Abstract
The disappointing results in bench-to-bedside translation of neuroprotective strategies caused a certain shift in stroke research towards enhancing the endogenous recovery potential of the brain. One reason for this focus on recovery is the much wider time window for therapeutic interventions which is open for at least several months. Since recently two large clinical studies using d-amphetamine or fluoxetine, respectively, to enhance post-stroke neurological outcome failed again it is a good time for a critical reflection on principles and requirements for stroke recovery science. In principal, stroke recovery science deals with all events from the molecular up to the functional and behavioral level occurring after brain ischemia eventually ending up with any measurable improvement of various clinical parameters. A detailed knowledge of the spontaneously occurring post-ischemic regeneration processes is the indispensable prerequisite for any therapeutic approaches aiming to modify these responses to enhance post-stroke recovery. This review will briefly illuminate the molecular mechanisms of post-ischemic regeneration and the principle possibilities to foster post-stroke recovery. In this context, recent translational approaches are analyzed. Finally, the principal and specific requirements and pitfalls in stroke recovery research as well as potential explanations for translational failures will be discussed.
Keywords: chronic stroke, combination therapy, recovery, regeneration, translation
Molecular basis of post-ischemic regeneration
Immediately after ischemic stroke a multitude of genes in the brain but also in peripheral organs are induced. This post-ischemic genetic response comprises both the activation of protective and adverse pathways with respect to the final functional outcome. The situation is complicated by the fact that timing of these events plays a critical role since the same gene product may exert protective effects in the acute phase but hinder regenerative effects in the subacute and chronic phase. For example, in experimental models matrix metalloproteinase-9 (MMP-9) has been shown to cause blood-brain barrier damage in the first days after stroke1,2 but later on it promotes angiogenesis and functional recovery.3 MMP-9 is even indispensable for physiological plastic processes like hippocampal late-phase long-term potentiation and memory.4 Similarly, in human stroke patients higher serum levels of MMP-9 are thought to be a predictor of worse outcome whereas at later time points MMP-9 levels could be positively correlated with clinical recovery.5 The effects of MMP9 in the context of stroke become more complicated since tPA treatment additionaly elevates MMP9 levels and increases the risk of hemorrhagic transformation but on the other side it may potentially enhance regenerative processes in the long run (for an extensive review on matrix metalloproteinases and stroke see Rosell and Lo).6 However, knowledge on the detailed temporal sequencing of MMP9 effects beyond the acute stroke phase is fragmentary yet. Nevertheless, delayed treatment with a virus mediated hypoxia-controlled MMP9 hyperexpression starting one week after transient middle cerebral artery occlusion (MCO) in mice demonstrated enhanced behavioral and structural recovery.3,7 These two papers also highlight the need for a common definition of post stroke phases, since the two different terms “subacute” and “chronic” are used for the same postischemic timepoint of one week. The major players for successful post-stroke recovery induced by the ischemic event are neurogenesis, axonal sprouting, dendritic branching, synaptogenesis, oligodendrogenesis, angiogenesis, inflammation, neurotransmitter receptor regulation and white matter remodeling. About a quarter-century ago the post-ischemic regulation of single genes or gene families has been investigated to elucidate the molecular mechanisms of post-stroke pathophysiology.8,9 With the development of the microarray technology allowing a high throughput analysis of gene expression the transcriptional post-ischemic response or the post-ischemic “transcriptome” could be determined comprehensively.10–12 In the last decade the deciphering of the orchestration of the genomic response by epigenetic mechanisms like DNA-methylation, histone deacetylation, histone methylation and non-coding RNAs has become a major focus with promising translational options.13–16
Principle possibilities to foster post-stroke recovery
The rationale of all therapeutic interventions to improve post-stroke recovery is to either enhance spontaneously occurring regenerative processes and/or to eliminate or at least reduce processes impeding regeneration. Briefly, the following principal approaches are available (in more detailed discussed in recent reviews).17,18 The idea of cell-based strategies is to replace injured brain tissue by various stem or precursor cells thought to differentiate into functional brain cells. However, the main effect seems to be an indirect one e.g. the production of growth factors which promote survival and regeneration of preexisting brain cells.19,20 Many attempts have been undertaken both in models of experimental stroke and in humans with different pharmacological and bioactive substances. All the various components of the regenerative processes have been used as targets for a pharmacological intervention. Nearly any sort of growth factors has been tested with promising results in preclinical models. In particular the multimodal and overlapping effects of the various growth factors have generated high expectations concerning their regenerative potential.21 Amphetamine is one of the most prominent and long known examples from the group of neuromodulators and neuroenhancers in particular in combination with neurorehabilitative training paradigms.22 Great expectations were placed in the serotonin reuptake inhibitor fluoxetine23 which however could not hold up its promises in a recently published large clinical trial.24 Another principal possibility to eliminate recovery hindering processes is the targeted use of antibodies. Blockade of the growth-inhibiting activity of Nogo-A by a monoclonal antibody is one of the most developed approaches in this field.25 Interestingly, besides a boosting effect with sprouting of new fibers, Nogo-A blockade also promotes angiogenesis and vascular repair after stroke.26 Targeting noncoding RNAs may play a central role in the near future in stroke recovery. One of the advantages of this method is a multifaceted impact on all components of the regenerative machinery.27,28 The decryption of the epigenetic regulation thus potentially allowing epigenetic reprogramming of the genetic machinery including regeneration enhancers will become a major task for the next years and decades to potentially do a significant step towards successful regenerative therapy of stroke-damaged tissue (for overview, see Ref.29). Direct support of recovery processes can be achieved by inserting biomaterials such as hydrogels or living scaffolds which promote a permissive environment and/or may be used to deliver cells or bioactive molecules.30
Apart from these approaches where any substances or molecules are applicated targeted to directly interfere at the transcriptional or translational level of regenerative pathways other therapeutic strategies are aimed to indirectly enhance brain repair. In this category fall the various classic neurorehabilitative training paradigms which can be subdivided into voluntary vs. forced training with constraint-induced movement therapy as the most prominent representative.31,32 Enriched environment is an additional therapeutic strategy frequently combined with other approaches which exerts positive effects by improving many components of endogenous regeneration.33 Fascinating upcoming approaches are brain-computer interfaces and the use of artificial intelligence.34,35 Finally, there is a multitude of data on non-invasive brain stimulation modulating cortical excitability and thereby improving regeneration.36 Reasoned combination of these therapy principles covered above is thought to further enhance regenerative processes.17
Examples for translation of therapies
Pharmacological
One of the classical examples for post-stroke recovery enhancement represents the use of neurotransmitters or neurotransmitter modulating drugs. Particularly dopamine was studied early in stroke patients due to its clinical availability and its potency to improve motor function in other neurological diseases. Consequently, a classic and early dopamine trial reported 100 mg levodopa to be superior compared to physiotherapy alone with regard to enhancement of motor function in chronic stroke patients.37 These findings were confirmed in a smaller study, where 3 doses of 100 mg dopamine treatment combined to procedural motor learning of the paretic hand clearly improved motor function compared to placebo.38 Interestingly, experimental studies investigating the efficacy of dopamine with regards to enhancement of post-stroke recovery are quite rare, and translation can be interpreted as backward from clinical to bench. Indeed, in these studies dopamine was found to improve the recovery of sensorimotor function after transient occlusion of the middle cerebral artery without affecting the infarct volume.39,40 Due to this combined clinical and preclinical evidence dopamine was used off label in several rehabilitation units to enhance post-stroke motor dysfunction dependent on patients individual deficits and needs. Evidence was challenged again in a recent, randomized, large controlled trial of a 6-week continuous dopamine treatment (100 mg levodopa+12.5 mg carbidopa) in addition to standard physical and occupational therapy within 6 weeks after the index event.41 The results with respect to the relatively crude study endpoint “independent walking ability at 8 weeks” was negative, no surprise at all considering the mixture of stroke and deficit subtypes included in the study: almost 25% lacunar strokes, a variety of anterior circulation strokes, posterior circulation strokes, and even up to 17% of hemorrhages.
Another monoaminergic drug, the neuromodulator d-amphetamine, was combined to different modes of physical rehabilitation.42 Animals treated with amphetamine 10 min before motor training on day 2, 5 and 8 post-stroke and intensive rehabilitative training performed significantly better than any other treatment group (control environment, enriched environment) achieving complete motor recovery by 8 weeks post-stroke. These findings were translated into primates: using a cortical infarction model in the squirrel monkey a single dose of d-amphetamine on the first day of training initiated 10 days after stroke onset facilitated the rate of recovery and improved performance almost 3-fold compared monkeys treated with saline.43 With this positive experience from experimental studies, amphetamine was investigated in several smaller clinical studies. A Cochrane review including ten studies involving 287 patients reported inconclusive results with some indication for benefits on motor function.44 The largest clinical trial with 64 stroke patients treated with dextroamphetamine (10 mg) revealed no benefit on recovery of motor function compared with placebo. The study population consisted of a mixture of moderate to severe clinical syndromes in patients with different stroke subtypes (subcortical, cortical, brainstem). At least, treatment in this study was combined with a 1-hour physical therapy session beginning 1 hour after drug or placebo administration every 4 days for 6 sessions in addition to standard rehabilitation. Surprisingly, the study was performed between 2001 and 2003, analyzed 2015 and published 2018.45
The serotonin reuptake inhibitors (SSRI) such as fluoxetine were investigated early with respect to their capability to enhance poststroke recovery. These experimental studies show quite convincingly, that fluoxetine combined to physical training failed to promote sensorimotor recovery poststroke,46,47 although a few newer studies showed positive effects on motor recovery.48 Interestingly, treatment with fluoxetine in rodents enhanced neurogenesis and improved cognitive function post-stroke.49,50 How complex the situation indeed is shows a recent experimental study suggesting that fluoxetine can overcome the gradient of diminished responsiveness to motor training over the first week after stroke and maintain maximal levels of responsiveness to training even 7 days after stroke.51 In a randomized, controlled trial in 118 subacute stroke patients, fluoxetine showed surprisingly a significantly better motor outcome measured with the Fugl-Meyer motor scale (almost 45% improval) and exhibited less depressive symptoms compared to patients treated with standard physiotherapy alone.23 Cognitive function was not investigated. This study raised, however, eligible hope that fluoxetine treatment might be a promising candidate for stroke recovery enhancement. Unfortunately, the large randomized FOCUS trial showed in 3127 patients no difference in functional outcome between fluoxetine (20 mg per day for 6 months) and placebo measured by the relatively crude distribution of the modified Ranking Scale at 6 months.24 Although fluoxetine treatment reduced the occurrence of depression, it increased the frequency of bone fractures largely due to falls. Problematic with this large study is again the broad spectrum of stroke subtypes and clinical syndromes including patients with severe deficits and large territorial infarctions as well as patients with mild symptoms and lacunar infactions. These negative findings were very recently supported by two other large fluoxetine trials, the “Efficacy oF Fluoxetine randomisEd Controlled Trial in Stroke” (EFFECTS)52 and the “Assessment oF FluoxetINe In sTroke recovery” (AFFINITY)53 trial. Both nearly identical trials suffer from the above discussed methodological issues (again the inappropriate primary endpoint modified Ranking Scale), and support the finding of reduced poststroke depressions for the price of significantly increased bone fractures. These data are supported by a recent Cochrane review where no reliable evidence could be found for SSRIs enhancing poststroke recovery.54
Growth factors such as BDNF, GDNF, IGF, bFGF, EPO or G-CSF represent ideal candidates to promote poststroke recovery due to their natural biological function of regulation and control of neuronal growth and differentiation. While the effect on stroke recovery enhancement was demonstrated for numerous factors in various experimental models and paradigms, clinical efficacy could not be demonstrated so far. In cases where growth factor treatment was translated into stroke patients, therapy resulted either in increased intracranial bleeding rates and an increased mortality (EPO stroke trial)55 or was simply ineffective (AXIS-II G-CSF trial).56 Of all growth factors, only G-CSF was tested in the stroke recovery phase. In a pilot study a 10-day G-CSF treatment was compared to placebo in 41 chronic stroke patients undergoing a training paradigm of hand motor function and verbal learning.57 The study proofed feasibility, but failed to show significant benefits of the G-CSF treatment. A quite similar approach was recently replicated in a blinded 2x2 factorial design study, where G-CSF was applied (5 doses subcutaneously) together with moderate physical training (18 home based therapy sessions) and compared to no treatment or no training.58 This study failed again to show a significant treatment effect of the growth factor, although a transient improval of motor function at 90 days could be observed, which disappeared at the end of the observation period (1 year). The study suffered from small group sizes (n=13–17), and a too long time window for treatment initiation (about 1 year). In contrast to direct growth factor application, novel developments focus on the secretion of growth factors in addition to chemokines and cytokines by mesenchymal stem cells (MSCs) directly or by MSC derived exosomes. The advantage of this strategy is clearly the release of an array of different recovery inducing substances physiologically secreted by MSCs or MSC derived exosomes, which were shown in several experimental studies to induce and enhance poststroke recovery.59,60
Physical training
Another approach to enhance postroke recovery includes neurorehabilitative training paradigms. Training strategies can be in principle subdivided in task specific paradigms and unspecific ones such as general physical exercise or rehabilitative physiotherapy. Standard physiotherapy post-stroke either administered in rehabilitative units or in subsequent training session represents the current therapeutic standard. Although efficacy was not demonstrated in larger RCT, numerous smaller trials suggest a benefit of physical rehabilitation. Summarized evidence from these studies suggests physical rehabilitation to be more effective than usual care in improving motor function (12 studies, 887 participants), balance (five studies, 246 participants) and gait velocity (14 studies, 1126 participants).61 Interestingly, there might even be an effect on dose of intervention, indicating that a dose of 30 to 60 minutes delivered five to seven days a week provides significant benefit compared to less intensive training. This finding correlates to experimental data indicating that intensity might be a key point, providing the basis for better long-term functional outcome compared to low intensity of voluntary training modalities.31 One important example for such a controlled intensive training is the forced and specific training paradigm constraint induced movement therapy (CIMT), were the non-impaired extremity (typically the arm) is immobilized to foster mobility and training of the paretic arm. This approach was studied early in animals and has been successfully translated into patients, where constraint-induced therapy was associated with significant gains in motor outcome in 222 patients treated in the time window 3 to 9 months after stroke onset.62,63 Interestingly, parallel to animals,64–67 high doses of training too early after stroke proofed to be harmful: in the Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS) trial higher treatment intensity by CIMT applied within 1 month of stroke onset was associated with poorer motor outcome at 90 days.68 Certainly, the great advantage of CIMT and its translational success is based on the fact, that only patients with very specific syndromes (moderate paresis of the arm), limited infarction in the respective motor area and an a priori defined readout such as motor function of the arm measured with the Action Research Arm test and Wolf Motor Function test qualify for this therapy.
Basic requirements of recovery studies
Time window
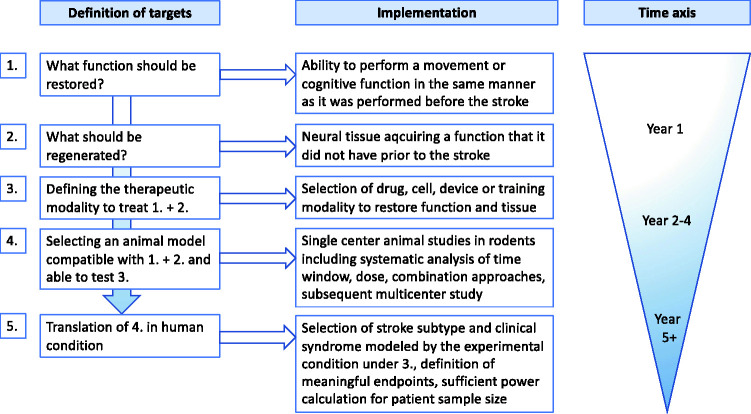
While the time window for recovery enhancing therapies might be open in animals up to 6 weeks, in humans up to 6 months and potentially longer,69 optimal timing appears to be crucial. Recent publications clearly show, early intensive training such as CIMT can impair functional outcome when initiated within minutes to hours after onset of cerebral ischemia both in rodents and humans.64–68 Importantly, such an early and intensive impulse on post-stroke recovery mechanisms impairs long-term functional outcome 6 weeks after cortical stroke independent of infarct evolution and final lesion volume.64,65 Clearly, the optimum time may be missed if the starting point is too late.70 Interestingly, comprehensive experimental studies investigating the time window for neurorehabilitative therapies are very rare, in fact none of the translated therapies exhibits conclusive time window studies (Table 1). The situation in patients is even more complex due to the different etiology, size and location of infarctions as well as severity and heterogeneity of clinical syndromes. Indeed, no formal evidence is available in any of the translated rehabilitative drug therapies comparing different time windows of efficacy in humans (Table 1). Nevertheless, putting together current knowledge of post-stroke events Bernhardt et al.71 suggested a framework of critical timepoints post stroke defining the temporal terms hyper-acute (0–24 hours), acute (1–7 days), early subacute (7 days–3 months), late subacute (3–6 months) and chronic (>6 months). This is a first step and considering these various phases together with neuroimaging biomarkers will potentially increase translational success.72 In times of precision medicine defining post stroke periods by specific blood or genetic biomarkers which may indicate the most promising therapy should be the ultimate goal. This may be achieved in the future by aligning “timing of putative predictive biomarker levels with phases of physical recovery”73 and with evaluation of therapeutic response. Comparing the biomarker levels between rodents and humans may eventually allow an adaption of the framework of post stroke recovery periods from mice to men and vice versa (Figure 1).
Table 1.
Reasons for translational failure.
| Experimental | Clinical |
|---|---|
| Time window | Time window |
| – Too late vs. too early | – Too late vs. too early |
| – Too short observation time points | – Too large range |
| No exploration of most effective therapeutic dose | No exploration of most effective therapeutic dose |
| Heterogenous stroke models – Different species – Intraluminal suture MCAO model – Craniectomy models – transient vs. permanent – Photothrombosis model – Endothelin-1 model – Embolic stroke models: – Thrombembolic clot models vs. microsphere/macrosphere stroke models – Spontaneous stroke models – Modeling comorbidity in stroke models |
Heterogenous stroke types – Lacunar – Cortical – Brainstem – Ischemic vs. hemorrhagic |
| Ignorance of the effects of aging and comorbidities | Mostly aged patients with comorbidities |
| Depending on the experimental models variability in the location of the ischemic lesion with respective variability in the neurological impairment; high degree of spontaneous recovery within a few weeks | Heterogeneous clinical syndromes of variable
severity – Hemiparesis motor, sensorimotor – Monoparesis arm, leg – Additional cognitive or neuropsychological deficits such as neglect or executive dysfunction |
| Multiple different testing methods with substantial variations impeding comparability | Multiple different measures resulting in impaired comparability; lack of defined endpoints both concerning function and timing |
| Lack of discrimination between recovery vs. compensation | Lack of discrimination between recovery vs. compensation |
| Undefined therapeutic combinations | Undefined therapeutic combinations |
| – Sequential vs. concurrent | – Sequential vs. concurrent |
| Preclinical study design | Clinical trial design |
| – Small and underpowered vs. large with the respective ethical issues | – Small and underpowered vs. large and heterogenous trials |
| – Lack of correct controls |
Figure 1.
Algorithm of targeted interventions for development of a regenerative therapy. Definition of targets (1-5) should precede interventions. The process should be developed as integrative whole box approach from the definition of the targeted functional deficit to the translation of experimental findings into the human situation. In the latter one, a selected human condition should be defined and carefully selected for translation.
Optimal dosage
Currently, knowledge of the optimal dosage which is defined by frequency, duration and intensity of the respective therapy, is more or less based on try and error approaches. From the literature available it becomes clear, that there exists a dosage maximum which by going beyond will result in less benefit. This holds true not only for pharmacological treatment but has also been convincingly demonstrated for rehabilitative therapy (Table 1). In the VECTORS study stroke patients with high-intensity CIMT therapy did worse compared to the standard CIMT group and even compared to a standard treatment control group.68 Theoretically infinite combinations are possible by varying the single parameters of the dosage of each single therapy. Furthermore, this variation is multiplied when combining priming and consolidating therapy (see below). In the future, stroke researchers should more involve biostatisticians and mathematicians to develop combination treatments with maximum benefit on a rational basis. However, also for single therapies such approaches are exceptionally rare.74
Combinations
A major challenge is the combination of therapeutic approaches with the goal to potentiate beneficial effects, achieve synergistic effects and avoid neutralizing or even worsening ones. In principle, combination treatments are possible among all potential modalities e.g. synergistically acting drug combinations including bioactive molecules and antibodies, stem cell grafting or other biomaterials plus drug treatment. The available post-stroke recovery approaches can be distinguished in “priming” and “consolidating” therapies.17 All therapies targeted to enhance spontaneously occurring recovery such as neurogenesis, angiogenesis, axonal sprouting, dendritic remodeling or synaptogenesis can be considered as “priming”. Therapies intended to exercise newly formed connections including all forms of training thereby stabilizing and strengthen them whereas non-used become pruned and eventually disappear would be classified as “consolidating”. Importantly, “consolidating” therapies can be combined with a pharmacotherapy e.g. with neuromodulators or neuro-enhancers to strengthen learning effects. All “priming” therapies build the biological basis for consolidation, may be started as early as possible after stroke and would be followed by “consolidating” approaches.17 Indeed, the most common approach represents the combination of a drug treatment to a specific rehabilitative training. Although this has been practiced in numerous experimental and clinical studies, direct evidence for superiority of this approach is scarce (for detailed review of single studies see Ref.17). For example, a sequential therapy with first neutralization of the growth inhibitory molecule Nogo-A for 2 weeks (“priming”) followed by subsequent skilled training paradigm (“consolidation”) resulted in nearly complete recovery while concurrent therapies failed.25 Importantly, the concurrent treatment group performed worse than all other groups due to hyper-innervation and aberrant sprouting.25 These findings correlate to combination studies with the growth factor G-CSF (“priming”) and forced training (CIMT, “consolidation”) in concurrent and sequential manner.75 While all combinations with G-CSF resulted in improved sensorimotor outcome, CIMT alone did not. Importantly, starting with CIMT followed by G-CSF failed to enhance recovery.75
Specific requirements and pitfalls in recovery studies
Principal problems of rodent models for stroke recovery
Recovery studies in animal models of stroke fall into two categories. In the first group, the spontaneous regeneration processes are characterized in a temporo-spatial manner to understand the biology underlying post-stroke brain plasticity. Manipulating these processes by knocking out some components of the repair machinery genetically, pharmacologically or functionally and correlating the resulting changes with the functional outcome is performed to get hints to the causal players in the recovery process. These experiments will be able to identify and characterize general principles and mechanisms of brain regeneration which may also play a role in humans. The results are also the basis for rational planning of therapeutic interventions aimed to enhance positive effects and inhibit adverse ones which can be tested in the respective animal models of stroke which represent the second group of stroke recovery studies. Major problems arise when trying to translate principally successful therapeutic approaches in rodents 1:1 into the patient. There are many publications problemizing the transfer of results from animals and especially from rodents into humans (for review see e.g. Ref.76,77). Differences concerning genetic and epigenetic background, size and anatomy of the brain, cerebral vascular anatomy, immune system, function and behavior are so fundamental that the translational road block is actually not really surprising.76 In light of the well-known deficiencies of rodent models of ischemic stroke and post-stroke recovery the use of non-human primate models may provide more relevant information. However, there is no such thing as a free lunch. The more close the experimental animal to the human situation the higher the arising ethical issues (for review, c.f. Ref.78).
Beside these principal issues which are reflected by nearly a complete lack of successful translation in post-stroke recovery79 there are some specific requirements and pitfalls which will be briefly discussed.
Poorly correlative studies
As mentioned above the great majority of data is purely correlative and only minor attempts have been performed to demonstrate a causal link between plastic remodeling and functional outcomes (for review see Ref.80). Nevertheless, there are sophisticated neuroimaging techniques to examine brain-wide remodeling, regional reorganization, neuronal circuit formation and activity. Furthermore, techniques to manipulate neuronal circuits and techniques to combine anatomical changes with molecular profiling to understand the causal underlying molecular changes are available.80,81
Long-term investigations and accompanying pitfalls in experimental studies
Apart from elegant experiments and the augmentation of scientific knowledge of the precise molecular mechanisms of regenerative post-stroke events, from the clinical perspective the only thing that matters for stroke patients is the amount of regained functional, cognitive as well as psychological and emotional health. Although these various parameters develop at different speeds all need a substantial amount of time. Concerning animal experiments the need for long-term investigations of the different factors is imperative for drawing any viable conclusions. Most data is published on the functional motor recovery. One principal problem with long-term investigations of post-stroke functional improvement in rodent models of ischemic stroke is that mice and rats show a rapid and frequently complete spontaneous recovery within a few weeks even after severe ischemia (Table 1).82,83 A battery of various functional tests has been analyzed with respect to their power to detect subtle deficits also in the long run. The situation is complicated by the fact that most tests for mice are just adaptions of tests primarily developed for rats. Selected tests ideal for testing of mice post stroke have been thoroughly reviewed by Balkaya et al.84 Using a mouse model of mild transient focal ischemia resulting in predominantly subcortical damage Balkaya et al.82 systematically compared established testing methods and introduced some novel ones focusing on motor recovery. The adhesive removal test which allows detection of somatosensory deficits was able to detect differences up to 4 weeks.82 In contrast, another systematic study using a mild focal ischemia model in rats did not show any differences between sham and ischemia groups at one or two months with this test.85 In a mouse model of 90 min MCAO only the corner test was able to significantly discriminate between ischemic and non-ischemic mice, both male and female.83 Finally, some tests such as the cylinder test rely on the natural exploratory behavior of animals, which sometimes loose interest overtime thus making the test worthless.85 Obviously, testing methods show substantial variation between studies impeding comparability (Table 1). Furthermore, there are already significant differences in behavioral deficits and patterns of recovery among different rat strains despite of the same ischemic model.86 These examples reveal the large amount of heterogeneity. Therefore, standardizing testing paradigms as well as time points, duration and endpoint of measurement procedures will be a central task in stroke recovery research to enable comparability between preclinical studies (Table 1; Figure 1). Similar to the efforts in acute neuroprotective stroke research international, randomized, blinded, and multicenter study design would be a promising approach.87 An interesting and controversially discussed approach would be submission of a research project with a detailed experimental plan for publication in a journal.88 These “pre-registered” studies are thought to reduce the bias for positive results and would be an important step in the stroke recovery field.
There are some specific problems with “milder” ischemia models. In animals with smaller ischemic lesions, lesion size and functional outcome frequently do not correlate.75,82 Lack of correlation between lesion size and behavioral deficits was also seen in two permanent MCAO models in the rat.89 Instead it is important to measure the volume of the remaining not directly damaged brain tissue.90,91 Shanina et al.92 could demonstrate that quantification of the remaining brain tissue is superior to quantification of the total lesion size and represents a better predictor of functional impairment. Additionally, detecting subtle changes requires large group sizes, which deserves a thorough group size calculation. On the other hand, large group sizes although necessary for obtaining significant valid results may become an ethical problem (Table 1).
Imaging the spontaneous or therapy-induced post stroke regeneration processes longitudinally and correlating the results with neurobehavioral tests is another challenge in stroke recovery science. In principle, functional magnetic resonance imaging (fMRI) would be ideal for testing and analyzing the same animal overtime. However, due to the need of anesthesia several groups of animals at individual time points are frequently used leading to a high variability between time points.93 Apart from the problem with anesthesia, resting-state fMRI technique is a step forward both towards reduction of variability in animals and improving the comparability between preclinical research and clinical studies due to reduced differences in the methodology used.81 Other noninvasive in vivo techniques such as multi-parametric Optical Coherence Tomography (OCT) platforms allow for longitudinal imaging of ischemic stroke in mice both in the acute and chronic phase and may increase our understanding of recovery mechanisms.94 In a rat photothrombotic stroke model with a chronic optical window, optical coherence tomography has been shown to allow 3D mapping of cerebral blood flow and cellular scattering offering an elegant technique to longitudinally evaluate treatment effects.95 In a study with non-human primates, longitudinal evaluation of size and location of the ischemic lesion by T2 scans up to 30 days post transient MCAO showed a strong correlation between lesion volume and lesion localization to NHPSS scores.96 This underlines the importance of correlating the exact lesion topography in the long run to more precisely predict the final behavioral outcome.
Longitudinal evaluation and endpoints in clinical studies
A general problem in clinical recovery trials is the uncertainty about endpoints and length of observation of the therapeutic effect (Table 1). In the acute stroke situation such key readouts are also not backed by primary evidence, but general agreement in the field build up over time resulting in the perception that an excellent or good functional outcome measured with the modified Rankin Scale is adequate when assessed 3 months after the stroke onset. These endpoints indeed proofed as measures for assessing and comparing the efficacy of recanalizing therapies.97,98 In the recovery field no such measures exist, nor is there an established agreement what kind of improvement would be functional meaningful. Clearly, a stroke patient would benefit from improvement of hand motor function restoring daily activities like writing or unbuttoning a shirt. Such functions were, however, not investigated in the recent large randomized trials. In the dopamine trial (DARS) assessment was focused on walking ability 8 weeks after stroke,41 and in the fluoxetine trial (FOCUS) gross functional outcome was measured by the modified Ranking Scale 6 months after stroke.24 The latter endpoint is even more incomprehensible with respect to the prior FLAME trial, where the fluoxetine treatment effect was assessed and effective on arm and leg motor function measured with the Fugl-Meyer Motor Scale 3 months after the stroke.23 As indicated from these studies, the time interval to endpoint assessment is also variable and ranges from 2 – 6 months to even longer time points of 12 or 24 months assessed in previous studies.58,63
Altogether, these data suggest a lack of basic requirements in clinical recovery studies in defining a functionally meaningful endpoint and the appropriate timing of that endpoint assessment (Table 1).
Recovery versus compensation
Another important issue is the unprecise use of the term recovery. In the landmark paper by Levin et al.99 clear definitions have been formulated to avoid any misunderstandings. At the neuronal level recovery is defined as “restoring function in neural tissue that was initially lost after injury”99 whereas compensation means “neural tissue acquires a function that it did not have prior to injury”.99 At the performance level recovery is defined as “restoring the ability to perform a movement in the same manner as it was performed before injury”99 whereas compensation means “performing an old movement in a new manner.”99 And at the functional level recovery is defined as “successful task accomplishment using limbs or end effectors typically used by nondisabled individuals”99 whereas compensation means “successful task accomplishment using alternate limbs or end effectors.”99 Taking this into consideration it is clear that most behavioral tests used are not able to reliably differentiate between true motor recovery and compensation (for thorough review c.f. Ref.100). Only kinematic analyses,101,102 automized tests84,103 or tests in combination with kinematic analyses are able to discriminate compensation.104 Apart from these problems many testing paradigms do no test for clinically relevant deficits or the tests are simply the wrong one for the respective ischemic lesion. Choosing the best experimental ischemia model for the respective scientific question seems to be a matter of course (Table 1). However, many experimental stroke studies suffer from carelessness concerning the choice of the experimental model.
Apart from the discrimination between true recovery and compensation differentiation between spontaneous and therapy-induced recovery is similarly difficult. In particular, in longitudinal investigations with multiple testing, the test procedure per se may act as an environmental enrichment or an interventional neurorehabilitative effect thus enhancing neurological outcome (for review see Ref.105). Provided the real therapy is an add-on effect, using the adequate control groups would allow a clear distinction.
Proportional recovery
It was well known for long that the majority of stroke patients develop some degree of spontaneous neurological recovery.106 A systematic analysis of patients with moderate post stroke hemiparesis revealed that most of them regained about 70% of their motor function of the upper limb within 3 months after stroke as assessed with Fugl-Meyer scale.107 This phenomenon of the so called proportional recovery has some important implications, since in the first 3 months after stroke obviously neurorehabilitative therapies have little or no effect.108 Whether this proportional recovery rule really holds true for other neurological systems is under discussion.109 On the other hand, a subset of patients with severe impairment does not recover at all and thus does not fit to this rule.107 Here, damage of the corticospinal tract seems to be causative for lack of spontaneous recovery.110 Nevertheless, based on these findings the urgent obvious goal would be to find prognostic and predictive biomarkers that allow discriminating between recoverers and non-recoverers early after stroke enabling a “more effective triage and stratification for neurorehabilitation.”108 However, since practically all patients with stroke obtain some form of neurorehabilitative treatment, it is very difficult to discriminate.
This proportional recovery rule has completely ignored for long in experimental models of stroke and thus may be a further component responsible for the lack of successful translation from mice to men. Most importantly, “preclinical models allow withholding of post-stroke rehabilitation, enabling the investigation of rehabilitation efficacy and the relationship between rehabilitative treatment and proportional recovery.”111 In a systematic retrospective analysis of a cohort of 593 male Sprague-Dawley rats, Jeffers et al.112 demonstrated that the proportional recovery holds also true for rats to a similar degree. Additionally, as for human stroke patients with severe impairments a subset of rats did not fit to the recovery rule.112 Importantly, depending on the intensity of training treatments a subset of non-fitters benefited from therapy demonstrating that rehabilitation in fact plays a role for post-stroke recovery. Development of an algorithm was able to calculate the dose of rehabilitation necessary for each rat and thus translating this concept into the clinic may be a promising approach to a personalized stroke therapy.112 Interesting and clinically so far not addressed is the finding, that periods of heightened responsiveness by a small cortical stroke itself but also by pharmacostimulation (Fluoxetine, see “III. Examples for translation of therapies, Pharmacological”, SSRIs) can enhance the response to training and mediate full recovery.51,113
Effects of aging
As for preclinical neuroprotection studies the effect of aging is frequently not investigated in recovery studies despite the fact the stroke mainly afflicts the elderly. Ignorance of this central issue may be one important factor for the frustrating lack of translational success. Preclinical studies in aged rodents on long-term post-stroke effects concerning structural and functional outcome are even more rare due to the higher mortality of aged stroke animals. There is accumulating evidence that the genomic post-stroke events differ substantially between young and aged individuals eventually resulting in impaired post-stroke structural regeneration and functional recovery in older rodents compared to younger ones (for review c.f. Ref.114). Changes of the immune response with a shift towards a proinflammatory state and alterations of microglial differentiation and function are thought to play a key role for the reduced regenerative capacity of older brains.115–117 There is even evidence for changes in the gut microbiota of aged mice followed by increased levels of systemic proinflammatory cytokines resulting in worse outcome after stroke.118 Not so surprisingly, there is proof that not only the endogenous regenerative capacity of the aged brain is reduced but also the response to various treatments when compared to younger animals. For example, post-stroke treatment with omega-3 polyunsaturated fatty acids after distal MCAO in 18 months vs. 2.5–3 months old mice attenuated brain damage and enhanced regenerative processes as well as sensorimotor function in both ages but aged mice benefitted less from this therapy.119 A more thrilling question arises in the context of cell-based stroke therapy whether the age of the donor cells may also have an influence on the therapeutic result. Yamaguchi et al.120 could demonstrate that age of the donor cell indeed matters. Transplantation of young vs. old human mesenchymal stem cells in a rat model of transient MCAO resulted in enhanced neurological outcome associated with increased production of trophic factors as well as enhanced anti-inflammatory effects, vessel maturation and neurogenesis.120 Similarly, using an experimental mouse model of heterochronic bone marrow chimeras, Ritzel et al.121 could show that aged animals reconstituted with young marrow developed reduced behavioral deficits compared to isochronic controls.
Finally, modelling risk factors and comorbidity such as diabetes mellitus, hypertension, atherosclerosis, hyperlipidemia, obesity or infection is of outmost importance to identify and disclose their impact on post-stroke recovery.76,122
Peripheral effects
Although it is not surprising that stroke recovery research focuses on the post-ischemic changes in the brain itself the importance of the peripheral aspect may have been underestimated yet and may contribute to the disappointing results in human stroke therapy. It has been shown that stroke induces substantial and prolonged changes in peripheral organs such as heart, kidney, liver, spleen or the microbiome of the gut which indirectly may modulate recovery processes.123,124
Conclusion and future directions
Actually, very ambitious goals have been formulated in the recovery field. The stroke recovery and rehabilitation roundtable (SRRR) taskforce e.g. claimed for a “radical new aim” for restitution and brain repair.71 In this context the first thrombolysis trials are recalled to strongly motivate the stroke community. However, while it was relatively clear that any way to open the occluded vessel would be a promising approach a similar clear and defined target is not in sight for rehabilitation. The idea of neural tissue engineering to find “an effective curative, rather than symptomatic, therapy”77 or reprogramming of the genome/epigenome to rebuild the brain125 ignores one major problem. Regeneration of brain tissue per se may not be sufficient to regain the pre-stroke situation since there is also more or less substantial loss of information depending on the exact localization of the ischemic brain loss. It is a little bit the same dilemma as in Alzheimer’s disease where the idea that removing beta-amyloid would enhance cognitive state failed since too much neurons had already been gone.126
Another major problem in stroke recovery research is a substantial incongruity between preclinical and clinical studies concerning the methods available to analyze the recovery mechanisms (Table 1). In particular the cellular level of regeneration processes in humans is largely a black box. Building up large stroke brain banks similar as we know it for neurodegenerative disorders would be a large step to be able to link findings from preclinical models to the human situation. “Better alignment of preclinical studies to clinical realities or constraints should be a priority for the field and funding bodies” as formulated by the Second Stroke Recovery and Rehabilitation Roundtable127 may cover only one side of the medal. Even if well and thoroughly designed, preclinical and here in particular rodent models have their inherent specifics and limitations. The unquestioned power of preclinical stroke research is the elucidation of general endogenous regeneration processes and the possibility to look for checkpoint events by manipulating spontaneous recovery. Therefore, these experiments should not be pushed in the background.
Nevertheless, we must not fall in nihilism. Choosing the right model at the preclinical level and designing clinical trials strictly focusing of nearly identical stroke cases concerning size and location may improve the success rate calling for a shift of our recovery therapy concepts to a more mechanistically and personalized approach (Figure 1). This process should be developed as integrative whole box approach from the definition of the targeted functional deficit to the translation of experimental findings into the human situation - which must be defined and carefully selected for translation (see suggested algorithm, Figure 1). That would mean that recovery approaches would strictly focus on lesion size and location similar as in trauma and reconstructive surgery, where a femoral neck fracture is treated differently than a fracture of the tibial plateau. Taking all these points into consideration will hopefully break through the translational roadblock in the stroke recovery field.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CJS and WRS are inventors on the patent application “Hematopoietic factors for treatment of neurological condition” including stroke and other diseases. Recently a part of the application (ALS) was granted. CJS and WRS transferred their rights to Sygnis and received a minor financial compensation upfront. In case of efficacy CJS and WRS participate in form of royalties. CJS and WRS neither hold nor receive stocks of Sygnis nor have direct interest in the company. WRS received compensation in form of honoraria in his function as PI of the AXIS I study as indicated in this particular publication.128
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; SO 908/3-1, SCH 787/5-1).
ORCID iD: Wolf-Rüdiger Schäbitz https://orcid.org/0000-0001-6333-1075
References
- 1.Bauer AT, Bürgers HF, Rabie T, et al. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab 2010; 30: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Leng Y, Tsai LK, et al. Valproic acid attentuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 2011; 31: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai H, Ma Y, Jiang L, et al. Hypoxia response element-regulated MMP-9 promotes neurological recovery via glial scar degradation and angiogenesis in delayed stroke. Mol Ther 2017; 25: 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagy V, Bozdagi O, Matynia A, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci 2006; 26: 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelnaseer MM, Elfauomy NM, Esmail EH, et al. Matrix metalloproteinase-9 and recovery of acute ischemic stroke. J Stroke Cerebrovasc Dis 2017; 26: 733–740. [DOI] [PubMed] [Google Scholar]
- 6.Rosell A, Lo EH.Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol 2008; 8: 82–89. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Mu Z, Jiang Z, et al. Hypoxia-controlled matrix metalloproteinase-9 hyperexpression promotes behavioral recovery after ischemia. Neurosci Bull 2015; 31: 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak TS, Jr, Jacewicz M.The heat shock/stress response in focal cerebral ischemia. Brain Pathol 1994; 4: 67–76. [DOI] [PubMed] [Google Scholar]
- 9.Kiessling M, Gass P.Stimulus-transcription coupling in focal cerebral ischemia. Brain Pathol 1994; 4: 77–83. [DOI] [PubMed] [Google Scholar]
- 10.Geschwind DH.DNA microarrays: translation of the genome from laboratory to clinic. Lancet Neurol 2003; 2: 275–282. [DOI] [PubMed] [Google Scholar]
- 11.VanGilder RL, Huber JD, Rosen CL, et al. The transcriptome of cerebral ischemia. Brain Res Bull 2012; 88: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Qiu Z, Gao S, et al. Intergrated analysis of expression profile based on differentially expressed genes in middle cerebral artery occlusion animal models. Int J Mol Sci 2016; 17: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweizer S, Meisel A, Märschenz S.Epigenetic mechanims in cerebral ischemia. J Cereb Blood Flow Metab 2013; 33: 1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z, Zhong B, Tan J, et al. The emerging role of epigenetics in cerebral ischemia. Mol Neurobiol 2017; 54: 1887–1905. [DOI] [PubMed] [Google Scholar]
- 15.Kassis H, Shehadah A, Chopp M, et al. Epigenetics in stroke recovery. Genes 2017; 8: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heydari E, Alishahi M, Ghaedrahmati F, et al. The role of non-coding RNAs in neuroprotection and angiogenesis following ischemic stroke. Metab Brain Dis 2020; 35: 31–43. [DOI] [PubMed] [Google Scholar]
- 17.Sommer CJ, Schäbitz W-R.Fostering poststroke recovery. Towards combination treatments. Stroke 2017; 48: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 18.Lin DJ, Finklestein SP, Cramer SC.New directions in treatments targeting stroke recovery. Stroke 2018; 49: 3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janowski M, Wagner DC, Boltze J.Stem cell-based tissue replacement after stroke: factual necessity of notorious fiction? Stroke 2015; 46: 2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surugiu R, Olaru A, Hermann DM, et al. Recent advances in mono- and combined stem cell therapies in animal models and humans. Int J Mol Sci 2019; 20: E6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bath PM, Sprigg N, England T.Colony stimulating factors (including erythropoietin, granulocyte colony stimulatin factor and analogues) for stroke. Cochrane Database Syst Rev 2013; 6: CD005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crisostomo EA, Duncan PW, Propst M, et al. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol 1988; 23: 94–97. [DOI] [PubMed] [Google Scholar]
- 23.Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trail. Lancet Neurol 2011; 10: 123–130. [DOI] [PubMed] [Google Scholar]
- 24.FOCUS Trial Collaboration. Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet 2019; 393: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahl AS, Omlor W, Rubio JC, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticoaspinal tract after stroke. Science 2014; 344: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 26.Rust R, Grönnert L, Gantner C, et al. Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc Natl Acad Sci USA 2019; 116: 14270–14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZG, Chopp M.Promoting brain remodeling to aid in stroke recovery. Trends Mol Med 2015; 21: 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henninger N, Mayasi Y.Nucleic acid therapies for ischemic stroke. Neurotherapeutics 2019; 16: 299–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez AM, Kang J.Regeneration enhancers: starting a journey to unravel regulatory events in tissue regeneration. Semin Cell Dev Biol 2020; 97: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolan F, Louca I, Heal C, et al. The potential of biomaterial-based approaches as therapies for ischemic stroke: a systematic review and meta-analysis of pre-clinial studies. Front Neurol 2019; 10: 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt A, Wellmann J, Schilling M, et al. Meta-analysis of the efficacy of different training strategies in animal models of ischemic stroke. Stroke 2014; 45: 239–247. [DOI] [PubMed] [Google Scholar]
- 32.Kwaggel G, Veerbeek JM, van Wegen EE, et al. Constraint-induced movement therapy after stroke. Lancet Neurol 2015; 14: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livingston-Thomas J, Nelson P, Karthikeyan S, et al. Exercise and environmental enrichment as enablers of task-specific neuroplasticity and stroke recovery. Neurotherapeutics 2016; 13: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friehs GM, Zerris VA, Qjakangas CL, et al. Brain-machine and brain-computer interfaces. Stroke 2004; 35: 2702–2705. [DOI] [PubMed] [Google Scholar]
- 35.Stefaniak JD, Halai AD, Ralph MAL.The neural and neurocomputational bases of recovery from post-stroke aphasia. Nat Rev Neuro 2020; 16: 43–55. [DOI] [PubMed] [Google Scholar]
- 36.Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014; 10: 597–608. [DOI] [PubMed] [Google Scholar]
- 37.Scheidtmann K, Fries W, Müller F, et al. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 2001; 358: 787–790. [DOI] [PubMed] [Google Scholar]
- 38.Rosser N, Heuschmann P, Wersching H, et al. Levodopa improves procedural motor learning in chronic stroke patients. Arch Phys Med Rehabil 2008; 89: 1633–1641. [DOI] [PubMed] [Google Scholar]
- 39.Ruscher K, Kuric E, Wieloch T.Levodopa treatment improves functional recovery after experimental stroke. Stroke 2012; 43: 507–513. [DOI] [PubMed] [Google Scholar]
- 40.Häggman Henrikson J, Pombo Antunes AR, Wieloch T, et al. Enhanced functional recovery by levodopa is associated with decreased levels of synaptogyrin following stroke in aged mice. Brain Res Bull 2020; 155: 61–66. [DOI] [PubMed] [Google Scholar]
- 41.Ford GA, Bhakta BB, Cozens A, et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2019; 18: 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadopoulos CM, Tsai S-Y, Guillen V, et al. Motor recovery and axonal plasticity with short-term amphetamine after stroke. Stroke 2009; 40: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbay S, Zoubina EV, Dancause N, et al. A single injection of D-amphetamine facilitates improvements in motor training following a focal cortical infarct in squirrel monkeys. Neurorehabil Neural Repair 2006; 20: 455–458. [DOI] [PubMed] [Google Scholar]
- 44.Martinsson L, Hårdemark H, Eksborg S.Amphetamines for improving recovery after stroke. Cochrane Database Syst Rev 2007; 1: CD002090. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein LB, Lennihan L, Rabadi MJ, et al. Effect of dextroamphetamine on poststroke motor recovery: a randomized clinical trial. JAMA Neurol 2018; 75: 1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Windle V, Corbett D.Fluoxetine and recovery of motor function after focal ischemia in rats. Brain Res 2005; 1044: 25–32. [DOI] [PubMed] [Google Scholar]
- 47.Zhao CS, Puurunen K, Schallert T, et al. Behavioral and histological effects of chronic antipsychotic and antidepressant drug treatment in aged rats with focal ischemic brain injury. Behav Brain Res 2005; 158: 211–220. [DOI] [PubMed] [Google Scholar]
- 48.Corbett AM, Sieber S, Wyatt N, et al. Increasing neurogenesis with fluoxetine, simvastatin and ascorbic acid leads to functional recovery in ischemic stroke. Recent Pat Drug Deliv Formul 2015; 9: 158–166. [DOI] [PubMed] [Google Scholar]
- 49.Li WL, Cai HH, Wang B, et al. Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J Neurosci Res 2009; 87: 112–122. [DOI] [PubMed] [Google Scholar]
- 50.Vahid-Ansari F, Albert PR.Chronic fluoxetine induces activity changes in recovery from poststroke anxiety, depression, and cognitive impairment. Neurotherapeutics 2018; 15: 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng KL, Gibson EM, Hubbard R, et al. Fluoxetine maintains a state of heightened responsiveness to motor training early after stroke in a mouse model. Stroke 2015; 46: 2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.EFFECTS Trial Collaboration. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2020; 19: 661–669. [DOI] [PubMed] [Google Scholar]
- 53.AFFINITY Trial Collaboration. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2020; 19: 651–660. [DOI] [PubMed] [Google Scholar]
- 54.Legg LA, Tilney R, Hsieh CF, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev 2019; 11: CD009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 2009; 40: e647–e656. [DOI] [PubMed] [Google Scholar]
- 56.Ringelstein EB, Thijs V, Norrving B, et al. Granulocyte colony-stimulating factor in patients with acute ischemic stroke: results of the AX200 for Ischemic Stroke trial. Stroke 2013; 44: 2681–2687. [DOI] [PubMed] [Google Scholar]
- 57.Floel A, Warnicke T, Duning T, et al. Granulocyte-colony stimulating factor (G-CSF) in stroke patients with concomitant vascular disease – a randomized controlled trial. PLoS One 2011; 6: e19767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sprigg N, ÓConnor R, Woodhouse L, et al. Granulocyte colony stimulating factor and physiotherapy after stroke: results of a feasibility randomised controlled trial: Stem Cell Trial of Recovery EnhanceMent after Stroke-3 (STEMS-3 ISRCTN16714730). PLos One 2016; 11: e0161359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkat P, Chen J, Chopp M.Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow Metab 2018; 38: 2165–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunningham CJ, Redondo-Castro E, Allan SM.The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab 2018; 38: 1276–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pollock A, Baer G, Campbell P, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev 2014; 4: CD001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. J Am Med Assoc 2006; 296: 2095–2104. [DOI] [PubMed] [Google Scholar]
- 63.Wolf SL, Winstein CJ, Miller JP, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol 2008; 7: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Müller HD, Hanumanthiah KM, Diederich K, et al. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke 2008; 39: 1012–1021. [DOI] [PubMed] [Google Scholar]
- 65.Schäbitz W-R, Berger C, Kollmar R, et al. Effect of BDNF treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke 2004; 35: 992–997. [DOI] [PubMed] [Google Scholar]
- 66.Bland ST, Pillai RN, Aronowski J, et al. Early overuse and disuse of the affected forelimb after moderately severe intraluminal suture occlusion of the middle cerebral artery in rats. Behav Brain Res 2001; 126: 33–41. [DOI] [PubMed] [Google Scholar]
- 67.Bland TS, Schallert T, Strong R, et al. Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats: functional and anatomic outcome. Stroke 2000; 31: 1144–1152. [DOI] [PubMed] [Google Scholar]
- 68.Dromerick AW, Lang CE, Birkenmeier RL, et al. Early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology 2009; 73: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ballester BR, Maier M, Duff A, et al. A critical time window for recovery extends beyond one-year post-stroke. J Neurophysiol 2019; 122: 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cassidy JM, Cramer SC.Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res 2017; 8: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation rountable taskforce. Int J Stroke 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
- 72.Boyd LA, Hayward KS, Ward NS, et al. Biomarkers of stroke recovery: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair 2017; 31: 864–876. [DOI] [PubMed] [Google Scholar]
- 73.Lai Y-J, Hanneman SK, Casarez RL, et al. Blood biomarkers for physical recovery in ischemic stroke: a systematic review. Am J Transl Res 2019; 11: 4603–4613. [PMC free article] [PubMed] [Google Scholar]
- 74.Schweighofer N, Han CE, Wolf SL, et al. A functional threshold for long-term use of hand and arm function can be determined: predictions from a computational model and supporting data from the Extremity Constraint-Induced Therapy Evaluation (EXCITE) Trial. Phys Ther 2009; 89: 1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diederich K, Quennet V, Bauer H, et al. Successful regeneration after experimental stroke by granulocyte-colony stimulating factor is not further enhanced by constraint-induced movement therapy either in concurrent or in sequential combination therapy. Stroke 2012; 43: 185–192. [DOI] [PubMed] [Google Scholar]
- 76.Sommer CJ.Ischemic stroke: experimental models and reality. Acta Neuropathol 2017; 133: 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsintou M, Dalamagkas K, Makris N.Taking central nervous system regenerative therapies to the clinic: curing rodents versus nonhuman primates versus humans. Neural Regen Res 2020; 15: 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cook DJ, Tymianski M.Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics 2012; 9: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stinear CM, Land CE, Zeiler S, et al. Advances and challenges in stroke rehabilitation. Lancet Neurol 2020; 19: 348–360. [DOI] [PubMed] [Google Scholar]
- 80.Wahl A-S.State-of-the-art techniques to causally link neural plasticity to functional recovery in experimental stroke research. Neural Plast 2018; 2018: 3846593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crofts A, Kelly ME, Gibson CL.Imaging functional recovery following ischemic stroke: clinical and preclinial fMRI studies. J Neuroimaging 2020; 30: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balkaya M, Kröber J, Gertz K, et al. Characterization of long-term funtional outcome in a murine model of mild brain ischemia. J Neurosci Meth 2013; 213: 179–187. [DOI] [PubMed] [Google Scholar]
- 83.Li X, Blizzard KK, Zeng Z.Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol 2004; 187: 94–104. [DOI] [PubMed] [Google Scholar]
- 84.Balkaya M, Kröber JM, Rex A, et al. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab 2013; 33: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trueman RC, Diaz C, Farr TD, et al. Systematic and detailed analysis of behavioural tests in the rat middle cerebral artery occlusion model of stroke: tests for long-term assessment. J Cereb Blood Flow Metab 2017; 37: 1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kunze A, Ziertath D, Drogomiretskiy O, et al. Variation in behavioral deficits and patterns of recovery after stroke among different rat strains. Transl Stroke Res 2014; 5: 569–576. [DOI] [PubMed] [Google Scholar]
- 87.Dirnagl U, Fisher M.International, multicenter randomized preclinical trials in translational stroke research: it's time to act. J Cereb Blood Flow Metab 2012; 32: 933–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allen C, Mehler DMA.Open science challenges, benefits and tips in early carrer and beyond. PLoS Biol 2019; 17: e3000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roof RL, Schielke GP, Ren X, et al. A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rat. Stroke 2001; 32: 2648–2657. [DOI] [PubMed] [Google Scholar]
- 90.Kozlowski DA, James DC, Schallert T.Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci 1996; 16: 4776–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chu CJ, Jones TA.Experience-dependent structural plasticity in cortex heterotopic to focal sensorimotor cortical damage. Exp Neurol 2000; 166: 403–414. [DOI] [PubMed] [Google Scholar]
- 92.Shanina EV, Redecker C, Reinecke S, et al. Long-term effects of sequential cortical infarcts on scar size, brain volume and cognitive function. Behav Brain Res 2005; 158: 69–77. [DOI] [PubMed] [Google Scholar]
- 93.Telzer EH, McCormick EM, Peters S, et al. Methodological considerations for developmental longitudinal fMRI research. Dev Cogn Neurosci 2018; 33: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Srinivasan VJ, Mandeville ET, Can A, et al. Multiparametric, longitudinal optical coherence tomography imaging reveals acute injury and chronic recovery in experimental ischemic stroke. PLoS One 2013; 8: e71478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang S, Liu K, Ding H, et al. Longitudinal in vivo intrinsic optical imaging of cortical blood perfusion and tissue damage in focal photothrombosis stroke model. J Cereb Blood Flow Metab 2019; 39: 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramirez-Garcia G, Harrison KA, Fernandez-Ruiz J, et al. Stroke longitudinal volumetric measures correlate with the behavioral score in non-human primates. Neuroscience 2019; 397: 41–55. [DOI] [PubMed] [Google Scholar]
- 97.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MacIsaac RL, Khatri P, Bendszus M, et al. A collaborative sequential meta-analysis of individual patient data from randomized trials of endovascular therapy and tPA vs. tPA alone for acute ischemic stroke: ThRombEctomy And tPA (TREAT) analysis: statistical analysis plan for a sequential meta-analysis performed within the VISTA-Endovascular collaboration. Int J Stroke 2015; 100: 136–144. [DOI] [PubMed] [Google Scholar]
- 99.Levin MF, Kleim JA, Wolf SL.What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehab Neural Repair 2009; 23: 313–319. [DOI] [PubMed] [Google Scholar]
- 100.Balkaya M, Cho S.Optimizing functional outcome endpoints for stroke recovery studies. J Cereb Blood Flow Metab 2019; 39: 2323–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Braun RG, Andrews EM, Kartje GL.Kinematic analysis of motor recovery with human adult bone marrow-derived somatic cell therapy in a rat model of stroke. Neurorehab Neural Rep 2012; 26: 898–906. [DOI] [PubMed] [Google Scholar]
- 102.Lai S, Panarese A, Spalletti C, et al. Quantitative kinematic characterization of reaching impairements in mice after stroke. Neurorehab Neural Repair 2015; 29: 382–392. [DOI] [PubMed] [Google Scholar]
- 103.Qin L, Jing D, Parauda S, et al. An adaptive role for BDNF Val66Met polymorphism in motor recovery in chronic stroke. J Neurosci 2014; 34: 2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baird AL, Meldrum A, Dunnett SB.The staircase test of skilled reaching in mice. Brain Res Bull 2001; 54: 243–250. [DOI] [PubMed] [Google Scholar]
- 105.Mering S, Jolkkonen J.Proper housing conditions in experimental stroke studies – special emphasis on environmental enrichment. Front Neurosci 2015; 9: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen R, Cohen LG, Hallett M.Nervous system reorganization following injury. Neuroscience 2002; 4: 761–773. [DOI] [PubMed] [Google Scholar]
- 107.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 2008; 22: 64–71. [DOI] [PubMed] [Google Scholar]
- 108.Krxakauer JW, Marshall RS.The proportional recovery rule for stroke revisited. Ann Neurol 2015; 78: 845–847. [DOI] [PubMed] [Google Scholar]
- 109.Kundert R, Goldsmith J, Veerbeek JM, et al. What the proportional recovery rule is (an is not): methodological and statistical considerations. Neurorehabil Neural Repair 2019; 33: 876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zarahn E, Alon L, Ryan SL, et al. Prediction of motor recovery using initial impairment and fMRI 48 h poststroke. Cereb Cortex 2011; 21: 2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jeffers MS, Karthikeyan S, Corbett D.Does stroke rehabilitation really matter? Part A: proportional stroke recovery in the rat. Neurorehabil Neural Repair 2018; 32: 3–6. [DOI] [PubMed] [Google Scholar]
- 112.Jeffers MS, Karhikeyan S, Gomez-Smith M, et al. Does stroke rehabilitation really matter? Part B: an algorithm for prescribing an effective intensity of rehabilitation. Neurorehabil Neural Repair 2018; 32: 73–83. [DOI] [PubMed] [Google Scholar]
- 113.Zeiler SR, Hubbard R, Gibson EM, et al. Paradoxical motor recovery from a first stroke after induction of a second stroke: reopening a postischemic sensitive period. Neurorehabil Neural Repair 2016; 30: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi L, Rocha M, Leak RK, et al. A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab 2018; 38: 2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buga AM, Di Napoli M, Popa-Wagner A.Preclinical models of stroke in aged animals with or without comorbidities: role of neuroinflammation. Biogerontology 2013; 14: 651–656. [DOI] [PubMed] [Google Scholar]
- 116.Suenaga J, Hu X, Pu H, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 2015; 272: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang L, Mu H, Xu F, et al. Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery. J Cereb Blood Flow Metab. Epub ahead of print 16 February 2020: DOI:10.1177/0271678X20902542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spychala MS, Venna VR, Jandzinski M, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol 2018; 84: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang X, Suenaga J, Pu H, et al. Post-stroke administration of omega-3 polyunsaturated fatty acids promotes neurovascular restoration after ischemic stroke in mice: efficacy declines with aging. Neurobiol Dis 2019; 126: 62–75. [DOI] [PubMed] [Google Scholar]
- 120.Yamaguchi S, Horie N, Satoh K, et al. Age of donor of human mesenchymal stem cells affects structural and functional recovery after cell therapy following ischaemic stroke. J Cereb Blood Flow Metab 2018; 38: 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ritzel RM, Lai Y-L, Crapser JD, et al. Aging alters the immunological response to ischemic stroke. Acta Neuropathol 2018; 136: 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Popa-Wagner A, Petcu EB, Capitanescu B, et al. Ageing as a risk factor for cerebral ischemia: underlying mechanisms and therapy in animal models and in the clinic. Mech Ageing Dev 2020; 190: 111312. [DOI] [PubMed] [Google Scholar]
- 123.Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med 2016; 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haley MJ, White CS, Roberts D, et al. Stroke induces prolonged changes in lipid metabolism, the liver and body composition in mice. Transl Stroke Res 2020; 11: 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barrero MJ, Izpisua Belmonte JC.Regenerating the epigenome. EMBO Rep 2011; 12: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008; 372: 216–223. [DOI] [PubMed] [Google Scholar]
- 127.Bernhardt J, Hayward KS, Dancause N, et al. A stroke recovery trial development framework: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Int J Stroke 2019; 14: 792–802. [DOI] [PubMed] [Google Scholar]
- 128.Schäbitz WR, Laage R, Vogt G, et al. AXIS: a trial of intravenous granulocyte colony-stimulating factor in acute ischemic stroke. Stroke 2010; 41: 2545–2551. [DOI] [PubMed]