmetry to patterns of social avoidance, negative affect, and anxiety in both children and adults (Reznik & Allen, 2018). In line with our hypothesis, we found that energy levels were asynchronous or negatively synchronized in dyads in which both peers exhibited mean or negative asymmetry scores (indicating right frontal EEG Alpha asymmetry). In contrast, the presence of left frontal EEG Alpha asymmetry in either member of the dyad was associated with significant positive synchrony. This effect was only present during the unstructured interaction, which relied on more active and spontaneous social interactions on the part of the dyadic pairs. Of course, this task always occurred first, which added to its novelty.
Our hypothesis was not supported for peer gaze, where we found that left frontal EEG Alpha asymmetry, rather than right, was associated with weaker synchrony during the unstructured task. To speculate broadly, left frontal EEG Alpha asymmetry is associated with approach behaviors, including impulsivity and dysregulation (Black et al., 2014; Degnan et al., 2014). It may be that, driven by the desire to successfully complete the structured task, the children may have prioritized task progression over behavioral alignment with their partner. Additional studies will be needed to probe this potential mechanism.
Recent work suggests that Delta-Beta coupling reflects individual variation in the coordination between cortical and subcortical networks involved in emotion regulation (Knyazev, 2012). We predicted that heightened, positive Delta-Beta coupling would be associated with weaker dyadic behavioral synchrony. However, a more complex relation emerged, evident only for peer gaze during the unstructured task.
Heightened, positive Delta-Beta coupling in behaviorally inhibited children was associated with greater gaze synchrony, regardless of the pattern of Delta-Beta coupling presented by their non-inhibited partner. However, a mismatch marked by negative Delta-Beta coupling for the behaviorally inhibited child and heightened, positive coupling in their non-inhibited peer was associated with asynchronous peer gaze. Negative Delta-Beta coupling is thought to reflect underregulated cortical-subcortical interactions, while extreme heightened, positive Delta-Beta coupling may reflect rigid and overcontrolling cortico-subcortical associations (De Pascalis et al., 2020; Miskovic & Schmidt, 2009). This mismatch, compounded by variation in temperamental profiles, may create a particularly difficult interaction when engaged in unstructured, unguided, and novel social settings.
The current findings should be assessed with some limitations in mind. First, the neural measures were captured during a sedentary baseline. Thus, it is not clear if patterns of EEG frontal Alpha symmetry and Delta-Beta coupling, in the moment, track variation in concurrent social behavior (Camacho et al., 2020). Rather, our measures may track underlying trait levels of approach-motivation and emotion regulation that may generally influence dyadic synchrony. Second, all measures were collected at a single timepoint. Longitudinal data will be needed to better understand the temperamental and neural antecedents that may impact subsequent patterns of social behavior. Third, we did not have a direct comparison group of non-inhibited dyads. The larger project was intentionally designed to examine social behavior among behaviorally inhibited children, thus the exclusive use of inhibited and non-inhibited pairings. However, this limits our understanding of the full breadth of dyadic relations that may emerge across the range of peers, and our understanding of the link between temperament, behavioral synchrony, and neural correlates of avoidance and regulation. Future studies can expand on this work by incorporating multiple combinations of dyad pairs (e.g., dyads with two non-inhibited or two inhibited peers). Fourth, the tasks used and the physical co-location of the children prevented us from coding mechanisms, such as joint attention, that likely impact intradyadic synchrony. Finally, the sample was relatively homogenous, fairly healthy, and engaged in only mildly stressful interactions. The patterns of behavior and neural relations noted here may differ across more diverse samples, children with more severe anxiety, or children engaged in highly stressful interactions.
In conclusion, the current study presents a novel approach to capturing individual variation in social behavior in the moment, a temperamental profile that colors social engagement, and underlying neural markers that reflect approach-avoidance motivation and emotion regulation. Uncovering subtle variation in social behavior associated with known risk factors across multiple levels of analysis may help us understand the active and dynamic mechanisms that act as conduits for previously noted broad-scale relations, such as the robust link between early behavioral inhibition and the later emergence of social reticence, withdrawal, and anxiety. This study served as an initial examination of constructs potentially associated with variation in dyadic synchrony. Building on this foundation, subsequent work may examine the consequences of variation in dyadic synchrony--that is, defining what constitutes “too much” or “not enough” synchrony with respect to specific socioemotional and relationship outcomes.
Supplementary Material
Highlights.
We examined behavioral synchrony between behaviorally inhibited children and unfamiliar peers.
EEG Asymmetry and Delta-Beta coupling were associated with synchrony in energy and peer gaze.
Synchronized energy levels and peer gaze varied in unstructured vs cooperative tasks.
Dynamic social behavior reflects dyadic matches in temperament and neural markers.
Acknowledgments:
This research was supported by an NIMH Diversity Supplement (R01 MH109692-02S1) and an NIH Blueprint Diversity Specialized Predoctoral to Postdoctoral Advancement in Neuroscience (D-SPAN) Award (1F99NS120411-01) to BA, an NIMH National Research Service Award (F31 MH121035) to AV, and an NIMH grant (BRAINS R01 MH094633) to KPE.
Footnotes
As part of the larger study, BI children completed a Baseline visit and returned to the lab after four weeks of an attention intervention to complete an Outcome visit. BI children completed the same dyad interaction at each visit, with a different unfamiliar BN peer each time. As a result, we randomly counterbalanced two different cooperation tasks (Lego and Sketch) for the Baseline and Outcome visits to account for any practice effects in the BI peer. As noted, the current study relies solely on data from the baseline visit.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JJB, Coan JA, & Nazarian M (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology, 67(1–2), 183–218. 10.1016/j.biopsycho.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Anaya B, Vallorani AM, & Pérez- Edgar K (2020). Individual dynamics of delta–beta coupling: Using a multilevel framework to examine inter- and intraindividual differences in relation to social anxiety and behavioral inhibition. Journal of Child Psychology and Psychiatry. 10.1111/jcpp.13319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson L, Ram N, & Stifter CA (2018). Using fishery models to examine self- and coregulation processes across multiple timescales. Structural Equation Modeling: A Multidisciplinary Journal, 25(6), 906–923. 10.1080/10705511.2018.1491313 [DOI] [Google Scholar]
- Bishop G, Spence SH, & Mcdonald C (2003). Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Development, 74(6), 1899–1917. 10.1046/j.1467-8624.2003.00645.x [DOI] [PubMed] [Google Scholar]
- Black CL, Goldstein KE, LaBelle DR, Brown CW, Harmon-Jones E, Abramson LY, & Alloy LB (2014). Behavioral approach system sensitivity and risk taking interact to predict left-frontal EEG asymmetry. Behavior Therapy, 45(5), 640–650. 10.1016/j.beth.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin G, Hagekull B, & Andersson K (2005). Inhibition as a precursor of peer social competence in early school age: The interplay with attachment and nonparental care. Merrill-Palmer Quarterly, 51(1), 1–19. [Google Scholar]
- Bornstein MH (2013). Mother-infant attunement: A multilevel approach via body, brain, and behavior. In Legerstee M, Haley DW, & Bornstein MH (Eds.), The infant mind: Origins of the social brain (pp. 266–298). The Guilford Press. [Google Scholar]
- Broeren S, & Muris P (2010). A psychometric evaluation of the behavioral inhibition questionnaire in a non-clinical sample of dutch children and adolescents. Child Psychiatry & Human Development, 41(2), 214–229. 10.1007/s10578-009-0162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, & McDoniel ME (2016). Improving the prediction of risk for anxiety development in temperamentally fearful children. Current Directions in Psychological Science, 25(1), 14–20. 10.1177/0963721415611601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho MC, Quiñones-Camacho LE, & Perlman SB (2020). Does the child brain rest?: An examination and interpretation of resting cognition in developmental cognitive neuroscience. NeuroImage, 212, 116688. 10.1016/j.neuroimage.2020.116688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry, 51(10), 1066–1075.e1. 10.1016/j.jaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology, 40(1), 106–114. 10.1111/1469-8986.00011 [DOI] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67(1–2), 7–50. 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, & McKnight PE (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology, 72(2), 198–207. 10.1016/j.biopsycho.2005.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C, Zapp DJ, Katherine Nelson S, & Pérez-Edgar K (2012). Speech presentation cues moderate frontal EEG asymmetry in socially withdrawn young adults. Brain and Cognition, 78(2), 156–162. 10.1016/j.bandc.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss MM, Shaw DS, & Ingoldsby EM (2003). Mother-son positive synchrony in middle childhood: Relation to antisocial behavior. Social Development, 12(3), 379–400. 10.1111/1467-9507.00239 [DOI] [Google Scholar]
- Davidson RJ (2004). What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biological Psychology, 67(1–2), 219–234. 10.1016/j.biopsycho.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Davis M, Bilms J, & Suveg C (2017). In sync and in control: A meta-analysis of parent-child positive behavioral synchrony and youth self-regulation. Family Process, 56(4), 962–980. 10.1111/famp.12259 [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Vecchio A, & Cirillo G (2020). Resting anxiety increases EEG delta–beta correlation: Relationships with the reinforcement sensitivity theory personality traits. Personality and Individual Differences, 156, 109796. 10.1016/j.paid.2019.109796 [DOI] [Google Scholar]
- Degnan KA, Almas AN, Henderson HA, Hane AA, Walker OL, & Fox NA (2014). Longitudinal trajectories of social reticence with unfamiliar peers across early childhood. Developmental Psychology, 50(10), 2311–2323. 10.1037/a0037751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan KA, & Fox NA (2007). Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology, 19(03), 729. 10.1017/S0954579407000363 [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, & Singer W (2001). Dynamic predictions: Oscillations and synchrony in top–down processing. Nature Reviews Neuroscience, 2(10), 704–716. 10.1038/35094565 [DOI] [PubMed] [Google Scholar]
- Farran DC, & Kasari C (1990). A longitudinal analysis of the development of synchrony in mutual gaze in mother-child dyads. Journal of Applied Developmental Psychology, 11(4), 419–430. 10.1016/0193-3973(90)90018-F [DOI] [Google Scholar]
- Feldman R (2007). Parent–infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science, 16(6), 340–345. 10.1111/j.1467-8721.2007.00532.x [DOI] [Google Scholar]
- Feldman R, Greenbaum CW, & Yirmiya N (1999). Mother–infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology, 35(1), 223–231. 10.1037/0012-1649.35.1.223 [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Long JM, & Stewart S (1995). Frontal activation asymmetry and social competence at four years of age. Child Development, 66(6), 1770. 10.2307/1131909 [DOI] [PubMed] [Google Scholar]
- Fox NA, Snidman N, Haas SA, Degnan KA, & Kagan J (2015). The relations between reactivity at 4 months and behavioral inhibition in the second year: Replication across three independent samples. Infancy, 20(1), 98–114. 10.1111/infa.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, & Pérez-Edgar K (2019). Threat-related attention bias in socioemotional development: A critical review and methodological considerations. Developmental Review, 51, 31–57. 10.1016/j.dr.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K, Kimura M, & Daibo I (2020). Rhythmic features of movement synchrony for bonding individuals in dyadic interaction. Journal of Nonverbal Behavior, 44(1), 173–193. 10.1007/s10919-019-00315-0 [DOI] [Google Scholar]
- Garcia-Coll C, Kagan J, & Reznick JS (1984). Behavioral inhibition in young children. Child Development, 55(3), 1005. 10.2307/1130152 [DOI] [Google Scholar]
- Glenn LE, Keeley LM, Szollos S, Okuno H, Wang X, Rausch E, Deros DE, Karp JN, Qasmieh N, Makol BA, Augenstein TM, Lipton MF, Racz SJ, Scharfstein L, Beidel DC, & De Los Reyes A (2019). Trained observers’ ratings of adolescents’ social anxiety and social skills within controlled, cross-contextual social interactions with unfamiliar peer confederates. Journal of Psychopathology and Behavioral Assessment, 41(1), 1–15. 10.1007/s10862-018-9676-4 [DOI] [Google Scholar]
- Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, Pine DS, Ernst M, Fox NA, & Nelson EE (2014). Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology, 26(01), 229–243. 10.1017/S0954579413000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, & Jarcho JM (2018). Neuroscience and peer relations. In Burkowski WM, Laursen B, & Rubin KH (Eds.), Handbook of Peer Interactions, Relationships, and Groups (2nd ed., pp. 177–199). Guilford Press. [Google Scholar]
- Hane AA, Fox NA, Henderson HA, & Marshall PJ (2008). Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology, 44(5), 1491–1496. 10.1037/a0012855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrewijn A, van der Molen MJW, van Vliet IM, Tissier RLM, & Westenberg PM (2018). Behavioral and EEG responses to social evaluation: A two-generation family study on social anxiety. NeuroImage: Clinical, 17, 549–562. 10.1016/j.nicl.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrewijn A, Van der Molen MJW, & Westenberg PM (2016). Putative EEG measures of social anxiety: Comparing frontal alpha asymmetry and delta–beta cross-frequency correlation. Cognitive, Affective, & Behavioral Neuroscience, 16(6), 1086–1098. 10.3758/s13415-016-0455-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrist AW, & Waugh RM (2002). Dyadic synchrony: Its structure and function in children’s development. Developmental Review, 22(4), 555–592. 10.1016/S0273-2297(02)00500-2 [DOI] [Google Scholar]
- Henderson HA, Fox NA, & Rubin KH (2001). Temperamental contributions to social behavior: The moderating roles of Frontal EEG asymmetry and gender. Journal of the American Academy of Child & Adolescent Psychiatry, 40(1), 68–74. 10.1097/00004583-200101000-00018 [DOI] [PubMed] [Google Scholar]
- Henderson HA, & Wilson MJG (2017). Attention processes underlying risk and resilience in behaviorally inhibited children. Current Behavioral Neuroscience Reports, 4(2), 99–106. 10.1007/s40473-017-0111-z [DOI] [Google Scholar]
- Hollenstein T, Lichtwarck-Aschoff A, & Potworowski G (2013). A model of socioemotional flexibility at three time scales. Emotion Review, 5(4), 397–405. 10.1177/1754073913484181 [DOI] [Google Scholar]
- Jarcho JM, & Guyer AE (2018). The neural mechanisms of behavioral inhibition. In Pérez-Edgar K & Fox NA (Eds.), Behavioral Inhibition (pp. 59–90). Springer International Publishing. 10.1007/978-3-319-98077-5_4 [DOI] [Google Scholar]
- Kagan J, Reznick JS, & Snidman N (1987). The physiology and psychology of behavioral inhibition in children. Child Development, 58(6), 1459. 10.2307/1130685 [DOI] [PubMed] [Google Scholar]
- Kellerman AM, Schwichtenberg AJ, Abu- Zhaya R, Miller M, Young GS, & Ozonoff S (2020). Dyadic synchrony and responsiveness in the first year: Associations with autism risk. Autism Research. 10.1002/aur.2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev GG (2012). EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neuroscience & Biobehavioral Reviews, 36(1), 677–695. 10.1016/j.neubiorev.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Schutter DJLG, & van Honk J (2006). Anxious apprehension increases coupling of delta and beta oscillations. International Journal of Psychophysiology, 61(2), 283–287. 10.1016/j.ijpsycho.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Launay J, Tarr B, & Dunbar RIM (2016). Synchrony as an adaptive mechanism for large-scale human social bonding. Ethology, 122(10), 779–789. 10.1111/eth.12528 [DOI] [Google Scholar]
- Levula A, Harré M, & Wilson A (2018). The association between social network factors with depression and anxiety at different life stages. Community Mental Health Journal, 54(6), 842–854. 10.1007/s10597-017-0195-7 [DOI] [PubMed] [Google Scholar]
- Lewis RS, Weekes NY, & Wang TH (2007). The effect of a naturalistic stressor on frontal EEG asymmetry, stress, and health. Biological Psychology, 75(3), 239–247. 10.1016/j.biopsycho.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Liu P, Taber-Thomas BC, Fu X, & Pérez-Edgar KE (2018). Biobehavioral markers of attention bias modification in temperamental risk for anxiety: A randomized control trial. Journal of the American Academy of Child & Adolescent Psychiatry, 57(2), 103–110. 10.1016/j.jaac.2017.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo FM, & Lunkenheimer E (2020). Understanding the parent-child coregulation patterns shaping child self-regulation. Developmental Psychology, 56(6), 1121–1134. 10.1037/dev0000926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenheimer E, Hamby CM, Lobo FM, Cole PM, & Olson SL (2020). The role of dynamic, dyadic parent–child processes in parental socialization of emotion. Developmental Psychology, 56(3), 566–577. 10.1037/dev0000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenheimer E, Ram N, Skowron EA, & Yin P (2017). Harsh parenting, child behavior problems, and the dynamic coupling of parents’ and children’s positive behaviors. Journal of Family Psychology, 31(6), 689–698. 10.1037/fam0000310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenheimer ES, Olson SL, Hollenstein T, Sameroff AJ, & Winter C (2011). Dyadic flexibility and positive affect in parent–child coregulation and the development of child behavior problems. Development and Psychopathology, 23(2), 577–591. 10.1017/S095457941100006X [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PC, Rynes KN, Aragón C, Caprihan A, Phillips JP, & Lowe JR (2014). Mother–infant mutual eye gaze supports emotion regulation in infancy during the still-face paradigm. Infant Behavior and Development, 37(4), 512–522. 10.1016/j.infbeh.2014.06.008 [DOI] [PubMed] [Google Scholar]
- MacNeill LA, Fu X, Buss KA, & Pérez-Edgar K (in press). Do you see what I mean?: Using mobile eye-tracking to capture parent-child dynamics in the context of anxiety risk. Development and Psychopathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo O, & Gordon I (2020). In and out of synchrony—Behavioral and physiological dynamics of dyadic interpersonal coordination. Psychophysiology, 57(6). 10.1111/psyp.13574 [DOI] [PubMed] [Google Scholar]
- Miskovic V, Moscovitch DA, Santesso DL, McCabe RE, Antony MM, & Schmidt LA (2011). Changes in EEG cross-frequency coupling during cognitive behavioral therapy for social anxiety disorder. Psychological Science, 22(4), 507–516. 10.1177/0956797611400914 [DOI] [PubMed] [Google Scholar]
- Miskovic V, & Schmidt LA (2009). Frontal brain oscillatory coupling among men who vary in salivary testosterone levels. Neuroscience Letters, 464(3), 239–242. 10.1016/j.neulet.2009.08.059 [DOI] [PubMed] [Google Scholar]
- Morales S, Ram N, Buss KA, Cole PM, Helm JL, & Chow S-M (2018). Age-related changes in the dynamics of fear-related regulation in early childhood. Developmental Science, 21(5), e12633. 10.1111/desc.12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K (2018). Attention mechanisms in behavioral inhibition: Exploring and exploiting the environment. In Pérez-Edgar K & Fox NA (Eds.), Behavioral Inhibition (pp. 237–261). Springer International Publishing. 10.1007/978-3-319-98077-5_11 [DOI] [Google Scholar]
- Poole KL, Anaya B, & Pérez-Edgar KE (2020). Behavioral inhibition and EEG delta-beta correlation in early childhood: Comparing a between-subjects and within-subjects approach. Biological Psychology, 149, 107785. 10.1016/j.biopsycho.2019.107785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole KL, & Schmidt LA (2019). Frontal brain delta- beta correlation, salivary cortisol, and social anxiety in children. Journal of Child Psychology and Psychiatry, 60(6), 646–654. 10.1111/jcpp.13016 [DOI] [PubMed] [Google Scholar]
- Poole KL, & Schmidt LA (2020). Positive shyness in the brain: Frontal electroencephalogram alpha asymmetry and delta–beta correlation in children. Child Development. 10.1111/cdev.13379 [DOI] [PubMed] [Google Scholar]
- Quiñones- Camacho LE, Fishburn FA, Camacho MC, Hlutkowsky CO, Huppert TJ, Wakschlag LS, & Perlman SB (2019). Parent–child neural synchrony: A novel approach to elucidating dyadic correlates of preschool irritability. Journal of Child Psychology and Psychiatry. 10.1111/jcpp.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik SJ, & Allen JJB (2018). Frontal asymmetry as a mediator and moderator of emotion: An updated review. Psychophysiology, 55(1), e12965. 10.1111/psyp.12965 [DOI] [PubMed] [Google Scholar]
- Rubin KH, Barstead MG, Smith KA, & Bowker JC (2018). Peer relations and the behaviorally inhibited child. In Pérez-Edgar K & Fox NA (Eds.), Behavioral Inhibition (pp. 157–184). Springer International Publishing. 10.1007/978-3-319-98077-5_8 [DOI] [Google Scholar]
- Rubin KH, Burgess KB, & Hastings PD (2002). Stability and social-behavioral consequences of toddlers’ inhibited temperament and parenting behaviors. Child Development, 73(2), 483–495. 10.1111/1467-8624.00419 [DOI] [PubMed] [Google Scholar]
- Schutter DJLG, & Knyazev GG (2012). Cross-frequency coupling of brain oscillations in studying motivation and emotion. Motivation and Emotion, 36(1), 46–54. 10.1007/s11031-011-9237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simony E, Honey CJ, Chen J, Lositsky O, Yeshurun Y, Wiesel A, & Hasson U (2016). Dynamic reconfiguration of the default mode network during narrative comprehension. Nature Communications, 7(1). 10.1038/ncomms12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunçgenç B, & Cohen E (2018). Interpersonal movement synchrony facilitates pro-social behavior in children’s peer-play. Developmental Science, 21(1), e12505. 10.1111/desc.12505 [DOI] [PubMed] [Google Scholar]
- Vacharkulksemsuk T, & Fredrickson BL (2012). Strangers in sync: Achieving embodied rapport through shared movements. Journal of Experimental Social Psychology, 48(1), 399–402. 10.1016/j.jesp.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass SV, Smith CG, Clackson K, Gibb C, Eitzenberger J, & Mirza FU (2019). Parents mimic and influence their infant’s autonomic state through dynamic affective state matching. Current Biology, 29(14), 2415–2422.e4. 10.1016/j.cub.2019.06.016 [DOI] [PubMed] [Google Scholar]
- Xavier J, Tilmont E, & Bonnot O (2013). Children’s synchrony and rhythmicity in imitation of peers: Toward a developmental model of empathy. Journal of Physiology-Paris, 107(4), 291–297. 10.1016/j.jphysparis.2013.03.012 [DOI] [PubMed] [Google Scholar]
- Zampella CJ, Csumitta KD, Simon E, & Bennetto L (2020). Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. Journal of Autism and Developmental Disorders, 50(9), 3195–3206. 10.1007/s10803-020-04412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.