Key Points
Question
Is the association between parent-reported habitual snoring and cognitive performance in children confounded by pertinent demographic, anthropometric, and socioeconomic variables?
Findings
In this cross-sectional study of 11 873 children aged 9 to 10 years participating in the Adolescent Brain and Cognitive Development study, the association between habitual snoring and cognitive performance was substantially attenuated after adjustments for demographic, anthropometric, and socioeconomic characteristics.
Meaning
The study’s findings suggest that incorporating appropriate adjustments for demographic, anthropometric, and socioeconomic characteristics is warranted when using epidemiologic surveys and clinical guidelines to assess or synthesize data associated with neurobehavioral outcomes among children with habitual snoring.
Abstract
Importance
Previous studies have identified an association between habitual snoring and lower cognitive performance in children. However, whether and to what extent this association is confounded by pertinent demographic, anthropometric, and socioeconomic characteristics is unknown.
Objective
To assess the extent to which potential confounding factors modify the association between parent-reported habitual snoring and cognitive outcomes among a large and diverse sample of typically developing preadolescent children.
Design, Setting, and Participants
This cross-sectional analysis used a baseline data set (version 2.0.1) from children enrolled in the ongoing Adolescent Brain Cognitive Development study between September 1, 2016, and October 15, 2018. Children aged 9 to 10 years without serious psychiatric or neurological comorbidities were recruited at 21 research sites in the US. Study recruitment was designed to approximate the racial and socioeconomic diversity of the US population. Data were analyzed from February 1 to March 31, 2020.
Exposures
Parent-reported habitual snoring in children that occurs 3 or more nights per week.
Main Outcomes and Measures
Associations between habitual snoring and cognitive performance were assessed using the Sleep Disturbance Scale for Children and the National Institutes of Health Toolbox Cognition Battery, which includes 7 domain-specific and 3 composite (total cognitive function, fluid cognition, and crystallized cognition) standard scores that are uncorrected for covariates. Cognitive performance was examined before and after adjustment for covariates, which included age, sex, body mass index percentile, annual household income before taxes, and highest educational level of caregiver. The extent of confounding was assessed by the effect size, represented by Cohen d, before and after inclusion of covariates using linear mixed-effects models.
Results
A total of 11 873 children aged 9 to 10 years (6187 boys [52.1%]; 6174 White [52.0%]) with available data were included in the study. Of those, habitual snoring (≥3 nights per week) was reported in 810 children (6.8%), and nonhabitual snoring (1-2 nights per week) was reported in 4058 children (34.2%). In the unadjusted models, the total cognitive function composite score among children who habitually snored was significantly lower compared with children who never snored (Cohen d, 0.35; 95% CI, 0.28-0.42). Differences were also identified in the crystallized cognition (Cohen d, 0.34; 95% CI, 0.26-0.41) and fluid cognition (Cohen d, 0.28; 95% CI, 0.21-0.35) composite scores. The association between habitual snoring and cognitive performance was substantially attenuated after adjustment for covariates (Cohen d, 0.16 [95% CI, 0.09 to 0.24] for total cognitive function, 0.14 [95% CI, 0.07 to 0.21] for crystallized cognition, and 0.13 [95% CI, 0.06 to 0.21] for fluid cognition). Similar mitigation was also observed for all domain-specific scores.
Conclusions
In this cross-sectional study, when adjusted for baseline demographic, anthropometric, and socioeconomic characteristics, the association between parent-reported habitual snoring and cognitive performance was substantially attenuated among children aged 9 to 10 years.
This cross-sectional study uses a baseline data set from the ongoing Adolescent Brain and Cognitive Development study to examine the extent to which potential confounding factors modify the association between parent-reported habitual snoring and cognitive outcomes among preadolescent children.
Introduction
Snoring is a primary symptom of sleep-disordered breathing in children, with an estimated overall prevalence of 5% to 10%.1 Snoring that occurs more than 2 nights per week is considered habitual.2,3 Most large cross-sectional studies worldwide have identified an association between habitual snoring and decreased cognitive performance in children.2,4,5,6,7,8 Cognitive deficits include attention,4 executive function,9 general intelligence,10 and school performance.11 These observations support the clinical guidelines from the American Academy of Pediatrics, in which parental reporting of snoring is part of the initial evaluation of children with symptoms of upper airway obstruction.12 Furthermore, parental reporting of children’s snoring has been reported to be superior to apnea hypopnea index measures derived from polysomnography for estimating neurobehavioral morbidity.10,13
The most recent American Academy of Pediatrics clinical guidelines for screening of obstructive sleep apnea syndrome, published in 2012, indicate that a discernible proportion of the negative association between sleep-disordered breathing and cognitive outcomes may be confounded, even when measured through polysomnography.12 Supporting data are found in studies of concurrent associations between socioeconomic status and lower cognitive outcomes14 and associations between socioeconomic status and sleep-disordered breathing.15 Most studies of these associations recruited children from urban areas with a higher prevalence of poverty and respiratory disorders, which complicated the assessment of the positive association between sleep-disordered breathing and cognitive performance. The robust control of these potential confounding variables justifies the need for diversity and representativeness in the study sample. Because of the lack of such population-based studies, delineating the presence and extent of confounding in the association between sleep-disordered breathing and lower cognitive performance in children remains challenging.
To address this gap in knowledge, we examined the association between habitual snoring and cognitive outcomes in detail and among a diverse population. Our objective was to assess potential confounders in the association between parent-reported snoring and cognitive performance among children. We hypothesized that the observed association between habitual snoring and lower cognitive performance is confounded by pertinent demographic, anthropometric, and socioeconomic characteristics.
Methods
The Adolescent Brain Cognitive Development (ABCD) study is an ongoing nationwide prospective observational assessment of brain development in children who were recruited at 21 research sites in the US. Children were enrolled at ages 9 to 10 years and are being followed up for 10 years. The rationale and design of the study are described elsewhere.16 The study was approved by the institutional review boards of the University of California, San Diego, and the University of Maryland, Baltimore. All caregivers provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies.
As the largest known investigation of brain development in children, the ABCD study was conceived with the goal of understanding how adverse experiences and exposures affect the developing brain and are associated with cognitive, social, emotional, and academic outcomes. Knowledge from this study may facilitate interventions to mitigate the consequences of childhood stressors.17 Children were included if they were aged 9 to 10 years, attended a public or private elementary school, or were home schooled. Exclusion criteria included lack of fluency in English; the presence of major medical, psychiatric, or neurological disorders; substantial traumatic brain injury; moderate or severe autism spectrum disorder; or current substance use.18
Data Source
The ABCD data set (version 2.0.1) used in the present study includes baseline data from 11 875 children enrolled between September 1, 2016, and October 15, 2018. Study variables included demographic and anthropometric characteristics along with cognitive and parent-reported sleep assessments. The ABCD study was designed to approximate the diversity of the US population with regard to sex, race and ethnicity, and socioeconomic status. The recruitment strategy replicated a multistage probability sample of eligible children derived from a national distribution of the eligible sites, the schools within the vicinity of each site, and the children recruited from those schools. The sample thus provides an unbiased representation of the US population and its major subpopulations by replicating the American Community Survey.19 The sample size ensures adequate power for the detection of small to medium effects for exploratory analyses and accounts for attrition of approximately 10%.20
Study variables in the present study included participant age (in months), sex assigned at birth, and race and ethnicity as reported by the parent at enrollment. In addition, the body mass index (calculated as weight in kilograms divided by height in meters squared) percentile score was calculated from the age- and sex-based growth charts from the Centers for Disease Control and Prevention.21 The combined annual household income before taxes was categorized as low (<$50 000), middle ($50 000-$100 000), and high (>$100 000) by combining the categories specified in the American Community Survey using intervals identical to other ABCD studies.22 The caregiver’s highest educational level was recorded and converted to approximate number of years of education.
Snoring Assessment
The frequency of snoring was assessed in the ABCD study using a portion of the Sleep Disturbance Scale for Children, which is a 27-item clinically validated inventory for the assessment of sleep disorders in children based on symptoms during the preceding 6 months.23 The survey includes a 5-point qualitative assessment of snoring, with 1 indicating no snoring, 2 indicating occasional snoring (1-2 nights per month), 3 indicating occasional snoring (1-2 nights per week), 4 indicating habitual snoring (>2 nights per week but not daily), and 5 indicating daily snoring.
Although the parent scale is not validated against polysomnography, we focused on a Likert-type rating of snoring that was similar to the rating system used in previous large cohorts.2,7,10 Consistent with previous studies,2,3 we grouped parent-reported snoring into no snoring, nonhabitual snoring (1-2 nights per week), and habitual snoring (>2 nights per week).
Cognitive Assessment
Cognitive performance in the ABCD cohort was assessed using the National Institutes of Health Toolbox (NIHTB) Cognition Battery, a readily-available, validated, and computer-based objective assessment of cognitive function in children.24 The NIHTB was developed as part of the NIH Blueprint for Neuroscience Research25 with the goals of (1) being comprehensive, (2) being suitable for longitudinal studies, (3) having relatively brief administration times, and (4) requiring less interaction between the test administrator and the participant.26 The NIHTB was formulated to be psychometrically sound by incorporating elements of consensus building, item response theory, and computerized adaptive testing. The cognitive battery was conceptualized using normative data generated from 5000 participants and has been used in hundreds of studies.26,27
The battery has excellent psychometric properties and includes 7 domain-specific and 3 composite standard scores, which are uncorrected for covariates. In brief, the Flanker Inhibitory Control and Attention Test assesses the inhibition of attention to irrelevant aspects of a task by presenting a central target with flanking stimuli to the left and right. The Dimensional Change Card Sort Test is a measure of cognitive flexibility that involves matching a target stimulus to 1 dimension (eg, shape), then switching to match the target stimulus to another dimension (eg, color). The List Sorting Working Memory Test is designed to assess the reproduction of visually and orally presented test items in order of size. The Picture Sequence Memory Test involves the presentation of a set of pictures in a fixed order accompanied by a verbal description, which the participant must then remember and reproduce. The Oral Reading Recognition Test assesses pronunciation of single words. The Picture Vocabulary Test asks participants to pair pictures with audible descriptions. The Pattern Comparison Processing Speed Test measures speed of processing by asking participants to discern whether 2 side-by-side pictures are the same or different.
In addition to the 7 individual domains, a fluid cognition composite score is derived using scores from 5 test domains (Flanker Inhibitory Control and Attention, Dimensional Change Card Sort, Picture Sequence Memory, List Sorting Working Memory, and Pattern Comparison Processing Speed), and a crystallized cognition composite score is derived using scores from 2 test domains (Picture Vocabulary and Oral Reading Recognition). The total cognitive function composite score is derived using scores from all 7 test domains.
Statistical Analysis
The baseline characteristics were described using numbers and percentages for categorical variables and means with 95% CIs for continuous variables. For the primary hypothesis, we fitted a linear mixed-effects model28 to the NIHTB scores as the dependent variable. A mixed-effects model extends the use of a linear regression analysis to hierarchical data, with variability arising from random factors, such as site of recruitment. The primary outcome was the total cognitive function composite score, and the secondary outcomes included the crystallized cognition and fluid cognition composite scores as well as the 7 domain scores. Missing values were identified and imputed by multivariate imputation using chained equations.29 All dependent variables were assessed for normality.
The association between cognitive outcomes measured by the NIHTB and the frequency of snoring was first assessed using a generalized linear mixed-effects model after incorporation of recruitment site as a random effect. Next, the marginal difference in effect size (Cohen d) between children who snored less than 3 nights per week (nonhabitual) and children who never snored was estimated by calculating the mean effect size over the random effect alone. This marginal effect was recalculated after inclusion of the covariates (age in months, biological sex at birth, race and ethnicity, body mass index percentile, annual household income level, and approximate number of years of caregiver education) in the mixed models. Similar differences were calculated for children who snored 3 or more nights per week, before and after incorporation of covariates, as described.
The linear mixed-effects model incorporated random-effects terms to account for variability arising from the 21 recruitment sites. The model also included the principal fixed effect, snoring frequency, which was categorized as no snoring, nonhabitual snoring (1-2 nights per week), and habitual snoring (≥3 nights per week). The other fixed effects (covariates) included age, biological sex at birth, race and ethnicity, body mass index percentile, annual household income stratified by tier (low, middle, or high), and approximate number of years of caregiver education. The base model comprised the principal fixed effect and the random-effects terms. The covariate-adjusted model included the covariates along with the principal fixed effect and the random-effects terms. The estimated marginal means of the dependent variable for each level of the principal fixed effect were calculated by adjusting for all other terms in the statistical model.
In addition, the effect size associated with the principal fixed effect and the random-effects terms, both before and after inclusion of the covariates, was measured by Cohen d and was categorized as negligible (<0.2), small (0.2-0.4), medium (0.5-0.7), and large (≥0.8).30 Confounding was considered substantial when the unadjusted effect size was mitigated by 15% or more after the addition of covariates.31 All statistical analysis was performed using R software, version 3.6, using the lme4 package (version 1.1) for the mixed-effects models and the emmeans package (version 1.4) for the estimation of marginal effects. Data were analyzed from February 1 to March 31, 2020.
Results
Among 11 875 children with available data, 2 children were excluded because of noncompletion of most of the NIHTB assessments. Habitual snoring was reported in 810 children (6.8%), nonhabitual snoring was reported in 4058 children (34.2%), and no snoring was reported in 7005 children (59.0%) (Table 1). A total of 6187 children (52.1%) were boys, 6174 children (52.0%) were White, and 1956 children (16.5%) were obese; 3222 families (27.1%) had annual household incomes less than $50 000, and 2043 caregivers (17.2%) had no college education.
Table 1. Baseline Characteristics of 11 873 Children Enrolled in the Adolescent Brain Cognitive Development Study.
| Characteristic | Participants, No. (%) |
|---|---|
| Age, mean (95% CI), mo | 118.9 (118.8-119.1) |
| Sexa | |
| Female | 5680 (47.8) |
| Male | 6187 (52.1) |
| Race/ethnicityb | |
| White | 6174 (52.0) |
| Black | 1777 (15.0) |
| Hispanic | 2407 (20.3) |
| Asian | 252 (2.1) |
| Otherc | 1245 (10.5) |
| BMI percentile, mean (95% CI) | 61.0 (60.4-61.5) |
| Obesed | 1956 (16.5) |
| Annual household income before taxes, $e | |
| <50 000 | 3222 (27.1) |
| 50 000-100 000 | 3070 (25.9) |
| >100 000 | 4563 (38.4) |
| Highest educational level of caregiverf | |
| <High school | 785 (6.6) |
| High school diploma or GED | 1258 (10.6) |
| Some college or associate degree | 3486 (29.4) |
| Bachelor’s degree | 3330 (28.0) |
| Postgraduate degree | 2994 (25.2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GED, general education development.
Data were missing for 6 participants.
Data were missing for 12 participants.
Specific races and ethnicities included in this category were not reported in the data set.
Obesity was defined as BMI higher than the 95th percentile.
Data were missing for 1012 participants.
Data were missing for 14 participants.
The Figure illustrates the comparison of NIHTB scores between children grouped by frequency of snoring. The unadjusted scores in Figure, A show the estimated marginal means from linear mixed-effects models, with snoring frequency as the principal fixed effect and recruitment site as a random effect. Children who snored 3 or more nights per week had lower cognitive test scores in 6 of 8 domain-specific scores and in all 3 composite scores. For example, the mean total cognitive function score was 83.8 (95% CI, 82.6-84.9) among children who snored at least 3 nights per week compared with 86.3 (95% CI, 85.7-86.9) among children who did not snore. The adjusted scores in Figure, B were derived from mixed-effects models that included snoring as well as other fixed-effect covariates (age, sex, race, body mass index percentile, highest educational level of caregiver, and annual household income), with recruitment site as a random effect. These models indicated that the differences between children grouped by snoring frequency were consistently mitigated by the inclusion of the pertinent demographic and socioeconomic variables.
Figure. Comparison of Unadjusted and Adjusted Models for the Association Between Parent-Reported Frequency of Snoring and Cognitive Test Scores Among Children Aged 9 to 10 Years.
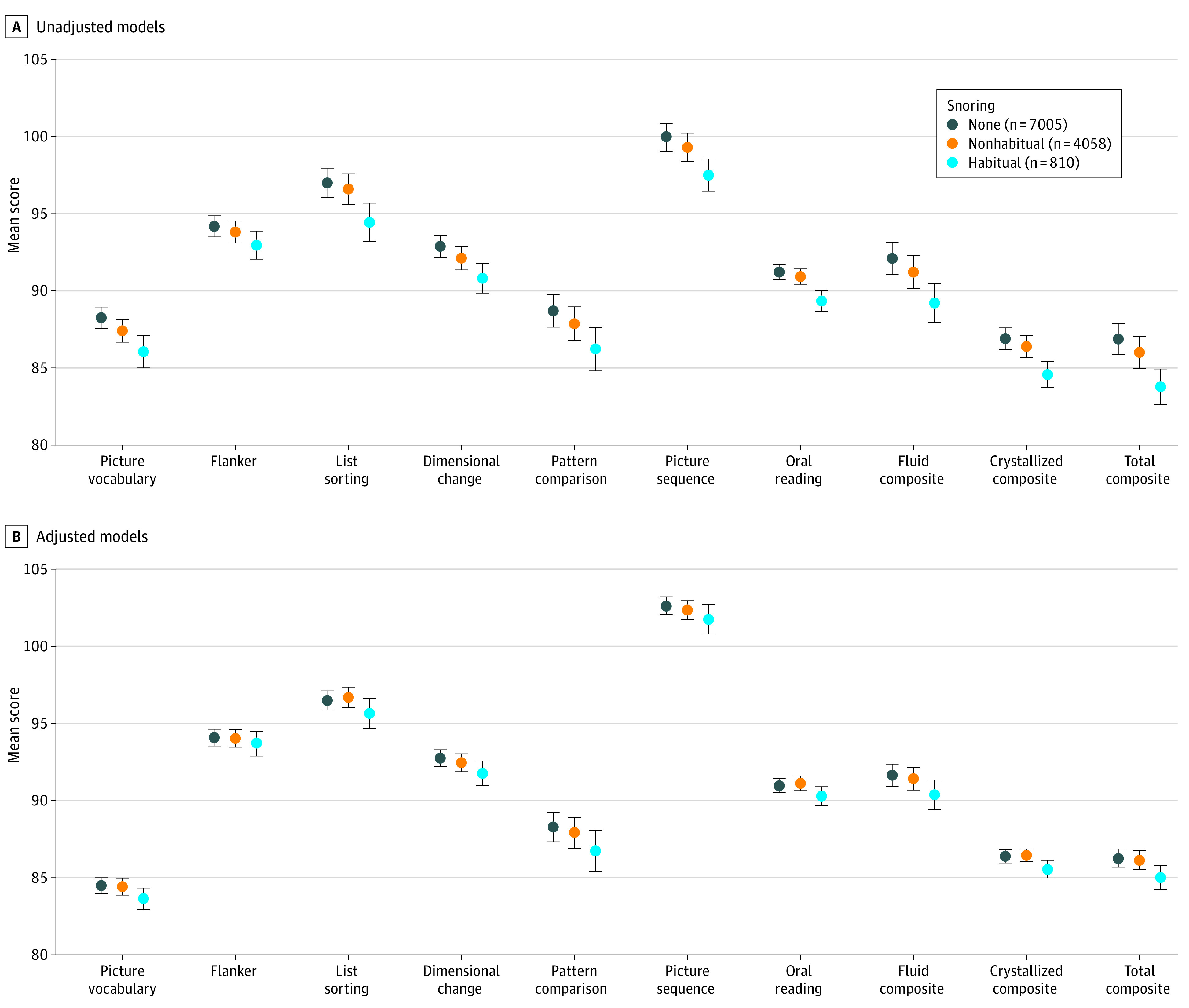
Estimated marginal mean scores of cognitive tests included in the National Institutes of Health Toolbox Cognitive Battery. Habitual snoring represents children who snore 3 or more nights per week, and nonhabitual snoring represents children who snore 1 to 2 nights per week. All outcome measures were derived from linear mixed-effects models. Span of vertical shading represents 95% CIs. Flanker indicates Flanker Inhibitory Control and Attention Test, which measures inhibitory control and attention. A, Unadjusted models. Models include only the principal fixed effect (snoring frequency), with recruitment site as a random effect. B, Adjusted models. Models include age, sex, race, body mass index (calculated as weight in kilograms divided by height in meters squared) percentile, highest educational level of caregiver, and annual household income, with recruitment site as a random effect.
Table 2 presents the group differences shown in the Figure, using Cohen d as a standardized effect size measure. The differences between children with nonhabitual snoring (1-2 nights per week) and children with no snoring were negligible both before adjustment (Cohen d for total cognitive function, 0.09; 95% CI, 0.06-0.13) and after adjustment (Cohen d for total cognitive function, 0.02; 95% CI, −0.03 to 0.06). The greatest difference was observed among children with habitual snoring (>2 nights per week) vs no snoring, with a Cohen d of 0.35 (95% CI, 0.28-0.42) for total cognitive function. Differences were also identified in crystallized cognition (Cohen d, 0.34; 95% CI, 0.26-0.41) and fluid cognition (Cohen d, 0.28; 95% CI, 0.21-0.35). The association between habitual snoring and cognitive performance was substantially attenuated after adjustment for covariates (Cohen d, 0.16 [95% CI, 0.09 to 0.24] for total cognitive function, 0.14 [95% CI, 0.07 to 0.21] for crystallized cognition, and 0.13 [95% CI, 0.06 to 0.21] for fluid cognition). Similar mitigation was also observed for all domain-specific scores.
Table 2. Differences in Cognitive Outcomes Among Children With Nonhabitual and Habitual Snoring vs Children Without Snoring Before and After Covariate Adjustment.
| NIHTB Cognition Battery outcome | Cohen d (95% CI) | |||
|---|---|---|---|---|
| Nonhabitual snoring vs no snoring | Habitual snoring vs no snoring | |||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Picture vocabulary | 0.08 (0.04 to 0.12) | 0.01 (−0.03 to 0.05) | 0.31 (0.23 to 0.38) | 0.13 (0.05 to 0.20) |
| Flanker inhibitory control and attention | 0.04 (0.00 to 0.08) | 0.01 (−0.04 to 0.04) | 0.14 (0.06 to 0.21) | 0.04 (−0.03 to 0.12) |
| List sorting working memory | 0.03 (−0.01 to 0.07) | −0.02 (−0.05 to 0.03) | 0.21 (0.14 to 0.28) | 0.07 (0.00 to 0.15) |
| Dimensional change card sort | 0.08 (0.04 to 0.12) | 0.03 (−0.01 to 0.07) | 0.22 (0.14 to 0.29) | 0.11 (0.03 to 0.18) |
| Pattern comparison processing speed | 0.06 (0.02 to 0.10) | 0.03 (−0.01 to 0.07) | 0.17 (0.10 to 0.24) | 0.11 (0.04 to 0.19) |
| Picture sequence memory | 0.07 (0.03 to 0.11) | 0.03 (−0.01 to 0.06) | 0.18 (0.11 to 0.26) | 0.08 (0.00 to 0.15) |
| Oral reading recognition | 0.04 (0.01 to 0.08) | −0.02 (−0.06 to 0.02) | 0.28 (0.20 to 0.35) | 0.11 (0.03 to 0.18) |
| Fluid cognition composite | 0.09 (0.05 to 0.12) | 0.02 (−0.02 to 0.06) | 0.28 (0.21 to 0.35) | 0.13 (0.06 to 0.21) |
| Crystallized cognition composite | 0.07 (0.03 to 0.11) | −0.01 (−0.04 to 0.04) | 0.33 (0.26 to 0.41) | 0.14 (0.07 to 0.21) |
| Total cognitive function composite | 0.09 (0.06 to 0.13) | 0.02 (−0.03 to 0.06) | 0.35 (0.28 to 0.42) | 0.16 (0.09 to 0.24) |
Abbreviation: NIHTB, National Institutes of Health Toolbox.
The differences between each adjusted and unadjusted effect size exceeded the 15% threshold for confounding effects. Seven of 10 NIHTB measures had small effect sizes, as measured by Cohen d. However, after adjustment, all group differences were negligible. Similar mitigation was also identified among children grouped by any snoring vs no snoring. No significant interactions were found between socioeconomic or demographic variables, and the association between snoring and cognition indicated that the extent of effect modification was similar for the different levels of socioeconomic status and the different categories of demographic variables.
Discussion
Previous studies of neurocognitive outcomes that were based on parental surveys of sleep-disordered breathing in children have indicated that children who habitually snore have substantially lower levels of cognitive performance compared with children who never snore.10,32,33,34 Results from the current study indicate that this association is confounded by their pertinent demographic, anthropometric, and socioeconomic characteristics. Notably, after adjustment for these characteristics, the association between parent-reported snoring and cognitive performance was negligible. In this study, we reported population-based data that necessitated the inclusion of appropriate demographic, anthropometric, and socioeconomic characteristics to accurately assess neurobehavioral outcomes among children with sleep-disordered breathing.
Habitual snoring has been reported to have negative associations with multiple facets of cognitive performance, including intelligence,9 memory,4 language and visuospatial performance,6 and academic performance.35,36 In 1 study, the difference in cognitive performance between children who habitually snored and those who never snored was almost 20%.5 These associations support guidelines recommending that children with snoring be referred for polysomnographic evaluation and adenotonsillectomy as the first line of clinical management for severe sleep-disordered breathing, such as breathing associated with substantial sleep disruption, lower quality of life, or behavioral problems.37 The role of habitual snoring in the evaluation and management of sleep-disordered breathing is further reinforced by studies reporting more significant associations between parent-reported habitual snoring and adverse cognitive outcomes compared with studies using the apnea hypopnea index derived from polysomnography.10,13
In the current study, the prevalence of habitual snoring was 6.8%, which is comparable to the estimated prevalence of 7.5% reported in a meta-analysis of 41 articles.1 The differences in cognitive scores between children who habitually snore vs those who never snore are smaller than those of other studies.6,13 These differences could be explained by the previous studies’ inclusion of less racially or geographically diverse participants, substantially smaller samples, and the use of polysomnography to quantify upper airway obstruction. Although the extent of the confounding effects observed in the present study is not directly comparable with findings from previous studies, the range of effect sizes is similar to the range observed in the behavioral outcomes and quality of life measures reported in rigorous clinical trials for the treatment of sleep-disordered breathing, such as the Childhood Adenotonsillectomy Trial.38
A 2012 technical report by the American Academy of Pediatrics identified a number of studies that evaluated an association between habitual snoring and lower cognitive performance in children, although causal associations were not established.12 Therefore, our findings need to be interpreted in the context of 2 types of previous studies: those using polysomnographic categories of sleep-disordered breathing and those using community-based parental surveys of symptoms. The first category of studies typically includes substantially smaller samples comprising children from a single site. The polysomnographic thresholds vary widely, with some studies using home polysomnographic testing to quantify the severity of obstruction. For example, Kennedy et al5 described a 20% difference between the primary neurocognitive outcome scores among 26 children grouped by the presence of snoring. Similar studies of 118 children by O’Brien et al6 and 205 children by Gottlieb et al9 reported large effect sizes.
However, even among studies that used polysomnography, the data regarding this association continues to be inconsistent. For example, Bourke et al39 studied 137 children and found that those categorized into various phenotypes of sleep-disordered breathing, based on polysomnographic results, indicated similarly impaired cognitive performance across subgroups. Brockmann et al36 reported similar findings among 114 children who habitually snored, with no substantial differences found between the phenotypes. Notably, 2 studies10,13 indicated that parent-reported habitual snoring is a more accurate measure for estimating adverse cognitive outcomes than polysomnographic thresholds. Several of the reported associations between snoring and cognitive outcomes were either not adjusted32,40 or only partially adjusted4,35 for family socioeconomic characteristics using other surrogate measures.
In contrast, population-based surveys have significantly more statistical power, although surveys are limited in the reliability and validity of the outcome measures used for cognitive performance and sleep-disordered breathing because they rely on parental reporting.8 Although the current study used well-validated cognitive assessments, it shares the disadvantages of previous survey studies, in which bias because of subjective parental reporting was a concern, and polysomnography was rendered impractical by large study samples.2,3
Our results extend the findings from previous smaller studies investigating the association of confounding factors, such as socioeconomic status, with standardized cognitive test results and school performance among children with sleep-disordered breathing. Children with lower socioeconomic status exhibit a greater frequency of sleep disturbances41 and lower scores on cognitive tests.14 Although causal inferences require prospective follow-up data, some putative confounders may be more pertinent than others. For example, both race and lower socioeconomic status were the most important factors associated with sleep-disordered breathing in a study of 146 children in elementary school.42 The association between socioeconomic status and snoring has been reported previously in both US and non-US populations,15,43,44 suggesting a potential causal association between household income and atopy, airway inflammation, and obesity, with subsequent increases in airflow resistance and snoring. Early childhood experiences are also augmented by higher household income, producing better outcomes within the domains of language, self-regulation, and working memory.45
Strengths and Limitations
This study has several strengths. The study has a wide geographic distribution for recruitment and a large sample. To our knowledge, the ABCD study is the largest study of children in which snoring history and objective cognitive assessments were collected. In the ABCD study, cognitive assessments were performed using the NIHTB rather than instruments such as the Neuropsychological Assessment (NEPSY), which was used in the Childhood Adenotonsillectomy Trial (CHAT).38 Although both instruments are designed to assess executive function, the use of the NIHTB within the domain of sleep-disordered breathing is novel, as it was developed after the CHAT was conducted. There are no fundamental differences between these 2 test batteries, with the exception of the NIHTB’s inclusion of a larger validation sample and more numerous cognitive test domains that assess all potential facets of cognitive dysfunction described in the literature.46 In the present study, the narrow range of enrollment age reduced heterogeneity, which is advantageous for assessing cognitive outcomes across subgroups; however, these findings may not be generalized to other age groups because cognitive deficits have also been reported in younger children with sleep-disordered breathing, including habitual snoring.47 Other studies have also reported that age did not moderate the association between snoring and cognition10 and that snoring was not associated with cognitive deficits in preschool children.48
The study also has several limitations. The principal weakness is the study’s cross-sectional design, which limits the ability to make causal inferences. Potential bias could occur from parental overreporting of sleep-associated problems among children with academic difficulties. Although habitual snoring has been reported to be a sensitive, albeit less specific, variable for estimating the presence of conditions such as obstructive sleep apnea, polysomnography could potentially have corroborated parental reporting and stratified the severity of obstruction. However, the use of parent-reported symptoms, such as snoring, reflects the approach used in most large cohorts worldwide, as the use of polysomnographic assessments among a large number of children (such as the sample included in this study) is impractical because of the costs involved and the resources needed.2,3,7,36,44 In addition, the results of the present study cannot be generalized to other outcomes that may be associated with sleep-disordered breathing, such as disease-specific, overall quality of life, daytime sleepiness, behavioral, and cardiovascular outcomes.
Conclusions
The study’s results indicate that, when controlled for baseline demographic and socioeconomic characteristics, the association between parent-reported habitual snoring and cognitive performance among children is negligible. The extent of the confounding effects highlights the importance of ensuring that representative samples are enrolled in studies of outcomes that are concurrently associated with socioeconomic status and sleep-disordered breathing. Strategies to mitigate unrecognized confounding factors include multicenter recruitment to enhance diversity, adequate sample size, and more reliable estimates of socioeconomic status or hardship. Future avenues of research include similar investigations of confounding variables in a younger population, inclusion of polysomnography or nocturnal oximetry to quantify the severity of sleep-disordered breathing, and assessment of potential confounding factors associated with other outcomes, such as problem behaviors.
References
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242-252. doi: 10.1513/pats.200708-135MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S, Jin X, Yan C, Wu S, Jiang F, Shen X. Habitual snoring in school-aged children: environmental and biological predictors. Respir Res. 2010;11(1):144. doi: 10.1186/1465-9921-11-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li AM, Au CT, So HK, Lau J, Ng PC, Wing YK. Prevalence and risk factors of habitual snoring in primary school children. Chest. 2010;138(3):519-527. doi: 10.1378/chest.09-1926 [DOI] [PubMed] [Google Scholar]
- 4.Blunden S, Lushington K, Kennedy D, Martin J, Dawson D. Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol. 2000;22(5):554-568. doi: 10.1076/1380-3395(200010)22:5;1-9;FT554 [DOI] [PubMed] [Google Scholar]
- 5.Kennedy JD, Blunden S, Hirte C, et al. Reduced neurocognition in children who snore. Pediatr Pulmonol. 2004;37(4):330-337. doi: 10.1002/ppul.10453 [DOI] [PubMed] [Google Scholar]
- 6.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44-49. doi: 10.1542/peds.114.1.44 [DOI] [PubMed] [Google Scholar]
- 7.Petry C, Pereira MU, Pitrez PMC, Jones MH, Stein RT. The prevalence of symptoms of sleep-disordered breathing in Brazilian schoolchildren. J Pediatr (Rio J). 2008;84(2):123-129. doi: 10.1590/S0021-75572008000200006 [DOI] [PubMed] [Google Scholar]
- 8.Galland B, Spruyt K, Dawes P, McDowall PS, Elder D, Schaughency E. Sleep disordered breathing and academic performance: a meta-analysis. Pediatrics. 2015;136(4):e934-e946. doi: 10.1542/peds.2015-1677 [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145(4):458-464. doi: 10.1016/j.jpeds.2004.05.039 [DOI] [PubMed] [Google Scholar]
- 10.Smith DL, Gozal D, Hunter SJ, Kheirandish-Gozal L. Frequency of snoring, rather than apnea-hypopnea index, predicts both cognitive and behavioral problems in young children. Sleep Med. 2017;34:170-178. doi: 10.1016/j.sleep.2017.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding R, Haszard JJ, Schaughency E, Drummond B, Galland B. Parent report of children’s sleep disordered breathing symptoms and limited academic progress in reading, writing, and math. Sleep Med. 2020;65:105-112. doi: 10.1016/j.sleep.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 12.Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576-584. doi: 10.1542/peds.2012-1671 [DOI] [PubMed] [Google Scholar]
- 13.Suratt PM, Peruggia M, D’Andrea L, et al. Cognitive function and behavior of children with adenotonsillar hypertrophy suspected of having obstructive sleep-disordered breathing. Pediatrics. 2006;118(3):e771-e781. doi: 10.1542/peds.2006-0173 [DOI] [PubMed] [Google Scholar]
- 14.Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773-778. doi: 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149(3):342-347. doi: 10.1016/j.jpeds.2006.04.061 [DOI] [PubMed] [Google Scholar]
- 16.Barch DM, Albaugh MD, Avenevoli S, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Dev Cogn Neurosci. 2018;32:55-66. doi: 10.1016/j.dcn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman EA, Clark DB, Orendain N, Hudziak J, Squeglia LM, Dowling GJ. Stress exposures, neurodevelopment and health measures in the ABCD study. Neurobiol Stress. 2019;10:100157. doi: 10.1016/j.ynstr.2019.100157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelini G, Barch DM, Tian Y, Watson D, Klein DN, Kotov R. Delineating and validating higher-order dimensions of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study. Transl Psychiatry. 2019;9(1):261. doi: 10.1038/s41398-019-0593-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heeringa SG, West BT, Berglund PA. Applied Survey Data Analysis. 2nd ed. CRC Press; 2017. Chapman & Hall/Statistics in the Social and Behavioral Sciences Series. [Google Scholar]
- 20.Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci. 2018;32:16-22. doi: 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1-27. [PubMed] [Google Scholar]
- 22.Marshall AT, Betts S, Kan EC, McConnell R, Lanphear BP, Sowell ER. Association of lead-exposure risk and family income with childhood brain outcomes. Nat Med. 2020;26(1):91-97. doi: 10.1038/s41591-019-0713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5(4):251-261. doi: 10.1111/j.1365-2869.1996.00251.x [DOI] [PubMed] [Google Scholar]
- 24.Weintraub S, Bauer PJ, Zelazo PD, et al. I. NIH Toolbox Cognition Battery (CB): introduction and pediatric data. Monogr Soc Res Child Dev. 2013;78(4):1-15. doi: 10.1111/mono.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NIH Blueprint for Neuroscience Research. National Institutes of Health. Accessed September 2020. https://neuroscienceblueprint.nih.gov
- 26.Luciana M, Bjork JM, Nagel BJ, et al. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018;32:67-79. doi: 10.1016/j.dcn.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akshoomoff N, Newman E, Thompson WK, et al. The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING). Neuropsychology. 2014;28(1):1-10. doi: 10.1037/neu0000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelman A, Hill J.. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; 2006. doi: 10.1017/CBO9780511790942 [DOI] [Google Scholar]
- 29.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40-49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 31.Vetter TR, Mascha EJ. Bias, confounding, and interaction: lions and tigers, and bears, oh my! Anesth Analg. 2017;125(3):1042-1048. doi: 10.1213/ANE.0000000000002332 [DOI] [PubMed] [Google Scholar]
- 32.Urschitz MS, Eitner S, Guenther A, et al. Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics. 2004;114(4):1041-1048. doi: 10.1542/peds.2003-1145-L [DOI] [PubMed] [Google Scholar]
- 33.Urschitz MS, Guenther A, Eggebrecht E, et al. Snoring, intermittent hypoxia and academic performance in primary school children. Am J Respir Crit Care Med. 2003;168(4):464-468. doi: 10.1164/rccm.200212-1397OC [DOI] [PubMed] [Google Scholar]
- 34.Brockmann PE, Bertrand P, Pardo T, Cerda J, Reyes B, Holmgren NL. Prevalence of habitual snoring and associated neurocognitive consequences among Chilean school aged children. Int J Pediatr Otorhinolaryngol. 2012;76(9):1327-1331. doi: 10.1016/j.ijporl.2012.05.028 [DOI] [PubMed] [Google Scholar]
- 35.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3 Pt 1):616-620. doi: 10.1542/peds.102.3.616 [DOI] [PubMed] [Google Scholar]
- 36.Brockmann PE, Urschitz MS, Schlaud M, Poets CF. Primary snoring in school children: prevalence and neurocognitive impairments. Sleep Breath. 2012;16(1):23-29. doi: 10.1007/s11325-011-0480-6 [DOI] [PubMed] [Google Scholar]
- 37.Mitchell RB, Archer SM, Ishman SL, et al. Clinical practice guideline: tonsillectomy in children (update). Otolaryngol Head Neck Surg. 2019;160(1_suppl)(suppl 1):S1-S42. doi: 10.1177/0194599818801757 [DOI] [PubMed] [Google Scholar]
- 38.Marcus CL, Moore RH, Rosen CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366-2376. doi: 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourke R, Anderson V, Yang JSC, et al. Cognitive and academic functions are impaired in children with all severities of sleep-disordered breathing. Sleep Med. 2011;12(5):489-496. doi: 10.1016/j.sleep.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 40.Goodwin JL, Kaemingk KL, Fregosi RF, et al. Clinical outcomes associated with sleep-disordered breathing in Caucasian and Hispanic children—the Tucson Children’s Assessment of Sleep Apnea study (TuCASA). Sleep. 2003;26(5):587-591. doi: 10.1093/sleep/26.5.587 [DOI] [PubMed] [Google Scholar]
- 41.Pagel JF, Forister N, Kwiatkowki C. Adolescent sleep disturbance and school performance: the confounding variable of socioeconomics. J Clin Sleep Med. 2007;3(1):19-23. [PubMed] [Google Scholar]
- 42.Chervin RD, Clarke DF, Huffman JL, et al. School performance, race, and other correlates of sleep-disordered breathing in children. Sleep Med. 2003;4(1):21-27. doi: 10.1016/s1389-9457(02)00243-5 [DOI] [PubMed] [Google Scholar]
- 43.Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. J Pediatr. 2011;158(5):789-795. doi: 10.1016/j.jpeds.2010.10.036 [DOI] [PubMed] [Google Scholar]
- 44.Urschitz MS, Guenther A, Eitner S, et al. Risk factors and natural history of habitual snoring. Chest. 2004;126(3):790-800. doi: 10.1378/chest.126.3.790 [DOI] [PubMed] [Google Scholar]
- 45.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651-659. doi: 10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115-1134. doi: 10.1093/sleep/29.9.1115 [DOI] [PubMed] [Google Scholar]
- 47.Beebe DW, Rausch J, Byars KC, Lanphear B, Yolton K. Persistent snoring in preschool children: predictors and behavioral and developmental correlates. Pediatrics. 2012;130(3):382-389. doi: 10.1542/peds.2012-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackman AR, Biggs SN, Walter LM, et al. Sleep-disordered breathing in preschool children is associated with behavioral, but not cognitive, impairments. Sleep Med. 2012;13(6):621-631. doi: 10.1016/j.sleep.2012.01.013 [DOI] [PubMed] [Google Scholar]


